Abstract
Cilia are microtubule-based, cell-surface projections whose machinery is evolutionarily conserved. In vertebrates, cilia are observed on almost every cell type and are either motile or immotile. Immotile sensory, or primary cilia, are responsive to extracellular ligands and signals. Cilia can be thought of as compartments, functionally distinct from the cell that provide an environment for signalling cascades. Hedgehog is a critical developmental signalling pathway which is functionally linked to primary cilia in vertebrates. The major components of the vertebrate Hedgehog signalling pathway dynamically localize to the ciliary compartment and ciliary membrane. Critically, G-protein coupled receptor (GPCR) Smoothened, the obligate transducer of the pathway, is enriched and activated in the cilium. While smoothened is the most intensely studied ciliary receptor, many GPCRs localize within cilia. Understanding the link between Smoothened and cilia defines common features, and distinctions, of GPCR signalling within the primary cilium.
The cilium is an ideal environment for G-protein coupled receptor signalling. The most intensively studied ciliary G-protein coupled receptor, Smoothened, provides a template for understanding principles governing receptor activation within the cilium and the resulting signalling cascade.
Graphical/Visual Abstract and Caption
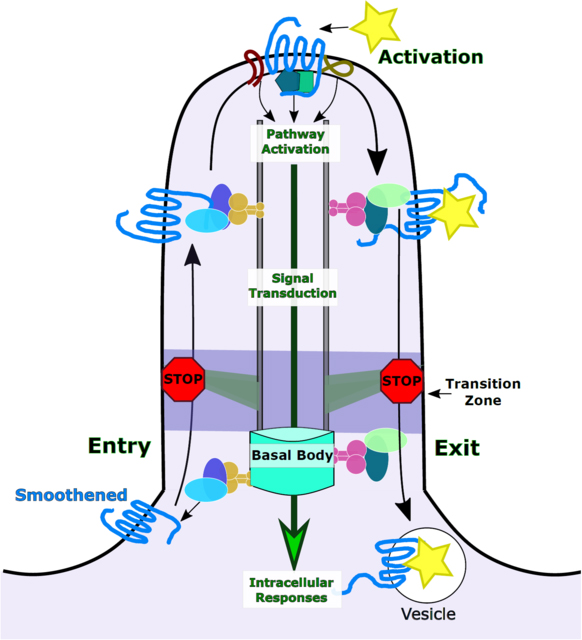
Introduction
Cilia are microtubule-based, cell-surface projections encased in a plasma membrane that is continuous with, yet biochemically distinct from, the membrane of the cell. Cilia are templated from a modified centriole, called the basal body (W. F. Marshall, 2008). The basic architecture and components of the cilium are highly conserved through evolution (G. J. Pazour et al., 2002; G. J. Pazour et al., 2000). Primary cilia, also called sensory or immotile cilia, are found on most cell types (Bangs, Schrode, Hadjantonakis, & Anderson, 2015). When cut in cross section, nine microtubule doublets are arranged around the periphery of a cilium. Motile cilia (and synonymous flagella), have an additional central pair of microtubules that are critical for the ciliary machinery that generates force, and thus motility (Fawcett & Porter, 1954; Manton & Clarke, 1952). Over the last two decades our understanding of primary cilia has shifted dramatically. While they were once thought to be vestigial organelles, cilia are now appreciated as fundamental cell organelles with essential roles in cell signalling throughout an organism (Barr & Sternberg, 1999; Huangfu et al., 2003; G. J. Pazour et al., 2000). Disruption of cilia proteins and processes leads to human diseases, collectively termed ciliopathies (see Sidebar below)(Baker & Beales, 2009).
Despite their connection to the cell body, cilia provide a small and exclusive compartment where receptors and mediators of signalling cascades rapidly interact to propagate intracellular responses. This is epitomized by G-protein coupled receptor (GPCR) signalling as multiple GPCRs display ciliary enrichment (Brailov et al., 2000; Corbit et al., 2005; Domire et al., 2011; Handel et al., 1999; Mukhopadhyay et al., 2013). In this review, we examine the relationship between cilia and signalling pathways through the lens of the Hedgehog pathway. Vertebrate Hedgehog (vHH) signalling requires the primary cilium for signal transduction (Huangfu & Anderson, 2005; Huangfu et al., 2003). It serves as an exemplar of cilia-dependent signalling as it requires activation of the GPCR, Smoothened (SMO), within the cilium and is intensely studied (Corbit et al., 2005; Rohatgi, Milenkovic, Corcoran, & Scott, 2009). Herein, we detail the structure and biochemical make-up of the primary cilium in order to discuss the environment it provides GPCRs like SMO for their activation and downstream signalling.
Cilia Structure
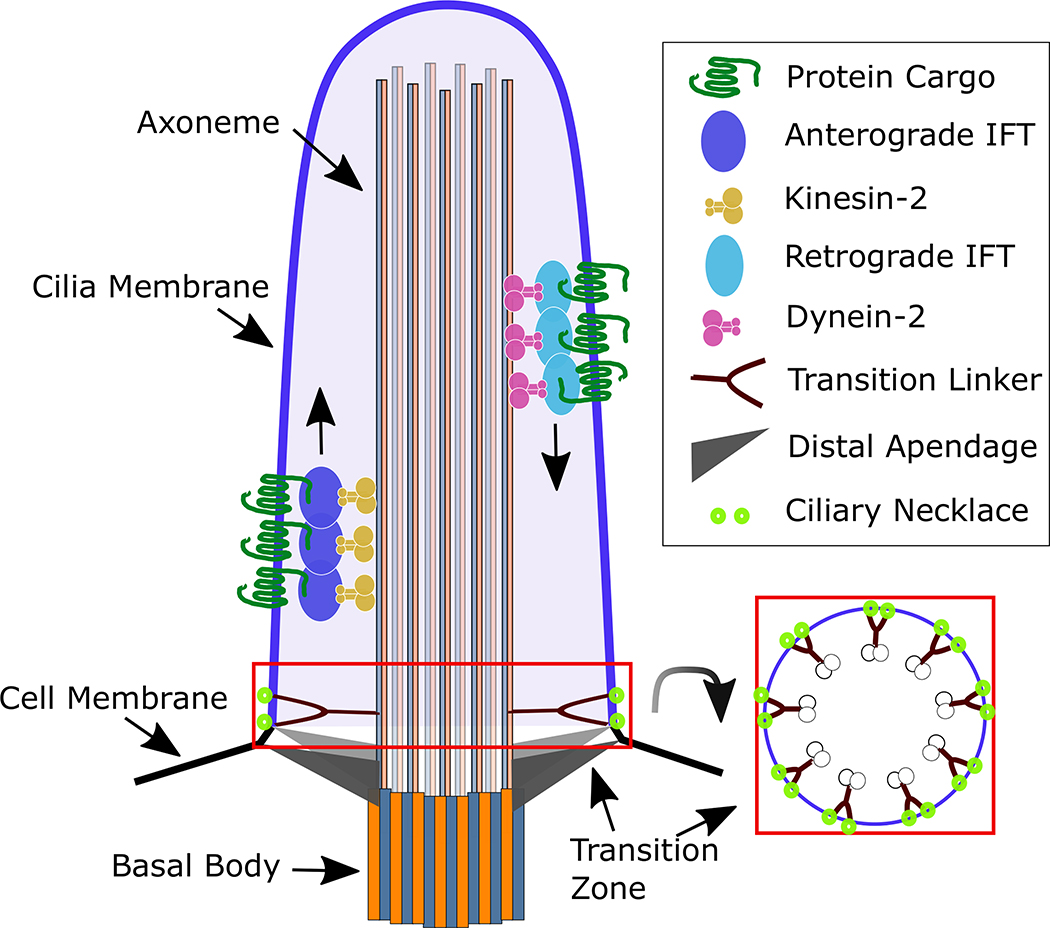
The axoneme is the microtubule backbone of the cilium, composed of αand β−tubulin polymers called protofilaments that are organized in doublets (Fawcett & Porter, 1954; Manton & Clarke, 1952). From within the cell, the basal body determines the position and orientation of the cilium on the cell surface, and it assists with the physical anchoring of cilium to the intracellular surface (K.-F. Lechtreck, Teltenkotter, & Grunow, 1999; W. F. Marshall, 2008). Immediately distal to the basal body is the transition zone that prevents proteins from freely entering or exiting the cilium. The transition zone is comprised of several proteins: Y-shaped proteins that link the axoneme to the ciliary membrane, the ciliary necklace that prevents protein diffusion along the membrane, and distal appendages that link the basal body to the cell membrane. Together, the proteins of the transition zone act as a diffusion barrier, excluding large or improperly targeted proteins from entering the cilium (Breslow, Koslover, Seydel, Spakowitz, & Nachury, 2013; Chih et al., 2011; Ishikawa, Kubo, Tsukita, & Tsukita, 2005; Kee et al., 2012; K.-F. Lechtreck et al., 1999; Reiter, Blacque, & Leroux, 2012; T. T. Yang et al., 2018). Thus, the transition zone separates the cell from the cilium, creating an exclusive ciliary environment (Figure 1).
Figure 1. Model of Ciliary Structure.
The cilium is a microtubule projection comprised of 9 microtubule doublets that make up the axoneme. The axoneme is anchored to the basal body, which resides in the cell. Trafficking into and out of cilia is regulated by the transition zone at the proximal end of the cilium. The transition zone is comprised of Y-shaped linker proteins, ciliary necklace membrane proteins, and distal and sub-distal appendages. Together, the transition zone acts as a diffusion barrier, preventing the free movement of proteins in to the cilium. Moreover, the transition zone regulates the active transport of proteins into and out of cilia. Finally, the transition zone provides structural support to the basal body and axoneme, anchoring the ciliary skeleton to the plasma membrane. Proteins are transported to the ciliary tip and back via anterograde and retrograde IFT, respectively. The ciliary membrane is contiguous to the cell’s plasma membrane but the ciliary membrane displays a distinct lipid composition being enriched for PI(4)P.
Role of Intraflagellar Transport (IFT)
Cilia do not contain the molecular machinery for protein translation and synthesis so depend on a transport mechanism called intraflagellar transport (IFT) for moving proteins to, within, and from cilia (Rosenbaum & Child, 1967). IFT refers to the transport process as well as to the class of proteins that mediate cargo transport. Initially described in the algae, Chlamydomonas reinhardtii, IFT is conserved in all ciliated species, including mammals (Kozminski, Johnson, Forscher, & Rosenbaum, 1993; G. J. Pazour et al., 2002; G. J. Pazour et al., 2000; Rosenbaum & Witman, 2002). A subcomplex of IFT proteins travels anterograde, to move ciliary cargos towards the distal tip of the cilium via a kinesin-2 motor, whereas a distinct subcomplex of IFT proteins treks retrograde, to return proteins from the tip using a dynein-2 motor (Kozminski, Beech, & Rosenbaum, 1995; G. J. Pazour, Wilkerson, & Witman, 1998). Therefore, IFT mediates the construction and maintenance of cilia (Kozminski et al., 1995; Kozminski et al., 1993). During ciliogenesis, α− and β−tubulin are added at the distal tip of the ciliary axoneme through IFT (Johnson & Rosenbaum, 1992). Disruptions in these processes lead to cilia anomalies. Cells lacking anterograde IFT lack cilia, whereas those lacking retrograde IFT display bulbous cilia due to a traffic jam of proteins trapped inside (Adams, Huang, & Luck, 1982; B. Huang, Rifkin, & Luck, 1977). When IFT stops, cilia shorten indicating IFT maintains cilia (Wallace F Marshall & Rosenbaum, 2001).
Sidebar
Sonic Hedgehog dependent patterning of the embryonic neural tube.
In the embryonic neural tube, SHH is expressed in the notochord (nc) and floorplate (fp) to specify the ventral cell fates of the embryonic neural tube. The concentration and duration of SHH is transformed into opposing gradients of GLI transcriptional activator (GliA) and repressor (GliR). Higher ratios of GliA: GliR ratios induce ventral fates expressing FOXA2, NKX2.2 and OLIG2 whereas high GliR production (absence of SHH signalling) permits dorsal cell fates expressing PAX6 and PAX7.
Vertebrate Hedgehog signalling requires cilia
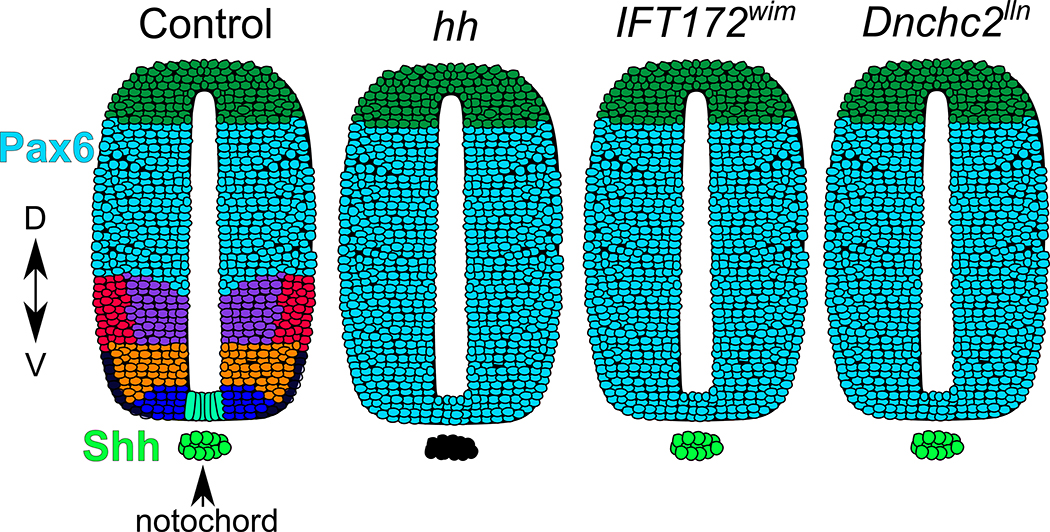
Cilia are essential to transduce HH signalling in vertebrates. Disruption of anterograde IFT in Ift172 null mice ablates cilia resulting in a loss of all ventral cell fates in the developing neural tube, a Sonic Hedgehog (SHH)-dependent process (Huangfu et al., 2003). The loss of ventral neural cell fates in Ift172 mutants phenocopies mutants lacking Shh, indicating IFT172 is required for SHH signalling (Chiang et al., 1996; Huangfu et al., 2003). Dnchc2lln mice, lacking a functional retrograde dynein motor display bulbous cilia due to their protein trafficking defects and also do not specify ventral neural cell fates (Huangfu & Anderson, 2005) (Figure 2). The simplest interpretation of defects in either anterograde or retrograde traffic leading to a loss of SHH response in the ventral neural tube is that the cilium itself is needed to transduce the pathway (Huangfu & Anderson, 2005; Huangfu et al., 2003).
