Abstract
Background:
PI3K pathway activation is common in endometrial cancer. We evaluated the safety and efficacy of the dual PI3K/mTOR inhibitor, LY3023414, in patients with advanced endometrial cancer harboring activating mutations in the PI3K pathway.
Methods:
We conducted a single-arm phase II study of monotherapy LY3023414. Eligible patients had advanced endometrial cancer of any grade, prior management with 1–4 cytotoxic lines, and PI3K pathway activation prospectively defined as a loss-of-function PTEN alteration or activating alteration in PIK3CA, AKT1, PIK3R1, PIK3R2, or MTOR. The primary objective was best overall response rate (ORR) per RECIST 1.1.
Results:
Twenty-eight patients were treated; histologies included endometroid (39%), carcinosarcoma (25%), serous (21%), and mixed (14%). Patients were heavily pretreated, with a median of 2 prior cytotoxic lines (range: 1–3). The most common alterations involved PIK3CA (68%), PTEN (43%), and PIK3R1 (32%). In the 25 efficacy-evaluable patients, the ORR was 16% (90% CI: 7%-100%), and the clinical benefit rate was 28% (90% CI: 16%-100%). Four patients had a confirmed partial response, and two responses lasted for >9 months. The median progression-free and overall survival were 2.5 months (95% CI: 1.2-3.0) and 9.2 months (95% CI: 5.0-15.9), respectively. The most common all-grade treatment-related adverse events were anemia (71%), hyperglycemia (71%), hypoalbuminemia (68%), and hypophosphatemia (61%). No correlation between molecular alterations and response was observed.
Conclusions:
In patients with heavily pretreated advanced endometrial cancer prospectively selected for tumors with PI3K pathway activating mutations, LY3023414 demonstrated modest single-agent activity and a manageable safety profile.
Keywords: endometrial cancer, dual PI3K/mTOR inhibitor, PI3K pathway, LY3023414, advanced
Precis:
We conducted a single-arm phase II study of LY3023414, a dual PI3K/mTOR inhibitor, in patients with advanced endometrial cancer who were prospectively selected to have activating PI3K pathway mutations. We found LY3023414 was tolerable and had a modest clinical response (overall response rate: 16% [90% CI: 7-100%]).
INTRODUCTION
The incidence and mortality of endometrial cancer are rising at a rate of 1.3% and 1.6% per year, respectively. This trend is expected to continue over the next decade, driven largely by the growing prevalence of obesity, a strong risk factor for this malignancy.1,2 Despite increasing burden of disease, approved therapies for treatment of advanced endometrial cancer are limited. With the exception of megestrol acetate for palliative treatment, pembrolizumab for treatment mismatch repair deficient (dMMR) previously treated endometrial cancer, and combination lenvatinib and pembrolizumab for advanced MMR-proficient endometrial cancer, there exists a tremendous unmet need,3,4 and novel therapeutic strategies that exploit underlying driver molecular alterations are needed.
Endometrial carcinomas are among the tumor types with the highest frequency of genomic activation of the PI3K/mTOR pathway.5–7 Specifically, alterations in PTEN, PIK3CA, and PIK3R1 occur in endometrial carcinomas at estimated frequencies of 65%, 50%, and 31%, respectively, with variation depending on various cohort factors.8 In addition to genetic alterations, PI3K pathway activation is also affected by hyperinsulinemia and increased insulin signaling.9 Specifically, increased binding of insulin receptors on endometrial cancer cells leads to further PI3K signaling pathway activation and promotion of tumorigenesis.10 Since the increase in endometrial cancer incidence is closely related to lifestyle factors, such as obesity and insulin resistance, strategies to lower insulin signaling and inhibit PI3K pathway activation are particularly attractive.
