Abstract
A combination of aged garlic, ginger, and chili peppers extracts (AGC) was studied by high-performance liquid chromatography, 2,2-diphenyl-1-picrylhydrazyl, and ferric-reducing antioxidant assays, and oxidative stress markers were analyzed in Aβ1-42-induced rats. The AGC was orally administered to Wistar rats at doses of 125, 250, and 500 mg/kg body weight (AGC125, AGC250, AGC500, respectively) for 64 days. At day 56, Aβ1-42 was injected via both sides of the lateral ventricles. The effects of the AGC on spatial and recognition memory were examined using a Morris water maze and novel object recognition tasks. Rats induced with Aβ1-42 exhibited obvious cognitive deficits, as demonstrated by their increased escape latency time (ET) and decreased retention time (RT) and percentage of discriminative index (DI). When compared with the control group, all AGC-treated rats showed significantly shorter ETs and higher DIs during the 5-min delay testing phase. Rats treated with AGC250 also had significantly longer RTs. Administration of Aβ1-42 significantly increased malondialdehyde (MDA) levels and decreased superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) levels in the rat brain homogenate. Pretreatment with the AGC caused significant increases in SOD, GPx, and CAT activities, as well as a significant decrease in MDA in the rat brain homogenates after Aβ-induced neurotoxicity. Our results suggested that an AGC may ameliorate cognitive dysfunction in Aβ-treated rats due to its role in the upregulation of SOD, GPx, and CAT.
Keywords: antioxidant, Morris water maze, novel object recognition, spatial memory
Introduction
Many types of spicy Thai food often contain ingredients that are high in antioxidants. Phad phrik khing is a popular Thai food that consists of a mixture of garlic, ginger, and chili peppers. These ingredients have been reported to be important antioxidants that may protect against memory impairment caused by oxidative stress. Extracts of garlic, ginger, and chili peppers have recently gained increased attention from researchers investigating various approaches to learning and memory [34, 36]. Aged garlic extract (AGE), an odorless preparation produced by subjecting garlic to an aging process, has demonstrated various pharmacological activities, including improvements with respect to learning and memory impairments [36], as well as neurotrophic and anti-aging effects [31]. Ginger (Zingiber officinale) is widely used as a food and medicine in countries in both Asia and the West to treat fever, digestive problems, and muscular discomfort [37]. Ginger has been reported to exhibit a wide range of neuroprotective effects, including cognitive enhancement and anti-5HT3-receptor effects [17]. Chili peppers are the fruits of plants in the Capsicum annuum family, which have been used medicinally for centuries. Capsaicin, the active component in chili peppers, has been shown to exhibit neuroprotective affects against brain-induced excitotoxicity in rats and to prevent memory deficit in rats with induced Alzheimer’s disease (AD) [45, 56]. Individually, these three herbs have been shown to enhance cognitive performance and to improve memory impairment. There have been no studies on whether the synergistic effect of these three ingredients in combination has anti-amnesic or cognitive-enhancing effects on memory and learning tasks. However, there are in vitro and in vivo data indicating that these three compounds exhibit high levels of antioxidant activities [22, 27, 40]. Oxidative stress plays a crucial role in the pathogenesis of neurodegenerative diseases including aging and Alzheimer’s disease [47]. β-amyloid (Aβ) peptide in the senile plaques of AD brains has been proposed as a mediator of oxidative stress and neurotoxicity by inducing protein oxidation and lipid peroxidation in vitro and in vivo [6, 57]. The damage to cellular components induced by reactive oxygen species can become more widespread due to the weakening of the brain’s cellular antioxidant defense mechanisms. Innate defenses may not be sufficient if oxidative stress is severe. Antioxidant compounds might act to prevent the propagation of cell damage and improve neurological outcomes. The combined extracts of AGE, ginger, and chili peppers may have more successful synergistic effects. Accordingly, the experiments presented here were designed to quantitatively evaluate the antioxidant activity in the combined extracts of AGE, ginger, and chili peppers (AGC) and to examine the effects of the AGC on learning and memory in young adult male rats by using the Morris water maze and novel object recognition tasks. The effects of the AGC on the alteration of malondialdehyde and three antioxidant enzymes, superoxide dismutase, catalase, and glutathione peroxidase, in the brain tissue were also investigated.
Materials and Methods
Plant materials and reagents
Aged garlic, ginger, and chili peppers extracts were prepared by the Center for Research and Development of Herbal Health Products (CRD-HHP), Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand. Fresh locally grown samples of each herb were macerated in a blender. The macerated plants were then extracted for 13 months in 30% ethanol (garlic), seven days in 50% ethanol (chili peppers), and seven days in 95% ethanol (ginger) in a closed conical flask at room temperature. Each crude ethanol extract was filtered through Whatman No. 1 qualitative filter paper and stored at −20°C until used. After filtration and evaporation, a dried AGE powder (Petty patent No. 3506, Thailand) containing 30.96 mg/g of S-allyl cysteine and 32 μg/g of allicin, a dried ginger powder containing 179.07 μg/mg of 6-gingerol, and a dried chili pepper powder containing 8.57 mg/g of capsaicin were obtained. The combination of aged garlic, ginger, and chili pepper extracts was prepared according to the proportions found in Phad phrik khing (2:2:1). All other chemicals were analytical grade.
Determination of antioxidant activity
Antiradical efficiency was assessed using the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) scavenging capacity assay [20, 44]. Each sample (AGE, ginger extract, chili pepper extract, and AGC; 2:2:1) was mixed with a methanol and DPPH methanol solution. The mixture was kept at room temperature for 15 min. The decrease in absorbance of the DPPH solution was measured at 517 nm using a UV-visible spectrophotometer. Vitamin C and vitamin E were used as the reference antioxidants. DPPH radical scavenging activity was expressed as inhibition % = [(A0 − A) / A0] × 100, where A0 was the absorbance of DPPH without the sample solution and A was the absorbance of DPPH with the sample solution under the same conditions. The ferric reducing antioxidant power (FRAP) assay based on the reduction of Fe3+TPTZ complex to blue colored Fe2+TPTZ solution by electron donating substances under acidic conditions [4] was also used to determine the antioxidation activity. Plant extract was allowed to react with the FRAP solution in the dark for 30 min. The absorbance of the assay solution was measured at 593 nm for four minutes at 37°C. The final absorbance was calculated using a standard curve. The data were expressed as μmol Fe2+/g crude extract. A high FRAP value indicated that the antioxidant activity of the sample was high.
