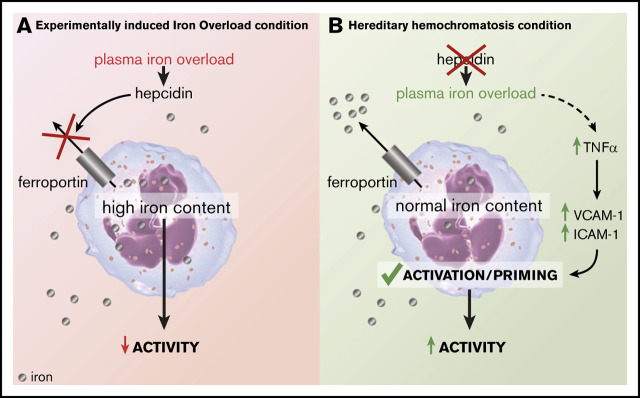
Key Points
Hepcidin was identified as a key player in iron-dependent regulation of neutrophil function.
Neutrophils from HH have a primed phenotype associated with an increased oxidative burst capacity and phagocytosis.
Abstract
Iron is required for the oxidative response of neutrophils to allow the production of reactive oxygen species (ROS). However, neutrophil function may be severely altered in conditions of iron overload, as observed in chronically transfused patients. Therefore, a tight regulation of neutrophil iron homeostasis seems to be critical for avoiding iron toxicity. Hepcidin is the key iron regulator in organisms; however, no studies have investigated its role in maintaining neutrophil iron homeostasis or characterized neutrophil function in patients with hereditary hemochromatosis (HH), a common iron overload genetic disorder that results from a defect in hepcidin production. To explore these issues, we studied 2 mouse models of iron overload: an experimentally induced iron overload model (EIO), in which hepcidin is increased, and a genetic HH model of iron overload with a deletion of hepatic hepcidin. We found that iron-dependent increase of hepatic hepcidin results in neutrophil intracellular iron trapping and consecutive defects in oxidative burst activity. In contrast, in both HH mouse models and HH patients, the lack of hepcidin expression protects neutrophils from toxic iron accumulation. Moreover, systemic iron overload correlated with a surprising neutrophil priming and resulted in a more powerful oxidative burst. Indeed, important factors in neutrophil priming and activation, such as tumor necrosis factor α (TNF-α), VCAM-1, and ICAM-1 are increased in the plasma of HH patients and are associated with an increase in HH neutrophil phagocytosis capacity and a decrease in L-selectin surface expression. This is the first study to characterize neutrophil iron homeostasis and associated functions in patients with HH.
Visual Abstract
Introduction
Because neutrophils are both the first line of innate defense and effectors of adaptive immunity, they play a crucial role in immune defense against infection and tissue inflammation.1,2 They respond to external stimuli by producing superoxide anions and hypochlorous acid via the phagocytic (reduced) nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex2,3 and myeloperoxidase (MPO), respectively, through a process called respiratory or oxidative burst. Iron is required for both neutrophil NADPH oxidase and MPO activities as a component of the catalytic site,3 but it can also participate in generating other reactive oxygen species (ROS) such as the hydroxyl radical (°OH) via the Fenton reaction during phagocytosis. Therefore, iron plays an important role in antimicrobial host defense and inflammation.4 However, studies extending back to 1959 have described the toxic effect of excess iron on MPO activity5 as well as on phagocytosis and bactericidal activity.6-8 Excess iron stimulates production of oxidant radicals that could be autotoxic for the neutrophil membranes with subsequent impairment of phagocytosis.7-9 Altered phagocytic functions of neutrophils have been reported in patients with chronically transfused hemodialysis10,11 and β-thalassemia major.12,13 Although other factors could contribute to impaired neutrophil functions in transfused patients, the improved neutrophil phagocytosis and bactericidal capacity after body iron stores are decreased by iron chelation11,14 provide good evidence for the negative effects of iron overload on neutrophil functions.
Since these seminal discoveries describing the negative effect of iron overload on neutrophil functions, iron homeostasis has been the focus of active research, which notably prompted the discovery of hepcidin as a key circulating hormone that maintains systemic iron homeostasis. However, no studies have investigated the impact of hepcidin on iron homeostasis in neutrophils. Hepcidin is a 25-amino-acid peptide produced mainly by the liver and secreted in the blood in response to iron overload or infection/inflammation.15 Because iron cannot be excreted physiologically, this peptide inhibits both iron absorption in the small intestine and the release of recycled iron from macrophages to avoid excess iron. Hepcidin acts by inhibiting cellular iron efflux by binding to ferroportin (FPN), the only known cellular iron exporter.16,17 Whether neutrophils express FPN and whether neutrophil intracellular iron content can be regulated by hepcidin is not known. Hereditary hemochromatosis (HH) or primary hemochromatosis is a common genetic disorder characterized by iron overload resulting from abnormally low hepcidin levels. HH is caused by mutations on genes involved in hepcidin regulation or, rarely, by mutations on the hepcidin gene itself.18,19 The most common mutations in HH patients are on the HFE gene, with the most frequent one being the C282Y substitution, which accounts for 90% of the HFE mutations.20 HH patients have high plasma iron levels, increased transferrin saturation, uncontrolled iron absorption, and iron accumulation in the parenchyma. Iron deposition leads to injuries in organs such as the liver, heart, or pancreas.21
Although iron overload in patients with primary hemochromatosis or HH is a consequence of hepcidin deficiency,22,23 patients with hematologic conditions that require multiple transfusions of red blood cells to treat anemia develop a secondary form of hemochromatosis associated with an increase in hepcidin in an attempt to counteract iron overload.
In this study, we hypothesized that the lack of hepcidin that causes iron overload in HH protects against neutrophil intracellular iron accumulation and consecutive iron-related impairment of defense functions. The first goal of this study was to elucidate the impact of hepcidin deficiency or increase on neutrophil iron content and oxidative burst activity in animal models. The second goal was to translate this model to humans and analyze the function of neutrophils isolated from patients with C282Y HH.
Methods
Animal studies
All animal studies were performed in 8- to 12-week-old C57BL/6J male mice and were reviewed and approved (Agreement No. 2017011914104034) by the President of the Ethical Committee for Animal Experimentation and are in accordance with the principles and guidelines established by the European Convention for the Protection of Laboratory Animals (Council of Europe, ETS 123, 1991).
The classic diet used contains 0.28 g of Fe per kilogram of food (Diet 103; Safe diets, Augy, France). When indicated, mice were fed an iron-rich diet (20 g/kg; Safe diet) or the associated control diet (0.2 g/kg; Safe diet).
Human samples
Blood samples from homozygous patients with C282Y hemochromatosis and healthy donors were collected after written informed consent was obtained by the Department of Adult Hematology (Hopital Necker‐Enfants Malades). Thirty-eight patients with hemochromatosis were recruited during the maintenance phlebotomy phase. Because of patient confidentiality, age and sex were blinded. Patients were randomly assigned to each of the assays to ensure that there was a representative and statistically significant number of patients in each group. Approval was obtained from the Ministry of Research (reference DC 2014-2272). Samples from hemochromatosis patients and healthy donors were compared on the same day. Blood samples from healthy donors were also obtained by the Etablissement Français du Sang in accordance with the principles and guidelines established by Convention No. 18/EFS/030.
