Abstract
Background:
Loss of residual limb volume degrades socket fit and may require accommodation.
Objectives:
To examine if either of two accommodation strategies executed during resting, socket release with full socket size return and socket release with partial socket size return, enhanced limb fluid volume retention during subsequent activity.
Study design:
Two repeated-measures experiments were conducted to assess the effects of socket release on limb fluid volume retention.
Methods:
Limb fluid volume was monitored while participants wore a socket with a single adjustable panel. Participants performed eight activity cycles that each included 10 minutes of sitting and 2 minutes of walking. The socket’s posterior panel and pin lock were released during the 5th cycle while participants were sitting. In one experiment (Full Return), the socket was returned to its pre-release size; in a second experiment (Partial Return), it was returned to 102% of its pre-release size. Short-term and long-term limb fluid volume retention were calculated and compared to a projected, No Intervention condition.
Results:
Partial Return and Full Return short-term retentions and Partial Return long-term retention were greater than those projected under the control condition (p<0.05).
Conclusion:
Socket release during resting after activity, particularly when the socket is returned to a slightly larger size, may be an effective accommodation strategy to reduce fluid volume loss in transtibial prosthesis users.
Clinical Relevance
This study suggests that existing prosthetic technologies adjustable sockets and locking pin tethers can be used in novel ways to help maintain residual limb fluid volume in active prosthesis users.
Keywords: Biomechanics of prosthetic/orthotic devices, biomechanics, prosthetic design, prosthetics, prosthetic interface mechanics, biomechanics, testing of prosthetic and orthotic components, amputee, adjustable socket, fluid volume
BACKGROUND
Prosthetic socket fit changes over time, in part because of volume fluctuations in the residual limb. Volume changes as low as 1.0% may induce clinically detectable changes in socket fit1. Limb volume fluctuation is considered the most significant challenge faced by people using prosthetic limbs2. Strategies to accommodate volume changes in the residual limb may therefore improve socket fit and enhance limb health.
One means for prosthesis users to overcome limb fluid volume loss is to conduct “socket release.” Socket release is the temporary reduction or removal of stresses on the residual limb so as to facilitate limb fluid volume recovery3. The intent is that relieving interface stresses will increase the volume of extracellular fluid in the residual limb and offset losses that occurred earlier in the day. A study on 16 participants with transtibial amputation showed that socket release executed by doffing for 30 minutes after 15 minutes of moderate activity caused on average a 5.5 (SD 3.0) percent increase in fluid volume in the posterior region of the residual limb. Participants demonstrated significant fluid volume retention (p<0.05) after both 5 and 15 minutes of additional activity. Shorter socket releases (10 minutes) using a different technology, releasing liquid from bladder-liners while leaving the prosthetic socket donned, induced significant fluid volume recovery, but did not produce significant fluid volume retention, after either 5 minutes or 15 minutes of additional activity4. While the shorter socket release time may have contributed to this result, leaving the user’s prosthetic liner pin locked in the shuttle lock during release may have been a more dominant contributor to the lack of meaningful fluid volume retention. The locked pin kept the residual limb deep in the sockets and may have caused the liner to hammock over the anterior distal end, increasing pressures and counteracting the fluid volume recovery benefits of socket release.
In addition to releasing the locking pin during socket release, another means for reducing fluid volume loss may be to return to a larger socket size than before socket release. Returning the socket to the same size after socket release in prior studies3,4,5 may partly explain why some of the recovered fluid was driven out of the residual limb once the prosthesis user stood and ambulated. Enlarging the socket slightly (i.e., small enough so that gait stability is not compromised) when re-donning may reduce subsequent fluid volume loss and improve retention.
The objective of this study was to determine if releasing socket panels and the distal pin lock during 10 minutes of socket release induced fluid volume recovery and retention during subsequent activity compared with no intervention. A second objective was to determine if returning the socket to a slightly larger size after socket release further enhanced fluid volume retention.
METHODS
Participants
Volunteer participants were included in this study if they were over 18 years of age, had a transtibial amputation at least 18 months prior, and regularly used a well-fitting definitive prosthesis. Participants were required to have a lock-and-pin suspension system. Participants were excluded if they had open wounds on their residual limb. Institutional review board approval was obtained and all individuals gave written informed consent before any test procedures were initiated.
