Abstract
Introduction
While the COVID-19 pandemic continues to have a significant global health impact, rates of maternal to infant vertical transmission remain low (<5%). Parenchymal changes of placentas from COVID-19 infected mothers have been reported by several groups, but the localization and relative abundance of SARS-CoV-2 viral proteins and cellular entry machinery has not been fully characterized within larger placental tissue cohorts.
Methods
An extended placental tissue cohort including samples from 15 COVID-19 positive maternal-fetal dyads (with n = 5 cases with evidence of fetal transmission) in comparison with 10 contemporary COVID-19 negative controls. Using comparative immunofluorescence, we examined the localization and relative tissue abundance of SARS-CoV2 spike glycoprotein (CoV2 SP) along with the co-localization of two SARS-CoV2 viral entry proteins angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2).
Results/conclusions
CoV2 SP was present within the villous placenta in COVID-19 positive pregnancies with and without evidence of fetal transmission. We further identified the predominance of ACE2 expression in comparison with TMPRSS2. Importantly, both CoV2 SP and ACE2 expression consistently localized primarily within the outer syncytiotrophoblast layer placental villi, a key physiologic interface between mother and fetus. Overall this study provides an important basis for the ongoing evaluation of SARS-CoV-2 physiology in pregnancy and highlights the importance of the placenta as a key source of primary human tissue for ongoing diagnostic and therapeutic research efforts to reduce the global burden of COVID-19.
Keywords: COVID-19, TMPRSS2, SARS-CoV-2, Placenta, ACE2, Vertical transmission
Highlights
-
•
SARS-CoV2 glycoprotein in the placenta does not vary with fetal transmission.
-
•
SARS-CoV-2 viral entry protein ACE2 is highly expressed in the villous placenta.
-
•
SARSS-CoV-2 viral entry protein TMPRSS2 has low expression in the villous placenta.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel infectious agent responsible for Coronavirus disease 2019 (COVID-19), has caused over ten million confirmed cases and has accounted for over 500,000 deaths worldwide in the six months since the first reported case in December 2019 [1]. Despite its severity in some populations (particularly older adults), studies to date have shown that COVID-19 does not cause increased morbidity or mortality in pregnant women compared to non-pregnant women [[2], [3], [4]]. There is a low rate of positive SARS-CoV-2 tests in infants born to mothers with COVID-19, with cohort studies reporting a range of 0–4.5% [5,6]. These rates have remained low even as more obstetrical and neonatal settings are implementing universal testing. Clinical manifestations in neonates related to SARS-CoV-2 exposure are still being fully characterized, but overall the majority of the literature has reported healthy neonates born to mothers with COVID-19 [2,3,[7], [8], [9], [10], [11], [12], [13], [14]]. However, Questions remain about fetal transmission of SARS-CoV-2 [4,5,[15], [16], [17], [18], [19], [20]] and the particular interface(s) dictating its pathogenesis during pregnancy. Established pregnancy transmission routes including placental, intrapartum (e.g. blood, vaginal secretions) or postpartum (e.g. breastfeeding) intervals all require additional examination for COVID-19 [21,22].
Cases of SARS-CoV-2 maternal infection in the current literature consistently report significant alterations of placental parenchyma, particularly within the villous and subchorionic compartments [19,[23], [24], [25], [26]]. Histopathological studies of placentas from several different cohorts have identified significant fibrosis, maternal vascular malperfusion, and intervillous thrombi among the most frequently reported pathological diagnoses [13,25,27,28]. These findings highlight that the placenta undergoes significant parenchymal changes secondary to maternal COVID-19 infection, yet SARS-CoV-2 transmission to the fetus is prevented in the majority of pregnancies, suggesting organ-specific antiviral mechanisms at the maternal-fetal interface.
As the primary anatomical and physiological interface between the mother and fetus during pregnancy, the placenta and its accompanying immune cell repertoire dictate blockade versus transmission of viral pathogens through highly interrelated pathways [22]. A unifying theme among most studies examining viral infections in pregnancy is the importance of the villous placenta and in particular the villous outer syncytiotrophoblast layer (sTB). This syncytial cell layer fed by underlying cytotrophoblast (cTB) cells remains in direct contact with the maternal blood space throughout pregnancy. sTB serve a variety of functions including bi-directional maternal-fetal trafficking and importantly, are known to express multiple key viral receptors for pathogens with varying rates of vertical transmission, such as HIV and Hepatitis C [22,[29], [30], [31], [32]].
