Abstract
Background
Stroke/thromboembolic events, infections, and death are all significantly increased by antipsychotics in dementia but little is known about why they can be harmful. Using a novel application of a drug repurposing paradigm, we aimed to identify potential mechanisms underlying adverse events.
Methods
Whole transcriptome signatures were generated for SH‐SY5Y cells treated with amisulpride, risperidone, and volinanserin using RNA sequencing. Bioinformatic analysis was performed that scored the association between antipsychotic signatures and expression data from 415,252 samples in the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) repository.
Results
Atherosclerosis, venous thromboembolism, and influenza NCBI GEO‐derived samples scored positively against antipsychotic signatures. Pathways enriched in antipsychotic signatures were linked to the cardiovascular and immune systems (eg, brain derived neurotrophic factor [BDNF], platelet derived growth factor receptor [PDGFR]‐beta, tumor necrosis factor [TNF], transforming growth factor [TGF]‐beta, selenoamino acid metabolism, and influenza infection).
Conclusions
These findings for the first time mechanistically link antipsychotics to specific cardiovascular and infectious diseases which are known side effects of their use in dementia, providing new information to explain related adverse events.
Keywords: amisulpride, antipsychotic, brain derived neurotrophic factor, cardiovascular, immune, platelet derived growth factor, risperidone, RNA‐seq, selenium, side effects, tumor necrosis factor
1. BACKGROUND
Atypical antipsychotics are a commonly used off‐label treatment for agitation, aggression, and psychosis in dementia. They are modestly effective but have a severe side effect profile which includes sedation, thromboembolic events, QTc prolongation, falls, fractures, infections, stroke, and all‐cause mortality. 1 , 2 The narrow margin of clinical benefit and the lack of alternative pharmacological agents makes investigation of drug safety a key priority. Antipsychotic therapeutic mechanism of action (MoA) is primarily via antagonism of serotonin receptor 2A (5‐HT2A) and/or dopamine receptors 2 and 3 (D2/3) but many also have significant antihistaminergic, anticholinergic, and antiadrenergic properties. It has long been hypothesized that this off‐target activity is a contributor to the side effect profile of antipsychotics in dementia. 1 , 3 , 4 , 5 , 6 It has also been suggested that generic mechanisms such as over‐sedation leading to dehydration, failure to clear the chest, and inactivity may be key mediating mechanisms. 1 Therefore, an important unanswered question is whether side effects are a primary result of perturbations to specific biological processes (eg, cardiovascular biology, immune response) or secondary consequences of more general mechanisms like sedation. Understanding the answer to this question will help enormously in the future development of safer antipsychotics and inform the safer prescribing of existing agents.
High‐throughput in silico screening approaches leveraging gene expression data may provide novel mechanistic insights into dementia‐related side effects. Such approaches rest on the principle that transcriptional activity represents a useful surrogate for disease states and are widely used to triage compounds in drug repurposing studies (exemplified by the Connectivity Map, Cmap). 7 , 8 , 9 , 10 , 11 A typical application would see a gene expression signature from a candidate disease screened against a compound expression database, with negative scores indicating possible therapeutic benefits (ie, evidence that the drug reverses the disease transcriptional signature). It follows that a positive score between a given compound and a condition that is a side effect of that compound would indicate a MoA which is linked to the condition. Thus, a key advantage of this approach in the examination of drug side effects is a more direct biological link to human disease side effects without testing in humans.
In the present study, our aim was to determine whether transcriptional perturbations derived in vitro could elucidate mechanisms underlying adverse effects of antipsychotic use in dementia. We generated gene expression signatures for three antipsychotics representing a range of mechanisms of action relevant to the current landscape of drug development and clinical use in dementia: amisulpride (primarily a D2/D3 antagonist), risperidone (primarily a 5HT2A/D2 antagonist), and volinanserin (highly selective 5HT2A inverse agonist). 12 , 13 , 14 We then used a high‐throughput bioinformatic scoring algorithm to test for association with human diseases. Specifically, we hypothesized that the antipsychotic signatures would score positively with conditions and diseases related to known side effects of their use in dementia.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature and the landscape of current drug development for neuropsychiatric symptoms in dementia using online searches and conference presentations. Antipsychotics are still commonly prescribed off label and are associated with a number of severe side effects in dementia. Many newer drugs are refinements of existing antipsychotic mechanisms of action but little is known about specific mechanisms underlying harm.
Interpretation: Our findings link antipsychotics to cardiovascular and infectious disease, suggesting that adverse effects of their use in dementia may be mediated by specific mechanisms as well as secondary to more general effects like sedation.
Future directions: These findings provide a collection of candidate adverse effect mechanisms. Future research should extend these to biological models of aging and frailty, epidemiological studies, and should explore the possibility of our approach in discovering new adverse events related to antipsychotics to improve safety screening in the drug development pipeline.
