Abstract
Mutations in CHMP2B, encoding a protein in the endosomal sorting complexes required for transport (ESCRT) machinery, causes frontotemporal dementia linked to chromosome 3 (FTD3). FTD, the second most common form of pre-senile dementia, can also be caused by genetic mutations in other genes, including TANK-binding kinase 1 (TBK1). How FTD-causing disease genes interact is largely unknown. We found that partial loss function of Ik2, the fly homologue of TBK1 also known as I-kappaB kinase ε (IKKε), enhanced the toxicity of mutant CHMP2B in the fly eye and that Ik2 overexpression suppressed the effect of mutant CHMP2B in neurons. Partial loss of function of Spn-F, a downstream phosphorylation target of Ik2, greatly enhanced the mutant CHMP2B phenotype. An interactome analysis to understand cellular processes regulated by Spn-F identified a network of interacting proteins including Spn-F, Ik2, dynein light chain, and Hook, an adaptor protein in early endosome transport. Partial loss of function of dynein light chain or Hook also enhanced mutant CHMP2B toxicity. These findings identify several evolutionarily conserved genes, including ik2/TBK1, cut up (encoding dynein light chain) and hook, as genetic modifiers of FTD3-associated mutant CHMP2B toxicity and implicate early endosome transport as a potential contributing pathway in FTD.
Subject terms: Evolution, Neuroscience
Introduction
Frontotemporal dementia (FTD) is an early onset dementia associated with frontotemporal lobar degeneration (FTLD)1. Identified causative loci, collectively representing ~ 40% of all FTD cases, reveal a genetic, pathological and mechanistic overlap with amyotrophic lateral sclerosis (ALS)2, 3. Extensive cell biological studies of these loci suggest RNA metabolism (TARDBP and FUS) and autophagy/endosomal-lysosomal function (CHMP2B, OPTN, p62, TBK1, Ubiquilin-2, VCP) as major contributors to neuronal pathology3, 4. However, how different disease genes interact with each other in ALS/FTD pathogenesis remains poorly understood.
CHMP2B encodes a subunit of the endosomal sorting complex required for transport III (ESCRT-III) complex that is recruited to the surface of early endosomes to participate in the final step in membrane scission during the formation of multivesicular bodies (MVBs)5. MVBs are the endosomal and autophagosomal entry point to the late endosome and eventual lysosomal degradation. A splicing site mutation in CHMP2B resulting in a C-terminal truncation of the protein was identified in a Danish FTD patient cohort6 and other mis-sense mutations have since been identified in FTD and ALS pedigrees7–12. It is proposed that the truncation of the C-terminal in the CHMP2BIntron5 mutant protein promotes an ‘open’ configuration, locking the protein into an association with its binding partner Snf7-2/CHMP4B13. This blockage in ESCRT-III disassembly results in endosomal accumulation and deficient cellular trafficking13–15.
Why neurons are particularly susceptible to mutant CHMP2B induced endosomal dysfunction is not currently understood. To address this question, we established a Drosophila model expressing CHMP2BIntron5 post-mitotically in the fly eye16. Genetic screening for enhancers and suppressors of neurodegeneration in this model have so far identified activated innate immune signaling16, autophagosomal dysfunction17, and endosomal signaling disruption18 leading to JNK/AP-1 mediated pro-apoptotic signaling18, 19. Here, using our fly eye model of CHMP2BIntron5 mediated neurodegeneration, we attempt to identify additional genetic modifiers of mutant CHMP2B toxicity and effectors of endosomal dysfunction. In particular, we focus on some other genes known to be involved in ALS/FTD pathogenesis.
