Abstract
Background
A variety of inflammatory and non-inflammatory indicators were increased in severe and critical Coronavirus disease-19 (COVID-19) and some of them were used to evaluate the severity and predict prognosis of community-acquired pneumonia. The aim of this study was to investigate the association of these indicators in COVID-19 with different severity.
Methods
Clinical data of 46 patients with severe COVID-19 and 31 patients with critical COVID-19 were collected. The general characteristics and comorbidities of the patients were retrospectively analyzed. The initial and peak concentrations of serum troponin I (cTnI), D-dimer (D-D), C-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT), initial and peak neutrophil counts and initial and trough lymphocyte counts were compared between two groups. The correlation between the variation of cTnI, D-D, CRP, IL-6, PCT, neutrophils, lymphocytes and the severity of the disease was analyzed. The efficacy of the initial concentrations of cTnI, D-D, CRP, IL-6, PCT, the initial neutrophil and lymphocyte counts in predicting critical COVID-19 were evaluated by receiver operating characteristic (ROC) curve.
Results
The initial and peak concentrations of cTnI, D-D, CRP, IL-6, PCT, initial and peak neutrophil counts in critical group were higher than those in severe group, the initial and trough counts of lymphocyte were lower than those in the severe group. Except for the initial level of PCT, the other differences were statistically significant (p < 0.05). The increase of cTnI, D-D, CRP, IL-6, PCT, neutrophils and the decrease of lymphocytes were related to the severity of the disease, OR values were 28.80, 2.20, 18.47, 10.80, 52.00, 9.60 and 21.08, respectively. Except for D-D, the other differences were statistically significant. The areas under ROC curves for predicting critical COVID-19 by initial concentrations of cTnI, D-D, CRP, IL-6, PCT, initial lymphocyte and neutrophil counts were 0.76, 0.78, 0.83, 0.95, 0.56, 0.68 and 0.62, respectively.
Conclusions
The severe and critical COVID-19 patients had significant differences in concentrations of serum cTnI, D-D, CRP, IL-6, PCT, neutrophil and lymphocyte counts. The increase of cTnI, CRP, IL-6, PCT, neutrophils and decrease of lymphocytes indicated severe condition. The initial IL-6 might be a good indicator of COVID-19 severity.
Keywords: COVID-19, Severity, Inflammatory indicators
1. Introduction
Coronavirus disease-19 (COVID-19) is pandemic and still raging all over the world. Most patients infected with SARS-CoV-2 have mild illness and present common symptoms such as fever, cough, and fatigue.(Huang et al., 2020) A small number of infected patients would progress to severe cases with acute respiratory distress syndrome. Some patients with severe and critical illness would worsen in a short period of time and die quickly due to multiple organ failure, especially in elderly patients with comorbidities.(Chen et al., 2020a) A variety of inflammatory and non-inflammatory indicators were increased in severe and critical COVID-19.(Huang et al., 2020; Chen et al., 2020a) Some of them were used to judge the severity and prognosis of community-acquired pneumonia.(Storisteanu et al., 2017; Menendez et al., 2008; Drewry et al., 2014; Ge et al., 2019) Thus, it is essential to figure out the differences between inflammatory and non-inflammatory of COVID-19 with diverse severity. We conducted an observational, retrospective study with severe and critical COVID-19 patients to evaluate the differences of inflammatory and non-inflammatory indicators in COVID-19 with different severity and their role in determining the severity of COVID-19.
2. Materials and methods
2.1. Patient involvement
According to the standards of the “COVID-19 Diagnosis and Treatment Program 7th Edition(China)”, the cases of severe and critical COVID-19 diagnosed from February 10, 2020 to March 30, 2020 in Guanggu Hospital of Tongji Medical College, Huazhong University of Science and Technology were searched. A total of 46 severe cases and 31 critical cases were included. Inclusion criteria: Diagnosis of severe and critical COVID-19 according to the “COVID-19 Diagnosis and Treatment Program 7th Edition(China)”. Diagnosis criteria: RT-PCR test positive for SARS-CoV-2 nucleic acid; viral gene sequence is highly homologous to known coronavirus; serum SARS-CoV-2 antibodies IgM, IgG are positive, IgG antibody changes from negative to positive or titer of IgG antibody in recovery period is 4 times higher and above than that in acute period. Severe COVID-19 criteria: Adults meet any of the followings: respiratory rate ≥ 30 breaths/min; resting oxygen saturation ≤ 93%; oxygenation index ≤300 mmHg; lung image lesions progress more than 50% within 24–48 h. Critical COVID-19 criteria: Adults meet any of the followings: with respiratory failure requires for mechanical ventilation; shock; with any other organ failure requires treatment in ICU. Exclusion criteria: age < 18 years old; with immunosuppressive disease; severe and critical condition not caused by COVID-19.
