Abstract
Study Objectives:
To evaluate whether dietary patterns are associated with sleep quality in Mexican midlife women.
Methods:
The study population included 4,467 Mexican women from a longitudinal study of teachers. In 2008, a semiquantitative food frequency questionnaire was administered. Principal components analysis identified 3 dietary patterns: Fruits and Vegetables, Western (meat and processed), and Modern Mexican (tortillas and soda, low in fiber and dairy). Starting in 2012, follow-up questionnaires included the Pittsburgh Sleep Quality Index, which yields a score ranging from 0 to 21 (higher scores = worse quality). Modified Poisson regression analyses examining the association between dietary patterns (categorized into quartiles) and poor sleep quality (score > 5) were conducted, adjusting for socio-demographic and lifestyle confounders and baseline comorbid conditions.
Results:
Women were 41.0 ± 7.1 years at baseline, with an average follow-up of 5.5 ± 0.7 years. In fully adjusted models, women in the least-healthy quartile of the Fruits and Vegetables pattern compared with the most were 21% more likely to have poor quality sleep at follow-up (95% confidence interval 1.06, 1.42), while those in the highest quartiles of the Modern Mexican pattern were 23% more likely to have poor quality sleep compared with the lowest quartiles (95% confidence interval 1.06 to 1.43, respectively).
Conclusions:
A fruit and vegetable-based dietary pattern was associated with higher sleep quality, while an unhealthier diet pattern was associated with worse sleep quality in midlife women.
Citation:
Jansen EC, Stern D, Monge A, et al. Healthier dietary patterns are associated with better sleep quality among midlife Mexican women J Clin Sleep Med. 2020;16(8):1321–1330.
Keywords: fruits, vegetables, processed, energy-dense, meat, dairy, sleep disturbance, insomnia, sleep latency
BRIEF SUMMARY
Current Knowledge/Study Rationale: Poor sleep quality is highly prevalent among women of perimenopausal age; thus, a critical need exists to uncover modifiable determinants of sleep quality in this population. Whether dietary patterns are predictive of sleep quality and its components remains unclear.
Study Impact: Less-healthy dietary patterns, including diets low in fruits, vegetables, and dairy and high in tortillas, soda, and red meat were associated with worse sleep quality 5 years later. Whether dietary changes could improve sleep quality among perimenopausal women deserves investigation in randomized trials.
INTRODUCTION
Sleep changes across the life span and certain life stages, including the menopausal transition, are marked by reduced quantity and quality of sleep.1,2 Suboptimal sleep represents a significant public health burden, as sleep is related to practically every aspect of health and daily function. Menopausal women with sleep disturbances have lower perceived health and quality of life, higher vasomotor symptoms, and higher probability of developing mental health problems and cardiometabolic disease.1,3,4 Thus, uncovering modifiable behaviors that could improve sleep quality during the menopausal transition is salient for public health.
Diet is one modifiable behavior that could affect sleep quality,5 as evidenced by studies that examine individual foods, nutrients, or other substances obtained through the diet. For example, caffeine and alcohol directly affect sleep onset and quality.6,7 Sources of tryptophan (the dietary precursor to melatonin, a sleep/wake regulatory hormone), such as milk, fruits including tart cherries and kiwifruit, and fatty fish are individual foods that have been related to earlier sleep onset or higher quality sleep.8–11
Beyond individual food items, how dietary patterns as a whole relate to sleep has garnered recent attention.12–16 Dietary pattern analysis takes into account the fact that in free-living populations, foods naturally cluster in the diet. Furthermore, there may be interactions between foods with respect to sleep (eg, milk and alcohol, which may affect sleep in counteracting ways, could be part of the same pattern). Recent studies in Australia12 and the United Kingdom16 reported associations between healthier diet patterns (characterized by fruits and vegetables, nuts, fish, whole grains) and longer sleep duration. A study among Chinese adults15 found that 2 distinct dietary patterns—1 marked by high wheat and low intake of rice and the other characterized by meat, fish, eggs, fruit, and dairy—were associated with fewer insomnia symptoms. Finally, a multiethnic US study14 showed that adults with a higher adherence to a Mediterranean dietary pattern score were less likely to have objectively-measured short sleep duration, as well as fewer insomnia symptoms.
While the previous studies overall support the premise that dietary patterns could have implications for sleep quality, there are some important limitations. First, findings may not be generalizable to women of perimenopausal age, as analyses were not stratified by age or reproductive stages, nor were they (except for 1 study12) stratified by sex. Second, most of the analyses were cross-sectional, and as a result could not disentangle the bidirectional relationships between sleep and diet. Third, Latin American populations remain underrepresented in previous analyses. Thus, using an established cohort of Mexican teachers in midlife, the aim of the present study was to evaluate whether dietary patterns were related to sleep quality assessed several years later, as measured by the Pittsburgh Sleep Quality Index (PSQI) adapted for Spanish speakers.
METHODS
Study population
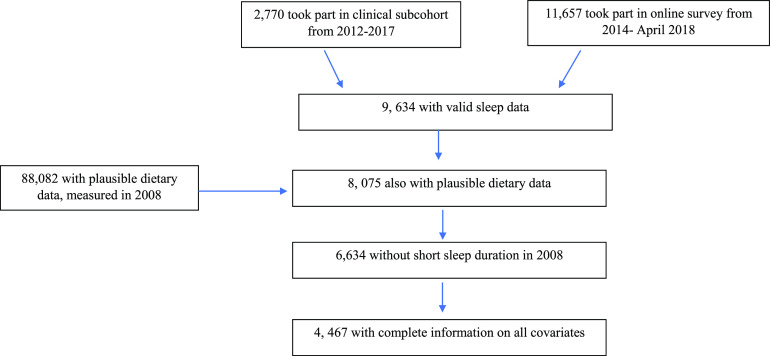
The Mexican Teachers’ Cohort is an ongoing prospective study of 115,314 female public-school teachers from 12 states in Mexico that was established between 2006 and 2008.17 Information about medical history, lifestyle (including diet and typical sleep duration), and health conditions was collected by self-administered questionnaires approximately every 3–4 years since baseline. Response to the baseline questionnaire was considered consent to participate in the study. This study was approved by the Institutional Review Board at National Institute of Public Health of Mexico. For the present analysis, the analytic sample relied on women who answered questions concerning sleep quality, which were included only at certain waves of follow-up. Specifically, sleep questions were included as a part of a clinical subcohort of women from 4 sites (Chiapas, Yucatan, Nuevo Leon, Mexico City) from 2012–2017, of which 2,770 women took part. In addition, starting in 2014, women were given the option to respond to an online questionnaire that also included sleep questions, and 11,657 participated as of April 2018. If women responded to both questionnaires, the questionnaire closer in time to dietary assessment (the clinical visit) was used.
