Abstract
Background:
The U.S. is currently experiencing the largest hepatitis A virus (HAV) outbreak since the introduction of a vaccine in 1996. More than 31,000 cases have been reported since 2016. While HAV had largely been considered a foodborne pathogen in recent years, this outbreak has been spread primarily through person-to-person transmission in urban settings and has been associated with homelessness and substance use. Michigan was one of the first states to report an outbreak, with 910 reported cases between August 2016 and December 2018.
Methods:
We analyzed surveillance and vaccination data from Michigan using a disease transmission model to investigate how vaccine timing and coverage influenced the spatiotemporal patterns of the outbreak, distinguishing between Southeast Michigan, where the outbreak began, and the rest of the state.
Results:
We estimated that vaccination had little impact in Southeast Michigan (3% cases averted (95% confidence interval (CI): 1%−8%)) but had a substantial impact in the rest of the state, preventing a larger outbreak (91% cases averted (95% CI: 85%−97%) lasting several more years.
Conclusions:
Our results emphasize the value of targeting populations where local transmission is not yet sustained rather than populations where transmission is already waning. Simulation modeling can aid in proactive rather than reactive decision making and may help direct the response to outbreaks emerging in other states.
Keywords: hepatitis A virus, outbreak, vaccination, mathematical model, Michigan, seasonality
Introduction
Hepatitis A incidence in the U.S. fell to historic lows after the introduction of vaccines in 1996 [1, 2]. Hepatitis A vaccines have high, durable seroconversion rates and efficacy [3–6]. Since then, most outbreaks of hepatitis A had been associated with point-source, foodborne transmission, consistent with its fecal–oral transmission route. However, since 2016, outbreaks of the highly contagious hepatitis A virus (HAV) have continued to emerge, starting in California, Utah, and Michigan. These outbreaks appear to be driven by person-to-person transmission among high-risk individuals, particularly those experiencing homelessness. By March 2020, more than 31,000 cases and 300 deaths have been reported across 30 states [7]. Other populations at high risk in this outbreak include people who use drugs (injection or non-injection), are or were recently incarcerated, and have chronic liver disease, as well as men who have sex with men (MSM) [7].
In Michigan, the first HAV cases appeared in August 2016 in the Detroit area of Southeast Michigan, before spreading to the rest of the state. Following preliminary investigation of the outbreak by the Michigan Department of Health and Human Services (MDHHS), vaccination clinics targeted to high-risk groups opened in 2017, about one year after the beginning of the outbreak. Vaccination is recommended for all infants as well as all adults who at high risk for HAV infection. The state legislature appropriated additional funds for outbreak response in December 2017. By the end of 2018, 910 cases had been reported state-wide, with only sporadic cases arising in 2019 [8]. In this paper, we present a mathematical model of HAV transmission to characterize patterns of spatial spread and the impact of vaccination on the magnitude and timing of the HAV outbreak in Michigan. In particular, we estimate the fraction of cases averted by the vaccination campaign in Southeast Michigan and the rest of the state.
METHODS
Data
The Michigan Disease Surveillance System (MDSS) is Michigan’s communicable disease surveillance system [9]. Cases of hepatitis A were ascertained through passive reporting. Surveillance cases of HAV are defined as people with symptoms of acute viral hepatitis (e.g., fever, dark urine, etc.) accompanied by a) jaundice or elevated bilirubin levels or b) elevated serum aspartate or alanine aminotransferase levels, in the absence of a more likely diagnosis [10]. All cases were submitted for lab confirmation; only confirmed positive cases were included in this analysis. An MDHHS interviewer attempted to follow up with cases to determine demographic information and high-risk indicators, including information about homelessness, substance use, incarceration/institutionalization, history of hepatitis B/C, and if they are men who have sex with men (MSM). MDHHS also collected information about case hospitalization and death. We used data on the 910 lab-confirmed cases ascertained between August 2016 and December 2018; 681 were recorded in Southeast Michigan and 229 outside of Southeast Michigan. Data are aggregated by week. In our analyses we distinguish between cases who reside in the Detroit area of Southeast Michigan (Detroit City and Macomb, Oakland, and Wayne Counties) and all other Michigan counties (Figure 1a and b). Weekly case data are available in eAppendix 1.
