Abstract
Mesenchymal stem cells (MSCs) are multipotent stem cells that have attracted increasing interest in the field of regenerative medicine. Previously, the differentiation ability of MSCs was believed to be primarily responsible for tissue repair. Recent studies have shown that paracrine mechanisms play an important role in this process. MSCs can secrete soluble molecules and extracellular vesicles (EVs), which mediate paracrine communication. EVs contain large amounts of proteins and nucleic acids, such as mRNAs and microRNAs (miRNAs), and can transfer the cargo between cells. The cargoes are similar to those in MSCs and are not susceptible to degradation due to the protection of the EV bimolecular membrane structure. MSC-EVs can mimic the biological characteristics of MSCs, such as differentiation, maturation, and self-renewal. Due to their broad biological functions and their ability to transfer molecules between cells, EVs have been intensively studied by an increasing number of researchers with a focus on therapeutic applications, especially those of EVs secreted by MSCs. In this review, we discuss MSC-derived EVs and their therapeutic potential in tissue regeneration.
Keywords: extracellular vesicles, exosomes, mesenchymal stem cells, tissue regeneration, tissue repair
Highlights
Paracrine secretion of mesenchymal stem cells plays an important role in tissue repair by sensing changes in the microenvironment and secretory factors and extracellular vesicles promoting tissue regeneration.
Extracellular vesicles contain abundant contents such as proteins and nucleic acids that can be transferred between cells as a new means of intercellular communication to regulate the homeostasis of recipient cells.
Extracellular vesicles have strong potential as a cell-free treatment for tissue regeneration because they can retain the therapeutic effects of their parent cells and do not have the safety concerns associated with cell therapy.
Introduction
Extracellular vesicles (EVs) are small vesicular nanoparticles secreted by cells. EVs have been proven to play a key role in cell-to-cell communication by transferring their cargoes, such as proteins, nucleic acids, and lipids1. Classically, EVs are divided into exosomes and microvesicles (MVs) according to their biogenesis, surface markers, and size. These particles have been found in most bodily fluids2, and numerous studies have shown that they play important roles in both normal physiology and disease, such as respiratory diseases3, cardiovascular diseases4, inflammatory diseases5, and cancers6–8. Due to the broad biological functions of EVs, such as maintaining physiological homeostasis9 and participating in the development of disease, and their ability to transfer molecules between cells, an increasing number of researchers have focused on the therapeutic applications of EVs. Specifically, EVs may have a therapeutic role in irreversible tissue injuries resulting from refractory disorders. For example, in a spinal cord injury model, EVs secreted by pericytes promoted angiogenesis and nerve regeneration10. Similarly, in graft-versus-host disease, EVs significantly ameliorated fibrosis of the skin, lungs, and liver through anti-inflammatory and immunomodulatory effects and prolonged survival11,12.
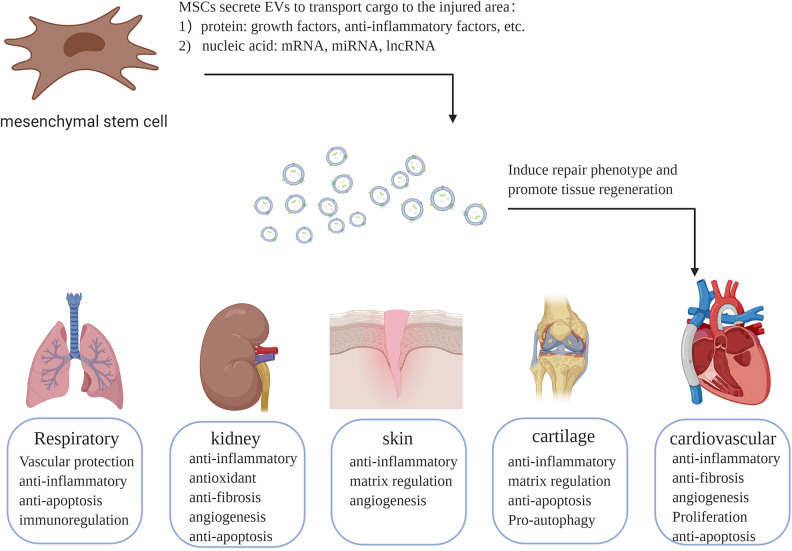
In recent decades, cell therapy has attracted increased attention. Mesenchymal stem cells (MSCs) have been broadly studied, especially in the field of regenerative medicine13. MSCs are multipotent stem cells commonly originating from bone marrow (BM), adipose tissue (AD), umbilical cord (UC) veins, and even solid organs, including the brain, lung, liver, spleen, kidney, thymus, and pancreas. MSCs show multiple differentiation potential with the ability to differentiate into both mesenchymal lineages and nonmesenchymal lineages, such as osteoblasts, chondrocytes, adipocytes, hepatocytes, and neuronal cells. In addition to their differentiation potential, MSCs can secrete vesicles and molecules such as growth factors, cytokines, and chemokines. Initially, the transdifferentiation or cell fusion potential of MSCs is believed to play a major role in the process of tissue repair. However, some studies have shown a low grafting rate and low survival rate of MSCs in injured areas, indicating that these two mechanisms of MSCs are inefficient in tissue repair14. Recently, the paracrine effects of MSCs were proposed to be primarily responsible for their regenerative potential. Many factors and EVs are found in the cell supernatant of MSCs, and in some animal models of disease, application of cell supernatant from MSCs resulted in a similar therapeutic effect to that of MSCs alone. In the following downstream experiments, it was confirmed that the components in the cell supernatant participated in tissue repair15,16. Increasing interest has focused on EVs and their cargo; the contents are rich in types and quantities and mainly include proteins and nucleic acids, which can avoid degradation under the protection of the bilayers, enabling long-distance transportation. MSC-EVs were first studied in a mouse model of myocardial ischemia-reperfusion injury (IRI) in 201017. Some studies have demonstrated that MSC-EVs can mimic the biological characteristics of MSCs, such as differentiation, maturation, and self-renewal18. Notably, an increasing number of reports have suggested that MSC-EVs have therapeutic effects in various conditions, including respiratory, kidney, cartilage, skin, and cardiovascular disorders (Fig. 1). MSC-EVs also transmit information between MSCs, and the cargoes of EVs participate in stem cell biology18. EVs from MSCs have anti-inflammatory, antiapoptotic, proangiogenic, and immunomodulatory effects similar to those of MSCs in various disease models. Currently, developing EV-based therapies is challenging due to the lack of standardized approaches for EV isolation and the lack of knowledge of the pharmacological properties and mechanisms of EVs. In this review, we elucidate these mechanisms with contemporary evidence, which could help develop novel therapeutic tactics to treat refractory tissue injury.
Fig. 1.
Therapeutic mechanisms of mesenchymal stem cell-derived extracellular vesicles in different diseases. Extracellular vesicles secreted by mesenchymal stem cells carry proteins and nucleic acids with repair functions to the injured area, induce repair phenotypes, and promote tissue regeneration. EVs: extracellular vesicles; lncRNA: long non-coding RNA; miRNA: microRNA; MSCs: mesenchymal stem cells.
