Abstract
Cardiovascular disease (CVD) is the leading cause of death worldwide and one key factor associated with the increased CVD risk is dyslipidemia. Statin therapy remains the first-line treatment to manage dyslipidemia, yet many patients do not achieve optimal low-density lipoprotein-cholesterol (LDL-C) levels even after taking moderate- or high-intensity statins; therefore, additional, non-statin therapy is often needed. Bempedoic acid is a prodrug that, once activated, decreases LDL-C levels by the inhibition of adenosine triphosphate citrate lyase in the liver. Five clinical trials have demonstrated the safety and efficacy of bempedoic acid and the bempedoic acid/ezetimibe combination in lowering LDL-C in patients with atherosclerotic CVD and heterozygous familial hypercholesterolemia and also in high-risk primary prevention, and statin-intolerant patients. Bempedoic acid has been demonstrated to lower LDL-C levels by 15–25% in clinical trials and up to 38% when combined with ezetimibe. In 2020, the FDA approved bempedoic acid. Furthermore, the combination of bempedoic acid with ezetimibe is FDA approved for the treatment of adults with heterozygous familial hypercholesterolemia or established atherosclerotic CVD who require additional LDL-C lowering after maximally tolerated statin therapy. The ongoing CLEAR OUTCOMES trial aims to evaluate whether bempedoic acid can reduce cardiovascular events in patients with statin intolerance and results will be available in the next 3 years. This outcomes trial will be pivotal for determining the role of bempedoic acid in the non-statin lipid-lowering armamentarium.
Keywords: bempedoic acid, cardiovascular events, dyslipidemia, hypercholesterolemia, low-density lipoprotein-cholesterol
Introduction
The leading cause of death in the United States continues to be cardiovascular disease (CVD).1 Dyslipidemia is one of the primary causal risk factors for the development of atherosclerotic CVD (ASCVD) and is one of the seven critical metrics that the American Heart Association (AHA) has used to define cardiovascular health in adults and children. The 2018 AHA/American College of Cardiology (ACC)/Multisociety Cholesterol Guidelines recommend the treatment of dyslipidemia for both the primary and secondary prevention of ASCVD.2 Statin therapy remains the first-line recommended treatment for dyslipidemia when the primary lipid abnormality is an increase in total and low-density lipoprotein-cholesterol (LDL-C), as an approach to prevent ASCVD in the primary and secondary prevention population. Extensive evidence over numerous trials supports the role of statins to reduce LDL-C levels and subsequently reduce major cardiovascular (CV) events in both primary and secondary prevention patients.3 Over the last 5 years, there have been other drug classes that have demonstrated further CV event lowering when added to statin therapy in the secondary prevention of the ASCVD population. Ezetimibe and proprotein convertase subtilisin/kexin 9 (PCSK9) inhibitors have been shown to lower the risk of CV events in patients with established ASCVD when added to statin therapy.4–6 Based on these findings, recommendations for the use of ezetimibe and/or PCSK9 inhibitors in addition to statin therapy in the high-risk and very high-risk ASCVD populations exist in the 2018 AHA/ACC/Multisociety Cholesterol Guidelines.2
Evidence continues to support the linear relationship of LDL-C and CVD.7–9 Because of this, there is continued research to evaluate the benefits of lowering LDL-C in ways beyond use of traditional medications with demonstrated benefit (e.g. statins, ezetimibe, PCSK9 inhibitors). The role of non-statin therapy in patients who develop stain intolerance is an additional reason to seek alternative approaches to lower LDL-C levels beyond the traditional first-line therapy with statins. The ultimate goal of further lowering LDL-C levels is to prevent CV events in primary and secondary prevention populations.
This narrative review aims to present and discuss the current evidence regarding the use of bempedoic acid for LDL-C lowering in patients with dyslipidemia and its potential role as an agent to lower CV events. We conducted an English language MEDLINE search through July 24th, 2020, using the search terms ‘bempedoic acid,’ ‘ETC-1002,’ ‘hypercholesterolemia,’ and ‘dyslipidemia.’ A manual search for references identified within these trials and review articles was performed to identify further relevant articles.
Mechanism of action
Bempedoic acid was developed under the name ETC-1002 and its chemical name is 8-hydroxy-2,2,14,14-tetramethylpentadecaned-ioic acid.10 It is a prodrug that, once activated, decreases LDL-C by inhibition of adenosine triphosphate-citrate lyase (ACL) in the liver. By inhibiting ACL, an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzme A reductase, bempedoic acid decreases the conversion of mitochondrial-derived citrate to cytosolic ACL, creating less substrate for cholesterol and fatty acid synthesis.11,12 This ultimately decreases liver cholesterol synthesis and decreases serum LDL-C levels by upregulating LDL receptors.10,13 Additionally, bempedoic acid activates adenosine monophosphate-activated protein kinase, which has been demonstrated in mice models to inhibit acetyl-CoA carboxylase and hydroxymethylglutaryl-CoA reductase, decreasing the synthesis of fatty acids and cholesterol.14 Bempedoic acid and its active metabolite, ESP15228, require activation by very long-chain acyl-CoA synthetase I (ACSVL1) to ETC-1002-CoA and ESP15228-CoA, respectively, in order to exert their therapeutic effects.10 The enzyme ACSVL1 is present in the liver but not in skeletal muscle, decreasing the risk for muscle-related adverse effects.12 The mechanism of action for cholesterol lowering with bempedoic acid versus statin therapy can be reviewed in Figure 1.