Figure 1:
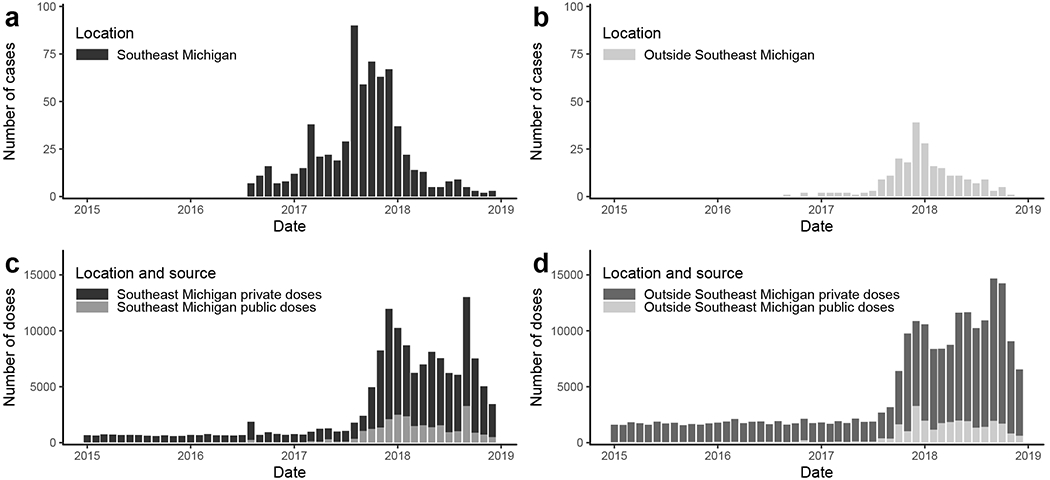
a) Reported cases of hepatitis A in Southeast Michigan (Macomb, Oakland, and Wayne Counties, including Detroit City). b) Reported cases of hepatitis A outside of Southeast Michigan (all other Michigan counties). c) Reported number of hepatitis A vaccine doses (private and public) administered in Southeast Michigan. d) Reported number of hepatitis A vaccine doses (private and public) administered outside of Southeast Michigan. Vaccine data is only recorded for those age 19 or older.
The Michigan Care Improvement Registry (MCIR) is Michigans child and adult immunization registry. The registry distinguishes between privately and publicly funded vaccine doses. The state of Michigan requires doses administered to ≤19 year-olds and all publicly funded doses be reported. There is no mandate to report adult data to MCIR, but it is strongly encouraged and has substantially increased since the advent of automated reporting in 2012. In our model, we estimated the impact of vaccination using weekly counts of immunizations administered to people aged ≥ 19 years from 201518. We assigned cases to Southeast Michigan or the rest of the state based on their residential address (Figure 1c and d). Because HAV vaccines are given in multiple doses, we only include an individual’s first dose. Weekly vaccination doses data are available in eAppendix 1.
The Institutional Review Boards of the University of Michigan and MDHHS determined this analysis to not be human subjects research.
Clinical staging assumptions
After a latent period of 10–12 days, infected people enter a presymptomatic infectious stage during which they are most likely to transmit the virus [11]. Symptoms typically appear about 4 weeks after infection, although substantial variation has been observed (15–50 days) [11, 12]. While HAV infection is often asymptomatic in children, between 70–80% of infected adults display symptoms of acute viral hepatitis [13]. Symptoms occur in two phases: a short prodromal phase, where symptoms are primarily fever and malaise, and a longer jaundice phase. The prodrome gives way to jaundice within a week; the jaundice period lasts less than 2 weeks in 85% of individuals, though it can persist for months [14]. Symptomatic individuals, while still infectious until about a week after the onset of jaundice, have lower concentrations of virus in their stool and are less likely to transmit than during the presymptomatic phase [11].
Mathematical model
We used a mathematical model to estimate transmission rates and the impact of vaccination on transmission the dynamics over the course of the outbreak. We employed a susceptible–infectious–recovered (SIR) type model, which was informed by existing models of hepatitis A transmission [15–20]. Based on the clinical and viral shedding literature described above, we assumed infected people progress through four stages: latent Li, presymptomatic infectious Ii, prodromal Pi, and jaundiced Ji. We also modeled susceptible Si and recovered/immune stages Ri. Since, we do not know the sizes of the underlying initially-at-risk populations, we model fractions of the population in different disease stages. Because we do not know what fraction of infectious cases were captured by surveillance, our model includes a reporting parameter, κi to relate the number of observed cases in the data to the unobserved total number of cases represented by the model. The reporting parameter accounts for the (unknown) size of the at-risk population as well as the reporting fraction. We used a two-patch metapopulation model to represent the outbreaks in the metro Detroit area of Southeast Michigan (population i = 1) and the rest of the state (population i = 2); we model transmission within each population separately as well as transmission from Southeast Michigan to the rest of the state (Figure 2).
