Abstract
BACKGROUND:
Amygdala overactivity has been frequently observed in patients with depression, as well as in nondepressed relatives of patients with depression. A remaining unanswered question is whether elevated amygdala activity in those with familial risk for depression is related to the presence of subthreshold symptoms or to a trait-level vulnerability for illness.
METHODS:
To examine this question, functional magnetic resonance imaging data were collected in nondepressed young adults with (family history [FH+]) (n = 27) or without (FH−) (n = 45) a first-degree relative with a history of depression while they viewed images of “looming” or withdrawing stimuli (faces and cars) that varied in salience by virtue of their apparent proximity to the subject. Activation of the amygdala and 2 other regions known to exhibit responses to looming stimuli, the dorsal intraparietal sulcus (DIPS) and ventral premotor cortex (PMv), were measured, as well as levels of resilience, anxiety, and psychotic and depressive symptoms.
RESULTS:
Compared with the FH− group, the FH+ group exhibited significantly greater responses of the amygdala, but not the dorsal intraparietal sulcus or ventral premotor cortex, to looming face stimuli. Moreover, amygdala responses in the FH+ group were negatively correlated with levels of resilience and unrelated to levels of subthreshold symptoms of psychopathology.
CONCLUSIONS:
These findings indicate that elevated amygdala activity in nondepressed young adults with a familial history of depression is more closely linked to poor resilience than to current symptom state.
Keywords: Amygdala, Depression, Familial risk, fMRI, Resilience, Youth
As the leading cause of disability worldwide (1), depression is a major worldwide health concern. With the increasing prevalence of depression (2,3), its economic and public health toll will likely escalate, as rising treatment needs strain already limited mental health resources. Further, many people receiving treatment for depression respond inadequately to available treatment options and develop persistent or recurrent depression (4). Therefore, to address the societal burden of depression, public health approaches such as early identification of at-risk individuals and prevention must be established. However, providing timely preventative measures or early treatment strategies relies on the identification of the most susceptible individuals (5). One vulnerable group is those individuals with a family history of depression (family history positive [FH+]), as they have a threefold greater likelihood of developing depression in their lifetime than those without such a family history (family history negative [FH−]) (6). However, further risk stratification within this group is necessary to develop cost-effective, prevention-focused public health initiatives. Thus, by examining both markers of risk and protective factors, we can more accurately identify individuals with the highest risk of developing depression who will benefit most from preventive interventions.
One general category of protective factors is referred to as emotional resilience (7,8). Resilience, as defined by the American Psychological Association, is a capacity to adapt well when facing adversity or significant sources of stress, or the constellation of skills or traits that enable one to “bounce back” from difficult experiences (9). It can also be defined operationally as a positive outcome following stressors (10). The building blocks of resilience are likely heterogeneous in terms of both the cognitive/affective processes and neurobiological mechanisms involved (7,11) and thus have been little studied using neuroimaging methods to date. However, given that self-report measures of resilience, such as the Connor-Davidson Resilience Scale (CD-RISC) (12), can predict positive outcomes following stressors (13,14), even cross-sectional investigations of the neurobiology of resilience may begin to shed light on the interplay between biological and environmentally determined aspects of resilience and psychopathology risk.
To investigate the neurobiology of depression risk and protective factors such as resilience, the networks of the brain most commonly implicated in the biology of depression must be examined. Much research on depression has focused on the amygdala (15). In addition, some current models of depression have linked attentional deficits and abnormal cognitive control of emotion to core symptoms of the illness (16). Evidence for attentional deficits in patients with depression includes reports of abnormal attentional shifting (17), slowed reaction times (18), and mood-congruent attentional biases (19). Supporting this model, both resting-state (20–22) and task-based (17,18,21) functional magnetic resonance imaging (fMRI) studies of depression have revealed involvement of circuitry supporting emotional processing and attention/cognitive control, such as the amygdale-centered and dorsal attention/frontoparietal networks, respectively. The functions of the amygdala-centered/limbic and attentional networks are closely linked; for example, the amygdala rapidly processes salient stimuli and then interacts with attention and cognitive control networks to influence behavioral responses (23–25). The dorsal attention network is involved in orienting attention to spatial cues and in cognitive control, and these processes can be influenced by input from the amygdala and other emotion-processing brain areas (26). Regions of the dorsal attention network, such as the dorsal intraparietal sulcus (DIPS) and ventral premotor cortex (PMv), support goal-directed attention and visuospatial processing (26), which are processes that are affected in depression (17).
Neuroimaging studies of depression have identified abnormalities in both networks, repeatedly detecting abnormalities in amygdala responses to emotional and neutral faces in patients with depression (27–29), as well as altered activation of frontoparietal regions of the dorsal attention network during visuospatial processing (29–31). One meta-analysis of functional connectivity studies of depressed individuals showed that regions of the dorsal attention network are among the brain regions most frequently affected in clinical depression (20). Similar findings within the amygdala and attentional regions have been reported for clinically remitted patients and in never-depressed FH+ individuals (32–36), indicating that such abnormal activation patterns may not represent state-dependent effects of depression. Furthermore, a recent meta-analysis (37) showed that cognitive (including attentional) deficits and deficits in visual scanning (38) are present in FH+ individuals when compared with control subjects. However, it remains unclear whether such changes in the function of the amygdala and attentional networks in unaffected relatives of individuals with depression reflect effects of subthreshold levels of symptoms, a marker of resilience (in the face of genetic vulnerability), or a heightened susceptibility for future illness.
To investigate this question further, in the current study we measured amygdala and frontoparietal cortical responses to dynamic social (faces) and nonsocial (cars) stimuli in young adults with or without a first-degree relative with a history of depression. Specifically, we used a paradigm that activates areas of the dorsal attentional network (dorsal parietal cortex and PMv) as well as the amygdala.
Therefore, first, we determined whether, in our cohort, the amygdala showed greater responses in nondepressed FH+ youths than in FH− youths, as others have reported. Second, we tested whether the FH+ group showed greater responses of the dorsal attentional network than the FH− group, consistent with prior findings in patients with depression. Third, we tested whether the predicted higher responsivity of these regions in the familial high-risk group was related to trait-like levels of emotional resilience or to subthreshold depressive or anxiety symptoms.