Determination of total phenolic content
The total phenolic content was measured using a modified Folin and Ciocalteu method [44], employing the reduction of a phosphowolframate-phosphomolybdate complex to blue products by phenolic compounds. The sample was dissolved in 50% ethanol at various concentrations (50 μl) and reacted with Folin-Ciocalteu reagent (80 μl) in a microplate for 4 min at room temperature. The absorbance was then measured at 650 nm, and the total phenolic content was expressed in gallic acid equivalents (GAE) in terms of mg/g of extract.
Animals and treatments
All experiments were conducted under the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of Khon Kaen University (Approval No. 0514.1.12.2/30). The behavior of 42 male Wistar rats (8 weeks of age) was assessed using Morris water maze (MWM) and Novel object recognition (NOR) tasks. The animals were kept at 25 ± 2°C with a relative humidity of 50–70% and maintained on a 12-h light/dark cycle with free access to commercial food pellets and drinking water. After one week of familiarization with the surroundings, all animals were trained with the MWM test for five days to assess their learning ability. The animals that were unable to learn how to relate the object with the surrounding environment were excluded from the experiments. The rats were divided into six groups (n=7) as follows: Group 1, the vehicle plus artificial cerebrospinal fluid (ACSF) group (Vehicle+ACSF), received distilled water orally and was injected with ACSF via the lateral ventricles. Group 2, the vehicle plus Aβ group (Vehicle+Aβ, received distilled water orally and was injected with amyloid-β (Aβ1-42) via the lateral ventricles. Group 3, the vitamin C plus Aβ group (Vit C+Aβ), received vitamin C (Blackmores, Australia) orally at 250 mg/kg body weight (b.w.) and was injected with Aβ1-42 via the lateral ventricles. Groups 4, 5, and 6, the combined extract of aged garlic, ginger, and chili (2:2:1) plus Aβ groups (AGC125+Aβ, AGC250+Aβ, AGC500+Aβ), received AGC orally at doses of 125, 250, and 500 mg/kg b.w., respectively, and were injected with Aβ1-42 via the lateral ventricles.
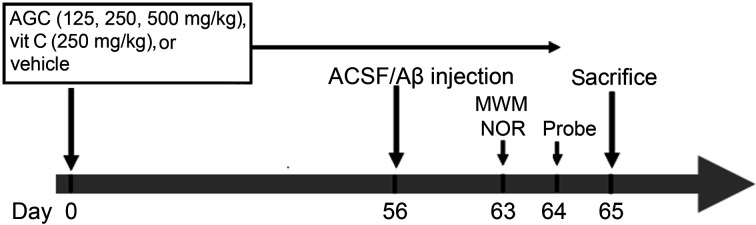
All treatments were performed by gastric gavage with biomedical needles at 8:00 to 9:00 a.m. for 64 consecutive days. At day 56, the rats in groups 2–6 received an injection of Aβ1-42 into both sides of the lateral ventricles, whereas the vehicle rats in the first group received a sham injection of ACSF. Seven days after Aβ1-42 injection, the rats were tested for behavioral performance using MWM and novel object recognition (NOR) tasks. A probe test was conducted 24 hours after each MWM test (Fig. 1).
Fig. 1.
Schematic diagram of drug treatment and behavioral tests. MWM, Morris water maze test; NOR, novel object recognition test; Aβ, β-amyloid (1-42); ACSF, artificial cerebrospinal fluid; vit C, vitamin C.
Aβ 1-42 treatment
All surgical procedures were conducted under aseptic conditions and sodium pentobarbital (35 mg/kg b.w., intraperitoneal (i.p.), Sigma-Aldrich, St. Louis, MO, USA) anesthesia. The Aβ1-42 (Enzo Life Sciences, Lausen, Switzerland) was dissolved in glacial acetic acid at a concentration of 1 mg/ml and stored at −18°C in aliquot tubes before use. It was then incubated at 37°C for 24 h, as previously described [35]. Rats were maintained in a stereotaxic holder. A midline sagittal incision was made in the scalp, and two holes were drilled in the skull over the lateral ventricles using the following coordinates: AP = −0.8 mm; L = ± 1.5 from the bregma [23]. The dura was perforated with the needle of a microsyringe, which was inserted 3.8 mm beneath the dura mater. Two microliters of either aggregated Aβ1-42 or ACSF was injected into both sides of the lateral ventricles at a rate of 0.2 μl/min with a 10-μl Hamilton syringe equipped with a 26s-gauge needle. After injection, the needle was left in place for 5 min and then slowly removed. After surgery, the animals were returned to their home cages. The doses of Aβ1-42 were chosen based on previous studies [9, 15] and our preliminary results.
Morris water maze
The water maze apparatus was a circular plastic pool, 180 cm in diameter and 60 cm in height, that was positioned in the center of the testing room. The pool was divided into four equal quadrants and filled with room-temperature (25 ± 2°C) water to a depth of 45 cm. A circular platform (10 cm in diameter) was always located 30 cm from the wall of a quadrant and hidden 2 cm below the water surface to allow the rats to climb onto it. White talcum was then sprinkled onto the surface of the water to make it opaque. Geometric objects such as circles, triangles, squares, and hexagons of varying colors were hung on the wall as visual spatial cues. The testing consisted of an acquisition phase and a probe phase. The acquisition phase was used to test the learning and short-term memory abilities of the rats, whereas the probe phase was used to test the long-term memory of the rats. On each test day, each rat underwent four trials. For each trial, each rat was allowed to swim for 60 s to find the hidden platform. When successful, it was allowed 15 s on the platform. If unsuccessful within 60 s, the rat was given a score of 60 s and then physically placed on the platform for 15 s. A 10-min interval was allowed between each trial. For the probe test, four trials (60 s each; 10 min apart) were conducted with no platform present 24 h after the last learning trial. Swimming paths were tracked via a camera linked to a computer monitoring system. The time to find the platform (escape latency time, ET) in the hidden platform test and time spent in the target quadrant (retention time, RT) in the probe test were recorded and analyzed using the free TRACK-ANALYZER program [54] to compare spatial learning and memory ability among groups.
