Abstract
Background
Globally, nearly a third of the population suffers from at least one form of malnutrition. Both over- and undernutrition are a growing concern in developing countries particularly among female adolescents. This study was aimed at assessing nutritional status and associated factors among female adolescents in secondary schools of Bahir Dar City, Amhara, Ethiopia, 2019.
Methods
A school-based cross-sectional study was conducted in secondary schools of Bahir Dar City among 682 female adolescent students in 2019. A simple random sampling technique with proportional allocation was used to select study participants. Data were collected using a self-administered questionnaire. Data were entered into Epi Info version 7.1 and analyzed by SPSS version 21. Underweight and overweight statuses of the participants were determined by using the WHO cutoff point. Bivariable and multivariable logistic regressions were used to identify the significance of association at a 95% confidence interval. P value < 0.05 was used to declare statistical significance.
Results
In this study, the prevalence of underweight, overweight, and obesity was 15%, 8.4%, and 4.7%, respectively. Female adolescents found between age groups of 14-16.5 years old (AOR: 1.7, 95% CI: 1.03-2.69), family size ≥ 4 (AOR: 2.8, 95% CI: 1.05-4.99), participants who did not eat meat once per week (AOR: 1.6, 95% CI: 1.90-2.82), and no onset of menarche (AOR: 4.4, 95% CI: 1.21-15.75) were found to be more likely underweight. In addition, adolescents with family monthly income above 6500 Ethiopian birr (AOR: 12.7, 95% CI: 2.47-65.62), who ate meat two times and more per week (AOR: 2.07, 95% CI: 1.47-9.14), and who ate fruit at least once a week (AOR: 0.20, 95% CI: 0.05-0.78) were more likely to be overweight compared with counterparts. Conclusion and Recommendation. The prevalence of underweight and overweight was found to be high. Design evidence-based adolescent nutritional intervention shall be emphasized by the government and other concerned bodies to avert the dual burden of malnutrition.
1. Background
Nutrition is recognized as a key determinant of health and well-being and a contributor to human capital development [1]. Poor nutrition starts before birth, continues into adolescence and adult life, and can span generations [2]. Body size during adolescence can be used as a proxy indicator for nutritional status, with overnutrition manifesting as overweight and obesity, while undernutrition can manifest as stunting and/or wasting. Adolescence is a period of rapid physiological, sexual, neurological, and behavioral changes which lays a foundation to adult role and responsibility [3]. Twenty percent of final adult height and 50% of adult weight are attained during the period of adolescence since it is the 2nd period of rapid growth next to the first one year following birth [4, 5]. Investment in adolescent nutrition could have economic and social benefits including saving health care costs, increased intellectual capacity, and adult productivity [6–8].
Globally, nearly one in three persons suffers from at least one form of malnutrition. Rapid nutrition and demographic transition resulted in change in living condition and dietary habit leading to a coexistence of over- and undernutrition [9]. The double burden of malnutrition, both underweight due to undernutrition and overweight due to overnutrition, are a growing concern among female adolescents in developing countries [8, 10–12]. A study conducted among school adolescents in Adama City, Central Ethiopia, indicated that 21.3% and 3.3% of adolescents were under and overweight, respectively [13]. Another study conducted in South Africa about predictors of weight status and obesity disclosed that combined overweight and obesity were found to be higher among female adolescents (15%) compared with male counterparts (4%) [8]. Malnutrition is a multifaceted problem influenced by biological, environmental, social, and behavioral factors [9]. Evidence from different literature indicated that poor dietary diversity score, place of residence, parental level of education, and life styles were predictors of malnutrition among female adolescents [12, 14].
Although malnutrition is a prevailing major public health problem [1], adolescent malnutrition is not well studied particularly in low- and middle-income countries. Little information is available in the Ethiopian context and in Bahir Dar in particular. This study was aimed at assessing malnutrition and its associated factors among female school adolescents in Bahir Dar City, Amhara Reginal State, Ethiopia, which might provide evidence for program implementers and policy makers for decision-making purposes.
2. Methods
2.1. Study Area, Design, and Population
A school-based cross-sectional study was conducted among female secondary school students in Bahir Dar City, Amhara, Ethiopia, from January to May 2019. In the city, there were 11 high schools (6 governmental and 5 private secondary schools) running a total of 16,644 students at the time of the study. Of these, 8364 of them were females. All secondary school female students in Bahir Dar City were the source population. While all female students in the selected schools were the study population, students who were not attending their education during data collection period, apparent physical deformity (like kyphoscoliosis), cognitive limitation, and those below 10 and above 19 years old were excluded from this study.
2.2. Sample Size Determination and Sampling Procedure
The sample size was determined by using a single population proportion formula with the prevalence of underweight 28% and overweight 5.2% [15]. Maximum prevalence was used to calculate the samples size. Other assumptions were 95% confidence (Z = 1.96), 5% margin error (d), design effect of 2, and nonresponse rate of 10% giving a final sample size of 622. Multistage random sampling technique was used. From a total of 11 secondary schools in Bahir Dar City, three secondary schools (2 governmental and 1 private) were selected using a simple random sampling technique after stratifying secondary schools as governmental and private. The number of schools selected for this study was determined by taking 20% from the total secondary schools as a rule of thumb. Students' rosters from each school were used as the sampling frame to select the study participants using a simple random sampling technique after proportional allocation of samples. Participants were selected by lottery method in each school. Hence, a total of 682 (583 from governmental and 99 from private schools) were selected to participate in this study.
2.3. Data Collection Procedure
A pretested structured questionnaire was used to collect the data through a self-administered method. The participants gave their response in a private setting after obtaining the consent from parents and participants. The questionnaire first prepared in English and was then translated into the local language Amharic and back into English by language experts to check consistency.
