Abstract
The influences of hyperhomocysteinemia on cardiovascular diseases (CVDs), stroke and new-onset hypertension are unclear. The aim of the study is to explore the associations of homocysteine levels with stroke, CVDs, and new-onset hypertension in Chinese individuals.
This retrospective cohort study included outpatients and inpatients from the Department of Geriatrics at Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine from January to December 2000. They were divided based on their homocysteine (Hcy) levels in 2000: Q1 (<10 μmol/L), Q2 (10–15 μmol/L), and Q3 (>15 μmol/L) and according to whether they had hypertension at baseline. Information about stroke, mortality and major adverse cardiac events, and newly onset hypertension was gathered in December each year until 2017. The effects of Hcy levels on the risk for stroke and CVDs among all patients, and new-onset hypertension among patients without hypertension at baseline were evaluated.
After adjustment for confounders, compared with the Q1 group (Hcy <10 μmol/L), when the Hcy increased to 10 to 15 μmol/L, the risks for stroke, CVDs, and new-onset hypertension significantly increased, and the hazard ratio and 95% confidence interval were 2.02 (1.35–3.05, P = .001), 2.22 (1.32–3.76, P = .003), and 7.20 (4.52–11.48, P < .001), respectively. Hcy improved the predictive capability of traditional risk factors for stroke. The optimal cut-off value of Hcy for predicting stroke was 13.4 μmol/L (sensitivity: 70.9%, specificity: 62.2%).
Hcy 10 to 15 μmol/L is significantly associated with the risks for stroke, mortality and major adverse cardiac events, and hypertension. The best cut-off point of Hcy for predicting stroke is 13.4 μmol/L.
Keywords: cardiovascular disease, homocysteine, hypertension, predictive value, stroke
1. Introduction
Cardiovascular diseases (CVDs) are among the most important threats to public health worldwide. There were 2.4 million deaths due to atherosclerotic CVDs in 2016 in China, representing 61% of deaths due to CVDs and 25% of all deaths.[1] Furthermore, the incidence and mortality of CVDs has been increasing from 1990 to 2016 in China,[1] probably due to lifestyle changes associated with the new economy.[2] Hypertension is an important independent risk factor for CVDs.[3] In China, 23.3% of Chinese adults (about 244 million persons) have hypertension and the condition is adequately controlled in only 15% of them.[4]
In addition to improving lifestyle and adopting drug therapies to prevent these diseases, the relationship between homocysteine (Hcy) and these diseases is gradually attracting attention.[5–7] Hcy is a sulphur-containing amino acid produced by methionine metabolism and is mainly cleared through the kidneys. The reference values of Hcy will be different in different races and populations, and will vary among different measurement methods. Typically, the normal reference range of Hcy is 5 to 15 μmol/L. Therefore, the definition of hyperhomocysteinemia (Hhcy) is controversial, but it is generally defined as plasma Hcy ≥15 μmol/L,[8,9] but it also has been known to be defined as plasma Hcy ≥10 μmol/L.[10–12] There is a large amount of epidemiological data demonstrating that elevated plasma Hcy is an independent risk factor for CVDs,[13–15] even when the plasma Hcy levels are only slightly increased (10–15 μmol/L).[16] Nevertheless, there are only a few long-term follow-up cohort studies available and there are currently no cohort studies that assessed the influence of Hhcy on cardiovascular events in patients with or without hypertension.
In recent years, many studies on different populations assessed the relationship between Hcy and hypertension and showed that Hcy is associated with the occurrence of hypertension and may interact with hypertension, affecting together the incidence of CVDs.[5,17] In China, due to the lack of folic acid and B vitamin intake in the diet and a high mutation rate of the methylenetetrahydrofolate reductase gene (the C677T single nucleotide polymorphism), which can cause an increase in Hcy, the proportion of patients with hypertension and Hhcy, which is known as H-type hypertension, is higher than that in other populations.[18–20] A study showed that Hhcy in Chinese hypertensive patients is associated with the risk of ischemic stroke, but not with the risk of coronary heart disease.[21] In Chinese populations, multivitamin supplementation containing folic acid decreases the risk of stroke, but not of coronary heart disease.[22–24]
Therefore, the aim of the present study was to explore the associations of Hcy with stroke and CVD in all patients, and with new-onset hypertension in patients without hypertension at baseline. The results could provide important insights in the pathogenesis of CVDs and hypertension associated with Hhcy, and may help in the management of the patients.
2. Material and methods
2.1. Study population
This retrospective cohort study included outpatients and inpatients from the Department of Geriatrics at Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine from January to December 2000. The study was approved by the ethics committee of Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine. Written informed consent was waived by the committee.
The inclusion criteria were:
-
1.
no new-onset stroke and CVDs in the past 1 year,
-
2.
no history of secondary hypertension,
-
3.
glomerular filtration rate >30 ml/min/1.73 m2, and
-
4.
did not take folic acid, vitamin B12, or other drugs that can influence the Hcy levels for the past 1 year.