Clinical experience with various PI3K pathway inhibitors in endometrial cancer, including pan-class 1 inhibitors and dual PI3K/mTOR inhibitors, have resulted in overall response rates (ORR) ranging from 0–6% and medication discontinuation secondary to side effects.11–13 AKT and mTOR inhibitors have also led to modest results, and trials with these agents have had limited retrospective biomarker analysis and/or no prospective biomarker selection strategies.14,15 It is noteworthy that the specific pattern of PI3K pathway activation in endometrial cancer presents a unique drug development challenge. For example, the high rate of PTEN alterations, which preferentially signal through PI3Kβ, suggest that isoform-selective PI3K inhibitors, which have been successful in the context of PIK3CA-mutant breast cancer, are unlikely to be efficacious in this setting.16,17 In addition, intricate feedback loops can activate compensatory signaling pathways which may contribute to the lack of efficacy of agents that selectively target terminal components of the pathway, such as mTOR.18,19 There is evidence suggesting that dual PI3K and mTOR inhibition may more potently inhibit the PI3K signaling pathway and provide more efficient pathway blockade.20
LY3023414 is a novel selective inhibitor of class I PI3K isoforms, mTORC1/2, and DNA-PK (DNA-dependent protein kinase).21 In the first-in-human phase I study of this agent, one of the longest treated participants was an endometrial cancer patient with dual activating PI3K pathway alterations (PTEN and PIK3R1); this patient achieved a partial response lasting >18 months.22 Based on both this experience and preclinical data supporting the use of this agent class in this setting, we conducted a single-arm, open-label phase II study of LY3023414 monotherapy in patients with advanced endometrial cancer (ClinicalTrials.gov, NCT02549989). We utilized a preselection strategy that involved prospective inclusion of patients with tumors harboring genomic alterations predicted to activate the PI3K pathway and exclusion of concurrent RAS alterations that may confer resistance to PI3K pathway targeting agents.
PATIENTS AND METHODS
Study Design
This trial was a single-center, single-arm, open-label phase II study of LY3023414 monotherapy in patients with advanced endometrial cancer. All patients received LY3023414 at the recommended dose of 200 mg orally twice daily, administered without interruption on a 21-day cycle. Patients were treated until disease progression, development of intolerable toxicity, or withdrawal of consent. Efficacy assessments were performed at baseline, and then every two cycles (approximately every six weeks) until disease progression or start of subsequent therapy.
The study protocol was approved by the institutional review board (IRB) at Memorial Sloan Kettering Cancer Center (MSK) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent prior to study enrollment. The study was registered at ClinicalTrials.gov, NCT02549989. Patients consented to genomic analysis of their tissue samples through a separate MSK IRB-approved protocol (ClinicalTrials.gov, NCT01775072).
Patients
Eligible adult (>18 years) patients included those with advanced endometrial cancer who had received at least one but no more than four prior lines of treatment. Adjuvant chemotherapy, chemotherapy and radiation, consolidation/maintenance therapy, or chemotherapy administered in conjunction with primary radiation as a radiosensitizer were counted as systemic therapy. All histologic subtypes of endometrial cancer, including carcinosarcoma (MMMT), were eligible. Eligible patients were required to have genomic activation of the PI3K pathway as defined as a loss-of-function mutation in PTEN or a known activating mutation in PIK3CA, AKT1, PIK3R1, PIK3R2, or MTOR in the tumor. Qualifying PTEN alterations included whole or partial gene deletions, frameshift mutations, or nonsense mutations; missense mutations in PTEN did not qualify. Patients were excluded if their tumor had a known concurrent activating RAS/RAF mutation or a loss-of-function alteration in NF1/NF2 predicted to result in MAP kinase pathway activation.23 Patients with prior mTOR, AKT, or PI3K inhibitor treatment were excluded. Mutational profiling was performed by a CLIA-approved sequencing assay either as standard of care or as part of a dedicated genomic profiling study. Other inclusion criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.124, adequate organ function, fasting blood glucose ≤135 mg/dL (7.49 mmol/L), and HbA1c ≤7.0%. Patients were excluded if they had diabetes that required insulin or more than one non-insulin hypoglycemic agent.
Study Endpoints
The primary objective was to assess the activity of LY3023414 in patients with PI3K pathway-activated, advanced endometrial cancer as measured by best ORR. The secondary objectives included assessment of the clinical benefit rate (CBR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. The CBR was defined as the percentage of patients with complete response (CR), partial response (PR), or stable disease (SD) lasting ≥12 weeks from the start of treatment. DOR was defined as the time from which initial criteria were met for CR or PR (whichever status was recorded first) until the first date of documented disease progression. PFS was defined as the duration from start of treatment to time of recurrence, progression, or death due to any cause (whichever occurred first). OS was defined as the duration from start of treatment until the date of death due to any cause.
Tumor response was evaluated based on RECIST 1.1 guidelines, with responses requiring confirmation.24 Serious and non-serious adverse events (AEs) and treatment-related toxicities were graded in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 and collected from time of consent until 30 days after discontinuation of LY3023414. Dose delays up to 14 days were allowed for treatment-related toxicity assessment.
