Abstract
PURPOSE
Liquid biopsy specimen genomic profiling is integrated in non–small-cell lung cancer (NSCLC) guidelines; however, data on the clinical relevance for ALK/ROS1 alterations are scarce. We evaluated the clinical utility of a targeted amplicon-based assay in a large prospective cohort of patients with ALK/ROS1-positive NSCLC and its impact on outcomes.
PATIENTS AND METHODS
Patients with advanced ALK/ROS1-positive NSCLC were prospectively enrolled in the study by researchers at eight French institutions. Plasma samples were analyzed using InVisionFirst-Lung and correlated with clinical outcomes.
RESULTS
Of the 128 patients included in the study, 101 were positive for ALK and 27 for ROS1 alterations. Blood samples (N = 405) were collected from 29 patients naïve for treatment with tyrosine kinase inhibitors (TKI) or from 375 patients under treatment, including 105 samples collected at disease progression (PD). Sensitivity was 67% (n = 18 of 27) for ALK/ROS1 fusion detection. Higher detection was observed for ALK fusions at TKI failure (n = 33 of 74; 46%) versus in patients with therapeutic response (n = 12 of 109; 11%). ALK-resistance mutations were detected in 22% patients (n = 16 of 74) overall; 43% of the total ALK-resistance mutations identified occurred after next-generation TKI therapy. ALK G1202R was the most common mutation detected (n = 7 of 16). Heterogeneity of resistance was observed. ROS1 G2032R resistance was detected in 30% (n = 3 of 10). The absence of circulating tumor DNA mutations at TKI failure was associated with prolonged median overall survival (105.7 months). Complex ALK-resistance mutations correlated with poor overall survival (median, 26.9 months v NR for single mutation; P = .003) and progression-free survival to subsequent therapy (median 1.7 v 6.3 months; P = .003).
CONCLUSION
Next-generation, targeted, amplicon-based sequencing for liquid biopsy specimen profiling provides clinically relevant detection of ALK/ROS1 fusions in TKI-naïve patients and allows for the identification of resistance mutations in patients treated with TKIs. Liquid biopsy specimens from patients treated with TKIs may affect clinical outcomes and capture heterogeneity of TKI resistance, supporting their role in selecting sequential therapy.
Context
Key Objective
To determine if liquid biopsy specimens are clinically relevant in patients with ALK- and ROS1-positive advanced NSCLC.
Knowledge Generated
In a multicenter cohort of 128 patients with ALK- and ROS1-positive advanced NSCLC, an amplicon-based ctDNA next-generation sequencing (NGS) liquid biopsy was feasible and clinically relevant; 67% of ALK and ROS1 fusions were detected in liquid biopsy specimens at diagnosis, and ALK- and ROS1-resistance mutations were identified in 22% of patients at TKI failure. This increased to 29% of patients at progression to second-generation TKI therapy.
Relevance
This amplicon-based, NGS liquid biopsy can enable molecular diagnosis for ALK/ROS1 fusions from specimens at diagnosis and, in patients treated with TKIs, allows for the identification of resistance mutations that may influence treatment selection and clinical outcomes.
INTRODUCTION
Since the discovery of driver oncogenic alterations in non–small-cell lung cancer (NSCLC), the treatment landscape has grown exponentially. Specific tyrosine kinase inhibitors (TKIs) targeting different alterations have impressively improved outcomes of patients with advanced NSCLC compared with chemotherapy.1,2 Molecular testing is recommended at the time of diagnosis3 and it should be also considered at disease progression (PD) in patients receiving TKI treatment to assess the resistance mechanisms that may support the selection of subsequent therapies. Tumor biopsy is the preferred approach for molecular testing, but in up to 30% of cases, the tissue quality is inadequate.4,5 Analysis of circulating tumor DNA (ctDNA) liquid biopsy specimens provides a noninvasive surrogate material for detecting somatic mutations and is currently integrated in NSCLC clinical guidelines.6,7
ALK and ROS1 fusions occur in approximately 5% and 2%, respectively, of cases of advanced NSCLC.8 Highly selective TKIs have enlarged the therapeutic arsenal. In patients positive for ALK, crizotinib9 was the first TKI approved as frontline treatment, but second-generation TKIs (ie, alectinib, ceritinib, or brigatinib)2 have improved frontline clinical outcomes, displacing crizotinib from the first-line TKIs, and third-generation TKIs, such as lorlatinib, with high activity in the crizotinib-resistance setting,10 are being tested as first-line therapy (ClinicalTrials.gov identifier: NCT03052608). Unfortunately, PD remains inevitable. ALK mutations are one of the main mechanisms of resistance to TKI, identified in up to 30% of the cases at PD after crizotinib therapy.11 Second- and third-generation TKIs can overcome resistance to crizotinib,12 and a sequential strategy has been established as the standard of care after TKI failure.3,13-15 Similarly, ROS1-positive NSCLC can develop crizotinib-resistance mutations in the ROS1 kinase domain, conferring variable degrees of sensitivity or resistance to next-generation TKIs.16-18 However, each TKI has a different spectrum of coverage for resistance mutations.12 Thus, detecting specific resistance mutations may influence the choice of the TKI sequence.
Liquid biopsy already provides an alternative option to tissue for molecular profiling in treatment-naïve patients with NSCLC.19 Although a few studies have recently reported the feasibility of using liquid biopsy specimens for profiling patients positive for ALK20-22 and ROS123 alternations, larger, real-life, prospective cohorts are needed to assess the relevance of this strategy.
Here, we evaluated the clinical utility of targeted, amplicon-based, NGS liquid biopsy in a large prospective cohort of patients positive for ALK/ROS1. Also, we assessed the clinical relevance of the detection of ALK/ROS1 fusions and resistance mutations on clinical outcomes and explored ctDNA as a potential predictive biomarker for efficacy of sequential TKIs.
