Abstract
Epithelial-mesenchymal transition (EMT), during which cancer cells lose the epithelial phenotype and gain the mesenchymal phenotype, has been verified to result in tumor migration and invasion. Numerous studies have shown that dysregulation of the Wnt/β-catenin signaling pathway gives rise to EMT, which is characterized by nuclear translocation of β-catenin and E-cadherin suppression. Wnt/β-catenin signaling was confirmed to be affected by microRNAs (miRNAs), several of which are down- or upregulated in metastatic cancer cells, indicating their complex roles in Wnt/β-catenin signaling. In this review, we demonstrated the targets of various miRNAs in altering Wnt/β-catenin signaling to promote or inhibit EMT, which may elucidate the underlying mechanism of EMT regulation by miRNAs and provide evidence for potential therapeutic targets in the treatment of invasive tumors.
Keywords: microRNA, Wnt/β-catenin signaling, epithelial-mesenchymal transition, cancer metastasis, cancer therapy
1. Introduction
Cancer metastasis has always been a challenge in the clinic, and is largely responsible for treatment failure and high mortality. It is known that invasive tumors undergo the EMT process where cells fail to maintain an epithelial phenotype and acquire a mesenchymal phenotype, thus transmitting from the primary tumor to other locations and forming secondary growths (1). To solve this problem, scientists have dedicated themselves to exploring the molecular mechanisms of this process. After four decades of work, various signaling pathways are known to participate in EMT including the transforming growth factor-β (TGF-β), Wnt/β-catenin, Hedgehog (Hh), Notch, fibroblast growth factor receptors (FGFRs), and nuclear factor kappa B (NF-κB) signaling pathways (2). Of these, the connections of cell adhesion, the Wnt/β-catenin pathway and EMT are more clearly studied.
The formation of adherens junctions needs cell-cell adhesion molecules, such as the cadherin superfamily, and nectins (3). Cadherins and nectins bind to their anchoring proteins, catenins and afadin, respectively, to form functional modules that affect the actin cytoskeleton (4). The nectin family, which is composed of four members, namely, nectin-1, −2, −3, and −4 (5), is involved in cell-cell adhesion in various cell types by forming a nectin-afadin complex (6,7). Previous findings have shown the significant role of the E-cadherin/β-catenin complex in maintaining stabilized cell-cell junctions (8). E-cadherin is regarded as the key component of the adherens junction complex (9), and invasive tumor is characterized by a marked decrease in E-cadherin expression (10). Several transcription factors such as Twist, Snail and zinc finger E-box-binding homeobox 1/2 (ZEB1/2) trigger EMT by directly binding to the E-box sequences of E-cadherin promoter, thus repressing its transcription (11). For example, Snail1 and Snail2 bind to CDH1 (gene of E-cadherin) promoter-based E-box DNA sequences and summon the polycomb repressive complex 2 (PRC2), resulting in CDH1 histone methylation and acetylation (12). Activation of these transcription factors is attributed to the translocation of β-catenin from the cytoplasm to the nucleus, which is considered to be the central event in EMT (13). β-catenin has demonstrated its crucial role in Wnt signal transduction (14). In the presence of Wnt signals, the phosphorylation of β-catenin by glycogen synthase kinase 3β (GSK3β) is inhibited, followed by β-catenin disassembly from the destruction complex and accumulation in the cytoplasm (15). Therefore, current research primarily focuses on the canonical Wnt signaling (β-catenin dependent) pathway in which the mechanisms are more clearly established.
MiRNAs are small noncoding molecules with 19–25 nucleotides, which regulate gene expression at the post-transcriptional level by inhibiting mRNA translation or facilitating mRNA degradation (16). Previous findings suggested that EMT is regulated by miRNAs through alteration of the Wnt/β-catenin pathway (17). However, the complex role of miRNA as an EMT inhibitor or promoter and its underlying mechanism need further clarification.
In this review, we focused on the interaction between miRNAs and the Wnt signaling pathway. Through literature retrieval, we summarized the distinct effects of miRNAs on Wnt signaling in the regulation of cancer metastasis, aiming to identify the mechanism of EMT regulation by miRNAs and potential therapeutic targets in invasive tumor treatment.
2. EMT and tumor metastasis
EMT comprises an essential biological process during which cells fail to maintain epithelial cell polarity and acquire the mesenchymal phenotype, thus increasing cell motility and invasion (18). EMT was reported to be involved in numerous biological activities such as embryogenesis (19), heart-valve (20) and neural crest formation (21). Scientists categorize EMT into three types including embryonic development and organ formation, wound healing and organ fibrosis, and cancer progression (22). The critical role of EMT in cancer has been extensively studied in recent years. It is generally accepted that EMT facilitates the invasion and metastasis of early stage tumors and contributes to cancer progression (23). The latest studies reveal that EMT-induced tumor progression is not only mediated by phenotypic change but also related to stemness (24), immune evasion (25), metabolic reprogramming (26), and therapeutic resistance (27) of cancer cells.
EMT is characterized by decreased expression of epithelial markers such as E-cadherin, γ-catenin and increased expression of mesenchymal markers such as N-cadherin, vimentin, Snail, Twist and ZEB (28). E-cadherin, a pivotal transmembrane adhesion protein in maintaining cell-cell junctions and polarity, was confirmed to stabilize cell junctions through forming the E-cadherin/β-catenin complex (8). The loss of E-cadherin, which contributes to the mesenchymal phenotype of cancer cells, is a basic event in tumor metastasis (10). As a result, the newly transformed mesenchymal cells detach from the primary tumor, invade into the circulation, and reform into epithelial cells through MET (29), thus leading to tumor formation at a distant secondary site (30).
EMT is regulated by various signaling pathways such as the TGF-β, Wnt/β-catenin, Hedgehog and Notch signaling pathway (18). These pathways trigger EMT by stimulating transcription factors including Snail, Twist, and ZEB1/2, which directly bind to the promoter-based E-box DNA sequences of E-cadherin and repress its transcription. In addition, Snail also facilitates the transcription of mesenchymal markers such as vimentin and N-cadherin (31). Among all the signaling pathways, the Wnt/β-catenin pathway shows its pivotal role in the regulation of EMT.
3. Wnt/β-catenin signaling pathway and EMT
Wnt (wingless and Int-1) signals are evolutionarily conserved consisting of secreted Wnt ligands, Frizzled (FZD) receptors and coreceptors, intracellular adaptors, and scaffolding proteins (32). The foremost roles of the Wnt signaling pathway in cell proliferation, differentiation, adhesion, invasion, migration, and stem cell self-renewal have been well established (17). Abnormal Wnt signaling is commonly correlated with multiple types of disease such as neural tube defects (33), rheumatoid arthritis (34), hepatic fibrosis (35), and cardiovascular disease (36). The Wnt signaling pathway is divided into two categories, a canonical pathway (β-catenin-dependent) and noncanonical pathway (β-catenin-independent), both of which are closely related to EMT (37). β-catenin is regarded as a key protein in Wnt signaling, since accumulation of β-catenin in the cytoplasm gives rise to its translocation and activation in the nucleus (15), further initiating the transcription of EMT-related genes (38).
When the Wnt signal is absent, β-catenin forms a destruction complex with Axin, adenomatous polyposis coli (APC), casein kinase I α (CKIα) and GSK3β (39). In this stage, β-catenin is phosphorylated by GSK3β and CKIα, forming β-catenin degradation by ubiquitination (40). In addition, Wnt signaling inhibitory factors such as Dickkopf (DKK) family, secreted frizzled-related protein (SFRP) family and Wnt inhibitory factor 1 (WIF1) contribute to the inactive status of β-catenin (41). Dkk, a small family of secreted glycoproteins, is comprised of four members, Dkk1-4. Dkk1 and Dkk2 bring about Wnt signal inhibition by binding to low-density lipoprotein receptor-related protein (LRP) 5/6 with high affinity (42). However, Dkk2 plays a dual role as an inhibitor or activator of the Wnt signaling pathway, depending on the cellular context (43,44). Dkk3 was reported to be different from other members of the DKK family as it does not bind LRP6 and does not affect Wnt signaling (45). In addition, there are some studies demonstrating the inhibitory effects of Xenopus Cerberus and Wise proteins on Wnt (46). Xenopus Cerberus binds to Wnt proteins via independent sites to inhibit the Wnt signaling pathway (47), whereas Wise may inhibit or activate Wnt signaling in a context-dependent manner (48). In addition to Wnt signaling inhibitory factors, E-cadherin also suppresses β-catenin by forming the E-cadherin/β-catenin complex to prevent nuclear translocation of β-catenin (49). When receptors receive Wnt ligands such as Wnt1 and Wnt3 binding to the FZD and LRP 5/6, LRP 5/6 and FZD form a complex to affect the stabilization of β-catenin and prevent its degradation, resulting in β-catenin accumulation in cytoplasm (50). As a consequence, β-catenin translocates to the nucleus and forms a complex with the T-cell factor/lymphoid enhancer factor (TCF/LEF), which promotes transcription of Wnt target genes including Twist, Snail and other oncogenes such as Cyclin D1, matrix metalloproteniase-7 (MMP-7) and cellular myelocytomatosis oncogene (c-Myc) (51), thus facilitating EMT (52).
4. miRNAs target the Wnt/β-catenin signaling pathway to regulate EMT
MicroRNAs are small noncoding molecules with 19–25 nucleotides that play fundamental roles in almost every cellular process such as cell differentiation and homeostasis (53) by regulating gene expression at the post-transcriptional level (54). MiRNA genes are transcribed into primary miRNA (pri-miRNA) by RNA polymerase II (55). Exportin 5 recognizes the 2-nucleotide overhang of the pre-miRNA and transports it to the cytoplasm, then pre-miRNA undergoes multistep biogenesis to become mature miRNAs (56). MiRNAs bind to the 3′-untranslated region (UTR) of target mRNAs to suppress their translation or accelerate degradation. It is reported that approximately 10–40% of mRNAs are regulated by miRNAs in humans (57) and dysregulation of miRNA may result in tumor metastasis (58). Research has increasingly focused on the interaction between miRNA and EMT as miRNAs affect multiple EMT-related signaling pathways such as Wnt/β-catenin, North, TGF-β pathway and their target genes (59). The role of miRNA as a tumor suppressor or oncogene during EMT has attracted much attention. Elucidating miRNA functions in the regulation of EMT may contribute to the finding of potential therapeutic targets.
MiRNA as an inhibitor of EMT
A large number of miRNAs were found to be downregulated in a wide spectrum of cancers (60,61), indicating their inhibitory effect on tumorigenesis and development. Furthermore, clinicopathological analysis also revealed that tumor migration and invasion was negatively correlated with a number of miRNAs (62). Numerous studies reported that miRNAs may function as EMT inhibitors by targeting the Wnt signaling pathway or its downstream transcription factors (Table I, Fig. 1); thus, overexpression of these miRNAs may be a therapeutic method to reverse EMT.
Table I.