Genomic Analysis
For the purposes of patient eligibility, mutational profiling via either MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets), a targeted DNA sequencing panel for somatic mutations in cancer-associated genes25, or another CLIA-certified platform was acceptable. Testing was performed on pretreatment archival tumor tissue for exploratory analysis of genomic correlates of response. Additionally, serial pre-, on-, and post-treatment blood samples were collected for cell-free tumor DNA (cfDNA) analysis. Sequencing was performed using a custom, ultra-deep coverage next-generation sequencing panel (MSK-ACCESS [Analysis of Circulating cfDNA to Evaluate Somatic Status]) that includes exons of 129 cancer-related genes and uses duplex unique molecular identifiers (UMIs) and dual index barcodes to minimize background sequencing errors. Consensus reads with representation from both strands of the original cfDNA duplex were used for de novo variant calling using VarDict (v1.5.1). Mutation calling required at least one collapsed read at a known oncogenic cancer hotspot or at least three collapsed reads at non-hotspot sites. All samples were sequenced to an average depth of approximately 20,000X coverage. Nonsynonymous somatic mutations were identified and quantified as variant allele frequencies.
Statistical Design and Analysis
To meet the definition of evaluable for efficacy, patients must have had measurable disease present at baseline, received at least one cycle of LY3023414 therapy, and been re-evaluated for response. Patients who did not meet these criteria were excluded from efficacy assessment and were replaced until a total of 25 efficacy-evaluable patients were enrolled. All patients were evaluable for toxicity from the start of their first treatment. Best overall response was analyzed as a binary endpoint, defined as complete response (CR) or partial response (PR) versus stable disease (SD) and progression of disease (PD). Clinical benefit rate (CBR) was defined as complete response (CR) or partial response (PR) or stable disease (SD) ≥12 weeks from the start of treatment. A sample size of 25 patients was determined to provide 90% power to test the hypothesis that the response rate is promising (defined as 30% or higher) against a non-promising rate of 10% or lower. This calculation was based on historical data from studies with ineffective agents in a similar patient population, which had an ORR<10% and a six-month PFS rate <20%26–29, and used an exact one-sample test for binomial proportion, with Type I error of 10%. The point estimates for ORR and CBR along with one-sided 90% confidence intervals (CIs) were calculated using exact binomial proportions. Median PFS, median OS, and DOR along with two-sided 95% CIs were estimated using the Kaplan-Meier method. Patients were censored at their last follow-up date. All statistical analyses were performed using SAS version 9.0 or higher. Genomic alterations for patients are reported in a binary fashion, and Fisher’s exact test was used to assess the correlation between genomic alterations and response. Cell-free tumor DNA data were assessed graphically.
RESULTS
Patient Characteristics
Between September 24, 2015, and October 16, 2018, 28 patients were enrolled and treated. Clinical and pathological characteristics are summarized in Table 1. Twelve patients (43%) had received one line of prior therapy, 13 patients (46%) had received two lines of prior therapy, and three patients (11%) had received three lines of prior therapy. Eleven (39%) patients had endometrioid histology, seven (25%) had carcinosarcoma, six (21%) had serous, and four (14%) had mixed histology. Twenty-eight patients were included in the toxicity assessment. Three patients were excluded from primary efficacy assessment due to medication nonadherence at cycle two (n=1), withdrawal of consent prior to cycle 1 completion (n=1), and treatment intolerance prior to first tumor re-evaluation (n=1). Thus, 25 patients were included in the final efficacy-evaluable population. At the time of data cut-off in October 2018, one (4%) patient remained on treatment, 18 (72%) patients discontinued treatment because of progression of disease, and six (24%) patients discontinued treatment because of AEs.
Table 1.