PATIENTS AND METHODS
Study Population
Patients ≥ 18 years old with ALK- and ROS1-fusion–positive advanced NSCLC were prospectively enrolled between October 2015 and August 2018 at Gustave Roussy (CEC-CTC study no. 2008-A00585-50), at Centre Léon Bérard, and six other French institutions (LIBIL study, ClinicalTrials.gov identifier: NCT02511288). All patients provided written informed consent for biomedical research and the institutional ethics committees approved the protocol. ALK or ROS1 fusion was determined by a validated test on tumor tissue (Data Supplement).
Sample Collection and ctDNA Analysis
Prospective samples were collected at any time point at diagnosis and/or at each disease radiologic evaluation (under response by RECIST v1.125 v progression). Patients receiving therapy with no previous sample collected at diagnosis were also enrolled; samples were collected at each radiologic evaluation. In blood samples, plasma was isolated and ctDNA analysis was centralized (Inivata, Cambridge, UK, and Research Triangle Park, NC) using InVisionFirst-Lung, which identifies single nucleotide variants, insertions and deletions, copy number variations, and fusions, with whole-gene and gene hotspots across a 36-gene panel (Data Supplement). Methods were as previously described24 (Data Supplement). Fusion load was evaluated using fusion reads normalized to control single nucleotide polymorphism primers across all replicates at each time point. Relative change in fusion load was calculated between time 0 (T0) and subsequent time points (TX) to give an estimate of fusion load over time relative to T0.
Statistical Analysis
Outcomes assessment is summarized in the Data Supplement. Survival curves were estimated with the Kaplan-Meier method and were compared by the log-rank test. All P values were two-sided and values < .05 were considered statistically significant.
The presence of one ALK mutation was defined as “single ALK,” the presence of two or more ALK resistance mutations was defined as “complex ALK”; the presence of other somatic mutations was defined as “others,” “non-ALK,” or non-ROS1,” and the absence of mutations in blood, included in the 36-gene panel, was defined as “negative ctDNA.” The prognostic value was measured in univariate analysis. For sensitivity analysis, referring to tissue data as the standard, the sensitivity was defined as true positive divided by the sum of true-positive and false-negative results.
Data were processed and analyzed using SPSS software, version 25.0.0 (IBM Corp., Armonk, NY). The numbers of all included patients and recorded variables were reported using descriptive statistics and the relationship between clinical characteristics and response was determined by Fisher exact test.
RESULTS
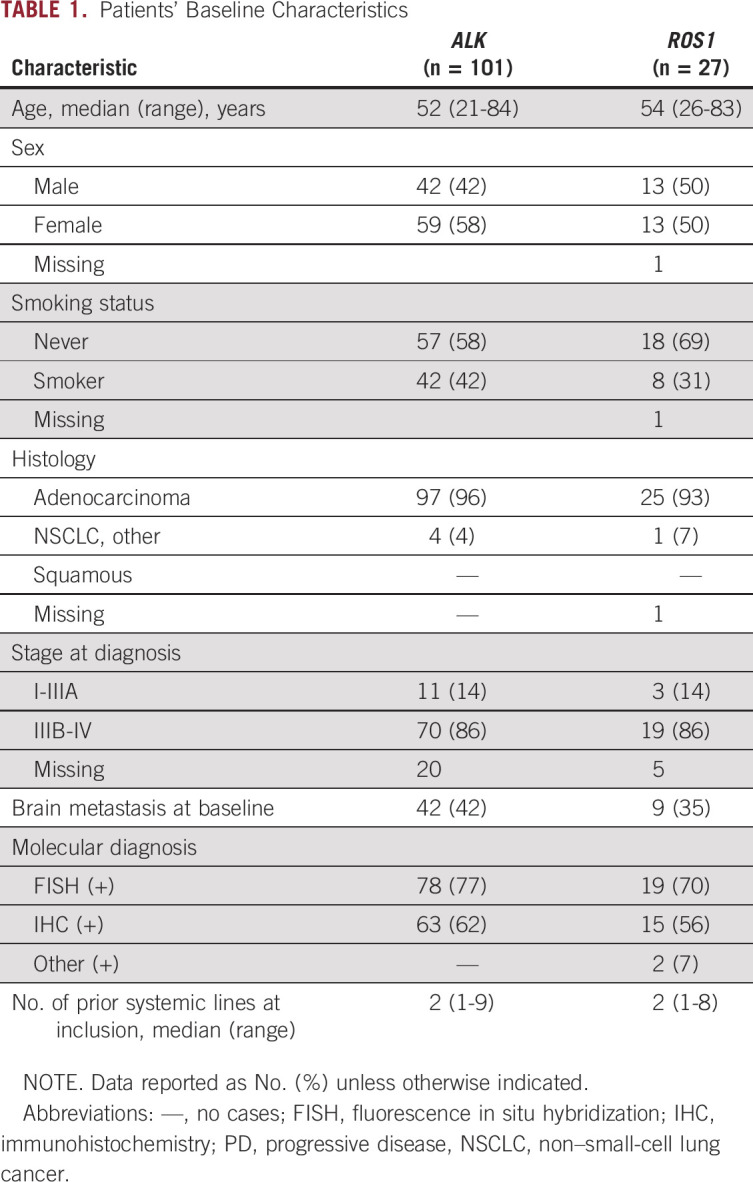
Of 128 enrolled patients, 101 had ALK-positive and 27 ROS1-positive NSCLC. Baseline characteristics are summarized in Table 1. Overall, 404 blood samples were collected: 29 in TKI-naïve patients (25 with ALK fusions, four with ROS1 fusions) and 375 in patients receiving treatment (Data Supplement). A median of two samples was collected per patient (range, 1 to 13 samples).
TABLE 1.
Patients’ Baseline Characteristics
Detection of ctDNA ALK/ROS1 Fusions
Treatment-naïve cohort.