Figure 1.
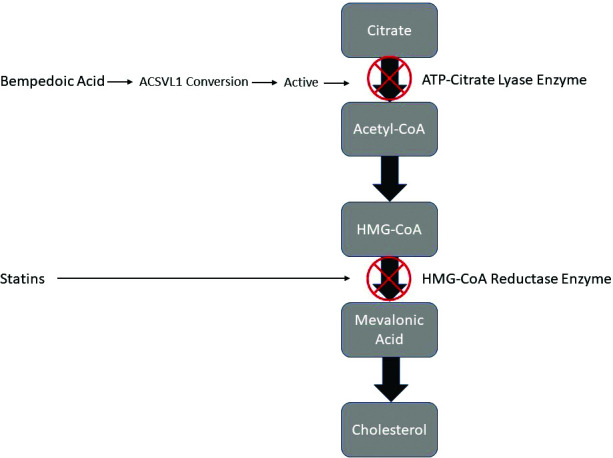
Mechanism of action of cholesterol lowering with bempedoic acid versus statins.
Phase I trials
Following completion of in vitro and in vivo animal studies, bempedoic acid was studied in humans in phase I and II clinical trials. Two phase Ia studies evaluated the effect of a single dose of bempedoic acid in healthy volunteers. In the first phase Ia study, 18 healthy volunteers were given a bempedoic acid dose of 2.5, 10, 45, 125, or 250 mg and pharmacokinetic data were collected.15 In the second phase Ia study, 6 healthy male volunteers were given a carbon-14 radiolabeled dose of bempedoic acid to evaluate the absorption, metabolism, and excretion of the drug.16 Two phase Ib multiple ascending dose studies evaluated the safety, tolerability, pharmacokinetics, and pharmacodynamics of bempedoic acid in patients with mild dyslipidemia (n=39) and healthy volunteers (n=18), respectively.17,18 Mild dyslipidemia in the first study was defined as a fasting LDL-C level of 100–160 mg/dL or fasting triglyceride levels of 100–350 mg/dL, and patients received the drug for either 14 or 28 days.17 The study of healthy volunteers demonstrated a mean decrease in LDL-C levels of 36% in the bempedoic acid group compared to a 4% increase in the placebo group (40% placebo-adjusted LDL-C reduction; p<0.0001).18,19 The phase I trials demonstrated that there were no adverse effects related to bempedoic acid dosing and bempedoic acid was overall determined to be safe to proceed with phase II trials.
Phase II trials
Phase II clinical trials of bempedoic acid were conducted in patients with dyslipidemia, with or without other common comorbidities, to assess the efficacy and safety of the drug in its intended population. Bempedoic acid was studied both as monotherapy and in combination with other lipid-lowering agents.
Monotherapy
The first phase II study evaluated 177 patients with dyslipidemia, defined as LDL-C levels of 130–220 mg/dL and triglyceride levels of either <150 mg/dL or 150–400 mg/dL, treated with bempedoic acid monotherapy or placebo for 12 weeks. Patients treated with bempedoic acid, 40, 80, or 120 mg, daily experienced a reduction in LDL-C levels of 18%, 25%, and 27%, respectively, compared with a 2% reduction in the placebo group (p<0.0001). Levels of atherogenic biomarkers apolipoprotein B (apo B), non-high-density lipoprotein C (non-HDL-C), and LDL particle number were significantly reduced in the bempedoic acid-treated patients compared to those who received placebo (p<0.0001). There was a trend of reduction in high-sensitivity C-reactive protein (hsCRP) levels in patients treated with bempedoic acid versus those treated with placebo (26% versus 2%), which was further amplified in patients with elevated hsCRP at baseline (43–64% versus 7%).20
A second phase II monotherapy study evaluated bempedoic acid specifically in patients with type 2 diabetes. Patients were randomized to placebo or bempedoic acid 80 mg daily with a run-in period of 2 weeks and a subsequent 2-week period of 120 mg daily. Bempedoic acid demonstrated a 43% reduction in LDL-C levels compared to a 4% reduction in the placebo arm at 4 weeks (p<0.0001). In patients who had a baseline LDL-C level of >100 mg/dL, 88% of those treated with bempedoic acid achieved an LDL-C level of <100 mg/dL compared to only 4% of those who received placebo (p<0.0001). Similar to the previous trial, hsCRP levels decreased by 41% in the bempedoic acid treatment arm compared to 11% in the placebo treatment arm. A 24-hour continuous glucose monitoring assessment demonstrated a non-significant trend toward improved glycemia in the bempedoic acid group.21
Lastly, bempedoic acid monotherapy was evaluated in 143 patients with both dyslipidemia and hypertension. Before study entry, all patients enrolled were taken off their blood pressure-lowering and lipid-lowering medications for a washout period. After 6 weeks, there was a statistically significant lowering of LDL-C compared to placebo, with a respective decrease of 21% versus an increase of 3% (p<0.0001). Further, levels of hsCRP increased by 20% in the placebo-treated patients compared to a 25% lower level in patients treated with bempedoic acid (p<0.0001). There was no change in blood pressure in the bempedoic acid group.22
Monotherapy in patients with statin intolerance