Test prostheses
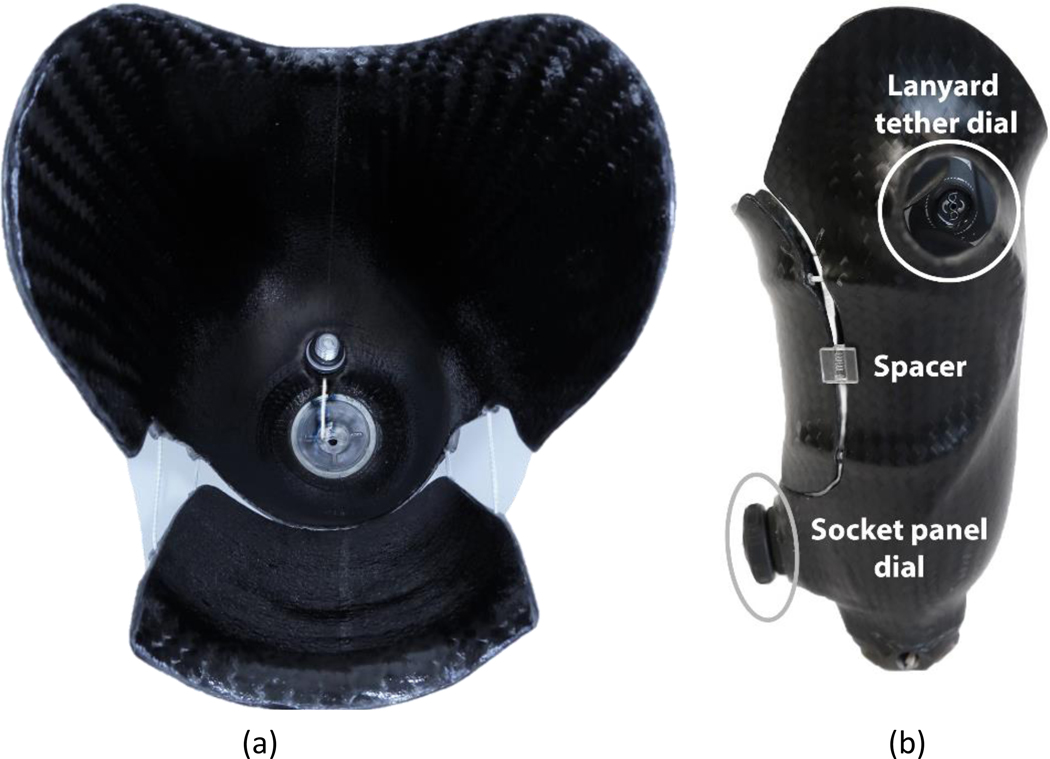
During an initial visit, the participant’s age, date of amputation, and medical history were recorded. The participant’s regular socket was scanned using an industrial coordinate measurement machine (FaroArm Platinum, FARO Technologies, Lake Mary, Florida). A duplicate socket was fabricated and modified to include a movable posterior panel and a tether to draw the liner’s distal locking pin into the shuttle lock (FIG. 1a), using the RevoFit VersaKit and RevoFit Lanyard ratcheting dial systems and recommended clinical procedures (Click Medical, Steamboat Springs, Colorado). A composite adhesive (Fabtech +PLUSeries, Everett, Washington) was used to adhere the dials onto the lateral and posterior aspects of the socket (FIG. 1b). Altogether the socket included four layers of carbon fiber and two layers of nyglass. The size of the posterior panel was chosen to maximize the area over the popliteal region while ensuring relief areas in the socket (e.g., the fibular head) were not disturbed. The test prosthesis was completed using prosthetic componentry (i.e., foot and pylon) similar to the participants’ regular componentry. Lab componentry was used so as to keep the test prostheses intact between sessions and maintain alignment. Setup, alignment, and clinical fitting of the test prosthesis was performed prior to testing by a licensed, certified research practitioner using usual clinical methods (i.e., limb inspection, observational gait analysis, and participant feedback).