Figure 2:
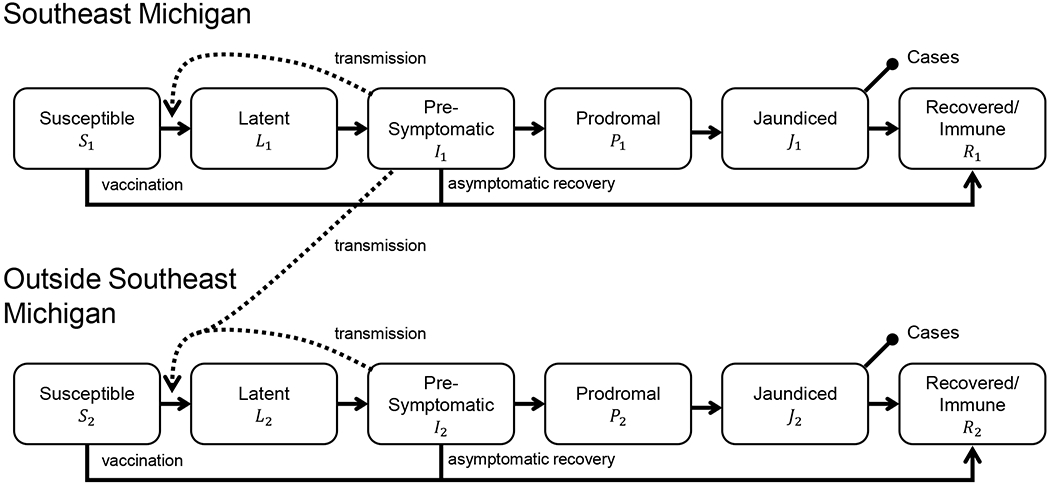
Schematic of the metapopulation infectious disease model for the Michigan hepatitis A outbreak. Solid arrows represent movement of individuals between model compartments (disease states) while dotted arrows show how infectious individuals contribute to new infections through disease transmission. The capped line segments indicate the compartments that correspond to the observed data.
We used the weekly number of doses of hepatitis A vaccine reported to the MCIR database administered in each population di(t) as part of the vaccination rate (Figure 1c and d). To convert the number of doses per week di(t) into a per-capita vaccination rate, we multiplied this by a vaccine coverage parameter ωi, which accounts for both the size of the at-risk population and the fraction of vaccine doses that are applied to at-risk individuals (as opposed to people who were not at-risk but received the vaccine). The coverage parameters are assumed to be constant in time and do not distinguish between private and public doses.
The model parameters are given in Table 1, and the model equations are given below:
| (1) |
where when S1(t) >0 and 0 otherwise, so that the vaccination campaign does not reduce the fraction of susceptible people below 0.
Table 1:
Parameters for the Michigan hepatitis A outbreak model (Figure 2)
| Parameter | Value | Units | Definition |
|---|---|---|---|
| σ | 0.64 | weeks−1 | Rate of leaving latent stage |
| ν | 0.41 | weeks−1 | Rate of leaving presymptomatic infectious stage |
| μ | 1.75 | weeks−1 | Rate of leaving prodromal stage |
| γ | 0.78 | weeks−1 | Rate of recovering from jaundice stage |
| β11 | – | weeks−1 | Baseline transmission rate within Southeast Michigan |
| β12 | – | weeks−1 | Baseline transmission rate from Southeast Michigan to the rest of the state |
| β22 | – | weeks−1 | Baseline transmission rate outside of Southeast Michigan |
| ωi | – | – | Vaccination coverage parameter |
| κi | – | – | Reporting parameter (population size times reporting fraction) |
| I0 | – | – | Initial condition I1(0) of infectious people in Southeast Michigan |
The number of reported new cases yi(t) for each population i in week tj is modeled as the reporting parameter κi times the cumulative fraction of people arriving in the jaundice state Ji in week tj,
| (2) |
Although hepatitis A is not generally considered to be seasonal as a foodborne pathogen, seasonality may be relevant for person-to-person transmission among these high-risk populations. We found that a model with seasonal transmission outperformed the model without; however, a wide range of the seasonality parameters representing the steepness of seasonal transition and the transition timing were found to be consistent with the data (see eAppendix 2). Representative seasonality parameters were selected for use in the main model; these parameters correspond to increased transmission when the temperature in Southeast Michigan is above 50°F, approximately April through October, with the transition from low to high transmission occurring over about one month. Methodological details and sensitivity analysis for seasonality are included in the supplementary material. In short, we estimate the estimate difference between baseline transmission in the cooler months and the peak transmission in warmer months δ.
Parameter identifiability, simulation, and parameter estimation
In order to achieve our objective of estimating the fraction of cases averted from the vaccination campaign, we must estimate the values of the model parameters by fitting the model to the data. However, in general, a model’s parameters may not be uniquely determined by its observed output (in this case, the trajectory of reported cases). For example, if we try to estimate the parameters of the linear model y = (m1 + m2)x + b by comparing it to the best-fit line through a set of (x, y) data, we will be able to uniquely determine b, but we cannot separately determine m1 and m2. Parameters that can be uniquely determined from model output are called identifiable. Parameters that are not identifiable may be part of an identifiable parameter combination, e.g., in the above example, m = m1 + m2 is an identifiable parameter combination. For models of even modest complexity, it is typically not obvious whether a given model parameter is identifiable or not. Structural identifiablity analysis [21, 22] is the process of determining whether each model parameter is identifiable from perfectly observed model outputs.
