The Lombardy region of Italy was the second epicenter of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after China, with Bergamo province being the most affected area. The Hospital Papa Giovanni XXIII, the tertiary referral center for inflammatory bowel disease (IBD) in the whole province, was reconverted into a coronavirus disease 2019 (COVID-19) point of care, with up to 500 beds dedicated to patients with COVID-19 and more than 1,500 admissions for severe COVID-19 during the early phase of the local epidemic. To prevent SARS-CoV-2 spreading, from March 7, 2020, the Italian government ordered a total lockdown (LD) to confine the viral diffusion. From May 4, 2020, when the basic reproduction number (R0) dropped below 1 in the whole country, the LD was converted into phase 2 (P2), which envisaged a gradual reopening of work activities, provided the maintenance of 1-meter social distancing and the use of face masks.
Previous reports suggested that patients with IBD have an uneventful course during the SARS-CoV-2 pandemic, including those on biologic treatment.1 , 2 Nonetheless, the rate of exposure to SARS-CoV-2 of these patients is currently unknown.
This study was designed to assess the seroprevalence of COVID-19 in patients with IBD undergoing biologic treatment during the main phase of the local epidemic.3 Furthermore, we investigated the risk factors for SARS-CoV-2 infection in this cohort of patients.
Patients and Methods
This study includes all patients with IBD (children and adults) undergoing biologic therapy (infliximab, adalimumab, golimumab, ustekinumab, and vedolizumab) followed at our center during the Italian COVID-19 outbreak.
The time period of enrollment was divided into 2 phases: LD (March 4–May 3) and P2 (May 4–July 10).
During LD, all patients were advised to continue their regular treatment and were screened for SARS-CoV-2 infection in the case of suspicious clinical symptoms or contact with ascertained infected patients.
From the beginning of P2, all patients were offered a rapid serologic test (VivaDiag COVID-19 immunoglobulin (Ig) G/IgM rapid lateral flow qualitative immunoassay, VivaChek Biotech, Hangzhou, China), performed by adding 10 μL of plasma and 2 drops of dilution buffer into the sample port. After 15 minutes, the results were evaluated by the same laboratory operator, who was unaware of the patient’s clinical and biochemical information.
The protocol was approved by the local ethical committee (protocol 141-20).
Results
A total of 103 patients with IBD on biologic treatment followed at our institution at the time of the study initiation completed a questionnaire exploring the presence of symptoms suggestive of SARS-CoV-2 infection as well as recent contacts with ascertained COVID-19 patients. Among them, 13 (12.6%) were younger than 18 years. All SARS-CoV-2–related symptoms reported by the patients were mild, and none required hospitalization for COVID-19 (Supplementary Table 1). Among them, 90 patients (87%) performed the serologic test, and 19 had positive results for IgG, IgM, or both. This resulted in a SARS-CoV-2 seroprevalence of 21%.
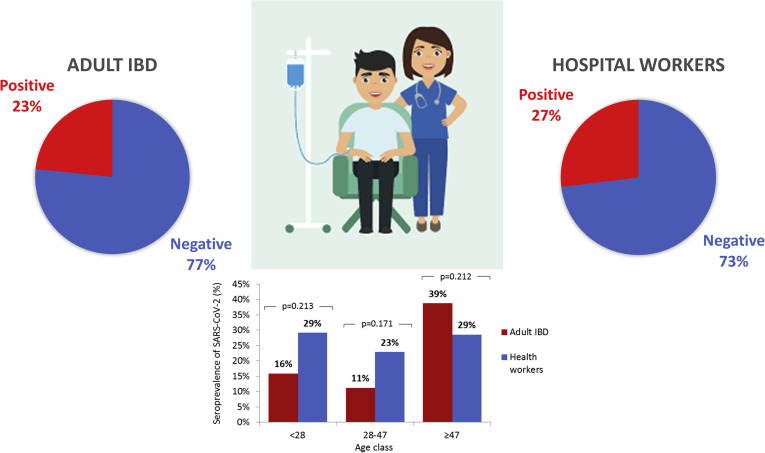
The comparison with a control group of health care personnel showed no significant difference in terms of global and age-stratified seroprevalence (P > .05) (Supplementary Figure 1).
