Abstract
Problem:
To determine the safety and efficacy of topical corticosteroid versus vehicle/moisturizer in children under 2 years old (<2y).
Eligibility Criteria:
A systematic review and meta-analysis searching PubMed MEDLINE, Embase, Web of Science, Cochrane Database of Controlled Trials, Cochrane Database of Systematic Reviews, DARE, NHS Economic Evaluation, CINAHL, GREAT, and Clinicaltrials.gov. We selected randomized controlled trials(RCTs) comparing topical corticosteroids to vehicle/moisturizer and included children <2y. Two authors extracted data.
Sample:
Only one study limited analyses to children <2y, so our review included participants older than 2 years. Twelve RCTs were included with 2224 participants. Ten studies were industry-sponsored.
Results:
The proportion of responders to topical corticosteroid across studies was 0.65 (95% CI, 0.54– 0.74), as compared to vehicle/moisturizer 0.32 (95% Confidence Interval (CI), 0.20–0.48). The proportion of adverse events were similar between groups (topical steroids 0.17 (95% CI, 0.08–0.33) vs. vehicle/moisturizer 0.12 (CI 0.02–0.42)). High heterogeneity in treatment response occurred across studies that could not be explained by potential moderators. Mild adrenal suppression occurred in 4 of 157 measured participants (3%) receiving topical corticosteroids. Limitations include the few RCTs on this topic, the inclusion of participants >2y and outcome measures and reporting methods rarely met CONSORT guidelines.
Conclusions:
Topical corticosteroids trended to being more effective and equally safe to vehicle/moisturizers, but generalizability is limited given the dearth of well-designed studies focused on children <2y. Adverse events from vehicle/moisturizer may be greater than topical corticosteroid due to under treatment.
Implications:
Further work is needed in this age group.
Keywords: atopic dermatitis, topical corticosteroids, pediatrics, moisturizer, placebo
Introduction
Atopic dermatitis (eczema, or AD) is one of the most common skin conditions in pediatrics, affecting 10%−20% of US children (Silverberg, 2017). The prevalence of AD is greatest in children under 2 years, as ~50% of AD is diagnosed in the first year of life (Kanchongkittiphon, Gaffin, & Phipatanakul, 2015). Topical corticosteroids are the mainstay of treatment and, after moisturizers, recommended as the first line treatment option in recent guidelines (Eichenfield et al., 2017). Steroid-sparing agents, such as calcineurin inhibitors and phosphodiesterase-4 (PDE4) inhibitors, are not FDA approved for children under 2 years. Adjunctive therapies such as wet wraps and bleach baths are also important options, which have been the subject of systematic reviews (Bath-Hextall, Birnie, Ravenscroft, & Williams, 2010; Gonzalez-Lopez, Ceballos-Rodriguez, Gonzalez-Lopez, Feito Rodriguez, & Herranz-Pinto, 2017). However, despite topical corticosteroids being first line treatment, they have not been systematically reviewed in children under 2 years old.
Under-treatment of AD in young children is common, in part because of the lack of guidelines specific to this age group and concerns about potential side effects. Infants are at the highest risk of having systemic absorption of topical corticosteroids, with potential risks including reduced linear growth due to their high ratio of body surface area (BSA) to body weight (Hengge, Ruzicka, Schwartz, & Cork, 2006). Although, “steroid phobia” does not correlate with disease severity and is often based on inaccurate perceptions of side effects, (Kojima et al., 2013) 80% of parents have concerns about the use of topical corticosteroids in their child (Morley & Dinulos, 2012). This is a common issue which parents discuss with their nursing providers, and it is important nurses are knowledgeable on this topic.
Under-treatment is a significant concern and puts patients at risk for repeated disease exacerbation. Given that scratching and rubbing when AD is not in control exacerbates the inflammation and barrier defect, early under-treatment can ultimately result in more overall topical corticosteroid usage, as higher potency corticosteroids might be needed. Additionally, continual flares from under-treatment has lead to an increased number of times that topical corticosteroids (even lower potency) have had to be used. Secondary signs (infection and other comorbidities associated with scratching and sleep disturbance) are also more common in undertreated AD (Fishbein et al., 2018; Fishbein et al., 2015; Furue, Chiba, & Takeuchi, 2011). It has also been suggested that early aggressive AD treatment might curb the development of food allergy (Leung & Guttman-Yassky, 2014). These factors highlight the importance of accurately triaging patients to moisturizer versus topical corticosteroid, and aggressively encouraging topical corticosteroid use when warranted. Furthermore, appropriate counseling on the relative efficacy and safety of topical corticosteroids in this age group is crucial.
Several randomized controlled trials (RCTs) have compared topical corticosteroid versus vehicle/moisturizer, but these have not been systematically reviewed with a focus on children <2 years old. The purpose of this study was to answer the primary question: What is the clinical response to topical corticosteroids vs. vehicle/moisturizer in children <2 years? Secondary questions included: 1) Is the adverse events profile different in the two treatment arms in this age group? And, 2) Do clinical characteristics moderate treatment effect (e.g. disease severity), demographic (e.g. age, race), or treatment (e.g. frequency, potency)?
Methods
The systematic review followed the guidelines and statement criteria established by Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (Liberati et al., 2009). We searched PubMed MEDLINE, Embase, Web of Science, Cochrane Database of Controlled Trials, Cochrane Database of Systematic Reviews, DARE, NHS Economic Evaluation, CINAHL, GREAT, and Clinicaltrials.gov databases from inception to 2/15/17. Database searches took place the week of 2/15/17. Search terms included “atopic dermatitis” and “eczema.” We used controlled vocabulary and RCT search filters when available. Titles and abstracts were searched with key words of either: atopic dermatitis OR eczema.