Figure 2. IFT mutants, like Shh mutants, fail to specify ventral neural cell fates.
Ventral neural tube patterning (colored cell fates) is an in vivo readout of Sonic Hedgehog (SHH) pathway activation. Loss of Shh results in a complete loss of SHH-dependent ventral cell fates. Disruption of anterograde or retrograde IFT in Ift172wim and Dnchc2lln mutants results in a loss of SHH-dependent cell fates and ventral expansion of PAX6 (blue), which marks SHH-independent cell fates. As these mutants phenocopy Shh−/− mutants, the cilium itself is required for transduction of the SHH signalling pathway.
The complexity of the mechanisms linking cilia and SHH signal transduction are highlighted by mutations in different cilia proteins impacting signal transduction in distinct ways. Despite most retrograde mutants displaying no ventral neural cell fates, Ift122 and Ift139 mutants show bulbous cilia and increased SHH signalling within the neural tube (Cortellino et al., 2009; Tran et al., 2008). Different cilia proteins impact the pathway to varying degrees or in multiple ways. Loss of the cilia-associated GTPase RAB23 leads to a dramatic expansion of ventral fates whereas loss of KIF7 results in a modest expansion of ventral fates (Eggenschwiler, Bulgakov, Qin, Li, & Anderson, 2006; Eggenschwiler, Espinoza, & Anderson, 2001); (Liem, He, Ocbina, & Anderson, 2009). Another ciliary GTPase, ARL13B, appears to both positively and negatively regulate SHH signaling as its loss causes both loss of the most ventral cell fates and expansion of the OLIG2-expressing domain (Caspary, Larkins, & Anderson, 2007). Importantly, all these roles are conserved among vertebrates and cilia ablation in fish, frogs and other vertebrates leads to the vHH defects indicating that all vertebrates rely on cilia for HH signal transduction (Chung et al., 2014; Chung et al., 2012; P. Huang & Schier, 2009).
Vertebrate Hedgehog components localize to cilia
The regulatory logic of the HH pathway was first worked out in flies and is conserved in vertebrates (Goodrich, Johnson, Milenkovic, McMahon, & Scott, 1996; Hahn et al., 1996; Nusslein-Volhard & Wieschaus, 1980). For clarity, we will distinguish general HH signalling from vertebrate (vHH) and invertebrate (ivHH) HH signalling throughout this review. In the off state, the HH receptor Patched (PTCH1 in vertebrates) inhibits SMO, limiting its GPCR activity (Rohatgi, Milenkovic, & Scott, 2007). The Ci transcription factor (GLI proteins in vertebrates) is cleaved into a repressor form, which inhibits the transcription of target genes, a process mediated by COS2 (KIF7 in vertebrates) (Kalderon, 2004; S. Y. Tay, Ingham, & Roy, 2005; W. Zhang et al., 2005). When HH ligand is present, it binds the PTCH1 receptor, alleviating PTCH1 mediated repression of SMO (Stone et al., 1996). SMO is subsequently activated and full-length Ci, instead of being cleaved, is activated through posttranslational modification, which then turns on target genes (Aza-Blanc, Ramirez-Weber, Laget, Schwartz, & Kornberg, 1997). While this basic logic of the pathway remains conserved in vertebrates, there are unanswered questions. For instance, the mechanism through which PTCH1 inhibits SMO remains unknown. The two proteins do not interact directly and PTCH1’s inhibition on SMO is not stochiometric (Taipale, Cooper, Maiti, & Beachy, 2002). It is possible that PTCH1 shields SMO from its ligand or that PTCH1 removes or sequesters SMO’s ligand (Deshpande et al., 2019; Kowatsch, Woolley, Kinnebrew, Rohatgi, & Siebold, 2019; Qi et al., 2019; Y. Zhang et al., 2018). It is also not clear what the endogenous ligand for SMO is- or exactly how the receptor is activated. We will discuss the PTCH1-SMO-cilia axis later in this review. For an in-depth, multi-organism review of HH see (Briscoe & Therond, 2013).
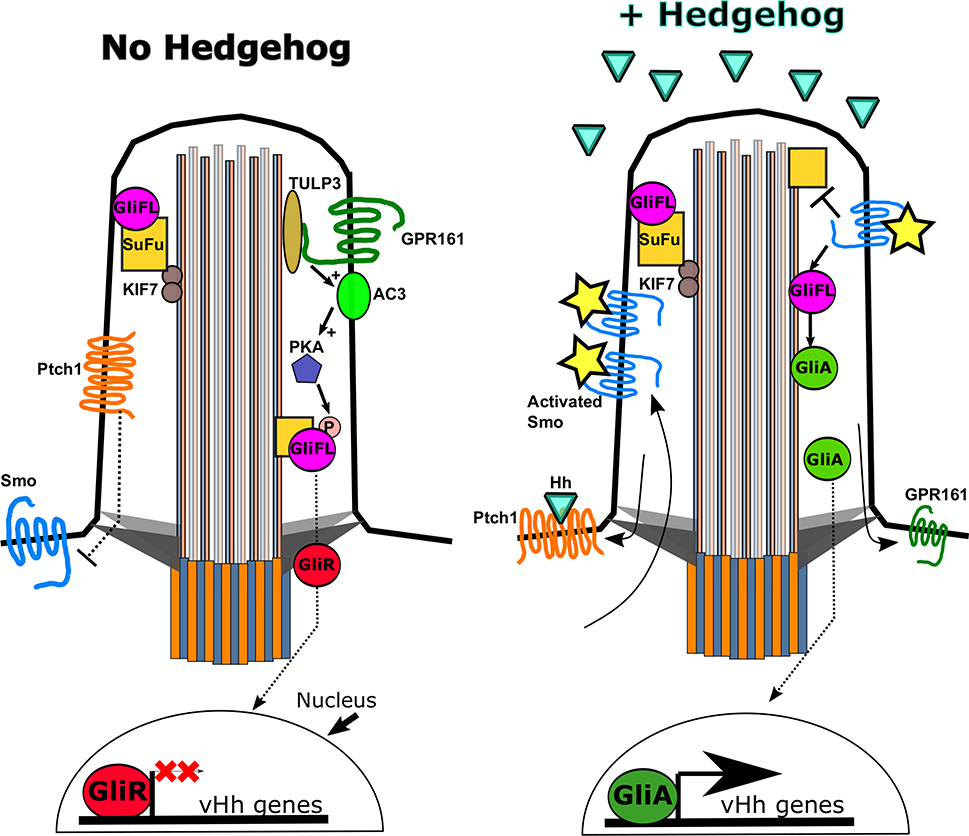
In vertebrates, the link between cilia and HH is reflected by the dynamic trafficking of the pathway’s core components in and out of cilia (Corbit et al., 2005; Haycraft et al., 2005; Rohatgi et al., 2007) (Figure 3). In the absence of HH, PTCH1 is visible along the ciliary membrane (Rohatgi et al., 2007). Upon stimulation with HH, ciliary PTCH1 becomes undetectable whereas SMO is visibly enriched in cilia (Corbit et al., 2005). This enrichment is necessary for subsequent activation of SMO (Rohatgi et al., 2009). As SMO enriches in cilia, GPR161, a GPCR and negative regulator of HH signalling exits cilia (Mukhopadhyay et al., 2013). Additional HH components regulate GLI transport and processing, mediating pathway activation. Kinesin family member 7 (KIF7) is responsible for moving GLI2 and GLI3 to the ciliary tip by IFT (Endoh-Yamagami et al., 2009; Haycraft et al., 2005; Liem et al., 2009; S. Y. Tay et al., 2005). The enrichment of the GLIs at the ciliary tip appears to be an essential step in SMO signalling to the GLIs, and thus for pathway activation (Haycraft et al., 2005; Liu, Wang, & Niswander, 2005).
Figure 3. The major components of the vertebrate Hedgehog pathway are dynamically localized to the primary cilium in response to Hedgehog ligand.
(Left) In the absence of vHH, the PTCH1 receptor is enriched in cilia, suppressing the ciliary enrichment and activation of SMO. KIF7 aids in shuttling the SUFU-GLI complex to the ciliary tip. Negative regulator GPR161 activates adenylyl cyclase 3 (AC3), facilitating PKA activation. PKA phosphorylates the full length GLI (GLIFL) which promotes the formation of GLI repressor (GLIR). GLIR translocates to the nucleus and suppresses vHH-dependent gene targets. (Right) vHH ligand binds PTCH1, which is subsequently removed from the cilium permitting ciliary SMO enrichment and activation (star). Activated SMO promotes the dissociation of the GLI-SUFU enabling activated GLI (GLIA) formation. GLIA translocates to the nucleus to promote HH-dependent gene transcription.
Processing of the 3 GLI proteins is complex. Distinct phosphorylation of the full length GLI proteins determines processing to an activator form or cleavage to a repressor form. That said, GLI1 lacks the cleavage site so functions solely as an activator. In the neural tube, GLI2 the predominant activator and GLI3 the prevalent repressor (Litingtung & Chiang, 2000; Matise, Epstein, Park, Platt, & Joyner, 1998). To process repressor in the absence of HH ligand, suppressor of fused (SUFU) forms an inhibitory complex with full-length GLI3 (Svard et al., 2006). The complex traffics out of the cilium where the kinases protein kinase A (PKA), GSK3β, and CKI phosphorylate full-length GLI3, to promote its cleavage to GLI3 repressor (GLIR) (Barzi, Berenguer, Menendez, Alvarez-Rodriguez, & Pons, 2010; Fumoto, Hoogenraad, & Kikuchi, 2006; Hammerschmidt, Bitgood, & McMahon, 1996; Tuson, He, & Anderson, 2011). To generate GLI2 activator when ligand is present, activated SMO promotes dissociation of the GLI2-SUFU complex and phosphorylation of full-length GLI2 to its activator form (GLIA) at which point it is shuttled out of the cilium (Humke, Dorn, Milenkovic, Scott, & Rohatgi, 2010; Kim, Kato, & Beachy, 2009; Tukachinsky, Lopez, & Salic, 2010; Wen et al., 2010). GLIA and GLIR translocate to the nucleus to influence HH-dependent gene targets.
Other HH pathway regulators are enriched within the cilium. TULP3 acts a negative regulator of the HH pathway and localizes with the ciliary tip (Mukhopadhyay et al., 2010; Norman et al., 2009). Loss of TULP3 causes a failure of cilia proteins ARL13B and INPP5E to localize with the cilium (Han et al., 2019). Both ARL13B and INPP5E are known for their influence over vHH pathway components (Caspary et al., 2007; Chavez et al., 2015; Constable, Long, Floyd, Schurmans, & Caspary, 2019; Garcia-Gonzalo et al., 2015; Larkins, Aviles, East, Kahn, & Caspary, 2011). Adenylyl cyclases (AC) are responsible for generating cyclic AMP (cAMP), the substrate required to activate protein kinase A (PKA), a negative HH regulator (Hammerschmidt et al., 1996). TULP3 is also responsible for recruiting the GPCR GPR161 to the cilium. GPR161 is coupled to stimulatory G-proteins that activate AC3 to increase cAMP levels (Bishop, Berbari, Lewis, & Mykytyn, 2007). Therefore, GPR161 acts as a negative regulator on vHH signalling through PKA. (Mukhopadhyay et al., 2013). GPR161 resides in cilia when the pathway is inactive. Ciliary SMO enrichment upon vHH activation promotes the removal of GPR161, and its inhibitory influence on the pathway, from cilia (Corbit et al., 2005; Mukhopadhyay et al., 2013; Pal et al., 2016). Curiously, BOC, GAS1, and CDO are well-established coreceptors with PTCH1 for the HH ligand, but have yet to be observed in cilia (Allen et al., 2011; Izzi et al., 2011).
In mutants lacking cilia or SHH, no ventral neural cell fates are specified because the relevant target genes are not induced. However, mutants lacking cilia die around E11.5 whereas those with no vHH signalling (Smo−/−) die at E9.0 (Caspary et al., 2002; Kasarskis, Manova, & Anderson, 1998; X. M. Zhang, Ramalho-Santos, & McMahon, 2001). This highlights the distinct mechanisms underlying the absence of SHH-signalling dependent gene transcription when cilia are lost (Chiang et al., 1996; Huangfu et al., 2003). Loss of cilia ablates the formation of both GLI activator (GLIA) and GLI repressor (GLIR), so that target genes are neither activated nor repressed (Huangfu & Anderson, 2005; Liu et al., 2005). In contrast, Smo single or Shh/Ihh double mutants only produce GliR so that target genes are repressed (Kasarskis et al., 1998; X. M. Zhang et al., 2001). Thus, Smo mutants display a more severe phenotype as they repress targets in non-neural tube tissue that are not repressed in the absence of cilia alone.