We performed structural identifiability analysis of the model assuming perfect observation of the trajectories of κ1J1 and κ2J2. The unfixed parameters are not individually identifiable from these data. Instead, we can estimate the following identifiable parameter combinations β11/κ1, β12/κ1, κ1ω1, and κ2ω2. This kind of unidentifiability is typical of models with a reporting parameter κ (here taking into account the at-risk population size and the reporting fraction). Similarly, the initial condition for the presymptomatic infectious compartment Ii(0) = I0 is not observable, but κ1I0 is; practically speaking, we can estimate the number of people in each compartment scaled by the reporting fraction, but we cannot estimate what fraction of the unknown at-risk population they represent.
To fit the model to data and determine the 95% confidence intervals for the parameter estimates, we use a likelihood function, which measures the goodness of fit of the model to the data as a function of the model parameters θ. We minimize the negative log-likelihood to achieve the maximum likelihood estimates . Model simulation and parameter estimation was done in R (v3.6.0) with packages deSolve for ODE simulation and Bhat for maximum likelihood estimation with the David–Fletcher–Powell algorithm [23, 24]. We determined likelihood-based 95% confidence intervals using parameter profile likelihoods [25]; that is, we determined the range of values at which each parameter could be fixed while still fitting the data within 95% likelihood thresholds (allowing the other parameters to vary). Technical details of the likelihood, likelihood thresholds and additional details are given in eAppendix 2.
Impact of the vaccination campaign
To quantify the impact of the vaccination campaign on the trajectory of the Michigan outbreak, we compared the observed outbreak to a counterfactual scenario in which there was no increase in the number of weekly doses of hepatitis A vaccine administered beyond the historical baseline. Specifically, in the counterfactual we set the rate of vaccine administration from August 2017 through December 2018 to the mean of the rate from January 2015 through July 2017. We ran the simulation with all parameters set to their maximum likelihood estimates, calculating the cumulative incidence Z (also called the attack ratio). We estimated the fraction of cases averted as .
RESULTS
Outbreak characteristics
There were 910 cases of hepatitis A in Michigan between August 2016 and December 2018, including 731 hospitalizations (80%) and 30 deaths (3%). The majority of cases were male (65%), and the median age of cases was 40. A majority of cases (66%) had one or more of the five pre-identified high-risk indicators identified through the follow-up interview (Table 2). This is likely an underestimate, both as the data are self-reported and as some cases, primarily near the beginning of the outbreak, were lost to follow-up (162, 18%). The most commonly reported risk indicators were substance use (intravenous (IV), non-IV, or both), history of hepatitis B or C, and institutional risk (including being a correctional facility, substance use rehab facility, or nursing/group home resident). Most risk factors were distributed homogeneously in time across the outbreak; the notable exception is cases in MSM, which were clustered at the end of 2017. We did not stratify by risk factor in the transmission model because there was not enough information support a model with the added complexity (e.g., size of each at-risk group, co-occurrence of risk factors, rates of contact between risk groups).
Table 2:
Outcome, demographic, and high-risk indicator characteristics of cases in the Michigan hepatitis A outbreak. Institutional risk includes being a correctional facility, substance use rehab facility, or nursing or group home resident.
| Characteristic | % (N) |
|---|---|
| Outcome | |
| All cases | 100% (910) |
| Hospitalized | 80% (731) |
| Died | 3% (30) |
| Demographic | |
| Male | 65% (594) |
| Median age | 40 years |
| High risk indicators | |
| Substance use | 47% (427) |
| Non-IV only | 19% (172) |
| IV only | 8% (71) |
| Both | 17% (159) |
| Unknown | 3% (25) |
| History of hepatitis B or C | 26% (233) |
| Homeless/transient housing | 13% (114) |
| Institutional risk | 11% (100) |
| Men who have sex with men | 9% (79) |
| None of the above reported* | 34% (311) |
includes 18% (162) lost to follow up.
Outbreak trajectory and parameter estimation
The metapopulation infectious disease model captures the dynamics of the outbreak in and outside of Southeast Michigan (Figure 3). The Southeast Michigan outbreak grew slowly from late 2016 to early 2017, accelerated in mid 2017 and peaked in late 2017. We estimate that the cumulative incidence in the at-risk population (i.e., attack ratio) was 44% (95% CI: 38%–54%). In contrast, the outbreak outside of Southeast Michigan consisted only of sporadic cases until mid 2017, at which time local transmission became sustained. We estimate that the cumulative incidence was substantially lower in this outbreak, only 5% (95% CI: 0.3%–20%).
Figure 3:
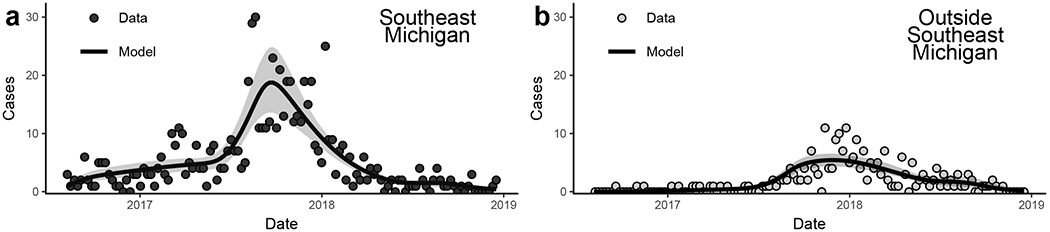
Model fit of the Michigan hepatitis A outbreak a) within Southeast Michigan and b) outside of Southeast Michigan. The ribbons give the confidence intervals for the maximum-likelihood trajectory using likelihood-based estimates of the 95% confidence parameter region.
