Abstract
Background:
The volume of a prosthesis user’s residual limb changes during the day, and may affect the fit of the prosthesis. These changes must be managed by the user to prevent discomfort, skin breakdown, and falls.
Objectives:
The objectives were to test how activity, time of day, and intermittent doffing affected residual limb fluid volume in people with transtibial amputation.
Study Design:
Standardized, repeated measure (A-B-A) out-of-laboratory protocol.
Methods:
Participants with transtibial amputation completed three 6-hour test sessions. Two sessions served as controls (A protocol) during which participants left their prosthesis donned, and one session was an intervention (B protocol) where participants doffed their prosthesis twice for 20 minutes during the 6 hours of testing. Within-socket fluid volume was measured using a custom portable bioimpedance analysis system.
Results:
Thirteen participants completed the study. The rate of limb fluid volume loss was higher early in the session compared with late in the session. Participants experienced less fluid volume loss during high-activity than low-activity. Socket users with pin suspension experienced less posterior fluid volume loss when they intermittently doffed their prosthesis. Intermittent doffing did not benefit limb fluid volume of mechanical vacuum and suction suspension users.
Conclusions:
High-activity may reduce fluid volume loss compared with low-activity. Intermittent doffing may provide volume accommodation for transtibial prosthesis users with pin suspension.
Clinical Relevance:
Prosthetists should query their patients about the intensity of activity they conduct when advising them on limb volume management. Patients using sockets with pin suspension may be able to offset limb fluid volume loss by periodically doffing the prosthesis.
Keywords: Prosthetic interface mechanics, biomechanics, prosthetic design, prosthetics, biomechanics of prosthetic/orthotic devices, biomechanics, skin stress, skin
Background
Residual limb volume loss is a common source of prosthetic fit problems for people with limb amputation. Volume loss may change how the residual limb fits within the prosthetic socket and induce interface stress concentrations that lead to soft tissue injury or an unstable gait.1, 2 These issues can adversely affect a prosthesis users’ health. A 1999 study found that the fit of the socket was the single most important issue related to successful use of a prosthesis.3
A prosthesis user’s residual limb changes volume throughout the day. Most users lose limb volume as the day progresses.4, 5 Fluid is driven out of the limb due to the continual stresses applied by the prosthetic socket to the soft tissues, both while weight-bearing (e.g., standing and walking) and non-weight-bearing (e.g., sitting).6 Factors such as vascular health, socket interface, and suspension are likely to influence the rate and magnitude of daily limb fluid volume loss. 4 It is unknown whether the rate of limb volume loss changes over the day or whether changes are consistent among prosthesis users. As such, deciding when to instruct prosthesis users to perform volume accommodation (e.g., adding a socket or adjusting socket size) may be difficult. One purpose of this study was therefore to compare rates of fluid volume loss early in the day with those later in the day.
Activity may also affect the rate of volume change.4 Many prosthesis users claim that they lose more volume when they are active than when they sit. Further, volume loss is suspected to be related to intensity of activity.7 However, a previous study found that many prosthesis users gained residual limb fluid volume during walking.6 Thus less volume loss is expected during periods with much walking than during periods with much sitting and intermitting walking. A second purpose of this study was to evaluate if users experienced lower fluid volume loss rates during periods of high-activity compared with periods of low-activity.
There are several methods available to prosthesis users to manage socket fit problems resulting from residual limb volume loss. Adding prosthetic socks 8 and using elevated vacuum sockets 7, 9, 10 are two commonly used strategies. However, they both have limitations. Adding socks tends to further decrease limb fluid volume,11 which may possibly be detrimental to the user’s long-term limb health. Further, it is often inconvenient to perform sock changes because a user must carry spare socks and remove pants to doff and re-don the socket. Elevated vacuum is a technology that is fault intolerant 12–14 since it can be difficult to maintain liners, sockets, and sleeves to ensure no leaks that cause loss of vacuum. An alternative strategy to socks and elevated vacuum is socket release, the temporary reduction of stresses on the residual limb, such as occurs during short-term doffing of the prosthesis. Laboratory testing showed that short-term doffing was effective – 30 minutes of doffing during sitting between 15 minute periods of activity helped to recover and retain limb fluid volume better than leaving the prosthesis donned during sitting.15 However, whether short-term doffing is effective over longer time periods and in less-controlled conditions outside of a laboratory setting is unknown. A third purpose of this study was to determine whether intermittent doffing reduced fluid volume losses in a controlled out-of-laboratory setting.
The purpose of this study was to test the following hypotheses on a group of people with transtibial limb loss: (1) The rate of limb fluid volume loss is greater earlier in the day compared with late in the day; (2) High-activity (much standing and walking; no sitting) induces less fluid volume loss than low-activity (little standing and walking; mainly sitting); and (3) Intermittently doffing the prosthesis for 20 minutes every 2 hours over the course of a 6-hour test session reduces morning-to-afternoon limb fluid volume loss compared with not doffing. Additional analysis was conducted to identify participant or prosthesis characteristics affecting fluid volume changes from intermittent doffing.
