Abstract
What does the best available balance of scientific evidence show is the optimum way to lose weight? Calorie density, water content, protein source, and other components significantly influence the effectiveness of different dietary regimes for weight loss. By “walling off your calories,” preferentially deriving your macronutrients from structurally intact plant foods, some calories remain trapped within indigestible cell walls, which then blunts the glycemic impact, activates the ileal brake, and delivers prebiotics to the gut microbiome. This may help explain why the current evidence indicates that a whole food, plant-based diet achieves greater weight loss compared with other dietary interventions that do not restrict calories or mandate exercise. So, the most effective diet for weight loss appears to be the only diet shown to reverse heart disease in the majority of patients. Plant-based diets have also been found to help treat, arrest, and reverse other leading chronic diseases such as type 2 diabetes and hypertension, whereas low-carbohydrate diets have been found to impair artery function and worsen heart disease, the leading killer of men and women in the United States. A diet centered on whole plant foods appears to be a safe, simple, sustainable solution to the obesity epidemic.
Keywords: plant-based diet, weight loss, obesity, ketogenic diet, low-carbohydrate diet
‘. . . thousands of americans continue to needlessly die each year from what we learned decades ago was a reversible condition.’
Might there already be a safe, simple, sustainable solution to the obesity epidemic? It may take an average of 17 years before research evidence makes it into day-to-day clinical practice.1 Take, for example, heart disease. Decades ago, Dr Dean Ornish and colleagues published evidence in The Lancet that our leading cause of death could be reversed with diet and lifestyle changes alone,2 yet his dietary approach has still not been widely adopted. Even now, hundreds of thousands of Americans continue to needlessly die each year from what we learned decades ago was a reversible condition.3
If a cure for the number-one killer of men and women could be overlooked or disregarded, what else might be buried in the medical literature that could help my patients? Uncovering such life-changing, life-saving information became my life’s mission and the reason I started a nonprofit organization, NutritionFacts.org, a noncommercial resource without ads or corporate sponsorship that provides free videos and articles almost daily on the latest in evidence-based nutrition.
So, what does the science show is the best way to lose weight? When it comes to making decisions as unquestionably important as the health and well-being of one’s self, one’s family, or one’s patients, the only question is: What does the best available balance of evidence say right now?
Isn’t weight loss as simple as eating less and moving more? Isn’t a calorie a calorie? The notion that a calorie from one source is just as likely to create an energy surplus that leads to weight gain as a calorie from any other is a trope broadcast by the food industry as a way to absolve itself of culpability, as illustrated by an advertisement by Coca-Cola emphasizing this “one simple common-sense fact.”4 As the current and past chairs of Harvard’s nutrition department put it, this “central argument” from industry is that the “overconsumption of calories from carrots would be no different from overconsumption of calories from soda.”5 If this were actually the case, why would it matter what kind of foods we consume if a calorie is just a calorie?
Calorie Density
Consider that example of carrots versus Coca-Cola. While it is true that in a tightly controlled laboratory setting, 240 calories of carrots, which is about 10 carrots, would have the same effect on calorie balance as the 240 calories in a bottle of Coke,6 this comparison fails in the real world. The 240 liquid calories could be swallowed in less than a minute, but eating 240 calories of carrots might take more than 2 hours and 30 minutes of constant chewing.7 What’s more, not only would this become tiresome, but many may not even be able to eat all 240 calories of carrots, as that is around 5 cups.8
The average American consumes about 3 pounds of food a day.9 The stomach capacity for volume is limited, and when a threshold volume is reached, stretch receptors in the stomach send a signal of fullness to the appetite regulation centers in the brain.10 Different foods provide varying calories for the same volume; some foods have more calories per cup, per pound, and per mouthful than others. This is the concept of calorie density, the number of calories per unit volume for a given amount of food.
Oil, for example, has a high calorie density, or a high calorie concentration. Drizzling just one tablespoon of oil on a dish adds more than a hundred calories,11 the same calories in about 2 cups of blackberries, for example, a food with a low calorie density.12 While one would not feel a difference in the stomach after swallowing a spoonful of oil, eating 2 cups of berries would initiate a satiety signal. That is why although a calorie is a calorie biochemically, but eating the same amount of calories in different forms can have different effects on satiety.
The average human stomach capacity is about 0.5 L, but it can expand to fit about a liter of food.13 The calorie intake for an entire day could be reached with just 1 L of strawberry ice cream.14 To get those same 2000 calories from strawberries themselves would necessitate eating 44 cups of berries,15 close to 11 L. Some foods are so low in calorie density that they are impossible to overeat; physically, one could not eat enough to maintain their weight.
In a laboratory, a calorie is a calorie, but in life, far from it.
Traditional weight-loss diets focus on decreasing portion size, but “eat less” approaches can leave individuals feeling hungry and unsatisfied. A more effective approach may be to shift the emphasis from restriction, which comes with a negative connotation, to a more positive message of “eat more” healthy, low-calorie-density foods.
Researchers in Hawaii tested this “eat more” approach by having subject consume a traditional Hawaiian diet with unlimited quantities of plant foods (fruits, vegetables, whole grains, and beans),and the study subjects lost an average of 17 lb in 21 days.16 Calorie intake dropped by 40%, compared with baseline, but not because subjects were eating less food—the subjects consumed an additional 4 lb of food while losing weight on the traditional Hawaiian diet. Whole plant foods tend to be so calorically dilute that weight gain will not occur even when unrestricted.
The most significant influence on calorie density is water content.17 Since water adds weight and bulk without adding calories, the most calorie-dense foods—and, as such, the most calorie-dense diets—tend to be those that are dry. In contrast, some vegetables are almost entirely composed of water. For example, cucumbers, celery, turnips, cooked napa cabbage, bok choi, summer squash, zucchini, bean sprouts, and bamboo shoots are approximately 95% water,18 essentially water in vegetable form. A large bowl of water-rich vegetables is essentially a large bowl of trapped water (with nutrients). The effect of water-rich foods on calorie density is so dramatic it has piqued the interest of the food industry, which wants to explore using nanotechnology to “structure a solid processed food similar to a celery stalk using self-assembled, water-filled, edible nanocells or nanotubes,”19 though Mother Nature beat them to it.
When dozens of common foods were investigated for their satiety value, the characteristic most predictive was not how little fat or how much protein in the food item, but how much water it contained.20 Most vegetables are more than 90% water by weight, followed by most fresh fruit, which are approximately 80% water. Starchier vegetables, whole grains, and canned beans are typically 70% water; thus about three-quarters of their weight8 is water. Generally, when considering how water-rich foods are, most whole plant foods rank highest, most animal foods hover somewhere in the middle, and most processed foods are the lowest in water.