Patient baseline characteristics
| Characteristic | All Patients (n=28) |
|---|---|
| Median age, years (range) | 65 (36–82) |
| <65 years (%) | 46% |
| >65 years (%) | 54% |
| ECOG Performance Score, n (%) | |
| 0 | 13 (46%) |
| 1 | 15 (54%) |
| Race, n (%) | |
| White | 26 (93%) |
| African American | 1 (3%) |
| Asian | 1 (3%) |
| Histology, n (%) | |
| Endometrioid Grade 1 | 3 (11%) |
| Endometrioid Grade 2 | 2 (7%) |
| Endometrioid Grade 3 | 6 (21%) |
| Serous | 6 (21%) |
| Carcinosarcoma | 7 (25%) |
| Mixed Histology | 4 (14%) |
| FIGO Stage at Initial Diagnosis, n (%) | |
| Stage I | 13 (46%) |
| Stage II | 1 (4%) |
| Stage III | 7 (25%) |
| Stage IV | 7 (25%) |
| No. Prior Chemotherapy Regimens, n (%) | |
| 1 | 12 (43%) |
| 2 | 13 (46%) |
| 3 | 3 (11%) |
FIGO: Federation of Gynecology and Obstetrics
Safety
Treatment-related AEs were reported in 96% (27/28) of patients (Table 2). The most common any-grade treatment-related AEs were anemia (71%), hyperglycemia (71%), hypoalbuminemia (68%), and hypophosphatemia (61%). Grade 3/4 treatment-related AEs were reported in 54% of patients, with decreased lymphocyte count (36%), hypophosphatemia (21%), anemia (18%), hypokalemia (18%), hyperglycemia (14%), and hyponatremia (14%) as the most common. Although not formally gradable per CTCAE, hyperinsulinemia was observed in 64% of patients.
Table 2.
Treatment-related adverse events
| Adverse Event | All Grades, N=28,n (%)* | Grade 3/4, N=28,n (%) |
|---|---|---|
| Anemia | 20 (71) | 5 (18) |
| Hyperglycemia | 20 (71) | 4 (14) |
| Hypoalbuminemia | 19 (68) | 1 (4) |
| Hypophosphatemia | 17 (61) | 6 (21) |
| Increased alanine aminotransferase | 16 (57) | 1 (4) |
| Decreased white blood cells | 15 (54) | 2 (7) |
| Hypomagnesemia | 14 (50) | 0 |
| Decreased platelets | 13 (46) | 3 (11) |
| Increased alkaline phosphatase | 12 (43) | 1 (4) |
| Increased aspartate aminotransferase | 11 (39) | 1 (4) |
| Decreased lymphocytes | 11 (39) | 10 (36) |
| Nausea | 11 (39) | 2 (7) |
| Hypocalcemia | 9 (32) | 2 (7) |
| Hypokalemia | 9 (32) | 5 (18) |
| Constipation | 8 (29) | 0 |
| Hyponatremia | 8 (29) | 4 (14) |
| Increased creatinine | 6 (21) | 0 |
| Fatigue | 6 (21) | 0 |
All-grade adverse events occurring in ≥20% of patients
Treatment-related AEs resulting in dose interruption/reduction occurred in 29% of patients, and permanent discontinuation in three patients (7%). The most common AEs leading to LY3023414 dose reduction were nausea (n=2), hypokalemia (n=2), liver function abnormalities (n=1), and drug reaction (n=1). Two deaths occurred while on study; one patient died from sepsis secondary to an Enterococcus faecalis infection, and a second patient died from a large retroperitoneal bleed that resulted in cardiac arrest. Both were considered unrelated to protocol treatment. There was an additional patient who had a prolonged admission and hospital course notable for fever and elevated creatine phosphokinase (CPK>3,000). No infectious source was identified, and this was considered to be possibly related to the study drug.
Response and Survival
The ORR was 4/25 (16%, 90% CI: 7-100%) (Table 3). Best overall response in the evaluable cohort included four patients with PR, ten patients with SD, and 11 patients with PD. The CBR was 7/25 (28%, 90% CI: 16-100%). The median PFS was 2.5 months (95% CI: 1.2-3.0 months). At the time of data cut-off, there were 16 deaths, and the median OS was 9.24 months (95% CI: 5.0-15.9 months).
Table 3.
Treatment efficacy and clinical response
| Efficacy Measure | Point Estimate | Confidence Interval (CI) |
|---|---|---|
| Best Overall Response (n) | ||
| Complete Response | 0 | N/A |
| Partial Response | 4 | N/A |
| Stable Disease | 10 | N/A |
| Progressive Disease | 11 | N/A |
| Not Evaluable | 3 | N/A |
| Overall Response Rate | 4/25 (16%) | 90% CI: 7-100% |
| Clinical Benefit Rate at 12 Weeks | 7/25 (28%) | 90% CI: 16-100% |
| Progression-Free Survival | 2.5 months | 95% CI: 1.2-3.0 months |
| Overall Survival | 9.2 months | 95% CI: 5.0-15.9 months |
| Duration of Response | 4.2 months | 95% CI: 1.4-9.6 months |
The median DOR was 4.2 months (95% CI: 1.4-9.6 months). However, two of the four patients who achieved a confirmed PR remained on therapy for >9 months. Two patients with SD also had durable responses; one patient remained on treatment for 11 months, and one remained on therapy for >21 months at the time of data cut off.