From the two waves of follow-up that asked about sleep, there were a total of 9,634 unique women for whom there was valid sleep data (Figure 1). Of the women with sleep information, 8,075 women also had valid dietary information collected in 2008. Of these women, a total of 1,441 women were excluded because they reported to sleep < 6 hours at the baseline visit. The purpose for this exclusion was to create a baseline sample for those who did not already have poor sleep quality (short sleep duration of < 6 hours was considered as a proxy for poor sleep quality), in an attempt to avoid temporal ambiguity between diet patterns and development of poor sleep quality. The final sample size was 4,467 women who had complete information on all potential confounders. In comparison with the full baseline sample, the women in the study sample was less likely to speak an indigenous language, had more education, higher socioeconomic status, were less likely to have certain comorbidities, but were slightly more likely to report hypercholesterolemia and depression (Table S1 in the supplemental material).
Figure 1. Flowchart of analytic sample.
Diet assessment and dietary patterns
Dietary information was collected at baseline (2006–2008) using a 139-item semiquantitative food frequency questionnaire derived from a previously validated 116-item food frequency questionnaire in Mexico City.18 Twenty-three food items were added to the questionnaire to capture regional differences and secular changes in food consumption. Participants were asked to specify the average frequency of consumption over the previous year of each food item in a commonly used unit or portion size. Ten multiple-choice frequencies of consumption were possible: ≥ 6 per day, 4 or 5 per day, 2 or 3 per day, 1 per day, 5 or 6 per week, 2 or 4 per week, 1 per week, 2 or 3 per month, ≤ 1 per month, and never. The US Department of Agriculture food-composition database19 and the database used in the National Health and Nutrition Survey in Mexico20 were used to calculate nutrient and energy intakes by multiplying the nutrient content of the predefined portion sizes by the frequency of consumption. Dietary variables were considered invalid if women reported energy intake < 500 or > 3,500 kcal/d,21 responded to ≤ 70 items on the dietary questionnaire (ie, less than half of the total number), or did not respond to the section regarding cereals/grains (a food group making up a substantial portion of the diet).
Dietary patterns in the larger cohort (n = 88,082) were derived using principal component analysis (PCA) as described previously.22 Briefly, food items in the food frequency questionnaire were collapsed into 37 food groups based on similarity of nutrient content. Food groups were standardized as percent of total energy and orthogonal transformation was used to rotate the components. The number of components retained was determined using a combination of the eigenvalues (> 1.5), the scree plot, and the interpretability of the components. Factor scores were calculated by summing the observed intakes of the component food items weighted by its factor loading. In the previous PCA of these data,22 the following 3 distinct dietary patterns were identified: 1) Fruits and Vegetables Pattern, characterized by positive loadings for green vegetables, tomatoes, carrots, other vegetables, fruits, and legumes and negative loadings for pastries; 2) Western Pattern, characterized by positive loadings for processed meats, fast foods, and red meat and negative loading for fruit and corn tortilla; and 3) Modern Mexican Pattern, characterized by positive loadings for corn tortillas, hot peppers, and sodas and negative loadings for whole grains, dairy, and fruits. A sensitivity analysis of PCA conducted only among the analytic sample showed that similar diet patterns were identified as those previously identified; thus, for comparability across analyses, we used the original dietary pattern scores generated from the larger cohort.
Sleep assessment
At the baseline visit, 1 question concerning typical sleep duration was asked. In later waves of follow-up (described above), sleep quality was evaluated with the PSQI.23 The PSQI was previously used in Spanish-speaking populations24 and has been shown to be a valid and reliable instrument. The PSQI differentiates low-quality sleep from good sleep by measuring 7 domains: self-reported sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. A summary score is obtained by adding all component scores (ranging from 0 to 21), with a total score of > 5 indicative of poor sleep quality. Sleep responses were considered invalid if the self-reported sleep duration was < 3 hours or > 18 hours or sleep efficiency was outside the range of 0–100%.
Covariates
Information on covariates was based on self-reports from the 2006–2008 Mexican Teachers’ Cohort questionnaire, unless otherwise stated. The questionnaire included information on age, state of residence (Baja California, Chiapas, Durango, Guanajuato, Hidalgo, Jalisco, Mexico City, State of Mexico State, Nuevo Leon, Sonora, Yucatan, and Veracruz), education level (last completed degree of education), health care provider used for important medical conditions (public, private, other), and indigenous background (woman or her parents spoke an indigenous language). The baseline questionnaire also asked participants about their ownership of 7 household assets as a marker of socioeconomic status25: telephone, car, computer, vacuum cleaner, microwave oven, cell phone, and internet access. On the basis of these assets, a socioeconomic status score was created. Physical activity was defined as self-reported minutes per week spent on moderate and vigorous recreational physical activity calculated from responses to 8 categories that ranged from none to > 10 hours per week. Total metabolic equivalent hours per week were calculated on the basis of the amount and intensity of reported physical activity. Menopausal status was based on self-reported information on whether they had experienced menopause and, if so, what was the cause (natural, oophorectomy, hysterectomy, radiation, or chemotherapy). Women were classified as premenopausal if they replied they had not experienced menopause and did not report a cause of menopause, were considered as postmenopausal if they reported to have experienced menopause for natural reasons or for oophorectomy, and were considered unknown if they replied they had not experienced menopause but listed a cause or if they replied they had experienced menopause because they had undergone a hysterectomy, radiation, or chemotherapy. If women were in the unknown category, but were older than 51 years of age, they were considered postmenopausal, while women in the unknown category younger than 40 years of age were considered premenopausal. Women were classified as never, past, or current smokers. Body mass index was calculated as the self-reported weight in kilograms over self-reported height in meters squared and categorized as normal, overweight, and obese. Self-reported height and weight have been shown to be valid proxies for measured height and weight in this cohort.26 For diabetes, hypertension, hypercholesterolemia, and depression, self-reported medical diagnosis of disease was used. As a proxy for perceived stress at baseline, a short form (4 questions) of the Spanish version of the Perceived Stress Scale-4 was used, which was administered during the follow-up visits (clinical and internet survey). The scale evaluates “the degree to which life in the past month has been experienced as unpredictable, uncontrollable, and overwhelming” on a 5-point response scale (0 = “never,” 1 = “almost never,” 2 = “sometimes,” 3 = “fairly often,” 4 = “very often”). Per standard practice, the Perceived Stress Scale-4 score was based on questions 2, 4, 5, and 10 of the original 10-question Perceived Stress Scale. Perceived Stress Scale-4 scores were obtained by reversing the scores on the 2 positive items (4, 5) and summing across all 4 items, with higher scores indicating higher levels of perceived stress (scores range from 0 to 16).