Results
Genetic interaction analysis identifies ik2 as a strong genetic modifier of mutant CHMP2B toxicity in Drosophila
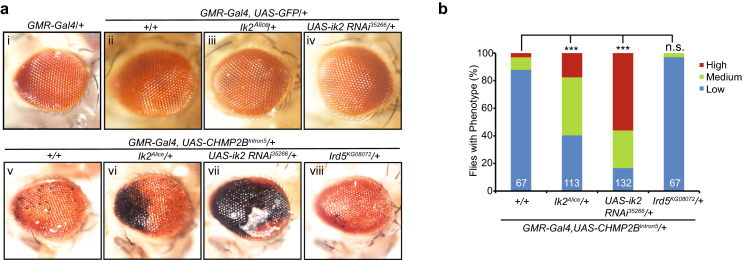
We previously generated a fly model of FTD-associated mutant CHMP2B neurotoxicity, in which expression of CHMP2BIntron5 but not CHMP2BWT driven by GMR-Gal4 produced a retinal degeneration phenotype characterized by a few small melanin deposits in the fly eye16. Here we first confirmed this eye phenotype in flies expressing CHMP2BIntron5 (Fig. 1a/v) but no phenotype when expressing GFP (Fig. 1a/i). To identify what other ALS/FTD genes may genetically interact with mutant CHMP2B, we tested mutants of ter94 and ik2, fly homologues of VCP and TBK1, respectively, for their ability to dominantly modify the phenotype of fly eyes expressing CHMP2BIntron5. We found partial loss of either ter94 (not shown) or ik2 gene were enhancers of mutant CHMP2B toxicity (Fig. 1a). The latter is the focus of the current study. The ik2 gene is also known as I-kappaB kinase ε (IKKε) in Flybase. A point mutation named Alice (ik2Alice) compromises the normal function of ik220. Ik2Alice heterozygous flies did not show any eye degeneration phenotype (Fig. 1a-iii). However, mutant CHMP2B toxicity was significantly enhanced in the ik2Alice heterozygous background (Fig. 1a/vi,b), suggesting that ik2 is a strong genetic modifier of mutant CHMP2B toxicity in the fly eye. This finding was further confirmed by ik2 RNAi knockdown. The UAS-ik2 RNAi35266 line was used and validated by others before21. Expression of ik2 RNAi by itself did not cause an eye phenotype (Fig. 1a/iv) but enhanced the toxicity of mutant CHMP2B (Fig. 1a/vii) to a much greater extent than the ik2Alice allele (Fig. 1b), presumably reflecting a greater knockdown of Ik2 activity. To demonstrate the specificity of the genetic interaction between ik2 and mutant CHMP2B, we also examined the effect of partial loss of IRD5 activity, another member of the Drosophila I kappa B kinase (IKK) family. The Ird5KG08072 allele did not modify the eye phenotype of mutant CHMP2B (Fig. 1a/viii,b). Thus, partial loss of Ik2 function in the presence of mutant CHMP2B may compromise the same cellular pathway leading to neurodegeneration.
Figure 1.
Genetic interactions between CHMP2BIntron5 and ik2, the fly homologue of mammalian TBK1, a gene mutated in a subset of ALS/FTD patients. (a) Compared to control flies (i, ii), the majority of 1-day-old GMR-Gal4,UAS-CHMP2BIntron5/CyO flies had a weak eye degeneration phenotype (v). Partial loss of Ik2 activity through the ik2Alice allele (iii) or RNAi knockdown (iv) did not cause an eye phenotype but significantly enhanced the CHMP2BIntron5 eye phenotype (vi, vii). In contrast, partial loss of function in ird5, another fly homologue of mammalian TBK1, had no additional effect (viii). The eye images were taken with a Nikon DS-Fi1 camera on a Nikon SMZ1500 stereomicroscope using the NIS-Element BR software version 3.10. This software requires purchase and is not freely available. (b) Quantification of eye phenotypes in flies of different genotypes in panel a by categorical data analysis. ***p < 0.001 by Chi-square test for three categorical variables (Low, Medium and High). n.s.: not significant.