The narrow confidence intervals for the incidence trajectory (Figure 3) reflects the relatively low uncertainty in the identifiable parameter combinations estimated by the model (Table 3). We can think of the β/κ combinations as an effective transmission rate, i.e., what the per person transmission rate would be assuming that we are observing all cases (100% reporting fraction). As expected, this observed transmission rate within Southeast Michigan is several orders of magnitude greater than the observed transmission from Southeast Michigan to the rest of the state, with observed transmission rate in areas outside of Southeast Michigan in between the two. The combination κω is the effective fraction of doses applied to at-risk individuals, scaled by the reporting fraction. We found that this effective vaccination coverage parameter for Southeast Michigan (κ1ω1) could be arbitrarily close to 0 and still fit the data. This result indicates that the end of the outbreak in Southeast Michigan was not influenced by the vaccination campaign, suggesting that the dynamics the outbreak’s end in this population could be explained solely by exhaustion of susceptible individuals in the at-risk population. For areas outside Southeast Michigan, the observed vaccination parameter did not approach 0, indicating that the vaccination campaign did impact the dynamics of that outbreak.
Table 3:
Estimated parameters and 95% confidence intervals for the Michigan hepatitis A outbreak model shown in Figure 2.
| Parameter | Estimate | 95% CI | Definition |
|---|---|---|---|
| β11/κ1 | 2.0×10−4 | (1.9, 2.1)×10−4 | Effective transmission rate within Southeast Michigan |
| β12/κ1 | 2.7×10−8 | (1.5, 4.9)×10−8 | Effective transmission rate from Southeast Michigan to the rest of the state |
| β22/κ2 | 4.3×10−6 | (4.0,5.1)×10−6 | Effective transmission rate outside of Southeast Michigan |
| κ1ω1 | 0.8×10−3 | (0.0, 3.5)×10−3 | Effective fraction of vaccine doses given to at-risk individuals for Southeast Michigan |
| κ2ω2 | 4.6×10−1 | (3.9, 5.4)×10−1 | Effective fraction of vaccine doses given to at-risk individuals outside of Southeast Michigan |
| κ1I0 | 2.5 | (1.7, 4.2) | Effective initial condition of infectious people in Southeast Michigan |
| δ | 1.6 | (1.4, 1.7) | Ratio of peak seasonal transmission to baseline tranmission |
Without knowing the size of the at-risk populations, the basic reproduction number is not identifiable from reported case data. Estimates of for HAV in the pre-vaccination era range from 1.1–1.6 [16]. In earlier work, it has been estimated that 60% of symptomatic hepatitis A infections are reported to a surveillance system [26]. Fixing the reporting fraction at this value, we can illustrate the relationship between the at-risk population size and the basic reproduction number (Figure 4). Basic reproduction numbers in the range 1.1–1.6 correspond to at-risk population sizes of 3,500–5,500 in Southeast Michigan and 170,000–270,000 in the rest of the state. Given that our model treats the rest of the state as a single well-mixed population, this latter estimate should be treated with caution. Nonetheless, it is helpful to have even preliminary estimates of the at-risk population sizes. For comparison, there are 670,000 people living in Detroit, 3.8 million across Southeast Michigan, and 10.0 million in the entire state of Michigan [27], with an estimated 8,351 people experiencing homelessness in Michigan (about 25% of whom are in the Detroit area) [28].
Figure 4:
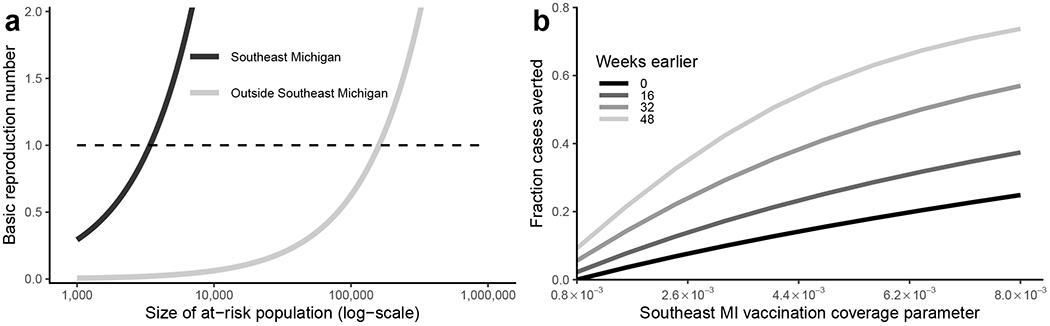
a) The estimated basic reproduction numbers depend on the size of the at-risk populations in Southeast Michigan (MI) and outside Southeast Michigan. b) The fraction of cases averted in the hepatitis A outbreak in Southeast Michigan depend on the timing of increased vaccination and vaccination coverage parameter for the at-risk population in Southeast Michigan.
