Abstract
Pancreatic ductal adenocarcinoma (PDAC) is plagued by a dismal 5-year survival rate, early onset of metastasis and limited efficacy of systemic therapies. This scenario highlights the need to fervently pursue novel therapeutic strategies to treat this disease. Recent research has uncovered complicated dynamics within the tumor microenvironment (TME) of PDAC. An abundant stroma provides a framework for interactions between cancer-associated fibroblasts, suppressive myeloid cells and regulatory lymphocytes, which together create an inhospitable environment for adaptive immune responses. This accounts for the poor infiltration and exhausted phenotypes of effector T cells within pancreatic tumors. Innovative studies in genetically engineered mouse models have established that with appropriate pharmacological modulation of suppressive elements in the TME, T cells can be prompted to regress pancreatic tumors. In light of this knowledge, innovative combinatorial strategies involving immunotherapy and targeted therapies working in concert are rapidly emerging. This review will highlight recent advances in the field related to immune suppression in PDAC, emerging preclinical data and rationale for ongoing immunotherapy clinical trials. In particular, we draw attention to foundational findings involving T-cell activity in PDAC and encourage development of novel therapeutics to improve T-cell responses in this challenging disease.
Keywords: T-lymphocytes, immunomodulation, immunotherapy, tumor escape
Immunosuppression and a harsh stromal microenvironment drive therapeutic resistance in pancreatic ductal adenocarcinoma (PDAC)
PDAC is a devastating malignancy in dire need of novel therapies. Single-agent immune checkpoint blockade has historically elicited almost no response in PDAC, outside of rare patients harboring genetic alterations impacting microsatellite instability.1–3 Similarly, vaccine or cellular therapies in PDAC demonstrate only modest effects, although these modalities remain in early stages.4–6 Many clinical challenges arise from rapid progression of PDAC, often presenting as metastatic disease.7 It is hypothesized that the aggressive nature of this disease and failure of many therapies can be attributed to dominant immunosuppressive features in the PDAC tumor microenvironment (TME).
The TME of PDAC has unique characteristics in comparison to other tumor types. It is dominated by a fibrotic and desmoplastic stroma containing diverse populations of cancer-associated fibroblasts and immunosuppressive myeloid cells, with sparse T-cell infiltration.8–10 This PDAC-associated stroma, often composing up to 90% of tumors by volume, presents a dynamic and insurmountable barrier to immunotherapy.9 11 12 In recent years, advanced murine models of PDAC and forward-thinking approaches have unveiled important mechanisms of immune suppression in PDAC. Additionally, our understanding of how effective antitumor responses can be generated in PDAC is advancing with a cautious optimism for successful application of immunotherapy in this deadly cancer. Here we describe recent findings related to immune suppression in PDAC, highlighting successful advances, and priority areas for future research and discovery.
Immune privilege of pancreatic cancer
T cells can intrinsically promote antitumor responses in coordination with a diverse array of cell types. Recent advances in immunohistochemistry (IHC) and microscopy, in addition to flow cytometry, have allowed for more precise quantification of immune infiltration in PDAC and revealed pancreatic tumors are largely devoid of effector T-cell infiltration and immune privilege.8–10 An eloquent study using multispectral IHC9 compared localization of T-cell and myeloid subsets in the stromal and tumor compartments in both melanoma and pancreatic cancer. The rationale for parallel analysis of these distinct tumor types was to compare differences in the infiltration of T cells and response to immune therapy. While comparing tissue of pancreatic cancer cases with poor or positive response to immunotherapy regimens would be preferential, the lack of immune response to PDAC necessitated this approach of comparing to immune responsive melanoma. Analysis of PDAC tissue revealed relatively few T-cell infiltrates as marked by CD3 and CD4 or CD8 staining compared with melanoma.9 This is certainly troublesome, since increased infiltration of CD4+ and CD8+ T cells in tumors is consistently associated with increased survival in patients.8 13–16 This observation parallels immune suppressive features of other tumors, including prostate and breast cancers. Certainly, emerging evidence in these other solid tumors points to a diverse array of complex intracellular mechanisms in the TME mediating T-cell inactivity, including T-regulatory activity and myeloid derived suppressor cell function.17–25 While this review focuses on PDAC, many observations described here will likely hold true for other ‘immunologically cold’ tumor types.
Low mutational burden and poor immunogenicity fails to induce T-cell infiltration
Lack of effector T-cell infiltration in PDAC has been hypothesized to be a product of poor tumor immunogenicity stemming, in part, from lower frequency of neoantigens.26 Attempts to directly interrogate the immunogenicity of PDAC have employed sophisticated techniques involving patient tissue and the genetically engineered KPC mouse model (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre), which recapitulates much of the microenvironment in human PDAC.27 28 Impressive efforts have employed novel methods to isolate and sequence neoplastic cells within pancreatic tumors while excluding stromal regions which may have confounded past studies.29 30 These reports indicate a complex and highly diverse mutational landscape in PDAC that challenges previous work.29 Certainly, recent clinical data from the ‘Know your Tumor’ initiative demonstrated that choice of personalized, targeted therapy based on genomic features can improve outcomes in PDAC.31 While PDAC is capable of appropriate antigen stimulation of T cells, these studies indicate release or presentation of antigen may be inhibited or obscured in cases with poor T-cell response.
Stromal barriers to T-cell infiltration at the margin of pancreatic tumors
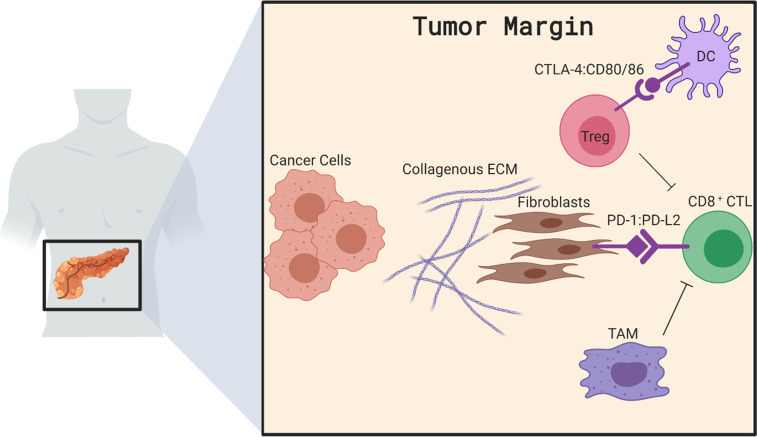
Perhaps the most unique aspect of pancreatic cancer is the overwhelming stroma which shapes the TME. The abundant stroma associated with PDAC has been long hypothesized to physically restrain T-cell and therapeutic drugs or antibodies due to the collagen, fibronectin and other extracellular matrix (ECM) components secreted by fibroblasts and cancer cells.32 However, research by two separate groups demonstrated no significant relationship between thickness of the desmoplasia, fibrotic content in the TME or presence of cancer-associated fibroblasts with the exclusion of infiltrating T cells from neoplastic lesions.8 33 While the stroma represents a barrier for T-cell infiltration into PDAC, these studies indicate this exclusion may occur through mechanisms more complicated than only a physical barrier. Indeed, research dissecting individual components of PDAC stroma and associated mechanisms reveals complex immunosuppressive mechanisms involving cancer-associated fibroblasts, T-regulatory cells (Tregs), tumor-associated macrophages (TAMs) and dendritic cells, each of which affect T-cell infiltration into tumors (figure 1).
Figure 1.
TME of pancreatic tumors encompasses heterogenous cell populations that collectively prevent T-cell infiltration of pancreatic tumors. Here we illustrate T-regulatory cells acting to directly suppress CD8 CTLs while also blocking T-cell priming by occupying dendritic cells. Multiple populations of fibroblasts produce extracellular matrix to drive fibrosis or express PD-L2, which sequesters T cells, while altering the balance of cytokines. TAMs also play a role in sequestering CD8+ CTLs at the tumor margin to prevent efficient infiltration. Together these TME interactions contribute to the immunologically ‘cold’ state of pancreatic tumors. CTL, cytotoxic lymphocyte; PD-L2, programmed death ligand 2; TAM, tumor-associated macrophage; TME, tumor microenvironment; CTLA-4, cytotoxic T-lymphocyte-associated protein 4.