Fig. 1. Test prosthesis.
(a) Top view of socket with panel opened. A tether connects to the locking pin and extends distally through a hole in the socket. (b) Side view of socket showing ratcheting dial posterior distally used to draw in the tether and lock the pin, and ratcheting dial lateral proximally to draw in the panel. Plastic spacers are shown that hold the socket at the Partial Return size.
Limb Fluid Volume Measurement
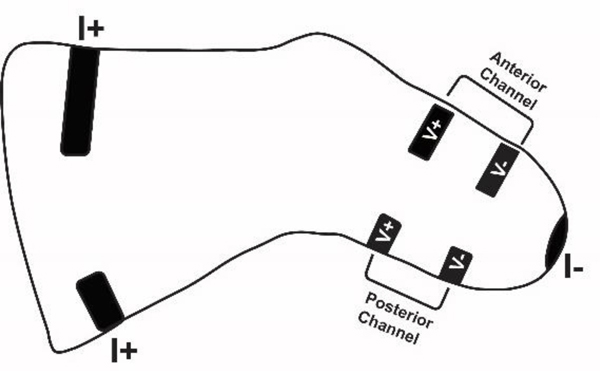
Residual limb fluid volume was monitored using a previously described instrument that implemented bioimpedance as a testing modality6. Custom thin-tape electrodes (ARCare 8881, Adhesives Research, Glen Rock, Pennsylvania) were placed on the proximal thigh and distal aspect of the residual limb to inject a small electrical current (~300μA peak-to-peak) at 24 frequencies ranging from 3 kHz to 1 MHz. Thirty frequency sweeps were applied every second. Other electrodes were placed on the anterior lateral surface and on the posterior surface to sense voltage (FIG. 2). Current and voltage data were demodulated and fit to a computational model to determine fluid resistance7. Fluid resistance was converted to extracellular fluid volume using an anthropometric model8,9. Error in the extracellular fluid volume measurement was less than 0.1% fluid volume6.
Fig. 2. Locations of electrode for fluid volume monitoring.
Current injection electrodes (I+ and I−) were positioned on the proximal thigh and distal limb (one electrode at each location). Volume sensing electrodes were positioned between the level of the fibular head and the distal end of the tibia.
Study Design
Two repeated-measures clinical experiments were conducted to examine the influence of socket release and return on limb fluid volume. In the first experiment, the socket was returned to its original size after socket release (Full Return). In the second experiment, the socket was returned to 102% of its original size (Partial Return). The experiments were conducted on different days. As part of this study, we created a strategy to mitigate the effects of day-to-day differences in participant fluid volume physiology from affecting study results – a modelling algorithm that used pre-intervention data to generate control (i.e., “projected”) data for the two clinical experiments described below. The projected data was based on results from a follow-up experiment (No Intervention) in which participants did not execute socket release.
Protocols for each experiment were similar. After arriving at the lab, participants sat with their prosthesis donned for 10 minutes to achieve a homeostatic condition. They then removed their regular prosthesis, and their residual limb was instrumented for limb fluid volume measurement as described above. The Full Return or Partial Return experiments were conducted within two weeks of each other in random order. The No Intervention experiment was conducted within two months of the second experiment. A longer time was allowed to return for the No Intervention experiment so as to minimize participant attrition.