Neutrophil purification
Neutrophils were purified from human whole blood collected in tubes with acid-citrate-dextrose anticoagulant by using the Dextran-Ficoll method as previously described.24 Purity of the preparation was assessed by cell counting with Türk hematologic staining and by cell viability with Trypan blue staining. The preparations used were above 95% purity and viability. Neutrophils were purified from mouse bone marrows by using the Miltenyi neutrophil magnetic isolation kit according to manufacturer’s instructions (Miltenyi Biotec).
Oxidative burst
Oxidative burst kinetics studies were performed on a TriStar LB942 Modular Multimode Microplate Reader using 1 × 105 cells resuspended in 200 µL Hank’s balanced salt solution (Thermo Fisher) and luminol (Sigma) and stimulated with the following agents: phorbol 12-myristate 13-acetate (PMA; 0.1 µg/mL; Sigma), N-formylmethionine-leucyl-phenylalanine (fMLF; 5 µM; Sigma), and opsonized zymosan (OZ; 20 mg/mL zymosan A from Saccharomyces cerevisiae; Sigma). The indexed maximal relative luminescence (in relative light units [RLU]) was calculated by using the following formula: indexed RLU max = ([hemochromatosis patient maximal RLU]/[healthy donor maximal RLU]) × 100.
Expression of neutrophil surface markers
After evaluation of the viability of the purified human neutrophil preparations (Thermo Fisher Aqua Live/Dead), the presence of the surface marker CD62L (using antibody against CD62L; BD Pharmingen 553152) was evaluated by using a BD LSRFortessa flow cytometer and was analyzed with FlowJo 10 software using median fluorescence intensity.
Evaluation of phagocytosis and quantification of plasma factors
Phagocytosis and acidification capacity of human neutrophils were quantified using the pHrodo Green Staphylococcus aureus BioParticles kit (Thermo Fisher) following the manufacturer’s instructions. Acquisition was performed using the BD LSRFortessa flow cytometer. Results were analyzed with FlowJo 10 software. Human plasma was analyzed with the V-plex Human Biomarker 40-Plex kit from Meso Scale Discovery (K15209D) according to the manufacturer’s recommendations.
Measurement of iron parameters
Mouse plasma iron parameters (plasma iron, transferrin, and ferritin) were quantified by using the biochemistry and metabolism platform at the Centre de Recherche sur l’Inflammation (INSERM Unite Mixte de Recherche 1149, Paris, France), and human plasma iron parameters were quantified by the Department of Automated Biological Diagnosis of Cochin Hospital. Neutrophil intracellular iron measurements were performed using electrothermal atomic absorption spectrometry on a SIMAA6100 spectrometer (Perkin Elmer, Villebon-sur-Yvette, France). Results are presented as iron content normalized by sodium content in 106 cells for each patient.
Western blot analysis
Total proteins were extracted from frozen samples with equal numbers of neutrophils. Triton X-100 lysis buffer (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES] 50 mM, phenylmethylsulfonyl fluoride 4 mM, leupeptin 0.4 mM, pepstatin 0.4 mM, orthovanadate 1 mM, EDTA 1 mM, EGTA 1 mM, dithiothreitol 1 mM, and Triton X-100 1%) was used to extract proteins. Western blotting was performed as previously described25 with a tris-glycin 10% acrylamide gel. The following antibodies were used for mouse samples: β actin (Sigma A5316), ferroportin (α Diagnostic MTP11-A), ferritin H (Cell Signaling Technology 4393), and TfR1 (Invitrogen 13-6800). The following antibodies were used for human samples: β actin (Sigma A5316), ferroportin (NOVUS NBP1-21502), and ferritin H (Cell Signaling Technology 4393). Band intensity was quantified using ImageJ imaging software and was expressed as fold change of the mean signal intensity over its own control.
Analysis with real-time quantitative polymerase chain reaction
Total RNA was extracted using TriReagent (Sigma) following the manufacturer’s protocol and was reverse transcribed into complementary DNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies). Real-time quantitative polymerase chain reaction was performed using The LightCycler 480 SYBR Green I Master (Roche) in a LightCycler480 detection system. All values were normalized against the cyclophilin A (CycloA) gene as a reference. The following primers were used: CycloA (forward: 5′ GTC AAC CCC ACC GTG TTC TT 3′; reverse: 5′ CTG CTG TCT TTG GGA CCT TGT 3′); Hamp1 (forward: 5′ CCT ATC TCC ATC AAC AGA T 3′; reverse: 5′ TGC AAC AGA TAC CAC ACT G 3′).
Statistical analysis
All statistical analyses were performed by using GraphPad Prism software. We evaluated the normality (α = 0.05) of each group before analysis to determine the most appropriate statistical test to use. For comparisons of normally distributed data between 2 groups, we used Student t tests; Mann-Whitney U tests were used when the normality was not accepted. A 1-way or 2-way analysis of variance followed by a Sidak test was used when more than 2 groups were compared. Spearman’s r test was used for correlations with 95% confidence intervals.
Results
Hepcidin deficiency protects neutrophils from intracellular iron excess
We assessed the level of iron-related proteins in neutrophils isolated from 2 models of iron overload: an experimentally induced iron overload (EIO) model in which hepcidin messenger RNA (mRNA) expression is increased, and a genetically induced iron overload model in which the hepatic hepcidin gene is inactivated by the Cre-LoxP system26 (Hamp1Δliver) (Figure 1A-B). The 2 models had similar plasma iron overload (Figure 1A-B) as well as similar neutrophil (Figure 1C) and white blood cell counts (supplemental Figure 1A). We first measured the expression of the iron storage molecule ferritin H (FTH), which is a surrogate for cellular iron content. Interestingly, although FTH expression was higher in the neutrophils isolated from the EIO model than it was in the control group, FTH expression was lower in the neutrophils from Hamp1Δliver mice compared with control littermates (Figure 1D-E). We next examined the expression of TfR1, which mediates cellular iron uptake from iron-loaded transferrin (holo-transferrin). As a consequence of the decrease in iron content, TfR1 was significantly increased in the neutrophils isolated from Hamp1Δliver mice compared with control littermates (Figure 1D-E). Indeed, TfR1 is posttranscriptionally regulated by the IRE-IRP system, in which under conditions of iron starvation, iron-regulatory proteins (IRPs) bind to iron-responsive elements (IREs) present in the 3′ untranslated region (UTR) of TfR1 mRNA and promote its stability. FPN expression level was found to be similar in both conditions (Figure 1D-E). These results show that the 2 mouse models share systemic iron overload but have either an increase in or a defect in hepcidin production and have different neutrophil iron content. This suggests that hepcidin has a role in managing neutrophil intracellular iron stores.
Figure 1.