METHODS AND MATERIALS
Overall Study Design
This study was conducted in a subset of subjects who participated in a larger study of mental health in college students (39), in which on-campus screenings were conducted at several Boston-area universities. During these screenings, self-report questionnaires measuring a range of symptoms were administered (39). One of the goals of the study was to further characterize young people with subthreshold symptoms of psychopathology, with mildly to moderately elevated scores on a measure of depression (the Beck Depression Inventory [BDI]) (40) and/or a measure of psychotic experiences (the Peters et al. Delusions Inventory [PDI]) (41). Students with elevated scores (BDI total score >5 or >0 on BDI item 9 [measuring suicidal ideation], or PDI total score >7) and a small number of students with no depressive symptoms (total BDI scores of 0) were invited to participate in 1) a brief clinical assessment (administered by a Ph.D.-or M.D.-level clinician) in which the mood module of the Structured Clinical Interview for DSM-IV Axis I Disorders was administered, 2) a baseline neuroimaging session, and 3) longitudinal follow-up assessments conducted at 6-month intervals (self-report scales completed online; analyses including the longitudinal data will be reported separately). The neuroimaging session included 1 T1 anatomical scan and 4 blood oxygen level–dependent (BOLD) scans (see Supplemental Methods for scan parameters), during which subjects viewed dynamic face and car stimuli and performed a low-level attentional task (24). Subjects with neurological disorders or serious medical illnesses, substance abuse or dependence, or contradictions to MRI scanning were excluded from participating in the scanning session. The presence or absence of a family history of depression in first-degree family members was assessed at baseline using 2 self-report questionnaires, which included questions about the psychiatric history of subjects’ family members. Resilience levels were measured using the CD-RISC (see Supplemental Methods for further details).
Participants
A total of 131 subjects were scanned. For the current study, the data of 2 groups of subjects within this cohort were examined: those with a first-degree relative with depression (family history postive [FH+]) (n = 29) and those without a first-degree relative with depression (family history negative [FH−]) (n = 47). Data of 4 subjects (2 FH+ and 2 FH−) were excluded following quality control procedures. See Supplemental Methods for additional information about the participants who were screened and the exclusion criteria.
All 72 subjects included in the analyses (27 FH+ and 45 FH−) did not meet DSM-IV criteria for depression at the time of scanning, based on the Structured Clinical Interview for DSM-IV Axis I Disorders. FH− subjects reported that their first-degree relatives were without a history of any mental illness. FH+ subjects reported the presence of a family history of unipolar depression in at least one first-degree relative.
The study protocol, including informed consent procedures, was approved by the Partners HealthCare and Harvard University Institutional Review Boards, and written informed consent was obtained from all subjects.
Functional MRI Paradigm
As described in Holt et al. (24), during each functional scan, subjects viewed 2 types of stimuli: images of human faces (with neutral expressions) and images of cars. Each stimulus appeared to be moving toward or away from the subject at the speed of walking (112 cm/s) (Supplemental Figure S1). This paradigm involves visuospatial processing of approaching stimuli, which robustly activates frontoparietal regions of the dorsal attentional network (23,24). Images of faces with neutral expressions were presented because some previous work has identified significant differences 1) between control subjects and patients with depression (42) and 2) between individuals with and without familial risk for mood disorders (43) in amygdala responses only to neutral (not to emotional) faces. Each of 4 conditions (Face Approach, Face Withdrawal, Car Approach, Car Withdrawal) was presented for 16 seconds. In each run, subjects viewed 2 blocks of each of the 4 stimuli (8 blocks total), randomly presented. During each of the 4 conditions, subjects performed a simple dot-detection task; to distribute spatial attention evenly across the approach and withdrawal conditions, subjects were asked to press a button, while maintaining fixation, whenever a dot appeared at a random location on the screen. The percentage of responses was calculated for each condition, in each subject. A run was excluded if the subject responded to <40% of the dots during that run, as in Holt et al. (24).
MRI Data Analysis
Data were analyzed using the FreeSurfer analysis stream (http://surfer.nmr.mgh.harvard.edu), with standard preprocessing methods and quality assessment procedures (see Supplemental Methods for details). Images were spatially smoothed with a 6-mm Gaussian kernel (full width at half maximum) and a 3-dimensional spatial filter.
Anatomical Region-of-Interest Analysis.
In the primary, hypothesis-testing analysis of this study, 3 a priori anatomical regions of interest (ROIs) (the DIPS, PMv, and amygdala) (Supplemental Figure S2) were defined in each subject’s T1 anatomical scan, using an automated parcellation method that relies on well-known anatomical landmarks (44). We focused on the amygdala because of the extensive prior literature demonstrating amygdala abnormalities in patients with depression (15,45) and their first-degree relatives (34,35). The DIPS and PMv were also examined because these two regions are central nodes of the dorsal attention network (which has shown abnormalities in patients with depression and in first-degree relatives of patients with depression, as described above) and because these two regions are robustly engaged by the task used here, showing significantly greater responses to approaching (i.e., appearing to “loom” toward the subject), compared with withdrawing, face stimuli in prior studies (24,46). Hereafter, we refer to the approaching versus withdrawing response or contrast as the looming response.
BOLD responses were extracted from each ROI for each condition. The average BOLD response to each face and car condition was compared with the BOLD response to the crosshair baseline condition. The two contrasts of interest were 1) approach versus withdrawal (the looming response) for each stimulus type (faces, cars) and 2) all faces versus all cars. These contrasts were chosen based on prior work demonstrating that the DIPS and PMv regions respond preferentially to looming stimuli, particularly faces (24), and that the amygdala responds preferentially to face stimuli, compared with nonface objects such as car stimuli (47,48). A repeated-measures analysis of variance, using a 3 region (DIPS, PMv, amygdala) × 2 hemisphere (left, right) × 2 condition (approach, withdrawal) × 2 stimuli (faces, cars) × 2 group (FH−, FH+) factorial design, was performed, to test our predictions that compared with the FH− group, the FH+ group would show 1) greater amygdala responses to faces (both to looming faces and to all faces vs. all cars) and 2) greater DIPS and PMv response to looming faces.