Methods
A repeated measures (A-B-A design) study was conducted. To be considered for this study, volunteers must have had a transtibial amputation at least 18 months prior and considered by the research practitioner, through traditional clinical evaluation techniques, to be a Medicare Functional Classification Level (K-level) of 2 (limited community ambulator) or higher. Participants were to be using a properly fitting socket, as deemed by the research prosthetist, for at least 6 hours a day by self-report, and not to be actively undergoing socket revision. A socket was deemed properly fitting based on individual evaluation of characteristics such as sock ply, skin health, and satisfaction. Presence of skin breakdown excluded a participant from this study. Participants were selected based on availability (i.e., convenience sampled). They were recruited locally from our registry of past research participants and by referral through flyers placed in local clinics. A University of Washington Institutional Review Board approved the procedures, and informed consent was obtained from each participant before beginning the protocol. Any clinical determinations and actions were conducted by a single research prosthetist.
An initial visit was conducted to ensure the participant met all inclusion criteria and to collect participant health history, date of amputation, height, weight, limb dimensions, and prosthesis componentry data. Vascular status, including the presence of peripheral arterial disease (PAD), was determined from a medical history questionnaire. Limb type (i.e. bony, fleshy, or muscular) was determined by the research prosthetist through visual inspection and limb palpation. Bony limbs were noted by limited soft tissue. Limbs with substantial soft tissue were further segmented by muscular definition. During the initial visit, the participant’s residual limb was instrumented for limb fluid volume measurement,16 and a brief 30-min test was conducted to ensure signal quality during ambulation. Participants were asked not to consume caffeine or alcohol before all test sessions, and to keep prosthetic componentry and diet consistent for all three test days.
Three test visits were scheduled for each participant, two control sessions (A protocol) and one experimental session (B protocol), arranged in an A-B-A structure with about seven days between each session. After arriving at the laboratory for testing, the participant sat for 10 minutes to achieve a homeostatic condition while the research practitioner noted any changes in participant health, socket fit, or componentry changes to the prosthesis. The participant then doffed the socket, and the residual limb was instrumented with electrodes for limb fluid volume monitoring (i.e., bioimpedance analysis) using a portable bioimpedance analysis system. The system was a portable version of an instrument created previously,16 except that it monitored from only two residual limb regions (anterior, posterior), and a calibration procedure was executed on each participant’s data (Appendix 1). Performance testing demonstrated a root mean square (RMS) error of approximately 0.4% limb fluid volume.
Researchers placed six electrodes on the participant’s limb.15 A small electrical current (<300 μA) over a range of frequencies between 3 kHz and 1 MHz was injected across the proximal to distal electrodes while voltage was measured across electrode pairs positioned on the anterior and posterior surfaces. Session raw data was calibrated using a look-up table (Appendix 1). Anthropometric models were used to convert extracellular fluid resistance to extracellular fluid volume.17, 18 The portable system weighed 400g and was positioned within a waist pack with wires running inside the user’s shorts or pants to the electrodes on the residual limb (Figure 1).
Figure 1.
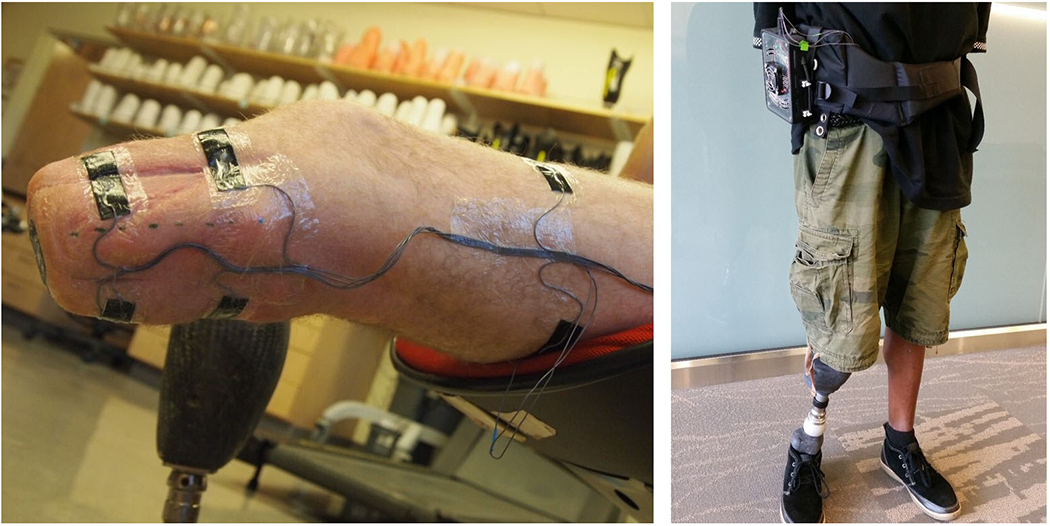
Electrode layout (left) and participant wearing portable bioimpedance monitoring system (right).
A controlled activity protocol was conducted outside of the laboratory (i.e., in and around the building) at each visit. The protocol was about 6 hours in length and included three cycles separated by two, 20-minute rest periods. The first two intervals in each cycle were of low-activity (L1, L2), and the third was of high-activity (H) (Figure 2, Appendix 2). Participants were asked to stand with equal weight on each limb for several seconds at the beginning and end of each interval, and before and after each period of sitting. Data collected during these brief standing periods, a total of 27 data collection points during each cycle, were used for limb fluid volume data analysis. Between each cycle (i.e., during rest periods), participants were instructed to doff (B protocol) or not doff (A protocol) their prosthesis but not their liner. A low-sodium, controlled lunch was provided to the participants at the same time for all three visits, typically during the first rest (Rest 1). Bottled water was provided during lunch and throughout the session as requested by the participant.
Figure 2.