2.2. Data collection
Data of gender, age, co-existing diseases, the initial concentrations of cTnI, D-D, CRP, IL-6, PCT, the initial lymphocytes and neutrophil counts within 24 h after admission, as well as the peak concentrations of cTnI, D-D, CRP, IL-6, PCT, peak neutrophil counts and the trough lymphocyte counts in the course of disease for severely and critically ill patients were collected retrospectively.
2.3. Laboratory test
Patient pharyngeal swab specimens were collected for the SARS-CoV-2 nucleic acid detection using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay. Detailed protocol was described somewhere else.(Wang et al., 2020) IL-6 was determined on a Rocher COBAS E602 electrochemiluminescence immunoassay analyzer (Roche Diagnostics, Basel,Switzerland) by using double antibody sandwich IL-6 assay (Roche Diagnostics, Switzerland), the reference range was 0-14 ng/l. PCT levels were measured on a Rocher COBAS E602 electrochemiluminescence immunoassay analyzer(Roche Diagnostics, Basel, Switzerland) by using double antibody sandwich PCT assay(Roche Diagnostics, Switzerland), the reference range was 0–0.05 ng/ml. cTnI levels were measured on a Radiometer AQT90 FLEX analyzer(Radiometer,Copenhagen, Denmark)by using time-resolved immunofluorescence cTnI assay(Radiometer,Turku, Finland), the reference range was 0-7 pg/ml. CRP levels were measured on Beckman AU5800 automatic biochemical analyzer(Beckman Coulter, California, USA)by using immunoturbidimetry CRP assay (Beckman Coulter, Suzhou, China), the reference range was 0-5 mg/l. D-D levels was determined on CS5100 automatic coagulation analyzer (Sysmex, Kobe, Japan) by using a latex-enhanced photometric immunoassay (Siemens, Marburg, Germany), the reference range was 0–0.55 mg/l. Neutrophil and lymphocyte counts were measured on Sysmex XS500i blood cell analyzer(Sysmex, Kobe, Japan) by using flow cytometry of semiconductor laser nucleic acid fluorescence staining, the reference ranges were (1.8–6.3) × 109/l and (1.1–3.2) × 109/l. All medical laboratory data were generated by the clinical laboratory of Guanggu Hospital.
2.4. Statistical analyses
Continuous variables were expressed as mean ± standard deviation or median and interquartile range (IQR) if the distribution was not satisfied by the Kolmogorov- Smirnov test. The measurement data were analyzed with t-test or Wilcoxon test, and the enumeration data were analyzed with χ2 test or Fisher's exact test. The odds ratio (OR) and its 95% confidence interval (95% CI) were used to analyze the correlation between the changes of the concentrations of cTnI, D-D, CRP, IL-6, PCT, lymphocyte counts, neutrophil counts and the severity of the disease. Receiver operating characteristic (ROC) curve was used to evaluate the efficacy of D-D, CRP, IL-6, PCT, lymphocytes and neutrophils in predicting critical COVID-19. All tests were two-tailed and a p-value <0.05 was considered statistically significant. Analysis were performed by using IBM SPSS Statistics for Windows, Version 22.0.
3. Results
3.1. Patient characteristics
There were 27 males and 19 females in the severely ill group, 22 males and 9 females in the critically ill group, and there was no significant difference between the two groups. The average age of the critically ill group was older than that of the severe group, and the difference between the groups was statistically significant [(70.26 ± 9.96)vs(63.78 ± 11.94), p = 0.02]. Except for cardiovascular disease, the differences of other coexisting diseases were not statistically significant (Table 1 ).
Table 1.