2.4. Study Variables
In this study, the dependent variables were underweight and overweight, measured based on WHO criteria, whereas the independent variables were sociodemographic characteristics of the respondents and parents (age of the respondent, type of school, level of grade, marital status of the respondents, family size, religion, ethnicity, sex of head of the household, occupational status of the father, occupational status of the mother, educational level of the father, educational level of the mother, monthly family income, and means of transport), diet-related information (staple diet, meals per day, vegetable intake per day, fruit intake per week, meat intake per week, snack intake per day, hunger in the last 30 days due to shortage of food, and additional food consume), behavior, life style, and health status (hard physical activities per week, moderate physical activities, sport done (gymnastic, swimming), time taken to school, alcohol drinking habit, cigarette smoking habit, availability of latrine, any illness in the past 2 weeks, and sources of drinking water).
2.5. Variable Measurements
Nutritional status of female adolescents (both underweight and overweight) was assessed using anthropometric measurement. A two-day training was given for 6 health officers and 2 human nutrition professionals about the procedures of the data collection instrument and measurements. Weight was measured by a calibrated and portable personal weight scale. During measurement, any heavy clothes, shoes, socks, and items from their pockets were removed from participants. Before measurement, the scale was zeroed, and the study participants stand in the centre without any support until the result is recorded. Zero adjustment on the scale was also made before taking the next measurement [16, 17]. Height was measured using a calibrated stadiometer with head board. During height measurement, the data collector wiped to clean the stadiometer and explained to the study participant about the measurement. Participants were asked to take off their shoes, wear light clothes, and stand with their back to the stadiometer and look forward directly with their arms hanging loosely at their sides. The back of their feet, calves, buttocks, shoulders, and the back of their head were made to be in contact with the stadiometer. Finally, the participants were asked to take a deep breath and hold [18, 19]. All measurements were taken to the nearest 0.1 kg and 0.1 cm for weight and height measurements, respectively. One measurement was taken by two data collectors and a third measurement was taken if the two differed by ≥0.5 kg and ≥0.5 cm for the weight and height, respectively [17, 18].
BMI-for-age Z-score values were used to determine the nutritional status of the participants by using the WHO Anthro Plus software. Based on the Z-score value, obesity is defined as greater than +2SD, overweight is greater than +1SD, normal weight is between less than +1SD and greater than -2SD, and underweight is less than -2SD [20]. In this study, adolescence is age groups between 10 and 19years old, secondary school is from grades 9 to 12, and physical activity is a total of one hour per day of moderate- to high-intensity physical activities.
2.6. Data Processing and Analysis
Data were entered and coded by Epi Info version 7.1 software. The data cleaning and analysis were performed by SPSS version 21 software. Descriptive and analytical statistics were computed. Bivariable and multivariable logistic regressions were conducted to identify the predictors of undernutrition and overnutrition among female adolescent students. P value < 0.2 was used to select candidate variables for multivariable analysis. P value < 0.05 with a 95% CI was used to decide statistically significant association.
2.7. Ethical Clearance
Ethical clearance was obtained from the Research Review Committee of the College of Medicine and Health Sciences, Bahir Dar University. The permission letter was taken from Amhara Regional State Education Bureau, Bahir Dar City Education Department, and respective secondary schools in the city. Informed written consent was received from parents and each study participant. Confidentiality of the information was also maintained.
3. Results
3.1. Sociodemographic Characteristics of the Respondents
A total of 682 female adolescent secondary school students were included in this study with a response rate of 100%. The mean ages of the respondents were 17.2 ± 1.4 years old. Most of the participants, 617 (90.5%) and 668 (97.9%), were single and Amhara in ethnicity, respectively. The majority, 432 (63.5%), of the study participants reported living in a household with 4-6 family members. Only a third of participants reported to use vehicle as a means of transport on a daily basis (Table 1).
Table 1.
Socioeconomic and demographic profile of adolescent school girls in Bahir Dar City, Northwest Ethiopia (n = 682).
| Characteristics | Frequency | Percent |
|---|---|---|
| Age of the respondents | ||
| 14–16.5 | 231 | 39.9 |
| 16.6–19 | 451 | 66.1 |
| Type of school | ||
| Government | 583 | 85.5 |
| Private | 99 | 14.5 |
| Level of grade | ||
| 9th | 248 | 36.4 |
| 10th | 134 | 19.6 |
| 11th | 125 | 18.3 |
| 12th | 175 | 25.7 |
| Marital status of the respondents | ||
| Married | 59 | 8.7 |
| Single | 617 | 90.5 |
| Separated | 6 | 8.8 |
| Family size | ||
| ≤3 | 101 | 14.8 |
| 4-6 | 432 | 63.3 |
| ≥7 | 149 | 21.8 |
| Religion | ||
| Orthodox | 543 | 79.6 |
| Protestant | 35 | 5.1 |
| Muslim | 101 | 14.8 |
| Others | 3 | 0.4 |
| Ethnicity | ||
| Amhara | 668 | 97.9 |
| Tigray | 8 | 1.2 |
| Others | 6 | 0.9 |
| Sex of head of the household | ||
| Male | 556 | 81.5 |
| Female | 126 | 18.5 |
| Occupational status of the father | ||
| Farmer | 89 | 15.3 |
| Government employee | 298 | 43.7 |
| Private employee (NGO, merchant, self-employed) | 260 | 44.8 |
| Others | 29 | 4.3 |
| Occupational status of the mother | ||
| Farmer | 44 | 6.5 |
| Government employee | 91 | 13.3 |
| Private employee (NGO, merchant, self-employed) | 167 | 24.5 |
| Housewife | 358 | 52.5 |
| Others | 22 | 3.2 |
| Educational level of the father | ||
| Illiterate | 82 | 12.1 |
| Primary school | 166 | 24.5 |
| Secondary school | 136 | 20.1 |
| Above secondary school | 292 | 42.8 |
| Educational level of the mother | ||
| Illiterate | 158 | 23.2 |
| Primary school | 197 | 28.9 |
| Secondary school | 180 | 26.4 |
| Above secondary school | 147 | 21.5 |
| Monthly family income(Ethiopian birr) | ||
| ≤2500 | 187 | 27.4 |
| 2500-3999 | 125 | 18.3 |
| 4000-6000 | 203 | 29.8 |
| ≥6001 | 167 | 24.5 |
| Vehicle use to come & back to school | ||
| Yes | 226 | 33.1 |
| No | 456 | 66.9 |
3.2. Nutrition- and Diet-Related Characteristics of the Study Participants
Majority of the respondents, 667 (97.8%), used teff as a staple diet. Around two-thirds, 432 (63.3%), of them consume three meals per day. Nearly half of the study participants reported that they consume vegetables and fruits once a day and once a week, respectively. More than half, 387 (56.7%), of them did not eat snacks. Most of the participants, 585 (85.8%), did not face hunger related to shortage of food in the last one month (Table 2).
