Abstract
Background:
The benefit of intravascular ultrasound (IVUS) use with percutaneous coronary intervention (PCI) in real world is uncertain.
Methods:
We identified Medicare patients who underwent PCI from 2009 to 2017 and evaluated the association of IVUS use with long-term risk of mortality, myocardial infarction (MI) and repeat revascularization. We used propensity score matching and inverse probability weighting (IPW) to adjust for baseline characteristics. To account for hospital effects, patients undergoing IVUS guided PCI were matched to non-IVUS patients in the same hospital and year. Sensitivity analyses comparing outcomes with and without IVUS in stable coronary artery disease and acute coronary syndrome, PCI with bare-metal stents and drug-eluting stents, complex and non-complex PCI, facilities with 1–5%, 5–10% and >10% IVUS utilization were performed.
Results:
Overall, IVUS was utilized in 5.6% of all PCI patients (105,787 out of 1,877,177 patients). Patients with IVUS-guided PCI had a higher prevalence of most comorbidities. In the propensity matched analysis, IVUS-guided PCI was associated with lower 1-year mortality (11.5% versus 12.3%), MI (4.9% versus 5.2%) and repeat revascularization (6.1% versus 6.7%) (P<0.001 for all). In IPW analysis with a median follow-up of 3.7 years (IQR 1.7–6.4 years), IVUS-guided PCI was associated with a lower risk of mortality (aHR 0.903, 95% CI 0.885–0.922), MI (aHR 0.899, 95% CI 0.893–0.904), and repeat revascularization (aHR 0.893, 95% CI 0.887–0.898) (P<0.001 for all). The above findings were consistent in all subgroups in sensitivity analyses.
Conclusion:
In this contemporary US Medicare cohort, the use of IVUS guidance in PCI remains low. Use of IVUS is associated with lower long-term mortality, MI and repeat revascularization.
Keywords: Intravascular ultrasound, percutaneous coronary intervention, mortality, myocardial infarction
CONDENSED ABSTRACT
Among 1,877,177 Medicare patients who underwent percutaneous coronary intervention (PCI) from 2009 to 2017, intravascular ultrasound (IVUS) was used in 105,787 patients (5.6%). Using propensity score matched analysis, IVUS-guided PCI was associated with lower 1-year mortality (11.5% versus 12.3%), MI (4.9% versus 5.2%) and repeat revascularization (6.1% versus 6.7%) (P<0.001 for all). In IPW analysis with a median follow-up of 3.7 years (IQR 1.7–6.4 years), IVUS-guided PCI was associated with lower risk of mortality (aHR 0.903, 95% CI 0.885–0.922), MI (aHR 0.899, 95% CI 0.893–0.904), and repeat revascularization (aHR 0.893, 95% CI 0.887–0.898) (P<0.001 for all).
INTRODUCTION
In contemporary interventional cardiology, percutaneous coronary intervention (PCI) is being performed in many patients with more complex coronary lesions than previously.(1) Intravascular ultrasound (IVUS) imaging is commonly used to optimize procedural results of contemporary PCI, particularly with the use of drug-eluting stents.(2) It carries a class IIb recommendation in the 2011 American College of Cardiology / American Heart Association (ACC/AHA) guidelines.(3)
In the “Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-Comers” Coronary Lesions” (ULTIMATE) prospective randomized trial,(4) use of IVUS was associated with a lower risk of target lesion failure at follow up compared to angiography alone. However, that study was not powered for assessment for mortality benefit with IVUS use. More recently, a meta-analysis of randomized controlled trials showed that the use of IVUS is associated with reduction in major adverse cardiac events.(5)
Despite the reported benefits with IVUS use in PCI in observational and randomized trials, the utilization of IVUS in real world practice remains low. In recent studies, IVUS use during PCI in the United States (U.S.) ranged from 5–7%.(6,7) Causes of underutilization of IVUS use are unclear. The lack of belief in its cost-effectiveness, lack of experience, cost of the equipment, debate about generalizability of findings of randomized trials and/or the time spent to perform the imaging are plausible explanations.
The aim of this study is to explore if IVUS use in real world patients is associated with improved long-term outcomes of PCI.
METHODS
Study cohort
Medicare patients enrolled in Fee-For-Service who underwent PCI from January 2009 to December 2017 were identified from the 100% Medicare Provider and Analysis Review (MEDPAR) Part A files. These files include all nationwide hospital admissions for Medicare beneficiaries during a given year and were obtained from the Center for Medicare and Medicaid Services (CMS). Patients undergoing PCI and the use of IVUS were identified using ICD-9-CM and ICD-10 procedure codes; before and after September 2015, respectively. We excluded patients who underwent optical coherence tomography (OCT) due to the low prevalence of use during the study period. Patient characteristics including age, sex, race, and comorbidities were derived from Medicare enrollment data and inpatient claims during the two years prior to the PCI admission. We used comorbidity algorithms originally defined by Elixhauser et al (8), as well as additional conditions that are relevant to our cohort. These included the following: presenting conditions which were defined as ST-segment elevation myocardial infarction [STEMI], non-ST-segment elevation MI [NSTEMI], or unstable angina [UA] using ICD-9 and ICD-10 diagnosis codes validated in prior studies (Supplemental Table 1) (9,10). If these three conditions were not present in any diagnosis in the claim, presentation was defined as stable coronary artery disease [CAD]). Important procedure characteristics such as use of drug-eluting stent (DES), bare-metal stent (BMS), number of stents placed, number of vessels treated, bifurcation lesion PCI, and chronic total occlusion (CTO) were defined from surgical procedure and diagnosis codes on the same admission (Supplemental Table 1). The number of stents and number of vessels were missing in 6% and 14.9% of the cohort respectively.
