Abstract
The 2011 EFSA opinion on Campylobacter was updated using more recent scientific data. The relative risk reduction in EU human campylobacteriosis attributable to broiler meat was estimated for on‐farm control options using Population Attributable Fractions (PAF) for interventions that reduce Campylobacter flock prevalence, updating the modelling approach for interventions that reduce caecal concentrations and reviewing scientific literature. According to the PAF analyses calculated for six control options, the mean relative risk reductions that could be achieved by adoption of each of these six control options individually are estimated to be substantial but the width of the confidence intervals of all control options indicates a high degree of uncertainty in the specific risk reduction potentials. The updated model resulted in lower estimates of impact than the model used in the previous opinion. A 3‐log10 reduction in broiler caecal concentrations was estimated to reduce the relative EU risk of human campylobacteriosis attributable to broiler meat by 58% compared to an estimate larger than 90% in the previous opinion. Expert Knowledge Elicitation was used to rank control options, for weighting and integrating different evidence streams and assess uncertainties. Medians of the relative risk reductions of selected control options had largely overlapping probability intervals, so the rank order was uncertain: vaccination 27% (90% probability interval (PI) 4–74%); feed and water additives 24% (90% PI 4–60%); discontinued thinning 18% (90% PI 5–65%); employing few and well‐trained staff 16% (90% PI 5–45%); avoiding drinkers that allow standing water 15% (90% PI 4–53%); addition of disinfectants to drinking water 14% (90% PI 3–36%); hygienic anterooms 12% (90% PI 3–50%); designated tools per broiler house 7% (90% PI 1–18%). It is not possible to quantify the effects of combined control activities because the evidence‐derived estimates are inter‐dependent and there is a high level of uncertainty associated with each.
Keywords: Campylobacter, Control, Broiler, primary production, biosecurity, population‐attributable fraction, modelling
Summary
In 2011, EFSA published an opinion on ‘Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain’. In 2018, the European Commission requested the Panel on Biological Hazards to deliver a scientific opinion updating and reviewing control options for Campylobacter in broilers, focussing on primary production. In particular, the Panel was requested to review, identify and rank the possible control options at the primary production level, considering and, if possible, quantifying the expected efficiency in reducing human campylobacteriosis cases. Advantages and disadvantages of different options at primary production should be assessed, as well as the possible synergic effect of combined control options.
The update of the previous opinion was carried out by reviewing the scientific literature published since then and by estimating the relative risk reduction, expressed as the percentage reduction in human campylobacteriosis in the EU associated with the consumption of broiler meat that could be achieved by implementing control options at primary production of broilers. The relative risk was estimated for on‐farm control options using population attributable fractions (PAF) for interventions that reduce Campylobacter flock prevalence, updating the modelling approach for interventions that reduce caecal concentrations and reviewing the scientific literature. The effect of control options that reduce the prevalence of Campylobacter spp. in broilers was estimated by calculating PAF, derived from epidemiological risk factors studies, and assuming a proportionate relation between flock prevalence prior to slaughter and the associated public health risk. The effect of control options that reduce Campylobacter spp. concentration in broilers was estimated by using a regression model, associating concentrations in the caeca and on skin samples, combined with a consumer phase and a dose response model. For some control options, the relative risk reduction could not be calculated by these methods and their effect was estimated using evidence from the scientific literature.
The PAF were calculated for six control options from several studies and included hygienic anteroom; effective rodent control; having no animals in close proximity to the broiler house; addition of disinfectant to drinking water; employing few and well‐trained staff and avoiding drinkers that allow standing water. The variation was greater between the different control options than for the same control options in different studies, which increased the confidence in the extrapolation potential of the results to the European Union (EU).
According to the PAF analyses, the mean relative risk reductions that could be achieved by adoption of each of these six control options individually are estimated to be substantial but the width of the confidence intervals of all control options indicates a high degree of uncertainty in the specific risk reduction potentials. For example, the mean estimate of the relative risk reduction for the control option ‘Addition of disinfectants to drinking water’ was between 5 (95% CI 0.6–8.2) and 32% (95% CI 6.0–54.9) based on three available studies.
The modelling approach for relative risk reductions achieved by a reduction of Campylobacter concentrations in the caeca, previously used in the 2011 opinion, was updated. A wider variety of consumer phase models and a newly published dose response model were also included. Furthermore, newly and more extensive published data on the relationship between Campylobacter concentrations in the caeca and corresponding broiler carcass skin samples were used. The updated model resulted in lower estimates of the slope of the linear regression line describing the relation between concentrations in caecal contents and on skin. As a result of the decrease of this slope, lower estimates were obtained for the effectiveness of control options directed at a reduction in the caecal concentrations. For example, for a 2‐log10 reduction in caecal concentrations, the median estimate was now a relative risk reduction of campylobacteriosis attributable to the consumption of broiler meat produced in the EU of 42% (95% CI 11–75%), whereas in the previous opinion, this relative risk reduction was 76–98% based on data from four Member States (MSs). Similarly, a 3‐log10 reduction in broiler caecal concentrations was estimated to reduce the relative EU risk of human campylobacteriosis attributable to broiler meat by 58% (95% CI 16–89%), compared to a relative risk reduction estimate of more than 90% in four MSs, which was found previously.
Overall, the ranking of control options was informed by three different evidence streams: effect of control options to reduce flock prevalence (supported by PAF calculations based on literature data), effect of control options to reduce the concentrations in broiler caeca (supported by estimates obtained by a combination of models) and effect of control options directly obtained from literature (not supported by either PAF or modelling). Also, the evidence from regional studies and laboratory experiments had to be translated into EU wide effects in field conditions, and the current application of control measures, as well as the modelling assumptions, had to be taken into account when assessing the effectiveness of the control options. Therefore, expert judgement was required for ranking the control options considering the associated uncertainties. The Panel agreed on the use of a structured approach, based on EFSA's (2014) guidance on expert knowledge elicitation (EKE), to ensure that all the identified evidence and uncertainties were considered in a balanced way and to improve the rigour and reliability of the judgements involved.
The effectiveness of 20 control options if implemented by all broiler farms in the EU, taking into account the current level of implementation, was estimated using a two‐step EKE process informed by the results from modelling of the updated scientific evidence, literature review (including the previous EFSA opinion) and also the experts’ knowledge and experience. Within the time frame of this opinion, experts made selections through the first step where all the options were considered and for the second step where eight control options were prioritised for further assessment of the magnitude of their effects.
In the first step of the EKE, for each of the control options, experts (i.e. working group members and selected EFSA staff) individually estimated the probability that the relative risk reduction would be larger than 10%. This 10% was chosen for its discriminative power in differentiating between the effectiveness of control options. The relative risk reduction was judged to have a higher probability to be larger than 10% for 12 control options: hygienic anterooms at broiler house entrance; no animals in close proximity of the broiler houses; employing few and well‐trained staff; addition of disinfectants to drinking water; avoiding drinkers that allow standing water; effective cleaning and disinfection between flocks; reduced slaughter age; discontinued thinning; designated tools for each broiler house; feed and water additives; bacteriophages and vaccination. The remaining eight control options, which were judged to have a lower probability to give more than 10% relative risk reduction included: effective rodent control; adjusting downtime between flocks; fly screens and keeping insects out of the broiler house; clean or amended litter; stocking density and flock size; the number of houses on site; selective breeding and feed structure.
From the 12 selected control options, eight options were selected for risk prioritisation based on the quality of evidence available and practical feasibility in the implementation of the control option.
The median values of the relative risk reduction of the eight prioritised control options were judged to be as follows; vaccination 27% (90% probability interval (PI) 4–74%); feed and water additives 24% (90% PI 4–60%); discontinued thinning 18% (90% PI 5–65%); employing few and well‐trained staff 16% (90% PI 5–45%); avoiding drinkers that allow standing water 15% (90% PI 4–53%); addition of disinfectants to drinking water 14% (90% PI 3–36%); hygienic anterooms at broiler house entrance 12% (90% PI 3–50%); designated tools per broiler house 7% (90% PI 1–18%). It was not possible to rank the selected control options according to effectiveness based on the EKE judgements because there is a substantial overlap of the probability intervals, due to the large uncertainties involved.
There are advantages and disadvantages associated with each control option. The advantages include ease of application (e.g. hygiene barrier, adding additives to feed), improved bird health (e.g. biosecurity actions), better broiler welfare (e.g. discontinued thinning), cross‐protection against other pathogens (e.g. drinking water treatments, feed additives). The disadvantages for a given control option may include a requirement for investment (e.g. if structural changes are required to install an anteroom), lack of control (e.g. the farmer may not own the fields adjacent to the broiler house and therefore cannot prevent other animals being close by), reduced broiler growth due to decreased consumption of feed and/or water (e.g. if an additive affected the sensory (odour, taste or appearance) properties making the feed or water less palatable).
Multiple control activities are expected to have a higher effect preventing Campylobacter spp. from entering the broiler house and infecting the birds. To minimise the risk of Campylobacter colonisation, all control activities relating to biosecurity would have to be implemented in full. It is not possible to reliably assess the effect of combined control activities because they are inter‐dependent and there is a high level of uncertainty associated with each. Some control options enhance while others reduce the effect of others. Combining two control measures targeting prevalence and concentration, respectively, may result in an additive effect, if their specific targets are unrelated.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The 2012 EFSA opinion on the public health hazards to be covered by inspection of poultry meat1 identified the need to address Campylobacter spp. as a high priority. Since 2005, Campylobacter spp. was the most frequently reported food‐borne pathogen in the EU (more than 200,000 confirmed cases per year). The reported number of confirmed cases of campylobacteriosis represented almost 70% of the 13 reported confirmed human zoonoses in the EU in 2016. Most patients are young children and elderly people. Its occurrence was high in broiler flocks (27.3%) and in fresh meat from broilers (36.7%) in Europe. In 2010, EFSA published an opinion on ‘Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU’2 and estimated that broiler meat may account for 20–30% of campylobacteriosis cases in humans, while 50–80% of these may be attributed to the chicken reservoir as a whole.
In April 2011, EFSA published an opinion on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’.3 Taking into account EFSA's recommendations on poultry meat inspection, the Commission proposed the introduction of a process hygiene criterion for Campylobacter spp. on poultry carcases to be respected in slaughterhouses. If not respected, the criterion leads to corrective measures taken to improve both slaughter hygiene and farm biosecurity.
Secondly, the revision of poultry meat inspection includes enhanced control of Campylobacter spp. (and Salmonella spp.), in line with the high priority set by the EFSA opinion on poultry meat inspection. Competent authorities must sample themselves for these pathogens or carefully verify the implementation of the process hygiene criterion by the operator.
According to the 2011 EFSA opinion, the public health benefits of controlling Campylobacter spp. in primary broiler production are expected to be greater than control later in the chain as the bacteria may also spread from farms to humans by other pathways than broiler meat. Nevertheless, limited information was available about such pathways, and quantification of the impact of interventions at farm level was done only for broiler meat‐related cases.
Since 2011, new scientific information is available on this matter (e.g. CAMCON, CAMPYBRO, CAMPYSAFE, CAMPYLOW projects). Thus, the time is right to request an update of the assessment of the impact of interventions at farm level and identify effective control options at primary production.
Examples of some relevant projects are provided below:
-
–
CAMCON project (https://cordis.europa.eu/project/rcn/95053 en.html), looking at Campylobacter control at primary production level (2010–2015), with participants from different countries. Publications derived from this project are available at https://www.vetinst.no/camcon-eu;
-
–
CAMPYBRO project (http://campybro.eu), looking at Campylobacter control at primary production through nutrition and vaccination, with several partners including producers.
-
–
CAMCHAIN project (http://gtr.rcuk.ac.uk/projects?ref=BB%2FK004514%2F1) looking at transmission of Campylobacter at primary production level;
-
–
CAMPYSAFE and CAMPYLOW projects looking at the use of probiotics to control Campylobacter populations;
-
–
Other scientific papers are also reported in the literature since 2011.
Terms of Reference (ToR)
To further support food business operators in the fight against Campylobacter at farm level, in accordance with Article 29(1) (a) of Regulation (EC) No 178/2002, the Commission requests EFSA to provide an update of the scientific opinion on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’, more in particular to review, identify and rank the possible control options at primary production level, taking into account, and if possible quantifying, the expected efficiency in reducing human campylobacteriosis cases. Advantages and disadvantages of different options at primary production should be assessed, as well as the possible synergic effect of combined control options.
1.2. Interpretation of the Terms of Reference
In 2011, EFSA published a scientific opinion on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’. The aim of this mandate was interpreted to be to review and update the 2011 opinion but focussing on control options at primary production of broiler chickens in the EU/EEA.
Moreover, it was considered that the mandate included a quantitative assessment of the effectiveness of control options at primary production using risk assessment models and expressing the relative risk reduction in terms of the number of cases of human campylobacteriosis attributable to the consumption of broiler meat from the EU that could be avoided if a specific control option is implemented in all farms across Member States in EU. While the cost of implementation or feasibility of control options was outside of this remit, the advantages and disadvantages of each were discussed. Throughout the opinion, control options were assessed in terms of effectiveness. The efficiency was considered in the section discussing advantages and disadvantages of control options. ‘Biosecurity’ was defined as in the previous opinion to be, ‘a set of preventative measures implemented to reduce the risk of transmission of infectious disease from reservoirs of the infectious agent to the target host’.
To address the different parts of the ToR, assessment questions (AQ) have been formulated as:
AQ: What new scientific evidence about control options has become available since the previous opinion of 2011 and what is their relative risk reduction on campylobacteriosis?
AQ: What is the ranking in terms of effectiveness of the selected control options in reducing human campylobacteriosis cases at the primary production level?
AQ: What are the advantages and disadvantages of the selected control options?
AQ: What would be the effect of combining control options?
1.3. Additional information
1.3.1. Previous scientific opinions of the BIOHAZ Panel
In 2008, the European Commission requested EFSA to deliver a scientific opinion on the quantification of the risk posed by broiler meat to human campylobacteriosis in the EU, which was expressed as a percentage of the total number of human campylobacteriosis cases. It was concluded that handling, preparation and consumption of broiler meat may account for 20% to 30% of human cases of campylobacteriosis, while 50% to 80% may be attributed to the chicken reservoir as a whole. However, the conclusions of this scientific opinion had to be interpreted with caution because, as stated in that opinion, data for source attribution in the EU were limited and unavailable for the majority of Member States (MS) and there were indications that the epidemiology of human campylobacteriosis differed between regions. Among several recommendations, the BIOHAZ Panel recommended the establishment of active surveillance of campylobacteriosis in all MS and also to quantify the level of under‐ascertainment and underreporting of the disease, in order to more precisely estimate the burden of the disease and facilitate evaluation of the human health effects of any interventions (EFSA BIOHAZ Panel, 2010).
In 2011, EFSA delivered a scientific opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. A quantitative microbiological risk assessment (QMRA) model was used to estimate the impact on human campylobacteriosis arising from the presence of Campylobacter spp. in the broiler meat chain. The model also used available quantitative data to rank/categorise selected intervention strategies in the farm to fork continuum. At the primary production level, the quantitative risk assessment concluded that there was a proportionate relationship between the prevalence of Campylobacter spp. in broiler flocks and public health risk from broiler meat. The opinion described how reducing either the level of Campylobacter spp. in chicken caeca or the prevalence of positive flocks could reduce the risk to humans. The risk reduction associated with interventions in primary production was expected to vary considerably between MS. Reducing the numbers of Campylobacter spp. in the caeca at slaughter by 3 log10 units was estimated to result in a reduction of the public health risk by at least 90%. However, no feasible intervention that would achieve a reduction in the level of Campylobacter spp. in chicken caeca was identified. The models calculated that in one MS (among four MS used in the model), a 50–90% risk reduction could be achieved using fly screens in conjunction with other strict biosecurity measures. Primary production interventions assessed included fly screens (in one MS), biosecurity (in one MS), earlier slaughter and discontinued thinning. Their impact was estimated as a reduced incidence of campylobacteriosis in humans attributable to the consumption of broiler meat. However, data were sparse, introducing uncertainty in the estimates and the applicability for all EU MS was also uncertain. Thus, it was recommended that individual MS pilot any control measure before full implementation to assess the efficiency in that specific environment (EFSA BIOHAZ Panel, 2011).
Following the previous opinions, a request from the European Commission in 2010 asked EFSA to deliver a scientific opinion on the public health hazards (biological and chemical, respectively) to be covered by inspection of poultry meat and to consider any implications for animal health and animal welfare of any changes proposed to current meat inspection methods. For biological hazards, a decision tree was developed and used for risk ranking poultry meat‐borne hazards. The ranking was based on the magnitude of the human health impact, the severity of the disease in humans, the proportion of human cases that can be attributed to the handling, preparation and consumption of poultry meat and the occurrence of the hazards in poultry flocks and carcasses. Campylobacter spp. and Salmonella spp. were considered to have high public health relevance for poultry meat inspection. As none of the main biological hazards of public health relevance and associated with poultry meat can be detected by traditional visual meat inspection methods, the BIOHAZ Panel proposed the establishment of an integrated food safety assurance system including improved food chain information (FCI) and risk‐based interventions (EFSA BIOHAZ Panel, 2012). A series of recommendations were made regarding biological hazards in relation to data collection, interpretation of monitoring results, future evaluations of the meat inspection system and hazard identification/ranking, training of all parties involved in the poultry carcass safety assurance system and needs for research on optimal ways to use FCI and approaches for assessing the public health benefits.
1.3.2. Legal background
A Process Hygiene Criterion (PHC) (Commission Regulation (EU) 2017/1495 of 23 August 2017 amending Regulation (EC) No 2073/2005) for Campylobacter spp. came into effect in January 2018. The objective of the PHC is to control contamination of carcases during the slaughtering process through monitoring and taking corrective actions when the mandated targets are breached. These actions include, in the case of unsatisfactory results (from 1.1.2020, if 15 out of 50 samples of carcasses after chilling have counts > 1,000 CFU/g) improvements in the slaughter hygiene, the review of process controls and improvement in the biosecurity measures in the farms of origin.
1.3.3. Approach to answer the term of reference
A literature search focussing on the time period after the previous scientific opinion (i.e. between 2011 and 2019) was carried out to update the scientific opinion on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain.’
The effectiveness of control options was estimated using two different modelling approaches for (a) control options reducing the Campylobacter spp. prevalence in broiler flocks sent to slaughter and (b) control options reducing the Campylobacter spp. concentration in their caecal content. The modelling steps used to estimate the effect on public health by reducing Campylobacter spp. flock prevalence (1–2) and concentrations in caecal content (3–5) are illustrated in Figure 1.
Figure 1.
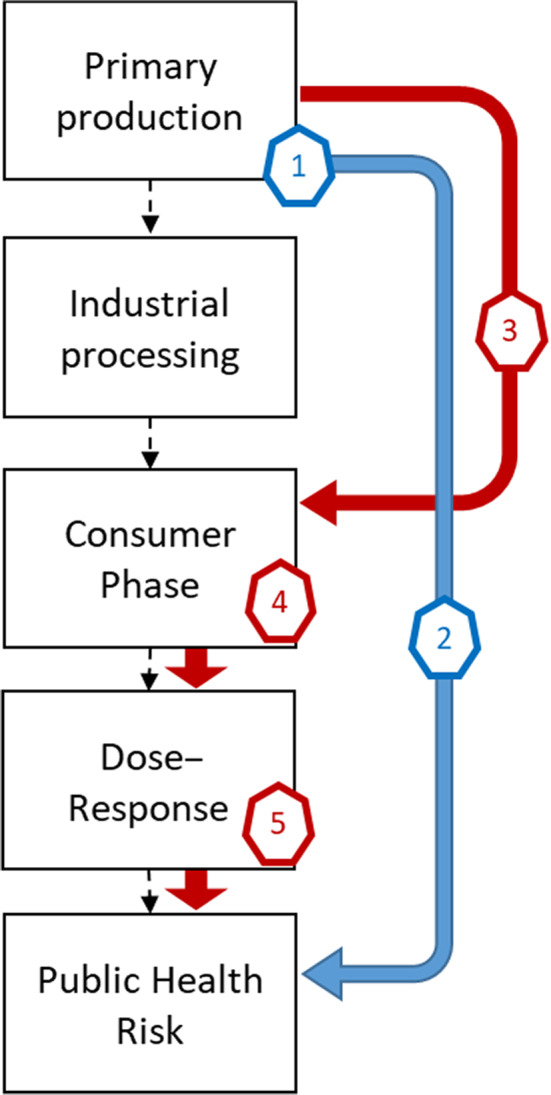
Modelling steps used to estimate the effect on public health by reducing Campylobacter spp. flock prevalence (blue arrow) and concentrations in caecal content (red arrows) in broilers. The numbers show the steps used in the two models, respectively
The reduction of flock prevalence was estimated by using (1) population attributable fractions (PAF)4 derived from epidemiological risk factors studies and (2) assuming a proportionate relationship between flock prevalence and public health risk (see Section 2.2 for details).
The effect of reduced Campylobacter spp. concentrations was modelled by (3) a regression model relating the concentration in the caeca with the concentration on the broiler meat, (4) a consumer phase model describing the effect of food preparation and (5) a dose response (DR) model (see Section 2.3 for details). In both approaches, the effectiveness of the control options is expressed as the relative risk reduction, that is the relative reduction in the incidence of human campylobacteriosis caused by the consumption of broiler meat, if the control option is implemented at all farms in the EU:
where
-
–
RRR is the relative risk reduction,
-
–
Inccurr is the current incidence of human campylobacteriosis attributable to broiler meat in the EU,
-
–
Incint is the new estimated incidence of human campylobacteriosis attributable to the consumption of broiler meat after implementation of the control option at all farms in the EU.
The ranking of control options is based on the assessment of their potential effectiveness, using Expert Knowledge Elicitation (EKE) to combine the different streams of evidence and consider their associated uncertainties.
Advantages and disadvantages of selected control options were assessed using literature search and expert judgement.
The impact of combinations of two or more selected control options, including the potential for synergism, was assessed based on the literature search and expert judgement.
2. Data and methodologies
2.1. Literature search
A literature search was undertaken, focusing on papers published between 2011 and 2019 (inclusive) on Campylobacter, risk factors and control options on broiler farms. In addition, manual searching of the reference list of these documents was performed to identify additional relevant information. This was supplemented by relevant published studies identified by the members of the Working Group (WG) and EFSA Biological Hazards (BIOHAZ) Panel throughout the term of the mandate until the WG members were satisfied that a thorough coverage of the subject had been achieved.
2.2. Modelling the effect of control options to reduce the prevalence of Campylobacter spp. in broilers
2.2.1. Literature search to inform calculation of population‐attributable fractions (PAF)
For the analysis of the PAF, the timeframe searched was expanded to 2004–2019 (inclusive) because epidemiological risk factor studies are limited and PAF analyses had not been carried out in the previous opinion. Only those studies that contained all of the following were included: [1] presentation of multi‐variable model outputs including estimates to calculate adjusted odds ratio (OR) and confidence intervals; [2] descriptive data showing the proportion of farms/flocks in the different categories, [3] data on biosecurity/on‐farm practices and [4] the sample size was ≥ 35 study units.
The search string used was: ((campylobacter AND (risk AND factor*) AND (farm* OR husbandry* OR (primary AND production*)) AND (chicken* OR broiler* OR poultry OR Gallus gallus)) and yielded 245 studies.
Abstracts of these papers were screened and, if no multivariable analysis was reported, the studies were excluded because, if the model does not adjust for confounding effects, where farming practices interact and are associated with each other (Stafford et al., 2008), the risk factor specific estimates are likely to be highly distorted. Overall, 31 studies were retained.
Full papers of these 31 studies were assessed and excluded if they did not contain all of the following: [1] presentation of multi‐variable model outputs including estimates to calculate adjusted OR and confidence intervals; [2] descriptive data showing the proportion of farms/flocks in the different categories and [3] data on biosecurity/on‐farm practices. Studies were also excluded if the sample size was less than 35 study units. This resulted in 17 studies in 15 articles for inclusion in the PAF analysis (see Table 6 in chapter 3.3.1).
Table 6.
Overview of the selected studies for calculation of the population attributable fraction for control options to reduce the prevalence of Campylobacter spp. in the primary production of broilers
| Reference | Country | Year of study | Number of flocks/farms | Campylobacter spp. prevalence |
|---|---|---|---|---|
| Allain et al. (2014) | France | 2008 | 121/121a | 71.9 |
| Barrios et al. (2006) | Iceland | 2001–2003 | 1091/36 | 15.4 |
| Borck Høg et al. (2016) | Denmark | 2010–2012 | 3328/104 | 9.5 |
| Borck Høg et al. (2016) | Norway | 2010–2012 | 1381/173 | 3.5 |
| Bouwknegt et al. (2004) | Netherlands | 1997–2000 | 495/461a | 26.3 |
| Chowdhury et al. (2012) | Denmark | 2009–2010 | 2835/187 | 14 |
| Ellis‐Iversen et al. (2009) | United Kingdom | 2003–2006 | 603/137 | 34.2 |
| Hansson et al. (2010) | Sweden | 2005 | 37/37 | 56.8 |
| Jonsson et al. (2012) | Norway | 2002–2007 | 18488/623 | 4.7 |
| Lyngstad et al. (2008) | Norway | 2005 | 131/131 | n.a. |
| McDowell et al. (2008) | Northern Ireland | 2001–2002 | 388/88 | 42 |
| Näther et al. (2009) Summer | Germany | 2004–2005 | 146/146 | 53 |
| Näther et al. (2009) Winter | Germany | 2004–2005 | 146/146 | 34 |
| Refregier‐Petton et al. (2001) | France | 2001 | 75/75 | 42.7 |
| Torralbo et al. (2014) | Spain | 2010–2012 | 291/134 | 62.5 |
Estimated from information provided in the articles.
2.2.2. Calculation of population attributable fractions (PAF)
Control measures to reduce the prevalence in broilers are aimed at preventing the introduction of Campylobacter spp. into the broiler house and the subsequent colonisation of the birds. A wide variety of measures and practices improve hygiene and biosecurity and are described in the literature review (Section 3.3). To assess the magnitude of control obtained by an intervention, studies that measure the prevalence with and without a control option are required. Intervention studies on the effect of improving biosecurity and changing farmer behaviour are limited. Assessing the effect of changes to multiple practices, with large variability in behaviours before and after interventions, varying compliance levels and interaction between practices, requires a very large sample size and repeated sampling to identify even large effects of interventions (Ellis‐Iversen et al., 2008). Therefore, most studies on biosecurity practises and their association with Campylobacter status are designed as risk factor studies providing a cross‐sectional picture of what happens more often during rearing of positive flocks than during rearing of negative flocks. Such epidemiological risk factor studies allow for multivariate modelling and the possibility to adjust for confounding factors and thereby provide an estimate of risk of a specific behaviour or practice.
Epidemiological risk factor studies are by their nature field studies. The data include the heterogeneity, diversity and variability in human and animal populations, e.g. different genetics or behavioural differences, differences in biosecurity practices, etc., reflecting real life. This contrasts with the standardisation used for experimental studies, where the study design usually removes diversity and minimises variability.
The strength of association derived from epidemiological risk factor studies is often much smaller than the measure of the effect of the same control option in an experimental study, due to the variability and heterogeneity of the population in the epidemiological study. Epidemiological risk factor studies are not able to detect small associations, which fully controlled experimental studies may be able to identify with statistical significance. On the other hand, the effects measured in the epidemiological risk factor studies include the heterogeneity of the farm environment, human factors and variety in practices and the results can be extrapolated to ‘real life’ situations with higher certainty than experimental studies in a small designed setting.
To interpret the effect of changing farm management practices on the risk of colonisation of broiler flocks with Campylobacter spp., the ORs can be transformed into PAF. This provides a measure of the proportion of cases (positive flocks) estimated to be linked to a specific risk factor. The World Health Organisation defines PAF as ‘the proportional reduction in population disease or mortality that would occur if exposure to a risk factor were reduced to an alternative ideal exposure’. It is assumed that the interventions are applied on the new farms to a similar degree as on farms that already applied the intervention at the time of the risk factor study. PAF is also applied to food‐borne zoonotic diseases in public health and has been calculated in at least two published studies on Campylobacter spp. in chicken (Georgiev et al., 2017; Rosner et al., 2017). For the scope of this opinion, population disease or mortality is interpreted as colonised broiler flocks, and only modifiable risk factors, for which control options within the farmer's control exist, were considered for the PAF analysis. Non‐modifiable risk factors such as the season or factors related to the geographical location of the farm were excluded. Only control options identified in at least two MS were included.
In epidemiological studies exploring associations with risk factors, there is a marked heterogeneity not only in the risk factors included in the analysis but also in the definition or wording of the risk factors related to the explanatory variables. As an example, a French study (Allain et al., 2014) found ‘No rodent control outside the house’ to be significantly associated with the occurrence of Campylobacter spp. in the broiler flocks while ‘presence of rodents in the poultry house’ was identified as a risk factor in a Spanish study (Torralbo et al., 2014).
Although these definitions relate to two different aspects, they are assessing the presence of the same underlying risk (i.e. presence of rodents); hence, ‘Having an effective rodent control program in place’ is the control practice reducing the risk recommended in both studies. Widening the wording of the control option to address several narrowly defined risk factors may also help account for variability in farming systems within and between MS.
PAF estimates need to be as precise as possible for the control option, despite farming practices which often interact or are associated with each other (confounding) (Stafford et al., 2008). In epidemiological risk factor studies, confounding is adjusted for by statistical modelling, usually by regression analysis, providing adjusted ORs to reduce this bias. In this opinion, we have only used adjusted OR.
From the selected studies, we extracted the following variables: (1) country, (2) number of units in study, (3) year of data collection. We also extracted the following parameters for each modifiable risk factor:
Wording of risk practice
Baseline practice (comparison)
Adjusted OR + confidence intervals
% positive in exposed group
% positive in non‐exposed group
% in study carrying out risk practice
If any of the above estimates were not directly available in the publication, they were calculated by inversing ‘protective ORs’ into ‘risk ORs’ including their confidence intervals or estimated from other model outputs, e.g. the standard error (SE).
After the extraction, adjusted OR and their confidence intervals were transformed to adjusted Relative Risk with confidence intervals using the formula (Equation (1)) from Zhang and Kai (1998).
| (1) |
with P0 the Campylobacter spp. prevalence in the group without implementation of the control option and ORadj the adjusted OR.
If a risk factor was identified as significant and data needed in the equation were available from the literature, the PAF was estimated using Levin's formula (Equation (2), Levin, 1953):
| (2) |
where pexp is the proportion of the population exposed to the risk factor and RRadj is the calculated adjusted relative risk between colonised and non‐colonised flocks as described above (Equation (1)).