Baseline characteristics of patients with COVID-19.
| Characteristic | Severe group |
Critical group |
Statistic | p-Value |
|---|---|---|---|---|
| (n = 46) | (n = 31) | |||
| Male [(n,%)] | 27(58.70) | 22(53.66) | χ2 = 1.42 | 0.233 |
| Age [year ] | 63.78 ± 11.94 | 70.26 ± 9.96 | t = 2.49 | 0.015 |
| Comorbidities [(n,%)] | ||||
| Cardiovascular disease | 5(10.87) | 9(29.03) | χ2 = 4.11 | 0.043 |
| Cerebrovascular disease | 2(4.35) | 2(6.45) | χ2 = 0.17 | 0.683 |
| Diabetes | 16(34.78) | 7(22.58) | χ2 = 1.32 | 0.251 |
| Malignant tumor | 2(4.35) | 1(3.23) | χ2 = 0.06 | 0.803 |
| COPD | 1(2.17) | 2(6.45) | χ2 = 0.91 | 0.341 |
COPD: Chronic obstructive pulmonary disease.
3.2. Comparison of the concentrations of cTnI, D-D, CRP, IL-6, PCT, lymphocyte counts and neutrophil counts between severe and critical group
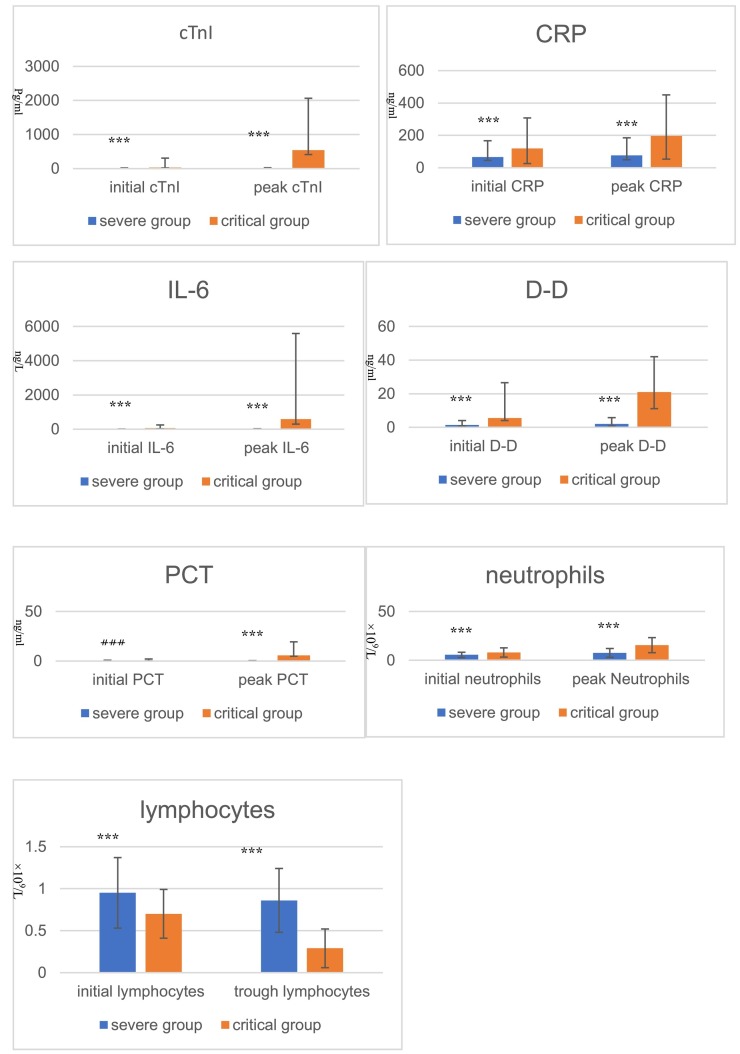
The initial and peak concentrations of cTnI, D-D, CRP, IL-6, PCT and peak neutrophil counts in critical group were higher than those in the severe group, and the initial and trough counts of lymphocyte were lower than those in the severe group. Except for the initial PCT, the other differences were statistically significant (p < 0.05) (Table 2 , Fig. 1 ).
Table 2.