Premeal Loading With Foods Low in Calorie Density
In a famous series of experiments, researchers at Pennsylvania State University served pasta to study subjects and instructed them to eat as much or as little as they wanted.21 On average, subjects consumed about 900 calories of pasta. The researchers then gave them, as a first course, 100 calories of salad (composed largely of lettuce, carrots, cucumbers, celery, and cherry tomatoes) to determine if the subjects would consume the same amount of pasta, resulting in 1,000-calorie lunch of 100 calories from the starter salad plus 900 pasta calories, or if they would eat 100 fewer calories of pasta, effectively canceling out the added calories from the salad.
In fact, the subjects consumed more than 200 fewer calories of pasta after the salad course. They consumed 100 calories from the salad and reduced pasta intake by 200 calories. In essence, one could say the salad had a net result of “negative” 100 calories. The Penn State study showed that preloading a meal with high-water, low-calorie-density vegetables can effectively reduce 100 calories from a meal.
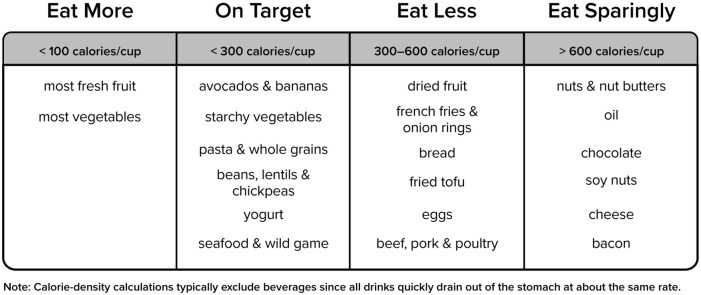
The researchers repeated the experiment, adding a fatty dressing and extra shredded cheese, which quadrupled the salad’s calorie density. Eating this higher fat salad as a first course did not result in a reduction in calorie consumption. Instead, the total calories consumed was over 1000. Studies on preloading show that eating about a cup of food with 100 calories before a meal decreases subsequent intake by about 100 calories. As such, to achieve a “negative calorie” effect, the first course would need to contain fewer than 100 calories per cup. Most fresh fruits and vegetables fall into this range, but a dinner roll, for example, does not. Figure 1 indicates the calorie density of some common foods.
Figure 1.
Calories per cup.
When study subjects were given a large apple to eat before that same pasta meal, rather than consuming about 200 fewer calories, they consumed more than 300 fewer calories.22 In effect, this means that the caloric “value” of an apple depends on when it is eaten. If consumed before a meal, it could essentially have a negative 200 calories due to the reduced intake in the main meal.
A similar result occurred when subjects were given vegetable soup as a first course: premeal soup consumption resulted in a dramatic decrease in calories consumed in the main meal. What’s more, one study that tracked people’s intake throughout the day found that overweight subjects randomized to prelunch vegetable soup not only consumed less at lunch, but intake at dinner 7 hours later was reduced by 100 calories as well.23
It has also been found that drinking 2 cups of plain water immediately before a meal resulted in a 20% reduction in food intake, resulting in more than 100 fewer calories consumed,24 and overweight men and women randomized to 2 cups of water before each meal lost weight 44% faster than the control group.25 So, “negative calorie” preloading—simply starting a meal with foods containing fewer than 100 calories per cup, such as many fruits, vegetables, soups, salads, or just a tall glass of water—may be able to accelerate the loss of body fat.
Foods That Enhance Weight Loss
How might that first-course salad boost weight loss even further? With the activation of an enzyme known as the “fat controller.”26 The discovery of 5′ AMP-activated protein kinase (AMPK) is considered one of the most important medical breakthroughs in the past few decades.27 The enzyme can be activated through exercise,28 fasting,27 and nicotine,29 and the pharmaceutical industry is investigating drugs than may activate the enzyme. Some persons with obesity may be “unwilling to perform even a minimum of physical activity,” wrote a group of pharmacologists, “thus, indicating that drugs mimicking endurance exercise are highly desirable.”30 As such, according to a researcher, “it is crucial that oral compounds with high bioavailability are developed to safely induce chronic AMPK activation [for] long-term weight loss and maintenance . . .”31 However, such a compound does not need to be developed. Vinegar is already readily available at any grocery store.
When the acetic acid in vinegar is absorbed and metabolized, a natural AMPK boost is achieved. In a randomized, double-blind, placebo-controlled trial on the effects of vinegar intake on the reduction of body fat in overweight men and women,32 subjects were randomized to drink a daily beverage containing either 1 or 2 tablespoons of apple cider vinegar or a placebo developed to taste the same as the vinegar drinks but prepared with a different type of acid. Three months into the study, the placebo group gained body fat, whereas the 2 vinegar groups significantly lost body fat, as determined by computed tomography scan. Daily doses of only 1 or 2 tablespoons of vinegar (at a cost of pennies per day) led to significant weight loss without dietary change. For this reason, one might suggest 2 teaspoons of vinegar with each meal to accelerate weight loss, on salad or added to tea with lemon juice, for example.
The vinegar studies were not just randomized controlled trials, but placebo-controlled, as well. Some studies are not controlled at all. For instance, women asked to consume a ripe tomato before lunch every day for a month lost 2 lb, but without a control group, it is unknown what role, if any, the tomato played in the weight loss.33 Simply being enrolled in a weight-loss study and knowing they will be weighed again in a month, for example, can motivate subjects to modify their diets.34 Thus, although it is certainly possible that eating a tomato before a meal may contribute to weight loss, since it is 95% water and, as such, would effectively fill a fist-sized portion of the stomach with only about 15 calories,35 a better study design is needed to assess its impact on weight loss.
More rigorous study designs include control groups. Subjects on a weight-loss diet randomized to 1 or 2 cups of low-sodium vegetable juice lost more weight than those given none,36 for example, and when subjects in another study were randomized into 2 groups with 1 group given about 2 tablespoons of goji berries a day, those in the goji group lost 2.5 inches in waist circumference in 45 days, while the control group lost none.37 Since the control group received no placebo, though, the placebo effect cannot be ruled out. However, some food items can be encapsulated, making double-blind, placebo-controlled trials easy to employ: spices.
For example, can garlic cause weight loss? Researchers gave subjects either garlic powder compressed into tablets or placebo pills and found that garlic resulted in a drop in both weight and waist circumference within 6 weeks.38 The dosage used in the study was about 0.5 teaspoon of garlic powder a day, which would cost less than 4 cents. Even 2 cents’ worth of garlic powder a day, 0.25 teaspoon, affected weight loss. About 100 overweight men and women were randomized to a quarter teaspoon of garlic powder a day or placebo, and those unknowingly taking the garlic lost about 6 lb of body fat over the next 15 weeks, significantly more than the placebo group (P = .02).39
Other common spices that have been found to facilitate weight loss include red pepper powder and ground ginger. Cayenne pepper can counteract the metabolic adaptation that results in a lower resting metabolic rate that accompanies weight loss40 and also accelerate lipolysis,41,42 and more than a dozen randomized controlled trials starting at only a quarter teaspoon of ground ginger daily showed significantly decreased body weight for just pennies a day.43 Although ginger powder’s efficacy has been demonstrated in placebo-controlled trials, these data remain relatively unknown, likely due to a lack of commercial potential.