Risk factors from all studies were then grouped by the practice or action needed to control the risk. For each control measure the representativeness was considered and factors were only included in the final modelling if PAF estimates were available from at least two MS.
2.2.3. The proportionate relationship between flock prevalence and public health risk
The model assumes that the relative reduction in flock prevalence caused by an intervention at primary production translates directly into a relative reduction in risk from the broiler meat to humans. Hence, with Inccurr and Incint as defined in Section 1.3.3; and FPcurr being the current flock prevalence and FPint being the flock prevalence after implementation of a control option, it is assumed that Incint/Inccurr = FPint/FPcurr.
This assumption is also made in several previous published risk assessment models and in the previous EFSA opinion (Nauta et al., 2009; EFSA BIOHAZ Panel, 2011). The underlying argument is that analyses performed by these risk assessment models suggest that meat from flocks that are not colonised does not add significantly to the public health risk. Even though cross‐contamination between carcases of birds from flocks is known to occur during slaughter, it is assumed to only have a small effect on the public health risk, because Campylobacter spp. concentrations on meat from cross‐contaminated flocks will be several orders of magnitude lower than those from flocks colonised at primary production (Havelaar et al., 2007; Nauta et al., 2009; EFSA BIOHAZ Panel, 2011).
In this opinion, the PAF values were interpreted as the proportional reduction in flock prevalence that would occur if exposure to a risk factor was eliminated (i.e. the associated control option was applied throughout the EU). This means that the PAF is equivalent to: 1−FPint/FPcurr and also to the estimated RRR defined in Section 1.3.3.
2.3. Modelling the effect of control options to reduce the concentration of Campylobacter spp. in broilers
The modelling approach applied for the effect of control options to reduce the concentration of Campylobacter spp. is explained below; details are given in Appendix A.
2.3.1. Literature search
A generic literature search has been carried out to identify studies that analysed the association between concentrations in the caeca of broilers and broiler meat products after industrial processing (see Appendix B).
2.3.2. Linear regression for concentration in caecal content and on skin of broiler meat
A model for the effect of control options that reduce the caecal concentration of Campylobacter spp. in broilers at primary production should describe how the caecal concentrations relate to concentrations on the meat. Two approaches have been used to describe this relationship: (i) the use of QMRA models that describe the dynamics of transfer and survival of Campylobacter spp. through the broiler meat production chain after primary production (Nauta et al., 2009; Chapman et al., 2016); (ii) the study of the association between concentrations of Campylobacter spp. found in the caecal content (usually called ‘caecal samples’) and on the end products (whole carcass, skin or meat samples) after the industrial processing, by means of linear regression. When the two approaches (QMRA and regression models) were compared, the results were similar, even though the QMRA models predicted the relation between the two concentrations as not linear, but J‐shaped (Nauta et al., 2016).
As in the previous opinion (EFSA BIOHAZ Panel, 2011), a linear regression approach was applied using a regression model to translate a change in bacterial concentration in the chicken caeca into a change in concentration on broiler skin, which assumes to represent a change in concentration on the meat. The approach allows the use of observational data from different European studies and does not require assumptions and data interpretation on the complex dynamics of transfer and survival of Campylobacter spp. during industrial processing. As explained in Appendix A, the slope of the regression line is needed to estimate the change in concentrations on broiler skin after industrial processing, given that the mean effect of an intervention (in terms of log reduction) and its standard deviation (expressing the variability in effect) are known. Data on Campylobacter spp. concentration on broiler skin were obtained from the EU baseline survey from 2008 (EFSA, 2010). Although these data are more than 10 years old and do not take into account the improvements in Campylobacter control arising from the measures implemented in MSs since then, they are, none‐the‐less, the only data available for all MSs, obtained using a harmonised sampling strategy.
The results of the literature review for the association between concentrations in the caeca and on skin samples or broiler meat after industrial processing are summarised in Appendix B and Table 1. In total, 15 studies were retrieved, that used different analytical methods for enumeration. Six of those studies reported no significant correlation and nine found values of the slope of the regression line between 0.21 and 1.15. In one of the six largest studies, with more than 50 batches analysed, Reich et al., 2018, found no positive correlation; the four studies publishing a regression line found slopes varying between 0.21 and 0.32. In this assessment, the uncertainty of the slope was expressed as a BetaPert distribution with minimum of 0, maximum of 0.7 and a most likely value of 0.27. This last value corresponds to the slope value obtained by linear regression on the largest data set, from the APHA/FSA monitoring programme for Campylobacter spp. in broiler flocks and broiler carcases in the UK (2012–2017) (FS241051, FS101126). The data set included paired caeca–skin microbiological data (enumeration according to ISO 10272‐2) for 1752 batches slaughtered in different abattoirs in UK.
Table 1.
Summary table of regression lines found in the literature
| Reference | Significant correlation?(a) | Slope | r or r2(a) | Number of batches | Number of slaughter plants |
|---|---|---|---|---|---|
| Allen et al. (2007) | No | – | – | 26 | 4 |
| Boysen et al. (2016) | Yes | 0.70 | r2 = 0.72 | 15 | 3 |
| Brena et al. (2013) | Yes | 0.21 | r2 = 0.23 | 76 | 3 |
| Duffy et al. (2014) | No | – | – | 4 | 2 |
| Elvers et al. (2011) | No | – | – | 5 | – |
| Hue et al. (2011) | Yes | 0.32 | r = 0.33 | 425 (297 pos.) | 58 |
| Laureano et al. (2013) | Yes | 0.28 | r2 = 0.26 | 80 | 10 |
| Malher et al. (2011) | Yes | ND | r = 0.28 | 140 (91 pos.) | 3 |
| Nauta et al. (2009) | No | – | – | 22 | – |
| Reich et al. (2008) | Yes | ND | r = 0.64 | 40 | 1 |
| Reich et al. (2018) | No | – | – | 365 | 3 |
| Rodgers (2020) | Yes | 0.27 | r2 = 0.48 | 1,146 | 19 |
| Rosenquist et al. (2006) | Yes | 1.15 | – | 6 | 2 |
| Stern and Robach (2003) | No | – | – | 20 | 1 |
| Vinueza‐Burgos et al. (2018) | Yes | 0.50 | r2 = 0.57 | 15 | 3 |
An extended version of this table is given in Appendix B.
As reported in the given reference.
r = linear correlation coefficient; r2 = coefficient of determination.
– = not determined or no value given.
2.3.3. Consumer Phase model (CPM)
The consumer phase is the last part in the food chain, where exposure to Campylobacter spp. occurs. As explained in more detail in Appendix A, it describes how the concentration on the skin or on the meat is linked to the dose ingested by the consumer. Food preparation and associated cross‐contamination of other foods have an important effect on the exposure. Modelling the consumer phase is challenging because data are scarce and difficult to obtain, the variability in food handling practices between (groups of) consumers is large and the effect on transfer and survival of Campylobacter spp. is not easily described.
As reviewed by Chapman et al. (2016), a large variety of CPMs are available that may include different routes of Campylobacter spp. transfer (cross‐contamination) and undercooking. Recently, another model estimating the effects of consumer habits during preparation of chicken meat has been proposed (Poisson et al., 2015; ANSES, 2018). From an initial level of contamination of 1.4 log10 CFU/g, the authors estimated a risk reduction of 50% if 100% of consumers adopted best practice during preparation or the initial load is reduced to 0.4 log10 CFU/g. A comparative analysis, Nauta and Christensen (2011) studied eight different CPMs and compared their performance in terms of the predicted effect of intervention measures before the consumer phase on the risk estimates. It was found that the difference between the relative risk estimates of the different models is often small. A CPM that performs intermediately (i.e. providing results falling in the range of the others) is based on the observational data described by Nauta et al. (2008) and has been applied in the previous EFSA opinion as the ‘classic +’ DR model (EFSA BIOHAZ Panel, 2011; Nauta et al., 2012).
In this opinion, the eight available CPMs (Christensen et al., 2001; Mylius et al., 2007; van Asselt et al., 2008; Brynestad et al., 2008; Calistri and Giovannini, 2008; Lindqvist and Lindblad, 2008; Nauta et al., 2008; WHO, 2009) that were compared by Nauta and Christensen (2011) were used to evaluate the uncertainty due to the choice of the CPMs. These CPMs apply a broad range of different cross‐contamination scenarios and use different data on food handling practices, routes of cross‐contamination, transfer rates and meat product and/or cross contaminated food product. None of the models specifically addresses undercooking, although it is implicitly included in the Nauta et al. (2008) model, which is solely based on observational data. No additional CPMs were included as the selected eight already represent a large variety of models and it appeared that the choice of the CPM had only limited impact on the uncertainty of the results (Nauta and Christensen (2011); see also the tornado plots for correlation coefficients in Appendix C).
2.3.4. Dose response model
A dose response model describes how an ingested dose relates to the probability of infection and/or the probability of illness in humans. Until now, the majority of Campylobacter QMRA studies, including the previous EFSA opinion (EFSA BIOHAZ Panel, 2011), have applied the ‘classic’ dose response model published by Teunis and Havelaar (2000), based on a human challenge study (Black et al., 1988) in which the strain A3249 was used. After an analysis of several additional data sets from both human and primate challenge studies, as well as a set of outbreak studies, the choice of this model was recently criticised by Teunis et al. (2018) as ‘an unfortunate choice’ as the default model for many risk assessments for Campylobacter spp., because A3249 seems to be of low virulence and is therefore a non‐representative strain. Therefore, next to the classic dose response model, two additional dose response models were used that were considered representative for the challenge studies and the outbreak studies reported by Teunis et al. (2018), as explained in Appendix A.
2.3.5. Selection of control options
A set of control options affecting the concentrations in the caecal content was selected, based on the literature study as described in Section 2.1.
Control options to reduce Campylobacter spp. concentration in the caeca were primarily selected based on new information that became available since the EFSA 2011 Opinion was published. From these, several control interventions (vaccines, prebiotic/other feed additives and bacteriophages) were selected for inclusion in model analysis based on: [1] evidence that the control activity had a reductive effect on the Campylobacter spp. concentrations in the caeca and/or faeces; [2] type of study (experimental and field trials using broilers); [3] inclusion of control (untreated) birds in the study; [4] sufficient data including mean log reduction and standard error (or equivalent); [5] number of birds included in the trial and frequency of sampling (statistically‐ based experimental design), [6] samples tested (at least caecal content samples); and [7] the length of the trial including the period of sampling (close to field practices, 35–42 days).
From the selected studies, the most and least effective values of different control options found (i.e. for vaccines and feed additives) were selected for modelling. The values were used to indicate the range of potential beneficial effects found in the literature, but references to specific vaccines or feed additives are not made, as the reproducibility of the quantitative results obtained by simulations in the present opinion, if applied in field conditions, remains unknown.
2.3.6. Model description and implementation
Appendix A provides a more detailed description of the modelling approach for the effect of control options to reduce the concentration of Campylobacter spp. in broilers. The linear regression model, consumer phase model and DR model are combined and implemented in an Excel spreadsheet (Nauta, 2020), using the model implementation approach described by Nauta and Christensen (2011).
The model allows an estimation of the RRR to be made based on a mean and standard deviation of the effect of the intervention in terms of log reduction in caecal concentration (d and sd); the regression line slope (a); the choice of an EU MS or the EU average (for current concentrations of broiler skin after processing (mskin and sskin)); a value of the log difference between skin samples and meat (τ); the choice of a CPM; the choice of a DR model. A version of the model implemented in @Risk version 7.6 allows analysis of the uncertainty by providing the uncertainty of the input parameters a, d, and τ, as well as the uncertainty in the choice of the MS (mskin and sskin), the choice of the CPM and the choice of the DR. Variability is included into the model analytically, uncertainty is modelled by Monte Carlo simulation, using 250.000 iterations per simulation.
Table 2 shows the options included in this model. For the uncertainty analysis of the model that informed the EKE, the difference between skin concentrations found in different MSs was not included, as the effect of control options is expressed at EU level.
Table 2.
Overview of the default model values chosen, if uncertainty is not included, and the way the uncertainty about the choice of the model or model parameter is described, if uncertainty is included. The options given in bold are used for the uncertainty analysis that informed the Expert Knowledge Elicitation (the reference model)
| Default values | Reference | Uncertainty | Reference | |
|---|---|---|---|---|
| Slope of regression line caeca – skin samples | a = 0.27 | Rodgers (2020) | BetaPert (0, 0.27, 0.7) | This opinion |
| Log difference between concentration on skin and meat | τ = 1 | EFSA BIOHAZ Panel (2011) | Uniform (0,3) | Nauta et al. (2012) |
| Member States (skin concentrations measured in EU baseline 2008) | EU‐weighted mean | Nauta et al. (2012) (derived from EU baseline study 2008) | Randomly select 1 Member State | Nauta et al. (2012) (derived from EU baseline study 2008) |
| Consumer phase model | Nauta et al. (2008) | EFSA BIOHAZ Panel (2011) | Randomly select one of eight models | Nauta and Christensen (2011) |
| Dose response model | Classic model | EFSA BIOHAZ Panel (2011) | Randomly select ‘classic’ or ‘median challenge’ model | This opinion |
A sensitivity analysis was performed to evaluate the effect of different modelling choices on the estimated effects of the selected control options.
2.4. Ranking and Uncertainty assessment
The ToR required a ranking of the possible control options at primary production level, and, if possible, quantifying the expected effectiveness of control actions on the broiler farm in terms of reducing human campylobacteriosis cases. Uncertainties affecting both the effectiveness estimates and the ranking of the different control options were considered by application of the framework provided by EFSA's uncertainty guidance (EFSA Scientific Committee, 2018).
For some control options, the expected reduction in campylobacteriosis cases could be estimated in one of two ways: calculate the PAF for control options that reduce the prevalence of Campylobacter spp. in broilers (Section 2.2); or by means of quantitative probabilistic modelling for control options aimed at reducing the level of intestinal Campylobacter spp. concentrations in broilers (Section 2.3). Statistical uncertainty affecting these estimates was described and quantified by confidence intervals for the PAF and sensitivity analysis for the stochastic model (See Appendix C). Other uncertainties are addressed as described below.
The expected effect of the control options in reducing the incidence of human campylobacteriosis in EU by all MS was made by means of expert judgement; thus taking into account sources of systematic uncertainty arising when trying to infer the analytical evidence from the PAF analysis and simulation model at EU level. Expert judgement was also used to take additional evidence on these control options from the 2011 Opinion and subsequent publications (for which PAF could not be calculated or quantitative effects could not be simulated) into account.
Ranking of control options was therefore informed by three different evidence streams: effect of control options to reduce prevalence (supported by PAF and literature), effect of control options to reduce the level of contamination in broilers (supported by probabilistic modelling) and the effect of control options from the scientific literature (not supported by either PAF or probabilistic modelling). Expert judgement was therefore essential for ranking the control options, both for weighing and integrating the different streams of evidence and for considering their associated uncertainties. The BIOHAZ Panel agreed on the use of a structured approach, based on EFSA's (2014) guidance on expert knowledge elicitation (EKE), to ensure that all the identified evidence and uncertainties were considered in a balanced way and to improve the rigour and reliability of the judgements involved.
Due to the large number of control options to be considered within the time available, the EKE was conducted in two steps to allow for greater focus on the control options that were more likely to be effective. In the first step, experts assessed the probability that each control option would, if implemented by all EU broiler producers, reduce the incidence of campylobacteriosis in the EU by at least 10%. This step provided an initial ranking that was used to identify a subset of control options for more detailed assessment in the second step.
In the second step, experts assessed the magnitude of reduction in campylobacteriosis in the EU that each of the prioritised control options would achieve, if implemented by all EU broiler producers. In both steps, each control option was assessed separately, assuming all other control options remained at their present level of implementation. The detailed process is described in Section 3.5, together with the results and in Appendix D.
In both steps, the expert judgements were made by members of the EFSA Working Group and EFSA staff that were involved in the drafting of this Opinion. When assessing the effects of control options, all the relevant evidence available to the Working Group was considered. This included evidence from the 2011 Opinion, evidence from scientific literature published since 2011 and results from both the modelling approaches. The sources of systemic uncertainty identified by the Working Group as relevant to the assessment of the control options were also considered. To help experts take all the relevant evidence and uncertainties into account in a balanced way, the Working Group prepared two tables summarising key aspects of the evidence on control options affecting concentrations and prevalence, and a third table summarising the identified sources of uncertainty (Appendix D).
2.4.1. Step 1. Screening of all control options
A list of 21 control options was agreed to be considered for ranking. This list resulted from the review of the 2011 opinion and updating the information provided (Section 2.1), the PAF analysis (Section 2.2) and modelling (Section 2.3):
Reduced slaughter age
Discontinued thinning
Employing few and well‐trained staff
Vaccination
No animals in close proximity to the broiler houses
Feed and water additives
Avoiding drinkers that allow standing water
Bacteriophage
Addition of disinfectants to drinking water
Designated tools per broiler house
Effective cleaning and disinfection
Hygienic anterooms at broiler house entrance
Selective breeding
Effective rodent control
Fly screens and keeping insects out of the broiler house
Stocking density and flock size
Downtime between flocks
Feed structure
The number of houses on site
Clean litter
Litter amendments
In the first step of EKE, for each control option, each expert assessed the probability that it would reduce the campylobacteriosis incidence in humans in the EU associated with the preparation and consumption of broiler meat by 10% or more, if implemented by all broiler farmers in the EU. The threshold was set at 10% to obtain sufficient discriminatory power in the EKE. If a lower threshold was used (i.e. 5%), most of the control options would have been considered as potentially effective, undermining the identification of the most promising control options that would be subject to detailed assessment in step 2. Note that the 10% was not meant to indicate a threshold level in terms of effectiveness and does not imply that 10% would be a sufficient reduction or not. To ensure that questions for eliciting probability judgements were well defined (EFSA, 2014), it was agreed to define the question for step 1 in more detail as follows:
What is the probability that, if the specified control option was implemented by all broiler producers in the EU that are not currently using it, the average annual incidence of campylobacteriosis cases in the whole EU population caused by Campylobacter spp. in broiler meat produced from chickens raised in the EU would reduce by more than 10% (compared to the current level), all other things being equal?
The following subsidiary definitions were used:
The meaning of ‘campylobacteriosis cases’ was clear for the experts and does not require further definition.
‘Other things being equal’ includes other control options remaining at the current level of implementation, production and processing practices remain unaltered and no change in the consumption in the EU of meat from broilers raised in the EU.
If a control option acts on both prevalence and concentration, both should be considered when answering the question.
For each control option, the experts answered the question assuming that, of the specific practices for this control option which are referred to in this Opinion (e.g. different vaccines, or different methods of rodent control), the practice that would, on its own, achieve the largest reduction in campylobacteriosis would be implemented by all EU broiler producers.
Experts expressing their judgement as precise or ranges of probabilities (see Table 3) (EFSA Scientific Committee, 2018).
Table 3.
Approximate probability scale adopted for harmonised use in EFSA (EFSA Scientific Committee, 2018)
| Probability term | Subjective probability range | Additional options | |
|---|---|---|---|
| Almost certain | 99–100% | More likely than not: > 50% |
Unable to give any probability: range is 0–100% Report as ‘inconclusive’, ‘cannot conclude’ or ‘unknown’ |
| Extremely likely | 95–99% | ||
| Very likely | 90–95% | ||
| Likely | 66–90% | ||
| About as likely as not | 33–66% | ||
| Unlikely | 10–33% | ||
| Extremely unlikely | 1–5% | ||
| Almost impossible | 0–1% | ||
2.4.2. Step 2. Detailed assessment of selected control options
The subset of the control options to be prioritised for detailed assessment in step 2 was informed by experts’ discussion upon results of step 1, the quality of evidence available, practical feasibility in the implementation of the control option and unequivocal definition of the control measure.
Selection resulted in eight control options (i.e. discontinued thinning, employing few and well‐trained staff, vaccination, feed and water additives, avoiding drinkers that allow standing water, addition of disinfectants to drinking water, designated tools per broiler house, hygienic anterooms at broiler house entrance) for which it was considered that the second step of EKE process could be done within the time frame of this opinion.
The question for the experts to consider in the second step was:
If the specified control option is implemented by all broiler producers in the EU that are not currently using it, what will be the resulting percentage reduction (compared to the current level of implementation) in average annual incidence of campylobacteriosis cases in the whole EU population caused by Campylobacter spp. in broiler meat produced from chickens raised in the EU, other things being equal?
The individual experts were asked to quantify their uncertainty about the percentage reduction in the form of a probability distribution, elicited by a version of the Sheffield or SHELF protocol (EFSA, 2014; Oakley and O'Hagan, 2019) adapted to the needs of the current assessment. Each probability distribution was assessed by estimating by a median, lower bound and upper bound, and the two remaining quartiles.
Thereafter, the judgements of the different experts were aggregated for each control option by calculating the equal‐weighted linear pool of the distributions providing the best fits to the individual judgements, using the SHELF software app for multiple experts.5 The linear pool distributions were plotted together with the individual expert distributions (See Appendix D) and discussed in the expert group. Following the discussions, the group of experts decided on a consensus distribution for the effect of each of the eight control options considered.
Finally, the resulting consensus distributions reflecting the uncertainty of the estimated effectiveness of each of the prioritised control options were presented in figures and tables that included uncertainty around each estimate (See Section 3.5).
3. Assessment
3.1. Update on broiler production
The EFSA BIOHAZ Panel (2011) describes the broiler meat production chain in the EU in detail.
Production of poultry meat in the EU increased from ~ 12 million tons in 2008 to ~ 15 million tonnes in 2018, comprising largely broilers (75% in 2008 and 83% in 2018), followed by turkeys (16% in 2008 and 13% in 2018) and ducks (~ 3%). Based on FAO and OECD data from 2017, the EU is the third largest poultry meat producer in the world (US 21.3 million tonnes, followed by China 17.0 million tonnes and EU 15.9 million tonnes, at an estimated world production of 118.1 million tonnes) (Damme et al., 2017).
Export of poultry meat increased from 1.3 million tonnes in 2014 to 1.5 million tonnes in 2017 (Table 4). The level of imported poultry meat remained the same during this time period (0.82 million tonnes in 2014 and 0.83 million tonnes in 2017). The rate of self‐sufficiency was estimated at 104.7% (2017) (AVEC, 2018).6
Table 4.
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|---|---|
| Gross production in the EU | 12,716 | 12,805 | 13,263 | 13,788 | 14,495 | 14,570 | 15,248 | 15,557f |
| Export | 1,324 | 1,311 | 1,361 | 1,508 | 1,679 | 1,671 | 1,780 | n.a. |
| Import | 841 | 791 | 821 | 875 | 902 | 807 | 813 | n.a. |
| Consumption | 12,233 | 12,285 | 12,719 | 13,254 | 13,831 | 13,827 | 14,457 | 14,761b |
| Consumption per head, kg | 21.3 | 21.3 | 22.1 | 22.9 | 23.9 | 24.1 | 25.0a | 25.5a |
n.a.: data not available.
Estimated.
Forecast.
Poultry meat consumption per capita in the EU was 24.1 kg in 2017 (22.1 kg in 2014) with large differences between Member States (e.g. 36.2 kg in Portugal vs. 20.8 kg in Italy). Broiler meat consumption dominated (19.4 kg per head), followed by turkey meat (4.0 kg per head) (Damme et al., 2017).
Six Member States account for 71% of the total EU poultry meat production (PL‐16%, UK 13%, FR 11%, ES 11%, DE 10%, IT 9%) (Figure 2) (Eurostat, 2018).8
Figure 2.
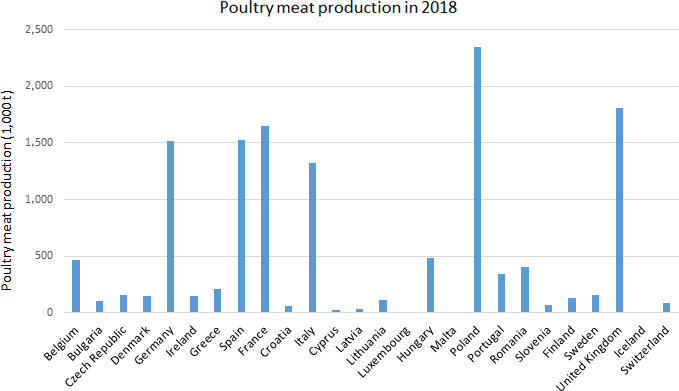
Poultry meat production in 2018 (source: Eurostat)5 (Estimated: Croatia; Provisional: Spain, France; Confidential: Estonia, The Netherlands, Austria, Slovakia; Not available: Liechtenstein, Norway)
The EU statistics showed a 14% yearly increase in the overall organic poultry production between 2005 and 2015 (European Commission, 2016).9 An EU‐wide baseline survey on Campylobacter spp. in broiler batches and on broiler carcasses was carried out in 2008 (results are given and analysed in EFSA BIOHAZ Panel, 2010). Although these data are out of date and pre‐date major Campylobacter control initiatives in many MSs, it is nonetheless the most recent EU‐wide study. The prevalence of Campylobacter spp. colonisation in broiler batches was 71% (determined from caecal contents) and the prevalence of Campylobacter‐contaminated broiler carcasses was 75.8% (based on neck and breast skin samples).
In general, large differences were seen among MSs, with prevalence in broiler batches ranging from 2.0% to 100.0%, and prevalence on broiler carcasses ranging from a 4.9% to 100.0%.
Table 5 shows the prevalence of Campylobacter spp. in broiler meat and broilers based on data reported in the annual European Union summary reports on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks. It appears that the Campylobacter spp. prevalence in broilers and on broiler meat has remained quite constant over the years. However, since the number of countries reporting prevalence data changed over the years, the data are not directly comparable.
Table 5.
Campylobacter spp. prevalence (% positive (total tested)) in the EU, 2010–2018
| Year | Campylobacter spp. prevalence % (n) | |||
|---|---|---|---|---|
| Fresh broiler meat at retail | Fresh broiler meat at processing plant | Broilers (flock based) | Reference | |
| 2018 | 37.5 (7,441)a | 26.0 (13,636) | EFSA and ECDC (2019) | |
| 2017 | 37.4 (13,445)a | 12.3 (10,077) | EFSA and ECDC (2018) | |
| 2016 | 36.7 (11,495)a | 27.3 (13,558) | EFSA and ECDC (2017) | |
| 2015 | 59 (3,652) | 37.7 (297) | 15.3 (7,033) | EFSA and ECDC (2016) |
| 2014 | 36.4 (1,570) | 9.9 (1,248) | 27.2 (9,907) | EFSA and ECDC (2015a) |
| 2013 | 25.2 (3,102) | 12.0 (1,904) | 15.1 (n. n.) | EFSA and ECDC (2015b) |
| 2012 | 24.9 (3,495) | 15.8 (2,049) | 13.2 (6,001) | EFSA and ECDC (2014) |
| 2011 | 34 (5,059) | 29.3 (1,260) | 17.8 (6,656) | EFSA and ECDC (2013) |
| 2010 | 22.9 (3,508) | 44.65 (1,010) | 18.2 (9,212) | EFSA and ECDC (2012) |
Not specified in the corresponding report.
3.2. Update on risk factors for Campylobacter spp. in broiler production
There are multiple sources and dissemination routes for Campylobacter spp. on broiler farms. The EFSA ‘Campylobacter in broiler meat’ Opinion (EFSA BIOHAZ Panel, 2011) identified several risk factors for Campylobacter spp. in primary production. All those risk factors were reviewed and, when there was new information (since 2011), updated. Factors for which there was more recent information included vertical transmission, slaughter age, season, thinning, Campylobacter‐contaminated drinking water, a previous Campylobacter‐positive flock in the house (carry‐over) and the use of therapeutic antimicrobials for treatment. The remaining risk factors either did not have new information or were discussed in terms of control options (Section 3.3).
3.2.1. Vertical transmission
Vertical transmission is the internal contamination of the egg within the genital tract and before intact shell deposition. As stated in the last EFSA opinion (EFSA BIOHAZ Panel, 2011), vertical transmission does not appear to be an important risk factor for broiler colonisation with Campylobacter spp. Recent articles reported no evidence of vertical transmission of Campylobacter spp. from hatching eggs to commercial flocks (Battersby et al., 2016b; Tangkham et al., 2016; Colles et al., 2019).
3.2.2. Slaughter age
Most conventionally housed broilers test Campylobacter‐negative for the first 21 days of rearing (EFSA BIOHAZ Panel, 2011). This may be due to maternal antibodies and/or the inability of current testing methods to detect low concentrations of the organism in a small subset of the flock population. Slaughter age is still found among the risk factors identified in epidemiological investigations with conventionally produced birds almost always having higher prevalence and caecal concentration towards the end of the production cycle (Sommer et al., 2013; Legaudaite‐Lydekaitiene et al., 2017; Higham et al., 2018). Higham et al. (2018) showed that age was significantly positively associated with Campylobacter spp.
3.2.3. Season
It is still generally agreed that season is a risk factor with increased prevalence in broilers in the summer months. This was reflected in the EU baseline survey which suggested an increased risk in the period of July–September as compared to January–March (EFSA BIOHAZ Panel, 2011). Based on data collected in six EU MSs, the CAMCON project (Sommer et al., 2016a) found that seasonality affected prevalence values and so did the monthly mean temperatures reported in the study. Although the reason for this observation is not clear, and different studies report conflicting findings, enhanced survival of Campylobacter spp. in the farm environment during the warmer months, more water consumption by the birds, heat stress of the birds and/or increased numbers of insects and dust from the neighbouring environment entering the broiler house (facilitated by increased additional ventilation), may be some of the factors contributing to the increased prevalence of Campylobacter spp. in broilers during the warmer months.
3.2.4. Thinning
Thinning or partial depopulation is a practice where a subset of the birds is removed for slaughter and processing, leaving the remaining birds to grow to the required size (Allen et al., 2008). Sometimes several rounds of thinning are performed in a flock (Millman et al., 2017). The 2011 EFSA Opinion highlighted thinning as a major risk factor and this is supported by research since then. Lawes et al. (2012) and Millman et al. (2017) reported thinning as a risk factor for Campylobacter spp. infection as it breaches the biosecurity barrier and catching crews, cages, modules and trucks visit multiple farms. Catching crews bring Campylobacter spp. into the house on their boots, forklift trucks or broiler harvester machines, trolley wheels, crates etc. (Ellis‐Iversen et al., 2012; Smith et al., 2016; Georgiev et al., 2017). In addition to breaching biosecurity, thinning also stresses the birds and the presence of stress hormones in the gastrointestinal tract may promote the growth, proliferation and virulence of Campylobacter spp. (Aroori et al., 2014). Thus, Campylobacter spp. introduced at thinning, spread rapidly throughout the flock reaching levels in the birds of up to 108 cells per gram of caecal matter within 4–5 days after thinning (Koolman et al., 2014). In a recent investigation, Higham et al. (2018) confirmed previous findings and showed that thinned houses had a 309% increase in the odds of being highly contaminated and concluded that further investigations are required to elucidate farm and individual factors related to thinning (Higham et al., 2018).