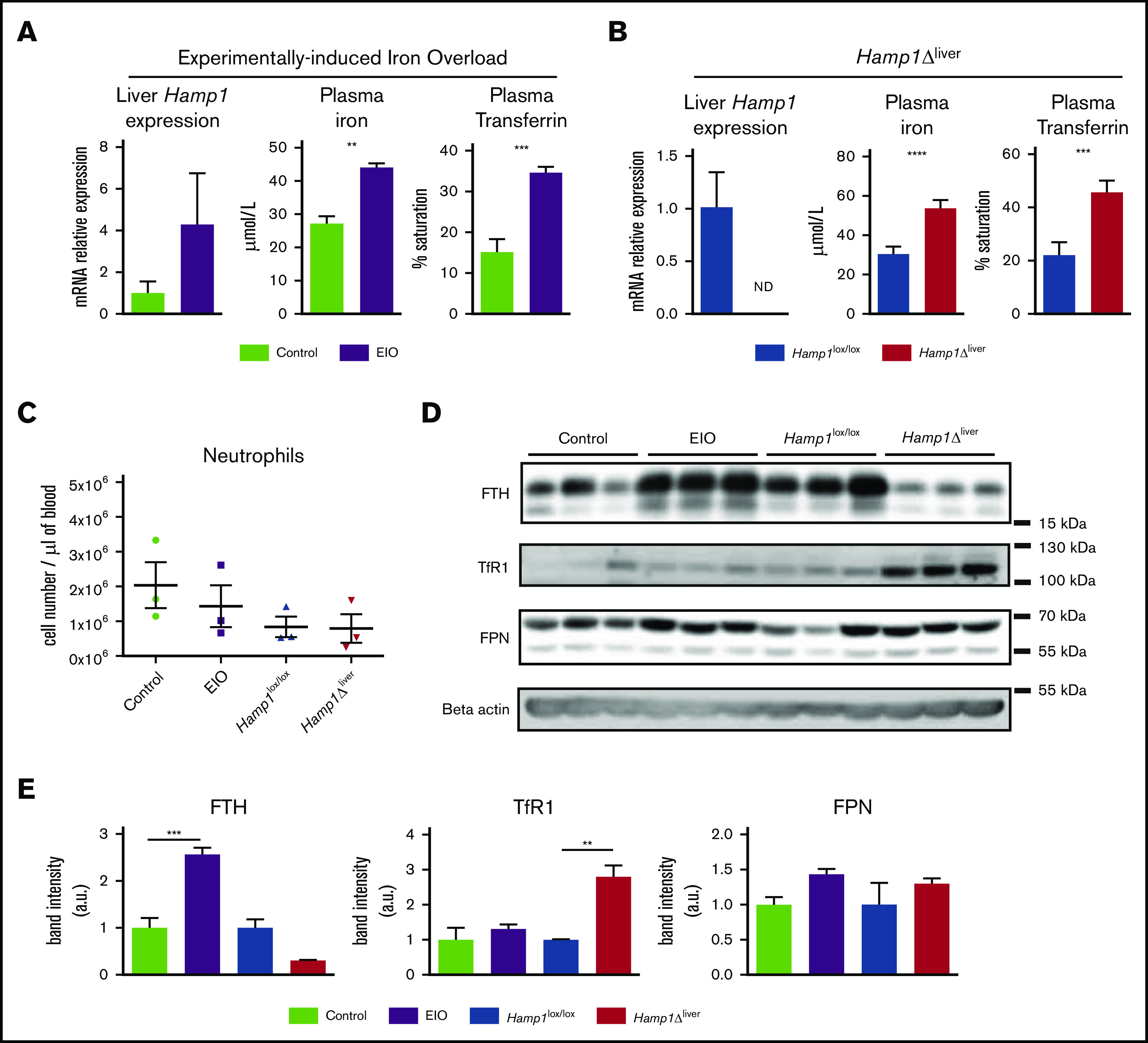
Hepcidin deficiency protects neutrophils from intracellular iron overload. Liver hepcidin (Hamp1) expression evaluated by real-time quantitative polymerase chain reaction, plasma iron, and transferrin saturation in mice fed an iron-rich diet for 6 weeks (EIO model) vs controls (A) or Hamp1Δliver vs Hamp1lox/lox mice (B) (n ≥ 3 mice per group). (C) Blood neutrophil cell count in the EIO model vs control and Hamp1Δliver vs Hamp1lox/lox mice (n = 3 mice per group). (D) Western blot detection of FTH, TfR1, FPN and β-actin on neutrophils isolated from the different mouse models as indicated (n = 3 mice per condition). Data are representative of 2 independent experiments. (E) Quantification of the band intensity expressed in arbitrary units (a.u.). All results are shown as mean ± standard error of the mean (SEM). **P < .01; ***P < .001; ****P < .0001.
Neutrophils from Hamp1Δliver mice displayed increased burst activity
We next tested whether the differences in neutrophil iron content between the 2 models translated into differences in oxidative burst activity. Neutrophils isolated from the EIO model had a significant defect in ROS production compared with controls when they were exposed to bacterial-type fMLF, yeast-type (OZ), or synthetic (PMA) agents (Figure 2A), confirming the decrease in neutrophil activity previously described in patients with secondary iron overload.10-13 Loading neutrophils with holo-transferrin in vitro decreased their oxidative burst capacity (supplemental Figure 1B), further confirming the detrimental effect of excess iron.8,27 In sharp contrast, neutrophils isolated from the genetically induced iron overload model unexpectedly showed a significant increase in their oxidative burst in comparison with neutrophils isolated from control littermates (Figure 2B). Iron-deficient neutrophils have been associated with a decrease rather than an increase in oxidative burst activity.28,29 This raises a question about how neutrophils isolated from hepcidin-deficient mice are able to show augmented burst activity.
Figure 2.
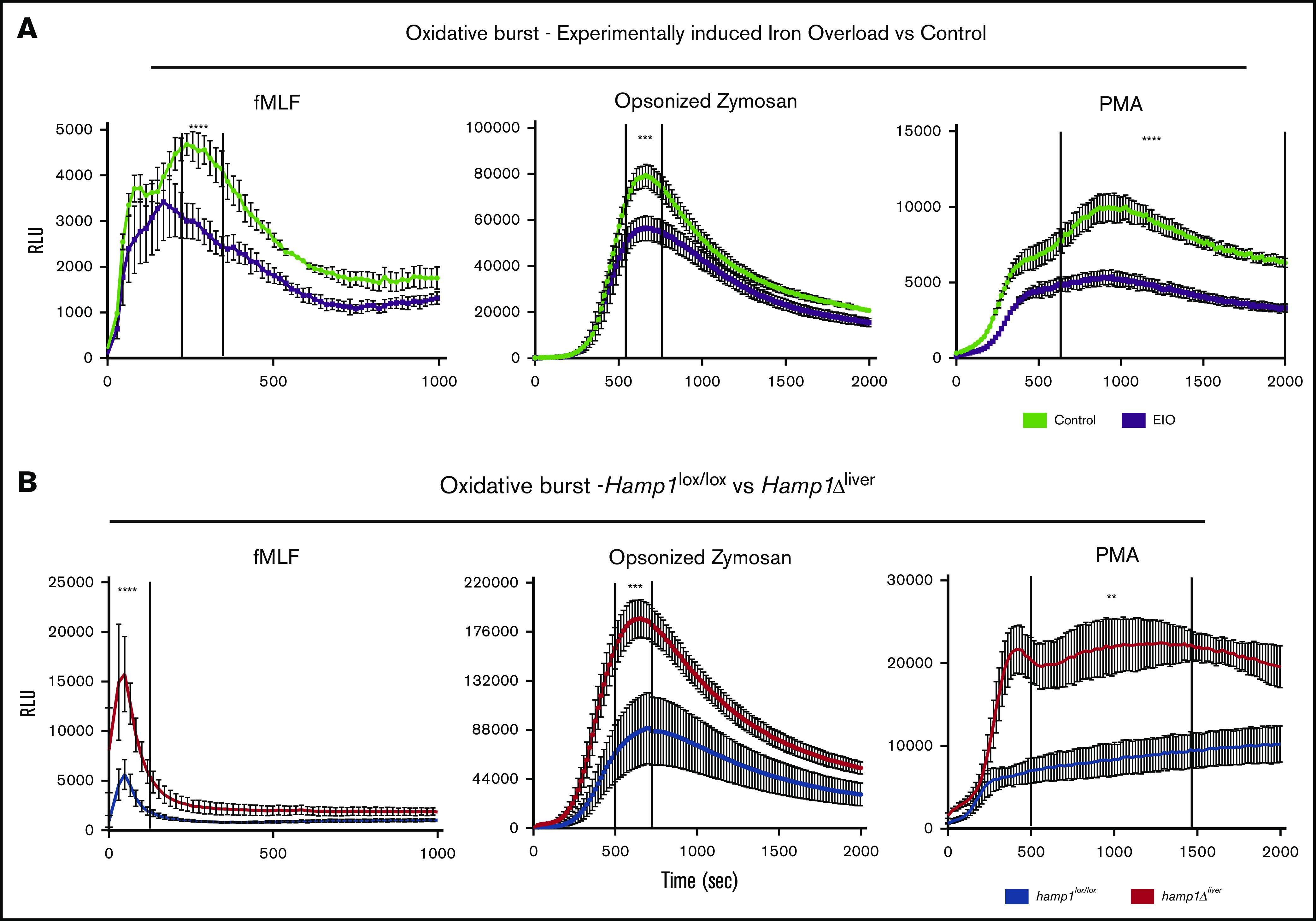
Decreased vs increased oxidative burst response in neutrophils isolated from Hamp1Δlivermice or from the EIO model, respectively. Kinetics of the oxidative burst response to fMLF, OZ, or PMA of neutrophils isolated from mice fed an iron-rich diet for 6 months (EIO) vs control mice (A) (n = 3 per group) or Hamp1Δliver vs Hamp1lox/lox mice (B) (n ≥ 3 per group). Data are representative of 3 independent experiments. Statistical analysis was performed using a 2-way analysis of variance (ANOVA) followed by a Sidak test. **P < .01; ***P < .001; ****P < .0001.