Search strategy developed by our study team (including reference librarian Ms. Patricia Smith) was published in PROSPERO and can be accessed at http://www.crd.york.ac.uk/prospero/display_record.asp?src=trip&ID=CRD42017056060. Ms. Patricia Smith conducted the database searches and de-duplicated articles. The abstracts were then reviewed by two authors independently for the following criteria. Briefly, inclusion criteria consisted of the following: RCT’s, sample with at least some children < 2 years, and AD defined using Hanifin and Rajka criteria or other established criteria (Vakharia, Chopra, & Silverberg, 2018). Eight articles defined AD using Hanifin and Rajka or modified Hanifin and Rajka criteria, three articles used clinician-based diagnosis, and one article used the UK Working Party’s diagnostic criteria. Abstracts and studies which provided no data on response of topical corticosteroids vs. vehicle/moisturizer were excluded. Although there was no exclusion by year of publication, many older articles did not have placebo comparators and were therefore not included.
The process of quality appraisal, data extraction and analysis was guided by National Institute for Health and Care Excellence (NICE, 2014). Two authors independently reviewed all eligible articles in order to select only those fulfilling the inclusion criteria. Any disagreement during this process was resolved by consultation with a third reviewer. Any article written in a language other than English was translated by an additional reviewer fluent in that language. With the final set of articles, two authors extracted the relevant data into a pre-formed extraction data document. If the article was unclear or necessary information for our primary outcome was not included, contact with the author was attempted. Biases within the studies were assessed with the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) system (Guyatt et al., 2008).
To answer the primary research question, co-primary outcomes for this study were treatment response to topical corticosteroid vs. vehicle/moisturizer. Three potential indicators were used to index treatment response: 1. Study-designated “good response”; 2. The number of participants with at least a 50% reduction in AD severity; 3. Eczema rated as cleared or controlled. These indicators were chosen based off National Institute for Health and Care Excellence published standards for atopic dermatitis. Outcomes were assessed at the final time point in the study. Most studies did not report all three of the potential indicators of treatment response. As such, we used the maximum response reported within a study, if multiple indices of response were described. Details on the class of topical corticosteroid, type of vehicle/moisturizer and duration of treatment were recorded.
To answer the research question regarding frequency and nature of adverse events, adverse events were coded when possible for number of patients with the adverse event and total number of subjects in which the adverse event was recorded. We also captured details regarding severity of events, likelihood it was related to topical corticosteroid, and nature of the adverse event. Coded adverse events included general (adrenal suppression, gastrointestinal, fever, headache, respiratory, generalized infection, psychiatric, other) or skin specific (striae, atrophy, telangiectasia, ulcer, acne, skin irritation, skin infection, skin itch, urticaria, folliculitis). Baseline clinical characteristics were also recorded in detail related to disease severity (change in disease severity, disease severity scale used) and demographics (age, race and sex). Secondary outcomes also considered included changes in pruritus, quality of life and sleep disturbance.
To aggregate results across studies, a binomial-normal model for the meta-analysis of proportions was used. This was fit using a random effects logistic regression model, with a study-specific random intercept (Stijnen, Hamza, & Ozdemir, 2010). Analyses were conducted using the metafor package in the R statistical environment (Viechtbauer, 2010). The initial model included only the main effect for the study, consistent with the primary research question. In the event that significant cross-study heterogeneity of treatment effect was observed, potential confounders and modifiers were added, including topical corticosteroid potency (above or below class IV); mean age (in months); and trial length (in days), consistent with the secondary research questions. Topical corticosteroid potency of class IV was chosen based off studied reviewed, also to differentiate efficacy expected with much higher potency topical corticosteroids. Analyses were conducted separately for treatment effect and for number of individuals reporting adverse events.