Bempedoic acid has been hypothesized to yield little-to-no risk of muscle-related side effects. The rationale for this hypothesis is that bempedoic acid does not get converted to the active form in skeletal muscle by the enzyme ACSVL1, and only gets converted to its active form in the liver.12 Two phase II trials of patients with dyslipidemia and a history of statin intolerance aimed to explore this. Thompson et al.23 randomized 56 patients with dyslipidemia and a history of statin intolerance (defined as new myalgia, muscle cramps, muscle aches, or muscle weakness that developed during statin treatment and resolved within 4 weeks of statin discontinuation) to either bempedoic acid or placebo for 8 weeks. Patients in the bempedoic acid group received increasing doses of 60, 120, 180, and 240 mg for 2 weeks each during the course of the study. A reduction in LDL-C levels from baseline to week 8 was the designated primary endpoint. Patients treated with bempedoic acid had lowered LDL-C levels by a mean of 32% compared to 3% in patients treated with placebo (p<0.0001). None of the patients treated with bempedoic acid dropped out of the study due to a muscle-related adverse effect. Similar to the previous phase II studies,20,21 hsCRP levels decreased by 42% in the bempedoic acid group compared to no change in the group that received placebo (p=0.0022).23
Another study by Thompson et al.24 compared bempedoic acid versus ezetimibe versus the combination of the two agents in 177 patients with a history of statin intolerance (n=177) and in 171 patients without a history of statin intolerance. Statin intolerance was defined as an intolerance to ≥2 statins, with at least one at the lowest therapeutic dose. Patients were stratified 1:1 by history of statin intolerance and then randomized 4:4:4:1 to bempedoic acid 120 mg daily, bempedoic acid 180 mg daily, ezetimibe 10 mg daily, bempedoic acid 120 mg daily plus ezetimibe 10 mg daily, or bempedoic acid 180 mg daily plus ezetimibe 10 mg daily. In the bempedoic acid monotherapy groups, the reduction in LDL-C levels was 27.5% for 120 mg daily and 30.1% for 180 mg daily (p=0.15) and this reduction was similar in statin-intolerant versus statin-tolerant patients. The reduction in LDL-C was 21.2% for the ezetimibe monotherapy group. There were significantly greater LDL-C reductions in the bempedoic acid plus ezetimibe groups (43.1% for the 120 mg daily group and 47.7% for the 180 mg daily group), with the decrease being approximately equal to a sum of each individual drug’s LDL-C lowering ability. There was no difference noted in the incidence of adverse effects between bempedoic acid and ezetimibe. More statin-intolerant patients experienced an adverse effect that led to discontinuation (n=7) compared to statin-tolerant patients (n=3). Muscle-related adverse effects were less frequent and caused fewer study discontinuations in the bempedoic acid monotherapy group and were more common in statin-intolerant patients. The authors concluded that bempedoic acid monotherapy (or in combination with ezetimibe) is a safe and efficacious treatment in patients with or without a statin intolerance.24
Combination therapy with statins
Bempedoic acid has been studied in two phase II trials where it was added to statin therapy in patients with dyslipidemia. In one 8-week study, bempedoic acid in escalating doses (60 mg daily for 2 weeks, 120 mg daily for 2 weeks, 180 mg daily for 2 weeks, and 240 mg daily for 2 weeks) was added to atorvastatin 10 mg daily and compared against daily placebo added to atorvastatin 10 mg daily. LDL-C levels were lowered by 22% in the bempedoic acid arm and no reduction was seen in the placebo arm (p<0.0001).19,25 In a study by Ballantyne et al.,26 134 patients with LDL-C levels between 115 and 220 mg/dL while taking atorvastatin ≤20 mg daily, pravastatin ≤40 mg daily, rosuvastatin ≤10 mg daily, or simvastatin ≤20 mg daily were randomized to receive bempedoic acid 120 mg, 180 mg, or placebo daily for 12 weeks. Patients who had bempedoic acid added onto concurrent statin therapy experienced an LDL-C reduction of 17% (120 mg daily) and 24% (180 mg daily) compared to 4% with placebo (p=0.0055 and p<0.0001, respectively). Bempedoic acid also decreased apo B, non-HDL-C, and total cholesterol (TC) levels to a greater extent than placebo (p<0.05). The overall incidence of adverse effects was similar between groups and muscle-related adverse effects were more common in the placebo group (13%) than in either bempedoic acid group (2–5%). The authors concluded that in patients with elevated LDL-C levels despite statin therapy, bempedoic acid was an efficacious and safe addition to therapy for additional lipid lowering.26
Phase III trials
Following successful completion of phase II clinical trials, bempedoic acid was studied in the phase III trial series named ‘CLEAR’ (Table 1).