The ciliary membrane
The ciliary membrane is contiguous with the cell’s plasma membrane, yet the distinct lipid composition of the ciliary membrane provides a specialized environment for signalling pathways. Phospholipids comprise the most abundant lipid on the plasma and ciliary membranes (Saarikangas, Zhao, & Lappalainen, 2010). The composition of the ciliary membrane is particularly enriched for phosphoinositol-4- phosphate (PI(4)P) compared to the plasma membrane. This distinction is mediated, in part, by the inositol polyphosphate −5-phosphatase, INPP5E. INPP5E is trafficked to cilia in a complex containing the regulatory GTPase ARL13B and the phosphodiesterase PDE6D (Humbert et al., 2012; Nozaki et al., 2017). Defects in proteins critical to the transition zone result in INPP5E not localizing to cilia, causing changes to the ciliary membrane phospholipid composition (Garcia-Gonzalo et al., 2015; Slaats et al., 2016).
INPP5E is enriched in cilia and removes the 5-phosphate from PI(3,4,5)P3 (preferred substrate) and PI(4,5)P2 (hereafter called PIP3 and PIP2 respectively) (Kisseleva, Wilson, & Majerus, 2000). Loss of Inpp5e results in a loss of ciliary PI(4)P and an increase in ciliary PIP2 (Bielas et al., 2009; Chavez et al., 2015; Garcia-Gonzalo et al., 2015; Jacoby et al., 2009). This causes PIP2 and PIP3 substrates to accumulate at the transition zone, disrupting the organization of transition zone scaffolding proteins. These data suggest that PIP composition may be critical to regulating protein entry and/or exit at the transition zone (Dyson et al., 2017). Moreover, TULP3 is a PIP2 binding protein so increased ciliary PIP2 promotes TULP3 and its associated GPR161 to localize within cilia (Badgandi, Hwang, Shimada, Loriot, & Mukhopadhyay, 2017; Garcia-Gonzalo et al., 2015). In vitro, this results in a dampened vHH response (Chavez et al., 2015; Garcia-Gonzalo et al., 2015). In vivo, Inpp5e mutants display increased SHH response in the caudal neural tube which corrects over time (Constable et al., 2019). It is unclear whether these differences are due to tissue-specific context, the duration of signalling or another factor. Nonetheless, the biochemical composition of the ciliary membrane is critical for vHH signal transduction.
Sidebar
Ciliopathies and cilia
Cilia biology benefits enormously from human genetics and visa-versa. Indeed, several of the major protein complexes in cilia are named due to the disease through which the relevant proteins got identified. These include the BBSome, named for Bardet-Biedl syndrome; the NPHP complex derived from nephronophthisis, the MKS complex which originated from Meckel-Gruber syndrome. As complete ablation of cilia is embryonic lethal in mammals, ciliopathies are often caused by missense mutations that create hypomorphic alleles (Reiter & Leroux, 2017; Waters & Beales, 2011). In addition to such disease-causing mutations being hugely informative to understand the diverse and specific functions of cilia proteins, ciliopathies present an interesting lack of genotype-phenotype correlation. In families in which distinct ciliopathy patients carry the same mutation, they can display quite different phenotypes. Oligogenicity was proposed to play a role but whole genome sequencing revealed that ciliopathies are Mendelian disorders with autosomal recessive mutations driving the disease (Shaheen et al., 2016). The variation in penetrance is likely due to modifiers. Thus, cilia biology and cilia-associated disease inform one another.
Evolution of the Hedgehog pathway and cilia
The initial link between vHH signalling and cilia surprised the field primarily because ivHH signalling was well studied in Drosophila, where the pathway was identified (Nusslein-Volhard & Wieschaus, 1980). While flies do have cilia, subsequent studies in fly IFT mutants did not reveal any defects in ivHH signalling. Planaria (Schmidtea mediterranea) possess motile cilia and HH signalling but they are not functionally linked (Rink, Gurley, Elliott, & Sanchez Alvarado, 2009). In evolutionary terms, the earliest link between cilia and HH is in sea urchin which use motile cilia to transduce HH signalling in developing muscle tissue (Walton, Warner, Hertzler, & McClay, 2009; Warner, McCarthy, Morris, & McClay, 2014). Subsequently, within deuterostomes, all members within the vertebrate lineage require primary cilia for HH signalling (Glazer et al., 2010; P. Huang & Schier, 2009; T. J. Park, Haigo, & Wallingford, 2006; Shang Yew Tay et al., 2010). It remains unclear whether vertebrates use motile cilia, in addition to primary cilia, for HH signalling.
In addition to testing individual organisms for functional links of HH and cilia, several clues come from examining when proteins that link the processes evolved. IFT25 and IFT27 regulate vHH but are unique among IFT components as they are lost in C. elegans and Drosophila, suggesting they may not play roles in cilia. Mice without Ift25, ciliate normally, but die shortly after birth with phenotypes consistent with low HH signalling (Keady et al., 2012). Ift27 mouse mutants display similar phenotypes. IFT25 and IFT27 are required for the export of SMO and PTCH1 out of cilia so may have evolved specifically to traffic HH components (Eguether et al., 2014; Liew et al., 2014). ARL13B also regulates the ciliary traffic of HH components in vertebrates (Larkins et al., 2011). ARL13 is an ancient protein that is lost in species lacking cilia. In species lacking HH signalling but that possess cilia such as C. elegans and C. reinhardtii, ARL13 mutants display cilia defects. Mouse Arl13b mutants also show cilia defects indicating ARL13/ARL13B has a conserved role in ciliary function (Cevik et al., 2010; Cevik et al., 2013; Gotthardt et al., 2015; Miertzschke, Koerner, Spoerner, & Wittinghofer, 2014; Warburton-Pitt, Silva, Nguyen, Hall, & Barr, 2014). ARL13 duplicated to ARL13A and ARL13B in the urochordates, about the time in the deuterostome lineage that HH became functionally linked to primary cilia (East, Bowzard, Dacks, & Kahn, 2012; Kahn et al., 2008; Li et al., 2004; Logsdon & Kahn, 2004; Schlacht, Mowbrey, Elias, Kahn, & Dacks, 2013). Thus, ARL13B may be critical to the mechanism that solidified HH signalling using the cilia within the deuterostomes. In fact, a cilia-excluded form of ARL13B is sufficient to mediate vertebrate HH signalling, suggesting that ARL13B’s function may solely be to traffic vHH components to the cilium (Gigante, Taylor, Ivanova, Kahn, & Caspary, 2019).
As the HH pathway evolved to depend on the cilium, components of the HH signalling pathway also evolved. Vertebrates have two HH ligands in addition to SHH: Desert (DHH) and Indian (IHH), which play tissue-specific roles. DHH is limited to germ cell differentiation (Bitgood, Shen, & McMahon, 1996). IHH is best known for roles in skeletal development (St-Jacques, Hammerschmidt, & McMahon, 1999). Similarly, the transcriptional mediator, Ci expanded to GLI1, GLI2 and GLI3 in vertebrates (Alexandre, Jacinto, & Ingham, 1996; Hui, Slusarski, Platt, Holmgren, & Joyner, 1994; Litingtung & Chiang, 2000; Matise et al., 1998; H. Park et al., 2000). Components such as FUSED and SUFU exist in invertebrates and vertebrates but their functional importance is swapped as they evolved (Cooper et al., 2005; Ruel, Rodriguez, Gallet, Lavenant-Staccini, & Thérond, 2003; J Svard, 2006). KIF7 seems to carry out similar function in vertebrates to Costal-2 in invertebrates despite their poor sequence homology (Farzan et al., 2008; Liem et al., 2009). The exception is SMO, relatively unchanged; it is the sole, obligate transducer of the HH pathway in both invertebrate and vertebrate HH signalling (Alcedo, Ayzenzon, Von Ohlen, Noll, & Hooper, 1996). (See (Briscoe & Therond, 2013) for a comparison of vHH and ivHH components).
G-Protein coupled receptor, SMOOTHENED.
SMO is a member of the Frizzled (F-class) GPCR family. F-class GPCRs are classic seven transmembrane GPCRs, uniquely defined by their large extracellular N-terminal cysteine rich domain (CRD) (Alcedo et al., 1996). In FRIZZLED (FZD) receptors, this domain readily associates with the WNT ligand (Dann et al., 2001; Janda, Waghray, Levin, Thomas, & Garcia, 2012). Despite sharing a receptor class with FZD receptors SMO does not directly bind to the HH ligand. Instead, HH binds PTCH1 which removes PTCH1 from inhibiting SMO and permits SMO activation (Taipale et al., 2002). Currently, the endogenous ligand responsible for SMO activation is unknown, leading SMO to be classified as an orphan receptor.
Smoothened has the structural hallmarks of a GPCR
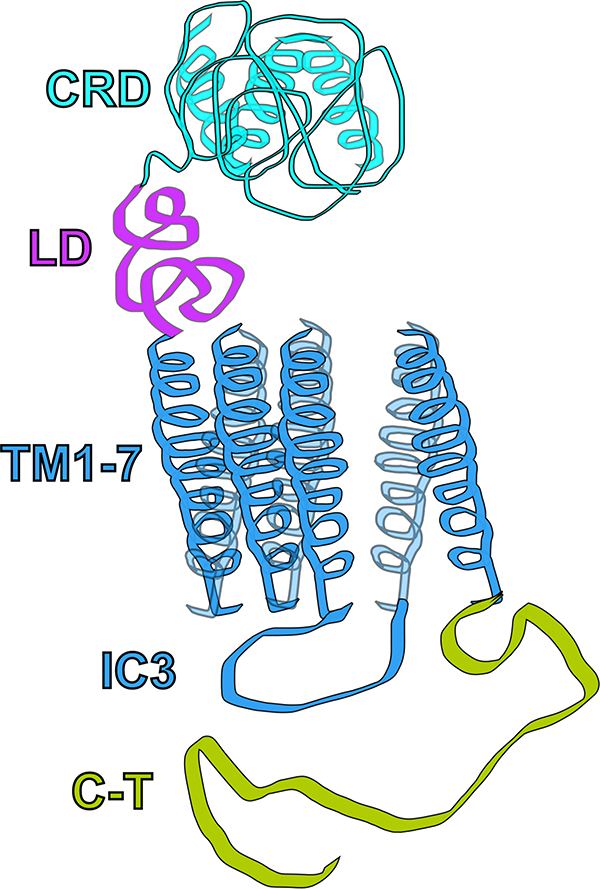
There are over 800 different GPCR genes, of which more than half encode olfactory GPCRs and others are critical for vision and taste (Takeda, Kadowaki, Haga, Takaesu, & Mitaku, 2002). GPCRs are typically integrated in the plasma membrane and stimulated by binding of an extracellular ligand. SMO contains all the hallmarks of a GPCR. It has an N-terminal extracellular domain, 7 transmembrane α−helices, and a C-terminal intracellular domain (Figure 4). Unique to SMO and other F-class receptors is the large N-terminal cysteine rich domain (CRD) that is connected to the transmembrane domains by a smaller linker domain. Like all GPCRs, SMO is thought to undergo a conformational change when activated. This conformational change alters the orientation of the CRD relative to the transmembrane domains through a bend in the linker domain (Byrne et al., 2016; P. Huang et al., 2018; Zhao, Tong, & Jiang, 2007). The CRD plays an essential role in the activation of SMO and may be involved in the translocation and enrichment of SMO in cilia (Aanstad et al., 2009; Myers, Neahring, Zhang, Roberts, & Beachy, 2017).
Figure 4. G-protein coupled receptor Smoothened.
Smoothened is an F-class G-protein coupled receptor (GPCR) defined by the N-terminal cysteine rich domain (CRD) containing small α-helices and ligand binding domains. A linker domain (LD) connects the CRD with the 7 transmembrane (TM1–7) domain of the receptor. The intracellular loop 3 (IC3) associates with intracellular Gαi G-proteins that are activated following the conformational change. The intracellular C-terminal (C-T) tail is critical for SMO transport and enrichment in cilia. Mutations to the C-T affect ciliary SMO traffic (Y. Chen et al., 2011; Corbit et al., 2005).
Conformational changes can promote the dissociation of intracellular G-proteins for signal transduction. Inactive GPCRs associate with intracellular G-proteins, and it is through these heterotrimeric complexes that the receptors influence intracellular pathways. The heterotrimeric complex can be separated into three G-protein subunit families Gα, Gβ, and Gγ. Together the heterotrimeric complex, Gαβγ, binds the intracellular loops of GPCRs. In an inhibitory state, the Gβγ complex serves to directly inhibit the Gα subunit (Lambright et al., 1996). When a GPCR is bound by an agonist and activated, the receptor undergoes a conformational shift that promotes the dissociation of the G-protein complex from the receptor. After which, the Gα subunit separates from the βγ subunits through the exchange of GDP for GTP. The dissociation of the Gα subunit from the Gβγ subunit frees both to influence downstream signalling in second messenger pathways. The Gα proteins can be sub-divided into four families, each with different signalling properties. To overcome the homology among the Gα subunits, individual GPCRs are selective of which G-proteins they bind to for their appropriate signal transduction (Flock et al., 2017; Van Eps et al., 2018). This allows them to stimulate molecules, which affect adenylyl cyclases, phospholipases, and phosphokinases, in turn activating second messenger pathways such as cyclic AMP (cAMP), PIP3, arachidonic acid, and calcium.