SARS-CoV-2 and its viral entry machinery have been examined in previous case studies and historical data sets from healthy placental tissues. Case reports reporting histology from limited tissue cohorts (n = 1–4) contain conflicting reports correlating the presence of placental COVID-19 viral RNA and protein with evidence of fetal transmission [23,25]. Angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) are two key enzymes of the SARS CoV-2 viral entry machinery [33,34]. The bulk of literature published on ACE2 and TMPRSS2 in the placenta has come from healthy tissues obtained prior to the current pandemic [35,36]. Historical single cell RNAseq data sets from healthy placental tissue have identified the expression of both ACE2 and TMPRSS2 mRNA in villous sTB and cTB and in some studies, a comparatively higher ACE2 mRNA signal as comparted to TMPRSS2 [[37], [38], [39]]. However, no study to date has surveyed the localization of these important viral entry proteins in primary placental tissues from pregnancies maternal COVID-19 infection.
We hypothesized that SARS-CoV-2 invades the villous placental compartment irrespective of fetal transmission and that ACE2 and TMPRSS2 are localized within key anatomical areas of the placenta. To address this hypothesis, we examined an extended cohort of placental tissues from 15 mother-infant dyads with maternal COVID-19 infection (5 with evidence of fetal transmission) along with 10 contemporary controls. Utilizing quantitative immunofluorescence, we surveyed the placental localization and comparative expression of the SARS-CoV-2 spike glycoprotein (CoV2 SP), ACE2 and TMPRSS2 in these tissues.
2. Materials and methods
Sample Collection: This study was conducted at an urban safety net hospital in Boston, MA between April and May 2020 during a period of peak admissions. Universal testing for SARS- CoV-2 by PCR of nasopharyngeal swabs was instituted at our hospital in mid-April 2020 for all women admitted in labor. We collected placental samples from women who tested positive at the time of delivery, and contemporary controls who tested negative. Selected patient demographic data and institutional pathology reports were collected for all cases and controls. Approval was obtained from the Boston University Medical Campus Institutional Review Board, with an informed consent waiver obtained for this study.
Tissue Processing: Tissues were placed in 4% buffered formalin at time of delivery followed by formal grossing, staging and histological evaluation by board-certified pathologists. Full thickness placental biopsies (including decidua, villous tissue and chorionic plate) were then dissected from the remaining formalin fixed placental tissues, soaked in 18% sucrose for 1 week followed by embedding in Tissue-Tek O·C.T. solution (ThermoFisher, Waltham, MA) and frozen at −80 °C. Tissue blocks were then cryo-sectioned at 10 μm thickness for subsequent immunostaining.
Immunohistochemistry: Placental tissue sections were subjected to antigen retrieval with 10 mM Sodium citrate, 0.05% Tween 20, pH 6, boiling for 20 min. Following a series of washes with PBS 0.05% Tween 20 (PBS-T), slides were then incubated for 1 h at room temperature in PBS with blocking/permeabilization solution 0.2% Triton X-100 along with serum of host species for all correlate secondary antibodies (Sigma-Aldrich, St. Louis, MO). Slides were then incubated at 4 °C overnight using the following primary antibodies at 1:100 diluted in PBS-T: single labeling: SARS CoV-2 spike glycoprotein (rabbit anti-human, ab272504 Abcam, Cambridge, MA), double labeling: ACE-2 (goat anti-human, AF933 R&D systems, Minneapolis, MD) and TMPRSS2 (rabbit anti-human, ab109131 Abcam). SARS-CoV-2 spike glycoprotein was visualized in a subset of slides first with anti-rabbit AEC development kit (Histostain-Plus, ThermoFisher) and then with the full tissue cohort via immunofluorescence with Alexa 647 goat anti-rabbit secondary (Abcam) at 1:500 for 1 h at room temperature. Double immunofluorescence for ACE-2 and TMPRSS2 was performed with secondary antibodies donkey anti goat Alexa 647 and donkey anti rabbit Alexa 594 (Abcam) at 1:500 for 1 h at room temperature. For control staining of placental tissues, slides were incubated with only secondary antibodies (without primary antibodies). Following a final series of washes, slides were cover slipped with either aqueous mounting medium for AEC staining (Abcam) or Prolong Gold with DAPI (ThermoFisher) and cured overnight prior to imaging. To ensure consistency for comparative analysis, all cohort slides were stained in bulk for each of the single and double immunohistochemistry protocols and all slides were imaged within 24–48 h of staining.