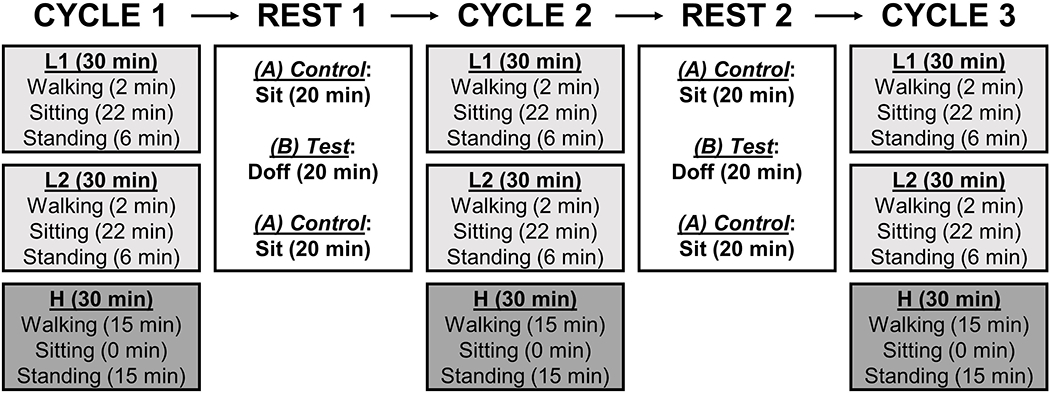
Study protocol. Each cycle consisted of two low-activity intervals (L1 and L2) and one high-activity interval (H).
Limb fluid volumes during equal-weight bearing stands were tracked over the test session. Since the three test protocols were identical up to the end of the first cycle, where the participant subsequently either doffed or did not doff the prosthesis, fluid volume during the last stand at the end of Cycle 1 was used as a reference for each test session. This strategy was used previously to evaluate the influence of an intervention.15 Fluid volume (VmL) at each time point (t) was expressed as a percentage (V%) of each reference fluid volume (VmL,ref):
An assessment of the rate of fluid volume change over time (%/h) during each of the three cycles was calculated using a least squares method. Rates of change from the two control test sessions (A protocol) for each participant were averaged for each cycle. A Friedman’s two-way analysis of variance by ranks with a post hoc all pairwise comparison with Bonferroni correction was conducted to compare rates of fluid volume change between Cycle 1 and Cycle 2, Cycle 1 and Cycle 3, and Cycle 2 and Cycle 3 (IBM SPSS Statistics, Version 23.0, Armonk, New York). This method provided multiple comparison tests.19 Non-parametric statistical tests were used in all analyses due to the limited sample size and the inability to verify underlying assumptions of parametric statistical tests.
To analyze the influence of activity on limb fluid volume, we compared the rates of fluid volume change during the second 30-minute low-activity segment (L2) to rates during the subsequent 30-minute high-activity segment (H) within each cycle. The basis for using L2 rather than L1 in this comparison is that the preceding 30 minutes of activity was the same for L2 and H, i.e. 30 minutes of low-activity. All equal-weight bearing stand data within the segment was used to calculate rate of change. Each participant’s rates of change from the two control test sessions (A protocol) were calculated for each cycle’s L2 and H segments, and an average for each cycle’s L2 and H calculated. A Wilcoxon signed-rank test was used to compare rates of fluid volume change in L2 to rates in H in each of the three cycles.
To analyze the influence of doffing the prosthesis during the rest periods on limb fluid volume change, we calculated a short-term (1.5 h) and long-term (3.5 h) fluid volume change for each of the three test sessions. The short-term change was defined as the percentage fluid volume during the last stand of Cycle 2 minus the reference fluid volume (which was 0.0%) divided by the elapsed time between them. The long-term fluid volume change was defined as the fluid volume during the last stand of Cycle 3 minus the reference fluid volume divided by the time between them. We chose not to use linear regressions for these calculations because there were often large fluid volume increases right after the rest periods which made the relationships between fluid volume change and time non-linear. A Friedman test was conducted to determine if there were differences in the rates of fluid volume change between sessions.
We lastly conducted an exploratory analysis to identify participant or prosthesis characteristics that affected results. We divided the participants into three groups based on how their fluid volumes changed during the experimental session (B protocol) relative to those from the control sessions (A protocol): (1) “Benefit” Group: Experimental session rate of change higher (less negative) than both control session rates of change. B>A1 and B>A2; (2) “No Effect” Group: Experimental session rate of change in between control session rates of change. B>A1 and B<A2, or B<A1 and B>A2; and (3) “Detriment” Group: Experimental session rate of change lower (more negative) than both control session rates of change. B<A1 and B<A2. We analyzed collected data by comparing the number of participants in each group.
Results
Thirteen individuals with a transtibial amputation participated in the study (Table 1). All participants executed the protocols as stated except Participants #1 and #2 had 15-minute rest periods instead of 20 minutes because of researcher time-management error.
Table 1.