Comparison of cTnI, D-D, CRP, IL-6, PCT, lymphocyte counts and neutrophil counts between severe and critical group.
| Variable category | Severe group(n=46) | Critical group(n=31) | Statistic | p-value |
|---|---|---|---|---|
| cTnI[ng/L(IQR)] | ||||
| Initial concentration | 6.10(3.13-13.88) | 30.40(11.80-278.20) | Z=4.59 | <0.001 |
| Peak concentration | 8.7(3.6-16.8) | 539.65(128.48-1523.85) | Z=6.00 | <0.001 |
| D-D[mg/L(IQR)] | ||||
| Initial concentration | 1.45(0.65-2.63) | 5.51(1.45-21) | Z=4.16 | 0.001 |
| Peak concentration | 2.02(1.21-3.8) | 21(9.83-21) | Z=5.54 | <0.001 |
| CRP[mg/L(IQR)] | ||||
| Initial concentration | 65.50(20.10-101) | 118.60(92.4-188.53) | Z=3.32 | <0.001 |
| Peak concentration | 76.9(27.35-108.2) | 196(142.7-253.9) | Z=6.51 | <0.001 |
| IL-6[pg/ml(IQR)] | ||||
| Initial concentration | 7.38(4.41-12.74) | 64.78(39.67-180.60) | Z=3.26 | 0.001 |
| Peak concentration | 8.76(3.11-23.37) | 590.9(293.85-5000) | Z=5.84 | <0.001 |
| PCT[ng/ml(IQR)] | ||||
| Initial concentration | 0.17(0.07-0.50) | 0.31(0.15-0.85) | Z=1.70 | 0.089 |
| Peak concentration | 0.12(0.07-0.32) | 5.63(1.65-13.75) | Z=5.88 | <0.001 |
| Neutrophils [×109/l] | ||||
| Initial counts | 5.54±2.75 | 7.95±4.72 | t=2.85 | 0.006 |
| Peak counts | 7.65±4.5 | 15.5±7.81 | t=6.88 | <0.001 |
| Lymphocytes[×109/l] | ||||
| Initial counts | 0.95±0.42 | 0.7±0.29 | t=2.82 | 0.006 |
| Trough counts | 0.86±0.38 | 0.29±0.23 | t=5.39 | <0.001 |
cTnI: Troponin I, D-D:D-dimer, CRP:C-reactive protein, IL-6: Interleukin-6, PCT: Procalcitonin
Fig. 1.
Comparison of cTnI, D-D, CRP, IL-6, PCT,lymphocytes and neutrophils between severe and critical group ⁎⁎⁎p < 0.05 ###p = 0.089.
3.3. The relationship between the variation of the concentrations of cTnI, D-D, CRP, IL-6, PCT, lymphocyte counts, neutrophil counts and the severity of the disease
According to the variation of the concentrations of cTnI, cTnI, D-D, CRP, IL-6, PCT, neutrophil counts and lymphocyte counts in the course of disease, the increase rate of the concentrations of cTnI, D-D, CRP, IL-6, PCT and neutrophil counts in the critical group were higher than those in the severe group, and the decrease rate of lymphocyte counts in the critical group was higher than that in the severe group. Except for D-D, the other differences were statistically significant. The final OR (95% CI) of critically ill patients were 28.80 (6.85–121.07), 2.20 (0.77–6.30), 18.47 (5.02–68.02), 10.80 (1–117), 52.00 (4.74–570.53), 9.60 (2.02–45.58) and 21.08 (5.34–83.19) respectively for patients with elevated cTnI, cTnI, D-D, CRP, IL-6, PCT or neutrophils and for patients with reduced lymphocytes compared with other patients (Table 3 ).
Table 3.
The relationship between the variation of the concentrations of cTnI, D-D, CRP, IL-6, PCT, lymphocyte counts, neutrophil counts and the severity of the disease.
| Variable category | Severe group (n = 46) | Critical group (n = 31) | Statistic | p-Value | OR (95%CI) |
|---|---|---|---|---|---|
| cTnI[elevation(n/%)] | 4(11.11) | 18(54.55) | χ2 = 27.06 | <0.001 | 28.80(6.85–121.07) |
| D-D[elevation(n/%)] | 14(38.89) | 14(58.33) | χ2 = 23.99 | 0.139 | 2.20(0.77–6.30) |
| CRP[elevation(n/%)] | 9(20.45) | 19(82.61) | Fisher's exact test | <0.001 | 18.47(5.02–68.02) |
| IL-6[elevation(n/%)] | 5(45.45) | 9(90.00) | χ2 = 25.18 | 0.043 | 10.80(1–117) |
| PCT[elevation(n/%)] | 1(7.69) | 13(81.25) | χ2 = 10.41 | <0.001 | 52.00(4.74–570.53) |
| Neutrophils[elevation(n/%)] | 25(55.55) | 24(92.31) | χ2 = 2.19 | 0.001 | 9.60(2.02–45.58) |
| Lymphocytes[reduction(n/%)] | 12(26.67) | 23(88.46) | Fisher's exact test | <0.001 | 21.08(5.34–83.19) |
cTnI: Troponin I, D-D:D-dimer, CRP:C-reactive protein, IL-6: Interleukin-6, PCT: Procalcitonin.
