Abstract
The dance between microbes and the immune system takes place in all biological systems, including the human body, but this interaction is especially complex in the primary gateway to the body: the oral cavity. Recent advances in technology have enabled deep sequencing and analysis of members and signals of these communities. In a healthy state, the oral microbiome is composed of commensals, and their genes and phenotypes may be selected by the immune system to survive in symbiosis. These highly regulated signals are modulated by a network of microbial and host metabolites. However, in a diseased state, host-microbial networks lead to dysbiosis and considerable burden to the host prior to systemic impact that extends beyond the oral compartment. Interestingly, we presented data demonstrating similarities between human and mice immune dysbiosis and discussed how this affects the host response to similar pathobionts. The host and microbial signatures of a number of disease states are currently being examined to identify potential correlations. How the oral microbiome interacts with inflammation and the immune system to cause disease remains an area of active research. In this review, we summarize recent advancements in understanding the role of oral microbiota in mediating inflammation and altering systemic health and disease. In line with these findings, it is possible that existing conditions may be resolved by targeting specific immune-microbial markers in a positive way.
Keywords: inflammation, systems biology, cytokines, dysbiosis, resolution, interactome
Introduction
The oral cavity is a complex microbial ecosystem that provides the gateway to the human body. The human oral microbiome contains upward of 2,000 bacterial, archaeal, viral, and fungal species (Sampaio-Maia et al. 2016). The majority of oral microbiota are considered to be commensals, although the oral cavity can also harbor opportunistic pathobionts (Yumoto et al. 2019). There has been increased interest in the role of the microbiome in human disease with the completion of the Human Microbiome Project (Human Microbiome Project Consortium 2012). While the majority of microbiome research has initially focused on the gut microbiome, there has been increasing research into other regions, including the skin, vaginal, and oral microbiomes, as well as recent recognition of oral-systemic axes of crosstalk, including the oral-gut axis (Schmidt et al. 2019; Carr et al. 2020). Within the oral microbiome, there has been significant interest in the role of oral dysbiosis in causing disease. Indeed, dysbiosis of the oral host-microbiome has been associated with local diseases (e.g., dental caries, periodontitis; Belstrøm et al. 2017), oral cancers (Gholizadeh et al. 2016), and systemic disease, including Alzheimer’s disease (Laugisch et al. 2018), preterm birth (Cobb et al. 2017), cardiovascular diseases (Mesa et al. 2019), and colorectal, pancreatic, and other cancers (Komiya et al. 2019; Chung et al. 2020).
Growing evidence suggests that dysbiotic inflammation precedes the development of chronic conditions through defective or exacerbated signaling networks. Inflammation is a protective response against infections and injury that ultimately promotes tissue repair and regeneration (Furman et al. 2019). The acute (or homeostatic) phase of the inflammatory response is carefully orchestrated and timed, where upregulation of activation mechanisms also triggers their resolution networks. However, low-grade inflammation that fails to resolve can progress to chronic inflammatory conditions, including periodontal, metabolic syndrome, cardiovascular, nonalcoholic fatty liver, chronic kidney, and autoimmune diseases. The prevalence of chronic inflammatory diseases is rising, representing an important threat to global health, and such diseases are the largest cause of death worldwide when combined, accounting for >50% of mortality (Roth et al. 2018). While the role of low-grade chronic inflammation has been increasingly recognized as being significant in the development of these comorbid noncommunicable diseases, we have yet to fully elucidate the exact inflammatory pathways that trigger or sustain this chronic inflammatory state.
The most common human chronic inflammatory diseases present in the oral cavity are gingivitis and periodontitis, which affect 42% of the US adult population (Eke et al. 2018). There is clear evidence indicating that host-microbiome dysbiosis affect oral diseases, including dental caries, periodontitis, and oral cancers (Curtis et al. 2011), with increasing evidence for a role in systemic disease (Konkel et al. 2019; Yumoto et al. 2019). However, the oral cavity maintains a robust microbiome in healthy individuals, and the exact mechanisms that trigger a shift to oral dysbiosis and disease remain unclear. As a mucosal barrier heavily exposed to various environmental stimuli, the oral cavity must maintain immune tolerance to its commensal microbiota and provide immune surveillance against possible microbial threats (Belkaid and Harrison 2017). This critical role of oral cavity immune surveillance has only recently been appreciated (Moutsopoulos and Konkel 2018; Konkel et al. 2019) and sheds light on the novel hypothesis of immune diversity in host-microbial commensalism, in which immune cells are highly heterogeneous. It continues to be debated how exactly changes in the oral microbiome reach the tipping point for disease; however, it is clear that an inflammatory immune response is involved (Curtis et al. 2011; Pan et al. 2019; Yumoto et al. 2019). Further mechanistic research is necessary to elucidate the directionality, to map the immune cell landscape, to understand how inflammation critically mediates the interplay between microbiome dysbiosis and disease, locally and systemically.
There has been recent progress in understanding the role of the oral microbiome in local and systemic disease. However, many unresolved questions remain, including how immune dysbiosis affects microbial community perturbations, whether inflammation is a result or cause of microbial dysbiosis, and whether host-microbial interactions in the oral cavity are similar to other mucosal environments or unique.
In this review, we critically examine the role of the oral microbiome in systemic human health and disease. We review current knowledge of oral homeostasis, inflammation, and its resolution, with emphasis on the role of oral immune dysbiosis in disease. We further focus on exciting new developments in the field, including utilizing multi-omic techniques to better assess the oral microbiome in health and disease and the potential to harness inflammation resolution to treat disease. In the midst of the COVID19 pandemic, the same virus (SARS-CoV-2) causes different diseases. Each individual presents unique levels of inflammation and dysbiotic networks toward the virus can aggravate, leading to death. Research focused on host repose and inflammatory networks is urgently needed.
Oral Homeostasis
In healthy individuals, the oral cavity exists in a carefully balanced state of homeostasis without inflammation (Belkaid and Harrison 2017; Caton et al. 2018). The oral cavity is a highly complex mucosal interface with varied environmental niches, each hosting its own microbial community, including the surfaces of the tongue, cheeks, palate, tonsils, and teeth (where oral biofilms can accumulate), as well as copious amounts of saliva (~750 mL/d in healthy individuals; Curtis et al. 2011). In addition to various microbial communities, immune cells are present in oral tissue, acting in routine surveillance. Neutrophils, monocytes, T lymphocytes (including Th17 cells), and B lymphocytes have been observed in healthy periodontal tissue alone; the transition to diseased tissues results in increased immune cell clonal expansion and abundance due to periphery cell migration from blood, including macrophages and dendritic cells (Graves et al. 2019; Konkel et al. 2019; Pan et al. 2019). The oral cavity is a place of enormous heterogeneity, from tissue composition to the microbiota to human immune cells—with each cell populations and subpopulations containing diverse members and interaction networks suited to particular niches, environments, and functions.