Participant and prosthesis characteristics. All participants used a gel liner. Bony limbs had limited soft tissue. Non-bony limbs were further separated by muscular definition.
| ID # | Suspension | Amputation Etiology | Limb Shape | Bony or Non-Bony (fleshy, muscular) | Gender | Tobacco/PAD | Residual Limb Length (cm) | Mid-Limb Circ. (cm) | Age (years) | Since Amp. (years) | Mass (kg) | Height (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Locking pin | Trauma | Conical | Bony | M | n/n | 12.5 | 32.1 | 68.8 | 41.2 | 91.7 | 185.4 |
| 2 | Suction | Trauma | Cylindrical | Non-bony (fleshy) | M | y/n | 16.2 | 28.4 | 51.3 | 22.5 | 80.6 | 181.6 |
| 3 | Mechanical Vacuum | Trauma | Cylindrical | Non-bony (fleshy) | M | n/n | 16.5 | 33.7 | 65.9 | 13.2 | 106.5 | 180.3 |
| 4 | Mechanical Vacuum | Vascular Disease | Conical | Bony | M | n/y | 17.0 | 26.4 | 79.0 | 8.2 | 68.5 | 177.8 |
| 5 | Locking pin | Trauma | Cylindrical | Non-bony (fleshy) | M | y/n | 18.0 | 33.1 | 29.4 | 10.0 | 108.2 | 172.7 |
| 6 | Locking pin | Trauma | Conical | Bony | M | n/n | 13.8 | 28.7 | 45.6 | 29.3 | 84.5 | 184.2 |
| 7 | Locking pin | Trauma | Conical | Non-bony (muscular) | M | n/n | 10.0 | 34.2 | 51.9 | 25.3 | 100.0 | 182.9 |
| 8 | Locking pin | Trauma | Conical | Non-bony (fleshy) | F | n/n | 16.0 | 30.5 | 34.3 | 30.3 | 102.2 | 152.4 |
| 9 | Locking pin | Trauma | Conical | Bony | M | n/n | 18.1 | 27.0 | 72.9 | 36.5 | 80.8 | 180.3 |
| 10 | Locking pin | Trauma | Cylindrical | Non-bony (muscular) | M | n/n | 17.0 | 30.4 | 61.3 | 7.6 | 84.4 | 177.8 |
| 11 | Mechanical Vacuum | Cancer | Cylindrical | Non-bony (fleshy) | M | n/n | 20.5 | 28.5 | 60.4 | 1.5 | 89.5 | 185.4 |
| 12 | Locking pin | Congenital Condition | Conical | Bony | F | n/n | 12.0 | 24.6 | 70.9 | 5.3 | 54.3 | 154.9 |
| 13 | Locking pin | Trauma | Cylindrical | Non-bony (muscular) | M | y/n | 12.5 | 30.4 | 40.8 | 2.2 | 76.5 | 175.3 |
| Mean | 15.4 | 29.8 | 56.3 | 17.9 | 86.7 | 176.2 | ||||||
| SD | 3.0 | 2.9 | 15.5 | 13.6 | 15.5 | 10.7 | ||||||
| Median | 16.2 | 30.4 | 60.4 | 13.2 | 84.5 | 180.3 | ||||||
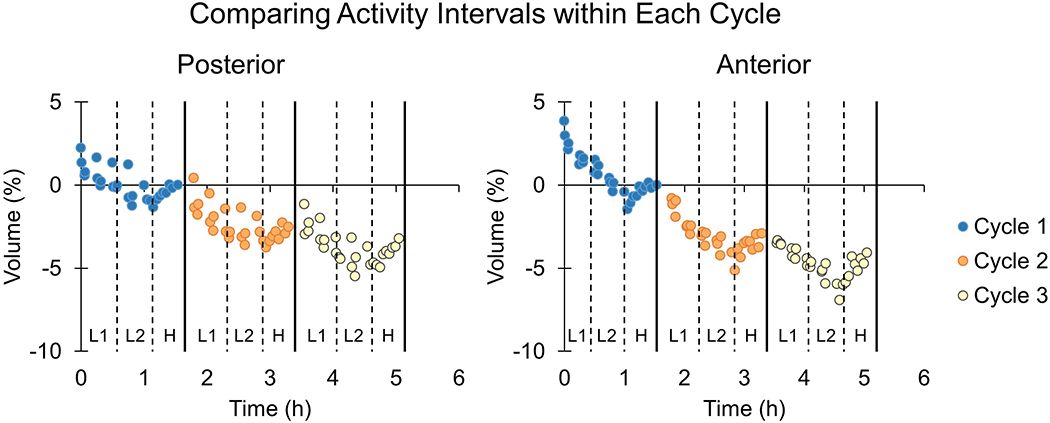
Median and mean fluid volume losses were greater in earlier cycles than later cycles for both the posterior and anterior regions (Figure 3, Appendix 3). While a majority of participants demonstrated greater losses in earlier cycles than in later cycles for both the anterior and posterior regions (Table 2), the only statistically significant difference was for the posterior region when comparing Cycles 3 and 1. Friedman test results for the anterior region showed no significant differences between cycles (p=0.06).
Figure 3.