Table 1.
Summary of dyslipidemia phase III trials with bempedoic acid.
| Trial | Design | Participants | Duration, weeks | Intervention, total daily mg (n) | Primary outcome | Secondary outcome |
|---|---|---|---|---|---|---|
| CLEAR HARMONY (2019)27 | MC, OL | ASCVD and/or HeFH on MTS with LDL-C ≥70 mg/dL | 52 | BA 180 (1488), P (742) | Number of participants with treatment-related adverse events (78.7% P versus 78.5% BA; p=0.91) | Percentage change in LDL-C at 52 weeks (1.6% P versus −16.5% BA; p<0.001) |
| CLEAR WISDOM (2019)28 | MC, DB, PC | ASCVD and/or HeFH on MTS with LDL-C ≥70 mg/dL | 52 | BA 180 (522), P (257) | Percentage change in LDL-C at 12 weeks (2.4% P versus −15.1% BA; p<0.001) | Percentage change in LDL-C at 24 weeks (2.7% P versus −12.1% BA; p<0.001) significant reductions in non-HDL-C, TC, Apo B, and hsCRP at 12 weeks |
| CLEAR SERENITY (2019)29 | MC, DB, PC | Primary prevention with LDL-C ≥130 mg/dL or HeFH with LDL-C ≥100 mg/dL or ASCVD with a history of statin intolerance | 24 | BA 180 (234), P (111) | Percentage change in LDL-C at 12 weeks (−1.3% P versus −23.6% BA; p<0.001) | Percentage change in non-HDL-C at 12 weeks (−0.4% P versus −19.0% BA; p<0.001) Percentage change in TC at 12 weeks (−0.6% P versus −16.1% BA; p<0.001) Percentage change in Apo B at 12 weeks (−0.2% P versus −15.5% BA; p<0.001) Percentage change in hsCRP at 12 weeks (2.7% P versus −25.4% BA; p<0.001) |
| CLEAR TRANQUILITY (2018)30 | MC, DB, PC | LDL-C ≥100 mg/dL with a history of statin intolerance on stable LLT | 12 | BA 180 + E 10 (181), P+E 10 (88) | Percentage change in LDL-C at 12 weeks (5.0% P+E versus −22.5% BA+E; p<0.001) | Percentage change in non-HDL-C at 12 weeks (5.2% P+E versus −18.4% BA+E; p<0.001) Percentage change in TC at 12 weeks (2.9% P+E versus −15.1% BA+E; p<0.001) Percentage change in Apo B at 12 weeks (4.7% P+E versus −14.6% BA+E; p<0.001) Percentage change in hsCRP at 12 weeks (2.1% P+E versus −32.5% BA+E; p<0.001) |
| Ballantyne et al., (2020)31 | MC, DB, PC | ASCVD or HeFH or multiple CV risk factors on MTS | 12 | BA 180 + E 10 (108), BA 180 (111), E 10 (109), P (55) | Percentage change in LDL-C (1.6% P versus −36.2% BA+E; p<0.001), (−23.2% E versus −36.2% BA+E; p<0.001), (−17.2% BA versus −36.2% BA+E; p<0.001) | Percentage change in hsCRP (21.6% P versus −35.1% BA+E; p<0.001), (−8.2% E versus −35.1% BA+E; p=0.002), (−31.9% BA versus −35.1% BA+E; NS) |
| CLEAR OUTCOMES (anticipated completion 2022) | RC, MC, DB, PC | ASCVD or high CV risk with LDL-C ≥100 mg/dL with a history of statin intolerance | Anticipated 3.75 years | BA 180, P (14,014 enrolled) | First occurrence of CV death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina, or coronary revascularization | Percentage change in LDL-C, non-HDL-C, TC, Apo B, hsCRP |
Apo B = apolipoprotein B; ASCVD, atherosclerotic cardiovascular disease; BA, bempedoic acid; CLEAR, Cholesterol Lowering via Bempedoic Acid, an ACL-inhibiting Regimen; CV, cardiovascular; DB, double blind; E, ezetimibe; HDL-C, high-density lipoprotein-cholesterol; HeFH, heterozygous familial hypercholesterolemia; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein-cholesterol; MC, multicenter; MTS, maximum tolerated statin; NS, not significant; OL, open label; PC, placebo controlled; R, randomized; TC, total cholesterol.