Inflammation and Resolution
Any environmental insult, including bacterial infection, trauma, and chemical cues, has the potential to elicit an inflammatory response (heat, pain, redness, swelling, and loss of function). Acute inflammation is protective for the host against infection or injury, involving an influx of immune cells, as well as migration, priming, cell activation, and synthesis of inflammatory mediators, such as proinflammatory cytokines (Feehan and Gilroy 2019). By nature, acute inflammation returns to homeostasis following resolution. Inflammation resolution is an active cellular process that includes removal of the inflammatory stimuli and involves specialized immunoresolvent mediators (e.g., resolvins, lipoxins, maresins, and protectins). These proresolution mediators bind to specific immune cell receptors as agonists, activating signaling, phagocytosis, and efferocytosis to resolve inflammation and restore tissue integrity and function (Serhan et al. 2008; Freire, Dalli, et al. 2017; Serhan and Levy 2018; Feehan and Gilroy 2019). Understanding the proresolution signals that restore the oral microbiome to its symbiotic (rather than dysbiotic) state may provide novel insights into host and microbial mechanisms of interaction in health and disease.
When cells fail to resolve acute inflammation to restore homeostasis, prolonged dysbiosis and low-grade unresolved inflammation develop, potentially leading to microbial changes in addition to chronic inflammatory disease complications. Chronic inflammation is mainly driven by delayed activity of innate immune cells, continuous challenge, and saturated response from the adaptive immune system. Neutrophils, normally present in periodontal tissues with sentinel capacity, are key immune cells for initial inflammation and its resolution, and neutrophil abnormalities—including impaired adhesion, cytokine signaling, and phagocytosis—are becoming increasingly recognized as being important to chronic inflammatory disease development (Alba-Loureiro et al. 2007; Curtis et al. 2011; Hotamisligil 2017; Serhan and Levy 2018; Feehan, Dalli, and Gilroy 2019). A recent transcriptomics study showed that chronic inflammation in type 2 diabetes correlated with reduced neutrophil gene expression as compared with healthy patients (Kleinstein et al. 2019), similar to observations in gut dysbiosis (Hunt et al. 2008). The main role of neutrophils in the inflammatory response is to clear tissue debris or microbes, which is partially completed by releasing neutrophil extracellular traps (NETs; Silvestre-Roig et al. 2019). NETs are primarily composed of decondensed neutrophil DNA, which is extruded from neutrophils to physically entangle bacteria, releasing antibacterial molecules and eliciting a proinflammatory response (Silvestre-Roig et al. 2019).
Activation of inflammatory pathways is a complex and generalized host response, mediated by various immune cells and signaling molecules such as cytokines. As signaling molecules that are secreted by mononuclear phagocytes, antigen-presenting cells, lymphocytes, and neutrophils, cytokines control the phenotypes seen in the tissue milieu. The most well-established proinflammatory cytokines are in the IL-1, IL-6, and TNF families, which are secreted after pathogen introduction during the oral tissue responses (Pan et al. 2019). These cytokines can act in cascades to broadly activate inflammation further and specifically recruit immune cell subsets, including differentiating T and B cells. The recruitment of immune cells is key to control tissue cells—such as fibroblasts, osteoclasts, and osteoblasts—through activation of a key regulator, the NLRP3 inflammasome, and have been associated with oral and systemic diseases (García-Hernández et al. 2019; Konkel et al. 2019; Pan et al. 2019). Inflammasomes are part of inflammatory complexes that respond to pathogens or cellular damage by maturing proinflammatory cytokines, and inflammasomes represent a key component of the innate immune response (Tsai et al. 2020). As cytokines are secreted by and recruit immune cells, they can act in a feedback loop that amplifies inflammation until resolution halts the process. Importantly in periodontitis, proinflammatory cytokines, along with enzymes, can also result in tissue destruction, while some cytokines, including IL-11 and IL-27, have anti-inflammatory activity, though pro- or anti-inflammatory cytokine activity is often concentration or stimuli dependent (Pan et al. 2019). We have further data indicating that specific cytokine levels are increased in type 2 diabetes, a chronic inflammatory disease, relative to healthy states in humans and mice (Fig. 1). The effects of bacteria on cytokine production and immune modulation have been shown for a variety of inflammatory mediators and bacteria, including Fusobacterium nucleatum and Porphyromonas gingivalis (Table). Overall, the complex interaction of cytokines, feedback between pro- and anti-inflammatory circuitry, and exactly how this interplay contributes to disease is still emerging.
Figure 1.
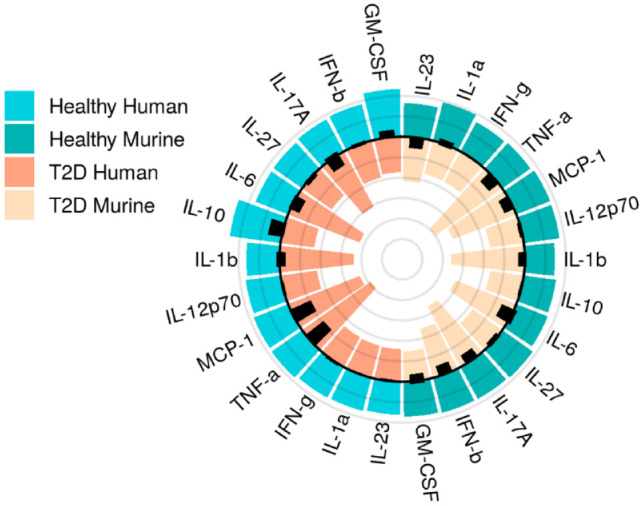
Dysbiotic inflammation is present in human and murine cytokine levels in disease (type 2 diabetes [T2D]). Original cytokine data are displayed to exemplify dysbiosis in the immune response, placing the host at risk of more aggressive microbial behavior. Mean levels (pg/mL) of 13 cytokines in mice (right side of circle, n = 5 healthy, n = 5 diabetic) and humans (left side of circle, n = 5 healthy, n = 5 diabetic). Health, green; type 2 diabetes, orange. Data show similar trends between human and mice cytokine levels, with higher expression among diseased versus healthy subjects. Radar lines mark 25 pg/mL; black bars show standard deviation.
Table.
Examples of Inflammatory Mediators of Microbial-Induced Disease.