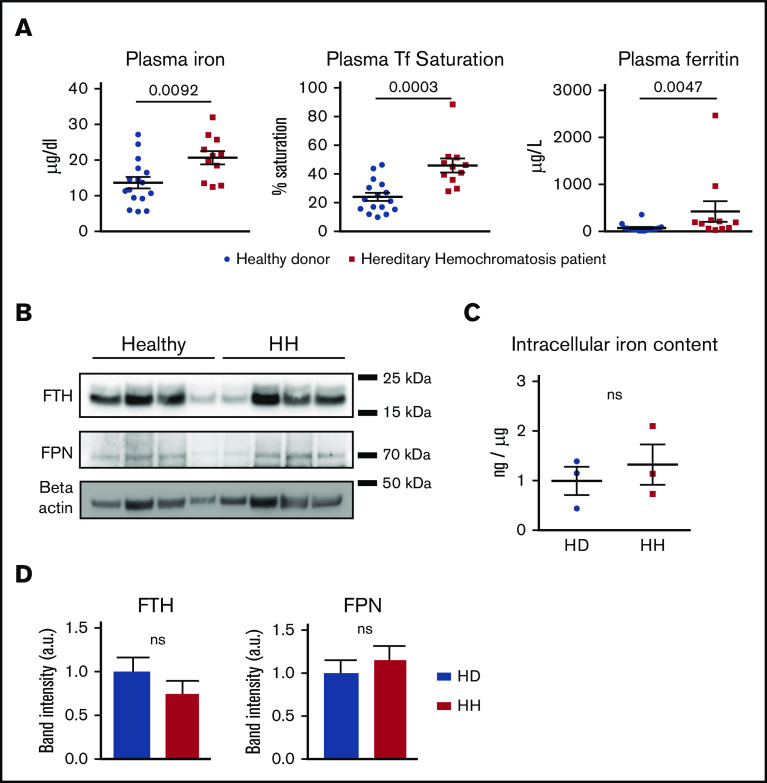
Despite systemic iron overload, neutrophils from HH patients are not iron loaded
To extrapolate these findings to studies in humans who have altered iron homeostasis, we studied a cohort of healthy donors and patients with HH who received maintenance phlebotomies that harbored homozygous HFE mutations (C282Y). Plasma iron and ferritin levels as well as transferrin saturation in the HH cohort were significantly increased compared with those in healthy donors (Figure 3A). No differences were observed in white blood cell count between the 2 groups (supplemental Figure 2A).
Figure 3.
Neutrophils from HH patients are not iron loaded. (A) Plasma iron, transferrin (Tf) saturation, and ferritin in healthy donors (n = 16) and HH patients (n = 11). (B) Western blot of FPN, FTH, and β-actin on total protein extracts from neutrophils isolated from healthy donors and HH patients. (C) Neutrophil intracellular iron content quantification by atomic absorption (ETAAS) normalized by sodium content in healthy donors (n = 3) versus HH patients (n = 3). (D) Western blot band intensity quantification for FPN and FTH normalized by the healthy donor mean band intensity and expressed in arbitrary units. All results are shown as mean ± SEM. ns, not significant.
In contrast to the decrease of ferritin observed in the neutrophils of Hamp1Δliver mice, neutrophils from HH patients had levels of ferritin similar to those of healthy donors (Figure 3B,D). We confirmed by electrothermal atomic absorption spectrometry that the neutrophil iron content was not significantly different between healthy donors and HH patients (Figure 3C). This could be explained by the residual amount of hepcidin in HH patients. Indeed, although systemic hepcidin is totally absent in the genetic HH mouse model,26 hepcidin levels in HH patients, although abnormally low in regard to the iron status, do not significantly differ from those in controls.30 Therefore, in contrast to the hepcidin knockout model, the inhibition of cellular iron export caused by hepcidin deficiency in HH patients may be milder and thus, it may not lead to iron-deficient neutrophils. FPN levels were not significantly changed between the 2 groups (Figure 3B,D). Altogether, these results suggest that neutrophils from HH patients, similar to those in the HH mouse model, do not show intracellular iron excess.
Oxidative burst capacity of HH neutrophils is positively correlated with plasma transferrin saturation
In agreement with our mouse model of HH, the oxidative burst was increased in neutrophils from HH patients compared with that in healthy donors upon stimulation with fMLF, OZ, or PMA (Figure 4A-B). To determine whether the iron status of HH patients was involved in the modulation of neutrophil activity, we correlated transferrin saturation with the level of ROS production in each patient. We found a significant positive correlation between transferrin saturation and the oxidative burst in response to fMLF (Spearman’s r = 0.51; P = .0188), OZ (Spearman’s r = 0.51; P = .0177), and PMA (Spearman’s r = 0.49; P = .0238) (Figure 4C). In contrast, plasma ferritin was not correlated with the oxidative burst response (Figure 4D). Collectively, these findings suggest that neutrophils from HH patients are protected from intracellular iron overload and that the increase in the activity of neutrophils from HH patients correlates with systemic iron overload.
Figure 4.