CLEAR HARMONY
The Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) HARMONY trial was a phase III trial of bempedoic acid that assessed its safety and efficacy over 1 year.27 The randomized, placebo-controlled trial enrolled 2230 patients with ASCVD, heterozygous familial hypercholesterolemia (HeFH), or both. The mean age was 66.1 years with a baseline mean LDL-C level of 103.2 mg/dL. Patients were randomized to bempedoic acid 180 mg daily or placebo with two bempedoic acid participants for every one placebo participant. Patients had to be receiving maximally tolerated statin therapy with an LDL-C level of at least 70 mg/dL. The primary endpoint assessed the number of participants with treatment-related adverse events. No difference in the incidence of adverse events was seen between the bempedoic acid group (78.5%, 1167/1487) and the placebo group (78.7%, 584/742) (p=0.91). The secondary endpoint of change in LDL-C at 12 weeks demonstrated a reduction in LDL-C levels by 19.2 mg/dL in the bempedoic acid arm and lowered LDL-C levels by 16.5% more than placebo (p<0.001). Additional secondary endpoints of non-HDL-C, TC, and apo B demonstrated statistically significant improvements compared to placebo. Similar rates of serious adverse events occurred in bempedoic acid and placebo groups (14.5 and 14.0%, respectively). There was a slightly higher rate of discontinuation in the bempedoic acid group (10.9%) compared to that in the placebo group (7.1%). Of note, there was a small, but statistically significant increase in uric acid in the bempedoic acid group compared to placebo. Overall, the positive safety and LDL-C efficacy findings of CLEAR HARMONY led to the 2020 FDA approval of bempedoic acid in addition to statin therapy to further lower LDL-C in patients with HeFH or established ASCVD.
CLEAR WISDOM
The Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) WISDOM trial was a phase III trial of bempedoic acid that assessed its efficacy over 1 year.28 The randomized, double-blind, placebo-controlled trial enrolled 1049 patients with ASCVD, HeFH, or both. The mean age was 64.3 years with a baseline mean LDL-C level of 120.4 mg/dL. Patients were randomized to bempedoic acid 180 mg daily or placebo with two bempedoic acid participants for every placebo participant. Patients had to be receiving maximally tolerated statin therapy with an LDL-C of at least 70 mg/dL. A reduction in LDL-C levels at 12 weeks was the primary endpoint. The bempedoic acid treatment arm demonstrated a 15.1% reduction in LDL-C levels versus a 2.4% reduction with placebo (p<0.001). The secondary endpoints of non-HDL-C (−13.0%; p<0.001), TC (−11.2%; p<0.001), apo B (−13.0%; p<0.001), and hsCRP (−8.7%, p=0.04) levels demonstrated statistically significant improvements compared to placebo. Common adverse events included hyperuricemia (4.2% bempedoic acid versus 1.9% placebo), nasopharyngitis (5.2% bempedoic acid versus 5.1% placebo), and urinary tract infections (5.0% bempedoic acid versus 1.9% placebo). Overall, the findings of CLEAR WISDOM aligned with those of CLEAR HARMONY, demonstrating the LDL-C-lowering ability of bempedoic acid in a high CV risk population.
CLEAR SERENITY
The Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) SERENITY trial was a phase III trial of bempedoic acid that assessed its efficacy and safety over 24 weeks.29 The double-blind, placebo-controlled trial enrolled 345 patients with hypercholesterolemia and a history of intolerance to at least two statins with one at the lowest available dose. Both primary and secondary prevention patients were enrolled. Primary prevention patients needed to have an LDL-C level of ≥130 mg/dL. Patients with HeFH or ASCVD needed to have an LDL-C level of ≥100 mg/dL to be enrolled. The mean age was 65.2 years with a baseline mean LDL-C level of 157.6 mg/dL and 93% of patients had a history of statin-associated muscle symptoms. As in the previous CLEAR trials, patients were randomized to bempedoic acid 180 mg daily or placebo with double the number of patients enrolled in the bempedoic acid arm compared to the placebo arm. The primary endpoint was percent change in LDL-C levels from baseline at 12 weeks. The bempedoic acid treatment arm demonstrated a 23.6% reduction in LDL-C levels versus a 1.3% reduction in the placebo treatment arm (p<0.001). The additional secondary endpoints of non-HDL-C (−18.6%; p<0.001), TC (−15.5%; p<0.001), apo B (−15.3%; p<0.001), and hsCRP (−28.1%, p<0.001) levels demonstrated statistically significant improvements compared to placebo. Myalgia was the most common muscle-related adverse event and occurred in 4.7% of patients treated with bempedoic acid compared to 7.2% in patients treated with placebo. The findings of CLEAR SERENITY demonstrated that bempedoic acid could be considered a safe and effective LDL-C-lowering therapy for patients with a history of statin intolerance.
CLEAR TRANQUILITY
The Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) TRANQUILITY trial was a phase III trial of bempedoic acid plus ezetimibe that assessed its efficacy and safety over 24 weeks.30 The multicenter, randomized, double-blind, placebo-controlled trial enrolled 269 patients with a history of statin intolerance and an LDL-C level of ≥100 mg/dL on stable lipid-lowering therapy. The mean age was 63.8 years with a baseline LDL-C level of 127.6 mg/dL and 25% had established ASCVD. All patients received a 4-week run-in with ezetimibe 10 mg daily and were then randomized to bempedoic acid 180 mg daily or placebo. Percent change in LDL-C from baseline at 12 weeks was the primary efficacy endpoint. The bempedoic acid treatment arm demonstrated a 23.5% reduction in LDL-C levels versus a 5.0% increase in the placebo treatment arm (p<0.001). The additional secondary endpoints of non-HDL-C (−23.6%; p<0.001), TC (−18.0%; p<0.001), apo B (−19.3%; p<0.001), and hsCRP (−34.5%, p<0.001) levels demonstrated statistically significant improvements compared to placebo. Muscle-related treatment-emergent adverse events occurred in 3.3% of patients treated with bempedoic acid and in 3.4% of those treated with placebo. The findings of CLEAR TRANQUILITY additionally support the fact that bempedoic acid can be safely used in patients with a history of statin intolerance who need LDL-C lowering.