We divided the study cohort into two groups based on IVUS use during the procedure. The dates of beneficiary Medicare enrollment and death were obtained from the 100% Beneficiary Summary File for the same period. Patients were excluded if they had been enrolled in Medicare fee-for-service for less than two years prior to the PCI.
Outcomes
The main outcome of our study was long-term all-cause mortality. Secondary outcomes included hospitalization for MI and repeat revascularization. These outcomes were assessed at 1-year post PCI and at long-term follow up. Patients were censored due to death, Medicare disenrollment, or at the end of the study period. Data on mortality were available till September 1, 2018, and on readmissions were available till December 31, 2017. The Institutional Review Board of the University of Iowa approved the study with a waiver for individual informed consent.
Statistical analysis
The temporal trends of IVUS use with PCI were examined over the 9 year period of the study, as well as the variability in IVUS utilization between PCI facilities in the U.S. Facilities were classified as non-IVUS capable, infrequent (<1%), moderate (1–5%), frequent (5–10%) and very frequent (>10%) IVUS facility, as a percentage out of all inpatient PCI’s performed.
We compared baseline characteristics between patients undergoing IVUS-guided PCI and non-IVUS PCI with analysis of variance or Wilcoxon test as appropriate for continuous variables and Chi-Square test for categorical variables. Next, we created Kaplan-Meier (KM) curves with 95% Confidence intervals (CI’s) to determine the cumulative proportion of patients with events as a function over time which were compared using log-rank or Generalized Wilcoxon statistic. To compare outcomes between patients who underwent PCI with vs. without IVUS guidance, we conducted a propensity-matched analysis. Propensity scores were created using a non-parsimonious logistic regression model with the dependent variable of IVUS use and a list of 53 covariates as the independent variables (Supplemental Table 2). Patients who underwent IVUS-guided PCI were matched 1:1 with patients who underwent PCI without IVUS use using propensity scores and a greedy matching algorithm with a caliper width of 0.01. To account for hospital effects, a patient who underwent IVUS guided PCI was matched with a similar patient who underwent non-IVUS guided PCI in the same hospital and the same year. Robustness of the matching algorithm was assessed by comparing standardized differences with a cut off value of 0.1 (Supplemental Table 2). A total of 103,558 pairs of patients were successfully matched. To account for the matching design, we utilized McNemar’s test for comparison of short-term outcomes and a conditional logistic regression to compare long-term outcomes.
We also performed survival time inverse probability weighing (IPW) propensity score analysis for the study outcomes to limit confounding by indication through estimation of the average treatment effects.(11) First, the unstabilized inverse probability weights of getting IVUS for the whole cohort were derived from the propensity scores. Then, multivariable survival models adjusting for age, sex, race, preexisting comorbidities, clinical presentation, and procedure characteristics were created using the inverse probability weights to determine the adjusted effect of IVUS use on the primary and secondary outcomes. The model was generated using a robust sandwich covariance matrix estimate and robust standard error estimates to account for the clustering of patients within hospitals.(12) For the secondary outcomes of MI and repeat revascularization, multivariable survival analyses were performed by competing risk regression analysis to account for death as a competing risk, using the Fine-Gray proportional sub hazards model, and subdistribution hazard ratios (sHRs) were calculated, along with 95% CIs.(13)
Sensitivity Analyses
We conducted a range of pre-specified subgroup analyses to evaluate the robustness of our findings. We compared study outcomes between IVUS-guided and non-IVUS PCIs in subgroups based on a) presentation (stable CAD or acute coronary syndrome (ACS)); b) type of stents utilized (DES) or (BMS); c) complexity of PCI (complex PCI defined as ≥3 stents placed and/or ≥ 2 vessels treated and/or intervention on a bifurcation or CTO lesion). This definition is similar to a definition used in prior studies (14). However, we lacked information on stent length. For each subgroup analysis, propensity scores were re-estimated using logistic regression models similar to the one described for the main analysis. The above analyses were performed with IPW method. We also analyzed study outcomes in centers with moderate, frequent and very frequent IVUS separately.
All analysis was performed with SAS version 9.4 (SAS Institute, Cary, North Carolina) and R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
The study population included 1,877,177 patients who underwent PCI during the study period. Overall, IVUS was used in 5.6% of the cohort (n= 105,787). The IVUS-guided PCI group had higher prevalence of most comorbidities including heart failure (28.6% versus 25.8%), prior CAD (22.8% versus 21.8%), prior stroke (5.1% versus 4.2%), chronic kidney disease (21.1% versus 19.3%), chronic lung disease (22.9% versus 20.9%) and pulmonary hypertension (4.8% versus 3.9%) (P<0.001 for all). IVUS-guided PCI group also had higher prevalence of complex PCI as evident by higher number of stents placed, higher number of vessels treated, as well as higher number of bifurcation and CTO lesions. However, patients who received non IVUS-guided PCI were more likely to present with MI (56.2% versus 48.8%, p<0.001). Table 1 summarizes the baseline characteristics. After propensity score matching, all variables were well balanced between both groups (Supplemental Table 2). Table 2&3 summarize the clinical presentations, procedure characteristics and in-hospital complications of the two groups.