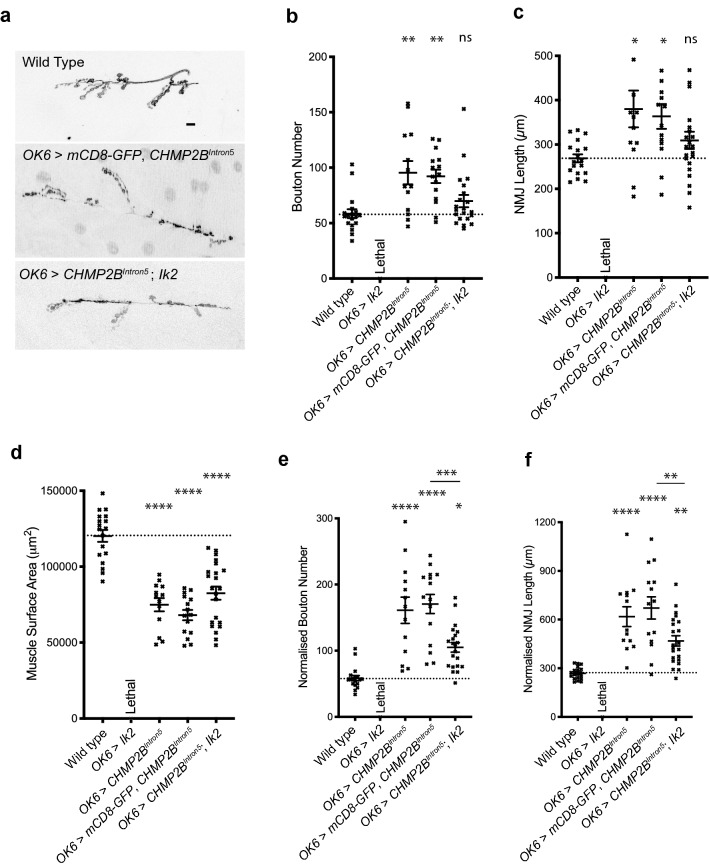
Ik2 shares a higher identity with TBK1 (35.5%) than with other human kinases such as IKK-epsilon and IKK-beta (34.5% and 18.7%, respectively). Human TBK1 shares 35.5% and 18.5% identity with Ik2 and IRD5, respectively. Thus, Ik2 appears to be the Drosophila homologue of human TBK1. Partial loss of TBK1 activity causes both ALS and FTD22, 23. To further examine the genetic interaction between Ik2/TBK1 and mutant CHMP2B, we also used the Drosophila neuromuscular junction (NMJ) as the experimental system. Previously we reported that pan-neuronal expression of mutant CHMP2B resulted in a significant overgrowth at the Drosophila third instar larval NMJ18, 19. Here we demonstrate that this synaptic overgrowth phenotype was also observed when CHMP2BIntron5 is expressed specifically in Drosophila motor-neurons, under the control of the OK6-Gal4 motorneuron driver. Motor-neuronal expression of CHMP2BIntron5 resulted in a significant increase in bouton number and NMJ length coupled with a significant decrease in muscle surface area (Fig. 2a–d) and decline in overall motor function (Supplementary 2). While motor-neuronal expression of UAS-ik2 resulted in lethality, co-expression of Ik2 with CHMP2BIntron5 was not lethal, presumably because the dilution of Gal4 by two UAS elements leading to a lower level of ectopic Ik2 expression. IK2 expression was sufficient to rescue CHMP2BIntron5 dependent synaptic overgrowth, but co-expression of mCD8-GFP with CHMP2BIntron5 had no effect. Normalization of NMJ lengths and bouton numbers against muscle surface area reveals that Ik2 rescue of the CHMP2BIntron5 phenotypes appears to be independent of altered muscle surface area (Fig. 2e,f).
Figure 2.
Co-expression of Ik2 alleviates CHMP2BIntron5-dependent overgrowth at the Drosophila larval neuromuscular junction (NMJ). (a) Representative micrographs of NMJs at muscle 6/7, hemi-segment A3 in 3rd instar larvae. Scale bar: 10 μm. Leica MM AF Premier Version 1.5.0 software (https://www.leica-microsystems.com/products/microscope-software/) was used for imaging. Graphs were made in Graphpad prism 8 (https://www.graphpad.com/scientific-software/prism/). Both softwares are not free to use. (b,c) Increase in synaptic bouton number (b) and NMJ length (c) associated with expression of UAS-CHMP2BIntron5 in motor neurons can be ameliorated through co-expression of UAS-ik2, but not UAS-mCD8-GFP. Muscle 6/7, hemi-segment A3, 3rd instar larvae. ANOVA with Tukey’s post-hoc multiple comparison test**p < 0.01 by ANOVA with Tukey’s post-hoc multiple comparison test. (d) Co-expression of UAS-ik2 has no effect on the reduced muscle sizes in larvae expressing UAS-CHMP2BIntron5 in motor neurons. Muscle 6/7, hemi-segment A3, 3rd instar larvae. ****p < 0.0001 by ANOVA with Tukey’s post-hoc multiple comparison test. (e,f) Normalization of bouton number (e) and NMJ length (f) to account for significantly reduced muscle sizes. Muscle 6/7, hemi-segment A3, 3rd instar larvae. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by ANOVA with Tukey’s post-hoc multiple comparison test.
