Abstract
The recent outbreak of the betacoronavirus SARS-CoV-2 has become a significant concern to public health care worldwide. As of August 19, 2020, more than 22,140,472 people are infected, and over 781,135 people have died due to this deadly virus. In the USA alone, over 5,482,602 people are currently infected, and more than 171,823 people have died. SARS-CoV-2 has shown a higher infectivity rate and a more extended incubation period as compared to previous coronaviruses. SARS-CoV-2 binds much more strongly than SARS-CoV to the same host receptor, angiotensin-converting enzyme 2 (ACE2). Previously, several methods to develop a vaccine against SARS-CoV or MERS-CoV have been tried with limited success. Since SARS-CoV-2 uses the spike (S) protein for entry to the host cell, it is one of the most preferred targets for making vaccines or therapeutics against SARS-CoV-2. In this review, we have summarised the characteristics of the S protein, as well as the different approaches being used for the development of vaccines and/or therapeutics based on the S protein.
Keywords: COVID-19, Therapeutic, SARS-CoV-2, Vaccine, Spike protein
1. Introduction
In December 2019, Wuhan in the Hubei province of China became the center of an outbreak of pneumonia of unknown cause, which raised intense attention not only within China but internationally. Later, it was confirmed that the causative agent for this pneumonia-like disease is a coronavirus (CoV) belonging to the family Coronaviridae (Jiang et al., 2020a; C. C. Wang et al., 2020; Q. Wang et al., 2020; F. F. Wu et al., 2020; Y. Wu et al., 2020; Zhou et al., 2020). On February 11, 2020, the World Health Organization named the virus SARS-CoV-2 and renamed the syndrome from 2019-nCoV to COVID-19, short for coronavirus disease 2019 (“WHO. Novel Coronavirus(2019-nCoV) Situation Report – 22. February 11, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20,200,211-sitrep-22-ncov.pdf. Accessed May27, 2020.,” n.d.). COVID-19, a respiratory disease, has led to more than 22,140,472 confirmed cases and over 781,135 deaths globally as of August 19, 2020. In the USA alone, over 5,482,602 people are currently infected with SARS-CoV-2, which has caused more than 171,823 deaths (“Johns Hopkins University: https://coronavirus.jhu.edu/map.html. Accessed on August 19, 2020.,” n.d.)
The first coronavirus pandemic of the 21 st century occurred in November 2002, when an infectious agent caused outbreaks of atypical pneumonia in Guangdong Province, southern China, and Southeast Asia, North America, and Europe. By the end of February 2003, the virus had spread to neighboring regions and countries. The disease caused by the virus was named severe acute respiratory syndrome (SARS), for which a global alert was issued by WHO on March 13, 2003 (Lee et al., 2003; Tsang et al., 2003; Zhong et al., 2003). SARS caused a global pandemic in 2002–2003, with approximately 10 % (774/8098) case fatality rate (CFR) ((Jiang et al., 2020a; Lee et al., 2003). The SARS-causing coronavirus SARS-CoV has not circulated in humans since 2004.
Another coronavirus, named MERS-CoV after the disease it causes (Middle East Respiratory Syndrome), was first reported in Saudi Arabia in 2012 and has continued to infect humans with restricted human-to-human transmission. WHO has reported a CFR of approximately 34.4 % (858/2494) in 27 countries for MERS-CoV (“WHO. Middle East respiratory syndrome coronavirus (MERS-CoV). August 2, 2019. https://www.who.int/emergencies/mers-cov/en/. Accessed on May 27, 2020.,” n.d.).
The coronavirus was first identified as a cause of the common cold in 1960 (Al-Osail and Al-Wazzah, 2017). Coronaviruses belong to the subfamily Coronavirinae in the family Coronaviridae and the order Nidovirales (International Committee on Taxonomy of Viruses). Based on phylogenetic and genome structure, this subfamily is further divided into four genera Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus (de Wilde et al., 2018). Alphacoronaviruses and betacoronaviruses usually cause respiratory illness in humans and gastroenteritis in animals. These two families include seven strains of human CoVs. The four viruses HCoV-NL63, HCoV-229E, HCoV−OC43, and HKU1 induce only mild upper respiratory diseases in immunocompetent hosts; however, some of them may cause severe complications in infants, young children and older people (Cui et al., 2019; Su et al., 2016). The other three highly pathogenic viruses, SARS-CoV, MERS-CoV, and SARS-CoV-2, cause severe respiratory syndrome, pneumonia, bronchiolitis, rhinitis, pharyngitis, sinusitis, and other systemic symptoms such as occasional watery diarrhea in humans of all ages. Based on current sequence databases, all human coronaviruses have animal origins. Five of them, SARS-CoV-2, SARS-CoV, MERS-CoV, HCoV-NL63, and HCoV-229E, originated from a bat, and the remaining two, HCoV−OC43 and HKU1, originated in rodents(Paules et al., 2020; Su et al., 2016).
The coronavirus genome is a single-stranded RNA of about 30 Kb, the largest among the RNA viruses (Masters, 2006). Coronaviruses usually infect their hosts in a species‐specific manner, resulting in acute or persistent infections. The RNA genome contains a 5′ cap and 3′ poly-A tail, making it suitable for direct translation into a large size polyprotein. Structural protein occupies 10 kb of the genome, whereas non-structural protein occupies two-third of the genome. The virus has a spherical shape with a diameter of approximately 125 nm. The most striking feature of the coronavirus is the club-shaped spike protein projecting from the virion surface. These spike proteins serve as attachment proteins for the virus to enter host cells (Fehr and Perlman, 2015; Neuman et al., 2006).
The viral genome of the coronavirus encodes four structural proteins named spike (S), envelope (E), membrane (M), and nucleocapsid (N), 16 non-structural proteins (nsp1–16), and 8 accessory proteins (Jiang et al., 2020b; Siu et al., 2008). The S protein plays the most critical role in viral attachment, fusion, and entry into the target cell (D. E. C.J. Gordon et al., 2020;D.E. Gordon et al., 2020; Jiang et al., 2020b, 2020b)). It is a trimeric class I fusion protein that exists in a metastable prefusion conformation following translation. A substantial structural rearrangement of the S protein is required to fuse the viral membrane with the host cell membrane (Bosch et al., 2003; Hulswit et al., 2016). The size of the densely glycosylated S protein ranges between CoV species from approximately 1100–1600 amino acids length, with an estimated molecular mass of up to 220 kDa (Hulswit et al., 2016; Li, 2016). CoV particles are decorated with the club-shaped trimers of the S protein, which is 8–23-nm long (Hofmann et al., 2004). The S protein not only determines the host tropism but also is the crucial target for neutralizing antibodies produced by the immune system of the infected host (Hofmann et al., 2004; Walls et al., 2020).
The S protein is divided into S1 and S2 subunits. The S1 subunit contains two domains termed the N-terminal domain (NTD) and the C-terminal domain (CTD). The receptor-binding domain (RBD) is in the CTD. The S2 subunit contains the necessary elements required for membrane fusion, including an internal membrane fusion peptide (FP), two 7-peptide repeats (HR), a membrane-proximal external region (MPER), and a transmembrane domain (TM) (Li, 2016). The S1 and S2 subunits are separated by a cleavage site that is recognized by host proteases and undergoes proteolytic cleavage (Hoffmann et al., 2020; Li et al., 2003; Wrapp et al., 2020b). The S protein is primed by cellular protease TMPRSS2 (Glowacka et al., 2011; Matsuyama et al., 2010; Meng et al., 2020; Shulla et al., 2011), which cleaves the S protein at the S1/S2 and S2′ sites, leading to conformational changes of the S2 unit to allow fusion between the virus and host cell membranes to release viral RNA into the cytoplasm (Hoffmann et al., 2020; Meng et al., 2020). The S protein is also an excellent target for the development of therapeutic entry inhibitors, vaccines, and therapeutic antibodies (Amanat and Krammer, 2020; “An updated guide to the coronavirus drugs and vaccines in development,” 2020; Du et al., 2009a; Kim et al., 2020; Zhao et al., 2018).
The S proteins of different coronaviruses are so unique that some phylogenetically similar spike proteins bind to different receptors. In contrast, some phylogenetically different spike proteins can bind to a similar receptor (Li, 2015). MERS-CoV requires dipeptidyl peptidase 4 (DPP4) as its receptor (Li et al., 2003; Raj et al., 2013). Murine hepatitis virus (MHV), a β-coronavirus, uses carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) for entry (Dveksler et al., 1991; Williams et al., 1991). A few coronaviruses, including bovine coronavirus, recognize sugar for attachment and entry (Schultze et al., 1991). Some CoVs, including porcine respiratory coronavirus (PRCV), require recognition of aminopeptidase N (APN) (Godet et al., 1994), whereas transmissible gastroenteritis virus (TGEV) requires recognition of APN in addition to sugar for virus entry (Delmas et al., 1992; Krempl et al., 1997). The human angiotensin-converting enzyme 2 (ACE2) is the common receptor for SARS-CoV, SARS-CoV-2, and NL63, although the NL63 RBD has a structure significantly different from those of SARS-CoV and CoV-2 (Hofmann et al., 2005, p. 63; Letko et al., 2020; Li et al., 2003, p. 2; Lin et al., 2008; Tai et al., 2020). It has been shown that the receptor-binding domain of SARS-CoV-2 binds more strongly to the ACE2 receptor than that of SARS-CoV, making it a more suitable target for the development of virus attachment inhibitors, neutralizing antibodies, and vaccines (Tai et al., 2020; Q. C. Wang et al., 2020; Q. Wang et al., 2020). Strong binding of SARS-CoV-2 RBD to the ACE2 receptor is also a key reason for the higher infection rate in SARS-CoV-2 as compared to SARS-CoV and MERS-CoV ((Tai et al., 2020; Wrapp et al., 2020a) However, a recent paper by Shang and colleagues suggests that the full-length spike protein of SARS-CoV-2 has similar or lower affinity to hACE2 than SARS-CoV. This suggests that SARS-CoV-2 RBD is less exposed than SARS-CoV-RBD, and it is also less dependent on target cell protease since it is preactivated by proprotein convertase furin (Shang et al., 2020).
2. SARS-CoV-2 and the S protein as a potential vaccine target
The recent outbreak of SARS-CoV-2 is much more challenging to control because of its long incubation period. Recent studies have shown that the incubation time could range from 2 to 14 days with the median incubation period of 6.4 days (Backer et al., 2020; Lauer et al., 2020; Linton et al., 2020). It is estimated that only around 101 of every 10,000 people who contract SARS-CoV-2 are likely to develop symptoms after 14 days (Lauer et al., 2020). Therefore, most regulatory agencies suggest quarantine for at least 14 days. Some virus-carrying individuals do not develop any disease symptoms, but they can transmit the virus. One investigation in China suggested that the R0 of SARS-CoV-2 was as high as 4.7–6.6 before any infection control measures were applied (Sanche et al., 2020), suggesting that SARS-CoV-2 is highly contagious and more infectious than previously estimated. These findings are also supported by the fact that the SARS-CoV-2-RBD demonstrates 10–20 fold higher affinity to the human ACE2 receptor than SARS-CoV (Tai et al., 2020; Wrapp et al., 2020a).
To date, there is no approved drug for treatment of COVID-19, but an experimental antiviral drug with a known safety profile is currently being used for treatment of COVID-19 patients. It has been shown that the nucleotide analog Remdesivir can inhibit SARS-CoV and MERS-CoV in tissue culture and nonhuman animal models (Martinez, 2020). A clinical trial of Remdesivir is underway for treatment of Ebola virus infection. Recently clinical trials for Remdesivir have been started for SARS-CoV-2 as well (Cao et al., 2020; George Sakoulas, 2020; C. J. C.J. Gordon et al., 2020; D.E. Gordon et al., 2020). Despite much research and effort, there is no vaccine available for SARS-CoV-2 or any other coronaviruses. The diversity of the S-protein also renders any vaccines and nAbs unlikely to be cross-protective between existing and emerging CoVs.
3. Structure of the SARS-CoV-2 spike protein RBD bound with ACE2
The role of the S protein in binding with host receptors makes it a perfect target for vaccine and antiviral therapeutic development. Previous studies on SARS-CoV and MESR-CoV have revealed that vaccines based on the S protein can induce antibodies to block virus binding and fusion or neutralize virus infection ((Du et al., 2009a). Since the S protein plays a role in both virus entry and viral RNA release into the cytoplasm, a vaccine against the S protein should induce antibodies to block viral entry (Fig. 1 ). Previous reports suggest that immunization of mice with a trimeric form of MERS-CoV S protein RBD domain elicits potent neutralizing antibodies against MERS CoV (Coleman et al., 2017; Du et al., 2013; Mou et al., 2013; Tai et al., 2016);. Other studies show that immunization of rabbits with an RBD-Fc fusion protein elicits robust antibody responses, and the antisera can completely block SARS-CoV infection at different serum dilution (Chen et al., 2014; Coleman et al., 2014; Du et al., 2009b; He et al., 2004). Another study showed that immunization of mice with the S1 subunit of MERS-CoV actively induces neutralizing antibody production. Using the same strategy, the S1 subunit of SARS-CoV-2 can also induce S1-specific IgG in mice. However, further studies are required to verify the potency of the antibodies (Kim et al., 2020). Also, development of a vaccine targeting the S protein is always challenging, as a vaccine based on neutralizing antibodies may lead to antibody-dependent enhancement of secondary virus infection and vaccine-enhanced disease.
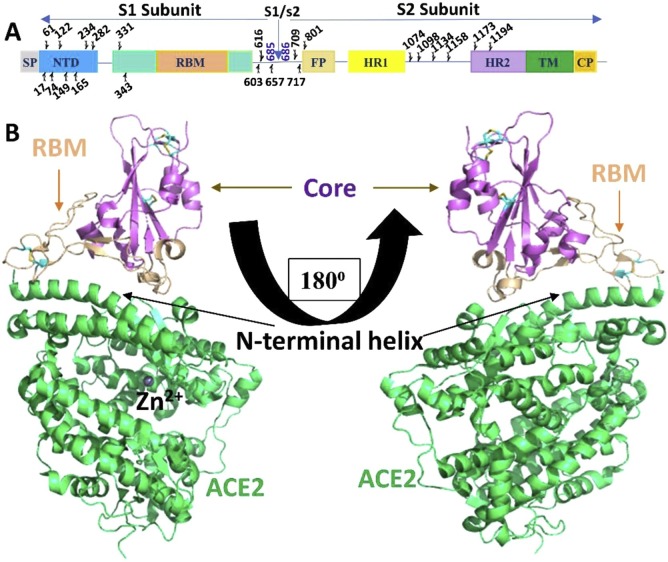
Fig. 1.
Structure of SARS-CoV-2 Spike protein RBD bound with ACE2A. Schematic representation of SARS-CoV-2 Spike protein. Linear representation of the SARS-CoV-2 spike monomer. Its S1 subunit contains the N-terminal domain (NTD; 14–305 aa), receptor binding domain (RBD; 319–541 aa), and receptor binding motif (RBM; 437–508 aa). Its S2 subunit contains fusion peptide (FP; 788–806 aa), heptad repeat 1 (HR1;12–984 aa), heptad repeat 2 (HR2;1163–1213 aa), transmembrane domain (TM;1214–1237 aa), and cytoplasmic domain (CP; 1238–1273). The predicted glycosylation sites are indicated above the domain bars. B. Overall structure of the SARS-CoV-2 RBD bound to ACE2. ACE2 is shown in lime green. The SARS-CoV-2 RBD core is shown in purple and RBM in tint colour. The N-terminal helix of ACE2 responsible for binding is labelled (Lan et al., 2020).