Inhibition of EMT by miRNAs.
| miRNA | Cancer type | Molecular targets | (Refs.) |
|---|---|---|---|
| miR-125b-5p | Hepatocellular carcinoma | STAT3 | (62) |
| miR-200 family | Gastric adenocarcinoma | ZEB1/2, β-catenin | (73,74) |
| Hepatocellular carcinoma | β-catenin | (76) | |
| Colonic adenocarcinoma | ZEB1/2 | (75) | |
| miR-122 | Hepatocellular carcinoma | Wnt1, Snail1/2 | (83,96) |
| miR-3127-5p | Non-small-cell lung cancer | FZD4 | (100) |
| miR-136 | Melanoma | PMEL | (122) |
| miR-708 | Melanoma | LEF1 | (84) |
| miR-203 | Breast cancer | DKK1 | (178) |
| miR-490-3p | Colorectal cancer | FRAT1 | (103) |
| miR-34b/c | Prostate cancer | β-catenin | (93) |
| miR-148a | Hepatocellular carcinoma | Wnt1 | (97) |
| Pancreatic cancer | Wnt10b | (98) | |
| miR-34a | Prostate cancer | LEF1 | (85) |
| miR-101 | Colon cancer | EZH2 | (179) |
| miR-22 | Melanoma | FMNL2 | (121) |
| miR-33b | Lung adenocarcinoma | ZEB1 | (80) |
| miR-145 | Lung cancer | Oct4 | (92) |
| miR-194 | Hepatocellular carcinoma | PRC1 | (118) |
| miR-300 | Pancreatic cancer | CUL4B | (110) |
| miR-338 | Gastric cancer | EphA2 | (114) |
| miR-495 | Non-small-cell lung cancer | TCF4 | (88) |
| miR-506 | Nasopharyngeal carcinoma | LHX2 | (116) |
| miR-3619-5p | Bladder carcinoma | β-catenin, CDK2, p21 | (94) |
| miR-15a-3p | Prostate cancer | SLC39A7 | (104) |
| miR-29c | Colorectal carcinoma | GNA13, PTP4A | (123) |
| miR-33a | Pancreatic cancer | β-catenin | (95) |
| miR-302b | Gastric cancer | EphA2 | (113) |
| miR-340 | Ovarian cancer | FHL2 | (180) |
| miR-375 | Gastric cancer | YWHAZ | (120) |
| miR-377 | Ovarian cancer | CUL4A | (111) |
| miR-378 | Colon cancer | SDAD1 | (60) |
| miR-498 | Liver cancer | ZEB2 | (81) |
| miR-504 | Glioblastoma | FZD7 | (101) |
| miR-516a-3p | Breast cancer | Pygo2 | (87) |
| miR-519d | Gastric cancer | Twist1 | (82) |
| miR-770 | Non-small-cell lung cancer | JMJD6 | (124) |
| miR-876-5p | Gastric cancer | Wnt5A and MITF | (89) |
| miR-33a-5p | Hepatocellular carcinoma | PNMA1 | (181) |
| miR-383 | Pancreatic carcinoma | ROBO3 | (182) |
| miR-371-5p | Colorectal cancer | SOX2 | (183) |
| miR-370-3p | Bladder cancer | Wnt7a | (99) |
STAT3, signal transducer and activator of transcription 3; ZEB, zinc finger E-box-binding homeobox; FZD, Frizzled; PMEL, Premelanosome Protein; LEF1, lymphoid enhancer factor 1; DKK1, Dickkopf 1; FRAT1, frequently rearranged in advanced T-cell lymphomas 1; EZH2, enhancer of zeste homolog 2; FMNL2, formin like 2; Oct4, octamer-binding protein 4; PRC1, protein regulator of cytokinesis 1; CUL4B, cullin 4B; EphA2, EPH receptor A2; TCF4, T-cell factor 4; LHX2, LIM homeobox 2; CDK2, cyclin-dependent kinase 2; PTP4A, protein tyrosine phosphatase 4A2; GNA13, G protein subunit alpha 13; FHL2, four and a half LIM domains 2; CUL4A, Cullin 4A; SDAD1, SDA1 Domain Containing 1; Pygo2, Pygopus2; JMJD6, Jumonji Domain Containing 6; MITF, melanogenesis-associated transcription factor; PNMA1, paraneoplastic ma sntigen 1; ROBO3, roundabout guidance receptor 3; SOX2, SRY-box transcription factor 2.
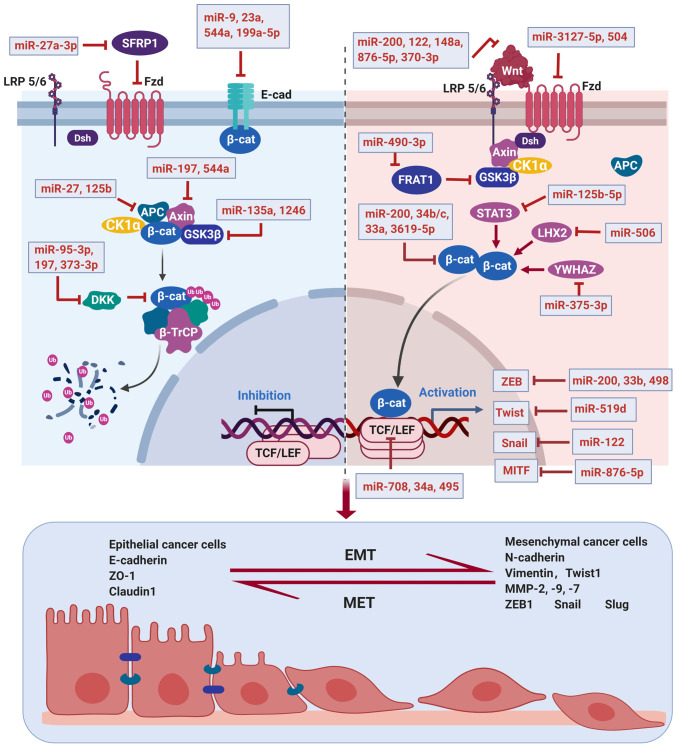
Figure 1.
Regulation of Wnt/β-catenin signaling by miRNAs. Left panel: miRNAs targeting inactive Wnt/β-catenin signaling to initiate EMT. In the absence of Wnt ligands, β-catenin is phosphorylated by GSK3β by forming a destruction complex with Axin, APC, CKIα and GSK3β, forming β-catenin degradation by ubiquitin. MiRNAs facilitate EMT by targeting Wnt/β-catenin suppressors. Right panel: miRNAs targeting activated Wnt/β-catenin signaling to inhibit EMT. When receptors received Wnt ligands, the phosphorylation of β-catenin by GSK3β was inhibited, followed by β-catenin disassembly from the destruction complex and accumulation in cytoplasm. Then, β-catenin translocated to the nucleus and formed a complex with TCF/LEF, which promoted transcription of Wnt target genes such as Twist and Snail, thus facilitating EMT. MiRNAs block EMT by targeting various components of the Wnt/β-catenin signaling pathway.
Targeting Wnt signaling downstream transcription factors
The EMT-induction transcription factors, most of which are downstream of the Wnt signaling pathway, have been studied extensively, including E-cadherin suppressors such as ZEB, Twist and Snail, which are considered to be regulated by miRNAs in various types of cancer (10).
The miR-200 family, comprising of 5 miRNA sequences (miR-200a, miR-200b, miR-200c, miR-141 and miR-429), is believed to play a significant role in EMT (63). The EMT initiated in several types of cancer has been shown to be correlated with the underexpression of the miR-200 family such as bladder cancer (64), breast cancer (65), melanoma (66), ovarian cancer (67), gastric cancer (68), and prostate cancer (69). Gregory et al found the levels of the miR-200 family were significantly reduced following TGF-β-mediated EMT in invasive breast cancer since the low level of miR-200 leads to the absence of E-cadherin, indicating miR-200 as a negative regulator of EMT (70). A mechanism study demonstrated that miR-200 inhibits Wnt signaling by targeting transcription factors ZEB1/2 and binding to β-catenin mRNA to suppress its translation (71). The ZEB family, containing two members ZEB1 and ZEB2, binds to the promoter-based E-box DNA sequences of E-cadherin thus repressing its transcription and facilitating the activation of mesenchymal genes (72). The inhibitory effects of miR-200 on β-catenin and ZEB1/2 were further confirmed in gastric adenocarcinoma (73,74), colonic adenocarcinoma (75), and hepatocellular carcinoma (HCC) (76). Overexpression of miR-200 results in E-cadherin upregulation by targeting ZEB1 and ZEB2, thereby inhibiting EMT and restoring the epithelial phenotype of cancer cells (77). However, components of Wnt signaling can inversely affect the miR-200 family. Tian et al reported that a downstream target of Wnt signaling, Achaete scute-like 2 (Ascl2) negatively regulates miR-200 family expression, thus inhibition of Ascl2 obviously restores miR-200 expression and suppresses EMT, making Ascl2 a promising target to reverse EMT (75). In addition to EMT inhibition, restoring the level of miR-200 can also induce cancer cell apoptosis and increase the sensitivity of cancer cells to chemotherapeutic drugs. For instance, niclosamide potentially induces the apoptosis of colon cancer cells by upregulating the miR-200 family members (78). Another study demonstrated that the overexpression of miR-200b could inhibit cell proliferation and enhance apoptosis and then reverse docetaxel chemoresistance of lung adenocarcinoma (LAD) cells by directly targeting E2F transcription factor 3 (E2F3), which were also verified in tissues of LAD patients (79).
In addition to the miR-200 family, miR-33b binds to 3′-UTR of ZEB1 and inhibits ZEB1 expression in lung adenocarcinoma cells, thus blocking Wnt/β-catenin signaling and suppressing tumor growth and EMT in vitro and in vivo (80). Research by Zhang et al identified miR-498 which was downregulated in liver cancer, and suppressed the growth and metastasis of liver cancer cells partly by directly targeting ZEB2, making miR-498 a potential biomarker for diagnosis and a promising therapeutic target for liver cancer treatment (81). Snail and Twist are also targeted by miRNAs in the regulation of EMT. Yue et al demonstrated that miR-519d directly binds to 3′-UTR of Twist1 to facilitate its degradation in gastric cancer cells, suggesting miR-519d as a potential therapeutic target for gastric cancer treatment (82). Jin et al reported that miR-122 inhibits EMT in HCC by targeting Snail1 and Snail2 to suppress the Wnt/β-catenin signaling pathway (83).
The translocation of β-catenin in the nucleus is followed by the activation of TCF/LEF-Legless-Pygo DNA binding proteins, which triggers transcription of many oncogenes such as extracellular matrix receptor III (CD44), c-Myc, MMP-7, and cyclin D1 (52). LEF1, a pivotal transcription factor in the Wnt signaling pathway, was reported to be a target of miR-708 and miR-34a by directly binding to 3′-UTR of LEF1, suggesting miR-708 and miR-34a as EMT inhibitors in melanoma and prostate cancer, respectively (84,85). Pygopus2 (Pygo2) is regarded as a tumor promoter in various types of cancer due to its combination with free β-catenin to cause abnormal activation of downstream oncogenes (86). A study demonstrated that miR-516a-3p inhibits breast cancer cell growth, metastasis and EMT by binding to 3′-UTR of Pygo2 mRNA, resulting in blockage of the Pygo2/Wnt pathway (87). In addition, transcription factor 4 (TCF4) has been found to promote the occurrence and development of several cancers by recognizing β-catenin to initiate the transcription of Wnt target genes (38). MiR-495 was reported to bind to the 3′-UTR of TCF4, thereby suppressing the migration, invasion, and proliferation of non-small-cell lung cancer (NSCLC) by inactivating the Wnt/β-catenin pathway (88).
Some other transcription factors were also reported to be affected by miRNA in the regulation of Wnt/β-catenin signaling. Melanogenesis-associated transcription factor (MITF), as one of the representative target genes of β-catenin, plays a carcinogenic role in gastric cancer. A study reported that miR-876-5p was able to bind to 3′-UTR of MITF and downregulate its expression, thus suppressing viability and migration of gastric cancer cells, and inducing cell apoptosis (89). Octamer-binding protein 4 (Oct4), an octamer motif-binding transcription factor, has been confirmed to exhibit an oncogenic effect in several types of cancer (90,91). A study by Ling et al demonstrated that miR-145 suppresses EMT in lung cancer cells by targeting Oct4 to inactivate the Wnt/β-catenin signaling pathway (92).
Targeting key proteins of the Wnt/β-catenin signaling pathway
Accumulation of β-catenin in cytoplasm is the central event in Wnt signaling activation; thus, miRNAs targeting β-catenin may act as EMT inhibitors. In addition to the miR-200 family mentioned above, miR-34b/c suppress β-catenin mRNA expression by targeting the 3′-UTR of β-catenin in prostate cancer (93). MiR-3619-5p directly binds to 3′-UTR of β-catenin and causes its downregulation in bladder carcinoma (94). Similarly, miR-33a targets the 3′-UTR of β-catenin to block EMT in human pancreatic cancer cells (95).