Results
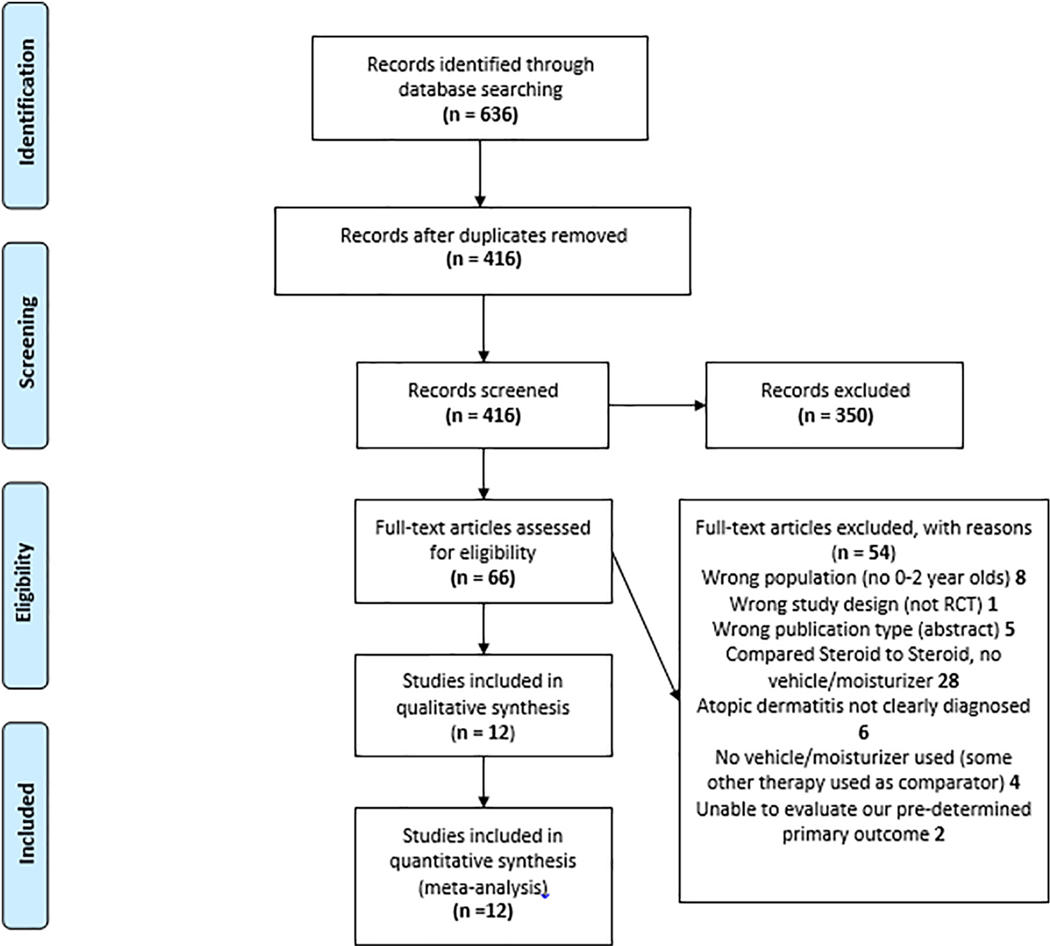
After the initial systematic search, 636 studies were identified (see Figure I for PRISMA flow diagram). After de-duplication, 416 unique articles were identified and screened. Of these, 12 studies met all inclusion criteria; all were in English. Table I provides details of the 12 RCT’s analyzed. The sample sizes (Intention to treat analysis (ITT)) ranged from 8 to 582 participants. The years of publication ranged from 1981 to 2013. Although patients under 2 years(y) were included in all 12 studies, only 1 study limited age of recruitment to ˂2y (Wu, Chen, Liu, Wu, & Dong, 2013). Table I includes participants of mixed ages, as studies did not separate results of children <2y. There was an overall mixed range of disease severity, as well as overall mixed race/ethnic/gender background. Most studies reported a duration of treatment ranging from 7–29 days. One study lasted up to 140 days (Hanifin, Gupta, & Rajagopalan, 2002). Five of the 12 studies involved daily dosing (one of those re-assigned subjects to more intermittent dosing as the study continued if subjects were controlled), whereas the seven others involved more frequent dosing. The potency of topical corticosteroids used ranged from class VII to class II. Only two studies appeared to be non-industry sponsored. Sponsorship occurred from manufacturers of moisturizer (n=2) and topical corticosteroid (n=8). A true moisturizer (as opposed to vehicle) was only clearly used in the 2 moisturizer manufacturer-sponsored studies (Sugarman & Parish, 2009; Udompataikul & Limpa-o-vart, 2012).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flow Diagram This diagram details the search strategy and methodology for choosing appropriate articles which were included in the final analyses.
Table I.
Studies included in the systematic review
| References | Study Design (SC or MC, RCT, blinded, other information) | Treatment: Name of steroid, potency and vehicle (class) | Placebo: Vehicle/moisturizer | Frequency of application | Duration of treatment (days) | Number ITT, n per protocol In final analysis (n intervention, n control group), | Age Range in months (mean), comments | AD severity, % male, % Race/Ethnicity | Potential Conflicts of Interest | Number of Responders to therapy Intervention, Control; Primary outcomes assessed (1,2 or 3) & primary outcome reported in this column)$ | Number of Participants >=1 AE Intervention, Control |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Udompatai kul and Limpa-o-vart 2012) | SC Thailand, RCT, single blind participant, comparative effectiveness, placebo & steroid on different arms at same time | Hydrocortisone 1% ointment (VII) | 5% Dexapantheno l ointment | BID | 28 | 30, 26 (26,26) | 2–180 (86) | Mild/moderate, 38%, 100% Asian | Bayer provided dexpanthenol ointment | 26, 26 (1,2,3 & 1,2,3) | 0, 0 |
| (Sudilovsk y, Muir et al. 1981) | MC USA, RCT, double blind, comparison study, placebo & steroid on different arms at same time | Halcinonide 0.10% cream (II) | Vehicle | QD | 21 | 58, 58 (58,58) | 9–1032 (not reported) | Not reported | Funded by Squibb Institute | 33, 10 (1 & 1) | Not reported |
| Wu (Wu, Chen et al. 2013) | SC China, RCT, double blind, parallel group comparison study | Mometasone furoate 0.1% cream (II) | Vehicle mixed with distilled water in 1% DMSO | BID | 10 | 40, 36 (19,17) | 1–12 (4) | All severities, 64%, 100% Asian. | Funded by Medical Emphasis grant from government of Jiangsu Province, People’s republic of China. | 19, 17 (1,2,3 & 1,2,3) | 0, 0 |