Extracellular Vesicles
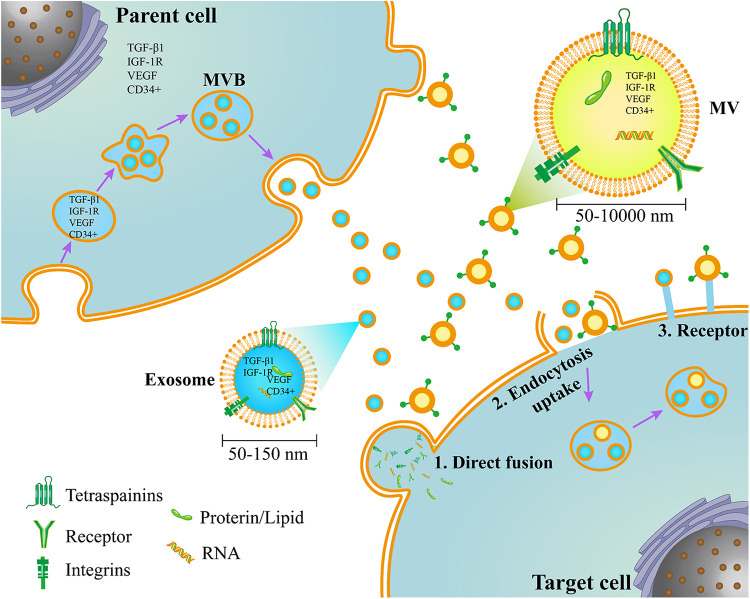
EVs were first observed in 1946 in normal plasma as a platelet-derived particle with a procoagulant function19 and were referred to as “platelet dust” in 196720. Since then, other cell-derived EVs have been discovered21–26, but the study of EVs was neglected due to insufficient research techniques until the 21st century. With the discovery that EVs contain proteins, mRNAs, and microRNAs (miRNAs)27,28, EVs are now regarded as a medium for cell–cell communication29 and have regained the interest of researchers. In general, based on current knowledge of their biogenesis, EVs can be divided into two main types: exosomes and MVs (Fig. 2)1,30.
Fig. 2.
The origins of extracellular vesicles and the interactions of target cells. Exosomes and MVs have two different pathways of origin. Exosomes are formed by the endosomal system, while MVs are formed by direct membrane budding. Both of them contain proteins, lipids, and nucleic acids and interact with target cells through three pathways. MVB: multivesicular bodies; MVs: microvesicles.
Exosomes are generated within the endosomal system as intraluminal vesicles (ILVs) and secreted during the fusion of multivesicular bodies (MVBs) with the cell membrane. Exosomes have diameters of 50 to 150 nm and are enriched in certain proteins, including tetraspanins (CD9, CD63, and CD81), heat shock proteins (HSP60, 70, and 90), major histocompatibility complex (MHC) class I and II, programmed cell death 6 interacting protein (Alix), and tumor susceptibility gene 101 (Tsg101)31,32. Exosome biogenesis involves two mechanisms: endosomal sorting complex required for transport (ESCRT)dependent and ESCRTindependent processes. ESCRT is involved in membrane shaping and scission and participates in the formation of MVBs and ILVs33. This complex also affects the cargo sorting of exosomes34. The ceramide and tetraspanin families, especially CD63, are involved in this process in an ESCRTindependent manner35,36. MVs, or microparticles, are formed by the outward budding and fission of the plasma membrane and the subsequent release of vesicles into the extracellular space. These particles range in size between 50 nm and 1 μm, and oncosomes can even reach 10 μm37,38. This biogenesis requires some molecular rearrangements within the plasma membrane, such as changes in lipid components, protein composition, and Ca2+ levels37. Although the biological origins of these two types of vesicles are known, the current methods to completely separate molecules with similar sizes are insufficient39.
Currently, most studies have focused on the functions and components of EVs. The components of EVs depend mainly on their cell origin and the stimuli they receive under different physiological or pathological conditions40,41. To date, some databases on EV composition, such as EVpedia, Exocarta, and Vesiclepedia42–44, have been established. EVs achieve their function primarily by transporting signaling molecules among cells. After being released into the extracellular space, EVs can deliver their cargo to recipient cells via three pathways (Fig. 2). They may enter cells via endocytic uptake or by direct fusion of the vesicles to the cell membrane. They may also transmit their contents through adhesion to the cell surface mediated by lipid ligand–receptor interactions. These interactions indicate the pivotal roles of EVs in cell-to-cell communication, which can affect physiological or pathological conditions45.
MSCs and MSC-EVs
MSCs are multipotent stem cells that reside mainly in mesodermal tissue and were first isolated from BM in the 1960s by Friedenstein et al.46. In addition to BM, MSCs can be found in other tissues or organs, such as the AD, UC, brain, lung, liver, spleen, kidney, thymus, and pancreas. In recent years, an increasing number of studies have been conducted on induced pluripotent stem cell-derived MSCs (iMSCs)47. To define MSCs for laboratory-based scientific investigations and preclinical studies, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has proposed a set of minimal criteria, including adherence to plastic in standard culture conditions; positive expression of most of the MSCs (≥95%) of surface molecules such as CD105, CD73, and CD90 and no expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA class II; and differentiation into osteoblasts, adipocytes, and chondroblasts under standard in vitro differentiating conditions48. MSCs have numerous unique features, including differentiation potential, colony formation, and self-renewal abilities. Homing and migration are also distinctive functions of MSCs. Some studies have reported the homing potential of MSCs to damage sites to exert their therapeutic effects49. Thus, MSCs may be a promising approach for tissue regeneration50.
However, there are some controversies about the tissue repair capability of MSCs. Injecting BM-MSCs directly into infarcted hearts failed to form new cardiomyocytes under in vivo conditions in mouse models of heart disease51. MSCs were found to be short-lived after systemic administration52. At the same time, some studies have suggested that their paracrine mechanisms play a major role in tissue repair16,53. In 2006, Gnecchi et al.54 found that injecting BM-MSC-conditioned medium (CM) into the ischemic heart significantly limited infarct size and improved ventricular function in rat models of myocardial infarction (MI). MSCs exert paracrine effects by secreting various growth factors and cytokines that induce angiogenesis, anti-inflammatory effects, immune modulation, and protection from apoptosis16. In addition to cytokines, MSCs can secrete EVs and MSC-EVs that promote tissue repair and regeneration. MSC-EVs express MSC surface molecules, such as CD44, CD73, and CD90, and do not express hematopoietic markers, such as CD34 and CD4555. MSC-EVs, as a new mediator of intercellular communication, contain various proteins, nucleic acids, and lipids. Mass spectrometry revealed 857 proteins in MSC-EVs56. A total of 2,089 miRNAs were found in MSCs and their EVs by miRNA microarray analysis, and most miRNAs (98.3%) showed the same expression in both MSCs and EVs57. MSC-EVs can transfer these cargoes to the recipient cells and thereby alter their activities.
