Abstract
Objective:
To compare the efficacy of contrast-enhanced ultrasound enabled reclassification of Breast Imaging Reporting and Data System (CEUS-BI-RADS) with MRI in the diagnosis of breast lesions with calcification.
Methods:
A total of 52 breast lesions with calcification from 51 patients were detected by ultrasound as hyperechoic foci and categorized as BI-RADS 3–5. The 51 patients further underwent CEUS scan and MRI. The ultrasound-BI-RADS combined with CEUS 5-point score system redefined the classification of BI-RADS which was called CEUS-BI-RADS. The diagnostic efficacy of three methods was assessed by receiver operating characteristic (ROC) curve analysis. Histopathological assessment used as the gold-standard.
Results:
The sensitivities of Ultrasound-BI-RADS, MRI classification of BI-RADS (MRI-BI-RADS) and CEUS-BI-RADS were 85%, 90% and 95% without significant difference among the three modalities (p > 0.05). The diagnostic specificities of ultrasound-BI-RADS, MRI-BI-RADS and CEUS-BI-RADS were 78.1%, 78.1% and 96.8%, respectively (p < 0.05); and the accuracy were 80.7%, 82.6% and 96.1% for ultrasound-BI-RADS, MRI-BI-RADS and CEUS-BI-RADS, respectively (p < 0.05). The area under ROC (AUROC) in differentiation of breast lesions with calcification was 0.945 for CEUS-BI-RADS, 0.907 for MRI-BI-RADS and 0.853 for ultrasound-BI-RADS, with no significant difference among the three modalities (p > 0.05).
Conclusion:
The CEUS-BI-RADS has a better diagnostic efficiency than MRI-BI-RADS in the differentiation of the breast lesions with calcification.
Advances in knowledge:
•CEUS is a better method in differentiation of breast lesions with calcification.
•CEUS-BI-RADS increases the efficiency of diagnosis compared to MRI.
Introduction
Breast cancer is a severe health threat of females worldwide, especially with tendency to younger females. Evidently, calcification is one of the hallmarks that indicate benign or malignant breast lesions, especially useful in prediction of malignancy. Since the occurrence of breast tumor cannot be prevented completely, early detection is of great significance.1 Breast calcifications are mineral deposits developed due to various causes. In breast tissues, the presence of microcalcifications plays an important role in early detection of breast cancer. In fact, nearly 50% of untouchable breast tumors are discovered by mammography through the recognition of the microcalcification, and this method can show up to 90% of in situ ductal carcinoma cases.2
In breast cancer screening, in order to further clarify the ambiguous lesions discovered by mammography, ultrasound is a primary screening tool for hereditary breast cancer.3 It is useful to evaluate tumor morphology and calcification, but difficult to differential diagnosis lesions in many cases with color Doppler ultrasound due to a variety of its own limitations, such as its intrinsic grayscale, overlapped findings4 and relatively lower sensitivity in the detection of blood flow of the tumor microvessels. CEUS is a huge break in ultrasound methodology in recent years by using a microbubble contrast agent. CEUS can describe the microscopic and macroscopic cycles of the targeted organ,5 and makes it possible to overcome the disadvantages of ultrasound. Besides, CEUS has been proposed being a reliable method in the differentiation of breast lesions, since it provides typical enhancement patterns.4,6 CEUS would help to visualize the microcirculation around the calcifications.7,8 Thus, as being reported, the sensitivity of CEUS to the characterization of breast lesions was 0.86 [95% confidence interval (0.83, 0.89)] and the specificity was 0.79 [95% confidence interval (0.75, 0.83)].9 Using paramagnetic contrast medium, MRI is able to evaluate breast lesion not only in its morphology, but also in the normal vascular and tumor vascular bed. MRI is also capable of showing different typical enhancement patterns of different types of breast lesions. As reported, malignant lesions tend to show early hyperenhancement in arterial phase and fast wash-out compare to benign lesions.10 According to these findings, breast MRI has been proved to have high sensitivity in discriminating different types of breast lesions, ranging from 94 to 99%, but with pretty lower specificity, ranging from 50 to 80%, consistent with previous reports.11,12 Moreover, MRI has a false-positive rate of 8% on dense breast screening, which increases the number of unnecessary biopsies correspondingly.13
The purposes of our study were to compare the diagnostic efficacy of CEUS-BI-RADS with MRI-BI-RADS in the differential diagnosis of breast lesions with calcification, hence to demonstrate the feasibility of using CEUS-BI-RADS to avoid unnecessary biopsies and to identify additional diagnostic efficacy against the conventional MRI-BI-RADS, in the evaluation of breast lesions with calcifications.