Diverse fibroblast populations contribute to immune suppression in PDAC
The fibroblast components of PDAC tumors are riddled by heterogeneity and plasticity. Previous research defined distinct populations of fibroblasts within PDAC possessing inflammatory or myofibroblastic properties termed inflammatory cancer associated fibroblasts (iCAFs) and myofibroblastic cancer associated fibroblasts (myCAFs), respectively.34 These cells have potential to modulate tumor growth and stromal composition and may alter immune responses to PDAC by contact-dependent and independent properties.34 The inflammatory iCAF subsets are characterized by production of soluble factors, such as interleukin (IL)-6, leukemia inhibitory factor and IL-11, with immune modulatory potential.34 35 myCAFs assume a more traditional activated fibroblast phenotype, secreting ECM components such as collagen and fibronectin.34–36 Work by Ohlund et al elucidated a dynamic interplay between tumor cell-derived interleukin-1-alpha (IL-1α) and transforming growth factor beta (TGFβ) within the stroma that significantly influences cancer associated fibroblast (CAF) fate.35 IL-1α from cancer cells polarized directly adjacent CAFs to a myCAF phenotype; however, IL-1α signaling can be disrupted by the presence of TGFβ in more distant stromal regions, promoting the inflammatory profile seen in iCAFs.35 Of note, TGFβ activation in the stroma has been linked to infiltration and activity of non-degranulated mast cells, which associate with CAFs, and whose infiltration has been linked with worse overall survival in tissue from previously untreated patients with resectable PDAC.37–40
Cross-species sequencing of pancreatic tumors in mice and humans has also revealed the existence of another interesting CAF population with the ability to present antigen.36 These antigen-presenting CAFs express both CD74 and major histocompatability complex-II (MHC-II), indicating a propensity to present antigen to CD4+ T cells in vivo, potentially resulting in increased activation of CD4+ T cells.36 The plasticity of these CAF populations and this ‘Jekyll and Hyde’ influence on the immune system present a complicated case for targeting the stroma to mediate immune activation in PDAC. Indeed, past challenges with pharmacological agents targeting stromal pathways such as sonic hedgehog have rightfully tempered enthusiasm for launching into clinical trials without rigorous data.41 Furthermore, two key reports have demonstrated that in vivo depletion of fibroblasts in murine models resulted in aggressive progression toward metastatic disease and that degree of stroma was inversely related to clinical outcome.42 43 Despite these data, tumors that arose in mice lacking α-SMA+ fibroblasts were exquisitely sensitive to immunotherapy, again implying the stroma restrains immune response to PDAC tumors. Taken together, these data indicate consideration of individual CAF subsets is likely necessary in designing approaches to treat PDAC.42–44
Intercellular dynamics mediating T-cell exclusion from PDAC
Cancer-associated fibroblasts have heterogeneous effects on T-cell activation
More recently, checkpoint-mediated interactions between CAFs and pancreatic cancer cells (PCCs) have been implicated as a mechanism by which T cells are trapped and killed or inactivated in the PDAC stroma.45 PDAC-associated CAFs display higher expression of programmed death ligand 1 (PD-L1) and programmed death ligand 2 than normal fibroblasts, with the latter more highly expressed. In vitro experiments demonstrate the ability of CAFs to upregulate programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and T-cell immunoglobulin and mucin domain-containing protein 3 on both CD4+ and CD8+ T cells, as well as lymphocyte-activation gene 3 on CD4+ T cells. This phenotypical shift eventually leads to decreased T-cell proliferation. Alternatively, fibroblast populations in the TME of PDAC can control immunity through contact-independent mechanisms such as secretion of cytokines and chemokines. In addition to secretory factors discussed previously, other investigations indicate a role for fibroblast-derived CXCL12 in facilitating T-cell exclusion in PDAC.46 Feig et al found CXCL12 from fibroblasts was responsible for excluding T cells in PDAC and mediating failure of both αPD-L1 and αCTLA-4 therapy.46 These data have led to clinical trials blocking the receptor for CXCL12 (CXCR4) with the Food and Drug Administration-approved drug plerixafor (NCT02179970). These results highlight the numerous complementary aspects of the PDAC stroma that drive T -cell exclusion from PDAC.
Duality of lymphocytes within the context of antitumor immunity
Interestingly, immunosuppressive Tregs and B cells with regulatory properties can localize to stromal areas of PDAC, rather than within foci of adenocarcinoma.9 47–50 These Tregs are most often characterized as CD4-positive, with high expression of the IL-2 receptor CD25 and the transcription factor Forkhead Box P3 (FOXP3). Definitive histological detection of these cells in tissue is challenging, and often their characterization omits CD25 for technical simplicity.51 52 Like effector CD4+ or CD8+ T cells, Tregs preferentially localize to stroma, rather than tumor foci in PDAC, but can be found in uninvolved and tumor compartments in equal proportion.9 53 However, the central location for the inhibitory action of these cells may be in peritumoral lymph nodes associated with PDAC. Indeed this is where the majority of Tregs in tumor-bearing mice are found.53 This research also revealed CTLA4/CD80 interactions between Tregs and dendritic cells (DCs) as essential molecular mediators of CD4 T-cell exclusion, but the specifics of how CD4 T cells are actually excluded as a result of these interactions are only now becoming clear. A novel observation by Jang et al describes prolonged interactions between Tregs and DCs in PDAC, demonstrating the ability for Tregs to outcompete CD8+ T cells and limit CD8+ T-cell interactions with DCs54 (figure 2). In this manner, Tregs limit T-cell priming in the periphery and significantly diminish cytotoxic T-cell responses to PDAC.53 54
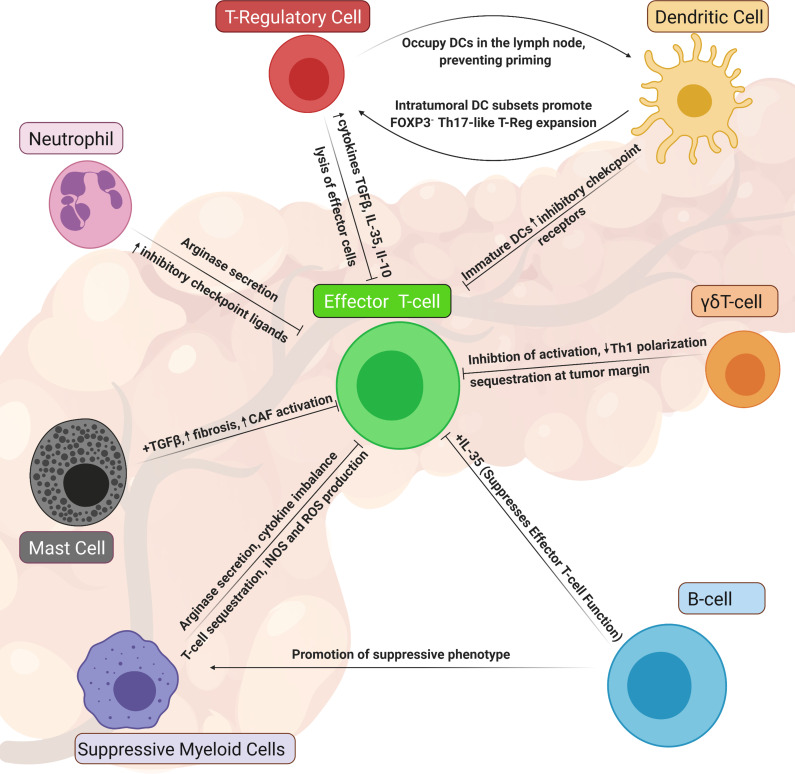
Figure 2.
Dismal T-cell responses observed in pancreatic cancer can be attributed, in part, to a multitude of inflammatory monocytes and suppressive lymphocytes within the tumor microenvironment of pancreatic tumors. Here, we highlight populations of immune suppressive cells in PDAC that have been understudied yet have been shown to directly and indirectly suppress effector T cells in PDAC. notably, many of the mechanisms highlighted here involve soluble mediators, such as chemokines, cytokines, growth factors and reactive nitrogen species and ROS. These cellular populations should be more commonly considered as we seek to develop novel therapeutic strategies to reinvigorate T-cell activity in PDAC. DC, dendritic cell; IL, interleukin; PDAC, pancreatic ductal adenocarcinoma; ROS, reactive oxygen species; TGFβ, transforming growth factor beta; cancer associated fibroblast, CAF; inducible Nitrous Oxide Synthase iNOS; myeloid-derived suppressor cell, MDSC.
Alternative regulatory T cells lacking FOXP3 expression contribute to immune suppression
Naturally occurring Tregs express the transcription factor FOXP351 55–57; however, CD4+ Tregs without canonical FOXP3 expression can also repress immune responses.58 59 In fact, a CD4+ Treg subset positive for IL-10 and IL-17 and negative for FOXP3 was identified in murine PDAC models.60 These FOXP3− Tregs promote tumor progression and have a similar phenotype to type I regulatory (Tr1) cells that develop from mature antigen-stimulated CD4+ T cells.60 61 Tr1 cells, identified over 30 years ago in patients, characteristically secrete large amounts of IL-10 and can negatively impact antigen-presenting myeloid cells.62 63 While natural Tregs traditionally develop in the thymus from naïve cells, Tr1 cells can be altered and differentiated in the TME of both mouse and human hosts, identified by their expression of CD49 and LAG3.60 61 64 A recent study by Barilla et al describes the influence of specialized DC subsets in skewing of CD4+ T cells to this Tr1 phenotype and the protumorigenic effect of this interaction60 (figure 2). Additionally, CD4+ T cells exposed to a suppressive DC subset from the PDAC TME shifted to a Th17 phenotype, including a population of Th17-like cells expressing FOXP3.60 Thus, DC subsets have the capacity to induce multiple regulatory T-cell subsets which suppress immune responses.60 The expansion of these cells from antigen-experienced CD4+ T cells also diminishes potential helper cells within the PDAC TME. Interestingly, DCs isolated from pancreata or spleens of naïve mice or from spleens of PDAC tumor-bearing mice do not have the same abilities, indicating PDAC exerts a unique influence over DCs in the TME.60 These data highlight the complex interactions mediating the presence of effector T-cell populations within the TME.