Table 2.
Nutrition- and diet-related characteristics of female school adolescents in Bahir Dar City, Northwest Ethiopia (n = 682).
| Characteristics | Frequency | Percent |
|---|---|---|
| Staple diet | ||
| Teff | 667 | 97.8 |
| Wheat | 2 | 0.3 |
| Maize | 4 | 0.6 |
| Sorghum | 2 | 0.3 |
| Others | 7 | 1.0 |
| Meals per day | ||
| One times | 6 | 0.9 |
| Two times | 72 | 10.6 |
| Three times | 432 | 63.3 |
| Four times and above | 172 | 25.2 |
| Vegetable intake per day | ||
| No intake | 89 | 13 |
| One times | 358 | 52.5 |
| Two times | 188 | 27.6 |
| Three times and above | 47 | 6.9 |
| Fruit intake per week | ||
| No intake | 158 | 23.2 |
| One times | 344 | 50.4 |
| Two times and above | 180 | 26.4 |
| Meat intake per week | ||
| No intake | 215 | 31.5 |
| One times | 158 | 23.2 |
| Two times and above | 309 | 45.3 |
| Snack intake per day | ||
| No intake | 387 | 56.7 |
| One times | 239 | 35.1 |
| Two times | 56 | 8.2 |
| Items taken for snack | ||
| Cereal & other grains | 165 | 55.7 |
| Fruit and vegetable | 129 | 43.6 |
| Meat, egg, and milk | 2 | 0.7 |
| Hunger in the last 30 days due to shortage of food | ||
| No hunger | 585 | 85.8 |
| One time | 54 | 7.9 |
| Two times | 28 | 4.1 |
| Three times | 8 | 1.2 |
| Four times and above | 7 | 1.0 |
| Additional food bought (consumed) | ||
| Cake | 126 | 18.5 |
| Biscuit | 310 | 45.5 |
| Ice cream | 57 | 8.4 |
| Chocolate | 159 | 23.3 |
| Others | 30 | 4.4 |
3.3. Behavior and Life Style
More than half, 385 (56.0%), of the study participants spent their time by doing moderate activities. A third of them spent their time by doing hard physical activities. The majority, 439 (64.4%), of the study participants reported they spend 30 minutes to go back and forth to school. More than sixty percent, 424 (62.2%), of the study participants had no illness in the past two weeks (Table 3).
Table 3.
Behavioral and life styles of female adolescents in Bahir Dar City, Northwest Ethiopia (n = 682).
| Variables | Frequency | Percent |
|---|---|---|
| Hard physical activities per week | ||
| None | 414 | 60.7 |
| One day | 110 | 16.1 |
| Two days | 66 | 9.7 |
| Three days | 31 | 4.5 |
| Four days and above | 61 | 8.9 |
| Moderate activities | ||
| Yes | 385 | 56.5 |
| No | 297 | 43.5 |
| Sport done (gymnastic, swimming) | ||
| Yes | 188 | 27.6 |
| No | 494 | 72.4 |
| Time taken to school | ||
| ≤15 min | 77 | 11.3 |
| 16-30 min | 439 | 64.4 |
| 31-60 min | 144 | 21.1 |
| ≥60 min | 22 | 3.2 |
| Do you drink alcohol | ||
| Yes | 45 | 6.6 |
| No | 637 | 93.4 |
| Did you smoke cigarette | ||
| Yes | 1 | 0.1 |
| No | 681 | 99.9 |
| Did you have a latrine | ||
| Yes | 671 | 98.4 |
| No | 11 | 1.6 |
| Did you have any illness in the past 2 weeks | ||
| Yes | 258 | 37.8 |
| No | 424 | 62.2 |
| Sources of drinking water | ||
| Tap water | 676 | 99.1 |
| Protected well | 6 | 0.9 |
3.4. Anthropometric Findings of the Participants
One hundred two (15%), 57 (8.4%), and 32 (4.7%) of secondary school adolescent girls were found to be underweight, overweight, and obese, respectively (Figure 1).
Figure 1.
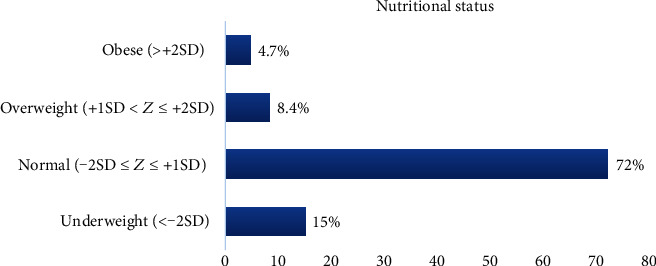
Nutritional status of adolescent school girls in Bahir Dar City, Northwest Ethiopia (n = 682).
In this study, high prevalence of overweight and obesity was found among private schools than among government schools (Figure 2).
Figure 2.