3.2.5. Campylobacter spp. contaminated drinking water
Contaminated water was previously considered an important source of Campylobacter spp. on farms (EFSA BIOHAZ Panel, 2011). Campylobacter spp. are commonly found in surface water on farms (Mughini‐Gras et al., 2016) and, if used without proper treatment, water may serve as a vehicle of transmission (Jonsson et al., 2012; Agunos et al., 2014; Allain et al., 2014; Torralbo et al., 2014; Borck Høg et al., 2016).
3.2.6. A previous Campylobacter‐positive flock in the house (carry‐over)
When a flock is Campylobacter‐positive the broiler house and surrounding environment is often heavily contaminated and, if not cleaned and disinfected properly between flocks, a previous positive flock will become an important source of Campylobacter spp. for the new flock (EFSA BIOHAZ Panel, 2011). More recent studies also support this conclusion. Damjanova et al. (2011) and Battersby et al. (2017) both demonstrated that inadequate cleaning and disinfection was a risk factor in the spread of Campylobacter spp. from one flock to the next. Other studies detected the same genotypes of Campylobacter spp. in broiler house samples before or during flock placement (Allen et al., 2011; Damjanova et al., 2011). Broiler house surroundings such as the tarmac apron, anteroom, house door, feeders, drinkers, walls, columns, barriers and bird scales may act as a source of direct or indirect infection resulting in carryover of persistent genotypes through several rearing cycles (Battersby et al., 2016b). The failure to eliminate Campylobacter spp. at these sites between flocks has been attributed to a range of factors including the design of feeders and drinkers, insufficient down time, a lack of knowledge or utilisation of proper cleaning methods and bacterial resistance to the disinfectants used (Agunos et al., 2014).
3.2.7. The use of therapeutic antimicrobials
The EFSA 2011 Opinion reported conflicting results on the effect of antibiotics on Campylobacter spp. carriage and shedding, with Herman et al. (2003) observing no effect while Refregier‐Petton et al. (2001) concluded that administering antibiotics to the birds decreased the risk of colonisation with this organism. Similar results were reported by Allain et al. (2014). These conflicting results may be due to the resistance of Campylobacter spp. to the antimicrobial administered or that Campylobacter spp. was introduced after the treatment. More recent research examining the effect of antibiotic treatments on the microbiome of the broiler gastrointestinal tract is similarly inconclusive (Allen and Stanton, 2014; Mancabelli et al., 2016). The use of antimicrobials to reduce Campylobacter spp. carriage and shedding may induce resistance in bacteria colonising the birds and is contrary to current EU policy to reduce antibiotic usage in animal husbandry. Therefore, it is not be considered further in this opinion.
3.2.8. Concluding remarks
New information was published since the EFSA 2011 Opinion that provides additional evidence indicating slaughter age, season, thinning, contaminated drinking water and carry‐over from a previous flock are still important risk factors for Campylobacter spp. colonisation of a broiler flock. Vertical transmission does not seem to be a relevant risk factor and the results on the use of antimicrobials are still inconclusive.
3.3. Control options to reduce the prevalence of Campylobacter spp. in broilers
In the following section, the updated knowledge on the expected effect of the individual biosecurity options that can be implemented at primary production level to control for the identified risk factor is summarised. In Section 3.3.1, the results of the control options associated with the risk factors for which PAF could be calculated are presented. In Section 3.3.2, the observed effects of the control options associated with the risk factors for which PAF could not be calculated are provided.
3.3.1. Calculation of population attributable fraction for control options
The systematic literature search resulted in 17 eligible studies published in 15 articles, as one of the articles (Borck Høg et al., 2016) presented two studies: one carried out in Norway and one in Denmark and another article presented both a summer and a winter study in Germany (Näther et al., 2009). Two studies were further excluded, because they investigated fewer than 10 farming practices resulting in difficulties excluding confounding or interaction with other known risk practices on the farms (Lawes et al., 2012; Sandberg et al., 2015). The remaining studies had examined multiple risk factors on more than 2,400 farms, calculated adjusted ORs and presented descriptive results to provide estimates for the PAF analyses. The 15 studies are described in Table 6.
Of the six main poultry producing MS, four were represented in the study data (FR, UK, ES, DE). Half of the studies were conducted in the Nordic countries, over‐representing this region of the EU that had a lower prevalence of Campylobacter spp. than the rest of the MS in the 2008 baseline survey (Table 5).
Modifiable risk factors from all studies were grouped by the practice or action needed to control the risk and for which PAF was calculated. Control options were only included in the final modelling if the risk factor had been identified in at least two MS.
The PAF analysis identified six control practices with sufficient evidence including [1] Hygienic anteroom at broiler house entrance; [2] effective rodent control; [3] no animals in close proximity of the poultry houses; [4] having few and well‐trained staff; [5] adding disinfectants to drinking water and [6] avoiding drinkers that allow standing water.
The PAF was calculated for each risk factor for each study and interpreted as the reduction factor, i.e. the level of reduction in prevalence, if all broiler farms in the EU implemented the control practice effectively. The results are presented in Figure 3 and discussed below.
Figure 3.
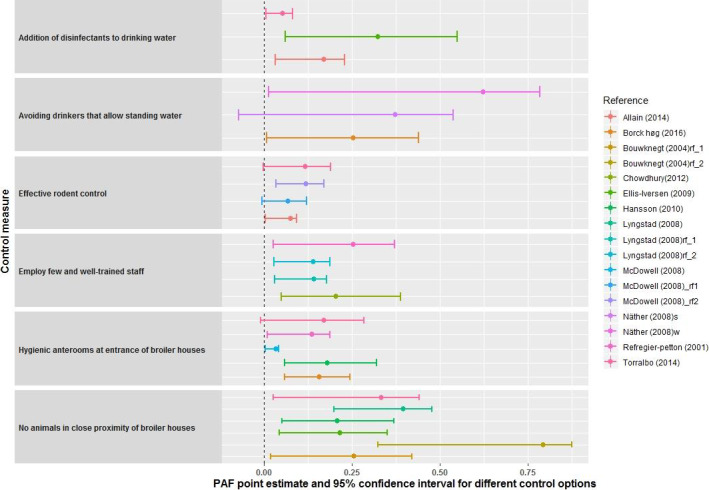
Population Attributable Fractions (PAF) point estimate values and 95% confidence intervals for six control measures with evidence for PAF calculation from the risk factor studies carried out in EU MSs
3.3.1.1. Addition of disinfectants to drinking water
Contaminated water was previously considered an important source of Campylobacter spp. on farms in contrast, to feed, where the lack of moisture creates a hostile environment for Campylobacter spp. (EFSA BIOHAZ Panel, 2011). Campylobacter spp. are commonly found in surface water on farms (Mughini‐Gras et al., 2016) and, if used without proper treatment, water may serve as a vehicle of transmission (Jonsson et al., 2012; Agunos et al., 2014; Allain et al., 2014; Torralbo et al., 2014; Borck Høg et al., 2016).
The PAF analysis suggested that adding organic acids, chlorine‐based biocides or hydrogen peroxide to the drinking water could reduce the risk of Campylobacter‐positive flocks up to 55% (Table 7). Mainly chlorination (UK, ES) and acidification (FR) were found to reduce the risk, but in Spain, hydrogen peroxide added to drinking water was also reported as common and effective. Other studies did not identify a significant effect and, again, this is probably due to the variation in practices such as concentration and type of additive, baseline cleanliness of water, temperature and many other factors. In addition, one French study (Refregier‐Petton et al., 2001) found that acidification of drinking water was a risk factor for the presence of Campylobacter spp., but this was most likely due to poor hygiene.
Table 7.
The effect of implementing the control option ‘Addition of disinfectants to drinking water’ on the Campylobacter spp. prevalence of broilers
| Estimated reduction in prevalence (PAF) | 95% confidence interval of PAF | Risk factors identified in studies in the EU | Study location | References |
|---|---|---|---|---|
| 32.4% | 6.0–54.9 | Non‐chlorinated water | United Kingdom | Ellis‐Iversen et al. (2009) |
| 5.3% | 0.6; 8.2 | Untreated water | Spain | Torralbo et al. (2014) |
| 17.1% | 3.3; 23.0 | No acid in drinking water | France | Allain et al. (2014) |
PAF: population attributable fraction.
3.3.1.2. Avoiding drinkers that allow standing water
Drinker types have been associated with a risk of positive poultry flocks, and studies suggest that removing cups, trays and other parts that allow standing water that may enhance the transmission, could reduce the risk up to 79% in Denmark and Germany (Table 8). PAFs could not be calculated for the recent multicountry (ES/UK/DK/NL/PL/NO) study (Sommer et al., 2016b); however, the authors identified drinkers with cups as significantly associated with increased risk. This finding is supported by Borck Høg et al. (2016) who also found that drinker nipples with cups were a risk factor, when compared to nipples without cups. Sommer et al. (2016a,b) estimated a reduction of up to 24% in human campylobacteriosis, if the broiler industry used drinking nipples without cups.
Table 8.
The effect of implementing the control option ‘Avoid drinkers that allow standing water’ on the Campylobacter spp. prevalence of broilers
| Estimated reduction in prevalence (PAF) | 95% confidence interval of PAF | Risk factors identified in studies in the EU | Study location | References |
|---|---|---|---|---|
| 37.2% | 0; 53.9 | Nipple drinker with tray | Germany | Näther et al. (2009) Summer |
| 62.4% | 1.2; 78.5 | Nipple drinker with tray | Germany | Näther et al. (2009) Winter |
| 25.4% | 0.8; 44.0 | Drinker with cups | Denmark | Borck Høg et al. (2016) |
PAF: population attributable fraction.
3.3.1.3. Effective rodent control
If effective rodent control was achieved on all broiler farms in the EU, the overall prevalence of Campylobacter spp. in EU flocks could decrease by up to 19% according to the studies in Table 9. The risk factors that were identified in Spain and Northern Ireland were farmers reporting the presence of rodents during the production cycle and/or rodent droppings were observed at a sampling visit. The French study measured whether rodent control was applied outside the house and found that lack of control was a risk factor.
Table 9.
The effect of implementing the control option ‘Effective Rodent Control’ on the Campylobacter spp. prevalence of broilers
| Estimated reduction in prevalence (PAF) | 95% confidence interval of PAF | Risk factors identified in studies in the EU | Study location | References |
|---|---|---|---|---|
| 7.5% | 0.4; 9.3 | No Rodent control outside house | France | Allain et al. (2014) |
| 11.8% | 3.4; 17.1 | Rodents seen by sampler | Northern Ireland | McDowell et al. (2008) |
| 6.7% | 0.0; 12.1 | Farmer reported rodents | Northern Ireland | McDowell et al. (2008) |
| 11.7% | 0.0; 18.9 | Presence of rodents in shed | Spain | Torralbo et al. (2014) |
PAF: population attributable fraction.
In a French study of outdoor reared birds (Huneau‐Salaun et al., 2007), rodent control carried out by contractors instead of farmers was found to be a risk factor, possibly due to the infrequent bait changes by the contractor. Since the study was for outdoor flocks, it was not included in the PAF analysis. In the CAMCON study, rodent control was not included in the final analyses due to too many missing values (Sommer et al., 2016a).
3.3.1.4. Employing few and well‐trained staff
Farm workers, maintenance personnel and catching crews who move between and within farms are a source of Campylobacter spp. dissemination via contaminated boots and poor hygiene practices. Thus, farm staff are often the vehicle of Campylobacter spp. carriage into the broiler house (Battersby et al., 2016a). In the study by Van Limbergen et al. (2018), the parameter ‘visitors and staff’ scored the lowest, suggesting that better education of the staff may help to improve the overall biosecurity on broiler farms in Europe. Similar research by Ansari‐Lari et al. (2011) using 100 flocks on 100 commercial broiler farms reported that the odds of flock infection decreased when the level of education of the farmers increased.
The PAF analysis suggested that restricting the number of employees and only using permanently or long‐term employed staff may reduce the prevalence by up to 40% (Table 10). Researchers in both France and Denmark found that having more than one person managing a flock increased the risk of Campylobacter spp. infection in the broilers. In Norway, the risk increased if the farmer hired staff and/or allowed external staff in the shed at bird delivery.
Table 10.
The effect of implementing the control option ‘Employing few and well‐trained staff’ on the Campylobacter spp. prevalence of broilers
| Estimated reduction in prevalence (PAF) | 95% confidence interval of PAF | Risk factors identified in studies in the EU | Study location | References |
|---|---|---|---|---|
| 14.2% | 2.9; 17.7 | Personnel in house at delivery | Norway | Lyngstad et al. (2008) |
| 13.9% | 2.7; 18.6 | Hired caretaker staff | Norway | Lyngstad et al. (2008) |
| 25.3% | 2.7; 37.1 | ≥ 2 persons taking care of broilers | France | Refregier‐Petton et al. (2001) |
| 20.5% | 5.0; 38.8 | ≥ 2 persons entering houses | Denmark | Chowdury et al. (2012) |
PAF: population attributable fraction.
3.3.1.5. Hygienic anterooms at broiler house entrance
Pre‐2011 research demonstrated the importance of anterooms. This describes a room between the outside door and the entry to the production area where the birds are housed. The anteroom may be separated into a ‘dirty’ zone (area closest to the outside door) and a clean zone (area closest to the door to the broiler production area) where the farmer changes footwear and puts on clean house‐specific overalls and washes hands before accessing the production area. Ensuring that anterooms are clean and function as an effective division, may result in between 5% and 13% reduction in the Campylobacter spp. flock prevalence.
The PAF analysis highlighted differences in the use and effectiveness of anterooms in different MS (Table 11). In Spain, for example, the absence of an anteroom in some broiler houses was identified as a risk factor. The studies from Denmark and Northern Ireland rated the anterooms and divided them into categories and in both countries; untidy and low‐quality anterooms were risk factors. In France, litter beetles in the changing room were associated with a higher risk of Campylobacter‐positive flocks. This observation was probably primarily a proxy for insect infestation within the broiler houses, but also a sign of unclean changing/anterooms. PAFs could not be calculated for all countries in the recent multi‐country (ES/UK/DK/NL/PL/NO) study (Sommer et al., 2016b), but the absence of an anteroom plus barrier (no effective biosecurity at the entrance of the houses) was identified as significantly associated with increased risk.
Table 11.
The effect of implementing the control option ‘Hygienic anterooms at shed entrance’ on the Campylobacter spp. prevalence of broilers
| Estimated reduction in prevalence (PAF) | 95% confidence interval of PAF | Risk factors identified in studies in the EU | Study location | References |
|---|---|---|---|---|
| 17.0% | 0.0; 28.4 | No anteroom in poultry house | Spain | Torralbo et al. (2014) |
| 3.3% | 0.4; 4.2 | Tidiness and cleanliness could be improved | Northern Ireland | McDowell et al. (2008) |
| 13.6% | 0.9; 18.8 | Presence of litter‐beetles in anterooms | France | Refregier‐Petton et al. (2001) |
| 15.6% | 5.9; 24.4 | Low quality anteroom | Denmark | Borck Høg et al. (2016) |
| 18% | 5.9;31.9 | General tidiness score reflecting a combination of a number of hygiene parameters | Sweden | Hansson et al. (2010) |
3.3.1.6. No animals in close proximity of the broiler houses
Animals in adjacent fields may be a source of Campylobacter spp. in broilers. Several studies have applied typing methods to test for the presence of the same clones, sequence types, etc. in broilers and other animals in the immediate vicinity of the poultry farm (Ellis‐Iversen et al., 2012; Mughini Gras et al., 2012; Sheppard and Maiden, 2015; Weis et al., 2016). In general, relatively small numbers of isolates are tested and even when the same strain is detected, the results are inconclusive as the direction of spread is unknown. Some Campylobacter strains are capable of colonising several different farm animal species (Ellis‐Iversen et al., 2012; Mughini Gras et al., 2012; Sheppard and Maiden, 2015; Weis et al., 2016) although not all studies find direct matches (Agunos et al., 2014).
While this might suggest that other livestock on the farm rarely act as a source of Campylobacter spp. in broilers, the inability to match strains between farm animal species could also be due to one flock, herd or even one animal carrying several genotypes of Campylobacter spp. at once and most studies normally look at only one isolate (Grove‐White et al., 2011; Sheppard and Maiden, 2015). In contrast, Ridley et al. (2011) used molecular typing to demonstrate similar Campylobacter strains in the broilers and in the cattle around the farm and, as these were first detected in the dairy herd, concluded that horizontal transmission from the cattle to the birds was a risk factor for colonisation of the broilers.
Nonetheless, epidemiological studies repeatedly identify other animals on or next to the farm as significant risk factors. It is likely that they play a role in maintaining the Campylobacter spp. between flocks. If other animals are kept away from broiler houses or surrounding areas, the risk of Campylobacter‐positive flocks can be reduced by up to 88%, but most likely up to 45%. This is a broad category as it includes both pets (Spain), pigs (NL), cattle (UK) or just a broad category of other livestock (NL; SE) on same or adjacent premises (Table 12). The PAF could not be calculated for the Norwegian study (Jonsson et al., 2012) investigating the presence of livestock farms within 2 km from poultry farms, but the variable was identified as a risk factor in the multivariate model.
Table 12.
The effect of implementing the control option ‘No animals in close proximity of broiler houses’ on the Campylobacter spp. prevalence of broilers
| Estimated reduction in prevalence (PAF) | 95% confidence interval of PAF | Risk factors identified in studies in the EU | Study location | References |
|---|---|---|---|---|
| 25.5% | 1.8; 42.1 | Other animal species on farm | Netherlands | Bouwknegt et al. (2004) |
| 79.3% | 32.3; 87.5 | Other animals within 1 km radius | Netherlands | Bouwknegt et al. (2004) |
| 21.5% | 4.4; 35.0 | Cattle adjacent | United Kingdom | Ellis‐Iversen et al. (2009) |
| 39.5% | 19.8; 47.8 | Pigs closer than 2 km | Norway | Lyngstad et al. (2008) |
| 33.4% | 2.6; 44.1 | Presence of dogs/cats at farm | Spain | Torralbo et al. (2014) |
| 20.9% | 5.0;37.0 | Other livestock on farm | Sweden | Hansson et al. (2010) |
3.3.2. Control options for which the population attributable fraction cannot be calculated
The PAF can only be estimated from studies that provide an adjusted risk estimate from a properly designed epidemiological risk factor. However, some other types of studies also identify 10 other risk factors that are summarised below.
3.3.2.1. Effective cleaning and disinfection
As described in Section 3.2.6, cleaning a broiler house after the flock has been harvested requires the removal of the waste materials, which are often heavily contaminated with Campylobacter spp. from the bird faeces. During this operation, the external area immediately adjacent to the main doors is often heavily contaminated. Effective cleaning and disinfection therefore require that the inside of the broiler house (including feeders and drinkers which are not designed to facilitate cleaning) and the concrete apron outside the main door to be effectively disinfected. This is often not done properly, resulting in a risk of carry‐over between flocks (EFSA BIOHAZ Panel, 2011; Damjanova et al., 2011; Battersby et al., 2016b).
There is a wide range of different cleaning agents and biocides commercially available for eliminating Campylobacter spp. in the broiler house between flocks. However, these vary in their effectiveness. Battersby et al. (2017) examined six different treatment combinations, applied as per manufacturer's instructions, and found that thermal fogging with the combination of potassium peroxymonosulfate, sulfamic acid and sodium chloride (5% v/v) or a glutaraldehyde and quaternary ammonium complex (0.3%, v/v) were effective treatments.
3.3.2.2. Downtime between flocks
The downtime between flocks is an important consideration linked to biosecurity. Pre‐2011, research data were contradictory on whether longer or shorter periods of time between cleaning and disinfection of the house and restocking reduced the risk of Campylobacter spp. infection in the subsequent flock (Hald et al., 2000; Lyngstad et al., 2008). In theory, longer periods should result in greater reduction of any Campylobacter spp. present as this organism is a poor competitor in the environment and sensitive to desiccation. However, longer periods also increase the chances of Campylobacter spp. ingress into the house, if strict biosecurity is not maintained. Based on the data reported by Borck Høg et al. (2016) and Georgiev et al. (2017), a downtime of more than 2 weeks would increase the risk of the next flock becoming infected with Campylobacter spp. Similarly, Sommer et al. (2016b) suggested a downtime of less than 10 days was best and would translate into a 2–8% reduction in the human incidence of campylobacteriosis if this was applied across the whole of the poultry industry.
3.3.2.3. Fly screens and keeping insects out of the broiler house
In previous studies, insects, especially flies, were considered as potential carriers of Campylobacter spp. into the broiler house (EFSA BIOHAZ Panel, 2011). Since then, there have been few, if any, new studies to either confirm or contradict this conclusion. The long‐term effect of fly screens in Denmark was studied by Bahrndorff et al. (2013) in a 4‐year intervention study with 10 fly‐screened and 10 control farms. They found that there was no effect of applying fly screens in winter but that, in fly screen houses, Campylobacter spp. prevalence did not peak during the summer. Royden et al. (2016) investigated the role of flies in the transmission of Campylobacter spp. to broilers and found that despite the low prevalence of Campylobacter spp. cultured from flies, the risk of transmission could be high, especially during summer when fly populations are greatest.
3.3.2.4. Clean litter and litter amendments
Dry clean litter was not considered to be a source of Campylobacter spp. although these organisms could survive in contaminated litter especially if it became wet (EFSA BIOHAZ Panel, 2011). Recent research supports this finding and fresh or dry litter is still not considered to be a source of Campylobacter spp. introduction into the broiler house, but wet litter may play a role in the survival and spread of these bacteria (Robyn et al., 2015).
Zhang et al. (2016) added sodium bisulfate to litter in order to reduce ammonia concentration and emissions. While multiple litter amendment applications significantly reduced ammonia emissions, there was no effect on Campylobacter spp. concentration. Similarly, applying a mixture of microorganisms (EM), Nuengjamnong and Luangtongkum (2014)reported that a product containing lactic acid bacteria, yeasts, Bacillus spp. and Actinomycetes was beneficial in terms of reducing ammonia in poultry houses, but there was no reduction in Campylobacter spp.
3.3.2.5. Stocking density and flock size
There is little new research on the association between flock Campylobacter positivity and stocking density or flock size that would contradict evidence previously reported (EFSA BIOHAZ Panel, 2011). Stocking density can influence the levels of stress experienced by the birds. Borck Høg et al. (2016) reported a reduced risk associated with a high stocking density in Denmark, but not in Norway, probably due to farms applying stricter management routines and a higher level of biosecurity.
3.3.2.6. The number of broiler houses on site
The 2011 EFSA Opinion reported that the risk of Campylobacter spp. colonisation increased with the number of broiler houses on site but the association between the degree of risk and the number of houses was unclear. Interestingly, adjacent flocks are still considered to be a risk for Campylobacter spp. transmission to new flocks (Newell et al., 2011; Agunos et al., 2014) and the range of influence was reported to range from 9 to 14 km between positive farms in Denmark (Chowdhury et al., 2013).
3.3.2.7. Reduced slaughter age
The prevalence of flock positivity is directly related to slaughter age and reducing the slaughter age could be an effective action to reduce Campylobacter spp. prevalence in flocks (EFSA BIOHAZ Panel, 2011). This conclusion was based on the analysis of the EU baseline data (EFSA, 2010) and suggested that the overall incidence of campylobacteriosis in the human population would be reduced by 21% to 43% if the slaughter age was reduced to 28 days. Berndtson et al. (1996) reported a twofold increase in flock positivity after 42–44 days and a fourfold increase after 48–61 days. Kalupahana et al. (2013) demonstrated that it is at least 14 to 21 days before Campylobacter spp. are detected even on broiler farms with poor biosecurity. The EFSA BIOHAZ Panel (2011) found that reducing the slaughter age to 35 days would not have any effect in one MS, where the current average slaughter age was 32 days, but in three other MSs, this was estimated to result in a relative risk reduction of 9–18%. Using a similar approach, in the CAMCON project, van Wagenberg et al. (2016) estimated that if all flocks were slaughtered by 35 days or less, there would be a reduction in human campylobacteriosis of 10–18%.
3.3.2.8. Discontinued thinning
Thinning presents a high risk of introducing Campylobacter spp. into the broiler house and stopping thinning would reduce this risk (EFSA BIOHAZ Panel, 2011). This is supported by Georgiev et al. (2017), who demonstrated that discontinuing thinning would reduce the number of contaminated broiler flocks by at least one‐third.
The EFSA BIOHAZ Panel (2011) estimated the effect of a ban on thinning in four MSs and found relative risk reductions between 2% and 25%. The effect of thinning could not be analysed using the data collected in the CAMCON project due to there being too many missing values (Sommer et al., 2016b), but using the approach applied by the EFSA BIOHAZ Panel (2011), the estimated relative risk reduction in the six countries involved varied between 3% and 13% (van Wagenberg et al., 2016).
3.3.2.9. Selective breeding
Selective breeding of Campylobacter‐resistant chickens was suggested by Kaiser et al. (2009) but given the prioritisation of high meat or egg production, it is considered a long‐term goal (EFSA BIOHAZ Panel, 2011). However, findings from a recent paper concluded that Campylobacter spp. load in broilers could have a low but significant heritability estimate (0.095 +/− 0.037), which indicated a limited genetic basis and that non‐genetic factors have a greater influence on the level of Campylobacter spp. found in the broiler chicken (Bailey et al., 2018). In another paper comparing Campylobacter spp. colonisation between four breeds (two broilers and two layers), it has been found lower prevalence and count of Campylobacter spp. and a quicker decrease in the shedding by the layer breeds than in broiler lines (Hankel et al., 2018).
3.3.2.10. Designated tools per broiler house
The presence and use of designated tools per broiler house, meaning that tools commonly used in broiler houses are applied in one broiler house only, were found to be an efficient control option in the CAMCON project (Sommer et al., 2016b). The estimated relative risk reduction of the application of designated tools in the six countries involved in this project varied between 1% and 16% (van Wagenberg et al., 2016). It was the most cost‐effective control option analysed in their study.
3.3.3. Concluding remarks
Compared to the 2011 opinion, the PAF approach was applied to convert results of epidemiological studies into quantitative control option effect estimates. European epidemiological risk factor studies on Campylobacter spp. in broilers were identified and when data were available, PAF was calculated for risk factors that could be associated with specific control options. This improved the interpretation of the available evidence basis and added new and more precise estimates of the effect of individual biosecurity practices to control Campylobacter spp.
PAF were calculated for six control options from several studies: hygienic anteroom, effective rodent control, having no animals in close proximity to the broiler houses, addition of disinfectant to drinking water, employing few and well‐trained staff and avoiding drinkers that allow standing water. The variation was greater between the different control options than for the same control options in different studies, which increased the confidence in the extrapolation potential of the results to the EU.
When PAF was calculated for independent studies across several locations in Europe, the majority of the point estimate values were of similar magnitude for control options such as rodent control, hygienic anterooms, employing few and trained staff and no animals in close proximity to the broiler houses in all broiler farms in the EU. This adds confidence to the validity of the associations and the potential of the control options to reduce Campylobacter spp. prevalence in the EU.
According to the PAF analyses, the mean relative risk reductions that could be achieved by adoption of each of these six control options individually are estimated to be substantial but the width of the confidence intervals of all control options indicates a high degree of uncertainty in the specific risk reduction potentials. For example, the mean estimate of the relative risk reduction for the control option ‘Addition of disinfectants to drinking water’ was between 5 (95% CI 0.6–8.2) and 32% (95% CI 6.0–54.9) based on three available studies.
3.4. Control options to reduce the concentration of Campylobacter spp. in broiler caeca
3.4.1. Literature update and selection of control options for model analysis
There are several papers reviewing potential Campylobacter control interventions on broiler farms to reduce the concentration of Campylobacter spp. in the birds. Several of these were described in the EFSA 2011 Opinion and new information when available is provided in this section including feed and water additives, bacteriophages, vaccination and feed structure. Information from relevant papers (Appendix E) was used for a final selection of studies to be included in the quantitative analyses.
3.4.1.1. Feed and water additives
A range of different feed and water additives, including biological cultures (probiotics/competitive exclusion), short chain fatty acids, organic acids, potassium sorbate, monocaprin (for water only) and chlorination (also water only) individually and in various combinations, were previously reviewed. They were not included in the quantitative models in the EFSA 2011 Opinion as the evidence behind the reductions achieved in Campylobacter spp. was too inconsistent to warrant consideration. Since then, several papers have been published on feed and water additives. Aguiar et al. (2013) concluded that probiotics may reduce the levels of Campylobacter spp. in broilers but only when administered early in the production period before exposure to Campylobacter spp. Other in vivo challenge studies have also observed reduced Campylobacter spp. caecal counts using probiotic cultures, including Bacillus spp. and Lactobacillus spp. although it was difficult to obtain consistent results (Ghareeb et al., 2013; Guyard‐Nicodème et al., 2016; Schneitz and Hakkinen, 2016; Manes‐Lazaro et al., 2017; Saint‐Cyr et al., 2017; Shrestha et al., 2017). Similar research has been undertaken using bacterial cultures that produce bacteriocins, a heterogenous group of mainly cationic, hydrophobic or amphiphilic peptides produced by a range of bacteria. These molecules cause pore formation in the bacterial cell membrane which usually results in cell lysis (Jozefiak and Sip, 2013). Umaraw et al. (2017) used bacteriocins in drinking water to combat Campylobacter spp. colonisation in broilers, while Messaoudi et al. (2011) reported that bacteriocin production by 3 different strains of Lactobacillus salivarius isolated from chicken caeca were active against both C. jejuni and C. coli. Other researchers had previously used L. salivarius strains in feed or drinking water to reduce the gut concentration of C. jejuni (Stern et al., 2006). Enterococcus durans and Enterococcus hirae also produce bacteriocins that may have potential application in broilers, but to date, no commercial bacteriocin products are available for controlling Campylobacter spp. in poultry (Svetoch et al., 2011) and it is difficult to extrapolate the results of small‐scale laboratory‐based studies to field conditions.