The exclusion criteria were:
-
1.
heart failure,
-
2.
systolic blood pressure (SBP) >185 mm Hg or diastolic blood pressure (DBP) >110 mm Hg,
-
3.
severe liver or kidney dysfunction needing replacement therapy,
-
4.
recent acute infection,
-
5.
family history of mental illness or use of psychotropic, or
-
6.
lost to follow-up.
2.2. Data collection
Clinical data (including demographic characteristics and chronic disease history) and results of laboratory test (including blood glucose, blood lipids, liver and kidney function, and Hcy) were collected from an electronic clinical information system at Ruijin Hospital at baseline. The patient data included age, gender, body mass index (BMI), history of smoking, history of drinking, history of diabetes, stroke history, blood pressure, family history of hypertension, and family history of stroke. Kidney dysfunction was defined as glomerular filtration rate <90 ml/min·1.73 m2.[25]
All patients underwent biochemistry examination, including fasting plasma glucose, triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase, and aspartate aminotransferase. Plasma folic acid and vitamin B12 levels were measured by electro-chemiluminescence method with an Elecsys 2010 system (Roche Diagnostics, Basel, Switzerland).
Plasma Hcy levels were determined at baseline by an enzyme-linked immunosorbent assay (ELISA) using a coda automatic plate type enzyme-labeled instrument (Bio-Rad, Hercules, CA). The patients were divided into 3 groups according to the Hcy level, the Q1 group (Hcy < 10 μmol/L), Q2 group (Hcy = 10–15 μmol/L) and Q3 group (Hcy > 15 μmol/L).[8–12]
2.3. Outcomes and follow-up
The primary outcome was the relationship between different Hcy levels and the risk for stroke, CVDs, and new-onset hypertension. The secondary outcome was the best cut-off value of Hcy for predicting stroke, CVDs, and newly onset hypertension.
All subjects participated in physical examinations at the Ruijin Hospital every year and they would see a doctor in our hospital if there was any discomfort. In addition, almost every surviving patient accepted follow-up for blood pressure and other examinations every 3 months for new-onset stroke, CVDs, and new-onset hypertension from the electronic clinical information system at Ruijin Hospital in December of each year until 2017.
New stroke and CVDs were recorded in all patients. Stroke was defined as a focal neurological deficit lasting >24 hours caused by intracerebral hemorrhage or infarction, and with computed tomography or magnetic resonance imaging evidence.[26] The CVDs included angina, myocardial infarction, percutaneous coronary intervention, and coronary artery bypass graft.[7,13,27] For patients without hypertension at baseline (n = 597), the incidence of new-onset hypertension was collected. Hypertension was defined as SBP ≥ 140 mm Hg or DBP ≥90 mm Hg.[28] Blood pressure measurements were performed in accordance with the National Guidelines for the Prevention and Control of Hypertension of China Basic Public Health Services.[28]
2.4. Statistical analysis
SPSS 20.0 (IBM, Armonk, NY) was used to manage and analyze the data. The continuous variables are described as mean ± standard deviation or median (interquartile range) after having been tested for normal distribution using the Kolmogorov-Smirnov test and were analyzed using the Student t test or ANOVA with the SNK post hoc test, as appropriate. Categorical variables are expressed as percentages, and were analyzed using the chi-square test. The occurrence of stroke, CVDs and new-onset hypertension were estimated using the Kaplan-Meier method and compared using the log-rank test. The receiver operating characteristic (ROC) curve was plotted and determined the optimal cut-off values of Hcy with the highest Youden index for predicting stroke, CVDs, and new-onset hypertension. The Cox proportional hazards regression model was applied to calculate the hazard ratio and corresponding 95% confidence interval (CI) to explore the effects of Hcy levels on the risk for stroke, CVDs, and new-onset hypertension. The dependent variable was the occurrence of stroke, CVDs, or new-onset hypertension. The variables that were significant (P < .05) in univariable analyses were entered in the Cox model (enter method).
3. Results
3.1. Baseline demographic and clinical characteristics
A total of 385 patients did not meet the inclusion criteria and 102 (7.7%) patients lost to follow-up were excluded from the analysis. Therefore, a total of 1226 patients were included in the final study cohort. The age range of the included patients was 42 to 97 years, with an average age of 70.2 ± 9.8 years. The study population included 1058 men (86.3%) and 168 women (13.7%). The 1226 subjects were directly divided into 3 groups according to the Hcy levels measured at baseline. On the other hand, the 1226 subjects were also stratified according to hypertension at baseline.
Table 1 presents the characteristics of the participants. There were 414 subjects (33.8%) in the Q1 group (Hcy <10 μmol/L), 393 (32.1%) in the Q2 group (Hcy = 10–15 μmol/L), and 419 (34.2%) in the Q3 group (Hcy >15 μmol/L). Mean age was 68.2 ± 11.1 years in the Q1 group, 71.2 ± 9.0 years in the Q2 group, and 71.4 ± 8.7 years in the Q3 group (P < .01). The percentages of males were 343 (82.9%), 352 (89.6%), and 363 (86.6%) in the Q1, Q2, and Q3 groups (P = .02), respectively. Meanwhile, compared with those in the Q1 group, those with a higher Hcy level had significantly higher rates of smoking, hypertension, diabetes mellitus, and renal function decline, while the levels of folic acid and vitamin B12 decreased gradually (all P < .05).