Correlational Analyses.
ROIs that showed significant between-group effects were then used to conduct correlational and regression analyses to test whether changes in brain function associated with having a family history of depression were correlated with resilience levels [measured using the CD-RISC (12)] and/or with levels of subsyndromal depressive, psychotic-like, and anxiety symptoms [measured using the BDI, PDI (41), and Spielberger State-Trait Anxiety Inventory (49), respectively].
Secondary, Voxelwise Analysis.
Subsequently, a secondary, voxelwise analysis was conducted for the purpose of further localizing the findings of the anatomical ROI analysis (see Results). This analysis was conducted using a Monte Carlo simulation (10,000 iterations) whole-brain correction, using 2 cluster-forming height thresholds of p = .001 and p = .05. In addition, percent signal change data extracted from these maps (limited to regions showing between-group differences in the anatomical ROI analysis) were used to confirm the findings of the regression and correlational analyses conducted using the anatomical ROIs.
RESULTS
Participant Characteristics and Performance on the Dot-Detection Task
There were no significant differences between the FH− and FH+ groups in age, gender, ethnicity, childhood adversity levels, or baseline symptom or resilience levels (Table 1).
Table 1.
Demographic Information and Symptom Levels of the Participants
| FH− (n = 45)a | FH+ (n = 27)a | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | SD | Mean | Min | Max | SD | |
| Age, Years | 19.49 | 18 | 22 | 1.31 | 19.59 | 18 | 24 | 1.60 |
| Depressive Symptomsb | 6.71 | 0 | 20 | 5.50 | 8.93 | 0 | 17 | 3.90 |
| Psychotic Experiencesc | 6.11 | 0 | 18 | 3.93 | 5.07 | 0 | 12 | 3.22 |
| Resilienced | 73.10 | 38 | 100 | 16.44 | 73.89 | 57 | 95 | 9.70 |
| State Anxietye | 28.58 | 0 | 56 | 16.72 | 32.85 | 0 | 62 | 18.27 |
| Trait Anxietyf | 31.31 | 0 | 59 | 17.70 | 34.41 | 0 | 57 | 17.35 |
| Childhood Adversityg | 30.11 | 0 | 48 | 8.93 | 31.96 | 0 | 77 | 17.47 |
| Gender, Male/Female, n | 12/33 | 9/18 | ||||||
| Race, % | ||||||||
| Caucasian | 51 | 44 | ||||||
| African American | 27 | 37 | ||||||
| Asian | 15 | 7 | ||||||
| Other | 8 | 12 | ||||||
| Ethnicity, Hispanic, % | 15 | 0 | ||||||
The FH− and FH+ groups showed no significant differences in age, education, gender, ethnicity, levels of symptoms of depression, psychotic experiences, suicidality, resilience, anxiety, and levels of childhood adversity, based on independent-sample t tests. There were no significant differences between groups in gender (Pearson’s χ21 = 0.3630, p = .547)
FH+, family history of depression; FH−, no family history of depression.
Resilience levels negatively correlated with depressive symptoms within the FH− group (r = −.61, p < .01) but not within the FH+ group (r = −.10, p = .61).
Measured using the Beck Depression Inventory.
Measured using the Peters et al. Delusions Inventory.
Measured using the Connor-Davidson Resilience Scale.
Measured using the Spielberger State Anxiety Inventory.
Measured using Spielberger Trait Anxiety Inventory.
Measured using the Childhood Trauma Questionnaire.
Analyses of subjects’ behavioral performance during scanning showed no main effects of group in response rates during the dot-detection task (F1,70 = 2.2, p = .14). A significant group × condition effect (F1,70 = 4.3, p = .04) was driven by greater accuracy of the FH+ group (vs. the FH− group) during the Car Withdrawal condition (t26 = 2.1, p = .04). Given that this result would not have any influence on our a priori hypotheses, it was not explored further.
Functional MRI
Hypothesis Testing: Anatomical ROI Analysis.
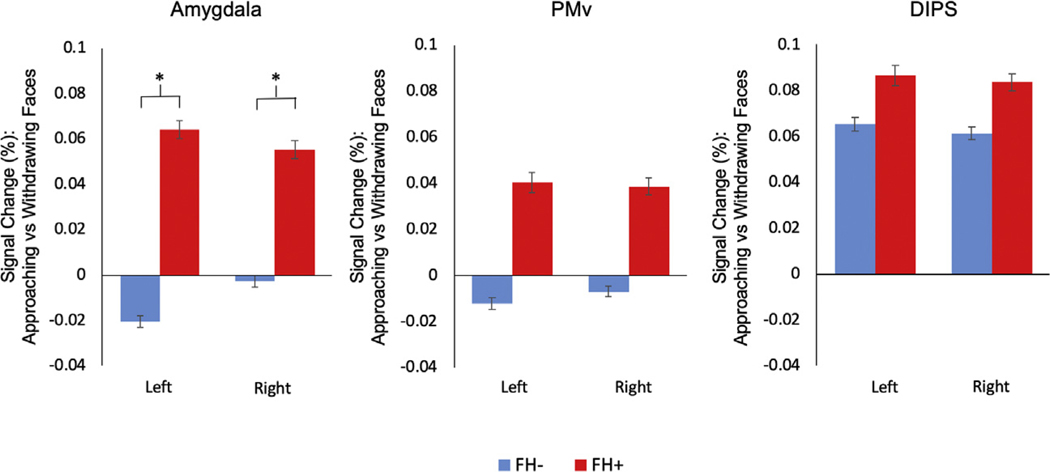
As expected, the repeated-measures analysis of variance revealed a main effect of condition (F1,70 = 12.88, p = .001) due to the significant activation to looming (Approach > Withdrawal) stimuli. Also, there was a significant 3-way interaction among group, condition, and stimulus type (F1,1 = 4.96, p = .029), which was due to a greater response of the FH+ group compared with the FH− group to looming faces in the bilateral amygdala (left [t70 = −2.89, p = 5 × 10−3], right [t70 = −2.02, p = .04]) (Figure 1 and Supplemental Table S1), with a trend toward a similar difference between the 2 groups in the bilateral PMv (left [t70 = −1.92, p = .06], right [t70 = −1.83, p = .07]) but not in the DIPS (left [t70 = −0.67, p = .50], right [t70 = −0.76, p = .45]). There was no group × stimulus or group × stimulus × region interaction, indicating that there was no between-group difference in the responses of the amygdala or of the other 2 regions to faces compared with cars. Also, for all 3 ROIs, there were no differences between the 2 groups in responses to looming cars.