2. MATERIALS AND METHODS
2.1. Antipsychotics
The following antipsychotic concentrations were used, based on previously published doses: 12 , 14 , 15 , 16 , 17 1 μM amisulpride (catalogue number CAY14619, Cambridge Bioscience, UK), 100nM risperidone (catalogue number ab120393, Abcam, UK), and 10 nM volinanserin (catalogue number CAY15936). Dimehtyl sulfoxide (DMSO) was used as the vehicle for all compounds.
2.2. Cell culture
SH‐SY5Y human neuroblastoma cells (P13) were cultured in media (DMEM/F‐12, GlutaMAX Supplement; catalogue number 11514436, Fisher Scientific, UK) containing filtered 10% fetal bovine serum (Gibco Fetal Bovine Serum, heat inactivated; catalogue number 11550356). Cells were maintained at 37°C, 5% CO2, and atmospheric O2 in a humidified incubator. Cells were seeded at a density of ≈70% in six‐well plates the day before experimentation and grown in the same media. On the day of the experiment, cells were treated with filter sterilized media containing the antipsychotic compounds or vehicle at desired concentration for 24 hours. No cell death was observed at the drug doses tested. Four individual culture well replicates were collected for each compound and vehicle.
2.3. RNA extraction
To preserve RNA in SH‐SY5Y cells, media was removed and 500 µl of Trizol (Invitrogen Trizol reagent; catalogue number 15596026) was applied to each well. Cells were mixed thoroughly with the reagent and collected for RNA extraction. RNA was purified using an RNA kit (Direct‐zol RNA MiniPrep w/ Zymo‐Spin IIC Columns [Capped]; catalogue number R2052) as shown in the instruction manual and stored at –80°C. After RNA purification, the concentration of RNA was measured by Qubit 3.0 Fluorometer using Qubit high sensitivity RNA kit (Qubit RNA HS Assay Kit; catalogue number Q32852, ThermoFisher Scientific, UK). The quality of purified RNA was tested using Agilent 2200 TapeStation system and RNA ScreenTape Assay (RNA ScreenTape; catalogue number 5067‐5576, RNA ScreenTape Sample Buffer; catalogue number 5067‐5577, Agilent, UK). The mean RNA Integrity Number (RIN, an indicator of RNA quality ranging from 1 to 10) value across all samples was 9.87 (minimum: 9.6, maximum: 10). RNA samples were diluted at the desired concentration for polyA‐tail library preparation and sequencing.
2.4. RNA sequencing
Illumina HiSeq 2500 standard mode sequencing system was used to sequence RNA samples (poly‐A tail library preparation, 125bp paired end, 20 million reads per sample). Quality control using FastQC was performed to remove low‐quality reads. To compare the expression profile of samples, STAR (version 2.6.1a) was used to align the RNA reads to the reference human genome (hg38). To create and sort bam files, samtools (version 0.1.16) and to index and assign mapped reads to genomic features, featureCounts (version 1.6.1) was used.
2.5. Identification of differentially expression genes
To generate differentially expressed genes (DEGs), DESeq2 (version 1.16.1) was applied to read counts from each compound versus control (n = 4 for each group), identifying significant changes based on a negative binomial distribution. Statistical filtering based on the log2 of 1.5‐fold change and a false discovery rate adjusted P‐value (P FDR) <.05 were used to generate the gene lists used in subsequent analysis. A 1.5‐fold change cut‐off was applied so that genes perturbed due to off target (which may be relevant to side effects) as well as therapeutic actions of the compounds were captured.
2.6. High‐throughput screening of antipsychotic drug signatures against dementia‐related side effects
To establish whether antipsychotic gene expression signatures were associated with gene expression of conditions representing known side effects, we first conducted a high‐throughput in silico screen against gene expression data from 415,252 human samples from 11,305 experimental series in the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) repository using the Searchable Platform Independent Expression Database (SPIED, www.spied.org.uk). 18 , 19 The SPIED tool facilitates querying of publicly available gene expression data from NCBI GEO with user‐defined transcriptional signatures. 8 , 9 , 11 A major barrier to high‐throughput in silico interrogation of human disease gene expression samples is that in many NCBI GEO series the case/control assignment of individual samples is not clear without manually curating the data (thus it is not practical to determine relative expression change across many hundreds or thousands of series). SPIED overcomes this by calculating an effective fold (EF) change at each probe in a sample, defined as the expression level of each individual array probe relative to the experimental series average. 19
In SPIED, association testing between the query antipsychotic signatures and NCBI GEO sample data are done via a Fisher exact test on 2 × 2 contingency table of up‐ and downregulated genes. A score is assigned to each sample to reflect the relationship with antipsychotic expression. This score is defined as the sum of the number of genes perturbed in the same direction subtracted from the sum of number of genes perturbed in the opposite direction, divided by the total number of genes common to antipsychotic and sample profiles. Possible scores therefore range between –1 (all genes perturbed in the opposite direction) and 1 (all genes perturbed in the same direction), thus quantifying the relationship between an individual sample and query signature. If an NCBI disease series is associated with an antipsychotic then by definition individual samples within that series will positively score with the drug. This initial screen thus provides a first indication of association, which can then be followed up. Specifically, highly scoring samples from NCBI GEO series assaying diseases or conditions of interest can then be manually assigned case/control status and tested for enrichment of positive scores among cases relative to controls. Thus, using SPIED, we followed the workflow described in detail in Williams 19 and broadly comprising the following stages (graphically summarized in Figure 1):
Generate a statistically filtered list of DEGs for each antipsychotic (described in Section 2.4).