Statistical analysis
To reduce the threat of reverse causation bias (poor sleep quality affecting dietary quality), women who reported < 6 hours of sleep at baseline were excluded from all analyses. First, continuous covariates were summarized as means and standard deviations (± SD) and categorical variables as percentages by PSQI category. The main exposures were the three major dietary patterns derived from PCA. Individuals were categorized into quartiles of each dietary pattern using the lowest category as the referent. Age and multivariable-adjusted modified Poisson regression models were used to estimate the prevalence ratios and 95% confidence intervals (CIs) of poor sleep quality (PSQI > 5) by quartiles of each dietary pattern (mutually adjusted for each other). The modified Poisson regression approach was used rather than log binomial models due to issues with model convergence.27
All models of diet patterns and sleep quality were run in a stepwise fashion as follows: Model 1 was adjusted for age, indigenous language spoken (yes/no), state (indicator variables), graduate education (< or ≥ graduate education), number of assets (continuous; proxy of socioeconomic status), health insurance (private, public, other), and menopause (premenopausal, postmenopausal, and unknown). Model 2 was further adjusted for diabetes (yes/no), hypertension (yes/no), hypercholesterolemia (yes/no), body mass index (continuous), smoking (never, past, current), physical activity (continuous), and depression (yes/no). In sensitivity analyses, we further adjusted for stress (continuous). To evaluate the presence of effect modification by menopausal status, interaction tests were run between each of the dietary patterns and menopausal status, and models stratified by menopausal status were run. As sensitivity analyses, models were run among those with short sleep duration at baseline.
To examine whether diet was associated with particular aspects of sleep quality, associations between dietary patterns and each sleep quality component were examined with modified Poisson regression models. Each component score (which each ranges from 0 to 3) was dichotomized based on the distribution of the data and were as follows: self-reported sleep quality rated as fairly bad or very bad (score > 1), sleep latency score > 2, sleep duration score > 1 (< 6 hours per night), sleep efficiency score >1 (proportion of time spent asleep out of time in bed < 75%), sleep disturbances score > 1, use of sleep medication score > 0 (any use in the past month), and daytime dysfunction score > 1. Sleep quality component models were also run with individual food groups that loaded strongly with 1 of the dietary patterns or that have been previously linked to sleep (eg, coffee and alcohol). All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
The mean ± SD age of the women at baseline (2006–2008) was 41.0 ± 7.1 years, and the majority were premenopausal (81%). The average length of follow-up was 5.5 ± 0.7 years. At follow-up, the mean ± SD sleep quality score was 4.9 ± 2.9 and 34% were classified as having poor quality sleep (PSQI score > 5). At follow-up, 72% were classified as premenopausal. Bivariate associations between baseline sociodemographic and lifestyle characteristics and sleep quality at follow-up are shown in Table 1. Overall, women classified as having poor quality sleep were more likely to have baseline comorbid conditions, including Type 2 diabetes, hypertension, hypercholesterolemia, and depression than women not defined as having poor quality sleep. Women with poor quality sleep at follow-up were also more likely to be overweight/obese and a smoker at baseline, as well as to have higher self-rated stress scores.
Table 1.
Sleep quality of 4,467 Mexican teachers, according to baseline sociodemographic, lifestyle, and chronic disease markers.
| Baseline Characteristics | N | PSQI ≤ 5, % N = 2,938 | PSQI > 5, % N = 1,529 | P, Difference |
|---|---|---|---|---|
| Age, years (mean ± SD) | 40.8 ± 7.2 | 41.2 ± 6.8 | .03 | |
| Indigenous language spoken | 245 | 5.4 | 5.7 | .66 |
| State | .44 | |||
| Baja California | 313 | 7.4 | 6.2 | |
| Chiapas | 253 | 5.8 | 5.5 | |
| Mexico City | 802 | 17.2 | 19.4 | |
| Durango | 202 | 4.4 | 4.8 | |
| Guanajuato | 517 | 11.4 | 11.9 | |
| Hidalgo | 299 | 6.7 | 6.7 | |
| Jalisco | 235 | 5.5 | 4.9 | |
| State of Mexico | 463 | 10.0 | 11.1 | |
| Nuevo Leon | 265 | 5.9 | 6.0 | |
| Sonora | 201 | 4.9 | 3.7 | |
| Veracruz | 733 | 16.6 | 16.0 | |
| Yucatan | 184 | 4.2 | 4.0 | |
| Graduate education obtained | 1,020 | 23.7 | 21.1 | .06 |
| SES, number of assets | .21 | |||
| Tertile 1, 0–4 | 1,155 | 25.3 | 27.0 | |
| Tertile 2, 5 | 804 | 18.0 | 17.9 | |
| Tertile 3, 6, or 7 | 2,508 | 56.7 | 55.1 | |
| Health insurance | .04 | |||
| Public | 2,774 | 61.3 | 63.7 | |
| Private | 1,246 | 28.1 | 27.6 | |
| Other | 447 | 10.7 | 6.7 | |
| Menopausal status | .01 | |||
| Premenopausal | 3,633 | 82.2 | 80.0 | |
| Postmenopausal | 471 | 10.5 | 10.7 | |
| Unknown | 363 | 7.4 | 9.6 | |
| Hypertension self-reported diagnosis | 501 | 10.0 | 13.6 | .0003 |
| Type 2 diabetes self-reported diagnosis | 166 | 3.3 | 4.6 | .03 |
| Hypercholesterolemia self-reported diagnosis | 745 | 14.8 | 20.2 | < .0001 |
| Depression self-reported diagnosis | 278 | 4.0 | 10.5 | < .0001 |
| Smoking | ||||
| Never | 3,407 | 77.4 | 74.0 | .007 |
| Past | 651 | 14.1 | 15.5 | |
| Current | 409 | 8.5 | 10.5 | |
| BMI, kg/m2 | < .0001 | |||
| Normal | 1,690 | 40.0 | 33.6 | |
| Overweight | 1,821 | 39.6 | 43.0 | |
| Obese | 956 | 20.4 | 23.4 | |
| Continuous BMI (mean ± SD) | 26.8 ± 4.6 | 27.2 ± 2.5 | .0001 | |
| Physical Activity, METs/wk (mean ± SD) | 37 ± 32 | 36 ± 31 | .15 | |
| Perceived stress score (mean ± SD) | 3.2 ± 2.4 | 5.1 ± 2.8 | < .0001 |
aStress measures were from the follow-up visit, but were considered as a proxy for stress at baseline. bRepresents % of women in sleep quality category, unless specified as mean ± standard deviation (SD). cAssets refers to telephone, car, computer, vacuum cleaner, microwave oven, cell phone, and internet access. dFrom the Perceived Stress Scale, short form. BMI = body mass index, MET = metabolic equivalents, PSQI = Pittsburgh Sleep Quality Index, SES = socioeconomic status.