PDAC-associated B cells limit cytotoxic T-cell activity
B lymphocytes, or B cells, can have immunosuppressive activity in several tumor types.47–50 B cells can associate with CD8+ T cells in both murine PDAC models and pancreatic intraepithelial neoplasias (PanIN) of patients.48 50 Several studies have uncovered B-cell phenotypes in PDAC, and targeting these cells improves immune responses in PDAC.48–50 Emerging reports highlight a role for B-cell-derived IL-35, as well as B-cell control of macrophage polarization to a tumor-promoting phenotype. While separate studies have alternatively defined tumor-promoting B-cell subsets, it should be noted that growth of orthotopic pancreatic tumors in B-cell-deficient mice (μMT) was severely diminished.50 Further, depletion of B cells in mice with PanIN significantly inhibited progression.48 Several pathways such as IL-35 secretion, dynamic fluctuation of hypoxia-inducible factor 1-alpha and Bruton tyrosine kinase activation are potential targets for inhibiting B cells in PDAC48–50 (figure 2). Balancing these immune suppressive properties of B cells in PDAC are other strong data demonstrating B cells can cluster in tertiary lymphoid tissues (TLTs). Further, B-cell clustering is significantly correlated with improved T-cell activity in murine models65 and more favorable outcomes for patients with PDAC.66 67 While TLTs have recently emerged as an interesting feature of potent antitumor responses,68 their presence and make-up in PDAC tissues is quite understudied. Indeed, the role of B cells and TLT in PDAC progression deserves further exploration.
More than M1/M2: complex interactions of TAMs suppress T-cell responses to PDAC
Macrophages represent a sizeable proportion of cells in the PDAC TME and have complex characteristics. Available evidence suggests these cells are either derived from circulating monocytes, or established in the organ during embryonic development.69 Through a set of elegant preclinical experiments, the role of these two macrophage lineages in the PDAC TME has been elucidated. These studies revealed embryonically derived, TAMs promote fibrosis and tumor growth, while monocyte-derived TAMs directly influence immune suppression.69 Embryonically-derived TAMs are distinguished by expression of colony-stimulating factor one receptor (CSF1R) in mice and CXCR4 in humans, and expand during tumor development.69 In contrast to established roles for these TAM subsets, dynamic imaging microscopy showed prolonged interactions between TAMs and CD8+ T cells in murine pancreatic tumors.69 These interactions were localized to dense stromal regions of tumors, whereby T cells were trapped and prevented from infiltrating tumors.69 These data indicate a dual role for embryonically derived TAMs in promoting fibrosis and tumor growth and also preventing the infiltration of cytotoxic T cells into tumors.
In comparison, monocyte-derived TAMs express high levels of MHC-II and are more adept at sampling and presenting antigen. Monocyte-derived TAMs in circulation can infiltrate into pancreatic tumors or tumor-draining lymph nodes, by virtue of interactions with chemokine receptor 2 (CCR2).24 70–73 A recent study characterizing extratumoral Ly6Clow F4/80+ macrophages (monocytic phenotype markers) demonstrates these cells act outside of tumors to drive CD4+ T-cell-specific exclusion from PDAC.74 Clodronate depletion of macrophages from mice with spontaneously arising PDAC increased infiltration of CD4+ T cells into tumors.74 As with studies of Tregs, the effects of macrophages on CD4+ T-cell exclusion are localized to extratumoral locations, as clodronate had no effect on macrophages in the TME.74
Taken together, the available data indicate investigation should extend to regions outside of tumor tissue, to consider how local and distal mechanisms influence immune suppression in PDAC. In addition to T-cell exclusion, there are numerous immune interactions in PDAC mediating suppression of T-cell activation, which we highlight in figure 2.
Therapeutic approaches to ameliorate T-cell exclusion and inactivation in PDAC
The extent to which T cells are both excluded and suppressed in PDAC may seem disheartening; however, as the obstacles become more well defined, our therapeutic strategies continue to improve in their sophistication. Recent advances have furthered our characterization and understanding of how T cells are inhibited from eliminating pancreatic tumors. A fibrotic and desmoplastic TME, inadequate exposure to tumor-associated antigens and numerous suppressive cells represent areas of investigation. While these factors bear weight on T-cell responses, they also uncover opportunities to dismantle specific aspects of immune suppression. Cellular and targeted agents aimed at blocking cell–cell interactions or crosstalk mediated by soluble factors have promise for re-engaging T-cell responses to PDAC. Here, we discuss a series of select therapeutic strategies being leveraged to advance immunotherapy in PDAC.
Combinatorial approaches with immune checkpoint inhibition (ICI)
ICI, specifically targeting CTLA-4 or the PD-1/programmed death ligand 1 (PD-L1) axis, has gained traction due to success in several oncological settings. Unfortunately, these drugs have shown little promise as single agents for patients with PDAC.2 75 Equipped with new and emerging knowledge of immune suppression in PDAC, many groups are pursuing combinatorial approaches to alleviate immune suppression and enhance ICI in PDAC. These include neutralization of growth factors or cytokines, inhibition of chaperone proteins and kinases, simultaneous blockade of multiple immune checkpoints and a host of others. Many strategies have emanated from encouraging preclinical results into first in human clinical trials in the setting of PDAC (table 1). It is worth noting that the effects of radiation and chemotherapy, which are commonly incorporated into combination therapeutic strategies (table 1), have not been fully characterized with respect to their immune influence. Recent studies addressing the effects of radiation have revealed this approach to control local tumor growth but with detrimental effects on antitumor immunity within the TME. Results from these studies indicate an influx in suppressive macrophages, Tregs and increases in inducible Nitic Oxide Synthase (iNOS) release by cancer-associated fibroblasts that together result in poor T-cell activity and sparse infiltration of pancreatic tumor tissues.76–78 In contrast, chemotherapy is hypothesized to increase T-cell priming, which we discuss further briefly, and data from our group and others indeed suggest immune changes elicited by chemotherapy are significant.76 79 Thus, considering how immunotherapy approaches can be strategically combined with radiation or chemotherapy will be key in moving forward.
Table 1.