NOR
The NOR apparatus consisted of an open box (48 × 48 cm square with 30-cm-high walls) and plastic objects of various shapes and colors. The box and the objects were cleaned with 20% ethanol between trials and before the first trial to minimize odor cues. The procedure consisted of three sessions: habituation, training, and retention. Habituation entailed exposing the animal to the experimental apparatus for 5 min in the absence of objects before training. During the training phase, two identical objects were placed in two locations. The animals were allowed to explore these objects for 5 min and were then returned to their cages. In the final session, one of the objects was replaced with a novel object. Animals were placed again in the apparatus to explore for another 5 min after a retention interval of 5 min and a 24-h delay. Exploratory behavior, head orientation or sniffing, and deliberate contact with each object (either at a distance or when in contact with the nose) were analyzed [3]. Each group’s ability to recognize the novel object was determined using a discrimination index (DI) calculated for each animal using the formula N − F/N + F [2], which corresponds to the difference between the time exploring the novel (N) and the familiar object (F), corrected for total time exploring both objects. The result varies between +1 and −1, with a positive score indicating more time spent with the novel object, a negative score indicating more time spent with the familiar object, and a score of 0 indicating a null preference.
Determination of malondialdehyde (MDA) level and antioxidant enzymes
Tissue preparation: All rats were anesthetized by injection with sodium pentobarbital (60 mg/kg b.w., i.p., Sigma-Aldrich) and transcardially perfused with 0.9% normal saline solution. After euthanization, the whole brain of each rat was immediately collected on ice, and the cortical tissue was separated from the white matter on an ice-cold surface. Cortical tissue homogenate was then immediately prepared [7]. A 10% (w/v) homogenate of brain samples in an ice-cold medium containing 0.04 M sodium phosphate buffer (pH 7.4) was prepared. The homogenate was centrifuged at 15,000 g at 4°C for 10 min. An aliquot of the homogenate and supernatant was stored at −80°C until the assays of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), enzyme activities, and MDA levels could be conducted. The protein content of the brain supernatant was determined using bovine serum albumin as a standard [26].
MDA level: The MDA level was indirectly estimated by determining the accumulation of thiobarbituric acid reactive substances in the supernatant of brain homogenate and was used as an oxidative damage indicator [19]. A working solution of 0.75 ml thiobarbituric acid (0.8% w/v) and 0.75 ml acetic acid (20%, v/v) was added to 0.2 ml of supernatant, and the samples were heated at 95°C for 1 h. After cooling (10 min in ice water bath), the absorbance was recorded at 532 nm. Using 1,1,3,3-tetraethoxypropane, a standard curve was prepared, and the MDA value of the supernatant was determined from this curve. The results were expressed as nmol/mg protein.
SOD: The SOD activity was assayed based on inhibition of superoxide-dependent reactions [29]. The reaction mixture contained 216 mM potassium phosphate buffer (pH 7.8), 10.7 mM ethylenediaminetetraacetic acid, 1.1 mM cytochrome C, 0.54 mM Xanthine, and 20 µl of supernatant in phosphate buffer in a final volume of 0.22 ml. The reaction was initiated by adding 10 µl of 50 units xanthine oxidase. The activity of SOD in the supernatant was measured at 550 nm on a microplate reader (Galapagos V1.1.2). A standard curve was prepared, and the value for the supernatant was determined from this curve. The results were expressed in SOD units/mg protein.
GPx: The activity of GPx was determined through an indirect method [14], using 2.0 mM reduced glutathione and 0.042% (w/w) hydrogen peroxide (H2O2) as a substrate. In the presence of glutathione reductase at a concentration 0.75 mU and 0.25 mM of NADPH, the oxidized glutathione was immediately converted to a reduced form with concomitant oxidation of NADPH to NAD+. The decrease in absorbance was then measured at 415 nm and 37°C. This assay was performed on the supernatant. The necessary enzyme activity to convert one μmol of NADPH to NADP in 1 min was defined as the activity of GPx, and the results were expressed as GPx units/mg protein. The levels of GPx were calculated from standard curves constructed from standard GPx.
CAT: The CAT activity was determined based on the method described by Goldblith and Proctor [16]. Briefly, 10 µl of supernatant was added into a 96-well plate. The reaction was initiated by adding of 50 µl of H2O2 0.036% (w/w), which was incubated at 37°C for 1 min. The reaction was stopped by the addition of 5N sulfuric acid reagent and 0.005 N potassium permanganate solution for 5 min. A deep pink color developed during the reaction and was measured at an absorbance of 490 nm. Control solutions were also processed in parallel. The difference in absorbance per unit was used as the measure of catalase activity. The data were expressed as units/mg protein.
Statistical analysis: All data were expressed as the mean ± SEM. Statistical analysis of the experimental data was carried out using GraphPad Prism (version 5). Significance of differences among groups were analyzed using a one-way ANOVA and a Newman-Keuls post hoc test. The criterion for statistical significance was P<0.05.