| Marker | Bacteria | Function | Reference |
|---|---|---|---|
| Immune activation: exacerbates disease | |||
| IL-1β | Aggregatibacter actinomycetemcomitans | Inflammasome cytokine activation. Leukotoxin-induced macrophage cell death. | Kelk et al. (2011) |
| IL-18 | A. actinomycetemcomitans | Inflammasome cytokine activation. Leukotoxin-induced macrophage cell death. | Kelk et al. (2011) |
| Th17 | A. actinomycetemcomitans | Proinflammatory; immune evasion; promoted Th17 activation and induced atherosclerotic lesions | Jia et al. (2015) |
| IL-6 | Fusobacterium nucleatum | FadA adhesin/invasin of F. nucleatum is a key virulence factor; implicated in oral infections, adverse pregnancy outcomes, gastrointestinal disorders, etc. | Han (2015) |
| IL-8 | F. nucleatum | FadA adhesin/invasin of F. nucleatum is a key virulence factor; implicated in oral infections, adverse pregnancy outcomes, gastrointestinal disorders, etc. | Han (2015) |
| TNF-α | F. nucleatum | FadA adhesin/invasin of F. nucleatum is a key virulence factor; implicated in oral infections, adverse pregnancy outcomes, gastrointestinal disorders, etc. | Han (2015) |
| Th1 | Klebsiella pneumoniae, K. aeromobilis | Proinflammatory; oral Klebsiella promoted Th1 proliferation and gut inflammation and disrupted tissue homeostasis (murine) | Atarashi et al. (2017) |
| IL-1β | Porphyromonas gingivalis | Proinflammatory; P. gingivalis enhanced expression/secretion; correlated with periodontitis; periodontitis treatment decreased IL-1β levels | Hamedi et al. (2009); Gilowski et al. (2014) |
| IL-17 | P. gingivalis | Proinflammatory; produced in response to P. gingivalis; increased Th17 cells in mesenteric lymph nodes; aggravated articular injury in arthritis, arthritic bone destruction | de Aquino et al. (2014); Sato et al. (2017) |
| IL-18 | P. gingivalis | Proinflammatory; P. gingivalis upregulated; stimulated MMP-8 production; leads to inflammatory bone loss | Johnson and Serio (2005); Hamedi et al. (2009) |
| IL-23 | P. gingivalis | Proinflammatory; secreted by myeloid antigen-presenting cells in response to P. gingivalis; promoted Th17 pathogenicity, suppressed anti-inflammatory IL-10; associated with periodontal tissue damage | McGeachy et al. (2007); Himani et al. (2014) |
| IL-33 | P. gingivalis | Proinflammatory; drove differentiation/polarization of myeloid and lymphoid cells; may induce alveolar bone destruction via RANKL | Malcolm et al. (2015); Tada et al. (2016); Tada et al. (2017) |
| IL-17 | Prevotella nigrescens | Proinflammatory; produced in response to P. nigrescens; increased Th17 cells in mesenteric lymph nodes; aggravated articular injury in arthritis, arthritic bone destruction | de Aquino et al. (2014) |
| IFN-γ | Streptococcus mutans | S. mutans induced in the liver to exacerbate colitis/digestive disease | Kojima et al. (2012) |
| MMP-9 | S. mutans | Induced MMP-9 expression to exacerbate cerebral hemorrhage in stroke | Nakano et al. (2011) |
| Immune protective: promotes health | |||
| MMP-3 | Lactobacillus casei | Decreased neutrophil elastase and MMP-3 activities in GCF | Staab et al. (2009) |
| MPO | L. casei | Gingival inflammation was lower in the group consuming the probiotic product, as measured by MPO activity after a 4-d period of experimental gingivitis. | Staab et al. (2009) |
| scFV | Lactobacillus paracasei | Synthetic expression of functional scFV antibody binding to the surface of P. gingivalis; decreased P. gingivalis-related phenotypes | Marcotte et al. (2006) |
| IL-12 | P. gingivalis | Anti-inflammatory; role in bacterial clearance; secreted by mononuclear phagocytes/dendritic cells; promoted IFN-γ production/Th1 differentiation; inhibited osteoclastogenesis/bone resorption | Horwood et al. (2001); Johnson and Serio (2005) |
| IL-37 | P. gingivalis | Anti-inflammatory; Treg secretion suppressed NK cell function; can suppress osteoclast formation; downregulated in GCF of chronic periodontitis. | Offenbacher et al. (2018); Sarhan et al. (2018) |
| IFN-γ | P. gingivalis | Protective against P. gingivalis induced osteoclastogenesis / bone resorption. | Horwood et al. (2001) |
Table sorted by immune function (exacerbating or ameliorating disease) and biological factor (marker).
GCF, gingival crevicular fluid; IFN, interferon; IL, interleukin; MMP, matrix metalloproteinase; NK, natural killer; scFV, single-chain variable fragment; Th, T helper.
Oral Microbiome and Inflammation
The correlation of oral microbiome dysbiosis with oral disease is well established: selected oral biofilms can lead to gingivitis, periodontitis, and peri-implantitis (Curtis et al. 2011; Freire, Devaraj, et al. 2017). Microbial dysbiosis in periodontal diseases is generally characterized by the oral microbiota being enriched for Gram-negative pathogenic bacteria expressing virulence factors, rather than Gram-positive commensal bacteria (Kirst et al. 2015; Lamont et al. 2018). Sokransky’s “red complex” comprises a cluster of oral bacteria with pathogenic behavior (P. gingivalis, Tannerella forsythia, and Treponema denticola) that have been associated with pathogenic biofilm formation and periodontitis, though the exact triggers of disease conditions remain unclear, as these species have also been found in healthy oral sites at low levels (Byrne et al. 2009; Curtis et al. 2011; Bartold and Van Dyke 2019). Gingivitis and periodontitis involve inflammation of oral tissues; however, while inflammation in gingivitis is reversible, chronic signaling in periodontitis destroys tissues irreversibly (Caton et al. 2018). The mechanisms of progression from oral health to gingivitis to periodontitis and why some individuals never progress to severe forms of the disease remain to be fully elucidated, though evidence clearly indicates a complex network of interactivity between oral bacteria and the host immune system.
Increasingly, links have been established between oral pathogens and oral cancer (Gholizadeh et al. 2016). In particular, F. nucleatum, a Gram-negative obligate anaerobe, has been implicated in periodontitis and gingivitis (Teles et al. 2013), as well as in oral cancers (Han 2015; Holt and Cochrane 2017; Yost et al. 2018). More broadly, changes in relative microbial abundance in the oral microbiome, including the decrease of commensal Rothia and abundance of Streptococcus, have been linked to the development of oral cancer (Schmidt et al. 2014; Zhao et al. 2017). A handful of recent longitudinal studies have tried to assess the oral microbiome role in caries development, including that in the context of head and neck cancer (Xu et al. 2018; Mougeot et al. 2019; Kahharova et al. 2020). These microbial abundances demonstrate that ecologic changes are happening in healthy versus disease states, yet functional and longitudinal studies are needed to capture the metabolic complexity of the immune systems and host-microbiome interactions.
Oral Dysbiosis in Systemic Disease
In addition to its local impact, the role of periodontal disease in systemic disease risk is appreciated for chronic inflammatory diseases, such as diabetes (García-Hernández et al. 2019) and cardiovascular disease (Pietiäinen et al. 2018). Oral microbiota may contribute to systemic disease through several routes: 1) by entering the circulatory system via perturbed periodontal tissues (oral-blood axis), leading to bacteremia; 2) through aspiration that can lead to respiratory diseases (oral-vascular axis); or 3) through the oral-gut axis (Schmidt et al. 2019), where dislodged portions of oral biofilms make their way through the digestive system to the gut, protected in the biofilm from the acidic stomach environment (Konkel et al. 2019; Yumoto et al. 2019). These microbial perturbations may be the result of dental procedures or routine activities, such as brushing and flossing (Konkel et al. 2019), though these activities alone are not enough to cause disease in healthy individuals. In fact, for disease causality, there must be an additional trigger, likely through oral dysbiosis and host inflammatory response interactions.