Dietary patterns
There were 3 PCA-derived dietary patterns based on baseline food frequency questionnaire data: Fruits and Vegetables, Western, and Modern Mexican. Table 2 shows average consumption of selected food items in the highest compared to lowest quartiles of each dietary pattern. Baseline sociodemographic variables according to categories of each of the dietary patterns are found in Table S2, Table S3, and Table S4. In general, women with higher education and higher socioeconomic status (proxied by number of assets) had higher intake of the Fruit and Vegetable pattern as well as higher Western but lower Modern Mexican diet pattern adherence.
Table 2.
Average food, beverage, and nutrient intakes according to categories (quartile 1 vs quartile 4) of dietary patterns.
| Fruits and Vegetable | Western | Modern Mexican | ||||
|---|---|---|---|---|---|---|
| Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Food/beverage categories, servings/d | ||||||
| Fruit | 2.11 ± 1.44 | 4.45 ± 2.79 | 3.96 ± 2.87 | 2.41 ± 1.60 | 4.59 ± 2.78 | 2.05 ± 1.38 |
| Green vegetables | 0.33 ± 0.27 | 1.31 ± 1.08 | 0.64 ± 0.62 | 0.77 ± 0.87 | 0.89 ± 0.86 | 0.56 ± 0.63 |
| Tomatoes | 0.62 ± 0.52 | 1.47 ± 1.13 | 1.05 ± 0.90 | 0.90 ± 0.82 | 0.97 ± 0.79 | 1.07 ± 1.03 |
| Carrots | 0.10 ± 0.11 | 0.43 ± 0.41 | 0.23 ± 0.26 | 0.22 ± 0.32 | 0.24 ± 0.26 | 0.21 ± 0.29 |
| Other vegetables | 0.98 ± 0.71 | 2.58 ± 1.69 | 1.79 ± 1.34 | 1.44 ± 1.27 | 1.74 ± 1.24 | 1.57 ± 1.34 |
| Starchy vegetables | 0.21 ± 0.17 | 0.40 ± 0.37 | 0.32 ± 0.28 | 0.27 ± 0.24 | 0.27 ± 0.23 | 0.33 ± 0.33 |
| Chilies | 0.65 ± 0.72 | 1.09 ± 1.23 | 0.91 ± 1.02 | 0.76 ± 0.97 | 0.48 ± 0.53 | 1.36 ± 1.32 |
| Whole grains | 0.34 ± 0.50 | 0.47 ± 0.53 | 0.35 ± 0.50 | 0.41 ± 0.54 | 0.64 ± 0.71 | 0.22 ± 0.33 |
| Pastries | 1.13 ± 0.96 | 0.35 ± 0.33 | 0.90 ± 0.90 | 0.44 ± 0.40 | 0.61 ± 0.66 | 0.65 ± 0.67 |
| Legumes | 0.37 ± 0.33 | 0.78 ± 0.74 | 0.81 ± 0.74 | 0.36 ± 0.32 | 0.44 ± 0.37 | 0.74 ± 0.75 |
| Alcohol | 0.07 ± 0.09 | 0.09 ± 0.19 | 0.05 ± 0.11 | 0.12 ± 0.22 | 0.07 ± 0.10 | 0.09 ± 0.21 |
| Coffee | 0.89 ± 1.02 | 1.31 ± 1.34 | 0.99 ± 1.08 | 1.18 ± 1.35 | 0.92 ± 1.05 | 1.24 ± 1.30 |
| Soda | 0.53 ± 0.70 | 0.19 ± 0.33 | 0.25 ± 0.38 | 0.43 ± 0.65 | 0.17 ± 0.27 | 0.58 ± 0.79 |
| Fast food | 0.14 ± 0.11 | 0.08 ± 0.06 | 0.07 ± 0.05 | 0.15 ± 0.13 | 0.09 ± 0.06 | 0.12 ± 0.10 |
| Red meat | 0.48 ± 0.30 | 0.38 ± 0.25 | 0.31 ± 0.20 | 0.54 ± 0.31 | 0.34 ± 0.22 | 0.53 ± 0.32 |
| Milk/yogurt | 1.18 ± 1.09 | 0.97 ± 0.86 | 1.13 ± 1.03 | 0.92 ± 0.86 | 1.80 ± 1.24 | 0.52 ± 0.49 |
| Macronutrients, g/d | ||||||
| Protein | 75.09 ± 23.44 | 75.38 ± 24.88 | 70.78 ± 21.96 | 76.14 ± 26.62 | 81.15 ± 25.75 | 69.71 ± 22.82 |
| Fats | 67.97 ± 24.07 | 53.87 ± 20.66 | 56.64 ± 21.87 | 60.98 ± 23.69 | 62.75 ± 23.85 | 56.35 ± 21.37 |
| Carbohydrates | 259.10 ± 93.63 | 269.50 ± 104.55 | 307.96 ± 106.05 | 206.45 ± 76.84 | 293.68 ± 102.98 | 234.83 ± 87.45 |
| Total energy intake, kcal | 1,917 ± 611 | 1,815 ± 608 | 1,968 ± 625 | 1,658 ± 571 | 2,010 ± 615 | 1,697 ± 566 |
Values are presented as mean ± standard deviation (SD). aDiet patterns derived from principal component analysis performed in a previous analysis.22 Q = quartile.