For 3 cents a day, there is black cumin, which has shown much promise for weight loss and other health benefits.”44 A meta-analysis of randomized controlled trials shows weight-loss efficacy with a daily quarter teaspoon.45 In addition to its weight-loss benefits, daily black cumin consumption has been shown in randomized controlled trials to significantly improve cholesterol and triglycerides,46 blood pressure,47 and blood sugar control.48 A simple suggested use for the spice is to put black cumin seeds in a pepper grinder and flavor foods as one might with black pepper.
More than a thousand articles have been published in the medical literature on black cumin, with some reporting lowering cholesterol levels as much as a statin drug.49 Why, then, are both the spice and its health benefits still relatively unknown? Why aren’t students learning about black cumin in medical school? One reason may be insufficient profit motive. Black cumin is a common, natural spice that cannot be patented, and a useful dose can be bought for less than 3 cents a day.
Black cumin is distinct from regular cumin, the second most popular spice on earth,50 which has also been found to be a weight-loss booster. Subjects randomized to a half teaspoon of cumin at both lunch and dinner over 3 months lost approximately 4 lb in weight and about an inch in waist circumference (P = .005).51 The spice was found comparable to the obesity drug known as orlistat,52 a drug that inhibits dietary fat digestion (and therefore absorption), which has the possible side effect of “anal leakage” if a low-fat diet is not consumed. The “anal leakage” drug sold as Alli, though its manufacturer describes the rectal discharge caused by orlistat as “fecal spotting”53 and suggests on its website that “it’s probably a smart idea to wear dark pants, and bring a change of clothes with you to work.”54 In contrast, cumin has not been found to cause people to soil themselves.
Thylakoids offer a more natural fat-blocking effect than orlistat. The source of nearly all known life and the oxygen we breathe, thylakoids are microscopic sac-like structures composed of chlorophyll-rich membranes concentrated in the leaves of plants in which photosynthesis takes place.55 When thylakoids are consumed, as when we bite into a leaf of spinach, for instance, those green leaf membranes do not get digested immediately. They can remain in our intestines for hours56 where they can bind to lipase, the enzyme our body uses to digest triglycerice. By binding the enzyme, fat absorption can slowed.57
However, slowing fat absorption does not eliminate fat absorption, which avoids the problem of anal leakage. If all the fat is absorbed eventually, what’s the benefit? Activation of what some refer to as the” ileal brake.” When undigested macronutrients are detected in the ileum, the last segment of the small intestine, gut peptide hormones are released, relaying a satiety signal to the appetite regulation centers in the brain.55
The ileal brake concept was illustrated in a randomized, single-blind placebo controlled study.58Thirteen subjects received safflower oil, casein, sucrose, or saline (control) infusion over a 90-minute period via a naso-ileal tubefeeding 30 minutes after breakfast. Calorie consumption at a subsequent ad libitum meal was significantly reduced by over 100 calories with 3 macronutrients compared to control (P < .001). Levels of the satiety gut peptides PYY and cholecystokinin were increased with the protein, carbohydrate and fat infusions compared with the control. By activating the ileal break, hunger lessens as a feeling of fullness is experienced while eating significantly less, which can then translate into weight loss.
In another study, overweight women were randomized to consume “green-plant membranes” (powdered spinach) or control group: the green-plant membranes group had significantly higher levels of postmeal gut peptide satiety hormones and a decreased urge for sweets compared to the control group.59 The green-plant membranes group also lost significantly more weight than the control group (P < .01), although the researchers used spinach powder, as many thylakoids can be delivered by consuming about a half cup of cooked greens.55 In the Journal of the Society of Chemical Industry, several food technologists argued that given their fat-blocking benefits, “thylakoid membranes could be incorporated in functional foods as a new promising appetite-reducing ingredient.”55 Or we can just eat our greens.
Which greens are the richest in thylakoids? Their appearance is revealing. Because chlorophyll is concentrated in thylakoids, the greener the leaves, the more potent the effect.56 Accordingly, the darkest-green greens, such as lacinato or dinosaur kale, have among the most thylakoids. Similarly, overcooking results in greens turning an olive-brown in color as the thylakoids physically degrade, whereas blanching them in steaming or boiling water for approximately 15 seconds causes them to become an even brighter green, which translates into increased potency and effectiveness.60
Gut Microflora
Thylakoids eventually break down in the gut, but fiber passes into the colon. Although fiber isn’t digested, our gut flora can metabolize it. Most of the cells in our body are bacteria,61 and our gut flora, which weigh as much as a kidney and are as metabolically active as the liver,62 have been called our “forgotten organ.”63 Fiber functions on microbiota-accessible carbohydrates (MACs),64 also known as prebiotics. Indeed, our good gut flora eat fiber.
Symbiotically, we feed fiber to our good bacteria, which in turn make short-chain fatty acids that then get absorbed into our bloodstream, circulate throughout the body, and reach the brain.65 In that way, the gut flora send satiety signals, reducing the appetite,66 increasing the lipolysis,67 and increasing the metabolic rate.68
When study subjects were put into a brain scanner and shown a high-calorie food, such as a donut, the reward centers in their brains instantly lit up. However, when short-chain fatty acids derived from fiber were delivered unknowingly directly into their colon prior to repeating the experiment, the reward center response was blunted and the subjects reported that the high-calorie foods seemed less appealing; subjects subsequently ate less of an all-you-can-eat meal.69 The fiber supplement psyllium does not elicit the same response,70 though, as it is nonfermentable - our gut bacteria cannot digest it. While such psyllium products can improve bowel regularity, they cannot be used by our gut flora to stimulate the satiety response and reduce the hedonic value of food, unlike consumption of real food.
When diets are deficient in fiber, we are in effect starving our microbial selves. Less than 5% of Americans reach the recommended minimum daily adequate intake of fiber.71 This is unsurprising given that the top fiber sources are beans and whole grains,72 and 96% of Americans do not meet the recommended minimum intake of legumes (beans, split peas, chickpeas, and lentils) and 99% do not meet the recommended minimum for whole grains.73
In fact, the majority of people do not know what fiber is. More than half of Americans surveyed believed steak to be a significant source of fiber.74 However, by definition, fiber is only found in plants.75 Meat, dairy, and eggs are completely devoid of fiber, and processed foods typically contain little or none.