It is not yet clear which Gα subunit(s) associate with SMO. There are data that SMO binds Gαi-inhibitory G-proteins, and does not bind to Gαs or Gα13 (DeCamp, Thompson, de Sauvage, & Lerner, 2000; Ogden et al., 2008; Polizio, Chinchilla, Chen, Manning, & Riobo, 2011; Qi et al., 2019; Riobo, Saucy, Dilizio, & Manning, 2006). Yet, GLI activity is influenced by Gαs, Gα13, and Gαi suggesting additional GPCRs that function through Gαs and Gα13 must be capable of modulating vHH responses in cilia (Barzi, Kostrz, Menendez, & Pons, 2011; Douglas et al., 2011; Rao, Salloum, Xin, & Lu, 2016). One candidate is GPR161, a cilia-associated Gαs-coupled GPCR, which negatively regulates HH signalling (Mukhopadhyay et al., 2013). While the details of SMO’s G-protein coupling remains uncertain, SMO-Gα fusion proteins are now a useful tool to for assaying SMO’s conformational state and thus activation indicating that SMO functions like other GPCRs (Myers et al., 2017).
Exogenous ligands inform our understanding of Smo mechanism
SMO is well studied as a drug target for cancer therapy purposes, therefore pharmacological compounds that alter its activity are informative in revealing mechanisms important for SMO activation (J. K. Chen, Taipale, Cooper, & Beachy, 2002; J. K. Chen, Taipale, Young, Maiti, & Beachy, 2002; Rohatgi et al., 2009). The caveat of these drugs is that they do not activate SMO through the endogenous mechanism, which involves the removal of PTCH1 mediated suppression by HH ligand. SMO, like other GPCRs, contains a heptahelical transmembrane domain to which several exogenous compounds are known to bind and either activate or inhibit SMO function. Two antagonists, SANT-1 and cyclopamine, block SHH-induced activation of SMO by binding in the ligand binding pocket of the heptahelical transmembrane domain and preventing activating conformational changes in SMO (J. K. Chen, Taipale, Cooper, et al., 2002; J. K. Chen, Taipale, Young, et al., 2002; Rohatgi et al., 2009). While both ligands antagonize SMO activation, SMO localization is distinctly modified under the two conditions. SANT1 prevents SMO ciliary enrichment whereas cyclopamine induces SMO ciliary enrichment (J. K. Chen, Taipale, Cooper, et al., 2002; Frank-Kamenetsky et al., 2002). These data indicate that ciliary SMO enrichment is not synonymous with SMO activation and demonstrate that the trafficking of SMO to cilia is distinct from its activation (Rohatgi et al., 2009).
There is further support for a model wherein SMO activation and SMO enrichment are separate, but linked processes. Smoothened agonist (SAG) binds the same ligand-binding pocket as the antagonists and promotes the conformational change necessary for SMO activation. SAG treatment results in ciliary SMO enrichment (J. K. Chen, Taipale, Young, et al., 2002). Moreover, an activated variant of SMO identified in basal cell carcinoma, SMOW539L, is highly enriched in cilia and results in high levels of HH response (Corbit et al., 2005; Taipale et al., 2000; Xie et al., 1998). This constitutively active form of SMO requires cilia to activate the SHH pathway (Ocbina & Anderson, 2008). Despite its constitutively active status, SMOW539L still requires normal cilia to activate the HH pathway. Indeed, disruption of PKA mediated SMO-Inversin associations at the base of the cilium is sufficient to block SMOW539L constitutive activation of the HH pathway (B. Zhang et al., 2019). Together, these data support a model wherein SMO is fully activated within cilia and that ciliary enrichment is necessary, but not sufficient, for SMO activation indicating that the process of SMO transport to cilia and its activation are mechanistically distinct (Rohatgi et al., 2009; Wilson, Chen, & Chuang, 2009).
Additional Ciliary GPCRs
SMO is one of many GPCRs demonstrated to enrich in primary cilia. Rhodopsin in the photoreceptor cells of the retina is the best-known example. This light-sensitive GPCR is found in disc-like membranes within the modified cilium of the rod cell (Jan & Revel, 1974; Röhlich, 1975). Olfactory receptors are uniquely regulated so that only one variety of receptor is expressed in any one olfactory neuron. Specific odorants activate the olfactory receptors to give a sense of smell (Buck & Axel, 1991; Chess, Simon, Cedar, & Axel, 1994). A host of additional GPCRs are also localized to primary cilia including: somatostatin receptor 3 (SSTR3), melanin-concentrating hormone receptor 1 (MCHR1), serotonin receptor 6 (5HT6), dopamine 1 receptor 1 (D1R), neuropeptide Y receptor 2 (NPY2R), kisspeptin receptor 1 (KISS1R) and GPR161 (Berbari, Lewis, Bishop, Askwith, & Mykytyn, 2008; Brailov et al., 2000; Domire et al., 2011; Hamon et al., 1999; Handel et al., 1999; Loktev & Jackson, 2013; Marley & von Zastrow, 2010; Mukhopadhyay et al., 2013; Schulz, Handel, Schreff, Schmidt, & Hollt, 2000; Shimada, Tritos, Lowell, Flier, & Maratos-Flier, 1998). Adenylyl cyclases, the GPCR signal transducers, are also found in cilia and are expressed in a tissue-specific manner. For example, neuronal cilia express adenylyl cyclase (AC3), whereas AC6 and AC5 are more common in bone and kidney, respectively(Bishop et al., 2007; Kwon, Temiyasathit, Tummala, Quah, & Jacobs, 2010; Q. Wang et al., 2018). While the distinct ligands contribute to the functional differences among ciliary GPCRs they also work via distinct second messenger cascades. For instance, MCHR1 and KISS1R are peptide receptors that influence neurotransmission and endocrine signalling (Berbari, Lewis, et al., 2008; Kotani et al., 2001; Yasuda et al., 1992). These receptors can signal through Gαq coupling, influencing both Ca2+ mobilization and phosphatidylinositol turnover (Fry et al., 2006; Kotani et al., 2001). In contrast, GPR161, 5HT6, and SSTR3 influence intracellular levels of cAMP, modulating PKA and its downstream targets (Law, Yasuda, Bell, & Reisine, 1993; Mukhopadhyay et al., 2013; Ruat et al., 1993). Importantly, several of these ciliary GPCRs change localization upon stimulation (Green et al., 2016; Pal et al., 2016; Ye, Nager, & Nachury, 2018).
GPCR localization in the cell and in cilia.
The compartmentalization and organization of GPCRs is an integral mechanism through which a cell can ensure the proper regulation of cell-specific functions. Cells control their responsiveness to endogenous ligands through the spatiotemporal regulation of GPCR expression and localization. A good example is the neural synapse, where GPCRs are concentrated to ensure response to an extracellular ligand. Activation of a neuron can trigger membrane remodelling by increasing or decreasing the presence of receptors in the membrane. For example, G-protein kinases phosphorylate open conformation GPCRs, allowing β-arrestin-mediated endocytosis through clathrin-coated pits (M. J. Lohse et al., 1992; Martin J Lohse, Benovic, Codina, Caron, & Lefkowitz, 1990). These endocytosed GPCRs are either returned to the intracellular pool of receptors or downregulated through lysosomal degradation. This is an effective means through which cells can regulate hyperactive signalling cascades (Eichel & von Zastrow, 2018).
Cilia as a specialized environment for GPCR signalling cascades
As a small compartment, the cilium is in many ways an ideal environment for GPCR signalling. Ciliary enrichment of GPCRs can be sensitive to pathway stimulation, which can ensure receptor availability-, or absence-, when needed. Given the high concentration of signalling molecules and small volume of the cilium, it likely requires much less G-protein activation to influence second messenger pathways (Nachury, 2014). Moreover, due to the compartmentalization of the cilium, GPCR-dependent signalling can locally influence changes in downstream pathways via cAMP and Ca2+ alterations within cilia.
GPCRs are well known to modulate intracellular cAMP levels through adenylyl cyclases. Within the cilium, GPCRs regulate AC3 through either activating or inhibiting mechanisms. By molecularly targeting cAMP reporters to cilia, several groups detect cAMP changes exclusively within cilia suggesting that ciliary cAMP changes are unable to influence cellular levels of cAMP (Marley et al 2013). This raises the potential that one function of cilia is to enhance the concentration and spatial localization of signalling cascades to either increase GPCR signalling efficiency or output (Nachury, 2014). It remains unclear whether cAMP in cilia is evenly distributed or is restricted into subcompartments within the cilium. There is crosstalk between some GPCRs that may suggest that cAMP levels are uniform within the cilium. For example, activation of SMO by SAG can attenuate the cAMP response via MCH receptors bound to MCH ligand (Bansal et al., 2019). This may be due to competition of G-proteins, one inhibitory and one excitatory, converging on cAMP production. More sensitive, cilia-specific biosensors to measure cAMP levels in cilia will aid in understanding the specificity and kinetics of GPCR-influenced fluctuations in ciliary cAMP levels (Mukherjee et al., 2016).
GPCR-dependent signalling also influences Ca2+ levels within cilia. Ciliary fluctuations in Ca2+ were first linked to defects in C. elegans carrying mutations in polycystin-1 and polycystin-2 (PC1 and PC2) (Barr & Sternberg, 1999). PC1 and PC2 are ciliary mechanosensory channels that facilitate Ca2+ entry into cilia in response to mechanic movement of the cilium (Nauli et al., 2003). In Chlamydomonas, Ca2+ levels in flagella fluctuate in response to mechanical stimuli, influencing the movement of IFT cargos (Collingridge, Brownlee, & Wheeler, 2013). In mice, mutations to Ift88 cause polycystic kidney disease and postnatal lethality (G. J. Pazour et al., 2000). Mutant Ift88 mice have short kidney cilia and abnormal increases in ciliary PC2 levels, resulting in changes to ciliary Ca2+ flux (G. J. Pazour et al., 2000; Gregory J Pazour, San Agustin, Follit, Rosenbaum, & Witman, 2002). In fact, almost any disruption to kidney cilia or IFT causes kidney disease (Jonassen, San Agustin, Follit, & Pazour, 2008; Ma, Tian, Igarashi, Pazour, & Somlo, 2013). Because of the low volume of the cilium compared to the volume of the cell body, small fluctuations in Ca2+ are likely sufficient for influencing downstream processes.
As with cAMP, several methods can detect Ca2+ flux within the cilium even though the cellular Ca2+ level in unchanged. For example, using in vivo calcium sensors, a Ca2+ flux can be observed within the cilia of the embryonic left-right organizer (LRO) that is distinct from Ca2+ flux in the cells of the LRO. PC2 receptors localize to these sensory cilia, suggesting a role of Ca2+ flux in the establishment of laterality (McGrath, Somlo, Makova, Tian, & Brueckner, 2003; Muller et al., 2012; Sakuma et al., 2002; Yoshiba et al., 2012; Yuan, Zhao, Brueckner, & Sun, 2015). In kidney epithelial cells, cilia sense fluid flow and activate Ca2+ influx that can be measured in the cell (X. Jin et al., 2014; Nauli et al., 2003; Su et al., 2013). This change in calcium is dependent on PC2-mediated calcium flow (X. Jin et al., 2014). While the roles of Ca2+ and cAMP in signalling pathways vary across tissues, advances in signalling cascade detection will help shed light on these differences (Lee et al., 2015).
Ciliary GPCR trafficking
Cilia GPCRs SSTR3, 5HT6, and MCHR1 all share a cilia localization sequence on their intracellular loops that is distinct from that in SMO (Berbari, Johnson, Lewis, Askwith, & Mykytyn, 2008; Corbit et al., 2005). The SSTR3, 5HT6, and MCHR1 receptors contain an AXXXQ targeting sequence on the intracellular loops that confers cilia localization. In contrast, the SMO localization sequence WR is found on the C-terminus. Moreover, these GPCR specific sequences differ from those of other cilia proteins. For example, VxPx motifs are necessary for ciliary enrichment of some proteins but dispensable for other cilia proteins containing the same motif (Geng et al., 2006; Mariani et al., 2016). However, a distinct cilia localization sequence in fibrocystin is sufficient to drive ciliary enrichment of GFP (Follit, Li, Vucica, & Pazour, 2010). The variety of cilia localization sequences suggest there are multiple pathways into the cilium, each using distinct machinery. Consistent with GPCRs using distinct trafficking mechanisms, TULP3 works with IFT to promote the trafficking of SSTR3, NPY2R and MCHR1 to cilia but does not affect SMO transport. (Mukhopadhyay et al., 2010). Studying the ciliary mechanisms that regulate GPCRs beyond SMO will shed new light on trafficking dynamics in cilia entry, exit, and retention of GPCRs. Moreover, because GPCRs serve to activate specific signalling cascades, careful focus should be paid to study cilia-GPCR dynamics in relevant contexts.
The BBSome is an octameric protein complex critical for ciliary trafficking of membrane proteins, including GPCRs. The BBSome interacts with IFT, expanding the ability of IFT to transport distinct cargo (K. F. Lechtreck et al., 2009). The BBSome recognizes the activated GPCRs through specific intracellular sequences that are revealed when the receptor adopts an active conformation (H. Jin et al., 2010; Klink et al., 2017). Therefore, the BBSome is responsible for binding and trafficking activated GPCRs across the transition zone (Ye et al., 2018). In fact, activated GPR161 and SSTR3 both require β-arrestin and BBSome-mediated transport across the transition zone for proper ciliary exit (Green et al., 2016; Pal et al., 2016; Ye et al., 2018). This is of critical importance to vHH signalling because GPR161 exits the cilium upon vHH stimulation as SMO enriches in cilia (Pal et al., 2016). Activated SMO exits cilia through ARL6-BBSome mediated transport, shuttling SMO to cross the barrier while still integrated in the plasma membrane (H. Jin et al., 2010; Nachury et al., 2007; Ye et al., 2018). Therefore, without proper trafficking components, GPCRs will linger in the cilium.