Microscopy: Images for brightfield microscopy were acquired on a Nikon TE2000 (Nikon, Melville, NY). Images for immunofluorescent microscopy were acquired on a Nikon deconvolution wide-field epifluorescence microscope using NIS-Elements Software (Nikon). For quantitative immunofluorescence decidua, villous tissue and chorionic plate areas were manually surveyed at 100× followed by automated acquisition at 200× of 4 tiled images from 5 randomized areas per slide. Exposure times were standardized for each target and remained constant throughout image acquisition to ensure accurate comparative quantification. Post-acquisition image processing was performed via ImageJ Fiji software package (imagej.net).
Quantitative Image Analysis: Image area and integrated density were measured via ImageJ software for each immunofluorescent 200× image (n = 20/slide) along with mean fluorescence values from 5 randomly selected background readings per image cohort. Images were measured by a total of three blinded reviewers (authors EST, YB, and KR). These values were then used to calculate a corrected total cell fluorescence (CTCF) per published protocols using the following calculation: CTCF = Integrated density – (Area of selected image x average mean background fluorescence) [[40], [41], [42]]. An average CTCF was also calculated from secondary only negative control slides (n = 3 per staining assay) and used to calculate a final Fluorescence Ratio: target antigen CTCF/secondary only control CTCF.
Statistical analysis: Demographics of the COVID-19 and control mother-infant dyads, and pathology diagnoses were compared using independent sample t-tests (normally distributed continuous data), Wilcoxon rank sum test (non-normally distributed continuous data), or the Fisher exact test (categorial data). CTCF values for immunofluorescence were compared using independent sample t-tests (normally distributed continuous data). Differences were considered significant at α = 0.05. All statistical analysis was performed using Prism 7 software (GraphPad, San Diego, CA).
3. Results
There were no significant demographic differences between cases with positive maternal SARS-CoV-2 testing (COVID-19Maternal) and control mother-infant dyads (Table 1 ). Placental pathology diagnoses, listed in Table 2 , were notable for the presence of fibrin deposition and signs of inflammation in COVID-19Maternal placentas, however this did not meet statistical significance. These diagnoses corresponded with gross pathology assessment of tissue biopsies obtained for the study, with notable intervillous and subchorionic fibrosis in the majority of COVID-19Maternal samples (Fig. 1 ).
Table 1.
Demographics of COVID vs control samples.
| Demographic | COVID (N = 15) | Control (N = 10) | P-Value |
|---|---|---|---|
| Maternal age (years) – Mean (SD) | 31.8 (5.5) | 30.1 (5.5) | 0.46 |
| Gestational age at birth (weeks) – Mean (SD) | 38.1 (1.7) | 39.3 (1.6) | 0.10 |
| Infant sex – N (%) | |||
| Male | 6 (40%) | 5 (50%) | 0.70 |
| Female | 9 (60%) | 5 (50%) | |
| Birth weight (grams) – Mean (SD) | 3319.9 (366.8) | 3182.7 (556.8) | 0.46 |
| Placental weight (grams) – Median (Range) | 484 (323–794) | 430 (370–570) | 0.35 |
| Infant COVID-19 nasal swab positive results | 5 (33.3%) | N/A | N/A |
| 24-h swab | 2 (13.3%) | ||
| 48-h swab | 2 (13.3%) | ||
| 5-day swab | 3 (20.0%) | ||
Table 2.
Pathology Diagnoses COVID vs control placentas.