Results
Antioxidant activity of AGC
According to the DPPH assay, AGC exhibited high antioxidant activity compared with standard vitamin C and vitamin E (4.78 ± 0.04 versus 3.11 ± 0.02 and 7.63 ± 0.26 μg/ml). Among the individual plant extracts, aged garlic and ginger showed the strongest antioxidative activities at 3.23 ± 0.05 and 3.24 ± 0.05, respectively, whereas that of chili peppers was the lowest at 10.36 ± 0.36 μg/ml. According to a FRAP assay with a calibration curve of standard ferrous sulfate (y = 0.2307× + 0.0872, R2 = 0.9995), the reducing ability of AGC was the higher than that of AGE, and that of the chili pepper extract was 241.50 ± 18.84 µmol Fe2+/g crude extract. However, ginger extract exhibited the highest activity at 345.35 ± 60.59 µmol Fe2+/g crude extract. In terms of total phenolic content as measured by the Folin-Ciocalteu method, ginger extract exhibited the highest concentration (154.75 ± 8.89 mg GAE/g), whereas AGC exhibited the second highest concentration at 92.44 ± 6.25 mg GAE/g (Table 1).
Table 1. Antioxidant activities, total amount of plant phenols, and flavonoids.
| Substances | DPPH IC50 (µg/ml) |
FRAP µmol of Fe2+/g |
Total phenols mg GAE/g |
|---|---|---|---|
| Vit C | 3.11 ± 0.02 | - | - |
| Vit E | 7.63 ± 0.26 | - | - |
| AGE | 3.23 ± 0.05 | 119.44 ± 18.79 | 56.02 ± 5.29 |
| Ginger | 3.24 ± 0.05 | 345.35 ± 60.59 | 154.75 ± 8.89 |
| Chili | 10.36 ± 0.36 | 142.27 ± 22.13 | 30.00 ± 2.01 |
| AGC (2:2:1) | 4.78 ± 0.04 | 241.50 ± 18.84 | 92.44 ± 6.25 |
Data are presented as the mean ± SD of three separate measurements. Vit C, vitamin C; Vit E, vitamin E.
Effect of AGC on Aβ1-42-induced memory impairment
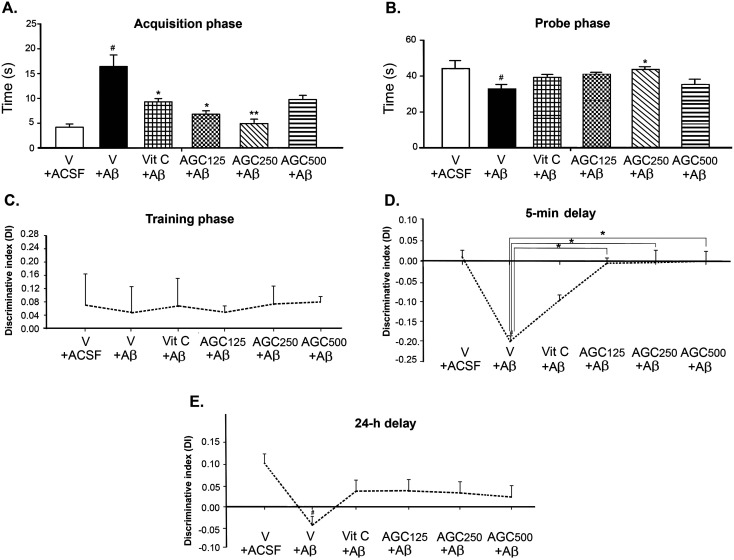
In the water maze test (Figs. 2A and B), post hoc analysis indicated that spatial learning and memory performance in the Aβ1-42-induced rats were significantly impaired as demonstrated by longer escape latency times and shorter retention times, compared with those of ACSF-treated controls (*P<0.05). Pretreatment with AGC125 and AGC250 for 56 days significantly improved learning and prevented short-term memory loss (*P<0.05 and P<0.01) compared with those of the Aβ-vehicle group, whereas there was no such improvement in the AGC500 groups compared with the Aβ-vehicle group. In the probe phase, rats treated with AGC at a dose of 250 mg/kg b.w. displayed significantly higher retention times than those in the vehicle plus Aβ group (*P<0.05). However, the rats given vitamin C, AGC125, and AGC500 had longer times spent in the target quadrant than those in the vehicle-Aβ group. The results of the recognition test showed no significant difference in the time spent exploring the two identical objects during training sessions due to both objects being novel (Fig. 2C). These data suggest that all groups had similar opportunities to become familiar with the appearance of the object used. In the 5-min delay period, only the animals that received Aβ1–42 and vitamin C still showed deficits in short-term memory retention. In contrast, rats in all treated AGC groups explored the novel object significantly more than they did the familiar object (*P<0.05; Fig. 2D), indicating recognition memory retrieval. However, in the long-term retention test (delay of 24 h), although all doses of AGC and vitamin C tended to increase the discrimination index, they did not reverse the toxic effect of Aβ1-42, as observed by the fact that there were no significant differences among the groups in time spent exploring the novel object. The group that received Aβ1-42 showed significant memory deficits when compared with the ACSF-treated rats (#P<0.05; Fig. 2E)
Fig. 2.
Effect of aged garlic, ginger, and chili peppers extracts (AGC) on spatial and recognition memory in Aβ-induced rats as measured by latency time to reach the platform (A) and time to spent in the target quadrant (B) during the Morris water maze (MWM) task, as well as the discrimination index (DI) in the training phase (C), after a 5-min delay (D), and after a 24-h delay (E) in the Novel object recognition (NOR) task. Data are presented as the mean ± SEM. #significant difference from the vehicle plus artificial cerebrospinal fluid (ACSF) group at P<0.05. *significant difference from the vehicle plus Aβ group at P<0.05. **significant difference from the vehicle plus Aβ group at P<0.01.