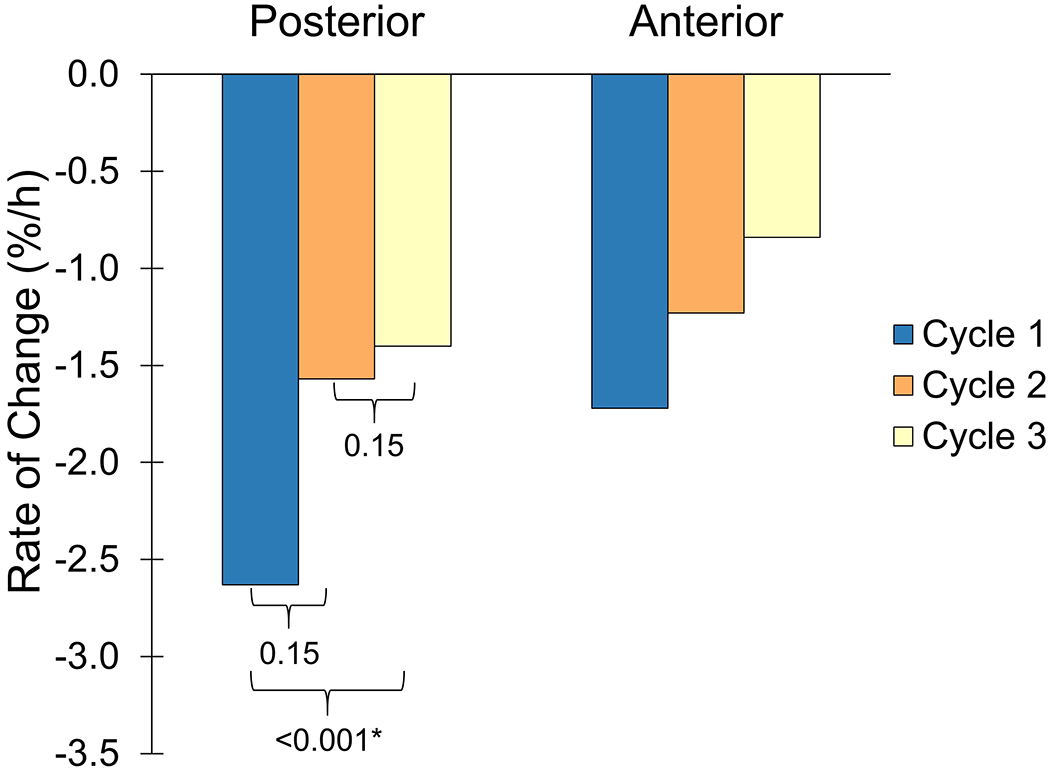
Rates of percent limb fluid volume change during each cycle for the posterior (left) and anterior (right) regions.
*Statistical significance.
Table 2.
Number of 13 participants who had higher fluid volume loss rates for early v. late cycles.
| Comparison of rates of fluid volume change | Posterior | Anterior |
|---|---|---|
| Cycle 1 > Cycle 2 | 10 | 9 |
| Cycle 1 > Cycle 3 | 13 | 10 |
| Cycle 2 > Cycle 3 | 10 | 9 |
In agreement with the hypothesis, participants experienced less fluid volume loss during intervals of high-activity than intervals of low-activity, for both the anterior and posterior regions (Figure 4, Appendix 4). A low number of participants, between 1 and 3 (of 13), demonstrated fluid volume changes contrary to the hypothesis (Table 3). There were statistically significant differences for all comparisons, both posterior and anterior, except posterior Cycle 2. An example result from one participant demonstrating the observed trend of a higher rate of fluid volume increase for H than L2 is shown in Figure 5.
Figure 4.
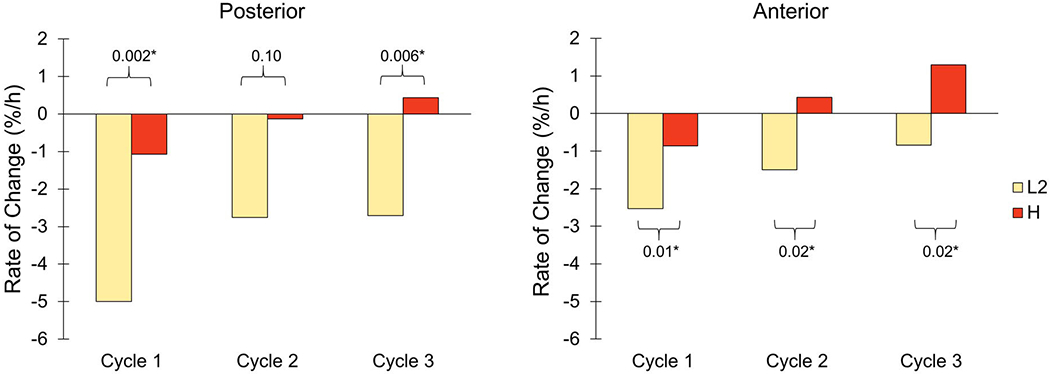
Rates of percent limb fluid volume change for low activity (L2) and high activity (H) for each cycle. Data from the posterior (left) and anterior (right) regions are shown. *Statistical significance.
Table 3.
Numbers of 13 participants for each of the 3 cycles who had higher rates of fluid volume loss during H than during L2.
| Cycle | Posterior | Anterior |
|---|---|---|
| Cycle 1 | 1 | 3 |
| Cycle 2 | 3 | 3 |
| Cycle 3 | 2 | 1 |
Figure 5.
Percent limb fluid volume change for a participant demonstrating fluid volume gains during high activity (H) and losses during low activity (L1, L2). Data from equal weight-bearing stand points are shown.
A Friedman test showed that there were no statistically significant differences in the rates of fluid volume change among the three sessions (two control sessions and one experimental session) in each region for both short-term (pant=0.29, ppost=0.12) and long-term changes (pant=0.09, ppost=0.37). An exploratory analysis followed to better understand how responses differed between participants.
We visually inspected the data by plotting results for each participant, and noted that the effect of intermittent doffing appeared to depend upon presence of suspension systems capable of creating negative pressure (i.e., suction or active mechanical vacuum systems) in the socket. Intermittent doffing during the rests generally benefited participants who used pin suspension, but only in their posterior regions (Figure 6). None of the mechanical vacuum or suction suspension users benefitted posteriorly from intermittent doffing either in the short-term or long-term. None of the suspension system groups demonstrated consistent benefit in the anterior region from intermittent doffing.