Ongoing and emerging clinical trials in PDAC using CAR-T therapy and novel combinations with ICI
| Interventions | Phases | Locations | NCT number | Status |
| Viral and vaccine-based therapies | ||||
| Pembrolizumab|wild-type reovirus | Phase II | Northwestern University, Chicago, Illinois, USA | NCT03723915 | Ongoing |
| GRT-C903|GRT-R904|nivolumab|ipilimumab | Phase I Phase II | Multicenter | NCT03953235 | Ongoing |
| Cyclophosphamide|nivolumab|ipilimumab|GVAX pancreas vaccine|CRS-207 | Phase II | Johns Hopkins SKCCC, Baltimore, Maryland, USA | NCT03190265 | Ongoing |
| Cyclophosphamide|nivolumab|GVAX pancreas vaccine|radiation: SBRT | Phase II | Multicenter | NCT03161379 | Ongoing |
| Epacadostat|pembrolizumab|CRS-207|CY|GVAX | Phase II | The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland, USA | NCT03006302 | Recruiting |
| Cyclophosphamide|GVAX|pembrolizumab|radiation: SBRT | Phase II | The Sidney Kimmel Comprehensive Cancer at Johns Hopkins, Baltimore, Maryland, USA | NCT02648282 | Ongoing |
| Cyclophosphamide|GVAX|pembrolizumab|IMC-CS4 | Early phase I | Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, USA | NCT03153410 | Recruiting |
| CAR-T or TIL-based therapies | ||||
| Activated CIK and CD3-MUC1 bispecific antibody in treating pancreatic cancer|procedure: cryotherapy | Phase II | Institutional Review Board of Guangzhou Fuda Cancer Hospital, Guangzhou, Guangdong, China | NCT03509298 | Ongoing |
| Anti-MUC1 CAR-pNK cells | Phase I Phase II | PersonGen BioTherapeutics (Suzhou) Co, Ltd, Suzhou, Jiangsu, China | NCT02839954 | Unknown status |
| Anti-MUC1 CAR-T cells | Phase I Phase II | PersonGen Biomedicine (Suzhou) Co, Ltd, Suzhou, Jiangsu, China | NCT02587689 | Unknown status |
| Anti-CEA CAR-T Cells| gemcitabine/nab paclitaxel| NLIR+FU/FA|capecitabine | Phase II Phase III | NCT04037241 | Not yet recruiting | |
| multiTAA specific T cells | Phase I Phase II | Baylor Clinic, Houston, Texas, USA|Houston Methodist Hospital, Houston, Texas, USA|Harris Health System, Smith Clinic, Houston, Texas, USA | NCT03192462 | Ongoing |
| BPX-601|rimiducid | Phase I Phase II | Multicenter | NCT02744287 | Ongoing |
| Young TIL|aldesleukin|cyclophosphamide|fludarabine|pembrolizumab (Keytruda) | Phase II | National Institutes of Health Clinical Center, 9000 Rockville Pike, Bethesda, Maryland, USA | NCT01174121 | Ongoing |
| TEW-7197 | Phase I Phase II | Samsung Medical Center, Seoul, Republic of Korea | NCT03666832 | Ongoing |
| Pegylated recombinant human hyaluronidase PH20|pembrolizumab | Phase II | M D Anderson Cancer Center, Houston, Texas, USA | NCT04058964 | Not yet recruiting |
| Targetted small mlecule and antibody-based therapies in combination with ICI | ||||
| Anti-SEMA4D monoclonal antibody VX15/2503|ipilimumab|nivolumab|procedure: surgery | Phase I | Emory University, Atlanta, Georgia, USA | NCT03373188 | Ongoing |
| APX005M|nivolumab|nab-paclitaxel|gemcitabine | Phase I Phase II | Multicenter | NCT03214250 | Active, not recruiting |
| XL888|pembrolizumab | Phase I | Emory University, Atlanta, Georgia, USA | NCT03095781 | Recruiting |
| Pembrolizumab|defactinib | Phase II | Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, USA | NCT03727880 | Ongoing |
| Antibiotics and pembrolizumab | Phase IV | NYU Langone Health, New York, New York, USA | NCT03891979 | Not yet recruiting |
| ENB003 plus pembrolizumab phase Ib/IIa in solid tumors | NCT04205227 | |||
| ENB003|pembrolizumab | Phase I Phase II | NCT04205227 | Not yet recruiting | |
| GB1275|nab-paclitaxel and gemcitabine|pembrolizumab | Phase I Phase II | Multicenter | NCT04060342 | Ongoing |
| Pembrolizumab|sonidegib | Phase I | Mayo Clinic in Arizona, Scottsdale, Arizona, United States|Mayo Clinic in Florida, Jacksonville, Florida, United States|Mayo Clinic, Rochester, Minnesota, USA | NCT04007744 | Ongoing |
| XmAb22841|pembrolizumab (Keytruda) | Phase I | Multicenter | NCT03849469 | Ongoing |
| FT500|nivolumab|pembrolizumab|atezolizumab|cyclophosphamide|fludarabine | Phase I | Multicenter | NCT03841110 | Ongoing |
| PEGPH20|pembrolizumab | Phase II | Multicenter | NCT03634332 | Ongoing |
| CPI-006| CPI-006+ciforadenant|CPI-006+pembrolizumab | Phase I | Multicenter | NCT03454451 | Ongoing |
| Pembrolizumab|paricalcitol|placebo | Phase II | Multicenter | NCT03331562 | Active, not recruiting |
| INT230-6|anti-PD-1 antibody|anti-CTLA-4 antibody | Phase I Phase II | Multicenter | NCT03058289 | Ongoing |
| CXCR4 Antagonist BL-8040| Pembrolizumab|Other: Pharmacological Study | Phase II | M D Anderson Cancer Center, Houston, Texas, USA | NCT02907099 | Active, not recruiting |
| Adoptive immunotherapy|aldesleukin|cyclophosphamide|other: laboratory biomarker analysis| pembrolizumab | Phase I | M D Anderson Cancer Center, Houston, Texas, USA | NCT02757391 | Active, not recruiting |
| Pembrolizumab|itacitinib|INCB050465 | Phase I | Multicenter | NCT02646748 | Active, not recruiting |
| Pegilodecakin|paclitaxel or docetaxel and carboplatin or cisplatin|FOLFOX (oxaliplatin/leucovorin/5-fluorouracil)|gemcitabine/nab-paclitaxel|capecitabine|pazopanib|pembrolizumab|paclitaxel|nivolumab| gemcitabine/carboplatin | Phase I | Multicenter | NCT02009449 | Active, not recruiting |
| Nivolumab|ipilimumab|tocilizumab|radiation: SBRT | Phase II | Herlev & Gentofte University Hospital, Denmark, Herlev, Denmark | NCT04258150 | Ongoing |
| BT5528|nivolumab | Phase I Phase II | Multicenter | NCT04180371 | Ongoing |
| KRAS peptide vaccine|nivolumab|ipilimumab | Phase I | Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, USA | NCT04117087 | Not yet recruiting |
| Nivolumab|radiation: radiation therapy|TLR9 agonist SD-101 | Phase I | University of California Davis Comprehensive Cancer Center, Sacramento, California, USA | NCT04050085 | Ongoing |
| Part 1 TPST-1120|part 2a TPST-1120+nivolumab|part 2b TPST-1120+docetaxel|part 2c TPST-1120+cetuximab|part 3 TPST-1120|part 4a TPST-1120+nivolumab|part 4b TPST-1120+docetaxel|part 4c TPST-1120+cetuximab | Phase I | Multicenter | NCT03829436 | Ongoing |
| Anetumab ravtansine|gemcitabine hydrochloride|ipilimumab|nivolumab | Phase I Phase II | Multicenter | NCT03816358 | Ongoing |
| Nivolumab|tadalafil|oral vancomycin | Phase II | National Institutes of Health Clinical Center, Bethesda, Maryland, USA | NCT03785210 | Ongoing |
| Radiation: SBRT|nivolumab|CCR2/CCR5 dual antagonist|GVAX | Phase I Phase II | Sidney Kimmel Comprehensive Cancer Center, Baltimore, Maryland, USA | NCT03767582 | Ongoing |
| FOLFIRINOX|losartan|nivolumab|radiation: SBRT|procedure: surgery | Phase II | Multicenter | NCT03563248 | Ongoing |
| Niraparib+nivolumab|niraparib+ipilimumab | Phase I Phase II | University of Pennsylvania, Abramson Cancer Center, Philadelphia, Pennsylvania, USA | NCT03404960 | Ongoing |
| Cabiralizumab|nab-paclitaxel|onivyde|nivolumab|fluorouracil|gemcitabine|oxaliplatin|leucovorin| irinotecan hydrochloride | Phase II | Multicenter | NCT03336216 | Active, not recruiting |
| Nivolumab|daratumumab | Phase I Phase II | Multicenter | NCT03098550 | Active, not recruiting |
| FPA008|BMS-936558 | Phase I | Multicenter | NCT02526017 | Active, not recruiting |
| BMS-813160|nivolumab|ab-paclitaxel|gemcitabine|5-fluorouracil|leucovorin|irinotecan | Phase I Phase II | Multicenter | NCT03184870 | Ongoing |
CAR-T, chimeric antigen receptor-expressing T cell; CCR2, chemokine receptor 2; ICI, immune checkpoint inhibition; PD-1, programmed cell death protein 1; PDAC, pancreatic ductal adenocarcinoma; SBRT, stereotactic body radiation; TIL, tumor-infiltrating lymphocyte.
IL-6 blockade has multicompartmental effects on antitumor immunity
The complicated cytokine and chemokine milieu of PDAC contributes to immune suppression in various ways. However, certain soluble factors are consistently upregulated by multiple cell subsets in PDAC. IL-6 represents one prominent cytokine consistently present within the PDAC TME. Although this cytokine can be derived from multiple cellular sources, it is transcribed in abundance by human pancreatic stellate cells and drives expansion of myeloid-derived suppressor cells (MDSC) in vitro80 (figure 3). The influence of IL-6 on myeloid cells has also been demonstrated in metastatic PDAC, where IL-6 signaling through serum amyloid A1 and A2 promotes myeloid cell recruitment and a prometastatic niche in the liver.81 IL-6 can also polarize T-cell responses away from Th1 immunity, characteristic of effective antitumor responses, and regulate balance of Th17 or T regs in a context-dependent manner82–84(figure 3). Using murine PDAC models, in vivo blockade of IL-6 enhanced efficacy of anti-PD-L1 antibodies in a CD8+ T-cell-dependent manner.85 The combination of blocking IL-6 and PD-L1 has since been extended to models of brain, colon, non-small cell lung cancer and others.86–88 These preclinical data provide rationale for an ongoing early phase clinical trial at our institution that encompasses a robust series of correlative studies on immune and stromal biomarkers in paired biopsies (NCT04191421). Exciting studies testing IL-6R blockade in combination with chemotherapy (NCT02767557) and immunotherapy/radiotherapy combinations (NCT04258150) have also recently opened at other institutions for patients with metastatic PDAC.