Twenty-seven samples were eligible for analysis. In 18 patients, the fusion was detected in blood (n = 16 patients with ALK; n = 2 with ROS1), with a sensitivity of 67% for ALK and ROS1 fusion (Data Supplement). In the ALK cohort, eight variant 1, two variant 2, and six variant 3 fusions were detected. In the ROS1 cohort, one CD74-ROS1 and one SLC34A2-ROS1 fusion were detected.
Fusion detection in blood was associated with a high number of metastatic sites and visceral involvement (Data Supplement). The metastatic pattern was not related to ALK variants or ROS1 partner gene. Plasma NGS was performed on 25 samples, of which 11 (44%) had concurrent gene aberrations: TP53 mutation (24%; n = 6), followed by NRAS, STK11, and CDKN2A mutations, and EGFR amplification (Data Supplement). One patient had an ALKL1196Q mutation at diagnosis.
Radiologic response.
A total of 143 samples collected at the time of confirmed objective response by RECIST, version. 1.1, were evaluable for fusion analysis (ALK, n = 109; ROS1, n = 34). Fusions were detected in 14 samples (10%): 12 of 109 (11%) for patients with ALK and two of 34 (6%) for ROS1 patients (Data Supplement).
A total of 121 samples were collected at the time of PD on systemic therapies (eg, chemotherapy, TKIs; ALK, n = 96; ROS1, n = 15). Among them, 74 were collected at TKI failure, with a detection rate of 45% (n = 33 of 74) for ALK and 30% (n = 3 of 10) for ROS1 fusions (Data Supplement). The detection rate was higher in patients with visceral and bone metastases (Data Supplement).
Detection of ctDNA Mutations at TKI Failure
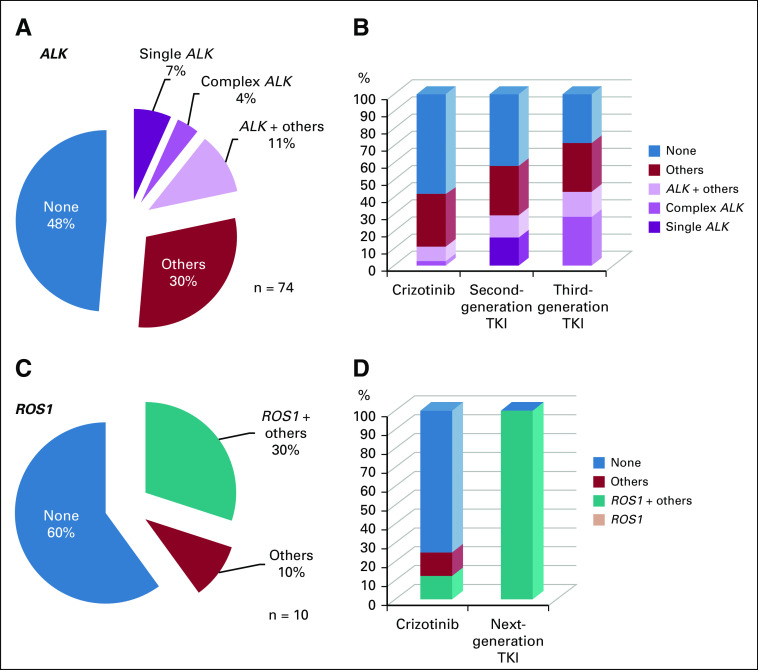
ALK mutations were detected in 22% of samples (n = 16 of 74) collected at TKI failure, including five samples with single ALK mutations, three with complex ALK mutations, and eight with ALK and other genes mutations (Fig 1A).
FIG 1.
Somatic mutations detected in liquid biopsies at progressive disease to tyrosine kinase inhibitor (TKI; A, C) and according to exposure to prior TKI (B, D) in ALK (A, B) and ROS1 (C, D) positive patients.
ALK mutations were more frequently detected in patients with bone or liver progression (75% to 80%) compared with exclusive CNS or thoracic progression (10%). Detection of ALK mutations at isolated CNS relapse was 10% (n = 3 of 29); 0% after crizotinib (n = 0 of 11) versus 18% after therapy with a next-generation TKI (n = 3 of 17). In 55%, no mutations were detected (n = 16 of 29). ALK variant 3 was associated with ALK mutations (37%; n = 6 of 16), compared with variant 2 (13%; n = 2 of 16) and variant 1 (none).
At PD, ALK mutations with third-generation TKIs were detected in 43% (n = 7) of samples compared with 29% in samples from patients treated with second-generation TKIs (n = 31) and 11% with crizotinib (n = 36; Fig 1B). ALK G1202R was detected as a single mutation (n = 3) or concurrent with other ALK mutations (n = 4) and was the most common resistance mutation (n = 1 after crizotinib therapy; n = 6 after treatment with next-generation TKIs).
Non-ALK mutations were detected in 41% of samples (n = 30 of 74); 27% (n = 8 of 30) were concurrent with ALK mutations. TP53 was the most common (n = 26 of 74), 54% (n = 14 of 26) as the unique mutation, 27% (n = 7 of 26) concurrent with ALK mutations, and in 19% of samples (n = 5 of 26) associated with other mutations. Other mutations detected included KRAS plus PI3KCA, PTEN plus PI3KCA, MET, STK11, and CDKN2A (Data Supplement).
In 21 patients, 57 longitudinal samples during sequential therapies were available. In five patients, de novo ALK mutations emerged after TKI (Data Supplement).