4. Antibody-dependent enhancement
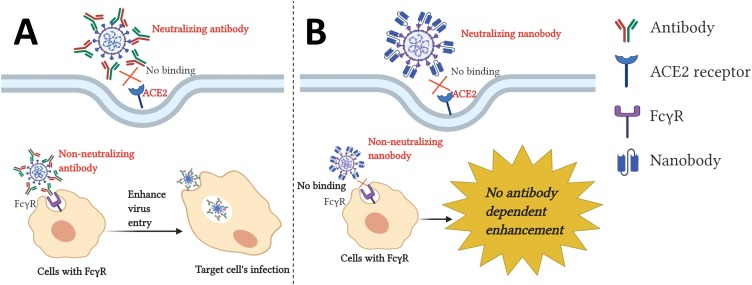
It has been observed that COVID-19 pandemic intensity is different among regions. A country or region suffering the most may have been primed by exposure to one or more coronaviruses and encountered the effects of antibody-dependent enhancement (ADE) due to antigenic epitope heterogeneity (Tetro, 2020). ADE is a phenomenon in which pre-existing non- or poorly neutralizing antibodies enhance the viral entry and replication in the host cell. ADE is more common in flaviviruses, especially in the dengue virus, which has four serotypes. It has been shown that when patients are infected with dengue virus for first time, specific neutralizing antibodies are produced. However, if they are later infected with a different serotype of dengue virus, the pre-existing antibodies from the previous infection do not have enough potency to neutralize the new virus, even though they can bind with the virus. These partially neutralizing antibodies help the virus to enter cells via an Fc-receptor (FcR)-mediated mechanism in addition to their natural entry route (Fig. 2 A). The antibody-bound viruses bind via the antibody Fc-domain to FcR expressed on the surface of several immune cells, leading to enhancement of the virus entry and replication (Katzelnick et al., 2017; Khandia et al., 2018). ADE has also been reported in other viruses like HIV, Ebola, HCV, and Zika ((Gorlani and Forthal, 2013; Meyer et al., 2008; Takada et al., 2001; Willey et al., 2011). Vaccine-induced enhancement was first reported during the vaccine trial of RSV in 1969. In this trial, the administration of the formalin-inactivated vaccine RSV (FI-RSV) to naïve infants resulted in a much higher incidence rate of severe illness. It led to increased hospitalization in 80 % among vaccinated infants, compared to only 5% of the non-immunized infants (Kim et al., 1969). Unfortunately, two vaccinated infants also died because of enhanced RSV infections (Kim et al., 1969).
Fig. 2.
Schematic representation of antibody-dependent enhancement and protection through nanobodies. A. Virus particles are detected by heterotypic antibodies from previous infection. This complex then binds to the Fcγ receptor on the surface of immune cells and is internalized. Further virus replication leads to an increased viral load. B. The nanobody lacks an Fc portion and is unable to bind to the Fcγ receptor, which protects the cell from antibody-dependent enhancement (created with BioRender.com).
ADE has also been reported in SARS-CoV and MERS-CoV. It has been shown that anti-spike sera, which can neutralize SARS-CoV pseudo viral particles, can initiate virus entry in cells that do not express the ACE2 receptor for SARS-CoV. ADE is based on the expression of FcR on cells (Jaume et al., 2011; Wang et al., 2014). Another report suggests that ADE occurs through FcR in macrophages, but there are no changes in gene profiling, and virus replication has been shown to be abortive (Yip et al., 2016). A recent study has shown that MERS-CoV RBD-specific MAb and the SARS-CoV RBD-specific MAb can mediate the entry of their respective coronaviruses into FcR-expressing human cells. This study also suggests that a fully neutralizing antibody can also mediate ADE by functionally mimicking viral receptors from host cells (Wan et al., 2019). It has also been shown that passive transfer of anti-S IgG or serum from previously infected animals caused more severe lung injury in animal models (Liu et al., 2019a,b).
There are huge theoretical concerns of vaccine-induced ADE which can occur in several scenarios. It is possible that vaccine-induced antibodies against SARS-CoV-2 may bind to the virus without neutralizing it. Currently mutations on the viral Spike protein have been found in circulating clinical SARS-CoV-2 strains (Korber et al., 2020; Lu et al., 2020). If this happen, the non-neutralizing antibodies could produce ADE effect and enhance the viral entry into cells (Garber, 2020; Iwasaki and Yang, 2020; Tetro, 2020; Ulrich et al., 2020). In addition, significant sequence variations between the spike proteins of SARS-CoV and SARS-CoV-2 may cause ADE (Tetro, 2020). Moreover, it is also possible that a future SARS-related (SARSr)-CoV causes ADE in patients vaccinated with SARS-CoV-2, if the future SARSr-CoV shows significant sequence variations from SARS-CoV-2. Furthermore, insufficient concentration of neutralizing Ab may also cause ADE (Renner et al., 2018; Wan et al., 2019). Given the fact that neutralizing antibodies declined rapidly in some of COVID-19 patients (Long et al., 2020; Seow et al., 2020), this latter scenario is more troublesome. Nevertheless, based on the knowledge from research on SARS-CoV, several vaccine trials are ongoing for SARS-CoV-2.
5. Use of nanobodies against coronaviruses
Soon after the discovery of the nanobody in the camelid family by C. Hamers-Casterman and colleagues in 1993 (Hamers-Casterman et al., 1993), it became a potent tool as a neutralizing antibody. Several nanobodies have been screened and validated for binding against different targets ranging from human melanoma cells to viruses. A nanobody against human melanoma cells targeting endothelin receptor type B has been recently identified, which will be helpful in the future to treat human melanoma (Ji et al., 2020). Besides, several nanobodies have been identified to block the ligand-receptor binding for primary immune cells. For example, nanobodies inhibiting PD1-PDL1 interaction, LIN28:let-7 interaction, and T cell marker CD3 are all promising candidates for targeting and activating cytotoxic T lymphocytes (CTLs) to induce anti-tumor responses. GRP78, CD38, and GPC3 expression on cancer cells have also been targeted for nanobody expression and purification, which provide useful cancer cell markers and potential therapeutic markers (Aghamollaei et al., 2020, p. 78; Hambach et al., 2020; Li et al., 2020; Moradi-Kalbolandi et al., 2020; L. L. Xia et al., 2020; S. Xia et al., 2020; C. J. Yu et al., 2020;C. Yu et al., 2020).
One drawback of use of nanobody is the absence of antibody-dependent cellular cytotoxicity (ADCC). Since nanobody lacks the Fc portion so it is unable to bind to immune cells to provide ADCC effect. It has been shown that non protective antibodies help in protection from HIV (Forthal et al., 2001; Haynes et al., 2012), herpes simplex virus (Kohl et al., 2000; Kohl and Loo, 1982, 1982), Ebola virus (Gunn et al., 2018; Saphire et al., 2018), and influenza virus (Greenberg et al., 1977; Jegaskanda et al., 2014), and SARS-CoV (Yasui et al., 2014) by mechanism of ADCC. Therefore, the use of nanobody has both benefits and drawbacks. Nanobody provides protection against ADE, however, it lacks ADCC. It is very important to balance the adverse effect of ADE and beneficial effect of ADCC. To this end, it is important to note that nanobody can be easily engineered to add different effector function such as Fc regions, toxins, liposomes, and other ligand binding scaffolds (Harmsen et al., 2005; Laursen et al., 2018; Schriewer et al., 2020). Recently, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have approved a bivalent nanobody, caplacizumab, for treatment of patients with thrombotic thrombocytopenic purpura (Hanlon and Metjian, 2020; Scully et al., 2019).
Several nanobodies have been reported to act as potential therapeutic agents against different viruses. Nanobodies against viruses work on the principle of inhibiting the ligand-receptor interaction (Fig. 2 A&B). A nanobody against HCV has been reported which blocks the E2-CD81 interaction and stops the virus-cell to cell transmission (Tarr et al., 2013). A replication-inhibiting nanobody against porcine reproductive and respiratory syndrome virus (PRRSV), which is a significant threat to the swine industry, has been identified and provides a novel antiviral drug for inhibition of PRRSV replication and controlling PRRSV disease (Duan et al., 2020; Wang et al., 2019). Another intracellular antibody directed towards human papillomavirus (HPV) oncoprotein E7 has shown great potential for therapy of HPV16-associated disease (Li et al., 2019). Nanobodies have also exhibited good antiviral effects against bovine viral diarrhea virus (Li et al., 2017).
Only limited studies have been performed on nanobodies against coronaviruses. A few groups investigated nanobody activity against the MERS-CoV S protein and successfully found nanobodies that could neutralize MERS-CoV in pseudo-viral particles and in a mouse model (Raj et al., 2018; Zhao et al., 2018). Recently, nanobodies against SARS-CoV have also been identified which have shown cross-neutralization activity, and therefore have excellent therapeutic potential against the SARS-CoV-2 virus (Wrapp et al., 2020a). Several other nanobodies have been identified recently that act against the SARS-CoV-2 receptor-binding domain (Chi et al., 2020; Walter et al., 2020; Y. F. Wu et al., 2020; Y. Wu et al., 2020).
6. Different vaccine strategies using the S protein against SARS-CoV-2
Protein-based subunit vaccines are probably one of the safest formats of vaccine, but immune responses generated with this kind of vaccine are heavily dependent on the adjuvant used. The S protein in different form including full-length S Protein, S1 RBD, RBD-Fc, and N-terminal, have shown nAb responses and protection in multiple animal models and nonhuman primates (Deng et al., 2018; Lan et al., 2015; Tse et al., 2020; Wang et al., 2017; Zhang et al., 2016).
7. DNA vaccines
DNA vaccines are composed of plasmid DNA molecules encoding one or more immunogens. A DNA vaccine is better than an mRNA vaccine in terms of the formulation required for its stability and ease of delivery. However, there is a risk that integration of the vaccine into the host genome may create mutations(Ledwith et al., 2000; Liu, 2019). Passive transfer of sera from mice immunized with DNA encoding the MERS-CoV S domain has been shown to protect naïve mice from MERS-CoV infection (Chi et al., 2017). Other research showed that the DNA encoding the S1 domain is superior to that with full length S protein in eliciting antibody and Th1/Th2 responses. Both DNAs encoding the S1 and S proteins were shown to induce cross-neutralizing Abs against multiple strains of MERS-CoV from human and camel origins (Al-amri et al., 2017). Another study showed that sera from mice immunized with S protein-encoding DNA detects SARS-CoV S protein (Zhao et al., 2004). Mice immunized with S protein-encoding DNA and booted with CD4+ and CD8 + T cell-specific peptides improves the expression of several cytokines (Huang et al., 2007). Another report shows that DNA encoding the S protein from SARS-CoV elicits neutralizing antibodies, as measured by a Pseudotyped lentiviral vector reporter neutralization assay and S protein-specific CD4+ and CD8 + T-cell responses in all immunized volunteer (Martin et al., 2008). Recently, Yu and colleagues reported that DNA vaccine encoding the SARS-CoV-2 S protein can protect rhesus macaques after challenge with SARS-CoV-2 (J. J. Yu et al., 2020; C. Yu et al., 2020). Currently, two SARS-CoV-2 DNA vaccines are under development. Inovio Pharmaceuticals developed a DNA vaccine NCT043364, which is undergoing Phase 1 clinical trial. Several other companies are involved in the development of DNA vaccines, which is mentioned S protein-based DNA vaccine (Table 1 ).
Table 1.
S protein-based DNA vaccines.
| Manufacturer | Strategy | Vaccine Name/Stage | Ref |
|---|---|---|---|
| Inovio Pharmaceuticals/International Vaccine Institute | DNA plasmid vaccine injected by intradermal (ID) injection followed by electroporation (EP) using a CELLECTRA® 2000 device in healthy adult volunteers | INO-4800/ Phase 1/2 NCT04336410 NCT04447781 |
(“Inovio Pharmaceuticals/International Vaccine Institute. http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-and-IVI-Partner-with-Seoul-National-University-Hospital-to-Start-Phase-12-Clinical-Trial-of-INOVIOs-COVID-19-DNA-Vaccine-INO-4800-in-South-Korea/default.aspx. Accessed July 29, 2020,” n.d.) |
| Osaka University/AnGes/Takara Bio | DNA plasmid vaccine + adjuvant | Phase 1/2 NCT04463472 |
(“Osaka University/AnGes/Takara Bio. https://www.takarabio.com/about/bioview-blog/current-events/dethroning-king-coronavirus-with-novel-vaccines. Accessed July 29, 2020,” n.d.) |
| Karolinska Institute / Cobra Biologics (OPENCORONA Project) | DNA vaccine delivered to patient’s muscle by electroporation to generate a viral antigen on which the immune system then reacts | Pre-Clinical | (“Cobra Biologics. Cobra Biologics and the Karolinska Institute collaborate to develop COVID-19 vaccine. 30th Mar 2020. https://www.cobrabio.com/News/March-2020/Cobra-Karolinska-Institutet-COVID-19-Vaccine. Accessed May 21, 2020,” n.d.) |
| Takis/Applied DNA Sciences/Evvivax | DNA vaccine candidates created using the structure of the S protein | Pre-Clinical | (“Takis. Takis, a biotech company in Castel Romano, Rome, announces that it is ready to test its Covid-19 vaccine on pre-clinical models. 17 March 2020. http://www.takisbiotech.it/index.php/en/news/209-rome-17-march-2020-takis-a-biotech-company-in-castel-romano-rome-announces-that-it-is-ready-to-test-its-covid-19-vaccine-on-pre-clinical-models. Accessed on May 21, 2020.,” n.d.) |
| Immunomic Therapeutics, Inc./EpiVax, Inc./PharmaJet, Inc. | Plasmid DNA delivered via PharmaJet’s Tropis Needle-free Injection System, which precisely targets delivery of vaccine to the intradermal tissue depth | Pre-Clinical | (“Pharmajet: Pharmajet partner with ITI and EpiVax to develop and deliver COVID-10 Vaccine. April 15, 2020. https://www.immunomix.com/pharmajet-partners-with-iti-and-epivax-to-develop-and-deliver-covid-19-vaccine/. Accessed May 21, 2020.,” n.d.) |
| Zydus Cadila | DNA plasmid vaccine against the major viral membrane protein responsible for the cell entry of the novel coronavirus | Phase 1/2 CTRI/2020/07/026,352 |
(“Zydus Cadila. Zydus Cadila accelerates COVID-19 vaccine research. 8 April 2020 https://www.pharmatutor.org/pharma-news/2020/zydus-cadila-accelerates-covid-19-vaccine-research. Accessed on May 21, 2020.,” n.d.) |
| Genexine Consortium | DNA VVaccine (GX-19) | Phase 1/2 NCT04445389 |
(“Genexine Consortium. https://pharmashots.com/press-releases/korean-biotech-firm-genexines-covid-19-deoxyribonucleic-acid-vaccine-pipeline-gx-19-is-ready-for-clinical-testing-as-soon-as-it-applies-to-the-drug-ministry-for-human-testing/. Accessed July 29, 2020,” n.d.) |
| BioNet Asia | GENE-based vaccine (COVIGEN) encoding the SARS-CoV-2 S protein | Pre-Clinical | (“BioNet. BioNet in Thailand is developing a COVID-19 GENE-based vaccine (COVIGEN) encoding the S (Spike) protein of SARS-CoV-2. http://www.bionet-asia.com/media/news-events/. Accessed on May 21, 2020.,” n.d.) |
| University of Waterloo | DNA vaccine using a bacteriophage-based approach allowing it to replicate within bacteria already present in the body, delivering the vaccine to cells in targeted tissues and triggering production of a virus-like particle (VLP) that will induce an immune response | Pre-Clinical | (“University of Waterloo. University of Waterloo developing DNA-based COVID-19 vaccine. April 16, 2020. https://uwaterloo.ca/stories/news/university-waterloo-developing-dna-based-covid-19-vaccine. Accessed May 21, 2020.,” n.d.) |
| Symvivo Corporation | bacTRL-Spike contains different concentrations of live Bifidobacterium longum, which has been engineered to deliver plasmids containing synthetic DNA encoding spike protein from SARS-CoV-2 | bacTRL-Spike/ Phase 1 (NCT04334980) |
(“Symvivo Corporation. Evaluating the Safety, Tolerability, and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19. April 6, 2020. https://clinicaltrials.gov/ct2/show/NCT04334980. Accessed May 21, 2020.,” n.d.) |
8. Viral vector-based vaccines
Viral vector-based vaccines depend on the delivery of one or more antigens encoded by a modified viral vector. This technique has several advantages over other techniques in use. This technology uses replicating or non-replicating vectors. Upon delivery, antigens are expressed in the host cells, and host cells generate both humoral and cell-based immune responses against the targeted pathogen (Bouard et al., 2009; Rauch et al., 2018). Viral vectors that can tolerate a large fragment insertion in their genome can be manipulated to encode any antigen of choice. Several viral vectors have been used recently in the generation of vaccine candidates against SARS-CoV. Also, a rabies virus-based vaccine expressing the SARS-CoV S protein has been found to induce the production of high levels of SARS-CoV-neutralizing antibodies in mice in a single vaccination (Faber et al., 2005).