Animal Versus Plant Protein Sources and Weight
Might one at least be satiated by the protein in a steak? Protein intake does not translate into subsequent reduction in consumption, which has been acknowledged by a review published by authors with ties to the meat, egg, and dairy industries.76 In contrast, eating a fiber-rich, whole-grain evening meal can cut calorie intake at lunch the next day, more than 12 hours later,77 as the gut flora are still fermenting the prior evening meal high in fiber and whole grains. The continued fermentation results in the same satiety at about a hundred fewer calories.
In addition to being completely devoid of fiber, animal protein today could be considered junk food. For more than a century, a major goal of animal agriculture has been to increase the carcass fat content of farm animals.78 Consider chicken, for example. A hundred years ago, the US Department of Agriculture determined that chicken was approximately 23% protein by weight and less than 2% fat.79 Today, due in part to genetic manipulation via selective breeding, chickens can have 10 times more fat.80
Weight gain is associated with consumption of meat in general,81 and poultry appears to contribute to weight gain.82,83 One daily ounce, which is about a single chicken nugget84 or 1 chicken breast every 10 days85 has been associated with weight gain compared with eating no chicken at all.83
In meat industry–funded studies on obesity and chicken consumption, researchers often chose foods such as cookies and “sugar-coated chocolates” for their comparisons.86 The strategy of elevating one product by using inappropriate controls should be well-known to anyone familiar with drug industry misconduct.87 When chicken is pitted against a meaningful comparator, such as chicken-free chicken, however, it fails. Both tofu and Quorn, a plant-based meat made from the mushroom kingdom, were found to have stronger satiating qualities than chicken.88 Four and a half hours after eating a lunch of chicken and rice, study subjects consumed 18% more calories at a dinner buffet than those who consumed a lunch of chicken-free chicken and rice.89,90
These findings are consistent with childhood obesity research that found meat consumption appears to double the odds of schoolchildren becoming overweight compared with the consumption of plant-based meat alternatives.91 What’s more, whole-food sources of plant protein, such as beans, have been associated with halving the odds of children becoming overweight.91 For these reasons, plant-based meat subsitutes should be considered a useful stepping stone toward a healthier diet, rather than the endgame ideal.
Plant-based meat substitutes may be less obesogenic in part because they result in lower insulin spikes. A meat-free chicken substitute like Quorn induces up to a 41% lower insulin spike than chicken,90 whereas animal protein causes almost as much insulin release as pure sugar.92 Simply adding egg whites to one’s diet can increase insulin output up to 60% within 4 days93 and fish may have an even greater effect.94
Adding tuna to mashed potatoes causes a spike in insulin levels95 while adding broccoli instead cuts the insulin response by about 40%.96 The difference in reaction is not due to fiber, since giving the same amount of broccoli fiber alone provided no significant benefit. Why does animal protein worsen insulin levels, but plant protein improves them?
Proteins from plants tend to be lower in branched-chain amino acids, which are associated with insulin resistance, the hallmark of type 2 diabetes.97 This has been shown experimentally. Vegan subjects given branched-chain amino acids became as insulin-resistant as omnivores,98 and after omnivores went through a “48-hour vegan diet challenge,” within 2 days they experienced significant improvements in metabolic health.99
Decreased consumption of branched-chain amino acids improves metabolic health. Subjects randomized to restrict their protein intake averaged hundreds more calories per day, yet they lost more body fat despite the greater caloric consumption. Restricting protein enabled them to eat more calories, while, at the same time, they lost more weight.100 How limiting was the “protein restriction?” The subjects consumed the recommended amount of protein.101 In this way, the “protein restriction” group could be considered the “normal protein” or “recommended protein” group, and the group eating protein levels more typical to the American diet102 and experiencing the resulting adverse metabolic consequences the “excess protein” group.
Given the metabolic harms of excess branched-chain amino acid exposure, leaders in the field have suggested the invention of drugs to block their absorption to “promote metabolic health and treat diabetes and obesity without reducing caloric intake.”103 Or one can simply reduce consumption of branched-chain amino acids, which are found mostly in meat, including chicken and fish, dairy products, and eggs. This may explain why animal protein has been associated with higher diabetes risk, whereas plant protein appears protective.104As such, defining the “appropriate upper limits” of animal protein intake “may offer a great chance for the prevention of [type 2 diabetes] and obesity.”105 Even an “intermittent vegan diet” may be beneficial.99
Plant Foods and Walling Off Calories
The one strategy that may best sum up the recommendations for safe, healthy, sustainable weight loss is to wall off your calories. Animal cells are encased in easily digestible membranes, which allow the enzymes in the gut to effortlessly liberate the calories within a chicken breast, for example, while plant cells have cell walls that are made of fiber, which can act as indigestible physical barriers so many of the calories remain trapped. Processed plant foods, however, such as fruit juice, sugar, refined grains, and even 100% whole grains that have been powdered into flour, have had their cellular structure destroyed and their calories are no longer trapped. In contrast, when eating structurally intact plant foods, no matter how well we chew, some calories will remain completely encapsulated by fiber, which then blunts the glycemic impact, activates the ileal brake, and delivers sustenance to the gut flora. We should therefore counsel our patients to try to source their macronutrients—their protein, carbohydrates, and fat—from whole, intact plant foods.
This strategy is rooted in our evolutionary legacy. Millions of years before we learned to sharpen spears, mill grains, or boil sugar cane, our entire physiology is presumed to have evolved in the context of eating what the rest of our great ape cousins eat—namely, plants.106 The Paleolithic period, when we started using tools, goes back approximately 2 million years, but we and other great apes have been evolving since the Miocene era, 20 million years ago.107 For the first 90% of our hominoid existence, our bodies evolved mostly on plants,108 which is why our bodies may thrive best on the diet we were designed to eat over tens of millions of years.
Whole Food Plant-Based Diets and Weight
With enough portion control or physical exertion, any amount of weight can be lost, but what is the most effective weight-loss regimen that does not involve calorie restriction or exercise? Based on the preponderance of randomized controlled trials so far published in the peer-reviewed medical literature, the single most successful strategy to date is a whole food, plant-based diet.109
We have known for more than 40 years that those eating predominantly plant-based diets weigh, on average, up to 30 lbs less than the general population.110 More recently, in 2017, a group of New Zealand researchers published the BROAD study, a 12-week randomized controlled trial in the poorest region of the country with the highest rates of obesity. Overweight individuals were randomized to receive either standard medical care or semiweekly classes offering advice and encouragement to eat a low-fat diet centered around fruits, vegetables, whole grains, and legumes—empowerment with knowledge. No meals were provided. The intervention group was merely informed about the benefits of plant-based eating and encouraged to incorporate it into their own lives.109
There was no significant change in body weight in the control group, but the plant-based group lost an average of 19 lbs by the end of the 3-month study despite being able to freely eat all the healthy foods they wanted without restrictions on portion sizes. At the end of the 12 weeks, the study concluded and no more instruction was given.