Table 1:
Baseline demographics and characteristics of the two study groups
| Variable | No IVUS | IVUS | P value |
|---|---|---|---|
| Number | 1,771,390 | 105,787 | |
| Age | 73.3±9.4 | 73±9.3 | <0.01 |
| Male sex | 60.1% | 60.0% | 0.6 |
| Black race | 8.3% | ||
| Diabetes mellitus | 38.4% | 38.7% | 0.04 |
| Hypertension | 80.1% | 82.5% | <0.001 |
| History of heart failure | 25.8% | 28.6% | <0.001 |
| Prior coronary artery disease | 21.8% | 22.8% | <0.001 |
| Prior revascularization | 19.2% | 20.1% | <0.001 |
| Prior bleeding | 4.7% | 5.3% | <0.001 |
| Prior GI bleed | 2.4% | 2.6% | <0.001 |
| Prior cerebral bleed | 0.2% | 0.2% | 0.8 |
| Prior ischemic stroke | 4.2% | 5.1% | <0.001 |
| Prior smoking | 24.4% | 25.8% | <0.001 |
| Pulmonary hypertension | 3.9% | 4.8% | <0.001 |
| Peripheral arterial disease | 14.4% | 16.4% | <0.001 |
| Liver disease | 1.4% | 1.8% | <0.001 |
| Chronic kidney disease | 19.3% | 21.1% | <0.001 |
| Prior permanent pacemaker | 3.4% | 3.8% | <0.001 |
| Prior intracardiac defibrillator | 1.9% | 2.4% | <0.001 |
| Preexisting atrial fibrillation | 15.4% | 16.8% | <0.001 |
| Sleep apnea | 7.5% | 9.4% | <0.001 |
| Underweight | 2.1% | 2.3% | <0.0001 |
| Obesity | 13.7% | 15.2% | <0.001 |
| Hypothyroid | 12.5% | 14.1% | <0.001 |
| Anemia deficiency | 13.8% | 15.9% | <0.001 |
| Rheumatoid arthritis and other connective tissue disease | 2.8% | 3.3% | <0.001 |
| Coagulopathy | 3.8% | 4.7% | <0.001 |
| Tumor without metastasis | 1.6% | 1.7% | 0.1 |
| Lung disease | 20.9% | 22.9% | <0.001 |
| Electrolytes abnormality | 16.9% | 18.4% | <0.001 |
| Depression | 7.3% | 8.5% | <0.001 |
Table 2:
Clinical presentation and procedure characteristics of the two study groups
| Variable | No IVUS | IVUS | P value |
|---|---|---|---|
| Number | 1,771,390 | 105,787 | |
| Stable coronary artery disease | 29.4% | 35.0% | <0.001 |
| Unstable angina | 15.0% | 16.9% | <0.001 |
| Non-ST elevation myocardial infarction (MI) | 34.5% | 33.1% | <0.001 |
| Anterior ST-elevation MI | 8.1% | 7.0% | <0.001 |
| Inferior ST-elevation MI | 13.6% | 8.7% | <0.001 |
| Cardiogenic shock | 4.6% | 4.5% | 0.03 |
| Cardiac arrest | 3.9% | 3.6% | <0.001 |
| Length of hospital stay (days), median (IQR) | 3 (2–5) | 3 (1–5) | <0.001 |
| Bare-metal stents | 23.1% | 17.5% | <0.001 |
| Drug-eluting stents | 78.3% | 84.1% | <0.001 |
| Bifurcation lesion | 2.2% | 4.0% | <0.001 |
| Chronic total occlusion | 10.6% | 10.1% | <0.001 |
| ≥4 | 3.1% | 5.0% | |
| ≥4 | 0.6% | 1.1% | |
| Complex percutaneous coronary intervention† | 31.2% | 39.3% |
6.1% of the values missing
14.9% of the values missing.
Defined as ≥3 stents placed and/or ≥ 2 vessels treated and/or intervention on a bifurcation/CTO lesion
Table 3:
One-year outcomes in both study groups in the whole cohort and in the propensity score-matched cohort
| variable | No IVUS | IVUS | P value |
|---|---|---|---|
| Before propensity score matching | |||
| Number | 1,771,390 | 105,787 | |
| 30-day mortality | 4.5% | 3.7% | <0.0001 |
| 1-year MI | 5.3% | 4.9% | <0.0001 |
| 1-year revascularization | 6.4% | 6.0% | <0.0001 |
| 1-year mortality | 12.2% | 11.5% | <0.0001 |
| After propensity score matching | |||
| Number | 103,558 | 103,558 | |
| 30-day mortality | 4.3% | 3.7% | <0.01 |
| 1-year MI | 5.2% | 4.9% | <0.01 |
| 1-year Revascularization | 6.7% | 6.1% | <0.01 |
| 1-year mortality | 12.3% | 11.5% | <0.01 |
| Events per 1000 person-years | |||
| Long-term mortality | 88.7 | 82.8 | <0.001 |
| Long-term MI | 35.8 | 33.5 | <0.001 |
| Long-term repeat revascularization | 39.9 | 35.8 | <0.001 |
IVUS Utilization
IVUS use with PCI increased from 3.0% in 2009 to 6.9% in 2017 (P for trend <0.01) (Supplemental Table 3). Out of 1934 PCI facilities, 1073 (55.5%) did not use any IVUS or used IVUS in <1% of PCI procedures. Among the remaining 861 facilities, there was large variability in IVUS utilization (median 5.7%, IQR 3.1%–10.7%) with 360 (33.6%), 261 (24.3%) and 240 (22.4%) facilities using IVUS in 1–5% (moderate), 5–10% (frequent) and >10% (very frequent) of PCI procedures, respectively (Central illustration). Facilities with >5% IVUS utilization were more likely to be a teaching hospital (29% versus 25%, P=0.05), and with higher bed capacity (median 302 beds versus 279 beds, P=0.03). The majority of facilities with >5% IVUS utilization were located in Southeast and West regions (25.6% and 24.5%), followed by Midwest (21.2%).