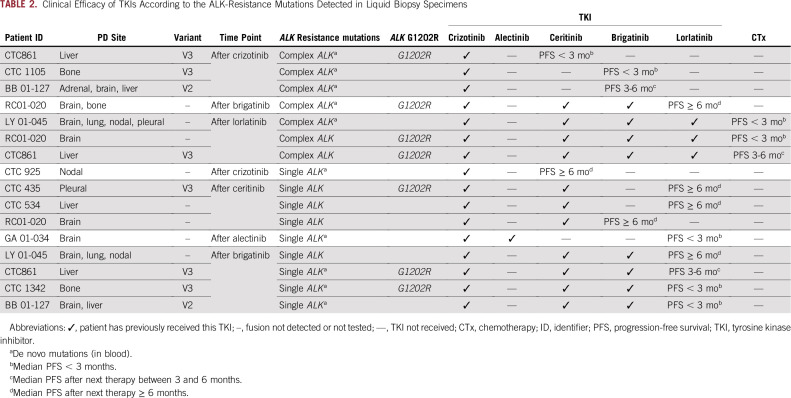
Paired tissue and liquid biopsy specimens with resistance mutations were available in six cases (in all cases, the ALK fusion was confirmed in tissue samples, Table 2). The three cases with single ALK mutation had 100% tissue- and liquid-sample concordance (n =2 ALK G1202R; n =1 ALK L1196M). The other three cases had complex ALK mutations, one was concordant between tissue and liquid biopsy specimens (ALK F1174V and ALK L1198F). However, some discordance was observed in two cases: one case with ALK G1202R plus ALK E1154K in the tissue biopsy specimen and ALK G1202R plus ALK I1268V in the liquid biopsy specimen; and one case with more mutations detected in the liquid biopsy specimen (tissue: ALK G1202R plus ALK F1174L; liquid: ALK G1202R plus ALK F1174L plus ALK C1156Y plus ALK G1269A plus ALK S1206F plus ALK T1151M).
TABLE 2.
Clinical Efficacy of TKIs According to the ALK-Resistance Mutations Detected in Liquid Biopsy Specimens
In the ROS1 cohort, among the 10 samples collected at the time of PD, three (30%) had the ROS1 G2032R-resistance mutation. All cases had concurrent mutations (CTNNB1, TP53, and TP53 plus CDKN2A). The ctDNA somatic mutations evidenced at TKI failure are depicted in Figure 1C and 1D. Three patients had sequential samples for assessing the emergence of mutations; in one case, we observed the emergence of ROS1 mutation at crizotinib failure (SLC34A2-ROS1 fusion; Data Supplement).
Clinical Outcomes in ALK-Positive Patients According to Liquid Biopsy Specimens
The absence of mutations in ctDNA was associated with improved overall survival (OS; n = 74 samples; n = 55 patients; Fig 2). The median OS was 58.5 (95% CI, 26.9 to not reached [NR]) months if one or more ALK mutations were detected versus 44.1 (95% CI, 21.7 to NR) months if non-ALK mutations were detected versus 105 (95% CI, 105.7 to NR) months in patients negative for ctDNA (P = .001). This effect was observed regardless of the number of lines of TKI received (more than TKIs v two or fewer TKIs; P = .01). This observation also held true when we assessed this outcome in the population exclusively treated with TKI as first-line therapy (n = 29; P = .04). The patients’ characteristics according to the ctDNA mutations are described in the Data Supplement.
FIG 2.
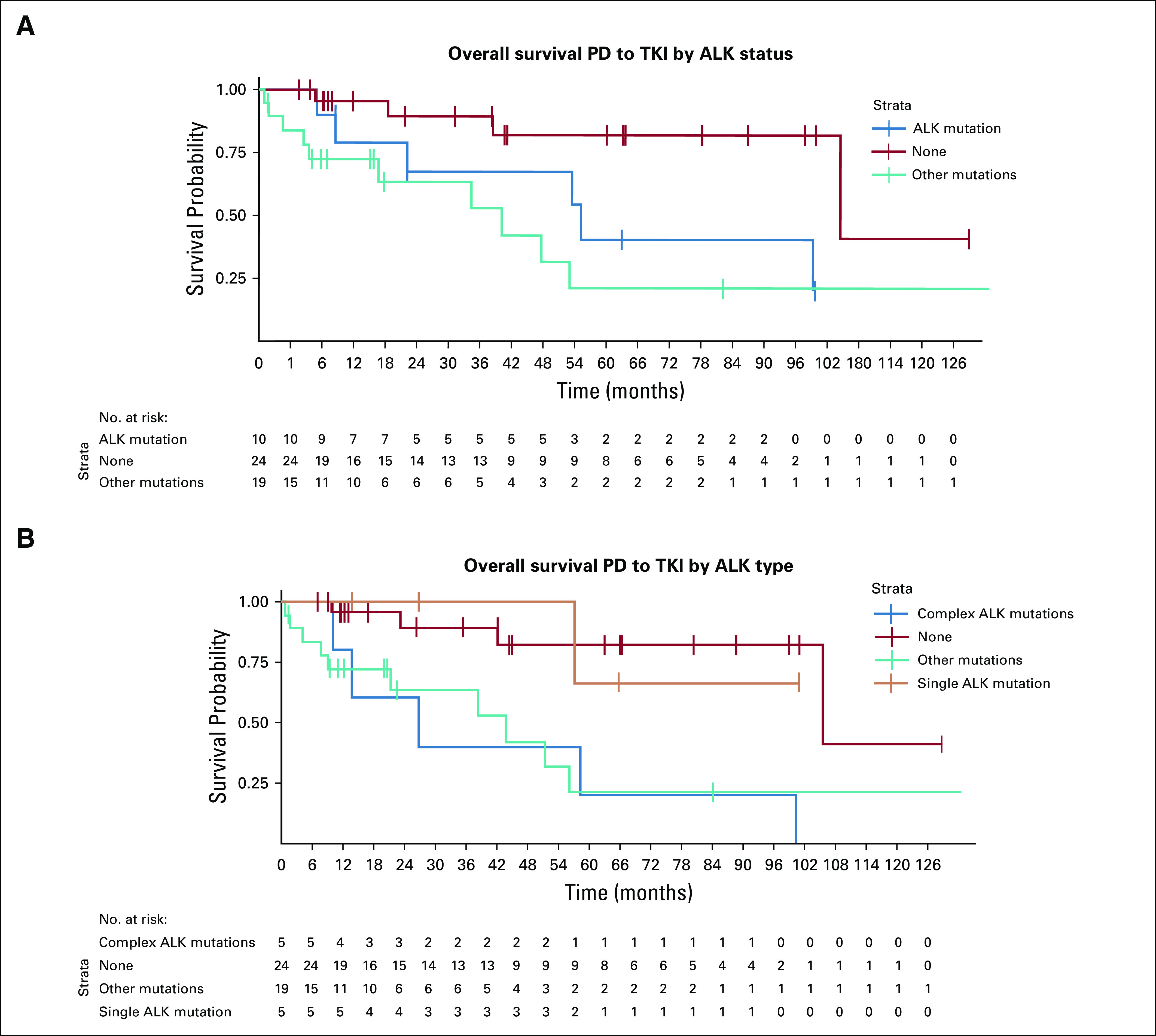
Overall survival according to the presence of ALK resistance mutations or other mutations in the liquid biopsy in ALK positive population. (A) PD while receiving TKI therapy, by ALK status. (B) PD while receiving TKI therapy, by ALK type. PD, disease progression; TKI, tyrosine kinase inhibitor.