Ballantyne CM, et al
Ballantyne et al.31 conducted a phase III trial of bempedoic acid plus ezetimibe that assessed their efficacy and safety over a 24-week trial. The multicenter, double-blind, placebo-controlled trial enrolled 301 patients with established ASCVD, HeFH, or multiple CV risk factors. The mean age was 64.3 years with most patients having a baseline LDL-C level of >130 mg/dL and 62.5% having established ASCVD and/or HeFH. All patients were randomized and received either bempedoic acid 180 mg daily and ezetimibe 10 mg daily (fixed dose combination), bempedoic acid 180 mg daily, ezetimibe 10 mg daily, or placebo. Percent change in LDL-C levels from baseline at 12 weeks was the primary efficacy endpoint. The bempedoic acid and ezetimibe treatment arm demonstrated a 36.2% reduction in LDL-C levels versus a 17.2% reduction in the bempedoic acid arm (p<0.001) versus a 23.2% reduction in the ezetimibe arm (p<0.001) versus a 1.0% increase in the placebo treatment arm (p<0.001). There was a 35.1% reduction in the secondary endpoint of hsCRP in the bempedoic acid and ezetimibe treatment arm compared to a reduction of 31.9% in the bempedoic acid arm (non-significant) compared to a reduction of 8.2% in the ezetimibe arm (p=0.002) compared to a 21.6% increase in the placebo treatment arm (p<0.001). Treatment-related adverse events were more common in the bempedoic acid plus ezetimibe (62.4%) and the bempedoic acid (65.9%) arms than in the ezetimibe (54.7%) or placebo (43.9%) arms. When assessing muscle-related adverse events, there was no difference between arms and the incidence was within 7–8%. Overall, the findings from this study support the LDL-C-lowering ability of the combination with bempedoic acid plus ezetimibe and did not elucidate any safety issues with this combination.
FDA-approved indication
The US Food and Drug Administration (FDA) approved bempedoic acid (Nexletol®) in February 2020, for the treatment of adults with HeFH or established ASCVD who require additional lowering of LDL-C. The FDA-approved dose combination with maximally tolerated statin therapy is 180 mg administered orally once daily. Following the approval of bempedoic acid by just a few days was the combination product of bempedoic acid and ezetimibe (Nexlizet®) as a single tablet. The combination product was approved by the FDA for the same indication as bempedoic acid.
Current guideline recommendations
No recommendations for the use of bempedoic acid are available for any of the major cholesterol guidelines from AHA/ACC, the National Lipid Association, or the European Society of Cardiology. It is anticipated that, with the recent FDA approval of bempedoic acid, there will be future recommendations for the role of bempedoic acid in managing dyslipidemia. Guideline recommendations should continue to be followed, within which the only non-statin therapy recommendations at this time are for the addition of ezetimibe and/or PCSK9 inhibitors to maximally tolerated statin therapy in patients with established ASCVD and/or FH considered high risk or very high risk. It is likely that future recommendations for the role of bempedoic acid will include recommendations for patients with statin intolerance.
Ongoing trials of bempedoic acid
CLEAR OUTCOMES
The Cholesterol Lowering via Bempedoic Acid, an ACL-Inhibiting Regimen (CLEAR) OUTCOMES trial is a phase III bempedoic acid trial.32 The multicenter, randomized, double-blind, placebo-controlled trial is designed to evaluate whether bempedoic acid can reduce the risk of CVD in patients who are statin intolerant. There are currently 14,014 patients enrolled with an anticipated completion date of December 2020.31
Patients are randomized to bempedoic acid 180 mg daily or placebo. Time to first occurrence of a major CV event, including CV death, non-fatal myocardial infarction, non-fatal stroke, or coronary revascularization, is the primary endpoint for the clinical trial. To be enrolled, patients need to be between 18 and 85 years of age and have established ASCVD or be high risk for ASCVD, a reported statin intolerance to at least two statins with one at a low dose, and a baseline LDL-C level of ≥100 mg/dL. Patients are excluded if they have severe hypertriglyceridemia, uncontrolled hypertension, uncontrolled diabetes, a history of severe heart failure, or have had a major CV event within 3 months of screening. The results of this trial will be instrumental in determining optimal treatment approaches for patients with established statin intolerance that will ultimately lower their risk of CV events through non-statin lipid-lowering therapy.