Methods and materials
Patients
Between January 2010 and September 2017, 51 patients (mean age, 44 ± 10.4 years; range 17–66 years) either with or without symptoms (mass, pain or nipple discharge) having 52 breast lesions (mean diameter, 2.52 cm ±1.25 cm; range 0.61–6.02 cm) were enrolled retrospectively in this study. All the calcifications showed on ultrasound were confirmed by mammography. With the detection of a solid breast lesion with calcification which categorized as BI-RADS 3–5 by ultrasound, all the subjects received both CEUS and MRI examination. Following patients were excluded from the study: pregnancy or lactation or previous therapy or interventional diagnosis (BI-RADS 6). All patients underwent biopsy or surgery to get the pathological results as the golden standard. The written informed consent was obtained from all patients and the study was approved by the institutional ethics committee of the First Affiliated Hospital, Sun Yat-sen University.
Ultrasound examination
Two-dimensional (2D) ultrasound imaging was performed with a 12–5-MHz linear probe by an iU22 (Philips Medical Systems, Bothell, WA) ultrasonic scanner. CEUS was performed on the same ultrasound system but with a 9–3-MHz linear transducer. The contrast agent SonoVue (Bracco SpA, Milan, Italy) was used in CEUS scan.
Multiplane scanning was used to showing all of the features accurately. Following planes under ultrasound were chosen to CEUS targeted section: with the richest blood supply or with the most irregular shape if no lesions with abundant blood. B-Mode pulse inversion harmonic imaging was used for CEUS. The mechanical index was set at 0.06. Side-by-side image mode was also applied to pinpoint the lesion precisely throughout the whole CEUS process. Compressing of the vessels was avoided by compressing the lesion lightly. After 4.8 ml of contrast agent was injected and followed by 5 ml saline irrigated with intravenous cannula, CEUS scan began immediately. Real-time side-by-side loops were stored for up to 180 s for further analysis.
Magnetic resonance imaging examination
Breast MRI was performed with a dedicated 16-channel breast on a Verio3.0 T magnet resonance scanner (Siemens, Munich, Germany), with patients in the prone position. According to the requirements of the ACR’s Breast MRI Certification Program,14 the standard sequences were obtained. Axial T1 weighted MRI images before contrast examination and five dynamic sequences after contrast examination were obtained within a 90-s interval. Reconstructed sagittal T1 weighted images were generated, basing on the axial T1 weighted images after contrast examination. Dynamic contrast-enhanced MRI (DCE-MRI) was performed by using a T1 weighted fat-suppression volume interpolated breath-hold sequence before and five times after a bolus injection of gadobenate dimeglumine (0.1 mmol/kg), followed by 15 ml of saline. Two experienced radiologists read MRI images of breast at the same time.
Image analysis
Ultrasonography and magnetic resonance imaging. All examinations of modalities were independently analyzed by two radiologists with more than 8 years of experience in performing and interpreting the breast ultrasound and radiology images. Both of them had no access to the surgical information, histopathology and other imaging findings.
According to the BI-RADS fifth edition, the lesions of ultrasound and MRI were divided into five categories: 3, 4A, 4B, 4C and 5. Most of categories 3 and 4A were benign (category 3 was <2% and category 4A was >2% to≤10% likelihood of malignancy), and categories 4B, 4C and 5 were considered as malignant in different likelihoods.1 Biopsy is recommended for 4A and beyond.15
CEUS 5-point score system. The CEUS 5-point score system was used as previous study by Xiao et al published earlier,16 score 1: no enhancement with a clear borderline; score 2: iso- and synchronous enhancement, with an unclear outline; score 3: earlier enhancement, regular shape with clear margin, size no larger than that on the 2D image; score 4: earlier enhancement, size larger than that on the 2D image, irregular with clear margin; score 5: earlier enhancement, heterogeneously enhanced, size larger than the corresponding 2D image, generally a typical crab claw-like enhancement with indistinct boundary. Scores ≤3 were regarded as benign, scores >3 were regarded as malignant.
CEUS-BI-RADS. A CEUS-BI-RADS score was determined according to the rules specified in previous study1 called “rerated BI-RADS”, with the help of the CEUS 5-point system. Table 1 shows how the CEUS-BI-RADS rerate the original ultrasound-BI-RADS categories 3–5 to make the categories either higher or lower.
Table 1.