MSCs have been proven to be effective in treating several human diseases, but safety concerns, such as pulmonary embolism, uncontrolled differentiation, and tumor formation, still exist58. As a new cell-free treatment, EVs have many unique advantages. Due to their bilayer membrane structure, EVs are stable in vivo and can be stored for a long time at –80 °C without losing their bioactivity59. In the following sections, we will review current research progress on the potential therapeutic value of MSC-EVs in regenerative medicine.
MSC-EVs in Respiratory Diseases
Several studies have reported that MSC-EVs have protective effects in respiratory injury models. By studying the mechanism of MSCs in the treatment of hypoxic-induced pulmonary hypertension (HP), Lee et al.60 found that MSCs exert therapeutic effects through paracrine mechanisms and that EVs act as the major paracrine anti-inflammatory and therapeutic mediators in the lung through inhibition of hypoxic signal transducer and activator of transcription-3 signaling. Tail vein injection of MSC-EVs could help prevent and reverse monocrotaline-induced right ventricle hypertrophy and pulmonary vascular remodeling by transferring specific miRNAs, such as miR-34a, miR-122, miR-124, and miR-127, which regulate anti-inflammatory/antiproliferative pathways61.
In several models of lung injury induced by ischemia or trauma, EVs have shown good repair effects. In a mouse model of hemorrhagic shock and laparotomy-induced lung injury, Potter et al.62 showed that injection of MSC-EVs protected mice from lung injury through vasculoprotective effects in a similar manner to MSCs in vivo. Phosphorylated protein expression profiling indicated that MSCs and EVs mainly reduced pulmonary vascular permeability by inhibiting the activation of the Rho GTPase pathway. Interestingly, in vitro MSCs but not EVs reduced endothelial cell permeability. This result suggested that there are differences between the molecular effects of MSCs and MSC-EVs, although their therapeutic effects are similar. The other two studies investigated the functions of the MSC-EV contents. One study showed that EV trafficking of miR-124-3p directly targeted purinergic receptor P2X ligand-gated ion channel 7 to exert anti-inflammatory effects to protect the lungs in a trauma-induced acute lung injury (ALI) model63. In another study, Li et al.64 found that in a murine lung ischemia/reperfusion model, intratracheal administration of MSC-Exo significantly reduced lung edema and dysfunction, M1 polarization of alveolar macrophages, and secretion of HMGB1, IL-8, IL-1β, IL-6, IL-17, and tumor necrosis factor (TNF)-α. The researchers further demonstrated that MSC-EVs ameliorated IRI by inhibiting both apoptosis pathways via transport of miR-21-5p, which targets phosphatase and tensin homolog (PTEN) and programmed cell death 4 (PDCD4).
In an Aspergillus hyphal extract-induced mouse model of allergic airway inflammation, the injection of EVs from human and murine MSCs through the tail vein was as effective as the cells themselves and revealed that EVs can exert immunomodulatory and anti-inflammatory effects, alleviating Th2/Th17-mediated airway hyperresponsiveness and lung inflammation65. In addition to airway inflammation, EVs can also repair lung damage caused by viral infections. By studying the therapeutic effect of EVs in influenza virus infection models, Khatri et al.66 found that EVs can inhibit influenza virus replication and virus-induced apoptosis of lung epithelial cells in vitro. In a virus-induced acute ALI pig model, MSC-EVs were found to reduce inflammatory factors (TNF-α, CXCL10, IL-10) in the lung by intratracheal administration.
Some studies have achieved encouraging results regarding the application of MSC-EVs in bronchopulmonary dysplasia (BPD). BPD, a serious long-term complication of prematurity, is a common chronic lung disease in premature infants that requires mechanical ventilation and oxygen therapy. Several serious complications are associated with BPD, such as pulmonary dysfunction, secondary HP, and brain injury. Willis et al.67 first explored the therapeutic role of MSC-EVs in BPD. The researchers found that UC-MSC-derived EVs can significantly improve pulmonary morphology, ameliorate pulmonary fibrosis and perivascular/vascular remodeling, and promote lung development by single-dose intravenous administration in a hyperoxia-induced BPD mouse model. Further RNA sequencing and gene ontology analysis indicated that EVs can attenuate hyperoxia-induced inflammatory responses, and in vitro and in vivo experiments demonstrated that EVs inhibit inflammation by modulating the macrophage phenotype67. In another study of BPD, a similar outcome was obtained by multiple injections of UC-MSC-derived EVs into the abdominal cavity, and the researchers also found that EVs can protect the heart and brain tissues from BPD. Mechanistic studies showed that TNF-α-stimulated gene-6 (TSG-6) in EVs exerted anti-inflammatory effects. Furthermore, the researchers demonstrated that EV-depleted MSC-CM, when injected into the BPD mouse model, did not have any protective effects, establishing that the protective factors are present in the EV fraction68.
MSC-EVs in Kidney Diseases
In recent years, EVs have also received increased attention in studies of kidney diseases, such as reports of emerging biomarkers in kidney cancers69, and as a new therapeutic approach in kidney injury. Most studies have focused on acute kidney injury (AKI), metabolic kidney disease, or chronic kidney disease (CKD). In a rat model of AKI induced by IRI, Kilpinen et al.70 found that the UC-MSC-derived EVs ameliorated kidney dysfunction and acute tubular necrosis. In vitro data showed that EVs can inhibit the proliferation of T cells, and in different inflammatory environments, protein components such as apolipoproteins (APOA1, APOA2, APOA4, APOC3), lipid-binding proteins (RBP4, SCP2, FABP6), and several complement-related proteins (C3, C4A, C5, CD93) in EVs secreted by MSCs might vary. In vivo, UC-MSC-derived EVs could significantly reduce serum markers of renal function, such as blood urea nitrogen, creatinine (Cr), and transaminase. Pathological findings suggested that the treatment improved necrosis, as shown by the analysis of the tubuloepithelial cells, tubular dilatation, and cast formation70. Some studies have further examined the mechanism by which EVs participate in repairing AKI. Analysis of the expression of inflammation-related proteins in EVs showed that C-C motif chemokine receptor-2 (CCR2) was highly expressed on the surface of the vesicles. Subsequent in vivo and in vitro experiments confirmed that CCR2-positive EVs can inhibit the migration and activation of macrophages by reducing the level of its ligand (CCL2), thereby promoting the repair of acute renal injury induced by ischemia-reperfusion71.