Several known oral bacteria have been implicated in systemic diseases. The periodontal pathogen P. gingivalis has been linked to cardiovascular diseases, lung disease, fetal loss, and rheumatoid arthritis, where local and systemic inflammation is suggested to act as the driving factor (Konkel et al. 2019). F. nucleatum, in addition to its role in bridging biofilms and in oral diseases, has been implicated in a range of systemic diseases, including gastrointestinal abscesses (George et al. 2016) and acute appendicitis (Swidsinski et al. 2011), intra-amniotic infection (Gauthier et al. 2011), colorectal cancers (Kelly et al. 2018; Brennan and Garrett 2019; Komiya et al. 2019), and pancreatic cancer (Mitsuhashi et al. 2015; Gaiser et al. 2019).
Oral streptococci are a common human commensal with >100 identified species, which are known to colonize early in life and act in initial microbiome development (Yumoto et al. 2019). However, Streptococcus pathogens are also opportunistic and play important roles in oral and systemic disease (Yumoto et al. 2019). Locally in the oral cavity, certain Streptococcus species have been associated with health, such as S. salivarius, while others were involved in dual health and disease states: S. sanguinis, S. mitis, S. gordonii, and S. oralis are considered common commensals that have been shown to be involved in initial biofilm formation and are associated with systemic disease risk (Yumoto et al. 2019). Other Streptococcus spp. have more clearly defined pathogenic roles: in particular, Streptococcus mutans can potentially degrade NETs to escape neutrophils and cause oral disease, from dental caries to cancer, as well as systemic diseases, including sepsis and endocarditis (Liu et al. 2017; Yumoto et al. 2019). Oral streptococci have been further associated with a range of systemic diseases—abscesses, arthritis, cardiovascular diseases, gastrointestinal diseases, and cerebrovascular diseases, among others—indicating their ability to survive, evade the immune system, and thrive throughout the body (Yumoto et al. 2019). Given the complex and often opposing roles of streptococci in health and disease, locally and systemically, it is clear that individual bacterial virulence factors (including adhesins, toxins, and colonization factors) and the host immune response, including inflammation, must play a role in disease development. Such study of specific organisms traditionally utilized defined culture systems to advance molecular knowledge. Unculturable bacteria represent a new field, and to capture the complexity of their immune functions, multi-omics techniques are needed.
Importantly, while periodontal diseases increase the risk of systemic diseases, the reverse is also true (Graves et al. 2019), indicating an ongoing interplay between inflammatory networks that signal through the tissues locally and systemically and that chronic immune dysfunction may also cause microbial dysbiosis (Figs. 2, 3). This dual increased risk between systemic and oral disease may be at least partially explained by the role of unresolved dysbiotic inflammatory networks in the development of a host of chronic inflammatory diseases across the body (e.g., the interplay of periodontitis and diabetes).
Figure 2.
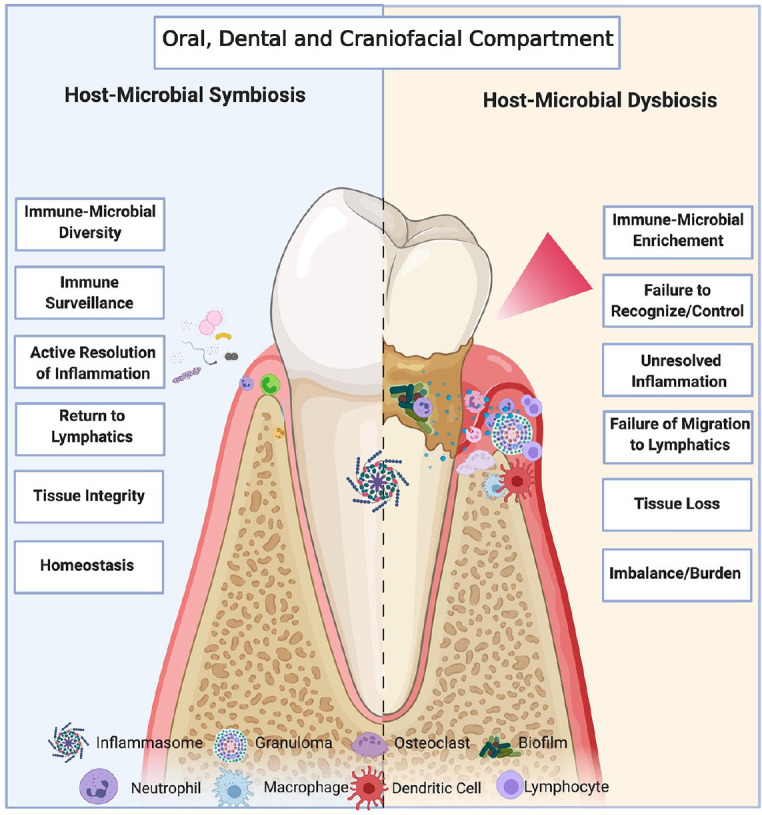
The oral microbiome interacts with host inflammatory networks, with oral dysbiosis inducing local disease. Schematic diagram of oral microbiome inflammatory interactions in health (left panel) and disease (right panel). In a healthy state, the oral microbiome and host immune system exist in a state of symbiosis, with commensal bacteria and sentinel host immune cells (neutrophils, inflammasomes) coexisting in oral tissue. This symbiosis is characterized by a diverse oral microbiome, with any inflammation (due to injury or insult) being acute and actively resolved to restore tissue integrity and homeostasis. However, should microbial dysbiosis occur, there is an enrichment of pathogenic over commensal bacteria and increased immune cell infiltration (neutrophils, macrophages, dendritic cells, other lymphocytes) in oral tissue, which can lead to biofilm and subsequent inflammasome formation. This failure to recognize or control bacterial dysbiosis, with immune cell infiltration, causes chronic low-grade inflammation, which can lead to tissue loss locally and systemic disease more broadly. Image created with BioRender.com.
Figure 3.