The rule for re-classified ultrasound-BI-RADS scores with CEUS
| Ultrasound-BI-RADS | CEUS score | CEUS-BI-RADS |
| 3, 4A | 1–3 | Unchanged with ultrasound-BI-RADS |
| 4 | Upgrade ultrasound-BI-RADS 3 to 4A | |
| Upgrade ultrasound-BI-RADS 4A to 4B | ||
| 5 | Upgrade ultrasound-BI-RADS 3 to 4B | |
| Upgrade ultrasound-BI-RADS 4A to 4C | ||
| 4B, 4C | 1–2 | Downgrade ultrasound-BI-RADS 4B to 3 |
| Downgrade ultrasound-BI-RADS 4C to 4A | ||
| 3 | Downgrade ultrasound-BI-RADS 4B to 4A | |
| Downgrade ultrasound-BI-RADS 4C to 4B | ||
| 4–5 | Upgrade ultrasound-BI-RADS 4B to 4C | |
| Upgrade ultrasound-BI-RADS 4C to 5 | ||
| 5 | 1–3 | Now CEUS-BI-RADS 4C |
| 4–5 | Stayed in CEUS-BI-RADS 5 |
CEUS: Contrast-enhanced ultrasound, CEUS-BI-RADS: contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System;Ultrasound-BI-RADS: conventional ultrasound classification of Breast Imaging Reporting and Data System.
Pathological diagnosis
Histologic confirmation was made by use of a core needle biopsy or surgical excision within one month after the examinations by the three imaging methods of ultrasound, CEUS and MRI. Histopathological assessment used as the gold-standard for diagnosis.
Statistical analysis
SPSS 22.0 software (SPSS, Inc., Chicago, IL) was used for recording data and statistical analysis. Firstly, 52 lesions were classified by pathological analysis. Secondly, McNemar's test was used to examine the diagnostic accuracy, specificity and sensitivity of different modalities. In addition, to compare the overall diagnostic performance of each method, Z-test was conducted to compare AUROCs of their each other. Thirdly, χ2 test was used to examine the patterns of ultrasound, CEUS and MRI. p < 0.05 was considered statistically significant in all of the statistical tests.
Results
Histopathology diagnosis and characteristics of breast lesions with calcification
52 breast lesions with calcification detected by ultrasound in 51 patients had surgical resection or biopsy. Histopathological diagnosis showed 32 benign and 20 malignant lesions (Table 2). The details for the characteristics of breast lesions with calcification were summarized in Supplementary Table 1. Both the size and the location of calcification within the breast lesions had significant statistical difference in distinguishing benign lesions from malignant (p < 0.05). Supplementary Tables 2-4 summarize the image patterns of ultrasound, CEUS and MRI between the benign and malignant breast solid lesions with calcification. Two representative cases are shown in Figures 1 and 2.
Table 2.
Histopathology diagnosis
| Pathology | Total |
|---|---|
| Malignant lesions | 20 |
| Invasive ductal carcinoma | 15 |
| Ductal carcinoma in situ | 3 |
| Invasive lobular carcinoma | 1 |
| Intraductal papillary carcinoma | 1 |
| Benign lesions | 32 |
| Fibroadenoma | 6 |
| Fibrocystic mastopathy | 17 |
| Intraductal papilloma | 5 |
| Chronic mastitis | 2 |
| Foreign body granuloma | 1 |
| Infiltrating syringomatous adenoma | 1 |
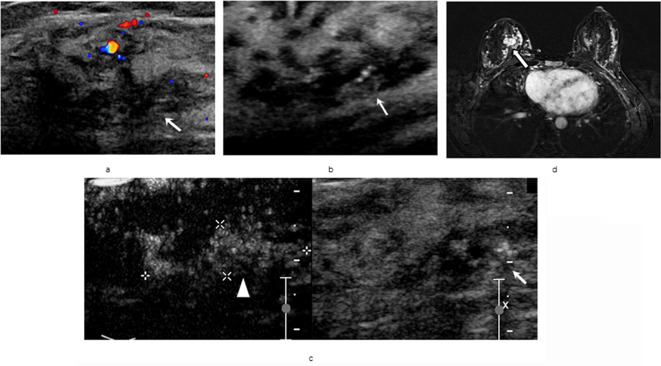
Figure 1.
A 45-years-old female with an invasive ductal carcinoma, diffuse spotty breast calcifications in the right side. (a/b) Ultrasound scans of the breast lesion categorized as ultrasound-BI-RADS 4A. (c) On CEUS dual image mode, lesion was reclassified as CEUS-BI-RADS 4B. (d) The lesion was categorized as MRI-BI-RADS 4B, all pointing to a malignant lesion. (lesion marked with‘↑’, area of CEUS enhancement was marked with white‘▲’). CEUS-BI-RADS, contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System.