Effect of AGC on MDA levels and antioxidant enzymes
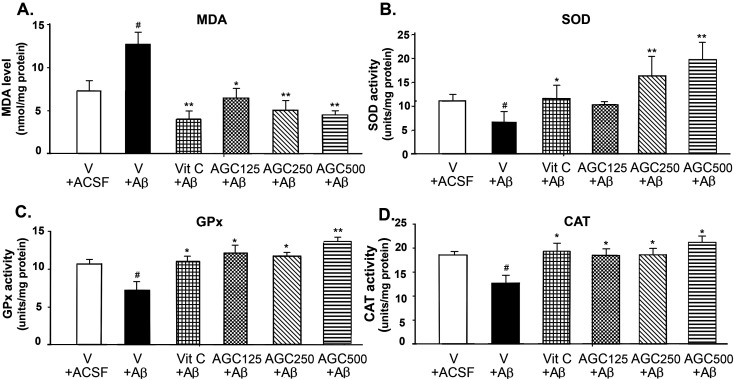
Aβ1-42 injection produced a significant increase in MDA levels (42.68% of the ACSF control group at P<0.05; Fig. 3A). Treatment with vitamin C and AGC at all doses resulted in significant reductions in MDA levels (P<0.05 and 0.01) in the Aβ-treated rats. The rats treated with Aβ1-42 showed significant decreases in brain SOD, GPx, and CAT activity (P<0.05) as compared with ACSF animals (Figs. 3B–D). One-way ANOVA followed by a post hoc test showed that all doses of AGC pretreatment were able to protect against the neurotoxicity of Aβ as observed by the fact that there were significant increases in SOD, GPx, and CAT activity in the brain supernatant (P<0.05 and 0.01). In addition, the standard drug, vitamin C, significantly increased the activities of all antioxidant enzymes (P<0.05).
Fig. 3.
Effects of aged garlic, ginger, and chili peppers extracts (AGC) on malondialdehyde (MDA) levels (A) and activation of antioxidant enzymes (superoxide dismutase (SOD) [B], glutathione peroxidase (GPx) [C], and catalase (CAT) [D]) in rat brain homogenates. Data are presented as the mean ± SEM. #significant difference from the vehicle plus artificial cerebrospinal fluid (ACSF) group at P<0.05. *significant difference from the vehicle plus Aβ group at P<0.05. **significant difference from the vehicle plus Aβ group at P<0.01.
Discussion
The potential of phytochemical antioxidants as health protectors that are safer than synthetic antioxidants has been widely demonstrated based on their ability to neutralize free radicals or oxidants [1]. Garlic, ginger, and chili peppers, which are commonly combined into a paste and used in Thai cuisine, have been reported to exhibit high levels of antioxidants [28, 33, 40]. The antioxidant activities of these ingredients were confirmed by DPPH assay, FRAP, and total phenolic content determination in this study. When these extracts were combined at an AGC ratio of 2:2:1, they were shown to have a stronger free-radical-scavenging effect than chili peppers and aged garlic extracts. This investigation is the first to report on the antioxidant property of these plants in combination. The high content of total phenols and the synergistic interactions in a combination extract might explain their strong antioxidant properties. To examine the beneficial effects of AGC, an MWM and NOR tasks were used to assess the learning and memory abilities of rats. The MWM involves rodents in a water-filled tank learning to navigate their way to a hidden platform and is a learning and memory paradigm that is well suited to investigation of specific mnemonic processes [5]. The NOR test, on the other hand, assesses the ability to discriminate between novel and familiar entities. The integrity of the hippocampus is necessary for processing of form recognition [24]. We observed that an injection of Aβ1-42 into both sides of the lateral ventricles caused significant impairment of spatial and recognition memory performance (Fig. 2), as has previously been reported [12, 55]. In the current study, pretreatment with AGC prevented the loss of short-term spatial and recognition memory in the Aβ-induced rats. However, only AGC 250 prevented impairment in long-term spatial memory, indicating that AGC had a neuroprotective effect on the Aβ1-42-injected rats. The ability of pretreatment with AGC to prevent short-term spatial and recognition memory loss in Aβ-induced rats is related to its antioxidant activity. Previous reports showed that Aβ can activate microglia to produce ROS, causing peroxidation of membrane lipids [30, 51]. In addition, Aβ also interacts directly with mitochondria and induces the production of free radicals, mitochondrial dysfunction, and cell death [42]. Malondialdehyde is an end product of lipid peroxidation and has been considered a late biomarker of oxidative stress and cellular damage [50]. Three antioxidant enzymes, SOD, GPx, and CAT, were monitored in this study. Biochemical data demonstrated that Aβ administration significantly increased MDA levels and decreased SOD, CAT, and GPx levels in the brain homogenate. Pretreatment with AGC caused significant increases in SOD, GPx, and CAT activities, as well as a significant decrease of MDA in rat brain homogenates after Aβ-induced neurotoxicity. Several previous reports have shown the association of phytochemical contents in AGC with antioxidation and neuroprotection. S-allylcysteine (SAC), a sulfur-containing compound, is an active ingredient of AGE with high antioxidant action [38]. Aged garlic extract and SAC have been demonstrated, both in vivo and in vitro, to reduce cerebral Aβ plaques through the reduction of tau protein phosphorylation and nitric oxide and peroxide levels [8, 10]. With its high polyphenolic content, ginger has been shown to exhibit strong antioxidant activity [49]. With regard to structure, several hydroxyl groups that bond to two or more benzene rings have been shown to interrupt, delay, or inhibit the initiation step or propagation step of lipid peroxidation [18]. The antioxidant activities of chili extract may be the result of the phenolic ring of capsaicinoids and ferulic acid ester [43]. In addition, chili extract has been found to prevent memory deficit by blocking tau phosphorylation and β-amyloid accumulation in rats with induced AD [56]. Although our combined extract exhibited strong antioxidant properties, treatment with the AGC containing high levels of antioxidants at a dose of 500 mg/kg b.w. had no neuroprotective effect. It is possible that capsaicin, a component of the AGC, at high concentrations inhibits the plasma membrane NADH oxidase and generates reactive oxygen species (ROS), which results in the degradation of mitochondrial membrane potential, followed by neuronal apoptosis [32, 39]. Therefore, we suggest reducing the proportion of chili peppers in the combined extract. Furthermore, our findings regarding the neuroprotective effects of AGC are consistent with those reports of individual extracts of ginger [17], aged garlic [35, 48], and chili [41] in rats. In agreement with this, histochemical results in our previous studies had demonstrated the neuroprotective effect of AGE, as it was observed that pretreatment with AGE can increase the neuron density in the CA1 and CA3 regions of Aβ-treated rats [48, 53], along with the presence of ChAT-positive neurons density in all regions of hippocampus. Pretreatment with AGE also inhibited the CD11b immunoreactivity in the cerebral cortex and hippocampus of Aβ-treated rats, suggesting an anti-inflammatory effect of AGE via a decrease in microglial activation [35]. Ginger extract was reported to increase the neuron density of the hippocampus in the stroke model of right common carotid artery occlusion [52] and Alzheimer’s model induced by AF64A in rats [46]. Capsaicin was also shown to rescue the ultrastructural features of Aβ-induced synaptic loss in the hippocampal CA1 area of mice [25]. However, AGC at 125 and 250 mg/kg b.w. was more effective than the AGE at equivalent doses and treatment durations [35]. An alternative possibility is that since AGC is a combination of three herbal extracts, its effects might be the result of a synergistic combination of all active elements in these extracts.