A live attenuated Modified Vaccinia Ankara (MVA) vector encoding a full-length S glycoprotein has been shown to generate a potent neutralizing antibody in mice, rabbit, and monkey (Chen et al., 2005). Other studies show that immunization of mice with recombinant adenovirus containing the S protein induces both neutralizing antibodies in serum as well as CD8 + T cells in lungs (Du et al., 2006, p.; Kobinger et al., 2007; Liu et al., 2005; Shim et al., 2012). Currently, different companies are involved in making replicating and non-replicating viral vector-based vaccines (Table 2, Table 3 , respectively). The University of Oxford’s non-replicating adenovirus-based ChAdOx1 nCoV-19 vaccine has shown protection in rhesus macaques after challenge with SARS-CoV-2(van Doremalen et al., 2020).
Table 2.
Replicating viral vector-based vaccines that encode for the S protein.
| Manufacturer | Strategy | Vaccine Name/Stage | Ref. |
|---|---|---|---|
| Zydus Cadila | Codon-optimised proteins of the new coronavirus, expressed by replicating measles viral vector, will use reverse genetics to stimulate long-term neutralizing antibodies that protect against the infection | Pre-Clinical | (“Zydus Cadila. Zydus Cadila looks to expedite Covid-19 vaccine development February 17, 2020. https://www.pharmaceutical-technology.com/news/zydus-cadila-covid-19-vaccine/, May 21, 2020.,” n.d.) |
| Institute Pasteur/Themis/ Univ. of Pittsburgh Center for Vaccine Research |
The vaccine candidate consists of a replicating measles viral vector | Pre-Clinical | (“CEPI. CEPI collaborates with the Institut Pasteur in a consortium to develop COVID-19 vaccine. March 19, 2020. https://cepi.net/news_cepi/cepi-collaborates-withthe- institut-pasteur-in-a-consortium-to-develop-covid-19- vaccine/. Accessed May 21, 2020.,” n.d.) |
| Tonix Pharma/ Southern Research | Horsepox vector expressing S protein | Pre-Clinical | (“Tonix Pharma. TNX-1800 Horsepox vector for S protein. April 26, 2020. https://covidvax.org/covid19-vaccine/Tonix/TNX-1800-Horsepox-vector-for-S-protein-Tonix-Pharma-Southern-Research. Accessed May 21, 2020.,” n.d.) |
| BiOCAD and IEM | This viral vector vaccine based on attenuated influenza virus backbone (intranasal) | Pre-Clinical | (“BIOCAD, 2020. https://gmpnews.net/2020/03/biocad-begins-developing-covid-19-vaccine/. Accessed May21, 2020.,” n.d.) |
| University of Hong Kong | Influenza vector expressing RBD on its surface to induce immunogenicity against the SARS-CoV-2 virus; notably, can be administered as a nasal spray | Pre-Clinical | (“University of Hong Kong. CEPI partners with University of Hong Kong to develop COVID-19 vaccine. March 18, 2020. https://cepi.net/news_cepi/cepi-partners-with-university-of-hong-kong-to-develop-covid-19-vaccine/. Accessed May 21, 2020.,” n.d.) |
| IAVI/Batavia | Replication-competent VSV chimeric virus technology (VSVΔG) delivering the SARS-CoV-2 Spike (S) glycoprotein | Pre-Clinical | (“IAVI and Batavia Biosciences Announce Collaboration on VSV-vector Based Epidemic Preparedness Vaccines: https://www.iavi.org/newsroom/press-releases/2020/iavi-and-batavia-announce-collaboration-vsv-vector-epidemic-preparedness-vaccines. March 5, 2020.,” n.d.) |
| University of Western Ontario | VSV-S (Vesicular Stomatitis Virus-S) previously successfully used for the development of a vaccine for MERS | Pre-Clinical | (“University of Ontario. Ontario Leading COVID-19 Research in Canada Province Announces First Phase of Research Projects to Fight COVID-19. May 21, 2020. https://news.ontario.ca/opo/en/2020/05/ontario-leading-covid-19-research-in-canada.html. Accessed May 21, 2020,” n.d.) |
Table 3.
Non-replicating viral vector-based vaccines that encode for the S protein.
| Manufacturer | Strategy | Vaccine Name/ Stage | Ref. |
|---|---|---|---|
| CanSino Biological Inc./ Beijing Institute of Biotechnology |
The Phase 2 clinical trial will evaluate immunogenicity and safety of Ad5-nCoV, which encodes for a full-length S protein of SARS-CoV-2 | Phase1 ChiCTR2000030906 Phase 2 NCT04313127 ChiCTR2000031781 |
(Zhu et al., 2020) |
| University of Oxford |
The ChAdOx1 platform consists of a transgenic nonreplicating chimp adenovirus-based vaccine that expresses, leading to host cell expression and display of the antigenic coronavirus spike protein upon immunization, thus prompting an immune response | COV001/ Phase 1/2 NCT04324606 Phase 2 2020-001,228-32 Phase 3 ISRCTN89951424 |
(Folegatti et al., 2020) |
| Gamaleya Research Institute | Adeno-based | Phase 1 NCT04436471 NCT04437875 |
(“Gamaleya Research Institute. https://www.trialsitenews.com/gamaleya-research-institute-developed-covid-19-vaccine-clinical-trial-commences-across-russia/. Accessed on JUly 29, 2020.,” n.d.) |
| GeoVax/BravoVax |
The aim of this vaccine is prevention/ control of SARS-CoV-2, using its GV-MVA-VLPTM vaccine platform | Pre-Clinical |
(“GeoVax. GeoVax Progresses in Coronavirus (COVID-19) Vaccine Development Program. March 18, 2020. https://www.globenewswire.com/news- release/2020/03/18/2,002,611/0/en/GeoVax-Progresses-in-Coronavirus-COVID-19-Vaccine-Development-Program.html. Accessed May21, 2020.,” n.d.) |
| Janssen Pharmaceutical Companies |
Ad26 (alone or with MVA boost); leverages Janssen’s AdVac® and PER.C6® technology, which provide the ability to rapidly upscale production of the optimal vaccine candidate | Pre-Clinical |
(“Johnson & Johnson. Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19; Landmark New Partnership with U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use. March 30, 2020. https://www.jnj.com/johnson-johnson-announces-a-lead-vaccine-candidate-for-covid-19-landmark-new-partnership-with-u-s-department-of-health-human-services-and-commitment-to-supply-one-billion-vaccines-worldwide-for-emergency-pandemic-use. Accessed on May 21, 2020.,” n.d.) |
| ReiThera |
This vaccine targets the spike protein of SARS-CoV-2. The vaccine technology is based on a novel ReiThera-proprietary simian adenoviral vector with strong immunological potency and low pre-existing immunity in humans | Pre-Clinical |
(“ReiThera, LEUKOCARE and Univercells. ReiThera, Leukocare and Univercells announce fast-track development of a COVID-19 vaccine. APRIL 23, 2020. https://www.univercells.com/newsroom/reithera-leukocare-and-univercells-announce-fast-track-development-of-a-covid-19-vaccine/. Accessed on May 21, 2020.,” n.d.) |
| DZIF – German Center for Infection Research | This vaccine will combine the spike protein with the MVA vector’s genetic information, resulting in a viral vector able to penetrate human cells and consequently produce spike proteins | Pre-Clinical |
(“SARS-CoV-2: DZIF scientists and the development of vaccines https://www.dzif.de/en/sars-cov-2-dzif-scientists-and-development-vaccines Mar 9 2020.,” n.d.) |
| Altimmune |
The new intranasal vaccine is based on NasoVAX, the company’s influenza vaccine candidate. Like NasoVAX, single intranasal dose would provide systemic immunity | Pre-Clinical | (“Hannah Balfour: Biotech and academia collaborate on intranasal COVID-19 vaccine development:https://www.drugtargetreview.com/news/59182/biotech-and-academia-collaborate-on-intranasal-covid-19-vaccine-development/ 2 April 2020,” n.d.) |
| Greffex |
This genetically modified adenovirus-based vector vaccine will exploit GreVac(TM) Plug-And-Play technology to expedite the production of vaccine candidates | AdCOVID/ Pre-Clinical |
(“Greffex. Greffex completes COVID-19 vaccine, prepares for FDA animal testing. March 12, 2020. https://www.innovationews.com/Greffex-completes-COVID-19-vaccine-prepares-for-FDA-testing/ Accessed May 21, 2020.,” n.d.) |
| Vaxart |
The candidate is based on the company’s VAASTT oral vaccines platform, which uses adenovirus type 5 (Ad5) as a delivery system for its treatment. Vaccine candidates will generate mucosal immune responses in addition to serum antibody responses | Pre-Clinical |
(“Vaxart. Vaxart Announces Additional Positive Pre-Clinical Data for its Oral COVID-19 Vaccine Program. April 30, 2020. https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-additional-positive-pre-clinical-data-its-oral/ Accessed May 21, 2020.,” n.d.) |
| Centro Nacional Biotecnología (CNB-CSIC), Spain | MVA poxvirus vectors expressing S protein | Pre-Clinical |
(“Luis Enjuanes, Isabel Sola y Sonia Zúñiga. NOVEL HUMAN PATHOGENIC CORONAVIRUS: SARS-CoV-2. March 26, 2020. Centro Nacional de Biotecnología (CNB-CSIC), Madrid (Spain),” n.d.) |
| University of Manitoba | Dendritic cell-based vaccine | Pre-Clinical | (“COVID-19 vaccine: Dendritic cell-based vaccine. https://covidvax.org/covid19-vaccine/ManitobaUni/Dendritic-cell-based-vaccine-University-of-Manitoba. Accessed May 26, 2020,” n.d.) |
9. Subunit vaccines
Subunit vaccines use one or more antigens suitable for eliciting a robust immune response. In theory, the subunit vaccine is a very easy and safe vaccine, but in practice, it requires a suitable adjuvant to stimulate the host immune response. Several previous attempts were partially successful with SARS-CoV. Immunization of animals with the S1 RBD domain fused with the IgG1 FC portion (RBD-FC) induced highly potent antibodies which could bind with the RBD domain of the S1 domain, completely neutralize SARS-CoV, and inhibit SARS-CoV entry into Vero E6 cells (Du et al., 2009b, 2007; He et al., 2004). A similar approach is being used to develop a vaccine against the SARS-CoV-2 virus. Several companies, including the University of Queensland, are developing subunit vaccines based on the “molecular clamp” technology (Takashima et al., 2011). Trimer-tag technology is being used by Clover Biopharmaceuticals, Inc. to develop a vaccine against SARS-CoV-2 using mammalian cell-expressed Trimeric S protein (“Improvement of Pharmacokinetic Profile of TRAIL via Trimer-Tag Enhances its Antitumor Activity in vivo. - PubMed - NCBI,” n.d.). Novavax, Inc. has several S protein-based nanoparticle vaccines which needs to be tested for its efficacy in the animal model. Johnson & Johnson, Pasteur Institute, and Chongqing Zhifei Biological Products Co., Ltd. have also started subunit vaccine development against SARS-CoV-2. Other companies involved in making subunit vaccines are listed in Table 4 .
Table 4.
Vaccines based on protein subunits.