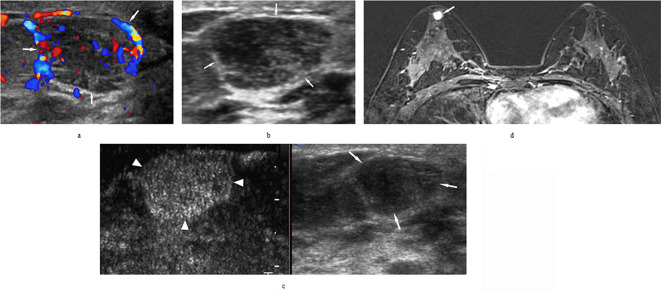
Figure 2.
A 33-years-old female with breast usual ductal hyperplasia, found breast lesion in the right side for 1 month after bilateral ovarian cancer resection. (a/b) Ultrasound scans of the breast lesion categorized as ultrasound-BI-RADS 4B. (c) On CEUS dual image mode, the lesion was reclassified as CEUS-BI-RADS 3. (d) The lesion was categorized as MRI-BI-RADS 4B by MRI. Both of ultrasound and MRI pointed to a malignant lesion. (lesion marked with‘↑’, area of CEUS enhancement was marked with white‘▲’). CEUS-BI-RADS, contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System.
Diagnostic performance
ROC curves were constructed to reflect the diagnostic efficacy of ultrasound-BI-RADS, CEUS-BI-RADS and MRI-BI-RADS in the differential diagnosis of breast lesions with calcification (Figure 3). The corresponding sensitivity, specificity, accuracy and AUROC of above three methods are shown in Table 3.
Figure 3.
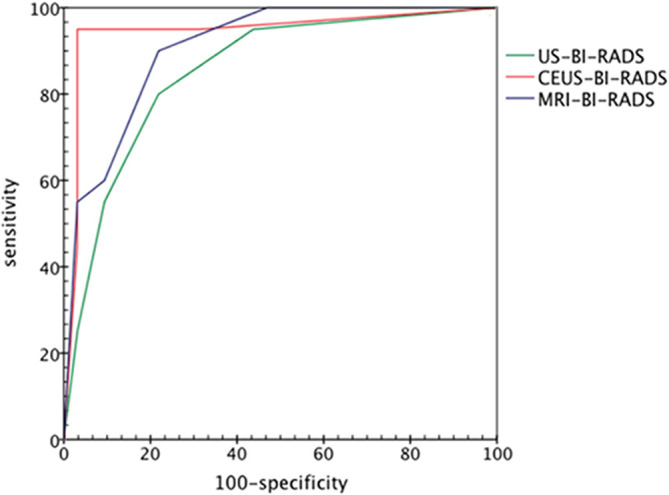
ROC curves representing the diagnostic efficacy of ultrasound-BI-RADS, CEUS-BI-RADS and MRI-BI-RADS for all of 52 breast lesions with calcification. The areas under the receiver operating characteristic curve for ultrasound-BI-RADS, CEUS-BI-RADS and MRI-BI-RADS were 0.853, 0.945 and 0.907, respectively.. CEUS-BI-RADS, contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System.
Table 3.
Diagnostic performance of the three modalities in differentiation of benign and malignant breast lesions with calcification
| Methods | Accuracy | Sensitivity | Specificity | AUROC |
|---|---|---|---|---|
| Ultrasound-BI-RADS | 80.7% | 85% | 78.1% | 0.853 |
| MRI-BI-RADS | 82.6% | 90% | 78.1% | 0.907 |
| CEUS- BI-RADS | 96.1% | 95% | 96.8% | 0.945 |
AUROC, Area under the receiver-operating characteristics curve; CEUS-BI-RADS, contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System;MRI-BI-RADS, Magnetic resonance imaging classification of Breast Imaging Reporting and Data System; US-BI-RADS, Conventional ultrasound classification of Breast Imaging Reporting and Data System.
The specificity of CEUS-BI-RADS compared with that of ultrasound-BI-RADS (p = 0.031) and MRI-BI-RADS (p = 0.031). .
The accuracy of CEUS-BI-RADS compared with that of US-BI-RADS (p = 0.028) and MRI-BI-RADS (p = 0.039)
Other comparisons were not statistically significant (p > 0.05).
CEUS-BI-RADS showed better diagnostic efficiency than either US-BI-RADS or MRI-BI-RADS in the diagnosis of breast lesions with calcification (AUC: 0.945 vs 0.853 and 0.907), with no statistically differences among them (p > 0.05 for all). The CEUS-BI-RADS had a considerably higher accuracy than that of ultrasound-BI-RADS and MRI-BI-RADS with statistical difference (96.1 vs 80.7 and 82.6%; p = 0.028 and p = 0.039, respectively). CEUS-BI-RADS also showed better specificity than that of ultrasound-BI-RADS and MRI-BI-RADS (96.8 vs 78.1 and 78.1%; p = 0.031 and p = 0.031, respectively). Compared with ultrasound-BI-RADS and MRI-BI-RADS, CEUS-BI-RADS still showed relatively higher sensitivity (95% vs 85 and 90%), without statistically differences (both p > 0.05) among them.