Ciliary Smo Trafficking
SMO transport into and out of cilia is highly regulated as illustrated by its mislocalization in numerous mutants including those involved in IFT, BBSome-trafficking and the transition zone. In mutants with defective retrograde cilia traffic, SMO visibly accumulates within cilia indicting that it normally exits via retrograde IFT (May et al., 2005; Ocbina & Anderson, 2008). When retrograde mutations are combined with hypomorphic mutations in anterograde IFT, slightly less ciliary SMO accumulates than in the single retrograde mutants consistent with SMO being trafficked within the cilium via IFT (Ocbina & Anderson, 2008). The unconventional IFT25 subunit facilitates SMO and Ptch export from cilia and facilitates GLI2 enrichment at the ciliary tip (Keady et al., 2012). Mutations in RPGRIP1L, which regulates transition zone structure, display a lack of SMO accumulation arguing that the integrity of the transition zone is needed for SMO ciliary entry or residency (Shi et al., 2017). In Arl13b and Bbs mutants, SMO accumulates in cilia in a ligand-independent manner, likely due to defective ciliary exit (Eguether et al., 2014; Larkins et al., 2011; Q. Zhang et al., 2011). The fact that mutations in proteins that control diverse aspects of cilia biology all show abnormal SMO enrichment reflects the multiple levels at which its trafficking is likely regulated.
In principle, the trafficking of SMO to cilia reflects the fact that GPCRs reside near the membrane at which they function, usually in vesicular pools, awaiting changes in membrane organization before localizing there. Indeed, by tagging SMO in live cells and looking at a subsequent timepoint, one group surmised that most SMO travels to the cilium in vesicles from the Golgi via IFT (Y. Wang, Zhou, Walsh, & McMahon, 2009). However, using a distinct tagging method, another group concluded that SMO is transported from the Golgi to the cell plasma membrane and then moves through lateral transport into the ciliary membrane (Milenkovic, Scott, & Rohatgi, 2009). They found SMO at the cell membrane turned over quickly and may have not been detected in the first study looking at a single timepoint. These data indicate at least one pool of SMO is available on the plasma membrane for cilia localization and subsequent activation.
Smoothened ciliary retention.
Once inside the cilium, SMO localization and retention depends on chaperone proteins that bind the SMO C-terminus. This region interacts with G protein-coupled receptor associated sorting protein 2 (GPRASP2) to assist with ciliary targeting (Jung et al., 2016), and Ellis-Van Creveld protein (EVC/EVC2) to promote interaction with the vHH pathway component SUFU (Dorn, Hughes, & Rohatgi, 2012; C. Yang, Chen, Chen, & Jiang, 2012). Moreover, SMO retention and SHH signalling is enhanced by the phospholipase A2, arachidonic acid pathway (Arensdorf et al., 2017). The necessity of chaperone proteins agrees with the idea of a gating function at the base of the cilium, likely mediated by the transition zone. The SMO C-terminus contains a cilia localization sequence that aids with targeting to cilia and ciliary retention (Corbit et al., 2005). Enrichment of the SMOW539L receptor corresponds with phosphorylation of the SMO C-terminus by kinases CK1α and GRK2 (Y. Chen et al., 2011).
The ciliary enrichment of an activated GPCR requires the receptor bypass the mechanisms that remove it from the cilium. The BBSome is known to promote the movement of MCHR1 and SSTR3 receptors across the transition zone and out of cilia (Berbari, Lewis, et al., 2008; H. Jin et al., 2010; Nachury et al., 2007; Ye et al., 2018). To achieve this, the BBSome recruits β-arrestin to facilitate the binding of activated GPCRs (Ye et al., 2018). This agrees with our understanding of β-arrestin function, that it preferentially binds activated GPCRs to facilitate their endocytosis, this includes activated SMO (W. Chen et al., 2004; Latorraca et al., 2018). Perhaps the cilium and BBSome have co-opted this endocytic pathway to shuttle activated receptors out of cilia (Green et al., 2016; Kovacs et al., 2008; Pal et al., 2016). An alternative exit strategy of activated GPCRs, including SMO, is exocytosis from the ciliary tip (Nager et al., 2017). Because GPCRs undergo conformational changes when activated, perhaps there are multiple pathways for moving a GPCR in and out of cilia, depending on the receptor conformation.
Endogenous activation of SMO
SMO is an orphan receptor as its endogenous ligand is yet to be identified. However, important clues emerge from the fact that SMO can be activated by synthetic oxysterol molecules that associate with the N-terminal cysteine rich domain (CRD) (Corcoran & Scott, 2006; Dwyer et al., 2007; Nachtergaele et al., 2012; Yavari et al., 2010). In fact, membrane-associated cholesterol, an abundant and endogenous sterol, binds to the SMO CRD and moderately activates the SHH pathway in cell lines (Byrne et al., 2016; P. Huang et al., 2016; P. Huang et al., 2018; Luchetti et al., 2016; Myers et al., 2013). Therefore, lowered cholesterol abundance can suppress SMO activation (Blassberg, Macrae, Briscoe, & Jacob, 2016). The SMO CRD contains a sterol-binding domain and the D99 residue is covalently modified by cholesterol. Mutating this aspartic acid residue to asparagine blocks cholesterol binding. SmoD99N/D99N embryos phenocopy Smo−/− embryos indicating that the residue is essential for SMO activation (Caspary et al., 2002; Kasarskis et al., 1998; Xiao et al., 2017; X. M. Zhang et al., 2001). Additionally, Smith-Lemli-Opitz patients carry mutations in the dehydrocholesterol reductase DHCR7 resulting in decreased freely available cholesterol and present with morphological abnormalities consistent with deficient SHH signalling (Blassberg et al., 2016).
Ligand binding alters GPCR conformation into either an activated or inhibited state (Zhao et al., 2007). When cholesterol binds to the SMO CRD, the receptor adopts an activated conformation in which the CRD shifts via an intermediate linker domain to form a new association with the SMO transmembrane domains (Byrne et al., 2016; Nachtergaele et al., 2013; Zhao et al., 2007). A mutation in the SMO linker domain is associated with decreased SHH signalling, suggesting dysfunction in SMO active conformation (Gigante, Long, Ben-Ami, & Caspary, 2018). That said, the CRD is not the only SMO sterol-binding site. Another site on the intracellular portion of the seven-transmembrane binding pocket can bind cholesterol and activate SMO (Deshpande et al., 2019; Hedger et al., 2019). This activation is independent of the CRD sterol-binding site. More than likely, this second sterol-binding site plays a modulatory role in SMO activation. SMO is not the only GPCR activated by cholesterol, and considering that the plasma membrane is a lipid rich environment, GPCRs likely all have their conformational states modulated by lipid dynamics (Hanson et al., 2008; Hanson et al., 2012). As discussed above, the ciliary membrane is a tightly regulated lipid rich environment, and contains sterols sufficient for activating SMO (Raleigh et al., 2018). As our knowledge of sterol driven SMO activation expands, models will soon need to examine the link between sterols, SMO, and the cilium.
The possibility that endogenous sterols activate SMO raises a question regarding whether PTCH1 regulates sterols to inhibit SMO. PTCH1 is long-known to bind cholesterolyated vHH (Kowatsch et al., 2019; Porter, Young, & Beachy, 1996; Rudolf et al., 2019). Recent data show the endogenous sterol 24,25-epoxycholesterol binds both PTCH1 and SMO, and activates vHH signalling (Qi et al., 2019). Curiously, these data also revealed SMO receptor coupling to the Gαi inhibitory subunit. An association between SMO and Gαi subunits has been previously reported, but the role of G-proteins in SMO activation of the HH cascade remained controversial (Polizio et al., 2011; Riobo et al., 2006). Perhaps oxysterols modulate SMO conformation, aiding the receptor to adopt new confirmations that promote transient G-protein coupling. This is in line with models that predict SMO activation is a multistep process. As the field shifts its focus towards the role of sterols in SMO activation, it will be paramount to define the role of the cilium in the steps of SMO activation- along with how PTCH1 regulates the sterols in the context of the cilium.
Conclusion
Clearly, the cilium has taken its rightful place as a fundamental cell organelle with critical roles in cell signalling. The intense focus of the past 15 years on vHH signalling has taught us several principles regarding cilia-dependent signalling. The cilium provides a privileged environment in which signalling components can interact. Importantly the machinery needed to build and maintain the ciliary axoneme and membrane is critical to the dynamic localization of signalling components. As a small exclusive organelle, the cilium is spatially ideal for facilitating a rapid response and the enrichment of many GPCRs, along with the distinct dynamics of cAMP and Ca2+ within the cilium and so suggest that it is being used this way.
Estimates put the cilium as about 1/4000 to 1/10,000th the volume of the cell and the field is rapidly adapting tools to study cilia biology (Nachury, 2014; Phua, Lin, & Inoue, 2015). For example, advances in microscopy are allowing researchers to observe cilia at a more detailed resolution than ever before (Milenkovic et al., 2015; T. T. Yang et al., 2018; Yoon et al., 2019). Recent advances in proximity labelling within cilia is enabling the identification of new ciliary proteins that may be playing critical roles in signalling (Mick et al., 2015; T Tony Yang, Tran, Chong, Huang, & Liao, 2019). Similarly, by adapting biosensors to the ciliary environment, the lipid composition of the membrane, Ca2+ flux and cAMP dynamics can be followed in vivo (Delling et al., 2016; Jiang, Falcone, Curci, & Hofer, 2019; Mukherjee et al., 2016; Su et al., 2013; Yuan et al., 2015). Optogenetic versions of such constructs can manipulate these same aspects of ciliary biology (Eickelbeck et al., 2019; Jansen et al., 2015). Finally, as the processes that regulate ciliary localization and signalling are better understood, in vivo gene editing techniques will enable researchers to target precise mutations that disrupt specific aspects of these processes, in contrast to removing cilia all together (Gigante et al., 2019). Such advances are key to understanding the details of how intertwined the cilium is with the signalling pathways that inhabit it.
For GPCR biology in particular, understanding the mechanics of how the cilium facilitates signalling will be fascinating. While it is possible that the cilium is simply a space where GPCR signalling can take place efficiently, it is also plausible that the cilium provides a space where the cell can run parallel signalling events. While we know many GPCRs are expressed in tissue-specific manners, it is not yet clear whether downstream steps in GPCR signalling exhibit tissue specificity. Armed with the tools to monitor and manipulate the ciliary membrane and components in the cilium, answers to such questions are within the field’s reach.
Acknowledgments
We thank Tim Rutkowski, Alyssa Long, Sarah Suciu, and Robby Van Sciver for discussion and comments.
Funding Information
NINDS : Tamara Caspary R01NS090029
NIGMS : Tamara Caspary R35GM122549
NINDS : Eduardo D. Gigante F31NS106755
Contributor Information
Eduardo D. Gigante, Graduate Program in Neuroscience, Department of Human Genetics, Emory University School of Medicine, Atlanta, GA, 30322, USA.
Tamara Caspary, Department of Human Genetics, Emory University School of Medicine, Atlanta, GA, 30322, USA.