In addition to their anti-inflammatory and immune regulatory effects, EVs showed antioxidant effects in repairing kidney damage72,73. Zhang et al.72 found that UC-MSC-derived EVs can improve renal function and alleviate histological damage through a single intravenous injection in a rat model of IRI. The elevation of malondialdehyde (MDA) and 8-OHdg induced by IRI was significantly reduced after the application of EVs, indicating that oxidative stress was alleviated. In vitro, EV treatment decreased reactive oxygen species (ROS) levels in the renal tubular duct epithelial cells of a hypoxic injury model by enhancing Nuclear factor E2-related factor 2/antioxidant response element activation72. In another study, in vitro models of renal injury were induced by kidney stones; after renal tubular epithelial cells were exposed to calcium oxalate monohydrate (COM) crystals and oxalate, the cell number and cell viability decreased, and the levels of lactate dehydrogenase, H2O2, MDA, and ROS were significantly elevated. After treatment with UC-MSC-EVs, all the effects induced by oxalate + COM were reversed73. The above results indicated that the antioxidative effect of EVs plays an important role in renal protection. Another study examined the effectiveness of EVs secreted by glomerular-derived MSCs (Gl-MSCs) for the treatment of AKI. Gl-MSCs were extracted from the renal cortex, and EVs were obtained from the cell supernatant by ultracentrifugation. Renal function, morphology, and tubular proliferation improved after the injection of Gl-MSC-derived EVs. Furthermore, RNase-treated EVs were ineffective in improving both kidney function and histological IRI recovery. Subsequently, the biodistribution of EVs was assessed by injecting PKH26-labeled EVs into IRI mice. EVs were detected in the kidneys 1 h after the injection, and 24 h after administration, only a few EVs could be observed in the tubules of IRI mice. Taken together, these results demonstrated that RNAs within the vesicles are primarily responsible for the repair of AKI and that the vesicles can quickly reach the damaged area by systemic administration74.
Eirin et al.75,76 examined the efficacy and mechanism of autologous AD-MSC-derived EVs in repairing renal injury in a novel porcine model of metabolic syndrome (MetS) with unilateral renal aortic stenosis (RAS). The researchers found that a single intrarenal administration could ameliorate renal hemodynamics and function, which were assessed with multidetector computed tomography. Medullary hypoxia and fibrosis were also significantly improved. In this MetS + RAS model, the macrophage subtype M1/M2 ratio in the kidney was increased; proinflammatory factors such as IL-6, IL-1β, and TNF-α in the renal vein were significantly elevated; and the anti-inflammatory factor IL-10 was decreased. These effects were reversed after the intrarenal administration of EVs, and the renal protection of EVs was blunted by IL-10 knockdown in MSCs75. Another study analyzed the mRNAs and protein cargo in EVs by next-generation mRNA sequencing and proteomic analysis. The results showed that there are several mRNAs and proteins that promote angiogenesis and regulate apoptosis and oxidative stress. Subsequently, in the MetS + RAS model, it was verified that EVs could improve renal microcirculation, inhibit apoptosis and oxidative stress, and repair renal function. Tracing the labeled EVs showed that the retention in the kidney peaked 2 days after intrarenal administration, and the fluorescent signal was found in the tubular cells and endothelial cells of the kidney until 4 weeks, indicating that EVs can be taken up, internalized by cells, and retained for a long time76.
In a mouse model of streptozotocin-induced diabetic nephropathy (DN), multiple (once a week for 4 weeks) intravenous injections of EVs were shown to improve renal function by reversing fibrosis. Array sequencing results showed that the EVs contained high levels of microRNAs, and bioinformatic analyses found that they mainly targeted the biological pathways involved in the profibrotic processes, such as transforming growth factor (TGF)-β, insulin like growth factor 1, EGFR, and PDGFR, which were consistent with the antifibrotic effects exerted by EVs in DN models77. In 2016, a clinical trial assessed the safety and therapeutic efficacy of allogeneic hUC-MSC-EVs in the treatment of CKD, including 40 grade III/IV CKD patients, who were randomly divided into two groups. The experimental group was administered two doses weekly at 100 μg/kg/dose, the first intravenously through the median cubital vein and the second intra-arterially targeting the diseased kidney under CT guidance. The control group was given only intravenous saline but no intra-arterial injections. During 1 year of follow-up, the inflammatory response was alleviated, renal function was improved, and no adverse reactions were observed, suggesting that the use of EVs to treat CKD is safe and effective in the short term78.
MSC-EVs in Skin Wounds
The application of EVs in wound healing has received increased attention in recent years. Researchers have focused on the contents of EVs and their anti-inflammatory effects, matrix regulation, and angiogenesis in the treatment of skin wounds79,80. In 2016, a study found that injection of AD-MSC-EVs through the tail vein could improve wound healing by optimizing the characteristics of fibroblasts at different stages. The results demonstrated that EVs might accelerate wound healing by enhancing the extracellular matrix (ECM) synthesis capacity of fibroblasts in the early stage of this process and reduce scar formation by inhibiting matrix synthesis in the late stage81. In a nude mouse model of full-thickness skin defects, Fang et al.82 found that scar formation was significantly reduced at 25 days by the injection of hydrogel-coated UC-MSC-EVs around the wound. Bioinformatics analysis and subsequent experiments demonstrated that miRNAs (miRNA-21, miRNA-23a, miRNA-125b, and miRNA-145) in EVs could reduce scar formation by inhibiting myofibroblast differentiation through the TGF-β/SMAD2 pathway. In another experiment, miRNA-205 in EVs extracted from AD-MSCs promoted keratinocyte and fibroblast migration and proliferation by activating the AKT pathway to promote wound healing83. Analysis of growth factor concentrations revealed that EVs contained high concentrations of cytokines associated with skin healing, among which the epidermal growth factor (EGF) had the highest concentration. Moreover, the researchers evaluated the permeation of EVs using human skin tissues. The results showed that labeled EVs permeated the outermost layer of the epidermis after 3 h and gradually permeated the epidermis after 18 h. At the same time, the expression of collagen I and elastin in the skin tissue also increased 3 days after the administration of EVs84. Because oral gingival wounds heal faster than skin wounds, Kou et al.85 tested the effect of gingiva-derived MSC-EVs in the treatment of gums and skin wounds. They found that the membrane surface of EVs contained high amounts of interleukin-1 receptor antagonist (IL-1RA), a natural inhibitor of the proinflammatory cytokine IL-1β; IL-1RA was found to exert anti-inflammatory effects and accelerate wound healing. In addition, the Fas/Fap-1/Cav-1 complex in gingiva-derived MSCs could promote the release of EVs containing IL-1RA under the activation of TNF-α.
Pretreatment can alter the contents of EVs and thus affect biological function during wound healing. Overexpression of miRNA-126-3P enhanced the proangiogenic effect of synovial MSC-derived EVs, which could promote vascular endothelial cell proliferation, migration, and tube formation in vitro. In a mouse model of diabetes, the overexpressed EVs accelerated the healing of full-thickness skin defects, and increased neovascularization was detected by μCT and histology86. Sung et al.87 compared the effects of different pretreatment methods, such as thrombin, H2O2, lipopolysaccharide (LPS), and hypoxia, on the production of EVs by UC-MSCs. The researchers found that all pretreatment methods, especially thrombin, increased the secretion of EVs. In this study, all pretreatments except LPS increased angiogenic growth factors, such as angiogenin, angiopoietin-1, HGF, and VEGF, within the EVs, while thrombin pretreatment optimally enhanced the angiogenic protein cargo content compared with the other methods. For in vivo cutaneous wound healing, significant attenuation of inflammatory responses and an improved closure rate were observed only with pretreatment of EVs with thrombin. Some studies have examined the administration of iMSC-EVs in wound healing. In a rat skin full-thickness defect model, topical administration of iMSC-EVs could promote matrix synthesis and angiogenesis in the wound area, which was further verified in vitro88. In another experiment, iMSC-EVs and UC-MSC-EVs produced similar outcomes in wound healing. Both of them can enhance the secretion of collagen in keratinocytes and fibroblasts. However, an increase in the fibronectin levels in fibroblasts was more apparent in the iMSC-EV group than in the UC-MSC-EV group, and only iMSC-EVs increased the phosphorylation of extracellular signal-regulated kinase (ERK)-1/289.