| Manufacturer | Strategy | Vaccine Name/Stage | Ref. |
|---|---|---|---|
| Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences | Adjuvant recombinant protein (RBD-Dimer | Phase 1 NCT04445194 Phase 2 NCT04466085 |
(“Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences. 2020https://covidvax.org/covid19-vaccine/AnhuiZhifei/Adjuvanted-recombinant-protein-Anhui-Zhifei-Longcom-Biopharma-Institute-of-Microbiology-Chinese-Acad. Accessed on July 29, 2020.,” n.d.) |
| Vaxine Pty Ltd/Medytox | Recombinant spike protein with Advax adjuvant | Phase 1 NCT04453852 |
(“Vaxine Pty Ltd/Medytox. https://www.biospectrumasia.com/news/37/16000/vaxine-announces-covid-19-vaccine-collaboration-with-medytox.html. Accessed on July 29, 2020.,” n.d.) |
| Kentucky Bioprocessing, Inc | RBD-Based | Phase 1 NCT04473690 |
(“Kentucky Bioprocessing, Inc. https://www.kentucky.com/news/coronavirus/article241709386.html. Accessed on July 29, 2020.,” n.d.) |
| AdaptVac (PREVENT-nCoV consortium) |
AdaptVac’s universal viral Capsid-Like Particle (CLP) will be employed to deliver an optimal vaccine against the SARS-CoV-2 virus (COVID-19) | Pre-Clinical |
(“ADPVAC, 2020. https://www.adaptvac.com/news. Accesses May 21, 2020.,” n.d.) |
| ExpreS2ion |
Drosophila S2 insect cell expression system VLPs. This vaccine uses AdaptVac’s capsid VLP technology and ExpreS2ion’s ExpreS2 technology, which has the potential to mimic a virus to the body’s immune system, giving the optimal stimulus to generate a fast, long-lasting immune response that offers a highly efficacious protection | Pre-Clinical |
(“ExpreS2ion. ExpreS2ion Announces Bavarian Nordic Enters Agreement with AdaptVac to Advance the COVID-19 Vaccine. May 06 2020. https://expres2ionbio.com/investor/international-press-releases/. Accessed May 21, 2020.,” n.d.) |
| IMV Inc |
DPX-COVID-19 is a formulation of the DPX platform with peptide epitopes from S proteins of the novel coronavirus (SARS-CoV-2) | DPX-COVID-19/ Pre-Clinical |
(“IMV. DPX-COVID-19 at a glance. April 15, 2020:https://imv-inc.com/clinical-trials/dpx-covid-19/. Accessed May 21, 2020.,” n.d.) |
| WRAIR/USAMRIID | Ferritin nanoparticle from H. pylori are used to attach a small part of the coronavirus S protein binding receptor onto the ferritin nanoparticle shell which is surrounded by proprietary lipid ring around the shell that acts as an accelerant or booster | Pre-Clinical | (“Eric Niiler: The US Army’s Virus Research Lab Gears Up to Fight Covid-19. April 01, 2020. https://www.wired.com/story/the-us-armys-virus-research-lab-gears-up-to-fight-covid-19/ Accessed on May21, 2020.,” n.d.) |
| National Institute of Infectious Disease, Japan | Vaccines rely on genetic engineering to create recombinant proteins that will serve as an antibody against the virus | Pre-Clinical | (“Shionogi Inc. Shionogi Accelerates Development of Potential COVID-19 Treatments and Vaccine. April 28, 2020. https://www.shionogi.com/us/en/news/2020/4/shionogi-accelerates-development-of-potential-covid-19-treatments-and-vaccine.html. Accessed May21, 2020.,” n.d.) |
| Osaka University/ BIKEN/ National Institutes of Biomedical Innovation, Japan | VLP-recombinant protein + Adjuvant | Pre-Clinical | (“BIKEN foundation, 2020. https://www.osaka-u.ac.jp/en/news/info/corona/corona_info/cooperation. Accesses May 21, 2020.,” n.d.) |
| Clover Biopharmaceuticals Inc./GSK/Dynavax | Clover uses Trimer-Tag© technology to produce an S-Trimer subunit vaccine (resembling the native trimeric viral spike via a rapid mammalian cell culture-based expression system) which will be combined with GSK’s pandemic adjuvant system to check vaccine efficacy | COVID-19 S-Trimer/ Phase 1 NCT04405908 |
(“Clover Biopharmaceuticals Inc./GSK/Dynavax. https://www.fiercebiotech.com/biotech/using-gsk-dynavax-tech-clover-kickstarts-covid-vax-trial-data-drop-august. Accessed on July 29, 2020.,” n.d.) |
| Univ. of Pittsburgh | The vaccine candidate is based on unique and patent protected signal peptide technology utilizing Vaxil’s proprietary VaxHit™ bioinformatics platform | Pre-Clinical | (“COVID-19 Vaccine Candidate Shows Promise: April 02, 2020. https://www.upmc.com/media/news/040,220-falo-gambotto-sars-cov2-vaccine. Accessed May21, 2020.,” n.d.) |
| Vaxil Bio |
The Vaccine Candidate is based on unique and patent protected signal peptide technology, utilizing Vaxil’s proprietary VaxHit™ bioinformatics platform. |
Pre-Clinical | (“Vaxil. Vaxil commences preclinical COVID-19 vaccine trial and files an additional COVD-19 patent. March 27, 2020. https://vaxil-bio.com/vaxil-commences-preclinical-covid-19-vaccine-trial-and-files-an-additional-covid-19-patent/ Accessed May 21, 2020.,” n.d.) |
| Biological E Ltd. | Adjuvanted protein subunit (RBD) | Pre-Clinical | (“Biological E Ltd, 2020. https://www.livemint.com/news/india/covid-19-six-indian-companies-working-on-coronavirus-vaccine-11587016987400.html. Accessed May 27, 2020.,” n.d.) |
| Flow Pharma Inc | FlowVax vaccines utilize Flow Pharma's patented Size Exclusion Antigen Presentation Control (SEAPAC(TM)) technology based on the benefits of making vaccine microspheres the same size as human white blood cells. This vaccine relies on killer T-cells rather than antibodies to fight virus infections | FlowVax Covid-19/ Pre-Clinical | (“Flow Pharma: Flow Pharma Announces Strategic Partnership with Oakwood Labs for GMP Manufacturing of FlowVax COVID-19 Vaccine. April 15, 2020. https://www.accesswire.com/585181/Flow-Pharma-Announces-Strategic-Partnership-with-Oakwood-Labs-for-GMP-Manufacturing-of-FlowVax-COVID-19-Vaccine. Accessed May 21, 2020.,” n.d.) |
| AJ Vaccines |
The vaccine candidate will use technology to raise strong immune responses while being well tolerated and to meet potential global demand in 2021 | Pre-Clinical |
(“AJ Vaccines, 2020. https://ajvaccines.com/news/. Accessed May 21, 2020.,” n.d.) |
| Generex/EpiVax | Generex Biotechnology Corporation will use EpiVax expertise and computational tools to predict epitopes that can be used to generate peptide vaccines against SARS-CoV-2 using the patented NuGenerex Immuno-Oncology (NGIO – Formerly Antigen Express) Ii-Key technology | Pre-Clinical | (“Generex. Generex Signs Contract with EpiVax to Develop Ii-Key Peptide Vaccines to Address the Coronavirus Pandemic. March 04, 202. https://epivax.com/news/press-release-generex-signs-contract-with-epivax-to-develop-ii-key-peptide-vaccines-to-address-the-coronavirus-pandemic. Accessed May 21, 2020.,” n.d.) |
| EpiVax/Univ. of Georgia | Sequence will be predicted based on EpiVax computational tool | Pre-Clinical | (“EpiVax. EpiVax Accelerates COVID-19 Vaccine Development with UGA’s Center for Vaccines and Immunology. May 04, 2020. https://epivax.com/news/press-release-epivax-accelerates-covid-19-vaccine-development-with-ugas-center-for-vaccines-and-immunology. Accessed May 21, 2020.,” n.d.) |
| Sanofi Pasteur/GSK |
Sanofi will contribute its recombinant DNA technology-based S-protein COVID-19 antigen expressed using baculovirus expression platform, and GSK will contribute its proven pandemic adjuvant technology | Pre-Clinical | (“Sanofi and GSK. Sanofi and GSK to join forces in unprecedented vaccine collaboration to fight COVID-19. April 14, 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-04-14-13-00-00. Accessed May 21, 2020.,” n.d.) |
| Novavax |
Full length recombinant SARS-CoV-2 glycoprotein nanoparticle vaccine adjuvanted with Matrix M | NVX-CoV2373/ Phase 1/2 NCT04368988 |
(“Novavax. https://ir.novavax.com/news-releases/news-release-details/novavax-initiates-phase-12-clinical-trial-covid-19-vaccine. Accessed on July 29, 2020.,” n.d.) |
| Heat Biologics/ Univ. Of Miami |
Heat's gp96 vaccine platform activates CD8 T cells, antigen presenting cells and natural killer cells, and induces mucosal immunity, which could make it an ideal vaccine for COVID-19 | Pre-Clinical | (“Heat Biologics. Heat Biologics Announces Research Collaboration with University of Miami to Develop Vaccine Designed to Protect Against COVID-19 Coronavirus. Match 05, 2020. https://www.heatbio.com/news-media/news-releases/detail/649/heat-biologics-announces-research-collaboration-with. May 21, 2020.,” n.d.) |
| University of Queensland /GSK/Dynavax |
Subunit vaccine consisting of a SARS-CoV-2 spike protein stabilized with a protein “molecular clam” |
ACTRN12620000674932p |
(“University of Queensland /GSK/Dynavax. https://advance.qld.gov.au/covid-19-fast-facts. Accessed on July 29, 2020.,” n.d.) |
| Baylor College of Medicine/Texas Children's Hospital/Path | Based on RBD of Spike protein | Pre-Clinical | (“Baylor College of Medicine, 2020https://www.bcm.edu/coronavirus-preparedness/covid-19-research. May 21, 2020.,” n.d.) |
| iBio/CC-Pharming | Subunit protein is produced in a plant. The aim of this company is to deliver vaccine candidates for rapid production at iBio’s FastPharming manufacturing facility | Pre-Clinical | (“Fast-Farming Pharma. How plants could speed up vaccine manufacture to tackle the COVID-19 pandemic? April 17, 2020. https://themedicinemaker.com/manufacture/fast-farming-pharma. Accessed May 21, 2020.,” n.d.) |
| Saint-Petersburg scientific research institute of vaccines and serums | Recombinant protein, nanoparticles (based on S-protein and other epitopes) | Pre-Clinical | (“Saint-Petersburg scientific research institute of vaccines and serums. May22, 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. Accessed May 25, 2020.,” n.d.) |
| Innovax/Xiamen Univ./GSK |
COVID-19′s series of truncated S will be screened during the preclinical testing process; ultimate lead candidate will be determined by immunogenicity data | COVID-19 XWG-03/ Pre-Clinical |
(“GSK. GSK Joins Xiamen Innovax Biotech to Support COVID-19 Vaccine Development & Expands Activity to Fight War against Pandemic. April 06, 2020. https://www.trialsitenews.com/gsk-joins-xiamen-innovax-biotech-to-support-covid-19-vaccine-development-expands-activity-to-fight-war-against-pandemic/. Accessed May 21, 2020.,” n.d.) |
| VIDO-InterVac, University of Saskatchewan |
Adjuvanted microsphere peptide-based vaccine | Pre-Clinical |
(“USask. USask VIDO-InterVac awarded $23 M for COVID-19 vaccine research. April 23, 2020. https://news.usask.ca/articles/research/2020/usask-vido-intervac-awarded-23m-for-covid-19-vaccine-research.php. Accessed May 21, 2020.,” n.d.) |
| OncoGen |
Concept of personalized vaccination. Multiepitope peptide vaccine candidates were identified against nCov that can potentially trigger both CD4+ and CD8 + T cell immune response | Pre-Clinical | (“OncoGen. OncoGen researchers propose personalized vaccinomics strategy for the novel China coronavirus: https://oncogen.ro/oncogen-vaccine-design-for-coronavirus/. Accessed May 21, 2020.,” n.d.) |
| MIGAL Galilee Research Institute | Oral, E. coli-based protein expression system of S and N proteins | Pre-Clinical | (“MIGAL’s Coronavirus Vaccine Project:https://www.migal.org.il/en/node/7010. Accessed May 21, 2020.,” n.d.) |
| University of Alberta | Spike-based | Pre-Clinical | (“WHO. Draft landscape of COVID-19 candidate vaccines. May 30, 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. Accessed May 30, 2020.,” n.d.) |
10. RNA vaccines
mRNA has been used as a template for endogenous expression of proteins selected as a vaccine candidate. An mRNA vaccine is a promising alternative because of its high potency, short production cycle, and low manufacturing cost. (Pardi et al., 2018; Yun et al., 2020). Currently, there is no mRNA vaccine in the market, so it will be challenging to pass the quality control and safety evaluation. Currently, mRNA-1273, a potential vaccine against
SARS-CoV-2 based on encoding for a prefusion stabilized form of the S protein, has been selected for evaluation by Moderna in collaboration with investigators at the NIAID Vaccine Research Center (VRC). This vaccine is currently the focus of a Phase 1 clinical trial (NCT04283461). Several other companies are also developing mRNA vaccines against SARS-CoV-2 (Table 5 ).
Table 5.
S protein-based RNA vaccines.
| Manufacturer | Strategy | Vaccine Name/ Stage | Ref |
|---|---|---|---|
| Fudan University /Shanghai JiaoTong University/RNACure Biopharma |
LNP-encapsulated mRNA cocktail encoding VLP for induction of neutralizing antibodies, as well as producing virus-like particles similar to SARS-CoV-2 | Pre-clinical | (“Fudan University. Towards an effective mRNA vaccine against 2019-nCoV: March 7, 2020. https://www.fudan.edu.cn/en/2020/0307/c1092a104281/page.htm. Accessed May 21, 2020.,” n.d.) |
| Moderna/NIAID | Lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine encoding a full-length, prefusion stabilized S protein of SARS-CoV-2 | mRNA-1273/ Phase 1 NCT04283461 Phase 2 NCT04405076 Phase 3 NCT04470427 |
(Jackson et al., 2020) |
| Centro Nacional Biotecnología (CNB-CSIC), Spain | Replicating defective SARS-CoV-2- derived RNAs | Pre-clinical | (“Centro Nacional Biotecnología (CNB-CSIC). TYPE: RNA vaccine:https://www.lykeo.com/ replicating-defective-sarscov-2-derived-rnas-centro-nacional-biotecnologia-cnb-csic-spain. Accessed May 21, 2020.,” n.d.) |
| University of Tokyo / Daiichi-Sankyo | LNP-encapsulated mRNA | Pre-clinical | (“University of Tokoyo and Daiichi Sankyo. Our Company’s Efforts to Limit the Spread of the Virus that Causes COVID-19 Accessed May 21, 2020.,” n.d.) |
| BIOCAD |
Liposome-encapsulated mRNA to produce disease-specific antigens and trigger a regular immune response | Pre-clinical | (“BIOCAD, 2020. https://gmpnews.net/2020/03/biocad-begins-developing-covid-19-vaccine/. Accessed May21, 2020.,” n.d.) |
| China CDC/Tongji University/Stermina | mRNA | Pre-clinical | |
| Arcturus/Duke-NUS | Self replication of mRNA with LUNAR®, a leading nanoparticle non-viral delivery system, to produce proteins inside the human body. Provokes a vaccine response at much lower doses compared to traditional mRNA vaccines |
Phase 1/2 NCT0448057 |
(“Arcturus/Duke-NUS, 2020https://www.duke-nus.edu.sg/about/media/media-releases/media-releases/arcturus-therapeutics-duke-nus-clinical-trials-for-covid-19-vaccine-candidate-approved-to-proceed. Accessed on July 29, 2020.,” n.d.) |
| BioNTech/Fosun Pharma/Pfizer | This includes four vaccine candidates, each representing a different combination of mRNA format and target antigen | Phase 1/22020−001038-36 ChiCTR2000034825 Phase 3 NCT04368728 |
(“BioNTech/Fosun Pharma/Pfizer. https://www.genengnews.com/covid-19-candidates/biontech-pfizer-and-fosun-pharma-bnt162/. Accessed on July 29, 2020.,” n.d.) |
| Imperial College London | Self-amplifying RNA vaccine will deliver genetic instructions to muscle cells to make the ‘S’ protein on the surface of the coronavirus. This should provoke an immune response and create immunity to COVID-19 | Phase 1 ISRCTN17072692 |
(“Imperial College London. https://www.clinicaltrialsarena.com/news/imperial-college-covid-vaccine/. Accessed on July 29, 2020.,” n.d.) |
| Curevac | mRNA | NCT04449276 | (“Curevac. https://www.curevac.com/news/curevac-receives-regulatory-approval-from-german-and-belgian-authorities-to-initiate-phase-1-clinical-trial-of-its-sars-cov-2-vaccine-candidate. Accessed on July 29, 2020.,” n.d.) |
| Saiba GmbH | Virus-like particle, based on RBD displayed on virus-like particles of plant virus Cucumber Mosaic Virus (CMV) | Pre-clinical | (“Saiba Biotech. Saiba proprietary technology has enabled scientists to generate a vaccine candidate against COVID-19 with preclinical proof-of-concept. https://www.saiba-biotech.com/. Accessed May 21, 2020.,” n.d.) |
11. Spike protein-based therapeutics
Two S-protein-based methods that show promise as therapeutics for SARS-CoV-2 include those facilitating peptide interference with RBD-ACE2 interaction, as well as those using mAbs that target the S-protein. A previous study has shown that hexapeptide 438YKYRYL443 from the S protein of SARS-CoV virus binds to ACE2 with a binding affinity of KD =46 μM and blocks the entry of SARS-CoV into Vero E6 cells (Struck et al., 2012). A peptide from the RBD domain of the S protein (aa 471–503) can inhibit the binding of ACE2 to SARS-CoV RBD and block SARS-CoV entry into Vero cells with an EC50 of 41.6 μM (Hu et al., 2005). Similarly, a combination of two peptides from ACE2 motif aa 22–44 and 351–357 linked together by glycine blocks entry of SARS pseudoparticles in HeLa cells expressing ACE2 protein with EC50 of 100 μM (Han et al., 2006). Recently it has been shown that a 23-mer spike binding peptide 1 (IEEQAKTFLDKFNHEAEDLFYQS) derived from human ACE2 a1 helix binds SARS-CoV-2-RBD with low nanomolar affinity (dissociation constant, KD = 47 nM), potentially inhibiting the entry of the virus into human cells (G. L. Zhang et al., 2020; G. Zhang et al., 2020). Another study showed that the lapidated form of peptide EK1, EK1C inhibits SARS-CoV-2 mediated cell-cell fusion at the concentration of 2.5 μM and with an IC50 of 48.1 nM. This study also suggests that lipidation, as well as linker length, plays an essential role in the overall activity of lipopeptides. (S. L. Xia et al., 2020; S. Xia et al., 2020). This would be a potential candidate for therapeutic development. However, its efficacy against SARS-CoV-2 still needs to be validated using an in vivo model (Table 6 ).