The diagnostic accuracy of CEUS-BI-RADS for lesions with BI-RADS category 4A and 4B was higher than that of ultrasound or MRI (Tables 4 and 5). Of the 52 breast lesions, 10 were misdiagnosed with ultrasound-BI-RADS (19.2%, 10/52), while the misdiagnosis rate for CEUS-BI-RADS was 3.8% (2/52).
Table 4.
Comparison of original BI-RADS category and CEUS-BI-RADS category with pathology results
| Ultrasound BI-RADS category(n) | CEUS BI-RADS category(n) | Pathology | |
|---|---|---|---|
| Benign (n = 32) | Malignant (n = 20) | ||
| 3 (19) | 3 (18) | 17 | 1 |
| 4A (1) | 1 | 0 | |
| 4B (0) | 0 | 0 | |
| 4A (9) | 3 (3) | 3 | 0 |
| 4A (4) | 4 | 0 | |
| 4B (2) | 0 | 2 | |
| 4B (10) | 3 (2) | 2 | 0 |
| 4A (2) | 2 | 0 | |
| 4B(1) | 0 | 1 | |
| 4C (5) | 0 | 5 | |
| 4C (8) | 4A (1) | 1 | 0 |
| 4C(2) | 0 | 2 | |
| 5 (5) | 1 | 4 | |
| 5 (6) | 4A (1) | 1 | 0 |
| 5 (5) | 0 | 5 | |
CEUS-BI-RADS: contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System; ultrasound-BI-RADS: conventional ultrasound classification of Breast Imaging Reporting and Data System.
Table 5.
Comparison of scores of ultrasound-BI-RADS, CEUS-BI-RADS and MRI-BI-RADS with pathology results
| BI-RADS category | Ultrasound-BI-RADS | CEUS-BI-RADS | MRI-BI-RADS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nn | Pathology | Accuracy | n | Pathology | Accuracy | n | Pathology | Accuracy | ||||
| (-) | (+) | (-) | (+) | (-) | (+) | |||||||
| 3 | 19 | 18 | 1 | 94.70% | 23 | 22 | 1 | 95.60% | 17 | 17 | 0 | 100% |
| 4A | 9 | 7 | 2 | 77.70% | 9 | 9 | 0 | 100% | 10 | 8 | 2 | 80.00% |
| 4B | 10 | 4 | 6 | 60.00% | 3 | 0 | 3 | 100% | 9 | 3 | 6 | 66.70% |
| 4C | 8 | 2 | 6 | 75.00% | 7 | 0 | 7 | 100% | 4 | 3 | 1 | 75.00% |
| 5 | 6 | 1 | 5 | 83.30% | 10 | 1 | 9 | 90.00% | 12 | 1 | 11 | 91.70% |
BI-RADS: Breast Imaging Reporting and Data System; CEUS-BI-RADS: contrast-enhanced ultrasound reclassification of Breast Imaging Reporting and Data System; MRI-BI-RADS: Magnetic resonance imaging classification of Breast Imaging Reporting and Data System; ultrasound-BI-RADS: conventional ultrasound classification of Breast Imaging Reporting and Data System.
Comparisons of ultrasound, CEUS and MRI imaging patterns between benign and malignant breast lesions with calcification
To analyze the ultrasound and CEUS features of breast lesions, the identification of breast solid lesions were confirmed through the combination of the pathologic results and all of the three imaging methods of ultrasound, CEUS and MRI. After exclusion of the 7 lesions with complex heterocyst and isolated calcification, 45 lesions were left. The characteristics of the 45 solid breast lesions were calculated, and shown in Supplementary Table 2-4. As shown in the tables, ultrasound features of the margin and vasculature showed statistic differences (p < 0.05). Malignant lesions usually featured with irregular shape, indistinct margin and rich vasculature while benign lesions with none or posterior enhancement and paucity vasculature (Supplementary Table 2).
Except the degree of enhancement and the type of margin, other CEUS patterns had significant difference in the differentiating benign solid breast lesions with calcification from that of malignant (p < 0.05 for all). The benign lesions showed the same size in both ultrasound and CEUS image and were characterized as homogeneous, iso- or hypoenhancement with no radial or penetrating vessels. The malignant lesions exhibited “classic” larger size in CEUS than that of US with hyperenhanced with radial or penetrating vessels (Supplementary Table 3).