Table 1.
Characteristics of the participants.

As presented in Table 2, when stratified according to hypertension, the smoking rate, folic acid levels, and vitamin B12 level distributions among the hypertension subgroup of the Q1, Q2, and Q3 groups were significantly different (all P < 0.05). In the nonhypertension subgroup, significant differences in age, smoking rate, folic acid, and vitamin B12 were observed among the Hcy groups.
Table 2.
Baseline characteristics according to hypertension.

3.1.1. Stroke, CVDs, and newly onset hypertension
After 17 years of follow-up in the 1226 participants, we recorded a total of 237 strokes, which included 141 in the hypertension subgroup and 96 in the nonhypertension subgroup. As shown in Figure 1A to C, the cumulative incidence of stroke increased significantly with the Hcy levels (P < .001), as well as in the nonhypertension and hypertension subgroups.
Figure 1.

Kaplan Meier analysis of stroke, CVDs, and new-onset hypertension according to different homocysteine groups. (A–C) Kaplan Meier analysis of stroke according to different homocysteine groups in all of subjects (A), in the nonhypertension subgroup (B), and in the hypertension subgroup (C). Log rank test P < .001. (D-F) Kaplan Meier analysis of CVDs according to different homocysteine groups in all of subjects (D), in the nonhypertension subgroup (E), and in the hypertension subgroup (F). (G) Kaplan Meier analysis for new-onset hypertension according to different homocysteine groups in the non-hypertension subgroup. CVDs = cardiovascular diseases.
Among all subjects, 145 (99 hypertensive subjects and 46 nonhypertensive subjects) had CVD during the 17 years of follow-up. For both subgroups, the Q2 subjects had a higher risk for CVD compared with Q1; Q3 had a significantly higher risk (Fig. 1D–F).
There were 236 cases of new-onset hypertension documented among the 629 nonhypertensive subjects at baseline (Fig. 1G). The risk of new-onset hypertension increased with Hcy levels (all P < .01).
3.1.2. Receiver operating characteristic analysis of the optimal cut-off value for predicting outcomes according to Hcy
Because the occurrence of stroke is influenced by multiple factors, we established a risk factor model for stroke with or without Hcy. As shown in Figure 2A to C, Hcy could improve the predictability of the risk factor model for stroke in all participants and in the nonhypertension and hypertension subgroups (Fig. 2A–C), with areas under the ROC curves (AUCs) of 0.740 (95% CI 0.707–0.773) vs 0.657 (95% CI 0.622–0.740), 0.768 (95% CI 0.720–0.815) vs 0.664 (95% CI 0.611–0.717), and 0.704 (95% CI 0.656–0.751) vs 0.638 (95% CI 0.589–0.687), respectively. In addition, to a certain extent, Hcy could increase the predictability of the risk factor model for CVDs and hypertension (Fig. 2D–G). In addition, the optimal cut-off values were determined using the Youden index. The corresponding sensitivities, specificities, Youden indexes, and hazard ratios (HRs) are presented in Table 3.
Figure 2.

ROC analysis for predicting stroke, CVDs, and new-onset hypertension. (A–C) ROC analysis for predicting stroke in all subjects (A), in the nonhypertension subgroup (B), and in the hypertension subgroup (C) during the 17-year follow-up. (D–F) ROC analysis for predicting CVDs in all subjects (D), in the nonhypertension subgroup (E), and in the hypertension subgroup (F) during the 17-year follow-up. (G) ROC analysis for predicting new-onset hypertension in the non-hypertension subgroup. CVDs = cardiovascular diseases, ROC = receiver operating characteristic.
Table 3.
Results for measurement cut-off value of Hcy in the prediction for stroke, CVDs, and new-onset hypertension based on the ROC analysis.

3.1.3. Univariable and multivariable Cox regressions for stroke, CVDs, and new-onset hypertension
Unadjusted and adjusted HRs for stroke and CVDs of the whole cohort are shown in Table 4. Variables that were significant (P < .05) in univariable analyses were included in the multivariable analysis. The multivariable analysis showed that age (HR = 1.04, 95% CI: 1.02–1.06, P < .001), Hcy (HR = 1.09, 95% CI: 1.07–1.11, P < .001), and history of hypertension (HR = 1.38, 95% CI: 1.04–1.81, P = .023), were independently associated with stroke. The second multivariable analysis showed that Hcy (HR = 1.07, 95% CI: 1.04–1.10, P < .001), history of diabetes (HR = 1.78, 95% CI: 1.22–2.59, P = .003), history of hypertension (HR = 1.70, 95% CI: 1.16–2.47, P = .006), and BMI (HR = 1.08, 95% CI: 1.02–1.13, P = .006) were independently associated with CVDs.
Table 4.
Univariable and multivariable Cox proportional hazard regression analyses of stroke and CVDs according to Hcy in all subjects.