Figure 1.
Results of the region-of-interest analysis. Bar plots of mean percent signal change (approaching vs. withdrawing [looming] faces) of the amygdala, ventral premotor cortex (PMv), and dorsal intraparietal sulcus (DIPS) regions of interest for the groups with (FH+) and without (FH−) a family history of depression are displayed. The FH+ group showed a significantly greater response to looming faces in the left and right amygdala compared with the FH− group (left [t70 = −2.89, p = 5 × 10−3], right [t70 = −2.02, p = .04]). *p < .05.
Follow-up repeated-measures analysis of variances conducted within each group revealed a significant interaction between region and stimulus for both the FH− and FH+ groups. This interaction was due to a significantly greater response to face compared with car stimuli in the left amygdala in both groups (FH− group [t44 = 3.50, p = .001], FH+ group [t26 = 2.50, p = .02]) and in the right amygdala in the FH− group, with a similar trend in the FH+ group (right: FH− group [t44 = 3.39, p = .002], FH+ group [t26 = 1.93, p = .06]).
Secondary, Voxelwise Analysis: Faces Approach Versus Withdrawal.
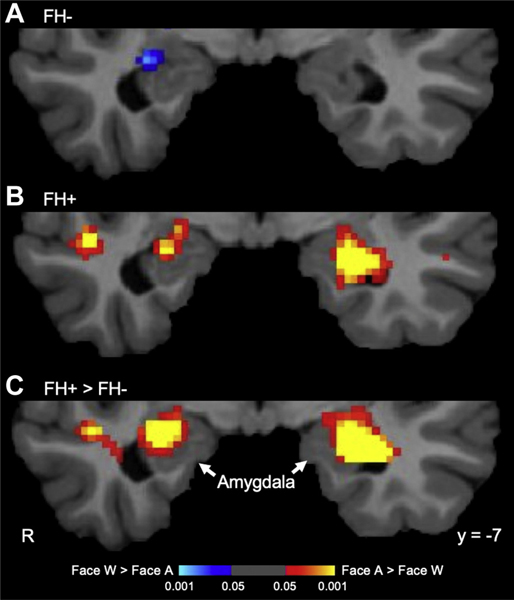
To localize the between-group difference observed in the amygdala in the anatomical ROI analysis, a secondary voxelwise analysis of the Faces Approach versus Faces Withdrawal contrast was conducted. No significant clusters were observed at the cluster-forming threshold of p = .001; however, at p = .05, a cluster within the left amygdala was present, owing to a significantly greater response of the FH+ group compared with the FH− group (Montreal Neurological Institute coordinates [x, y, z] of the peak voxel: −26, −5, −23 [z = 4.5, p = 6.6 × 10−6]). A similar (slightly weaker) pattern of findings was observed for the right amygdala (30, −8, −17 [z = 3.9, p = 1 × 10−4]) (Figure 2). Follow-up 1-sample t tests confirmed that the FH+ group, but not the FH− group, showed significant responses of the left amygdala to looming faces (−26, −5, −23 [z = 4.4, p = 1 × 10−5]). There was no significant activation at this threshold in the right amygdala in either group.
Figure 2.
Results of the voxelwise analyses. These whole brain–corrected maps (p < .05) display the amygdala responses to looming faces in (A) the group without a family history of depression (FH−) and (B) the group with a family history of depression (FH+) and (C) the comparison between the responses of the two groups. Only the FH+ group (not the FH− group) showed a significant response to looming faces, in the left amygdala. A, Approach condition; R, right; W, Withdrawal condition.
Regression Analysis
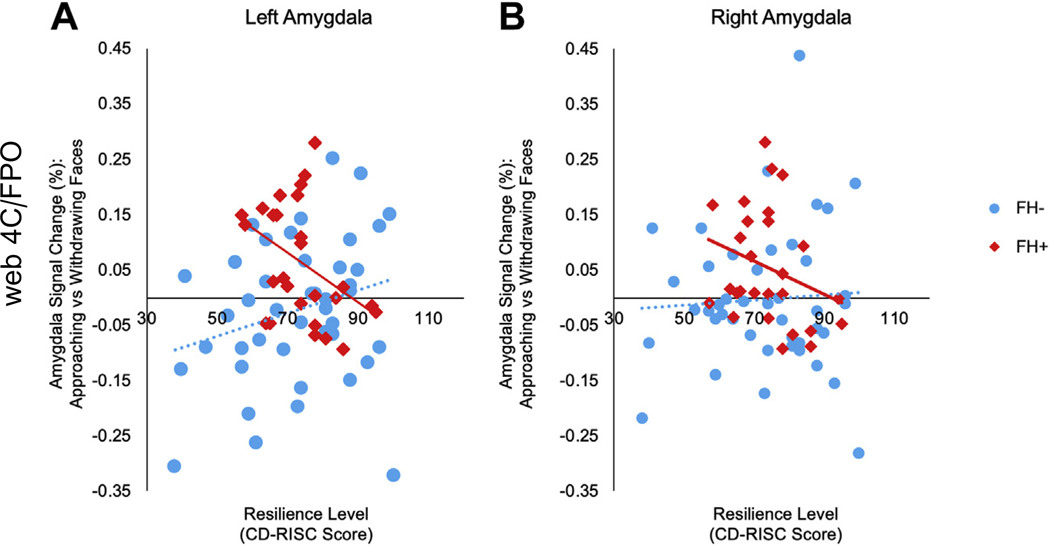
A regression analysis using a multivariate general linear model was then conducted to test whether changes in brain function associated with having a family history of depression were associated with resilience or symptom levels. The regression analysis was performed with amygdala BOLD response, extracted from the left and right amygdala anatomical ROIs, as the dependent variable, group (FH+/FH−) as a between-subjects factor, and resilience/symptom scores as covariates. Significant interactions between group and resilience or symptom levels on amygdala response were followed up with Pearson’s correlations (2-tailed). There was a significant interaction between group and resilience levels for the responses of the left, but not of the right, amygdala (left amygdala [F1,55 = 8.94, p = .004], right amygdala [F1,55 = 1.69, p = .20]). There were no significant interactions between group and levels of depressive (BDI), psychotic-like (PDI), or anxiety (Spielberger State-Trait Anxiety Inventory) symptoms for either the left or right amygdala responses. Follow-up correlations showed that levels of resilience were negatively correlated with responses of the left amygdala to looming faces in the FH+ group (r = −.41, p = .03) (Figure 3). This correlation remained significant after controlling for levels of subthreshold symptoms of depression and anxiety. In contrast, the FH− group did not show any significant correlations between amygdala responses to looming faces and resilience levels (left: p = .09; right: p = .71) (Figure 3). There were no correlations between resilience levels and amygdala responses to faces overall (compared with cars) in either the FH+ or FH− group (all p > .3).