Central illustration: Trends, variability and outcomes of IVUS use in PCI.
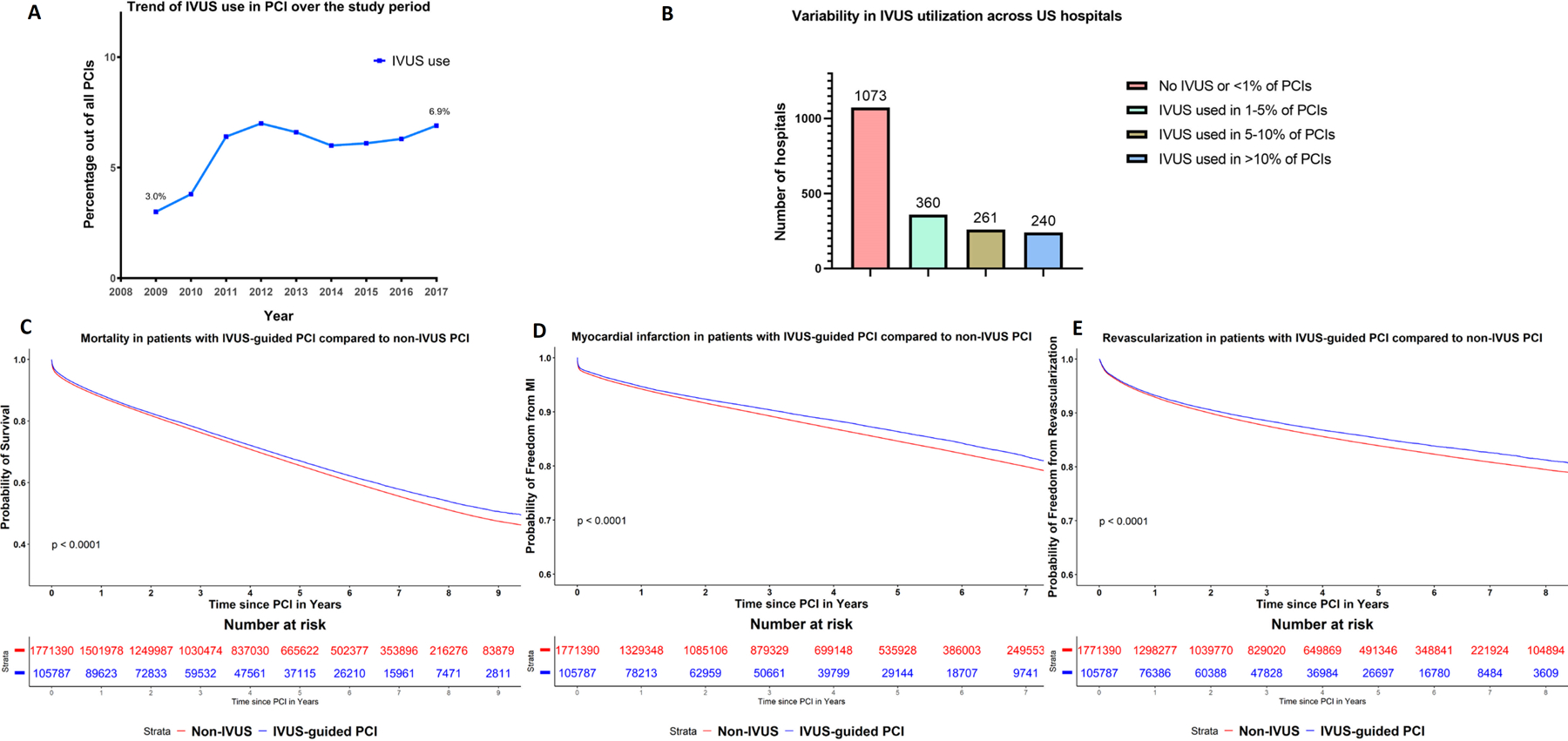
Panel A: Trend in intravascular ultrasound (IVUS) use as a percentage out of all percutaneous coronary interventions in Medicare patients in the study period. Panel B: Variability in IVUS utilization across US hospitals. Panel C-E: Kaplan Meier curves for all-cause mortality, myocardial infarction and revascularization between PCI with and without intravascular ultrasound in the overall cohort.
Short- and intermediate-term outcomes
Table 3 shows the unadjusted 1-year outcomes of IVUS-guided PCI and non-IVUS PCI. IVUS-guided PCI use was associated with a lower 1-year mortality compared to PCI without IVUS (11.5% versus 12.2%, p<0.001). IVUS use was similarly associated with lower rate of 1-year MI (4.9% versus 5.3%) and 1-year repeat revascularization (6.0% versus 6.4%), P<0.001 for both.
In the propensity score-matched cohort of patients, mortality was lower in the IVUS group compared to the non-IVUS group (3.7% versus 4.3%, P<0.001) at 30-days, while MI was numerically lower but not significantly different (2.1% versus 2.2%, P=0.2). At 6-months and one year, MI was lower in IVUS group compared to the non-IVUS group (3.6% versus 3.8%, P=0.03) and (4.8% versus 5.1%, P=0.01) respectively. IVUS remained associated with significantly lower 1-year mortality (11.5% versus 12.3%%, P<0.01). It was also associated with lower 1-year MI and 1-year repeat revascularization (4.9% versus 5.2% and 6.1% versus 6.7% respectively, P<0.01 for both).