-
Use SPIED to screen each antipsychotic signature against all human gene expression micro‐array data in the NCBI GEO repository. The resulting SPIED output is a "longlist" of the 500 top scoring NCBI GEO samples with a statistically significant (adjusted P‐value .05/11,305 NCBI GEO series = P < 4.42 × 10−6) score (either positive or negative). The list was then manually curated to shortlist samples from NCBI GEO series meeting the following criteria:
Sample is from a series assaying one of the following disease areas relevant to side effects of antipsychotic use in dementia: thromboembolic events, stroke, bone density/osteoporosis (relevant to fractures), pneumonia and other respiratory infections, urinary tract infections, and atherosclerosis/coronary artery disease.
Case/control design.
Manually annotate every sample in each shortlisted series as case or control according to their designation in NCBI GEO.
Test for enrichment of positive scores among cases relative to controls in each series using Fisher test. Given the correlation between the three antipsychotic signatures, a Bonferroni correction of 0.05/N shortlisted series was applied.
FIGURE 1.
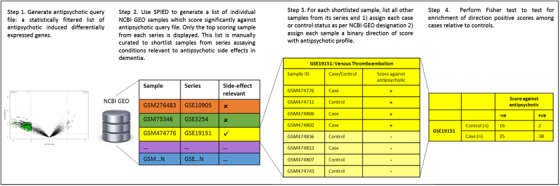
Graphical representation of the Searchable Platform Independent Expression Database (SPIED) screening method
3. RESULTS
3.1. Differentially expressed genes
In total, 10,841 genes were detected and used for differential gene expression analysis. Gene expression level and bidirectional distribution pattern of expression associated with each antipsychotic is illustrated in the volcano plots presented in Figure 2. Treatment of cells with volinanserin, amisulpride, and risperidone resulted in the activation of 2267 (1749 downregulated and 518 upregulated), 1026 (922 downregulated and 104 upregulated) and 809 (756 downregulated and 53 upregulated) genes, respectively (Figure 2, a full list of differentially expressed gene names and statistics is shown in Tables S1–S3 in supporting information). The three antipsychotic signatures were positively correlated with each other (amisulpride vs risperidone, Spearman test: rs = 0.76, amisulpride vs volinanserin: rs = 0.88, risperidone vs volinanserin: rs = 0.66). Raw sequence data are available in the GEO repository under GSE149611.
FIGURE 2.
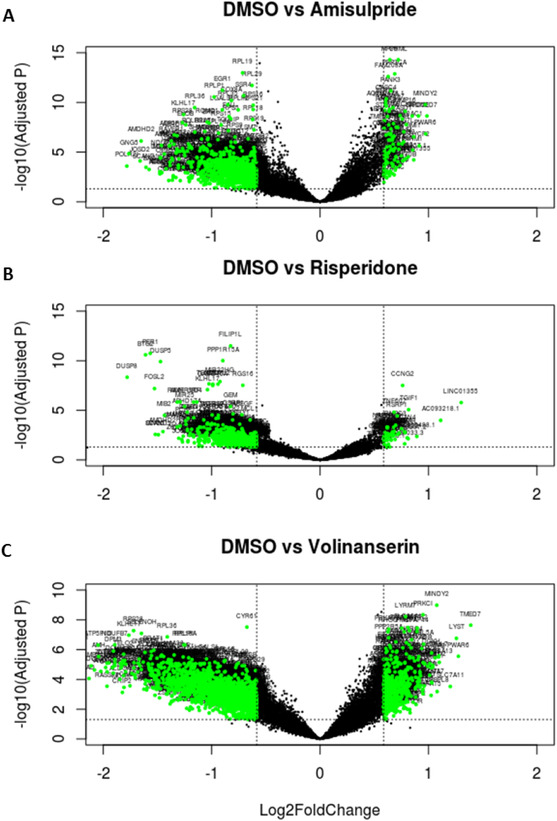
Volcano plots illustrating differentially expressed genes for amisulpride, risperidone, and volinanserin versus dimehtyl sulfoxide (DMSO). Dotted horizontal lines mark adjusted P‐value threshold of .05; dotted vertical lines mark log 1.5‐fold change threshold. Green markers indicate statistically significantly differentially expressed genes with > ±1.5‐fold change
3.2. Association between antipsychotic and dementia‐related side effects
Each antipsychotic signature was screened against the NCBI GEO repository using SPIED (Step 2, Figure 1). As this is a high‐throughput screen, we focused on the top 500 statistically significant (Bonferroni adjusted P‐value .05/11,305 NCBI GEO series: P < 4.42 × 10−6) scoring samples identified by SPIED for each drug. Of the 1500 total antipsychotic‐sample scores identified by SPIED, 817 were statistically significantly associated with at least two antipsychotics, leaving 683 unique samples in the long list. This list of samples along with associated scores, P‐value, and number of overlapping genes is shown in Table S4 in supporting information. Of these 683 unique samples, 18 were from series which assayed diseases/conditions relevant to side effects of antipsychotics in dementia (Step 3, Figure 1). Twelve of these were excluded as they were not case‐control designs (meaning testing for association between the score in individual samples and case/control status is not possible). Thus, six series were taken forward for further analysis: GSE13850 and GSE2208 (bone density), GSE23746 (atherosclerosis), GSE19151 (venous thromboembolism [VTE]), GSE7638 (coronary artery disease [CAD]), GSE17156 (respiratory infection, containing three conditions: influenza, rhinovirus, and respiratory syncytial virus, which were analyzed separately in this analysis). Individual sample‐level data showing the distributions of cases and controls in each series and their associated scores and P‐values are shown in Tables S5 to S27 in supporting information (Step 4, Figure 1).