Figure 3.
Correlations between amygdala responses and resilience levels. For these correlational analyses, responses of the left and right amygdala for each subject were extracted using anatomically defined regions of interest generated by FreeSurfer from the T1 scan of each subject. The scatter plot in (A) illustrates the significant correlation between resilience level (as measured by the Connor-Davidson Resilience Scale [CD-RISC]) and the response of the left amygdala to looming faces in the group with a family history of depression (FH+) (r = −.41, p = .03, n = 27), whereas there was no significant correlation between responses of the left amygdala and resilience levels in the group without a family history of depression (FH−) (r = .26, p = .09). Moreover, the correlations between responses of the left amygdala and resilience levels in the FH+ and FH− groups were significantly different from each other (z = −2.48, p = .02). (B) Responses of the right amygdala to looming faces were not significantly correlated with resilience levels in the FH+ (r = −.28, p = .16) or FH− (r = .05, p = .71) groups.
Because having a history of childhood adversity is linked to an increased risk for developing depression (50), we repeated this analysis controlling for levels of childhood adversity, measured using the Childhood Trauma Questionnaire (51). The correlation between resilience levels and amygdala responses to looming faces in the FH+ group remained significant after controlling for childhood adversity (r = −.42, p = .03). Also, there were no correlations between amygdala responses to looming faces and baseline levels of subsyndromal depression, anxiety, psychotic experiences, or levels of childhood adversity in the FH+ group (all ps > .20) (Supplemental Table S2).
Last, to confirm the above findings by repeating the analyses using a slightly different approach, we conducted these correlational analyses using 3 functionally defined amygdala ROIs, which were derived from between-group comparison maps that were generated using 3 thresholds (p = .05, p = .01, and p = .001). These analyses revealed significant negative correlations between resilience levels and amygdala responses, regardless of the ROI used (Table 2).
Table 2.
Correlations Between Amygdala Responses to Looming Faces and Levels of Resilience
| Region of Interest | ||||||
|---|---|---|---|---|---|---|
| Left Amygdala | Right Amygdala | Left + Right Amygdala | ||||
| Region of Interest Definition | Correlation Coefficient (r) | p Value | Correlation Coefficient (r) | p Value | Correlation Coefficient (r) | p Value |
| Anatomical (FreeSurfer Segmentation) | −.41 | .03 | −.28 | .16 | −.36 | .06 |
| Functional (Voxel Threshold .05) | −.52 | .01 | −.42 | .03 | −.50 | .01 |
| Functional (Voxel Threshold .01) | −.52 | .01 | −.36 | .06 | −.46 | .01 |
| Functional (Voxel Threshold .001) | −.49 | .01 | −.35 | .07 | −.45 | .02 |
Amygdala responses for each subject were extracted using regions of interest that were either defined anatomically (in the primary analysis) or defined using the between-group comparison (family history of depression vs. no family history of depression) voxelwise maps generated using 3 different thresholds (p = .05, p = .01, and p = .001). Significant correlations with resilience levels (measured using the Connor-Davidson Resilience Scale) in the family history–positive group [n = 27]) are observed regardless of the type of amygdala region of interest used to extract the functional magnetic resonance imaging data.
DISCUSSION
Summary of Main Findings
Consistent with prior work, this study demonstrated that nondepressed young adults with a first-degree relative who had a history of depression display greater amygdala responsivity than nondepressed young adults without such a family history (33–35). Further analyses revealed that this pattern of responses was not related to the presence of subthreshold symptoms of psychopathology but rather to low resilience levels. However, a parietofrontal cortical network involved in attention did not show the same pattern of significantly elevated responsivity as seen in the amygdala in the relatives, suggesting that attentional systems may be disrupted in depressive illness but not in an at-risk cohort.
Amygdala Dysregulation as a Marker of Low Resilience and Risk for Illness
Potentially related to these results, one recent study found that lower resting-state connectivity of the amygdala with the orbitofrontal cortex in adolescents at risk for depression predicted the later development of depression (52); another prior study found that higher basal levels of amygdala activity was associated with low, self-reported resilience in older adults (53). Taken together with the current results, these findings suggest that specific patterns of disrupted amygdala function (i.e., increases in basal or stimulus-elicited activity and reduced functional coupling with prefrontal cortical regions) may be linked to poor resilience and vulnerability to depression. However, exactly how poor resilience and depression risk are manifested and linked biologically remains to be understood.
Resilience
While the definition of resilience and its components continues to be debated, it is likely the result of a combination of a range of biological and environmental factors influencing one’s response to adversity (54). In previous studies, resilience as measured by the self-report scale used here, the CD-RISC, has been shown to moderate the relationship between adverse events and severity of subsequent symptoms of anxiety and depression (13,14), as well as predict treatment response in patients with depression (55) and posttraumatic stress disorder (56), irrespective of symptom severity. Together, these findings suggest that this self-report scale measures an aspect of resilience that predicts both risk for the development of psychopathology and the capacity for rapid recovery from it.