Table 6.
Inactivated Virus based vaccines.
| Manufacturer | Strategy | Vaccine Name/ Stage | Ref |
|---|---|---|---|
| Sinovac | Inactivated | Phase 3 NCT04456595 Phase 1 NCT04383574 NCT04352608 |
(Gao et al., 2020) |
| Wuhan Institue of Biological Products/Snopharm | Inactivated | Phase 3 ChiCTR2000034780 Phase1 ChiCTR2000031809 |
(“Wuhan Institue of Biological Products/Snopharm. https://www.fiercepharma.com/pharma-asia/china-s-sinopharm-touts100-antibody-response-for-covid-19-vaccine-it-s-already-giving. Accessed on July 29, 2020.,” n.d.) |
| BEJING Institute of Biological Products/Sinopharma | Inactivated | Phase 3 ChiCTR2000034780 Phase1 ChiCTR2000032459 |
(“BEJING Institute of Biological Products/Sinopharma, 2020https://www.fiercebiotech.com/research/inactivated-covid-19-vaccine-by-china-s-sinopharm-clears-animal-tests. Accessed on July 29, 2020.,” n.d.) |
| Institute of Medical Biology, Chinesee Academy of Medical Sciences | Inactivated | Phase 1/2 NCT04470609 Phase 1 NCT04412538 |
(“Institute of Medical Biology, Chinesee Academy of Medical Sciences. http://english.nmpa.gov.cn/2020-06/22/c_502093.htm. Accessed on July 29, 2020.,” n.d.) |
| Bharat Biotech | Inactivated (Whole- Virion Inactivated | (“Bharat Biotech, 2020https://www.deccanchronicle.com/lifestyle/health-and-wellbeing/270720/bharat-biotechs-covaxin-shows-encouraging-results-in-phase-i.html. Accessed on July 29, 2020.,” n.d.) |
Several monoclonal antibodies targeting the S protein against SARS-CoV have been identified that inhibit the virus entry to cells (Sui et al., 2004; ter Meulen et al., 2006, 2004; Traggiai et al., 2004; Zhu et al., 2007). Although RBD of the S protein is the prime target for neutralizing antibodies, antibodies can recognize different epitopes on RBD and work synergistically. The SARS-CoV neutralizing antibodies CR3014 and CR3022 bind noncompetitively to the SARS-CoV RBD and synergistically neutralize the virus (ter Meulen et al., 2006). A recent report revealed that although a SARS-CoV-neutralizing antibody m396 showed promising binding with SARS-CoV-2 RBD in computational modeling, it only showed slight binding at the highest measured concentration (2.0 μM) in an experimental condition. Another study found that plasma samples from SARS-CoV-convalescent patients can neutralize SARS-CoV, but cannot cross-neutralize SARS-CoV-2, even though they can bind SARS-CoV-2 (Lv et al., 2020). These results suggest that monoclonal antibodies that bind with SARS-CoV S protein are unable to bind SARS-CoV-2. These results suggest an urgent need to develop novel monoclonal antibodies that could bind specifically to SARS-CoV-2 (Tian et al., 2020).
11.1. Small molecule inhibitor
Another area of research which can provide immediate control of SARS-CoV-2 spreads is the small molecule inhibitors. Several previous reports have suggested that small molecule compound can successfully inhibit the interaction between SARS-CoV spike protein and ACE2, leading to block the virus entry into the cells (Adedeji et al., 2013; Huentelman Matthew J. et al., 2004; Kao et al., 2004). Similar study in SARS-CoV-2 suggest that small molecule can inhibit the interaction between SARS-CoV-2 and ACE2. Hanson et. al., have performed a drug repurposing screen against 3384 small molecule drugs using a proximity -based assay which measure the binding of SARS-CoV2 spike protein Domain (RBD) and ACE2, and found 25 high quality small molecule hits that can be evaluated for its efficacy in future (Hanson et al., 2020). Even though druggable pockets are absent in spike protein and ACE2 in their unbound states, bound states have well defined pockets for drug development. Using computer modeling, Patil et al. has shown that several antiviral compound against HCV and HIV viruses e.g. Atazanavir, Grazoprevir, Saquinavir, Simeprevir, Telaprevir and Tipranavir could be potential candidate inhibitors for immediate future investigation (Patil et al., 2020).
12. Conclusions and futures perspectives
The emergence of the COVID-19 pandemic has underscored the necessity to find therapeutics and/or vaccines to control the spread of infection and save infected people. From recent and previous studies of SARS-CoV, MERS-CoV, and SARS-CoV-2, it is well known that the S protein plays a vital role in virus-host interaction and its entry into human cells. Still, we need to know more about the fundamental nature of SARS-CoV-2, for example, mechanisms of pathogenesis, cellular and humoral immune response in the host after virus infection, etc., to make a successful therapeutics and/or vaccine.
In this review, we summarised different approaches using the S protein to generate a vaccine, therapeutics, or neutralizing antibodies targeting SARS-CoV-2. Several neutralizing antibodies have been detected that act against SARS-CoV or MERS-CoV, but with limited success. Many of the neutralizing antibodies or vaccines identified cause ADE or vaccine-enhanced disease, respectively. There is a rising concern about ADE effect by sub-optimal antibody generated by vaccine under development against SARS-CoV-2. Because of these risks, it is crucial to perform large-scale clinical trials to assess vaccine or therapeutic efficacy and safety before approving any for widespread use. It is also essential to find a neutralizing antibody which can neutralize different strain of viruses and yet not cause antibody-induced enhancement. Use of nanobodies is one of the options that can successfully avoid the ADE effect. Since the virus is still spreading globally, a vaccine is the preferred means to control COVID-19. Development, testing, and clinical trials are ongoing with a focus on identifying a potential drug/vaccine to prevent or treat SARS-CoV-2-associated disease.
Recent studies have shown that SARS-CoV-2 is actively evolving. Recently, it was found that a clinical strain of SARS-CoV-2 containing a D614 G mutation within the viral spike protein is now dominant in many countries (Korber et al., 2020). It has been reported in several studies that this mutation is not causing patient sicker but has higher rate of infectivity than the wild-type virus (Daniloski et al., 2020; Eaaswarkhanth et al., 2020; Ogawa et al., 2020;). D614 G did not alter the virus’s response to antibodies from patients infected with the wild-type virus, which suggests that vaccines currently under development based on the original viral spike protein will be still effective against the new strain (Grubaugh et al., 2020; L. L. Zhang et al., 2020; G. Zhang et al., 2020). However, in order to fully establish the safety, cross-challenging studies of the new strain in vaccinated animals should be performed to evaluate the biosafety of vaccines.
references
BioNet (2020), BioNTech/Fosun Pharma/Pfizer (2020), Centro Nacional Biotecnología (CNB-CSIC). (2020), CEPI (2020), Clover Biopharmaceuticals Inc (2020), Cobra Biologics (2020), COVID-19 Vaccine Candidate Shows Promise (2020), COVID-19 vaccine: Dendritic cell-based vaccine (2020), Curevac (2020), de Wilde et al. (2018d), EpiVax (2020), Eric Niiler (2020), ExpreS2ion (2020), Fast-Farming Pharma (2020), Flow Pharma (2020), Fudan University (2020), Gamaleya Research Institute (2020), Generex (2020), Genexine Consortium (2020), GeoVax et al. (2020), C.J. Gordon et al., 2020; D.E. Gordon et al., 2020, Greffex (2020), GSK (2020), ZZZZZ (2020), Heat Biologics (2020), Huentelman Matthew et al. (2004), IAVI and Batavia Biosciences Announce Collaboration on VSV-vector Based Epidemic Preparedness Vaccines (2020), Imperial College London (2020), Improvement of Pharmacokinetic (2020), IMV (2020), Inovio Pharmaceuticals/International Vaccine Institute (2020), Institute of Medical Biology and Chinesee Academy of Medical Sciences (2020), Johns Hopkins University (2020), Johnson and Johnson (2020), Kentucky Bioprocessing, Inc (2020), Enjuanes and Zúñiga (2020), MIGAL’s Coronavirus Vaccine (2020), Novavax (2020), OncoGen (2020), Osaka University/AnGes/Takara Bio (2020), Pharmajet et al., il 15 (2020), ReiThera (2020), Saiba Biotech (2020), Saint-Petersburg scientific research institute of vaccines and serums (2020), Sanofi and GSK (2020), SARS-CoV-2: DZIF, 9 2020, Shionogi Inc (2020), Symvivo Corporation (2020), Takis (2020), ter Meulen et al. (2004t), Tonix Pharma (2020), University of Hong Kong (2020), University of Ontario (2020), University of Queensland /GSK/Dynavax (2020), University of Tokoyo and Daiichi Sankyo (2020), University of Waterloo (2020), USask (2020), van Doremalen et al. (2020v), Vaxart (2020), Vaxil (2020), Vaxine Pty Ltd/Medytox (2020), C. Wang et al., 2020; Q. Wang et al., 2020, WHO, 2020a,b, 2019, WHO, 2020a,b, F. Wu et al., 2020; Y. Wu et al., 2020, Wuhan Institue of Biological Products/Snopharm (2020), L. Xia et al., 2020; S. Xia et al., 2020, J. Yu et al., 2020; C. Yu et al., 2020, L. Zhang et al., 2020; G. Zhang et al., 2020, Zydus Cadila, 2020a,b, Zydus Cadila, 2020a,b, Liu et al., 2019a,b, Walls et al. (2019) and Houser et al. (2017).
Acknowledgements
We thank Dr. McClive-Reed of Heath Research, Inc. for helpful suggestions. HL was partially supported by NIH grants AI131669, AI133219, AI140491, AI134568, AI140406, AI141178, and AI140726.
References
- Adedeji A.O., Severson W., Jonsson C., Singh K., Weiss S.R., Sarafianos S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J. Virol. 2013;87:8017–8028. doi: 10.1128/JVI.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADPVAC BARBARIAN NORDIC Enters Agreement With ADAPVAC to Advance COVID-19 Vaccine Program. May 6. 2020. https://www.adaptvac.com/news Accesses May 21, 2020., n.d.
- Aghamollaei H., Ghanei M., Rasaee M.J., Latifi A.M., Bakherad H., Fasihi-Ramandi M., Taheri R.A., Gargari S.L.M. Isolation and characterization of a novel nanobody for detection of GRP78 expressing cancer cells. Biotechnol. Appl. Biochem. 2020 doi: 10.1002/bab.1916. [DOI] [PubMed] [Google Scholar]
- AJ Vaccines AJ Vaccines to Develop Vaccine for COVID-19. March 06. 2020. https://ajvaccines.com/news/ Accessed May 21, 2020., n.d.
- Al-amri S.S., Abbas A.T., Siddiq L.A., Alghamdi A., Sanki M.A., Al-Muhanna M.K., Alhabbab R.Y., Azhar E.I., Li X., Hashem A.M. Immunogenicity of candidate MERS-CoV DNA vaccines based on the spike protein. Sci. Rep. 2017;7 doi: 10.1038/srep44875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Osail A.M., Al-Wazzah M.J. The history and epidemiology of Middle East respiratory syndrome corona virus. Multidiscip. Respir. Med. 2017;12 doi: 10.1186/s40248-017-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020 doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stat. 2020. 2020an updated guide to the coronavirus drugs and vaccines in development.https://www.statnews.com/2020/03/19/ an-updated-guide-to-the-coronavirus-drugs-and-vaccines-in-development/ URL (accessed 4.15.20). [Google Scholar]
- Anhui Zhifei Longcom Biopharmaceutical/Institute of Microbiology, Chinese Academy of Sciences. https://covidvax.org/covid19-vaccine/AnhuiZhifei/Adjuvanted-recombinant-protein-Anhui-Zhifei-Longcom-Biopharma-Institute-of-Microbiology-Chinese-Acad. Accessed on July 29, 2020., n.d.
- Arcturus/Duke-NUS. https://www.duke-nus.edu.sg/about/media/media-releases/media-releases/arcturus-therapeutics-duke-nus-clinical-trials-for-covid-19-vaccine-candidate-approved-to-proceed. Accessed on July 29, 2020., n.d.
- Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor College of Medicine New Partnership to Accelerate Research of COVID-19 Vaccine. May 5. 2020. https://www.bcm.edu/coronavirus-preparedness/covid-19-research May 21, 2020., n.d.
- BEJING Institute of Biological Products/Sinopharma.https://www.fiercebiotech.com/research/inactivated-covid-19-vaccine-by-china-s-sinopharm-clears-animal-tests. Accessed on July 29, 2020., n.d.
- Bharat Biotech. https://www.deccanchronicle.com/lifestyle/health-andwellbeing/270720/bharat-biotechs-covaxin-shows-encouraging-results-in-phase-i.html. Accessed on July 29, 2020., n.d.
- BIKEN foundation RIMD, And NIBIOHN: Joint Development of Drugs and Inspection Techniques for COVID-19. March 18. 2020. https://www.osaka-u.ac.jp/en/news/info/corona/corona_info/cooperation Accesses May 21, 2020., n.d.
- BIOCAD BIOCAD. Begins Developing COVID-19 Vaccine. March 19. 2020. https://gmpnews.net/2020/03/biocad-begins-developing-covid-19-vaccine/ Accessed May21, 2020., n.d.
- Biological E Ltd Covid 19: Six Indian Companies Working on Coronavirus Vaccine. April 16. 2020. https://www.livemint.com/news/india/covid-19-six-indian-companies-working-on-coronavirus-vaccine-11587016987400.html Accessed May 27, 2020., n.d.
- BioNet BioNet In Thailand Is Developing a COVID-19 GENE-based Vaccine (COVIGEN) Encoding the S (Spike) Protein of SARS-CoV-2. http://www.bionet-asia.com/media/news-events/ Accessed on May 21, 2020., n.d.
- BioNTech/Fosun Pharma/Pfizer. https://www.genengnews.com/covid-19-candidates/biontech-pfizer-and-fosun-pharma-bnt162/. Accessed on July 29, 2020., n.d.
- Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouard D., Alazard‐Dany N., Cosset F.-L. Viral vectors: from virology to transgene expression. Br. J. Pharmacol. 2009;157:153–165. doi: 10.1038/bjp.2008.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Wang L., Rowe R.G., Han A., Ji W., McMahon C., Baier A.S., Huang Y.-C., Marion W., Pearson D.S., Kruse A.C., Daley G.Q., Wu H., Sliz P. A nanobody targeting the LIN28:let-7 interaction fragment of TUT4 blocks uridylation of let-7. Proc. Natl. Acad. Sci. U.S.A. 2020;117:4653–4663. doi: 10.1073/pnas.1919409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Gotte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. jbc.RA120.013679. 2020 doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Deng Q., Dai S. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centro Nacional Biotecnología (CNB-CSIC). TYPE: RNA vaccine:https://www.lykeo.com/replicating-defective-sarscov-2-derived-rnas-centro-nacional-biotecnologia-cnb-csic-spain. Accessed May 21, 2020., n.d.
- CEPI CEPI Collaborates With the Institut Pasteur in a Consortium to Develop COVID-19 Vaccine. March 19. 2020. https://cepi.net/news_cepi/cepi-collaborates-withthe- institut-pasteur-in-a-consortium-to-develop-covid-19- vaccine/. Accessed May 21, 2020., n.d.