The findings that AGC at all doses exhibited antioxidative activity by decreasing MDA and increasing antioxidant enzymes, but that AGC at a high dose (500 mg/kg) failed to show a neuroprotective effect, indicate that the cognitive enhancing effect of AGC is not solely due to its antioxidative properties but is also due to some other mechanisms of action. Each part of the combination AGC may synergistically act to confer the beneficial effects. AGE has been reported to have an anti-inflammatory effect [35], ginger has been reported to induce vasodilation [21], and capsaicin has been reported to stimulate the biosynthesis of endocannabinoids [13], which may provide neuroprotection. Furthermore, AGC at dose of 250 mg/kg b.w. provides the most beneficial effect without the dose-dependent effect at the higher dose of 500 mg/kg b.w., and this may suggest the involvement of many factors. It is possible that exceeding the limit of gut absorption at the higher dose or the toxicity of some of the ingredients, such as capsaicin, may be involved, since capsaicin has been reported to trigger two distinct effects. At low concentration, it triggers calcium influx through vanilloid receptor type 1. At higher concentrations, it exhibits a neurotoxic effect by inhibiting calcium clearance from cytosol and causing damage of mitochondrial calcium buffering in CNS neurons [11]. However, to confirm this notion, further investigations are necessary.
Acknowledgments
This work was supported by the Higher Education Research Promotion and National Research, University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster of Khon Kaen University and the Center for Research and Development of Herbal Health Products (CRD-HHP), and Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand. We would like to thank Dylan Southard for his assistance with the English-language presentation of this manuscript.
References
- 1.Ansari A.Q., Ahmed S.A., Waheed M.A., Juned A.S.2013. Extraction and determination of antioxidant activity of Withania somnifera Dunal. Eur. J. Exp. Biol. 3: 502–507. [Google Scholar]
- 2.Antunes M., Biala G.2012. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process. 13: 93–110. doi: 10.1007/s10339-011-0430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arqué G., Fotaki V., Fernández D., Martínez de Lagrán M., Arbonés M.L., Dierssen M.2008. Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A). PLoS One 3: e2575. doi: 10.1371/journal.pone.0002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benzie I.F., Strain J.J.1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239: 70–76. doi: 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- 5.Brandeis R., Brandys Y., Yehuda S.1989. The use of the Morris Water Maze in the study of memory and learning. Int. J. Neurosci. 48: 29–69. doi: 10.3109/00207458909002151 [DOI] [PubMed] [Google Scholar]
- 6.Butterfield D.A., Drake J., Pocernich C., Castegna A.2001. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 7: 548–554. doi: 10.1016/S1471-4914(01)02173-6 [DOI] [PubMed] [Google Scholar]
- 7.Carrillo M.C., Kanai S., Nokubo M., Kitani K.1991. (-) deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci. 48: 517–521. doi: 10.1016/0024-3205(91)90466-O [DOI] [PubMed] [Google Scholar]
- 8.Chauhan N.B.2006. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer’s transgenic model Tg2576. J. Ethnopharmacol. 108: 385–394. doi: 10.1016/j.jep.2006.05.030 [DOI] [PubMed] [Google Scholar]
- 9.Chen X., Hu J., Jiang L., Xu S., Zheng B., Wang C., Zhang J., Wei X., Chang L., Wang Q.2014. Brilliant Blue G improves cognition in an animal model of Alzheimer’s disease and inhibits amyloid-β-induced loss of filopodia and dendrite spines in hippocampal neurons. Neuroscience 279: 94–101. doi: 10.1016/j.neuroscience.2014.08.036 [DOI] [PubMed] [Google Scholar]
- 10.Colín-González A.L., Santana R.A., Silva-Islas C.A., Chánez-Cárdenas M.E., Santamaría A., Maldonado P.D.2012. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid. Med. Cell. Longev. 2012: 907162. doi: 10.1155/2012/907162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dedov V.N., Mandadi S., Armati P.J., Verkhratsky A.2001. Capsaicin-induced depolarisation of mitochondria in dorsal root ganglion neurons is enhanced by vanilloid receptors. Neuroscience 103: 219–226. doi: 10.1016/S0306-4522(00)00540-6 [DOI] [PubMed] [Google Scholar]
- 12.Delobette S., Privat A., Maurice T.1997. In vitro aggregation facilities beta-amyloid peptide-(25-35)-induced amnesia in the rat. Eur. J. Pharmacol. 319: 1–4. doi: 10.1016/S0014-2999(96)00922-3 [DOI] [PubMed] [Google Scholar]
- 13.Di Marzo V., Lastres-Becker I., Bisogno T., De Petrocellis L., Milone A., Davis J.B., Fernandez-Ruiz J.J.2001. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur. J. Pharmacol. 420: 123–131. doi: 10.1016/S0014-2999(01)01012-3 [DOI] [PubMed] [Google Scholar]