Figure 6.
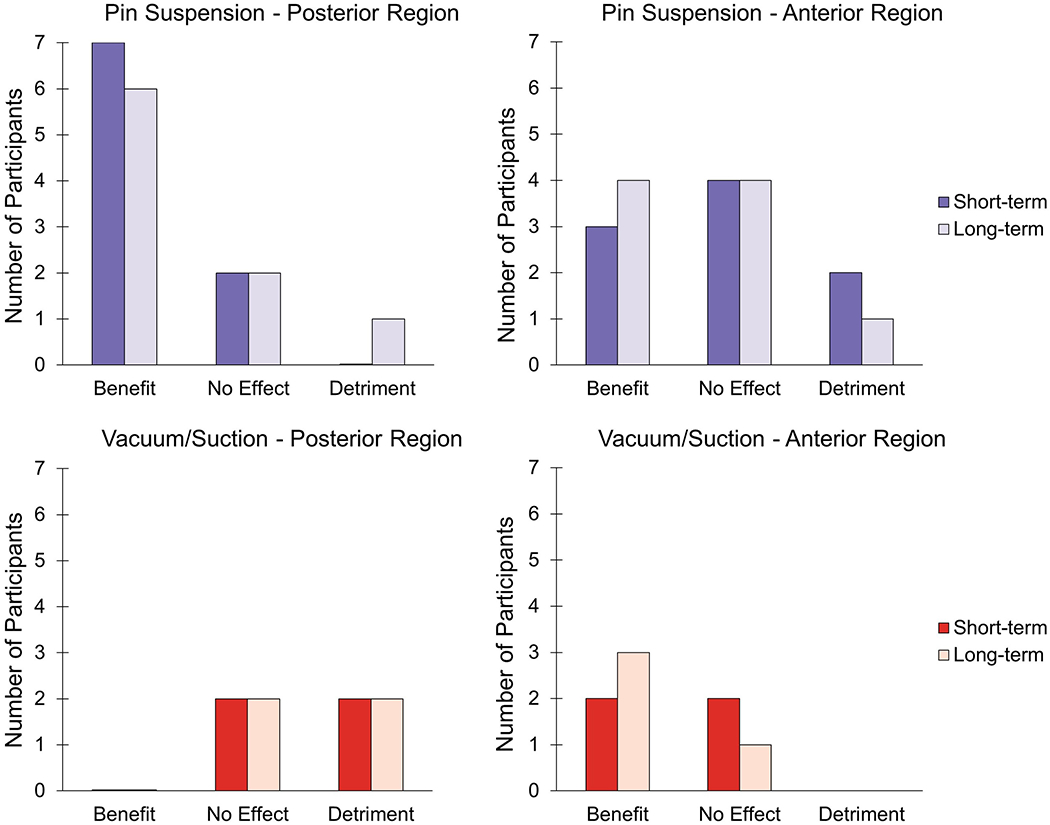
Short-term and long-term effect of intermittent doffing for posterior and anterior regions in sockets with pin (upper) and vacuum or suction (lower) suspension. “Benefit” occurred when the experimental session (B) rate of change was higher (less negative) than both control session rates of change (A1, A2), B > A1 and B > A2. “No Effect” was indicated by the experimental session rate of change in between control session rates of change, B > A1 and B < A2, or B < A1 and B > A2. When the experimental session rate of change was lower (more negative) than both control session rates of change, B < A1 and B < A2, this signified “Detriment.”
Discussion
Understanding factors that influence daily limb fluid volume loss may help people with limb amputation and their practitioners to better manage diurnal changes in socket fit. In this study of active people with transtibial limb loss, we found that rates of limb fluid volume loss were: greater earlier in the day compared with late in the day; lower during periods of high-activity compared with low-activity; and reduced posteriorly when intermittent doffing was executed while wearing a socket with pin suspension.
Hypothesis 1: Early v. late in the day
In a prior study, we proposed that residual limb soft tissue fluid volume recovery (which we termed “mechanical response”) to stress may be characterized as “unrecoverable” or “recoverable.” Tissue may be characterized as unrecoverable where it is soft, deformable, loses volume when a load is applied, and does not easily recover volume when the load is released. Alternatively, tissue may be characterized as “recoverable” where the tissue is relatively rigid and stiff, but recovers volume when an applied load is released.20 Further, we proposed that a residual limb tissue’s state at any given moment depends on how much fluid has already been expelled from the limb that day; greater fluid volume loss induces a tendency towards the recoverable state.20 In the present study, tissues were in their most unrecoverable state in Cycle 1 and their most recoverable state in Cycle 3, evidenced by the significantly greater extracellular fluid volume loss during Cycle 1 than Cycle 3. The clinical application of this result is that people with limb loss who live active lifestyles may anticipate greater fluid volume loss rates early compared with a few hours later in the day. As in our prior investigation,20 participants were still losing fluid volume late in the session (Cycle 3), and never reached zero loss rate.