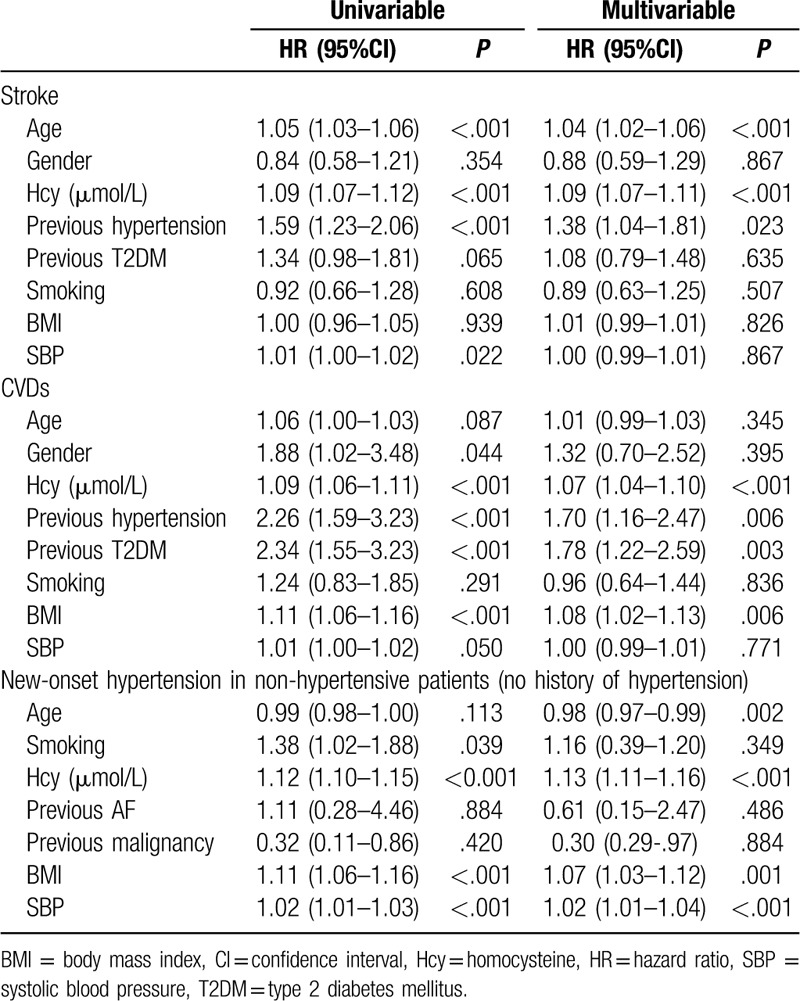
For all patients, the analyses were adjusted for age, smoking, previous AF, previous malignancy, BMI, and SBP. In the hypertension subgroup, the analyses were adjusted for smoking, LDL, and SBP. In the non-HP subgroup, the analyses were adjusted for age, smoking, previous malignancy, previous AF, BMI, and SBP.
Table 5 shows the HRs from the subgroup analysis of the hypersensitive population after adjustments for smoking, LDL, and SBP. The risk for stroke and CVDs was increased (P < .05) in the Q3 group (adjusted HR [95% CI]: 2.12 [1.39–3.24] and 2.24 [1.29–3.88], respectively), whereas no statistically significant increase was found in the Q2 group (Table 5). On the other hand, in the non-hypertension subgroup, after adjustments for age, smoking, previous malignancy, previous AF, BMI, and SBP, and using Hcy < 10 μmol/L as the reference group, the risk for stroke, CVD, and new-onset hypertension was significantly increased in Q2 and Q3 subjects (P < .05).
Table 5.
Cox proportional hazard regression analyses of stroke and CVDs for different levels of Hcy in subgroups.

4. Discussion
No cohort studies assessed the influence of Hhcy on cardiovascular events, stroke, and new-onset hypertension in Chinese subjects. Therefore, this study aimed to explore the associations of Hcy levels with stroke and CVDs in Chinese patients, and new-onset hypertension in patients without hypertension at baseline. The study, which included 1226 subjects with a 17-year follow-up, provides evidence that there is a significant association between elevated plasma Hcy (>10 μmol/L) and the risk of stroke, CVD, and new-onset hypertension among middle-age and old patients. Of note, we found that Hcy and hypertension jointly affected the risk of stroke and CVD. In addition, we attempted to provide a more accurate definition of Hhcy using ROC curves in order to improve its predictive capability for the risk of CVD.
There are few cohort studies with a long-term follow-up in Asian populations,[29–32] and their conclusions are controversial. An increase in plasma Hcy levels is associated with a variety of vascular diseases.[5,6,31] The possible mechanisms of vascular injury involve oxidative stress interfering with the balance of the nitric oxide synthase system, immune and inflammatory responses, dyslipidemia, and coagulation dysfunction.[33–37] A combination of multiple mechanisms eventually leads to the development of atherosclerosis and CVD.