Genomic Analysis
Overall, the distribution of PI3K pathway alterations was consistent with prior sequencing studies in advanced endometrial cancer.8 Sixty-eight percent of patients harbored a PIK3CA mutation, 32% had a PIK3R1 mutation, and one patient (4%) had an AKT1 mutation only. To explore the molecular correlates of response to dual PI3K/mTOR inhibition in PI3K-altered endometrial cancer, next-generation sequencing results were interrogated for 24/28 patients (Figure 1). There was no pattern of response by histology identified in the four patients with confirmed PR [serous (n=2) and grade 1 endometrioid (n=1)]. Additionally, no consistent pattern of genomic alterations was observed in the four patients with confirmed PR. Among these patients, two harbored concurrent PTEN/PIK3R1 alterations, and two had PIK3CA mutations. One of the responding PIK3CA-mutant patients had a concurrent ERBB2 amplification, while one responder had a hotspot FGFR3 mutation and two responders had concurrent TP53 mutations. Only one patient with an AKT1 mutation was enrolled (AKT1 [E17K]), and this patient achieved PD as their best response. There was no clear association between tumor mutational burden (TMB) and treatment outcome. Of note, only three patients’ tumors were identified as microsatellite instability-high (MSI-H), including one responder and two non-responders. Consistent with protocol eligibility, no patients with concurrent KRAS, BRAF, NF1, or NF2 alterations were identified on central analysis.
Figure 1. Integrated clinical and genomic analysis.
The waterfall plot shows the greatest percentage change from baseline in the target lesion as assessed per RECIST 1.1 criteria in all patients. Tumor histology and grade at diagnosis is indicated in the legend. The oncoprint shows (top to bottom): tumor mutational burden (TMB) measured in mutations per megabase (mutations/Mb), microsatellite instability (MSI) status, and top somatic non-synonymous mutations observed across the cohort.
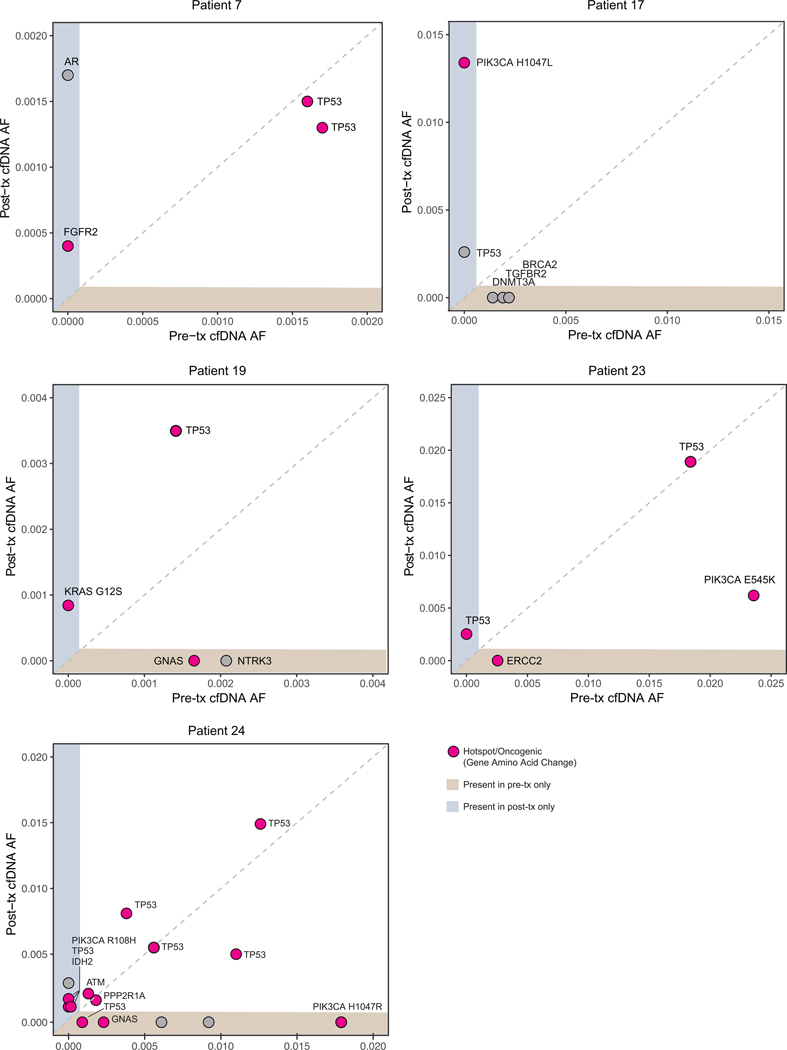
Among patients who achieved clinical benefit, and based on sample availability, paired cfDNA genomic analysis was performed on samples obtained at baseline (pretreatment) and upon disease progression or the most recently acquired time point (post-treatment) to evaluate potential mechanisms of acquired resistance. This analysis included three patients who achieved PR as well as two patients who had sustained SD, one of whom was still on treatment at the time of data cut-off. One patient with PR had only one cfDNA collection and was excluded from the analysis. The emergence of oncogenic mutations was observed in post-treatment time points, although no consistent post-treatment acquired resistance alterations were identified among the five patients (Figure 2 and Supplemental Table 1).