More recent research has also been reported on the potential application of organic and medium chain fatty acids. Haughton et al. (2013) reported reduced Campylobacter spp. concentrations in water treated with a commercial acidification product, but caecal counts were unchanged. In contrast, Jansen et al. (2014) achieved reduced caecal counts when organic acids were added to the drinking water but the Campylobacter spp. concentrations on the broiler carcasses obtained from these birds were unchanged. Other studies have also failed to observe a consistent positive effect (van Bunnik et al., 2012a,b). Hermans et al. (2012) added a mixture of medium chain fatty acids (caproic, caprylic, capric and lauric acid) to the drinking water in pilot studies. After 24 h, none of the 10 test birds that received a dose of 2 × 103 CFU C. jejuni were positive while 60% of the control birds were colonised. After 5 days, Campylobacter spp. colonisation or transmission was not reduced as all the birds were colonised with similar concentrations of Campylobacter spp. in the caeca. Thibodeau et al. (2015) developed and tested a feed additive based on a mixture of essential oils and organic acids which achieved a 0.7 log reduction in the caecal concentration of Campylobacter spp. without impacting on the gut microbiome. Robyn et al. (2013) reported that allicin (a compound produced when garlic is crushed or chopped) in drinking water eliminated Campylobacter spp. in vitro (laboratory studies), but did not have a similar impact in vivo (experimental trials) and attributed this to the presence of mucin‐containing mucus in the caecum. Within the ‘Campybro’ project, 24 different additives belonging to either organic acids, plant extracts or pre‐ and probiotics have been tested in experimental husbandries (Gracia et al., 2016a; Guyard‐Nicodème et al., 2016). Whilst no additive was able to prevent broiler colonisation by Campylobacter spp., some of them showed significant reductions reaching 3 log10 in one experimental trial. However, the main drawbacks found were the non‐reproducibility between trials and the high level of within‐batch variability (Gracia et al., 2016a,b; Guyard‐Nicodème et al., 2016). The same conclusions were reached after field trials using organic acids and a cation exchange clay‐based product in feed (Guyard‐Nicodème et al., 2016; Huneau‐Salaun et al., 2018). Other additives including ferric tyrosine (Skoufos et al., 2019) and a phytophenolic compound b‐resorcylic acid (Wagle et al., 2017) were found to reduce C. jejuni concentrations in the caeca. In the study of Wagle et al., 2017, 2.5 log10 and 1.7 log10 reductions in birds were observed, 14 days after treatment, when the feed additive was applied at 0.5% and 1%, respectively (Wagle et al., 2017). In the study reported by Skoufos et al. (2019), the authors showed a significant 2 log10 reduction in the treated group of broilers.
Considering the criteria reported in Section 2.3.5 on selection of control options, two studies were included in the model analysis. The one from ‘Campybro project’ (Guyard‐Nicodème et al., 2016) from which the least effective (referred to here as FA1, an organic acid) and most effective feed additive (FA2, a prebiotic) were selected, and the study reported by Skoufos et al. (2019) (FA3, an amino acid with iron) (Table 13). Raw data from Guyard‐Nicodème et al. (2016) were reanalysed by a maximum likelihood estimation method that allows distribution fitting in the presence of censored data (Lorimer and Kiermeier, 2007), to obtain estimates for the mean log reduction (d) and the variability therein between applications, as well as the uncertainty of the mean estimate. The result d = 2 found by Skoufos et al. (2019) can be used to compare the results obtained by the model applied here with the results that were obtained by the model that was applied in the previous opinion (EFSA BIOHAZ Panel, 2011).
Table 13.
Effect of three selected feed additives in terms of mean log10 reduction (d) of caecal concentration of Campylobacter spp. and the variability (standard deviation) in the log10 reduction (sd). The standard error of the mean (S.E.M) is used to express the uncertainty about the mean and is used in the uncertainty analysis. NA = Not Available
| Feed additive | d | sd | S.E.M. | Number of birds tested | Reference |
|---|---|---|---|---|---|
| FA1 | 1.23 | 0 | 0.66 | 15 | Guyard‐Nicodème et al. (2016) |
| FA2 | 3.25 | 2.2 | 0.64 | 15 | Guyard‐Nicodème et al. (2016) |
| FA3 | 2 | NA | NA | 36 (3 replicates of 12) | Skoufos et al. (2019) |
NA: Not Available.
3.4.1.2. Bacteriophages
Bacteriophages, also referred to as phages, are viruses that infect bacteria. Several pre‐2011 studies indicated that bacteriophages have potential use as a therapeutic rather than a preventive treatment to reduce the concentration of Campylobacter spp. carriage in caecal contents and faeces immediately before slaughter (Loc Carrillo et al., 2005; Wagenaar et al., 2005; Carvalho et al., 2010; Hermans et al., 2011). Robyn et al. (2015) describe the minimum requirements for a phage treatment to be effective including [1] having a broad spectrum host capable of killing multiple strains of C. jejuni and C. coli; [2] having an obligate lytic cycle; [3] being safe and cost‐effective and [4] being able to overcome host resistance. However, most phages are highly specific in the strains they can infect and as broiler flocks are infected with multiple strains of C. jejuni the results achieved are inconsistent. Kittler et al. (2013) applied a phage cocktail via drinking water. While a 3.2 log10 CFU of caecal contents reduction was achieved in one treatment group, the results were inconsistent and significant reductions were not obtained in other trials. More recent work by Richards et al. (2019) developed a phage cocktail that could achieve a Campylobacter spp. reduction of up to 2.4 log10 CFU of caecal material as compared to the untreated control birds. However, this reduction, achieved 2 days post treatment, was not maintained and the difference between the treated and control birds was reduced to 1.3 log10 CFU/g by 5 days post treatment (no data is provided beyond that time point). Although the authors reported a stable phage titre over time, approximately 10% of the C. jejuni population showed resistance. Sorensen et al. (2015) highlighted the fact that many phages rely on the highly diverse capsular polysaccharide (CPS) for infection that may be different in different Campylobacter spp. species and strains thus limiting the effectiveness of any phage‐based intervention if not developed using multiple phage isolation strains known to carry different CPS variants. In addition to the requirement for multiple phage populations, there are other limitations in phage application including safety (from the human and veterinary health perspective) and practical (effective administration) aspects, logistic problems associated with treatment immediately before harvest and the development of resistant strains. It was therefore concluded that this technology is in the early stages of development and requires more research, including field trials, before it can be considered a viable option for Campylobacter control in broilers. This control option was not included in the model analysis due to the non‐compliance with the selection criteria defined in Section 2.3.4.
3.4.1.3. Vaccination
Pre‐2011, the proof of principle that Campylobacter antibodies (induced by vaccination in chickens), has protective properties, had been demonstrated (Stern et al., 1990) and several vaccination regimes and strategies have previously been tested but without success (EFSA BIOHAZ Panel, 2011). More recently, a range of candidate vaccines have been developed and tested. using as antigens; whole cells, live‐attenuated strains, flagellin, an ATP‐binding cassette (ABC) transporter protein, an outer membrane protein, an aspartate/glutamate‐binding ABC transporter protein, a glutamine‐binding ABC transporter protein, a hemin‐uptake outer membrane protein, an outer membrane component of the CmeABC multidrug efflux pump and a periplasmic protein (Robyn et al., 2015). Although the majority of these achieved partial protection at best, there is no vaccine commercially available yet. However, work is ongoing, and several candidate molecules have shown promise. Okamura et al. (2012) immunised two groups of Jidori chicks subcutaneously with a formalin‐killed C. jejuni with two different adjuvants. Both vaccines induced high levels of anti‐Campylobacter IgG, but in challenge studies, the concentration of the organism in the caeca and faeces remained the same. Annamalai et al. (2013) used poly (lactide‐co‐glycolide) nanoparticle (NP) encapsulated outer membrane proteins (OMP) of C. jejuni that was administered subcutaneously. Seven days after vaccination, Campylobacter were not detected in any of the test groups of broilers. Neal‐McKinney et al. (2014) achieved a 2 log10 reduction in C. jejuni colonisation in specific pathogen‐free (SPF) chickens, using a vaccine based on recombinant surface‐exposed proteins involved in broiler gut colonisation, but field trial validation has yet to be undertaken. Riazi et al. (2013) found that orally administered C. jejuni specific single domain antibodies were effective in preventing C. jejuni colonisation of day‐old SPF broilers but future studies are required over the entirety of the growth cycle. Recently, Meunier et al. (2016) using reverse vaccinology identified 14 new antigens based on their subcellular localization, immunogenicity, B‐cell epitopes density and their sequence conservation among C. jejuni and C. coli strains. Six of them were tested on broilers in experimental husbandries and four candidates showed Campylobacter load decreases in caeca by 2–4 logs CFU/g correlated with the induction of specific humoral responses (Meunier et al., 2017). Liu et al. (2019) used a native protein microarray approach and identified 30 new immunogenic C. jejuni proteins but no information was provided about in vivo testing. Vandeputte et al. (2019) reported that in ovo‐vaccinated birds using vaccines based on maternal antibodies were not protected with the treated birds showing similar caecal C. Jejuni concentrations to the control birds. Adams et al. (2019) developed and tested live‐attenuated Salmonella‐vectored vaccines but found that oral vaccination of the broilers failed to trigger significant systemic and intestinal mucosal immune responses and hence did not confer protection against C. jejuni.
Considering the criteria reported in Section 2.3.4, only one study was included in the model analysis. From the ‘Campybro’ project (Meunier et al., 2017), the most effective (referred to here as VA2, YP9817 protein) and least effective vaccine (VA1, YP562 protein) in terms of caecal log reduction when birds were 42 days of age were selected (Table 14). Raw data from Meunier et al. (2017) were reanalysed by a maximum likelihood estimation method that allows distribution fitting in the presence of censored data (Lorimer and Kiermeier, 2007), to obtain estimates for the mean log reduction (d) and the variability therein between applications, as well as the uncertainty of the mean estimate.
Table 14.
Effect of two selected vaccines in terms of mean log reduction (d) of caecal concentration of Campylobacter spp. and the variability in the log reduction (sd). The standard error of the mean (S.E.M) is used to express the uncertainty about the mean, and is used in the uncertainty analysis
3.4.1.4. Feed structure
Coarse feed materials are retained in the gizzard where they are ground down and exposed to hydrochloric acid. Thus, any feeds that delay the passage of materials through the gizzard may reduce the passage of Campylobacter spp. further along the gastrointestinal tract. This was demonstrated by Moen et al. (2012) who reported that a diet supplemented with 15% oat/barley hulls increased gizzard weight and delayed the spread of Campylobacter spp. within the birds. They concluded that stimulating the bird's natural barriers was an effective means of reducing the spread of Campylobacter spp. in broiler flocks. However, within the Campybro EU project, it was concluded that, feed structure had no significant effect on Campylobacter colonisation (Gracia et al., 2016a,b) and most commercial broiler flocks are already fed a proportion of whole grain within the ration.
This control option was not included in the model analysis due to the lack of evidence on the effect and/or missing quantitative data.
3.4.2. Results of model analysis
The selected control options (feed additives and vaccination) affecting the concentration in the caecal content were analysed and results are shown in Figure 4.
Figure 4.
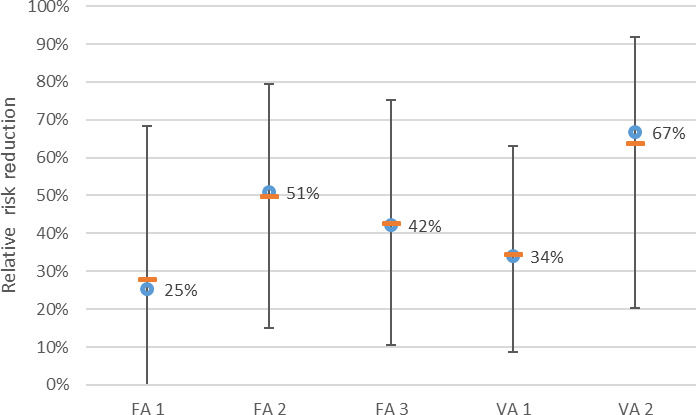
Estimated relative risk reductions achieved by Campylobacter control options ‘feed additives’ and ‘vaccination’, for the selected feed additives (FA) and vaccines (VA) (see Tables 13 and 14). Blue circle: median effect (numeric result given); orange line: mean effect; error bar: 95% confidence interval
According to the model, the most effective feed additive analysed (FA2) gave a median 51% risk reduction (95% CI 15–79%; mean 50%), FA3 gave a median 42% risk reduction (CI 11%–75%; mean 42%) and the low effective feed additive (FA1) gave median 25% risk reduction (CI 0%–68%; mean 28%). For the two vaccines studied, the most effective one studied (VA2) gave median 67% risk reduction (CI 20%–92%; mean 64%), whereas the less effective one (VA1) gave a median 34% risk reduction (CI 9%–63%; mean 34%).
Note that the effects of these interventions are less than estimated in the previous opinion (EFSA BIOHAZ Panel, 2011), where a 3 log10 reduction of the concentration of Campylobacter spp. in the caecal content was found to reduce the public health risk of campylobacteriosis associated with the consumption of broiler meat by at least 90%. With the model used in this opinion, the same hypothetical intervention would give a median 58% risk reduction at EU level (PI 16–89%; mean 56%). A 2 log10 reduction reduced the risk by 76–98% in four MSs in the 2011 opinion, which contrasts with the relative risk reduction estimated for FA3 that gave 2 log reductions. This decreased effect is mainly due to the update of the value of the slope of the regression line, based on an update of the literature (Table 2 and Appendix B), and a large unpublished data set (Rodgers, 2020).
3.4.3. Concluding remarks
The modelling approach used in the 2011 opinion was updated using newly published data on the relationship between Campylobacter spp. concentrations in caecal contents and on broiler carcass skin samples, a larger variety of consumer phase models and a newly published dose response model.
In a review of information available since 2011, new experimental in vivo studies were identified that reported estimates of the log reduction (and variation therein) that may be obtained by providing feed or water additives or through application of vaccination. However, no new information was available from field studies, which adds uncertainty to the actual log reductions that can be achieved in practice.
The updated model resulted in lower estimates of the slope of the linear regression line describing the relation between concentrations in caecal contents and on skin samples. Consequential to the decrease of this slope, lower estimates of the relative risk reduction were obtained for the effectiveness of control options directed at a reduction in the caecal concentrations. For example, for a 2‐log10 reduction in caecal concentrations, the median estimate of the relative risk reduction of campylobacteriosis attributable to the consumption of broiler meat produced in the EU is now 42% (95% CI 11–75%), whereas in the previous opinion, this relative risk reduction was 76–98% based on data from four MSs; similarly, a 3‐log10 reduction is now estimated to reduce this risk in the EU by 58% (95% CI 16–89%), compared to a relative risk reduction larger than 90% in four MSs, as found previously.
3.5. Ranking of control options
Within the time frame of this opinion, experts had to conduct a first‐stage selection (step 1), where all the 21 control options were considered, leading to the second step where eight selected control options were prioritised for further assessment of the magnitude of their effect. The 21 control options included in the screening step 1 of the EKE were collated and plotted in descending order of probability of controlling Campylobacter spp. with more than 10% in the EU from top to bottom, sorted by the mean of the midpoints of the experts’ ranges (Figure 5).
Figure 5.
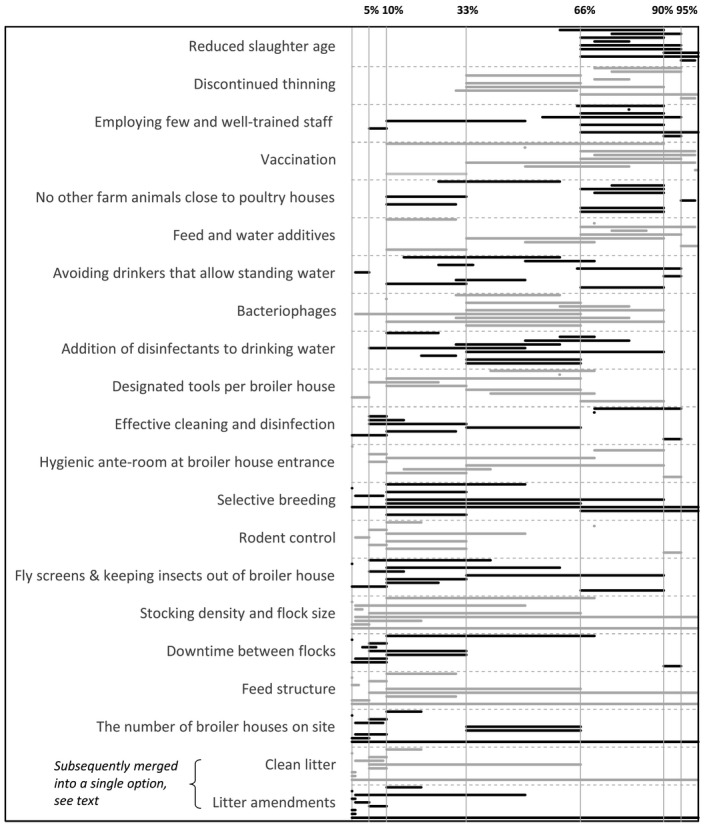
Results of step 1 of the ranking and uncertainty analysis. The horizontal axis is the probability, that, if the specified control option was implemented by all broiler producers in the EU, the average annual incidence of campylobacteriosis cases in the whole EU population caused by Campylobacter spp. in broiler meat produced from chickens raised in the EU would reduce by more than 10% (compared to the current level), assessed by expert judgement. For each control option, the set of horizontal bars shows the probability ranges given by the participating experts. Where a precise probability was given, the bar appears as a black dot. Dotted lines separate the control options. Black and grey bars are alternated to distinguish the different control options
Results of step 1, as shown in Figure 5, were collectively discussed until consensus was reached on which control options to take forward to Step 2.
The control options ‘clean litter’ and ‘litter amendments’ were combined into one ‘clean litter and litter amendments’ resulting in a final list of 20 possible control options.
After the first screening, the experts (i.e. the working group members and selected EFSA staff) agreed to exclude the eight control options in the lower part of Figure 5, up to and including ‘selective breeding’. Based on visual inspection of Figure 5, and further discussion of the rationales behind individual experts’ estimates, these eight control options were overall judged to have a rather low probability (less than about 30–40% as shown in Figure 5) to result in a relative risk reduction higher than 10%. In the remainder of this opinion, these eight options are identified as having a ‘lower probability to have more than 10% effect’. The other 12 control options are identified as having a ‘higher probability to have more than 10% effect’.
Of these 12 top ranked control options based on the probability of causing more than 10% risk reduction, four (4) were excluded from EKE step 2 for the following reasons:
No other farm animals in close proximity to the broiler houses: lack of feasibility of implementation of this control option (the adjacent fields may belong to a different farmer) and doubts about the evidence of direction of transfer of Campylobacter spp.
Effective cleaning and disinfection: The definition of ‘cleaning and disinfection’ was not precise enough for the experts to enable judgements, i.e. it combines a group of activities and evidence of effectiveness is based on a variety of different activities;
Reduced slaughter age: current practices vary largely between EU countries and welfare issues relating to fast‐growing birds might prevent application;
Bacteriophages: lack of convincing evidence of effectiveness in the field due to shortage of field studies.
Hence, the following eight control options were selected for further consideration:
Discontinued thinning
Employing few and well‐trained staff
Vaccination
Feed and water additives
Avoiding drinkers that allow standing water
Addition of disinfectants to drinking water
Designated tools per broiler house
Hygienic anterooms at broiler house entrance
The outcomes of step 2 of the EKE for the eight prioritised control options are sorted by the medians and presented in a boxplot (Figure 6). As the uncertainty around the effectiveness of each control option largely overlapped with the effectiveness of all other control options, a meaningful ranking of these eight control options was not considered possible. More detailed results are available in Appendix D.
Figure 6.

Results of step 2 of the ranking and uncertainty analysis. The horizontal axis is the relative risk reduction for each control option, assessed by expert judgement and expressed as % relative risk reduction in EU campylobacteriosis cases if the control option was implemented by all EU broiler producers. For each control option, the horizontal line shows the 95% probability interval for the estimated risk reduction (P2.5 and P97.5), the box shows the interquartile range (P25 and P75) and the vertical line shows the median (P50). The control options are ordered by the medians, but this should not be interpreted as a ranking due to the large degree of overlap between options
The consensus distributions obtained were based on discussions between the experts and the available evidence from the three evidence streams (effect of control options to reduce flock prevalence (supported by PAF calculations based on literature data), effect of control options to reduce the concentrations in broiler caeca (supported by estimates obtained by a combination of models) and effect of control options directly obtained from literature (not supported by either PAF or modelling). Table 15 summarises the main arguments.
Table 15.
Overview of arguments leading to the consensus distributions for the eight selected control options. Next to the arguments for low and high effectiveness and some general comments, the table shows the largest range of quantitative estimates as given in this opinion, the source of these estimates (i.e. the PAF analysis, the concentration model or literature data), and the relative risk reduction estimates obtained by EKE
| Control option | Arguments for low effectiveness | Arguments for high effectiveness | General comment | Largest range of quantitative estimates (%)a | Source of quantitative estimates | Median consensus (90% Probability interval) (%) |
|---|---|---|---|---|---|---|
| Vaccination |
Doubts about inconsistencies in field conditions after upscaling Despite many efforts, there is no vaccine (proven to be effective in practice) currently available |
Model results confirm large potential of vaccination The results are more consistent than for feed or water additives |
It is generally agreed that the wide distribution reflects existing uncertainty and differences in judgement between experts | 9–92 | Concentration model | 27 (4–74) |
| Feed and water additives |
Doubts about inconsistencies under field conditions; very little evidence of reproducibility Publication bias: negative results are not published |
Some additives show a large effect When effective, the risk reduction achieved is large |
Unclear which specific additive is chosen Large variability between additives and studies |
0–80 | Concentration model | 24 (4–60) |
| Discontinued thinning | Discontinued thinning will not reduce prevalence under poor biosecurity conditions |
Data suggest that flocks are rarely Campylobacter negative after thinning (Koolman et al., 2014) It is very difficult to maintain high biosecurity during thinning; undermines the motivation to, and effect of, carrying out good biosecurity |
Different experiences in different countries Differences between countries will be large Unclear how often thinning is currently applied in EU MSs; CAMCON project found that majority of flocks (> 80%) were thinned in large producer countries: ES, UK and PL |
2–25 | EFSA Opinion 2011; van Wagenberg et al. (2016) | 18 (5–65) |
| Employing few and well‐trained staff | Training and/or certification alone does not mean that good biosecurity is consistently implemented |
Key for good overall biosecurity Good biosecurity and husbandry are reliant on people knowing what they are doing and why |
Main peak reflects PAF results | 2.7–38.8 | PAF analysis | 16 (5–45) |
| Avoiding drinkers that allow standing water | Not clear why nipple drinkers are associated with lower prevalence of Campylobacter in the flock |
Standing water may facilitate cross‐infection between birds using the same drinker. Drinker cups are quickly contaminated with bedding materials and possibly feed providing a niche in which Campylobacter may survive and cross‐infect other birds Cups on drinkers may allow spillage onto the bedding resulting in a higher moisture content that supports Campylobacter survival |
Experts agreed on average (linear pool) of distributions based on individual judgements, without detailed discussion | 0–78.5 | PAF analysis | 15 (4–53) |
| Addition of disinfectants to drinking water | The birds may dislike the odour or taste of the water resulting in lower effectiveness |
Prevents water acting as a source of Campylobacter Effective way of administering anti‐Campylobacter agents ensuring all birds are treated |
Experts agreed on average (linear pool) of distributions based on individual judgements, without detailed discussion | 0.6–54.9 | PAF analysis | 14 (3–36) |
| Hygienic anterooms at broiler house entrance | Requires motivation on the part of the farmers |
A key part of any biosecurity system ensuring Campylobacter are not transmitted form a contaminated farmyard into the broiler house Easy to implement |
Experts agreed on average (linear pool) of distributions based on individual judgements, without detailed discussion | 0–31.9 | PAF analysis | 12 (3–50) |
| Designated tools per broiler house |
Will not be effective as stand‐alone measure Sharing tools indicates poor overall biosecurity |
There is evidence that it is effective, although the effect is small | 1–16 | CAMCON project | 7 (1–18) |
As presented in the opinion.
Apart from information on mean and percentiles of the consensus distribution, the probability distribution obtained in step 2 of the EKE also contains an estimate of the probability that the relative risk reduction is more than 10% (i.e. the percentile where the relative risk reduction as illustrated in Figure 6 is 10%). This estimate, which was implicitly agreed on after the discussions between the experts, can be compared with the estimates obtained in step 1 of the EKE, where individual experts were asked to assess the likelihood that the relative risk reduction would be more than 10% (Figure 5). Table 16 compares these estimates, where the ranges shown in Figure 5 are summarised by the mean of the midpoint estimates for each of the individual experts.
Table 16.
The estimated probability that the relative risk reduction is more than 10%, as obtained from the mean of the midpoints of the individual experts in step 1 of the EKE (Figure 5); and as derived from the probability distributions obtained in step 2 of the EKE, after discussion between the experts (Figure 6)
| Control options | Mean of midpoints of EKE step 1 | Estimate derived from probability distribution of EKE step 2 |
|---|---|---|
| Vaccination | 67% | 83% |
| Feed and water additives | 64% | 86% |
| Discontinued thinning | 70% | 81% |
| Employing few and well‐trained staff | 67% | 81% |
| Avoiding drinkers that allow standing water | 49% | 71% |
| Addition of disinfectants to drinking water | 45% | 70% |
| Hygienic anterooms at broiler house entrance | 38% | 62% |
| Designated tools per broiler house | 42% | 31% |
EKE: expert knowledge elicitation.
The comparison shows that the estimated probability of more than 10% relative risk reduction is considerably higher for all control options after step 2 of the EKE, except the designated tools per broiler house. It should be stressed that step 1 was an approximate screening step, while step 2 was more refined. It is therefore expected that the results differ to some degree, and more weight should be given to the step 2 results, as they followed more detailed consideration and thorough discussions between the experts. Other reasons for the differences between the estimated likelihoods might be the difference in the EKE questions at step 1 and step 2, where experts were asked to estimate the probability that the relative risk reduction is more than 10% (step 1) or to assess the relative risk reduction itself (step 2), and the choice of the mean of the midpoints (i.e. ignoring the uncertainty which was an explicit part of the question and may give a bias towards values for the midpoint closer to 50%).
3.5.1. Concluding remarks
The effectiveness of 20 control options if implemented by all broiler farms in the EU, taking into account the current level of implementation, was estimated by means of a two‐step expert knowledge elicitation (EKE) process that was informed by results from modelling of the updated scientific evidence and literature review (including previous opinion).
Eight control options, when considered separately, were judged to have a lower probability of achieving a reduction of at least 10% in the incidence of campylobacteriosis, as follows; effective rodent control, extended downtime between flocks, fly screens and keeping insects out of the broiler house, clean or amended litter, stocking density and flock size, the number of houses on site, selective breeding and feed structure.
The 12 other control options were judged as having a higher probability of achieving a reduction of at least 10% in the incidence of campylobacteriosis in humans: hygienic anteroom at broiler house entrance, no animals in close proximity to the broiler houses, employing few and well‐trained staff, addition of disinfectants to drinking water, avoiding drinkers that allow standing water, effective cleaning and disinfection, reduced slaughter age, discontinued thinning, designated tools per broiler house, feed and water additives, bacteriophages and vaccination.
From the 12 selected control options, eight options were selected for ranking their risk reduction efficiency, based on the quality of evidence available, practical feasibility in the implementation of the control option.
The median values of the relative risk reduction of eight control options were judged using EKE to be as follows: for vaccination 27% (90% Probability interval (PI) 4–74%); for feed and water additives 24% (90% PI 4–60%); for discontinued thinning 18% (90% PI 5–65%); for employing few and well‐trained staff 16% (90% PI 5–45%); for avoiding drinkers that allow standing water 15% (90% PI 4–53%); for addition of disinfectants to drinking water 14% (90% PI 3–36%); for hygienic anterooms at broiler house entrance 12% (90% PI 3–50%); for designated tools per broiler house 7% (90% PI 1–18%).
It was not possible to rank the selected control options according to effectiveness based on the EKE judgements because there is a large overlap between the probability intervals, due to the large uncertainties involved.
3.6. Advantages, disadvantages and availability of control options
After the first EKE step, the first 12 control options judged as having a higher probability of achieving a reduction of at least 10% in the incidence of campylobacteriosis are listed in Table 17 to consider further their advantages and disadvantages (apart from the effect on campylobacteriosis), if they are to be applied.
Table 17.
Advantages and disadvantages of the first 12 control options judged as having a higher probability of achieving a reduction of at least 10% in the incidence of campylobacteriosis
| Control option | Advantages | Disadvantages |
|---|---|---|
| Vaccination | Systems of multiple vaccines applied at the same time and mass vaccinations are available to which an anti‐Campylobacter vaccine could be added |
The effective vaccine does not yet exist and testing is still at experimental level No vaccines available that work consistently in field trials Unavailability of vaccines at commercial scale Consumers may prefer meat from birds that have not been vaccinated If there are side effects, there could be broiler welfare issues |
| Feed and water additives |
Feed and water additives are relatively easy to administer to the birds These could improve the birds gut microbiota resulting in healthier broilers and better welfare Relatively easy application |
At experimental level, there is still a lack of reproducibility among tests and large variability in effect between treated birds The effects observed at experimental level are lower or non‐existent at field level and there are not yet results reproducing the effect at the field level If the additive changes the odour or taste of the water, the birds may not drink |
| Discontinued thinning | Avoiding stress at thinning is likely to increase flock welfare | This may cause problems with birds in different sizes at slaughter increasing the risk of broken intestines and faecal contamination during evisceration in the processing plant |
| Employing few and well‐trained staff |
Permanency and higher skill levels will benefit the industry generally and may have positive impacts on broiler welfare, health, etc Well‐trained staff are more likely to dedicate sufficient time to hygiene and biosecurity in general and may improve the effectiveness of other control measures The lower the number and the higher the competency of staff the more likely biosecurity measures will be understood and consistently implemented |
Initially, there may be a lack of trained staff and the workload may be an issue During specific times (e.g. when bringing a new batch of birds into the broiler house), relatively high numbers of staff are required (which are not needed thereafter). At those times, a few well trained staff may not be sufficient |
| Avoiding drinkers that allow standing water |
Easy application and a relatively limited resource‐ demanding intervention Easier to clean and disinfect between flocks |
Requires modifications in broiler houses May result in a higher moisture content in the litter and therefore an increased occurrence and severity of contact dermatitis |
| Addition of disinfectants to drinking water |
Easy application May have protective effect against other pathogens and increase health and welfare and reduce the need for antimicrobials |
Low pH of water and palatability for birds could lead to issues for animal growth and welfare Varying effect depending on organic material and solubility in water Some treatments may dislodge biofilm within water lines which can block nipple drinkers The Campylobacter spp. may develop resistance May adversely affect the gut microbiota resulting in poorer broiler performance May be used to cover up poor operator performance in terms of biosecurity and hygiene |
| Hygienic anterooms at broiler house entrance |
Easy to use and serves as a reminder to practice biosecurity Facilitates other activities as the anteroom accommodates the temperature monitoring equipment, bird weight recording, feed and water supply monitoring equipment, etc May include a glass panel that allows inspection of the flock without always entering the production area |
May require structural changes to broiler houses and staff training Double barrier systems installed in small anterooms can lead to cramped conditions and increased risk of poor usage May promote a false sense of biosecurity resulting in other essential hygienic activities, such as disinfectant boot dips, being ignored Many are poorly designed and are ineffective |
| Designated tools per broiler house |
Easy and rapid implementation Relatively cheap to implement Does not require any specific training |
The farmer will have to purchase multiple sets of tools |
| Reduced slaughter age | Reduced probability of biosecurity breaches resulting in less opportunity to contract avian diseases |
The large heterogeneity in the industry complicates implementation. Some companies use slower growing breeds and consumer demands drive specific bird sizes. By slaughtering earlier, the industry may have to introduce faster growing birds and breeds, which may introduce welfare problems Smaller birds may lead to more carcass contamination due to gut rupture at slaughter if automated evisceration equipment is designed for larger birds |
| No animals in close proximity of the broiler houses |
Efforts and resources are focused on broiler houses Reducing the Campylobacter spp. load in the environment |
Farmers can restrict access of some animals (dogs and cats) to the broiler houses and choose not to have other animals close by but may have little control over animals on neighbouring farms or fields Even within a given farm, it may not be possible to place a ‘buffer biosecurity zone’ around the broiler houses unless the farmer has other land not currently in use |
| Bacteriophage | Relatively easy to administer to the birds |
Bacteriophages are specific of strains of Campylobacter spp., their use would not cover the diversity of strains prevalent in birds unless a comprehensive cocktail of phage is used Their effects are transient and do not last until the end of rearing Campylobacter spp. can develop a resistance to bacteriophages |
| Effective cleaning and disinfection | Allows the elimination of contamination between successive flocks |
Some disinfectants may need to be changed to avoid appearance of resistance to those used for a certain time Nearly impossible to clean everywhere for example feeders and drinkers are not designed to facilitate cleaning as there are parts that cannot be reached |
3.6.1. Concluding remarks
There are advantages and disadvantages associated with each control option.