MSC-EVs in Cartilage Injury
Many studies have shown that MSC-EVs could effectively treat cartilage injury caused by trauma or degenerative diseases. In a rat model of full-thickness cartilage defects, intra-articular injection of EVs derived from embryonic MSCs (EMSCs) caused complete restoration of the cartilage and subchondral bone90. For tissue repair, EVs can promote the proliferation and migration of chondrocytes by activating the AKT and ERK signaling pathways. EV treatment of defects showed an immunoregulatory role by regulating macrophage polarization, with a concomitant reduction in proinflammatory synovial cytokines91. Liu et al.92 made an acellular tissue patch by mixing hydrogels and EVs to treat rabbit cartilage defects. Compared with the simple application of EVs, the patch had a better repair effect by slowly releasing the vesicles in vivo. Chen and colleagues93 synthesized a 3D-printed bioink using decellularized ECM, BMSC-EVs, and gelatin methacrylate hydrogel to repair cartilage defects. The researchers found that this 3D print scaffold can restore mitochondrial dysfunction and oxidative stress damage in chondrocytes and polarize the synovial macrophage response toward an M2 phenotype.
In osteoarthritis (OA), MSC-EVs were found to exert cartilage protection mainly through anti-inflammatory effects94 and balancing the synthesis and catabolism of ECM95. In addition, EVs protected chondrocytes from apoptosis and regulated the polarization of macrophages in the synovium96. For analysis of the effect of ADMSC-EVs on OA cartilage inflammation, IL-1β-stimulated OA chondrocytes were treated with EVs. The results showed that EVs reduced the production of inflammatory mediators, such as TNF-α, IL-6, PGE 2, and NO, and that annexin A1 is overrepresented in EVs and exerts complex anti-inflammatory and proresolution effects by inhibiting the NF-κB signaling pathway97. Many studies have examined the function of noncoding RNAs in EVs in cartilage protection. Wu et al.98 found that miRNA-100-5p in the EVs derived from infrapatellar fat pad MSCs promoted the autophagy of chondrocytes via the inhibition of mTOR. Intra-articular injection of antagomir-miRNA-100-5p dramatically attenuated the EV-mediated protective effect on cartilage in vivo. The long non-coding RNA (lncRNA)-KLF3-AS1 axis in EVs could also restrain inflammation-induced chondrocyte apoptosis99. By measuring the gene expression levels, they found that Wnt5a and Wnt5b were enriched in synovial MSC-EVs. Wnt5a and Wnt5b carried by EVs activated yes associated protein (YAP) via the alternative Wnt signaling pathway and enhanced the proliferation and migration of chondrocytes with the side effect of significantly decreasing ECM secretion. Tao et al.100 overexpressed miRNA-140-5P in EVs to block this side effect by rescuing SOX9 through suppressing RalA. Another experiment successfully promoted chondrocyte proliferation and matrix synthesis by overexpressing miRNA-92a-3p in BMSC-EVs to target WNT5a in OA while promoting the chondrogenic differentiation of MSCs101. In an animal model of temporomandibular arthritis induced by monosodium iodoacetate in SD rats, EVs derived from EMSCs were injected weekly and could improve pain and alleviate cartilage degeneration through reduced inflammation and improvements in matrix expression and subchondral bone architecture. The cellular activities during EV-mediated cartilage repair of an in vitro model induced by IL-1β were attributed to adenosine activation of AKT, ERK, and AMPK signaling102. Zhu et al.103 aimed to compare the effectiveness of EVs secreted by synovial membrane MSCs and iMSCs on the treatment of OA. The injection of both EVs attenuated OA in the collagenase-induced OA model, but iMSC-EVs had a superior therapeutic effect. Similarly, chondrocyte migration and proliferation were stimulated by iMSC-EVs, which exerted a strong effect.
MSC-EVs in Cardiovascular Disorders
The cardiac repair capacity of MSC-EVs was discovered in an animal model of MI in 201017. In this rat model of MI, the myocardial protection of MSCs and their secreted EVs was directly compared. The results suggested that both of them could reduce inflammation, inhibit fibrosis, and improve cardiac function, and the effects of the MSC-EVs were significantly better than those of MSCs104. In recent years, many scholars have further explored the cardioprotective mechanism of EVs. MSC-EVs can reduce MI size by promoting angiogenesis and improving myocardial blood flow. In vitro, BMSC-EVs promoted the proliferation, migration, and tube formation of vascular endothelial cells105,106. By analyzing the protein and nucleic acid components in EVs, researchers found that extracellular matrix metalloproteinase inducer (EMMPRIN) and miRNA-210 play a major role in promoting angiogenesis107,108. Vrijsen et al.107 found that EVs contain high levels of VEGF, EMMPRIN, and MMP-9, which are related to angiogenesis. The reduced levels of EMMPRIN in EVs significantly attenuated the proangiogenic effects in vitro and in vivo. Similar research found that miRNA-210 was highly enriched in MSC-EVs and necessary for MSC-EV-induced angiogenesis through the target gene Ephrin-A3108.
In addition to promoting angiogenesis, EVs can also inhibit apoptosis, autophagy, and inflammatory regulation during cardioprotection. Luther et al.109 found that miRNA-21a-5p is the most abundant among several cardioprotective miRNAs through deep miRNA sequencing of MSC-EV cargo. Subsequent mechanistic studies found that miRNA-21 targeted the proapoptotic gene products PDCD4, PTEN, Peli1, and FasL in the myocardium, which in turn protected the myocardium by inhibiting ischemia-induced apoptosis. Enriched miRNA-21 was also found in endometrial MSC-derived EVs and shown to promote angiogenesis and inhibit apoptosis by targeting the PTEN/Akt pathway110. Transplantation of MSCs inhibited ischemia-induced autophagy by inducing an increase in the autophagy receptor P62 and a decrease in the autophagosome marker LC3-II, which improved myocardial recovery by impeding autophagy. This effect is mainly mediated through interference of the MSC-EV-transported miRNA-125-5p with the p53/Bnip3 signaling pathway111. In an MI model, direct intramyocardial injection of BMSC-EVs reduced infarct size and alleviated inflammation in the heart and serum by modulating the polarization of M1 macrophages to M2 macrophages. The miRNA sequencing of EVs and bioinformatics analysis identified miRNA-182 as a potent candidate mediator of macrophage polarization and Toll-like receptor 4 as a downstream target112.