References
- Aanstad P, Santos N, Corbit KC, Scherz PJ, Trinh le A, Salvenmoser W, . . . Stainier DY (2009). The extracellular domain of Smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr Biol, 19(12), 1034–1039. doi: 10.1016/j.cub.2009.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GM, Huang B, & Luck DJ (1982). Temperature-Sensitive, Assembly-Defective Flagella Mutants of CHLAMYDOMONAS REINHARDTII. Genetics, 100(4), 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, & Hooper JE (1996). The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell, 86(2), 221–232. doi: 10.1016/s0092-8674(00)80094-x [DOI] [PubMed] [Google Scholar]
- Alexandre C, Jacinto A, & Ingham PW (1996). Transcriptional activation of hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes & development, 10(16), 2003–2013. [DOI] [PubMed] [Google Scholar]
- Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, . . . McMahon AP (2011). Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell, 20(6), 775–787. doi: 10.1016/j.devcel.2011.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensdorf AM, Dillard ME, Menke JM, Frank MW, Rock CO, & Ogden SK (2017). Sonic Hedgehog Activates Phospholipase A2 to Enhance Smoothened Ciliary Translocation. Cell Rep, 19(10), 2074–2087. doi: 10.1016/j.celrep.2017.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber FA, Laget MP, Schwartz C, & Kornberg TB (1997). Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell, 89(7), 1043–1053. doi: 10.1016/s0092-8674(00)80292-5 [DOI] [PubMed] [Google Scholar]
- Badgandi HB, Hwang SH, Shimada IS, Loriot E, & Mukhopadhyay S (2017). Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol, 216(3), 743–760. doi: 10.1083/jcb.201607095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, & Beales PL (2009). Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet, 151C(4), 281–295. doi: 10.1002/ajmg.c.30231 [DOI] [PubMed] [Google Scholar]
- Bangs FK, Schrode N, Hadjantonakis AK, & Anderson KV (2015). Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol, 17(2), 113–122. doi: 10.1038/ncb3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Engle SE, Antonellis PJ, Whitehouse LS, Baucum AJ 2nd, Cummins TR, . . . Berbari NF (2019). Hedgehog Pathway Activation Alters Ciliary Signaling in Primary Hypothalamic Cultures. Front Cell Neurosci, 13, 266. doi: 10.3389/fncel.2019.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MM, & Sternberg PW (1999). A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature, 401(6751), 386–389. doi: 10.1038/43913 [DOI] [PubMed] [Google Scholar]
- Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, & Pons S (2010). Sonic-hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J Cell Sci, 123(Pt 1), 62–69. doi: 10.1242/jcs.060020 [DOI] [PubMed] [Google Scholar]
- Barzi M, Kostrz D, Menendez A, & Pons S (2011). Sonic Hedgehog-induced proliferation requires specific Galpha inhibitory proteins. J Biol Chem, 286(10), 8067–8074. doi: 10.1074/jbc.M110.178772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Johnson AD, Lewis JS, Askwith CC, & Mykytyn K (2008). Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell, 19(4), 1540–1547. doi: 10.1091/mbc.E07-09-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, & Mykytyn K (2008). Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A, 105(11), 4242–4246. doi: 10.1073/pnas.0711027105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, . . . Gleeson JG (2009). Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet, 41(9), 1032–1036. doi: 10.1038/ng.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, & Mykytyn K (2007). Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol, 505(5), 562–571. doi: 10.1002/cne.21510 [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, & McMahon AP (1996). Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol, 6(3), 298–304. doi: 10.1016/s0960-9822(02)00480-3 [DOI] [PubMed] [Google Scholar]
- Blassberg R, Macrae JI, Briscoe J, & Jacob J (2016). Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum Mol Genet, 25(4), 693–705. doi: 10.1093/hmg/ddv507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, & Verge D (2000). Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res, 872(1–2), 271–275. doi: 10.1016/s0006-8993(00)02519-1 [DOI] [PubMed] [Google Scholar]
- Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, & Nachury MV (2013). An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J Cell Biol, 203(1), 129–147. doi: 10.1083/jcb.201212024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, & Therond PP (2013). The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol, 14(7), 416–429. doi: 10.1038/nrm3598 [DOI] [PubMed] [Google Scholar]
- Buck L, & Axel R (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell, 65(1), 175–187. [DOI] [PubMed] [Google Scholar]
- Byrne EF, Sircar R, Miller PS, Hedger G, Luchetti G, Nachtergaele S, . . . Siebold C (2016). Structural basis of Smoothened regulation by its extracellular domains. Nature, 535(7613), 517–522. doi: 10.1038/nature18934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, Rakeman AS, . . . Anderson KV (2002). Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Current Biology, 12, 1628–1632. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, & Anderson KV (2007). The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell, 12(5), 767–778. doi: 10.1016/j.devcel.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Cevik S, Hori Y, Kaplan OI, Kida K, Toivenon T, Foley-Fisher C, . . . Blacque OE (2010). Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol, 188(6), 953–969. doi: 10.1083/jcb.200908133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevik S, Sanders AA, Van Wijk E, Boldt K, Clarke L, van Reeuwijk J, . . . Blacque OE (2013). Active transport and diffusion barriers restrict Joubert Syndrome-associated ARL13B/ARL-13 to an Inv-like ciliary membrane subdomain. PLoS Genet, 9(12), e1003977. doi: 10.1371/journal.pgen.1003977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, & Schiffmann SN (2015). Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Dev Cell, 34(3), 338–350. doi: 10.1016/j.devcel.2015.06.016 [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, & Beachy PA (2002). Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev, 16(21), 2743–2748. doi: 10.1101/gad.1025302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, & Beachy PA (2002). Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A, 99(22), 14071–14076. doi: 10.1073/pnas.182542899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, . . . Lefkowitz RJ (2004). Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science, 306(5705), 2257–2260. doi: 10.1126/science.1104135 [DOI] [PubMed] [Google Scholar]
- Chen Y, Sasai N, Ma G, Yue T, Jia J, Briscoe J, & Jiang J (2011). Sonic Hedgehog dependent phosphorylation by CK1alpha and GRK2 is required for ciliary accumulation and activation of smoothened. PLoS Biol, 9(6), e1001083. doi: 10.1371/journal.pbio.1001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, & Axel R (1994). Allelic inactivation regulates olfactory receptor gene expression. Cell, 78(5), 823–834. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, & Beachy PA (1996). Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature, 383(6599), 407–413. doi: 10.1038/383407a0 [DOI] [PubMed] [Google Scholar]
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, . . . Peterson AS (2011). A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol, 14(1), 61–72. doi: 10.1038/ncb2410 [DOI] [PubMed] [Google Scholar]
- Chung MI, Kwon T, Tu F, Brooks ER, Gupta R, Meyer M, . . . Wallingford JB (2014). Coordinated genomic control of ciliogenesis and cell movement by RFX2. Elife, 3, e01439. doi: 10.7554/eLife.01439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MI, Peyrot SM, LeBoeuf S, Park TJ, McGary KL, Marcotte EM, & Wallingford JB (2012). RFX2 is broadly required for ciliogenesis during vertebrate development. Dev Biol, 363(1), 155–165. doi: 10.1016/j.ydbio.2011.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge P, Brownlee C, & Wheeler GL (2013). Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Curr Biol, 23(22), 2311–2318. doi: 10.1016/j.cub.2013.09.059 [DOI] [PubMed] [Google Scholar]
- Constable S, Long AB, Floyd KA, Schurmans S, & Caspary T (2019). Ciliary phosphatidylinositol phosphatase Inpp5e plays positive and negative regulatory roles in Shh signaling. bioRxiv, 721399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AF, Yu KP, Brueckner M, Brailey LL, Johnson L, McGrath JM, & Bale AE (2005). Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development, 132(19), 4407–4417. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, & Reiter JF (2005). Vertebrate Smoothened functions at the primary cilium. Nature, 437(7061), 1018–1021. doi: 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- Corcoran RB, & Scott MP (2006). Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A, 103(22), 8408–8413. doi: 10.1073/pnas.0602852103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortellino S, Wang C, Wang B, Bassi MR, Caretti E, Champeval D, . . . Bellacosa A (2009). Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev Biol, 325(1), 225–237. doi: 10.1016/j.ydbio.2008.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann CE, Hsieh J-C, Rattner A, Sharma D, Nathans J, & Leahy DJ (2001). Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature, 412(6842), 86. [DOI] [PubMed] [Google Scholar]
- DeCamp DL, Thompson TM, de Sauvage FJ, & Lerner MR (2000). Smoothened activates Gαi-mediated signaling in frog melanophores. Journal of Biological Chemistry, 275(34), 26322–26327. [DOI] [PubMed] [Google Scholar]
- Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, & Clapham DE (2016). Primary cilia are not calcium-responsive mechanosensors. Nature, 531(7596), 656–660. doi: 10.1038/nature17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande I, Liang J, Hedeen D, Roberts KJ, Zhang Y, Ha B, . . . Manglik A (2019). Smoothened stimulation by membrane sterols drives Hedgehog pathway activity. Nature, 571(7764), 284–288. doi: 10.1038/s41586-019-1355-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, & Mykytyn K (2011). Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci, 68(17), 2951–2960. doi: 10.1007/s00018-010-0603-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn KV, Hughes CE, & Rohatgi R (2012). A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev Cell, 23(4), 823–835. doi: 10.1016/j.devcel.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AE, Heim JA, Shen F, Almada LL, Riobo NA, Fernandez-Zapico ME, & Manning DR (2011). The alpha subunit of the G protein G13 regulates activity of one or more Gli transcription factors independently of smoothened. J Biol Chem, 286(35), 30714–30722. doi: 10.1074/jbc.M111.219279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, & Parhami F (2007). Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem, 282(12), 8959–8968. doi: 10.1074/jbc.M611741200 [DOI] [PubMed] [Google Scholar]
- Dyson JM, Conduit SE, Feeney SJ, Hakim S, DiTommaso T, Fulcher AJ, . . . Mitchell CA (2017). INPP5E regulates phosphoinositide-dependent cilia transition zone function. J Cell Biol, 216(1), 247–263. doi: 10.1083/jcb.201511055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East MP, Bowzard JB, Dacks JB, & Kahn RA (2012). ELMO domains, evolutionary and functional characterization of a novel GTPase-activating protein (GAP) domain for Arf protein family GTPases. J Biol Chem, 287(47), 39538–39553. doi: 10.1074/jbc.M112.417477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Bulgakov OV, Qin J, Li T, & Anderson KV (2006). Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev Biol, 290(1), 1–12. doi: 10.1016/j.ydbio.2005.09.022 [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Espinoza E, & Anderson KV (2001). Rab23 is an essential negative regulator of the mouse Sonic Hedgehog Signalling pathway. Nature, 412(6843), 194–198. [DOI] [PubMed] [Google Scholar]
- Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, . . . Pazour GJ (2014). IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell, 31(3), 279–290. doi: 10.1016/j.devcel.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichel K, & von Zastrow M (2018). Subcellular Organization of GPCR Signaling. Trends Pharmacol Sci, 39(2), 200–208. doi: 10.1016/j.tips.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelbeck D, Karapinar R, Jack A, Suess ST, Barzan R, Azimi Z, . . . Gerwert K (2019). CaMello-XR enables visualization and optogenetic control of G q/11 signals and receptor trafficking in GPCR-specific domains. Communications biology, 2(1), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, . . . Peterson AS (2009). The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol, 19(15), 1320–1326. doi: 10.1016/j.cub.2009.06.046 [DOI] [PubMed] [Google Scholar]
- Farzan SF, Ascano M Jr, Ogden SK, Sanial M, Brigui A, Plessis A, & Robbins DJ (2008). Costal2 functions as a kinesin-like protein in the hedgehog signal transduction pathway. Current Biology, 18(16), 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, & Porter KR (1954). A study of the fine structure of ciliated epithelia. Journal of Morphology, 94(2), 221–281. [Google Scholar]
- Flock T, Hauser AS, Lund N, Gloriam DE, Balaji S, & Babu MM (2017). Selectivity determinants of GPCR-G-protein binding. Nature, 545(7654), 317–322. doi: 10.1038/nature22070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Li L, Vucica Y, & Pazour GJ (2010). The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. The Journal of cell biology, 188(1), 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Zhang XM, Bottega S, Oivin G, Wichterle H, Dudek H, . . . Porter JA (2002). Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. Journal of Biology, 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D, Dayton B, Brodjian S, Ogiela C, Sidorowicz H, Frost LJ, . . . Collins CA (2006). Characterization of a neuronal cell line expressing native human melanin-concentrating hormone receptor 1 (MCHR1). Int J Biochem Cell Biol, 38(8), 1290–1299. doi: 10.1016/j.biocel.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Fumoto K, Hoogenraad CC, & Kikuchi A (2006). GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J, 25(24), 5670–5682. doi: 10.1038/sj.emboj.7601459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G 3rd, Abedin M, Schurmans S, . . . Reiter JF (2015). Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev Cell, 34(4), 400–409. doi: 10.1016/j.devcel.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, & Somlo S (2006). Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci, 119(Pt 7), 1383–1395. doi: 10.1242/jcs.02818 [DOI] [PubMed] [Google Scholar]
- Gigante ED, Long AB, Ben-Ami J, & Caspary T (2018). Hypomorphic Smo mutant with inefficient ciliary enrichment disrupts the highest level of vertebrate Hedgehog response. Developmental biology, 437(2), 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante ED, Taylor MR, Ivanova AA, Kahn RA, & Caspary T (2019). Arl13b regulates Sonic Hedgehog signaling from outside primary cilia. bioRxiv, 711671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer AM, Wilkinson AW, Backer CB, Lapan SW, Gutzman JH, Cheeseman IM, & Reddien PW (2010). The Zn finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Developmental biology, 337(1), 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, & Scott MP (1996). Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev, 10(3), 301–312. doi: 10.1101/gad.10.3.301 [DOI] [PubMed] [Google Scholar]
- Gotthardt K, Lokaj M, Koerner C, Falk N, Giessl A, & Wittinghofer A (2015). A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. Elife, 4. doi: 10.7554/eLife.11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, Schmid CL, Bley E, Monsma PC, Brown A, Bohn LM, & Mykytyn K (2016). Recruitment of beta-Arrestin into Neuronal Cilia Modulates Somatostatin Receptor Subtype 3 Ciliary Localization. Mol Cell Biol, 36(1), 223–235. doi: 10.1128/MCB.00765-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H, Christiansen J, Wicking C, Zaphiropoulos PG, Chidambaram A, Gerrard B, . . . Wainwright B (1996). A mammalian patched homolog is expressed in target tissues of sonic hedgehog and maps to a region associated with developmental abnormalities. J Biol Chem, 271(21), 12125–12128. doi: 10.1074/jbc.271.21.12125 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Bitgood MJ, & McMahon AP (1996). Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev, 10(6), 647–658. doi: 10.1101/gad.10.6.647 [DOI] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, . . . Verge D (1999). Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology, 21(2 Suppl), 68S–76S. doi: 10.1016/S0893-133X(99)00044-5 [DOI] [PubMed] [Google Scholar]