It is generally recognized that Wnt ligands are regulated by various miRNAs. For example, Wnt1 is a direct target gene of miR-122 in HCC HepG2 and Huh7 cell lines, thus downregulation of miR-122 facilitates EMT in HCC cells by activating Wnt signaling (96). Another study confirmed that Wnt1 is a target gene of miR-148a in HCC cells, suggesting miR-148a acts as an HCC metastasis suppressor by blocking the Wnt signaling pathway (97). In addition, Peng et al reported that miR-148a suppresses EMT and invasion of pancreatic cancer cells by targeting Wnt10b and inhibiting the Wnt signaling pathway, making miR-148a a novel therapeutic target for pancreatic cancer treatment (98). Wnt5A was found to be targeted by miR-876-5p, which suppresses the viability and migration of gastric cancer cells and induces cell apoptosis (89). Moreover, Wnt7a, which activates Wnt signaling to promote EMT of bladder cancer, can be inhibited by miR-370-3p (99).
Wnt ligands transduce signals by binding to several receptors such as FZD and LRP5/6, this process was described to be regulated by miRNAs. When Wnt ligands bind to receptors, FZD and LRP5/6 form a complex on the surface of the cell membrane. Then, Dsh protein is recruited and constitutes a complex with Axin, which binds GSK3β and CKIα to release β-catenin, thus forming β-catenin accumulation in the cytoplasm (50). MiR-3127-5p was reported to block Wnt/β-catenin signaling by directly targeting FZD4 in NSCLC (100). Moreover, miR-504 negatively regulates the Wnt/β-catenin pathway by directly targeting FZD7, thus suppressing the mesenchymal phenotype of glioblastoma (101).
The phosphorylation of β-catenin by GSK3 is necessary for β-catenin degradation when the Wnt signal is absent. Researchers found that proto-oncogene frequently rearranged in advanced T-cell lymphomas 1 (FRAT1) belongs to the GSK3-binding protein family, which inhibits GSK3-mediated phosphorylation of β-catenin and positively regulates the Wnt signaling pathway (102). MiR-490-3p is identified to directly target FRAT1, suggesting its tumor suppressive effects (103). SLC39A7 (ZIP7), a zinc transporter essential for the activation of tyrosine kinase, is considered to be a potential target of Wnt/β-catenin. A study by Cui et al suggested that miR-15a-3p suppresses prostate cancer by targeting SLC39A7 to inhibit the Wnt/β-catenin signaling pathway (104). In addition, Nimmanon et al reported that activation of SLC39A7 drives the PI3K/Akt pathway (105). Thus, targeting SLC39A7 by miR-15a-3p to suppress cancer cell progression may also result from inhibition of the PI3K/Akt pathway.
Targeting other signaling pathways
MiRNAs may regulate Wnt signal transduction by crosstalk with other signaling pathways (106). Signal transducer and activator of transcription 3 (STAT3) has been confirmed to be associated with EMT via regulation of β-catenin (107). Guo et al reported that miR-125b-5p targeting STAT3 results in β-catenin phosphorylation and degradation in HCC cells, indicating the inhibitory effect of miR-125b-5p on β-catenin-mediated EMT (62). Cyclin-dependent kinase 2 (CDK2), a member of the Ser/Thr protein kinase family, plays a crucial role in cancer proliferation and metastasis (108). Zhang et al found miR-3619-5p directly targets CDK2 and β-catenin to suppress bladder carcinoma progression, while further studies revealed that miR-3619-5p inhibits Wnt signaling partly through the induction of p21 following CDK2 and β-catenin inhibition (94). Cullin 4B (CUL4B), a scaffold protein assembling the cullin-RING-based E3 ubiquitin-protein ligase complexes, plays a critical role in proteolysis and tumorigenesis (109). Zhang et al reported that CUL4B is a direct target of miR-300 in pancreatic cancer cells, and downregulation of CUL4B by miR-300 results in inhibition of the Wnt signaling pathway and EMT (110). Similarly, Cullin 4A (CUL4A), also known as a core component of multiple cullin-RING-based E3 ubiquitin-protein ligase complexes, is negatively regulated by miR-377, indicating the inhibitory effect of miR-377 on the Wnt signaling pathway (111). A member of the receptor tyrosine kinases (RTKs) family, EPH receptor A2 (EphA2) is highly expressed in solid tumors, suggesting its important role in tumor initiation, progression, and invasion (112). MiR-302b and miR-338 serve as EphA2 inhibitors to suppress gastric cancer tumorigenesis and metastasis by inactivating the Wnt/β-catenin pathway (113,114). LIM Homeobox 2 (LHX2), a member of the LIM homeobox family, is involved in elevated β-catenin level and cell proliferation in pancreatic ductal adenocarcinoma (115). Liang et al revealed that miR-506 targets LHX2 to repress EMT and lymph node metastasis in nasopharyngeal carcinoma. They also found decreased TCF4 following LHX2 inhibition is responsible for Wnt/β-catenin signaling inactivation (116). Moreover, protein regulator of cytokinesis 1 (PRC1) was reported to mediate early HCC formation, transfer, stemness and development through Wnt/β-catenin signaling (117) and miR-194 could target PRC1 to suppress EMT in HCC cells by inactivating the Wnt/β-catenin signaling pathway (118). Additionally, Chen et al found that YWHAZ (14-3-3ζ) regulates the EMT process by interaction with β-catenin in NSCLC (119). On this basis, Guo et al demonstrated miR-375-3p targets YWHAZ to inhibit migration, invasion, and the EMT processes of gastric cancer cells by blocking the Wnt/β-catenin signaling pathway (120).
Although miRNAs may regulate Wnt signaling by affecting other signaling pathways, the underlying mechanism on how they interact has not been clearly defined. For example, miR-22 targeting formin-like 2 (FMNL2) (121), miR-136 targeting premelanosome protein (PMEL) (122), miR-29c targeting protein tyrosine phosphatase 4A2 (PTP4A2) and G protein subunit alpha 13 (GNA13) (123), and miR-378 targeting SDAD1 (60) all participate in Wnt/β-catenin signaling inhibition; however, the relationship between these targets and Wnt signaling needs further exploration. Therefore, the study of miRNAs targeting Wnt/β-catenin signaling, not only reveals the complex process of EMT, but also gives us better understanding of the crosstalk between Wnt signaling and other signaling pathways. For instance, Zhang et al found that miR-770 functions as a tumor suppressor by directly targeting the Jumonji domain containing 6 (JMJD6) 3′-UTR and inhibiting the Wnt/β-catenin pathway in NSCLC, suggesting Wnt/β-catenin as the downstream signal of JMJD6 in NSCLC cells (124).
miRNA as promoter of EMT
MiRNAs upregulated in various types of cancer display their carcinogenic role in tumor progression, migration, and invasion (125,126). There is a smaller quantity of miRNAs as EMT promoters compared with EMT inhibitors by targeting the Wnt signaling pathway (Table II, Fig. 1), but exploration of these miRNAs as potential therapeutic targets is also meaningful in combating EMT (127).
Table II.
Promotion of EMT by miRNAs.
| MiRNA | Cancer type | Molecular targets | (Refs.) |
|---|---|---|---|
| miR-135 | Bladder cancer | GSK3β | (133) |
| miR-106b-3p | Esophageal squamous cell carcinoma | ZNRF3 | (145) |
| miR-26b | Colorectal cancer | PTEN, Wnt5A | (150) |
| miR-27a-3p | Oral squamous carcinoma stem cells | SFRP1 | (143) |
| miR-95-3p | Prostatic cancer | DKK3 | (140) |
| miR-191 | Lung cancer | BASP1 | (184) |
| miR-197 | Hepatocellular carcinoma | AXIN2, NKD1, DKK2 | (126) |
| miR-373 | Endometrial cancer | LATS2 | (185) |
| miR-374a | Breast cancer | WIF1, PTEN, Wnt5A | (15) |
| miR-9 | Synovial sarcoma | E-cadherin | (129) |
| miR-23a | Epithelial ovarian cancer | ST7L | (186) |
| Breast cancer | E-cadherin | (130) | |
| miR-25 | Hepatocellular carcinoma | RhoGDI1 | (187) |
| miR-27 | Gastric cancer | APC | (132) |
| miR-93-5p | Lacrimal gland adenoid cystic carcinoma | BRMS1L | (188) |
| miR-125b | Triple negative breast cancer | APC | (137) |
| miR-146b-5p | Thyroid cancer | ZNRF3 | (146) |
| miR-373-3p | Tongue squamous cell carcinoma | DKK1 | (141) |
| miR-429 | Hepatocellular carcinoma | PTEN | (189) |
| miR-483-5p | Lung adenocarcinoma | RhoGDI1, ALCAM | (190) |
| miR-496 | Colorectal cancer | RASSF6 | (191) |
| miR-544a | Gastric cancer | E-cadherin, AXIN2 | (125) |
| miR-675 | Gastric cancer | PITX1 | (192) |
| miR-1246 | Lung cancer | GSK3β | (134) |
| miR-192/215 | Gastric cancer | SMG-1 | (193) |
| miR-199a-5p | Gastric cancer | E-cadherin | (131) |
| miR-139 | Pancreatic cancer | TOP2A | (194) |
| miR-150 | Colorectal cancer | CREB1, EP300 | (195) |
| miR-421 | Non-small cell lung cancer | HOPX | (196) |
| miR-23a/24 | Pancreatic ductal adenocarcinoma | FZD5, TMEM92, HNF1B | (197) |
| miR-92a | Colorectal cancer | KLF4 | (148) |
GSK3β, glycogen synthase kinase 3β; ZNRF3, zinc and ring finger 3; PTEN, phosphatase and tensin homologue; SFRP1, secreted frizzled-related protein 1; BASP1, brain abundant membrane attached signal protein 1; LATS2, large tumor suppressor kinase 2; WIF1, Wnt inhibitory factor 1; APC, adenomatous polyposis coli; ALCAM, activated leukocyte cell adhesion molecule; RASSF6, Ras association domain family member 6; PITX1, paired-like homeodomain 1; TOP2A, DNA Topoisomerase II Alpha; CREB1, CAMP responsive element binding protein 1; EP300, E1A binding protein P300; HOPX, homeodomain only protein x; HNF1B, HNF1 homeobox B; KLF4, Kruppel-like factor 4.
E-cadherin is an important intercellular adhesion molecule in maintaining cell-cell junctions and polarity. It is known that suppression of E-cadherin may result in cell detachment, invasion, and metastasis (128). Therefore, miRNAs which target E-cadherin are involved in EMT initiation. According to research, miR-9 (129), miR-23a (130), miR-544a (125) and miR-199a-5p (131) suppress E-cadherin to trigger EMT in particular cancer types, indicating these miRNAs are potential targets in cancer therapy.
Axin, APC, and GSK3β are β-catenin suppressors that act by forming a destructive complex to anchor β-catenin thus making it degrade. Therefore, miRNAs which target Axin, APC, and GSK3β activate Wnt signaling to trigger EMT by stabilization of β-catenin in the nucleus (132). A study by Mao et al demonstrated that miR-135a activates the Wnt/β-catenin signaling pathway by directly targeting GSK3β to accelerate the EMT, invasion, and migration of bladder cancer cells (133). In addition, miR-1246 facilitates the Wnt/β-catenin pathway through targeting GSK3β, which partly contributes to lung cancer metastasis (134). However, there is another study demonstrating different effects and mechanisms of miRNA on GSK3β. GSK3β modulates the NF-κB signaling pathway as it facilitates NF-κB function through post-transcriptional regulation of the NF-κB complex (135). Liu et al found that GSK3β is a direct target of miR-377-3p and is upregulated by miR-377-3p. Consequently, miR-377-3p promotes cell proliferation and EMT by upregulating GSK-3β expression and activating the NF-κB pathway in human colorectal cancer (136). In addition, miR-197 was reported to directly target Axin2 in HCC, leading to activation of Wnt/β-catenin signaling and EMT (126). Similarly, miR-544a plays an oncogenic role by directly targeting Axin2 to trigger EMT of gastric cancer (125). Moreover, APC was identified as the direct and functional target of miR-27 (132) and miR-125b (137) in gastric cancer and breast cancer, respectively, making miR-27 and miR-125b promising therapeutic targets for invasive cancer treatment.