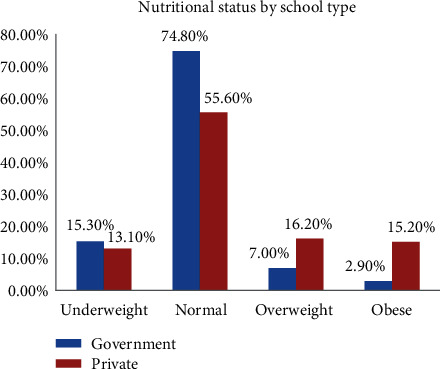
Nutritional status of adolescent school girls by school type in Bahir Dar City, Northwest Ethiopia.
3.5. Factors Associated with Underweight among Female School Adolescents
This study revealed that ages of the respondents, family size, meat intake per week, and onset of menarche were found to be associated with underweight. Adolescent age found between 14 and 16.5 years old were 1.7 times more likely to be underweight compared with counterparts (AOR: 1.7, 95% CI: 1.03-2.69). Respondents living with a family size of 4-6 were 2.8 times more likely to be underweight than those having a family size of ≤3 (AOR: 2.8, 95% CI: 1.05-4.99), whereas participants who did not eat meat once a week were1.6 times more likely to be underweight than those who ate meat two or more times per week (AOR: 1.6, 95% CI: 1.90-2.82). Moreover, girls with no onset of menarche were 4 times more likely to be underweight than the counterparts (AOR = 4.4, 95% CI: 1.21, 15.75 (Table 4).
Table 4.
Factors associated with underweight among female school adolescents in Bahir Dar City, Northwest Ethiopia (n = 682).
| Characteristics | Underweight | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Ages of the respondents | ||||
| 14–16.5 | 45 (6.6) | 186 (27.3) | 1.67 (1.1, 2.57)a | 1.66 (1.03, 2.69)b |
| 16.6–19 | 57 (8.4) | 394 (57.8) | Ref | Ref |
| Family size | ||||
| ≤3 | 10 (1.5) | 91 (13.3) | Ref | Ref |
| 4–6 | 72 (10.6) | 360 (52.8) | 1.82 (0.90, 3.70) | 2.83 (1.05, 4.99)b |
| ≥7 | 20 (2.9) | 129 (18.9) | 1.4 (0.60, 3.20) | 1.60 (0.64, 4.29 |
| Meat intake per week | ||||
| No intake | 50 (7.3) | 165 (24.2) | 2.09 (1.32, 3.33)a | 1.55 (1.90, 2.82)b |
| One time | 13 (1.9) | 145 (21.3) | 0.62 (0.32, 1.20) | 0.52 (0.26, 1.05) |
| Two times and above | 39 (5.7) | 270 (39.6) | Ref | Ref |
| Family income per month in ETB birr | ||||
| ≤2500 | 39 (5.7) | 148 (21.7) | 1.83 (1.03, 3.26)a | 1.13 (0.55, 2.31) |
| 2500-3999 | 22 (3.2) | 103 (15.1) | 1.49 (0.78, 2.84) | 1.2 (0.58, 2.49) |
| 4000-6000 | 20 (2.9) | 183 (26.8) | 0.76 (0.40, 1.46) | 0.67 (0.33, 1.36) |
| ≥6001 | 21 (3.1) | 146 (21.4) | Ref | Ref |
| Additional food bought (consumed) | ||||
| Cake | 12 (1.8) | 108 (15.8) | Ref | Ref |
| Biscuit | 64 (9.4) | 239 (35.0) | 2.83 (1.34, 5.73)a | 2.5 (0.30, 20.8) |
| Ice cream | 5 (0.7) | 45 (6.6) | 0.97 (0.29, 3.27) | 0.8 (0.70, 8.70) |
| Chocolate | 17 (2.5) | 136 (19.9) | 1.27 (0.55, 2.91) | 1.15 (0.13, 10.37) |
| Others (not much sweets) | 9 (1.3) | 47 (6.9) | 1.86 (0.69, 5.02) | 1.59 (0.17, 15.29) |
| Do you start menarche | ||||
| Yes | 217 (31.8) | 353 (51.8) | Ref | Ref |
| No | 44 (6.5) | 68 (10.0) | 4.22 (1.31, 13.56)a | 4.36 (1.21, 15.75)b |
N.B.: asignificant in the bivariable analysis; bsignificant in the multivariable analysis; Ref: reference.
3.6. Factors Affecting Female School Adolescents' Overweight
Monthly family income, meat intake per week, and fruit intake per week were found to be independently associated with overweight. Adolescents whose monthly family income was above 6500 Ethiopian birr were 12.7 times more likely to be overweight than adolescents having family monthly income below 2500 Ethiopian birr (AOR: 12.7, 95% CI: 2.47-65.62). Study participants who ate meat two times and more per week were 2.1 times more likely to be overweight than the counterparts (AOR: 2.07, 95% CI: 1.47-9.14), whereas the risk of being overweight was found to be low among adolescents who ate fruits at least once per week compared with those who did not eat fruit (AOR: 0.20, 95% CI: 0.05-0.78) (Table 5).
Table 5.
Factors associated with overweight/obesity among female school adolescents in Bahir Dar City, Northwest Ethiopia (n = 682).