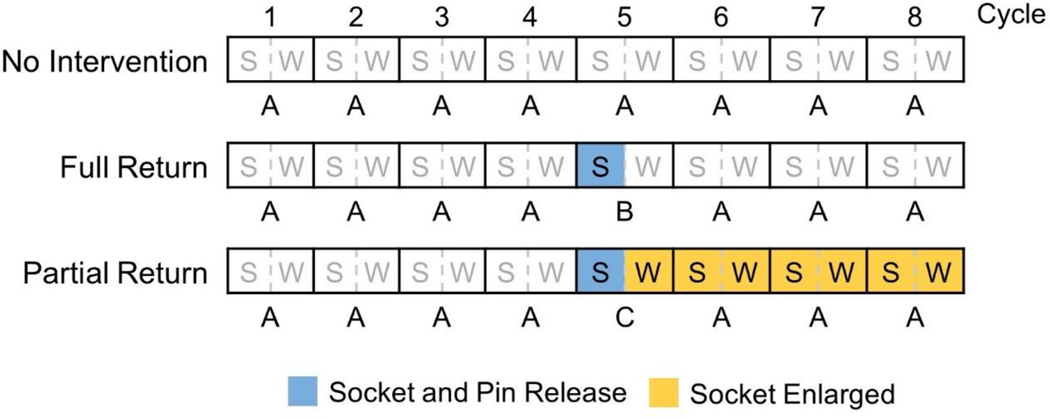
All three experiments (No Intervention, Full Return, Partial Return) included eight cycles of activity, arranged in AAAAAAAA, AAAABAAA, and AAAACAAA formats respectively, where A was no socket release, B was the Full Return intervention, and C was the Partial Return intervention (FIG. 3). Each A cycle included two actions – sitting in a chair for 10 minutes with the prosthesis donned and the feet supported by the floor; and walking for 2 minutes on a treadmill (Landice L7, Randolph, New Jersey) at a self-selected walking speed. The same walking speed was maintained for each participant in all test sessions. For the Full Return intervention (B), the participant immediately sat down, released the posterior panel ratcheting dial, released the ratcheting dial for the lanyard tether, and then partially withdrew his or her residual limb from the socket until the lanyard length (i.e., distal liner to the shuttle lock) reached 4 cm. A length of 4 cm was used because in pilot testing it proved to be sufficient to induce fluid volume recovery while still allowing for a comfortable sitting posture. The researcher monitored lanyard length using ink marks on the tether to ensure the 4 cm distance was achieved. After 10 minutes of sitting, the participant reset the pin in the lock by twisting the tether ratcheting dial to draw in the lanyard, and then returned the socket to the same size as before socket release by twisting the posterior panel ratchet. The participant then walked on the treadmill for 2 minutes. For the Partial Return intervention (C), the participant conducted the same procedure as B except that two 3mm-4mm thick spacers were inserted by the researcher between the panel and socket to create gaps so that when participants closed the panel, the socket was approximately 2% greater in volume than before socket release (Table 2). These spacers were kept in the socket for the remainder of the trial. Spacers of 4mm thickness were used unless participants considered the fit with them unacceptable; then 3mm spacers were used. The sockets were scanned both with and without spacers to quantify socket volumes as described below.
Fig. 3. Diagram illustrating protocols for the three experiments.
S = sit, W = walk.
Table 2.
Participant socket volumes – differences between Partial Return and Full Return.
| Participant | % Diff |
|---|---|
| 1 | 2.6% |
| 2 | 2.1% |
| 3 | 3.2% |
| 4 | 2.0% |
| 5 | 3.5% |
| 6 | 2.1% |
| 7 | 2.0% |
| 8 | 2.7% |
| 9 | 2.3% |
| 10 | 2.9% |
| 11 | 2.7% |
| 12 | 1.6% |
| 13 | 2.3% |
| 14 | 1.7% |
| 15 | 1.6% |
| Mean(SD) | 2.4% (0.6%) |
| Median | 2.3% |
| Max | 3.5% |
| Min | 1.6% |
Data Processing
For all three experiments (No Intervention, Full Return, Partial Return), limb extracellular fluid volume data during each walking cycle was segmented into steps. We noted that a local maxima occurred in each step towards the end of swing phase when rapid angular deceleration of the prosthesis occurred (right before prosthetic foot contact with the ground). The limb fluid volume data stream was segmented into steps using these local maxima (Matlab findpeaks function, v. R2016a, Mathworks, Natick, Massachusetts) and time interval windows determined from the participant’s treadmill walking speed. Selected peaks were visually inspected by an investigator to ensure they were properly selected. Within each step the minimum limb fluid volume was determined, and then the mean of all minima within a cycle was calculated. Results were expressed as a percentage of the mean from cycle 4, termed the reference limb fluid volume. This cycle was used as the reference since the protocols for all three experiments (No Intervention, Full Return, Partial Return) were identical up to that point. A similar method was used effectively in previous investigations3,4.