3.4. Areas under ROC
ROC curve analysis results showed that the areas under the curves of initial cTnI, D-D, CRP, IL-6, PCT, neutrophils and lymphocytes for predicting critically ill patients were 0.76, 0.78, 0.83, 0.95, 0.56, 0.68 and 0.62, respectively. Among them, the initial IL-6 level was the most effective, and the area under the curve was up to 0.95. The second was CRP, and the lymphocytes was the least effective (Fig. 2, Fig. 3 ).
Fig. 2.
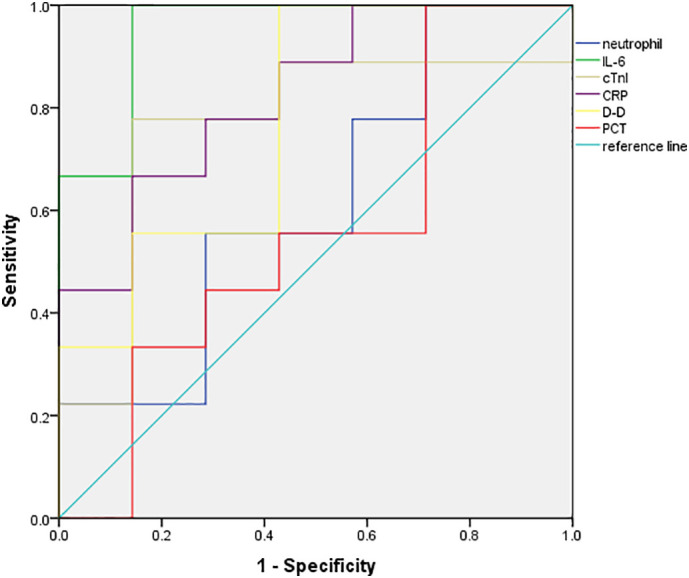
ROC curves of cTnI, D-D, CRP, IL-6, PCT, neutrophils in predicting critically ill patients.
Fig. 3.
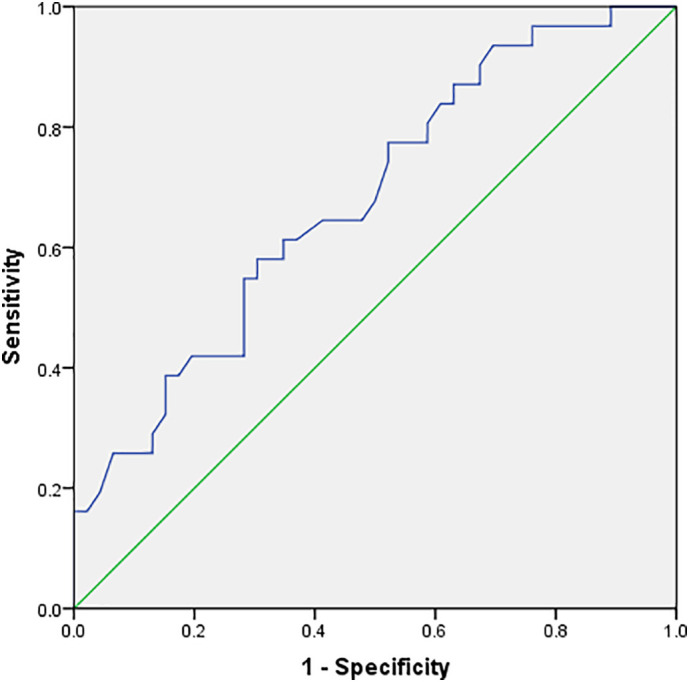
ROC curve of lymphocytes in predicting critically ill patients.