| (Wolkerstorfer, Visser et al. 2000) | SC Netherlands, RCT, unspecified blinding | Fluticasone propionate 0.05% cream diluted, open weave cotton wet wraps applied on top (V) | Vehicle | QD | 7–14 | 8, 8 (6,2) | 5–156 (not reported) | Moderate/severe , 48%, Not reported | Funding source not listed. | 5, 0 (2 & 2) | 2, 2 |
| (Sugarman and Parish 2009) | MC USA, RCT, triple blind | Fluticasone propionate 0.05%, cream (III) | Ceramide-dominant triple lipid barrier repair formulation (epiceram) | BID | 28 | 121, 112 (59,53) | 6–216 (91) | Moderate/severe , 40%, 26% White, 56% Black, 3% Asian, 9% Hispanic, 6% Other | Funded by Ceragenix corporation. | 27, 21 (3 & 3) | 0, 4 |
| (Abramovit s and Oquendo 2010) | MC USA, RCT, double blind | Hydrocortisone butyrate 0.1% lipocream (V) | Lipocream vehicle | BID | 21–29 | 264, 247 (131,133) | 3–204 (85) | Mild/moderate, Not reported | Sponsor initiated investigation | 82, 37 (1 & 1) | 29, 28 |
| (Matheson, Kempers et al. 2008) | MC USA, RCT, triple blind, parallel group | Hydrocortisone butyrate 0.1%, lotion (V) | Vehicle | BID | 28 | 284, 252 (139,145) | 3–216 (86), 33% <2 years | Mild/moderate, 50%, 65% White, 32% Black, 5% Asian, 1% Other* | Funded by Ferndale Laboratories, authors compensated for the study. | 68, 35 (1 & 1) | 48, 56 |
| (Hanifin, Gupta et al. 2002) | MC USA & Canada, RCT, double bind, we analyzed data only from maintenance phase of study when placebo compared | Fluticasone propionate 0.05%, cream (V) | Vehicle | QD (4 days/week for 4 weeks) then QD (2 days/week for 16 weeks) | 140 | 247, 231 (154,77) | 3–204 (85), 24% <2 years | Moderate/severe#, 44%, 64% White, 16% Black, 14% Asian, 6% Other | Funded by Glaxo Wellcome, Inc. | 112, 26 (1 & 1) | 71, 29 |
| (Stalder, Fleury et al. 1994) | SC France, RCT, double blind | Desonide 0.1%, cream (III) | Polyoxyethyle ne glycol mixed stearate with other additives | QD | 7 | 40, 39 (18,21) | 4–180 (40) | Not reported, 55%, Not reported | Funding source not specified, but one of the authors works at Pierre Fabre laboratories. | 12, 3 (3 & 3) | Not reported |
| (Rauschkol b, Bender et al. 1981) | MC USA, RCT, double blind, paired comparison, place & steroid on different arms at the same time | Halcinonide 0.025% cream (IV) | Placebo cream unspecified | TID | 14 | 86, 79 (79,79) | 7–180 (96) | Not reported, 38%, 70% White, 29% Black, 1% Asian | Funded by Squibb and Sons, Inc. | 64, 40 (1 & 1) | 4, 5 |
| (Hebert, Cook-Bolden et al. 2007) | RCT USA, MC, double blind, phase III study | Desonide 0.05%, gel (VI) | Hydrogel vehicle | BID | 28 | 582, 541 (425,157) | 3–216 (80), 30% <3years | Mild/Moderate, 45%, 53% White, 28% Black, 4% Asian, 12% Hispanic, 3% Other. | Funded by SkinMedica, Inc. | 166, 17 (1,3 & 1,3) | 85, 46 |
| (Eichenfield and Miller 2006) | RCT USA, MC, double blind, phase III study | Fluticasone propionate 0.05% lotion (V) | Lotion vehicle | QD | 28 | 438, not reported (221,217) | 3–192 (not reported), 6% ≤1 year | Moderate/Sever e, Not reported | Funded by GlaxoSmithKline. | 156, 64 (1,2 & 2) | 77, 82 |
Application site reactions= dermatitis, pruritus, irritation, burning
Numbers reported in article add up to great than 100%
moderate/severe prior to starting the stabilization phase, likely mild by the time randomized
Primary Outcomes Assessed: 1. Study-designated “good response”; 2. The number of participants with at least a 50% reduction in AD severity; 3. Eczema rated as cleared or controlled. Most studies did not report all three of these potential outcomes.
Adverse events were recorded in 10 of the studies, 2 did not provide details about adverse events. Secondary outcomes of itch, quality of life or sleep could not be analyzed in aggregate, as only 5 of the 12 studies reported on itch, 3 on quality of life and 1 on sleep.