It is possible that limb doffing prior to beginning the experimental session (i.e., to place electrodes) accentuated Cycle 1 fluid volume loss rates in this study. However, we do not expect that influence to be strong since doffing for electrode placement was conducted after sitting with the prosthesis donned instead of after walking, which would tend to minimize fluid volume gains during rest.21 Also, participants were active before coming to the laboratory for testing, traveling from home to the testing facility, thus their early morning limb fluid volume changes were not included within our measurements. If those changes had been included, Cycle 1 loss rates would likely have been greater, accentuating differences relative to the other cycles.
Hypothesis 2: High v. low-activity
Consistent with Hypothesis 2, we observed that greater activity led to lower rates of fluid volume loss. This result is consistent with prior investigation from a controlled laboratory study where we found that 16 of 24 participants gained limb fluid volume during walking.6 It is likely reflective of the enhanced vascular drive people experience during more intense dynamic activity. We note, however, that standing contributed to fluid volume loss.6 Prosthesis users generally comment that high-activity accentuates limb fluid volume loss, possibly because they do not distinguish standing from walking when they talk with their practitioner about activity. While more standing (15 minutes) was executed in H than L1 or L2, fluid volume gains during walking likely offset losses during standing. The extended sitting during L1 and L2 compared with H may also have contributed to the differences between H and L2 for some users. In a prior investigation, participants did not consistently demonstrate fluid volume gains or losses during sitting.6 There appear to be differences between sitting, standing, and walking from a limb fluid volume perspective, and practitioners should consider these differences when discussing activity and volume accommodation with their patients. The use of monitoring tools that distinguish types of activities (e.g., sit, stand, walk, doff) may facilitate clinical efforts.22, 23
Hypothesis 3: Intermittent doffing v. not doffing
Results from the present study, which showed that intermittent doffing improved limb fluid volume recovery and retention posteriorly for users with pin suspension, are consistent with prior findings from shorter-term studies. Doffing the socket for 30 minutes between two 15-minute periods of activity improved limb fluid volume retention compared with not doffing in all 16 transtibial prosthesis users tested.15 Thus, intermittent doffing should be considered by practitioners and prosthesis users as a useful accommodation strategy for limb fluid recovery and retention for people who experience daily limb fluid volume loss using pin suspension. Practitioners might recommend a certain number of doffs per day, or doffing at regular times during the day (e.g., during the patient’s lunch hour) to recover limb fluid volume. Doffing in the present study was conducted right after high-activity, as it was shown previously to substantially increase limb fluid volume gain when compared with doffing after sitting.21
Prosthesis users wearing mechanical vacuum or suction suspension appeared to not benefit from periodic doffing (Figure 6). Elevated vacuum has been shown to stabilize residual limb volume, which may explain why periodic doffing had a limited effect in those using vacuum suspesion.7, 9, 10 We also note, however, that doffing and donning vacuum sockets is mechanically strenuous to limb soft tissues, and that vacuum sockets are typically of smaller size than sockets made for pin suspension, thus it is possible that re-donning was responsible for reducing limb fluid volumes in protocol B of this study. It is also possible that the loose socket during ambulation right after donning (i.e., it takes about 30 steps to achieve full vacuum pressure using mechanical vacuum) further contributed to the observed outcome. Electronic vacuum systems are intended to apply consistent vacuum pressure and may produce different results, but were not tested here.
The present study included a strenuous 6-hour testing protocol that included several periods of high-activity. Therefore, it was likely achievable only by K-3 and K-4 level ambulators. Thus, it is not known if results from this study are applicable to less active individuals. Different doffing durations and frequencies may be more appropriate for K-1 and K-2 prosthesis users. The present study only included direct measurement of limb fluid volume using custom instrumentation. Field testing comparing clinical outcomes (e.g., self-report measures) for people who intermittently doff during rest and those who do not should be pursued.
Conclusion
Results from this study into how time of day, activity, and intermittent doffing affected residual limb fluid volume may be useful to practitioners advising patients how to manage limb volume fluctuations. Findings that fluid volume loss rates reduced after 4 hours of intermittent activity indicate that, as expected, practitioners should advise their patients to anticipate a greater need for accommodation early in a day’s activities than later on. Results that prosthesis users lost less fluid volume during high-activity than during low-activity indicate that practitioners should pay attention to the nature of their patients’ activities (e.g., if they mainly stand or mainly walk), not just if they do or do not conduct activities. Finally, intermittent doffing may be an effective accommodation alternative to adding socks for pin suspension users since it reduces overall volume loss.
Supplementary Material
Contributor Information
Robert T Youngblood, Department of Bioengineering, University of Washington.
Brian J Hafner, Department of Rehabilitation Medicine, University of Washington.
Katheryn J Allyn, Department of Bioengineering, University of Washington.
John C Cagle, Department of Bioengineering, University of Washington.
Paul Hinrichs, Department of Bioengineering, University of Washington.
Christian B Redd, Department of Bioengineering, University of Washington.
Andrew C Vamos, Department of Bioengineering, University of Washington.
Marcia A Ciol, Department of Rehabilitation Medicine, University of Washington.
Nathanial Bean, Department of Bioengineering, University of Washington.
Joan E Sanders, Department of Bioengineering, University of Washington.