At the 6-month mark, the researchers invited the subjects to get reweighed to see how much weight they gained back after being released from the study. The plant-based group had left the 3-month study 19 pounds lighter, but at 6 months were down about 27 lbs. The subjects in the intervention group reported experiencing benefits physically and mentally, had been able to stop taking many of their medications, had elected to remain on the diet after the study ended, and continued to lose weight.
And 1 year later? Even in studies with year-long durations during in which subjects are coached to remain on a particular diet the entire time, any initial weight lost in the first few months tends to be regained by the end of the year. The BROAD study, however, lasted only 3 months, yet those who had been randomized to the plant-based group not only lost significant weight, they kept it off. They achieved greater weight loss at 6 and 12 months than any other comparable trial, months after the study had already ended.
Given that any diet reducing calorie intake can result in weight loss, the issue is not losing weight but not regaining it. A key difference between plant-based nutrition and more traditional approaches to weight loss is that the former encourages people to eat ad libitum without counting calories or controlling portion sizes. The strategy is to improve the quality of food rather than restricting its quantity.
When subjects have been placed on a diet high in fruits, vegetables, whole grains, and beans and allowed to eat ad libitum, they consumed nearly 50% fewer calories than a higher calorie density diet,111 feeling just as full on half the calories. By eating more high-bulk, low-calorie density foods, such as vegetables, fruits, whole grains, and beans, and fewer calorie-dense foods, like meats, cheeses, sugars, and fats, they were satisfied even after cutting more than a thousand calories from their daily diets.
Beyond impact on calorie intake, those eating more plant-based diets appear to effectively burn more calories in their sleep. Their resting metabolic rate may be about 10% higher or more,112 and this boosted metabolism can translate into burning off hundreds of additional calories a day.113
Meal Timing
Having established the optimal weight-loss diet, how might further weight loss be boosted? Given that a calorie is not necessarily a calorie and a hundred calories of chickpeas have a different impact than a hundred calories of chicken, can the same foods eaten differently have different effects? Even if the same amount is consumed and the same amount absorbed, a calorie may still not be a calorie. It is not only what we eat, but how and when.
Calories consumed at breakfast, for example, are significantly less fattening than the same number of calories consumed at dinner. Indeed, more weight loss is achieved with a diet with a larger breakfast than the same diet with a larger dinner.114,115 As such, I recommend not eating after 7:00 pm because a snack consumed at night is more obesogenic than the exact same snack eaten in the daytime, due to our circadian rhythms, our chronobiology.
The Ketogenic Diet
What does the literature indicate about the use of ketogenic diets for weight loss? Although the keto diet appears to be effective, with subjects losing less than a pound a week on a regular diet and then experiencing a weight loss of 3.5 lbs within 7 days after switching to a ketogenic diet, weight loss doesn’t tell the whole story.116 On switching to a ketogenic diet, body fat loss slows by more than half because it appears that the body starts cannibalizing its own protein, which may help explain why the leg muscles of CrossFit trainees placed on a ketogenic diet can diminish as much as 8% within 2 months.117
What’s more, people whose diets tend to trend toward keto appear to live significantly shorter lives,118 but simply drifting in the direction of eating more healthy plant foods is associated with living longer.119 In contrast, individuals who consumed more plant-based diets then later added meat at least once a week not only appeared to double or triple their odds of diabetes, stroke, heart disease, and weight gain, but they may also suffer an associated 3.6-year drop in life expectancy.120
Low-carbohydrate diets have been shown to impair artery function121 and worsen heart disease,122 whereas whole food, plant-based diets, used by Ornish in his landmark study, have been shown to reverse heart disease.123
Conclusions
What appears to be the most effective weight-loss diet is the only diet shown to reverse heart disease in the majority of patients. If reversing the number-one killer of men and women was all a plant-based diet could do, shouldn’t it be considered the default diet until proven otherwise? Furthermore, it can also be effective in treating, arresting, and reversing other leading killers like type 2 diabetes124 and high blood pressure, making the case for plant-based eating simply overwhelming.
A diet centered on whole plant foods is the only diet that has ever been shown to reap such benefits. Our health need not be mortgaged to lose weight. The single healthiest diet also appears to be the most effective diet for weight loss.
Permanent weight loss requires permanent dietary change. More healthful habits simply need to become a way of life. The single best diet for weight loss may just so happen to be the safest, cheapest way to eat for the longest, most healthful life.
Acknowledgments
Much appreciation to Miyun Park for editorial assistance, Alissa Finley for fact-checking and formatting, and Dustin Kirkpatrick for the figure design.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
References
- 1. Balas EA, Boren SA. Managing clinical knowledge for health care improvement. In: Bemmel J, McCray AT, eds. Yearbook of Medical Informatics 2000: Patient-Centered Systems. Stuttgart, Germany: Schattauer; 2000:65-70. [PubMed] [Google Scholar]
- 2. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet. 1990;336:129-133. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Heart disease facts. https://www.cdc.gov/heartdisease/facts.htm. Accessed October 1, 2019.
- 4. The Coca-Cola Co. Coming Together [Video]. https://www.youtube.com/watch?v=oV2D0Zq124g. Published November 11, 2013. Accessed March 19, 2019.
- 5. Malik VS, Willett WC, Hu FB. The revised nutrition facts label: a step forward and more room for improvement. JAMA. 2016;316:583-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ha V, Cozma AI, Choo VL, Mejia SB, Souza RJ, Sievenpiper JL. Do fructose-containing sugars lead to adverse health consequences? Results of recent systematic reviews and meta-analyses. Adv Nutr. 2015;6:504S-511S. [Google Scholar]
- 7. Viskaal-van Dongen M, Kok FJ, de Graaf C. Eating rate of commonly consumed foods promotes food and energy intake. Appetite. 2011;56:25-31. [DOI] [PubMed] [Google Scholar]
- 8. United States Department of Agriculture, Agricultural Research Service. USDA Food Composition Databases. https://ndb.nal.usda.gov/ndb. Accessed April 1, 2019.
- 9. Grunwald GK, Seagle HM, Peters JC, Hill JO. Quantifying and separating the effects of macronutrient composition and non-macronutrients on energy density. Br J Nutr. 2001;86:265-276. [DOI] [PubMed] [Google Scholar]
- 10. Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. United States Department of Agriculture, Agricultural Research Service. Basic report: 04053, oil, olive, salad or cooking. https://ndb.nal.usda.gov/ndb/foods/show/04053. Accessed April 1, 2019.
- 12. United States Department of Agriculture, Agricultural Research Service. Basic report: 09042, blackberries, raw. https://ndb.nal.usda.gov/ndb/foods/show/301063. Accessed April 1, 2019.