Dietary patterns and poor sleep quality
In bivariate linear regression analysis, lower adherence to the Fruits and Vegetables pattern at baseline was associated with lower sleep quality at follow-up, whereas higher adherence to the Modern Mexican dietary patterns were associated with worse sleep quality at follow-up (Table 3). These associations held after adjusting for multiple potential confounders. In fully adjusted models, women in the lowest quartile of the Fruits and Vegetables pattern compared with the highest were 21% more likely to be classified as having poor quality sleep at follow-up (95% CI 1.04 to 1.20), whereas those in the highest quartiles of the Modern Mexican pattern were 23% more likely to be classified as having poor quality sleep compared to the lowest quartiles (95% CI 1.06 to 1.43, respectively). The association with the Western pattern was in the same direction, although not statistically significant at P < .05 (prevalence ratio = 1.16 with 95% CI 1.00 to 1.25). Results were not substantially altered upon additional adjustment for stress measured in 2011. There were not statistically significant interactions between dietary patterns and menopausal status (all P for interaction > .10), although stratified analyses suggested that the associations with Fruits and Vegetables were primarily found among premenopausal women, whereas the association with Modern Mexican diet was stronger among women of unknown status (potentially perimenopausal; Table S5). Analyses of women with short sleep duration at baseline revealed similar associations for the Fruits and Vegetables and the Western pattern, but there was no association with the Modern Mexican diet pattern (Table S6).
Table 3.
Sleep quality according to categories of dietary patterns among 4,476 Mexican teachers.
| Quartiles of Dietary Patterns | PSQI Score | Adjusted Prevalence Ratio of Poor Quality Sleep (95% CI) | ||
|---|---|---|---|---|
| Average ± SD | Percentage with poor quality sleep (PSQI > 5) | Adjusted for age and sociodemographic characteristics | Adjusted for sociodemographic, lifestyle and comorbidities | |
| Fruits and vegetables | ||||
| Q1 | 5.18 ± 2.86 | 37.7 | 1.22 (1.06, 1.42) | 1.21 (1.04, 1.40) |
| Q2 | 4.96 ± 2.86 | 35.1 | 1.14 (0.99, 1.32) | 1.15 (0.99, 1.33) |
| Q3 | 4.80 ± 2.92 | 33.7 | 1.10 (0.95, 1.28) | 1.11 (0.96, 1.29) |
| Q4 (healthiest) | 4.52 ± 2.81 | 30.4 | 1.00 | 1.00 |
| Western | ||||
| Q1 (healthiest) | 4.70 ± 2.80 | 32.5 | 1.00 | 1.00 |
| Q2 | 4.88 ± 2.85 | 35.3 | 1.12 (0.97, 1.29) | 1.12 (0.97, 1.30) |
| Q3 | 4.88 ± 2.92 | 33.4 | 1.07 (0.92, 1.24) | 1.07 (0.92, 1.24) |
| Q4 | 5.01 ± 2.92 | 35.7 | 1.16 (1.00, 1.35) | 1.16 (1.00, 1.25) |
| Modern Mexican | ||||
| Q1 (healthiest) | 4.65 ± 2.94 | 30.7 | 1.00 | 1.00 |
| Q2 | 4.73 ± 2.88 | 31.9 | 1.03 (0.89, 1.20) | 1.01 (0.87, 1.18) |
| Q3 | 4.89 ± 2.77 | 35.5 | 1.15 (0.99, 1.33) | 1.12 (0.97, 1.30) |
| Q4 | 5.21 ± 2.87 | 38.9 | 1.28 (1.11, 1.48) | 1.23 (1.06, 1.43) |
aFrom modified Poisson models with poor quality sleep (> 5 PSQI score) as a dichotomous outcome and indicator variables for quartiles of dietary patterns (mutually adjusted for each other). bAdjusted for continuous age, indigenous language spoken, state (indicator variables), graduate education, number of assets (continuous), health insurance (private, public, other), menopause (premenopausal, postmenopausal, and unknown). cAdjusted for all covariates listed above as well as Type 2 diabetes, hypertension, hypercholesterolemia, body mass index (continuous), smoking (never, past, current), physical activity (continuous), and depression. *Confidence interval (CI) does not encompass null value (1.00). PSQI = Pittsburgh Sleep Quality Index, Q = quartile.
Dietary patterns and individual components of sleep quality
To examine whether individual dietary patterns were associated with particular aspects of sleep quality, associations between dietary patterns and dichotomous sleep quality components were evaluated (Table 4). For the Fruits and Vegetables dietary pattern, there were associations with self-reported sleep quality, sleep latency, sleep efficiency, sleep disturbances, and daytime dysfunction. Specifically, women in the lowest quartile versus the highest quartile were more likely to rate their sleep quality as fairly bad or very bad, to have a long sleep latency, to have low sleep efficiency, to have sleep disturbances, and to have daytime dysfunction. For the Western pattern, those in the highest compared with the lowest quartile had longer sleep latency and greater reporting of sleep medication use. The associations of Modern Mexican dietary pattern-sleep quality component were of the highest magnitude for sleep disturbances, such that those in the highest quartile of this pattern had a 45% higher probability of having a sleep disturbance score > 1 compared with the lowest quartile (95% CI 1.18 to 1.79). There were no associations between any of the diet patterns and sleep duration.
Table 4.