The advantages include ease of application (e.g. hygiene barrier, adding additives to feed), improved bird health (e.g. biosecurity actions), better broiler welfare (e.g. discontinued thinning), cross‐protection against other pathogens (e.g. drinking water treatments, feed additives).
The disadvantages for a given control option may include a requirement for investment (e.g. if structural changes are required to install an anteroom), lack of control (e.g. the farmer may not own the fields adjacent to the broiler house and therefore cannot prevent other animals being close by), reduced broiler growth due to decreased consumption of feed and/or water (e.g. if an additive affects the sensory (odour, taste or appearance) properties making the feed or water unpalatable).
3.7. Combinations of control options
It is difficult to assess the effects of combined control options, as studies that systematically assess such effects (experimentally or by observation) are not available; the number of possible combinations is large and the dependence on effective performance of control options could not be included in the modelling approaches used, and poor application of one control measure may undermine the effectiveness of another. Inclusion of interaction in the epidemiological models would provide an estimate of the combined effects, but interaction is rarely examined, probably due to lack of statistical power in studies due to inadequate numbers of flocks being included. However, the epidemiological risk factor studies do attempt to adjust for confounding factors, or the risk posed by the other risk factors included in the same multi‐variable model (Pourhoseingholi et al., 2012). These models and studies provide relatively specific risk estimates and can be converted to specific risk reduction estimates. Nonetheless, applying control to one farming practice is likely to affect other practices and the relative reduction potential of each factor will change. In general, every control option applied correctly on a farm will further decrease the probability of colonisation of the broiler flock. For biosecurity control options, interaction is evident, as explained in the previous opinion (EFSA BIOHAZ Panel, 2011), because biosecurity depends on many interrelated local factors. In some cases, the interaction may be synergistic increasing the effects beyond the added effect of the individual measures. As an example, training staff in the importance of biosecurity is very likely to increase the effect of all other biosecurity measures, as the level of implementation of these in their daily routines should also improve. Using an anteroom correctly with good division between sections and corresponding biosecurity actions such as putting on house‐dedicated overalls, boots, using a boot dip, washing hands etc., reduces the risk more than just having, but not properly operating, an anteroom.
Another case illustrating synergistic effect is where control options prevent colonisation of the birds before thinning. If thinning is then also abolished, the opportunity for introduction of infection of the broilers is removed allowing continuation of the benefits of disease control activities.
In other situations, applying multiple control measures may result in less than the additive effect. For example, it is expected that if disinfectant was added to drinking water, the risk of standing water in drinkers would decrease thus and there would be no additional benefit to using nipple only drinkers.
Combining two control measures targeting prevalence and concentration, respectively, may result in a cumulative effect. Combining biosecurity measures with a future effective vaccine would for example provide a cumulative effect. Furthermore, a transient effect (e.g. a few days after treatment only) from a feed additive administered just before slaughter, combined with discontinued thinning, may also have a cumulative effect.
3.7.1. Concluding remarks
Multiple control activities are expected to have a higher effect preventing Campylobacter spp. from entering the broiler house and infecting the birds. To minimise the risk of Campylobacter colonisation, all control activities relating to biosecurity would have to be implemented in full.
It is not possible to estimate the effect of combined control options because the current estimates are inter‐dependent and there is a high level of uncertainty of their effect in the EU as a whole.
Combining two control options targeting prevalence and concentration, respectively, may result in a cumulative effect.
3.8. Comparison to the 2011 Opinion
As explained above, the 2011 EFSA opinion on ‘Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain’ was updated for control options at primary production, by a review of new evidence and, when possible, incorporating this evidence in a novel modelling approach applying the PAF or a new version of the model to assess the effectiveness of control options reducing the concentrations in caecal contents.
As in the 2011 opinion, biosecurity measures are considered essential to prevent flock colonisation with Campylobacter spp. However, the previous opinion did not assess the effectiveness of individual control options related to biosecurity, as it was argued that they depend on many interrelated local factors. Although interrelating factors are still complicated, the current opinion includes such an assessment. This was possible by consideration of the PAF as reported in various European studies, and by the application of the EKE where all available evidence was evaluated. By means of the EKE, the effect of application of the control options could be assessed for the whole EU, instead of only four MSs as in the previous opinion, and the uncertainty attending the effect estimates was quantified. Table 18 provides a comparative overview of the effects of individual interventions as described in the previous and the current opinion. It includes the control options with higher probability of producing more than 10% effect (First step of EKE; including all those for which the concentration model was applied), those for which a PAF analysis was performed (Section 3.3.1) and those specifically referred to in the 2011 opinion. When possible, quantitative estimates from the 2011 opinion are presented, otherwise quotes are given from the conclusions or descriptions of the control measures. Overall, the descriptive conclusions from the 2011 opinion are still considered appropriate.
Table 18.
Control options with higher probability to have more than 10% effect (1st step EKE), those for which a PAF analysis was performed (Section 3.3.1) and those specifically referred to in the 2011 opinion. Quotes from the 2011 opinion are given in italics
| Control option | Conclusion EFSA BIOHAZ Panel (2011) | Conclusions in this opinion | |
|---|---|---|---|
| Analysed by | Judged risk reduction effecta | ||
| Vaccination | Vaccination could reduce flock prevalence as well as numbers of Campylobacter spp. in the intestines. However, vaccination is still being developed and no evidence of effective vaccines was found | Concentration model; 2nd step EKE | 27 (4–74) |
| Feed and water additives | Inclusion of additives to feed or drinking water could reduce flock prevalence as well as numbers of Campylobacter spp. in the intestines. However, there is conflicting evidence regarding the effectiveness of additives. | Concentration model; 2nd step EKE | 24 (4–60) |
| Discontinued thinning | Thinning (partial depopulation) is a risk factor for flock colonisation. The public health impact of stopping this practice as estimated in four countries is expected to reduce the risk by up to 25% | EFSA Opinion 2011; 2nd step EKE | 18 (5–65) |
| Employing few and well‐trained staff | Not considered (part of biosecurity) | PAF analysis; 2nd step EKE | 16 (5–45) |
| Avoiding drinkers that allow standing water | Not considered | PAF analysis; 2nd step EKE | 15 (4–53) |
| Addition of disinfectants to drinking water | The effectiveness of drinking water decontamination as an individual biosecurity measure is unclear | PAF analysis; 2nd step EKE | 14 (3–36) |
| Hygienic anterooms at broiler house entrance |
The strict use of a hygiene barrier about 50% and seems especially important when there are other livestock on the farm Not considered as a separate control option (part of biosecurity) |
PAF analysis; 2nd step EKE | 12 (3–50) |
| Designated tools per broiler house | Not considered (part of biosecurity) | CAMCON Project; 2nd step EKE | 7 (1–18) |
| Reduced slaughter age | Restricting the broiler slaughter age of indoor flocks to 35 or 28 days, as estimated from four countries, would reduce the public health risk by 10–20% or up to 50%, respectively | EFSA Opinion 2011; 1st step EKE | Higher probability (mm = 83%) to have more than 10% effect |
| No animals in close proximity of the broiler houses | Recognised as a risk factor but not considered as a control option | PAF analysis; 1st step EKE | Higher probability (mm = 64%) to have more than 10% effect |
| Bacteriophages | The use of bacteriophages as currently envisaged has limited practicality | 1st step EKE | Higher probability (mm = 47%) to have more than 10% effect |
| Effective cleaning and disinfection | Not considered (part of biosecurity) | 1st step EKE | Higher probability (mm = 40%) to have more than 10% effect |
| Selective breeding | Selective breeding programmes, especially in poultry previously selected for high meat or egg production, must be considered as a very long term goal. Therefore, this potential intervention was not modelled | 1st step EKE | Lower probability (mm = 33%) to have more than 10% effect |
| Rodent control | Not considered (part of biosecurity) | PAF analysis; 1st step EKE | Lower probability (mm = 30%) to have more than 10% effect |
| Fly nets and keeping insects out | Where strict biosecurity measures are applied in indoor production, the use of fly screens effectively reduces flock colonisation in summer and thereby reduces public health risk by 50–90% as estimated in one MS | 1st step EKE | Lower probability (mm = 28%) to have more than 10% effect |
EKE: expert knowledge elicitation; PAF: population attributable fractions.
Numeric results indicate the median relative risk reductions (and 90% probability interval) as obtained in the 2nd step EKE; otherwise the result of the 1st step EKE is given, in terms of the probability to have achieve more than 10% relative risk reduction (‘lower’ and ‘higher’ probability are explained in Section 3.5; mm refers to the mean of the midpoint estimates for the probability to have more than 10% relative risk reduction, obtained from the individual experts).
3.8.1. Concluding remarks
In comparison with the previous opinion, new data and novel analyses allowed for a more informative estimate of the effectiveness of individual control options.
The effect estimates of vaccination and application of feed and/or water additives still rank high, but are less pronounced, due to the use of newly published data on the relationship between Campylobacter spp. concentrations in caecal contents and on skin samples, a larger variety of consumer phase models and a newly published dose response model, as well as inclusion of uncertainty when extrapolating from experimental data to field conditions.
The updated model resulted in lower effect estimates than the model used in the 2011 opinion. For a 2‐log10 reduction in caecal concentrations, the median estimate of relative risk reduction is 42% (95% CI 11–75%), compared to the previous opinion, where this relative risk reduction was 76–98% in four MSs. A 3‐log10 reduction is estimated to reduce the risk in the EU by 58% (95% CI 16–89%), compared to a relative risk reduction larger than 90% in four MS in the previous opinion.
For several control options related to biosecurity (i.e. employing few and well‐trained staff, hygienic anterooms at broiler house entrance, designated tools per broiler house), numerical estimates of the effectiveness were obtained after consideration of evidence obtained after the 2011 opinion was published.
4. Conclusions
To address the different parts of the ToR, the conclusions have been reformulated as answers to the following assessment questions:
Assessment question 1: What new scientific evidence about control options has become available since the previous opinion of 2011 and what is their relative risk reduction on campylobacteriosis?
New information was published since the EFSA 2011 Opinion that provides additional evidence that slaughter age, season, thinning, contaminated drinking water and carry‐over from a previous flock are important risk factors for Campylobacter spp. colonisation of a broiler flock.
New epidemiological evidence was analysed by the use of PAFs to obtain estimates of the effectiveness of several control options at primary production that reduce the flock prevalence.
PAFs were calculated for six control options from available studies including; hygienic anteroom, effective rodent control, having no animals in close proximity to the broiler house, addition of disinfectant to drinking water, employing few and well‐trained staff and avoiding drinkers that allow standing water.
According to the PAF analyses, the mean relative risk reductions that could be achieved by adoption of each of these six control options individually are estimated to be substantial but the width of the confidence intervals of all control options indicates a high degree of uncertainty in the specific risk reduction potentials. For example, the mean estimate of the relative risk reduction for the control option ‘Addition of disinfectants to drinking water’ was between 5 (95% CI 0.6–8.2) and 32% (95% CI 6.0–54.9) based on three available studies.
In a review of information available since 2011, new experimental studies were identified that reported estimates of the log reduction in caeca (and variation therein) that may be obtained by providing feed or water additives or through application of vaccination. However, no new information was available from field studies.
Newly published data on the relationship between Campylobacter spp. concentrations in caecal contents and on skin samples, a larger variety of consumer phase models and a newly published dose response model allowed an update of the modelling approach for interventions that reduce the Campylobacter spp. concentrations in broiler caeca. This led to lower effect estimates than with the model used in the 2011 opinion. For a 2‐log10 reduction in caecal concentrations, the median estimate of relative risk reduction is 42% (95% CI 11–75%), compared to the previous opinion, where this relative risk reduction was 76–98% in four MSs. A 3‐log10 reduction is estimated to reduce the risk in the EU by 58% (95% CI 16–89%), compared to a relative risk reduction larger than 90% in four MS in the previous opinion.
Assessment question 2: What is the ranking in terms of effectiveness of the selected control options in reducing human campylobacteriosis cases at the primary production level?
The effectiveness of 20 control options if implemented by all broiler farms in EU, taking into account the current level of implementation, was estimated by means of a two‐step expert knowledge elicitation (EKE) process informed by results from modelling of the updated scientific evidence, literature review (including the 2011 EFSA opinion) and experts’ experience.
The following eight control options were judged to have lower probability of achieving a reduction of at least 10% in the incidence of campylobacteriosis: effective rodent control, downtime between flocks, fly screens and keeping insects out of the broiler house, clean or amended litter, stocking density and flock size, the number of houses on site, selective breeding and feed structure.
The other 12 control options were judged as having a higher probability of achieving a reduction of at least 10% in the incidence of campylobacteriosis: hygienic anterooms at broiler house entrance, no animals in close proximity of the broiler houses, employing few and well‐trained staff, addition of disinfectants to drinking water, avoiding drinkers that allow standing water, effective cleaning and disinfection, reduced slaughter age, discontinued thinning, designated tools per broiler house, feed and water additives, bacteriophages and vaccination.
From the 12 selected control options, eight options were selected for further evaluation based on the quality of evidence available and practical feasibility in the implementation of the control option.
The median relative risk reduction for those eight control options was judged in the EKE process to be as follows; vaccination 27% (90% Probability interval (PI) 4–74%); feed and water additives 24% (90% PI 4–60%); discontinued thinning 18% (90% PI 5–65%); employing few and well‐trained staff 16% (90% PI 5–45%); avoiding drinkers that allow standing water 15% (90% PI 4–53%); addition of disinfectants to drinking water 14% (90% PI 3–36%); hygienic anterooms at broiler house entrance 12% (90% PI 3–50%); designated tools per broiler house 7% (90% PI 1–18%).
It was not possible to rank the selected control options according to their effectiveness based on the EKE judgements because there was a large overlap between the probability intervals, due to the large uncertainties involved.
Assessment question 3: What are the advantages and disadvantages of the selected control options?
The advantages include ease of application for some measures (e.g. hygiene barrier, adding additives to feed), improved bird health (e.g. biosecurity actions), better broiler welfare (e.g. discontinued thinning) or cross‐protection against other pathogens (e.g. drinking water treatments, feed additives).
The disadvantages for a given control option may include a requirement for investment (e.g. if structural changes are required to install an anteroom), lack of control (e.g. the farmer may not own the fields adjacent to the broiler house and therefore cannot prevent other animals being close by) or reduced broiler growth due to decreased consumption of feed and/or water (e.g. if an additive affected the sensory (odour, taste or appearance) properties making the feed or water unpalatable).
Assessment question 4: What would be the effect of combining control options?
Multiple control activities are expected to have a higher effect preventing Campylobacter spp. from entering the broiler house and infecting the birds. To minimise the risk of Campylobacter colonisation, all control activities relating to biosecurity would have to be implemented in full.
It is not possible to quantify the effects of combined control activities because the evidence‐derived estimates are inter‐dependent and there is a high level of uncertainty associated with each. Some control options enhance the effect of others and some could reduce the effect of other control options.
Combining two control options targeting prevalence and concentration, respectively, may result in a cumulative effect, if their targets are unrelated.
5. Recommendations
It is recommended to collect new data (new baseline study) for an update of the current Campylobacter spp. prevalence in the EU, including the quantification of Campylobacter spp. in caeca and on carcass skin samples of the same flocks, for a better estimation of the correlation between Campylobacter concentrations at caeca and carcases.
More field studies of sufficient scale and consistency to provide definitive findings at EU level should be encouraged, especially on the effect of vaccination, probiotics, water and feed additives and pre‐slaughter phage treatment on Campylobacter spp. in broilers because of the large uncertainty related to the extrapolation from experimental studies. Moreover, field studies should also be undertaken on different combinations of control options.
It is recommended to extend this update of the 2011 opinion to include control options throughout the whole broiler chain.
Abbreviations
- ABC
ATP‐binding cassettes
- AQ
Assessment question
- CFU
Colony‐forming units
- CI
Confidence Interval
- CPM
Consumer Phase model
- DR
Dose response
- EKE
Expert Knowledge Elicitation
- FA
Feed Additive
- FCI
Food chain intervention
- FP
Flock Prevalence
- Inc
Incidence
- MS
Member State
- NP
Nanoparticle
- OMP
Outer membrane proteins
- OR
Odds ratio
- PAF
Population attributable fractions
- PHC
Process Hygiene Criterion
- PI
Probability Interval
- QMRA
Quantitative microbiological risk assessment
- RR
Risk Reduction
- RRR
Relative risk reduction
- SD
Standard Deviation
- SE
Standard Error
- S.E.M.
Standard error of the mean
- SPF
Specific pathogen free
- ToR
Terms of Reference
- VA
Vaccine
- WG
Working Group
Appendix A – Modelling approach for control options affecting the concentration in the caecal content of broilers
1.
Control options affecting the concentration in the caecal content are assessed by in three steps using following models:
A regression model to describe the relation between a change in the Campylobacter concentration in the caecal content of broilers and a change in the Campylobacter concentration on the meat at the end of the slaughterhouse (i.e. the concentration on neck or breast skin, whole carcass rinses or otherwise, for carcasses after the industrial chilling process or chicken meat products);
A consumer phase model describing the effect of storage and food preparation (inactivation and cross contamination);
A dose response model.
First, an explanation is given of the regression model approach (See also Nauta et al. (2016)). The approaches for the consumer phase model and dose response model are explained next.
A.1. Regression model for the relation between caecal samples and skin samples
The regression model translates a change in Campylobacter concentration in the chicken caeca in batches of broiler chicken into a change in Campylobacter concentration in batches of broiler meat, in terms of change in distributions on skins of carcasses after chilling or whatever is measured as ‘contamination of the meat’ in the regression model.
Based on published data, different regression models were explored describing a linear relation between the log concentration on broiler skin (or meat) samples taken at the end of processing (log Cskin) and the log concentration found in the caeca (log Ccec).
| (A1) |
In this equation, a indicates the slope of the regression line and b is the intercept. It is assumed that caecal samples and skin samples are taken from the same slaughter batch. The possible pooling of samples is not considered in this analysis.
The regression model is applied to estimate the effect of reducing the concentration Ccec (by Δ log Ccec) on the concentration Cskin.
If the concentration on the skin after reduction of Ccec (due to intervention) is represented by Cskin’ and Equation (A1) is rewritten to Δ log10 Cskin = − a Δ log10 Ccec, then
| (A2) |
The EU baseline data from 2008 are used to describe initial concentration on broiler skin (log10 Cskin), being described by a normal distribution with mean mskin and standard deviation sskin. Nauta et al. (2012) describe the values for mskin and sskin in positive batches for each Member State (MS), along with the mean batch prevalence and the contribution to the EU production of the MS at the time of the baseline survey. These data are used in the analysis. From the data, it is derived that, for the EU including Norway and Switzerland, the weighted mean mskin = 2.44 log CFU/g skin; sskin = 1.24; the weighted flock prevalence for the whole EU is 67%.
The effect of the intervention, Δ log Ccec is implemented by a Normal distribution with mean d and standard deviation sd. Distributions for log Cskin’ can then be obtained analytically. The mean and sd after intervention are:
| (A3) |
With this model, the distribution of concentrations after implementation of an intervention is characterised by a mean log reduction d with standard deviation sd can be derived based on the slope of the regression line a.
The default value for a is set at a = 0.27, based on the largest data set, Rodgers (2020). In the uncertainty analysis, a BetaPert distribution with minimum 0, most likely 0.27 and maximum 0.7 is used, based on the results of the literature search (Section 2.3.1). This distribution is illustrated in Figure A.1.
Figure A.1.
The BetaPert (0, 0.27, 0.7) distribution used to describe the uncertainty about a, the slope of the regression line
A.2. Consumer phase models (CPM)
The eight CPMs described and analysed by Nauta and Christensen (2011) (Nauta and Christensen, 2011) are used (see Section 2.3.2). As in the previous opinion (EFSA BIOHAZ Panel, 2011), the model presented by Nauta et al. (2008), who collected observational data from Dutch consumers preparing a chicken salad, is used as the default CPM in simulations where the uncertainty about the choice of the CPM is not included (see Table 3; results not shown). Also an assumption made by all CPMs is the following relation between the concentration per gram of skin and the concentration per gram of meat
Based on expert opinion in EFSA Biohaz Panel (2011) (EFSA 2011), the default assumption is τ = 1. In the uncertainty analysis, a Uniform distribution of τ between 0 and 3 is used, as in Nauta et al. (2012).
All CPMs translate the distribution of concentrations of Campylobacter on the meat (Cmeat) in a distribution of ingested dose, by models that describe the transfer and survival of Campylobacter, and the variation therein, during the preparation of a meal including chicken meat, based on observational and/or experimental data obtained in different countries. Details of the models (as also implemented in this opinion) are presented in Nauta and Christensen (2011). All models apply the same serving sizes, based on observational data from Christensen et al. (2001) (a lognormal distribution with mean 189 g and standard deviation 127, with a maximum portion size of 1 kg).
A.3. Dose response models
Teunis et al. (2018) presented new dose response models for Campylobacter, which differ substantially from the one used in the previous opinion (the ‘classic’ DR model). Their paper presents an elaborated quantitative analysis of the uncertainty associated with the data and provides a variety of outbreaks and challenge studies. The outbreaks are four raw milk outbreak studies. The challenge studies include human and primate challenge studies for specific Campylobacter jejuni strains, and include the challenge study (Black et al., 1988) that is used for the classic model.
In this opinion, two alternative DR models derived from the Teunis et al. (2018) paper are compared to the classic DR model, by a comparative analysis of relative risk reduction estimates of interventions affecting the concentrations on the meat (similar to what Nauta and Christensen (2011) (Nauta and Christensen, 2011) did for CPMs).
The alternative DR models are those defined by the median estimates of the model parameters provided by Teunis et al. (2018) for the challenge studies and the outbreak studies. These median parameter values do not necessarily give a median DR relation, but some unreported trials suggested that they get close.
Table A.1.
The model parameter values for the models used are given below. For details, see Teunis et al. (2018)
| Pinf | Pill|inf | ||||
|---|---|---|---|---|---|
| α | ß | r | η | Constant | |
| Classic model | 0.145 | 7.59 | 0.33 | ||
| Median Challenge model | 0.44 | 0.51 | 0.06 | 0.88 | |
| Median Outbreak model | 0.38 | 0.51 | 0.76 | 0.0092 |
A comparison of the dose response curves for illness, using the median estimates of the model parameters, is given in the graph below.
For this opinion, the default choice for the DR model remains the ‘classic model’, for best comparison with the 2011 opinion. In the uncertainty analysis, a random choice is made between the ‘classic model’ and the ‘median challenge model’. The ‘median outbreak model’ is based on (potentially non representative) raw milk outbreaks and gives very high estimates of the probability of illness. It is therefore not included.
Appendix B – Studies on the association between caecal content samples and skin or meat samples
1.
| No | Reference | Study content | Tested matrices (caecal content) | Tested matrices (skin or meat) | Samples | Results |
|---|---|---|---|---|---|---|
| 01 | Allen et al. (2007) | Relation: contamination of carcasses during processing – degree of flock colonisation | Caeca after evisceration (caecal content) | Neck skin | 26 slaughter groups, originating from 22 single‐house flocks |
No correlation was found overall between the numbers of campylobacters detected in caeca and on carcasses All carcasses from high prevalence flocks were contaminated with campylobacters After chilling, they carried significantly (p < 0.001) higher numbers than carcasses originating from low prevalence flocks |
| 02 | Boysen et al. (2016) | Campylobacter spp. and Escherichia coli contamination of broiler carcasses across the slaughter line | 24 individual caeca per flock | 24 thigh skins per flock | 15 broiler flocks (from 3 slaughterhouses) | A significant positive correlation was found, r2 = 0.72, p < 0.0001, regression line y = 0.692 × − 2.45. This relationship was not dependent on slaughterhouse |
| 03 | Brena (2013) (PhD thesis Liverpool) | PhD thesis on Campylobacter in broiler slaughter plants | 10 caeca per flock | 10 neck skins per flock | 76 batches of broiler chickens (from 46 different farms); 3 slaughterhouses |
Significant correlation r2 = 0.23. y = 0.21 × + 1.13 p < 0.005 |
| 04 | Duffy et al. (2014) | Effect of processing practices on prevalence/concentration of Campylobacter correlation concentration of E. coli and Campylobacter | Caeca after evisceration (caecal content) | whole bird rinse technique | n = 240 |
Prevalence in caeca has influence on prevalence on carcasses (3/4 flocks) ‘Control of processing parameters may play more important role in reducing concentration on whole chickens than the initial concentration in caeca |
| 05 | Elvers et al. (2011) | Molecular tracking through processing | Caeca | Neck and breast skin | 5 Campylobacter‐positive flocks | ‘no obvious correlation …between the number of campylobacters in the ceca and those on the carcasses…’ There was also a significant association between flock bacterial load and sampling point (p =0.033). This suggests that Campylobacter numbers on the flock and at the point at which they were sampled combine to influence the overall numbers on the carcass |
| 06 | Hue et al. (2011) | Campylobacter and Salmonella contamination | Caeca from 10 birds per batch | 1 carcass per batch | 425 batches | A positive correlation (r = 0.59) between average number of Campylobacter in caeca and on carcasses (p < 0.001) (n = 425). Considering only positive batches (n = 297), correlation was significant (p < 0.001) and Spearman coefficient was lower (r = 0.33). Slope of the regression line shown in figure is 0.3243 |
| 07 | Laureano, Corujo and Van Gerwe (2013) | Correlation of different matrices with Campylobacter counts in neck skin of broiler carcasses | Two pooled samples of 5 caeca per flock | Two pooled samples of 5 neck skins | 80 flocks (from 10 slaughter plants) |
A significant positive correlation. r2 = 0.26; y = 0.276 × + 1.276 The introduction of the variable slaughterhouse as a random effect had no effect in the model |
| 08 | Malher et al. (2011) | Factors associated with carcass contamination | Pooled caeca (1 pooled sample per batch) | Pooled neck skin samples (1 pooled sample per batch) | 140 batches | When a carcass was contaminated …, the level of skin contamination appeared to be poorly correlated (r = 0.28) to the level of caecal contamination in the batches where the skin test was positive. No slope of regression line published |
| 09 | Nauta et al. (2009) | ‘testing and scheduling’ strategy | 10 caecal samples per flock | 5 (skinned) breast fillet samples | 62 flocks | Numbers of Campylobacter on breast fillets do not correlate with those of caecal contents |
| 10 | Reich et al. (2008) | Correlation caeca‐carcasses | 10 caeca per flock | 5 carcasses per flock (breast caps) | 40 flocks caeca/18 flocks carcasses |
Positive correlations between numbers in caeca and on carcasses and breast caps Positive correlation between mean numbers of Campylobacter in caecal contents and mean count on carcasses at various processing steps (correlation coefficients and corresponding p‐values were: 0.56 p = 0.058 for carcasses after scalding/defeathering, 0.81 p = 0.0013 for carcasses after evisceration and 0.64 p = 0.0196 for carcasses after chilling, 0.72 p = 0.0057 for breast caps). No slope of regression line for carcass after chilling of breast caps published |
| 11 | Reich et al. (2018) | Contamination in broilers and microbiological criteria | 10 caecal samples per batch (pooled) | 50 neck skin samples per batch (pooled into 5 neck skin samples) | 365 batches | Counts on neck skin were not predictable (from counts in caecal content) by linear regression due to the low r2‐values found (slaughterhouse A: 0.083; slaughterhouse B: 0.274; slaughterhouse C: 0.134, respectively) |
| 12 | Rodgers (2020) | Carcass‐caeca | 10 caecal samples per batch | Neck skin from 1 carcass per batch | 1,146 slaughter batches | Contamination of carcasses correlated to caecal colonisation |
| 13 | Rosenquist et al. (2006) | Influence of slaughter operation on contamination level | Caecal content (30 per flock) after evisceration | Neck skin samples (30 per flock) | 900 samples from 6 broiler flocks |
Regression analysis of concentrations in caecal material and on neck skin of carcasses after defeathering showed a correlation (α = 1.15; β = −5.29) between these two variables Mean concentrations in neck skin samples after defeathering can be explained by the mean concentration in the caecal samples |
| 14 | Stern and Robach. 2003 | Enumeration in faeces and corresponding carcasses | Faecal droppings (day before slaughter) | Carcass rinses | 20 broiler flocks |
No significant correlation between Campylobacter spp. levels recorded for the faecal samples and those recorded for the prechill carcasses No correlation (p > 0.05) between levels of Campylobacter spp. in faeces and those on fully processed carcasses was observed |
| 15 | Vinueza‐Burgos et al. (2018) | Contamination of broilers along the slaughter line | One pooled sample of 25 caeca per flock | 5 breast skin samples per flock | 15 batches (from 3 slaughterhouses) |
Data published in supplementary material, no correlation study performed Analysis of the published data gives a slope 0.498, r2 = 0.57, p = 0.001 |
Appendix C – Sensitivity analyses of the model for the effect of control options affecting the concentration in the caecal content of broilers
1.