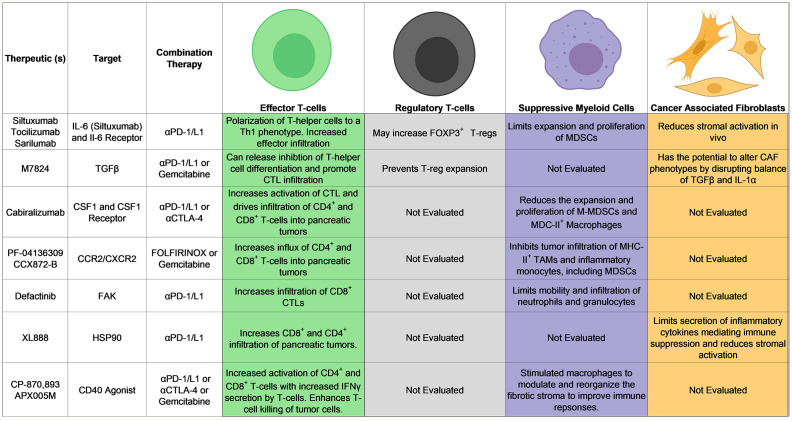
Figure 3.
Strategies to reinvigorate immune responses in PDAC target many unique pathways. Shown here are select antibody and small molecule therapeutics combined with ICI in current clinical trials for the treatment of pancreatic cancer. Their respective heterogenous effects on T cells, myeloid cells and CAFs in the TME are highlighted. Of note, many emerging therpaeutic strategies combined with ICI influence multiple cellular populations in the TME of pancreatic tumors. While not thoroughly evaluated, these combination strategies likely influence many other cellular subsets. Putative mechanisms of action are derived from published preclinical data and/or correlative research as part of a clinical trial. ICI, immune checkpoint inhibition; IL, interleukin; TAM, tumor-associated macrophage; TGFβ, transforming growth factor beta; TME, tumor microenvironment; cancer associated fibroblast, CAF; major histocompatability complex-II, MHC-II; CTL, cytotoxic T-lymphocyte.
Restoring balance of T-cell phenotypes by inhibiting TGFβ
Another potent mediator of tumor progression in PDAC is TGFβ, which is present in the stroma of pancreatic tumors.89 90 While TGFβ has a powerful influence on malignant cells, this cytokine also influences T-cell function and differentiation (figure 3). With respect to CD4+ T cells, TGFβ regulates expansion of cells with Th17 or regulatory phenotypes, depending on the presence of IL-6.91 TGFβ also influences the phenotype of CD4+ T ells by inhibiting expression of the transcription factors GATA binding protein 3, T-box protein expressed in T cells and subsequent signaling to prevent activation of inflammatory CD4+ T cells.92–94 More recently, evidence has emerged demonstrating TGFβ signaling in CD8+ T cells inhibits both trafficking into tumors and activation of CD8+ T cells.95 While dual blockade of TGFβ and PD-1 led to tumor regression in preclinical studies, activation of T cells both in the tumor and in the periphery demonstrates the ability of this combination to overcome broad immune suppression in these models.96 Currently, a TGFβ ligand trap (M7824) is in clinical trials alongside standard of care gemcitabine for patients with untreated PDAC (NCT03451773).
Modulating suppressive myeloid cells in PDAC by antibody blockade of CFS1/CSF1R
Investigation of tumor-associated myeloid populations has revealed important mechanisms of suppressed T-cell immunity in PDAC and new targets for therapy. Recently, colony-stimulating factor 1 (CSF1) and its receptor, colony-stimulating factor 1 receptor (CSF1R) have garnered attention in PDAC. The source of CSF1 is likely PCCs themselves, with CSF1R expression localized to the immediately adjacent stroma.97 Initial mouse studies blocking CSF1/CSF1R interactions revealed a significant impact on myeloid populations, reducing M-MDSCs and MHC-II expressing macrophages that can directly inhibit T-cell activity in PDAC97 (figure 3). Subsequent studies using dual blockade of CSF1R and PD-1 or CTLA-4 profoundly increased both CD4 and CD8 infiltration of tumors and resulted in tumor regression, including complete regression in about 30 percent of mice.97 This strategy has now led to a national phase Ia/b clinical trial (NCT02526017) incorporating αPD-1 blockade and cabiralizumab, an antibody against CSF1R.
CCR2/CXCR2 impacts myeloid populations in PDAC to reinvigorate T-cell responses
Blockade of the chemokine CCR2 has elicited similar, even redundant mechanisms of response to that of CSF1R blockade.70 CCR2 has been identified as a crucial mediator of macrophage migration and infiltration into various tumor types.24 72 73 While CCR2 inhibitors only modestly impact tumor growth, an impressive antitumor response was mounted in mice treated simultaneously with gemcitabine and CCR2 inhibitor (CCR2i).70 Following these promising studies, a clinical trial at Washington University (NCT01413022) and a multicenter trial (NCT02345408) treated advanced/metastatic patients with PDAC with the combination of fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and one of two distinct CCR2i.10 98 Both clinical trials elicited excitement with objective responses and prolonged overall survival compared with published results of FOLFIRINOX alone.10 98 Correlative studies from these trials revealed decreases in MDSCs, and increased CD4+ and CD8+ T-cell infiltration in tumor tissue, consistent with data reported in murine models71 (figure 3).
Focal adhesion kinase (FAK) disrupts the mobility of myeloid cells to restore T-cell infiltration into tumors
FAK inhibitors are being explored as a therapeutic option with similar mechanisms of action to CCR2i and CSF1Ri. Interest in FAK has been evident for some time, as the multifunctional protein has been studied for its role in invasion, migration, cell survival and proliferation.99–103 Emerging research has exposed a novel role for FAK in mediating immunosuppression.104 105 Infiltrating myeloid cells, specifically tumor-associated macrophages and neutrophils, depend on FAK to penetrate tumors with dense ECM.106 107 In line with these data, increased FAK activation in human PDAC samples correlates with decreased infiltration of CD8+ lymphocytes, increased neutrophils and CD15+ granulocytes104 (figure 3). These data indicate a correlation between increased FAK activation and cells with an immunosuppressive phenotype in human PDAC tissues. Indeed, FAK inhibition in murine models of PDAC successfully inverted this balance of cytotoxic lymphocytes and suppressive myeloid cells to favor regression of pancreatic tumors104 (figure 3). The combination of FAK inhibition with chemotherapy and PD-1 blockade improved survival in mice bearing spontaneous PDAC.104 These preclinical results culminated in a phase I clinical trial of gemcitabine, the PD-1 blocking antibody pembrolizumab, and the FAK inhibitor defactinib.108 Stable disease was observed in just over 50% of the patients in this trial, with no dose limiting toxicities reported in the dose expansion phase.108 This trial is now enrolling patients in a phase II expansion cohort (NCT03727880).
Inhibiting heat shock protein 90 (HSP90) has a beneficial multicellular impact on the TME
HSP90 is a chaperone protein at the crux of pathways associated with many hallmarks of cancer. These include assisting the folding of proteins such as BRAF, EGFR, fusion proteins like Bcr-Abl and other factors dysregulated in cancer.109–113 Immune-associated client proteins of HSP90 such as STAT3, STAT5, and C/EBPε are also of interest, given their involvement in expansion of myeloid cells with suppressive functions.114–118 Thus, HSP90 represents a target with a centralized role in many pathways regulating tumor progression while also contributing to a protumorigenic microenvironment. HSP90 inhibitors may also be leveraged for immune modulation.119–122 Preclinical studies demonstrate XL888, an HSP90 inhibitor, can elicit efficacy in murine PDAC models when combined with PD-1 blockade and can enhance tumorous infiltration of both CD4+ and CD8+ cells123 (figure 3). It is possible the improved response results from the impact of XL888 on the TME of these tumors. Specially, XL888 can limit activation and inflammatory cytokine secretion from pancreatic tumor-associated fibroblasts (figure 3). These preclinical studies complement an ongoing investigator-initiated phase Ib/II clinical trial (NCT03095781) of XL888 and pembrolizumab (anti-PD-1).
Reprogramming macrophages to modulate PDAC-associated stroma with CD40 agonists
Given the immunologically cold state of pancreatic tumors, licensing of antigen-presenting cells (APCs) to prime and activate T-cell responses is an area of growing attention. On recognition of antigen on APCs, interaction between CD40 ligand on T cells and CD40 receptor on APCs mediates priming of T cells, described as the transition of T cells to a ‘licensed’ state.124–126 This allows for efficient and potent activation of both CD4+ and CD8+ T cells. The development of CD40 agonists to boost this response was therefore identified as an auspicious therapeutic approach. This strategy was tested in a multicenter phase I dose escalation trial with patients receiving the combination of CD40 agonist and gemcitabine demonstrating improved OS and progression free survival(PFS) versus gemcitabine alone.127 Notably, this trial enrolled patients with newly diagnosed, resectable PDAC rather than advanced disease. Priming T-cell responses requires sufficient antigen presentation by APCs; therefore, patients were treated with gemcitabine with CD40 agonist delivered several days after the first dose of gemcitabine.127 This approach was used with the hypothesis that chemotherapy would elicit some tumor cell killing to provide APCs in the lymphoid organs with antigen released from dying tumors. These studies raise a particularly important question as to the timing of combination therapy.