| PATHOLOGY DIAGNOSIS | COVID (N = 15) | CONTROL (N = 10) | P-Value |
|---|---|---|---|
| Meconium Histiocytosis | 8 (53.3%) | 8 (80.0%) | 0.23 |
| Infarcts | 5 (33.3%) | 1 (10.0%) | 0.34 |
| Vasculopathy | 1 (6.7%) | 0 | – |
| Chorioamnionitis | 2 (13.3%) | 1 (10.0%) | 1.00 |
| Chronic villitis | 1 (6.7%) | 0 | – |
| Fibrin deposition | 7 (46.7%) | 1 (10.0%) | 0.09 |
| Fetal chorionic vessel vasculitis | 1 (6.7%) | 1 (10.0%) | 1.00 |
| Funisitis | 0 | 1 (10.0%) | – |
| Hypoplastic villi | 1 (6.7%) | 0 | – |
| Acute subchorionitis | 5 (33.3%) | 2 (20.0%) | 0.66 |
| Decidual vasculopathy | 0 | 1 (10.0%) | – |
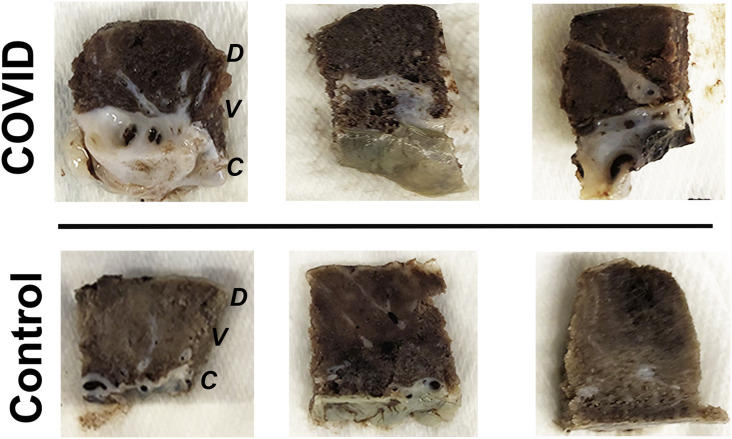
Fig. 1.
Gross subchorionic and intervillous fibrosis in placental tissues from pregnancies affected by COVID-19. Representative gross images of full thickness term placental biopsies. COVID: Placental tissues from pregnant women who were SARS-CoV-2 positive upon admission screening (full cohort, n = 15). Control: Placental tissues from pregnant women who were SARS-CoV-2 negative upon admission screening (full cohort, n = 10). D: Decidua, V: Villous tissue, C: Chorionic plate.
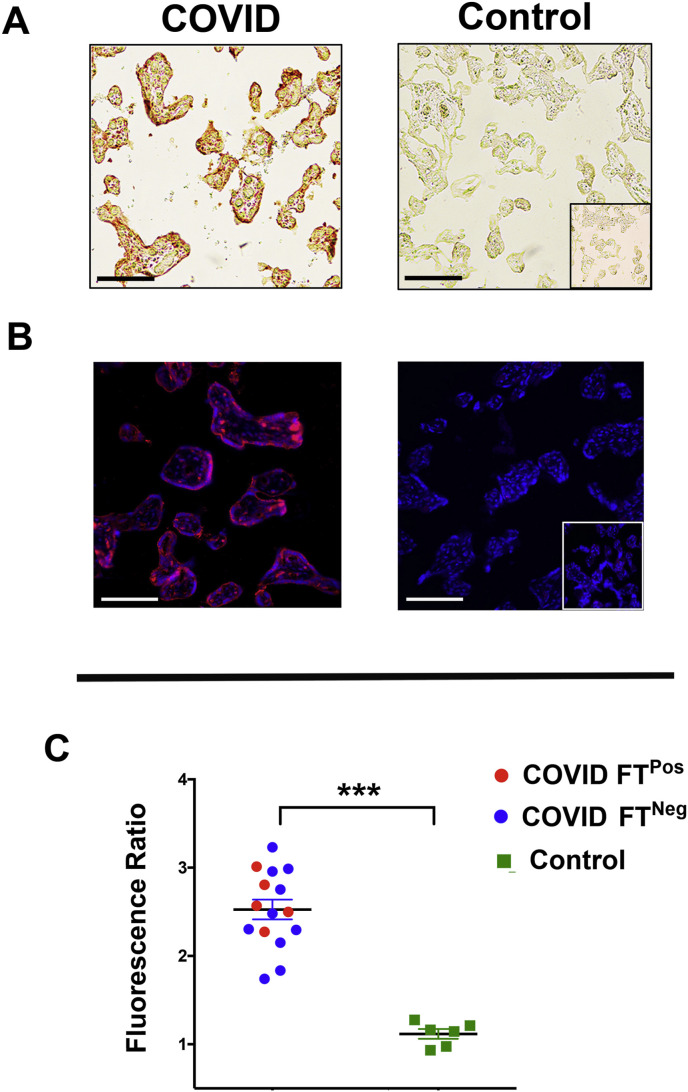
We next surveyed tissues for the presence and localization of SARS-Co-V-2 spike glycoprotein (CoV2 SP). This viral protein was present in the villous compartment of all COVID-19Maternal placentas and absent from control tissues, as visualized by non-fluorescent and fluorescent immunohistochemical techniques (Fig. 2 A and B). No expression was observed within chorionic plate or decidual tissues (data not shown). Within placental villi, CoV2 SP was consistently expressed within the sTB layer with intermittent localization to cTB among certain villi (Fig. 2B). Using quantitative microscopy, we then surveyed the presence of CoV2 SP among all tissues. COVID-19Maternal cases with evidence of fetal transmission did not have significant alterations of CoV2 SP in comparison to pregnancies with negative infant COVID-19 testing (Fig. 2C).