Figure 2. Genomic alterations in cell-free DNA (cfDNA) of responders.
Allele frequencies (AF) of non-synonymous somatic mutations detected in the cfDNA at pre-treatment (pre-tx) and post-treatment (post-tx) for five patients who had a clinical response.
DISCUSSION
In this signal-seeking study, LY3023414, a dual PI3K/mTOR inhibitor, was evaluated in advanced endometrial cancers harboring PI3K pathway alteration. LY3023414 also inhibits DNA-PK, a member of the PIKK superfamily that mediates AKT phosphorylation and activation and detection and repair of DNA double-strand breaks via the non-homologous end-joining pathway. Over-activation of DNA-PK in cancers can result in resistance to anticancer therapy.30 Inhibition of DNA-PK is a promising anticancer target30, and this additional activity rendered LY3023414 highly attractive to investigate in advanced endometrial cancer.
The RAS/RAF pathway, which is activated through mutations in KRAS, NRAS, and BRAF as well as through receptor tyrosine kinase activation, can promote increased proliferation and metastasis. Activation of this pathway has been implicated in resistance to PI3K-targeting agents, and activating mutations in KRAS (identified in 10–30% of endometrial cancers) have been shown to be predictive of resistance to PI3K inhibitors.31,32 Hence, patients with a concurrent activating RAS/RAF mutation or loss-of-function alteration in associated NF1/NF2 were excluded from this study.
Despite prospective selection of patients with genomic alterations predicted to activate the PI3K pathway and exclusion of patients with known concurrent MAPK pathway activating alterations, LY3023414 showed only modest monotherapy efficacy in patients with heavily pretreated endometrial cancer across histologic subtypes. Our data provide further evidence that baseline clinical characteristics and individual genomic biomarkers are still limited in their ability to reliably predict response to PI3K pathway inhibitors in endometrial cancer. These results also further illustrate the broader challenge of implementing clinical strategies to successfully target this pathway.