Modeling algorithm
The purpose of the modeling algorithm was to calculate projected no-intervention limb fluid volumes from pre-intervention data. To create the modeling algorithm, we used results from the No Intervention experiment, fitting the mean stance phase minima results (calculated as described above) from cycles 2 through 4 to a mathematical function. We then used that function to project fluid volumes at later time points in the session. Data from cycle 1 was not used in analysis because of its high variability, as noted previously10,11. Model data was generated using three different curve-fitting functions – linear, logarithmic, and logarithmic/tangent. Linear and logarithmic were fits to data for cycles 2 to 4, and then extension of the curves through to the end time of cycle 8. Logarithmic/tangent was a logarithmic fit to the data for cycles 2 through 4, and then a linear expression through to the end time of cycle 8. The slope of the linear portion was the derivative of the logarithmic portion of the curve at cycle 4 (i.e. the line tangent to the logarithmic curve). The basis for using this strategy was to avoid functions that changed sign of their slope beyond cycle 4. The curve-fitting strategy with the maximum p-value and the minimum least squares error between model and experimental data for all No Intervention data for cycles 5 through 8 was selected as the modeling algorithm. Results generated from application of the modeling algorithm were termed “projected data.”
Differences in percentage fluid volume between cycle 5 data and the reference (cycle 4), termed “short-term fluid volume retention,” were calculated for both the measured and projected data. Similarly, differences in percentage fluid volume between cycle 8 and the reference, termed “long-term fluid volume retention,” were calculated. A Shapiro-Wilk test was conducted to evaluate normality of data for both the measured and projected data for the Full Return and Partial Return studies. If data were normally distributed, then a paired t-test was conducted for all participants to evaluate differences between model and experimental data. If the data were not normally distributed, then a Wilcoxon signed-rank test was conducted instead.
RESULTS
A total of 15 individuals with transtibial limb loss (14 male and 1 female) participated in the study (Table 1). Three participants did not complete the No Intervention experiment (1, 8, and 13), and one participant did not complete the Full Return experiment (13).
Table 1.
Participant and socket characteristics.
| Number | Sex | Age (yr) | Age of Socket (months) | Time Since Amputation (months) | Circumf (cm) | Residual Limb Length (cm) | K Level | Mass (kg) | Reason for Amputation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 45 | 26 | 36 | 29.5 | 15.0 | 4 | 87.7 | Trauma |
| 2 | M | 54 | 89 | 326 | 34.4 | 11.5 | 3 | 99.5 | Trauma |
| 3 | F | 35 | 58 | 148 | 26.6 | 14.0 | 4 | 73.1 | Trauma |
| 4 | M | 55 | 4 | 110 | 27.1 | 13.3 | 3 | 77.7 | Trauma |
| 5 | M | 71 | 50 | 520 | 31.1 | 12.3 | 3 | 90.2 | Trauma |
| 6 | M | 71 | 144 | 586 | 29.7 | 21.8 | 3 | 93.7 | Trauma |
| 7 | M | 41 | 1 | 18 | 26.8 | 11.8 | 3 | 60.8 | Trauma |
| 8 | M | 36 | 64 | 57 | 37.1 | 16.2 | 3 | 119.9 | Trauma |
| 9 | M | 42 | 55 | 132 | 27.7 | 16.3 | 4 | 73.9 | Trauma |
| 10 | M | 75 | 97 | 464 | 28.3 | 18.1 | 3 | 79.5 | Trauma |
| 11 | M | 43 | 12 | 51 | 32.3 | 12.5 | 3 | 86.7 | Trauma |
| 12 | M | 56 | 211 | 153 | 30.6 | 21.2 | 3 | 129.4 | Dysvascular |
| 13 | M | 62 | 145 | 109 | 30.0 | 13.2 | 3 | 79.1 | Dysvascular |
| 14 | M | 57 | 42 | 399 | 27.0 | 18.5 | 3 | 79.3 | Trauma |
| 15 | M | 25 | 5 | 47 | 35.3 | 20.8 | 3 | 132.1 | Trauma |
| Mean (SD) |
51 (14) |
67 (59) |
210 (188) |
30.2 (3.2) |
15.8 (3.4) |
n/a n/a |
90.8 (20.4) |
||
| Median | 54 | 55 | 132 | 29.7 | 15.0 | 3 | 86.7 | ||
| Max | 75 | 211 | 586 | 37.1 | 21.8 | 4 | 132.1 | ||
| Min | 25 | 1 | 18 | 26.6 | 11.5 | 3 | 60.8 | ||
The logarithmic/tangent method produced the least error of the three mathematical functions tested for use as the modeling algorithm (Appendix 1). Measured data from the No Intervention experiment was not significantly different from the logarithmic/tangent projected data for either the short-term (p=0.972 for anterior, p=0.345 for posterior) or the long-term (p=0.249 for anterior, and p=0.701 for posterior). Errors for short-term and long-term projections are summarized in Appendix 2.