| Characteristics | Over weight | COR (95% CI) | AOR (95% CI) | |
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Meat intake per week | ||||
| No intake | 17 (2.5) | 198 (29.0) | Ref | Ref |
| One time | 18 (2.6) | 140 (20.5) | 1.50 (0.75, 3.01) | 4.31 (0.97, 19.27) |
| Two times and above | 58 (8.5) | 255 (37.4) | 2.47 (1.39, 4.39)a | 2.07 (1.47, 9.14)b |
| Fruit intake per week | ||||
| No intake | 17 (2.5) | 141 (20.7) | Ref | Ref |
| One time | 39 (5.7) | 305 (44.7) | 1.06 (0.58, 1.94) | 0.22 (0.06, 0.76)b |
| Two times and above | 33 (4.8) | 147 (21.6) | 1.86 (0.99, 3.49) | 0.2 (0.05, 0.78)b |
| Monthly family income in ETB birr | ||||
| ≤2500 | 11 (1.6) | 176 (25.8) | Ref | Ref |
| 2500-3999 | 9 (1.3) | 116 (17.0) | 1.24 (0.50, 3.09) | 2.48 (0.45, 13.83) |
| 4000-6000 | 25 (3.7) | 178 (26.1) | 2.25 (1.07, 4.71)a | 3.15 (0.62, 15.96) |
| ≥6001 | 44 (6.5) | 123 (18.0) | 5.72 (2.84, 11.52)a | 12.74 (2.47, 65.62)b |
| Use vehicle to travel to school | ||||
| Yes | 40 (5.9) | 186 (27.3) | 1.79 (1.14, 2.81)a | 0.86 (0.33, 2.25) |
| No | 49 (7.2) | 407 (59.7) | Ref | Ref |
N.B.: asignificant in the bivariable analysis; bsignificant in the multivariable analysis; Ref: reference.
4. Discussion
Adolescent malnutrition both overweight and underweight is the major global health challenge of the 21st century [21]. Adolescent girls are the most vulnerable group of the population, in which addressing the nutritional problems of this portion of the population is critical to save the future generations [22]. This study showed that adolescent girls were affected with the twin burden of malnutrition. In the current study, the prevalence of underweight was 102 (15%). The finding is found to be consistent with studies conducted in Poland [23] and Saudi Arabia [24]. However, the finding is lower than studies conducted in Sudan [25], India [26], and Bangladesh [27]. The difference could be due to the level of socioeconomic status (the aforementioned studies were conducted in rural and semiurban areas), the contrasting level of awareness of the community, and the study period.
In the current study, 54 (7.9%) adolescent girls face hunger at least once per month, a third of the girls did not eat meat at least once per week, around a quarter of female adolescents did not eat fruit per week, and only a quarter of them eats four and more meals per day. Despite this population group having rapid physical growth and the greatest nutritional need, the feeding practice was found to be poor. Inadequate nutritional intake in this population group might affect educational status, negatively affect physical growth, increase the risk of poor obstetric outcomes for teen mothers, jeopardize the healthy development of future children, and hurt future productivity and income generation potentials [21, 22]. Therefore, a design evidence-based female adolescent nutrition intervention shall be emphasized by the government and other concerned bodies to avert the effect of lifelong consequences of malnutrition.
Consistent with other studies [5, 11, 28–30], those in the early adolescent age group were 1.7 times more likely to be underweight than the counterparts. This might be related to the high nutrition demand of the body since it is a period of a growth spurt including physical, emotional, psychological, and sexual. On the contrary, in the late adolescent period, they might search food stuffs by themselves and reach maturation [22]. Therefore, avoiding constant dietary practice from childhood to early adolescent is very important, since the demand of food in amount and item in different age groups is different. Thus, creating awareness on adolescent feeding practice in the community shall be considered.
In line with studies [31, 32], those adolescents with late onset of menarche were 4 times more likely to be underweight than the counterparts. This might be due to girls needing adequate lean body fat and reaching a certain weight for menarche to occur. Studies showed that age at menarche is significantly associated with BMI and height of the girl. Those who have higher BMI are more likely to have menarche at lower ages [32–34]. On the other hand, those who are thin are more likely to have delayed menarche [32–34]. Similarly, those with short stature are likely to have delayed menarche [35]. Therefore, late onset of menarche might be a sign of undernutrition.
Adolescent girls living with more than four family members were 2.8 times more likely to be underweight than those having less than three. The finding is comparable with other studies [28, 36, 37]. This might be due to the presence of a large family size who might be sharing the available food stuffs. In addition, the finding which is explained by large family size is associated with poor economic and educational status [38].
In the present study, the prevalence of overweight and obesity was 8.4% and 4.7%, respectively. The finding is in line with studies conducted in Poland [23], Sudan [25], regional city of Nigeria [39], Addis Ababa (Ethiopia) [40], and India [41]. However, the finding is lower than a study conducted in Saudi Arabia [24] and higher than studies conducted in West Hararghe, Ethiopia [11], and Adama, Central Ethiopia [13]. The difference could be attributed to the variation in socioeconomic condition of the household and energy-related behavior including behavior of sedentary, physical activities, and eating behavior. Overnutrition in adolescent girls could predispose to different chronic medical illnesses such as cardiovascular, endocrine, gastrointestinal, neurological, psychosocial, and mental health problems [42–44]. Therefore, attention shall be given to overweight adolescents through implementing different strategies like creating awareness among the community on healthy feeding practice and physical activities and reducing sedentary life style. In addition, implementing a school-based nutrition program and screening adolescents for malnutrition shall be emphasized [45].
Participants who ate fruit at least once per week were less likely to be overweight. The finding is comparable with previous studies [46–50]. Therefore, encouraging fruit and vegetable intake is very important to reduce excessive weight gain. On the contrary, in this study, high meat intake per week was associated with weight again in a multivariable model. Those who ate meat more than two times per week were 2 times more likely to be overweight than the counterparts. The finding is in line with different previous studies [51–54]. This might be due to the fact that meat is one of the high energy dense foods and has high saturated fats [55].
Those with monthly family income more than 6500 Ethiopian birr were 12 times more likely to be overweight than those whose monthly income was below 2500 Ethiopian birr. The finding is consistent with different studies conducted in developing countries [56–59]. This could be due to those who had high monthly income in developing countries being more likely to take processed, westernized foods and use a vehicle for transport which are unhealthy practices. On the other hand, studies conducted in developed countries showed that poor socioeconomic status is associated with overweight [60–62].
This study shall be seen with the following limitations: First, using a cross-sectional study design might affect causal inference. Second, a dietary diversity score was not assessed. Third, we did not use any quantification for dietary assessment.