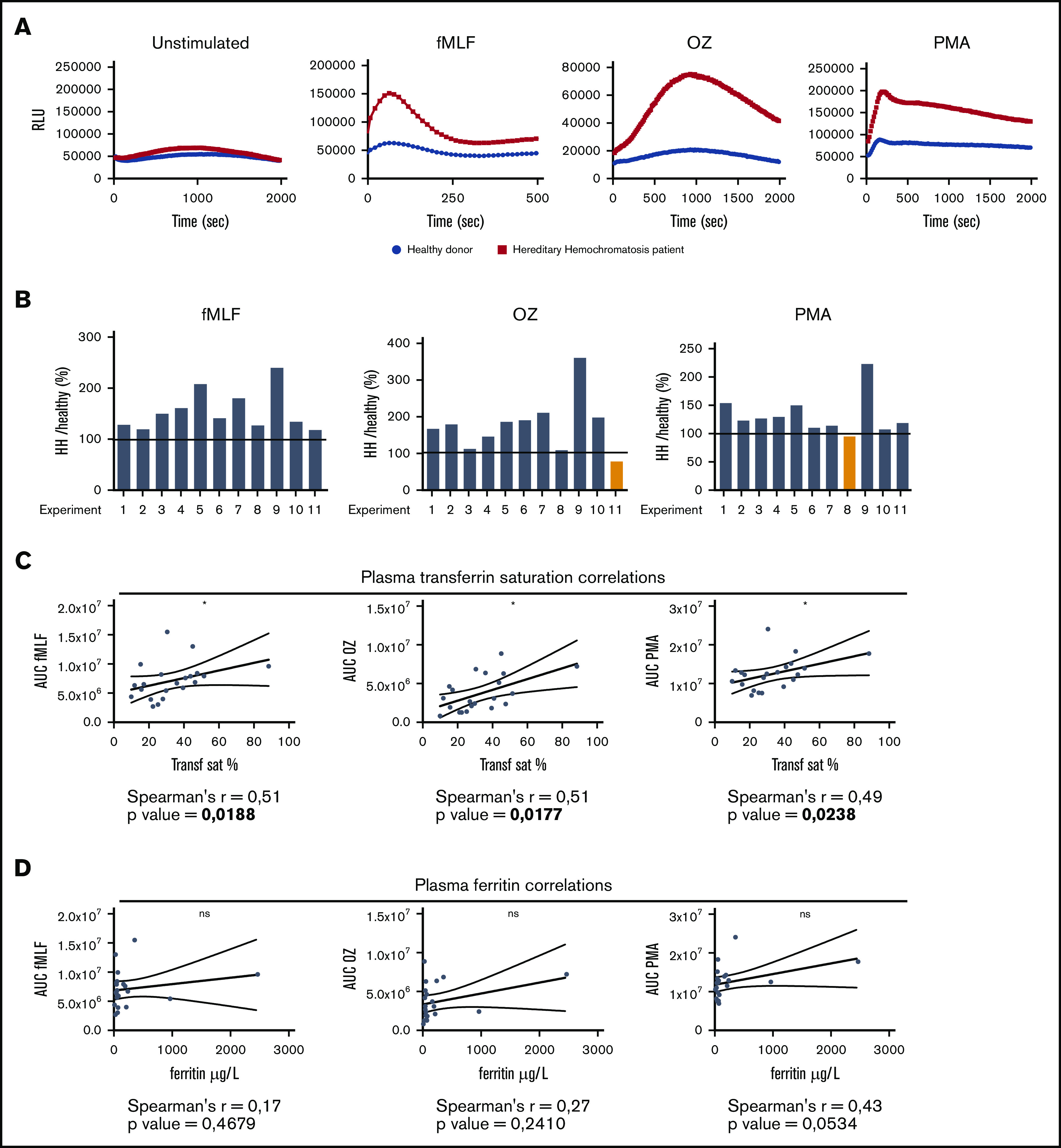
Oxidative burst in neutrophils from HH patients is positively correlated with plasma transferrin saturation. (A) Representative experiment of the oxidative burst assay at resting state or upon stimulation with fMLF, OZ, or PMA on neutrophils isolated from HH patients (red) and healthy donors (blue). (B) RLUmax HH patient:RLUmax healthy donor ratio for each independent experiment comparing each patient with their appropriate control. (C) Correlations between plasma transferrin saturation and area under the curve (AUC) upon stimulation with fMLF, OZ, or PMA for each patient (n = 21). (D) Correlations between plasma ferritin level and area under the curve for the same previous stimulating agents for each patient. *P < .05.
Plasma TNF-α from patients correlates with oxidative burst and transferrin saturation
To determine whether the indirect effect of systemic iron overload on the oxidative burst response could be linked to circulating factors known to be involved in neutrophil priming, we performed a multiplex analysis of 40 circulating factors that included cytokines, chemokines, angiogenesis, and vascular injury factors in the plasma of 26 HH patients and 22 healthy controls (Figure 5A; supplemental Figure 2B). Interestingly, tumor necrosis factor α (TNF-α) was among the most significantly increased factors in the plasma of HH patients compared with healthy donors. We found a positive correlation between plasma TNF-α levels and transferrin saturation (Figure 5B), suggesting that plasma TNF-α production is a likely consequence of iron overload. TNF-α plays an essential role in endothelial cell activation, mainly by increasing the number of adhesion molecules at the surface of the endothelium, such as intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1). Leukocyte-endothelial cell interaction is an important pro-priming environment for neutrophils. Consistent with the observed increase in TNF-α, both ICAM-1 and VCAM-1 were also found to be significantly increased in the plasma of HH patients.31,32 TNF-α is also one of the most effective priming agents for human neutrophils33-35 and significantly increases the neutrophil respiratory burst to a subsequent stimulus.36 Therefore, it may participate in neutrophil priming in HH patients. Indeed, plasma TNF-α also correlates with the oxidative capacity of neutrophils after fMLF, OZ, and PMA stimulation (Figure 5B), confirming the importance of this factor for neutrophil priming in these patients.
Figure 5.