Long-term outcomes
Overall, median follow up was 3.7 years (IQR 1.7–6.4 years) for mortality and 3.0 years (IQR 1.0–5.6 years) for other outcomes. On propensity score-matched analysis, IVUS-guided PCI was associated with lower long-term mortality compared to PCI without IVUS (82.8 versus 88.7 event per 1000 person-years, adjusted HR (aHR) 0.921, 95% CI 0.908–0.935, P<0.001). Similarly, IVUS-guided PCI was associated with lower long-term MI (33.5 versus 35.8 events per 1000 person-years, aHR 0.927, 95% CI 0.903–0.951, P<0.001), and repeat revascularization rates (35.8 versus 39.9 events per 1000 person-years, aHR 0.891, 95% CI 0.869–0.914, P<0.001). For mortality, on landmark analysis at one year, the risk of mortality was lower with IVUS in the first year (aHR 0.913, 95% CI 0.891–0.935, P<0.001) as well as after the first year (aHR 0.937, 95% CI 0.921–0.954, P<0.001).
On inverse probability weighting analysis, IVUS guided PCI remained associated with lower long-term mortality (aHR 0.903, 95% CI 0.885–0.922, P<0.001), MI (sHR 0.899, 95% CI 0.893–0.904, P<0.001), and repeat revascularization (sHR 0.893, 95% CI 0.887–0.898, P<0.001). (Central illustration and Supplemental Table 4).
Sensitivity analyses
IVUS remained associated with significantly lower risk of the long-term mortality, MI and repeat revascularization when analysis was done separately in patients who presented with ACS (N= 1,319,271) (Figure 1A–C), stable CAD (N=557,906) (Supplemental Figure), patients who were treated with BMS (N=402,009), and patients who were treated with DES (N=1,475,168), patients who underwent complex PCI (N= 594,003)and patients who underwent non-complex PCI (N=1,283,174), patients who were treated in moderate (N=661,112), frequent (N=353,224), and very frequent (N=287,840) IVUS facilities. Figure 2 shows interaction analysis with risk estimates in subgroup analyses.
Figure 1A–C:
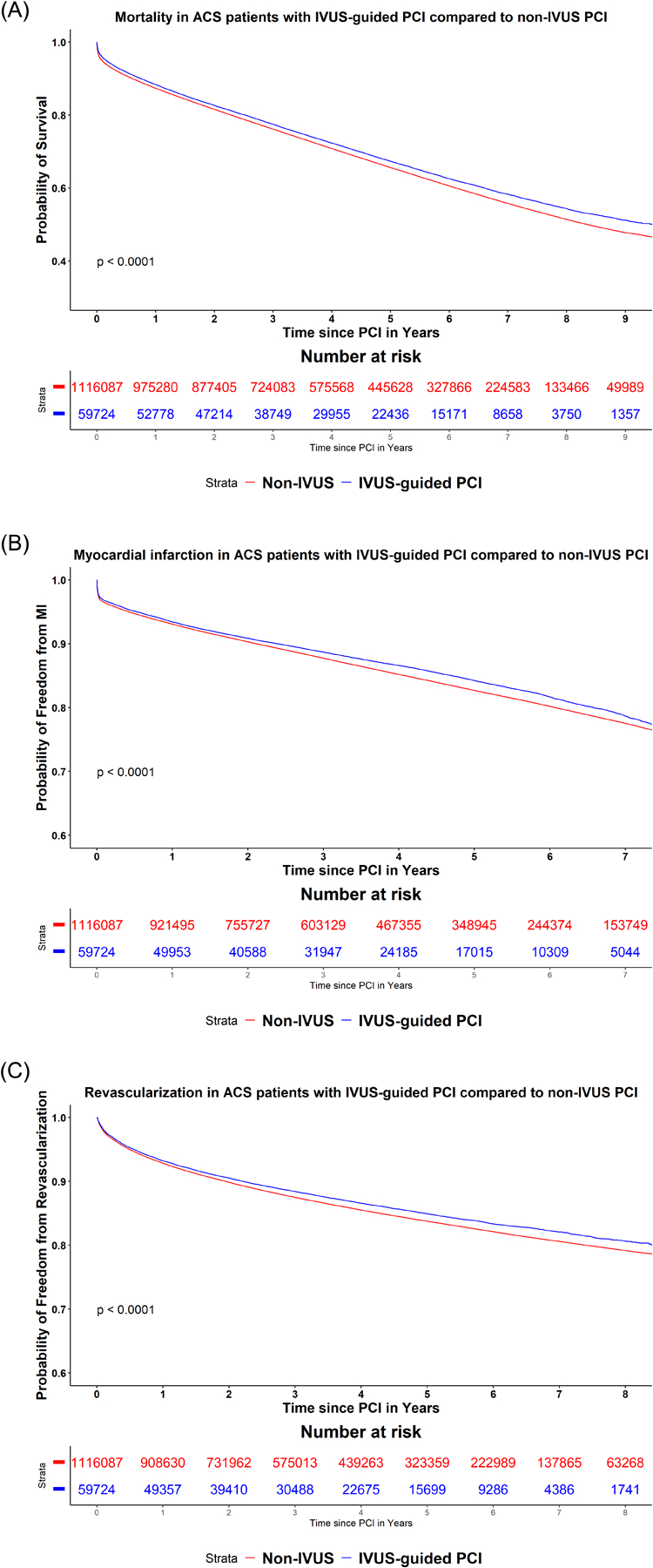
Kaplan Meier curves for the study outcomes A) all-cause mortality, B) myocardial infarction, C) repeat revascularization between PCI with and without intravascular ultrasound in patients presenting with acute coronary syndrome.