The presence of complex ALK mutations was associated with poor OS (median, 26.9 months; 95% CI, 13.9 months to NR) compared with single ALK mutation (median, NR; 95% CI, 57.0 months to NR; P = .003). This effect was also observed in the subgroup treated with upfront TKI (P = .038). The median OS in the four patients with emergence of ctDNA ALK G1202R was 59.5 (95% CI, 26.9 to NR) months.
The group with absence of ctDNA mutations had a median progression-free survival (PFS) to the therapy on which their disease progressed of 14.8 (95% CI, 8.1 to 23.1) months versus 9.6 (95% CI, 6.6 to 19.9) months if there was one or more ALK mutation or 7.8 (95% CI, 4.5 to 11.7) months if there were non-ALK mutations at TKI failure (P = .31). The median PFS of the four cases with emergence of ALK G1202R was 2.7 (95% CI, 2.03 to NR) months versus 8.6 (95% CI, 5.6 to 10.6) months in the remaining population (P = .05).
We then studied the PFS to the subsequent therapy according to ctDNA mutations detected in 56 samples. The median PFS was 20.7 (95% CI, 6.3 to NR) months in the negative ctDNA group versus 8 (95% CI, 2.8 to NR) months for non-ALK mutations versus 2.8 (95% CI, 1.2 to NR) months in the group with one or more ALK mutation detected (P = .03).
We further explored the PFS specifically in the subgroup harboring ALK mutations (n = 16; Data Supplement; Table 2). The ALK complex mutations were associated with poor efficacy, with a median PFS of 1.7 (95% CI, 0.9 to NR) months. In contrast, the ALK single mutation was more commonly associated with longer PFS, with a median of 6.3 (95% CI, 1.8 to NR) months (P = .003). The median PFS to the sequential therapy of the four cases with emergence of ALK G1202R was 3.7 (95% CI, 1.2 to NR) months versus 8.3 (95% CI, 4.9 to NR) months in overall population (P = .15).
The ctDNA dynamics were evaluated at TKI baseline and longitudinally in 34 patients. See the Data Supplement for a representation of the association between the fusion and the highest allelic frequency of any mutation detected in blood in ALK-positive patients and therapeutic response. The detection of fusion in blood and higher level of allelic frequency were correlated with PD at the time of response assessment.
The fusion load was calculated in nine patients. The clearance of the fusion was well correlated with the clearance of other somatic mutations detected in the same sample (Data Supplement) in response to therapy.
DISCUSSION
Herein, we report the clinical relevance of a targeted, amplicon-based NGS assay in a large, prospective, real-world cohort of 128 ALK/ROS1-positive patients. At the time of diagnosis, the sensitivity was 67%. At progression, ALK- and ROS1-resistance mutations were reported in 22% versus 30% of patients, respectively. We also describe the clinical relevance of liquid biopsy specimens on patient outcomes and the potential role of ctDNA as a predictive biomarker.
In our cohort, the sensitivity of amplicon-based NGS for the detection of ALK fusion was in line with the limited data reported to date on other blood-based approaches, such as hybrid-capture liquid biopsy (55%),26 RNA exosomes (63%),27 and reverse transcriptase polymerase chain reaction in platelets (65%).27 In the treatment-naïve population with ROS1, we found a sensitivity of 67%; a single study recently reported a detection rate of 50% in plasma at the time of PD, with no data for treatment-I patients.23 Sensitivity was correlated with higher number of metastatic sites and visceral involvement, reflecting the impact of tumor burden. Our sensitivity data are comparable with the detection rate of other mutations, for which liquid biopsy specimens are routinely used in cases where adequate tissue is unavailable.26
We showed the potential of ctDNA as a surrogate biomarker for therapeutic response, including a novel method for evaluating the relative fusion change: ALK/ROS1 fusion detection was higher in patients at TKI failure than in those whose disease was responding to TKI treatment (49% v 11%). ctDNA clearance was correlated to radiologic response, which should be further explored.
Concurrent non-ALK mutations were associated with poor outcomes, consistent with reports from studies using tissue testing.28,29 This emphasizes the clinical relevance of comprehensive genomic profiling to test for fusion and also mutations in other genes. Interestingly, we reported an ALK mutation at diagnosis that is typically detected at TKI failure; this has been previously reported in preclinical models.30 Its impact on clinical outcome remains unknown.
We found ALK mutations in 22% of samples at TKI failure, which is comparable to the 24% presented by Shaw et al.35 However, this is lower than the 30% reported in tissue12 and 50% in liquid biopsy specimens in smaller cohorts of patients treated with second-generation TKIs.20,21
As previously reported, we detected more ALK-resistance mutations after next-generation TKIs (≤ 43%). Interestingly, the complex ALK mutations, potentially associated with compound mutations after sequential exposure to TKIs,31 were detected in 45% of samples after crizotinib therapy and more likely were related to a polyclonality PD.32 In addition, liquid biopsy specimens can reveal molecular heterogeneity, particularly in cases of complex ALK mutations that were not observed in tissue testing.