Efficacy from Topical Corticosteroids
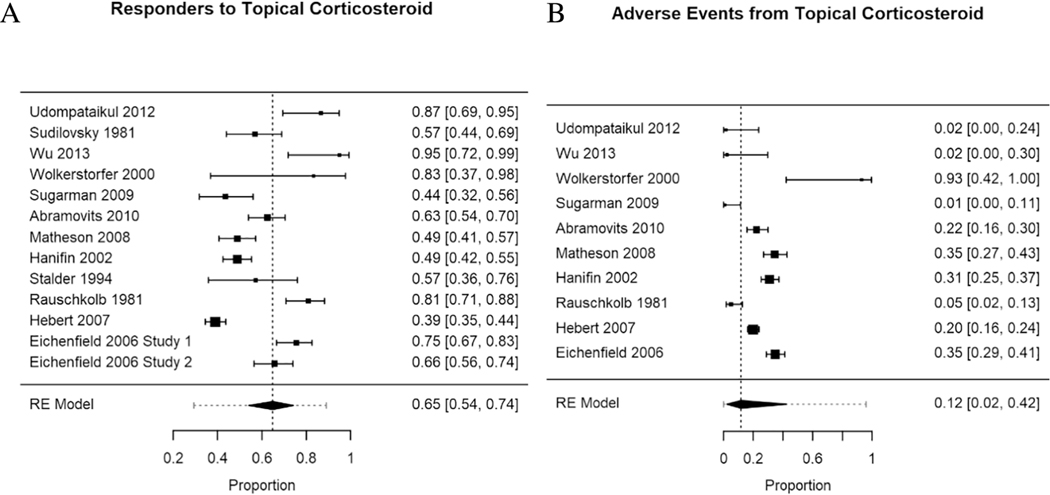
Topical corticosteroid response trended towards superiority over vehicle/moisturizer across the 12 studies, with the proportion of “responders” as 0.65 with a confidence interval from 0.54 to 0.74, as indicated in the forest plot (Figure 2a). Heterogeneity of response was not explained in subanalyses by potency of topical corticosteroid, average age of study participants, or length of the trial (QM(1) = 0.28 p = 0.60; residual heterogeneity Wald(11) = 104.58, p < 0.01; QM(1) = 0.51 p = 0.48; residual heterogeneity Wald(11) = 101.17, p < 0.01, and QM(1) < 0.01 p = 0.99; residual heterogeneity Wald(11) = 111.20, p < 0.01, respectively).
Figure 2.
Forest plot of proportions by study (solid square is the mean proportion), number on the right. 95% confidence interval (CI) represented by bars on graph, summarized in brackets on the right. Larger squares represent more participants in the study. RE (Random-Effects Model) summarizes data from all studies, with the dashed vertical line displaying the overall proportion of responders/events from the aggregate data, and the horizontal dashed line representing the 95% CI across studies.
Efficacy from Vehicle/Moisturizer
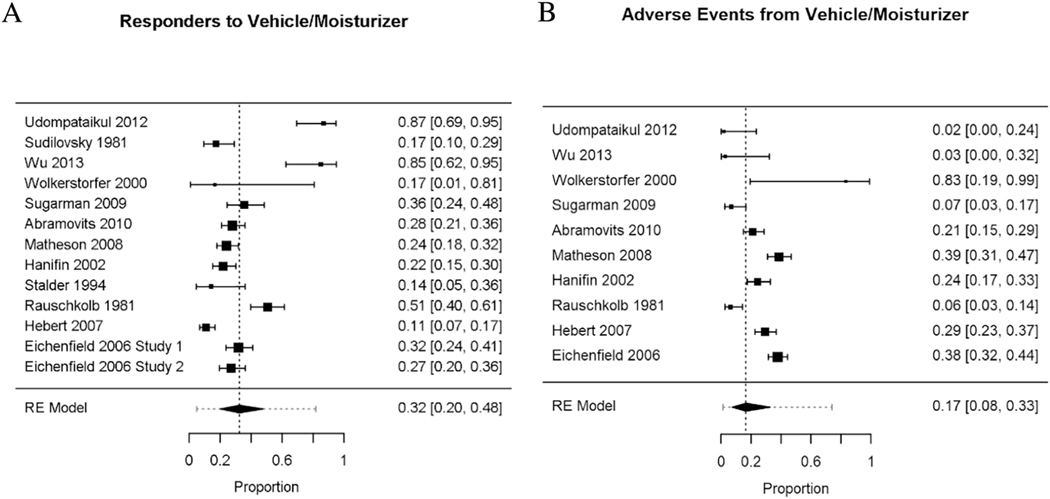
The overall proportion of responders to vehicle/moisturizer across the 12 studies was 0.32 with a 95% confidence interval (CI) from 0.20 to 0.48 (Figure 3a). As indicated in the forest plot, there was a large heterogeneity of response to vehicle/moisturizer. To determine heterogeneity, multiple factors were examined. Age and duration of treatment did not explain the heterogeneity (QM(1) = 0.01 p = 0.92; residual heterogeneity Wald(11) = 94.77, p < 0.01; and QM(1) = 0.34 p = 0.56; residual heterogeneity Wald(11) = 95.49, p < 0.01, respectively).
Figure 3.
Forest plot of proportions by study (solid square is the mean proportion), number on the right. 95% confidence interval (CI) represented by bars on graph, summarized in brackets on the right. Larger squares represent more participants in the study. RE (Random-Effects Model) summarizes data from all studies, with the dashed vertical line displaying the overall proportion of responders/events from the aggregate data, and the horizontal dashed line representing the 95% CI across studies.
Adverse Events from Topical Corticosteroids
The overall proportion of adverse events was 0.12, with a confidence interval from 0.02 to 0.42 (Figure 2b). Adverse event heterogeneity was not explained by age of participants or duration of therapy. However, steroid class significantly moderated the occurrence of adverse events (QM(1) = 7.2, p = 0.01). Though, even after accounting for this moderation, significant heterogeneity of adverse event reporting occurred (Wald(8) = 25.0, p = 0.002). Low-potency topical corticosteroids (class ≥V) actually had a slightly higher rate of adverse events (0.28) than the high-potency (classes II-IV) corticosteroid (0.01) (see statistical details in Supplemental table I). The frequency of side effects commonly associated with topical corticosteroids and vehicle/moisturizer in these studies is summarized in Table II.