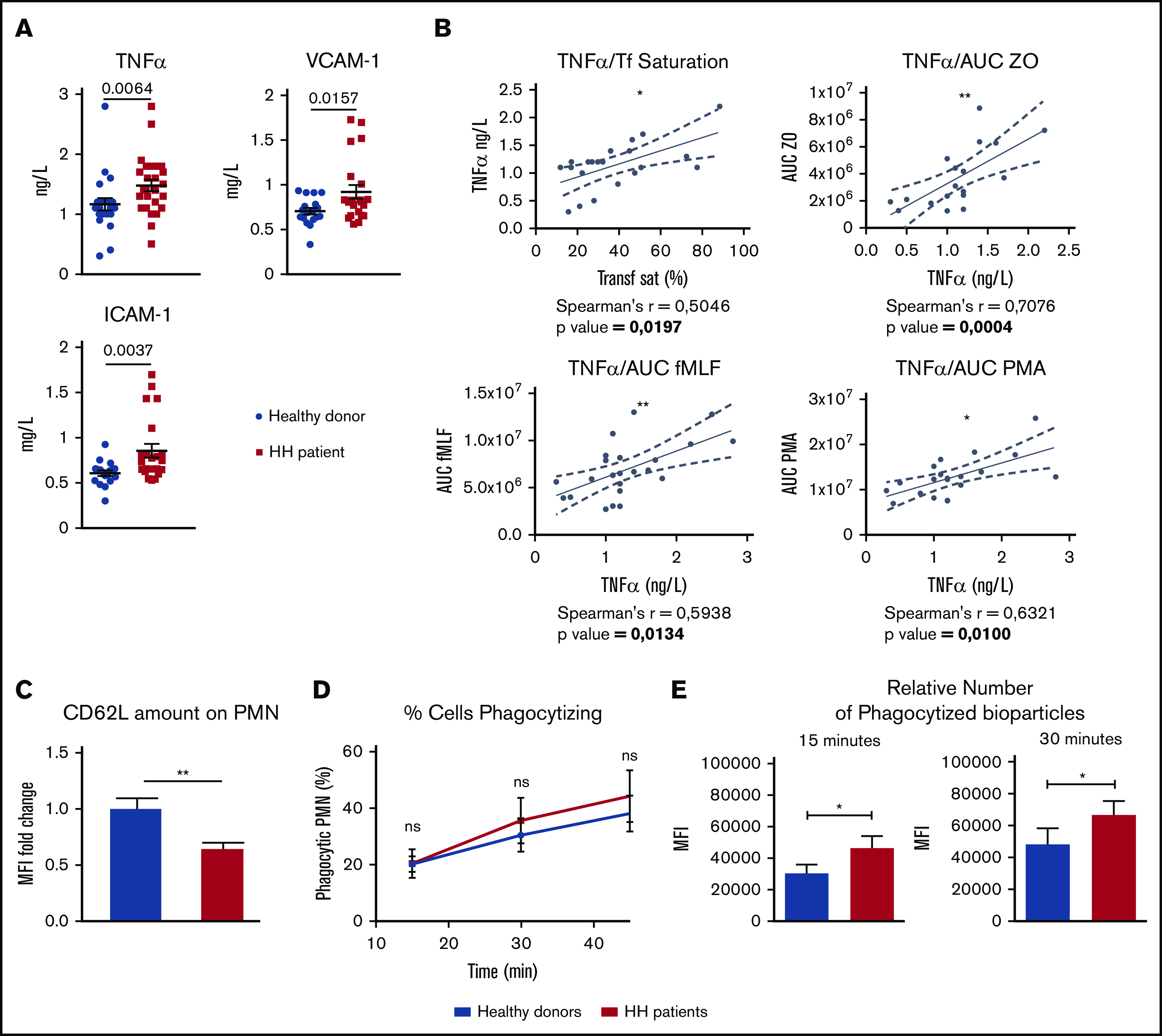
Neutrophils from HH patients are primed. (A) Plasma TNF-α, VCAM-1, and ICAM-1 in the plasma of HH patients (n = 23; red squares) vs healthy donors (n = 18; blue circles). (B) Correlations between plasma TNF-α and transferrin saturation (Transf Sat) or between plasma TNF-α and area under the curve (AUC) of the oxidative burst upon stimulation with fMLF, OZ, or PMA for each patient. (C) Analysis by flow cytometry of CD62L surface expression on neutrophils isolated from HH patients (n = 4) vs healthy donors (n = 3) as measured by mean fluorescence intensity (MFI) and expressed in fold change. (D-E) Evaluation of the phagocytosis capacity (number of phagocytized S aureus bioparticles) in neutrophils from HH patients (red) vs healthy donors (blue) (n ≥ 6; data from 3 independent experiments). (D) Analysis of the percentage of phagocytic neutrophils over time. (E) Comparison of the MFI between neutrophils from HH patients (red) vs healthy donors (blue). The normality of each data batch was assessed to determine the appropriate statistic test to use. Mann-Whitney U test was used for analysis of TNF-α and ICAM-1 in panel A, unpaired Student t test for panel E and VCAM-1 graph in panel A, and 2-way ANOVA followed by a Sidak test in panel D. All results are shown as mean ± SEM. *P < .05; **P < .01.
Neutrophils from HH patients are primed
To confirm the primed phenotype of neutrophils in the HH population, we measured 2 other classical parameters: surface L-selectin shedding and phagocytosis capacity.37 First, we used flow cytometry to determine the relative amount of L-selectin/CD62L on the surface of neutrophils. Compared with the amount of L-selectin/CD62L in healthy donors, it was observed to be decreased on the surface of neutrophils of HH patients (Figure 5C), confirming the priming state of neutrophil in this population.
Second, we analyzed the neutrophil phagocytosis capacity of both populations. We performed an in vitro experiment using S aureus bioparticles coupled to a fluorochrome. Although the recognition capacity of neutrophils toward the bioparticles was not affected (Figure 5D), flow cytometry analysis indicated that relative amounts of phagocytized fluorescein-labeled S aureus particles were higher in neutrophils isolated from HH patients compared with healthy donors, after either a 15-minute or a 30-minute incubation (Figure 5E).
Discussion
Iron is a key metal for neutrophil function. Because it is a highly reactive element, iron stores have to be tightly regulated. Surprisingly, nothing is known about the role of hepcidin in the maintenance of iron homeostasis in neutrophils, even in systemic iron overload diseases caused by a lack of circulating hepcidin such as in HH. By using neutrophils isolated from genetic mouse models of iron overload and from HH patients, we were able to investigate the impact of hepcidin deficiency on neutrophil iron homeostasis and function.
In this study, we found that the amounts of iron storing– and iron efflux–associated proteins were greatly influenced by hepcidin levels (see visual abstract). We showed that hepcidin deficiency protected neutrophils from iron excess. In the liver-specific hepcidin knockout model, neutrophils were iron deficient (as measured by decreased FTH levels). The basal differences of amount of FTH in neutrophils between mice in the control diet condition and Hamp1lox/lox may come from slight differences in iron content between the control diet and the classical diet (see “Methods”). The basal differences may also have come from the birthplace (Janvier Laboratories) of C57BL/6J mice that were fed either a control or an iron-rich diet. Nevertheless, regulation of iron homeostasis genes in response to intracellular iron levels is mediated at a posttranscriptional level by IRPs, which bind to IREs found in the UTRs of target mRNAs. High IRP-binding activity in response to low intracellular iron levels results in translational repression of mRNAs that contain an IRE in their 5′ UTR (such as FPN or FTH) or promotes mRNA stability if bound to mRNAs that contain an IRE in the 3′ UTR (such as TfR1). As a consequence of cellular iron deficiency, TfR1 levels were increased, and conversely FTH levels were decreased in the neutrophils isolated from Hamp1Δliver mice. At first, the absence of any FPN upregulation was intriguing, but it can be easily explained. Indeed, FPN may be stabilized at the protein level (by the lack of hepcidin) but be translationally repressed by the IRE-IRP system. This dual regulation could explain the absence of changes in FPN protein levels in neutrophils isolated from Hamp1Δliver mice compared with wild-type neutrophils. Conversely, in the EIO model, hepcidin-mediated FPN degradation may be compensated by FPN stabilization at the translational level through the IRE-IRP system.
Our study showed that intracellular iron trapping had a detrimental effect in neutrophils isolated from iron overload conditions in which hepcidin is increased, resulting in a significantly impaired ROS production. From a therapeutic perspective, these results suggest that the use of hepcidin mimetics or agonists to treat iron overload may have deleterious effects on neutrophil functions. In HH particularly, the use of hepcidin for treatment should be considered with caution, because it would increase intracellular iron levels in neutrophils and may decrease their activity. In the treatment phase, when systemic iron is still present, patients may be more susceptible to infections because of both iron excess and defective neutrophils. Hepcidin agonists are also clinically relevant in β-thalassemia. β-thalassemia major causes ineffective erythropoiesis and chronic anemia. It is associated with iron overload resulting from both transfused iron and increased iron absorption, the latter being mediated by suppression of the iron regulatory hormone hepcidin. In this case too, the therapeutic use of hepcidin should be considered with caution.