Fig. 2.
Consistent SARS-CoV-2 spike glycoprotein expression in placental tissues with and without evidence of fetal transmission. A. Representative 200× brightfield images of AEC immunohistochemistry for SARS-CoV-2. B. Representative images (200×) of SARS-CoV-2 immunofluorescence. C. Graphical analysis of comparative fluorescence quantitation. COVID and Control: tissue cohorts as described in Fig. 1. Fluorescence Ratio: ratio of corrected total cell fluorescence of target antigen/secondary only control. COVID FTPos: COVID-19 affected pregnancies with evidence of fetal transmission, COVID FTNeg: COVID-19 affected pregnancies without evidence of fetal transmission. ***p < 0.001. Scale bars: 50 μm.
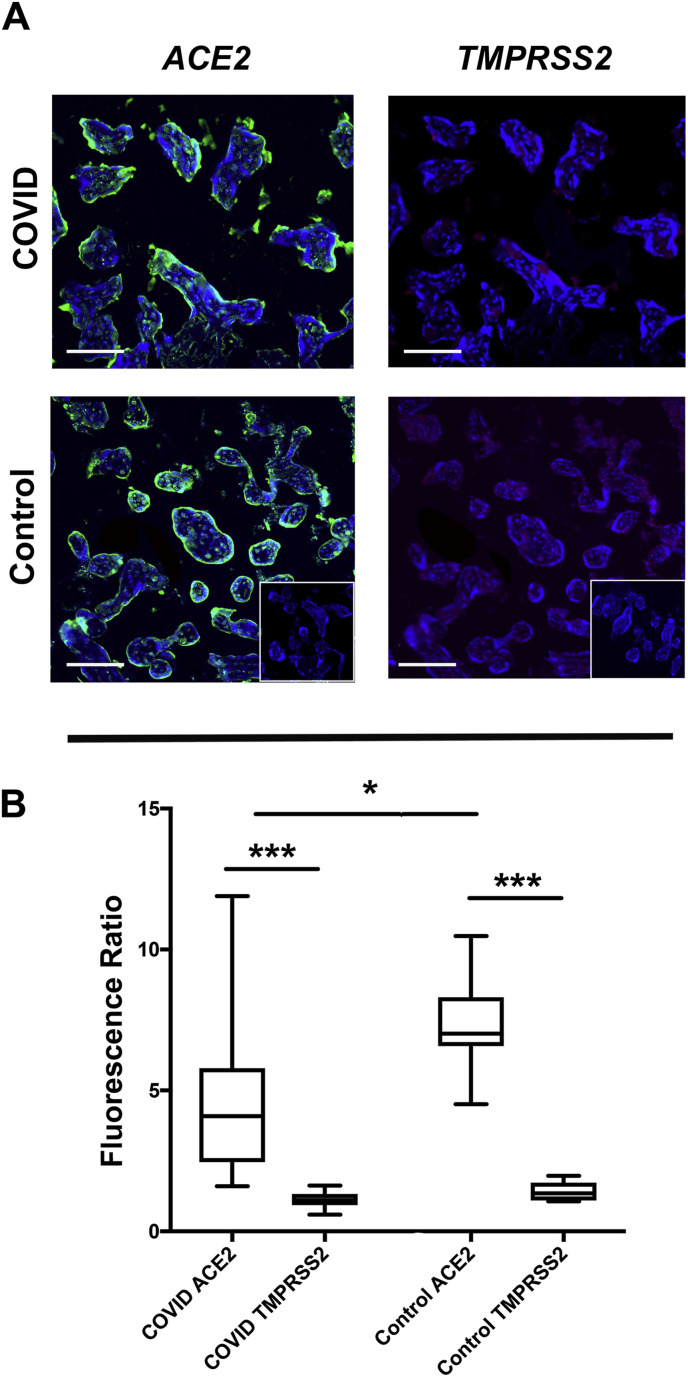
Comparative expression and localization of ACE2 and TMPRSS2 receptors was then evaluated using double immunofluorescence to simultaneously evaluate both proteins within the same tissue section. While ACE2 was consistently found in the sTB layer of all tissues surveyed (COVID-19Maternal and controls), TMPRSS2 expression was absent in both groups of placentas (Fig. 3 A and B). We also found a statistically significant reduction in ACE2 expression levels in COVID-19Maternal placental tissues as compared to controls, (Fig. 3B), but this finding should be interpreted with caution as the values from the ACE2 fluorescence within the COVID-19Maternal placental cohort had a wide distribution.