Table II.
Frequency of side effects commonly associated with topical steroids or vehicle/moisturizer
| topical steroid n(%) | vehicle/moisturizer n(%) | |
|---|---|---|
| total subjects* | 1337 | 954 |
| skin infection | 1 (0.1) | 5 (0.5) |
| folliculitis | 8 (0.6) | 3 (0.3) |
| skin irritation | 26 (1.9) | 33 (3.5) |
| telangiectasia | 1 (0.1) | 2 (0.2) |
| skin atrophy | 1 (0.1) | 0 |
| striae | 1 (0.1) | 0 |
| acne | 2 (0.1) | 0 |
includes only those in which side effects were considered
Five studies assessed the possibility of adrenal suppression secondary to topical corticosteroids in a total of 157 patients (Hanifin et al., 2002; Sugarman & Parish, 2009; Udompataikul & Limpa-o-vart, 2012; Wolkerstorfer, Visser, De Waard van der Spek, Mulder, & Oranje, 2000; Wu et al., 2013). Four patients (3%) had evidence of mild adrenal suppression, but the age of these participants was not provided. In the study by Woldersorfer et al., participants received dilute (50% on body and 10% on face) fluticasone propionate 0.05% cream under wet wraps (class V), with two patients having a 9am serum cortisol < 0.2 umol/L (0.09 and 0.03) after treatment for 7 days (Wolkerstorfer et al., 2000). Those participants used 957 ug/m2 and 1125 ug/m2 of steroid cream respectively. In a recent study reported by Hanifin et al., two patients did not have an adequate response to cortisol stimulation testing at the end of the study (Hanifin et al., 2002). Participants received intermittent fluticasone propionate cream (steroid class V), exact quantity used not reported. One participant received 345 days of treatment and had a cortisol stimulation level after treatment of 17 ug/dL (normal was >=18 ug/dL). The other participant was treated for 280 days and had a cortisol stimulation level of 9 ug/dL. Follow up testing to demonstrate resolution of the adrenal suppression in both of these studies was not available for these participants.
Adverse Events from Vehicle/Moisturizer
The overall proportion of adverse events across the remaining 10 studies was relatively low. The overall proportion was 0.17, with a 95% CI ranging from 0.08 to 0.33 (Figure 3b). As indicated by the wide confidence interval, significant heterogeneity in adverse events is exhibited. Mean age or treatment duration did not explain the heterogeneity in adverse events to vehicle (see statistical details in Supplemental table I).
Discussion
Few RCTs have included children 0–2 years to assess the efficacy and safety of topical corticosteroids versus vehicle/moisturizer. Despite the common topical corticosteroid usage in this age group, our systematic review identified only 12 articles that had sufficient detail to determine treatment response. Only 1 of these studies had analyses which were limited to children <2 years, so our meta-analysis included subjects ≥ 2 years old. Our meta-analysis demonstrated topical corticosteroids resulted in 2 out of 3 subjects having a response. We were not able to determine characteristics of topical corticosteroid responders versus non responders based off the studies available. Only 2 studies compared topical corticosteroid to a true moisturizer, while the rest used vehicle. Interestingly, many vehicle studies showed a high proportion of responders. Our findings are consistent with the NHS-sponsored systematic review of RCTs comparing topical corticosteroids versus placebo to treat AD across age groups, which reported a large treatment effect of topical corticosteroids, “without evidence of harm” (Hoare, Li Wan Po, & Williams, 2000; Nankervis et al., 2016).
With regards to adverse events, we found that the vehicle/moisturizer group had a slightly higher, but not significant, rate of adverse events versus the topical corticosteroid group (0.17 versus 0.12). Lower potency topical corticosteroids also showed a slightly higher rate of adverse events as compared to higher potency corticosteroids. This could be partly explained by the bias of the studies included in our review. Eight of the studies were funded by topical corticosteroid companies, and in industry funded moisturizer studies, lower potency corticosteroids were used as the comparator. However, inadequate treatment of AD appears to result in significant skin infections and side effects more often than topical corticosteroids. This is despite the fact that side effects of topical corticosteroids are more commonly feared. Local skin irritation was the most common side effect from both moisturizer and topical corticosteroids. Similar to previously published studies, less than 0.2% of subjects developed cutaneous side effects linked to topical corticosteroids (skin atrophy, striae, acne, telangiectasia) (Green, Colquitt, Kirby, & Davidson, 2005). In a cross-sectional observational study by Hong et al, use of topical corticosteroids appropriate for disease severity and body location minimized side effects. All seventy children, with 93% regularly using potent topical corticosteroids for ~10 months, had no evidence of skin atrophy (Hong, Smith, & Fischer, 2011). It is important to remember that almost all cutaneous side effects from topical steroids (except striae) are reversible, so high potency topical corticosteroids are appropriate to use as long as patients are closely monitored.