Sleep quality components according to categories of dietary patterns among 4,476 Mexican teachers.
| Quartiles of Dietary Patterns | Subjective Sleep Quality Score > 1, | Sleep Latency Score > 2, | Sleep Duration Score > 1 (< 6 h/night), | Sleep Efficiency Score > 1, | Sleep Disturbances Score > 1, | Use of Sleep Medication Score > 0 (Any Use in Past Month), | Daytime Dysfunction Score > 1, |
|---|---|---|---|---|---|---|---|
| Fruits and Vegetables | |||||||
| Q1 | 1.39 (1.12, 1.72) | 1.46 (1.07, 2.00) | 1.00 (0.72, 1.41) | 1.34 (1.02, 1.76) | 1.27 (1.03, 1.56) | 1.09 (0.84, 1.41) | 1.73 (1.30, 2.29) |
| Q2 | 1.29 (1.04, 1.60) | 1.49 (1.10, 2.02) | 0.82 (0.58, 1.16) | 1.23 (0.94, 1.62) | 1.30 (1.06, 1.59) | 1.02 (0.78, 1.33) | 1.51 (1.13, 2.01) |
| Q3 | 1.15 (0.92, 1.43) | 1.38 (1.01, 1.87) | 0.68 (0.47, 0.98) | 1.31 (1.00, 1.71) | 1.03 (0.83, 1.28) | 1.15 (0.90, 1.48) | 1.24 (0.92, 1.66) |
| Q4 (healthiest) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Western | |||||||
| Q1 (healthiest) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.03 (0.83, 1.27) | 1.01 (0.74, 1.37) | 1.18 (0.82, 1.70) | 1.05 (0.81, 1.37) | 1.28 (1.05, 1.57) | 1.41 (1.07, 1.86) | 1.16 (0.89, 1.51) |
| Q3 | 1.07 (0.87, 1.33) | 1.09 (0.81, 1.48) | 1.27 (0.88, 1.83) | 1.04 (0.80, 1.37) | 1.15 (0.93, 1.42) | 1.29 (0.98, 1.71) | 1.09 (0.83, 1.44) |
| Q4 | 1.14 (0.92, 1.42) | 1.42 (1.05, 1.92) | 1.28 (0.88, 1.86) | 1.14 (0.87, 1.50) | 1.19 (0.96, 1.48) | 1.49 (1.12, 1.97) | 1.02 (0.76, 1.36) |
| Modern Mexican | |||||||
| Q1 (healthiest) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.09 (0.88, 1.34) | 0.95 (0.69, 1.28) | 1.20 (0.84, 1.70) | 1.16 (0.89, 1.51) | 0.99 (0.80, 1.23) | 0.81 (0.63, 1.04) | 0.95 (0.72, 1.26) |
| Q3 | 1.04 (0.84, 1.29) | 0.97 (0.71, 1.31) | 1.12 (0.78, 1.60) | 1.15 (0.88, 1.50) | 1.16 (0.94, 1.44) | 0.87 (0.67, 1.11) | 1.24 (0.95, 1.62) |
| Q4 | 1.19 (0.96, 1.48) | 1.33 (0.99, 1.78) | 1.03 (0.71, 1.50) | 1.16 (0.88, 1.53) | 1.45 (1.18, 1.79) | 0.86 (0.66, 1.11) | 1.23 (0.93, 1.62) |
aSleep component scores each range from 0 to 3. bAll models adjusted for indigenous language spoken, state (indicator variables), graduate education, number of assets (continuous), health insurance (private, public, other), menopause (premenopausal, postmenopausal, and unknown), Type 2 diabetes, hypertension, hypercholesterolemia, body mass index (continuous), smoking (never, past, current), physical activity (continuous), and depression. *Confidence interval does not encompass null value (1.00). Q = quartile.
Individual foods and individual components of sleep quality
Finally, analyses examining individual foods were conducted (Table S7). Alcohol intake was associated the use of sleep medications; coffee intake was associated with longer sleep latency; soda intake was associated with longer sleep latency, higher sleep disturbances, and worse sleep quality; and red meat was associated with longer latency and use of sleep medications. In contrast, pastries were associated with shorter sleep latency and milk/yogurt intake was associated with longer sleep duration and fewer sleep disturbances.
DISCUSSION
In a sample of midlife Mexican women, low adherence to a fruit and vegetable-based dietary pattern and high adherence to a Modern Mexican dietary pattern was associated with lower sleep quality measured approximately 5.5 years later. Furthermore, each dietary pattern was related to distinct aspects of sleep quality. Women with lower adherence to the Fruit and Vegetable pattern had worse self-reported sleep quality scores, longer sleep latency, lower sleep efficiency, higher sleep disturbances, and higher daytime dysfunction at follow-up. Women with higher adherence to the Western dietary pattern were more likely to experience longer sleep latency and reported higher use of sleep medications at follow-up, and women with higher adherence to the Modern Mexican pattern had more sleep disturbances.
In this study, distinct dietary patterns were related to specific characteristics of sleep, a finding that could have clinical relevance as well as help identify biological mechanisms. The Fruits and Vegetables dietary pattern was related to multiple aspects of sleep quality: overall perception of sleep quality, sleep latency, sleep disturbances, and daytime dysfunction. Of note, prior studies have found that intake of certain fruits, including tart cherries and kiwifruit, associates with shorter onset latency or higher sleep quality.10,11 The Western dietary pattern was associated with symptoms often related to insomnia, including longer sleep latency, use of sleep medication, and daytime dysfunction. Foods within this pattern that appeared to help explain the associations included red meat and alcohol intake. Although a broad body of literature supports an alcohol and sleep quality relationship,7 an association with red meat is less well documented. However, red meat is high in saturated fat, which has been related to alterations of sleep architecture.28 The Modern Mexican dietary pattern was most strongly associated with sleep disturbances. Soda intake was positively correlated with this pattern and was also associated with higher sleep disturbances, an expected association.29 In contrast, milk/yogurt intake was inversely correlated with this pattern and was associated with fewer sleep disturbances. Some experimental evidence supports a link between malted milk or melatonin-enriched milk, especially at bedtime, with fewer movements during sleep.9 Consequently, although pastries loaded positively with the Modern Mexican pattern, pastry intake was associated with shorter sleep onset latency. This finding is in line with some results from small experimental studies, where intake of carbohydrate-rich foods several hours before bedtime has been associated with shorter sleep onset30 and shorter wake times during the sleep period.31 One possible reason is that carbohydrate intake induces insulin release, which promotes the uptake of tryptophan into the brain.5,9 Tryptophan is a precursor of serotonin and melatonin, the hormone produced in the pineal gland of the brain at night to trigger the onset of sleep.