4. Discussion
We found that the severe and critical COVID-19 patients had significant differences in concentrations of cTnI, D-D, CRP, IL-6, PCT, neutrophil counts and lymphocyte counts. The increase of cTnI, CRP, IL-6, PCT, neutrophils and decrease of lymphocytes indicated aggravated condition. The initial IL-6 might be a good indicator for severity of COVID-19.
ACE2 (angiotensin-converting enzyme 2), receptor of SARS-CoV-2, exists in multiple organs including the heart,(Chen et al., 2020b) so SARS-CoV-2 can directly attack the heart, leading to myocardial cell apoptosis and necrosis. For critical patients, the systemic viral load was larger, and the direct damage to myocardium was greater. CRP and IL-6 in the critically ill group were higher than those in the severe group, and the lung damage was usually more serious for critical patients, so the inflammatory response and myocardial hypoxia were greater for critically ill patients. What's more, the fluid supplementation for critical patients during treatment increased the cardiac load. All these factors above(Vita et al., 1992; Moammar et al., 2010; Frencken et al., 2019) resulted in the high level of serum troponin. At the same time, critical patients with more comorbidities had older average age, so they were prone to be complicated with acute myocardial infarction under conditions of ischemia and hypoxia.(Violi et al., 2017)
The levels of serum CRP and IL-6 were usually increased in the infection by various pathogens, such as bacteria, virus, fungi etc. But high serum PCT level usually indicates bacterial infection. Critical patients who had a long course of disease and often required respiratory support were susceptible to bacterial infection. Therefore, the peak PCT level, the initial and peak levels of CRP and IL-6 were significantly different between severe and critical group, and for initial PCT, no difference was found. Admission of CRP, IL-6, and PCT in serum can be used to evaluate the severity of pneumonia, and continuously high levels usually indicates poor treatment effect.(Menendez et al., 2008) The prognosis of pneumonia was also affected by many factors such as tumor, kidney disease and cardiovascular event. Pneumonia may be just an early warning signal of the above diseases. As sensitive indicators of systemic inflammation, CRP and IL-6 can reflect the changes of various diseases, so their overall affection for prognosis of pneumonia are usually superior.(Yende et al., 2008) Our research also showed that the areas of CRP and IL-6 under ROC curves were larger than other indicators.
In pulmonary infectious diseases, D-D level is directly related to the coagulation process during acute and chronic lung injury. Serious lung damage of critically ill patients induced excessive activation of fibrinolytic system and more breakdown of fibrin in the alveoli,(Jain et al., 2015; Arslan et al., 2010; Castro et al., 2001) leading to higher D-D level than severe patients. With the increment of D-D, patients with pneumonia may have enhanced inflammatory response in vivo,(Ge et al., 2019) aggravated illness, and poor prognosis.(Querol-Ribelles et al., 2004) So the change of D-D can be used to judge the severity of the disease. Our study showed that in prediction of critical COVID-19, the area under the ROC curve for D-D was even larger than that of PCT, which is consistent with the result of Ge et al.(Ge et al., 2019). However, there was no difference in the proportion of patients with increased D-D in the critically ill group compared with that in the severe group, which indicated the strong sensitivity of D-D in reflecting the variation of the disease.
The initial neutrophil counts of the severe and critical patients were different, but the mean numbers were all within the normal range, which might be because of the stronger stress reaction of the critically ill patients, and the difference in peak level was mostly associated with the secondary bacterial infection and the application of glucocorticoids in critically ill patients, which was also in accordance with the difference of PCT between the two groups. Lymphocytes are the main actors in adaptive immunity, and lymphopenia in CAP, mainly caused by a CD4+ depletion, has been found to be related to a dysregulated immune response with more inflammatory responses.(Mendez et al., 2019) Our study also showed that the inflammatory response of the critically ill group was stronger than that of the severely ill group, and the initial and trough lymphocyte counts in critically ill group were significantly lower than those of the severe group.
Limitations: firstly, the number of samples in this study was relatively small, which still needs to be further confirmed by a large sample study; secondly, only the initial and peak value of indicators were involved, if there were more kinetic values, the relationship between indicators and the change of the disease could be better reflected.
Conclusions: The severe and critical COVID-19 patients had significant differences in concentrations of serum cTnI, D-D, CRP, IL-6, PCT, neutrophil and lymphocyte counts. The increase of cTnI, CRP, IL-6, PCT, neutrophils and decrease of lymphocytes indicated severe condition. The initial IL-6 might be a good indicator for COVID-19 severity.