Stroke is the second leading cause of death in the world, and it is also one of the most common chronic noncommunicable diseases in China.[38,39] In addition to the traditional risk factors of stroke (such as blood lipids, blood pressure, and smoking), a number of studies have proposed that elevated plasma Hcy is closely related to stroke risk.[21,29,40,41] Similarly, the findings from some cross-sectional studies indicated that plasma Hcy levels in stroke patients are significantly higher than in individuals without stroke.[42,43] Recently, a number of studies have suggested that reducing the level of Hcy can effectively decrease the risk of stroke, which indirectly indicates that Hcy is a risk factor for stroke. In 2015, the China Stroke Primary Prevention Trial (a randomized, double-blind clinical trial) reported that among hypertensive adults without a history of stroke or myocardial infarction, enalapril-folic acid significantly reduced the risk for first stroke compared with enalapril after a median treatment duration of 4.5 years.[44] Nevertheless, there are still some studies indicating that Hcy-lowering therapy has no significant effect on reducing the risk of stroke.[23] To evaluate the association between Hcy and stroke risk, we conducted an observational study with a 17-year follow-up. As hypothesized, among the 1226 subjects, after adjustment for confounding factors, the risk for stroke (HR = 2.02) in subjects with Hcy of 10 to 15 μmol/L was higher than in subjects with Hcy < 10 μmol/L, and subjects with Hcy > 15 μmol/L had an HR of 3.44. Moreover, Hcy of 10 to 15 μmol/L was found to be independently associated with the risk of stroke in the nonhypertensive subjects. In the hypertension subgroup, the risk for stroke was significantly increased in the Hcy > 15 μmol/L group. Finally, ROC curve analysis was performed to evaluate the value of Hcy for predicting stroke. The results indicated that Hcy can significantly improve the predictive capability of traditional risk factors for stroke. The Youden index indicated that Hcy > 13.5 μmol/L could be considered a promising marker for the prediction of stroke, with 70.9% sensitivity and 62.2% specificity. Nevertheless, the mechanisms of Hcy in promoting stroke are still unclear. The most likely causes are cerebral vascular injury and atherosclerosis, but further research is needed.
CVDs impose an enormous financial burden to the society and is one of the main causes of death worldwide.[1] In addition to the traditional risk factors such as hyperlipidemia, smoking, hypertension, and diabetes, 15% to 20% of patients with CVD do not have these traditional risk factors and are not identified in time to prevent a primary event, thereby missing the opportunity for primary prevention. For these patients, Hcy can be considered to be one of the important potential risk factors of CVD.[45] Ma et al[46] showed that high levels of Hcy are significantly correlated with CAD categories (r = 0.286, P < .001). Compared to patients with stable angina pectoris, patients with acute myocardial infarction and unstable angina pectoris had higher Hcy levels by approximately 4 to 5 μmol/L.[46] In line with these results, Fu et al[47] reported that Hhcy plays a significant role in the prediction of long-term clinical outcomes, including all-cause mortality and major adverse cardiac events, in Chinese octogenarians with acute coronary syndrome. In the present study, by observing the occurrence of cardiovascular events at different Hcy levels over 17 years, we concluded that mildly elevated Hcy (>10 μmol/L) increased the risk for CVD events. For the nonhypertensive subjects, the risk for CVD was greater in those with Hcy of 10 to 15 μmol/L, for whom the predictive cut-off value was 10.10 μmol/L, but Hcy > 15 μmol/L was still a risk factor for CVD events in hypertensive patients. Taken together, the results suggest that Hcy > 10 μmol/L may be helpful for assessing the risk for CVDs and the need for Hcy-lowering therapy.
Furthermore, we also studied the relationship between Hcy levels and the risk for new-onset hypertension in the nonhypertension subgroup. The result showed that Hcy > 10 μmol/L significantly increased the risk for new-onset hypertension. ROC curve analysis indicated that the predictive cut-off value of Hcy for new-onset hypertension was 9.10 μmol/L with 94.9% sensitivity and 50.3% specificity. The pathogenesis of essential hypertension involves both genetic and environmental factors, and aside from well-established traditional predisposing factors such as obesity, alcohol consumption, high sodium and/or low potassium diet, lack of physical exercise and other environmental factors for the occurrence and development of hypertension, the role of Hcy has attracted an increasing amount of attention.[48] Elevated Hcy can increase oxidative stress, decrease vasodilation by nitric oxide, and stimulate the proliferation of vascular smooth muscle cells. As a result, blood pressure increases.[18,20] Many studies have shown that Hcy is associated with elevated blood pressure.[5,17,18,20] For every 5 μmol/L increase in Hcy, the SBP and DBP increase by 0.7/1.2 mm Hg and 0.5/0.7 mm Hg in women and men, respectively.[49] In addition, in 2016, a meta-analysis of 11 published epidemiological studies demonstrated that Hcy contributed to the risk of essential hypertension (OR = 1.36, 95% CI: 1.02–1.80, random-effects model). In summary, the early detection of elevated Hcy and timely intervention against elevated Hcy may provide a new therapeutic strategy to help strengthen primary and secondary prevention of essential hypertension and its complications.