The performance of the model to estimate the effect of control options to reduce the Campylobacter concentration in the caecal content of broilers was analysed by a sensitivity analysis to investigate the sources of the uncertainty in the estimated relative risk reduction.
The model was run for the reference model (as detailed in Table 3), and results obtained for the effects of five selected control options (three feed additives and two vaccines). Additionally, the model was run with additional uncertainty by including (1) a random choice of MSs instead of using the EU mean and (2) randomly selecting the median outbreak DR model, the classic DR model or the median challenge DR model, instead of only the last two, for the least effect vaccine (VA1).
The uncertainty distributions for the relative risk reductions obtained are presented in Figure C.1. It shows that the uncertainty is large in all cases, the added uncertainty for VA1 does not add much to it. The distributions are obtained from Monte Carlo simulations with 250.000 iterations.
Figure C.1.
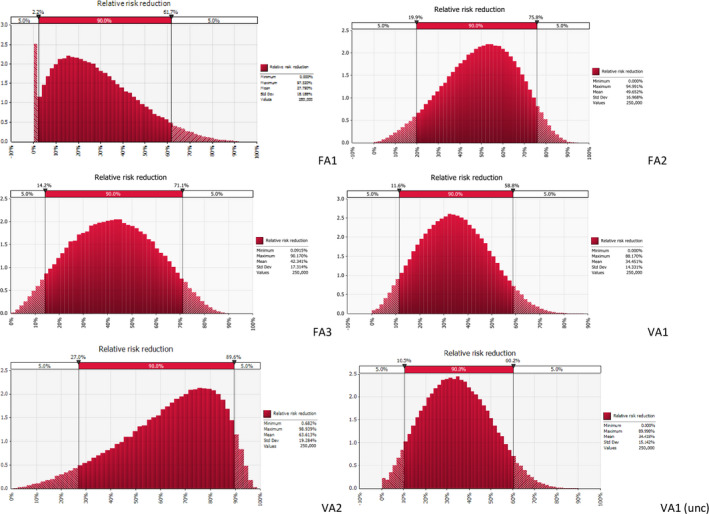
Uncertainty distributions for the relative risk reductions for the three feed additives (FA1, FA2 and FA3) and the two vaccines (VA1 and VA2) as well as VA1 with additional uncertainty, respectively
Figure C.2 presents the correlation of the uncertainty in the input distributions and the uncertainty in the relative risk reductions. It shows that in most cases the uncertainty in the slope is the main source of uncertainty, except in one case where the uncertainty of the effect of the control option (Δ log Ccec) dominates (FA1). An obvious explanation is that the impact of the uncertainty in the effect of the control option varies between the scenarios, and not only depends on this uncertainty itself, but also on the mean size and the variability of this effect. The results also show that the uncertainty about the choice of the CPM, the DR and the MS (i.e. the concentrations on the skin after processing) has a smaller impact on the total final.
Figure C.2.
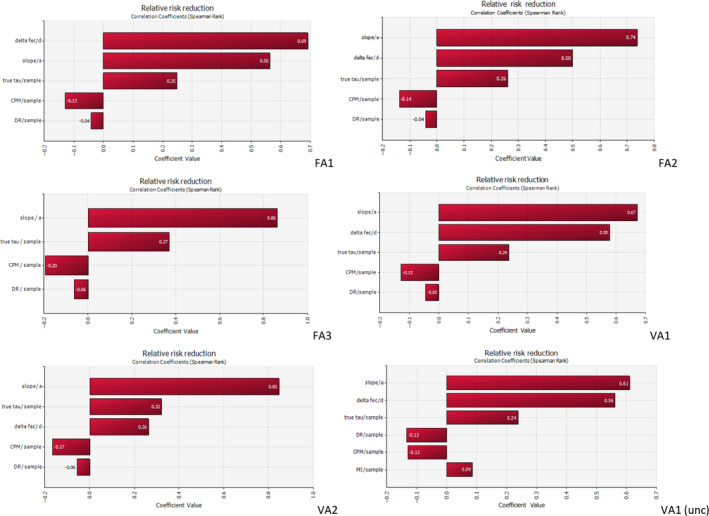
Tornado graphs showing the correlation of the uncertainty in the input distributions with the uncertainty in the relative risk reductions for the three feed additives (FA1, FA2 and FA3), the two vaccines (VA1 and VA2) and VA1 with additional uncertainty. A larger correlation coefficient indicates that the uncertainty in the input parameter has a larger impact on the uncertainty in the relative risk reductions found (and illustrated in Figure A
After these several alternative scenarios were run where some of the uncertainties or variabilities in the model were modified, to explore their effect on the relative risk reduction estimates. Results are shown graphically in Figure C.3. for (1) the reference scenario, and scenarios where (2) the uncertainty about the choice of the MS was added; (3) the slope was not uncertain, fixed at 0.27; (4)) the slope was not uncertain, fixed at 1 (as in EFSA Biohaz Panel, 2011) (EFSA 2011); (5) only the median outbreak DR model was used; (6) only the classic DR model was used; (7) only the median challenge DR model was used; (8) one of the three DR model was randomly selected; (9) all uncertainties (S.E.M.) in the effects of control options were set at zero; (10) all variabilities (sd) in the effects of control options were set at zero. These analyses confirm the importance of the lower values for the slope as obtained and applied in this opinion, compared to the previous one. They also show that the impact of other sources of uncertainty is less pronounced.
Figure C.3.
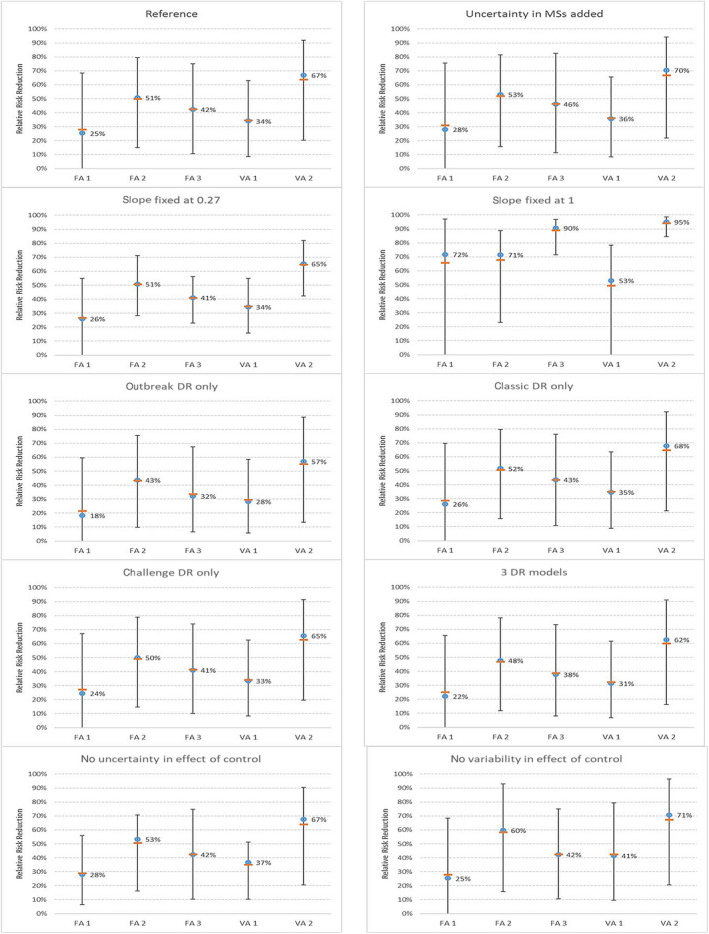
Relative risk reductions found in the reference scenario (as in Figure 4) and nine other scenarios. Blue circle: median effect (numeric result given); orange line: mean effect; error bar: 95% credible interval
This information was made available to inform the EKE and taken into account by the experts in their judgements.
Appendix D – Uncertainty analysis
D.1. Evidence table for control options affecting prevalence
| Specific factor studied | Estimated measure | Result (CI) | MS | Year (data) | Stud | Year (pub) | Specific strengths | Specific uncertainties | Uptake 2016 % |
|---|---|---|---|---|---|---|---|---|---|
| Hygienic anterooms at entrance of broiler houses |
– Five different epidemiological studies from different countries identified this as a risk. – repeatability is evidenced, ‘control’ always included, field studies, – includes two newer study incl. CAMCON – magnitude of PAF fairly similar – Two of the large producer countries are included – Effects are fairly similar across populations |
69–94%* CAMCON REPORT 51–73% Van Wagenberg et al. |
|||||||
| no anteroom in poultry house | PAF % | 17.1 (0*–28.4) | ES | 2010–2012 | Torralbo | 2014 | only carried out in Andalusia | ||
| Could be improved | PAF % | 3.3 (0.4–4.2) | NI | 2001–2002 | McDowell | 2008 | |||
| Litter‐beetles in anterooms | PAF % | 13.6 (0.9–18.8) | FR | 2001 | Refregier‐petton | 2001 | Only representative of 60% of French broilers | ||
| Low quality anteroom | PAF % | 15.6 (5.9–24.4) | DK/NO? | 2010–2012 | Borck høg | 2016 | |||
| General tidiness score reflecting a combination of a number of hygiene parameters | PAF % | 18 (5.9−31.9) | SW | 2005 | Hansson | 2010 | |||
| Non‐significant (i.e. explored in univariate analysis but not retained for the multivariate model) studies | PAF % | – | – |
2004–2005 1997–2000 2001–2004 2003–2004 2003–2006 2008 1999–2000 2005 |
Näther Bouwknegt Guerin Huneau‐salun Ellis‐Iversen Allain Sommer Lyngstad |
2008 2004 2007 2007 2009 2014 2013 2008 |
(Mention here if any of these studies are strong negatives) | ||
| * = Non‐significant in multivariate model | |||||||||
| No ‘anteroom and hygiene barrier in houses < 15 years old’ | RRR |
3 4 6 13 9 12 |
PL ES UK NL DK NO |
2012 |
CAMCON Sommer Van Wagenberg |
2016 2016 |
EU study |
No PAF calculated, as another type of data analysis was performed Based on only few (20) farms in ES, NL, PL and EK, 100 and 160 farms in DK and NO Data are imputed based on assumptions to provide significance and allow modelling Two risk factors in one |
|
| ADD ROWS FOR LIT STUDIES | |||||||||
| Effective rodent control |
– Three epidemiological studies finds similar results = repeatability – Similar PAFS – includes a newer study Includes large producer countries |
> 95% (professional 8–74%) CAMCON report |
|||||||
| No Rodent control outside house | PAF % | 7.5 (0.4–9.3) | FR | 2008 | Allain | 2014 | Only carried out in Brittany | ||
| Rodents seen by sampler | PAF % | 11.8 (3.3, 17.1) | NI | 2001–2002 | McDowell_rf1 | 2008 | |||
| Farmer reported rodents | PAF % | 6.7(0*–12) | NI | 2001–2002 | McDowell_rf2 | 2008 | |||
| presence of rodents in shed | PAF % | 11.7 (0*–18.9) | ES | 2010–2012 | Torralbo | 2014 | Only carried out in Andalusia | ||
| Non‐significant (i.e. explored in univariate analysis but not retained for the multivariate model) | PAF % | – | – |
2005 1997–2000 2001 2001–2004 2004–2005 |
Lyngstad Bouwknegt Refregier‐petton Guerin Näther |
2008 2004 2001 2007 2008 |
Huneau‐Salun (2007) reported the presence of control measures against rodents as significantly associated with risk increase | ||
| * = Non‐significant in multivariate model | |||||||||
| ADD ROWS FOR LIT STUDIES | |||||||||
| No animals in close proximity of broiler houses |
– Repeatability from five countries – PAFs (but one) fairly similar magnitude – Includes newer study – Supported by Jonsson et al. (2012), PAF for this study not calculated due to lack of information |
– Large variation in the risk factor measurements in individual studies |
Low (< 10%?) CAMCON REPORT |
||||||
| Other animal species on farm | PAF % | 25.5 (1.8–42.1) | NL | 1997–2000 | Bouwknegt rf_1 | 2004 | |||
| Other animals within 1 km radius | PAF % | 79.3 (32.3–87.5) | NL | 1997–2000 | Bouwknegt rf_2 | 2004 | Only 27 flocks (out of 457) did not have a farm within 1 km, lack of statistical power cannot be ruled out | ||
| Other livestock on farm | PAF % | 20.9 (5–37) | SE | 2005 | Hansson | 2010 | |||
| Cattle adjacent | PAF % | 21.5 (4.4–35) | UK | 2003–2006 | Ellis‐Iversen | 2009 | |||
| Pigs closer than 2 km | PAF % | 39.5 (19.8–47.8) | NO | 2005 | Lyngstad | 2008 | |||
| Presence of dogs/cats at farm | PAF % | 33.4 (2.6–44.1) | ES | 2010–2012 | Torralbo | 2014 | Only carried out in Andalusia | ||
| Non‐significant (i.e. explored in univariate analysis but not retained for the multivariate model) | PAF % | – | – |
1999–2000 2004–2005 2001 2008 2009–2010 2001–2002 |
Sommer Näther Refregier‐petton Allain Chowdhury McDowell |
2013 2008 2001 2014 2012 2008 |
|||
| ADD ROWS FOR LIT STUDIES | |||||||||
| Employing few and well‐trained staff |
– repeatability from four countries – fairly similar PAFs – supported by other study types |
– Nordic countries are over‐represented – newest data from 2009–2010 |
|||||||
| Personnel in house at delivery | PAF % | 14.2 (2.9–17.7) | NO | 2005 | Lyngstad rf_1 | 2008 | |||
| Hired caretaker staff | PAF % | 13.9 (2.7–18.6) | NO | 2005 | Lyngstad rf_2 | 2008 | |||
| ≥ 2 persons taking care of birds | PAF % | 25.3 (2.7–37.1) | FR | 2001 | Refregier‐petton | 2001 | |||
| ≥ 2 persons entering houses | PAF % | 20.5 (5–38.85) | DK | 2009–2010 | Chowdhury | 2012 | |||
| Non‐significant (i.e. explored in univariate analysis but not retained for the multivariate model) | PAF % | – | – |
2004–2005 1999–2000 |
Näther Sommer |
2008 | |||
| ADD ROWS FOR LIT STUDIES | |||||||||
| Addition of disinfectants to drinking water |
Repeatability in three countries Newer data included |
50–90% (CAMCON report) | |||||||
| Non‐chlorinated water | PAF % | 32.5 (6–54.9) | UK | 2003–2006 | Ellis‐Iversen | 2009 | |||
| Water not treated | PAF % | 5.3 (0.6–8.2) | ES | 2010–2012 | Torralbo | 2014 | Only carried out in Andalusia | ||
| No Acid in drinking water | PAF % | 17.1 (3.3–23) | FR | 2008 | Allain | 2014 | Only carried out in Brittany | ||
| Non‐significant (i.e. explored in univariate analysis but not retained for the multivariate model) studies | PAF % | – | – | 2003–2004 | Huneau‐salun | 2007 | Refregier‐Petton et al. (2001) found treating drinking water as significantly associated with risk increase | ||
| ADD ROWS FOR LIT STUDIES | |||||||||
| Avoid drinkers that allow standing water |
Supported by Sommer et al. (CAMCON) Newer data included |
– Only from DK and DE Two studies – same study |
15–49% (Van Wagenberg) | ||||||
| Nipple drinker with tray | PAF % | 37.2 (0*–53.9) | DE | 2004–2005 | Näther s | 2008 | These studies were convenience samples of farms: may not represent all of Germany and may be less variable than a representative sample | ||
| Nipple drinker with tray | PAF % | 62.4 (1.2–78.5) | DE | 2004–2005 | Näther w | 2008 | |||
| Drinker with cups | PAF % | 25.4 (0.8‐44) | DK/NO? | 2010–2012 | Borck høg | 2016 | |||
| Non‐significant studies (i.e. explored in univariate analysis but not retained for the multivariate model) | PAF % | – | – |
2001–2004 2001 2003–2006 1999–2000 2010–2012 |
Guerin Refregier‐petton Ellis‐Iversen Sommer Torralbo |
2007 2001 2009 2013 2014 |
|||
| * = Non‐significant in multivariate model | |||||||||
| No’ drinkers with drink nipples without cups in farmhouses < 15 years old’ | RRR |
5 8 12 14 24 24 |
PL ES UK NL DK NO |
2012 |
CAMCON Sommer Van Wagenberg |
2016 2016 |
EU study RR reduction DK estimate is similar to PAF for DK study (same data) |
No PAF calculated, as another type of data analysis is performed Based on only few (20) farms in ES, NL, PL and EK, 100 and 160 farms in DK and NO Data are imputed based on assumptions to provide significance and allow modelling No CI given |
|
| ADD ROWS FOR LIT STUDIES | |||||||||
| EXTRA OPTIONS FROM LIT REVIEW | |||||||||
| No ‘designated tools for each house in farmhouses < 15 years old’ | RRR |
1 6 11 16 2 6 |
PL ES UK NL DK NO |
2012 |
CAMCON Sommer Van Wagenberg |
2016 2016 |
EU study |
No PAF calculated, as another type of data analysis is performed Based on only few (20) farms in ES, NL, PL and EK, 100 and 160 farms in DK and NO Data are imputed based on assumptions to provide significance and allow modelling No CI given Not found in other studies |
23–93% (report) 63–98 (Van Wagenb.) |
| No ‘establishing a maximum downtime of 10 days between flocks including rodent control more than 6 times per year and clean‐ ing and disinfection between flocks’ | RRR |
8 10 2 7 7 42 |
PL ES UK NL DK NO |
2012 |
CAMCON Sommer Van Wagenberg |
2016 2016 |
EU study |
No PAF calculated, as another type of data analysis is performed Based on only few (20) farms in ES, NL, PL and EK, 100 and 160 farms in DK and NO Data are imputed based on assumptions to provide significance and allow modelling No CI given Not reported in other RF studies included |
|
| Farmhouses > 15 years old | RRR |
4 57 27 26 22 27 |
PL ES UK NL DK NO |
2012 |
CAMCON Sommer Van Wagenberg |
2016 2016 |
EU study |
No PAF calculated, as another type of data analysis is performed Based on only few (20) farms in ES, NL, PL and EK, 100 and 160 farms in DK and NO Data are imputed based on assumptions to provide significance and allow modelling No CI given Not reported in other RF studies |
|
| Stop thinning | RRR |
2 13 25 23 |
2011 | EFSA | Previous opinion | 37–97% (Van Wagenberg ea) | |||
|
Reduced slaughter age 35 days 28 days |
RRR |
0–18 22–43 |
2011 | EFSA | Previous opinion | ||||
| Table column | Explanation |
|---|---|
| Control options | General name for class of control practices |
| Specific factor studied | The specific practice or risk factor studied |
| Measure | The measure of effect on risk we use from that study, plus units (if multiple measures available from same study, take the most relevant or add multiple measures as separate rows in table). Key: RRR = Relative risk reduction |
| Result (CI) | The result from the study (and 95% confidence interval (CI) where available). If the result cannot be given as a number, describe it verbally but as concisely as possible. (To make more space for this, you could merge this cell with the ‘measure’ cell just for this study, if that helps) |
| MS | Member State(s) study was conducted in |
| Year | Year(s) study was conducted in (not year of publication) |
| Study | First author name (only include year if more than one study for same first author) |
| Strengths | Brief summary of notable strengths of this study. Exclude strengths that affect all studies using the same measure (e.g. PAF) |
| Specific uncertainties | Brief summary of notable uncertainties affecting this study. Exclude uncertainties that affect control options, as these will be listed in the general uncertainty table |
| Uptake 2016 % | Uptake of the control practice (i.e. the % of farms that do already apply the control option) in 2016; data from the CAMCON project (van Wagenberg et al., 2016) |
D.2. Evidence table for control options affecting concentration
| Control practice | Specific factor studied | Measure | Result (CI) | MS | Year | Study/model | Specific strengths | Specific uncertainties |
|---|---|---|---|---|---|---|---|---|
| Vaccination | 4 antigens | log reduction | 1.96 – 3.54 – 4.2 – 2.01 (ND) | France | 2015–2016 | Meunier et al. (2017) | New antigens found by the reverse vaccinology and tested on animals with a promising result of log reduction |
Lack of reproducibility between trials Experimental level, not tested on field |
| Feed additive | Probiotic | log reduction | 1.79 (1.18) | France | 2014–2015 | Guyard‐Nicodème et. al | Reduction measured at the end of the rearing of standard broilers (42 day), close to the field conditions | Lack of reproducibility between trials |
| Feed additive | Probiotic | Log reduction | 1.67 (1.2) | France | 2014–2015 | Guyard‐Nicodème et. al | Lack of reproducibility between trials | |
| Feed additive* | Prebiotic | Log reduction | 3.25 (2.2) | France | 2014–2015 | Guyard‐Nicodème et. al | Lack of reproducibility between trials | |
| Feed additive* | Organic acid | log reduction | 1.23 (0) | France | 2014–2015 | Guyard‐Nicodème et. al | Lack of reproducibility between trials | |
| Feed additive | Organic acid | log reduction | 0.9 | Spain | 2014–2015 | Lack of reproducibility between trials | ||
| Feed additive | Mixture of ion‐exchanged clay in feed and organic acid in water | log reduction | 0.82 | France | 2014–2015 | Reduction measured at the end of the rearing of free range broilers (78 day), close to the field conditions | ||
| Feed additive* | Amino acid with iron | log reduction | 2 | Greece | Skoufos et al. | Reduction measured at the end of the rearing of standard broilers (42 day) based on 3 replicates |
| Table column | Explanation |
|---|---|
| Control practices | General name for class of control practices |
| Specific factor studied | The specific practice or risk factor studied or modelled |
| Measure | The measure of effect on risk we use from that study/model, plus units (if multiple measures available from same study/model, take the most relevant or add multiple measures as separate rows in table) |
| Result (CI) | The result from the study/model (and 95% confidence interval where available). If the result cannot be given as a number, describe it verbally but as concisely as possible. (To make more space for this, you could merge this cell with the ‘measure’ cell just for this study, if that helps) |
| MS | Member State(s) study was conducted in, or model refers to. If EU model, enter ‘EU’ |
| Year | Year(s) study was conducted in (not year of publication). For models, enter year of publication |
| Study/model | First author name (only include year if more than one study/model for same first author) |
| Strengths | Brief summary of notable strengths of this study/model. Exclude strengths that affect all studies using the same measure or model |
| Specific uncertainties | Brief summary of notable uncertainties affecting this study/model result. Exclude uncertainties that affect all control options, or all results from same model, as these will be listed in the general uncertainty table |
| Uptake 2016 % | % uptake of the control practice in 2016 from countries studied in CAMCON project. Can we list all the countries and values in the space available in this column? If not, would it be sufficient to show the range? |
| * | Analysed by the C‐model |
D.3. Uncertainty table
| Risk factor, parameter or model feature affected by the uncertainty | One sentence description of the cause of uncertainty affecting this risk factor, parameter or model feature (one row per cause of uncertainty) | One sentence description of how this source of uncertainty might lead to incorrect ranking of control options, or why that might be possible | Which types of model or study does this uncertainty affect? (e.g. PAFs, C model, etc.) |
|---|---|---|---|
| Risk factors for broiler infection with Campylobacter spp. | Small numbers of broiler farms are a cause of uncertainty in many studies | Sources of Campylobacter on broiler farms are many and varied and may depend on the farms studied. Thus, the findings of a given study, which often includes small numbers of farms, may not be applicable on other farms and the ranking will reflect the choice of farms in published studies and might be different if the observations were made using a larger number of farms | PAFs |
| Risk factors for broiler infection with Campylobacter spp. | The difference in key climate, broiler husbandry practices etc. in different countries is a cause of uncertainty | Many studies on the risk factors for Campylobacter in broilers are undertaken in a single country or a few countries and the data generated and conclusions may not be applicable to other countries in the EU, where, for example, the climate and broiler farm practices may be different | PAFs – representativeness of all of the EU is uncertain |
| Risk factors for broiler infection with Campylobacter spp. | Sample size is a cause of uncertainty |
Sample size may also be an issue with many studies using 1 g or 10 g samples, which may or may not be representative of the flock or broiler house. For example, a 10 g sample of broiler faeces or 10 cloacal swabs from a flock of 30,000 may or may not be representative of the flock as a whole The result may be false‐negative flocks |
PAFs |
| Risk factors for broiler infection with Campylobacter spp. | Seasonal effects are a cause of uncertainty | Seasonality greatly affects Campylobacter survival in the environment, sources and dissemination routes and data from studies that do not include a seasonal consideration may not be applicable throughout the year | PAFs |
| Risk factors for broiler infection with Campylobacter spp. | Assumptions about the direction of Campylobacter spread is a cause on uncertainty | Contrary to the conclusion in some papers, the detection of similar Campylobacter genotypes in farm animals adjacent to the broiler house before they appear in the birds should not be indicative of horizontal transmission form these animals to the birds. It is equally possible that these animals were infected by Campylobacter from previous broiler flocks and/or the levels in the birds were below the level of detection if tested at the same time as the farm animals | PAFs |
| Risk factor and control options | No/little adjustment for confounding or interaction of all other factors | Measured effects or risks may actually be caused by other factors, unless these are deliberately adjusted for – either by exclusion (laboratory trials) or statistically (epidemiological studies) | All‐except for epistudies and carefully designed experimental studies |
| Control options for preventing broiler infection with Campylobacter spp. | Unrepresentative laboratory studies are a cause of uncertainty | Laboratory studies may not accurately reflect the conditions encountered in the broiler house and there is considerable uncertainty in laboratory data with respect to its application on commercial broiler farms | The model including the impact of interventions on Campylobacter count reduction? |
| Control options for preventing broiler infection with Campylobacter spp. | Unrepresentative field trials are a cause of uncertainty | Field trials may not reliably predict the impact of specific interventions under real‐world conditions where there may be up to 30,000 birds in a shed with a stocking density of 20 birds per m2 | The model including the impact of interventions on Campylobacter count reduction? |
| Control options for preventing broiler infection with Campylobacter spp. | Human behaviour is a cause of uncertainty | In biosecurity studies, the participants are often volunteers who are aware they are being assessed and may behave differently to their normal practices | Minor for PAFs as variability – incl. Compliance is included and thus accounted for in epi‐studies |
| Control options for preventing broiler infection with Campylobacter spp. | Broiler farm selection is a cause of uncertainty | Many studies are undertaken on a relatively low number of broiler farms that may not be representative of broiler farms in general. Moreover, their selection is often agreed with their contract processor, who is more likely to suggest farms that have a good Campylobacter performance record | ? |
| Control options for preventing broiler infection with Campylobacter spp. | The specificity of bacteriophage is a cause of uncertainty | The high specificity of phage in terms of the Campylobacter strains they can infect introduces great uncertainty in phage research especially when extrapolating to a real‐world situation where there may be multiple Campylobacter strains within a given bird or flock | ? |
| Control options for preventing broiler infection with Campylobacter spp. | The age and lineage of the test birds is a source of uncertainty | When evaluating probiotic bacteria as a feed additive, a range of factors including the age and lineage of the birds and the mode of administration greatly influence the outcome of the experiments. Moreover, if the birds are exposed to Campylobacter first, the probiotic strain is considerably less likely to have a positive effect | ? |
| Control options for preventing broiler infection with Campylobacter spp. | Lack of reproducibility between trials Experimental level, not tested on field | In one trial, the product can have a very significant effect in terms of reduction but when repeating the same conditions in a second trial, no significant effect can be obtained. This loss of significance between two trials is often (but not the only reason) the variability of Campylobacter counts between (control) animals | C‐model |
| Risk factors for broiler infection with Campylobacter spp. Control options for preventing broiler infection with Campylobacter spp. | The ability of Campylobacter to enter the viable but not culturable (VBNC) state is a cause of uncertainty | Campylobacter can enter a viable but not culturable (VBNC) state which may greatly affect the data obtained, especially in studies designed to reduce or eliminate these pathogens. An alternative is to use PCR‐based methods, but the presence of Campylobacter DNA does not indicate the presence of viable cells | ? |
| C‐model: Use of caecal concentration | It is not necessarily the caeca that contaminate the carcass, it may also be leaking faeces, which has a lower concentration | This increases the variability in the data and the uncertainty about the regression line and the uncertainty of the ranking. (In general: The ranking between measures within the C‐model is not altered, only the ranking compared to the P‐model measures) | C‐model |
| C‐smodel: Use of caecal concentration | It need not be the caecal count that is used to measure the impact of the intervention | The regression line is not representative for the evaluated intervention if no caecal counts are used, it increases the overall uncertainty of the ranking | C‐model |
| C‐model: In reality, the relation between caecal concentration and carcass concentration is probably not linear | If the slope at high caecal concentrations is larger than the slope at low values (i.e. there is not really a linear relation, it is j‐shaped), the regression line may overestimate the effect of the intervention at low concentrations | The effect of the intervention may be slightly overestimated if the regression is based on high concentrations | C‐model |
| C‐model: Censored data on caeca and/or skins | Concentration data usually suffer from the presence of data below (or above) a limit of quantification, so‐called censored data. The way these are traditionally dealt with (taking a fixed value, the lower limit or so; or simply omitting the data) may have high large impact on the regression line obtained. Methods for regression with censored data may help (Tobit regression), but there is no established method to deal with regression and censored data in both the dependent and independent variable | This impacts the uncertainty of the slope obtained | C‐model |
| C‐model: assumption that τ = 1: the contamination of the meat is always 1 log less than the skin | Lack of data led to an expert estimate of the Campylobacter WG in 2010 | This impacts the effect estimate (in terms of relative risk reduction), it is included in the model | C‐model |
| C‐model: CPM used (with assumptions on cross contamination) is representative for all prepared chicken meals | Consumer food preparation is highly variable, not under control and hard to measure or influence | Increases the overall uncertainty; this is studied by comparing different CPMs | C‐model |
| C‐model: DR model used: actual dose response for Campylobacter on chicken is highly uncertain | The DR relations are based on one or a few strains; not from chicken meat; are for healthy adults or primates that are not immune | Increases the overall uncertainty; this is studied by comparing different DRs | C‐model |
| C‐model: use of 2008 baseline data on skin samples | These are old data, but collected all over Europe in a harmonised way. In the meantime, action may have been taken to reduce these concentrations | Not really sure how this impacts the results; we may overestimate the (relative) risks. The high incidence of human infection suggests that Campylobacter prevalence at farm and slaughter at has not improved since 2008 | C‐model |
| C‐model: implementation effect of control options | The variation between log reductions as observed in the (raw) data is interpreted both as variability (the st. dev in the data) and the uncertainty (the s.e.m.), whilst it actually is a combination of both | Both variability and uncertainty are overestimated, which leads to underestimation of the effect and overestimation of the uncertainty. The impact of this phenomenon can be assessed by considering the scenarios where either the variability or uncertainty are omitted, in the sensitivity analysis | C‐model |
| Risk factors (all) for PAF calculation | PAFs are calculated from adjusted Relative Risks, different studies adjust for different confounders in the final multivariate model | Impact on the estimates of the multivariate model and thus on the size of PAFs | PAF |
| Risk factors (all) for PAF calculation | Few studies considered the effect of including non‐significant (but potentially confounding) factors in the final multivariate model | Impact on the estimates of the multivariate model and thus on the size of PAFs | PAF |
| Risk factors (all) for PAF calculation | Heterogeneity in the biological samples that are collected on farm/slaughter (Pool of different number of caeca samples, cloacal swabs, sock samples) and analysed to define a flock as ‘positive’. Possible differences in sensitivity between methods | Probably little impact under the assumption that within‐flock‐prevalence is typically considered to by high in infected flocks | PAF |
| All risk factors | Underreporting of non‐significant effects | If no significant effect is found for a risk factor or control option, it may not be published. This means the ‘mean published’ effect size of a potential control option may be larger than it actually is | All |
| Control measure: ‘Avoid presence of standing water in drinkers’ | Two of the studies were convenience samples of farms and may not represent all of Germany. Furthermore, a convenience sample may have less variability than a representative sample | Introduces more uncertainty about the effect in all of EU and the altered variance may have over‐ or underestimated the PAF | PAF |
| Effective hygiene barrier at broiler house entrance | One of the studies was only representative of 60% of French broilers and the Spanish study was only carried out in Andalusia | This introduces more uncertainty about the control effect of all of EU and the biased variability may over‐ or underestimate the PAF | PAF |
| Effective rodent control | Two of the studies were carried out in regions (Brittany and Andalusia) and are unlikely to be nationally representative | This introduces uncertainty about the control effect of all of EU and the biased variability may over‐ or underestimate the PAF | PAF |
| No animals in close proximity | This control estimate was derived from a broad range of risk factors | This adds uncertainty around the precision of the PAF estimate | PAF |
| Have few and permanent staff | PAF | ||
| Additives to drinking water | One of the studies was carried out using a convenience sample and another was from Brittany | This introduces uncertainty about the control effect in all of EU and a biased variability may over‐ or underestimate the PAF | PAF |
D.4. Report on expert knowledge elicitation, 19–20 November 2019
The ranking and quantification of control options reported in the Opinion was conducted in two steps:
-
•
Step 1: Experts assessed the probability that each of 22 (later reduced to 20) control options would, if implemented by all EU broiler producers, reduce the incidence of campylobacteriosis in the EU by at least 10%.