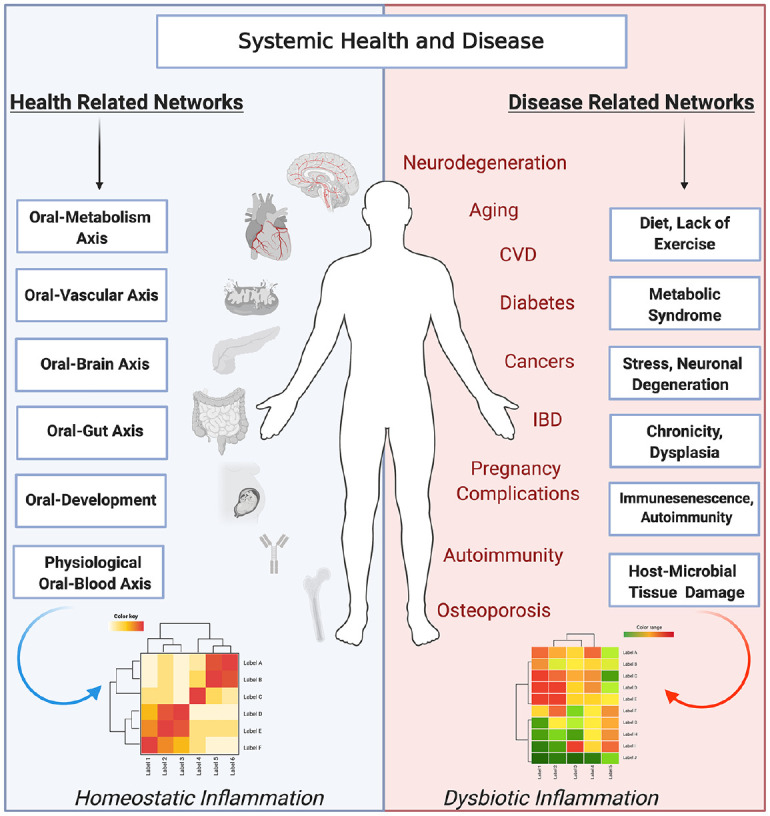
Dynamic interactions of the oral microbiome and host inflammatory networks play a role in oral dysbiosis and the oral impact on systemic diseases. The oral microbiome plays a key role in health and systemic disease. In a healthy state, homeostatic interactions of systemic and oral tissues lead to health. These driving factors affect systemic health through the oral-metabolism, oral-vascular, oral-brain, oral-gut, oral-development, and oral-blood axes. In contrast, oral dysbiosis can act through these axes to activate pathologic inflammatory networks, leading to chronic inflammation and, in conjunction with environmental factors, affecting systemic organs and aggravating systemic diseases, including diabetes, cardiovascular diseases, autoimmune diseases, age-related ailments, lung diseases, cancers, osteoporosis, pregnancy complications, and neurodegenerative diseases. Image created with BioRender.com. CVD, cardiovascular disease; IBD, inflammatory bowel disease.
Advances in Multi-omics Research of the Oral Microbiome
The capacity of multi-omics to expand scientific knowledge across fields is rapidly being appreciated. Interactions between human hosts and their microbiota are complex and require novel multimodal techniques. Recent multi-omics investigations into periodontal diseases are helping to reveal microbial biofilm composition, including its role in disease-causing microbial dysbiosis and subsequent commensal restoration following dental treatments (Califf et al. 2017). In addition, such multi-omics research with 16S ribosomal RNA sequencing has greatly expanded available knowledge of the bacterial role in oral cancer (Robledo-Sierra et al. 2019).
Identification of bacteria has traditionally relied on laboratory culturing methods (which are limited by bacterial growth in available media and conditions) and sequencing of the bacterial 16S ribosomal RNA through universal primers and polymerase chain reaction. While 16S sequencing has greatly improved bacterial detection over traditional culturing methods, including that for the oral microbiome (Dewhirst et al. 2010), it remains difficult to detect low-abundance species, and it has limited ability to detect complex microbiome community interactions. Advances in sequencing technologies have enabled deep sequencing for more comprehensive bacterial detection in samples (Varoni et al. 2019), including identification of microbial communities in deep periodontal pockets (Curtis et al. 2011) and supragingival plaque biofilms (Espinoza et al. 2018). In addition, binary logistic regression prediction models to detect severe periodontitis provided increased reliability when combining biomarkers from an oral rinse (albumin, MMP-8, chitinase, protease) with a tailored self-reported oral health questionnaire (Verhulst et al. 2019). These noninvasive measures combined with multi-omic molecular measures provide an opportunity to enhance the accuracy of diagnosis and precision medicine and dentistry.
Meta-transcriptomics represents an opportunity to investigate the microbial composition and mRNA expression through microbial profiling at the individual species and community levels. A recent meta-transcriptomics study of the oral microbiome was able to identify an association with F. nucleatum and oral squamous cell carcinoma where F. nucleatum had higher overall numbers of transcripts in tumor sites (vs. healthy tissue), but microbial communities also expressed clear metabolic signatures in disease (including enrichment of chemotaxis, iron update, and protease activities), regardless of composition (Yost et al. 2018).
Meta-transcriptomics studies have also been recently applied to specific oral cavity regions, including investigating the saliva microbiome in healthy subjects (enriched for carbohydrate metabolism genes) as compared with those with dental disease (dental caries or periodontitis); P. gingivalis and Filifactor alocis were associated with periodontitis, while S. mutans and Lactobacillus fermentum were associated with caries, lending evidence to the pathogenic nature of these bacteria (Belstrøm et al. 2017). A study of S. mutans global transcriptomics with whole-genome microarrays showed differential gene expression during biofilm dispersal, including that of a key virulence nuclease (deoC; Liu et al. 2017). Studies such as these highlight the potential of functional meta-transcriptomics and multi-omics to tease apart the relative contributions of specific microbes, including disease progression and severity. Meta-transcriptomics research also provides functional information about commensal-pathobiont transitions, including expression levels of microbial virulence factors (e.g., O-antigens, leukotoxin, lipopolysaccharides, Fap2) and whether a specific taxon or multiple pathobionts will provide “disease” signals to the niche.
Host-Microbial Interactions: Inflammation and Disease
Microbial biofilms contain not only bacteria but also their products, including polysaccharides and proteins, as well as bacterial and host DNA (Yumoto et al. 2019). This extracellular host DNA (eDNA) is involved in biofilm formation, maturation, and structural maintenance and has emerged as a potential drug target to treat pathogenic biofilms, as the exact quantity of eDNA is tightly regulated: a certain threshold is necessary for biofilm formation, but extremely high eDNA concentrations will cause the biofilm to collapse or detach (Okshevsky et al. 2015; Yumoto et al. 2019). While eDNA immunomodulation of the oral biofilm and other biofilm regions can ameliorate local microbial dysbiotic phenotypes, further research on its systemic effects are needed (Freire, Devaraj, et al. 2017; Devaraj et al. 2018). It is plausible to hypothesize that detachment of bacteria liberated from oral biofilms could cause secondary site infections through potential mechanisms, including resistomes within the oral-gut axis (Carr et al. 2020). This detached biofilm can be swallowed and affect the gut axis (Schmidt et al. 2019). Unraveling the molecular links of biofilm formation and stabilization with immune evasion is necessary to determine how to best co-opt this dispersion mechanism for the treatment of human disease.
Biofilm formation and eDNA incorporation are not the only mechanism that bacteria use to escape the host immune system. As experts at incorporating and modulating the host response, pathogenic oral bacteria, including P. gingivalis and Aggregatibacter actinomycetemcomitans, acquired the capability to “copy” host functions through molecular mimicry. For example, A. actinomycetemcomitans induced host citrullination, while pathogenic transitions of P. gingivalis demonstrated increased expression of citrullinated antigens, leading to autoantibody production via epigenetics in a mouse model (Konkel et al. 2019). The presence of these bacteria mimicking self-antigens has been proposed as being causal in autoimmune diseases such as rheumatoid arthritis, as well as potentially related to development of cardiovascular disease and pregnancy complications, where cross-reactive bacterial epitopes lead to aberrant immune responses (Konkel et al. 2019).