Overall, the finding that a healthier dietary pattern was associated with higher sleep quality while unhealthier dietary patterns related to poorer sleep quality is in line with previous studies conducted in the United Kingdom,16 Australia,12 China,15 and Japan13 that have examined empirically derived dietary patterns in relation to sleep duration and/or insomnia symptoms. For example, 2 separate dietary patterns—1 high in wheat and the other high in animal foods, eggs, fresh fruit, and dairy products—were associated with approximately 10–15% lower odds of insomnia (comparing those in the highest quartile to the lowest) in a study of 481,242 Chinese adults.15 Similarly, Japanese working adults with the highest scores on a pattern characterized by vegetables, mushrooms, potatoes, seaweed, soy, and eggs had a 25% lower odds of difficulty initiating sleep at least once a week compared with those with the lowest scores.13 In addition, a study among Australian adults reported that each standard deviation higher traditional dietary pattern score, which was characterized by vegetables and fruits (both men and women), fish and nuts (women only), and beef and whole grains (men only), was associated with 13% lower odds of short sleep duration (< 7 h/night).12
The present findings on diet and sleep quality corroborate previous observations, but they also represent an important extension for several reasons. First, prior studies were not specifically focused on women of perimenopausal age. Although 72% of the women in the present study were still classified as premenopausal by the end of the study, the fact that they were on average 46.5 years old indicates most of the sample was likely in the early stages of the menopausal transition (the estimated median age of menopause in Mexico City is 46.5 years32). Uncovering modifiable factors that could contribute to worse or better sleep quality is particularly relevant at this life stage, as it tends to coincide with worsening sleep quality, which in turn may affect a myriad of other chronic health conditions and quality of life.1 A second unique aspect of the present report is that the food recall and sleep quality information were collected at separate points in time, with food intake assessed at baseline. Although sleep and dietary measures may remain fairly stable over time, one advantage to the temporally separated measurements is that any reporting biases may be independent. Finally, this analysis represents the first study to examine dietary patterns in relation to sleep in the context of a Latin American population. This is relevant in light of the population-level changes in diet that are still ongoing in many Latin American countries, where traditional diets high in fruits and vegetables are replaced by diets high in meat and animal products, processed, and energy-dense foods (the “nutrition transition”).33 From a public health perspective, these dietary changes have been linked to higher rates of obesity and chronic disease. Given the known relationship between poor sleep quality and risk of obesity, it is possible that sleep quality also plays an intermediary role in dietary pattern-obesity relationships observed in populations in the midst of nutrition transitions.
In contrast to previous studies, no associations were observed between dietary patterns and short sleep duration. One possible reason could have to do with the fact the sleep duration may be tightly constrained by family and work schedules,34 such that diet would play a very minimal role in determining amount of sleep obtained, especially on the weekdays. Another potential reason is that actual time spent sleeping is hard to recall with accuracy, whereas issues related to sleep quality—having a hard time falling asleep, waking up in the night, being excessively sleepy during the day—may be more memorable and thus easier to recall.
There are both strengths and limitations in the present study. Strengths include the use of a longitudinal cohort and the ability to account for a wide range of important potential confounders. Additional strengths are a large sample size, representing women teachers from all states in Mexico, as well as a wide breadth of foods included in the dietary analysis. One of the main limitations was the lack of sleep quality information at baseline, which precluded exclusion of those with poor sleep quality at baseline (and thus examining development of poor sleep quality over the follow-up period). Nonetheless, there was information about typical sleep duration at baseline, which allowed the exclusion of women with short sleep as a way to eliminate some threat of reverse causation bias. Another limitation was that perceived stress was not measured at baseline and was therefore proxied by perceived stress measured at the same time as sleep quality. Therefore, including it as a confounder could induce over-adjustment bias35 if perceived stress is a mediator between dietary patterns and sleep quality, which is the rationale for adjusting for perceived stress only as a sensitivity analysis. A substantial portion of the sample had to be excluded due to missing information on covariates, which could lead to bias and may affect the generalizability, since the women included in the study were more highly educated and of a higher socioeconomic status than the overall cohort. Nonetheless, sensitivity analyses showed their dietary patterns were similar to the larger cohort. It is also important to note that this study is ongoing, with additional recruitment of women who have not yet responded to sleep questionnaires underway, thus allowing for a larger examination of sleep and diet in the future. A final limitation is the reliance on self-report for dietary recall, which is subject to both random and systematic measurement error; given the categorization of diet patterns into quartiles, the effect of this error is difficult to predict. Sleep quality, assessed over the previous month, was also self-reported and subject to the same types of potential biases. Finally, the fact that menopausal status was based on self-report of menopausal status and the cause of menopause rather than STRAW+10 criteria36 means that some women’s true menopausal status may have been misclassified.
In summary, this study found that dietary patterns of midlife Mexican women were related to sleep quality in distinct ways. While a dietary pattern characterized by fruits and vegetables was associated with higher perceived sleep quality and less daytime dysfunction, dietary patterns marked by Western foods (red meat and alcohol) and modern Mexican foods (soda consumption and lower intake of fiber and dairy) were related to longer sleep onset latency, use of sleep medications, and sleep disturbances. From a clinical perspective, these findings suggest that women with specific sleep disturbances may be advised to follow or avoid particular dietary patterns. Future randomized interventions should test whether dietary modifications can alter sleep in perimenopausal women.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the National Institute of Public Health in Mexico. Dr. López-Ridaura and Dr. Lajous have a nonrestricted investigator-initiated grant from AstraZeneca. Dr. Jansen was supported by funding from National Institute of Diabetes and Digestive and Kidney Diseases (T32DK071212) and National Heart, Lung, and Blood Institute (T32HL110952). This work is also supported by the American Institute for Cancer Research (05B047), and Consejo Nacional de Ciencia y Tecnología (S0008-2009-1:000000000115312). The authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGMENTS
The authors gratefully acknowledge Antonio García-Anaya, Adrian Cortés, and Eduardo Ortiz-Panozo for data management expertise and assistance. The authors thank Victor Sastré from Secretaria de Educación Pública and ISSSTE (Social Security and Services Institute for Employees of the State) for technical and administrative support. We also thank the participants of the Mexican Teachers’ Cohort. Without their participation, this study would not have been possible.