Table 1 shows that atherosclerosis cases (GSE23746) were enriched for positive scores for all three antipsychotics (Fisher exact test amisulpride, P = .002; risperidone, P = 6.98 × 10−5; volinanserin, P = 5.5 × 10−3). VTE cases (GSE19151) were enriched for positive scores for risperidone (P = 8.13 × 10−7) and volinanserin (P = .002). Finally, influenza cases (GSE7638) were enriched for positive scores for amisulpride (P = .002).
TABLE 1.
Association between antipsychotic and side effect gene expression profiles
| Amisulpride | Risperidone | Volinanserin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Side effect | NCBI GEO Series | Array | Case/ control | Negative score (n) | Positive score (n) | P | Negative score (n) | Positive score (n) | P | Negative score (n) | Positive score (n) | P |
| Atherosc‐lerosis | GSE23746 |
Sentrix humanref ‐8 expression beadchip |
Control (n) Case (n) |
16 35 |
2 38 |
.002 |
16 30 |
1 43 |
6.98 × 10−5 |
15 37 |
2 38 |
5.5 × 10−3 |
| VTE | GSE19151 |
Affymetrix human ‐ genome U133A 2.0 |
Control (n) Case (n) |
‐ ‐ |
‐ ‐ |
‐ |
30 17 |
5 36 |
8.13 × 10−7 |
39 28 |
17 39 |
.002 |
| Influenza | GSE17156 |
Affymetrix human ‐ genome U133A 2.0 |
Control (n) Case (n) |
8 1 |
2 10 |
.002 |
0 2 |
1 6 |
1 |
7 2 |
2 10 |
.009 |
| Bone density | GSE2208 |
Affymetrix ‐ human genome U133A |
Control (n) Case (n) |
6 2 |
1 4 |
.103 |
6 2 |
1 6 |
0.041 |
7 2 |
1 6 |
.041 |
| CAD | GSE7638 |
Affymetrix human ‐ genome U133A 2.0 |
Control (n) Case (n) |
18 27 |
19 51 |
.159 |
18 22 |
18 44 |
0.137 |
22 32 |
19 60 |
.056 |
| Bone density | GSE13850 |
Affymetrix ‐ human genome U133A |
Control (n) Case (n) |
9 11 |
8 7 |
.738 |
9 11 |
8 6 |
0.728 |
10 13 |
10 7 |
.523 |
| Rhinovirus | GSE17156 |
Affymetrix human ‐ genome U133A 2.0 |
Control (n) Case (n) |
0 1 |
10 5 |
.375 |
4 0 |
2 0 |
1 |
2 2 |
14 13 |
1 |
| Respiratory‐ syncytial virus | GSE17156 |
Affymetrix human ‐ genome U133A 2.0 |
Control (n) Case (n) |
16 12 |
2 5 |
.228 |
9 3 |
0 1 |
.308 |
16 14 |
4 3 |
1 |
Abbreviations: CAD, coronary artery disease; NCBI GEO, National Center for Biotechnology Information Gene Expression Omnibus; VTE, venous thromboembolism.
Notes: Raw P values of Fisher exact test on 2 × 2 table are shown, statistically significant values after Bonferroni correction (0.05/8 = 0.00625) are highlighted in bold.
‘‐’ denotes test not done as no individual VTE samples were correlated with amisulpride in the high throughput screen stage.
'Positive score: the number of individual samples in each NCBI GEO series with a positive score for each antipsychotic.
'Negative score: the number of individual samples in each NCBI GEO series with a negative score for each antipsychotic.