Some studies have modified or pretreated MSCs to alter the composition of EVs and improve myocardial repair. Ma et al.113 overexpressed AKT in MSCs, leading to the upregulation of platelet-derived growth factor D in the EVs. These modified EVs showed efficacy in MI therapy through promoting angiogenesis. Similarly, EVs derived from SDF1-overexpressing MSCs improved cardiac protection by activating the PI3 K signaling pathway, which can inhibit apoptosis and autophagy while promoting angiogenesis in MI114. EVs from hypoxia-treated MSCs resulted in improved myocardial protection against MI. Hypoxic treatment increased the expression of miRNA-125 in EVs and significantly ameliorated cardiomyocyte apoptosis by targeting the proapoptotic genes P53 and BAK1115. Hypoxia also increased the expression of VEGF, EGF, FGF, and their receptors, significantly increasing the angiogenic ability of MSC-EVs116.
Conclusion
In the past few decades, studies on EVs have improved our understanding of the origin, content, and biological functions of EVs. The application of MSC-derived EVs has resulted in good experimental outcomes in antitumor immune therapy117,118 and regenerative therapies. However, there are still many challenges to the future clinical applications of EVs. First, the selection of an appropriate source of MSCs for the production of EVs is important. Different cell sources can affect the composition of EVs and then affect the therapeutic effects of different diseases. It is also important to select cells of an appropriate “age” for EV collection. As MSCs undergo senescence, the composition of the EVs also changes119. Furthermore, researchers need to develop and formulate a unified method for collecting EVs. Ultracentrifugation is the most common method, and some laboratories use other methods, such as commercial kits or flow field separation. However, the amount and purity of EVs isolated using these methods are not sufficient for clinical applications. Currently, some articles have reported that the application of bioreactors for cell culture can substantially increase the production of EVs120,121. The pharmacological characteristics, such as biodistribution, bioavailability, pharmacokinetics, and pharmacodynamics, of EVs in the body remain unknown. Therefore, it is necessary to develop an excellent EV tracking technology in vivo.
A total of 473 clinical trials were found in the clinical trials database (https://clinicaltrials.gov/) using the keywords extracellular vesicle, exosome, and microvesicles, and approximately 200 were clinical trials of EVs. Most of these trials used EVs as biomarkers for disease diagnosis and monitoring, mainly in tumor research. A total of eight clinical trials on the application of EVs for disease treatment were identified (Table). However, they have not yet obtained clinical trial results. Numerous experiments are needed to verify biosafety before clinical application. As an emerging therapeutic product, EVs used in clinical trials need to show “purity, identity, quantity, potency, and sterility”122. Among these parameters, purity and sterility should be closely studied. The conventional enrichment methods, such as ultracentrifugation, density gradient centrifugation, and size exclusion chromatography, cannot easily remove copurifying non-EV components. The composition and function of these copurifying components should be further investigated. In addition to the purity issue of EV-based products, a primary concern is the high congruence between EVs and virus123. Most purification techniques originate from virus or virus-like particle collection methods. Therefore, any virus in the CM will be enriched in the final product, increasing the potential risk. Rigorous virus testing is especially important at every step of the production process.
Table.
Clinical Trials of EV-Based Therapies.
| NCT number/Ref | Disease | Phase | EV source | Status |
|---|---|---|---|---|
| NCT03857841 | BPD | 1 | BM-MSC | Recruiting |
| NCT03437759 | Macular holes | 1 | UC-MSC | Recruiting |
| NCT03384433 | Stroke | 1/2 | Allogenic-MSC | Not yet recruiting |
| NCT02565264 | Cutaneous ulcers | 1 | Plasma | Enrolling by invitation |
| NCT02138331 | Diabetes mellitus type 1 | 2/3 | UC-MSC | Unknown |
| NCT01854866 | Malignant pleural effusion/ascites | 2 | Tumor cell | Unknown |
| NCT01294072 | Colon cancer | 1 | Plant | Not yet recruiting |
| NCT01159288 | Non-small cell lung cancer | 2 | Dendritic cell | Completed |
| 117 | Melanoma | 1 | Dendritic cell | Completed |
| 118 | Colon cancer | 1 | Autologous ascites | Completed |
BM: bone marrow; BPD: bronchopulmonary dysplasia; EV: extracellular vesicle; MSC: mesenchymal stem cell; NCT: national clinical trial; UC: umbilical cord.
EVs contain large amounts of cargo and can be used as a natural drug delivery tool to simulate cell therapy without the drawback of proliferation. Currently, most research focuses on the function of miRNAs in tissue regeneration. Functional studies on other noncoding RNAs, such as long noncoding RNA and circular RNA, are scarce. This field is still in its infancy, but research to optimize EV production and to dissect the complex biology, content, and function of EVs is ongoing.
Acknowledgments
We thank American Journal Experts (AJE) for English language editing.
Footnotes
Author Contributions: Bocheng Zhang and Xiaoyuan Tian authors contributed equally to this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bocheng Zhang  https://orcid.org/0000-0003-1084-4947
https://orcid.org/0000-0003-1084-4947
References
- 1. Yanez Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4(1):27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guiot J, Struman I, Louis E, Louis R, Malaise M, Njock MS. Exosomal mirnas in lung diseases: from biologic function to therapeutic targets. J Clin Med. 2019;8(9):E1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jaquenod De Giusti C, Santalla M, Das S. Exosomal non-coding rnas (exo-ncrnas) in cardiovascular health. J Mol Cell Cardiol. 2019;137:143–151. [DOI] [PubMed] [Google Scholar]
- 5. Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–171. [DOI] [PubMed] [Google Scholar]
- 6. Vu LT, Peng B, Zhang DX, Ma V, Mathey Andrews CA, Lam CK, Kiomourtzis T, Jin J, McReynolds L, Huang L, Grimson A, et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microrna-125b. J Extracell Vesicles. 2019;8(1):1599680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15(10):617–638. [DOI] [PubMed] [Google Scholar]
- 8. Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. 2017;120(10):1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda). 2019;34(3):169–177. [DOI] [PubMed] [Google Scholar]
- 10. Yuan X, Wu Q, Wang P, Jing Y, Yao H, Tang Y, Li Z, Zhang H, Xiu R. Exosomes derived from pericytes improve microcirculation and protect blood-spinal cord barrier after spinal cord injury in mice. Front Neurosci. 2019;13:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. Msc-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28(4):970–973. [DOI] [PubMed] [Google Scholar]
- 12. Lai P, Chen X, Guo L, Wang Y, Liu X, Liu Y, Zhou T, Huang T, Geng S, Luo C, Huang X, et al. A potent immunomodulatory role of exosomes derived from mesenchymal stromal cells in preventing CGVHD. J Hematol Oncol. 2018;11(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther. 2015;151:107–120. [DOI] [PubMed] [Google Scholar]
- 14. Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol Biol. 2016;1416:123–146. [DOI] [PubMed] [Google Scholar]
- 15. Witwer KW, Van Balkom BWM, Bruno S, Choo A, Dominici M, Gimona M, Hill AF, De Kleijn D, Koh M, Lai RC, Mitsialis SA, et al. Defining mesenchymal stromal cell (msc)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles. 2019;8(1):1609206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, et al. Exosome secreted by msc reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. [DOI] [PubMed] [Google Scholar]
- 18. Nawaz M, Fatima F, Vallabhaneni KC, Penfornis P, Valadi H, Ekstrom K, Kholia S, Whitt JD, Fernandes JD, Pochampally R, Squire JA, et al. Extracellular vesicles: Evolving factors in stem cell biology. Stem Cells Int. 2016;2016:1073140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–197. [PubMed] [Google Scholar]
- 20. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. [DOI] [PubMed] [Google Scholar]