Similar results were observed in KPC mice treated with gemcitabine and the CD40 agonist FGK45.127 In an interesting mechanistic twist, murine studies in KPC mice revealed macrophages, rather than T cells, as indispensable for FGK45-induced tumor regression, as this CD40 agonist converted the cytokine profile and activity of macrophages to elicit stromal degradation and lysis of pancreatic tumor cells127 (figure 3). The ability of CD40 agonists to stimulate stromal modulation and reorganization via macrophage activity suggests other therapeutic modalities could be enhanced without the immunosuppressive stroma characteristic of pancreatic tumors. In melanoma, αCD40 agonists have been combined with CTLA-4 blockade to significantly enhance both T-cell priming and activation with survival benefits for patients (NCT01103635).128 Employing a similar strategy, a Phase Ia/b clinical trial (NCT03214250) of gemcitabine plus nab-paclitaxel, the CD40 agonist APX005M, and nivolumab (αPD-1) demonstrated safety and tolerability with promising antitumor activity. Based on encouraging initial data, this combination has proceeded to phase II dose escalation.
Vaccine and cellular therapies
Very recent advances in technology and understanding of T-cell biology have catalyzed development of vaccine and cellular therapies such as autologous cell transfer and chimeric antigen receptor-expressing T cells (CAR-Ts). There are a number of active clinical trials using vaccine therapies in PDAC with novel strategic targets. These trials include vaccines directing immune responses to tumor-expressed human guanylyl cyclase C and mutated KRAS (NCT04111172 and NCT04117087, respectively), as well as several personalized peptide-based vaccines (NCT03794128 and NCT02600949). The granulocyte-macrophage colony-stimulating factor-based vaccine GVAX is being tested in clinical trials in combination with ICI for PDAC (table 1). Another unique vaccine therapy uses patient-derived, mature DCs, which are directed against mutant KRAS by pulsation with mutant KRAS peptides (NCT03592888). These cells are then used for autologous transfer back into patients to elicit adaptive immune responses.
Adoptive cell therapies have had success in multiple cancer types and are under development for PDAC. These approaches include administration of TIL products and autologous approaches involving T-cell receptor (TCR) or chimeric antigen receptor T cell therapy. While TIL expansion from PDAC tumors can be challenging, CAR-T or TCR-based therapy is governed by identification of appropriate antigens toward which immune responses should be directed.129–132 While several tumor-associated antigens continually emerge as targets in pancreatic cancer, the investigation and identification of viable TCR or CAR-T targets is ongoing. Current clinical trials using CAR-T therapy are targeting a variety of antigens, including carcinoembryonic antigen, mesothelin, CD133, CD70, human epidermal growth factor receptor 2, epithelial cell adhesion molecule and many others, which we have previously reviewed.133 Additionally, prostate stem-cell antigen (PSCA) targeting CAR-T therapy is currently in clinical trials (NCT02744287) and has shown promising interim results. The broad heterogeneity of antigen presentation by cells within solid tumors makes CAR-T design extremely challenging. An innovative approach by Marker Therapeutics is testing a T-cell product directed against up to five tumor-associated antigens simultaneously, which is currently in phase I/II clinical trials (NCT03192462). Additionally, with the discovery of T-cell subsets with high potency against tumors, the field of cellular therapy for PDAC is rapidly expanding. Further, these cellular therapies will encompass significant advances in innovation, such as cytokine, antibody or chemokine receptor engineering that may be advantageous for notoriously hard-to-treat diseases such as PDAC.134
Conclusions
Our knowledge of immune suppression in PDAC is quickly expanding and providing new therapeutic opportunities in the realm of this disease. The field is now rich with numerous emerging combination therapies that elicit effects across multiple cellular components of the TME to promote antitumor T cell-mediated immune responses (figure 3). Rapidly growing technology in the field of antibodies, small molecules, vaccines, gene therapy and engineered T cells show promise for pancreatic cancer, among other aggressive diseases. Furthermore, our continually improving ability to dissect molecular and genetic mechanisms mediating tumor progression provides opportunity for individualized targeted and immune therapy. With careful attention to complex cell–cell interactions in the PDAC TME, we can certainly improve our ability to invigorate T-cell responses to these recalcitrant tumors.
Footnotes
Twitter: @BwareRedHair, @LesinskiLab
Contributors: MBW and GBL wrote and drafted the manuscript. BFE-R reviewed and edited the manuscript and provided consultation and advisement for clinical trial descriptions and evaluation.
Funding: Supported by NIH grants R01CA208253, R01CA228406 and P30CA138292.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests: GBL has consulted for ProDa Biotech, LLC and received compensation, and received research funding through a sponsored research agreement between Emory University and Merck and Co, Bristol-Myers Squibb, Boerhinger-Ingelheim, and Vaccinex. BFE-R has been on the advisory board for Ipsen, Natera, AstraZeneca, and Bristol-Myers Squibb, Inc, and on the Data Safety Monitoring Board for Exelixis and Erytech and has been a consultant to Merck and Co. and receives compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. BFE-R has received research funding through a sponsored research agreement between Emory University and Bristol-Myers Squibb, Boston Biomedical, Novartis, Merck and Co, Bayer, Exelixis, Pfizer, AstraZeneca, Xencor, and EUSA.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.O'Reilly EM, Oh D-Y, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol 2019;5:1431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828–33. 10.1097/CJI.0b013e3181eec14c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty GL, O'Hara MH, Nelson AM, et al. Safety and antitumor activity of chimeric antigen receptor modified T cells in patients with chemotherapy refractory metastatic pancreatic cancer. JCO 2015;33:3007 10.1200/jco.2015.33.15_suppl.3007 [DOI] [Google Scholar]
- 5.Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36:382–9. 10.1097/CJI.0b013e31829fb7a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol 2015;33:1325–33. 10.1200/JCO.2014.57.4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin 2019;69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 8.Carstens JL, Correa de Sampaio P, Yang D, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun 2017;8:15095. 10.1038/ncomms15095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blando J, Sharma A, Higa MG, et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights vista as a potential target in pancreatic cancer. Proc Natl Acad Sci U S A 2019;116:1692–7. 10.1073/pnas.1811067116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with Folfirinox in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1B trial. Lancet Oncol 2016;17:651–62. 10.1016/S1470-2045(16)00078-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2008;6:1155–61. 10.1016/j.cgh.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 12.Torphy RJ, Wang Z, True-Yasaki A, et al. Stromal content is correlated with tissue site, contrast retention, and survival in pancreatic adenocarcinoma. JCO Precis Oncol 2018;2018 10.1200/PO.17.00121. [Epub ahead of print: 16 Jan 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-Infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 2012;30:2678–83. 10.1200/JCO.2011.37.8539 [DOI] [PubMed] [Google Scholar]
- 14.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 15.Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 2019;576:465–70. 10.1038/s41586-019-1836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005;353:2654–66. 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 17.Ai L, Mu S, Wang Y, et al. Prognostic role of myeloid-derived suppressor cells in cancers: a systematic review and meta-analysis. BMC Cancer 2018;18:1220. 10.1186/s12885-018-5086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidsson S, Andren O, Ohlson A-L, et al. FOXP3+ regulatory T cells in normal prostate tissue, postatrophic hyperplasia, prostatic intraepithelial neoplasia, and tumor histological lesions in men with and without prostate cancer. Prostate 2018;78:40–7. 10.1002/pros.23442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dysthe M, Parihar R. Myeloid-Derived suppressor cells in the tumor microenvironment. Adv Exp Med Biol 2020;1224:117–40. 10.1007/978-3-030-35723-8_8 [DOI] [PubMed] [Google Scholar]
- 20.Khaled YS, Ammori BJ, Elkord E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients. J Immunol Res 2014;2014:879897. 10.1155/2014/879897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudensky AY. Regulatory T cells and FOXP3. Immunol Rev 2011;241:260–8. 10.1111/j.1600-065X.2011.01018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santegoets SJ, Stam AG, Lougheed SM, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer 2014;2:31. 10.1186/s40425-014-0031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh K, Lee O-Y, Shon SY, et al. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res 2013;15:R79. 10.1186/bcr3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian B-Z, Li J, Zhang H, et al. Ccl2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222–5. 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu J, Du W, Yan F, et al. Myeloid-Derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013;190:3783–97. 10.4049/jimmunol.1201449 [DOI] [PubMed] [Google Scholar]