This interaction between bacteria and the host immune response is critical: it is now understood that host variation in inflammatory genes, including IL-17, can increase the risk of periodontitis and systemic diseases (Bedoya et al. 2013; Dutzan et al. 2018; Borilova Linhartova et al. 2019). Furthermore, we have shown evidence for dysregulation of neutrophil gene expression and cytokines in type 2 diabetes as compared with health (Kleinstein et al. 2019). Dissecting interaction between specific signals from bacteria and inflammation is key to understanding the development of chronic inflammatory diseases.
Further understanding the role of inflammation in microbial dysbiosis can provide relevant treatment options. As inflammation resolution is a natural process endogenously, recent research has investigated the possibility of treating inflammatory diseases (oral and systemic) with an exogenous dose of a proresolving small molecule lipid ligand mediator, such as resolvin E1 (Lee et al. 2016; Chiang et al. 2017; Werz et al. 2018). There is compelling evidence that treatment of inflammation with proresolving molecules can actually restore microbial commensalism from dysbiosis (Lee et al. 2016). Yet gaps remain in our mechanistic understanding of how host-microbial dysbiosis is dependent on each environmental compartment. It also remains unclear how inherited, acquired, or triggered dysbiotic resolution signals select for specific microbial and immune networks, acutely and chronically.
Conclusion
The microbiome is composed of a community of organisms that act synergistically and antagonistically. Research suggests that disruptions in the composition, quantity, and function of host-microbial networks result in local and systemic consequences. Evidence that the presence of a particular “keystone pathogen” drives most prevalent chronic oral diseases—rather than ecologic and functional dysbiosis linking local oral, dental, and craniofacial and systemic compartments—has proved questionable (Bartold and Van Dyke 2019). This highlights the limitations of our current knowledge, including the potential for spurious results in host-microbiome research, emphasizing the necessity of robust research and validation of associations. In this review, we have explored the role of inflammatory signals in linking oral microbial and immune dysbiosis to systemic human disease, which we believe acts as a critical component of this process. Chronic, low-grade, and unresolved inflammation has been shown to underlie the development of a variety of chronic inflammatory diseases across the body, including periodontal diseases, and this link is important for investigation. Many questions about the role of the oral microbiome in disease remain to be addressed, including fully elucidating whether disease is caused by community compositional changes, metabolites, or host-microbial networks and whether the oral dysbiosis is a cause or symptom of the underlying deficiency of resolution. Future research of the oral cavity microbiome and systemic axes as a whole (e.g., oral-brain, oral-gut, oral-respiratory, oral-blood vessels; Fig. 3) and specific oral surfaces (including expanded deep sequencing, multi-omics, and longitudinal studies) is critically needed to address the important role of host-microbiome network specificity and heterogeneity in health and disease.
Author Contributions
S.E. Kleinstein, contributed to conception, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; K.E. Nelson, contributed to design, critically revised the manuscript; M. Freire, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
We thank Ryan Eveloff for excellent technical assistance in generating analysis of cytokine data set and Mathew Ramsey, PhD, for helpful discussions.
Footnotes
This work was supported in part by US Public Health Service grant DE025383 from the National Institutes of Dental and Craniofacial Research and by the J. Craig Venter Institute.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: S.E. Kleinstein  https://orcid.org/0000-0002-9714-5155
https://orcid.org/0000-0002-9714-5155
M. Freire  https://orcid.org/0000-0003-4906-7698
https://orcid.org/0000-0003-4906-7698
References
- Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, Sannomiya P. 2007. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol. 40(8):1037–1044. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, et al. 2017. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 358(6361):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold PM, Van Dyke TE. 2019. An appraisal of the role of specific bacteria in the initial pathogenesis of periodontitis. J Clin Periodontol. 46(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedoya SK, Lam B, Lau K, Larkin J. 2013. Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013:986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Harrison OJ. 2017. Homeostatic immunity and the microbiota. Immunity. 46(4):562–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm D, Constancias F, Liu Y, Yang L, Drautz-Moses DI, Schuster SC, Kohli GS, Jakobsen TH, Holmstrup P, Givskov M. 2017. Metagenomic and metatranscriptomic analysis of saliva reveals disease-associated microbiota in patients with periodontitis and dental caries. NPJ Biofilms Microbiomes. 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borilova Linhartova P, Poskerova H, Tomandlova M, Bartova J, Kankova K, Fassmann A, Izakovicova Holla L. 2019. Interleukin-1 gene variability and plasma levels in Czech patients with chronic periodontitis and diabetes mellitus. Int J Dent. 2019:6802349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CA, Garrett WS. 2019. Fusobacterium nucleatum—symbiont, opportunist and oncobacterium. Nat Rev Microbiol. 17(3):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SJ, Dashper SG, Darby IB, Adams GG, Hoffmann B, Reynolds EC. 2009. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol Immunol. 24(6):469–477. [DOI] [PubMed] [Google Scholar]
- Califf KJ, Schwarzberg-Lipson K, Garg N, Gibbons SM, Caporaso JG, Slots J, Cohen C, Dorrestein PC, Kelley ST. 2017. Multi-omics analysis of periodontal pocket microbial communities pre- and posttreatment. mSystems. 2(3):e00016-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VR, Witherden EA, Lee S, Shoaie S, Mullany P, Proctor GB, Gomez-Cabrero D, Moyes DL. 2020. Abundance and diversity of resistomes differ between healthy human oral cavities and gut. Nat Commun. 11(1):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS. 2018. A new classification scheme for periodontal and peri-implant diseases and conditions—introduction and key changes from the 1999 classification. J Clin Periodontol. 45 Suppl 20:S1–S8. [DOI] [PubMed] [Google Scholar]
- Chiang N, de la Rosa X, Libreros S, Serhan CN. 2017. Novel resolvin D2 receptor axis in infectious inflammation. J Immunol. 198(2):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M, Zhao N, Meier R, Koestler DC, Del Castillo E, Wu G, Paster BJ, Charpentier K, Izard J, Kelsey KT, et al. 2020. Oral, intestinal, and pancreatic microbiomes are correlated and exhibit co-abundance in patients with pancreatic cancer and other gastrointestinal diseases. medRxiv. doi: 10.1101/2020.01.30.20019752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CM, Kelly PJ, Williams KB, Babbar S, Angolkar M, Derman RJ. 2017. The oral microbiome and adverse pregnancy outcomes. Int J Womens Health. 9:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Zenobia C, Darveau RP. 2011. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe. 10(4):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, Walgreen B, Helsen MM, van den Bersselaar LA, de Molon RS, et al. 2014. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1–driven Th17 response. J Immunol. 192(9):4103–4111. [DOI] [PubMed] [Google Scholar]