- 13. Ebbeling CB, Garcia-Lago E, Leidig MM, Seger-Shippee LG, Feldman HA, Ludwig DS. Altering portion sizes and eating rate to attenuate gorging during a fast food meal: effects on energy intake. Pediatrics. 2007;119:869-875. [DOI] [PubMed] [Google Scholar]
- 14. United States Department of Agriculture, Agricultural Research Service. Ice cream strawberries & cream, UPC: 079893092959. https://fdc.nal.usda.gov/fdc-app.html#/food-details/450582/nutrients. Accessed February 28, 2020.
- 15. United States Department of Agriculture, Agricultural Research Service. Basic report: 09316, strawberries, raw. https://ndb.nal.usda.gov/ndb/foods/show/301283. Accessed April 1, 2019.
- 16. Shintani TT, Hughes CK, Beckham S, O’Connor HK. Obesity and cardiovascular risk intervention through the ad libitum feeding of traditional Hawaiian diet. Am J Clin Nutr. 1991;53(6 suppl):1647S-1651S. [DOI] [PubMed] [Google Scholar]
- 17. Smethers AD, Rolls BJ. Dietary management of obesity: cornerstones of healthy eating patterns. Med Clin North Am. 2018;102:107-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. United States Department of Agriculture, Agricultural Research Service. Food composition databases show nutrients list. https://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=208&nutrient2=&fg=4&subset=0&sort=f&measureby=g. Accessed April 1, 2019.
- 19. Robson AA. Food nanotechnology: water is the key to lowering the energy density of processed foods. Nutr Health. 2011;20:231-236. [DOI] [PubMed] [Google Scholar]
- 20. Holt SH, Miller JC, Petocz P, Farmakalidis E. A satiety index of common foods. Eur J Clin Nutr. 1995;49:675-690. [PubMed] [Google Scholar]
- 21. Rolls BJ, Roe LS, Meengs JS. Salad and satiety: energy density and portion size of a first-course salad affect energy intake at lunch. J Am Diet Assoc. 2004;104:1570-1576. [DOI] [PubMed] [Google Scholar]
- 22. Flood-Obbagy JE, Rolls BJ. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite. 2009;52:416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Himaya A, Louis-Sylvestre J. The effect of soup on satiation. Appetite. 1998;30:199-210. [DOI] [PubMed] [Google Scholar]
- 24. Corney RA, Sunderland C, James LJ. Immediate pre-meal water ingestion decreases voluntary food intake in lean young males. Eur J Nutr. 2016;55:815-819. [DOI] [PubMed] [Google Scholar]
- 25. Dennis EA, Dengo AL, Comber DL, et al. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity (Silver Spring). 2010;18:300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinberg GR, Macaulay SL, Febbraio MA, Kemp BE. AMP-activated protein kinase—the fat controller of the energy railroad. Can J Physiol Pharmacol. 2006;84:655-665. [DOI] [PubMed] [Google Scholar]
- 27. López M. EJE PRIZE 2017. Hypothalamic AMPK: a golden target against obesity? Eur J Endocrinol. 2017;176:R235-R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Musi N, Fujii N, Hirshman MF, et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921-927. [DOI] [PubMed] [Google Scholar]
- 29. Novak CM, Gavini CK. Smokeless weight loss. Diabetes. 2012;61:776-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niederberger E, King TS, Russe OQ, Geisslinger G. Activation of AMPK and its impact on exercise capacity. Sports Med. 2015;45:1497-1509. [DOI] [PubMed] [Google Scholar]
- 31. Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. 2013;366:194-203. [DOI] [PubMed] [Google Scholar]
- 32. Kondo T, Kishi M, Fushimi T, Ugajin S, Kaga T. Vinegar intake reduces body weight, body fat mass, and serum triglyceride levels in obese Japanese subjects. Biosci Biotechnol Biochem. 2009;73:1837-1843. [DOI] [PubMed] [Google Scholar]
- 33. Vinha AF, Barreira SV, Costa AS, Alves RC, Oliveira MB. Pre-meal tomato (Lycopersicon esculentum) intake can have anti-obesity effects in young women? Int J Food Sci Nutr. 2014;65:1019-1026. [DOI] [PubMed] [Google Scholar]
- 34. Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P; Behavioural Weight Management Review Group. Weight change among people randomized to minimal intervention control groups in weight loss trials. Obesity (Silver Spring). 2016;24:772-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. United States Department of Agriculture. Tomatoes, fresh. Household USDA Foods Fact Sheet. https://www.choosemyplate.gov/usda-foods-fact-sheets. Accessed April 17, 2019.
- 36. Shenoy SF, Poston WS, Reeves RS, et al. Weight loss in individuals with metabolic syndrome given DASH diet counseling when provided a low sodium vegetable juice: a randomized controlled trial. Nutr J. 2010;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Souza Zanchet MZ, Nardi GM, de Oliveira Souza Bratti L, Filippin-Monteiro FB, Locatelli C. Lycium barbarum reduces abdominal fat and improves lipid profile and antioxidant status in patients with metabolic syndrome. Oxid Med Cell Longev. 2017;2017:9763210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharifi F, Sheikhi AK, Behdad M, Mousavinasab N. Effect of garlic on serum adiponectin and interleukin levels in women with metabolic syndrome. Int J Endocrinol Metab. 2010;8:68-73. [Google Scholar]
- 39. Soleimani D, Paknahad Z, Askari G, Iraj B, Feizi A. Effect of garlic powder consumption on body composition in patients with nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled trial. Adv Biomed Res. 2016;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smeets AJ, Janssens PL, Westerterp-Plantenga MS. Addition of capsaicin and exchange of carbohydrate with protein counteract energy intake restriction effects on fullness and energy expenditure. J Nutr. 2013;143:442-447. [DOI] [PubMed] [Google Scholar]
- 41. Ludy MJ, Mattes RD. The effects of hedonically acceptable red pepper doses on thermogenesis and appetite. Physiol Behav. 2011;102:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Snitker S, Fujishima Y, Shen H, et al. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr. 2009;89:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maharlouei N, Tabrizi R, Lankarani KB, et al. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59:1753-1766. [DOI] [PubMed] [Google Scholar]
- 44. Ahmad A, Husain A, Mujeeb M, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3:337-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mousavi SM, Sheikhi A, Varkaneh HK, Zarezadeh M, Rahmani J, Milajerdi A. Effect of Nigella sativa supplementation on obesity indices: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2018;38:48-57. [DOI] [PubMed] [Google Scholar]
- 46. Sahebkar A, Beccuti G, Simental-Mendía LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;106:37-50. [DOI] [PubMed] [Google Scholar]
- 47. Sahebkar A, Soranna D, Liu X, et al. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J Hypertens. 2016;34:2127-2135. [DOI] [PubMed] [Google Scholar]
- 48. Daryabeygi-Khotbehsara R, Golzarand M, Ghaffari MP, Djafarian K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: a systematic review and meta-analysis. Complement Ther Med. 2017;35:6-13. [DOI] [PubMed] [Google Scholar]
- 49. Ibrahim RM, Hamdan NS, Mahmud R, et al. A randomised controlled trial on hypolipidemic effects of Nigella Sativa seeds powder in menopausal women. J Transl Med. 2014;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mnif S, Aifa S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem Biodivers. 2015;12:733-742. [DOI] [PubMed] [Google Scholar]
- 51. Zare R, Heshmati F, Fallahzadeh H, Nadjarzadeh A. Effect of cumin powder on body composition and lipid profile in overweight and obese women. Complement Ther Clin Pract. 2014;20:297-301. [DOI] [PubMed] [Google Scholar]
- 52. Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. Effect of the cumin cyminum L. intake on weight loss, metabolic profiles and biomarkers of oxidative stress in overweight subjects: a randomized double-blind placebo-controlled clinical trial. Ann Nutr Metab. 2015;66:117-124. [DOI] [PubMed] [Google Scholar]
- 53. Fox M, Thumshirn M, Menne D, Stutz B, Fried M, Schwizer W. The pathophysiology of faecal spotting in obese subjects during treatment with orlistat. Aliment Pharmacol Ther. 2004;19:311-321. [DOI] [PubMed] [Google Scholar]
- 54. myalli.com. Before you begin with alli. https://web.archive.org/web/20080821141135/http://www.myalli.com:80/howdoesitwork/treatmenteffects.aspx. Accessed March 31, 2019.