Differences between Partial Return and Full Return socket volumes ranged from 1.6% to 3.5% with a median of 2.3% and a mean of 2.4% (SD 0.6%). Results for each participant are included in Table 2.
Measured and projected data were normally distributed for the Partial Return test but not for the Full Return test. We report both mean and median results in the analyses below. We used a Wilcoxon signed-rank test for all statistical comparisons.
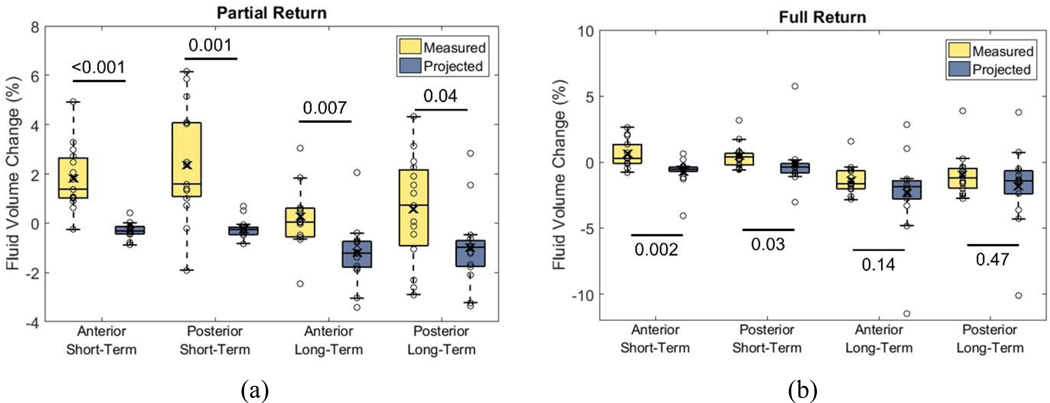
Mean differences between experimental and projected percent limb fluid volume for the Partial Return intervention were 2.1% (SD 1.4%, median 1.8%) anteriorly and 2.6% (SD 2.5%, median 2.4%) posteriorly for the short-term, and 1.5% (SD 1.8%, median 1.9%) anteriorly and 1.6% (SD 2.7%, median 2.2%) posteriorly for the long-term. All four differences were statistically significant (p<0.05) (FIG. 4a).
Fig. 4a,b. Percent limb fluid volume differences between measured and projected data.
a: Partial Return. b: Full Return. Circles are data for each participant. Box limits indicate the 25th and 75th quartile. Line within boxes is the median. “X” inside boxes represents the mean. Graphical presentation of data from all participants is included in Appendix 2.
Mean differences between measured and projected percent limb fluid volume for the Full Return intervention were 1.4% (SD 1.5%, median 0.6%) anteriorly and 0.6% (SD 2.5%, median 0.8%) posteriorly for the short-term, and 0.9% (SD 3.1%, median 0.8%) anteriorly and 0.8% (SD 4.2%, median 0.0%) posteriorly for the long-term. Only short-term differences were statistically significant (FIG. 4b). Partial Return and Full Return experimental results for all participants are shown in Appendix 3.