- 21. Anderson HC. 1969. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 41(1):59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stegmayr B, Ronquist G. Promotive effect on human sperm progressive motility by prostasomes. Urol Res. 1982;10(5):253–257. [DOI] [PubMed] [Google Scholar]
- 23. Taylor DD, Homesley HD, Doellgast GJ. Binding of specific peroxidase-labeled antibody to placental-type phosphatase on tumor-derived membrane fragments. Cancer Res. 1980;40(11):4064–4069. [PubMed] [Google Scholar]
- 24. Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–263. [PubMed] [Google Scholar]
- 25. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. [DOI] [PubMed] [Google Scholar]
- 26. Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mrna and protein delivery. Leukemia. 2006;20(5):847–856. [DOI] [PubMed] [Google Scholar]
- 28. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 29. Nawaz M, Fatima F. Extracellular vesicles, tunneling nanotubes, and cellular interplay: synergies and missing links. Front Mol Biosci. 2017;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. [DOI] [PubMed] [Google Scholar]
- 31. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8): E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles. 2013;2(1):20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by escrt complexes. Nature. 2010;464(7290):864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of escrt functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. [DOI] [PubMed] [Google Scholar]
- 35. Kajimoto T, Okada T, Miya S, Zhang L, Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. [DOI] [PubMed] [Google Scholar]
- 36. van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin cd63 regulates escrt-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meehan B, Rak J, Di Vizio D. Oncosomes - large and small: what are they, where they came from? J Extracell Vesicles. 2016;5(1):33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tkach M, Kowal J, Thery C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci. 2018;373(1737): 20160479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hinger SA, Cha DJ, Franklin JL, Higginbotham JN, Dou Y, Ping J, Shu L, Prasad N, Levy S, Zhang B, Liu Q, et al. Diverse long rnas are differentially sorted into extracellular vesicles secreted by colorectal cancer cells. Cell Rep. 2018;25(3):715–725.e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma H, Chinnappan M, Agarwal S, Dalvi P, Gunewardena S, O’Brien-Ladner A, Dhillon NK. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered mirna cargo in response to hiv infection and substance abuse. FASEB J. 2018;32(9):5174–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim DK, Lee J, Simpson RJ, Lotvall J, Gho YS. Evpedia: a community web resource for prokaryotic and eukaryotic extracellular vesicles research. Semin Cell Dev Biol. 2015;40:4–7. [DOI] [PubMed] [Google Scholar]
- 43. Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, et al. Exocarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S. Vesiclepedia 2019: a compendium of rna, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1): D516–D519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luria EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2(2):83–92. [PubMed] [Google Scholar]
- 47. Jiang D, Xiong G, Feng H, Zhang Z, Chen P, Yan B, Chen L, Gandhervin K, Ma C, Li C, Han S, et al. Donation of mitochondria by ipsc-derived mesenchymal stem cells protects retinal ganglion cells against mitochondrial complex i defect-induced degeneration. Theranostics. 2019;9(8):2395–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 49. Yin Y, Li X, He XT, Wu RX, Sun HH, Chen FM. Leveraging stem cell homing for therapeutic regeneration. J Dent Res. 2017;96(6):601–609. [DOI] [PubMed] [Google Scholar]
- 50. Bateman ME, Strong AL, Gimble JM, Bunnell BA. Concise review: using fat to fight disease: a systematic review of nonhomologous adipose-derived stromal/stem cell therapies. Stem Cells. 2018;36(9):1311–1328. [DOI] [PubMed] [Google Scholar]
- 51. Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. [DOI] [PubMed] [Google Scholar]
- 52. Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70(20):3871–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for AKT-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20(6):661–669. [DOI] [PubMed] [Google Scholar]
- 55. Ramos TL, Sanchez-Abarca LI, Muntion S, Preciado S, Puig N, Lopez-Ruano G, Hernandez-Hernandez A, Redondo A, Ortega R, Rodriguez C, Sanchez-Guijo F, et al. Msc surface markers (cd44, cd73, and cd90) can identify human msc-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK. Proteolytic potential of the msc exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics. 2012;2012:971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zou XY, Yu Y, Lin S, Zhong L, Sun J, Zhang G, Zhu Y. Comprehensive mirna analysis of human umbilical cord-derived mesenchymal stromal cells and extracellular vesicles. Kidney Blood Press Res. 2018;43(1):152–161. [DOI] [PubMed] [Google Scholar]
- 58. Heslop JA, Hammond TG, Santeramo I, Tort Piella A, Hopp I, Zhou J, Baty R, Graziano EI, Proto Marco B, Caron A, Skold P, et al. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med. 2015;4(4):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reis M, Ogonek J, Qesari M, Borges NM, Nicholson L, Preussner L, Dickinson AM, Wang XN, Weissinger EM, Richter A. Recent developments in cellular immunotherapy for hsct-associated complications. Front Immunol. 2016;7(9):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, Goldberg LR, Baird GL, Ventetuolo CE, Quesenberry PJ, Klinger JR. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110(3):319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Potter DR, Miyazawa BY, Gibb SL, Deng X, Togaratti PP, Croze RH, Srivastava AK, Trivedi A, Matthay M, Holcomb JB, Schreiber MA, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg. 2018;84(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li QC, Liang Y, Su ZB. Prophylactic treatment with msc-derived exosomes attenuates traumatic acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2019;316(6): L1107–L1117. [DOI] [PubMed] [Google Scholar]
- 64. Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic mir-21-5p. Eur J Pharmacol. 2019;852:68–76. [DOI] [PubMed] [Google Scholar]
- 65. Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, Antunes M, Robinson KL, Mitsialis SA, Kourembanas S, Thane K, et al. Systemic administration of human bone marrow-derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4(11):1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197(1):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chaubey S, Thueson S, Ponnalagu D, Alam MA, Gheorghe CP, Aghai Z, Singh H, Bhandari V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor tsg-6. Stem Cell Res Ther. 2018;9(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrom K, Wang X, Principe S, Shah N, Ashraf NM, Fatima F, Neder L, et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol. 2014;11(12):688–701. [DOI] [PubMed] [Google Scholar]
- 70. Kilpinen L, Impola U, Sankkila L, Ritamo I, Aatonen M, Kilpinen S, Tuimala J, Valmu L, Levijoki J, Finckenberg P, Siljander P, et al. Extracellular membrane vesicles from umbilical cord blood-derived msc protect against ischemic acute kidney injury, a feature that is lost after inflammatory conditioning. J Extracell Vesicles. 2013;2(1):21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, Qiu J, Fan Y. Ccr2 positive exosome released by mesenchymal stem cells suppresses macrophage functions and alleviates ischemia/reperfusion-induced renal injury. Stem Cells Int. 2016;2016(1):1240301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang G, Zou X, Huang Y, Wang F, Miao S, Liu G, Chen M, Zhu Y. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing nrf2/are activation in rats. Kidney Blood Press Res. 2016;41(2):119–128. [DOI] [PubMed] [Google Scholar]