- 55. Östbring K, Sjöholm I, Sörenson H, Ekholm A, Erlanson-Albertsson C, Rayner M. Characteristics and functionality of appetite-reducing thylakoid powders produced by three different drying processes. J Sci Food Agric. 2018;98:1554-1565. [DOI] [PubMed] [Google Scholar]
- 56. Erlanson-Albertsson C, Albertsson PÅ. The use of green leaf membranes to promote appetite control, suppress hedonic hunger and loose [sic] body weight. Plant Foods Hum Nutr. 2015;70:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Albertsson PA, Köhnke R, Emek SC, et al. Chloroplast membranes retard fat digestion and induce satiety: effect of biological membranes on pancreatic lipase/co-lipase. Biochem J. 2007;401:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Avesaat M, Troost FJ, Ripken D, Hendriks HF, Masclee AA. Ileal brake activation: macronutrient-specific effects on eating behavior? Int J Obes (Lond). 2015;39:235-243. [DOI] [PubMed] [Google Scholar]
- 59. Montelius C, Erlandsson D, Vitija E, Stenblom EL, Egecioglu E, Erlanson-Albertsson C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite. 2014;81:295-304. [DOI] [PubMed] [Google Scholar]
- 60. Östbring K, Rayner M, Sjöholm I, et al. The effect of heat treatment of thylakoids on their ability to inhibit in vitro lipase/co-lipase activity. Food Funct. 2014;5:2157-2165. [DOI] [PubMed] [Google Scholar]
- 61. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009;11:307-313. [DOI] [PubMed] [Google Scholar]
- 63. O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab. 2014;20:779-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18:18-31. [DOI] [PubMed] [Google Scholar]
- 66. Nilsson A, Johansson E, Ekström L, Björck I. Effects of a brown beans evening meal on metabolic risk markers and appetite regulating hormones at a subsequent standardized breakfast: a randomized cross-over study. PLoS One. 2013;8:e59985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Beek CM, Canfora EE, Lenaerts K, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond). 2016;130:2073-2082. [DOI] [PubMed] [Google Scholar]
- 68. Canfora EE, van der Beek CM, Jocken JWE, et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Byrne CS, Chambers ES, Alhabeeb H, et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sayer RD, Amankwaah AF, Tamer GG, et al. Effects of dietary protein and fiber at breakfast on appetite, ad libitum energy intake at lunch, and neural responses to visual food stimuli in overweight adults. Nutrients. 2016;8:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clemens R, Kranz S, Mobley AR, et al. Filling America’s fiber intake gap: summary of a roundtable to probe realistic solutions with a focus on grain-based foods. J Nutr. 2012;142:1390S-1401S. [DOI] [PubMed] [Google Scholar]
- 72. Burkitt DP, Meisner P. How to manage constipation with high-fiber diet. Geriatrics. 1979;34:33-35, 38-40. [PubMed] [Google Scholar]
- 73. Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wick JY. Diverticular disease: eat your fiber! Consult Pharm. 2012;27:613-618. [DOI] [PubMed] [Google Scholar]
- 75. US Institute of Medicine. Dietary Reference Intakes: Proposed Definition of Dietary Fiber. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 76. Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S-1329S. [DOI] [PubMed] [Google Scholar]
- 77. Ibrügger S, Vigsnæs LK, Blennow A, et al. Second meal effect on appetite and fermentation of wholegrain rye foods. Appetite. 2014;80:248-256. [DOI] [PubMed] [Google Scholar]
- 78. Eaton SB. Humans, lipids and evolution. Lipids. 1992;27:814-820. [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Lehane C, Ghebremeskel K, Crawford MA. Modern organic and broiler chickens sold for human consumption provide more energy from fat than protein. Public Health Nutr. 2010;13:400-408. [DOI] [PubMed] [Google Scholar]
- 80. United States Department of Agriculture, Agricultural Research Service. Basic report: 05123, chicken, stewing, meat and skin, raw. https://ndb.nal.usda.gov/ndb/foods/show/05123. Accessed April 1, 2019.
- 81. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Vergnaud AC, Norat T, Romaguera D, et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study. Am J Clin Nutr. 2010;92:398-407. [DOI] [PubMed] [Google Scholar]
- 83. Gilsing AM, Weijenberg MP, Hughes LA, et al. Longitudinal changes in BMI in older adults are associated with meat consumption differentially, by type of meat consumed. J Nutr. 2012;142:340-349. [DOI] [PubMed] [Google Scholar]
- 84. United States Department of Agriculture, Agricultural Research Service. Chicken nuggets, UPC: 011110819659. https://fdc.nal.usda.gov/fdc-app.html#/food-details/410103/nutrients. Accessed February 28, 2020.
- 85. United States Department of Agriculture, Agricultural Research Service. Basic report: 05057, chicken, broilers or fryers, breast, meat and skin, raw. https://ndb.nal.usda.gov/ndb/foods/show/05057. Accessed April 1, 2019.