Despite the overall disappointing results, the CBR of 24% achieved in this study compares somewhat favorably to recent studies of the other PI3K pathway inhibitors in endometrial cancer. Specifically, a recent phase II study of BKM-120, a pan-class 1 PI3K inhibitor, in advanced endometrial cancer showed an ORR of 0% and excessive toxicity that necessitated a recruitment hold and dose reduction; 21% of patients discontinued BKM120 for toxicity.11 Similarly, studies of apitolisib (a dual PI3K/mTOR inhibitor) and pilaralisib (a pan-class 1 PI3K inhibitor) in advanced endometrial cancer both failed to meet their primary efficacy endpoints (6% ORR in both studies).12,13 While neither of these studies required PI3K pathway alteration for enrollment, it is noteworthy that all responders (n=3) in the apitolisib study and all responders (n=4) in the pilaralisib study harbored at least one PI3K pathway activating alteration. Similar to our findings, the responding patients in these studies harbored mixed cell type or serous histology. Separately, a phase II trial of the dual PI3K/mTOR inhibitor gedatolisib that stratified patients by stathmin (a hypothesized marker of PI3K activation) resulted in an ORR of 16% and a CBR of 53% in the stathmin-low arm (vs. CBR of 26% in the stathmin-high arm); however, nausea, mucositis, anorexia, diarrhea, and fatigue were common, requiring dose delays (45%) and dose reductions (60%).33
In mouse models, including patient-derived xenograft models of advanced endometrial cancer, treatment with multiple PI3K inhibitors has been shown to result in insulin feedback, ultimately leading to hyperglycemia and hyperinsulinemia.19 This systemic glucose-insulin feedback is sufficient to reactivate PI3K signaling, even in the presence of PI3K inhibitors.19 Consistent with this observation, we observed hyperinsulinemia in 64% of patients treated in our study. It is important to underscore that in these models, strong suppression of the insulin feedback loop had the potential to increase PI3K inhibitor efficacy.19 Thus, combining PI3K pathway inhibitors with interventions that lower circulating insulin may be a promising strategy to improve efficacy in patients. Indeed, data from mouse studies suggest both a ketogenic diet and sodium-glucose cotransporter 2 (SGLT2) inhibitors may be able to circumvent the glucose-insulin feedback induced by PI3K inhibitors.19
Such a combined approach is particularly relevant to endometrial cancer given the very high rate of PI3K pathway alterations and the strong link between insulin resistance and hyperglycemia and development of endometrial cancer.34,35 Even without PI3K pathway inhibition, endometrial cancer patients often have underlying dysregulation of insulin signaling. This dysregulation has been implicated in the development of endometrial cancer and in the development of metabolic syndrome, insulin resistance, and hyperglycemia.
In summary, despite a precision selection strategy in patients with advanced and heavily pretreated endometrial cancer, LY3023414 treatment resulted in only a modest clinical response. To enhance efficacy, future studies evaluating the combination PI3K pathway inhibitors with a ketogenic diet or SGLT2 inhibitors in PI3K pathway altered endometrial carcinomas and carcinosarcomas are warranted and planned.
Supplementary Material
Acknowledgments
Funding statement: This research was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. Drs. O’Cearbhaill and Sabbatini are supported by NIH P01 CA190174.
Footnotes
Other notes: Previously presented at AACR – Targeting PI3K/mTOR Signaling, December 2018, Poster Presentation
Conflict of interest statement: MMR, IC, Helen Won, KS, KS, JG, SDS, CZ, and JT have no conflicts of interest.
DMH reports personal fees from Chuigai Pharma, CytomX Therapeutics, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer Pharmaceuticals, Genentech/Roche, and Fount. He has ownership interest in Fount and receives research funding from Loxo Oncology, PUMA Biotechnology, AstraZeneca, and Bayer Pharmaceuticals.
AI reports personal fees from Mylan.
WPT reports personal fees from Up To Date and Millenium.
REO reports personal fees from Tesaro, Clovis, and GlaxoSmithKline.
RNG reports personal fees from Mateon, Clovis, and Regeneron. She has research funding from Cycle for Survival, OCFRA, and Conquer Cancer Foundation.
MLH reports personal fees from Janssen, Tesaro, Lily, and Up To Date. Her spouse is employed by Sanofi.
TT-S reports personal fees from Bio Ascend.
PS has received research funding from Bristol-Myers Squibb.
CA reports personal fees from Tesaro, Immunogen, Clovis, Mateon Therapeutics, and Cerulean Pharma. She has received research funding from Clovis, Genentech, AbbVie, and AstraZeneca.
VM reports personal fees from Eisai, Merck, Karyopharm and Boston Biomedical. Her research has received funding from AstraZeneca, Eisai, Merck, Lilly, Karyopharm, Takeda, and Genentech.
REFERENCES
- 1.SEER Cancer Stat Facts: Uterine Cancer. 2017; https://seer.cancer.gov/statfacts/html/corp.html.