Implications for practice
The recent FDA approval of bempedoic acid and the fixed dose combination of bempedoic acid with ezetimibe for patients with established ASCVD or HeFH on maximally tolerated statins needing further LDL-C lowering will impact future lipid management. With five clinical trials demonstrating LDL-C-lowering ability across patient populations, including ASCVD, HeFH, high risk for ASCVD, and statin intolerant patients, there is a need and role for this addition to the non-statin therapy options in maximizing CV risk-reduction therapy. A recent pooled analysis of all the phase III bempedoic acid trials has been published.33 This pooled analysis reinforced that bempedoic acid is safe and efficacious to lower LDL-C levels compared to placebo when added to statin therapy or in those patients with statin intolerance. Overall, there remains a need to assess patients’ statin therapy with coadministration for several reasons. First, has the statin therapy been maximized to the highest tolerable dose to optimize LDL-C levels based on indication? Secondly, does the patient have a history of statin intolerance and can the patient tolerate any dose of statin? If they cannot tolerate any dose of statin, then the patient will likely need to utilize multiple non-statin approaches (e.g. bempedoic acid plus ezetimibe) to achieve an LDL-C lowering that matches a moderate-intensity statin LDL-C-lowering range. This combination has been demonstrated in clinical trials to lower LDL-C levels by 36% and, when given as monotherapy, bempedoic acid and ezetimibe have been respectively shown to lower LDL-C levels by 15–23% and by 13–20%.31,34 Thirdly, if the patient is on simvastatin or lovastatin, the dose limits are of no more than 20 mg daily for simvastatin or 40 mg daily for lovastatin while coadministered with bempedoic acid. If following the 2018 AHA/ACC/Multisociety Cholesterol Guidelines, ezetimibe remains the first add-on to maximally tolerated statin therapy in patients with established ASCVD needing additional LDL-C-lowering therapy. This is based on the efficacy of ezetimibe demonstrated in the IMPROVE-IT trial and the low cost of ezetimibe (need IMPROVE-IT reference). The estimated cost of a 30-day supply of bempedoic acid is US$396 based on average wholesale pricing, which is much greater than ezetimibe. Bempedoic acid and PCSK9 inhibitors could be considered as additional lipid-lowering therapies. PCSK9 inhibitors have been demonstrated, in clinical trials, to lower LDL-C levels by 40–72% in monotherapy and in combination with statin therapy.35,36 Based on their high degree of LDL-C lowering, the choice to initiate therapy with a PCSK9 will depend on how far the patient is from their LDL-C threshold, if there exists a history of intolerance to lipid-lowering therapy, and on medication accessibility (e.g. cost, formulary inclusion).
Conclusion
Bempedoic acid and the combination of bempedoic acid with ezetimibe are approved by the FDA for the treatment of adults with HeFH or established ASCVD who require additional LDL-C lowering after maximally tolerated statin therapy. Five clinical trials have demonstrated the safety and efficacy of bempedoic acid and the bempedoic acid/ezetimibe combination in lowering LDL-C in patients with ASCVD or HeFH, in high-risk primary prevention, and in statin-intolerant patients. The ongoing CLEAR OUTCOMES trial aims to evaluate whether bempedoic acid can reduce CV events in patients with statin intolerance and results will be available in the next 3 years. This outcomes trial will be pivotal for determining the role of bempedoic acid in the non-statin lipid-lowering armamentarium.
Acknowledgements
None.
Footnotes
Contributions: All authors contributed equally to the preparation of this review. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2020/08/dic.2020-6-5-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2020 Marrs JC, Anderson SL. https://doi.org/10.7573/dic.2020-6-5. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Virani AA, Alonso A, Benjamin EJ, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/AGS? APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 5.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular Disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Steg PG, Szarek M, et al. ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarcho JA, Keaney JF., Jr Proof that lower is better: LDL cholesterol and IMPROVE-IT. N Engl J Med. 2015;372:2448–2450. doi: 10.1056/NEJMe1507041. [DOI] [PubMed] [Google Scholar]
- 9.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis [published correction appears in JAMA. 2018 Oct 2;320(13):1387] JAMA. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nexletol (bempe acid) [package insert] Ann Arbor, MI: Esperion Therapeutics, Inc; 2020. [Accessed June 5, 2020]. https://pi.esperion.com/nexletol/nexletol-pi.pdf. [Google Scholar]
- 11.Lemus HN, Mendivil CO. Adenosine triphosphate citrate lyase: emerging target in the treatment of dyslipidemia. J Clin Lipidol. 2015;9(3):384–389. doi: 10.1016/j.jacl.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Ruscica M, Banach M, Sahebkar A, Corsini A, Sirtori CR. ETC-1002 (Bempedoic acid) for the management of hyperlipidemia: from preclinical studies to phase 3 trials. Expert Opin Pharmacother. 2019;20(7):791–803. doi: 10.1080/14656566.2019.1583209. [DOI] [PubMed] [Google Scholar]
- 13.Pathy K. Bempedoic acid a small molecule drug process and synthesis, innovation and/or advantages, development status and/or regulatory status. Sur Cas Stud Op Acc J. 2019;3(1):222–227. doi: 10.32474/scsoaj.2019.03.000154. [DOI] [Google Scholar]
- 14.Samsoondar JP, Burke AC, Sutherland BG, et al. Prevention of diet-induced metabolic dysregulation, inflammation, and atherosclerosis in ldlr−/− mice by treatment with the atp-citrate lyase inhibitor bempedoic acid. Arterioscler Thromb Vasc Biol. 2017;37:647–656. doi: 10.1161/ATVBAHA.116.308963. [DOI] [PubMed] [Google Scholar]
- 15.Esperion Therapeutics I. Form 10-K – annual report United States Securities and Exchange Commission. Mar 10, 2015. [Accessed June 5, 2020]. http://www.sec.gov/Archives/edgar/data/1434868/000104746915001908/0001047469-15-001908-index.htm.