- 73. Li D, Zhang D, Tang B, Zhou Y, Guo W, Kang Q, Wang Z, Shen L, Wei G, He D. Exosomes from human umbilical cord mesenchymal stem cells reduce damage from oxidative stress and the epithelial-mesenchymal transition in renal epithelial cells exposed to oxalate and calcium oxalate monohydrate. Stem Cells Int. 2019;2019:6935806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M, Biancone L, Gontero P, Frea B, Camussi G. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther. 2017;8(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92(1):114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eirin A, Zhu XY, Jonnada S, Lerman A, van Wijnen AJ, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles improve the renal microvasculature in metabolic renovascular disease in swine. Cell Transplant. 2018;27(7):1080–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G, Brizzi MF. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9(1):4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fatima F, Ekstrom K, Nazarenko I, Maugeri M, Valadi H, Hill AF, Camussi G, Nawaz M. Non-coding rnas in mesenchymal stem cell-derived extracellular vesicles: deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front Genet. 2017;8:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nawaz M, Shah N, Zanetti BR, Maugeri M, Silvestre RN, Fatima F, Neder L, Valadi H. Extracellular vesicles and matrix remodeling enzymes: the emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells. 2018;7(10):E167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L. Exosomes derived from human adipose mesenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2016;6:32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal micrornas suppress myofibroblast differentiation by inhibiting the transforming growth factor-beta/smad2 pathway during wound healing. Stem Cells Transl Med. 2016;5(10):1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferreira ADF, Cunha PDS, Carregal VM, da Silva PC, de Miranda MC, Kunrath-Lima M, de Melo MIA, Faraco CCF, Barbosa JL, Frezard F, Resende V, et al. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate akt pathway in human keratinocytes and fibroblasts independently of mir-205 activity. Stem Cells Int. 2017;2017:9841035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kim YJ, Yoo SM, Park HH, Lim HJ, Kim YL, Lee S, Seo KW, Kang KS. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun. 2017;493(2):1102–1108. [DOI] [PubMed] [Google Scholar]
- 85. Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y, Giraudo C, Le A, Shi S. The fas/fap-1/cav-1 complex regulates il-1ra secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018;10(432):eaai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan wound dressings incorporating exosomes derived from microrna-126-overexpressing synovium mesenchymal stem cells provide sustained release of exosomes and heal full-thickness skin defects in a diabetic rat model. Stem Cells Transl Med. 2017;6(3):736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sung DK, Chang YS, Sung SI, Ahn SY, Park WS. Thrombin preconditioning of extracellular vesicles derived from mesenchymal stem cells accelerates cutaneous wound healing by boosting their biogenesis and enriching cargo content. J Clin Med. 2019;8(4):E533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived mscs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;3(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kim S, Lee SK, Kim H, Kim TM. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):E3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24(12):2135–2140. [DOI] [PubMed] [Google Scholar]
- 91. Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. Msc exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. [DOI] [PubMed] [Google Scholar]
- 92. Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9(13):4430–4438. [DOI] [PubMed] [Google Scholar]
- 93. Chen P, Zheng L, Wang Y, Tao M, Xie Z, Xia C, Gu C, Chen J, Qiu P, Mei S, Ning L, et al. Desktop-stereolithography 3d printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration in vitro. Theranostics. 2018;8(4):906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7(1):16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tofino-Vian M, Guillen MI, Perez Del Caz MD, Silvestre A, Alcaraz MJ. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell Physiol Biochem. 2018;47(1):11–25. [DOI] [PubMed] [Google Scholar]
- 98. Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, Chen H, Huang S, Fu Z, Li J, Liu R, et al. Mir-100-5p-abundant exosomes derived from infrapatellar fat pad mscs protect articular cartilage and ameliorate gait abnormalities via inhibition of mtor in osteoarthritis. Biomaterials. 2019;206:87–100. [DOI] [PubMed] [Google Scholar]
- 99. Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal klf3-as1 from hmscs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475(22):3629–3638. [DOI] [PubMed] [Google Scholar]
- 100. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from mir-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from mir-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting wnt5a. Stem Cell Res Ther. 2018;9(1):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. Msc exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. [DOI] [PubMed] [Google Scholar]
- 103. Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, Xiao P, Meng Q, Geng YJ, Yu XY, Li Y. Mirna-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. Biomed Res Int. 2017;2017:4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014;92(4):387–397. [DOI] [PubMed] [Google Scholar]
- 106. Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37(6):2415–2424. [DOI] [PubMed] [Google Scholar]
- 107. Vrijsen KR, Maring JA, Chamuleau SA, Verhage V, Mol EA, Deddens JC, Metz CH, Lodder K, van Eeuwijk EC, van Dommelen SM, Doevendans PA, et al. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via emmprin. Adv Healthc Mater. 2016;5(19):2555–2565. [DOI] [PubMed] [Google Scholar]
- 108. Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, Zhou F, Yang X, Yang J, Zeng C, Wang WE. Mesenchymal stem cells-derived extracellular vesicles, via mir-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):2085–2092. [DOI] [PubMed] [Google Scholar]
- 109. Luther KM, Haar L, McGuinness M, Wang Y, Lynch Iv TL, Phan A, Song Y, Shen Z, Gardner G, Kuffel G, Ren X, et al. Exosomal mir-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol. 2018;119:125–137. [DOI] [PubMed] [Google Scholar]
- 110. Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microrna-21. Stem Cells Transl Med. 2017;6(1):209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xiao C, Wang K, Xu Y, Hu H, Zhang N, Wang Y, Zhong Z, Zhao J, Li Q, Zhu D, Ke C, et al. Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of mir-125b. Circ Res. 2018;123(5):564–578. [DOI] [PubMed] [Google Scholar]
- 112. Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through mir-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X, Qian H, Xu W, Zhu W. Exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor d. Stem Cells Transl Med. 2017;6(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gong XH, Liu H, Wang SJ, Liang SW, Wang GG. Exosomes derived from sdf1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J Cell Physiol. 2019;234(8):13878–13893. [DOI] [PubMed] [Google Scholar]
- 115. Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M, Xu L, Zhang HX, Qiu XT, Li CC, Wang KK, et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through mir-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8(22):6163–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through vegf/vegf-r. Int J Biochem Cell Biol. 2019;109:59–68. [DOI] [PubMed] [Google Scholar]
- 117. Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (dc) derived-exosomes: Results of thefirst phase i clinical trial. J Transl Med. 2005;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase i clinical trial of autologous ascites-derived exosomes combined with gm-csf for colorectal cancer. Mol Ther. 2008;16(4):782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, Ren W, Guo H, Zhang L, Wang H, Chen Z, et al. Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells. Theranostics. 2017;7(10):2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yan IK, Shukla N, Borrelli DA, Patel T. Use of a hollow fiber bioreactor to collect extracellular vesicles from cells in culture. Methods Mol Biol. 2018;1740:35–41. [DOI] [PubMed] [Google Scholar]
- 122. Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K, Rohde E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci. 2017;18(6):E1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21(6):581–592. [DOI] [PubMed] [Google Scholar]