ABBREVIATIONS
- CI
confidence interval
- PCA
principal components analysis
- PSQI
Pittsburgh Sleep Quality Index
- SD
standard deviation
REFERENCES
- 1.Baker FC, De Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: Prevalence, impact, and management challenges. Nat Sci Sleep. 2018;1073–95. 10.2147/NSS.S125807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Santos C, Saura CB, Lucas JAR, Castell P, Madrid JA, Garaulet M. Menopause status is associated with circadian- and sleep-related alterations. Menopause. 2016;23(6):682–690. 10.1097/GME.0000000000000612 [DOI] [PubMed] [Google Scholar]
- 3.Lampio L, Polo-Kantola P, Himanen SL, et al. Sleep during menopausal transition: A 6-year follow-up. Sleep. 2017;40(7):zsx090. 10.1093/sleep/zsx090 [DOI] [PubMed] [Google Scholar]
- 4.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: A community survey of sleep and the menopausal transition. Menopause. 2003;1019–28. 10.1097/00042192-200310010-00005 [DOI] [PubMed] [Google Scholar]
- 5.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res. 2012;32(5):309–319. 10.1016/j.nutres.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 6.Drake C, Roehrs T, Shambroom J, Roth T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9(11):1195–1200. 10.5664/jcsm.3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colrain IM, Nicholas CL, Baker FC. Alcohol and the sleeping brain. Handb Clin Neurol. 2014;125415–431. 10.1016/B978-0-444-62619-6.00024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Brutto OH, Mera RM, Ha JE, Gillman J, Zambrano M, Castillo PR. Dietary fish intake and sleep quality: a population-based study. Sleep Med. 2016;17126–128. https://doi.org/10.1016/j.sleep.2015.09.021 [DOI] [PubMed] [Google Scholar]
- 9.St-Onge M-P, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. 2016;7(5):938–949. 10.3945/an.116.012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losso JN, Finley JW, Karki N, et al. Pilot study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am J Ther. 2018;25(2):e194–e201. 10.1097/MJT.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H-H, Tsai P-S, Fang S-C, Liu J-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac J Clin Nutr. 2011;20(2):169–174. 10.6133/apjcn.2011.20.2.05 [PubMed] [Google Scholar]
- 12.Mondin TC, Stuart AL, Williams LJ, Jacka FN, Pasco JA, Ruusunen A. Diet quality, dietary patterns and short sleep duration: a cross-sectional population-based study. Eur J Nutr. 2019;58(2):641–651. 10.1007/s00394-018-1655-8 [DOI] [PubMed] [Google Scholar]
- 13.Kurotani K, Kochi T, Nanri A, et al. Dietary patterns and sleep symptoms in Japanese workers: The Furukawa Nutrition and Health Study. Sleep Med. 2015;16(2):298–304. 10.1016/j.sleep.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 14.Castro-Diehl C, Wood AC, Redline S, et al. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41(11):zsy158. 10.1093/sleep/zsy158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C, Shi Z, Lv J, et al. Dietary patterns and insomnia symptoms in Chinese adults: The China Kadoorie Biobank. Nutrients. 2017;9(3):232. 10.3390/nu9030232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almoosawi S, Palla L, Walshe I, Vingeliene S, Ellis JG. Long sleep duration and social jetlag are associated inversely with a healthy dietary pattern in adults: Results from the UK national diet and nutrition survey rolling programme Y1–4. Nutrients. 2018;10(9):1131. 10.3390/nu10091131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lajous M, Ortiz-Panozo E, Monge A, et al. Cohort profile: The Mexican Teachers’ Cohort (MTC). Int J Epidemiol. 2017;46(2):e10. 10.1093/ije/dyv123 [DOI] [PubMed] [Google Scholar]
- 18.Hernández-Avila M, Romieu I, Parra S, Hernández-Avila J, Madrigal H, Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998;40(2):133–140. 10.1590/S0036-36341998000200005 [DOI] [PubMed] [Google Scholar]
- 19.US Department of Agriculture, Agricultural Research Service . 2009 USDA National Nutrient Database for Standard Reference, Release 22. http://www.ars.usda.gov/nea/bhnrc/mafc
- 20.Zubiran S. Composición de Alimentos Mexicanos. Mexico City, Mexico: Composición Química Aminoácidos Cálculo de Aportes Dietarios; 1999 [Google Scholar]
- 21.Rhee JJ, Sampson L, Cho E, Hughes MD, Hu FB, Willett WC. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. 2015;181(4):225–233. 10.1093/aje/kwu308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monge A, Lajous M, Ortiz-Panozo E, Rodríguez BL, Góngora JJ, López-Ridaura R. Western and Modern Mexican dietary patterns are directly associated with incident hypertension in Mexican women: a prospective follow-up study. Nutr J. 2018;17(1):21. 10.1186/s12937-018-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 24.Hita-Contreras F, Martínez-López E, Latorre-Román PA, Garrido F, Santos MA, Martínez-Amat A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol Int. 2014;34(7):929–936. 10.1007/s00296-014-2960-z [DOI] [PubMed] [Google Scholar]
- 25.Hirko KA, Lajous M, Ortiz-Panozo E, et al. Socioeconomic position and markers of adiposity among female teachers in Mexico. J Epidemiol Community Health. 2017;71(10):999–1004. 10.1136/jech-2017-209179 [DOI] [PubMed] [Google Scholar]
- 26.Ortiz-Panozo E, Yunes-Díaz E, Lajous M, Romieu I, Monge A, López-Ridaura R. Validity of self-reported anthropometry in adult Mexican women. Salud Publica Mex. 2017;59(3):266. 10.21149/7860 [DOI] [PubMed] [Google Scholar]
- 27.Barros AJD, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3(1):21. 10.1186/1471-2288-3-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Onge MP, Roberts A, Shechter A, Choudhury AR. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med. 2016;12(1):19–24. 10.5664/jcsm.5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark I, Landolt HP. Coffee, caffeine, and sleep: A systematic review of epidemiological studies and randomized controlled trials. Sleep Med Rev. 2017;3170–78. 10.1016/j.smrv.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 30.Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007;85(2):426–430. 10.1093/ajcn/85.2.426 [DOI] [PubMed] [Google Scholar]
- 31.Lindseth G, Murray A. Dietary macronutrients and sleep. West J Nurs Res. 2016;38(8):938–958. 10.1177/0193945916643712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrido-Latorre F, Lazcano-Ponce EC, López-Carrillo L, Hernández-Avila M. Age of natural menopause among women in Mexico city. Int J Gynecol Obstet. 1996;53159–166. 10.1016/0020-7292(96)02655-0 [DOI] [PubMed] [Google Scholar]
- 33.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. 10.1111/j.1753-4887.2011.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30(9):1085–1095. 10.1093/sleep/30.9.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.