- Han S, Miyoshi K, Shikada S, Amano G, Wang Y, Yoshimura T, & Katayama T (2019). TULP3 is required for localization of membrane-associated proteins ARL13B and INPP5E to primary cilia. Biochemical and biophysical research communications, 509(1), 227–234. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, . . . Hollt V (1999). Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience, 89(3), 909–926. doi: 10.1016/s0306-4522(98)00354-6 [DOI] [PubMed] [Google Scholar]
- Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EY, . . . Stevens RC (2008). A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure, 16(6), 897–905. doi: 10.1016/j.str.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, . . . Stevens RC (2012). Crystal structure of a lipid G protein-coupled receptor. Science, 335(6070), 851–855. doi: 10.1126/science.1215904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, & Yoder BK (2005). Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet, 1(4), e53. doi: 10.1371/journal.pgen.0010053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger G, Koldsø H, Chavent M, Siebold C, Rohatgi R, & Sansom MS (2019). Cholesterol interaction sites on the transmembrane domain of the hedgehog signal transducer and class FG protein-coupled receptor smoothened. Structure, 27(3), 549–559. e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Rifkin MR, & Luck DJ (1977). Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J Cell Biol, 72(1), 67–85. doi: 10.1083/jcb.72.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Nedelcu D, Watanabe M, Jao C, Kim Y, Liu J, & Salic A (2016). Cellular Cholesterol Directly Activates Smoothened in Hedgehog Signaling. Cell, 166(5), 1176–1187 e1114. doi: 10.1016/j.cell.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, & Schier AF (2009). Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development, 136(18), 3089–3098. doi: 10.1242/dev.041343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Zheng S, Wierbowski BM, Kim Y, Nedelcu D, Aravena L, . . . Salic A (2018). Structural Basis of Smoothened Activation in Hedgehog Signaling. Cell, 174(2), 312–324 e316. doi: 10.1016/j.cell.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, & Anderson KV (2005). Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A, 102(32), 11325–11330. doi: 10.1073/pnas.0505328102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, & Anderson KV (2003). Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature, 426(6962), 83–87. doi: 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- Hui C-C, Slusarski D, Platt KA, Holmgren R, & Joyner AL (1994). Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm-and mesoderm-derived tissues suggests multiple roles during postimplantation development. Developmental biology, 162(2), 402–413. [DOI] [PubMed] [Google Scholar]
- Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, & Seo S (2012). ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A, 109(48), 19691–19696. doi: 10.1073/pnas.1210916109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humke EW, Dorn KV, Milenkovic L, Scott MP, & Rohatgi R (2010). The output of Hedgehog signaling is controlled by the dynamic association between Suppressor of Fused and the Gli proteins. Genes Dev, 24(7), 670–682. doi: 10.1101/gad.1902910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Kubo A, Tsukita S, & Tsukita S (2005). Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol, 7(5), 517–524. doi: 10.1038/ncb1251 [DOI] [PubMed] [Google Scholar]
- Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, . . . Charron F (2011). Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell, 20(6), 788–801. doi: 10.1016/j.devcel.2011.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, . . . Schurmans S (2009). INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet, 41(9), 1027–1031. doi: 10.1038/ng.427 [DOI] [PubMed] [Google Scholar]
- Jan LY, & Revel J-P (1974). Ultrastructural localization of rhodopsin in the vertebrate retina. The Journal of cell biology, 62(2), 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda CY, Waghray D, Levin AM, Thomas C, & Garcia KC (2012). Structural basis of Wnt recognition by Frizzled. Science, 337(6090), 59–64. doi: 10.1126/science.1222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen V, Alvarez L, Balbach M, Strunker T, Hegemann P, Kaupp UB, & Wachten D (2015). Controlling fertilization and cAMP signaling in sperm by optogenetics. Elife, 4. doi: 10.7554/eLife.05161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Falcone JL, Curci S, & Hofer AM (2019). Direct visualization of cAMP signaling in primary cilia reveals up-regulation of ciliary GPCR activity following Hedgehog activation. Proceedings of the National Academy of Sciences, 116(24), 12066–12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, . . . Nachury MV (2010). The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell, 141(7), 1208–1219. doi: 10.1016/j.cell.2010.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, & Nauli SM (2014). Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci, 71(11), 2165–2178. doi: 10.1007/s00018-013-1483-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, & Rosenbaum JL (1992). Polarity of flagellar assembly in Chlamydomonas. J Cell Biol, 119(6), 1605–1611. doi: 10.1083/jcb.119.6.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen JA, San Agustin J, Follit JA, & Pazour GJ (2008). Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol, 183(3), 377–384. doi: 10.1083/jcb.200808137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Padula D, Burtscher I, Landerer C, Lutter D, Theis F, . . . Lickert H (2016). Pitchfork and Gprasp2 Target Smoothened to the Primary Cilium for Hedgehog Pathway Activation. PLoS One, 11(2), e0149477. doi: 10.1371/journal.pone.0149477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Bruford E, Inoue H, Logsdon JM Jr., Nie Z, Premont RT, . . . Cassel D (2008). Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol, 182(6), 1039–1044. doi: 10.1083/jcb.200806041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D (2004). Hedgehog signaling: Costal-2 bridges the transduction gap. Curr Biol, 14(2), R67–69. doi: 10.1016/j.cub.2003.12.047 [DOI] [PubMed] [Google Scholar]
- Kasarskis A, Manova K, & Anderson KV (1998). A phenotype-based screen for embryonic lethal mutations in the mouse. Proc Natl Acad Sci U S A, 95(13), 7485–7490. doi: 10.1073/pnas.95.13.7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, . . . Pazour GJ (2012). IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell, 22(5), 940–951. doi: 10.1016/j.devcel.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee HL, Dishinger JF, Blasius TL, Liu CJ, Margolis B, & Verhey KJ (2012). A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat Cell Biol, 14(4), 431–437. doi: 10.1038/ncb2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kato M, & Beachy PA (2009). Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A, 106(51), 21666–21671. doi: 10.1073/pnas.0912180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva MV, Wilson MP, & Majerus PW (2000). The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. Journal of Biological Chemistry, 275(26), 20110–20116. [DOI] [PubMed] [Google Scholar]
- Klink BU, Zent E, Juneja P, Kuhlee A, Raunser S, & Wittinghofer A (2017). A recombinant BBSome core complex and how it interacts with ciliary cargo. Elife, 6. doi: 10.7554/eLife.27434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, . . . Parmentier M (2001). The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem, 276(37), 34631–34636. doi: 10.1074/jbc.M104847200 [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, . . . Lefkowitz RJ (2008). Beta-arrestin-mediated localization of smoothened to the primary cilium. Science, 320(5884), 1777–1781. doi: 10.1126/science.1157983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatsch C, Woolley RE, Kinnebrew M, Rohatgi R, & Siebold C (2019). Structures of vertebrate Patched and Smoothened reveal intimate links between cholesterol and Hedgehog signalling. Current opinion in structural biology, 57, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, & Rosenbaum JL (1995). The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol, 131(6 Pt 1), 1517–1527. doi: 10.1083/jcb.131.6.1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, & Rosenbaum JL (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A, 90(12), 5519–5523. doi: 10.1073/pnas.90.12.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, & Jacobs CR (2010). Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. The FASEB journal, 24(8), 2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, & Sigler PB (1996). The 2.0 A crystal structure of a heterotrimeric G protein. Nature, 379(6563), 311–319. doi: 10.1038/379311a0 [DOI] [PubMed] [Google Scholar]
- Larkins CE, Aviles GD, East MP, Kahn RA, & Caspary T (2011). Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell, 22(23), 4694–4703. doi: 10.1091/mbc.E10-12-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorraca NR, Wang JK, Bauer B, Townshend RJL, Hollingsworth SA, Olivieri JE, . . . Dror RO (2018). Molecular mechanism of GPCR-mediated arrestin activation. Nature, 557(7705), 452–456. doi: 10.1038/s41586-018-0077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SF, Yasuda K, Bell GI, & Reisine T (1993). Gi alpha 3 and G(o) alpha selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem, 268(15), 10721–10727. [PubMed] [Google Scholar]
- Lechtreck K-F, Teltenkotter A, & Grunow A (1999). A 210 kDa protein is located in a membrane-microtubule linker at the distal end of mature and nascent basal bodies. Journal of cell science, 112(11), 1633–1644. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, . . . Witman GB (2009). The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol, 187(7), 1117–1132. doi: 10.1083/jcb.200909183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, & Jacobs CR (2015). The primary cilium functions as a mechanical and calcium signaling nexus. Cilia, 4, 7. doi: 10.1186/s13630-015-0016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kelly WG, Logsdon JM Jr., Schurko AM, Harfe BD, Hill-Harfe KL, & Kahn RA (2004). Functional genomic analysis of the ADP-ribosylation factor family of GTPases: phylogeny among diverse eukaryotes and function in C. elegans. FASEB J, 18(15), 1834–1850. doi: 10.1096/fj.04-2273com [DOI] [PubMed] [Google Scholar]
- Liem KF Jr., He M, Ocbina PJ, & Anderson KV (2009). Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci U S A, 106(32), 13377–13382. doi: 10.1073/pnas.0906944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, . . . Nachury MV (2014). The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell, 31(3), 265–278. doi: 10.1016/j.devcel.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, & Chiang C (2000). Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci, 3(10), 979–985. doi: 10.1038/79916 [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, & Niswander LA (2005). Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development, 132(13), 3103–3111. doi: 10.1242/dev.01894 [DOI] [PubMed] [Google Scholar]
- Logsdon JM Jr., & Kahn RA (Eds.). (2004). Arf Family GTPases. In: Kahn RA, editor.
- Lohse MJ, Andexinger S, Pitcher J, Trukawinski S, Codina J, Faure JP, . . . Lefkowitz RJ (1992). Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. J Biol Chem, 267(12), 8558–8564. [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, & Lefkowitz RJ (1990). beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science, 248(4962), 1547–1550. [DOI] [PubMed] [Google Scholar]
- Loktev AV, & Jackson PK (2013). Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep, 5(5), 1316–1329. doi: 10.1016/j.celrep.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Luchetti G, Sircar R, Kong JH, Nachtergaele S, Sagner A, Byrne EFX, . . . Rohatgi R (2016). Cholesterol activates G-protein coupled receptor Smoothened to promote Hedgehog signaling. Elife, 5, e20304. doi: 10.7554/eLife.20304.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Tian X, Igarashi P, Pazour GJ, & Somlo S (2013). Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet, 45(9), 1004–1012. doi: 10.1038/ng.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton I, & Clarke B (1952). An electron microscope study of the spermatozoid of Sphagnum. Journal of experimental botany, 265–275. [Google Scholar]
- Mariani LE, Bijlsma MF, Ivanova AI, Suciu SK, Kahn RA, & Caspary T (2016). Arl13b regulates Shh signaling from both inside and outside the cilium. Mol Biol Cell. doi: 10.1091/mbc.E16-03-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley A, & von Zastrow M (2010). DISC1 regulates primary cilia that display specific dopamine receptors. PLoS One, 5(5), e10902. doi: 10.1371/journal.pone.0010902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF (2008). Basal bodies platforms for building cilia. Curr Top Dev Biol, 85, 1–22. doi: 10.1016/S0070-2153(08)00801-6 [DOI] [PubMed] [Google Scholar]
- Marshall WF, & Rosenbaum JL (2001). Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol, 155(3), 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, & Joyner AL (1998). Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development, 125(15), 2759–2770. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, . . . Peterson AS (2005). Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol, 287(2), 378–389. doi: 10.1016/j.ydbio.2005.08.050 [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, & Brueckner M (2003). Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell, 114(1), 61–73. [DOI] [PubMed] [Google Scholar]
- Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, & Nachury MV (2015). Proteomics of Primary Cilia by Proximity Labeling. Dev Cell, 35(4), 497–512. doi: 10.1016/j.devcel.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miertzschke M, Koerner C, Spoerner M, & Wittinghofer A (2014). Structural insights into the small G-protein Arl13B and implications for Joubert syndrome. Biochem J, 457(2), 301–311. doi: 10.1042/BJ20131097 [DOI] [PubMed] [Google Scholar]
- Milenkovic L, Scott MP, & Rohatgi R (2009). Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol, 187(3), 365–374. doi: 10.1083/jcb.200907126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic L, Weiss LE, Yoon J, Roth TL, Su YS, Sahl SJ, . . . Moerner WE (2015). Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proc Natl Acad Sci U S A, 112(27), 8320–8325. doi: 10.1073/pnas.1510094112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Jansen V, Jikeli JF, Hamzeh H, Alvarez L, Dombrowski M, . . . Wachten D (2016). A novel biosensor to study cAMP dynamics in cilia and flagella. Elife, 5. doi: 10.7554/eLife.14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, & Jackson PK (2010). TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev, 24(19), 2180–2193. doi: 10.1101/gad.1966210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, & Jackson PK (2013). The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell, 152(1–2), 210–223. doi: 10.1016/j.cell.2012.12.026 [DOI] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, & Schier AF (2012). Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science, 336(6082), 721–724. doi: 10.1126/science.1221920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BR, Neahring L, Zhang Y, Roberts KJ, & Beachy PA (2017). Rapid, direct activity assays for Smoothened reveal Hedgehog pathway regulation by membrane cholesterol and extracellular sodium. Proc Natl Acad Sci U S A, 114(52), E11141–E11150. doi: 10.1073/pnas.1717891115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, . . . Beachy PA (2013). Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell, 26(4), 346–357. doi: 10.1016/j.devcel.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, & Rohatgi R (2012). Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol, 8(2), 211–220. doi: 10.1038/nchembio.765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtergaele S, Whalen DM, Mydock LK, Zhao Z, Malinauskas T, Krishnan K, . . . Rohatgi R (2013). Structure and function of the Smoothened extracellular domain in vertebrate Hedgehog signaling. Elife, 2, e01340. doi: 10.7554/eLife.01340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV (2014). How do cilia organize signalling cascades? Philos Trans R Soc Lond B Biol Sci, 369(1650). doi: 10.1098/rstb.2013.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, . . . Jackson PK (2007). A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell, 129(6), 1201–1213. doi: 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, & Nachury MV (2017). An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell, 168(1–2), 252–263 e214. doi: 10.1016/j.cell.2016.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, . . . Zhou J (2003). Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet, 33(2), 129–137. doi: 10.1038/ng1076 [DOI] [PubMed] [Google Scholar]