Spn-F is a strong genetic modifier of mutant CHMP2B toxicity
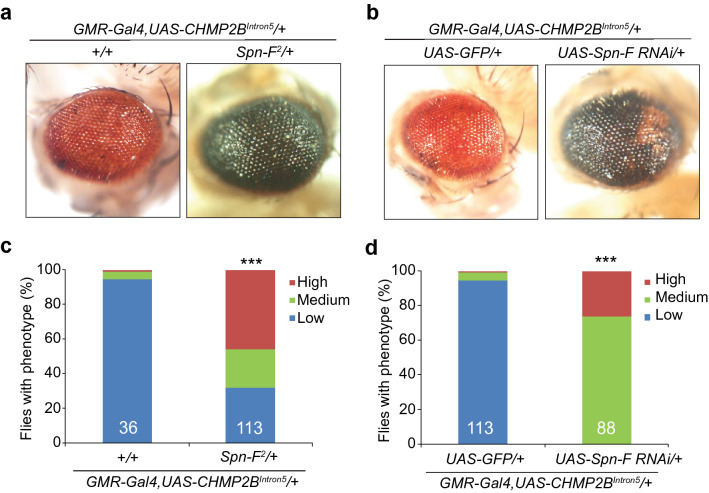
Spindle F (Spn-F) is a major phosphorylation target of Ik2, and these two proteins form a complex that regulates several developmental processes in Drosophila24–26. Therefore, we examined whether Spn-F is also a genetic modifier of mutant CHM2B toxicity. Indeed, the toxicity of mutant CHMP2B in the eye was greatly enhanced in the Spn-F2 heterozygous background (Fig. 3a,c); this finding was further confirmed by RNAi-mediated reduction of Spn-F activity (Fig. 3b,d), while flies with Spn-F RNAi knockdown did not show eye degeneration phenotypes (Supplementary 3).
Figure 3.
Partial loss of function of Spn-F, a phosphorylation target of Ik2, enhances the toxicity of CHMP2BIntron5 in vivo. (a) Partial loss of Spn-F activity greatly enhanced the CHMP2BIntron5 eye phenotype, as judged by comparison with control flies. (b) RNAi mediated knockdown of Spn-F activity also enhanced the CHMP2BIntron5 eye phenotype. The eye images were taken with a Nikon DS-Fi1 camera on a Nikon SMZ1500 stereomicroscope using the NIS-Element BR software version 3.10. This software requires purchase and is not freely available. (c,d) Quantification of the eye phenotypes in panel a (c) and panel b (d) by categorical data analysis. ***p < 0.001 by Chi-square test for three categorical variables (Low, Medium and High). n.s.: not significant.
Hook interacts with Spn-F
To determine which cellular pathways regulated by the Ik2-Spn-F complex are involved in mutant CHMP2B toxicity, we sought to identify other interacting proteins by immunoprecipitation of Spn-F followed by mass spectrometry. We expressed UAS-EGFP-Spn-F using actin-Gal4 and used GFP antibody to pull down Spn-F from fly head lysates. We identified several proteins that bind to Spn-F (Table 1). The most abundant was Ik2, validating the experimental approach, which also identified Hook and dynein light chain 1 (Dlc1), which is encoded by the gene cut up (ctp). Although the functional homolog of Spn-F in mammals is unknown, both Hook and Dlc1 are highly conserved evolutionarily27, 28. Spn-F directly interacts with Dlc1 in flies29 and Hook family proteins activate dynein adaptors in mammalian early endosome transport30. Thus, our interactome analysis identified Hook as another protein that interacts with Spn-F.
Table 1.