"Case/control": the case/control status of each sample in each NCBI GEO series
3.2.1. Pathway analysis
Pathway analysis was then performed to elucidate more specific biological mechanisms underlying the reported associations. As this study is focused on side effects rather than therapeutic action, a pruned gene list for each antipsychotic was created; this comprised only genes which were also differentially expressed in cases relative to controls in the series in Table 1. Thus, the first step was to create a list of DEGs for atherosclerosis, VTE, and influenza. This was done using the NCBI GEO analyzer tool using a P FDR < .05 threshold (gene lists for each signature are shown in Tables S28 to S30). DEGs in each antipsychotic signature which were not also present in any of the side effects signatures were excluded, creating three pruned gene lists.
For amisulpride, risperidone, and volinanserin, query lists for pathway analysis comprised 547, 435, and 1218 genes, respectively (ie, those genes overlapping with atherosclerosis, VTE, or influenza). Genes in each of these three pruned antipsychotic lists were ranked in descending order by the log‐fold change associated with the antipsychotic and tested for enrichment using the g:Profiler tool, which is well suited to pruned lists. 20 Gene set enrichment analyses included the following gene ontology (GO) and biological pathway sources: GO molecular function (MF), GO cellular components (CC), GO biological processes (BP), Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome, and WikiPathways. Any annotations not curated manually (therefore being less reliable) were excluded. g:Profiler's multiple testing correction was applied (known as "g:SCS" and developed specifically for pathway analysis). A g:SCS‐adjusted P‐value threshold of 0.05 was used. 21 Outputs were filtered to exclude pathway gene sets with <10 or >200 genes and with <3 overlapping genes in the input list.
3.2.2. Biological pathways
Genes from 39, 23, and 44 GO terms and pathways were enriched in amisulpride, risperidone, and volinanserin, respectively (Figure 3, with detailed results in Table S31).
FIGURE 3.
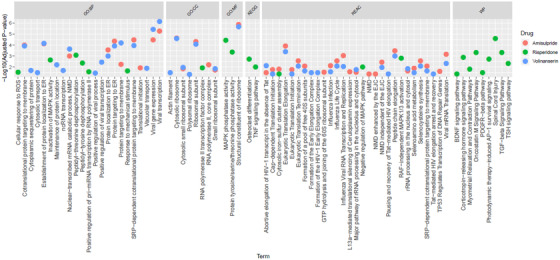
Plot of gene ontology (GO) terms and pathways statistically significantly enriched in amisulpride, risperidone, and volinanserin. Abbreviations: BP, biological processes; CC, cellular component; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes; REAC, Reactome; WP, WikiPathways; NMD, nonsense‐mediated decay; ER, endoplasmic reticulum; MAPK, mitogen‐activated protein kinase; EJC, exon junction complex; GTP, guanosine‐5'‐triphosphate; ROS, reactive oxygen species; TNF, tumor necrosis factor; RAF, rapidly accelerated fibrosarcoma; TGF, transforming growth factor; TSH, thyroid stimulating hormone; PDGFR, platelet derived growth factor receptor; BDNF, brain‐derived neurotrophic factor
Twenty‐three and 21 Reactome pathways were enriched in amisulpride and volinanserin, respectively. A number of these related to infectious disease pathways (eg, viral mRNA transcription: volinanserin, g:SCS adjusted P = 6.75x−4, amisulpride P = .005; influenza life cycle: amisulpride, P = .003, volinanserin, P = .009). Two pathways linked to the essential amino acid selenium were also enriched in both amisulpride and volinanserin: selenocysteine synthesis (amisulpride, P = .003, volinanserin, P = .009) and selenoamino acid metabolism (amisulpride, P = .01, volinanserin, P = .03).
For risperidone, 14 pathways across the KEGG (n = 2), Reactome (n = 3), and WikiPathways (n = 9) databases were identified. The Reactome pathways were linked to MAPK (RAF‐independent MAPK1/3 activation, P = .002; negative regulation of MAPK pathway, P = .01). KEGG and WikiPathways enriched in risperidone were linked to cell growth/differentiation, with some growth factor pathways linked to the cardiovascular system and inflammation: brain derived neurotrophic factor (BDNF) signaling pathway, P = .045; platelet derived growth factor receptor (PDGFR)‐beta signaling, P = .034; osteoclast differentiation, P = .002; inflammation; oncostatin M signaling, P = .0005; tumor necrosis factor (TNF) signaling pathway, P = .01; transforming growth factor (TGF) beta signaling, P = 4.6x−4.
3.2.3. GO terms
All GO terms enriched in the three antipsychotic lists are shown in Figure 3, with detailed results in Table S31. Removing redundant terms using Revigo 22 showed that the amisulpride gene list was primarily enriched for GO terms related to viral transcription (P = 1.29x−6), signal recognition particle (SRP)‐dependent co‐translational protein targeting to membrane (P = 3.43E‐05), cystolic ribosome (P = 2.2x−5), and structural constituent of ribosome (P = 1.29x−6).
Risperidone was enriched for terms relating to peptidyl‐threonine dephosphorylation (P = 8.43x−4), response to mechanical stimulus (P = .02), positive regulation of pri‐miRNA transcription from RNA polymerase II promotor (P = .03), RNA polymerase II transcription factor complex (P = .01), MAP kinase phosphatase activity (P = 3.5x−5).