In our sample, average resilience levels did not differ between the FH+ and FH− groups. However, only the FH+ subjects with lower resilience levels showed elevated amygdala responses to looming faces. Thus, in future studies, amygdala responses to salient stimuli and/or self-reported resilience levels could be tested as potential components of a tool for identifying individuals, among those with genetic risk for depression (conferring a threefold increase in risk), who have a particularly elevated risk for depression due to other, perhaps nonfamilial, factors.
Specific Effect on Amygdala Responses to Looming Faces
Interestingly, in the current study, we did not find evidence for overall amygdala dysfunction in those with familial risk for depression, as amygdala responses to faces overall (in comparison with responses to cars) was not elevated in this group, nor were amygdala responses to faces (compared with cars) negatively correlated with resilience levels in either group. This may be due to the robustness of face-elicited responses of the amygdala (as compared with amygdala responses to nonface stimuli) in most individuals; there may not be sufficient variance in these responses to reveal a dimensional relationship with resilience levels, in contrast to the variance observed in amygdala responses to salient [e.g., approaching and on a collision course with the body, or emotional (24,57)] versus nonsalient or safe-appearing (e.g., withdrawing or emotionally neutral) face images.
Task Performance
There were no differences between the 2 groups in performance of the dot-detection task that was performed while the approaching and withdrawing face stimuli were presented, suggesting that differences between the 2 groups in levels of attention to the stimuli were not present and thus cannot account for the between-group differences in amygdala responses. The FH+ group did perform significantly better on the task than the FH− group during the Withdrawing Cars condition, perhaps reflecting a generally greater level of vigilance that was only detectable during a rather nonsalient condition.
Dorsal Attention Network
We found no significant between-group differences in the responses of the DIPS, and only a trend toward an elevated response of the PMv, in the FH+ compared with the FH− individuals. Thus, these results do not provide support for the hypothesis that primary abnormalities in the dorsal attentional system are responsible for attention-related abnormalities in individuals at risk for depression. One possible interpretation of this pattern of findings is that attentional abnormalities in depression and depression risk are primarily due to consequences of alterations in amygdala function (or in an amygdala-centered network of regions), rather than due to fundamental changes in the neural circuitry that controls attention. However, it is also important to note that the task used here did not engage all circuitry involved in attentional control; for example, the ventral “bottom-up” attention network was not examined. Thus, additional studies will be needed to fully evaluate the neural systems mediating the various forms of attention in individuals with risk factors for developing depression.
Elevated Responses of the Amygdala as a Transdiagnostic Risk Factor
Current models of depression (8,58) propose that a vulnerability to depression may remain dormant for years, without leading to a depressive episode. Thus, a combination of factors, including environmental events and hormonal changes, as well as protective biological and psychosocial factors, may determine whether clinically impairing depressive symptoms emerge in vulnerable individuals. Notably, recent evidence suggests that this model of depression may in fact correspond to a more general model of psychopathology; some neural “vulnerability markers,” such as heightened amygdala responses to salient social stimuli, may not be specific to depression risk (59–63). For example, two studies have shown that the response of the amygdala to threatening stimuli prior to a traumatic event predicts the later development of posttraumatic stress disorder symptoms (59,64), and a third study showed that amygdala responses to negatively valenced faces predicted the later development of internalizing symptoms (65). The current data are generally in line with this and other evidence for amygdala dysfunction as a transdiagnostic marker of psychopathology risk. Specifically, taken together with prior work, these results suggest that reactivity of the amygdala in response to salient social stimuli may be a marker of vulnerability for the context-dependent emergence of psychopathology related to a reduced capacity to tolerate stress (i.e., low resilience).
Limitations and Future Directions
Resilience is a complex construct with no agreed-upon operational definition. Thus, one limitation of this study is that resilience was measured using only one self-report questionnaire. Future studies can include additional measures that have been linked to resilience, such as physiologic and epigenetic markers, and test whether those and the putative marker of resilience described here, low or “normal” amygdala reactivity to salient social stimuli, can be used to prospectively predict outcomes (i.e., resilient or not) following stressful events. Follow-up work can also determine whether specific resilience-boosting interventions can influence amygdala function, psychological responses to environmental stressors, and psychiatric outcomes. Future studies can also expand on the experimental design of the functional MRI paradigm used here, including increasing the representation of different ethnicities in the social (face) stimuli, as well as the types of social and nonsocial stimuli.
Lastly, it is also important to emphasize that it is not possible to distinguish here, given the design of the current study, between an effect of greater loading for genetic variants associated with depression (6) and the influence of growing up with a depressed relative and the associated environmental stressors that may accompany that experience. Follow-up studies can investigate whether aversive childhood experiences associated with the stress of familial depression are closely linked to elevated amygdala responses in at-risk individuals, or whether there is instead (or in addition) a strongly genetic basis for this phenotype.
Conclusions
These findings show that a specific pattern of amygdala function (higher responses to salient social information) in those with familial risk for depression is linked to lower resilience within this group. In the future these results could potentially provide a component of a quantitative tool that could be used to stratify individuals with a family history of depression into high and low risk groups. Such a tool is a prerequisite for the development of objective screening strategies, individualized risk profiles and targeted preventive interventions.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by a Dupont Warren fellowship (to TB), a Stuart T. Hauser Research Training Program in Biological and Social Psychiatry T32 award (to TB), internal support from the Massachusetts General Hospital Department of Psychiatry for neuroimaging research in psychiatry, and Grant No. RO1MH109562 (Principal investigator, DJH).
We are very grateful to Tammy Moran, Marisa Hollinshead, and other staff of the Center for Brain Science of Harvard University for their invaluable assistance with data acquisition.