- 26.Knudsen ES, Vail P, Balaji U, et al. Stratification of pancreatic ductal adenocarcinoma: combinatorial genetic, stromal, and immunologic markers. Clin Cancer Res 2017;23:4429–40. 10.1158/1078-0432.CCR-17-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. 10.1016/j.ccr.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 28.Lee JW, Komar CA, Bengsch F, et al. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+);LSL-Trp53(R172H/+);Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Curr Protoc Pharmacol 2016;73:39–20. 10.1002/cpph.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network. Electronic address: andrew_aguirre@dfci.harvard.edu, Cancer Genome Atlas Research Network . Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 2017;32:e113. 10.1016/j.ccell.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801–6. 10.1126/science.1164368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the know your tumor registry trial. Lancet Oncol 2020;21:508–18. 10.1016/S1470-2045(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miksch RC, Schoenberg MB, Weniger M, et al. Prognostic impact of tumor-infiltrating lymphocytes and neutrophils on survival of patients with upfront resection of pancreatic cancer. Cancers 2019;11. 10.3390/cancers11010039. [Epub ahead of print: 03 Jan 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–96. 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biffi G, Oni TE, Spielman B, et al. Il1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 2019;9:282–301. 10.1158/2159-8290.CD-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elyada E, Bolisetty M, Laise P, et al. Cross-Species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 2019;9:1102–23. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang DZ, Ma Y, Ji B, et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res 2011;17:7015–23. 10.1158/1078-0432.CCR-11-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karamitopoulou E, Shoni M, Theoharides TC. Increased number of non-degranulated mast cells in pancreatic ductal adenocarcinoma but not in acute pancreatitis. Int J Immunopathol Pharmacol 2014;27:213–20. 10.1177/039463201402700208 [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Hwang RF, Logsdon CD, et al. Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Cancer Res 2013;73:3927–37. 10.1158/0008-5472.CAN-12-4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porcelli L, Iacobazzi RM, Di Fonte R, et al. CAFs and TGF-β signaling activation by mast cells contribute to resistance to Gemcitabine/Nabpaclitaxel in pancreatic cancer. Cancers 2019;11. 10.3390/cancers11030330. [Epub ahead of print: 07 Mar 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.JI M. Update from phase 2 study of Saridegib plus gemcitabine in patients with metastatic pancreatic cancer, 2012. [Google Scholar]
- 42.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–47. 10.1016/j.ccr.2014.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457–61. 10.1126/science.1171362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorchs L, Fernández Moro C, Bankhead P, et al. Human Pancreatic Carcinoma-Associated Fibroblasts Promote Expression of Co-inhibitory Markers on CD4+ and CD8+ T-Cells. Front Immunol 2019;10:847. 10.3389/fimmu.2019.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212–7. 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Affara NI, Ruffell B, Medler TR, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 2014;25:809–21. 10.1016/j.ccr.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunderson AJ, Kaneda MM, Tsujikawa T, et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov 2016;6:270–85. 10.1158/2159-8290.CD-15-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee KE, Spata M, Bayne LJ, et al. Hif1A deletion reveals Pro-Neoplastic function of B cells in pancreatic neoplasia. Cancer Discov 2016;6:256–69. 10.1158/2159-8290.CD-15-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-Producing B cells promote the development of pancreatic neoplasia. Cancer Discov 2016;6:247–55. 10.1158/2159-8290.CD-15-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003;4:330–6. 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 52.Fontenot JD, Rasmussen JP, Williams LM, et al. Regulatory T cell lineage specification by the forkhead transcription factor FOXP3. Immunity 2005;22:329–41. 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 53.Bengsch F, Knoblock DM, Liu A, et al. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol Immunother 2017;66:1609–17. 10.1007/s00262-017-2053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang J-E, Hajdu CH, Liot C, et al. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep 2017;20:558–71. 10.1016/j.celrep.2017.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor FOXP3. J Exp Med 2003;198:1875–86. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FOXP3. Science 2003;299:1057–61. 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 57.Kasprowicz DJ, Smallwood PS, Tyznik AJ, et al. Scurfin (FOXP3) controls T-dependent immune responses in vivo through regulation of CD4+ T cell effector function. J Immunol 2003;171:1216–23. 10.4049/jimmunol.171.3.1216 [DOI] [PubMed] [Google Scholar]
- 58.Vieira PL, Christensen JR, Minaee S, et al. Il-10-Secreting regulatory T cells do not express FOXP3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 2004;172:5986–93. 10.4049/jimmunol.172.10.5986 [DOI] [PubMed] [Google Scholar]
- 59.Zohar Y, Wildbaum G, Novak R, et al. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest 2017;127:3913. 10.1172/JCI97015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barilla RM, Diskin B, Caso RC, et al. Specialized dendritic cells induce tumor-promoting IL-10+IL-17+ FoxP3neg regulatory CD4+ T cells in pancreatic carcinoma. Nat Commun 2019;10:1424 10.1038/s41467-019-09416-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Buanec H, Gougeon M-L, Mathian A, et al. IFN-α and CD46 stimulation are associated with active lupus and skew natural T regulatory cell differentiation to type 1 regulatory T (Tr1) cells. Proc Natl Acad Sci U S A 2011;108:18995–9000. 10.1073/pnas.1113301108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 1997;389:737–42. 10.1038/39614 [DOI] [PubMed] [Google Scholar]
- 63.Magnani CF, Alberigo G, Bacchetta R, et al. Killing of myeloid APCS via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur J Immunol 2011;41:1652–62. 10.1002/eji.201041120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013;19:739–46. 10.1038/nm.3179 [DOI] [PubMed] [Google Scholar]
- 65.Johansson-Percival A, He B, Li Z-J, et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol 2017;18:1207–17. 10.1038/ni.3836 [DOI] [PubMed] [Google Scholar]
- 66.Castino GF, Cortese N, Capretti G, et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology 2016;5:e1085147. 10.1080/2162402X.2015.1085147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hiraoka N, Ino Y, Yamazaki-Itoh R, et al. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer 2015;112:1782–90. 10.1038/bjc.2015.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin L, Hu X, Zhang H, et al. Tertiary lymphoid organs in cancer immunology: mechanisms and the new strategy for immunotherapy. Front Immunol 2019;10:1398. 10.3389/fimmu.2019.01398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y, Herndon JM, Sojka DK, et al. Tissue-Resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 2017;47:e326 10.1016/j.immuni.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128–41. 10.1158/0008-5472.CAN-12-2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nywening TM, Belt BA, Cullinan DR, et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 2018;67:1112-1123. 10.1136/gutjnl-2017-313738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res 2013;19:3404–15. 10.1158/1078-0432.CCR-13-0525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao L, Lim SY, Gordon-Weeks AN, et al. Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 2013;57:829–39. 10.1002/hep.26094 [DOI] [PubMed] [Google Scholar]
- 74.Beatty GL, Winograd R, Evans RA, et al. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology 2015;149:201–10. 10.1053/j.gastro.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamath SD, Kalyan A, Kircher S, et al. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist 2020;25:e808-e815. 10.1634/theoncologist.2019-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farren MR, Sayegh L, Ware MB, et al. Immunologic alterations in the pancreatic cancer microenvironment of patients treated with neoadjuvant chemotherapy and radiotherapy. JCI Insight 2020;5. 10.1172/jci.insight.130362. [Epub ahead of print: 16 Jan 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pereira PMR, Edwards KJ, Mandleywala K, et al. iNOS regulates the therapeutic response of pancreatic cancer cells to radiotherapy. Cancer Res 2020;80:1681–92. 10.1158/0008-5472.CAN-19-2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seifert L, Werba G, Tiwari S, et al. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology 2016;150:e1655. 10.1053/j.gastro.2016.02.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Principe DR, Narbutis M, Kumar S, et al. Long-Term gemcitabine treatment reshapes the pancreatic tumor microenvironment and sensitizes murine carcinoma to combination immunotherapy. Cancer Res 2020. 10.1158/0008-5472.CAN-19-2959. [Epub ahead of print: 01 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 2013;73:3007–18. 10.1158/0008-5472.CAN-12-4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JW, Stone ML, Porrett PM, et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 2019;567:249-252. 10.1038/s41586-019-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 2002;39:531–6. 10.1016/s0161-5890(02)00210-9 [DOI] [PubMed] [Google Scholar]