In DCE-MRI images, lesions were classified as mass and non-mass-like enhancing patterns according to the first version of the MRI-BI-RADS in 2003. Due to no lesions shown in those types, four distribution patterns (ductal, regional, multiple regions and diffuse) and two internal characteristics (homogeneous, reticular/dendritic) were not included (Supplementary Table 4) . Of the mass, except the shapes, there were significant differences in margin and internal enhancement between the benign and malignant lesions (p < 0.05). In this study, it was noticed that in the non-mass-like enhancement, no significant difference were found between malignant and benign lesions (p > 0.05). For enhancement kinetic descriptors, benign lesions showed “slow in” and persistent enhancement throughout the entire scan while malignant lesions expressed “fast in” and “wash out” enhancement, of which there was statistically significant difference between them (p < 0.05).
Discussion
As we all know, it is the first time of a study employing the CEUS 5-point score system and reclassifying the ultrasound-BI-RADS categories to CEUS-BI-RADS, with the help of CEUS to assess the breast lesions with calcification initially detected by ultrasound. It aimed to compare the diagnosis efficacy between CEUS-BI-RADS and other conventional methods. The study showed that diagnostic efficacy of CEUS-BI-RADS was markedly superior to both of ultrasound-BI-RADS and MRI-BI-RADS. In terms of the diagnosis of breast lesions with calcification, the CEUS-BI-RADS improved the diagnosis in comparison with MRI-BI-RADS.
Ultrasound is useful in estimating lesion appearance, such as shape, tumor size, boundary, echo and calcification, which had been demonstrated to be helpful in breast lesions feature by many studies.17–19 Ultrasound is also able to detect most of microcalcifications (a maximum diameter of 1 mm), which are often the first indication of breast cancer.20 This study showed that almost three-quarters of benign lesions had microcalcifications, and the microcalcifications exist in breast lesions no matter benign or malignant. In addition, the detection of flow in tiny vessels by ultrasound is relatively low in respect of the low speed and the interference with breathing or heartbeat of the patient.21 So, ultgrasound diagnostic accuracy is limited in the diagnosis of breast lesions with calcification.
In the previous study by Xiao et al,16 a CEUS 5-point score system was established and it was named as rerated BI-RADS that focused on the solid breast lesions regardless of the presence of the calcification. However, the microvasculature, the size and distribution of calcification are both vital in the differential diagnosis of breast lesions. In Table 4, of the nine breast lesions initially categorized as ultrasound-BI-RADS 4A, including two misdiagnosed malignancies, but they were all later recategorized to CEUS-BI-RADS 4B after CEUS. Of the 10 ultrasound-BI-RADS 4B lesions, four benign lesions were over diagnosed and were later downgraded to CEUS-BI-RADS 4A or 3 after CEUS scans. In Table 5, the diagnosis accuracy of CEUS-BI-RADS for both BI-RADS category 4A and 4B lesions were 100%, but the diagnostic accuracy were significantly lower at 77.7%, 60% for ultrasound-BI-RADS and 80.0%, 66.7% for MRI-BI-RADS. In view of this, the diagnostic value of CEUS-BI-RADS was higher in comparison with ultrasound or MRI alone. Compared with ultrasound and MRI, the specificity and accuracy of CEUS-BI-RADS were significantly higher (p < 0.05 for all), especially in diagnosing the CEUS-BI-RADS 4A and 4B lesions. Besides, CEUS-BI-RADS has higher sensitivity (95%) which was consistent to the previous studies (96.9%, reported by Xiao et al1). One benign lesion was over estimated and one malignant lesion was underestimated in our study, they were infiltrating syringomatous adenoma and ductal carcinoma in situ (DCIS) with scattered calcification, respectively in pathology. Even if not enough more substantial angiogenesis supporting, Low-grade carcinoma in situ still can grow under the situation that the surrounding capillary network can be able to nourish its growth. The occurrence of misdiagnosis due to scattered macrocalcification and the lack of malformed neovascular.22 All of the findings from this study require further investigations.
DCE-MRI is more sensitive in the detection of breast cancer, with a negative predictive value ranging between 89 and 99%, however the weak side is that it has a large variations in specificity ranging from 47 to 97%,23 which was in accordance with this study. Tozaki et al reported that a linear enhancement distribution was found in half of those benign lesions in non-mass-like enhancement.24 Liberman et al also depicted that among non-mass lesions, segmental enhancement was shown frequently in carcinoma (with 67% chance of carcinoma),25 however no significant difference was found in our study, possibly due to a small sample size. A typical enhancement pattern which shows washout or plateau after peak enhancement is common feature of the invasive breast cancer. Thus, significant difference in initial phase was shown between benign and malignant breast lesions (p < 0.05 for all). Here, only the lesions showed rapid enhancement and washout quickly can the differential diagnosis be used more confidently.24 Small malignant lesion may be different from typical large malignant lesion in characteristics. Therefore, applying more or multimodalities would help to evaluate the small malignant lesions by acquiring more information hence lead to better judgment.