References
- 1.Sanders JE, Zachariah SG, Jacobsen AK and Fergason JR. Changes in interface pressures and shear stresses over time on trans-tibial amputee subjects ambulating with prosthetic limbs: comparison of diurnal and six-month differences. J Biomech. 2005; 38: 1566–73. [DOI] [PubMed] [Google Scholar]
- 2.Hoaglund FT, Jergesen HE, Wilson L, Lamoreux LW and Roberts R. Evaluation of problems and needs of veteran lower-limb amputees in the San Francisco Bay Area during the period 1977-1980. J Rehabil Res Dev. 1983; 20: 57–71. [PubMed] [Google Scholar]
- 3.Legro MW, Reiber G, del Aguila M, Ajax MJ, Boone DA, Larsen JA, Smith DG and Sangeorzan B. Issues of importance reported by persons with lower limb amputations and prostheses. J Rehabil Res Dev. 1999; 36: 155–63. [PubMed] [Google Scholar]
- 4.Sanders JE and Fatone S. Residual limb volume change: systematic review of measurement and management. J Rehabil Res Dev. 2011; 48: 949–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders JE, Youngblood RT, Hafner BJ, Ciol MA, Allyn KJ, Gardner D, Cagle JC, Redd CB and Dietrich CR. Residual limb fluid volume change and volume accommodation: Relationships to activity and self-report outcomes in people with trans-tibial amputation. Prosthet Orthot Int. 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders JE, Cagle JC, Allyn KJ, Harrison DS and Ciol MA. How do walking, standing, and resting influence transtibial amputee residual limb fluid volume? J Rehabil Res Dev. 2014; 51: 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Board WJ, Street GM and Caspers C. A comparison of trans-tibial amputee suction and vacuum socket conditions. Prosthet Orthot Int. 2001; 25: 202–9. [DOI] [PubMed] [Google Scholar]
- 8.Nelson VS, Flood KM, Bryant PR, Huang ME, Pasquina PF and Roberts TL. Limb deficiency and prosthetic management. 1. Decision making in prosthetic prescription and management. Arch Phys Med Rehabil. 2006; 87: S3–9. [DOI] [PubMed] [Google Scholar]
- 9.Goswami J, Lynn R, Street G and Harlander M. Walking in a vacuum-assisted socket shifts the stump fluid balance. Prosthet Orthot Int. 2003; 27: 107–13. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JE, Harrison DS, Myers TR and Allyn KJ. Effects of elevated vacuum on in-socket residual limb fluid volume: Case study results using bioimpedance analysis. J Rehabil Res Dev. 2011; 48: 1231–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders JE, Harrison DS, Allyn KJ, Myers TR, Ciol MA and Tsai EC. How do sock ply changes affect residual-limb fluid volume in people with transtibial amputation? J Rehabil Res Dev. 2012; 49: 241–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klute GK, Berge JS, Biggs W, Pongnumkul S, Popovic Z and Curless B. Vacuum-Assisted Socket Suspension Compared With Pin Suspension for Lower Extremity Amputees: Effect on Fit, Activity, and Limb Volume. Arch Phys Med Rehabil. 2011; 92: 1570–5. [DOI] [PubMed] [Google Scholar]
- 13.Traballesi M, Delussu AS, Fusco A, Iosa M, Averna T, Pellegrini R and Brunelli S. Residual limb wounds or ulcers heal in transtibial amputees using an active suction socket system. A randomized controlled study. Eur J Phys Rehab Med. 2012; 48: 613–23. [PubMed] [Google Scholar]
- 14.Gholizadeh H, Lemaire ED and Eshraghi A. The evidence-base for elevated vacuum in lower limb prosthetics: Literature review and professional feedback. Clin Biomech. 2016; 37: 108–16. [DOI] [PubMed] [Google Scholar]
- 15.Sanders JE, Hartley TL, Phillips RH, Ciol MA, Hafner BJ, Allyn KJ and Harrison DS. Does temporary socket removal affect residual limb fluid volume of trans-tibial amputees? Prosthet Orthot Int. 2016; 40: 320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders JE, Moehring MA, Rothlisberger TM, Phillips RH, Hartley T, Dietrich CR, Redd CB, Gardner DW and Cagle JC. A Bioimpedance Analysis Platform for Amputee Residual Limb Assessment. IEEE Trans Biomed Eng. 2016; 63: 1760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenech M and Jaffrin MY. Extracellular and intracellular volume variations during postural change measured by segmental and wrist-ankle bioimpedance spectroscopy. IEEE Trans Biomed Eng. 2004; 51: 166–75. [DOI] [PubMed] [Google Scholar]
- 18.Hanai T Electrical properties of emulsions In: Sherman P, (ed.). Emulsion Science. London, England: Academic Press, 1968, p. 354–477. [Google Scholar]
- 19.Dunn OJ. Multiple Comparisons Using Rank Sums. Technometrics. 1964; 6: 241–52. [Google Scholar]
- 20.Sanders JE, Cagle JC, Harrison DS, Myers TR and Allyn KJ. How does adding and removing liquid from socket bladders affect residual-limb fluid volume? J Rehabil Res Dev. 2013; 50: 845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders JE, Harrison DS, Cagle JC, Myers TR, Ciol MA and Allyn KJ. Post-doffing residual limb fluid volume change in people with trans-tibial amputation. Prosthet Orthot Int. 2012; 36: 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redfield MT, Cagle JC, Hafner BJ and Sanders JE. Classifying prosthetic use via accelerometry in persons with transtibial amputations. J Rehabil Res Dev. 2013; 50: 1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders JE, Redd CB, Larsen BG, Vamos AC, Brzostowski JT, Hafner BJ, Allyn KJ, Henrikson KM, McLean JB, Hinrichs P. A novel method for assessing prosthesis use and accommodation practices of people with trans-tibial amputation. J Prosthet Orthot. 2018; 30(4):214–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