Volinanserin was enriched for viral transcription (P = 6.94x−7), SRP‐dependent co‐translational protein targeting to membrane (P = 1.07E‐04), cystolic ribosome (P = 2.46x−5), and structural constituent of ribosome (P = 2.03x−6).
4. DISCUSSION
This study aimed to elucidate mechanisms underlying side effects associated with antipsychotic use in dementia. To our knowledge we provide the first evidence mechanistically linking antipsychotics with specific cardiovascular and infectious diseases that are common side effects of their use in dementia. Supporting our hypothesis, the initial high‐throughput screen identified three conditions related to known side effects which were associated with the antipsychotics; atherosclerosis cases were enriched for positive scores for all three antipsychotics, VTE cases were enriched with positive scores for risperidone and volinanserin, and influenza cases were enriched with positive scores for amisulpride. Supplementing these drug–disease associations, a number of biological pathways related to cardiovascular biology, infectious disease, and inflammation/immune system were enriched across antipsychotic signatures. These findings suggest specific cardiovascular and immune processes may underlie some harmful effects of antipsychotics and for the first time provide a number of candidates which can now be prioritized for further investigation.
Notable pathways enriched in risperidone include BDNF, PDGFR‐beta, TNF, and TGF‐beta signaling. Findings from previous in vitro and in vivo studies strongly implicate PDGFR‐beta in atherosclerosis and cardiovascular disease, providing a possible mechanism to explain the positive association between the three antipsychotics and atherosclerosis and VTE observed in this study. 23 Similarly, BDNF also plays a role in cardiovascular disease (as well as neuroplasticity and development) 24 , 25 and is expressed in a variety of blood cells, the heart, and vasculature. 26 It is also noteworthy that previous studies have demonstrated that part of risperidone's pro‐cognitive therapeutic MoA may be via BDNF. 27 It is evident from our findings that more work must be done to untangle this complex element of antipsychotic MoA, where BDNF is plausibly related to both beneficial and detrimental effects of antipsychotics, which is highly relevant to dementia in which the margin between clinical benefit and harm is so narrow. Two pathways linked to the essential amino acid selenium were enriched in amisulpride and volinanserin. Selenium plays a role in preventing oxidative stress and has been widely linked in observational studies to cardiovascular disease and atherosclerosis. 28 Moreover, one study in patients with schizophrenia implicated selenium deficiency in the adverse cardiac effects of clozapine, though it was not clear whether the deficiency was caused by the drug or the schizophrenia itself. 29 Our findings bring greater clarity to this previous work by providing evidence that antipsychotics directly act on selenium pathways. This has particular relevance to neurodegeneration in which selenium deficiency in AD brain tissue has been observed and is hypothesized to play a role in cardiovascular side effects in Parkinson's disease. 30 , 31 Our findings provide a clear indication for prioritizing study of selenium deficiency and its interaction with antipsychotics in people with neurodegenerative disease to understand if it may be a clinically useful marker.
Infectious disease and immune pathways were also enriched across all three antipsychotics. These included a range of viral and influenza‐linked GO terms in amisulpride and volinanserin, and TNF and TGF‐beta in risperidone. Consistent with this, a recent study showed a considerable global suppression of immune response in mice treated with risperidone, indicated by reduction in a number of cytokines during treatment. 32 Our findings suggest that this impact extends to other antipsychotics and so underscore the need to prioritize investigation of immune response in people with dementia. They also suggest that susceptibility to infection associated with antipsychotics is not solely secondary to more general effects of antipsychotics like sedation‐induced inactivity or failure to clear the chest.
Although more work needs to be done to build on the candidate mechanisms highlighted in this study, their initial identification is an important step which could ultimately have important implications for clinical decision making. For example, the incorporation of more formal cardiovascular history screening, with a particular focus on thrombosis risk or selenium deficiency, into clinical decision making could result in greater harm reduction.