MF has received research support from following: Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid; Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; Biohaven; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clarus Funds; Clintara, LLC; Covance; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; HoffmanLaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia and Depression; National Center for Complementary and Alternative Medicine; National Coordinating Center for Integrated Medicine; National Institute of Drug Abuse; National Institute of Mental Health; Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; Otsuka Pharmaceutical Development, Inc.; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); SanofiAventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute; Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceuticals; Tal Medical; VistaGen; and Wyeth-Ayerst Laboratories. DJH has received research support from Forum Pharmaceuticals, Inc., and Janssen Scientific Affairs. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at http://doi.org/10.1016/j.bpsc.2019.10.010.
REFERENCES
- 1.World Health Organization (2017): Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Weinberger AH, Gbedemah M, Martinez AM, Nash D, Galea S, Goodwin RD (2018): Trends in depression prevalence in the USA from 2005 to 2015: Widening disparities in vulnerable groups. Psychol Med 48:1308–1315. [DOI] [PubMed] [Google Scholar]
- 3.Akushevich I, Kravchenko J, Yashkin AP, Yashin AI (2018): Time trends in the prevalence of cancer and non-cancer diseases among older U. S. adults: Medicare-based analysis. Exp Gerontol 110:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghaemi SN (2008): Why antidepressants are not antidepressants: STEP-BD, STAR*D, and the return of neurotic depression. Bipolar Disord 10:957–968. [DOI] [PubMed] [Google Scholar]
- 5.Garber J, Clarke GN, Weersing VR, Beardslee WR, Brent DA, Gladstone TRG, et al. (2009): Prevention of depression in at-risk adolescents: A randomized controlled trial. JAMA 301:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan PF, Neale MC, Kendler KS (2000): Genetic epidemiology of major depression: Review and meta-analysis. Am J Psychiatry 157:1552–1562. [DOI] [PubMed] [Google Scholar]
- 7.Charney DS (2004): Psychobiological mechanisms of resilience and vulnerability: Implications for successful adaptation to extreme stress. Am J Psychiatry 161:195–216. [DOI] [PubMed] [Google Scholar]
- 8.Southwick SM, Vythilingam M, Charney DS (2005): The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annu Rev Clin Psychol 1:255–291. [DOI] [PubMed] [Google Scholar]
- 9.American Psychological Association. The road to resilience. Available at: http://www.apa.org/helpcenter/road-resilience.aspx. Accessed June 29, 2018.
- 10.Kalisch R, Baker DG, Basten U, Boks MP, Bonanno GA, Brummelman E, et al. (2017): The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav 1:784–790. [DOI] [PubMed] [Google Scholar]
- 11.Hermans EJ, Fernández G (2015): Heterogeneity of cognitive-neurobiological determinants of resilience. Behav Brain Sci 38:e103. [DOI] [PubMed] [Google Scholar]
- 12.Connor KM, Davidson JRT (2003): Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety 18:76–82. [DOI] [PubMed] [Google Scholar]
- 13.Peng L, Zhang J, Li M, Li P, Zhang Y, Zuo X, et al. (2012): Negative life events and mental health of Chinese medical students: The effect of resilience, personality and social support. Psychiatry Res 196:138–141. [DOI] [PubMed] [Google Scholar]
- 14.Campbell-Sills L, Cohan SL, Stein MB (2006): Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav Res Ther 44:585–599. [DOI] [PubMed] [Google Scholar]
- 15.Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H (2002): Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry 7:234–242. [DOI] [PubMed] [Google Scholar]
- 16.Disner SG, Beevers CG, Haigh EAP, Beck AT (2011): Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 12:467–477. [DOI] [PubMed] [Google Scholar]
- 17.Schock L, Schwenzer M, Sturm W, Mathiak K (2011): Alertness and visuospatial attention in clinical depression. BMC Psychiatry 11:78.21554705 [Google Scholar]
- 18.Pardo JV, Pardo PJ, Humes SW, I Posner M (2006): Neurocognitive dysfunction in antidepressant-free, non-elderly patients with unipolar depression: Alerting and covert orienting of visuospatial attention. J Affect Disord 92:71–78. [DOI] [PubMed] [Google Scholar]
- 19.de Fockert JW, Cooper A (2013): Higher levels of depression are associated with reduced global bias in visual processing. Cogn Emot 28:541–549. [DOI] [PubMed] [Google Scholar]
- 20.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015): Large-scale network dysfunction in major depressive disorder: Meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambataro F, Visintin E, Doerig N, Brakowski J, Holtforth MG, Seifritz E, Spinelli S (2017): Altered dynamics of brain connectivity in major depressive disorder at-rest and during task performance. Psychiatry Res Neuroimaging 259:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Geng X, Xu J, Liu B, Shi Y (2018): Multivariate classification of major depressive disorder using the effective connectivity and functional connectivity. Front Neurosci 12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cléry J, Hamed SB (2018): Frontier of self and impact prediction. Front Psychol 9:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt DJ, Cassidy BS, Yue X, Rauch SL, Boeke EA, Nasr S, et al. (2014): Neural correlates of personal space intrusion. J Neurosci 34:4123–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graziano MSA, Cooke DF (2006): Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44:2621–2635. [DOI] [PubMed] [Google Scholar]
- 26.Corbetta M, Shulman GL (2002): Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- 27.Jaworska N, Yang X-R, Knott V, MacQueen G (2015): A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry 16:448–471. [DOI] [PubMed] [Google Scholar]
- 28.Singh MK, Gotlib IH (2014): The neuroscience of depression: Implications for assessment and intervention. Behav Res Ther 62:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desseilles M, Schwartz S, Dang-Vu TT, Sterpenich V, Ansseau M, Maquet P, Phillips C (2011): Depression alters “top-down” visual attention: A dynamic causal modeling comparison between depressed and healthy subjects. NeuroImage 54:1662–1668. [DOI] [PubMed] [Google Scholar]
- 30.Beevers CG, Clasen P, Stice E, Schnyer D (2010): Depression symptoms and cognitive control of emotion cues: A functional magnetic resonance imaging study. Neuroscience 167:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rive MM, Koeter MWJ, Veltman DJ, Schene AH, Ruhé HG (2016): Visuospatial planning in unmedicated major depressive disorder and bipolar disorder: Distinct and common neural correlates. Psychol Med 46:2313–2328. [DOI] [PubMed] [Google Scholar]