Supplementary Figure 1.
Comparison of the seroprevalence of SARS-CoV-2 between patients with IBD and the health care personnel of the Hospital Papa Giovanni XXIII. Data of seroprevalence in adult patients with IBD were compared with those of 4,395 health care workers screened between May and July 2020.
Remarkably, in univariate analysis, female sex, older age, and presence of reported symptoms were associated with a significant increased rate of positive serology (Table 1 ). The multivariate analysis confirmed the protective role of male sex, and older age had a 5-fold increased risk of positive serology result compared to younger age (47 years [older] vs 28 years [younger]). No positive pediatric cases were recorded. Eleven (58%) patients with a positive serology result reported no symptoms during the study period. Two patients, who had persistent fever and were eventually found to be positive on serology testing, underwent chest computed tomography that returned negative results for pulmonary involvement.
Table 1.
Characteristics of Patients With IBD Undergoing Biologic Therapy According to Serology Test Results
| Characteristics | n | Serology test results |
P value | |
|---|---|---|---|---|
| Negative (n = 71) | Positive (n = 19) | |||
| Sex, n (%) | ||||
| Female | 90 | 29 (40.8) | 13 (68.4) | .032a |
| Male | 42 (59.2) | 6 (31.6) | ||
| Age, y, median (IQR) | 90 | 34.0 (19.0–47.0) | 50.0 (28.0–57.0) | .020a |
| <28a | 28 (39.4) | 4 (21.1) | .019a | |
| 28–47a | 24 (33.8) | 3 (15.8) | ||
| ≥47a | 19 (26.8) | 12 (63.2) | ||
| IBD type, n (%) | ||||
| Ulcerative colitis | 90 | 30 (42.3) | 5 (26.3) | .21 |
| Crohn’s disease | 41 (57.7) | 14 (73.7) | ||
| Biologic therapy, n (%) | ||||
| Anti-TNF, n (%) | 90 | 59 (83.1) | 15 (78.9) | 0.67 |
| Adalimumab | 30 (42.3) | 10 (52.6) | .42 | |
| Infliximab | 28 (39.4) | 5 (26.3) | .29 | |
| Golimumab | 1 (1.4) | 0 (0.0) | .99 | |
| Anti-integrin (vedolizumab) , n (%) | 90 | 8 (11.3) | 1 (5.3) | .68 |
| Anti-IL12/23 (ustekinumab) , n (%) | 90 | 4 (5.6) | 3 (15.8) | .16 |
| Monotherapy/combination therapy, n (%) | 90 | 13 (18.3) | 5 (26.3) | 0.44 |
| Contact, n (%) | 86 | 2 (3.0) | 2 (10.5) | 0.21 |
| Symptoms, n (%) | 87 | 4 (5.9) | 8 (42.1) | <.001a |
| Fever | 12 | 2 (50.0) | 7 (87.5) | 0.24 |
| Cough | 12 | 3 (75.0) | 3 (37.5) | 0.55 |
| Dysgeusia/anosmia | 12 | 0 (0.0) | 5 (62.5) | .081 |
NOTE. Data are expressed as column percentages. Percentages may not reach 100% because of rounding
IL, interleukin; IQR, interquartile range; TNF, tumor necrosis factor.
Tertiles of age distribution.
Discussion
After more than 2 months from the first COVID-19 case in our region, we found that nearly one quarter of adult patients with IBD treated with biologics has been exposed to SARS-CoV-2 infection and that this figure is comparable to that of health care personnel working in their tertiary referral center. This suggests that the previously described uneventful course of IBD patients was not due to sheltering from the infection.1
More than half of patients with positive SARS-CoV-2 serology results were completely asymptomatic during LD, and the others reported only mild symptoms. All biologic treatments were safely administered, even at high doses for the induction of remission. In this cohort, immunosuppressive treatment did not affect SARS-CoV-2 infection outcome.