We note that there were differences in the overlapping side effect profiles and pathways enriched between antipsychotics; however. it would not be appropriate to draw direct comparison between them at the specific pathway level or interpret differences as clinically relevant. This is because these experiments were conducted in vitro, so cellular responses will be affected by dosing and duration of exposure to each compound; similarly, equivalent doses and bioavailability of drugs in humans will differ. At a broader level however, it is worth noting that associations between antipsychotics and side effects, and enrichment of relevant biological pathways were observed across all compounds, despite their differing MoAs. Further comparison in different biological models, including those in which aging and frailty can be incorporated, and epidemiological studies is now warranted. 33 This line of investigation could have important implications for AD, Parkinson's disease, and elderly people with schizophrenia for which clinical trials ofamisulpride and pimavanserin (a highly selective 5‐HT2A inverse agonist) have recently been published and more antipsychotic‐like drugs are in development. 2 , 34 , 35 , 36
The overall trend toward downregulation of genes in this experiment is also worth comment. This pattern was particularly notable in risperidone, for which 53 genes were upregulated and 756 were downregulated. However, although notable this is not without precedent. One study, with a similar design, which treated SK‐N‐SH neuroblastoma cell lines with risperidone for 24 hours showed 80% of genes were downregulated in analysis of microarray data. 12
With regard to limitations, the design and analysis of this study follows the same principles as Cmap and therefore the same caveats apply. These include the comparison between cell line–derived signatures and human studies, specifically that it would be premature to draw concrete conclusions on the clinical profile of compounds based on these data alone. However, as with Cmap, the trade‐off is an experimental design which provides a high‐throughput low‐cost screen, analogous to a drug repurposing experiment in which thousands of licensed compounds are triaged against a single disease signature. Similarly, in this study, screening three antipsychotic signatures against thousands of diseases showed that mechanisms underlying VTE, atherosclerosis, and infection may be relevant to the side effect profiles of antipsychotics, providing a clear rationale for prioritizing their investigation in different biological models and epidemiological studies. In doing so, this study also represents an important step toward safety screening for compounds in development of neuropsychiatric symptoms in AD. This study highlights molecular‐level links between cardiovascular and infectious diseases and antipsychotics, suggesting that adverse effects of their use in dementia may be mediated by specific mechanisms as well as secondary to more general effects like sedation. These findings provide a collection of candidate adverse effect mechanisms which may have important implications for use of existing compounds in clinical practice and the development of safer drugs for dementia in the future.
FUNDING INFORMATION
The funders of the study had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
CONFLICTS OF INTEREST
CB has received grants and personal fees from ACADIA Pharmaceuticals and Lundbeck, and personal fees from Heptares, Roche, Lilly, Otsuka, Orion, GlaxoSmithKline, and Pfizer. DAC is an employee of Eli Lilly and Company Ltd.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
This work was generously supported by the Wellcome Trust Institutional Strategic Support Award (204909/Z/16/Z) and in part through the MRC Proximity to Discovery: Industry Engagement Fund (External Collaboration, Innovation and Entrepreneurism: Translational Medicine in Exeter 2 (EXCITEME2) ref. MC_PC_16072.
Malekizadeh Y, Williams G, Kelson M, et al. Whole transcriptome in silico screening implicates cardiovascular and infectious disease in the mechanism of action underlying atypical antipsychotic side effects. Alzheimer's Dement. 2020;6:e12078 10.1002/trc2.12078
REFERENCES
- 1. Ballard CHR. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci. 2006;7:492‐500. [DOI] [PubMed] [Google Scholar]
- 2. Creese B, Da Silva MV, Johar I, Ballard C. The modern role of antipsychotics for the treatment of agitation and psychosis in Alzheimer's disease. Expert Rev Neurother. 2018;18:461‐467. [DOI] [PubMed] [Google Scholar]
- 3. Kleijer BC, Van Marum RJ, Egberts ACG, Jansen PAF, Knol W, Heerdink ER. Risk of cerebrovascular events in elderly users of antipsychotics. J Psychopharmacol. 2009;23:909‐914. [DOI] [PubMed] [Google Scholar]
- 4. Herrmann N, Lanctôt KL. Do atypical antipsychotics cause stroke? CNS Drugs. 2005;19:91‐103. [DOI] [PubMed] [Google Scholar]
- 5. Smith DA, Beier MT. Association between risperidone treatment and cerebrovascular adverse events: examining the evidence and postulating hypotheses for an underlying mechanism. J Am Med Dir Assoc. 2004;5:129‐132. [PubMed] [Google Scholar]
- 6. De Clerck F, Somers Y, Mannaert E, Greenspan A, Eerdekens M. In vitro effects of risperidone and 9‐hydroxy‐risperidone on human platelet function, plasma coagulation, and fibrinolysis. Clin Ther. 2004;26:1261‐1273. [DOI] [PubMed] [Google Scholar]
- 7. Lamb J, Crawford ED, Peck D, et al. The connectivity map: using gene‐expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929‐1935. [DOI] [PubMed] [Google Scholar]