Figure 2:
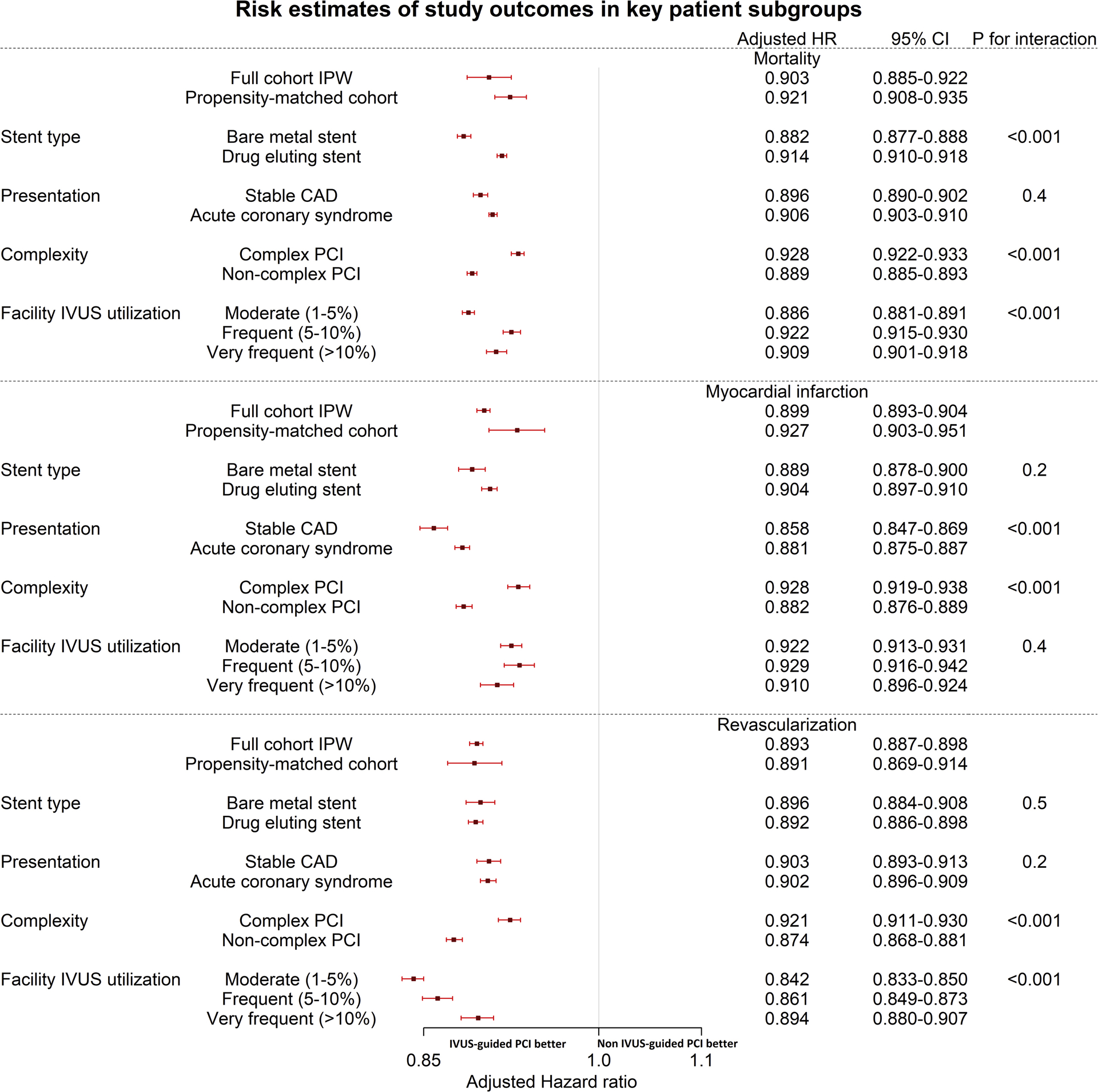
Risk estimates in key patient subgroups and interaction analysis.
DISCUSSION
In this study from Medicare database we demonstrate several findings. First, overall IVUS utilization during PCI remains low in the U.S. during our study period with a wide variation in its use among different facilities. Second, the use of IVUS during PCI in the U.S. was associated with a lower risk of mortality, MI and hospitalization for repeat revascularization compared to patients in whom IVUS was not used during PCI (Central illustration). The favorable outcomes with IVUS were evident in facilities regardless of high or low IVUS use. Third, the beneficial impact of IVUS use during PCI on clinical outcomes was consistent across various subgroups defined by presentation (ACS versus stable CAD), type of stent implanted (DES vs BMS), or the complexity of PCI.
The prevalence of IVUS use in PCI during the study period was relatively low (5.6%) and it increased modestly over the nine years of the study. This is consistent with prior studies published from national registries in the U.S.(6,7) Despite its potential benefits, IVUS remains underutilized in the U.S. Studies from other countries report the prevalence of IVUS use ranging from 22% to more than 90% of PCI cases.(15,16) Furthermore, there is a high variability of IVUS use in PCI between different hospitals in the US.(6) The use of IVUS in optimizing stent implantation currently carries a class IIb recommendation in the American College of Cardiology/American Heart Association (ACC/AHA) 2011 guidelines and carries a class IIa in the European Society of Cardiology (ESC) 2018 guidelines for PCI (3,17). IVUS use in PCI offers several potential advantages including more accurate vessel sizing, improved stent expansion, larger minimum stent lumen area, better strut apposition and identification of complications such as edge dissections or intramural hematomas.(18) This subsequently leads to reduction in the rates of in-stent restenosis and stent thrombosis,(18) and is considered the plausible mechanism for reduced MI and repeat revascularization with IVUS use.