- Chen Z., Zhang L., Qin C., Ba L., Yi C.E., Zhang F., Wei Q., He T., Yu W., Yu J., Gao H., Tu X., Gettie A., Farzan M., Yuen K.-Y., Ho D.D. Recombinant modified vaccinia virus Ankara expressing the spike glycoprotein of severe acute respiratory syndrome coronavirus induces protective neutralizing antibodies primarily targeting the receptor binding region. J. Virol. 2005;79:2678–2688. doi: 10.1128/JVI.79.5.2678-2688.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.-H., Du L., Chag S.M., Ma C., Tricoche N., Tao X., Seid C.A., Hudspeth E.M., Lustigman S., Tseng C.-T.K., Bottazzi M.E., Hotez P.J., Zhan B., Jiang S. Yeast-expressed recombinant protein of the receptor-binding domain in SARS-CoV spike protein with deglycosylated forms as a SARS vaccine candidate. Hum. Vaccin. Immunother. 2014;10:648–658. doi: 10.4161/hv.27464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H., Zheng X., Wang X., Wang C., Wang H., Gai W., Perlman S., Yang S., Zhao J., Xia X. DNA vaccine encoding Middle East respiratory syndrome coronavirus S1 protein induces protective immune responses in mice. Vaccine. 2017;35:2069–2075. doi: 10.1016/j.vaccine.2017.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Liu X., Wang C., Zhang X., Ren L., Jin Q., Wang J., Yang W. Humanized single domain antibodies neutralize SARS-CoV-2 by targeting spike receptor binding domain (preprint) Microbiology. 2020 doi: 10.1101/2020.04.14.042010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clover Biopharmaceuticals Inc./GSK/Dynavax. https://www.fiercebiotech.com/biotech/using-gsk-dynavax-tech-clover-kickstarts-covid-vax-trial-data-drop-august. Accessed on July 29, 2020., n.d.
- Cobra Biologics Cobra Biologics and the Karolinska Institute Collaborate to Develop COVID-19 Vaccine. 30th Mar. 2020. https://www.cobrabio.com/News/March-2020/Cobra-Karolinska-Institutet-COVID-19-Vaccine Accessed May 21, 2020, n.d.
- Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., Glenn G.M., Smith G.E., Frieman M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C.M., Venkataraman T., Liu Y.V., Glenn G.M., Smith G.E., Flyer D.C., Frieman M.B. MERS-CoV spike nanoparticles protect mice from MERS-CoV infection. Vaccine. 2017;35:1586–1589. doi: 10.1016/j.vaccine.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Vaccine Candidate Shows Promise: April 02, 2020. https://www.upmc.com/media/news/040220-falo-gambotto-sars-cov2-vaccine Accessed May21, 2020., n.d. [Google Scholar]
- COVID-19 vaccine: Dendritic cell-based vaccine. https://covidvax.org/covid19-vaccine/ManitobaUni/Dendritic-cell-based-vaccine-University-of-Manitoba. Accessed May 26, 2020, n.d.
- Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curevac. https://www.curevac.com/news/curevac-receives-regulatory-approval-from-german-and-belgian-authorities-to-initiate-phase-1-clinical-trial-of-its-sars-cov-2-vaccine-candidate. Accessed on July 29, 2020., n.d.
- Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., O’Meara M.J., et al. A SARS-CoV-2-Human protein-Protein interaction map reveals drug targets and potential drug-repurposing (preprint) Syst. Biol. (Stevenage) 2020 doi: 10.1101/2020.03.22.002386. [DOI] [Google Scholar]
- Daniloski Z., Guo X., Sanjana N.E. The D614G mutation in SARS-CoV-2 Spike increases transduction of multiple human cell types. bioRxiv. 2020;2020 doi: 10.1101/2020.06.14.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. In: Roles of Host Gene and Non-Coding RNA Expression in Virus Infection, Current Topics in Microbiology and Immunology. Tripp R.A., Tompkins S.M., editors. Springer International Publishing; Cham: 2018. Host factors in coronavirus replication; pp. 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L’Haridon R., Vogel L.K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Lan J., Bao L., Huang B., Ye F., Chen Y., Yao Y., Wang W., Qin C., Tan W. Enhanced protection in mice induced by immunization with inactivated whole viruses compare to spike protein of middle east respiratory syndrome coronavirus. Emerg. Microbes Infect. 2018;7:60. doi: 10.1038/s41426-018-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Wang Y., Zhang H., Ma S., Wong C.K.L., Wu S.H.W., Ng F., Huang J.-D., Yuen K.-Y., Jiang S., Zhou Y., Zheng B.-J. Recombinant adeno-associated virus expressing the receptor-binding domain of severe acute respiratory syndrome coronavirus S protein elicits neutralizing antibodies: Implication for developing SARS vaccines. Virology. 2006;353:6–16. doi: 10.1016/j.virol.2006.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., He Y., Guo Y., Zheng B.-J., Jiang S., Zhou Y. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine. 2007;25:2832–2838. doi: 10.1016/j.vaccine.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Chan C.C.S., Sun S., Chen M., Liu Z., Guo H., He Y., Zhou Y., Zheng B.-J., Jiang S. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. Coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393:144–150. doi: 10.1016/j.virol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Kou Z., Ma C., Tao X., Wang L., Zhao G., Chen Y., Yu F., Tseng C.-T.K., Zhou Y., Jiang S. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H., Ma Z., Xu L., Zhang A., Li Z., Xiao S. A novel intracellularly expressed NS5B-specific nanobody suppresses bovine viral diarrhea virus replication. Vet. Microbiol. 2020;240 doi: 10.1016/j.vetmic.2019.108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G.S., Pensiero M.N., Cardellichio C.B., Williams R.K., Jiang G.S., Holmes K.V., Dieffenbach C.W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J. Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaaswarkhanth M., Al Madhoun A., Al-Mulla F. Could the D614G substitution in the SARS-CoV-2 spike (S) protein be associated with higher COVID-19 mortality? Int. J. Infect. Dis. 2020;96:459–460. doi: 10.1016/j.ijid.2020.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuanes Luis, Zúñiga Isabel Solay Sonia. Centro Nacional de Biotecnología (CNB-CSIC); Madrid (Spain): 2020. NOVEL HUMAN PATHOGENIC CORONAVIRUS: SARS-CoV-2. March 26. n.d. [Google Scholar]
- EpiVax EpiVax Accelerates COVID-19 Vaccine Development With UGA’s Center for Vaccines and Immunology. May 04. 2020. https://epivax.com/news/press-release-epivax-accelerates-covid-19-vaccine-development-with-ugas-center-for-vaccines-and-immunology Accessed May 21, 2020., n.d.
- Eric Niiler The US Army’s Virus Research Lab Gears Up to Fight Covid-19. April 01. 2020. https://www.wired.com/story/the-us-armys-virus-research-lab-gears-up-to-fight-covid-19/ Accessed on May21, 2020., n.d.
- ExpreS2ion ExpreS2ion Announces Bavarian Nordic Enters Agreement With AdaptVac to Advance the COVID-19 Vaccine. May 06. 2020. https://expres2ionbio.com/investor/international-press-releases/ Accessed May 21, 2020., n.d.
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M., Lamirande E.W., Roberts A., Rice A.B., Koprowski H., Dietzschold B., Schnell M.J. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J. Gen. Virol. 2005;86:1435–1440. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast-Farming Pharma How Plants Could Speed up Vaccine Manufacture to Tackle the COVID-19 Pandemic? April 17. 2020. https://themedicinemaker.com/manufacture/fast-farming-pharma Accessed May 21, 2020., n.d.
- Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flow Pharma Flow Pharma Announces Strategic Partnership With Oakwood Labs for GMP Manufacturing of FlowVax COVID-19 Vaccine. April 15. 2020. https://www.accesswire.com/585181/Flow-Pharma-Announces-Strategic-Partnership-with-Oakwood-Labs-for-GMP-Manufacturing-of-FlowVax-COVID-19-Vaccine Accessed May 21, 2020., n.d.
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;0 doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal D.N., Landucci G., Daar E.S. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudan University Towards an Effective mRNA Vaccine Against 2019-nCoV: March 7. 2020. https://www.fudan.edu.cn/en/2020/0307/c1092a104281/page.htm Accessed May 21, 2020., n.d.
- Zhang G., Pomplun S., Loftis A.R., Loas A., Pentelute B.L. The first-in-class peptide binder to the SARS-CoV-2 spike protein (preprint) Bioengineering. 2020 doi: 10.1101/2020.03.19.999318. [DOI] [Google Scholar]
- Gamaleya Research Institute. https://www.trialsitenews.com/gamaleya-research-institute-developed-covid-19-vaccine-clinical-trial-commences-across-russia/. Accessed on JUly 29, 2020., n.d.
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. Coronavirus vaccine developers wary of errant antibodies. Nat. Biotechnol. 2020 doi: 10.1038/d41587-020-00016-w. [DOI] [PubMed] [Google Scholar]
- Generex Generex Signs Contract With EpiVax to Develop Ii-key Peptide Vaccines to Address the Coronavirus Pandemic. March 04, 202. https://epivax.com/news/press-release-generex-signs-contract-with-epivax-to-develop-ii-key-peptide-vaccines-to-address-the-coronavirus-pandemic Accessed May 21, 2020., n.d.
- Genexine Consortium. https://pharmashots.com/press-releases/korean-biotech-firm-genexines-covid-19-deoxyribonucleic-acid-vaccine-pipeline-gx-19-is-ready-for-clinical-testing-as-soon-as-it-applies-to-the-drug-ministry-for-human-testing/. Accessed July 29, 2020, n.d.
- George Sakoulas M.D. Remdesivir: a promising antiviral against coronaviruses. NEJM Journal Watch. 2020;2020 doi: 10.1056/nejm-jw.NA50889. [DOI] [Google Scholar]
- GeoVax GeoVax Progresses in Coronavirus (COVID-19) Vaccine Development Program. March 18. 2020. https://www.globenewswire.com/news- release/2020/03/18/2002611/0/en/ GeoVax-Progresses-in-Coronavirus-COVID-19-Vaccine-Development-Program.html. Accessed May21, 2020., n.d.
- Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godet M., Grosclaude J., Delmas B., Laude H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994;68:8008–8016. doi: 10.1128/jvi.68.12.8008-8016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlani A., Forthal D.N. Antibody-dependent enhancement and the risk of HIV infection. Curr. HIV Res. 2013;11:421–426. doi: 10.2174/1570162x113116660062. [DOI] [PubMed] [Google Scholar]
- Greenberg S.B., Criswell B.S., Six H.R., Couch R.B. Lymphocyte cytotoxicity to influenza virus-infected cells. II. Requirement for antibody and non-T lymphocytes. J. Immunol. 1977;119:2100–2106. [PubMed] [Google Scholar]
- Greffex Greffex Completes COVID-19 Vaccine, Prepares for FDA Animal Testing. March 12. 2020. https://www.innovationews.com/Greffex-completes-COVID-19-vaccine-prepares-for-FDA-testing/ Accessed May 21, 2020., n.d.
- Grubaugh N.D., Hanage W.P., Rasmussen A.L. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell. 2020 doi: 10.1016/j.cell.2020.06.040. S0092867420308175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSK GSK Joins Xiamen Innovax Biotech to Support COVID-19 Vaccine Development & Expands Activity to Fight War Against Pandemic. April 06. 2020. https://www.trialsitenews.com/gsk-joins-xiamen-innovax-biotech-to-support-covid-19-vaccine-development-expands-activity-to-fight-war-against-pandemic/ Accessed May 21, 2020., n.d.
- Gunn B.M., Yu W.-H., Karim M.M., Brannan J.M., Herbert A.S., Wec A.Z., Halfmann P.J., Fusco M.L., Schendel S.L., Gangavarapu K., Krause T., Qiu X., He S., Das J., Suscovich T.J., Lai J., Chandran K., Zeitlin L., Crowe J.E., Lauffenburger D., Kawaoka Y., Kobinger G.P., Andersen K.G., Dye J.M., Saphire E.O., Alter G. A role for fc function in therapeutic monoclonal antibody-mediated protection against ebola virus. Cell Host Microbe. 2018;24:221–233. doi: 10.1016/j.chom.2018.07.009. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambach J., Riecken K., Cichutek S., Schütze K., Albrecht B., Petry K., et al. Targeting CD38-Expressing multiple myeloma and burkitt lymphoma cells in vitro with nanobody-based chimeric antigen receptors (Nb-CARs) Cells. 2020;9 doi: 10.3390/cells9020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hammers C., Songa E.B., Bendahman N., Hammers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- Han D.P., Penn-Nicholson A., Cho M.W. Identification of critical determinants on ACE2 for SARS-CoV entry and development of a potent entry inhibitor. Virology. 2006;350:15–25. doi: 10.1016/j.virol.2006.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon A., Metjian A. Caplacizumab in adult patients with acquired thrombotic thrombocytopenic purpura. Ther. Adv. Hematol. 2020;11 doi: 10.1177/2040620720902904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson Q.M., Wilson K.M., Shen M., Itkin Z., Eastman R.T., Shinn P., Hall M.D. Targeting ACE2-RBD interaction as a platform for COVID19 therapeutics: development and drug repurposing screen of an AlphaLISA proximity assay. bioRxiv. 2020 doi: 10.1101/2020.06.16.154708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen M.M., Van Solt C.B., Fijten H.P.D., Van Setten M.C. Prolonged in vivo residence times of llama single-domain antibody fragments in pigs by binding to porcine immunoglobulins. Vaccine. 2005;23:4926–4934. doi: 10.1016/j.vaccine.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Haynes B.F., Gilbert P.B., McElrath M.J., Zolla-Pazner S., Tomaras G.D., Alam S.M., Evans D.T., Montefiori D.C., Karnasuta C., Sutthent R., Liao H.-X., DeVico A.L., Lewis G.K., Williams C., Pinter A., Fong Y., Janes H., DeCamp A., Huang Y., Rao M., Billings E., Karasavvas N., Robb M.L., Ngauy V., de Souza M.S., Paris R., Ferrari G., Bailer R.T., Soderberg K.A., Andrews C., Berman P.W., Frahm N., De Rosa S.C., Alpert M.D., Yates N.L., Shen X., Koup R.A., Pitisuttithum P., Kaewkungwal J., Nitayaphan S., Rerks-Ngarm S., Michael N.L., Kim J.H. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M., Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heat Biologics Heat Biologics Announces Research Collaboration With University of Miami to Develop Vaccine Designed to Protect Against COVID-19 Coronavirus. Match 05. 2020. https://www.heatbio.com/news-media/news-releases/detail/649/heat-biologics-announces-research-collaboration-with May 21, 2020., n.d.
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Hattermann K., Marzi A., Gramberg T., Geier M., Krumbiegel M., Kuate S., Uberla K., Niedrig M., Pöhlmann S. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004;78:6134–6142. doi: 10.1128/JVI.78.12.6134-6142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Pyrc K., Hoek Lvander, Geier M., Berkhout B., Pöhlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. PNAS. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser K.V., et al. Enhanced inflammation in New Zealand white rabbits when MERS-CoV reinfection occurs in the absence of neutralizing antibody. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006565. PPATHOGENS-D-17-00350 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Li L., Kao R.Y., Kou B., Wang Z., Zhang L., et al. Screening and identification of linear B-cell epitopes and entry-blocking peptide of severe acute respiratory syndrome (SARS)-associated coronavirus using synthetic overlapping peptide library. J. Comb. Chem. 2005;7:648–656. doi: 10.1021/cc0500607. [DOI] [PubMed] [Google Scholar]
- Huang J., Cao Y., Du J., Bu X., Ma R., Wu C. Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses. Vaccine. 2007;25:6981–6991. doi: 10.1016/j.vaccine.2007.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huentelman Matthew J., Zubcevic Jasenka, Hernández Prada, Jose A., Xiaodong Xiao, Dimitrov Dimiter S., Raizada Mohan K., Ostrov David A. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44:903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- Hulswit R.J.G., de Haan C.A.M., Bosch B.-J. In: Advances in Virus Research, Coronaviruses. Ziebuhr J., editor. Academic Press; 2016. Chapter Two - coronavirus spike protein and tropism changes; pp. 29–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IAVI and Batavia Biosciences Announce Collaboration on VSV-vector Based Epidemic Preparedness Vaccines:https://www.iavi.org/newsroom/press-releases/2020/iavi-and-batavia-announce-collaboration-vsv-vector-epidemic-preparedness-vaccines. March 5, 2020., n.d.