- 32.Miskowiak KW, Glerup L, Vestbo C, Harmer CJ, Reinecke A, Macoveanu J, et al. (2015): Different neural and cognitive response to emotional faces in healthy monozygotic twins at risk of depression. Psychol Med 45:1447–1458. [DOI] [PubMed] [Google Scholar]
- 33.Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, et al. (2015): Functional and structural brain correlates of risk for major depression in children with familial depression. NeuroImage Clin 8:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, et al. (2008): Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry 165:90–98. [DOI] [PubMed] [Google Scholar]
- 35.Swartz JR, Williamson DE, Hariri AR (2015): Developmental change in amygdala reactivity during adolescence: Effects of family history of depression and stressful life events. Am J Psychiatry 172:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samara Z, Evers EAT, Peeters F, Uylings HBM, Rajkowska G, Ramaekers JG, Stiers P (2018): Orbital and medial prefrontal cortex functional connectivity of major depression vulnerability and disease. Biol Psychiatry Cogn Neurosci Neuroimaging 3:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKenzie LE, Uher R, Pavlova B (2019): Cognitive performance in first-degree relatives of individuals with vs without major depressive disorder: A meta-analysis. JAMA Psychiatry 76:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh MK, Leslie SM, Bhattacharjee K, Gross M, Weisman EF, Soudi LM, et al. (2018): Vulnerabilities in sequencing and task switching in healthy youth offspring of parents with mood disorders. J Clin Exp Neuropsychol 40:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farabaugh A, Bitran S, Nyer M, Holt DJ, Pedrelli P, Shyu I, et al. (2012): Depression and suicidal ideation in college students. Psychopathology 45:228–234. [DOI] [PubMed] [Google Scholar]
- 40.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961): An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- 41.Peters ER, Joseph SA, Garety PA (1999): Measurement of delusional ideation in the normal population: Introducing the PDI (Peters et al. Delusions Inventory). Schizophr Bull 25:553–576. [DOI] [PubMed] [Google Scholar]
- 42.Oliveira L, Ladouceur CD, Phillips ML, Brammer M, Mourao-Miranda J (2013): What does brain response to neutral faces tell us about major depression? Evidence from machine learning and fMRI. PLoS One 8: e60121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mourão-Miranda J, Oliveira L, Ladouceur CD, Marquand A, Brammer M, Birmaher B, et al. (2012): Pattern recognition and functional neuroimaging help to discriminate healthy adolescents at risk for mood disorders from low risk adolescents. PLoS One 7:e29482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- 45.Abler B, Erk S, Herwig U, Walter H (2007): Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res 41:511–522. [DOI] [PubMed] [Google Scholar]
- 46.Holt DJ, Boeke EA, Coombs G, DeCross SN, Cassidy BS, Stufflebeam S, et al. (2015): Abnormalities in personal space and parietal-frontal function in schizophrenia. NeuroImage Clin 9:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rolls ET (1984): Neurons in the cortex of the temporal lobe and in the amygdala of the monkey with responses selective for faces. Hum Neurobiol 3:209–222. [PubMed] [Google Scholar]
- 48.Leonard CM, Rolls ET, Wilson FA, Baylis GC (1985): Neurons in the amygdala of the monkey with responses selective for faces. Behav Brain Res 15:159–176. [DOI] [PubMed] [Google Scholar]
- 49.Spielberger CD, Gorsuch RL (1970): STAI Manual for the State-trait Anxiety Inventory (“Self-evaluation Questionnaire”). Washington, DC: Consulting Psychologists Press. [Google Scholar]
- 50.Heim C, Nemeroff CB (2001): The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry 49:1023–1039. [DOI] [PubMed] [Google Scholar]
- 51.Pennebaker JW, Susman JR (1988): Disclosure of traumas and psychosomatic processes. Soc Sci Med 26:327–332. [DOI] [PubMed] [Google Scholar]
- 52.Fischer AS, Camacho MC, Ho TC, Whitfield-Gabrieli S, Gotlib IH (2018): Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry 75:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leaver AM, Yang H, Siddarth P, Vlasova RM, Krause B, St Cyr N, et al. (2018): Resilience and amygdala function in older healthy and depressed adults. J Affect Disord 237:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R (2014): Resilience definitions, theory, and challenges: Interdisciplinary perspectives. Eur J Psychotraumatology 5:25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Min J-A, Lee N-B, Lee C-U, Lee C, Chae J-H (2012): Low trait anxiety, high resilience, and their interaction as possible predictors for treatment response in patients with depression. J Affect Disord 137:61–69. [DOI] [PubMed] [Google Scholar]
- 56.Davidson J, Stein DJ, Rothbaum BO, Pedersen R, Szumski A, Baldwin DS (2012): Resilience as a predictor of treatment response in patients with posttraumatic stress disorder treated with venlafaxine extended release or placebo. J Psychopharmacol 26:778–783. [DOI] [PubMed] [Google Scholar]
- 57.Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, et al. (2012): Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: A voxelbased meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol J 22:100–113. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan V, Nestler EJ (2008): The molecular neurobiology of depression. Nature 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA (2014): Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety 31:834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton JP (2015): Amygdala reactivity as mental health risk endophenotype: A tale of many trajectories. Am J Psychiatry 172:214–215. [DOI] [PubMed] [Google Scholar]
- 61.Pinkham AE, Loughead J, Ruparel K, Overton E, Gur RE, Gur RC (2011): Abnormal modulation of amygdala activity in schizophrenia in response to direct- and averted-gaze threat-related facial expressions. Am J Psychiatry 168:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR (2012): Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry 69:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carlisi CO, Robinson OJ (2018): The role of prefrontal-subcortical circuitry in negative bias in anxiety: Translational, developmental and treatment perspectives. Brain Neurosci Adv 2:2398212818774223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T (2009): Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci U S A 106:14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swartz JR, Knodt AR, Radtke SR, Hariri AR (2015): A neural biomarker of psychological vulnerability to future life stress. Neuron 85:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.