With advances in DES design and the associated significant reduction in adverse events such as in-stent restenosis and thrombosis, it was proposed that IVUS use might not provide any additional benefit to angiographic guidance in regard to improving outcomes. However, several studies have demonstrated the benefit of IVUS guidance, even with use of DES in reducing adverse cardiac events.(19,20) In our study, IVUS use with PCI utilizing both BMS and DES was associated with lower mortality, MI and revascularization in follow up.
In a recent study in which 1,448 patients undergoing PCI with DES were randomized to IVUS guided versus angiographically guided PCI found a significantly lower risk of a composite endpoint of cardiac death, target vessel MI and target vessel revascularization at follow up among patients treated with an IVUS guided approach.(4) However, there was no significant difference in the individual endpoints of cardiac death or MI, likely due to the limited sample size in that study. Previous observational studies in patients with left main disease have shown reduction in mortality in IVUS-guided unprotected left main stenting.(21,22) To our knowledge, the current study is the first to report a significant association of IVUS use with long-term mortality, MI and repeat revascularization in an “all-comer” patient population and the above association was consistent among key patient subgroups. The separation of line estimates noted on the Kaplan Meir curves during follow up indicates the hazard was proportional between the two groups and constant during the follow up. At 30 days, the difference in mortality was significant. For MI, events were numerically higher at 30-days but not significantly different. At 6 months and at one-year, MI became significantly lower with IVUS-guided PCI. This is similar to the findings from prior reports such as the MATRIX registry.(ref PMID 21939937)
It is important to note that the use of IVUS in the current study was less common in patients with acute MI. Several plausible factors for this finding include reluctance of operators to perform intravascular imaging in the presence of intra-luminal thrombus and increased risk of spasm. IVUS use in MI patients can help identify the true culprit lesion and the severity of coronary remodeling and hence allow optimum choice of stent size.
In patients undergoing CTO PCI, IVUS can be used to detect the exact site of occlusion and facilitate guide wire crossing especially in patients with an ambiguous proximal cap, and to help re-direction of the wire into the true lumen from the subintimal space.(23) Despite these potential benefits, prior studies have reported conflicting results about the role of IVUS in improving success rates and outcomes after CTO PCI, however, results were limited with small patient populations.(24) Another important group of patients are those undergoing complex PCI. Optimizing stent deployment and expansion, in the setting of multiple and overlapping stents in heavily calcified vessels, or at bifurcation locations could potentially drive lower rates of adverse cardiac events in these patients.(18) However, In a prior randomized controlled trial, IVUS use during complex PCI failed to show benefit in reducing adverse cardiac events in follow up.(25) This study was limited with the small number of patients (N=284) and short follow up period (24 months).(25) Similar to our study, a recent prospective registry study by Choi and colleagues showed that IVUS use in complex procedures was associated with a significantly lower mortality, MI and revascularization.(26)
Our study is one of the largest to date to report long-term outcomes of IVUS guided PCI, however, our study has several limitations. First, we lacked information on stent or vessel diameter, stent or lesion length, all factors known to affect risk of in-stent restenosis and thrombosis, with subsequent need for repeat revascularization and/or MI. Second, it is unclear whether IVUS imaging changed the management in the IVUS-guided PCI group or it is just a marker of more sophisticated PCI and operator skills. However, in prior randomized trials and registry studies, IVUS use changed management and was associated with post-stenting optimization in >80% of cases. (2,4) Third, we lacked information on ejection fraction (EF), and EF is known to have an important prognostic role in mortality of patients with CAD. Fourth, we included PCI procedures that were done as an inpatient procedure, we did not have access to PCI procedures that were done in an outpatient setting, extrapolation of our results to such procedures might be limited. Fifth, Fifth, we could not ascertain if a repeat revascularization procedure was in the same treated vessel or a new vessel, thus patients with staged procedures or progression of disease in previously untreated segments could not be distinguished from those with in-stent restenosis or thrombosis. Last, as this is a retrospective observational study, selection bias and unmeasured confounders could not be completely eliminated despite robust statistical adjustments.
CONCLUSION
Among Medicare patients, the contemporary use of IVUS in the U.S. remains low and highly variable across hospitals. Our study, as well as other observational and randomized trials, demonstrate that the use of IVUS during PCI is associated with lower long-term mortality, MI and repeat-revascularization compared to conventional angiography guided PCI. Such observations remained evident regardless of various clinical and procedural variabilities.
Supplementary Material
PERSPECTIVES.
WHAT IS KNOWN?
Intravascular ultrasound (IVUS) use during percutaneous coronary intervention (PCI) was shown to reduce major adverse cardiac events in randomized controlled trials.
WHAT IS NEW?
In the Medicare population in the US, IVUS use in PCI remains low in current practice. IVUS use was associated with lower risk of long-term mortality, myocardial infarction and revascularization in patients presenting with stable coronary artery disease or acute coronary syndrome.
WHAT IS NEXT?
Randomized controlled adequately powered trials may be needed to confirm the favorable impact of IVUS use with PCI on mortality.
Funding:
Dr. Mentias received support from National Institute of Health NRSA institutional grant (T32 HL007121) to the Abboud Cardiovascular Research Center. Dr. Sarrazin is supported by funding from the National Institute on Aging (NIA R01AG055663-01), and by the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs.