The ALK G1202R mutation was the most commonly detected (44%), generally after second-generation TKI, but also seen after crizotinib. ALK-resistance mutations were most frequently associated with ALK variant 3, including all cases of ALK G1202R, as previously reported from tissue biopsy specimens.33 We observed the emergence of some de novo mutations in longitudinal analyses during sequential TKI therapy, including the emergence of ALK G1202R after crizotinib, or the accumulation of ALK mutations during sequential TKI therapy, as reported by Yoda et al.31 We identified ROS1-resistance mutations in 30% of cases, comparable to the 33% recently reported by Dagogo-Jack et al.23
At TKI failure, no mutation was detected in approximately half of the liquid biopsy specimens, as previously reported34,35; this can be related to the sites of metastatic disease (ie, in case of isolated CNS or thoracic PD), with lower detection rate reported.36 Systemic bone and liver PD was associated with higher detection than was isolated CNS or thoracic PD (≤ 80% v 10%). Thus, the pattern of metastatic disease should be considered when interpreting data from liquid biopsy specimens in the context of resistance. More prospective data are required to draw definitive recommendations.
Other non-ALK and non-ROS1 mutations were detected in 30% of ALK and 10% of ROS1 samples at resistance. One hypothesis is that they could represent bypass mechanisms in some cases, reported as the second main cause of TKI resistance.12,37 KRAS, PI3KCA, or PTEN mutations identified potentially could be related to bypass mechanisms.
In an exploratory analysis in the ALK population, the absence of ctDNA mutations was associated with improved outcomes; this could be related to a lower tumor burden or a less heterogeneous tumor. In contrast, the complex ALK mutations were associated with the worst survival outcome, probably reflecting polyclonal and resistant tumors or compound mutations. In the ROS1 population, similar findings were observed. Patients with absence of ctDNA mutations at TKI failure had an improved outcome, and all patients with ROS1 G2032R experienced rapid progression (< 3 months) to subsequent TKI therapy.
Our study has some limitations. Although the amplicon-based NGS approach used is highly sensitive for mutations, it is limited to the detection of known fusion partners, which cover 90% to 95% of the ALK/ROS1 population.38 Second, the sample size is limited and the heterogeneity of the samples collected in different time points is high, though, to our knowledge, it is the largest, real-world, prospective cohort of ALK/ROS1 studied. In addition, in the ALK population, only 51% of the samples were collected after next-generation TKI, the current standard of care; however the information derived from cases after crizotinib therapy was also informative (ie, detection of ALK G1202R, uncommon after crizotinib therapy, and complex mutations that did not respond to the next TKI). Finally, patients were included either as they were diagnosed with advanced NSCLC or at any time of treatment, which may be associated with a bias in recruitment of patients with long survival. Prospective clinical trials are required in context of next-generation TKI therapies39 that showed promising activity against the ALKG1202R and ROS1G2032R mutations, which are resistant to the majority of other available TKIs.
In conclusion, our clinical experience of an amplicon-based, NGS liquid biopsy in a large, real-world, prospective cohort of ALK/ROS1-positive patients with NSCLC provide evidence of the clinical utility of this approach at the time of diagnosis as well as at the time of PD for detection of resistance mutations. Liquid biopsy specimens in TKI-treated patients capture heterogeneity of TKI resistance, supporting the role of liquid biopsy in selecting sequential therapy.
ACKNOWLEDGMENT
We thank the team of the Liquid Biopsy Program at Gustave Roussy and the secretary team of the Thoracic Oncology Group for the support, as well as the Biobank at Centre Léon Bérard (BB-0033-00050) and the CLB Clinical Research Team.
Presented at American Association for Cancer Research Annual Meeting 2018, Chicago, IL, April 14-18, 2018; ASCO Annual Meeting 2018, Chicago, IL, June 1-5, 2018; 19th World Congress on Lung Cancer, Toronto, ON, Canada, September 23-26, 2018; ASCO Annual Meeting 2019, Chicago, IL, May 31-June 4, 2019.
Supported by funding from the Ligue Nationale contre le Cancer (Drôme and Puy-de-Dôme Committees; S.O.-C.), the National Cancer Institute (INCa PRTK-17-154; P.S.), Aviesan ITMO Cancer (18CN044-00; P.S.), the Integrated Cancer Research Site LYriCAN (INCa-DGOS-Inserm_12563), and a research grant from AstraZeneca (S.O.-C. and P.S.). Inivata provided funding for cost of analyses.