Impact of vaccination
In Southeast Michigan, we estimated that only 3% of cases were averted by vaccination (95% CI: 1%–8%), equal to about 20 cases. When considering only those cases reported in August 2017 or later (when vaccination rates increased above baseline), the estimated impact increased by one percent to 4% (95% CI: 1%–11%)). However, we estimate that vaccination averted a potentially much greater outbreak in the rest of the state, with 91% of potential cases averted (95% CI: 85%–97%), equivalent to about 2,300 cases. In the counterfactual scenario, the outbreak in the rest of the state lasted an additional three years.
Our results indicate that that increased vaccination coverage earlier in the outbreak would have been necessary to have had a substantial impact on the size of the Southeast Michigan outbreak (Figure 4b). At the best-fit observed vaccination coverage parameter (0.8×10−3), we find that earlier vaccination timing alone would have had at most a modest impact on the number of cases (about 9% of cases averted with vaccination starting 48 weeks earlier, about one month after detecting the first cases). With the actual vaccination timing and with the vaccination coverage parameter increased by an order of magnitude (8.0×10−3), we estimate approximately 25% of cases would have been averted. Together, starting vaccination 48 weeks earlier and with the increase in vaccine coverage, we observe synergistic increase the fraction of cases averted, up to about 75%. Increasing the vaccination coverage parameter could be interpreted as increasing the coverage fraction itself (through better targeting), or increasing the number of doses d(t) administered.
DISCUSSION
Despite efforts to target high-risk populations in Southeast Michigan, vaccination did not reach enough at-risk individuals in time to avert a substantial number of cases in that local outbreak. This result is not surprising given the logistic and resource challenges of increasing vaccine coverage in a relatively short time. The more effective delivery of vaccines to at-risk people outside of Southeast Michigan, therefore, was aided by a later start of sustained transmission. Our model estimates suggest that this vaccination activity potentially prevented a massive outbreak lasting several more years.
Our results emphasize that proactive rather than reactive control strategies [29] will more effectively control future outbreaks. Specifically, continuous vaccination of high-risk groups—particularly in urban centers, which can serve as hubs for disease spread—prior to the emergence of an outbreak will most effectively curb transmission, although we recognize that this strategy is not always possible when resources are limited. Once cases arise, local transmission may already be sustained and thus harder to interrupt. If outbreaks do occur, the most effective strategy may be to target vaccination and control strategies to localities and populations where the disease has just been introduced, but where transmission is not yet sustained. Although the level of herd immunity needed to prevent sustained disease transmission is not known with certainty, and will vary by population, previous work has estimated that is likely somewhere between 65 and 80% [15, 20, 30]. Indeed, it has been suggested that herd immunity was responsible for limiting the spread of hepatitis A into the MSM population in the California outbreak in San Diego [30].
These results have important implications for disease control policy moving forward and could influence control strategies for nascent outbreaks. The Michigan outbreak is part of a widespread series of outbreaks that began in 2016 and have been ongoing, with almost 29,000 cases reported in 30 states by December 2019. Outbreaks of hepatitis A are expected to continue to emerge in other states; since the beginning of 2019, six states have declared outbreaks.
The Michigan outbreak is notable for its high hospitalization (80%) and death (3%) rates; the nationwide average of the 2016–2019 outbreaks estimates a 60% hospitalization and 1% death rate [7]. Moreover, according to data from the National Notifiable Disease Surveillance System between 2009–16, the average hospitalization rate for cases of hepatitis A was 44% and the average death rate was 0.8% (author analysis of data available in [31]). Although these values are likely underestimates (clinical case characteristics are typically only available in 50–60% of cases), the rates in the Michigan outbreak are substantially higher. This high death rate may be, in part, an artifact reflecting Michigan’s known diligence in checking death certificates, but it may also suggest a higher percentage of people more vulnerable to more severe outcomes. The risk indicators in this outbreak, namely substance use, history of liver disease, homelessness or transient housing, and institutional risk mirror those nationally and globally for person-to-person transmission [32]. However, the relative importance of these risk factors will vary by state and over time.
More work is needed to understand the modes of transmission at work in this outbreak and the relationship to the high risk indicators. Hepatitis A has a fecal–oral transmission route, and hand-washing and personal hygiene campaigns have been a vital part of the public health response in Michigan and other states. While some other outbreaks (e.g., San Diego [33], Philadelphia [34]) have been linked to the unsheltered homeless or open defecation, the Michigan outbreak appears to be strongly linked to substance use. It is unclear whether and how hepatitis A transmission takes advantage of substance use as a mode of transmission, or whether substance use is a confounder. While bloodborne transmission is possible [35], it is unclear whether this pathway is epidemiologically relevant. About a quarter of all cases in the Michigan outbreak (and half of cases who were substance users) reported some sort of intravenous (IV) drug use, although underreporting is likely. Nevertheless, the question has prompted renewed public health interest in needle exchange programs and should be further investigated.