The Wnt/β-catenin signaling pathway could be negatively regulated by antagonist molecules, therefore miRNAs targeting antagonists of the Wnt signaling have been regarded as EMT drivers. The DKK gene family, composed of DKK1-4 (138), was found to inhibit tumor invasion and migration by negative regulation of β-catenin (139). Some studies focused on the DKK family and found that miR-95-3p targeting DKK3 in prostatic cancer (140), miR-197 targeting DKK2 in HCC (126), and miR-373-3p targeting DKK1 in tongue squamous cell carcinoma (141) are responsible for the activation of Wnt/β-catenin signaling and EMT. Secreted frizzled-related protein 1 (SFRP1) acts as an antagonist of Wnt signaling by binding to Wnt proteins through its CRD domain against the transmembrane frizzled receptor (142). MiR-27a-3p was confirmed to promote EMT in oral squamous carcinoma stem cells by targeting SFRP1 (143). Zinc and ring finger 3 (ZNRF3) belongs to the E3 ubiquitin ligase family, which negatively regulates Wnt/β-catenin signaling by promoting the turnover of FZD and LRP6 (144). Qiao et al found that miR-106b-3p promotes cell proliferation and invasion by directly targeting ZNRF3, thus triggering EMT of esophageal squamous cell carcinoma (ESCC) (145). In addition, miR-146b-5p induces EMT in thyroid cancer by silencing of ZNRF3 (146). KLF4 (Kruppel-like factor 4), highly expressed in the adult intestine, is another negative regulator of Wnt signaling by interacting with β-catenin (147). Chen et al showed that miR-92a acts as an oncogene by directly targeting KLF4, thus affecting Wnt/β-catenin pathway and participating in colorectal cancer progression (148). A study by Parenti et al also demonstrated that Mesalazine treatment suppresses the expression of miR-130a and miR-135b, which target KLF4 mRNA, to mediate β-catenin inhibition in colon cancer (149). Furthermore, it was identified that miR-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis by targeting multiple negative regulators of Wnt including WIF1, PTEN, and Wnt5A (15). Similarly, downregulation of PTEN and Wnt5A by miR-26b also results in colorectal cancer metastasis (150). However, the effect of the miR-29 family on WIF1 in NSCLC is completely opposite, as miR-29 positively regulates WIF1 expression by inhibiting the methylation of its promoter, thus inhibiting the Wnt signaling pathway (151).
5. Use of miRNAs to regulate EMT
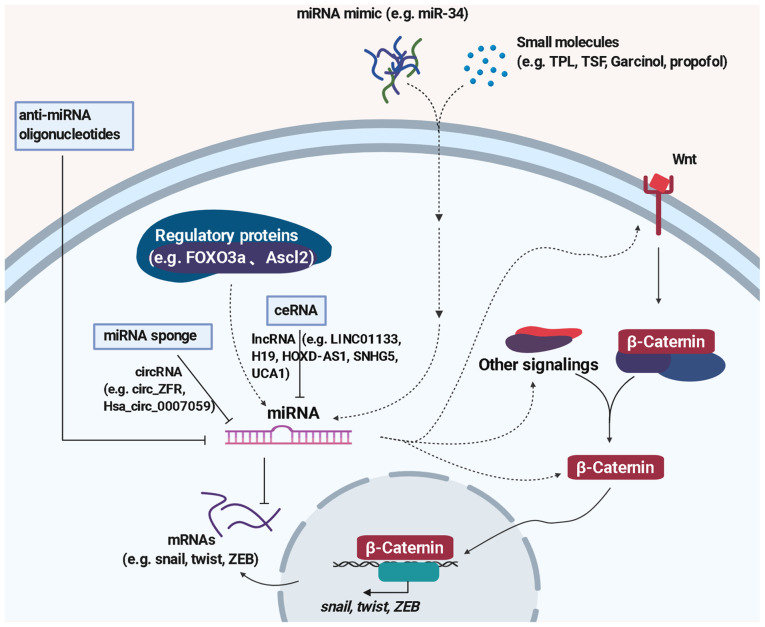
Since miRNAs play important roles in the regulation of EMT by activating or inhibiting the Wnt signaling pathway, miRNA-based therapies including those inhibiting miRNA function or restoring miRNA expression have been suggested as efficient strategies in cancer treatment (152) (Fig. 2). Delivering miRNA mimics contributes to the restoration of tumor-suppressive miRNA, while miRNA sponges, anti-miRNA oligonucleotides, small molecule inhibitors are useful approaches to block tumor promotive miRNA (153).
Figure 2.
Use of miRNAs to combat EMT. MiRNAs regulate Wnt/β-catenin signaling by targeting downstream transcription factors and key proteins of Wnt signaling or crosstalk with other signaling pathways. The strategies of using miRNAs to combat EMT include delivering miRNA mimic, anti-miRNA oligonucleotides or small molecule inhibitors. In addition, circRNA as miRNA sponge, lncRNA as ceRNA, and targeting regulatory proteins may constitute new prospective therapeutic strategies for cancer treatment.
The first miRNA-based therapy for cancer is MRX34, which was designed to deliver miR-34 mimic to cancer cells. MiR-34, which exerts a suppressive effect on Wnt signaling and tumor metastasis, is downregulated in various types of cancer including colon cancer, liver cancer, NSCLC, and cervical cancer (154). Several preclinical studies demonstrated that delivery of miR-34 mimic has promising effects against liver cancer (155), lung cancer (156), and prostate cancer (157). MRX34 encapsulated in lipid is under clinical testing (NCT01829971) in several solid and haematological malignancies (158). In addition, miravirsen, a locked nucleic acid (LNA)-based antisense oligonucleotide targeting miR-122, reached phase II trials for treating hepatitis (127). Recently, LNA-modified miR-92a inhibitor MRG-110 and miR-29 mimic MRG-201 are under phase I clinical trials by miRagen Therapeutics, Inc. (159). RGLS5579, which targets miR-10b, demonstrated statistically significant improvements in survival in an orthotopic glioblastoma multiforme animal model, and the addition of a single dose of RGLS5579 combined with temozolomide led to a >2-fold improvement in survival compared to TMZ alone (https://www.sec.gov/Archives/edgar/data/1505512/000162828020003483/rgls20191231-10k.htm). Moreover, replenishing tumor-suppressive miRNAs such as miR-200, miR-26a, miR-506, miR-520, miR-15/16 and inhibiting tumor-stimulating miRNAs such as miR-10b, miR-221, miR-155, miR-630 have also been included in preclinical studies (160).
Efforts have been made to explore small molecular compounds targeting EMT-related miRNAs. A natural compound isolated from Tripterygium wifordii Hook F, namely, Triptolide (TPL), was reported to exert anti-colorectal cancer properties by downregulating miR-191, thus blocking NF-κB and Wnt/β-catenin signaling activation (161). Toosendanin (TSN), a triterpenoid extracted from the bark or fruits of Melia toosendan Sieb et Zucc, suppresses gastric cancer proliferation, invasion, and migration by targeting miR-200a to downregulate β-catenin (162). In addition, another study demonstrated that Garcinol exerts antineoplastic effects on aggressive breast cancer due to reversal of the mesenchymal phenotype, which is mediated by miR-200s and let-7s targeting NF-κB and Wnt signaling (163). Moreover, Du et al showed that propofol can inhibit the proliferation and EMT of MCF-7 cells by targeting miR-21 to regulate the PI3K/Akt and Wnt/β-catenin pathway (164). These findings not only provide promising compounds against EMT but also reveal the mechanism of miRNAs as targets in Wnt signaling regulation.
6. Results and Discussion
As shown in Fig. 1, Wnt/β-catenin signaling is under solid regulation by miRNAs to prevent EMT prior to tumor metastasis. Dysregulation of miRNAs is involved in multiple types of invasive cancer due to their effects on gene expression at the post-transcriptional level (59). A single miRNA can target many genes, similarly a specific gene is regulated by multiple miRNAs, indicating the complex biological effects caused by a small change of miRNA (15,84,85). In this review, we divided miRNAs into two classes including EMT suppressors or stimulators; however, certain miRNAs may play dual roles in different types or different stages of cancer. For example, miR-374a acts as an EMT suppressor in early-stage NSCLC (stages I and II) by targeting cyclin D1 but switches to an EMT promoter in more advanced stages by targeting PTEN (165). Another study demonstrated the paradoxical effects of miR-145 on SW480 and SW620. Ectopic expression of miR-145 suppresses the proliferation, migration and invasion in SW480 but enhances these traits in its metastatic counterpart, SW620, which may be mediated through the downregulation of SIP1 but differential tuning of Wnt signaling and EMT-mediators (166). Interestingly, the dual effects of Wnt/β-catenin signaling also add to the complexity of EMT regulation. Li et al reported miR-630 inhibits EMT of gastric cancer by activating the Wnt/β-catenin pathway (167). Moreover, there is an exceptional case where a high level of Wnt3A suppresses melanoma growth and metastasis although β-catenin is active (168), indicating the opposite effects of activated Wnt/β-catenin signaling in response to different Wnt ligands. These results suggest that miRNAs and Wnt signaling may act as double-edged swords, and the level of miRNA affects gene expression in a cell and tissue context-dependent manner (169). Therefore, the dual functions of miRNA and the strategies of using miRNA targeting Wnt to overcome EMT need further investigation. We should take cancer type, clinical stage, tissue context, and tumor microenvironment into consideration. Additionally, recent studies have elucidated the regulatory effects of miRNAs on EMT-induced cancer drug resistance. For example, Wang et al found that miR-200c-3p suppression contributes to the acquired resistance of NSCLC to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors via a mediating EMT process (170). Cochrane et al reported that miR-200c could inhibit EMT and reinstate sensitivity to chemotherapeutic drugs in endometrial, breast, and ovarian cancer cells (171). These results indicated that regulation of EMT by miRNAs also plays a role in drug sensitivity, and comprehensive studies of miRNA's effects in all respects are required to combat cancer.
There are numerous studies demonstrating the upstream regulators of miRNA including key proteins, circular RNA (circRNA), and long non-coding RNA (lncRNA) (Fig. 2). For example, Forkhead box-O 3a (FOXO3a) inhibits β-catenin through transactivating miR-34b/c (93). As mentioned above, Ascl2 negatively regulates the miR-200 family which belongs to tumor suppressors, making Ascl2 a potential target to reverse EMT (75). Hsa_circ_0007059 blocks the Wnt/β-catenin and ERK1/2 pathways by targeting miR-378 in A549 and H1975 cells (172). MiR-106a-3p is a direct target of lncRNA LINC01133 which suppresses gastric cancer metastasis by acting as a competitive endogenous RNA (ceRNA) for miR-106a-3p to regulate APC in Wnt/β-catenin signaling (173). Furthermore, LncRNA H19 (174), lncRNA HOXD-AS1 (175), lncRNA SNHG5 (176) and lncRNA UCA1 (177) were confirmed to regulate the Wnt signaling pathway by targeting miRNAs. All the evidence indicate that multiple miRNA-mediated signal transductions participate in the regulation of EMT. Revealing the connections of miRNAs and their upstream regulators may give us new prospective therapeutic strategies for cancer treatment.
Since miRNA has established its role in EMT, the strategy of utilizing miRNA to overcome cancer metastasis has increasingly gained attention. Although the complex mechanism of EMT regulation by miRNA has not been fully defined, miRNAs are still regarded as potential therapeutic implements in cancer (Fig. 2). On the one hand, various methods of directly switching the level of miRNA by miRNA mimics, miRNA sponges, or anti-miRNA oligonucleotides, which are under study for different phases, have been shown to be effective. On the other hand, indirect regulation of miRNAs by affecting upstream regulators (protein, circRNA, lncRNA) or crosstalk with other signaling pathways are also useful approaches to inhibit EMT. Currently, a number of miRNA-based therapies are in clinical trials to treat cancer or other diseases. However, safety concerns regarding miRNA therapy always exist. Off-target side-effects, toxicity, and carcinogenicity of miRNA are important challenges in the development of miRNA therapy. Seeking effective delivery systems for miRNA is also a dilemma, so further research may focus on these issues to improve the utilization value of miRNA therapy.