5. Conclusion
The prevalence of underweight and overweight was found to be high. Age of respondents, family size, meat intake per week, and onset of menarche were found to be associated with underweight, whereas monthly family income, meat intake, and fruit intake were found to be predictors of overweight. Designing an evidence-based nutritional intervention shall be emphasized by the government and other stakeholders to avert the dual burden of malnutrition.
Acknowledgments
We would like to acknowledge the College of Health Sciences, Bahir Dar University, for funding the data collection cost and approving the ethical process of this study. We would like to acknowledge the Amhara Regional Education Bureau and Bahir Dar City Education Department for giving the permission letter to collect the data. Lastly, we would like to thank the study participants. The data collection fee was covered by the College of Medicine and Health Sciences, Bahir Dar University.
Abbreviations
- BMI:
Body mass index
- CI:
Confidence interval
- OR:
Odds ratio
- SD:
Standard deviation.
Data Availability
The datasets used in this study are available from the corresponding author and can be accessed through request.
Ethical Approval
Ethical clearance was obtained from the Research Review Committee of the College of Medicine and Health Sciences, Bahir Dar University. Permission letter was taken from the Amhara Regional State Education Bureau, Bahir Dar City Education Department, and respective secondary schools in the city. Informed written consent was received from parents and each study participant before the start of the data collection process. The confidentiality of the information was also maintained.
Disclosure
The funding body has no role in the design, data collection, write-up, and manuscript preparation.
Conflicts of Interest
The authors declares they have no competing interests.
Authors' Contributions
WT, SB, MM, and TA have contributed to this study in the design, data collection, data analysis, thesis write-up, and manuscript development and edition. The final manuscript was read and approved by all the authors.
References
- 1.WHO African Region. Nutrition in the WHO African Region. 2017. https://www.afro.who.int/publications/nutrition-who-african-region.
- 2.Deshmukh P. R., Gupta S. S., Bharambe M. S., et al. Nutritional status of adolescents in rural Wardha. The Indian Journal of Pediatrics. 2006;73(2):139–141. doi: 10.1007/bf02820204. [DOI] [PubMed] [Google Scholar]
- 3.Das J. K., Salam R. A., Thornburg K. L., et al. Nutrition in adolescents: physiology, metabolism, and nutritional needs. Annals of the New York Academy of Sciences. 2017;1393(1):21–33. doi: 10.1111/nyas.13330. [DOI] [PubMed] [Google Scholar]
- 4.Abudayya A. H., Stigum H., Shi Z., Abed Y., Holmboe-Ottesen G. Sociodemographic correlates of food habits among school adolescents (12–15 year) in north Gaza Strip. BMC Public Health. 2009;9(1) doi: 10.1186/1471-2458-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulugeta A., Hagos F., Stoecker B., et al. Nutritional status of adolescent girls from rural communities of Tigray, Northern Ethiopia. Ethiopian Journal of Health Development. 2009;23(1) doi: 10.4314/ejhd.v23i1.44831. [DOI] [Google Scholar]
- 6.Delisle H., Chandra-Mouli V., de Benoist B. Should adolescents be specifically targeted for nutrition in developing countries: to address which problems, and how? World Health Organization/International Nutrition Foundation for Developing Countries; 2000. Http: //Www Who Int/Childadolescent-Health/New_Publications/NUTRITION/Adolescent_ nutrition_paper Pdf. [Google Scholar]
- 7.WHO. Nutrition in adolescence: issues and challenges for the health sector: issues in adolescent health and development. WHO discussion papers on adolescence. 2005. https://apps.who.int/iris/bitstream/handle/10665/43342/9241593660_eng.pdf;jsessionid=71C6AB1EEE44CD393BD3AB6180816C35?sequence=1.
- 8.Kimani-Murage E. W., Kahn K., Pettifor J. M., Tollman S. M., Klipstein-Grobusch K., Norris S. A. Predictors of adolescent weight status and central obesity in rural South Africa. Public health nutrition. 2011;14(6):1114–1122. doi: 10.1017/s1368980011000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO [World Health Organization] The double burden of malnutrition-policy brief WHO. 2016. https://www.who.int/nutrition/publications/doubleburdenmalnutrition-policybrief/en/
- 10.Branca F., Piwoz E., Schultink W., Sullivan L. M. Nutrition and health in women, children, and adolescent girls. BMJ. 2015 doi: 10.1136/bmj.h4173. [DOI] [PubMed] [Google Scholar]
- 11.Damie T., Kbebew M., Teklehaymanot A. Nutritional status and associated factors among school adolescent in Chiro Town, West Hararge, Ethiopia. Gaziantep Medical Journal. 2015;21(1):p. 32. doi: 10.5455/gmj-30-169505. [DOI] [Google Scholar]
- 12.Wassie M. M., Gete A. A., Yesuf M. E., Alene G. D., Belay A., Moges T. Predictors of nutritional status of Ethiopian adolescent girls: a community based cross sectional study. BMC Nutrition. 2015;1(1) doi: 10.1186/s40795-015-0015-9. [DOI] [Google Scholar]
- 13.Roba K. T., Abdo M. Nutritional status and its associated factors among school adolescent girls in Adama city, Central Ethiopia. Journal of Nutrition & Food Sciences. 2016;6(3) doi: 10.4172/2155-9600.1000493. [DOI] [Google Scholar]
- 14.Aounallah-Skhiri H., Romdhane H. B., Traissac P., et al. Nutritional status of Tunisian adolescents: associated gender, environmental and socio-economic factors. Public health nutrition. 2008;11(12):1306–1317. doi: 10.1017/s1368980008002693. [DOI] [PubMed] [Google Scholar]
- 15.Wolde T., Amanu W., Mekonnin D., et al. Nutritional status of adolescent girls living in southwest of Ethiopia. Food Science and Quality Management. 2014;34 [Google Scholar]
- 16.WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO report expert committee. WHO Technical report series. 1995 https://apps.who.int/iris/bitstream/handle/10665/37003/WHO_TRS_854.pdf?sequence=1. [PubMed]
- 17.Stewart A., Marfell-Jones M., Olds T., Hans De Ridder J. International standards for anthropometric assessment. Australia International society for the advancement of kinanthropometry. 2006:9–59. https://trove.nla.gov.au/work/26414250. [Google Scholar]
- 18.CDC. Anthropometry procedures manual. 2004. https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/BM.pdf.