- 14.Eyer P., Podhradský D.1986. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman’s reagent. Anal. Biochem. 153: 57–66. doi: 10.1016/0003-2697(86)90061-8 [DOI] [PubMed] [Google Scholar]
- 15.Giovannelli L., Casamenti F., Scali C., Bartolini L., Pepeu G.1995. Differential effects of amyloid peptides β-(1-40) and β-(25-35) injections into the rat nucleus basalis. Neuroscience 66: 781–792. doi: 10.1016/0306-4522(94)00610-H [DOI] [PubMed] [Google Scholar]
- 16.Goldblith S.A., Proctor B.E.1950. Photometric determination of catalase activity. J. Biol. Chem. 187: 705–709. [PubMed] [Google Scholar]
- 17.Gomar A., Hosseini A., Mirazi N.2014. Memory enhancement by administration of ginger (Zingiber officinale) extract on morphine-induced memory impairment in male rats. J. Acute Dis. 3: 212–217. doi: 10.1016/S2221-6189(14)60047-0 [DOI] [Google Scholar]
- 18.Gülçin İ., Elmastaş M., Aboul-Enein H.Y.2012. Antioxidant activity of clove oil – A powerful antioxidant source. Arab. J. Chem. 5: 489–499. doi: 10.1016/j.arabjc.2010.09.016 [DOI] [Google Scholar]
- 19.Gupta R., Gigras P., Mohapatra H., Goswami V.K.2003. Microbial α-amylases: a biotechnological perspective. Process Biochem. 38: 1599–1616. doi: 10.1016/S0032-9592(03)00053-0 [DOI] [Google Scholar]
- 20.Katsube T., Tabata H., Ohta Y., Yamasaki Y., Anuurad E., Shiwaku K., Yamane Y.2004. Screening for antioxidant activity in edible plant products: comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J. Agric. Food Chem. 52: 2391–2396. doi: 10.1021/jf035372g [DOI] [PubMed] [Google Scholar]
- 21.Krüth P., Brosi E., Fux R., Mörike K., Gleiter C.H.2004. Ginger-associated overanticoagulation by phenprocoumon. Ann. Pharmacother. 38: 257–260. doi: 10.1345/aph.1D225 [DOI] [PubMed] [Google Scholar]
- 22.Kumar O.A., Rao S.A., Tata S.S.2010. Phenolics quantification in some genotypes of capsicum annuum L. J. Phytol. 2: 87–90. [Google Scholar]
- 23.Lin H.B., Yang X.M., Li T.J., Cheng Y.F., Zhang H.T., Xu J.P.2009. Memory deficits and neurochemical changes induced by C-reactive protein in rats: implication in Alzheimer’s disease. Psychopharmacology (Berl.) 204: 705–714. doi: 10.1007/s00213-009-1499-2 [DOI] [PubMed] [Google Scholar]
- 24.Logothetis N.K., Sheinberg D.L.1996. Visual object recognition. Annu. Rev. Neurosci. 19: 577–621. doi: 10.1146/annurev.ne.19.030196.003045 [DOI] [PubMed] [Google Scholar]
- 25.Long C., Zhilin H., Yehong D., Min F., Huili H., Yutian W., Zhifang D.2017. Capsaicin Attenuates Amyloid-β-Induced Synapse Loss and Cognitive Impairments in Mice. PLoS One 12: e0172477. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J.1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 27.Masuda Y., Kikuzaki H., Hisamoto M., Nakatani N.2004. Antioxidant properties of gingerol related compounds from ginger. Biofactors 21: 293–296. doi: 10.1002/biof.552210157 [DOI] [PubMed] [Google Scholar]
- 28.Materska M., Perucka I.2005. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L). J. Agric. Food Chem. 53: 1750–1756. doi: 10.1021/jf035331k [DOI] [PubMed] [Google Scholar]
- 29.McCord J.M., Fridovich I.1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244: 6049–6055. [PubMed] [Google Scholar]
- 30.Meda L., Cassatella M.A., Szendrei G.I., Otvos L., Jr, Baron P., Villalba M., Ferrari D., Rossi F.1995. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 374: 647–650. doi: 10.1038/374647a0 [DOI] [PubMed] [Google Scholar]
- 31.Moriguchi T., Saito H., Nishiyama N.1996. Aged garlic extract prolongs longevity and improves spatial memory deficit in senescence-accelerated mouse. Biol. Pharm. Bull. 19: 305–307. doi: 10.1248/bpb.19.305 [DOI] [PubMed] [Google Scholar]
- 32.Morré D.J., Sun E., Geilen C., Wu L.Y., de Cabo R., Krasagakis K., Orfanos C.E., Morré D.M.1996. Capsaicin inhibits plasma membrane NADH oxidase and growth of human and mouse melanoma lines. Eur. J. Cancer 32A: 1995–2003. doi: 10.1016/0959-8049(96)00234-1 [DOI] [PubMed] [Google Scholar]
- 33.Mošovská S., Nováková D., Kaliňák M.2015. Antioxidant activity of ginger extract and identification of its active components. Acta Chim. Slov. 8: 115–119. doi: 10.1515/acs-2015-0020 [DOI] [Google Scholar]
- 34.Mukherjee D., Banerjee S.2013. Learning and memory promoting effects of crude garlic extract. Indian J. Exp. Biol. 51: 1094–1100. [PubMed] [Google Scholar]
- 35.Nillert N., Pannangrong W., Welbat J.U., Chaijaroonkhanarak W., Sripanidkulchai K., Sripanidkulchai B.2017. Neuroprotective effects of aged garlic extract on cognitive dysfunction and neuroinflammation induced by β-amyloid in rats. Nutrients 9: 24. doi: 10.3390/nu9010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishiyama N., Moriguchi T., Saito H.1997. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Exp. Gerontol. 32: 149–160. doi: 10.1016/S0531-5565(96)00062-9 [DOI] [PubMed] [Google Scholar]
- 37.Ojewole J.A.O.2006. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother. Res. 20: 764–772. doi: 10.1002/ptr.1952 [DOI] [PubMed] [Google Scholar]