- Imperial College London. https://www.clinicaltrialsarena.com/news/imperial-college-covid-vaccine/. Accessed on July 29, 2020., n.d.
- Improvement of Pharmacokinetic Profile of TRAIL via Trimer-Tag Enhances its Antitumor Activity in vivo. - PubMed - NCBI [WWW Document], n.d. URL https://www.ncbi.nlm.nih.gov/pubmed/28827692 (accessed 5.6.20).
- IMV DPX-COVID-19 at a glance. April 15. 2020. https://imv-inc.com/clinical-trials/dpx-covid-19/ Accessed May 21, 2020., n.d.
- Inovio Pharmaceuticals/International Vaccine Institute. http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-and-IVI-Partner-with-Seoul-National-University-Hospital-to-Start-Phase-12-Clinical-Trial-of-INOVIOs-COVID-19-DNA-Vaccine-INO-4800-in-South-Korea/default.aspx. Accessed July 29, 2020, n.d.
- Institute of Medical Biology, Chinesee Academy of Medical Sciences. http://english.nmpa.gov.cn/2020-06/22/c_502093.htm. Accessed on July 29, 2020., n.d.
- Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 2020;20:339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020 doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N. Engl. J. Med. 2020;0 doi: 10.1056/NEJMoa2022483. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaume M., Yip M.S., Cheung C.Y., Leung H.L., Li P.H., Kien F., Dutry I., Callendret B., Escriou N., Altmeyer R., Nal B., Daëron M., Bruzzone R., Peiris J.S.M. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegaskanda S., Reading P.C., Kent S.J. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J. Immunol. 2014;193:469–475. doi: 10.4049/jimmunol.1400432. [DOI] [PubMed] [Google Scholar]
- Ji L., Dong C., Fan R., Qi S. A high affinity nanobody against endothelin receptor type B: a new approach to the treatment of melanoma. Mol. Biol. Rep. 2020;47:2137–2147. doi: 10.1007/s11033-020-05313-w. [DOI] [PubMed] [Google Scholar]
- Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg. Microbes Infect. 2020;9:275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends in Immunology S1471490620300570. 2020 doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University: https://coronavirus.jhu.edu/map.html. Accessed on August 19, 2020., n.d.
- Johnson & Johnson Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19; Landmark New Partnership With U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use. March 30. 2020. https://www.jnj.com/johnson-johnson-announces-a-lead-vaccine-candidate-for-covid-19-landmark-new-partnership-with-u-s-department-of-health-human-services-and-commitment-to-supply-one-billion-vaccines-worldwide-for-emergency-pandemic-use Accessed on May 21, 2020., n.d.
- Kao R.Y., Tsui W.H.W., Lee T.S.W., Tanner J.A., Watt R.M., Huang J.-D., Hu L., Chen G., Chen Z., Zhang L., He T., Chan K.-H., Tse H., To A.P.C., Ng L.W.Y., Wong B.C.W., Tsoi H.-W., Yang D., Ho D.D., Yuen K.-Y. Identification of novel small-molecule inhibitors of severe acute respiratory syndrome-associated coronavirus by chemical genetics. Chem. Biol. 2004;11:1293–1299. doi: 10.1016/j.chembiol.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C., Kuan G., Gordon A., Balmaseda A., Harris E. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358:929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentucky Bioprocessing, Inc. https://www.kentucky.com/news/coronavirus/article241709386.html. Accessed on July 29, 2020., n.d.
- Khandia R., Munjal A., Dhama K., Karthik K., Tiwari R., Malik Y.S., Singh R.K., Chaicumpa W. Modulation of Dengue/Zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in zika virus infection. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.W., Canchola J.G., Brandt C.D., Pyles G., Chanock R.M., Jensen K., Parrott R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kim E., Erdos G., Huang S., Kenniston T.W., Balmert S.C., Carey C.D., Raj V.S., Epperly M.W., Klimstra W.B., Haagmans B.L., Korkmaz E., Falo L.D., Gambotto A. Microneedle array delivered recombinant coronavirus vaccines: immunogenicity and rapid translational development. EBioMedicine. 2020:102743. doi: 10.1016/j.ebiom.2020.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobinger G.P., Figueredo J.M., Rowe T., Zhi Y., Gao G., Sanmiguel J.C., Bell P., Wivel N.A., Zitzow L.A., Flieder D.B., Hogan R.J., Wilson J.M. Adenovirus-based vaccine prevents pneumonia in ferrets challenged with the SARS coronavirus and stimulates robust immune responses in macaques. Vaccine. 2007;25:5220–5231. doi: 10.1016/j.vaccine.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Loo L.S. Protection of neonatal mice against herpes simplex virus infection: probable in vivo antibody-dependent cellular cytotoxicity. J. Immunol. 1982;129:370–376. [PubMed] [Google Scholar]
- Kohl S., Charlebois E.D., Sigouroudinia M., Goldbeck C., Hartog K., Sekulovich R.E., Langenberg A.G.M., Burke R.L. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J. Infect. Dis. 2000;181:335–339. doi: 10.1086/315208. [DOI] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C., Angyal A., Brown R.L., Carrilero L., Green L.R., Groves D.C., Johnson K.J., Keeley A.J., Lindsey B.B., Parsons P.J., Raza M., Rowland-Jones S., Smith N., Tucker R.M., Wang D., Wyles M.D. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020 doi: 10.1016/j.cell.2020.06.043. S0092867420308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempl C., Schultze B., Laude H., Herrler G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997;71:3285–3287. doi: 10.1128/jvi.71.4.3285-3287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L., Teng Q., Chen Q., Zhang F. Preparation and characterization of Anti-GPC3 nanobody against hepatocellular carcinoma. Int. J. Nanomedicine. 2020;15:2197–2205. doi: 10.2147/IJN.S235058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jackson C.B., Mou H., Ojha A., Rangarajan E.S., Izard T., Farzan M., Choe H. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv. 2020;2020 doi: 10.1101/2020.06.12.148726. 06.12.148726. [DOI] [Google Scholar]
- Lan J., Yao Y., Deng Y., Chen H., Lu G., Wang W., Bao L., Deng W., Wei Q., Gao G.F., Qin C., Tan W. Recombinant receptor binding domain protein induces partial protective immunity in Rhesus macaques against middle east respiratory syndrome coronavirus challenge. EBioMedicine. 2015;2:1438–1446. doi: 10.1016/j.ebiom.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen N.S., Friesen R.H.E., Zhu X., Jongeneelen M., Blokland S., Vermond J., et al. Universal protection against influenza infection by a multidomain antibody to influenza hemagglutinin. Science. 2018;362:598–602. doi: 10.1126/science.aaq0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledwith B.J., Manam S., Troilo P.J., Barnum A.B., Pauley C.J., Griffiths T.G., Harper L.B., Schock H.B., Zhang H., Faris J.E., Way P.A., Beare C.M., Bagdon W.J., Nichols W.W. Plasmid DNA vaccines: assay for integration into host genomic DNA. Dev. Biol. (Basel) 2000;104:33–43. [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J.Y. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Receptor recognition mechanisms of coronaviruses: a decade of structural studies. J. Virol. 2015;89:1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Huang M., Xiao H., Zhang G., Ding J., Wu P., Zhang H., Sheng J., Chen C. Selection and characterization of specific nanobody against bovine virus diarrhea virus (BVDV) E2 protein. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhang W., Jiang K., Shan H., Shi M., Chen B., Hua Z. Nanobody against the E7 oncoprotein of human papillomavirus 16. Mol. Immunol. 2019;109:12–19. doi: 10.1016/j.molimm.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Li S., Jiang K., Wang T., Zhang W., Shi M., Chen B., Hua Z. Nanobody against PDL1. Biotechnol. Lett. 2020;42:727–736. doi: 10.1007/s10529-020-02823-2. [DOI] [PubMed] [Google Scholar]
- Lin H.-X., Feng Y., Wong G., Wang L., Li B., Zhao X., Li Y., Smaill F., Zhang C. Identification of residues in the receptor-binding domain (RBD) of the spike protein of human coronavirus NL63 that are critical for the RBD–ACE2 receptor interaction. J. Gen. Virol. 2008;89:1015–1024. doi: 10.1099/vir.0.83331-0. [DOI] [PubMed] [Google Scholar]
- Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.-M., Yuan B., Kinoshita R., Nishiura H. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.A. A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines (Basel) 2019;7 doi: 10.3390/vaccines7020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.-Y., Wu L.-Z., Huang B.-J., Huang J.-L., Zhang Y.-L., Ke M.-L., Wang J.-M., Tan W.-P., Zhang R.-H., Chen H.-K., Zeng Y.-X., Huang W. Adenoviral expression of a truncated S1 subunit of SARS-CoV spike protein results in specific humoral immune responses against SARS-CoV in rats. Virus Res. 2005;112:24–31. doi: 10.1016/j.virusres.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z., Wu T., Cheung K.-W., Chan K.-H., Alvarez X., Qin C., Lackner A., Perlman S., Yuen K.-Y., Chen Z. Anti–spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4 doi: 10.1172/jci.insight.123158. 123158 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., Hu J.-L., Xu W., Zhang Y., Lv F.-J., Su K., Zhang F., Gong J., Wu B., Liu X.-M., Li J.-J., Qiu J.-F., Chen J., Huang A.-L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Wu N.C., Tsang O.T.-Y., Yuan M., Perera R.A.P.M., Leung W.S., So R.T.Y., Chan J.M.C., Yip G.K., Chik T.S.H., Wang Y., Choi C.Y.C., Lin Y., Ng W.W., Zhao J., Poon L.L.M., Peiris J.S.M., Wilson I.A., Mok C.K.P. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. bioRxiv. 2020 doi: 10.1101/2020.03.15.993097. 2020.03.15.993097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J.E., Louder M.K., Holman L.A., Gordon I.J., Enama M.E., Larkin B.D., Andrews C.A., Vogel L., Koup R.A., Roederer M., Bailer R.T., Gomez P.L., Nason M., Mascola J.R., Nabel G.J., Graham B.S., VRC 301 Study Team A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. 2008;26:6338–6343. doi: 10.1016/j.vaccine.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.A. Compounds with therapeutic potential against novel respiratory 2019 coronavirus. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00399-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P.S. Advances in Virus Research. Academic Press; 2006. The molecular biology of coronaviruses; pp. 193–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T., Cao H., Zhang H., Kang Z., Xu D., Gong H., Wang J., Li Z., Cui X., Xu H., Wei H., Pan X., Zhu R., Xiao J., Zhou W., Cheng L., Liu J. The insert sequence in SARS-CoV-2 enhances spike protein cleavage by TMPRSS. bioRxiv. 2020;2020 doi: 10.1101/2020.02.08.926006. 02.08.926006. [DOI] [Google Scholar]
- Meyer K., Ait-Goughoulte M., Keck Z.-Y., Foung S., Ray R. Antibody-dependent enhancement of hepatitis C virus infection. J. Virol. 2008;82:2140–2149. doi: 10.1128/JVI.01867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIGAL’s Coronavirus Vaccine Project:https://www.migal.org.il/en/node/7010. Accessed May 21, 2020., n.d.
- Moradi-Kalbolandi S., Sharifi-K A., Darvishi B., Majidzadeh-A K., Jalili N., Sadeghi S., Mosayebzadeh M., Sanati H., Salehi M., Farahmand L. Evaluation the potential of recombinant anti-CD3 nanobody on immunomodulatory function. Mol. Immunol. 2020;118:174–181. doi: 10.1016/j.molimm.2019.12.017. [DOI] [PubMed] [Google Scholar]
- Mou H., Raj V.S., van Kuppeveld F.J.M., Rottier P.J.M., Haagmans B.L., Bosch B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 2013;87:9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Adair B.D., Yoshioka C., Quispe J.D., Orca G., Kuhn P., Milligan R.A., Yeager M., Buchmeier M.J. Supramolecular Architecture of Severe Acute Respiratory Syndrome Coronavirus Revealed by Electron Cryomicroscopy. J. Virol. 2006;80:7918–7928. doi: 10.1128/JVI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novavax. https://ir.novavax.com/news-releases/news-release-details/novavax-initiates-phase-12-clinical-trial-covid-19-vaccine. Accessed on July 29, 2020., n.d.
- Ogawa J., Zhu W., Tonnu N., Singer O., Hunter T., Ryan A.L., (Firth), Pao G.M. The D614G mutation in the SARS-CoV2 Spike protein increases infectivity in an ACE2 receptor dependent manner. bioRxiv. 2020;2020 doi: 10.1101/2020.07.21.214932. 07.21.214932. [DOI] [Google Scholar]
- OncoGen. OncoGen researchers propose personalized vaccinomics strategy for the novel China coronavirus: https://oncogen.ro/oncogen-vaccine-design-for-coronavirus/. Accessed May 21, 2020., n.d.
- Osaka University/AnGes/Takara Bio. https://www.takarabio.com/about/bioview-blog/current-events/dethroning-king-coronavirus-with-novel-vaccines. Accessed July 29, 2020, n.d.
- Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil S., Hofer J., Ballester P.J., Fattakhova E., DiFlumeri J., Campbell A., Oravic M. 2020. Drug Repurposing for Covid-19: Discovery of Potential Small-Molecule Inhibitors of Spike Protein-ACE2 Receptor Interaction Through Virtual Screening and Consensus Scoring. [DOI] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Pharmajet: Pharmajet partner with I.T.I. and EpiVax to develop and deliver COVID-10 Vaccine. April 15, 2020. https://www.immunomix.com/pharmajet-partners-with-iti-and-epivax-to-develop-and-deliver-covid-19-vaccine/. Accessed May 21, 2020., n.d.
- Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y., Wang Qisheng, Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.03.045. S009286742030338X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H.W., Müller M.A., Dijkman R., Muth D., Demmers J.A.A., Zaki A., Fouchier R.A.M., Thiel V., Drosten C., Rottier P.J.M., Osterhaus A.D.M.E., Bosch B.J., Haagmans B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Okba N.M.A., Gutierrez-Alvarez J., Drabek D., Dieren B., van Widagdo W., et al. Chimeric camel/human heavy-chain antibodies protect against MERS-CoV infection. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aas9667. eaas9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch S., Jasny E., Schmidt K.E., Petsch B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReiThera LEUKOCARE And Univercells. ReiThera, Leukocare and Univercells Announce Fast-track Development of a COVID-19 Vaccine. APRIL 23. 2020. https://www.univercells.com/newsroom/reithera-leukocare-and-univercells-announce-fast-track-development-of-a-covid-19-vaccine/ Accessed on May 21, 2020., n.d.
- Renner M., Flanagan A., Dejnirattisai W., Puttikhunt C., Kasinrerk W., Supasa P., Wongwiwat W., Chawansuntati K., Duangchinda T., Cowper A., Midgley C.M., Malasit P., Huiskonen J.T., Mongkolsapaya J., Screaton G.R., Grimes J.M. Characterization of a potent and highly unusual minimally-enhancing antibody directed against dengue virus. Nat. Immunol. 2018;19:1248–1256. doi: 10.1038/s41590-018-0227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiba Biotech. Saiba proprietary technology has enabled scientists to generate a vaccine candidate against COVID-19 with preclinical proof-of-concept. https://www.saiba-biotech.com/. Accessed May 21, 2020., n.d.