Author contributions
MW drafted the manuscript, MW, YD, QZ, and LL collected the data, JF analyzed the data, MW and YD designed the study and interpreted the data, MX polished the manuscript.
Declaration of competing interest
None of the authors have any conflict of interest to declare.
References
- Arslan S., Ugurlu S., Bulut G., et al. The association between plasma D-dimer levels and community-acquired pneumonia. Clinics (Sao Paulo). 2010;65(6):593–597. doi: 10.1590/S1807-59322010000600006. 2010-06-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro D.J., Perez-Rodriguez E., Montaner L., et al. Diagnostic value of D dimer in pulmonary embolism and pneumonia. Respiration. 2001;68(4):371–375. doi: 10.1159/000050529. 2001-01-20. [DOI] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. 2020-02-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li X., Chen M., et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. 2020-05-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry A.M., Samra N., Skrupky L.P., et al. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391. doi: 10.1097/SHK.0000000000000234. 2014-11-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frencken J.F., van Baal L., Kappen T.H., et al. Myocardial injury in critically Ill patients with community-acquired pneumonia. A cohort study. Ann. Am. Thorac. Soc. 2019;16(5):606–612. doi: 10.1513/AnnalsATS.201804-286OC. 2019-05-01. [DOI] [PubMed] [Google Scholar]
- Ge Y.L., Liu C.H., Wang N., et al. Elevated plasma D-dimer in adult community-acquired pneumonia patients is associated with an increased inflammatory reaction and lower survival. Clin. Lab. 2019;65:1. doi: 10.7754/Clin.Lab.2018.180720. 2019-01-01. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. 2020-02-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Khera R., Suneja M., et al. Role of D-dimer assays in the diagnostic evaluation of pulmonary embolism. Am. J. Med. Sci. 2015;350(6):501–507. doi: 10.1097/MAJ.0000000000000405. 2015-12-01. [DOI] [PubMed] [Google Scholar]
- Mendez R., Menendez R., Amara-Elori I., et al. Lymphopenic community-acquired pneumonia is associated with a dysregulated immune response and increased severity and mortality. J. Inf. Secur. 2019;78(6):423–431. doi: 10.1016/j.jinf.2019.04.006. 2019-06-01. [DOI] [PubMed] [Google Scholar]
- Menendez R., Cavalcanti M., Reyes S., et al. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax. 2008;63(5):447–452. doi: 10.1136/thx.2007.086785. 2008-05-01. [DOI] [PubMed] [Google Scholar]
- Moammar M.Q., Ali M.I., Mahmood N.A., et al. Cardiac troponin I levels and alveolar-arterial oxygen gradient in patients with community-acquired pneumonia. Heart Lung Circ. 2010;19(2):90–92. doi: 10.1016/j.hlc.2009.08.009. 2010-02-01. [DOI] [PubMed] [Google Scholar]
- Querol-Ribelles J.M., Tenias J.M., Grau E., et al. Plasma d-dimer levels correlate with outcomes in patients with community-acquired pneumonia. Chest. 2004;126(4):1087–1092. doi: 10.1378/chest.126.4.1087. 2004-10-01. [DOI] [PubMed] [Google Scholar]
- Storisteanu D.M., Pocock J.M., Cowburn A.S., et al. Evasion of neutrophil extracellular traps by respiratory pathogens. Am. J. Respir. Cell Mol. Biol. 2017;56(4):423–431. doi: 10.1165/rcmb.2016-0193PS. 2017-04-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F., Cangemi R., Falcone M., et al. Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin. Infect. Dis. 2017;64(11):1486–1493. doi: 10.1093/cid/cix164. 2017-06-01. [DOI] [PubMed] [Google Scholar]
- Vita J.A., Treasure C.B., Yeung A.C., et al. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation. 1992;85(4):1390–1397. doi: 10.1161/01.cir.85.4.1390. 1992-04-01. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. 2020-02-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yende S., D’Angelo G., Kellum J.A., et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 2008;177(11):1242–1247. doi: 10.1164/rccm.200712-1777OC. 2008-06-01. [DOI] [PMC free article] [PubMed] [Google Scholar]