-
•
Step 2: Experts assessed the magnitude of reduction in incidence of campylobacteriosis in the EU that each of the prioritised control options would achieve, if implemented by all EU broiler producers.
The methods and results for the first step are reported in the opinion and were used to prioritise eight control options to be considered in step 2. Methods and results for step 2 are summarised in the opinion and documented in more detail in this appendix.
Note: the names used for control options in this appendix are those used during the elicitation workshop.
Methods for step 2 of elicitation process
The precise question to be addressed in step 2 was specified as follows: If the specified control option is implemented by all broiler producers in the EU that are not currently using it, what will be the resulting % reduction (compared to the current level of implementation) in average annual incidence of campylobacteriosis cases in the whole EU population caused by campylobacter in broiler meat produced from chickens raised in the EU, other things being equal?
When addressing this question, the following definitions were applied:
The meaning of ‘campylobacteriosis cases’ was clear for the experts and did not require further definition.
‘Other things being equal’ includes other control options remaining at the current level of implementation, production and processing practices remain unaltered and no change in the consumption in the EU of meat from broilers raised in the EU.
If a control option acts on both prevalence and concentration, both should be taken into account when answering the question.
For each control option, the experts will answer the question assuming that, of the specific practices for this control option which are referred to in this Opinion (e.g. different vaccines, or different methods of rodent control), the practice that would, on its own, achieve the largest reduction in campylobacteriosis will be implemented by all EU broiler producers.
The experts comprised all members of the Working Group developing the Opinion, plus three EFSA scientists who were supporting the WG.
Expert judgements on the question were elicited in the course of a one day (midday to midday) expert knowledge elicitation (EKE) workshop. The elicitation method was based on the Sheffield or SHELF protocol (EFSA 2014, Oakley and O'Hagan, 2019), which first elicits judgements from each expert individually and then seeks to elicit a consensus judgement from the group, integrating their individual judgements by a process of discussion (behavioural aggregation, EFSA 2014) rather than calculation. The elicitation was conducted by a facilitator and rapporteur who are experienced in use of the Sheffield method. The experts were trained on the approach to making both the individual and consensus judgements using an example question unrelated to the Opinion.
Two modifications were made to the normal Sheffield protocol (EFSA, 2014) in order to complete the exercise within the time allocated. First, experts made individual judgements on all eight of the selected control options during a single session, before proceeding to the consensus process. Second, the unweighted linear pool of the individual judgements was used as a starting point to be discussed by the group and modified as necessary to reflect their consensus judgement, rather than eliciting consensus quantiles and fitting a distribution.
Evidence and sources of uncertainty relevant to answering the question had been collated and summarised in the course of the work on the Opinion, as described in the main text. The experts were familiar this information, having used it for their judgements on the question for Step 1 during the preceding 2 weeks.
The experts completed their individual judgements in a period of 40 min at the end of the first half of the workshop. Overnight, the facilitator and rapporteur collected and processed the individual judgements, using a downloaded copy of the SHELF app for multiple experts (http://www.jeremy-oakley.staff.shef.ac.uk/project/elicitation/). For each control option considered in step 2, the app was used to plot, on a single graph, a separate distribution fitted to the judgements of each expert (selecting the option ‘best‐fitting’ in the app) plus an additional distribution representing the unweighted linear pool of the individual expert distributions, together with the median and 95% probability interval for the linear pool. An example of this for one control option is shown in Figure D.1.
Figure D.1.

Example of the initial results of the elicitation for the control option ‘discontinued thinning’, prior to the consensus process. The dashed curves represent the best‐fitting distributions for the individual experts and the solid line represents the unweighted linear pool (average) of those distributions
A boxplot was produced, comparing the linear pool distributions for all eight control options. The first version of this, depicting the results prior to the consensus process, is shown in Figure D.2.
Figure D.2.
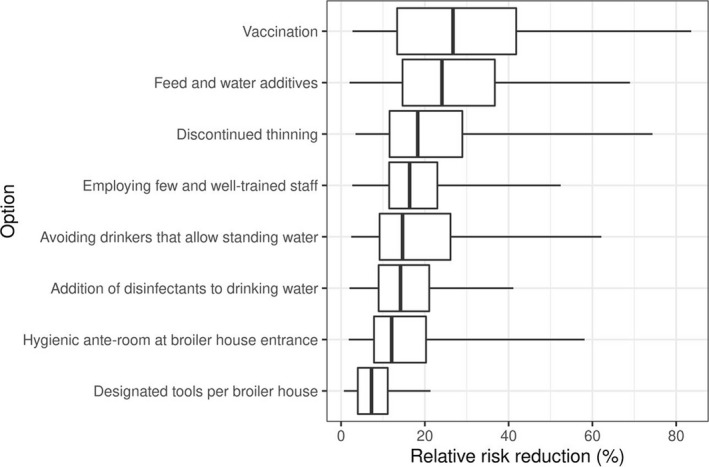
Boxplots comparing the initial unweighted linear pool distributions for all eight control options considered in step 2, prior to the consensus process. For each control option, the horizontal line represents the 95% probability interval of the linear pool distribution, the box represents the interquartile range (covering 50% probability) and the vertical bar within the box is the median
At the beginning of the second half‐day of the workshop, the initial results (Figure D.2 plus a version of Figure D.1 for each control option) were displayed and discussed by the group. In view of the limited time, the group prioritised five options (designated tools; additives; discontinued thinning; few and well‐trained staff; and vaccination) to discuss in detail for developing consensus distributions. For each of these options in turn, the facilitator asked experts with contrasting individual distributions to summarise the reasoning behind their judgements. For three control options (designated tools; discontinued thinning; few and well‐trained staff), this led to agreement by the group to accept the unweighted linear pool as representing their consensus judgement. For additives, the discussion led one expert to suggest reducing the weight given to this expert's distribution. A linear pool giving zero weight to this expert was displayed and the group unanimously agreed to accept this as their consensus judgement for additives. For vaccination, the discussion led three experts to revise their individual judgements, after which the group agreed to accept the revised linear pool as their consensus.
After agreeing the consensus for each of the five options that were discussed in detail, the group briefly summarised the principal evidence and reasoning behind it, as shown in Table D.1.
Table D.1.
Rapporteur's notes summarising the expert group's collective rationale for the consensus distributions for the five control options that were discussed in detail
| Control option | Summary of rationale as recorded by rapporteur |
|---|---|
| Designated tools per broiler house | Reason why it is unlikely that designated tools would have more than 10% effect: very little evidence that the tools are the main risk; sharing tools is such a bad biosecurity sign that unlikely to be more than 10% effect just by changing this practice; available evidence points to less than 10% effect |
| Feed and water additives | Consensus distribution represents experts’ judgement of difference from model estimates due to likelihood of unreported negative studies, expectation of less effects in field, expectation that effect will not happen always |
| Discontinued thinning | Start from old opinion (4 Member States between 2% and 25%, CANCOM between 3% and 13%, but various biases), but long tail due to uncertainty about differences between countries and differing experiences in different countries) |
| Employing few and well‐trained staff | Main peak reflects the PAF results, but the tail reflects variation of judgement about how large the bonus biosecurity effect would be |
| Vaccination | Diversity of views about role of strain specificity and about transfer to the field is key to the wide distribution of uncertainty with some experts close to the model results and others much lower |
Finally, the experts reviewed a revised set of boxplots, which had been updated with the modified consensus distributions for additives and vaccination. The group accepted this plot as representing its consensus judgement about all eight control options considered in step 2, including the three which it had not been possible to discuss in detail (hygienic anterooms; avoiding drinkers that allow standing water; and disinfectants added to drinking water). This final set of boxplots representing the group's consensus judgements are shown in Figure 6 in the main text of the opinion. Selected quantiles for the consensus distributions (including those shown in the boxplots) are shown in Table D.2.
Table D.2.
Selected quantiles of the consensus distribution for each of the control options considered in step 2. The P2.5 and P97.5 provide the 95% probability interval for each option, while the P16.7 and P83.3 provide a 66.6% probability interval (corresponding to the category ‘Likely’ in EFSA's approximate probability scale, (EFSA Scientific Committee, 2018)
| Control options | P2.5 | P5 | P16.7 | P25 | P50 | P75 | P83.3 | P95 | P97.5 |
|---|---|---|---|---|---|---|---|---|---|
| Vaccination | 3 | 4 | 10 | 13 | 27 | 42 | 50 | 74 | 84 |
| Feed and water additives | 2 | 4 | 11 | 15 | 24 | 37 | 43 | 60 | 69 |
| Discontinued thinning | 3 | 5 | 9 | 12 | 18 | 29 | 39 | 65 | 74 |
| Few and well‐trained staff | 3 | 5 | 9 | 11 | 16 | 23 | 28 | 45 | 52 |
| Avoiding drinkers that allow standing water | 2 | 4 | 7 | 9 | 15 | 26 | 34 | 53 | 62 |
| Addition of disinfectants to drinking water | 2 | 3 | 7 | 9 | 14 | 21 | 25 | 36 | 41 |
| Hygienic anterooms at broiler house entrance | 2 | 3 | 6 | 8 | 12 | 20 | 26 | 50 | 58 |
| Designated tools per broiler house | 1 | 1 | 3 | 4 | 7 | 11 | 13 | 18 | 21 |
Appendix E – Studies on effects of control options on broiler farms to reduce the concentration of Campylobacter in the birds
1.
Numbers in bold are obtained after re‐analysing the raw data with a method that allows inclusion of censored data (Lorimer and Kiermeier, 2007).
| Intervention | Specification | Type of study | Mean log reduction | SD log reduction | S.E. of mean | Sample size | Reference |
|---|---|---|---|---|---|---|---|
| Vaccination | Recombinant (DNA + protein) new antigen 1 | Experimental on broilers | 1.96 2.03 | 1.83 | 0.49 | 15 birds for each vaccine test | Meunier et al. (2017) |
| Recombinant (DNA + protein) new antigen 2 | Experimental on broilers | 3.54 3.47 | 2.14 | 0.63 | 15 birds for each vaccine test | Meunier et al. (2017) | |
| Recombinant (DNA + protein) new antigen 3 | Experimental on broilers | 4.2 4.15 | 1.63 | 0.51 | 15 birds for each vaccine test | Meunier et al. (2017) | |
| Recombinant (DNA + protein) new antigen 4 | Experimental on broilers | 2.01 2.09 | 1.82 | 0.5 | 15 birds for each vaccine test | Meunier et al. (2017) | |
| Feed additive | Probiotic | Experimental on broilers (45–49 birds) | 1.88 (at 35 day) 1.79 1.88 | 1.18 | 0.42 | 15 birds | Guyard‐Nicodème et al. (2016) |
| Probiotic | Experimental on broilers (45–49 birds) | 1.70 (at 42 day) 1.68 | 1.2 | 0.65 | 15 birds | Guyard‐Nicodème et al. (2016) | |
| prebiotic | Experimental on broilers (45–49 birds) | 3.17 (at 42 day) 3.25 | 2.2 | 0.64 | 15 birds | Guyard‐Nicodème et al. (2016) | |
| Organic acid | Experimental on broilers | 2.13 (at 42 day) 1.23 | 0 | 0.66 | 15 birds | Guyard‐Nicodème et al. (2016) | |
| Organic acid | Experimental on broilers (45–49 birds) | 0.9 (at 42 day) | 0.206 | 10 birds | Gracia et al. (2016a,b) | ||
| Microencapsulated mixture of short chain fatty acids and phenolic essential oils, 2,000 ppm | Chickens inoculated with a mixture of 2 strains of C, jejuni (10,000 CFU) | 0.7 log reduction in treated birds (after 35 days) | Not given | Not given | Not given | Thibodeau et al. (2015) | |
| Ferric tyrosine | Experimental on broilers | 2 (at 42 day) | 36 birds (3 replicates of 12) | Skoufos et al. (2019) | |||
| Mixture of ion‐exchanged clay in feed and organic acid in water | Experimental on free range broilers (78 day) | 0.82 | 0.25 | 15 birds | Guyard‐Nicodème et al. (2017) | ||
| Bacteriophage | 4 phage cocktail applied via drinking water, 5.8–7.5 log pfu dose per bird, 6–7 days before slaughter | 3 field trials using broilers under commercial conditions. Data (control and trial groups) have been provided for the 3 field trials |
Trials 1, 2 & 2 Treatment groups (trial number, days after application, mean faecal count t plus SD in brackets) 1, 0, 2.4 (0.6) 1, 1, < 1.8 (0) 1, 6, 3.8 (0.5) 2, 0, 3.1 (0.8) 2, 1, 4.6 (0.4) 2, 6, 5.9 (0.2) 3, 0, 0.5 (0.7) 3, 1, 4.6 (1.0) 3,7, 0, 6.7 (0.2) |
Corresponding data for control groups 1, 0, 1.2 (0.6) 1, 1, 2.6 (0.7) 1, 6, 7.0 (0.2) 2, 0, 4.6 (0.7) 2, 1, 4.7 (0.6) 2, 6, 4.4 (1.0) 3, 0, 2.7 (0.9) 3, 1, 5.6 (0.6) 3,7, 0, 4.5 (0.4) |
9 birds per each of the 3 trials | Kittler et al. (2013) | |
| Water additive | Commercial additive composed of short chain organic acids and medium chain fatty acids |
Commercial flocks. broilers‐3 consecutive rearing cycles. Caecal reductions did not result in lower carcass counts |
Treated birds 35 days mean count (SD) 5.67 (1.28) 0.85 (0) 0.85 (0) Treated birds (42 days 4.19 (1.59) 0.85 (0) 0.85 (0) |
Control birds 35 days) 2.95 (3.39) 7.72 (0.92) 0.85 (0) 6.26 (1.19) 5.09 (2.14) 7.36 (0.92) |
Jansen et al. (2014) | ||
| Thinning | Naturally contaminated broilers before and after thinning | Commercial flocks – Data provided for prevalence and caecal counts for 14 commercial flocks | Mean counts and SD provided | Too much data to be included here but well presented in Table 1 (page 878) of the paper | Koolman et al. (2014) (incl. Bolton) | ||
| Vaccination | 2 vaccines both formalin killed C. jejuni but in 2 different adjuvants (oil and aluminium hydroxide gel) | Field‐based trial using Jidori chicks |
26 days post challenge: oil: 7.0 (1.2) Aluminium: 7.5 (0.7) Control: 7.0 (1.1) 56 days post challenge: Oil: 6.8 (1.2) Aluminium: 6.6 (2.2) Control: 7.2 (1.1) |
17 | Okamura et al. (2012) | ||
| Feed additive | MCFA | Applied test conditions. Birds experimentally infected with C. jejuni at 15 days old and tested at 27 days old |
Control: 7.0 (0.8) Tylosin 2.8 (1.5) Caproic acid 7.3 (0.8) Caprylic acid 7.8 (0.7) |
10 | Hermans et al. (2010) | ||
| Bacteriophage | Ross 308 broilers were given 7 log CFU C. jejuni after 20 days rearing | Birds were housed in a commercial environment in individual pens | The control was birds treated with a placebo or carrier alone | 1 log reduction after 1 day, 2.4 log reduction after 2 days, 1.9 log reduction after 3 days, 1.4 log reduction after 4 days & 1.6 reduction after 6 days | Provided, see Figure 1 | 5 birds tested per sampling time | Richards et al. (2019) |
| At 24 days, the birds were given a 2 phage mixture, & log PFU | |||||||
| 5 chickens were tested on each day from 25 to 29 days and C. jejuni enumerated in ceca |
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Alter T, Crotta M, Ellis‐Iversen J, Hempen M, Messens W and Chemaly M, 2020. Update and review of control options for Campylobacter in broilers at primary production. EFSA Journal 2020;18(4):6090, 89 pp. 10.2903/j.efsa.2020.6090
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00676
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: The BIOHAZ Panel wishes to thank the following for the support provided to this scientific output: Peter Craig, Andrew Hart, Katrin Bote, Maria Francesca Iulietto and John Rodgers.
Adopted: 18 March 2020
Notes
EFSA Journal 2012;10(6):2741.
EFSA Journal 2010; 8(1):1437.
EFSA Journal 2011;9(4):2105, 141 pp. https://doi.org/10.2903/j.efsa.2011.2105
http://www.avec-poultry.eu/wp-content/uploads/2018/10/8.-WF-28-09-2018-AVEC-annual-report-2018.pdf
Eurostat database: http://ec.europa.eu/eurostat/data/database (last accessed 22 January 2020).
Eurostat database: http://ec.europa.eu/eurostat/data/database (last accessed 17 January 2019).
References
- Adams LJ, Zeng X and Lin J, 2019. Development and evaluation of two live salmonella‐vectored vaccines for campylobacter control in broiler chickens. Foodborne Pathog Dis, 16, 399–410. [DOI] [PubMed] [Google Scholar]
- Aguiar VF, Donoghue AM, Arsi K, Reyes‐Herrera I, Metcalf JH, de los Santos FS, Blore PJ and Donoghue DJ, 2013. Targeting motility properties of bacteria in the development of probiotic cultures against Campylobacter jejuni in broiler chickens. Foodborne Pathogens and Disease, 10, 435–441. [DOI] [PubMed] [Google Scholar]
- Agunos A, Waddell L, Léger D and Taboada E, 2014. A systematic review characterizing on‐farm sources of campylobacter spp. for broiler chickens. PLoS ONE, 9, e104905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain V, Chemaly M, Laisney MJ, Rouxel S, Quesne S and Le Bouquin S, 2014. Prevalence of and risk factors for Campylobacter colonisation in broiler flocks at the end of the rearing period in France. British Poultry Science, 55, 452–459. [DOI] [PubMed] [Google Scholar]
- Allen HK and Stanton TB, 2014. Altered egos: antibiotic effects on food animal microbiomes. Annual Review of Microbiology, 68, 297–315. [DOI] [PubMed] [Google Scholar]
- Allen VM, Bull SA, Corry JEL, Domingue G, Jørgensen F, Frost JA, Whyte R, Gonzalez A, Elviss N and Humphrey TJ, 2007. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. International Journal of Food Microbiology, 113, 54–61. [DOI] [PubMed] [Google Scholar]
- Allen VM, Weaver H, Ridley AM, Harris JA, Sharma M, Emery J, Sparks N, Lewis M and Edge S, 2008. Sources and spread of thermophilic Campylobacter spp. during partial depopulation of broiler chicken flocks. Journal of Food Protection, 71, 264–270. [DOI] [PubMed] [Google Scholar]
- Allen VM, Ridley AM, Harris JA, Newell DG and Powell L, 2011. Influence of production system on the rate of onset of Campylobacter colonization in chicken flocks reared extensively in the United Kingdom. British Poultry Science, 52, 30–39. [DOI] [PubMed] [Google Scholar]
- Annamalai T, Pina‐Mimbela R, Kumar A, Binjawadagi B, Liu Z, Renukaradhya GJ and Rajashekara G, 2013. Evaluation of nanoparticle‐encapsulated outer membrane proteins for the control of Campylobacter jejuni colonization in chickens. Poultry Science, 92, 2201–2211. [DOI] [PubMed] [Google Scholar]
- Ansari‐Lari M, Hosseinzadeh S, Shekarforoush SS, Abdollahi M and Berizi E, 2011. Prevalence and risk factors associated with campylobacter infections in broiler flocks in Shiraz, southern Iran. International Journal of Food Microbiology, 144, 475–479. [DOI] [PubMed] [Google Scholar]
- ANSES , 2018. Contamination des poulets de chair par Campylobacter ‐ Avis de l'Anses Collective Expert Appraisal Report. É. scientifique.
- Aroori SV, Cogan TA and Humphrey TJ, 2014. Effect of noradrenaline on the virulence properties of campylobacter species. Int J Microbiol, 2014, 279075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asselt ED, de Jong AE, de Jonge R and Nauta MJ, 2008. Cross‐contamination in the kitchen: estimation of transfer rates for cutting boards, hands and knives. Journal of Applied Microbiology, 105, 1392–1401. [DOI] [PubMed] [Google Scholar]
- AVEC , 2018. Available online: http://www.avec-poultry.eu/wp-content/uploads/2018/10/8.-WF-28-09-2018-AVEC-annual-report-2018.pdf
- Bahrndorff S, Rangstrup‐Christensen L, Nordentoft S and Hald B, 2013. Foodborne disease prevention and broiler chickens with reduced campylobacter infection. Emerging Infectious Diseases, 19, 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RA, Kranis A, Psifidi A, Watson KA, Rothwell L, Hocking PM, Kaiser P, Stevens MP and Avendano S, 2018. Colonization of a commercial broiler line by Campylobacter is under limited genetic control and does not significantly impair performance or intestinal health. Poultry Science, 97, 4167–4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios PR, Reiersen J, Lowman R, Bisaillon J‐R, Michel P, Fridriksdóttir V, Gunnarsson E, Stern N, Berke O, McEwen S and Martin W, 2006. Risk factors for Campylobacter spp. colonization in broiler flocks in Iceland. Preventive Veterinary Medicine, 74, 264–278. [DOI] [PubMed] [Google Scholar]
- Battersby T, Whyte P and Bolton D, 2016a. Protecting broilers against Campylobacter infection by preventing direct contact between farm staff and broilers. Food Control, 69, 346–351. [Google Scholar]
- Battersby T, Whyte P and Bolton DJ, 2016b. The pattern of Campylobacter contamination on broiler farms; external and internal sources. Journal of Applied Microbiology, 120, 1108–1118. [DOI] [PubMed] [Google Scholar]
- Battersby T, Walsh D, Whyte P and Bolton D, 2017. Evaluating and improving terminal hygiene practices on broiler farms to prevent Campylobacter cross‐contamination between flocks. Food Microbiology, 64, 1–6. [DOI] [PubMed] [Google Scholar]
- Berndtson E, Emanuelson U, Engvall A and Danielsson‐Tham ML, 1996. A 1‐year epidemiological study of campylobacters in 18 Swedish chicken farms. Preventive Veterinary Medicine, 26, 167–185. [Google Scholar]
- Black RE, Levine MM, Clements ML, Hughes TP and Blaser MJ, 1988. Experimental Campylobacter jejuni Infection in Humans. The Journal of Infectious Diseases, 157, 472–479. [DOI] [PubMed] [Google Scholar]
- Borck Høg B, Sommer HM, Larsen LS, Sørensen AIV, David B, Hofshagen M and Rosenquist H, 2016. Farm specific risk factors for Campylobacter colonisation in Danish and Norwegian broilers. Preventive Veterinary Medicine, 130, 137–145. [DOI] [PubMed] [Google Scholar]
- Bouwknegt M, van de Giessen AW, Dam‐Deisz WD, Havelaar AH, Nagelkerke NJ and Henken AM, 2004. Risk factors for the presence of Campylobacter spp. in Dutch broiler flocks. Preventive veterinary medicine, 62, 35–49. [DOI] [PubMed] [Google Scholar]
- Boysen L, Nauta M and Rosenquist H, 2016. Campylobacter spp. and Escherichia coli contamination of broiler carcasses across the slaughter line in Danish slaughterhouses. Microbial Risk Analysis, 2–3, 63–67. [Google Scholar]
- Brena MC, 2013. Effect of different poultry production methods on Campylobacter incidence and transmission in the broiler meat food chain. Doctor in Philosophy, University of Liverpool. [Google Scholar]
- Brynestad S, Braute L, Luber P and Bartelt E, 2008. Quantitative microbiological risk assessment of campylobacteriosis cases in the German population due to consumption of chicken prepared in homes. International Journal of Risk Assessment and Management, 8, 194–213. [Google Scholar]
- van Bunnik BA, Hagenaars TJ, Bolder NM, Nodelijk G and de Jong MC, 2012a. Interaction effects between sender and receiver processes in indirect transmission of Campylobacter jejuni between broilers. BMC Veterinary Research, 8, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bunnik BA, Katsma WE, Wagenaar JA, Jacobs‐Reitsma WF and de Jong MC, 2012b. Acidification of drinking water inhibits indirect transmission, but not direct transmission of Campylobacter between broilers. Preventive Veterinary Medicine, 105, 315–319. [DOI] [PubMed] [Google Scholar]
- Calistri P and Giovannini A, 2008. Quantitative risk assessment of human campylobacteriosis related to the consumption of chicken meat in two Italian regions. International Journal of Food Microbiology, 128, 274–287. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Gannon BW, Halfhide DE, Santos SB, Hayes CM, Roe JM and Azeredo J, 2010. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiology, 10, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Otten A, Fazil A, Ernst N and Smith BA, 2016. A review of quantitative microbial risk assessment and consumer process models for Campylobacter in broiler chickens. Microbial Risk Analysis, 2–3, 3–15. [Google Scholar]
- Chowdhury S, Sandberg M, Themudo GE and Ersboll AK, 2012. Risk factors for Campylobacter infection in Danish broiler chickens. Poultry Science, 91, 2701–2709. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Themudo GE, Sandberg M and Ersbøll AK, 2013. Spatio‐temporal patterns of Campylobacter colonization in Danish broilers. Epidemiology and Infection, 141, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B, Sommer H, Nielsen N and Rosenquist H, 2001. Risk Assessment of Campylobacter jejuni in Chicken Products. Danish Veterinary and Food Administration, Copenhagen, Denmark. [Google Scholar]
- Colles FM, Preston SG, Barfod KK, Flammer PG, Maiden MCJ and Smith AL, 2019. Parallel sequencing of porA reveals a complex pattern of Campylobacter genotypes that differs between broiler and broiler. Scientific Reports, 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damjanova I, Jakab M, Farkas T, Meszaros J, Galantai Z, Turcsanyi I, Bistyak A, Juhasz A, Paszti J, Kiss I and Kardos G, 2011. From farm to fork follow‐up of thermotolerant campylobacters throughout the broiler production chain and in human cases in a Hungarian county during a ten‐months period. International Journal of Food Microbiology, 150, 95–102. [DOI] [PubMed] [Google Scholar]
- Damme K, Muth F and Mayer A, 2017. Geflügeljahrbuch 2018 ‐ Schwerpunkt: Deutschland im internationalen Vergleich. Suttgart, Deutschland, Eugen Ulmer. [Google Scholar]
- Duffy LL, Blackall PJ, Cobbold RN and Fegan N, 2014. Quantitative effects of in‐line operations on Campylobacter and Escherichia coli through two Australian broiler processing plants. International Journal of Food Microbiology, 188, 128–134. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Analysis of the baseline survey on the prevalence of Campylobacter in broiler batches and of Campylobacter and Salmonella on broiler carcasses in the EU, 2008 ‐ Part A: Campylobacter and Salmonella prevalence estimates. EFSA Journal 2010;8(3):1503 10.2903/j.efsa.2010.1503 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Scientific Opinion on Quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA Journal 2010;8(1):1437, 89 pp. 10.2903/j.efsa.2010.1437 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on Campylobacter in broiler meat production: control options and performance objectives and/or targets at different stages of the food chain. EFSA Journal 2011;9(4):2105, 141 pp. 10.2903/j.efsa.2011.2105 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) and EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2012. Scientific Opinion on the public health hazards to be covered by inspection of meat (poultry). EFSA Journal 2012;10(6):2741, 179 pp. 10.2903/j.efsa.2012.2741 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2012. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2010. EFSA Journal 2012;10(3):2597, 442 pp. 10.2903/j.efsa.2012.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2013. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2011. EFSA Journal 2013;11(4):3129, 250 pp. 10.2903/j.efsa.2013.3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2014. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food‐borne Outbreaks in 2012. EFSA Journal 2014;12(2):3547 10.2903/j.efsa.2014.3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015a. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2014. EFSA Journal 2015;13(12):4329 10.2903/j.efsa.2015.4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):e04634 10.2903/j.efsa.2016.e04634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal 2017;15(12):e05077 10.2903/j.efsa.2017.e05077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2017. EFSA Journal 2018;16(12):5500 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019. The European Union One Health 2018 Zoonoses Report. EFSA Journal 2019;17(12):e05926 10.2903/j.efsa.2019.e05926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2015b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2013. EFSA Journal 2015;13(1):3991 10.2903/j.efsa.2015.3991 [DOI] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck R, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten L, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis‐Iversen J, Smith R, Van Winden S, Paiba G, Watson E, Snow L and Cook A, 2008. Farm practices to control E‐coli O157 in young cattle ‐ a randomised controlled trial. Veterinary Research, 39. [DOI] [PubMed] [Google Scholar]
- Ellis‐Iversen J, Jorgensen F, Bull S, Powell L, Cook AJ and Humphrey TJ, 2009. Risk factors for Campylobacter colonisation during rearing of broiler flocks in Great Britain. Preventive Veterinary Medicine, 89, 178–184. [DOI] [PubMed] [Google Scholar]
- Ellis‐Iversen J, Ridley A, Morris V, Sowa A, Harris J, Atterbury R, Sparks N and Allen V, 2012. Persistent environmental reservoirs on farms as risk factors for Campylobacter in commercial poultry. Epidemiology and Infection, 140, 916–924. [DOI] [PubMed] [Google Scholar]
- Elvers KT, Morris VK, Newell DG and Allen VM, 2011. Molecular tracking, through processing, of campylobacter strains colonizing broiler flocks. Applied and Environmental Microbiology, 77, 5722–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2016. Available online: https://ec.europa.eu/info/sites/info/files/food-farming-fisheries/farming/documents/organic-agriculture-2015_en.pdf
- Eurostat , 2018. Available online: http://ec.europa.eu/eurostat/data/database (last accessed 17 January 2019).