- 83.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol 2009;130:27–33. 10.1016/j.clim.2008.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korn T, Mitsdoerffer M, Croxford AL, et al. Il-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A 2008;105:18460–5. 10.1073/pnas.0809850105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mace TA, Shakya R, Pitarresi JR, et al. Il-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018;67:320-332. 10.1136/gutjnl-2016-311585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamano JB, Lamano JB, Li YD, et al. Glioblastoma-Derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes tumor growth. Clin Cancer Res 2019;25:3643–57. 10.1158/1078-0432.CCR-18-2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li J, Xu J, Yan X, et al. Targeting interleukin-6 (IL-6) sensitizes anti-PD-L1 treatment in a colorectal cancer preclinical model. Med Sci Monit 2018;24:5501–8. 10.12659/MSM.907439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res 2018;78:5011–22. 10.1158/0008-5472.CAN-18-0118 [DOI] [PubMed] [Google Scholar]
- 89.Bardeesy N, Cheng K-H, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 2006;20:3130–46. 10.1101/gad.1478706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chu GC, Kimmelman AC, Hezel AF, et al. Stromal biology of pancreatic cancer. J Cell Biochem 2007;101:887–907. 10.1002/jcb.21209 [DOI] [PubMed] [Google Scholar]
- 91.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 2006;441:235–8. 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 92.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 2002;195:1499–505. 10.1084/jem.20012076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ivanoiu A, Adam S, Van der Linden M, et al. Memory evaluation with a new cued recall test in patients with mild cognitive impairment and Alzheimer's disease. J Neurol 2005;252:47–55. 10.1007/s00415-005-0597-2 [DOI] [PubMed] [Google Scholar]
- 94.Lin JT, Martin SL, Xia L, et al. Tgf-Beta 1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall: differential involvement of STAT4 and T-bet. J Immunol 2005;174:5950–8. 10.4049/jimmunol.174.10.5950 [DOI] [PubMed] [Google Scholar]
- 95.Principe DR, DeCant B, Mascariñas E, et al. Tgfβ signaling in the pancreatic tumor microenvironment promotes fibrosis and immune evasion to facilitate tumorigenesis. Cancer Res 2016;76:2525–39. 10.1158/0008-5472.CAN-15-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Principe DR, Park A, Dorman MJ, et al. Tgfβ blockade augments PD-1 inhibition to promote T-cell-mediated regression of pancreatic cancer. Mol Cancer Ther 2019;18:613–20. 10.1158/1535-7163.MCT-18-0850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014;74:5057–69. 10.1158/0008-5472.CAN-13-3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Linehan D, Noel MS, Hezel AF, et al. Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: correlation with blood monocyte counts. JCO 2018;36:92 10.1200/JCO.2018.36.5_suppl.92 [DOI] [Google Scholar]
- 99.Frisch SM, Schaller M, Cieply B. Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis. J Cell Sci 2013;126:21–9. 10.1242/jcs.120907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsia DA, Lim S-T, Bernard-Trifilo JA, et al. Integrin alpha4beta1 promotes focal adhesion kinase-independent cell motility via alpha4 cytoplasmic domain-specific activation of c-Src. Mol Cell Biol 2005;25:9700–12. 10.1128/MCB.25.21.9700-9712.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005;6:56–68. 10.1038/nrm1549 [DOI] [PubMed] [Google Scholar]
- 102.Sulzmaier FJ, Jean C, Schlaepfer DD. Fak in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014;14:598–610. 10.1038/nrc3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tancioni I, Uryu S, Sulzmaier FJ, et al. Fak inhibition disrupts a β5 integrin signaling axis controlling anchorage-independent ovarian carcinoma growth. Mol Cancer Ther 2014;13:2050–61. 10.1158/1535-7163.MCT-13-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jiang H, Liu X, Knolhoff BL, et al. Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut 2020;69:122–32. 10.1136/gutjnl-2018-317424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serrels A, Lund T, Serrels B, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell 2015;163:160–73. 10.1016/j.cell.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kasorn A, Alcaide P, Jia Y, et al. Focal adhesion kinase regulates pathogen-killing capability and life span of neutrophils via mediating both adhesion-dependent and -independent cellular signals. J Immunol 2009;183:1032–43. 10.4049/jimmunol.0802984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Owen KA, Pixley FJ, Thomas KS, et al. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol 2007;179:1275–87. 10.1083/jcb.200708093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang-Gillam A, Lockhart AC, Tan BR, et al. Phase I study of defactinib combined with pembrolizumab and gemcitabine in patients with advanced cancer. JCO 2018;36:1 10.1200/JCO.2018.36.4_suppl.380 [DOI] [Google Scholar]
- 109.Ahsan A, Ramanand SG, Whitehead C, et al. Wild-Type EGFR is stabilized by direct interaction with Hsp90 in cancer cells and tumors. Neoplasia 2012;14:670–1. 10.1593/neo.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.da Rocha Dias S, Friedlos F, Light Y, et al. Activated B-Raf is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res 2005;65:10686–91. 10.1158/0008-5472.CAN-05-2632 [DOI] [PubMed] [Google Scholar]
- 111.Sawai A, Chandarlapaty S, Greulich H, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res 2008;68:589–96. 10.1158/0008-5472.CAN-07-1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu LX, Xu JH, Zhang KZ, et al. Disruption of the Bcr-Abl/Hsp90 protein complex: a possible mechanism to inhibit Bcr-Abl-positive human leukemic blasts by novobiocin. Leukemia 2008;22:1402–9. 10.1038/leu.2008.89 [DOI] [PubMed] [Google Scholar]
- 113.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 114.Kim HL, Cassone M, Otvos L, et al. Hif-1Alpha and STAT3 client proteins interacting with the cancer chaperone Hsp90: therapeutic considerations. Cancer Biol Ther 2008;7:10–14. 10.4161/cbt.7.1.5458 [DOI] [PubMed] [Google Scholar]
- 115.Moulick K, Ahn JH, Zong H, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol 2011;7:818–26. 10.1038/nchembio.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shyamsunder P, Shanmugasundaram M, Mayakonda A, et al. Identification of a novel enhancer of CEBPE essential for granulocytic differentiation. Blood 2019;133:2507–17. 10.1182/blood.2018886077 [DOI] [PubMed] [Google Scholar]
- 117.Su Y-L, Banerjee S, White SV, et al. Stat3 in tumor-associated myeloid cells: multitasking to disrupt immunity. Int J Mol Sci 2018;19. 10.3390/ijms19061803. [Epub ahead of print: 19 Jun 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waight JD, Netherby C, Hensen ML, et al. Myeloid-Derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest 2013;123:4464–78. 10.1172/JCI68189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Graner MW. Hsp90 and immune modulation in cancer. Adv Cancer Res 2016;129:191–224. 10.1016/bs.acr.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 120.Haggerty TJ, Dunn IS, Rose LB, et al. Heat shock protein-90 inhibitors enhance antigen expression on melanomas and increase T cell recognition of tumor cells. PLoS One 2014;9:e114506. 10.1371/journal.pone.0114506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mbofung RM, McKenzie JA, Malu S, et al. Hsp90 inhibition enhances cancer immunotherapy by upregulating interferon response genes. Nat Commun 2017;8:451. 10.1038/s41467-017-00449-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Proia DA, Kaufmann GF. Targeting heat-shock protein 90 (Hsp90) as a complementary strategy to immune checkpoint blockade for cancer therapy. Cancer Immunol Res 2015;3:583–9. 10.1158/2326-6066.CIR-15-0057 [DOI] [PubMed] [Google Scholar]
- 123.Mohammad Zaidi YZ, Ware MB, Farren MR, et al. Abstract 4074: heat shock protein 90 inhibitors alter pancreatic stellate cell cytokine production and enhances the efficacy of immune checkpoint blockade in pancreatic cancer. Cancer Research 2019;79. [Google Scholar]
- 124.Bennett SR, Carbone FR, Karamalis F, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 1998;393:478–80. 10.1038/30996 [DOI] [PubMed] [Google Scholar]
- 125.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 1998;393:474–8. 10.1038/30989 [DOI] [PubMed] [Google Scholar]
- 126.Schoenberger SP, Toes RE, van der Voort EI, et al. T-Cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998;393:480–3. 10.1038/31002 [DOI] [PubMed] [Google Scholar]
- 127.Beatty GL, Chiorean EG, Fishman MP, et al. Cd40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–6. 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bajor DL, Mick R, Riese MJ, et al. Long-Term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology 2018;7:e1468956. 10.1080/2162402X.2018.1468956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hall M, Liu H, Malafa M, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer 2016;4:61. 10.1186/s40425-016-0164-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sakellariou-Thompson D, Forget M-A, Creasy C, et al. 4-1BB Agonist Focuses CD8+ Tumor-Infiltrating T-Cell Growth into a Distinct Repertoire Capable of Tumor Recognition in Pancreatic Cancer. Clin Cancer Res 2017;23:7263–75. 10.1158/1078-0432.CCR-17-0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ma S, Li X, Wang X, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci 2019;15:2548–60. 10.7150/ijbs.34213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poschke IC, Hassel JC, Rodriguez-Ehrenfried A, et al. The Outcome of Ex Vivo TIL Expansion Is Highly Influenced by Spatial Heterogeneity of the Tumor T-Cell Repertoire and Differences in Intrinsic In Vitro Growth Capacity between T-Cell Clones. Clin Cancer Res 2020. 10.1158/1078-0432.CCR-19-3845. [Epub ahead of print: 17 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 133.Akce M, Zaidi MY, Waller EK, et al. The potential of CAR T cell therapy in pancreatic cancer. Front Immunol 2018;9:2166. 10.3389/fimmu.2018.02166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans 2016;44:412–8. 10.1042/BST20150291 [DOI] [PMC free article] [PubMed] [Google Scholar]