The results of this analysis are based on the reported case data, the structure of the model, and our extraction of the natural history parameters from the literature. Each of these components have strengths and weaknesses. First, although we did incorporate a reporting factor, we do not know if and how reporting rates may have varied in space and time and cannot account for this potential bias. Second, the results do depend to some extent on the model’s specification (e.g., the results will be at least slightly different if we include more or fewer disease stages). Also, while the well-mixed assumptions within and outside Southeast Michigan appear to capture the overall dynamics well, we are not capturing heterogeneities in local outbreaks. Finally, while the parameters extracted from the literature are based on clinical data, we do not know how representative they are for this strain in this population.
Our lack of information about the size of the at-risk populations within and outside of Southeast Michigan was the primary sources of uncertainty in our parameter estimation. More information is needed about the prevalence and distribution of risk factors. As a first step, conducting a case–control study to investigate the differential prevalence of risk factors in people who were or were not infected in the outbreak would inform how effective targeting high-risk groups for vaccination is likely to be in practice. Finally, neither contact nor molecular tracing was available in this outbreak, either of which could have provided information to trace the spread of the disease through subpopulations, allowing for a more granular analysis of transmission [12, 36]. Molecular tracing could provide additional information about how and when outbreaks are transmitted between regions.
Supplementary Material
1. eAppendix 1. Weekly case data and weekly vaccination dose data.
2. eAppendix 2. Technical modeling details.
Acknowledgments
This work was supported by grant U01GM110712 from the National Institute of General Medical Sciences of the National Institutes of Health. We thank Jeremy Kuo (MDHHS) for initially coordinating this collaboration. We thank Sarah Lyon-Callo (MDHHS), Robert Swanson (MDHHS), Jevon McFadden (CDC), Sarah Davis (MDHHS), Jim Collins (MDHHS), Cole Burkholder (MDHHS), and Monique Foster (CDC) for providing feedback on this project.
References
- [1].Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. Journal of the American Medical Association. 2005;294(2):194–201. [DOI] [PubMed] [Google Scholar]
- [2].Murphy TV, Denniston MM, Hill HA, McDonald M, Klevens MR, Elam-Evans LD, et al. Progress Toward Eliminating Hepatitis A Disease in the United States. Morbidity and Mortality Weekly Report Supplements. 2016;65(1):29–41. [DOI] [PubMed] [Google Scholar]
- [3].Ashur Y, Adler R, Rowe M, Shouval D. Comparison of immunogenicity of two hepatitis A vaccines - VAQTA® and HAVRIX® - in young adults. Vaccine. 1999;17(18):2290–2296. [DOI] [PubMed] [Google Scholar]
- [4].Demicheli V, Tiberti D. The effectiveness and safety of hepatitis A vaccine: A systematic review. Vaccine. 2003;21(19-20):2242–2245. [DOI] [PubMed] [Google Scholar]
- [5].Sharapov UM, Bulkow LR, Negus SE, Spradling PR, Homan C, Drobeniuc J, et al. Persistence of hepatitis A vaccine induced seropositivity in infants and young children by maternal antibody status: 10-year follow-up. Hepatology. 2012;56(2):516–522. [DOI] [PubMed] [Google Scholar]
- [6].Raczniak GA, Bulkow LR, Bruce MG, Zanis CL, Baum RL, Snowball MM, et al. Long-term immunogenicity of hepatitis a virus vaccine in alaska 17 years after initial childhood series. Journal of Infectious Diseases 2013;207(3):493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention. Widespread outbreaks of hepatitis A across the United States; 2020. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Updated January 6, 2020. Accessed March 6, 2020.
- [8].Michigan Department of Health and Human Services. Michigan Hepatitis A Outbreak; 2019. https://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed August 5, 2019.
- [9].Michigan Department of Health and Human Services. 2019 Reportable Diseases in Michigan—By Pathogen; 2019. https://www.michigan.gov/documents/mdch/Reportable_Diseases_Michigan_by_Pathogen Accessed September 9, 2019.
- [10].Centers for Disease Control and Prevention. Hepatitis A, Acute 2012 Case Definition; 2012. https://wwwn.cdc.gov/nndss/conditions/hepatitis-a-acute/case-definition/2012. Accessed August 1, 2019.