- 16.Esperion Therapeutics. Single Radiolabeled Dose Study to investigate the absorption, metabolism and excretion of [14C]-ETC-1002. [Accessed June 5, 2020]. https://clinicaltrials.gov/ct2/show/record/NCT02044627. NLM identifier: NCT02044627.
- 17.Espirion Therapeutics. A Multiple Ascending Dose Study of ETC-1002 in subjects with mild dyslipidemia. [Accessed June 5, 2020]. https://clinicaltrials.gov/ct2/show/NCT01105598. NLM identifier: NCT01105598.
- 18.Esperion Therapeutics. A Multiple Ascending Dose Study of ETC-1002 in healthy subjects. [Accessed June 5, 2020]. https://clinicaltrials.gov/ct2/show/NCT01485146. NLM identifier: NCT01485146.
- 19.Bilen O, Ballantyne CM. Bempedoic acid (ETC-1002): an Investigational Inhibitor of ATP Citrate Lyase. Curr Atheroscler Rep. 2016;18(10):61. doi: 10.1007/s11883-016-0611-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballantyne CM, Davidson MH, Macdougall DE, et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. J Am Coll Cardiol. 2013;62:1154–1162. doi: 10.1016/j.jacc.2013.05.050. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–683. doi: 10.1161/ATVBAHA.113.302677. [DOI] [PubMed] [Google Scholar]
- 22.Espirion Therapeutics. Evaluation of ETC-1002 in patients with hypercholesterolemia and hypertension. [Accessed June 5, 2020]. https://clinicaltrials.gov/ct2/show/NCT02178098. NLM identifier: NCT02178098.
- 23.Thompson PD, Rubino J, Janik MJ, et al. Use of ETC-1002 to treat hypercholesterolemia in patients with statin intolerance. J Clin Lipidol. 2015;9:295–304. doi: 10.1016/j.jacl.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PD, Macdougall DE, Newton RS, et al. Treatment with ETC-1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol. 2016;10:556–567. doi: 10.1016/j.jacl.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Esperion Therapeutics. A study of the safety, pharmacokinetic drug interaction and efficacy of ETC-1002 and atorvastatin in subjects with hypercholesterolemia. [Accessed June 5, 2020]. https://clinicaltrials.gov/ct2/show/study/NCT01779453. NLM identifier: NCT01779453.
- 26.Ballantyne CM, McKenney JM, MacDougall DE, et al. Effect of ETC-1002 on serum low-density lipoprotein cholesterol in hypercholesterolemic patients receiving statin therapy. Am J Cardiol. 2016;117(12):1928–1933. doi: 10.1016/j.amjcard.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380(11):1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom Randomized Clinical Trial [published correction appears in JAMA. 2020 Jan 21;323(3):282] JAMA. 2019;322(18):1780–1788. doi: 10.1001/jama.2019.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laufs U, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8(7):e011662. doi: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballantyne CM, Banach M, Mancini GBJ, et al. Efficacy and safety of bempedoic acid added to ezetimibe in statin intolerant patients with hypercholesterolemia: a randomized, placebo-controlled study. Atherosclerosis. 2018;277:195–203. doi: 10.1016/j.atherosclerosis.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol. 2020;27(6):593–603. doi: 10.1177/2047487319864671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esperion Therapeutics. Evaluation of major cardiovascular events in patients with, or at high risk for, cardiovascular disease who are statin intolerant treated with bempe acid (ETC-1002) or placebo (CLEAR Outcomes) [Accessed June 5, 2020]. https://clinicaltrials.gov/ct2/show/NCT02993406. NLM identifier: NCT02993406.
- 33.Banach MJ, Duell PB, Gotto AM, Jr, et al. Association of bempe acid administration with atherogenic lipid levels in phase 3 randomized clinical trials of patients with hypercholesterolemia. JAMA Cardiol. 2020:e202314. doi: 10.1001/jamacardio.2020.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zetia (ezetimibe) package insert] Whitehouse Station, NJ: Merck & Co Inc; 2013. [Accessed June 5, 2020]. https://www.merck.com/product/usa/pi_circulars/z/zetia/zetia_pi.pdf. [Google Scholar]
- 35.Repatha (evolocumab) [package insert] Thousand Oaks, CA: Amgen, Inc; 2019. [Accessed June 5, 2020]. https://www.pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/repatha_pi_hcp_english.pdf. [Google Scholar]
- 36.Praluent (alirocumab) [package insert] Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2020. [Accessed June 5, 2020]. https://www.regeneron.com/sites/default/files/Praluent_PI.pdf. [Google Scholar]