7. Conclusion
In conclusion, miRNAs regulate Wnt/β-catenin signaling through targeting transcription factors and key proteins of Wnt signaling or crosstalk with other signaling pathways. However, the complicated role of miRNA as either a tumor suppressor or an oncogene and its underlying mechanism need further exploration. This review, not only provides potential applications of miRNAs as molecular targets in invasive tumor treatment, but also helps us gain a better understanding of the complexity of the EMT process and crosstalk between Wnt/β-catenin and other signaling pathways.
Acknowledgements
The authors would like to thank Professor Dong-Mei Zhang (College of Pharmacy, Jinan University) and Jun-Shan Liu (Traditional Chinese Medicine, Southern Medical University) for their guidance.
Funding
This review was supported by the National Natural Science Foundation of China (grant nos. 81803790, 81573918, and 81703975), National Natural Science Foundation of Guangdong (grant no. 2020A1515011090), Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (grant no. 20181069), the Fundamental Research Funds for the Central Universities (grant no. 21618336), and Public Health Research Projects of Futian District, Shenzhen (grant no. FTWS2019064)
Availability of data and materials
Not applicable.
Authors' contributions
EXZ and LJD designed the study and revised the manuscript. YHL, LC and GZ searched the literature and drafted the manuscript. AYS, BL, JYS and CFZ were also involved in the conception of the study. JW, XL, CFY and YYC assisted with the critical revision of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Pasquier J, Abu-Kaoud N, Al Thani H, Rafii A. Epithelial to mesenchymal transition in a clinical perspective. J Oncol. 2015;2015:792182. doi: 10.1155/2015/792182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 3.Okumura N, Kagami T, Fujii K, Nakahara M, Koizumi N. Involvement of nectin-afadin in the adherens junctions of the corneal endothelium. Cornea. 2018;37:633–640. doi: 10.1097/ICO.0000000000001526. [DOI] [PubMed] [Google Scholar]
- 4.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 5.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 6.Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11:1125–1132. doi: 10.1111/j.1365-2443.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 7.Okabe N, Shimizu K, Ozaki-Kuroda K, Nakanishi H, Morimoto K, Takeuchi M, Katsumaru H, Murakami F, Takai Y. Contacts between the commissural axons and the floor plate cells are mediated by nectins. Dev Biol. 2004;273:244–256. doi: 10.1016/j.ydbio.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coopman P, Djiane A. Adherens Junction and E-Cadherin complex regulation by epithelial polarity. Cell Mol Life Sci. 2016;73:3535–3553. doi: 10.1007/s00018-016-2260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo F, Parker Kerrigan BC, Yang D, Hu L, Shmulevich I, Sood AK, Xue F, Zhang W. Post-transcriptional regulatory network of epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions. J Hematol Oncol. 2014;7:19. doi: 10.1186/1756-8722-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Wu Y, Wang Y, Wang C, Kang T, Rychahou PG, Chi YI, Evers BM, Zhou BP. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene. 2013;32:1351–1362. doi: 10.1038/onc.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Q, Qin W. DKK3 blocked translocation of β-catenin/EMT induced by hypoxia and improved gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J Cell Mol Med. 2015;19:2832–2841. doi: 10.1111/jcmm.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao JH, Luo Y, Jiang YG, He DL, Wu CT. Knockdown of β-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1α. Cancer Invest. 2011;29:377–382. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 15.Cai J, Guan H, Fang L, Yang Y, Zhu X, Yuan J, Wu J, Li M. MicroRNA-374a activates Wnt/β-catenin signaling to promote breast cancer metastasis. J Clin Invest. 2013;123:566–579. doi: 10.1172/JCI65871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 17.Wu C, Zhuang Y, Jiang S, Liu S, Zhou J, Wu J, Teng Y, Xia B, Wang R, Zou X. Interaction between Wnt/beta-catenin pathway and microRNAs regulates epithelial-mesenchymal transition in gastric cancer (Review) Int J Oncol. 2016;48:2236–2246. doi: 10.3892/ijo.2016.3480. [DOI] [PubMed] [Google Scholar]
- 18.Zaravinos A. The Regulatory Role of MicroRNAs in EMT and Cancer. J Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Xing T, Yang Z, Dudek R, Lu Q, Chen YH. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J Clin Med. 2017;7:1. doi: 10.3390/jcm7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang RR, Gui YH, Wang X. Role of the canonical Wnt signaling pathway in heart valve development. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17:757–762. (In Chinese) [PubMed] [Google Scholar]
- 21.Ahsan K, Singh N, Rocha M, Huang C, Prince VE. Prickle1 is required for EMT and migration of zebrafish cranial neural crest. Dev Biol. 2019;448:16–35. doi: 10.1016/j.ydbio.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paolillo M, Serra M, Schinelli S. Integrins in glioblastoma: Still an attractive target? Pharmacol Res. 2016;113:55–61. doi: 10.1016/j.phrs.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Santoro R, Zanotto M, Carbone C, Piro G, Tortora G, Melisi D. MEKK3 sustains EMT and stemness in pancreatic cancer by regulating YAP and TAZ transcriptional activity. Anticancer Res. 2018;38:1937–1946. doi: 10.21873/anticanres.12431. [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Tian X, Li Y, Yang T, Han Z, An J, Kong L, Li Y. Epithelial-mesenchymal transition may be involved in the immune evasion of circulating gastric tumor cells via downregulation of ULBP1. Cancer Med. 2020;9:2686–2697. doi: 10.1002/cam4.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang H, Kim H, Lee S, Youn H, Youn B. Role of Metabolic Reprogramming in Epithelial-Mesenchymal Transition (EMT) Int J Mol Sci. 2019;20:2042. doi: 10.3390/ijms20082042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg M. Epithelial plasticity, autophagy and metastasis: Potential modifiers of the crosstalk to overcome therapeutic resistance. Stem Cell Rev Rep. 2020 doi: 10.1007/s12015-019-09945-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Mai W, Chen M, Hu J, Zhuo Z, Lei X, Deng L, Liu J, Yao N, Huang M, et al. Arenobufagin inhibits prostate cancer epithelial-mesenchymal transition and metastasis by down-regulating β-catenin. Pharmacol Res. 2017;123:130–142. doi: 10.1016/j.phrs.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Derynck R, Muthusamy BP, Saeteurn KY. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr Opin Cell Biol. 2014;31:56–66. doi: 10.1016/j.ceb.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 32.Astudillo P. Wnt5a Signaling in Gastric Cancer. Front Cell Dev Biol. 2020;8:110. doi: 10.3389/fcell.2020.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Marco P, Capra V, Kibar Z. Update on the Role of the Non-Canonical Wnt/Planar Cell Polarity Pathway in Neural Tube Defects. Cells. 2019;8:1198. doi: 10.3390/cells8101198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cici D, Corrado A, Rotondo C, Cantatore FP. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci. 2019;20:5552. doi: 10.3390/ijms20225552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang GR, Wei SJ, Huang YQ, Xing W, Wang LY, Liang LL. Mechanism of combined use of vitamin D and puerarin in anti-hepatic fibrosis by regulating the Wnt/β-catenin signalling pathway. World J Gastroenterol. 2018;24:4178–4185. doi: 10.3748/wjg.v24.i36.4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gay A, Towler DA. Wnt signaling in cardiovascular disease: Opportunities and challenges. Curr Opin Lipidol. 2017;28:387–396. doi: 10.1097/MOL.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villarroel A, Del Valle-Perez B, Fuertes G, Curto J, Ontiveros N, Garcia de Herreros A, Duñach M. Src and Fyn define a new signaling cascade activated by canonical and non-canonical Wnt ligands and required for gene transcription and cell invasion. Cell Mol Life Sci. 2020;77:919–935. doi: 10.1007/s00018-019-03221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: A prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 42.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 43.Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol. 2002;22:6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr Biol. 2000;10:1611–1614. doi: 10.1016/S0960-9822(00)00868-X. [DOI] [PubMed] [Google Scholar]
- 45.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/S0378-1119(02)01106-X. [DOI] [PubMed] [Google Scholar]
- 46.Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 49.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 50.Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3:117–136. [PMC free article] [PubMed] [Google Scholar]
- 51.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/beta-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–1649. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Guo Y, Xiao L, Sun L, Liu F. Wnt/beta-catenin signaling: A promising new target for fibrosis diseases. Physiol Res. 2012;61:337–346. doi: 10.33549/physiolres.932289. [DOI] [PubMed] [Google Scholar]
- 53.Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20:21–37. doi: 10.1038/s41580-018-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 55.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada C, Yamashita E, Lee SJ, Shibata S, Katahira J, Nakagawa A, Yoneda Y, Tsukihara T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–1279. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- 57.Dalmay T. Mechanism of miRNA-mediated repression of mRNA translation. Essays Biochem. 2013;54:29–38. doi: 10.1042/bse0540029. [DOI] [PubMed] [Google Scholar]
- 58.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moyret-Lalle C, Ruiz E, Puisieux A. Epithelial-mesenchymal transition transcription factors and miRNAs: ‘Plastic surgeons’ of breast cancer. World J Clin Oncol. 2014;5:311–322. doi: 10.5306/wjco.v5.i3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeng M, Zhu L, Li L, Kang C. miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell Mol Biol Lett. 2017;22:12. doi: 10.1186/s11658-017-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian L, Zhao Z, Xie L, Zhu J. MiR-361-5p inhibits the mobility of gastric cancer cells through suppressing epithelial-mesenchymal transition via the Wnt/β-catenin pathway. Gene. 2018;675:102–109. doi: 10.1016/j.gene.2018.06.095. [DOI] [PubMed] [Google Scholar]