- 38.Okada Y., Tanaka K., Fujita I., Sato E., Okajima H.2005. Antioxidant activity of thiosulfinates derived from garlic. Redox Rep. 10: 96–102. doi: 10.1179/135100005X38851 [DOI] [PubMed] [Google Scholar]
- 39.Okun J.G., Lümmen P., Brandt U.1999. Three classes of inhibitors share a common binding domain in mitochondrial complex I (NADH:ubiquinone oxidoreductase). J. Biol. Chem. 274: 2625–2630. doi: 10.1074/jbc.274.5.2625 [DOI] [PubMed] [Google Scholar]
- 40.Park J.H., Park Y.K., Park E.2009. Antioxidative and antigenotoxic effects of garlic (Allium sativum L.) prepared by different processing methods. Plant Foods Hum. Nutr. 64: 244–249. doi: 10.1007/s11130-009-0132-1 [DOI] [PubMed] [Google Scholar]
- 41.Pegorini S., Braida D., Verzoni C., Guerini-Rocco C., Consalez G.G., Croci L., Sala M.2005. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br. J. Pharmacol. 144: 727–735. doi: 10.1038/sj.bjp.0706115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy P.H.2006. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J. Neurochem. 96: 1–13. doi: 10.1111/j.1471-4159.2005.03530.x [DOI] [PubMed] [Google Scholar]
- 43.Shetty K.2004. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: a review. Process Biochem. 39: 789–804. doi: 10.1016/S0032-9592(03)00088-8 [DOI] [Google Scholar]
- 44.Sripanidkulchai B., Fangkrathok N.2014. Antioxidant, antimutagenic and antibacterial activities of extracts from Phyllanthus emblica branches. Songklanakarin J. Sci. Technol. 36: 669–674. [Google Scholar]
- 45.Suganuma H., Hirano T., Inakuma T.1999. Amelioratory effect of dietary ingestion with red bell pepper on learning impairment in senescence-accelerated mice (SAMP8). J. Nutr. Sci. Vitaminol. (Tokyo) 45: 143–149. doi: 10.3177/jnsv.45.143 [DOI] [PubMed] [Google Scholar]
- 46.Sutalangka C., Wattanathorn J.2017. Neuroprotective and cognitive-enhancing effects of the combined extract of Cyperus rotundus and Zingiber officinale. BMC Complement. Altern. Med. 17: 135. doi: 10.1186/s12906-017-1632-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tahirovic I., Sofic E., Sapcanin A., Gavrankapetanovic I., Bach-Rojecky L., Salkovic-Petrisic M., Lackovic Z., Hoyer S., Riederer P.2007. Brain antioxidant capacity in rat models of betacytotoxic-induced experimental sporadic Alzheimer’s disease and diabetes mellitus. J. Neural Transm. Suppl. (72): 235–240. [DOI] [PubMed] [Google Scholar]
- 48.Thorajak P., Pannangrong W., Welbat J.U., Chaijaroonkhanarak W., Sripanidkulchai K., Sripanidkulchai B.2017. Effects of aged garlic extract on cholinergic, glutamatergic and GABAergic systems with regard to cognitive impairment in Aβ-induced rats. Nutrients 9: E686. doi: 10.3390/nu9070686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tohma H., Gülçin İ., Bursal E., Gören A.C.2017. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 11: 556–566. doi: 10.1007/s11694-016-9423-z [DOI] [Google Scholar]
- 50.Vaca C.E., Wilhelm J., Harms-Ringdahl M.1988. Interaction of lipid peroxidation products with DNA. A review. Mutat. Res. 195: 137–149. doi: 10.1016/0165-1110(88)90022-X [DOI] [PubMed] [Google Scholar]
- 51.Valencia A., Morán J.2004. Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic. Biol. Med. 36: 1112–1125. doi: 10.1016/j.freeradbiomed.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 52.Wattanathorn J., Jittiwat J., Tongun T., Muchimapura S., Ingkaninan K.2011. Zingiber officinale mitigates brain damage and improves memory impairment in focal cerebral ischemic rat. Evid. Based Complement. Alternat. Med. 2011: 429505 doi: 10.1155/2011/429505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wichai T., Pannangrong W., Welbat J.U., Chaichun A., Sripanidkulchai K., Sripanidkulchai B.2019. Effects of aged garlic extract on spatial memory and oxidative damage in the brain of amyloid-β induced rats. Songklanakarin J. Sci. Technol. 41: 311–318. [Google Scholar]
- 54.Wolfer D.P., Lipp H.P.1992. A new computer program for detailed off-line analysis of swimming navigation in the Morris water maze. J. Neurosci. Methods 41: 65–74. doi: 10.1016/0165-0270(92)90124-V [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi Y., Kawashima S.2001. Effects of amyloid-beta-(25-35) on passive avoidance, radial-arm maze learning and choline acetyltransferase activity in the rat. Eur. J. Pharmacol. 412: 265–272. doi: 10.1016/S0014-2999(01)00730-0 [DOI] [PubMed] [Google Scholar]
- 56.Yang H.J., Kwon D.Y., Kim M.J., Kang S., Moon N.R., Daily J.W., Park S.2015. Red peppers with moderate and severe pungency prevent the memory deficit and hepatic insulin resistance in diabetic rats with Alzheimer’s disease. Nutr. Metab. (Lond.) 12: 9. doi: 10.1186/s12986-015-0005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yatin S.M., Varadarajan S., Link C.D., Butterfield D.A.1999. In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid beta-peptide (1-42). Neurobiol. Aging 20: 325–330, discussion 339–342. [DOI] [PubMed] [Google Scholar]