- Devaraj A, Buzzo J, Rocco CJ, Bakaletz LO, Goodman SD. 2018. The DNABII family of proteins is comprised of the only nucleoid associated proteins required for nontypeable Haemophilus influenzae biofilm structure. MicrobiologyOpen. 7(3):e00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, et al. 2018. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 10(463):eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. 2018. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009–2014. J Am Dent Assoc. 49(7):576–588e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza JL, Harkins DM, Torralba M, Gomez A, Highlander SK, Jones MB, Leong P, Saffery R, Bockmann M, Kuelbs C, et al. 2018. Supragingival plaque microbiome ecology and functional potential in the context of health and disease. mBio. 9(6):e01631-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan KT, Gilroy DW. 2019. Is resolution the end of inflammation? Trends Mol Med. 25(3):198–214. [DOI] [PubMed] [Google Scholar]
- Freire M, Devaraj A, Young A, Navarro J, Downey J, Chen C, Bakaletz L, Zadeh H, Goodman S. 2017. A bacterial biofilm induced oral osteolytic infection can be successfully treated by immuno-targeting an extracellular nucleoid associated protein. Mol Oral Microbiol. 32(1):74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MO, Dalli J, Serhan CN, Van Dyke TE. 2017. Neutrophil resolvin E1 receptor expression and function in type 2 diabetes. J Immunol Baltim Md 1950. 198(2):718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, et al. 2019. Chronic inflammation in the etiology of disease across the life span. Nat Med. 25(12):1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiser RA, Halimi A, Alkharaan H, Lu L, Davanian H, Healy K, Hugerth LW, Ateeb Z, Valente R, Fernández Moro C, et al. 2019. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut. 68(12):2186–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Hernández AL, Muñoz-Saavedra ÁE, González-Alva P, Moreno-Fierros L, Llamosas-Hernández FE, Cifuentes-Mendiola SE, Rubio-Infante N. 2019. Upregulation of proteins of the NLRP3 inflammasome in patients with periodontitis and uncontrolled type 2 diabetes. Oral Dis. 25(2):596–608. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Tétu A, Himaya E, Morand M, Chandad F, Rallu F, Bujold E. 2011. The origin of Fusobacterium nucleatum involved in intra-amniotic infection and preterm birth. J Matern Fetal Neonatal Med. 24(11):1329–1332. [DOI] [PubMed] [Google Scholar]
- George N, Flamiatos E, Kawasaki K, Kim N, Carriere C, Phan B, Joseph R, Strauss S, Kohli R, Choi D, et al. 2016. Oral microbiota species in acute apical endodontic abscesses. J Oral Microbiol. 8:30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh P, Eslami H, Yousefi M, Asgharzadeh M, Aghazadeh M, Kafil HS. 2016. Role of oral microbiome on oral cancers: a review. Biomed Pharmacother. 84:552–558. [DOI] [PubMed] [Google Scholar]
- Gilowski Ł, Wiench R, Płocica I, Krzemiński TF. 2014. Amount of interleukin-1β and interleukin-1 receptor antagonist in periodontitis and healthy patients. Arch Oral Biol. 59(7):729–734. [DOI] [PubMed] [Google Scholar]
- Graves DT, Corrêa JD, Silva TA. 2019. The oral microbiota is modified by systemic diseases. J Dent Res. 98(2):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamedi M, Belibasakis GN, Cruchley AT, Rangarajan M, Curtis MA, Bostanci N. 2009. Porphyromonas gingivalis culture supernatants differentially regulate interleukin-1β and interleukin-18 in human monocytic cells. Cytokine. 45(2):99–104. [DOI] [PubMed] [Google Scholar]
- Han YW. 2015. Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol. 23:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himani GS, Prabhuji MLV, Karthikeyan BV. 2014. Gingival crevicular fluid and interleukin-23 concentration in systemically healthy subjects: their relationship in periodontal health and disease. J Periodontal Res. 49(2):237–245. [DOI] [PubMed] [Google Scholar]
- Holt RA, Cochrane K. 2017. Tumor potentiating mechanisms of Fusobacterium nucleatum, a multifaceted microbe. Gastroenterology. 152(4):694–696. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Elliott J, Martin TJ, Gillespie MT. 2001. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 166(8):4915–4921. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. 2017. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 47(3):406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature. 486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KA, Zhernakova A, Turner G, Heap GAR, Franke L, Bruinenberg M, Romanos J, Dinesen LC, Ryan AW, Panesar D, et al. 2008. Novel celiac disease genetic determinants related to the immune response. Nat Genet. 40(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia R, Hashizume-Takizawa T, Du Y, Yamamoto M, Kurita-Ochiai T. 2015. Aggregatibacter actinomycetemcomitans induces Th17 cells in atherosclerotic lesions. Pathog Dis. 73(3):ftu027. [DOI] [PubMed] [Google Scholar]
- Johnson RB, Serio FG. 2005. Interleukin-18 concentrations and the pathogenesis of periodontal disease. J Periodontol. 76(5):785–790. [DOI] [PubMed] [Google Scholar]
- Kahharova D, Brandt BW, Buijs MJ, Peters M, Jackson R, Eckert G, Katz B, Keels MA, Levy SM, Fontana M, et al. 2020. Maturation of the oral microbiome in caries-free toddlers: a longitudinal study. J Dent Res. 99(2):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelk P, Abd H, Claesson R, Sandström G, Sjöstedt A, Johansson A. 2011. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D, Yang L, Pei Z. 2018. Gut microbiota, fusobacteria, and colorectal cancer. Diseases. 6(4):E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst ME, Li EC, Alfant B, Chi Y-Y, Walker C, Magnusson I, Wang GP. 2015. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl Environ Microbiol. 81(2):783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinstein SE, McCorrison J, Ahmed A, Hasturk H, Van Dyke TE, Freire M. 2019. Transcriptomics of type 2 diabetic and healthy human neutrophils. medRxiv. doi: 10.1101/19011353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima A, Nakano K, Wada K, Takahashi H, Katayama K, Yoneda M, Higurashi T, Nomura R, Hokamura K, Muranaka Y, et al. 2012. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci Rep. 2:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, Shimomura Y, Higurashi T, Sugi Y, Arimoto J, Umezawa S, Uchiyama S, Matsumoto M, Nakajima A. 2019. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut. 68(7):1335–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel JE, O’Boyle C, Krishnan S. 2019. Distal consequences of oral inflammation. Front Immunol. 10:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Koo H, Hajishengallis G. 2018. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 16(12):745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugisch O, Johnen A, Maldonado A, Ehmke B, Bürgin W, Olsen I, Potempa J, Sculean A, Duning T, Eick S. 2018. Periodontal pathogens and associated intrathecal antibodies in early stages of Alzheimer’s disease. J Alzheimers Dis. 66(1):105–114. [DOI] [PubMed] [Google Scholar]