L.M. and A.S. contributed equally to this work. P.S. and B.B. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Laura Mezquita, Aurélie Swalduz, Sandra Ortiz-Cuaran, David Planchard, Samuel Woodhouse, Clive Morris, Emma Green, Benjamin Besse, Pierre Saintigny
Financial support: Sandra Ortiz-Cuaran
Administrative support: Solène Marteau, Emma Green
Provision of study material or patients: Laura Mezquita, Sandra Ortiz-Cuaran, Luc Odier, Claudio Nicotra, Clive Morris, Christophe Massard, Maurice Pérol, Benjamin Besse
Collection and assembly of data: Laura Mezquita, Aurélie Swalduz, Cécile Jovelet, Karen Howarth, Virginie Avrillon, Solène Marteau, Jose Carlos Benitez, Ludovic Lacroix, Luc Odier, Pierre Fournel, Claire Tissot, Julien Adam, Claudio Nicotra, Jordi Remon, Clive Morris, Emma Green, Maurice Pérol, Benjamin Besse, Pierre Saintigny
Data analysis and interpretation: Laura Mezquita, Aurélie Swalduz, Sandra Ortiz-Cuaran, Karen Howarth, David Planchard, Gonzalo Recondo, Frank De Kievit, Vincent Plagnol, Ludovic Lacroix, Etienne Rouleau, Caroline Caramella, Edouard Auclin, Jordi Remon, Emma Green, Christophe Massard, Maurice Pérol, Luc Friboulet, Benjamin Besse, Pierre Saintigny
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Laura Mezquita
Consulting or Advisory Role: Roche, Roche Diagnostics, Takeda
Speakers’ Bureau: Roche, Bristol-Myers Squibb, Tecnofarma
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb
Aurélie Swalduz
Honoraria: Roche, Bristol-Myers Squibb, Takeda, AZD
Consulting or Advisory Role: Eli Lilly, Pfizer, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Takeda, Pfizer, Boehringer Ingelheim, Roche
Karen Howarth
Employment: Inivata
Stock and Other Ownership Interests: Inivata
Research Funding: Inivata
Patents, Royalties, Other Intellectual Property: Patent pending
David Planchard
Honoraria: Prime Oncology, Peer CME
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Novartis, Roche, Pfizer, MSD Oncology, Celgene, MedImmune, BeiGene
Research Funding: AstraZeneca/MedImmune (Inst),, Bristol-Myers Squibb (Inst),, Boehringer Ingelheim (Inst), Eli Lilly (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), Sanofi/Aventis (Inst), Taiho Pharmaceutical (Inst), Daiichi Sankyo (Inst), AbbVie (Inst)
Gonzalo Recondo
Consulting or Advisory Role: Roche, Amgen, Pfizer
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
Frank De Kievit
Employment: Inivata
Travel, Accommodations, Expenses: Inivata
Vincent Plagnol
Employment: Inivata, Genomics
Stock and Other Ownership Interests: Inivata, Genomics
Patents, Royalties, Other Intellectual Property: Inivata patents
Etienne Rouleau
Honoraria: AstraZeneca (Inst), Roche (Inst), Bristol-Myers Squibb (Inst), AstraZeneca
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Bristol-Myers Squibb
Pierre Fournel
Consulting or Advisory Role: Bristol-Myers Squibb, Amgen, MSD Oncology, Eli Lilly, AstraZeneca, Pfizer, AbbVie, Takeda, Roche, AstraZeneca, Amgen, Eli Lilly, Pfizer, Bristol-Myers-Squibb, MSD Oncology
Research Funding: Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Amgen (Inst), Pfizer (Inst), Pfizer/EMD Serono (Inst), Ipsen (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, Eli Lilly, Pfizer, Boehringer Ingelheim, AstraZeneca, Amgen, Bristol-Myers Squibb, MSD Oncology
Caroline Caramella
Honoraria: Bristol-Myers Squibb
Honoraria: MSD Oncology, Pfizer
Claire Tissot
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, AstraZeneca, MSD Oncology
Julien Adam
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, AstraZeneca, Merck Sharp & Dohme
Research Funding: Pierre Fabre (Inst), Merck Sharp & Dohme (Inst)
Samuel Woodhouse
Employment: Inivata
Patents, Royalties, Other Intellectual Property: Patents related to fusion technology used by Inivata and in this article
Edouard Auclin
Honoraria: Sanofi Genzyme, Mundipharma
Jordi Remon
Consulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, MSD Oncology, AstraZeneca, Roche, Inivata, OSE Immunotherapeutics
Clive Morris
Employment: Inivata
Leadership: Inivata
Stock and Other Ownership Interests: Inivata
Emma Green
Employment: Inivata
Stock and Other Ownership Interests: Inivata
Christophe Massard
Consulting or Advisory Role: Amgen, Astellas Pharma, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Debiopharm Group, Roche, Ipsen, Janssen, Eli Lilly, MSD Oncology, Novartis, Pfizer, Sanofi, Orion, Tahio, Blueprint Medicines, Innate Pharma, PharmaMar
Maurice Pérol
Consulting or Advisory Role: Eli Lilly, Roche, Pfizer, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, Amgen, Takeda, Chugai Pharma
Research Funding: AstraZeneca (Inst), Roche (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer
Benjamin Besse
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Eli Lilly (Inst), Onxeo (Inst), Bristol-Myers Squibb (Inst), Inivata (Inst), AbbVie (Inst), Amgen (Inst), Biogen (Inst), Blueprint Medicines (Inst), Celgene (Inst), GlaxoSmithKline (Inst), Ignyta (Inst), Ipsen (Inst), Merck (Inst), MSD Oncology (Inst), Nektar (Inst), PharmaMar (Inst), Sanofi (Inst), Spectrum Pharmaceuticals (Inst), Takeda (Inst), Tiziana Therapeutics (Inst)
Pierre Saintigny
Honoraria: HTG Molecular Diagnostics, Inivata, ArcherDx, Bristol-Myers Squibb, Roche Molecular Diagnostics, Roche, AstraZeneca, Novartis, Bristol-Myers Squibb Foundation, Illumina
No other potential conflicts of interest were reported.