DISCUSSION
For transtibial prosthesis users, implementation of socket release during resting using adjustable sockets may be an effective means to facilitate the recovery and retention of fluid volume driven out of the residual limb earlier in the day. Unlike accommodation strategies that reduce socket size and promote additional limb fluid volume loss (e.g., adding prosthetic socks)12, socket release is intended to increase limb fluid volume and contribute towards a more stable overall daily limb size. A more stable daily limb volume may be more favorable towards maintaining tissue health and socket fit.
Compared with a prior socket release investigation where liquid was released from bladder-liners for 10 minutes4, the present study demonstrated greater short-term benefit from releasing the socket and locking pin during rest and then bringing it back to the same size afterwards (Full Return experiment). Median retention in the posterior region in the present study was 0.6% and 0.9% fluid volume at 5 and 15 minutes, respectively, both statistically significant, and in the prior bladder-liner study was 0.0% and 0.01% fluid volume, respectively, neither statistically significant. Though the time interval for socket release was the same (10 minutes), there were a number of variables that differed between the present and prior investigation that may explain this result. These included the technology used to adjust socket size (socket panel v. bladder-liner), the total duration of ambulation (12 minutes v. 30 minutes), and the locking pin configuration during socket release (unlocked v. locked). We suspect that the locking pin configuration was the most important of these variables towards fluid volume recovery and retention. However, this hypothesis would need to be tested through rigorous scientific investigation.
The duration of socket release may be an important factor determining the duration of limb fluid volume retention when the socket is brought back to its original size after release. A prior study using a 30-minute socket release time (achieved by doffing) demonstrated significant fluid volume retention for at least 15 minutes of activity, unlike the present study that showed no significant difference after 15 minutes of activity (Full Return experiment). A longer duration of socket release may allow slow transport mechanisms to effectively increase interstitial fluid. The movement of interstitial fluid and ground substance in soft tissue is slow and viscous and occurs over a longer time scale13–15 compared with vascular fluid transport within the vessels13,16. Though this interstitial fluid moves at a slower rate, it may contribute to improved limb volume retention17.
Releasing the socket during rest and then bringing it back to a slightly larger socket size afterwards (Partial Return experiment) retained limb fluid well, resulting in significant percent fluid volume retention at 15 minutes in both anterior and posterior regions. It is possible that the 2% larger sockets accentuated limb fluid volume recovery during subsequent walking periods because they reduced resistance to fluid entering the residual limb. Further, results from a prior study suggested that participants without vascular disease tended to gain limb fluid volume during walking10; the larger socket may have further accentuated this recovery.
There were several limitations in this study. Most (13 of 15) participants had their amputation due to trauma. The results may or may not generalize to people with etiology other than trauma; those other etiologies would need to be explored in future studies. Similarly, most (14 of 15) participants were male, thus results may or may not generalize to females. There was a strict protocol of activity and duration of activity in this study. Benefits of the interventions may or may not occur in users’ free-living environments if they use their socket differently. The studies were of short duration, thus it is unknown is participants would or would not have perceived socket discomfort later in the day after execution of the Partial Return intervention. The clinical relevance of this study is that posterior-panel adjustable sockets and pin tethers, which are both currently available technologies in the prosthetics industry, may offer novel ways for prosthesis users to maintain their residual limb fluid volume during the day. Limb fluid volume losses are a common source of socket fit problems, thus strategies that reduce volume loss have the potential to improve the quality of life of people with lower-limb amputation.
CONCLUSION
Socket and pin lock release during resting after activity reduced subsequent short-term fluid volume loss in transtibial prosthesis users. Returning the socket to a slightly larger size (102%) after socket and pin release reduced subsequent long-term fluid volume loss.
Supplementary Material
Contributor Information
Jacob T Brzostowski, Department of Bioengineering, University of Washington, Seattle.
Brian G Larsen, Department of Bioengineering, University of Washington, Seattle.
Robert T Youngblood, Department of Bioengineering, University of Washington, Seattle.
Marcia A Ciol, Department of Rehabilitation Medicine, University of Washington, Seattle.
Brian J Hafner, Department of Rehabilitation Medicine, University of Washington, Seattle.
Clement J Gurrey, Department of Bioengineering, University of Washington, Seattle.