- 62.Guo R, Wu Z, Wang J, Li Q, Shen S, Wang W, Zhou L, Wang W, Cao Z, Guo Y. Development of a Non-Coding-RNA-based EMT/CSC Inhibitory Nanomedicine for In Vivo Treatment and Monitoring of HCC. Adv Sci (Weinh) 2019;6:1801885. doi: 10.1002/advs.201801885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–165. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 64.Wiklund ED, Bramsen JB, Hulf T, Dyrskjøt L, Ramanathan R, Hansen TB, Villadsen SB, Gao S, Ostenfeld MS, Borre M, et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int J Cancer. 2011;128:1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 65.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 66.Elson-Schwab I, Lorentzen A, Marshall CJ. MicroRNA-200 family members differentially regulate morphological plasticity and mode of melanoma cell invasion. PLoS One. 2010;5:e13176. doi: 10.1371/journal.pone.0013176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, Mutch DG, Grigsby PW, Powell SN, Wang X. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 68.Shinozaki A, Sakatani T, Ushiku T, Hino R, Isogai M, Ishikawa S, Uozaki H, Takada K, Fukayama M. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. 2010;70:4719–4727. doi: 10.1158/0008-5472.CAN-09-4620. [DOI] [PubMed] [Google Scholar]
- 69.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 71.Saydam O, Shen Y, Wurdinger T, Senol O, Boke E, James MF, TannousB A, Stemmer-Rachamimov AO, Yi M, Stephens RM, et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol. 2009;29:5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, Engel P, Postigo A. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29:3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- 73.Cong N, Du P, Zhang A, Shen F, Su J, Pu P, Wang T, Zjang J, Kang C, Zhang Q. Downregulated microRNA-200a promotes EMT and tumor growth through the wnt/β-catenin pathway by targeting the E-cadherin repressors ZEB1/ZEB2 in gastric adenocarcinoma. Oncol Rep. 2013;29:1579–1587. doi: 10.3892/or.2013.2267. [DOI] [PubMed] [Google Scholar]
- 74.Su J, Zhang A, Shi Z, Ma F, Pu P, Wang T, Zhang J, Kang C, Zhang Q. MicroRNA-200a suppresses the Wnt/β-catenin signaling pathway by interacting with β-catenin. Int J Oncol. 2012;40:1162–1170. doi: 10.3892/ijo.2011.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian Y, Pan Q, Shang Y, Zhu R, Ye J, Liu Y, Zhong X, Li S, He Y, Chen L, et al. MicroRNA-200 (miR-200) cluster regulation by achaete scute-like 2 (Ascl2): Impact on the epithelial-mesenchymal transition in colon cancer cells. J Biol Chem. 2014;289:36101–36115. doi: 10.1074/jbc.M114.598383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J, Ruan B, You N, Huang Q, Liu W, Dang Z, Xu W, Zhou T, Ji R, Cao Y, et al. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the beta-catenin pathway in hepatic oval cells. PLoS One. 2013;8:e79409. doi: 10.1371/journal.pone.0079409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suliman MA, Zhang Z, Na H, Ribeiro AL, Zhang Y, Niang B, Hamid AS, Zhang H, Xu L, Zuo Y. Niclosamide inhibits colon cancer progression through downregulation of the Notch pathway and upregulation of the tumor suppressor miR-200 family. Int J Mol Med. 2016;38:776–784. doi: 10.3892/ijmm.2016.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng B, Wang R, Song HZ, Chen LB. MicroRNA-200b reverses chemoresistance of docetaxel-resistant human lung adenocarcinoma cells by targeting E2F3. Cancer. 2012;118:3365–3376. doi: 10.1002/cncr.26560. [DOI] [PubMed] [Google Scholar]
- 80.Qu J, Li M, An J, Zhao B, Zhong W, Gu Q, Cao L, Yang H, Hu C. MicroRNA-33b inhibits lung adenocarcinoma cell growth, invasion, and epithelial-mesenchymal transition by suppressing Wnt/β-catenin/ZEB1 signaling. Int J Oncol. 2015;47:2141–2152. doi: 10.3892/ijo.2015.3187. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X, Xu X, Ge G, Zang X, Shao M, Zou S, Zhang Y, Mao Z, Zhang J, Mao F, et al. miR498 inhibits the growth and metastasis of liver cancer by targeting ZEB2. Oncol Rep. 2019;41:1638–1648. doi: 10.3892/or.2018.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yue H, Tang B, Zhao Y, Niu Y, Yin P, Yang W, Zhang Z, Yu P. MIR-519d suppresses the gastric cancer epithelial-mesenchymal transition via Twist1 and inhibits Wnt/β-catenin signaling pathway. Am J Transl Res. 2017;9:3654–3664. [PMC free article] [PubMed] [Google Scholar]
- 83.Jin Y, Wang J, Han J, Luo D, Sun Z. MiR-122 inhibits epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Snail1 and Snail2 and suppressing WNT/β-cadherin signaling pathway. Exp Cell Res. 2017;360:210–217. doi: 10.1016/j.yexcr.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 84.Song XF, Wang QH, Huo R. Effects of microRNA-708 on epithelial-mesenchymal transition, cell proliferation and apoptosis in melanoma cells by targeting lef1 through the wnt signaling pathway. Pathol Oncol Res. 2019;25:377–389. doi: 10.1007/s12253-017-0334-z. [DOI] [PubMed] [Google Scholar]
- 85.Liang J, Li Y, Daniels G, Sfanos K, De Marzo A, Wei J, Li X, Chen W, Wang J, Zhong X, et al. LEF1 Targeting EMT in Prostate Cancer Invasion Is Regulated by miR-34a. Mol Cancer Res. 2015;13:681–688. doi: 10.1158/1541-7786.MCR-14-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Luo Q, Yuan Y, Huang X, Cai W, Li C, Wei T, Zhang L, Yang M, Liu Q, et al. Pygo2 associates with MLL2 histone methyltransferase and GCN5 histone acetyltransferase complexes to augment Wnt target gene expression and breast cancer stem-like cell expansion. Mol Cell Biol. 2010;30:5621–5635. doi: 10.1128/MCB.00465-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chi Y, Wang F, Zhang T, Xu H, Zhang Y, Shan Z, Wu S, Fan Q, Sun Y. miR-516a-3p inhibits breast cancer cell growth and EMT by blocking the Pygo2/Wnt signalling pathway. J Cell Mol Med. 2019;23:6295–6307. doi: 10.1111/jcmm.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng HE, Wang G, Song J, Liu Y, Li YM, Du WP. MicroRNA-495 inhibits the progression of non-small-cell lung cancer by targeting TCF4 and inactivating Wnt/beta-catenin pathway. Eur Rev Med Pharmacol Sci. 2018;22:7750–7759. doi: 10.26355/eurrev_201811_16398. [DOI] [PubMed] [Google Scholar]
- 89.Xu Z, Yu Z, Tan Q, Wei C, Tang Q, Wang L, Hong Y. MiR-876-5p regulates gastric cancer cell proliferation, apoptosis and migration through targeting WNT5A and MITF. Biosci Rep. 2019;39:BSR20190066. doi: 10.1042/BSR20190066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Zhang X, Wang X, Gan L, Yu G, Chen Y, Liu K, Li P, Pan J, Wang J, Qin S. Inhibition of LDH-A by lentivirus-mediated small interfering RNA suppresses intestinal-type gastric cancer tumorigenicity through the downregulation of Oct4. Cancer Lett. 2012;321:45–54. doi: 10.1016/j.canlet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Iida H, Suzuki M, Goitsuka R, Ueno H. Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int J Oncol. 2012;40:71–79. doi: 10.3892/ijo.2011.1207. [DOI] [PubMed] [Google Scholar]
- 92.Ling DJ, Chen ZS, Zhang YD, Liao QD, Feng JX, Zhang XY, Shi TS. MicroRNA-145 inhibits lung cancer cell metastasis. Mol Med Rep. 2015;11:3108–3114. doi: 10.3892/mmr.2014.3036. [DOI] [PubMed] [Google Scholar]
- 93.Liu H, Yin J, Wang H, Jiang G, Deng M, Zhang G, Bu X, Cai S, Du J, He Z. FOXO3a modulates WNT/beta-catenin signaling and suppresses epithelial-to-mesenchymal transition in prostate cancer cells. Cell Signal. 2015;27:510–518. doi: 10.1016/j.cellsig.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q, Miao S, Han X, Li C, Zhang M, Cui K, Xiong T, Chen Z, Wang C, Xu H. MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21. Cell Death Dis. 2018;9:960. doi: 10.1038/s41419-018-0986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang C, Wang Z, Li YY, Yu BH, Zhang F, Li HY. miR-33a suppresses the nuclear translocation of beta-catenin to enhance gemcitabine sensitivity in human pancreatic cancer cells. Tumour Biol. 2015;36:9395–9403. doi: 10.1007/s13277-015-3679-5. [DOI] [PubMed] [Google Scholar]
- 96.Wang N, Wang Q, Shen D, Sun X, Cao X, Wu D. Downregulation of microRNA-122 promotes proliferation, migration, and invasion of human hepatocellular carcinoma cells by activating epithelial-mesenchymal transition. Onco Targets Ther. 2016;9:2035–2047. doi: 10.2147/OTT.S92378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan H, Dong X, Zhong X, Ye J, Zhou Y, Yang X, Shen J, Zhang J. Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol Carcinog. 2014;53:960–969. doi: 10.1002/mc.22064. [DOI] [PubMed] [Google Scholar]
- 98.Peng L, Liu Z, Xiao J, Tu Y, Wan Z, Xiong H, Li Y, Xiao W. MicroRNA-148a suppresses epithelial-mesenchymal transition and invasion of pancreatic cancer cells by targeting Wnt10b and inhibiting the Wnt/beta-catenin signaling pathway. Oncol Rep. 2017;38:301–308. doi: 10.3892/or.2017.5705. [DOI] [PubMed] [Google Scholar]
- 99.Huang X, Zhu H, Gao Z, Li J, Zhuang J, Dong Y, Shen B, Li M, Zhou H, Guo H, Huang R, Yan J. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J Biol Chem. 2018;293:6693–6706. doi: 10.1074/jbc.RA118.001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang Y, Sun Y, Wu Y, Tang D, Ding X, Xu W, Su B, Gao W. Downregulation of miR-3127-5p promotes epithelial-mesenchymal transition via FZD4 regulation of Wnt/β-catenin signaling in non-small-cell lung cancer. Mol Carcinog. 2018;57:842–853. doi: 10.1002/mc.22805. [DOI] [PubMed] [Google Scholar]
- 101.Liu Q, Guan Y, Li Z, Wang Y, Liu Y, Cui R, Wang Y. miR-504 suppresses mesenchymal phenotype of glioblastoma by directly targeting the FZD7-mediated Wnt-β-catenin pathway. J Exp Clin Cancer Res. 2019;38:358. doi: 10.1186/s13046-019-1370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin R, Liu W, Menezes S, Yue F, Zheng M, Kovacevic Z, Richardson DR. The metastasis suppressor NDRG1 modulates the phosphorylation and nuclear translocation of β-catenin through mechanisms involving FRAT1 and PAK4. J Cell Sci. 2014;127:3116–3130. doi: 10.1242/jcs.147835. [DOI] [PubMed] [Google Scholar]
- 103.Zheng K, Zhou X, Yu J, Li Q, Wang H, Li M, Shao Z, Zhang F, Luo Y, Shen Z, et al. Epigenetic silencing of miR-490-3p promotes development of an aggressive colorectal cancer phenotype through activation of the Wnt/β-catenin signaling pathway. Cancer Lett. 2016;376:178–187. doi: 10.1016/j.canlet.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 104.Cui Y, Yang Y, Ren L, Yang J, Wang B, Xing T, Chen H, Chen M. miR-15a-3p Suppresses Prostate Cancer Cell Proliferation and Invasion by Targeting SLC39A7 Via Downregulating Wnt/β-Catenin Signaling Pathway. Cancer Biother Radiopharm. 2019;34:472–479. doi: 10.1089/cbr.2018.2722. [DOI] [PubMed] [Google Scholar]
- 105.Nimmanon T, Ziliotto S, Morris S, Flanagan L, Taylor KM. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics. 2017;9:471–481. doi: 10.1039/C6MT00286B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao X, Lu Y, Nie Y, Fan D. MicroRNAs as critical regulators involved in regulating epithelial- mesenchymal transition. Curr Cancer Drug Targets. 2013;13:935–944. doi: 10.2174/15680096113136660099. [DOI] [PubMed] [Google Scholar]
- 107.Huang H, Wang C, Liu F, Li HZ, Peng G, Gao X, Dong KQ, Wang HR, Kong DP, Qu M, et al. Reciprocal network between cancer stem-like cells and macrophages facilitates the progression and androgen deprivation therapy resistance of prostate cancer. Clin Cancer Res. 2018;24:4612–4626. doi: 10.1158/1078-0432.CCR-18-0461. [DOI] [PubMed] [Google Scholar]