This study shows that Hcy can serve as a marker for predicting risks for stroke, CVDs, and hypertension. MicroRNAs – a class of small, endogenous, single-stranded, non-coding RNAs – have also been found to be innovative biomarkers for facilitating diagnosis and predicting prognosis of stroke, CVD, and hypertension.[50–52] In comparison, microRNAs might offer several advantages over Hcy. Firstly, microRNAs are comparatively stable in both plasma and serum,[53] whereas Hcy can be easily affected by a range of factors. Secondly, it has been revealed that particular microRNA species could be associated with specific etiology of stroke,[51,54] while there has been no evidence for Hcy in this respect. Further, results from bioinformatics analysis show that in stroke some genes are noted to be targeted by microRNAs, which indicates a potential therapeutic target for the disease.[51] However, several problems with microRNAs need to be resolved prior to their widespread application in clinical practice. Above all, compared to Hcy, detection of microRNAs is expensive and more complex thus necessitating exploration of an improved technique for analysis.[50–52] The great number of species as well as their varying nature also brings about challenges in determining cut-off value for diagnosis.[51] Additionally, the pathophysiological mechanism is not clear, and the therapeutic target is uncertain.[50]
Lipoprotein(a) has been demonstrated to be an independent risk factor for CVDs and niacin is therefore suggested as a possible treatment to reduce its level. However, there has been no success in robustly lowering its level in human study as yet.[55,56]
Adipokines including leptin, resistin, protein 4 (FABP4), and retinol binding protein 4(RBP4),[57,58] have also been considered as risk factors associated with CVDs, stroke and hypertension.[59–62] They are reported to be capable of inducing tissue factor, producing C-reactive protein,[59,60] promoting insulin resistance[63] and stimulating inflammatory reaction, oxidative stress, atherogenesis and thrombosis, resulting in endothelial dysfunction, arterial stiffness, and atherosclerotic plaques.
Lifestyle modifications as well as medications such as statins and hypoglycemics may be helpful in reducing adipokines levels.[64] In this sense, adipokines are also promising tools for diagnosing CVDs that enable subsequent exploration of novel pharmacological interventions so long as a better appreciation of their function and molecular targets can be achieved.[65]
There are several limitations to our study. First, Hcy levels are affected by a variety of factors and we excluded subjects with a history of taking folic acid, vitamin B12, and other drugs that can influence Hcy levels. Second, as an observational study, we only measured the level of Hcy in 2000, so there is a lack of evaluation of the changes in the Hcy levels over the 17-year follow-up. Nevertheless, all participants were from the same geographical area and we were able to collect the information about stroke, CVDs, and hypertension. Third, the subjects were mostly males and elderly, and were from the Department of Geriatrics in Ruijin Hospital (Shanghai, China) and do not represent the whole population. Though, our analysis adjusted for confounders, majority of the included participants were of males and recent evidence suggests that the Hcy levels found be more in males compared to females. Therefore, the results should be for reference only and they need further confirmation. Finally, we did not further analyze the interaction between Hcy and hypertension.
5. Conclusions
Mildly elevated Hcy levels (10–15 μmol/L) are significantly associated with an increased risk for stroke and CVD events in this retrospective cohort study, and with new-onset hypertension without hypertension at baseline. Furthermore, Hcy improves the predictive capability of traditional risk factors for stroke. The optimal cut-off value of Hcy for predicting stroke, CVD, and hypertension was 13.4, 12.1, and 9.1 μmol/L, respectively, which might serve as reference values for future studies.
Author contributions
Conceptualization: Wei Wang, Jiumei Cao.
Investigation: Yuanyuan Feng, Kai Kang, Qiqi Xue, Yafen Chen.
Methodology: Yuanyuan Feng, Kai Kang, Qiqi Xue, Yafen Chen.
Writing – original draft: Yuanyuan Feng, Jiumei Cao.
Writing – review & editing: Wei Wang, Jiumei Cao.
Footnotes
Abbreviations: CI = confidence interval, CVDs = cardiovascular diseases, DBP = diastolic blood pressure, Hhcy = hyperhomocysteinemia, LDL-C = low-density lipoprotein cholesterol, ROC = receiver operating characteristic, SBP = systolic blood pressure, TC = total cholesterol.
How to cite this article: Feng Y, Kang K, Xue Q, Chen Y, Wang W, Cao J. Value of plasma homocysteine to predict stroke, cardiovascular diseases, and new-onset hypertension: a retrospective cohort study. Medicine. 2020;99:34(e21541).
Yuanyuan Feng: Huangpu District Dapuqiao Community Health Center, Shanghai, 200023, China.