In this study, one benign lesion was over diagnosed as a malignant lesion by all of the modalities. Ultrasound scan showed an obscure boundary, irregular shape or hypoechoic lesion with shadowing and rich blood, while on CEUS, it appeared larger size in enhancement and hyperenhanced, radial or penetrating vessels. All of the ultrasound and CEUS features led to an impression of malignant. Examining the area of nipple and periareolar under ultrasound is known to be challenging, and it is necessarily to use a standoff pad, and various compression techniques.26 It is difficult to visualize the microcalcifications on ultrasound, particularly a surface structure, such as a nipple.
There were a few limitations in our study. First, it is difficult for convincing all the enrolled patients to accept both MRI and CEUS because of time-wasting and high-costs. Second, the number of cases and the types of breast lesions were limited. Third, quantitative measurements of CEUS was not used, instead, we just used only one image with the abundant blood flow to evaluate enhancement mode. Also, two readers evaluated the images together and had reached an agreement on the all kinds of imaging characteristics of breast lesions, so it was impossible to evaluate interobserver errors among the ultrasound-BI-RADS, CEUS and MRI-BI-RADS image readings.
Conclusions
In conclusion, the CEUS-BI-RADS has an obvious advantage in the diagnostic performance than MRI and conventional ultrasound in terms of diagnosing the breast lesions with calcification.
Footnotes
Acknowledgment: The scientific guarantor of this research is Yanling Zheng. The authors have no conflicts of interest with any persons or business. The authors state that this study received no funding. This study has obtained approval by Institutional Review Board.There were no study subjects or cohorts ever reported elsewhere.
Contributor Information
Jiamin Pan, Email: 263464446@qq.com.
Wenjuan Tong, Email: yaya09wjtong@126.com.
Jia Luo, Email: luojia7@mail.sysu.edu.cn.
Jinyu Liang, Email: ljyu@mail.sysu.edu.cn.
Fushun Pan, Email: panfushun123@163.com.
Yanling Zheng, Email: zhyanl@mail.sysu.edu.cn.
REFERENCES
- 1.Xiao X, Dong L, Jiang Q, Guan X, Wu H, Luo B. Incorporating contrast-enhanced ultrasound into the BI-RADS scoring system improves accuracy in breast tumor diagnosis: a preliminary study in China. Ultrasound Med Biol 2016; 42: 2630–8. doi: 10.1016/j.ultrasmedbio.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 2.Bonfiglio R, Scimeca M, Toschi N, Pistolese CA, Giannini E, Antonacci C, et al. Radiological, histological and chemical analysis of breast microcalcifications: diagnostic value and biological significance. J Mammary Gland Biol Neoplasia 2018; 23(1-2): 89–99. doi: 10.1007/s10911-018-9396-0 [DOI] [PubMed] [Google Scholar]
- 3.Delorme S. Ultrasound in oncology: screening and staging. Internist 2012; 53: 271–81. doi: 10.1007/s00108-011-2958-5 [DOI] [PubMed] [Google Scholar]
- 4.Du J, Wang L, Wan C-F, Hua J, Fang H, Chen J, et al. Differentiating benign from malignant solid breast lesions: combined utility of conventional ultrasound and contrast-enhanced ultrasound in comparison with magnetic resonance imaging. Eur J Radiol 2012; 81: 3890–9. doi: 10.1016/j.ejrad.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Xu H-X. Contrast-Enhanced ultrasound: the evolving applications. World J Radiol 2009; 1: 15–24. doi: 10.4329/wjr.v1.i1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci P, Cantisani V, Ballesio L, Pagliara E, Sallusti E, Drudi FM, et al. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med 2007; 28: 57–62. doi: 10.1055/s-2006-927226 [DOI] [PubMed] [Google Scholar]
- 7.Leng X, Huang G, Ma F, Yao L, Ultrasonography RC-E. CEUS) characteristics of breast cancer and correlation with microvessel density (MVD. Med Sci Monit 2017; 23: 3428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidner N. Semple Jp Fau - Welch WR, Welch Wr Fau - Folkman J, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. 0028-4793. [DOI] [PubMed] [Google Scholar]