- 2.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. The New England journal of medicine. 2003;348(17):1625–1638. [DOI] [PubMed] [Google Scholar]
- 3.Diaz LA, Marabelle A, Delord J-P, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. 2017;35(15_suppl):3071–3071. [Google Scholar]
- 4.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. The Lancet Oncology. 2019;20(5):711–718. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research N, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soumerai TE, Donoghue MTA, Bandlamudi C, et al. Clinical Utility of Prospective Molecular Characterization in Advanced Endometrial Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(23):5939–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers AP. New Strategies in Endometrial Cancer: Targeting the PI3K/mTOR Pathway—The Devil Is in the Details. 2013;19(19):5264–5274. [DOI] [PubMed] [Google Scholar]
- 9.Wang CF, Zhang G, Zhao LJ, et al. Overexpression of the insulin receptor isoform A promotes endometrial carcinoma cell growth. PloS one. 2013;8(8):e69001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Poloz Y, Stambolic V. Obesity and cancer, a case for insulin signaling. Cell Death &Amp; Disease. 2015;6:e2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heudel PE, Fabbro M, Roemer-Becuwe C, et al. Phase II study of the PI3K inhibitor BKM120 in patients with advanced or recurrent endometrial carcinoma: a stratified type I-type II study from the GINECO group. British journal of cancer. 2017;116(3):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makker V, Recio FO, Ma L, et al. A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study). Cancer. 2016;122(22):3519–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matulonis U, Vergote I, Backes F, et al. Phase II study of the PI3K inhibitor pilaralisib (SAR245408; XL147) in patients with advanced or recurrent endometrial carcinoma. Gynecologic oncology. 2015;136(2):246–253. [DOI] [PubMed] [Google Scholar]
- 14.Trédan O, Treilleux I, Wang Q, et al. Predicting everolimus treatment efficacy in patients with advanced endometrial carcinoma: a GINECO group study. 2013;8(4):243–251. [DOI] [PubMed] [Google Scholar]
- 15.Myers AP, Broaddus R, Makker V, et al. Phase II, two-stage, two-arm, PIK3CA mutation stratified trial of MK-2206 in recurrent endometrial cancer (EC). 2013;31(15_suppl):5524–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juric D, Castel P, Griffith M, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518(7538):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. The New England journal of medicine. 2019;380(20):1929–1940. [DOI] [PubMed] [Google Scholar]
- 18.Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nature Reviews Cancer. 2014;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560(7719):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Reilly KE, Rojo F, She Q-B, et al. mTOR Inhibition Induces Upstream Receptor Tyrosine Kinase Signaling and Activates Akt. 2006;66(3):1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MC, Mader MM, Cook JA, et al. Characterization of LY3023414, a Novel PI3K/mTOR Dual Inhibitor Eliciting Transient Target Modulation to Impede Tumor Growth. Molecular cancer therapeutics. 2016;15(10):2344–2356. [DOI] [PubMed] [Google Scholar]
- 22.Bendell JC, Varghese AM, Hyman DM, et al. A First-in-Human Phase 1 Study of LY3023414, an Oral PI3K/mTOR Dual Inhibitor, in Patients with Advanced Cancer. Clinical Cancer Research. 2018. [DOI] [PubMed] [Google Scholar]
- 23.Chappell WH, Steelman LS, Long JM, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2(3):135–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England : 1990). 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 25.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fracasso PM, Blessing JA, Molpus KL, Adler LM, Sorosky JI, Rose PG. Phase II study of oxaliplatin as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103(2):523–526. [DOI] [PubMed] [Google Scholar]
- 27.Schilder RJ, Blessing JA, Pearl ML, Rose PG. Evaluation of irofulven (MGI-114) in the treatment of recurrent or persistent endometrial carcinoma: A phase II study of the Gynecologic Oncology Group. Invest New Drugs. 2004;22(3):343–349. [DOI] [PubMed] [Google Scholar]
- 28.Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol. 2008;111(1):22–26. [DOI] [PubMed] [Google Scholar]
- 29.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pospisilova M, Seifrtova M, Rezacova M. Small molecule inhibitors of DNA-PK for tumor sensitization to anticancer therapy. J Physiol Pharmacol. 2017;68(3):337–344. [PubMed] [Google Scholar]
- 31.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65(23):10669–10673. [DOI] [PubMed] [Google Scholar]
- 32.Hayes MP, Wang H, Espinal-Witter R, et al. PIK3CA and PTEN mutations in uterine endometrioid carcinoma and complex atypical hyperplasia. Clin Cancer Res. 2006;12(20 Pt 1):5932–5935. [DOI] [PubMed] [Google Scholar]
- 33.Del Campo JM, Birrer M, Davis C, et al. A randomized phase II non-comparative study of PF-04691502 and gedatolisib (PF-05212384) in patients with recurrent endometrial cancer. Gynecologic oncology. 2016;142(1):62–69. [DOI] [PubMed] [Google Scholar]
- 34.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: A significant risk factor of endometrial cancer. Gynecologic oncology. 2012;125(3):751–757. [DOI] [PubMed] [Google Scholar]
- 35.Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer causes & control : CCC. 2011;22(8):1163–1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.