Adrenal suppression is an important concern for providers and patients, in this systematic review we identified a rate of 3% for HPA suppression (adrenal suppression) from topical corticosteroid (although it is not known if the children were <2y). The long-term use of topical corticosteroid and HPA suppression was not addressed in any articles included in our review. In the studies which noted adrenal suppression, one used a wet wrap method of occluding topical corticosteroid on the skin (unknown funding source) and the other used class V steroid for prolonged use (steroid-company sponsored). Similarly, in a post marketing survey, the FDA did not find any cases of adrenal insufficiency in children using class VI-VII topical corticosteroids (Hengge et al., 2006). Another review found no HPA axis suppression in studies using Class VVII steroids, (Levin, Gupta, Butler, Chiang, & Koo, 2014) and that the mild early adrenal suppression from use of higher potency corticosteroids spontaneously normalized despite continued corticosteroid use. This is presumably because reduction in AD severity and barrier improvement reduced overall corticosteroid absorption after topical application. Although the reviewed articles had limited information regarding adrenal suppression, it appears that adrenal suppression is a very rare complication and close clinical monitoring of children on topical corticosteroids is adequate to monitor for this side effect. Wet wraps can enhance steroid absorption, and in a small number of patients temporarily cause adrenal suppression, but in larger reviews on this topic, this side effect has been found to generally be reversible (Andersen, Thyssen, & Maibach, 2015). More long-term studies on this topic are needed.
Limitations to this review are significant. Primary limitations include the small number of studies included, and varying classes of topical corticosteroid. There was large heterogeneity in vehicle/moisturizers used, with varying moisturizing properties (van Zuuren, Fedorowicz, & Arents, 2017). We were unable to limit our analyses only to children under 2y, and 50% of studies had less than 100 total participants. Study design was really inconsistent, 25% of studies used participants as their own control. Outcomes were not reported in a standardized manner, i.e. different severity assessments and itch assessments were used across studies. Some studies evaluated change in certain individual clinical scores (erythema, oozing, lichenification, etc.), whereas others reported on a global score changes, such as the SCoring of Atopic Dermatitis (SCORAD) assessment. Five studies reported on >=1 of our primary outcomes. Nine studies reported on the number of participants with a “good response.” Besides overall frequency of adverse events, we were not able to report on our secondary outcomes. Specifically, outcomes of itch, quality of life and sleep disturbance were assessed in only 5, 3 and 1 article respectively. Inconsistent formatting was noted in reporting secondary outcomes as some graphs had unclear numbers reported and data had to be extrapolated.
With regards to application to nursing practice, these findings can be interpreted to mean that more research is needed on this topic. However, nurses can reassure families that topical steroids are generally safe and effective, and side effects are rare and almost always reversible.
Conclusions
We suggest that AD trials going forward refer to Harmonizing Outcomes Measures for Eczema to standardize outcome measures and thereby improve interpretability of findings (Charman, Chambers, & Williams, 2003; Schmitt et al., 2014). Further studies in children under 2 with AD are desperately needed to adequately characterize topical corticosteroid responders versus those who only need moisturizer, and determine the risk of adverse events by treatment group.
Supplementary Material
Highlights.
Atopic Dermatitis is a common pediatric problem which is frequently cared for by nurses.
There are limited randomized control trials which assess the safety and efficacy of topical corticosteroids in children <2 years old.
Although further work is needed, topical corticosteroids trended to being more effective and equally safe to vehicle/moisturizers.
Acknowledgments
Funding Source: This work was supported in part by grant number K12HS023011 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
This study was approved by the Ann & Robert H. Lurie Children’s Hospital IRB.
Abbreviations:
- AD
atopic dermatitis
- RCTs
randomized controlled trials
- y
years
- CI
confidence interval
- ITT
intention to treat analysis
- RE
random effects model
- DARE
Database of Abstracts of Reviews of Effects
- NHS
National Health Service
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- GREAT
Global Research for EczemA Trials
- CONSORT
CONsolidated Standards Of Reporting Trials
Footnotes
Financial Disclosure: none
Conflict of Interest: none
Prospero Protocol registration: PROSPERO 2017:CRD42017056060
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RM, Thyssen JP, & Maibach HI (2015). The role of wet wrap therapy in skin disorders - a literature review. Acta Derm Venereol, 95(8), 933–939. doi: 10.2340/00015555-2134 10.2340/00015555-3134 [DOI] [PubMed] [Google Scholar]
- Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, & Williams HC (2010). Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol, 163(1), 12–26. doi: 10.1111/j.1365-2133.2010.09743.x [DOI] [PubMed] [Google Scholar]
- Charman C, Chambers C, & Williams H. (2003). Measuring atopic dermatitis severity in randomized controlled clinical trials: what exactly are we measuring? J Invest Dermatol, 120(6), 932–941. doi: 10.1046/j.1523-1747.2003.12251.x [DOI] [PubMed] [Google Scholar]