Fig. 3.
ACE2 over TMPRSS2 predominance in villous placental compartment. A. Representative images (200×) of ACE2 and TMPRSS2 co-immunofluorescence. B. Graphical analysis of comparative fluorescence quantitation. COVID and Control: tissue cohorts as described in Fig. 1. Fluorescence Ratio: ratio of corrected total cell fluorescence of target antigen/secondary only control. ***p < 0.001, *p < 0.05. Scale bars: 50 μm.
4. Discussion
We identified that CoV2 SP is present in the villous compartment of placental tissues in pregnancies with and without evidence of fetal transmission in an extended cohort of maternal-fetal dyads affected by COVID-19 infection. We also identified the predominance of the ACE2 viral entry protein in comparison with TMPRSS2. Importantly, in these tissues both CoV2 SP and ACE2 expression consistently localized within the outer sTB layer of placental villi juxtaposed with the maternal blood space.
Despite the rapid publication of multiple studies addressing SARS CoV2 in pregnancy, several key points regarding COVID-19 infection at the maternal-fetal interface still remain unclear. First is whether the prominent parenchymal alterations observed within placentas from COVID-19 infected women are a consequence of systemic maternal inflammation or localized placental invasion of SARS-CoV-2. We identified extensive fibrosis and subchorionic thickening consistent with previous reports [13,25,27,28] along with consistent localization of CoV2 SP in all COVID-19Maternal placental tissues. However, more detailed analyses on the alterations of the molecular pathways in COVID infected tissues are required to determine the ultimate source of these parenchymal changes. Second is the question of variation in SARS CoV2 placental infection in relation to fetal transmission [4,5,[15], [16], [17], [18], [19], [20]]. We identified the presence of SARS CoV-2 viral proteins within the villous placental compartment regardless of fetal transmission, helping clarify this key point.
A final key gap in the literature has been characterization of SARS CoV-2 viral entry machinery in placental tissues from primary COVID-19 affected pregnancies, rather than historical data sets. Our localization of ACE2 within placental villous sTB is consistent with prior work [35,36], and through comparative analysis we noted that ACE2 expression levels had a small but significant decrease in COVID-19Maternal placental tissues as compared to non-infected controls. While it is possible that the placenta could be downregulating ACE2 in COVID infection, the findings from this limited descriptive analysis are not sufficient to make any true conclusion on this point. Further, our data illustrating ACE2 over TMPRSS2 predominance in this cohort is consistent with prior reports on single cell RNA seq data sets in healthy placental tissues [[37], [38], [39]]. While this finding requires further validation in subsequent studies, it does suggest that SARS-CoV-2 may be using alternative cellular entry pathways molecules for viral entry in the placenta, as has been identified for intestinal enterocytes [43].
The current study has several limitations. First, this is a descriptive analysis with quantification based on a single experimental technique in tissue biopsies that encompass <10% of the tissue surface of the placental organ. Additional analysis with larger cohorts with additional quantitative techniques such as western blotting and qPCR/RNAseq analysis will be required to more fully characterize the expression of CoV-SP, ACE2 and TMPRSS2 and other potential viral entry machinery in these tissues. Finally, our cohort only encompassed pregnancies affected by third trimester COVID-19 infections. As pandemic continues, comparative analysis on the effects of COVID-19 infection in all trimesters will be key in understanding the pathophysiology and consequences for mother and fetus throughout gestation.
Overall this study provides an important basis for ongoing study of SARS-CoV-2 infection at the maternal-fetal interface. Analysis of placental tissues also allows a critical viewpoint of SARS CoV-2 physiology in primary human tissue, which may be informative for other organ systems. The placenta shares many developmental and physiological similarities with the lung [44] and as mucosal immune interface, placental leukocyte populations have many well-recognized parallels with the immune repertoire of the small and large intestine [45]. Thus ongoing study of COVID-19 physiology within the placenta can significantly inform management and surveillance of pregnant women and infants during this pandemic, while providing a key source of primary tissue for development of ongoing diagnostic and therapeutic targets critical in the effort to reduce the global burden of SARS-CoV-2.