- 19.Centre NSBR. Procedure for measuring adult height. pp. 2–4. https://www.uhs.nhs.uk/Media/Southampton-ClinicalResearch/Procedures/BRCProcedures/Procedure-for-adult-height. pdf.
- 20.WHO. Growth reference 5-19 years: BMI-for-age. 2020. https://www.who.int/growthref/who2007_bmi_for_age/en/
- 21.Larson N., Neumark-Sztainer D. Adolescent nutrition. Pediatrics in Review. 2009;30(12):494–496. doi: 10.1542/pir.30-12-494. [DOI] [PubMed] [Google Scholar]
- 22.USAID. Multi-sectoral nutrition strategy global learning and evidence exchange, east and southern Africa regional meeting. 2010. https://www.fantaproject.org/sites/default/files/Multi-Sectoral-Nutrition-Strategy-2014-2025.pdf.
- 23.Przysławski J., Stelmach M., Grygiel-Górniak B., Mardas M., Walkowiak J. Dietary habits and nutritional status of female adolescents from the great Poland region. Polish Journal of Food and Nutrition Sciences. 2011;61(1):73–78. doi: 10.2478/v10222-011-0008-6. [DOI] [Google Scholar]
- 24.Al-Jaaly E., Lawson M., Hesketh T. Overweight and its determinants in adolescent girls in Jeddah City, Saudi Arabia. International Journal of Food, Nutrition and Public Health. 2011;4(2) [Google Scholar]
- 25.Mahgoub H. M., Fadlelseed O. E., Khamis A. H., Bilal J. A., Adam I. Nutritional and micronutrient status of adolescent schoolgirls in eastern Sudan: a cross-sectional study. F1000Research. 2017;6 doi: 10.12688/f1000research.12721.1. [DOI] [Google Scholar]
- 26.Saxena Y., Saxena V. Nutritional status in rural adolescent girls residing at hills of Garhwal in India. Internet Journal of Medical Update. 2009;6(3):3–8. [Google Scholar]
- 27.Alam N., Roy S. K., Ahmed T., Ahmed A. M. S. Nutritional status, dietary intake, and relevant knowledge of adolescent girls in rural Bangladesh. Journal of Health, Population, and Nutrition. 2010;28(1):86–94. doi: 10.3329/jhpn.v28i1.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebregyorgis T., Tadesse T., Atenafu A. Prevalence of thinness and stunting and associated factors among adolescent school girls in Adwa town, North Ethiopia. International Journal of Food Science. 2016;2016, article 8323982:1–8. doi: 10.1155/2016/8323982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prista A., Maia J. A. R., Damasceno A., Beunen G. Anthropometric indicators of nutritional status: implications for fitness, activity, and health in school-age children and adolescents from Maputo, Mozambique. The American Journal of Clinical Nutrition. 2003;77(4):952–959. doi: 10.1093/ajcn/77.4.952. [DOI] [PubMed] [Google Scholar]
- 30.Arage G., Assefa M., Worku T. Socio-demographic and economic factors are associated with nutritional status of adolescent school girls in Lay Guyint Woreda, Northwest Ethiopia. Open Medicine. 2019;7:p. 205031211984467. doi: 10.1177/2050312119844679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juliyatmi R. H., Handayani L. Nutritional status and age at menarche on female students of junior high school. International Journal of Evaluation and Research in Education (IJERE) 2015;4(2):p. 71. doi: 10.11591/ijere.v4i2.4494. [DOI] [Google Scholar]
- 32.de Siqueira Barros B., Kuschnir M. C. M. C., Bloch K. V., da Silva T. L. N. ERICA: idade da menarca e sua associaçao com o estado nutricional. Jornal de Pediatria. 2019;95(1):106–111. doi: 10.1016/j.jped.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Pejhan A., Moghaddam H. Y., Najjar L. The relationship between menarche age and anthropometric indexes of girls in Sabzevar, Iran. World Applied Sciences Journal. 2011;14(11):1748–1753. [PubMed] [Google Scholar]
- 34.Adesina A. F. Age at menarche and body mass index (BMI) among adolescent secondary school girls in Port Harcourt, Nigeria. IOSR Journal of Dental and Medical Sciences. 2013;3(5):41–46. doi: 10.9790/0853-0354146. [DOI] [Google Scholar]
- 35.Onland-Moret N. C., Peeters P. H. M., van Gils C. H., et al. Age at menarche in relation to adult height: the EPIC study. American Journal of Epidemiology. 2005;162(7):623–632. doi: 10.1093/aje/kwi260. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharyya H., Barua A. Nutritional status and factors affecting nutrition among adolescent girls in urban slums of Dibrugarh, Assam. National Journal of Community Medicine. 2013;4(1):35–39. [Google Scholar]
- 37.Assefa H., Belachew T., Negash L. Socioeconomic factors associated with underweight and stunting among adolescents of Jimma zone, South West Ethiopia: a cross-sectional study. ISRN Public Health. 2013;2013:7. doi: 10.1155/2013/238546.238546 [DOI] [Google Scholar]
- 38.Bonney M. E. Relationships between social success, family size, socio-economic home background, and intelligence among school children in grades III to V. Sociometry. 1944;7(1):p. 26. doi: 10.2307/2785535. [DOI] [Google Scholar]
- 39.Adesina A. F., Peterside O., Anochie I., Akani N. A. Weight status of adolescents in secondary schools in port Harcourt using body mass index (BMI) Italian Journal of Pediatrics. 2012;38(1):p. 31. doi: 10.1186/1824-7288-38-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebreyohannes Y. Nutritional status of adolescents in selected government and private secondary schools of Addis Ababa, Ethiopia. International Journal of Nutrition and Food Sciences. 2014;3(6):p. 504. doi: 10.11648/j.ijnfs.20140306.13. [DOI] [Google Scholar]
- 41.Choudhary S. K., Kumar S., Bharati D. R., Kumar Rajak B., Kumari S., Shree V. Problem of obesity among school going adolescent in rural practice area of Indira Gandhi Institute of Medical Sciences, Patna. International Journal of Scientific Study. 2017;5(3) [Google Scholar]