- Eurostat , 2020. Available online: http://ec.europa.eu/eurostat/data/database (last accessed 22 January 2020).
- Georgiev M, Beauvais W and Guitian J, 2017. Effect of enhanced biosecurity and selected on‐farm factors on Campylobacter colonization of chicken broilers. Epidemiology and Infection, 145, 553–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghareeb K, Awad WA, Mohnl M, Schatzmayr G and Bohm J, 2013. Control strategies for Campylobacter infection in poultry production. Worlds Poultry Science Journal, 69, 57–76. [Google Scholar]
- Gracia MI, Millan C, Sanchez J, Guyard‐Nicodeme M, Mayot J, Carre Y and Medel P, 2016a. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period: part B. Poultry Science, 95, 886–892. [DOI] [PubMed] [Google Scholar]
- Gracia MI, Sánchez J, Millán C, Casabuena Ó, Vesseur P, Martín Á, García‐Peña FJ and Medel P, 2016b. Effect of feed form and whole grain feeding on gastrointestinal weight and the prevalence of Campylobacter jejuni in broilers orally infected. PLoS ONE, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove‐White D, Leatherbarrow A, Cripps P, Diggle P and French N, 2011. Molecular epidemiology and genetic diversity of Campylobacter jejuni in ruminants. Epidemiology & Infection, 139, 1661–1671. [DOI] [PubMed] [Google Scholar]
- Guyard‐Nicodème M, Keita A, Quesne S, Amelot M, Poezevara T, Le Berre B, Sanchez J, Vesseur P, Martin A, Medel P and Chemaly M, 2016. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poultry Science, 95, 298–305. [DOI] [PubMed] [Google Scholar]
- Guyard‐Nicodème M, Huneau‐Salaün A, Tatone FA, Skiba F, Quentin M, Quesne S, Poezevara T and Chemaly M, 2017. Effect of feed additives on productivity and Campylobacter spp. loads in broilers reared under free range conditions. Frontiers in Microbiology, 8, 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald B, Wedderkopp A and Madsen M, 2000. Thermophilic Campylobacter spp. in Danish broiler production: a cross‐sectional survey and a retrospective analysis of risk factors for occurrence in broiler flocks. Avian Pathology, 29, 123–131. [DOI] [PubMed] [Google Scholar]
- Hankel J, Popp J, Meemken D, Zeiger K, Beyerbach M, Taube V, Klein G and Visscher C, 2018. Influence of lauric acid on the susceptibility of chickens to an experimental Campylobacter jejuni colonisation. PLoS ONE, 13(9), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson I, Engvall EO, Vagsholm I and Nyman A, 2010. Risk factors associated with the presence of Campylobacter‐positive broiler flocks in Sweden. Preventive Veterinary Medicine, 96, 114–121. [DOI] [PubMed] [Google Scholar]
- Haughton PN, Lyng J, Fanning S and Whyte P, 2013. Potential of a commercially available water acidification product for reducing Campylobacter in broilers prior to slaughter. British Poultry Science, 54, 319–324. [DOI] [PubMed] [Google Scholar]
- Havelaar AH, Mangen M‐JJ, De Koeijer AA, Bogaardt M‐J, Evers EG, Jacobs‐Reitsma WF, Van Pelt W, Wagenaar JA, De Wit GA, Van Der Zee H and Nauta MJ, 2007. Effectiveness and efficiency of controlling campylobacter on broiler chicken meat. Risk Analysis, 27, 831–844. [DOI] [PubMed] [Google Scholar]
- Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I and De Zutter L, 2003. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiology and Infection, 131, 1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Martel A, Van Deun K, Verlinden M, Van Immerseel F, Garmyn A, Messens W, Heyndrickx M, Haesebrouck F and Pasmans F, 2010. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium‐chain fatty acids. Poultry science, 89, 1144–1155. [DOI] [PubMed] [Google Scholar]
- Hermans D, Van Deun K, Messens W, Martel A, Van Immerseel F, Haesebrouck F, Rasschaert G, Heyndrickx M and Pasmans F, 2011. Campylobacter control in poultry by current intervention measures ineffective: Urgent need for intensified fundamental research. Veterinary Microbiology, 152, 219–228. [DOI] [PubMed] [Google Scholar]
- Hermans D, Martel A, Garmyn A, Verlinden M, Heyndrickx M, Gantois I, Haesebrouck F and Pasmans F, 2012. Application of medium‐chain fatty acids in drinking water increases Campylobacter jejuni colonization threshold in broiler chicks. Poultry Science, 91, 1733–1738. [DOI] [PubMed] [Google Scholar]
- Higham LE, Scott C, Akehurst K, Dring D, Parnham A, Waterman M and Bright A, 2018. Effects of financial incentives and cessation of thinning on prevalence of Campylobacter: a longitudinal monitoring study on commercial broiler farms in the UK. Veterinary Record, vetrec‐2017‐104823. [DOI] [PubMed]
- Hue O, Allain V, Laisney M‐J, Le Bouquin S, Lalande F, Petetin I, Rouxel S, Quesne S, Gloaguen P‐Y, Picherot M, Santolini J, Bougeard S, Salvat G and Chemaly M, 2011. Campylobacter contamination of broiler caeca and carcasses at the slaughterhouse and correlation with Salmonella contamination. Food Microbiology, 28, 862–868. [DOI] [PubMed] [Google Scholar]
- Huneau‐Salaun A, Denis M, Balaine L and Salvat G, 2007. Risk factors for Campylobacter spp. colonization in French free‐range broiler‐chicken flocks at the end of the indoor rearing period. Preventive Veterinary Medicine, 80, 34–48. [DOI] [PubMed] [Google Scholar]
- Huneau‐Salaun A, Guyard‐Nicodème M, Benzoni G, Gautier X, Quesne S, Poezevara T and Chemaly M, 2018. Randomized control trial to test the effect of a feed additive on Campylobacter contamination in commercial broiler flocks up to slaughter. Zoonoses and Public Health, 65, 404–411. [DOI] [PubMed] [Google Scholar]
- Jansen W, Reich F and Klein G, 2014. Large‐scale feasibility of organic acids as a permanent preharvest intervention in drinking water of broilers and their effect on foodborne Campylobacter spp. before processing. Journal of Applied Microbiology, 116, 1676–1687. [DOI] [PubMed] [Google Scholar]
- Jonsson ME, Chriél M, Norström M and Hofshagen M, 2012. Effect of climate and farm environment on Campylobacter spp. colonisation in Norwegian broiler flocks. Preventive Veterinary Medicine, 107, 95–104. [DOI] [PubMed] [Google Scholar]
- Jozefiak D and Sip A, 2013. Bacteriocins in poultry nutrition ‐ a review. Annals of Animal Science, 13, 449–462. [Google Scholar]
- Kaiser P, Howell MM, Fife M, Sadeyen JR, Salmon N, Rothwell L, Young J, Poh TY, Stevens M, Smith J, Burt D, Swaggerty C and Kogut M, 2009. Towards the selection of chickens resistant to Salmonella and Campylobacter infections. Bulletin et mémoires de l'Académie royale de médecine de Belgique, 164, 17–25. [PubMed] [Google Scholar]
- Kalupahana RS, Kottawatta KS, Kanankege KS, van Bergen MA, Abeynayake P and Wagenaar JA, 2013. Colonization of Campylobacter spp. in broiler chickens and laying hens reared in tropical climates with low‐biosecurity housing. Applied and Environment Microbiology, 79, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler S, Fischer S, Abdulmawjood A, Glunder G and Klein G, 2013. Effect of bacteriophage application on Campylobacter jejuni loads in commercial broiler flocks. Applied and Environmental Microbiology, 79, 7525–7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolman L, Whyte P and Bolton DJ, 2014. An investigation of broiler caecal Campylobacter counts at first and second thinning. Journal of Applied Microbiology, 117, 876–881. [DOI] [PubMed] [Google Scholar]
- Laureano L, Corujo A and Van Gerwe T, 2013. Correlation of different matrix with Campylobacter counts in neck skin of broiler carcasses Organisms. Aberdeen, UK. [Google Scholar]
- Lawes JR, Vidal A, Clifton‐Hadley FA, Sayers R, Rodgers J, Snow L, Evans SJ and Powell LF, 2012. Investigation of prevalence and risk factors for Campylobacter in broiler flocks at slaughter: results from a UK survey. Epidemiology and Infection, 140, 1725–1737. [DOI] [PubMed] [Google Scholar]
- Legaudaite‐Lydekaitiene V, Serniene L, Vismantaite V, Malakauskas M and Kudirkiene E, 2017. Broiler health status has a major negative impact on broiler flock contamination with Campylobacter Spp. In Lithuania. Annals of Animal Science, 17, 799–817. [Google Scholar]
- Levin ML, 1953. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum, 9, 531–541. [PubMed] [Google Scholar]
- Lindqvist R and Lindblad M, 2008. Quantitative risk assessment of thermophilic Campylobacter spp. and cross‐contamination during handling of raw broiler chickens evaluating strategies at the producer level to reduce human campylobacteriosis in Sweden. International Journal of Food Microbiology, 121, 41–52. [DOI] [PubMed] [Google Scholar]
- Liu J, Parrish JR, Hines J, Mansfield L and Finley RL Jr, 2019. A proteome‐wide screen of Campylobacter jejuni using protein microarrays identifies novel and conformational antigens. PLoS ONE, 14, e0210351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc Carrillo C, Atterbury RJ, El‐Shibiny A, Connerton PL, Dillon E, Scott A and Connerton IF, 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Applied and Environmental Microbiology, 71, 6554–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer MF and Kiermeier A, 2007. Analysing microbiological data: Tobit or not Tobit? International Journal of Food Microbiology, 116, 313–318. [DOI] [PubMed] [Google Scholar]
- Lyngstad TM, Jonsson ME, Hofshagen M and Heier BT, 2008. Risk factors associated with the presence of Campylobacter species in Norwegian broiler flocks. Poultry Science, 87, 1987–1994. [DOI] [PubMed] [Google Scholar]
- Malher X, Simon M, Charnay V, des Deserts RD, Lehebel A and Belloc C, 2011. Factors associated with carcass contamination by Campylobacter at slaughterhouse in cecal‐carrier broilers. International Journal of Food Microbiology, 150, 8–13. [DOI] [PubMed] [Google Scholar]
- Mancabelli L, Ferrario C, Milani C, Mangifesta M, Turroni F and Duranti S, 2016. Insights into the biodiversity of the gut microbiota of broiler chickens. Environmental Microbiology, 18, 4727–4738. [DOI] [PubMed] [Google Scholar]
- Manes‐Lazaro R, Van Diemen PM, Pin C, Mayer MJ, Stevens MP and Narbad A, 2017. Administration of Lactobacillus johnsonii FI9785 to chickens affects colonisation by Campylobacter jejuni and the intestinal microbiota. British Poultry Science, 58, 373–381. [DOI] [PubMed] [Google Scholar]
- McDowell SW, Menzies FD, McBride SH, Oza AN, McKenna JP, Gordon AW and Neill SD, 2008. Campylobacter spp. in conventional broiler flocks in Northern Ireland: epidemiology and risk factors. Preventive Veterinary Medicine, 84, 261–276. [DOI] [PubMed] [Google Scholar]
- Messaoudi S, Kergourlay G, Rossero A, Ferchichi M, Prevost H, Drider D, Manai M and Dousset X, 2011. Identification of lactobacilli residing in chicken ceca with antagonism against Campylobacter. International microbiology, 14, 103–110. [DOI] [PubMed] [Google Scholar]
- Meunier M, Guyard‐Nicodème M, Hirchaud E, Parra A, Chemaly M and Dory D, 2016. Identification of novel vaccine candidates against campylobacter through reverse vaccinology. Journal of Immunology Research, 20165715790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Guyard‐Nicodème M, Vigouroux E, Poezevara T, Beven V, Quesne S, Bigault L, Amelot M, Dory D and Chemaly M, 2017. Promising new vaccine candidates against Campylobacter in broilers. PLoS ONE, 12, e0188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman C, Christley R, Rigby D, Dennis D, O'Brien SJ and Williams N, 2017. “Catch 22”: Biosecurity awareness, interpretation and practice amongst poultry catchers. Preventive Veterinary Medicine, 141, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen B, Rudi K, Svihus B and Skanseng B, 2012. Reduced spread of Campylobacter jejuni in broiler chickens by stimulating the bird's natural barriers. Journal of Applied Microbiology, 113, 1176–1183. [DOI] [PubMed] [Google Scholar]
- Mughini Gras L, Smid JH, Wagenaar JA, de Boer AG, Havelaar AH, Friesema IH, French NP, Busani L and van Pelt W, 2012. Risk factors for campylobacteriosis of chicken, ruminant, and environmental origin: a combined case‐control and source attribution analysis. PLoS ONE, 7, e42599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughini‐Gras L, Penny C, Ragimbeau C, Schets FM, Blaak H, Duim B, Wagenaar JA, de Boer A, Cauchie HM, Mossong J and van Pelt W, 2016. Quantifying potential sources of surface water contamination with Campylobacter jejuni and Campylobacter coli. Water Research, 101, 36–45. [DOI] [PubMed] [Google Scholar]
- Mylius SD, Nauta MJ and Havelaar AH, 2007. Cross‐contamination during food preparation: a mechanistic model applied to chicken‐borne Campylobacter. Risk Analysis, 27, 803–813. [DOI] [PubMed] [Google Scholar]
- Näther G, Alter T, Martin A and Ellerbroek L, 2009. Analysis of risk factors for Campylobacter species infection in broiler flocks. Poultry Science, 88, 1299–1305. [DOI] [PubMed] [Google Scholar]
- Nauta M, 2020. Modelling approach for control options affecting the Campylobacter concentration in the caecal content of broilers. Zenodo. 10.5281/zenodo.3742196 [DOI] [Google Scholar]
- Nauta M and Christensen B, 2011. The impact of consumer phase models in microbial risk analysis. Risk Analysis, 31, 255–265. [DOI] [PubMed] [Google Scholar]
- Nauta MJ, Fischer AR, van Asselt ED, de Jong AE, Frewer LJ and de Jonge R, 2008. Food safety in the domestic environment: the effect of consumer risk information on human disease risks. Risk Analysis, 28, 179–192. [DOI] [PubMed] [Google Scholar]
- Nauta M, Hill A, Rosenquist H, Brynestad S, Fetsch A, van der Logt P, Fazil A, Christensen B, Katsma E, Borck B and Havelaar A, 2009. A comparison of risk assessments on Campylobacter in broiler meat. International Journal of Food Microbiology, 129, 107–123. [DOI] [PubMed] [Google Scholar]
- Nauta MJ, Sanaa M and Havelaar AH, 2012. Risk based microbiological criteria for Campylobacter in broiler meat in the European Union. International Journal of Food Microbiology, 158, 209–217. [DOI] [PubMed] [Google Scholar]
- Nauta M, Johannessen G, Laureano Adame L, Williams N and Rosenquist H, 2016. The effect of reducing numbers of Campylobacter in broiler intestines on human health risk. Microbial Risk Analysis, 2–3, 68–77. [Google Scholar]
- Neal‐McKinney JM, Samuelson DR, Eucker TP, Nissen MS, Crespo R and Konkel ME, 2014. Reducing Campylobacter jejuni colonization of poultry via vaccination. PLoS ONE, 9, e114254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I, Pearson D, Salvat G and Allen VM, 2011. Biosecurity‐based interventions and strategies to reduce campylobacter spp. on poultry farms. Applied and Environmental Microbiology, 77, 8605–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuengjamnong C and Luangtongkum T, 2014. Effects of effective microorganisms on growth performances, ammonia reduction, hematological changes and shedding of Salmonella enterica and Campylobacter spp. in broilers. Thai Journal of Veterinary Medicine, 44, 15–22. [Google Scholar]
- Oakley JE and O'Hagan A, 2019. SHELF: the Sheffield Elicitation Framework (version 4). School of Mathematics and Statistics. University of Sheffield, UK. Available online: http://tonyohagan.co.uk/shelf [Google Scholar]
- Okamura M, Tominaga A, Ueda M, Ohshima R, Kobayashi M, Tsukada M, Yokoyama E, Takehara K, Deguchi K, Honda T and Nakamura M, 2012. Irrelevance between the induction of anti‐campylobacter humoral response by a bacterin and the lack of protection against homologous challenge in Japanese Jidori Chickens. Journal of Veterinary Medical Science, 74, 75–78. [DOI] [PubMed] [Google Scholar]
- Poisson S, Gauchard F, Sanaa M and Guillier L, 2015. Quantitative risk assessment of human campylobacteriosis related to the consumption of chicken meat in France: focus on the consumer phase European Symposium on the Quality of Poultry Meat and Eggs and Egg Products. Nantes, World's Poultry Science Association. [Google Scholar]
- Pourhoseingholi MA, Baghestani AR and Vahedi M, 2012. How to control confounding effects by statistical analysis. Gastroenterology and hepatology from bed to bench, 5, 79–83. [PMC free article] [PubMed] [Google Scholar]
- Refregier‐Petton J, Rose N, Denis M and Salvat G, 2001. Risk factors for Campylobacter spp. contamination in French broiler‐chicken flocks at the end of the rearing period. Preventive Veterinary Medicine, 50, 89–100. [DOI] [PubMed] [Google Scholar]
- Reich F, Atanassova V, Haunhorst E and Klein G, 2008. The effects of Campylobacter numbers in caeca on the contamination of broiler carcasses with Campylobacter. International Journal of Food Microbiology, 127, 116–120. [DOI] [PubMed] [Google Scholar]
- Reich F, Valero A, Schill F, Bungenstock L and Klein G, 2018. Characterisation of Campylobacter contamination in broilers and assessment of microbiological criteria for the pathogen in broiler slaughterhouses. Food Control, 87, 60–69. [Google Scholar]
- Riazi A, Strong PC, Coleman R, Chen W, Hirama T, van Faassen H, Henry M, Logan SM, Szymanski CM, Mackenzie R and Ghahroudi MA, 2013. Pentavalent single‐domain antibodies reduce Campylobacter jejuni motility and colonization in chickens. PLoS ONE, 8, e83928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards PJ, Connerton PL and Connerton IF, 2019. Phage biocontrol of campylobacter jejuni in chickens does not produce collateral effects on the gut microbiota. Frontiers in Microbiology, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AM, Morris VK, Cawthraw SA, Ellis‐Iversen J, Harris JA, Kennedy EM, Newell DG and Allen VM, 2011. Longitudinal molecular epidemiological study of thermophilic campylobacters on one conventional broiler chicken farm. Applied and Environmental Microbiology, 77, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyn J, Rasschaert G, Hermans D, Pasmans F and Heyndrickx M, 2013. Is allicin able to reduce Campylobacter jejuni colonization in broilers when added to drinking water? Poultry Science, 92, 1408–1418. [DOI] [PubMed] [Google Scholar]
- Robyn J, Rasschaert G, Pasmans F and Heyndrickx M, 2015. Thermotolerant Campylobacter during broiler rearing: risk factors and intervention. Comprehensive Reviews in Food Science and Food Safety, 14, 81–105. [DOI] [PubMed] [Google Scholar]
- Rodgers JD, 2020. APHA/FSA monitoring programme for Campylobacter in broiler flocks and broiler carcases in the UK (2012‐2017) (FS241051, FS101126). [Data set]. Zenodo. 10.5281/zenodo.3742190 [DOI]
- Rosenquist H, Sommer HM, Nielsen NL and Christensen BB, 2006. The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. International Journal of Food Microbiology, 108, 226–232. [DOI] [PubMed] [Google Scholar]
- Rosner BM, Schielke A, Didelot X, Kops F, Breidenbach J, Willrich N, Golz G, Alter T, Stingl K, Josenhans C, Suerbaum S and Stark K, 2017. A combined case‐control and molecular source attribution study of human Campylobacter infections in Germany, 2011‐2014. Scientific Reports, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royden A, Wedley A, Merga JY, Rushton S, Hald B, Humphrey T and Williams NJ, 2016. A role for flies (Diptera) in the transmission of Campylobacter to broilers? Epidemiology and Infection, 144, 3326–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint‐Cyr MJ, Haddad N, Taminiau B, Poezevara T, Quesne S, Amelot M, Daube G, Chemaly M, Dousset X and Guyard‐Nicodème M, 2017. Use of the potential probiotic strain Lactobacillus salivarius SMXD51 to control Campylobacter jejuni in broilers. International Journal of Food Microbiology, 247, 9–17. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Sorensen LL, Steenberg B, Chowdhury S, Ersboll AK and Alban L, 2015. Risk factors for Campylobacter colonization in Danish broiler flocks, 2010 to 2011. Poultry Science, 94, 447–453. [DOI] [PubMed] [Google Scholar]
- Schneitz C and Hakkinen M, 2016. The efficacy of a commercial competitive exclusion product on Campylobacter colonization in broiler chickens in a 5‐week pilot‐scale study. Poultry Science, 95, 1125–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SK and Maiden MC, 2015. The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harbor Perspectives in Biology, 7, a018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Arsi K, Wagle BR, Donoghue AM and Donoghue DJ, 2017. Ability of select probiotics to reduce enteric Campylobacter colonization in broiler chickens. International Journal of Poultry Science, 16, 37–42. [Google Scholar]
- Skoufos I, Tzora A, Giannenas I, Bonos E, Tsinas A, McCartney E, Lester H, Christaki E, Florou‐Paneri P, Mandavi J and Soultanas P, 2019. Evaluation of in‐field efficacy of dietary ferric tyrosine on performance, intestinal health and meat quality of broiler chickens exposed to natural Campylobacter jejuni challenge. Livestock Science, 221, 44–51. [Google Scholar]
- Smith S, Messam LL, Meade J, Gibbons J, McGill K, Bolton D and Whyte P, 2016. The impact of biosecurity and partial depopulation on Campylobacter prevalence in Irish broiler flocks with differing levels of hygiene and economic performance. Infection Ecology and Epidemiology, 6, 31454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer HM, Heuer OE, Sorensen AIV and Madsen M, 2013. Analysis of factors important for the occurrence of Campylobacter in Danish broiler flocks. Preventive Veterinary Medicine, 111, 100–111. [DOI] [PubMed] [Google Scholar]
- Sommer HM, Høg BB, Larsen LS, Sørensen AIV, Williams N, Merga JY, Cerdà‐Cuéllar M, Urdaneta S, Dolz R, Wieczorek K, Osek J, David B, Hofshagen M, Jonsson M, Wagenaar JA, Bolder N and Rosenquist H, 2016a. Analysis of farm specific risk factors for Campylobacter colonization of broilers in six European countries. Microbial Risk Analysis, 2–3, 16–26. [Google Scholar]
- Sommer HM, Nauta M and Rosenquist H, 2016b. Translation of risk factor estimates into on‐farm interventions and their effect on Campylobacter broiler flock prevalence. Microbial Risk Analysis, 2–3, 27–37. [Google Scholar]
- Sorensen MCH, Gencay YE, Birk T, Baldvinsson SB, Jackel C, Hammerl JA, Vegge CS, Neve H and Brondsted L, 2015. Primary isolation strain determines both phage type and receptors recognised by campylobacter jejuni bacteriophages. PLoS ONE, 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford RJ, Schluter PJ, Wilson AJ, Kirk MD, Hall G, Unicomb L and OzFoodNet Working G, 2008. Population‐attributable risk estimates for risk factors associated with Campylobacter infection, Australia. Emerging Infectious Diseases, 14, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern NJ and Robach MC, 2003. Enumeration of Campylobacter spp. in broiler feces and in corresponding processed carcasses. Journal of Food Protection, 66, 1557–1563. [DOI] [PubMed] [Google Scholar]
- Stern NJ, Meinersmann RJ and Dickerson HW, 1990. Influence of antibody treatment of Campylobacter jejuni on the dose required to colonize chicks. Avian Diseases, 34, 595–601. [PubMed] [Google Scholar]
- Stern NJ, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich EV, Mitsevich IP, Pokhilenko VD, Levchuk VP, Svetoch OE and Seal BS, 2006. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrobial Agents and Chemotherapy, 50, 3111–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetoch EA, Eruslanov BV, Levchuk VP, Perelygin VV, Mitsevich EV, Mitsevich IP, Stepanshin J, Dyatlov I, Seal BS and Stern NJ, 2011. Isolation of Lactobacillus salivarius 1077 (NRRL B‐50053) and characterization of its bacteriocin, including the antimicrobial activity spectrum. Applied and Environmental Microbiology, 77, 2749–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangkham W, Janes M and LeMieux F, 2016. Prevalence and Distribution of Campylobacter jejuni in Small‐Scale Broiler Operations. Journal of Food Protection, 79, 75–81. [DOI] [PubMed] [Google Scholar]
- Teunis PF and Havelaar AH, 2000. The Beta Poisson dose‐response model is not a single‐hit model. Risk Analysis, 20, 513–520. [DOI] [PubMed] [Google Scholar]
- Teunis PFM, Bonačić Marinović A, Tribble DR, Porter CK and Swart A, 2018. Acute illness from Campylobacter jejuni may require high doses while infection occurs at low doses. Epidemics, 24, 1–20. [DOI] [PubMed] [Google Scholar]
- Thibodeau A, Fravalo P, Yergeau E, Arsenault J, Lahaye L and Letellier A, 2015. Chicken caecal microbiome modifications induced by campylobacter jejuni colonization and by a non‐antibiotic feed additive. PLoS ONE, 10, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralbo A, Borge C, Allepuz A, Garcia‐Bocanegra I, Sheppard SK, Perea A and Carbonero A, 2014. Prevalence and risk factors of Campylobacter infection in broiler flocks from southern Spain. Preventive Veterinary Medicine, 114, 106–113. [DOI] [PubMed] [Google Scholar]
- Umaraw P, Prajapati A, Verma AK, Pathak V and Singh VP, 2017. Control of campylobacter in poultry industry from farm to poultry processing unit: A review. Critical Reviews in Food Science and Nutrition, 57, 659–665. [DOI] [PubMed] [Google Scholar]
- Van Limbergen T, Dewulf J, Klinkenberg M, Ducatelle R, Gelaude P, Mendez J, Heinola K, Papasolomontos S, Szeleszczuk P and Maes D and Prohealth consortium , 2018. Scoring biosecurity in European conventional broiler production. Poultry Science, 97, 74–83. [DOI] [PubMed] [Google Scholar]
- Vandeputte J, Martel A, Van Rysselberghe N, Antonissen G, Verlinden M, De Zutter L, Heyndrickx M, Haesebrouck F, Pasmans F and Garmyn A, 2019. In ovo vaccination of broilers against Campylobacter jejuni using a bacterin and subunit vaccine. Poultry Science, 98, 5999–6004. [DOI] [PubMed] [Google Scholar]
- Vinueza‐Burgos C, Cevallos M, Cisneros M, Van Damme I and De Zutter L, 2018. Quantification of the Campylobacter contamination on broiler carcasses during the slaughter of Campylobacter positive flocks in semi‐industrialized slaughterhouses. International Journal of Food Microbiology, 269, 75–79. [DOI] [PubMed] [Google Scholar]
- Wagenaar JA, Van Bergen MA, Mueller MA, Wassenaar TM and Carlton RM, 2005. Phage therapy reduces Campylobacter jejuni colonization in broilers. Veterinary Microbiology, 109, 275–283. [DOI] [PubMed] [Google Scholar]
- van Wagenberg CPA, van Horne PLM, Sommer HM and Nauta MJ, 2016. Cost‐effectiveness of Campylobacter interventions on broiler farms in six European countries. Microbial Risk Analysis, 2–3, 53–62. [Google Scholar]
- Wagle BR, Upadhyay A, Arsi K, Shrestha S, Venkitanarayanan K, Donoghue AM and Donoghue DJ, 2017. Application of beta‐resorcylic acid as potential antimicrobial feed additive to reduce campylobacter colonization in broiler chickens. Frontiers in Microbiology, 8, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis AM, Storey DB, Taff CC, Townsend AK, Huang BC, Kong NT, Clothier KA, Spinner A, Byrne BA and Weimer BC, 2016. Genomic comparison of campylobacter spp. and their potential for zoonotic transmission between birds, primates, and livestock. Applied and Environmental Microbiology, 82, 7165–7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO , 2009. Risk assessment of Campylobacter spp. in broiler chickens: technical report. Microbiological Risk Assessment Series, 12. [Google Scholar]
- Zhang J and Kai FYJJ, 1998. What's the relative risk?: A method of correcting the odds ratio in cohort studies of common outcomes. JAMA, 280, 1690–1691. [DOI] [PubMed] [Google Scholar]
- Zhang C, Weiss A, Lin C, Li H, Joerger R and Chiu P, 2016. Effects of multiple litter amendment applications in commercial broiler houses on ammonia emissions and litter microflora. Transactions of the ASABE, 59, 1393–1401. [Google Scholar]