Second, our findings showed that neutrophils from HH patients have a primed phenotype. Two things led us hypothesize that systemic iron overload could trigger increased oxidative burst mediated by a TNF-α priming effect: (1) the positive correlation between transferrin saturation and oxidative burst and (2) transferrin saturation and TNF-α. Indeed, an increase in TNF-α levels has already been associated with iron overload pathologies.37-40 Moreover, several studies showed that circulating TNF-α results in neutrophil priming, oxidative response, and adhesion.33 In endothelial cells, TNF-α induces the release of chemokines and leads to the enhanced expression of VCAM-1 and ICAM-1,41 also elevated in our HH cohort, which confirms our previous results.42 It has also been reported that interferon-γ (IFN-γ) could result in increased ICAM-1 expression,43 but there is no correlation between IFN-γ and iron parameters or between IFN-γ and amount of ICAM-1 in plasma (data not shown).
Neutrophil oxidative burst represents a pivotal component of innate immune defense against invading microorganisms, but it is also causes collateral tissue damage via the extracellular effects of ROS. There may be several consequences of neutrophil priming in HH patients. Hemochromatosis in its advanced stages has been associated with diabetes mellitus, arthropathy, and severe cardiac complications (including congestive heart failure, premature coronary artery disease, and cardiac arrhythmias). Activated neutrophils play a crucial role in these manifestations. First, the elevation of ROS production by neutrophils has been shown to be an intensifying factor in diabetic-related complications.44,45 Second, neutrophils play an important role in the pathophysiology of arthritis via the release of ROS, and neutrophil depletion can greatly inhibit the development of arthritis.46,47 Finally, although the pathophysiology of cardiac dysfunction in HH has not been well characterized, some have speculated that enhanced production of ROS is responsible for tissue damage.48 There is also substantial evidence that neutrophils induce tissue injury (both myocardial and microvascular) in many forms of acute heart failure.49 Altogether, activation of neutrophil priming could be a contributing factor in the development of the pathologies associated with hemochromatosis.
It is generally accepted that iron overload disorders cause increased susceptibility to infections. Among the potential explanations for this phenomenon, it is possible that the increased availability of iron stimulates bacterial growth and increases bacterial virulence. Concerning HH, case reports have described an increased prevalence of certain infections with intracellular bacteria such as Yersinia enterocolitica,50,51 Y pseudotuberculosis,50,52 Listeria monocytogenes,53 and Vibrio vulnificus.54 These pathogens require higher iron concentrations for growth. In some cases, it was not clear whether the observed infection was a direct consequence of the iron overload or whether it was secondary to coexisting liver cirrhosis, liver carcinoma, or liver transplantation surgery. Thus, the real impact of iron overload in increasing susceptibility to infection is a matter of debate, especially in view of several studies that have emphasized the deleterious effect of excess iron on leukocytes.10-14 Thus, our study, which points out an unexpected neutrophil priming in HH patients, may explain the absence of a drastic increase in morbidity related to infection in these patients, despite the iron overload.
In conclusion, our study characterizes iron homeostasis and associated proteins in neutrophils for the first time. In these key cells of innate immunity, we showed that hepcidin expression strongly influences neutrophil iron stores, especially in systemic iron overload conditions. Furthermore, we showed that hepcidin deficiency in HH patients protects neutrophils from detrimental iron excess, emphasizing the key role of hepcidin in iron-dependent regulation of neutrophil function. In a larger context, our study highlights an unexpected priming of neutrophils in HH patients that may explain some of the collateral tissue damage related to the disease. It also raises questions about relative protection against infection in patients with HH as opposed to other iron overload diseases, such as chronically transfused patients. Further studies will be required to test these different hypotheses, which could open a new area of research.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Véronique Witko-Sarsat and Sylvie Chollet-Martin for scientific discussions and technical advice, Sophia Sangiorgio (University of California at Los Angeles) for the English proofreading, and the HistIM and animal facilities of Institut Cochin.
This work was supported by a grant from the European Research Council under the European Community’s Seventh Framework Program (FP7/2011-2015, grant No. 261296), the Fondation pour la Recherche Médicale (DEQ20160334903), and the Laboratory of Excellence GR-Ex (reference ANR-11-LABX-0051) funded by the Investissements d’Avenir program of the French National Research Agency (reference ANR-11-IDEX-0005-02).
Footnotes
For data sharing requests, please send an e-mail to Carole Peyssonnaux at carole.peyssonnaux@inserm.fr.
Authorship
Contribution: C.R., S.L., S.C., N.B., J.-C.D., J.P., and K.B. performed the experiments and analyzed the data; P.M.-C.D., J.E.-B., S.M., S.V., F.L, and D.B.. provided essential reagents and scientific advice; C.P. and C.R. designed the experiments and analyzed the data; C.P. supervised the project; and C.P. and C.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carole Peyssonnaux, Université de Paris, INSERM, Institut Cochin, CNRS, 24 Rue du Faubourg Saint Jacques, 75014 Paris, France; e-mail: carole.peyssonnaux@inserm.fr.