- 108.Arai K, Eguchi T, Rahman MM, Sakamoto R, Masuda N, Nakatsura T, Calderwood SK, Kozaki K, Itoh M. A Novel high-throughput 3D screening system for EMT inhibitors: A pilot screening discovered the EMT inhibitory activity of CDK2 Inhibitor SU9516. PLoS One. 2016;11:e0162394. doi: 10.1371/journal.pone.0162394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang X, Chen Z. Knockdown of CUL4B Suppresses the Proliferation and Invasion in Non-Small Cell Lung Cancer Cells. Oncol Res. 2016;24:271–277. doi: 10.3727/096504016X14666990347473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang JQ, Chen S, Gu JN, Zhu Y, Zhan Q, Cheng DF, Chen H, Deng XX, Shen BY, Peng CH. MicroRNA-300 promotes apoptosis and inhibits proliferation, migration, invasion and epithelial-mesenchymal transition via the Wnt/β-catenin signaling pathway by targeting CUL4B in pancreatic cancer cells. J Cell Biochem. 2018;119:1027–1040. doi: 10.1002/jcb.26270. [DOI] [PubMed] [Google Scholar]
- 111.Yu R, Cai L, Chi Y, Ding X, Wu X. miR377 targets CUL4A and regulates metastatic capability in ovarian cancer. Int J Mol Med. 2018;41:3147–3156. doi: 10.3892/ijmm.2018.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paraiso KH, Das Thakur M, Fang B, Koomen JM, Fedorenko IV, John JK, Tsao H, Flaherty KT, Sondak VK, Messina JL, et al. Ligand-independent EPHA2 signaling drives the adoption of a targeted therapy-mediated metastatic melanoma phenotype. Cancer Discov. 2015;5:264–273. doi: 10.1158/2159-8290.CD-14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang J, He Y, McLeod HL, Xie Y, Xiao D, Hu H, Chen P, Shen L, Zeng S, Yin X, et al. miR-302b inhibits tumorigenesis by targeting EphA2 via Wnt/β-catenin/EMT signaling cascade in gastric cancer. BMC Cancer. 2017;17:886. doi: 10.1186/s12885-017-3875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song B, Lin HX, Dong LL, Ma JJ, Jiang ZG. MicroRNA-338 inhibits proliferation, migration, and invasion of gastric cancer cells by the Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:1290–1296. doi: 10.26355/eurrev_201803_14470. [DOI] [PubMed] [Google Scholar]
- 115.Zhou F, Gou S, Xiong J, Wu H, Wang C, Liu T. Oncogenicity of LHX2 in pancreatic ductal adenocarcinoma. Mol Biol Rep. 2014;41:8163–8167. doi: 10.1007/s11033-014-3716-2. [DOI] [PubMed] [Google Scholar]
- 116.Liang TS, Zheng YJ, Wang J, Zhao JY, Yang DK, Liu ZS. MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2. J Exp Clin Cancer Res. 2019;38:97. doi: 10.1186/s13046-019-1023-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Chen J, Rajasekaran M, Xia H, Zhang X, Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL, et al. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut. 2016;65:1522–1534. doi: 10.1136/gutjnl-2015-310625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang H, Zhao H, Yu ZY, Feng X, Fu BS, Qiu CH, Zhang JW. MicroRNA-194 inhibits cell invasion and migration in hepatocellular carcinoma through PRC1-mediated inhibition of Wnt/β-catenin signaling pathway. Dig Liver Dis. 2019;51:1314–1322. doi: 10.1016/j.dld.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Chen CH, Chuang SM, Yang MF, Liao JW, Yu SL, Chen JJ. A novel function of YWHAZ/beta-catenin axis in promoting epithelial-mesenchymal transition and lung cancer metastasis. Mol Cancer Res. 2012;10:1319–1331. doi: 10.1158/1541-7786.MCR-12-0189. [DOI] [PubMed] [Google Scholar]
- 120.Guo F, Gao Y, Sui G, Jiao D, Sun L, Fu Q, Jin C. miR-375-3p/YWHAZ/β-catenin axis regulates migration, invasion, EMT in gastric cancer cells. Clin Exp Pharmacol Physiol. 2019;46:144–152. doi: 10.1111/1440-1681.13047. [DOI] [PubMed] [Google Scholar]
- 121.Shi L, Huo JW, Chen SS, Xue JX, Gao WY, Li XY, Song YH, Xu HT, Zhu XW, Chen K. MicroRNA-22 targets FMNL2 to inhibit melanoma progression via the regulation of the Wnt/β-catenin signaling pathway and epithelial-mesenchymal transition. Eur Rev Med Pharmacol Sci. 2019;23:5332–5342. doi: 10.26355/eurrev_201906_18200. [DOI] [PubMed] [Google Scholar]
- 122.Wang JJ, Li ZF, Li XJ, Han Z, Zhang L, Liu ZJ. Effects of microRNA-136 on melanoma cell proliferation, apoptosis, and epithelial-mesenchymal transition by targetting PMEL through the Wnt signaling pathway. Biosci Rep. 2017;37:BSR20170743. doi: 10.1042/BSR20170743. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 123.Zhang JX, Mai SJ, Huang XX, Wang FW, Liao YJ, Lin MC, Kung HF, Zeng YX, Xie D. MiR-29c mediates epithelial-to-mesenchymal transition in human colorectal carcinoma metastasis via PTP4A and GNA13 regulation of β-catenin signaling. Ann Oncol. 2014;25:2196–2204. doi: 10.1093/annonc/mdu439. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Z, Yang Y, Zhang X. MiR-770 inhibits tumorigenesis and EMT by targeting JMJD6 and regulating WNT/β-catenin pathway in non-small cell lung cancer. Life Sci. 2017;188:163–171. doi: 10.1016/j.lfs.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Yanaka Y, Muramatsu T, Uetake H, Kozaki K, Inazawa J. miR-544a induces epithelial-mesenchymal transition through the activation of WNT signaling pathway in gastric cancer. Carcinogenesis. 2015;36:1363–1371. doi: 10.1093/carcin/bgv106. [DOI] [PubMed] [Google Scholar]
- 126.Hu Z, Wang P, Lin J, Zheng X, Yang F, Zhang G, Chen D, Xie J, Gao Z, Peng L, Xie C. MicroRNA-197 Promotes Metastasis of Hepatocellular Carcinoma by Activating Wnt/β-Catenin Signaling. Cell Physiol Biochem. 2018;51:470–486. doi: 10.1159/000495242. [DOI] [PubMed] [Google Scholar]
- 127.Gebert LF, Rebhan MA, Crivelli SE, Denzler R, Stoffel M, Hall J. Miravirsen (SPC3649) can inhibit the biogenesis of miR-122. Nucleic Acids Res. 2014;42:609–621. doi: 10.1093/nar/gkt852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petrova YI, Schecterson L, Gumbiner BM. Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell. 2016;27:3233–3244. doi: 10.1091/mbc.E16-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu XZ, Li XA, Luo Y, Liu JF, Wu HW, Huang G. MiR-9 promotes synovial sarcoma cell migration and invasion by directly targeting CDH1. Int J Biochem Cell Biol. 2019;112:61–71. doi: 10.1016/j.biocel.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 130.Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren Y, Li Z, Pang D, Qian C. MiR-23a promotes TGF-β1-induced EMT and tumor metastasis in breast cancer cells by directly targeting CDH1 and activating Wnt/β-catenin signaling. Oncotarget. 2017;8:69538–69550. doi: 10.18632/oncotarget.18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao X, He L, Li T, Lu Y, Miao Y, Liang S, Guo H, Bai M, Xie H, Luo G, et al. SRF expedites metastasis and modulates the epithelial to mesenchymal transition by regulating miR-199a-5p expression in human gastric cancer. Cell Death Differ. 2014;21:1900–1913. doi: 10.1038/cdd.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Z, Liu S, Shi R, Zhao G. miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer Genet. 2011;204:486–491. doi: 10.1016/j.cancergen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 133.Mao XW, Xiao JQ, Li ZY, Zheng YC, Zhang N. Effects of microRNA-135a on the epithelial-mesenchymal transition, migration and invasion of bladder cancer cells by targeting GSK3β through the Wnt/β-catenin signaling pathway. Exp Mol Med. 2018;50:e429. doi: 10.1038/emm.2017.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang F, Xiong H, Duan L, Li Q, Li X, Zhou Y. MiR-1246 Promotes Metastasis and Invasion of A549 cells by Targeting GSK-3betaMediated Wnt/β-Catenin Pathway. Cancer Res Treat. 2019;51:1420–1429. doi: 10.4143/crt.2018.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 136.Liu WY, Yang Z, Sun Q, Yang X, Hu Y, Xie H, Gao HJ, Guo LM, Yi JY, Liu M, Tang H. miR-377-3p drives malignancy characteristics via upregulating GSK-3β expression and activating NF-κB pathway in hCRC cells. J Cell Biochem. 2018;119:2124–2134. doi: 10.1002/jcb.26374. [DOI] [PubMed] [Google Scholar]
- 137.Nie J, Jiang HC, Zhou YC, Jiang B, He WJ, Wang YF, Dong J. MiR-125b regulates the proliferation and metastasis of triple negative breast cancer cells via the Wnt/β-catenin pathway and EMT. Biosci Biotechnol Biochem. 2019;83:1062–1071. doi: 10.1080/09168451.2019.1584521. [DOI] [PubMed] [Google Scholar]
- 138.Barrantes Idel B, Montero-Pedrazuela A, Guadano-Ferraz A, Obregon MJ, Martinez de Mena R, Gailus-Durner V, Fuchs H, Franz TJ, Kalaydjiev S, Klempt M, et al. Generation and characterization of dickkopf3 mutant mice. Mol Cell Biol. 2006;26:2317–2326. doi: 10.1128/MCB.26.6.2317-2326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.CAN-03-1952. [DOI] [PubMed] [Google Scholar]
- 140.Xi M, Cheng L, Hua W, Zhou YL, Gao QL, Yang JX, Qi SY. MicroRNA-95-3p promoted the development of prostatic cancer via regulating DKK3 and activating Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci. 2019;23:1002–1011. doi: 10.26355/eurrev_201902_16987. [DOI] [PubMed] [Google Scholar]
- 141.Weng J, Zhang H, Wang C, Liang J, Chen G, Li W, Tang H, Hou J. miR-373-3p Targets DKK1 to promote EMT-induced metastasis via the Wnt/β-catenin pathway in tongue squamous cell carcinoma. Biomed Res Int. 2017;2017:6010926. doi: 10.1155/2017/6010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elzi DJ, Song M, Hakala K, Weintraub ST, Shiio Y. Wnt antagonist SFRP1 functions as a secreted mediator of senescence. Mol Cell Biol. 2012;32:4388–4399. doi: 10.1128/MCB.06023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qiao B, He BX, Cai JH, Tao Q, King-Yin Lam A. MicroRNA-27a-3p modulates the Wnt/β-catenin signaling pathway to promote epithelial-mesenchymal transition in oral squamous carcinoma stem cells by targeting SFRP1. Sci Rep. 2017;7:44688. doi: 10.1038/srep44688. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Zebisch M, Xu Y, Krastev C, MacDonald BT, Chen M, Gilbert RJ, He X, Jones EY. Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin. Nat Commun. 2013;4:2787. doi: 10.1038/ncomms3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qiao G, Dai C, He Y, Shi J, Xu C. Effects of miR106b3p on cell proliferation and epithelialmesenchymal transition, and targeting of ZNRF3 in esophageal squamous cell carcinoma. Int J Mol Med. 2019;43:1817–1829. doi: 10.3892/ijmm.2019.4107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang X, Fan Y. MiR-146b-5p promotes metastasis and induces epithelial-mesenchymal transition in thyroid cancer by targeting ZNRF3. Cell Physiol Biochem. 2015;35:71–82. doi: 10.1159/000369676. [DOI] [PubMed] [Google Scholar]
- 147.Zhang W, Chen X, Kato Y, Evans PM, Yuan S, Yang J, Rychahou PG, Yang VW, He X, Evers BM, Liu C. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006;26:2055–2064. doi: 10.1128/MCB.26.6.2055-2064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen E, Li Q, Wang H, Yang F, Min L, Yang J. MiR-92a promotes tumorigenesis of colorectal cancer, a transcriptomic and functional based study. Biomed Pharmacother. 2018;106:1370–1377. doi: 10.1016/j.biopha.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 149.Parenti S, Montorsi L, Fantini S, Mammoli F, Gemelli C, Atene CG, Losi L, Frassineti C, Calabretta B, Tagliafico E, et al. KLF4 Mediates the Effect of 5-ASA on the β-Catenin Pathway in Colon Cancer Cells. Cancer Prev Res (Phila) 2018;11:503–510. doi: 10.1158/1940-6207.CAPR-17-0382. [DOI] [PubMed] [Google Scholar]
- 150.Fan D, Lin X, Zhang F, Zhong W, Hu J, Chen Y, Cai Z, Zou Y, He X, Chen X, et al. MicroRNA 26b promotes colorectal cancer metastasis by downregulating phosphatase and tensin homolog and wingless-type MMTV integration site family member 5A. Cancer Sci. 2018;109:354–362. doi: 10.1111/cas.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tan M, Wu J, Cai Y. Suppression of Wnt signaling by the miR-29 family is mediated by demethylation of WIF-1 in non-small-cell lung cancer. Biochem Biophys Res Commun. 2013;438:673–679. doi: 10.1016/j.bbrc.2013.07.123. [DOI] [PubMed] [Google Scholar]