REFERENCES
- 1. Soria J-C, Ohe Y, Vansteenkiste J, et al: Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 11 378:113-125, 2018. [DOI] [PubMed]
- 2.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 3. Planchard D, Popat S, Kerr K, et al: Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv192-237, 2018, (suppl 4) [DOI] [PubMed]
- 4.Goldman JW, Noor ZS, Remon J, et al: Are liquid biopsies a surrogate for tissue EGFR testing? Ann Oncol 29:i38-46, 2018 (suppl 1). [DOI] [PubMed]
- 5.Zugazagoitia J, Ramos I, Trigo JM, et al. Clinical utility of plasma-based digital next-generation sequencing in patients with advance-stage lung adenocarcinomas with insufficient tumor samples for tissue genotyping. Ann Oncol. 2019;30:290–296. doi: 10.1093/annonc/mdy512. [DOI] [PubMed] [Google Scholar]
- 6.Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018;36:1631–1641. doi: 10.1200/JCO.2017.76.8671. [DOI] [PubMed] [Google Scholar]
- 7.Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): A statement paper from the IASLC. J Thorac Oncol. 2018;13:1248–1268. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Barlesi F, Mazieres J, Merlio J-P, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. doi: 10.1016/S0140-6736(16)00004-0. [DOI] [PubMed] [Google Scholar]
- 9.Solomon BJ, Mok T, Kim D-W, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 10.Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 11.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–481. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 12.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK Inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: A single-grulticenterentre, phase 2 trial. Lancet Oncol. 2016;17:234–242. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crinò L, Ahn M-J, De Marinis F, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: Results from ASCEND-2. J Clin Oncol. 2016;34:2866–2873. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 15.Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017;18:1590–1599. doi: 10.1016/S1470-2045(17)30680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song A, Kim TM, Kim D-W, et al. Molecular changes associated with acquired resistance to crizotinib in ROS1-rearranged non-small cell lung cancer. Clin Cancer Res. 2015;21:2379–2387. doi: 10.1158/1078-0432.CCR-14-1350. [DOI] [PubMed] [Google Scholar]
- 17.Awad MM, Katayama R, McTigue M, et al. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;368:2395–2401. doi: 10.1056/NEJMoa1215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facchinetti F, Loriot Y, Kuo M-S, et al. Crizotinib-resistant ROS1 mutations reveal a predictive kinase inhibitor sensitivity model for ROS1- and ALK-rearranged lung cancers. Clin Cancer Res. 2016;22:5983–5991. doi: 10.1158/1078-0432.CCR-16-0917. [DOI] [PubMed] [Google Scholar]
- 19. Mezquita L, Jovelet C, Lacroix L, et al: An amplicon-based liquid biopsy for detecting ALK and ROS1 fusions and resistance mutations in advanced non-small cell lung cancer (NSCLC) patients. J Clin Oncol 36:9095-9095, 2018 (suppl 15) [DOI] [PMC free article] [PubMed]
- 20. Dagogo-Jack I, Rooney MM, Lin JJ, et al: Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res 25:6662-6670, 2019. [DOI] [PMC free article] [PubMed]
- 21.McCoach CE, Blakely CM, Banks KC, et al. Clinical utility of cell-free DNA for the detection of ALK fusions and genomic mechanisms of ALK inhibitor resistance in non-small cell lung cancer. Clin Cancer Res. 2018;24:2758–2770. doi: 10.1158/1078-0432.CCR-17-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dagogo-Jack I, Brannon AR, Ferris LA, et al: Tracking the evolution of resistance to ALK tyrosine kinase inhibitors through longitudinal analysis of circulating tumor DNA. JCO Precis Oncol 2:1-14, 2018. [DOI] [PMC free article] [PubMed]
- 23.Dagogo-Jack I, Rooney M, Nagy RJ, et al. Molecular analysis of plasma from patients with ROS1-positive NSCLC. J Thorac Oncol. 2019;14:816–824. doi: 10.1016/j.jtho.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plagnol V, Woodhouse S, Howarth K, et al. Analytical validation of a next generation sequencing liquid biopsy assay for high sensitivity broad molecular profiling. PLoS One. 2018;13:e0193802. doi: 10.1371/journal.pone.0193802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Cui S, Zhang W, Xiong L, et al. Use of capture-based next-generation sequencing to detect ALK fusion in plasma cell-free DNA of patients with non-small-cell lung cancer. Oncotarget. 2017;8:2771–2780. doi: 10.18632/oncotarget.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson RJA, Karachaliou N, Berenguer J, et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget. 2016;7:1066–1075. doi: 10.18632/oncotarget.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopoulos P, Dietz S, Kirchner M, et al. Detection of TP53 mutations in tissue or liquid rebiopsies at progression identifies ALK+ lung cancer patients with poor survival. Cancers (Basel) 2019;11:E124. doi: 10.3390/cancers11010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, Ou Q, Wu X, et al. Concomitant resistance mechanisms to multiple tyrosine kinase inhibitors in ALK-positive non-small cell lung cancer. Lung Cancer. 2019;127:19–24. doi: 10.1016/j.lungcan.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Lucena-Araujo AR, Moran JP, VanderLaan PA, et al. De novo ALK kinase domain mutations are uncommon in kinase inhibitor-naïve ALK rearranged lung cancers. Lung Cancer. 2016;99:17–22. doi: 10.1016/j.lungcan.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8:714–729. doi: 10.1158/2159-8290.CD-17-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 33.Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36:1199–1206. doi: 10.1200/JCO.2017.76.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw AT, Martini J-F, Besse B, et al. Abstract CT044: Efficacy of lorlatinib in patients (pts) with advanced ALK-positive non-small cell lung cancer (NSCLC) and ALK kinase domain mutations. Cancer. 2018;78(suppl 13):CT044. [Google Scholar]
- 35.Shaw AT, Solomon BJ, Besse B, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37:1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aldea M, Hendriks L, Mezquita L, et al: Circulating tumor DNA analysis for patients with oncogene-addicted NSCLC with isolated central nervous system progression. J Thorac Oncol . [epub ahead of print on December 13, 2019] [DOI] [PubMed] [Google Scholar]
- 37. Gainor JF, Tseng D, Yoda S, et al: Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol 10.1200/PO.17.00063. [DOI] [PMC free article] [PubMed]
- 38.Soda M, Isobe K, Inoue A, et al. A prospective PCR-based screening for the EML4-ALK oncogene in non-small cell lung cancer. Clin Cancer Res. 2012;18:5682–5689. doi: 10.1158/1078-0432.CCR-11-2947. [DOI] [PubMed] [Google Scholar]
- 39.Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent- front mutations. Cancer Discov. 2018;8:1227–1236. doi: 10.1158/2159-8290.CD-18-0484. [DOI] [PubMed] [Google Scholar]