Declaration of competing interest
None.
Acknowledgements
We would like to thank the Department of Pathology at Boston Medical Center for their support and collaboration, particularly Elizabeth Duffy, Charline Mack, and Cheryl Spencer. We would also like to thank the Boston University Medical Campus Alumni Library, the Boston University School of Medicine Cellular Imaging Core and everyone in the Finland Laboratory for Pediatric Infectious Disease at Boston Medical Center, particularly lab manager Yazdan Dasthagrasaheb for all of his support, and laboratory technician Loc Truong. This work was funded by the Boston University Clinical and Translational Science Institute COVID-19 Pilot Grant Program (UL1TR001430), NIH T32 1T32HD098061-01 (EST) and the Boston University School of Medicine Medical Student Summer Research Program.
References
- 1.Map C.- Johns Hopkins Coronavirus Resource Center; 2020. COVID-19 Map. [Google Scholar]
- 2.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D., Chen S., Zeng W., Feng L., Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study, the Lancet. Infectious diseases. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., Liao J., Yang H., Hou W., Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshafeey F., Magdi R., Hindi N., Elshebiny M., Farrag N., Mahdy S., Sabbour M., Gebril S., Nasser M., Kamel M., Amir A., Maher Emara M., Nabhan A. A systematic scoping review of COVID-19 during pregnancy and childbirth. Int. J. Gynaecol. Obstet.: the official organ of the International Federation of Gynaecology and Obstetrics. 2020;150(1):47–52. doi: 10.1002/ijgo.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duran P., Berman S., Niermeyer S., Jaenisch T., Forster T., Gomez Ponce de Leon R., De Mucio B., Serruya S. COVID-19 and newborn health: systematic review. Rev. Panam. Salud Públic. 2020;44:e54. doi: 10.26633/RPSP.2020.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huntley B.J.F., Huntley E.S., Di Mascio D., Chen T., Berghella V., Chauhan S.P. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet. Gynecol. 2020;136(2):303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 7.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. Jama. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J., Feng L. A case report of neonatal 2019 coronavirus disease in China, clinical infectious diseases. An official publication of the Infectious Diseases Society of America. 2020;71(15):853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., Yue L., Li Q., Sun G., Chen L., Yang L. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America; 2020. Maternal and Neonatal Outcomes of Pregnant Women with COVID-19 Pneumonia: a Case-Control Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. Jama. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., Liu Y. Infants born to mothers with a new coronavirus (COVID-19) Frontiers in pediatrics. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y., Nie X., Huang B.X. Pregnancy with new coronavirus infection: clinical characteristics and placental pathological analysis of three cases. Zhonghua bing li xue za zhi = Chin. J. Pathol. 2020;49(5):418–423. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 14.Yang H., Sun G., Tang F., Peng M., Gao Y., Peng J., Xie H., Zhao Y., Jin Z. Clinical features and outcomes of pregnant women suspected of coronavirus disease 2019. J. Infect. 2020;81(1):e40–e44. doi: 10.1016/j.jinf.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am. J. Obstet. Gynecol. 2020;223(1):36–41. doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dashraath P., Wong J.L.J., Lim M.X.K., Lim L.M., Li S., Biswas A., Choolani M., Mattar C., Su L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020;222(6):521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamouroux A., Attie-Bitach T., Martinovic J., Leruez-Ville M., Ville Y. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am. J. Obstet. Gynecol. 2020;223(1):91.e1–91.e4. doi: 10.1016/j.ajog.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z., Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: A systematic review. Am. J. Perinatol. 2020;37(10):1055–1060. doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. Jama. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection Be acquired in utero?: More definitive evidence is needed. Jama. 2020 doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 21.Muldoon K.M., Fowler K.B., Pesch M.H., Schleiss M.R. SARS-CoV-2: Is it the newest spark in the TORCH? J. Clin. Virol. : Off. Publ. Pan Am. Soc. Clin. Virol. 2020;127:104372. doi: 10.1016/j.jcv.2020.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor G., Aldo P., Alvero A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017;17(8):469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- 23.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. American journal of obstetrics & gynecology MFM; 2020. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth; p. 100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J., Roman A.S. American journal of obstetrics & gynecology MFM; 2020. Detection of SARS-COV-2 in placental and fetal membrane samples; p. 100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B., Casanovas-Massana A., Vijayakumar P., Geng B., Odio C.D., Fournier J., Brito A.F., Fauver J.R., Liu F., Alpert T., Tal R., Szigeti-Buck K., Perincheri S., Larsen C.P., Gariepy A.M., Aguilar G., Fardelmann K.L., Harigopal M., Taylor H.S., Pettker C.M., Wyllie A.L., Dela Cruz C.S., Ring A.M., Grubaugh N.D., Ko A.I., Horvath T.L., Iwasaki A., Reddy U.M., Lipkind H.S. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020 doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Algarroba G.N., Rekawek P., Vahanian S.A., Khullar P., Palaia T., Peltier M.R., Chavez M.R., Vintzileos A.M. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020;223(2):275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baergen R.N., Heller D.S. Placental pathology in covid-19 positive mothers: Preliminary findings, pediatric and developmental pathology. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol.Soc. 2020;23(3):177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giugliano S., Petroff M.G., Warren B.D., Jasti S., Linscheid C., Ward A., Kramer A., Dobrinskikh E., Sheiko M.A., Gale M., Jr., Golden-Mason L., Winn V.D., Rosen H.R. Hepatitis C virus sensing by human trophoblasts induces innate immune responses and recruitment of maternal NK cells: Potential implications for limiting vertical transmission. J. Immunol. 2015;195(8):3737–3747. doi: 10.4049/jimmunol.1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorsamy V., Vallen C., Haffejee F., Moodley J., Naicker T. The role of trophoblast cell receptor expression in HIV-1 passage across the placenta in pre-eclampsia: an observational study. BJOG. 2017;124(6):920–928. doi: 10.1111/1471-0528.14311. [DOI] [PubMed] [Google Scholar]
- 31.Fazely F., Fry G.N., Thirkill T.L., Hakim H., King B.F., Douglas G.C. Kinetics of HIV infection of human placental syncytiotrophoblast cultures: an ultrastructural and immunocytochemical study. AIDS Res. Hum. Retrovir. 1995;11(9):1023–1030. doi: 10.1089/aid.1995.11.1023. [DOI] [PubMed] [Google Scholar]
- 32.Arora N., Sadovsky Y., Dermody T.S., Coyne C.B. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21(5):561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pringle K.G., Tadros M.A., Callister R.J., Lumbers E.R. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32(12):956–962. doi: 10.1016/j.placenta.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Valdés G., Neves L.A., Anton L., Corthorn J., Chacón C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27(2–3):200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Singh M., Bansal V., Feschotte C. 2020. A Single-Cell RNA Expression Map of Human Coronavirus Entry Factors, bioRxiv : the Preprint Server for Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pique-Regi R., Romero R., Tarca A.L., Luca F., Xu Y., Alazizi A., Leng Y., Hsu C.D., Gomez-Lopez N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife. 2020;9 doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PloS One. 2020;15(4) doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrejeva G., Gowan S., Lin G., Wong Te Fong A.L., Shamsaei E., Parkes H.G., Mui J., Raynaud F.I., Asad Y., Vizcay-Barrena G., Nikitorowicz-Buniak J., Valenti M., Howell L., Fleck R.A., Martin L.A., Kirkin V., Leach M.O., Chung Y.L. De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy. Autophagy. 2020;16(6):1044–1060. doi: 10.1080/15548627.2019.1659608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenan S., Liang H., Goodman H.J., Jacobs A.J., Chan A., Grande D.A., Levin A.S. 5-Aminolevulinic acid tumor paint and photodynamic therapy for myxofibrosarcoma: an in vitro study. J. Orthop. Surg. Res. 2020;15(1):94. doi: 10.1186/s13018-020-01606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nocera A.L., Meurer A.T., Singleton A., Simons C., BuSaba J., Tara Gass N., Han X., Bleier B.S. Intact soluble P-glycoprotein is secreted by sinonasal epithelial cells. Am. J. Rhinol. Aller. 2016;30(4):246–249. doi: 10.2500/ajra.2016.30.4330. [DOI] [PubMed] [Google Scholar]
- 43.Zang R., Gomez Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., Diamond M.S., Ciorba M.A., Whelan S.P.J., Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47) doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taglauer E., Abman S.H., Keller R.L. Recent advances in antenatal factors predisposing to bronchopulmonary dysplasia. Semin. Perinatol. 2018;42(7):413–424. doi: 10.1053/j.semperi.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erlebacher A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003. [DOI] [PubMed] [Google Scholar]