- 152.Tokarz P, Blasiak J. The role of microRNA in metastatic colorectal cancer and its significance in cancer prognosis and treatment. Acta Biochim Pol. 2012;59:467–474. doi: 10.18388/abp.2012_2079. [DOI] [PubMed] [Google Scholar]
- 153.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bader AG. miR-34-a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Slaby O, Laga R, Sedlacek O. Therapeutic targeting of non-coding RNAs in cancer. Biochem J. 2017;474:4219–4251. doi: 10.1042/BCJ20170079. [DOI] [PubMed] [Google Scholar]
- 156.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther. 2011;19:1116–1122. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang L, Liao Y, Tang L. MicroRNA-34 family: A potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gallant-Behm CL, Piper J, Dickinson BA, Dalby CM, Pestano LA, Jackson AL. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen. 2018;26:311–323. doi: 10.1111/wrr.12660. [DOI] [PubMed] [Google Scholar]
- 160.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 161.Qi Y, Li J. Triptolide inhibits the growth and migration of colon carcinoma cells by down-regulation of miR-191. Exp Mol Pathol. 2019;107:23–31. doi: 10.1016/j.yexmp.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 162.Wang G, Huang YX, Zhang R, Hou LD, Liu H, Chen XY, Zhu JS, Zhang J. Toosendanin suppresses oncogenic phenotypes of human gastric carcinoma SGC7901 cells partly via miR200amediated downregulation of β-catenin pathway. Int J Oncol. 2017;51:1563–1573. doi: 10.3892/ijo.2017.4139. [DOI] [PubMed] [Google Scholar]
- 163.Ahmad A, Sarkar SH, Bitar B, Ali S, Aboukameel A, Sethi S, Li Y, Bao B, Kong D, Banerjee S, et al. Garcinol regulates EMT and Wnt signaling pathways in vitro and in vivo, leading to anticancer activity against breast cancer cells. Mol Cancer Ther. 2012;11:2193–2201. doi: 10.1158/1535-7163.MCT-12-0232-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Du Q, Zhang X, Zhang X, Wei M, Xu H, Wang S. Propofol inhibits proliferation and epithelial-mesenchymal transition of MCF-7 cells by suppressing miR-21 expression. Artif Cells Nanomed Biotechnol. 2019;47:1265–1271. doi: 10.1080/21691401.2019.1594000. [DOI] [PubMed] [Google Scholar]
- 165.Zhao M, Xu P, Liu Z, Zhen Y, Chen Y, Liu Y, Fu Q, Deng X, Liang Z, Li Y, et al. Dual roles of miR-374a by modulated c-Jun respectively targets CCND1-inducing PI3K/AKT signal and PTEN-suppressing Wnt/β-catenin signaling in non-small-cell lung cancer. Cell Death Dis. 2018;9:78. doi: 10.1038/s41419-017-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 166.Sathyanarayanan A, Chandrasekaran KS, Karunagaran D. microRNA-145 downregulates SIP1-expression but differentially regulates proliferation, migration, invasion and Wnt signaling in SW480 and SW620 cells. J Cell Biochem. 2018;119:2022–2035. doi: 10.1002/jcb.26365. [DOI] [PubMed] [Google Scholar]
- 167.Li D, Tian B, Jin X. miR-630 Inhibits Epithelial-to-Mesenchymal Transition (EMT) by Regulating the Wnt/β-Catenin Pathway in Gastric Cancer Cells. Oncol Res. 2018;27:9–17. doi: 10.3727/096504018X15178732625479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci USA. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ha TY. MicroRNAs in human diseases: From cancer to cardiovascular disease. Immune Netw. 2011;11:135–154. doi: 10.4110/in.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang HY, Liu YN, Wu SG, Hsu CL, Chang TH, Tsai MF, Lin YT, Shih JY. MiR-200c-3p suppression is associated with development of acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in EGFR mutant non-small cell lung cancer via a mediating epithelial-to-mesenchymal transition (EMT) process. Cancer Biomark. 2020;28:351–363. doi: 10.3233/CBM-191119. [DOI] [PubMed] [Google Scholar]
- 171.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8:1055–1066. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gao S, Yu Y, Liu L, Meng J, Li G. Circular RNA hsa_circ_0007059 restrains proliferation and epithelial-mesenchymal transition in lung cancer cells via inhibiting microRNA-378. Life Sci. 2019;233:116692. doi: 10.1016/j.lfs.2019.116692. [DOI] [PubMed] [Google Scholar]
- 173.Yang XZ, Cheng TT, He QJ, Lei ZY, Chi J, Tang Z, Liao QX, Zhang H, Zeng LS, Cui SZ. LINC01133 as ceRNA inhibits gastric cancer progression by sponging miR-106a-3p to regulate APC expression and the Wnt/beta-catenin pathway. Mol Cancer. 2018;17:126. doi: 10.1186/s12943-018-0874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ding D, Li C, Zhao T, Li D, Yang L, Zhang B. LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on wnt signaling. Mol Cells. 2018;41:423–435. doi: 10.14348/molcells.2018.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang Y, Dun Y, Zhou S, Huang XH. LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2017;96:1216–1221. doi: 10.1016/j.biopha.2017.11.096. [DOI] [PubMed] [Google Scholar]
- 176.Li Y, Guo D, Zhao Y, Ren M, Lu G, Wang Y, Zhang J, Mi C, He S, Lu X. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-26a-5p/GSK3β signal pathway. Cell Death Dis. 2018;9:888. doi: 10.1038/s41419-018-0882-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen X, Gao J, Yu Y, Zhao Z, Pan Y. Long non-coding RNA UCA1 targets miR-185-5p and regulates cell mobility by affecting epithelial-mesenchymal transition in melanoma via Wnt/β-catenin signaling pathway. Gene. 2018;676:298–305. doi: 10.1016/j.gene.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 178.Taube JH, Malouf GG, Lu E, Sphyris N, Vijay V, Ramachandran PP, Ueno KR, Gaur S, Nicoloso MS, Rossi S, et al. Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci Rep. 2013;3:2687. doi: 10.1038/srep02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Strillacci A, Valerii MC, Sansone P, Caggiano C, Sgromo A, Vittori L, Fiorentino M, Poggioli G, Rizzello F, Campieri M, Spisni E. Loss of miR-101 expression promotes Wnt/β-catenin signalling pathway activation and malignancy in colon cancer cells. J Pathol. 2013;229:379–389. doi: 10.1002/path.4097. [DOI] [PubMed] [Google Scholar]
- 180.Huang Z, Li Q, Luo K, Zhang Q, Geng J, Zhou X, Xu Y, Qian M, Zhang JA, Ji L, Wu J. miR-340-FHL2 axis inhibits cell growth and metastasis in ovarian cancer. Cell Death Dis. 2019;10:372. doi: 10.1038/s41419-019-1604-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu P, Chen B, Gu Y, Liu Q. PNMA1, regulated by miR-33a-5p, promotes proliferation and EMT in hepatocellular carcinoma by activating the Wnt/β-catenin pathway. Biomed Pharmacother. 2018;108:492–499. doi: 10.1016/j.biopha.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 182.Han S, Cao C, Tang T, Lu C, Xu J, Wang S, Xue L, Zhang X, Li M. ROBO3 promotes growth and metastasis of pancreatic carcinoma. Cancer Lett. 2015;366:61–70. doi: 10.1016/j.canlet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 183.Li Y, Lv Z, He G, Wang J, Zhang X, Lu G, Ren X, Wang F, Zhu X, Ding Y, et al. The SOX17/miR-371-5p/SOX2 axis inhibits EMT, stem cell properties and metastasis in colorectal cancer. Oncotarget. 2015;6:9099–9112. doi: 10.18632/oncotarget.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xu W, Ji J, Xu Y, Liu Y, Shi L, Liu Y, Lu X, Zhao Y, Luo F, Wang B, et al. MicroRNA-191, by promoting the EMT and increasing CSC-like properties, is involved in neoplastic and metastatic properties of transformed human bronchial epithelial cells. Mol Carcinog. 2015;54(Suppl 1):E148–161. doi: 10.1002/mc.22221. [DOI] [PubMed] [Google Scholar]
- 185.Li Y, Sun D, Gao J, Shi Z, Chi P, Meng Y, Zou C, Wang Y. MicroRNA-373 promotes the development of endometrial cancer by targeting LATS2 and activating the Wnt/β-Catenin pathway. J Cell Biochem. 2018 doi: 10.1002/jcb.28149. [DOI] [PubMed] [Google Scholar]
- 186.Yang Z, Wang XL, Bai R, Liu WY, Li X, Liu M, Tang H. miR-23a promotes IKKα expression but suppresses ST7L expression to contribute to the malignancy of epithelial ovarian cancer cells. Br J Cancer. 2016;115:731–740. doi: 10.1038/bjc.2016.244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 187.Wang C, Wang X, Su Z, Fei H, Liu X, Pan Q. MiR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1. Oncotarget. 2015;6:36231–36244. doi: 10.18632/oncotarget.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hao J, Jin X, Shi Y, Zhang H. miR-93-5p enhance lacrimal gland adenoid cystic carcinoma cell tumorigenesis by targeting BRMS1L. Cancer Cell Int. 2018;18:72. doi: 10.1186/s12935-018-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tang J, Li L, Huang W, Sui C, Yang Y, Lin X, Hou G, Chen X, Fu J, Yuan S, et al. MiR-429 increases the metastatic capability of HCC via regulating classic Wnt pathway rather than epithelial-mesenchymal transition. Cancer Lett. 2015;364:33–43. doi: 10.1016/j.canlet.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 190.Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen J, Zhang Y, Lai P, Fan X, Zhou X, et al. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 191.Wang H, Yan B, Zhang P, Liu S, Li Q, Yang J, Yang F, Chen E. MiR-496 promotes migration and epithelial-mesenchymal transition by targeting RASSF6 in colorectal cancer. J Cell Physiol. 2020;235:1469–1479. doi: 10.1002/jcp.29066. [DOI] [PubMed] [Google Scholar]
- 192.Liu L, Tian YC, Mao G, Zhang YG, Han L. MiR-675 is frequently overexpressed in gastric cancer and enhances cell proliferation and invasion via targeting a potent anti-tumor gene PITX1. Cell Signal. 2019;62:109352. doi: 10.1016/j.cellsig.2019.109352. [DOI] [PubMed] [Google Scholar]
- 193.Zhang X, Peng Y, Huang Y, Yang M, Yan R, Zhao Y, Cheng Y, Liu X, Deng S, Feng X, et al. SMG-1 inhibition by miR-192/-215 causes epithelial-mesenchymal transition in gastric carcinogenesis via activation of Wnt signaling. Cancer Med. 2018;7:146–156. doi: 10.1002/cam4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pei YF, Yin XM, Liu XQ. TOP2A induces malignant character of pancreatic cancer through activating β-catenin signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:197–207. doi: 10.1016/j.bbadis.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 195.Guo YH, Wang LQ, Li B, Xu H, Yang JH, Zheng LS, Yu P, Zhou AD, Zhang Y, Xie SJ, et al. Wnt/β-catenin pathway transactivates microRNA-150 that promotes EMT of colorectal cancer cells by suppressing CREB signaling. Oncotarget. 2016;7:42513–42526. doi: 10.18632/oncotarget.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liang H, Wang C, Gao K, Li J, Jia R. MuicroRNA-421 promotes the progression of nonsmall cell lung cancer by targeting HOPX and regulating the Wnt/β-catenin signaling pathway. Mol Med Rep. 2019;20:151–161. doi: 10.3892/mmr.2019.10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Listing H, Mardin WA, Wohlfromm S, Mees ST, Haier J. MiR-23a/-24-induced gene silencing results in mesothelial cell integration of pancreatic cancer. Br J Cancer. 2015;112:131–139. doi: 10.1038/bjc.2014.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.