Preliminary data suggest that immunosuppression is not an additional risk factor for the development of severe forms of SARS-CoV-2 infection, because it was also shown for the previous coronaviruses pandemics, SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome).4
The only study assessing COVID-19 prevalence in patients with IBD was conducted in California5 and reported a 3% prevalence of disease, which was comparable to that of the general population. This retrospective study included patients who underwent SARS-CoV-2 nasopharyngeal testing mainly because of COVID-19–related symptoms; therefore, it cannot provide information on the rate of exposure to the infection of patients with IBD. Preliminary data of SARS-CoV-2 serology screening carried out by the Italian government found a 24% prevalence in the general population of the Bergamo province (https://www.istat.it/it/files//2020/08/ReportPrimiRisultatiIndagineSiero.pdf), which is comparable to the prevalence we found in our patients with IBD and health care personnel.
The high prevalence of patients with IBD having an asymptomatic course after SARS-CoV-2 infection is a matter of concern when it comes to scheduling hospital admissions for infusions, follow-up visits, or endoscopy in regions hit by SARS-CoV-2 and suggests the need for preadmission screening tests to prevent intrahospital dispersal of the virus. Previous findings suggesting nonadherence to biologic treatment6 are not confirmed in our cohort, where only 1 patient delayed the infusion because of fear of attending the hospital during the epidemic.
COVID-19 is mediated by an important cytokine storm.7 In COVID-19 patients, plasma concentrations of several cytokines, including tumor necrosis factor α, were remarkably increased compared to healthy control individuals, especially in those with more severe disease.8 According to that evidence and our findings, drugs aiming at blocking tumor necrosis factor α and interferon γ signaling pathways, if not beneficial, appear to be not harmful during SARS-CoV-2 infection.
Acknowledgments
The authors acknowledge the following collaborators: Salvatore Greco, MD, and Arianna Ghirardi.
CRediT Authorship Contributions
Lorenzo Norsa, MD, PhD (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Writing – original draft: Lead); Paola Cosimo, PhD (Conceptualization: Equal; Data curation: Equal); Amedeo Indriolo, MD (Data curation: Equal; Formal analysis: Equal); Naire Sansotta, MD (Data curation: Equal; Investigation: Equal; Validation: Equal); Lorenzo D’Antiga, MD (Conceptualization: Supporting; Supervision: Equal; Validation: Equal; Writing – review & editing: Lead); Annapaola Callegaro, MD (Conceptualization: Lead; Investigation: Lead; Methodology: Lead; Supervision: Lead; Validation: Lead; Writing – review & editing: Lead).
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at https://doi.org/10.1053/j.gastro.2020.08.046.
Supplementary Material
Supplementary Table 1.
Characteristics of the Enrolled Cohort
| Characteristics | Value |
|---|---|
| Total number | 103 |
| Female sex, n (%) | 50 (48) |
| Age, y, median (range) | 40 (22–50) |
| Disease, n (%) | |
| Crohn’s disease | 63 (61) |
| Ulcerative colitis | 40 (39) |
| Biologic treatment, n (%) | |
| Infliximab | 36 (35) |
| Adalimumab | 44 (43) |
| Vedolizumab | 12 (11) |
| Ustekinumab | 8 (8) |
| Golimumab | 3 (3) |
| Concomitant immunosuppressive treatment, n (%) | |
| Prednisone | 2 (2) |
| Azathioprine | 21 (20) |
| Treatment regimen, n (%) | |
| Induction | 11 (11) |
| Regular | 88 (85) |
| Delayed | 4 (4) |
| COVID-19 reported symptoms, n (%) | 13 (13) |
| Fever | 10 (9) |
| Cough | 6 (6) |
| Dysgeusia/anosmia | 5 (5) |
| Contacts with COVID-19–ascertained patients, n (%) | 4 (4) |
References
- 1.Norsa L. Gastroenterology. 2020;159:371–372. doi: 10.1053/j.gastro.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner E.J. Gastroenterology. 2020;159:481–491. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J. Int Immunopharmacol. 2020;88:106861. doi: 10.1016/j.intimp.2020.106861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 5.Gubatan J. Gastroenterology. 2020;159:1141–1144. doi: 10.1053/j.gastro.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan N. Gastroenterology. 2020;159:1592–1594.e1. doi: 10.1053/j.gastro.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]