- 9.Hu Q, Wang XY, Zhu SY, Kang LK, Xiao YJ, Zheng HY. Meta-Analysis of contrast-enhanced ultrasound for the differentiation of benign and malignant breast lesions. Acta Radiol 2015; 56: 25–33. doi: 10.1177/0284185113517115 [DOI] [PubMed] [Google Scholar]
- 10.Drudi FM, Cantisani V, Gnecchi M, Malpassini F, Di Leo N, de Felice C. Contrast-Enhanced ultrasound examination of the breast: a literature review. Ultraschall Med 2012; 33: E1–7. doi: 10.1055/s-0031-1299408 [DOI] [PubMed] [Google Scholar]
- 11.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999; 213: 881–8. doi: 10.1148/radiology.213.3.r99dc01881 [DOI] [PubMed] [Google Scholar]
- 12.Al-Khawari H, Athyal R, Kovacs A, Al-Saleh M, Madda JP. Accuracy of the Fischer scoring system and the breast imaging reporting and data system in identification of malignant breast lesions. Ann Saudi Med 2009; 29: 280–7. doi: 10.4103/0256-4947.55310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 2019; 381: 2091–102. doi: 10.1056/NEJMoa1903986 [DOI] [PubMed] [Google Scholar]
- 14.Covington MF, Young CA, Appleton CM. American College of radiology accreditation, performance metrics, reimbursement, and economic considerations in breast MR imaging. Magn Reson Imaging Clin N Am 2018; 26: 303–14. doi: 10.1016/j.mric.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Xu P, Yang M, Liu Y, Li Y-P, Zhang H, Shao G-R. Breast non-mass-like lesions on contrast-enhanced ultrasonography: feature analysis, breast image reporting and data system classification assessment. WJCC 2020; 8: 700–122307-8960 (Print). doi: 10.12998/wjcc.v8.i4.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao X, Ou B, Yang H, Wu H, Luo B. Breast contrast-enhanced ultrasound: is a scoring system feasible? A preliminary study in China. PLoS One 2014; 9: e105517. doi: 10.1371/journal.pone.0105517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E-K, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol 2008; 190: 1209–15. doi: 10.2214/AJR.07.3259 [DOI] [PubMed] [Google Scholar]
- 18.Hong AS, Rosen EL, Soo MS, Baker JA. Bi-Rads for sonography: positive and negative predictive values of sonographic features. AJR Am J Roentgenol 2005; 184: 1260–5. doi: 10.2214/ajr.184.4.01841260 [DOI] [PubMed] [Google Scholar]
- 19.Lee H-J, Kim E-K, Kim MJ, Youk JH, Lee JY, Kang DR, et al. Observer variability of breast imaging reporting and data system (BI-RADS) for breast ultrasound. Eur J Radiol 2008; 65: 293–8. doi: 10.1016/j.ejrad.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Henrot P, Leroux A, Barlier C, Génin P. Breast microcalcifications: the lesions in anatomical pathology. Diagn Interv Imaging 2014; 95: 141–52. doi: 10.1016/j.diii.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 21.Kettenbach J, Helbich TH, Huber S, Zuna I, Dock W. Computer-Assisted quantitative assessment of power Doppler us: effects of microbubble contrast agent in the differentiation of breast tumors. Eur J Radiol 2005; 53: 238–44. doi: 10.1016/j.ejrad.2004.04.017 [DOI] [PubMed] [Google Scholar]
- 22.Xiao X, Jiang Q, Wu H, Guan X, Qin W, Luo B. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound-a preliminary study in China. Eur Radiol 2017; 27: 2443–50. doi: 10.1007/s00330-016-4628-4 [DOI] [PubMed] [Google Scholar]
- 23.Pinker K, Helbich TH, Morris EA. The potential of multiparametric MRI of the breast. Br J Radiol 2017; 90: 20160715. doi: 10.1259/bjr.20160715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal G, Su M-Y, Nalcioglu O, Feig SA, Chen J-H. Significance of breast lesion descriptors in the ACR BI-RADS MRI lexicon. Cancer 2009; 115: 1363–80. doi: 10.1002/cncr.24156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberman L, Morris EA, Lee MJ-Y, Kaplan JB, LaTrenta LR, Menell JH, et al. Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol 2002; 179: 171–8. doi: 10.2214/ajr.179.1.1790171 [DOI] [PubMed] [Google Scholar]
- 26.AlSharif S, Tremblay F, Omeroglu A, Altinel G, Sun S, Mesurolle B. Infiltrating syringomatous adenoma of the nipple: sonographic and mammographic features with pathologic correlation. J Clin Ultrasound 2014; 42: 427–9. doi: 10.1002/jcu.22150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.