- 86. Mahon AK, Flynn MG, Stewart LK, et al. Protein intake during energy restriction: effects on body composition and markers of metabolic and cardiovascular health in postmenopausal women. J Am Coll Nutr. 2007;26:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mann H, Djulbegovic B. Comparator bias: why comparisons must address genuine uncertainties. J R Soc Med. 2013;106:30-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Williamson DA, Geiselman PJ, Lovejoy J, et al. Effects of consuming mycoprotein, tofu or chicken upon subsequent eating behaviour, hunger and safety. Appetite. 2006;46:41-48. [DOI] [PubMed] [Google Scholar]
- 89. Burley VJ, Paul AW, Blundell JE. Influence of a high-fibre food (myco-protein) on appetite: effects on satiation (within meals) and satiety (following meals). Eur J Clin Nutr. 1993;47:409-418. [PubMed] [Google Scholar]
- 90. Bottin JH, Swann JR, Cropp E, et al. Mycoprotein reduces energy intake and postprandial insulin release without altering glucagon-like peptide-1 and peptide tyrosine-tyrosine concentrations in healthy overweight and obese adults: a randomised-controlled trial. Br J Nutr. 2016;116:360-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sabaté J, Wien M. Vegetarian diets and childhood obesity prevention. Am J Clin Nutr. 2010;91:1525S-1529S. [DOI] [PubMed] [Google Scholar]
- 92. Nuttall FQ, Mooradian AD, Gannon MC, Billington C, Krezowski P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care. 1984;7:465-470. [DOI] [PubMed] [Google Scholar]
- 93. Remer T, Pietrzik K, Manz F. A moderate increase in daily protein intake causing an enhanced endogenous insulin secretion does not alter circulating levels or urinary excretion of dehydroepiandrosterone sulfate. Metabolism. 1996;45:1483-1486. [DOI] [PubMed] [Google Scholar]
- 94. Pal S, Ellis V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br J Nutr. 2010;104:1241-1248. [DOI] [PubMed] [Google Scholar]
- 95. Gulliford MC, Bicknell EJ, Scarpello JH. Differential effect of protein and fat ingestion on blood glucose responses to high- and low-glycemic-index carbohydrates in noninsulin-dependent diabetic subjects. Am J Clin Nutr. 1989;50:773-777. [DOI] [PubMed] [Google Scholar]
- 96. Ballance S, Knutsen SH, Fosvold ØW, Wickham M, Trenado CD, Monro J. Glyceamic and insulinaemic response to mashed potato alone, or with broccoli, broccoli fibre or cellulose in healthy adults. Eur J Nutr. 2018;57:199-207. [DOI] [PubMed] [Google Scholar]
- 97. Tian S, Xu Q, Jiang R, Han T, Sun C, Na L. Dietary protein consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Nutrients. 2017;9:E982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gojda J, Rossmeislová L, Straková R, et al. Chronic dietary exposure to branched chain amino acids impairs glucose disposal in vegans but not in omnivores. Eur J Clin Nutr. 2017;71:594-601. [DOI] [PubMed] [Google Scholar]
- 99. Draper CF, Vassallo I, Di Cara A, et al. A 48-hour vegan diet challenge in healthy women and men induces a BRANCH-chain amino acid related, health associated, metabolic signature. Mol Nutr Food Res. 2018;62. [DOI] [PubMed] [Google Scholar]
- 100. Fontana L, Cummings NE, Arriola Apelo SI, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16:520-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621-1630. [DOI] [PubMed] [Google Scholar]
- 102. US Department of Agriculture, Agricultural Research Service. Nutrient intakes from food: mean amounts and percentages of calories from protein, carbohydrate, fat, and alcohol, one day, 2005-2006. 2008. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/0506/Table_2_NIF_05.pdf. Accessed February 28, 2020.
- 103. Cummings NE, Williams EM, Kasza I, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol (Lond). 2018;596:623-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Isanejad M, Lacroix AZ, Thomson CA, et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women’s Health Initiative. Br J Nutr. 2017;117:1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes. 2012;3:38-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jenkins DJ, Kendall CW. The garden of Eden: plant-based diets, the genetic drive to store fat and conserve cholesterol, and implications for epidemiology in the 21st century. Epidemiology. 2006;17:128-130. [DOI] [PubMed] [Google Scholar]
- 107. Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283-289. [DOI] [PubMed] [Google Scholar]
- 108. Anderson JW, Konz EC, Jenkins DJ. Health advantages and disadvantages of weight-reducing diets: a computer analysis and critical review. J Am Coll Nutr. 2000;19:578-590. [DOI] [PubMed] [Google Scholar]
- 109. Wright N, Wilson L, Smith M, Duncan B, McHugh P. The BROAD study: a randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr Diabetes. 2017;7:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Sacks FM, Castelli WP, Donner A, Kass EH. Plasma lipids and lipoproteins in vegetarians and controls. N Engl J Med. 1975;292:1148-1151. [DOI] [PubMed] [Google Scholar]
- 111. Duncan KH, Bacon JA, Weinsier RL. The effects of high and low energy density diets on satiety, energy intake, and eating time of obese and nonobese subjects. Am J Clin Nutr. 1983;37:763-777. [DOI] [PubMed] [Google Scholar]
- 112. Toth MJ, Poehlman ET. Sympathetic nervous system activity and resting metabolic rate in vegetarians. Metabolism. 1994;43:621-625. [DOI] [PubMed] [Google Scholar]
- 113. Montalcini T, De Bonis D, Ferro Y, et al. High vegetable fats intake is associated with high resting energy expenditure in vegetarians. Nutrients. 2015;7:5933-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Halberg F, Haus E, Cornélissen G. From biologic rhythms to chronomes relevant for nutrition. In: Marriott B, ed. Not Eating Enough: Overcoming Underconsumption of Military Operational Rations. Washington, DC: National Academy Press; 1995:361-372. [PubMed] [Google Scholar]
- 115. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21:2504-2512. [DOI] [PubMed] [Google Scholar]
- 116. Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kephart WC, Pledge CD, Roberson PA, et al. The three-month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in crossfit trainees: a pilot study. Sports (Basel). 2018;6:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Noto H, Goto A, Tsujimoto T, Noda M. Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One. 2013;8:e55030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kim H, Caulfield LE, Rebholz CM. Healthy plant-based diets are associated with lower risk of all-cause mortality in US adults. J Nutr. 2018;148:624-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Singh PN, Arthur KN, Orlich MJ, et al. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am J Clin Nutr. 2014;100(suppl 1):359S-364S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schwingshackl L, Hoffmann G. Low-carbohydrate diets impair flow-mediated dilatation: evidence from a systematic review and meta-analysis. Br J Nutr. 2013;110:969-970. [DOI] [PubMed] [Google Scholar]
- 122. Fleming RM. The effect of high-protein diets on coronary blood flow. Angiology. 2000;51:817-826. [DOI] [PubMed] [Google Scholar]
- 123. Esselstyn CB. A plant-based diet and coronary artery disease: a mandate for effective therapy. J Geriatr Cardiol. 2017;14:317-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Anderson JW, Ward K. High-carbohydrate, high-fiber diets for insulin-treated men with diabetes mellitus. Am J Clin Nutr. 1979;32:2312-2321. [DOI] [PubMed] [Google Scholar]