- Eichenfield LF, Ahluwalia J, Waldman A, Borok J, Udkoff J, & Boguniewicz M. (2017). Current guidelines for the evaluation and management of atopic dermatitis: A comparison of the Joint Task Force Practice Parameter and American Academy of Dermatology guidelines. J Allergy Clin Immunol, 139(4s), S49–s57. doi: 10.1016/j.jaci.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Fishbein AB, Mueller K, Kruse L, Boor P, Sheldon S, Zee P, & Paller AS (2018). Sleep disturbance in children with moderate/severe atopic dermatitis: A case-control study. J Am Acad Dermatol, 78(2), 336–341. doi: 10.1016/j.jaad.2017.08.043 [DOI] [PubMed] [Google Scholar]
- Fishbein AB, Vitaterna O, Haugh IM, Bavishi AA, Zee PC, Turek FW, . . . Paller AS (2015). Nocturnal eczema: Review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J Allergy Clin Immunol, 136(5), 1170–1177. doi: 10.1016/j.jaci.2015.08.028 [DOI] [PubMed] [Google Scholar]
- Furue M, Chiba T, & Takeuchi S. (2011). Current status of atopic dermatitis in Japan. Asia Pac Allergy, 1(2), 64–72. doi: 10.5415/apallergy.2011.1.2.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lopez G, Ceballos-Rodriguez RM, Gonzalez-Lopez JJ, Feito Rodriguez M, & Herranz-Pinto P. (2017). Efficacy and safety of wet wrap therapy for patients with atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol, 177(3), 688–695. doi: 10.1111/bjd.15165 [DOI] [PubMed] [Google Scholar]
- Green C, Colquitt JL, Kirby J, & Davidson P. (2005). Topical corticosteroids for atopic eczema: clinical and cost effectiveness of once-daily vs. more frequent use. Br J Dermatol, 152(1), 130–141. doi: 10.1111/j.1365-2133.2005.06410.x [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, & Schunemann HJ (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj, 336(7650), 924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanifin J, Gupta AK, & Rajagopalan R. (2002). Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol, 147(3), 528–537. [DOI] [PubMed] [Google Scholar]
- Hengge UR, Ruzicka T, Schwartz RA, & Cork MJ (2006). Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol, 54(1), 1–15; quiz 16–18. doi: 10.1016/j.jaad.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Hoare C, Li Wan Po A, & Williams H. (2000). Systematic review of treatments for atopic eczema. Health Technol Assess, 4(37), 1191. [PMC free article] [PubMed] [Google Scholar]
- Hong E, Smith S, & Fischer G. (2011). Evaluation of the atrophogenic potential of topical corticosteroids in pediatric dermatology patients. Pediatr Dermatol, 28(4), 393–396. doi: 10.1111/j.15251470.2011.01445.x [DOI] [PubMed] [Google Scholar]
- Kanchongkittiphon W, Gaffin JM, & Phipatanakul W. (2015). Child with Atopic Dermatitis. Ann Allergy Asthma Immunol, 114(1), 6–11. doi: 10.1016/j.anai.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima R, Fujiwara T, Matsuda A, Narita M, Matsubara O, Nonoyama S, . . . Matsumoto K. (2013). Factors associated with steroid phobia in caregivers of children with atopic dermatitis. Pediatr Dermatol, 30(1), 29–35. doi: 10.1111/j.1525-1470.2012.01808.x [DOI] [PubMed] [Google Scholar]
- Leung DY, & Guttman-Yassky E. (2014). Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol, 134(4), 769–779. doi: 10.1016/j.jaci.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E, Gupta R, Butler D, Chiang C, & Koo JY (2014). Topical steroid risk analysis: differentiating between physiologic and pathologic adrenal suppression. J Dermatolog Treat, 25(6), 501–506. doi: 10.3109/09546634.2013.844314 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, . . . Moher D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology, 62(10), e1–34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Morley KW, & Dinulos JG (2012). Update on topical glucocorticoid use in children. Current Opinion in Pediatrics, 24(1), 121–128. doi: 10.1097/MOP.0b013e32834ef53d [DOI] [PubMed] [Google Scholar]
- Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, & Williams HC (2016). Programme Grants for Applied Research In Scoping systematic review of treatments for eczema. Southampton (UK): NIHR Journals Library Copyright (c) Queen’s Printer and Controller of HMSO 2016; This work was produced by Nankervis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. [PubMed] [Google Scholar]
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, . . . Williams HC (2014). The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol, 134(4), 800–807. doi: 10.1016/j.jaci.2014.07.043 [DOI] [PubMed] [Google Scholar]
- Silverberg JI (2017). Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatologic Clinics, 35(3), 283–289. doi: 10.1016/j.det.2017.02.002 [DOI] [PubMed] [Google Scholar]
- Stijnen T, Hamza TH, & Ozdemir P. (2010). Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Statistics in Medicine, 29(29), 3046–3067. doi: 10.1002/sim.4040 [DOI] [PubMed] [Google Scholar]
- Sugarman JL, & Parish LC (2009). Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol, 8(12), 1106–1111. [PubMed] [Google Scholar]
- Udompataikul M, & Limpa-o-vart D. (2012). Comparative trial of 5% dexpanthenol in water-in-oil formulation with 1% hydrocortisone ointment in the treatment of childhood atopic dermatitis: a pilot study. J Drugs Dermatol, 11(3), 366–374. [PubMed] [Google Scholar]
- Vakharia PP, Chopra R, & Silverberg JI (2018). Systematic Review of Diagnostic Criteria Used in Atopic Dermatitis Randomized Controlled Trials. Am J Clin Dermatol, 19(1), 15–22. doi: 10.1007/s40257-017-0299-4 [DOI] [PubMed] [Google Scholar]
- van Zuuren EJ, Fedorowicz Z, & Arents BWM (2017). Emollients and moisturizers for eczema: abridged Cochrane systematic review including GRADE assessments. 177(5), 1256–1271. doi: 10.1111/bjd.15602 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. (2010). Conducting Meta-Analyses in R with the metafor Package. 2010, 36(3), 48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Wolkerstorfer A, Visser RL, De Waard van der Spek FB, Mulder PG, & Oranje AP (2000). Efficacy and safety of wet-wrap dressings in children with severe atopic dermatitis: influence of corticosteroid dilution. Br J Dermatol, 143(5), 999–1004. [DOI] [PubMed] [Google Scholar]
- Wu SH, Chen XQ, Liu B, Wu HJ, & Dong L. (2013). Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol, 168(1), 172–178. doi: 10.1111/j.1365-2133.2012.11177.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.