- 8. Fletcher EJR, Jamieson AD, Williams G, Doherty P, Duty S. Targeted repositioning identifies drugs that increase fibroblast growth factor 20 production and protect against 6‐hydroxydopamine‐induced nigral cell loss in rats. Sci Rep. 2019;9:8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams G, Gatt A, Clarke E, et al. Drug repurposing for Alzheimer's disease based on transcriptional profiling of human iPSC‐derived cortical neurons. Transl Psychiatry. 2019;9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mittal S, Bjørnevik K, Im DS, et al. β2‐Adrenoreceptor is a regulator of the α‐synuclein gene driving risk of Parkinson's disease. Science 2017;357:891‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rivera AD, Butt AM. Astrocytes are direct cellular targets of lithium treatment: novel roles for lysyl oxidase and peroxisome‐proliferator activated receptor‐γ as astroglial targets of lithium. Transl Psychiatry. 2019;9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mas S, Gassó P, Bernardo M, Lafuente A. Functional analysis of gene expression in risperidone treated cells provide new insights in molecular mechanism and new candidate genes for pharmacogenetic studies. Eur Neuropsychopharmacol. 2013;23:329‐337. [DOI] [PubMed] [Google Scholar]
- 13. Schoemaker H, Claustre Y, Fage D, et al. Neurochemical characteristics of amisulpride, an atypical dopamine D2/D3 receptor antagonist with both presynaptic and limbic selectivity. J Pharmacol Exp Ther. 1997;280:83‐97. [PubMed] [Google Scholar]
- 14. Kehne JH, Baron BM, Carr AA, et al. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5‐HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968‐981. [PubMed] [Google Scholar]
- 15. Park SW, Seo MK, Cho HY, et al. Differential effects of amisulpride and haloperidol on dopamine D2 receptor‐mediated signaling in SH‐SY5Y cells. Neuropharmacology. 2011;61:761‐769. [DOI] [PubMed] [Google Scholar]
- 16. Marek GJ, Aghajanian GK. Excitation of interneurons in piriform cortex by 5‐hydroxytryptamine: blockade by MDL 100,907, a highly selective 5‐HT2A receptor antagonist. Eur J Pharmacol. 1994;259:137‐141. [DOI] [PubMed] [Google Scholar]
- 17. Aghajanian GK, Marek GJ. Serotonin, via 5‐HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825:161‐171. [DOI] [PubMed] [Google Scholar]
- 18. Williams G. A searchable cross‐platform gene expression database reveals connections between drug treatments and disease. BMC Genomics. 2012;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams G. SPIEDw: a searchable platform‐independent expression database web tool. BMC Genomics. 2013;14:2‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reimand J, Isserlin R, Voisin V, et al. Pathway enrichment analysis and visualization of omics data using g:profiler, GSEA, cytoscape and enrichmentmap. Nat Protoc. 2019;14:482‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reimand J, Kull M, Peterson H, Hansen J, Vilo J. G:Profiler‐a web‐based toolset for functional profiling of gene lists from large‐scale experiments. Nucleic Acids Res. 2007;35:193‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Supek F, Bošnjak M, Škunca N, Šmuc T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237‐254. [DOI] [PubMed] [Google Scholar]
- 24. Krebs MO, Guillin O, Bourdel MC, et al. Brain derived neurotrophic factor (BDNF) gene variants association with age at onset and therapeutic response in schizophrenia. Mol Psychiatry. 2000;5:558‐562. [DOI] [PubMed] [Google Scholar]
- 25. Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: Effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135‐144. [DOI] [PubMed] [Google Scholar]
- 26. Pius‐sadowska, Ewa ; Machaliński B. BDNF—A key player in cardiovascular system. J Mol Cell Cardiol. 2017;110:54‐60. [DOI] [PubMed] [Google Scholar]
- 27. Yu W, Zhu M, Fang H, et al. Risperidone reverses the downregulation of BDNF in hippocampal neurons and MK801‐induced cognitive impairment in rats. Front Behav Neurosci. 2019;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu H, Xu H, Huang K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics. 2017;9:21‐37. [DOI] [PubMed] [Google Scholar]
- 29. Vaddadi KS, Soosai E, Vaddadi G. Low blood selenium concentrations in schizophrenic patients on clozapine. Br J Clin Pharmacol. 2003;55:307‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Varikasuvu SR, Prasad VS, Kothapalli J, Manne M. Brain selenium in Alzheimer's disease (BRAIN SEAD Study): a systematic review and meta‐analysis. Biol Trace Elem Res. 2019;189:361‐369. [DOI] [PubMed] [Google Scholar]
- 31. Lertxundi U, Hernández R, Medrano J, Domingo‐Echaburu S, García M, Aguirre C. Clozapine‐induced cardiomyopathy in parkinson's disease. Mov Disord Clin Pract. 2017;4:643‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. May M, Beauchemin M, Vary C, Barlow D, Houseknecht KL. The antipsychotic medication, risperidone, causes global immunosuppression in healthy mice. PLoS One. 2019:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Viana J, Wildman N, Hannon E, et al. Clozapine‐induced transcriptional changes in the zebrafish brain. npj Schizophr. 2020;6:3 10.1038/s41537-019-0092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson's disease psychosis : a randomised, placebo‐controlled phase 3 trial. Lancet. 2014;383:533‐540. [DOI] [PubMed] [Google Scholar]
- 35. Howard R, Cort E, Bradley R, et al. Antipsychotic treatment of very late‐onset schizophrenia‐like psychosis (ATLAS): a randomised, controlled, double‐blind trial. Lancet Psychiatry. 2018;5:553‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ballard C, Banister C, Khan Z, et al. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer's disease psychosis: a phase 2, randomised, placebo‐controlled, double‐blind study. Lancet Neurol. 2018;17:213‐222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