Jake B McLean, Department of Bioengineering, University of Washington, Seattle.
Katheryn J Allyn, Department of Bioengineering, University of Washington, Seattle.
Joan E Sanders, Department of Bioengineering, University of Washington, Seattle.
REFERENCES
- 1.Sanders JE, Severance MR, Allyn KJ. Computer-socket manufacturing error: How much before it is clinically apparent? J Rehabil Res Dev. 2012;49(4):567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legro MW, Reiber G, del Aguila M, Ajax MJ, Boone DA, Larsen JA, Smith DG, Sangeorzan B. Issues of importance reported by persons with lower limb amputations and prostheses. J Rehabil Res Dev. 1999;36(3):155–163. [PubMed] [Google Scholar]
- 3.Sanders JE, Hartley TL, Phillips RH, Ciol MA, Hafner BJ, Allyn KJ, Harrison DS. Does temporary socket removal affect residual limb fluid volume of trans-tibial amputees? Prosthet Orthot Int. 2016;40(3):320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders JE, Redd CB, Cagle JC, Hafner BJ, Gardner D, Allyn KJ, Harrison DS, Ciol MA. Preliminary evaluation of a novel bladder-liner for facilitating residual limb fluid volume recovery without doffing. J Rehabil Res Dev. 2016;53(6):1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youngblood RT, Hafner BJ, Allyn KJ, Cagle JC, Hinrichs P, Redd CB, Vamos AC, Ciol MA, Bean N, Sanders JE. Effects of activity intensity, time, and intermittent doffing on daily limb fluid-volume change in people with transtibial amputation. Prosthet Orthot Int. 2018;43(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinrichs P, Cagle J, Sanders JE. A portable bioimpedance instrument for monitoring residual limb fluid volume in people with transtibial limb loss: A technical note. Med Eng Phys. 2019; 68:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lorenzo A, Andreoli A, Matthie J, Withers P. Predicting body cell mass with bioimpedance by using theoretical methods: a technological review. J Appl Physiol. 1997;82(5):1542–1558. [DOI] [PubMed] [Google Scholar]
- 8.Hanai T Electrical properties of emulsions In: Sherman P, ed. Emulsion Science. London, England: Academic Press; 1968:354–477. [Google Scholar]
- 9.Fenech M, Jaffrin MY. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans Biomed Eng. 2004;51(1):166–175. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JE, Cagle JC, Allyn KJ, Harrison DS, Ciol MA. How do walking, standing, and resting influence transtibial amputee residual limb fluid volume? J Rehabil Res Dev. 2014;51(2):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JE, Cagle JC, Harrison DS, Myers TR, Allyn KJ. How does adding and removing liquid from socket bladders affect residual-limb fluid volume? J Rehabil Res Dev. 2013;50(6):845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders JE, Harrison DS, Allyn KJ, Myers TR, Ciol MA, Tsai EC. How do sock ply changes affect residual-limb fluid volume in people with transtibial amputation? J Rehabil Res Dev. 2012;49(2):241–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daly CH, Chimoskey JE, Holloway GA, Kennedy D. The effect of pressure loading on the blood flow rate in human skin. J Tissue Viability. 2006;16(4):17–21. [DOI] [PubMed] [Google Scholar]
- 14.Guyton AC, Granger HJ, Taylor AE. Interstitial fluid pressure. Physiol Rev. 1971;51(3):527–563. [DOI] [PubMed] [Google Scholar]
- 15.Kenyon DE. A mathematical model of water flux through aortic tissue. Bull Math Biol. 1979;41(1):79–90. [DOI] [PubMed] [Google Scholar]
- 16.Reddy NP. Mechanical Stress and Viability of Skin and Subcutaneous Tissue In: Hargens AR, ed. Tissue Nutrition and Viability. New York, Berlin, Heidelberg, Tokyo: Springer; 1986:215–241. [Google Scholar]
- 17.Zachariah SG, Saxena R, Fergason JR, Sanders JE. Shape and volume change in the transtibial residuum over the short term: preliminary investigation of six subjects. J Rehabil Res Dev. 2004;41(5):683–694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.