This work was supported by a grant from Shanghai Jiaotong University School of Medicine (TM201805). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol 2019;16:203–12. [DOI] [PubMed] [Google Scholar]
- [2].Wu Y, Chen Z, Zhang L, et al. Prevention and control of cardiovascular disease in the rapidly changing economy of China. Circulation 2016;133:2545–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lawes CM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet (London, England) 2008;371:1513–8. [DOI] [PubMed] [Google Scholar]
- [4].Wang Z, Vander Hoorn S, Rodgers A, et al. Status of hypertension in China: results from the China Hypertension Survey, 2012–2015. Circulation 2018;137:2344–56. [DOI] [PubMed] [Google Scholar]
- [5].Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nut J 2015;14:6.doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baggott JE, Tamura T. Homocysteine, iron and cardiovascular disease: a hypothesis. Nutrients 2015;7:1108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marti-Carvajal AJ, Sola I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev 2017;8:Cd006612.doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang B, Fan S, Zhi X, et al. Prevalence of hyperhomocysteinemia in China: a systematic review and meta-analysis. Nutrients 2014;7:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang C, Wu Q, Zhang L, et al. Elevated total plasma homocysteine levels are associated with type 2 diabetes in women with hypertension. Asia Pacific J Clin Nutr 2015;24:683–91. [DOI] [PubMed] [Google Scholar]
- [10].Skeete J, DiPette DJ. Relationship between homocysteine and hypertension: new data add to the debate. J Clin Hypertens (Greenwich Conn) 2017;19:1171–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yun L, Xu R, Li G, et al. Homocysteine and the C677T gene polymorphism of its key metabolic enzyme MTHFR are risk factors of early renal damage in hypertension in a Chinese Han population. Medicine 2015;94:e2389.doi: 10.1097/MD.0000000000002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation 2006;113:e409–49. [PubMed] [Google Scholar]
- [13].Catena C, Colussi G, Nait F, et al. Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. Am J Hypertens 2015;28:943–50. [DOI] [PubMed] [Google Scholar]
- [14].Catena C, Colussi G, Url-Michitsch M, et al. Subclinical carotid artery disease and plasma homocysteine levels in patients with hypertension. J Am Soc Hypertens 2015;9:167–75. [DOI] [PubMed] [Google Scholar]
- [15].Zhong C, Xu T, Xu T, et al. Plasma homocysteine and prognosis of acute ischemic stroke: a gender-specific analysis from CATIS randomized clinical trial. Mol Neurobiol 2017;54:2022–30. [DOI] [PubMed] [Google Scholar]
- [16].Kundi H, Kiziltunc E, Ates I, et al. Association between plasma homocysteine levels and end-organ damage in newly diagnosed type 2 diabetes mellitus patients. Endocr Res 2017;42:36–41. [DOI] [PubMed] [Google Scholar]
- [17].Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet 2008;49:267–82. [DOI] [PubMed] [Google Scholar]
- [18].Li J, Jiang S, Zhang Y, et al. H-type hypertension and risk of stroke in chinese adults: a prospective, nested case-control study. J Transl Intern Med 2015;3:171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Momin M, Fan F, Li J, et al. Associations of plasma homocysteine levels with peripheral systolic blood pressure and noninvasive central systolic blood pressure in a community-based Chinese population. Sci Rep 2017;7:6316.doi: 10.1038/s41598-017-06611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang J, Liu Y, Wang A, et al. Association between H-type hypertension and asymptomatic extracranial artery stenosis. Sci Rep 2018;8:1328.doi: 10.1038/s41598-018-19740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han L, Wu Q, Wang C, et al. Homocysteine, ischemic stroke, and coronary heart disease in hypertensive patients: a population-based, prospective cohort study. Stroke 2015;46:1777–86. [DOI] [PubMed] [Google Scholar]
- [22].Mark SD, Wang W, Fraumeni JF, Jr, et al. Lowered risks of hypertension and cerebrovascular disease after vitamin/mineral supplementation: the Linxian Nutrition Intervention Trial. Am J Epidemiol 1996;143:658–64. [DOI] [PubMed] [Google Scholar]
- [23].Hu S, Ren L, Wang Y, et al. Homocysteine-lowering therapy and early functional outcomes of ischemic patients with H-type hypertension: a retrospective analysis of CNSR. Aust Phys Eng Sci Med 2015;38:785–91. [DOI] [PubMed] [Google Scholar]
- [24].Zhao M, Wang X, He M, et al. Homocysteine and stroke risk: modifying effect of methylenetetrahydrofolate reductase C677T polymorphism and folic acid intervention. Stroke 2017;48:1183–90. [DOI] [PubMed] [Google Scholar]
- [25].National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39: 2 Suppl 1: S1–266. [PubMed] [Google Scholar]
- [26].Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet (London, England) 2014;383:1899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].National guidelines for the prevention and control of hypertension for Basic public health services: Nantional center for cardiovascular disease. 2017:1-32. [Google Scholar]
- [29].Iso H, Moriyama Y, Sato S, et al. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation 2004;109:2766–72. [DOI] [PubMed] [Google Scholar]
- [30].Sun Y, Chien KL, Hsu HC, et al. Use of serum homocysteine to predict stroke, coronary heart disease and death in ethnic Chinese. 12-year prospective cohort study. Circ J 2009;73:1423–30. [DOI] [PubMed] [Google Scholar]
- [31].Fallon UB, Elwood P, Ben-Shlomo Y, et al. Homocysteine and ischaemic stroke in men: the Caerphilly study. J Epidemiol Commu Health 2001;55:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang W, Sun K, Chen J, et al. High plasma homocysteine levels contribute to the risk of stroke recurrence and all-cause mortality in a large prospective stroke population. Clin Sci (London, England: 1979) 2009;118:187–94. [DOI] [PubMed] [Google Scholar]