Disclosures:
Dr. Horwitz receives grant support from Edwards Lifesciences and Boston Scientific. The remaining authors do not have any conflicts of interest or financial disclosures.
The remaining authors have nothing to disclose.
ABBREVIATIONS AND ACRONYMS
- ACS
Acute coronary syndrome
- BMS
Bare metal stent
- CTO
Chronic total occlusion
- DES
Drug-eluting stent
- IPW
Inverse probability weighting
- IVUS
Intra-vascular ultrasound
- MI
Myocardial infarction
- NSTEMI
non-ST elevation myocardial infarction
- PCI
Percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Landes U, Bental T, Levi A et al. Temporal trends in percutaneous coronary interventions thru the drug eluting stent era: Insights from 18,641 procedures performed over 12-year period. Catheter Cardiovasc Interv 2018;92:E262–E270. [DOI] [PubMed] [Google Scholar]
- 2.Witzenbichler B, Maehara A, Weisz G et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation 2014;129:463–70. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–122. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Gao X, Kan J et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol 2018;72:3126–3137. [DOI] [PubMed] [Google Scholar]
- 5.di Mario C, Koskinas KC, Raber L. Clinical Benefit of IVUS Guidance for Coronary Stenting: The ULTIMATE Step Toward Definitive Evidence? J Am Coll Cardiol 2018;72:3138–3141. [DOI] [PubMed] [Google Scholar]
- 6.Smilowitz NR, Mohananey D, Razzouk L, Weisz G, Slater JN. Impact and trends of intravascular imaging in diagnostic coronary angiography and percutaneous coronary intervention in inpatients in the United States. Catheter Cardiovasc Interv 2018;92:E410–E415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elgendy IY, Ha LD, Elbadawi A et al. Temporal Trends in Inpatient Use of Intravascular Imaging Among Patients Undergoing Percutaneous Coronary Intervention in the United States. JACC Cardiovasc Interv 2018;11:913–915. [DOI] [PubMed] [Google Scholar]
- 8.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 9.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel AB, Quan H, Welsh RC et al. Validity and utility of ICD-10 administrative health data for identifying ST- and non-ST-elevation myocardial infarction based on physician chart review. CMAJ Open 2015;3:E413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee EW, Wei L, Amato DA, Leurgans S. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. Survival analysis: state of the art: Springer, 1992:237–247. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association 1999;94:496–509. [Google Scholar]
- 14.Giustino G, Chieffo A, Palmerini T et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J Am Coll Cardiol 2016;68:1851–1864. [DOI] [PubMed] [Google Scholar]
- 15.Kuno T, Numasawa Y, Sawano M et al. Real-world use of intravascular ultrasound in Japan: a report from contemporary multicenter PCI registry. Heart Vessels 2019. [DOI] [PubMed] [Google Scholar]
- 16.Kim N, Lee JH, Jang SY et al. Intravascular modality-guided versus angiography-guided percutaneous coronary intervention in acute myocardial infarction. Catheter Cardiovasc Interv 2019. [DOI] [PubMed] [Google Scholar]
- 17.Neumann FJ, Sousa-Uva M, Ahlsson A et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 18.Mahtta D, Elgendy AY, Elgendy IY, Mahmoud AN, Tobis JM, Mojadidi MK. Intravascular Ultrasound for Guidance and Optimization of Percutaneous Coronary Intervention. Interv Cardiol Clin 2018;7:315–328. [DOI] [PubMed] [Google Scholar]
- 19.Nerlekar N, Cheshire CJ, Verma KP et al. Intravascular ultrasound guidance improves clinical outcomes during implantation of both first- and second-generation drug-eluting stents: a meta-analysis. EuroIntervention 2017;12:1632–1642. [DOI] [PubMed] [Google Scholar]
- 20.Shin DH, Hong SJ, Mintz GS et al. Effects of Intravascular Ultrasound-Guided Versus Angiography-Guided New-Generation Drug-Eluting Stent Implantation: Meta-Analysis With Individual Patient-Level Data From 2,345 Randomized Patients. JACC Cardiovasc Interv 2016;9:2232–2239. [DOI] [PubMed] [Google Scholar]
- 21.Andell P, Karlsson S, Mohammad MA et al. Intravascular Ultrasound Guidance Is Associated With Better Outcome in Patients Undergoing Unprotected Left Main Coronary Artery Stenting Compared With Angiography Guidance Alone. Circ Cardiovasc Interv 2017;10. [DOI] [PubMed] [Google Scholar]
- 22.Park SJ, Kim YH, Park DW et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv 2009;2:167–77. [DOI] [PubMed] [Google Scholar]
- 23.Galassi AR, Sumitsuji S, Boukhris M et al. Utility of Intravascular Ultrasound in Percutaneous Revascularization of Chronic Total Occlusions: An Overview. JACC Cardiovasc Interv 2016;9:1979–1991. [DOI] [PubMed] [Google Scholar]
- 24.Karacsonyi J, Alaswad K, Jaffer FA et al. Use of Intravascular Imaging During Chronic Total Occlusion Percutaneous Coronary Intervention: Insights From a Contemporary Multicenter Registry. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chieffo A, Latib A, Caussin C et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J 2013;165:65–72. [DOI] [PubMed] [Google Scholar]
- 26.Choi KH, Song YB, Lee JM et al. Impact of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention on Long-Term Clinical Outcomes in Patients Undergoing Complex Procedures. JACC Cardiovasc Interv 2019;12:607–620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