References
- 1.Kruger P, Saffarzadeh M, Weber AN, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210(7):1283-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181-189. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90(1):1-37. [DOI] [PubMed] [Google Scholar]
- 5.Schultz J, Rosenthal S. Iron (II) inactivation of myeloperoxidase. J Biol Chem. 1959;234:2486-2490. [PubMed] [Google Scholar]
- 6.Cantinieaux B, Hariga C, Clumeck N, et al. The role of excess iron in the pathogenesis of disturbed neutrophil functions in cirrhotic patients (neutrophil functions in cirrhotic patients). Acta Clin Belg. 1987;42(3):153-167. [DOI] [PubMed] [Google Scholar]
- 7.van Asbeck BS, Marx JJ, Struyvenberg A, van Kats JH, Verhoef J. Deferoxamine enhances phagocytic function of human polymorphonuclear leukocytes. Blood. 1984;63(3):714-720. [PubMed] [Google Scholar]
- 8.van Asbeck BS, Marx JJ, Struyvenberg A, van Kats JH, Verhoef J. Effect of iron (III) in the presence of various ligands on the phagocytic and metabolic activity of human polymorphonuclear leukocytes. J Immunol. 1984;132(2):851-856. [PubMed] [Google Scholar]
- 9.Cantinieaux B, Boelaert JR, De Meuleneire J, Kerrels V, Fondu P. Neutrophils from patients with secondary haemosiderosis contain excessive amounts of autotoxic iron. Eur J Haematol. 1993;51(3):161-165. [DOI] [PubMed] [Google Scholar]
- 10.Cantinieaux B, Boelaert J, Hariga C, Fondu P. Impaired neutrophil defense against Yersinia enterocolitica in patients with iron overload who are undergoing dialysis. J Lab Clin Med. 1988;111(5):524-528. [PubMed] [Google Scholar]
- 11.Waterlot Y, Cantinieaux B, Hariga-Muller C, De Maertelaere-Laurent E, Vanherweghem JL, Fondu P. Impaired phagocytic activity of neutrophils in patients receiving haemodialysis: the critical role of iron overload. Br Med J (Clin Res Ed). 1985;291(6494):501-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantinieaux B, Hariga C, Ferster A, De Maertelaere E, Toppet M, Fondu P. Neutrophil dysfunctions in thalassaemia major: the role of cell iron overload. Eur J Haematol. 1987;39(1):28-34. [DOI] [PubMed] [Google Scholar]
- 13.Skoutelis AT, Lianou E, Papavassiliou T, Karamerou A, Politi K, Bassaris HP. Defective phagocytic and bactericidal function of polymorphonuclear leucocytes in patients with beta-thalassaemia major. J Infect. 1984;8(2):118-122. [DOI] [PubMed] [Google Scholar]
- 14.Cantinieaux B, Hariga C, Ferster A, Toppet M, Fondu P. Desferrioxamine improves neutrophil phagocytosis in thalassemia major. Am J Hematol. 1990;35(1):13-17. [DOI] [PubMed] [Google Scholar]
- 15.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131(8):899-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093. [DOI] [PubMed] [Google Scholar]
- 18.Barton JC. Hemochromatosis and iron overload: from bench to clinic. Am J Med Sci. 2013;346(5):403-412. [DOI] [PubMed] [Google Scholar]
- 19.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33(1):21-22. [DOI] [PubMed] [Google Scholar]
- 20.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998;95(4):1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loréal O. Haemochromatosis. Nat Rev Dis Primers. 2018;4(1):18016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolas G, Viatte L, Lou DQ, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34(1):97-101. [DOI] [PubMed] [Google Scholar]
- 23.Pietrangelo A. Genetics, genetic testing, and management of hemochromatosis: 15 years since hepcidin. Gastroenterology. 2015;149(5):1240-1251.e4. [DOI] [PubMed] [Google Scholar]
- 24.Kuhns DB, Long Priel DA, Chu J, Zarember KA. Isolation and functional analysis of human neutrophils. Curr Protoc Immunol. 2015;111:7.23.1-7.23.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumerle S, Mathieu JR, Delga S, et al. Targeted disruption of hepcidin in the liver recapitulates the hemochromatotic phenotype. Blood. 2014;123(23):3646-3650. [DOI] [PubMed] [Google Scholar]
- 27.van Asbeck BS, Marx JJ, Struyvenberg A, Verhoef J. Functional defects in phagocytic cells from patients with iron overload. J Infect. 1984;8(3):232-240. [DOI] [PubMed] [Google Scholar]
- 28.Kurtoglu E, Ugur A, Baltaci AK, Mogolkoc R, Undar L. Activity of neutrophil NADPH oxidase in iron-deficient anemia. Biol Trace Elem Res. 2003;96(1-3):109-115. [DOI] [PubMed] [Google Scholar]
- 29.Mackler B, Person R, Ochs H, Finch CA. Iron deficiency in the rat: effects on neutrophil activation and metabolism. Pediatr Res. 1984;18(6):549-551. [DOI] [PubMed] [Google Scholar]
- 30.Gehrke SG, Kulaksiz H, Herrmann T, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102(1):371-376. [DOI] [PubMed] [Google Scholar]
- 31.Kong DH, Kim YK, Kim MR, Jang JH, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 2018;19(4):1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106(2):584-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273(1):180-193. [DOI] [PubMed] [Google Scholar]
- 34.Elbim C, Bailly S, Chollet-Martin S, Hakim J, Gougerot-Pocidalo MA. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect Immun. 1994;62(6):2195-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McColl SR, Beauseigle D, Gilbert C, Naccache PH. Priming of the human neutrophil respiratory burst by granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha involves regulation at a post-cell surface receptor level. Enhancement of the effect of agents which directly activate G proteins. J Immunol. 1990;145(9):3047-3053. [PubMed] [Google Scholar]
- 36.Wewers MD, Rinehart JJ, She ZW, et al. Tumor necrosis factor infusions in humans prime neutrophils for hypochlorous acid production. Am J Physiol. 1990;259(4 pt 1):L276-L282. [DOI] [PubMed] [Google Scholar]
- 37.Bhowmik A, Ojha D, Goswami D, et al. Inositol hexa phosphoric acid (phytic acid), a nutraceuticals, attenuates iron-induced oxidative stress and alleviates liver injury in iron overloaded mice. Biomed Pharmacother. 2017;87:443-450. [DOI] [PubMed] [Google Scholar]
- 38.Ige AO, Ongele FA, Adele BO, Emediong IE, Odetola AO, Adewoye EO. Pathophysiology of iron overload-induced renal injury and dysfunction: Roles of renal oxidative stress and systemic inflammatory mediators. Pathophysiology. 2019;26(2):175-180. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Hou Y, Zhang S, et al. Excess iron undermined bone load-bearing capacity through tumor necrosis factor-α-dependent osteoclastic activation in mice. Biomed Rep. 2013;1(1):85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maras JS, Das S, Sharma S, et al. Iron-Overload triggers ADAM-17 mediated inflammation in Severe Alcoholic Hepatitis. Sci Rep. 2018;8(1):10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203-1211. [DOI] [PubMed] [Google Scholar]
- 42.Norris S, White M, Mankan AK, Lawless MW. Highly sensitivity adhesion molecules detection in hereditary haemochromatosis patients reveals altered expression. Int J Immunogenet. 2010;37(2):125-133. [DOI] [PubMed] [Google Scholar]
- 43.Oexle H, Kaser A, Möst J, et al. Pathways for the regulation of interferon-gamma-inducible genes by iron in human monocytic cells. J Leukoc Biol. 2003;74(2):287-294. [DOI] [PubMed] [Google Scholar]
- 44.Ridzuan N, John CM, Sandrasaigaran P, et al. Preliminary study on overproduction of reactive oxygen species by neutrophils in diabetes mellitus. World J Diabetes. 2016;7(13):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong RK, Pettit AI, Davies JE, Ng LL. Augmentation of the neutrophil respiratory burst through the action of advanced glycation end products: a potential contributor to vascular oxidant stress. Diabetes. 2002;51(9):2846-2853. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119(2):195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601-1608. [DOI] [PubMed] [Google Scholar]
- 48.Gulati V, Harikrishnan P, Palaniswamy C, Aronow WS, Jain D, Frishman WH. Cardiac involvement in hemochromatosis. Cardiol Rev. 2014;22(2):56-68. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, Chirkov YY, Horowitz JD. Neutrophil-initiated myocardial inflammation and its modulation by B-type natriuretic peptide: A potential therapeutic target. Int J Mol Sci. 2018;20(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott M, Galloway A, Cunningham JL. Haemochromatosis presenting with a double Yersinia infection. J Infect. 1986;13(2):143-145. [DOI] [PubMed] [Google Scholar]
- 51.Olesen LL, Ejlertsen T, Paulsen SM, Knudsen PR. Liver abscesses due to Yersinia enterocolitica in patients with haemochromatosis. J Intern Med. 1989;225(5):351-354. [DOI] [PubMed] [Google Scholar]
- 52.Conway SP, Dudley N, Sheridan P, Ross H. Haemochromatosis and aldosterone deficiency presenting with Yersinia pseudotuberculosis septicaemia. Postgrad Med J. 1989;65(761):174-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinkovics JG, Cormia F, Plager C. Hemochromatosis and Listeria infection. Arch Intern Med. 1980;140(2):284. [PubMed] [Google Scholar]
- 54.Bullen JJ, Spalding PB, Ward CG, Gutteridge JM. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151(8):1606-1609. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.