- [33].Stamler JS, Osborne JA, Jaraki O, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Investig 1993;91:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Au-Yeung KK, Woo CW, Sung FL, et al. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circ Res 2004;94:28–36. [DOI] [PubMed] [Google Scholar]
- [35].Ovechkin AV, Tyagi N, Sen U, et al. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am J Physiol Lung Cell Mol Physiol 2006;291:L905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sen U, Tyagi SC. Homocysteine and hypertension in diabetes: does PPARgamma have a regulatory role? PPAR Res 2010;2010:806538.doi: 10.1155/2010/806538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang XC, Sun WT, Yu CM, et al. ER stress mediates homocysteine-induced endothelial dysfunction: modulation of IKCa and SKCa channels. Atherosclerosis 2015;242:191–8. [DOI] [PubMed] [Google Scholar]
- [38].Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2016;387:251–72. [DOI] [PubMed] [Google Scholar]
- [40].Bostom AG, Rosenberg IH, Silbershatz H, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med 1999;131:352–5. [DOI] [PubMed] [Google Scholar]
- [41].Bots ML, Launer LJ, Lindemans J, et al. Homocysteine and short-term risk of myocardial infarction and stroke in the elderly: the Rotterdam Study. Arch Intern Med 1999;159:38–44. [DOI] [PubMed] [Google Scholar]
- [42].Wang C, Han L, Wu Q, et al. Association between homocysteine and incidence of ischemic stroke in subjects with essential hypertension: a matched case-control study. Clin Exp Hypertens (New York, N Y: 1993) 2015;37:557–62. [DOI] [PubMed] [Google Scholar]
- [43].Salem-Berrabah OB, Mrissa R, Machghoul S, et al. Hyperhomocysteinemia, C677T MTHFR polymorphism and ischemic stroke in Tunisian patients. La Tunisie medicale 2010;88:655–9. [PubMed] [Google Scholar]
- [44].Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015;313:1325–35. [DOI] [PubMed] [Google Scholar]
- [45].Gopinath B, Flood VM, Rochtchina E, et al. Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults. Eur J Prev Cardiol 2012;19:1420–9. [DOI] [PubMed] [Google Scholar]
- [46].Ma Y, Peng D, Liu C, et al. Serum high concentrations of homocysteine and low levels of folic acid and vitamin B12 are significantly correlated with the categories of coronary artery diseases. BMC Cardiovasc Disord 2017;17:37.doi: 10.1186/s12872-017-0475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fu Z, Qian G, Xue H, et al. Hyperhomocysteinemia is an independent predictor of long-term clinical outcomes in Chinese octogenarians with acute coronary syndrome. Clin Interv Aging 2015;10:1467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rodrigo R, Passalacqua W, Araya J, et al. Homocysteine and essential hypertension. J Clin Pharmacol 2003;43:1299–306. [DOI] [PubMed] [Google Scholar]
- [49].Lim U, Cassano PA. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2002;156:1105–13. [DOI] [PubMed] [Google Scholar]
- [50].Pordzik J, Pisarz K, De Rosa S, et al. The potential role of platelet-related microRNAs in the development of cardiovascular events in high-risk populations, including diabetic patients: a review. Front Endocrinol 2018;9:74.doi: 10.3389/fendo.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Eyileten C, Wicik Z, De Rosa S, et al. MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke – a comprehensive review and bioinformatic analysis. Cells 2018;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].De Rosa S, Indolfi C. Circulating microRNAs as Biomarkers in Cardiovascular Diseases. Experientia Supplementum (2012). Exp Suppl 2015;106:139–49. [DOI] [PubMed] [Google Scholar]
- [53].Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res 2017;120:381–99. [DOI] [PubMed] [Google Scholar]
- [54].Sepramaniam S, Tan JR, Tan KS, et al. Circulating microRNAs as biomarkers of acute stroke. Int J Mol Sci 2014;15:1418–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J Am Coll Cardiol 2013;62:1575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bregar U, Jug B, Keber I, et al. Extended-release niacin/laropiprant improves endothelial function in patients after myocardial infarction. Heart vessels 2014;29:313–9. [DOI] [PubMed] [Google Scholar]
- [57].Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nature reviews. Immunology 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939–49. [DOI] [PubMed] [Google Scholar]
- [59].De Rosa S, Cirillo P, Pacileo M, et al. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J Vasc Res 2009;46:609–17. [DOI] [PubMed] [Google Scholar]
- [60].Cirillo P, Angri V, De Rosa S, et al. Pro-atherothrombotic effects of leptin in human coronary endothelial cells. Thromb Haemost 2010;103:1065–75. [DOI] [PubMed] [Google Scholar]
- [61].Calabro P, Cirillo P, Limongelli G, et al. Tissue factor is induced by resistin in human coronary artery endothelial cells by the NF-kB-dependent pathway. J Vasc Res 2011;48:59–66. [DOI] [PubMed] [Google Scholar]
- [62].Zachariah JP, Quiroz R, Nelson KP, et al. Prospective relation of circulating adipokines to incident metabolic syndrome: the framingham heart study. J Am Heart Assoc 2017;6:e004974.doi: 10.1161/JAHA.116.004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes 2002;51:2450–8. [DOI] [PubMed] [Google Scholar]
- [64].Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin 2018;39:1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fasshauer M, Bluher M. Adipokines in health and disease. Trends Pharmacol Sci 2015;36:461–70. [DOI] [PubMed] [Google Scholar]