- Saint-Petersburg scientific research institute of vaccines and serums. May22, 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. Accessed May 25, 2020., n.d.
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. The novel coronavirus, 2019-nCoV, is highly contagious and more infectious than initially estimated (preprint) Epidemiology. 2020 doi: 10.1101/2020.02.07.20021154. [DOI] [Google Scholar]
- Sanofi and GSK Sanofi and GSK to Join Forces in Unprecedented Vaccine Collaboration to Fight COVID-19. April 14. 2020. https://www.sanofi.com/en/media-room/press-releases/2020/2020-04-14-13-00-00 Accessed May 21, 2020., n.d.
- Saphire E.O., Schendel S.L., Fusco M.L., Gangavarapu K., Gunn B.M., Wec A.Z., et al. Systematic analysis of monoclonal antibodies against Ebola virus GP defines features that contribute to protection. Cell. 2018;174:938–952. doi: 10.1016/j.cell.2018.07.033. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARS-CoV-2: DZIF scientists and the development of vaccines https://www.dzif.de/en/sars-cov-2-dzif-scientists-and-development-vaccines Mar 9 2020., n.d.
- Schriewer L., Schütze K., Petry K., Hambach J., Fumey W., Koenigsdorf J., et al. Nanobody-based CD38-specific heavy chain antibodies induce killing of multiple myeloma and other hematological malignancies. Theranostics. 2020;10:2645–2658. doi: 10.7150/thno.38533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze B., Gross H.J., Brossmer R., Herrler G. The S protein of bovine coronavirus is a hemagglutinin recognizing 9-O-acetylated sialic acid as a receptor determinant. J. Virol. 1991;65:6232–6237. doi: 10.1128/jvi.65.11.6232-6237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully M., Cataland S.R., Peyvandi F., Coppo P., Knöbl P., Hovinga J.A.K., Metjian A., Rubia J., de la, Pavenski K., Callewaert F., Biswas D., Winter H.D., Zeldin R.K. Caplacizumab treatment for acquired thrombotic thrombocytopenic Purpura. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa1806311. [DOI] [Google Scholar]
- Seow J., Graham C., Merrick B., Acors S., Steel K.J.A., Hemmings O., et al. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection (preprint) Infectious Diseases (except HIV/AIDS). 2020 doi: 10.1101/2020.07.09.20148429. [DOI] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. PNAS. 2020 doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim B.-S., Stadler K., Nguyen H.H., Yun C.-H., Kim D.W., Chang J., Czerkinsky C., Song M.K. Sublingual immunization with recombinant adenovirus encoding SARS-CoV spike protein induces systemic and mucosal immunity without redirection of the virus to the brain. Virol. J. 2012;9:215. doi: 10.1186/1743-422X-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionogi Inc Shionogi Accelerates Development of Potential COVID-19 Treatments and Vaccine. April 28. 2020. https://www.shionogi.com/us/en/news/2020/4/shionogi-accelerates-development-of-potential-covid-19-treatments-and-vaccine.html Accessed May21, 2020., n.d.
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S.M., Bruzzone R., Nal B. The M, E, and N Structural Proteins of the Severe Acute Respiratory Syndrome Coronavirus Are Required for Efficient Assembly, Trafficking, and Release of Virus-Like Particles. J. Virol. 2008;82:11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck A.-W., Axmann M., Pfefferle S., Drosten C., Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012;94:288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Li W., Murakami A., Tamin A., Matthews L.J., Wong S.K., Moore M.J., Tallarico A.S.C., Olurinde M., Choe H., Anderson L.J., Bellini W.J., Farzan M., Marasco W.A. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2536–2541. doi: 10.1073/pnas.0307140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symvivo Corporation Evaluating the Safety, Tolerability, and Immunogenicity of bacTRL-Spike Vaccine for Prevention of COVID-19. April 6. 2020. https://clinicaltrials.gov/ct2/show/NCT04334980 Accessed May 21, 2020., n.d.
- Tai W., Zhao G., Sun S., Guo Y., Wang Y., Tao X., Tseng C.-T.K., Li F., Jiang S., Du L., Zhou Y. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology. 2016;499:375–382. doi: 10.1016/j.virol.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020 doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A., Watanabe S., Okazaki K., Kida H., Kawaoka Y. Infectivity-enhancing antibodies to ebola virus glycoprotein. J. Virol. 2001;75:2324–2330. doi: 10.1128/JVI.75.5.2324-2330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y., Osaki M., Ishimaru Y., Yamaguchi H., Harada A. Artificial molecular clamp: a novel device for synthetic polymerases. Angew. Chemie Int. Ed. 2011;50:7524–7528. doi: 10.1002/anie.201102834. [DOI] [PubMed] [Google Scholar]
- Takis Takis, a Biotech Company in Castel Romano, Rome, Announces That It Is Ready to Test Its Covid-19 Vaccine on Pre-clinical Models. 17 March. 2020. http://www.takisbiotech.it/index.php/en/news/209-rome-17-march-2020-takis-a-biotech-company-in-castel-romano-rome-announces-that-it-is-ready-to-test-its-covid-19-vaccine-on-pre-clinical-models Accessed on May 21, 2020., n.d.
- Tarr A.W., Lafaye P., Meredith L., Damier-Piolle L., Urbanowicz R.A., Meola A., Jestin J.-L., Brown R.J.P., McKeating J.A., Rey F.A., Ball J.K., Krey T. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology. 2013;58:932–939. doi: 10.1002/hep.26430. [DOI] [PubMed] [Google Scholar]
- ter Meulen J., Bakker A.B.H., van den Brink E.N., Weverling G.J., Martina B.E.E., Haagmans B.L., et al. Human monoclonal antibody as prophylaxis for SARS coronavirus infection in ferrets. Lancet. 2004;363:2139–2141. doi: 10.1016/S0140-6736(04)16506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Meulen J., van den Brink E.N., Poon L.L.M., Marissen W.E., Leung C.S.W., Cox F., et al. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes and infection. Special issue on the new coronavirus causing the COVID-19 outbreak. 2020;22:72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonix Pharma TNX-1800 Horsepox Vector for S Protein. April 26. 2020. https://covidvax.org/covid19-vaccine/Tonix/TNX-1800-Horsepox-vector-for-S-protein-Tonix-Pharma-Southern-Research Accessed May 21, 2020., n.d.
- Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- Tse L.V., Meganck R.M., Graham R.L., Baric R.S. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front. Microbiol. 2020;11:658. doi: 10.3389/fmicb.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich H., Pillat M.M., Tárnok A. Dengue fever, COVID-19 (SARS-CoV-2), and antibody-dependent enhancement (ADE): a perspective. Cytom. Part A. 2020;97:662–667. doi: 10.1002/cyto.a.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Hong Kong CEPI Partners With University of Hong Kong to Develop COVID-19 Vaccine. March 18. 2020. https://cepi.net/news_cepi/cepi-partners-with-university-of-hong-kong-to-develop-covid-19-vaccine/ Accessed May 21, 2020., n.d.
- University of Ontario Ontario Leading COVID-19 Research in Canada Province Announces First Phase of Research Projects to Fight COVID-19. May 21. 2020. https://news.ontario.ca/opo/en/2020/05/ontario-leading-covid-19-research-in-canada.html Accessed May 21, 2020, n.d.
- University of Queensland /GSK/Dynavax. https://advance.qld.gov.au/covid-19-fast-facts. Accessed on July 29, 2020., n.d.
- University of Tokoyo and Daiichi Sankyo. Our Company’s Efforts to Limit the Spread of the Virus that Causes COVID-19 Accessed May 21, 2020., n.d.
- University of Waterloo University of Waterloo Developing DNA-based COVID-19 Vaccine. April 16. 2020. https://uwaterloo.ca/stories/news/university-waterloo-developing-dna-based-covid-19-vaccine Accessed May 21, 2020., n.d.
- USask USask VIDO-InterVac Awarded $23M for COVID-19 Vaccine Research. April 23. 2020. https://news.usask.ca/articles/research/2020/usask-vido-intervac-awarded-23m-for-covid-19-vaccine-research.php Accessed May 21, 2020., n.d.
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V., Bushmaker T., Flaxman A., Ulaszewska M., Feldmann F., Allen E.R., Sharpe H., Schulz J., Holbrook M., Okumura A., Meade-White K., Pérez-Pérez L., Bissett C., Gilbride C., Williamson B.N., Rosenke R., Long D., Ishwarbhai A., Kailath R., Rose L., Morris S., Powers C., Lovaglio J., Hanley P.W., Scott D., Saturday G., de Wit E., Gilbert S.C., Munster V.J. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques (preprint) Microbiology. 2020 doi: 10.1101/2020.05.13.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaxart Vaxart Announces Additional Positive Pre-clinical Data for Its Oral COVID-19 Vaccine Program. April 30. 2020. https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-additional-positive-pre-clinical-data-its-oral/ Accessed May 21, 2020., n.d.
- Vaxil Vaxil Commences Preclinical COVID-19 Vaccine Trial and Files an Additional COVD-19 Patent. March 27. 2020. https://vaxil-bio.com/vaxil-commences-preclinical-covid-19-vaccine-trial-and-files-an-additional-covid-19-patent/ Accessed May 21, 2020., n.d.
- Vaxine Pty Ltd/Medytox. https://www.biospectrumasia.com/news/37/16000/vaxine-announces-covid-19-vaccine-collaboration-with-medytox.html. Accessed on July 29, 2020., n.d.
- Walls A.C., et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176:1026–1039. doi: 10.1016/j.cell.2018.12.028. e1015, doi:S0092-8674(18)31642-31648 [pii] 10.1016/j.cell.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and Antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J.D., Hutter C.A.J., Zimmermann I., Earp J., Egloff P., Sorgenfrei M., Hürlimann L.M., Gonda I., Meier G., Remm S., Thavarasah S., Plattet P., Seeger M.A. Synthetic nanobodies targeting the SARS-CoV-2 receptor-binding domain. bioRxiv. 2020;2020 doi: 10.1101/2020.04.16.045419. 04.16.045419. [DOI] [Google Scholar]
- Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q., He L., Chen Y., Wu J., Shi Z., Zhou Y., Du L., Li F. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 2019;94:e02015–02019. doi: 10.1128/JVI.02015-19. /jvi/94/5/JVI.02015-02019.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-F., Tseng S.-P., Yen C.-H., Yang J.-Y., Tsao C.-H., Shen C.-W., Chen K.-H., Liu F.-T., Liu W.-T., Chen Y.-M.A., Huang J.C. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tai W., Yang J., Zhao G., Sun S., Tseng C.-T.K., Jiang S., Zhou Y., Du L., Gao J. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum. Vaccin. Immunother. 2017;13:1615–1624. doi: 10.1080/21645515.2017.1296994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang L., Huang B., Li K., Hou G., Zhao Q., Wu C., Nan Y., Du T., Mu Y., Lan J., Chen H., Zhou E.-M. A nanobody targeting viral nonstructural protein 9 inhibits porcine reproductive and respiratory syndrome virus replication. J. Virol. 2019;93 doi: 10.1128/JVI.01888-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Middle East Respiratory Syndrome Coronavirus (MERS-CoV). August 2. 2019. https://www.who.int/emergencies/mers-cov/en/ Accessed on May 27, 2020., n.d.
- WHO Draft Landscape of COVID-19 Candidate Vaccines. May 30. 2020. https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines Accessed May 30, 2020., n.d.
- WHO Novel Coronavirus(2019-nCoV) Situation Report – 22. February 11. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf Accessed May27, 2020., n.d.
- Willey S., Aasa-Chapman M.M., O’Farrell S., Pellegrino P., Williams I., Weiss R.A., Neil S.J. Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection. Retrovirology. 2011;8:16. doi: 10.1186/1742-4690-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.K., Jiang G.S., Holmes K.V. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. PNAS. 1991;88:5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Van Breedam W., Roose K., et al. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies (preprint) Microbiology. 2020 doi: 10.1101/2020.03.26.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhan Institue of Biological Products/Snopharm.https://www.fiercepharma.com/pharma-asia/china-s-sinopharm-touts-100-antibody-response-for-covid-19-vaccine-it-s-already-giving. Accessed on July 29, 2020., n.d.
- Wu Y., Li C., Xia S., Tian X., Kong Y., Wang Z., Gu C., Zhang R., Tu C., Xie Y., Yang Z., Lu L., Jiang S., Ying T. Identification of human single-domain antibodies against SARS-CoV-2. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui F., Kohara M., Kitabatake M., Nishiwaki T., Fujii H., Tateno C., Yoneda M., Morita K., Matsushima K., Koyasu S., Kai C. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology. 2014;454–455:157–168. doi: 10.1016/j.virol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M.S., Leung H.L., Li P.H., Cheung C.Y., Dutry I., Li D., Daëron M., Bruzzone R., Peiris J.S., Jaume M. Antibody-dependent enhancement of SARS coronavirus infection and its role in the pathogenesis of SARS. Hong Kong Med. J. 2016;22:25–31. [PubMed] [Google Scholar]
- Yun S., Ning W., Quanming Z. Progress and challenge of vaccine development against 2019 novel coronavirus (2019-nCoV) Chinese Journal of Preventive Medicine. 2020;54 doi: 10.3760/cma.j.cn112150-20200317-00366. E029–E029. [DOI] [PubMed] [Google Scholar]
- Zhang N., Channappanavar R., Ma C., Wang L., Tang J., Garron T., Tao X., Tasneem S., Lu L., Tseng C.-T.K., Zhou Y., Perlman S., Jiang S., Du L. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell. Mol. Immunol. 2016;13:180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Ke J.-S., Qin Z.-L., Ren H., Zhao L.-J., Yu J.-G., Gao J., Zhu S.-Y., Qi Z.-T. DNA vaccine of SARS-Cov S gene induces antibody response in mice. Acta Biochim. Biophys. Sin. (Shanghai) 2004;36:37–41. doi: 10.1093/abbs/36.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., He L., Sun S., Qiu H., Tai W., Chen J., et al. A novel nanobody targeting middle east respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J. Virol. 2018;92 doi: 10.1128/JVI.00837-18. e00837-18, /jvi/92/18/e00837-18.atom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N.S., Zheng B.J., Li Y.M., Poon L.L.M., Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P., Liu X.Q., Xu J., Li D.X., Yuen K.Y., Peiris J.S.M., Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X., Hensley L.E., Prabakaran P., Rockx B., Sidorov I.A., Corti D., Vogel L., Feng Y., Kim J.-O., Wang L.-F., Baric R., Lanzavecchia A., Curtis K.M., Nabel G.J., Subbarao K., Jiang S., Dimitrov D.S. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. PNAS. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zydus Cadila Zydus Cadila Accelerates COVID-19 Vaccine Research. 8 April. 2020. https://www.pharmatutor.org/pharma-news/2020/zydus-cadila-accelerates-covid-19-vaccine-research Accessed on May 21, 2020., n.d.
- Zydus Cadila Zydus Cadila Looks to Expedite Covid-19 Vaccine Development February 17. 2020. https://www.pharmaceutical-technology.com/news/zydus-cadila-covid-19-vaccine/ May 21, 2020., n.d.
- Hannah Balfour: Biotech and academia collaborate on intranasal COVID-19 vaccine development:https://www.drugtargetreview.com/news/59182/biotech-and-academia-collaborate-on-intranasal-covid-19-vaccine-development/ 2 April 2020, n.d.