- Lee C-T, Teles R, Kantarci A, Chen T, McCafferty J, Starr JR, Brito LCN, Paster BJ, Van Dyke TE. 2016. Resolvin E1 reverses experimental periodontitis and dysbiosis. J Immunol 1950. 197(7):2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sun L, Liu W, Guo L, Liu Z, Wei X, Ling J. 2017. A nuclease from Streptococcus mutans facilitates biofilm dispersal and escape from killing by neutrophil extracellular traps. Front Cell Infect Microbiol. 7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm J, Awang RA, Oliver-Bell J, Butcher JP, Campbell L, Adrados Planell A, Lappin DF, Fukada SY, Nile CJ, Liew FY, et al. 2015. IL-33 exacerbates periodontal disease through induction of RANKL. J Dent Res. 94(7):968–975. [DOI] [PubMed] [Google Scholar]
- Marcotte H, Kõll-Klais P, Hultberg A, Zhao Y, Gmür R, Mändar R, Mikelsaar M, Hammarström L. 2006. Expression of single-chain antibody against RgpA protease of Porphyromonas gingivalis in Lactobacillus. J Appl Microbiol. 100(2):256–263. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. 2007. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat Immunol. 8(12):1390–1397. [DOI] [PubMed] [Google Scholar]
- Mesa F, Magan-Fernandez A, Castellino G, Chianetta R, Nibali L, Rizzo M. 2019. Periodontitis and mechanisms of cardiometabolic risk: novel insights and future perspectives. Biochim Biophys Acta Mol Basis Dis. 1865(2):476–484. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, et al. 2015. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 6(9):7209–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougeot J-LC, Stevens CB, Almon KG, Paster BJ, Lalla RV, Brennan MT, Mougeot FB. 2019. Caries-associated oral microbiome in head and neck cancer radiation patients: a longitudinal study. J Oral Microbiol. 11(1):1586421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Konkel JE. 2018. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 39(4):276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, Kojima A, Naka S, Muranaka Y, Thura M, et al. 2011. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun. 2:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Jiao Y, Kim SJ, Marchesan J, Moss KL, Jing L, Divaris K, Bencharit S, Agler CS, Morelli T, et al. 2018. GWAS for interleukin-1β levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation. Nat Commun. 9(1):3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okshevsky M, Regina VR, Meyer RL. 2015. Extracellular DNA as a target for biofilm control. Curr Opin Biotechnol. 33:73–80. [DOI] [PubMed] [Google Scholar]
- Pan W, Wang Q, Chen Q. 2019. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 11(3):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietiäinen M, Liljestrand JM, Kopra E, Pussinen PJ. 2018. Mediators between oral dysbiosis and cardiovascular diseases. Eur J Oral Sci. 126 Suppl 1:26–36. [DOI] [PubMed] [Google Scholar]
- Robledo-Sierra J, Ben-Amy DP, Varoni E, Bavarian R, Simonsen JL, Paster BJ, Wade WG, Kerr R, Peterson DE, Frandsen Lau E. 2019. World Workshop on Oral Medicine VII: targeting the oral microbiome. Part 2: current knowledge on malignant and potentially malignant oral disorders. Oral Dis. 25 Suppl 1:28–48. [DOI] [PubMed] [Google Scholar]
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. 2018. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392(10159):1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Maia B, Caldas IM, Pereira ML, Pérez-Mongiovi D, Araujo R. 2016. The oral microbiome in health and its implication in oral and systemic diseases. Adv Appl Microbiol. 97:171–210. [DOI] [PubMed] [Google Scholar]
- Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, Curtsinger J, Davis Z, Zhang B, Cooley S, et al. 2018. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol Res. 6(7):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Takahashi N, Kato T, Matsuda Y, Yokoji M, Yamada M, Nakajima T, Kondo N, Endo N, Yamamoto R, et al. 2017. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci Rep. 7(1):6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BL, Kuczynski J, Bhattacharya A, Huey B, Corby PM, Queiroz ELS, Nightingale K, Kerr AR, DeLacure MD, Veeramachaneni R, et al. 2014. Changes in abundance of oral microbiota associated with oral cancer. PloS One. 9(6):e98741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TS, Hayward MR, Coelho LP, Li SS, Costea PI, Voigt AY, Wirbel J, Maistrenko OM, Alves RJ, Bergsten E, et al. 2019. Extensive transmission of microbes along the gastrointestinal tract. eLIFE. 8:e42693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Levy BD. 2018. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 128(7):2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre-Roig C, Fridlender ZG, Glogauer M, Scapini P. 2019. Neutrophil diversity in health and disease. Trends Immunol. 40(7):565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab B, Eick S, Knöfler G, Jentsch H. 2009. The influence of a probiotic milk drink on the development of gingivitis: a pilot study. J Clin Periodontol. 36(10):850–856. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Dorffel Y, Loening-Baucke V, Theissig F, Ruckert JC, Ismail M, Rau WA, Gaschler D, Weizenegger M, Kuhn S, et al. 2011. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut. 60(1):34–40. [DOI] [PubMed] [Google Scholar]
- Tada H, Matsuyama T, Nishioka T, Hagiwara M, Kiyoura Y, Shimauchi H, Matsushita K. 2016. Porphyromonas gingivalis gingipain-dependently enhances IL-33 production in human gingival epithelial cells. PLoS One. 11(4):e0152794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Suzuki R, Nemoto E, Shimauchi H, Matsushita K, Takada H. 2017. Increases in IL-33 production by fimbriae and lipopeptide from Porphyromonas gingivalis in mouse bone marrow-derived dendritic cells via Toll-like receptor 2. Biomed Res. 38(3):189–195. [DOI] [PubMed] [Google Scholar]
- Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. 2013. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000. 62(1):95–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-M, Riestra AM, Ali SR, Fong JJ, Liu JZ, Hughes G, Varki A, Nizet V. 2020. Siglec-14 enhances NLRP3-inflammasome activation in macrophages. J Innate Immun. 12(4):333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoni EM, Bavarian R, Robledo-Sierra J, Ben-Amy DP, Wade WG, Paster B, Kerr AR, Peterson DE, Lau EF. 2019. World Workshop on Oral Medicine VII: targeting the microbiome for oral medicine specialists. Part 1: a methodological guide. Oral Dis. 25(S1):12–27. [DOI] [PubMed] [Google Scholar]
- Verhulst MJL, Teeuw WJ, Bizzarro S, Muris J, Su N, Nicu EA, Nazmi K, Bikker FJ, Loos BG. 2019. A rapid, non-invasive tool for periodontitis screening in a medical care setting. BMC Oral Health. 19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, Chiang N, Serhan CN. 2018. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun. 9(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Tian J, Hao W, Zhang Q, Zhou Q, Shi W, Qin M, He X, Chen F. 2018. Oral microbiome shifts from caries-free to caries-affected status in 3-year-old Chinese children: a longitudinal study. Front Microbiol. 9:2009. doi: 10.3389/fmicb.2018.02009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost S, Stashenko P, Choi Y, Kukuruzinska M, Genco CA, Salama A, Weinberg EO, Kramer CD, Frias-Lopez J. 2018. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int J Oral Sci. 10(4):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto H, Hirota K, Hirao K, Ninomiya M, Murakami K, Fujii H, Miyake Y. 2019. The pathogenic factors from oral streptococci for systemic diseases. Int J Mol Sci. 20(18):E4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chu M, Huang Z, Yang X, Ran S, Hu B, Zhang C, Liang J. 2017. Variations in oral microbiota associated with oral cancer. Sci Rep. 7(1):11773. [DOI] [PMC free article] [PubMed] [Google Scholar]