- Norman RX, Ko HW, Huang V, Eun CM, Abler LL, Zhang Z, . . . Eggenschwiler JT (2009). Tubby-like protein 3 (TULP3) regulates patterning in the mouse embryo through inhibition of Hedgehog signaling. Hum Mol Genet, 18(10), 1740–1754. doi: 10.1093/hmg/ddp113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki S, Katoh Y, Terada M, Michisaka S, Funabashi T, Takahashi S, . . . Nakayama K (2017). Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E. J Cell Sci, 130(3), 563–576. doi: 10.1242/jcs.197004 [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, & Wieschaus E (1980). Mutations affecting segment number and polarity in Drosophila. Nature, 287(5785), 795–801. [DOI] [PubMed] [Google Scholar]
- Ocbina PJ, & Anderson KV (2008). Intraflagellar transport, cilia, and mammalian Hedgehog signaling: analysis in mouse embryonic fibroblasts. Dev Dyn, 237(8), 2030–2038. doi: 10.1002/dvdy.21551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, & Robbins DJ (2008). G protein Galphai functions immediately downstream of Smoothened in Hedgehog signalling. Nature, 456(7224), 967–970. doi: 10.1038/nature07459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K, Hwang SH, Somatilaka B, Badgandi H, Jackson PK, DeFea K, & Mukhopadhyay S (2016). Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol, 212(7), 861–875. doi: 10.1083/jcb.201506132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Bai C, Platt K, Matise M, Beeghly A, Hui C, . . . Joyner A (2000). Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development, 127(8), 1593–1605. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, & Wallingford JB (2006). Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet, 38(3), 303–311. doi: 10.1038/ng1753 [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, . . . Besharse JC (2002). The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol, 157(1), 103–113. doi: 10.1083/jcb.200107108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, & Cole DG (2000). Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol, 151(3), 709–718. doi: 10.1083/jcb.151.3.709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, & Witman GB (2002). Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current Biology, 12(11), R378–R380. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, & Witman GB (1998). A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J Cell Biol, 141(4), 979–992. doi: 10.1083/jcb.141.4.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua SC, Lin Y-C, & Inoue T (2015). An intelligent nano-antenna: primary cilium harnesses TRP channels to decode polymodal stimuli. Cell calcium, 58(4), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizio AH, Chinchilla P, Chen X, Manning DR, & Riobo NA (2011). Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal, 4(200), pt7. doi: 10.1126/scisignal.2002396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Young KE, & Beachy PA (1996). Cholesterol modification of hedgehog signaling proteins in animal development. Science, 274(5285), 255–259. [DOI] [PubMed] [Google Scholar]
- Qi X, Liu H, Thompson B, McDonald J, Zhang C, & Li X (2019). Cryo-EM structure of oxysterol-bound human Smoothened coupled to a heterotrimeric G i. Nature, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh DR, Sever N, Choksi PK, Sigg MA, Hines KM, Thompson BM, . . . Garcia-Gonzalo FR (2018). Cilia-associated oxysterols activate smoothened. Molecular cell, 72(2), 316–327. e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Salloum R, Xin M, & Lu QR (2016). The G protein Galphas acts as a tumor suppressor in sonic hedgehog signaling-driven tumorigenesis. Cell Cycle, 15(10), 1325–1330. doi: 10.1080/15384101.2016.1164371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, Blacque OE, & Leroux MR (2012). The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep, 13(7), 608–618. doi: 10.1038/embor.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, & Leroux MR (2017). Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol, 18(9), 533–547. doi: 10.1038/nrm.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink JC, Gurley KA, Elliott SA, & Sanchez Alvarado A (2009). Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science, 326(5958), 1406–1410. doi: 10.1126/science.1178712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riobo NA, Saucy B, Dilizio C, & Manning DR (2006). Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A, 103(33), 12607–12612. doi: 10.1073/pnas.0600880103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Corcoran RB, & Scott MP (2009). Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci U S A, 106(9), 3196–3201. doi: 10.1073/pnas.0813373106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, & Scott MP (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science, 317(5836), 372–376. doi: 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Röhlich P (1975). The sensory cilium of retinal rods is analogous to the transitional zone of motile cilia. Cell and tissue research, 161(3), 421–430. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, & Child FM (1967). Flagellar regeneration in protozoan flagellates. J Cell Biol, 34(1), 345–364. doi: 10.1083/jcb.34.1.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, & Witman GB (2002). Intraflagellar transport. Nat Rev Mol Cell Biol, 3(11), 813–825. doi: 10.1038/nrm952 [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Arrang JM, Tardivel-Lacombe J, Diaz J, Leurs R, & Schwartz JC (1993). A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem Biophys Res Commun, 193(1), 268–276. doi: 10.1006/bbrc.1993.1619 [DOI] [PubMed] [Google Scholar]
- Rudolf AF, Kinnebrew M, Kowatsch C, Ansell TB, El Omari K, Bishop B, . . . Qian M (2019). The morphogen Sonic hedgehog inhibits its receptor Patched by a pincer grasp mechanism. Nature chemical biology, 15(10), 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel L, Rodriguez R, Gallet A, Lavenant-Staccini L, & Thérond PP (2003). Stability and association of Smoothened, Costal2 and Fused with Cubitus interruptus are regulated by Hedgehog. Nature cell biology, 5(10), 907. [DOI] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, & Lappalainen P (2010). Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev, 90(1), 259–289. doi: 10.1152/physrev.00036.2009 [DOI] [PubMed] [Google Scholar]
- Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, . . . Hamada H (2002). Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells, 7(4), 401–412. [DOI] [PubMed] [Google Scholar]
- Schlacht A, Mowbrey K, Elias M, Kahn RA, & Dacks JB (2013). Ancient complexity, opisthokont plasticity, and discovery of the 11th subfamily of Arf GAP proteins. Traffic, 14(6), 636–649. doi: 10.1111/tra.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Handel M, Schreff M, Schmidt H, & Hollt V (2000). Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol Paris, 94(3–4), 259–264. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Szymanska K, Basu B, Patel N, Ewida N, Faqeih E, . . . Aldahmesh MA (2016). Characterizing the morbid genome of ciliopathies. Genome Biol, 17(1), 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Garcia III G, Van De Weghe JC, McGorty R, Pazour GJ, Doherty D, . . . Reiter JF (2017). Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nature cell biology, 19(10), 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, & Maratos-Flier E (1998). Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature, 396(6712), 670–674. doi: 10.1038/25341 [DOI] [PubMed] [Google Scholar]
- Slaats GG, Isabella CR, Kroes HY, Dempsey JC, Gremmels H, Monroe GR, . . . Kumar SA (2016). MKS1 regulates ciliary INPP5E levels in Joubert syndrome. Journal of medical genetics, 53(1), 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, & McMahon AP (1999). Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev, 13(16), 2072–2086. doi: 10.1101/gad.13.16.2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, . . . Rosenthal A (1996). The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature, 384(6605), 129–134. doi: 10.1038/384129a0 [DOI] [PubMed] [Google Scholar]
- Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, . . . Inoue T (2013). Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods, 10(11), 1105–1107. doi: 10.1038/nmeth.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svard J (2006). Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell, 10, 187–197. [DOI] [PubMed] [Google Scholar]
- Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, . . . Teglund S (2006). Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell, 10(2), 187–197. doi: 10.1016/j.devcel.2005.12.013 [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, . . . Beachy PA (2000). Effects of Oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature, 406, 1005–1009. [DOI] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, & Beachy PA (2002). Patched acts catalytically to suppress the activity of Smoothened. Nature, 418(6900), 892–897. doi: 10.1038/nature00989 [DOI] [PubMed] [Google Scholar]
- Takeda S, Kadowaki S, Haga T, Takaesu H, & Mitaku S (2002). Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett, 520(1–3), 97–101. doi: 10.1016/s0014-5793(02)02775-8 [DOI] [PubMed] [Google Scholar]
- Tay SY, Ingham PW, & Roy S (2005). A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development, 132(4), 625–634. doi: 10.1242/dev.01606 [DOI] [PubMed] [Google Scholar]
- Tay SY, Yu X, Wong KN, Panse P, Ng CP, & Roy S (2010). The iguana/DZIP1 protein is a novel component of the ciliogenic pathway essential for axonemal biogenesis. Developmental Dynamics, 239(2), 527–534. [DOI] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, . . . Shah JV (2008). THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nature genetics, 40(4), 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukachinsky H, Lopez LV, & Salic A (2010). A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol, 191(2), 415–428. doi: 10.1083/jcb.201004108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuson M, He M, & Anderson KV (2011). Protein kinase A acts at the basal body of the primary cilium to prevent Gli2 activation and ventralization of the mouse neural tube. Development, 138(22), 4921–4930. doi: 10.1242/dev.070805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eps N, Altenbach C, Caro LN, Latorraca NR, Hollingsworth SA, Dror RO, . . . Hubbell WL (2018). Gi- and Gs-coupled GPCRs show different modes of G-protein binding. Proc Natl Acad Sci U S A, 115(10), 2383–2388. doi: 10.1073/pnas.1721896115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Warner J, Hertzler PH, & McClay DR (2009). Hedgehog signaling patterns mesoderm in the sea urchin. Dev Biol, 331(1), 26–37. doi: 10.1016/j.ydbio.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Cobo-Stark P, Patel V, Somlo S, Han P-L, & Igarashi P (2018). Adenylyl cyclase 5 deficiency reduces renal cyclic AMP and cyst growth in an orthologous mouse model of polycystic kidney disease. Kidney international, 93(2), 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhou Z, Walsh CT, & McMahon AP (2009). Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci U S A, 106(8), 2623–2628. doi: 10.1073/pnas.0812110106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton-Pitt SR, Silva M, Nguyen KC, Hall DH, & Barr MM (2014). The nphp-2 and arl-13 genetic modules interact to regulate ciliogenesis and ciliary microtubule patterning in C. elegans. PLoS genetics, 10(12), e1004866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JF, McCarthy AM, Morris RL, & McClay DR (2014). Hedgehog signaling requires motile cilia in the sea urchin. Mol Biol Evol, 31(1), 18–22. doi: 10.1093/molbev/mst176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, & Beales PL (2011). Ciliopathies: an expanding disease spectrum. Pediatr Nephrol, 26(7), 1039–1056. doi: 10.1007/s00467-010-1731-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Lai CK, Evangelista M, Hongo JA, de Sauvage FJ, & Scales SJ (2010). Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol, 30(8), 1910–1922. doi: 10.1128/MCB.01089-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Chen MH, & Chuang PT (2009). Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS One, 4(4), e5182. doi: 10.1371/journal.pone.0005182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Tang JJ, Peng C, Wang Y, Fu L, Qiu ZP, . . . Song BL (2017). Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling. Mol Cell, 66(1), 154–162 e110. doi: 10.1016/j.molcel.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, . . . de Sauvage FJ (1998). Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature, 391(6662), 90–92. doi: 10.1038/34201 [DOI] [PubMed] [Google Scholar]
- Yang C, Chen W, Chen Y, & Jiang J (2012). Smoothened transduces Hedgehog signal by forming a complex with Evc/Evc2. Cell Res, 22(11), 1593–1604. doi: 10.1038/cr.2012.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Chong WM, Wang WJ, Mazo G, Tanos B, Chen Z, . . . Liao JC (2018). Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat Commun, 9(1), 2023. doi: 10.1038/s41467-018-04469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Tran MNT, Chong WM, Huang C-E, & Liao J-C (2019). Single-particle tracking localization microscopy reveals nonaxonemal dynamics of intraflagellar transport proteins at the base of mammalian primary cilia. Molecular biology of the cell, 30(7), 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, & Bell GI (1992). Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem, 267(28), 20422–20428. [PubMed] [Google Scholar]
- Yavari A, Nagaraj R, Owusu-Ansah E, Folick A, Ngo K, Hillman T, . . . Banerjee U (2010). Role of lipid metabolism in smoothened derepression in hedgehog signaling. Dev Cell, 19(1), 54–65. doi: 10.1016/j.devcel.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Nager AR, & Nachury MV (2018). BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol, 217(5), 1847–1868. doi: 10.1083/jcb.201709041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Comerci CJ, Weiss LE, Milenkovic L, Stearns T, & Moerner W (2019). Revealing nanoscale morphology of the primary cilium using super-resolution fluorescence microscopy. Biophysical journal, 116(2), 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, . . . Hamada H (2012). Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science, 338(6104), 226–231. doi: 10.1126/science.1222538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Zhao, Brueckner, & Sun. (2015). Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr Biol, 25(5), 556–567. doi: 10.1016/j.cub.2014.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhuang T, Lin Q, Yang B, Xu X, Xin G, . . . Zhang T (2019). Patched1–ArhGAP36–PKA–Inversin axis determines the ciliary translocation of Smoothened for Sonic Hedgehog pathway activation. Proceedings of the National Academy of Sciences, 116(3), 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, . . . Sheffield VC (2011). Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci U S A, 108(51), 20678–20683. doi: 10.1073/pnas.1113220108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao Y, Tong C, Wang G, Wang B, Jia J, & Jiang J (2005). Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell, 8(2), 267–278. doi: 10.1016/j.devcel.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, & McMahon AP (2001). Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell, 106(2), 781–792. [PubMed] [Google Scholar]
- Zhang Y, Bulkley DP, Xin Y, Roberts KJ, Asarnow DE, Sharma A, . . . Beachy PA (2018). Structural Basis for Cholesterol Transport-like Activity of the Hedgehog Receptor Patched. Cell, 175(5), 1352–1364 e1314. doi: 10.1016/j.cell.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tong C, & Jiang J (2007). Hedgehog regulates smoothened activity by inducing a conformational switch. Nature, 450(7167), 252–258. doi: 10.1038/nature06225 [DOI] [PubMed] [Google Scholar]