- 42.McClanahan K. K., Huff M. B., Omar H. A. Overweight children and adolescents: impact on psychological and social development. Pediatrics Faculty Publications; 2009. https://uknowledge.uky.edu/pediatrics_facpub/140. [Google Scholar]
- 43.van Vuuren C. L., Wachter G. G., Veenstra R., et al. Associations between overweight and mental health problems among adolescents, and the mediating role of victimization. BMC Public Health. 2019;19(1):p. 612. doi: 10.1186/s12889-019-6832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raj M., Kumar R. K. Obesity in children & adolescents. The Indian Journal of Medical Research. 2010;135(5):598–607. http://www.ijmr.org.in/text.asp?2010/132/5/598/73409. [PMC free article] [PubMed] [Google Scholar]
- 45.Bonsergent E., Agrinier N., Thilly N., et al. Overweight and obesity prevention for adolescents: a cluster randomized controlled trial in a school setting. American Journal of preventive medicine. 2013;44(1):30–39. doi: 10.1016/j.amepre.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 46.Schwingshackl L., Hoffmann G., Kalle-Uhlmann T., Arregui M., Buijsse B., Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PLOS ONE. 2015;10(10):p. e0140846. doi: 10.1371/journal.pone.0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma S., Chung H., Kim H., Hong S. Paradoxical effects of fruit on obesity. Nutrients. 2016;8(10):p. 633. doi: 10.3390/nu8100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nour M., Lutze S., Grech A., Allman-Farinelli M. The relationship between vegetable intake and weight outcomes: a systematic review of cohort studies. Nutrients. 2018;10(11):p. 1626. doi: 10.3390/nu10111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlesinger S., Neuenschwander M., Schwedhelm C., et al. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Advances in Nutrition. 2019;10(2):205–218. doi: 10.1093/advances/nmy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mytton O. T., Nnoaham K., Eyles H., Scarborough P., Ni Mhurchu C. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health. 2014;14(1) doi: 10.1186/1471-2458-14-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grosso G., Micek A., Godos J., et al. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: a comprehensive meta-analysis. PLOS ONE. 2017;12(8):p. e0183787. doi: 10.1371/journal.pone.0183787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teshome T., Singh P., Moges D. Prevalence and associated factors of overweight and obesity among high school adolescents in urban communities of Hawassa, Southern Ethiopia. Current Research in Nutrition and Food Science Journal. 2013;1(1):23–36. doi: 10.12944/crnfsj.1.1.03. [DOI] [Google Scholar]
- 53.Wang Y., Beydoun M. A. Meat consumption is associated with obesity and central obesity among US adults. International Journal of Obesity. 2009;33(6):621–628. doi: 10.1038/ijo.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z., Zhang B., Zhai F., et al. Fatty and lean red meat consumption in China: differential association with Chinese abdominal obesity. Nutrition, Metabolism and Cardiovascular Diseases. 2014;24(8):869–876. doi: 10.1016/j.numecd.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams P. Nutritional composition of red meat. Nutrition & Dietetics. 2007;64(s4 The Role of):S113–S119. doi: 10.1111/j.1747-0080.2007.00197.x. [DOI] [Google Scholar]
- 56.Liu W., Liu W., Lin R., et al. Socioeconomic determinants of childhood obesity among primary school children in Guangzhou, China. BMC Public Health. 2016;16(1) doi: 10.1186/s12889-016-3171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmad A., Zulaily N., Shahril M. R., Syed Abdullah E. F. H., Ahmed A. Association between socioeconomic status and obesity among 12-year-old Malaysian adolescents. PLOS ONE. 2018;13(7):p. e0200577. doi: 10.1371/journal.pone.0200577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adetunji A. E., Adeniran K. A., Olomu S. C., et al. Socio-demographic factors associated with overweight and obesity among primary school children in semi-urban areas of mid-western Nigeria. PLOS ONE. 2019;14(4):p. e0214570. doi: 10.1371/journal.pone.0214570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fruhstorfer B. H., Mousoulis C., Uthman O. A., Robertson W. Socio-economic status and overweight or obesity among school-age children in sub-Saharan Africa - a systematic review. Clinical Obesity. 2016;6(1):19–32. doi: 10.1111/cob.12130. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y., Zhang Q. Are American children and adolescents of low socioeconomic status at increased risk of obesity? Changes in the association between overweight and family income between 1971 and 2002. The American Journal of Clinical Nutrition. 2006;84(4):707–716. doi: 10.1093/ajcn/84.4.707. [DOI] [PubMed] [Google Scholar]
- 61.Manios Y., Panagiotakos D. B., Pitsavos C., Polychronopoulos E., Stefanadis C. Implication of socio-economic status on the prevalence of overweight and obesity in Greek adults: the ATTICA study. Health Policy. 2005;74(2):224–232. doi: 10.1016/j.healthpol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Luhar S., Mallinson P. A. C., Clarke L., Kinra S. Do trends in the prevalence of overweight by socio-economic position differ between India’s most and least economically developed states? BMC Public Health. 2019;19(1):p. 783. doi: 10.1186/s12889-019-7155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are available from the corresponding author and can be accessed through request.