- [11].Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th Ed. Hamborsky J, Kroger A, Wolfe S, editors. Washington, D.C.: Public Health Foundation; 2015. [Google Scholar]
- [12].Lemon SM, Ott JJ, Van Damme P, Shouval D. Type A viral hepatitis: A summary and update on the molecular virology, epidemiology, pathogenesis and prevention. Journal of Hepatology. 2018;68(1):167–184. [DOI] [PubMed] [Google Scholar]
- [13].Tong MJ, El-Farra NS, Grew MI. Clinical manifestations of hepatitis a: Recent experience in a community teaching hospital. Journal of Infectious Diseases. 1995;171(Suppl 1). [DOI] [PubMed] [Google Scholar]
- [14].Koff RS. Clinical manifestations and diagnosis of hepatitis A virus infection. Vaccine. 1992;10(SUPPL. 1):15–17. [DOI] [PubMed] [Google Scholar]
- [15].Regan DG, Wood JG, Benevent C, Ali H, Smith LW, Robertson PW, et al. Estimating the critical immunity threshold for preventing hepatitis A outbreaks in men who have sex with men. Epidemiology and infection. 2016;144(7):1528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Van Effelterre TP, Zink TK, Hoet BJ, Hausdorff WP, Rosenthal P. A Mathematical Model of Hepatitis A Transmission in the United States Indicates Value of Universal Childhood Immunization. Clinical Infectious Diseases. 2006;43(2):158–164. [DOI] [PubMed] [Google Scholar]
- [17].Van Effelterre T, Marano C, Jacobsen KH. Modeling the hepatitis A epidemiological transition in Thailand. Vaccine. 2016;34(4):555–562. [DOI] [PubMed] [Google Scholar]
- [18].Van Effelterre T, Guignard A, Marano C, Rojas R, Jacobsen KH. Modeling the hepatitis A epidemiological transition in Brazil and Mexico. Human Vaccines and Immunotherapeutics. 2017;13(8):1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lin KY, Sun HY, Chen YH, Lo YC, Hsieh SM, Sheng WH, et al. Effect of a hepatitis A vaccination campaign during a hepatitis A outbreak in Taiwan, 20152017: a modeling study. Clinical Infectious Diseases. 2019;. [DOI] [PubMed] [Google Scholar]
- [20].Zhang XS, Charlett A. Bayesian modelling of a hepatitis A outbreak in men who have sex with men in Sydney, Australia, 1991/1992. Epidemiology and Infection. 2019;147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cobelli C, DiStefano JJ. Parameter and structural identifiability concepts and ambiguities: a critical review and analysis. The American Journal of Physiology. 1980;239(1):R7–24. [DOI] [PubMed] [Google Scholar]
- [22].Audoly S, Bellu G, D’Angìo L, Saccomani MP, Cobelli C. Global identifiability of nonlinear models of biological systems. IEEE transactions on bio-medical engineering. 2001;48(1):55–65. [DOI] [PubMed] [Google Scholar]
- [23].Soetaert K, Setzer RW, Petzoldt T. Package ‘deSolve’; 2019. [Google Scholar]
- [24].Luebeck G, Meza R. Package ‘Bhat’; 2015. [Google Scholar]
- [25].Raue A, Kreutz C, Maiwald T, Bachmann J, Schilling M, Klingmüller U, et al. Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics. 2009;25(15):1923–1929. [DOI] [PubMed] [Google Scholar]
- [26].Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. American Journal of Public Health. 2014;104(3):482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].U S Department of Commerce. U.S. Census Bureau: Quick Facts; 2018. https://www.census.gov/quickfacts/. Accessed August 5, 2019.
- [28].United States Interagency Council on Homelessness. Michigan Homelessness Statistics; 2018. https://www.usich.gov/homelessness-statistics/mi/. Accessed November 7, 2019.
- [29].Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438(7066):355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Smith D, Huynh C, Moore AJ, Frick A, Anderson C, Porrachia M, et al. Herd Immunity Likely Protected the Men Who Have Sex With Men in the Recent Hepatitis A Outbreak in San Diego, California. Clinical Infectious Diseases. 2019;68(7):1228–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Centers for Disease Control and Prevention. Viral Hepatitis Surveillance — United States; 2019. https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm. Accessed July 17, 2019.
- [32].Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, et al. Hepatitis A virus: Host interactions, molecular epidemiology and evolution. Infection, Genetics and Evolution. 2014;21:227–243. [DOI] [PubMed] [Google Scholar]
- [33].Peak CM, Stous SS, Healy JM, Hofmeister MG, Lin Y, Ramachandran S, et al. Homelessness and Hepatitis A San Diego County, 20162018. Clinical Infectious Diseases. 2019;p. 2016–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Blumgart Jake. Hepatitis A is spreading through poop on the streets. Philly is betting public bathrooms can stem the outbreak.; 2019. https://whyy.org/articles/hepatitis-a-is-spreading-through-poop-on-the-streets-philly-is-betting-public-bathrooms-can-stem-the-outbreak/.
- [35].Lemon SM. The Natural History of Hepatitis A: The Potential for Transmission by Transfusion of Blood or Blood Products. Vox Sanguinis. 1994;67(Suppl 4):19–23. [PubMed] [Google Scholar]
- [36].Eames KTD, Keeling MJ. Contact tracing and disease control. Proceedings of the Royal Society B: Biological Sciences. 2003;270(1533):2565–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. eAppendix 1. Weekly case data and weekly vaccination dose data.
2. eAppendix 2. Technical modeling details.