Spn-F interacting proteins identified by mass spectrometry.
| Protein name | Flybase symbol | Accession number | Molecular weight (kDa) | Total reads | |
|---|---|---|---|---|---|
| GFP-SpnF | GFP | ||||
| Spindle-F | spn-F | A0A0B4KI68 | 42 | 52 | 0 |
| I-kappaB kinase ε | IKKε | Q7KJQ4 | 81 | 17 | 0 |
| Hook | Hook | Q24185 | 77 | 5 | 0 |
| Cut up | cpt | Q24117 | 10 | 4 | 0 |
| Yolk protein 1 | Yp1 | P02843 | 49 | 3 | 0 |
| Serine-arginine protein 55 | B52 | P26686 | 43 | 2 | 0 |
| Histone H4 | His4 | P84040 | 11 | 3 | 0 |
| Triosephosphate isomerase | Tpi | P29613 | 27 | 2 | 0 |
| Cytochrome c oxidase subunit 4 | CoI4 | Q9VIQ8 | 21 | 2 | 0 |
Hook and ctp are also strong genetic modifiers of mutant CHMP2B toxicity
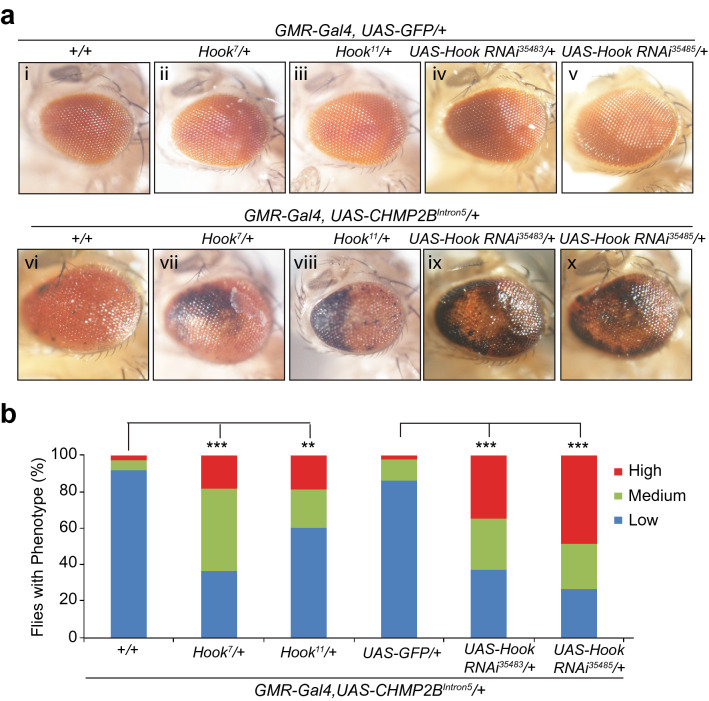
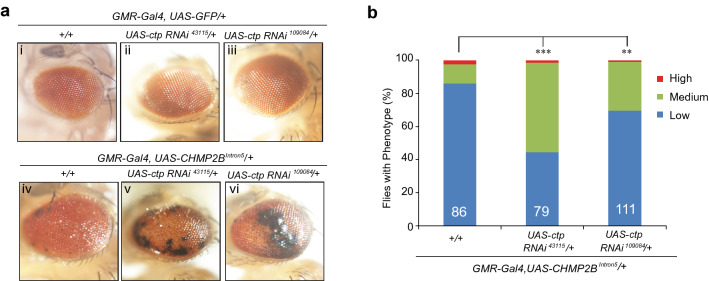
Because Hook has a specific role in early endosome transport30, we sought to determine whether this cellular pathway contributes to the toxicity of mutant CHMP2B. For this analysis, we used two hook mutant alleles (hook7 and hook11) and two hook-specific RNAi lines (Fig. 4a). As judged by comparison with control flies (Fig. 4a/i), Hook heterozygous mutant flies (Fig. 4a/ii, iii) or flies expressing hook RNAi (Fig. 4a/iv,v) appeared to have normal morphology. However, partial loss of Hook activity, through genetic alleles (Fig. 4a/vii,viii) or RNAi knockdown (Fig. 4a/ix,x), greatly enhanced the retinal degeneration phenotype caused by mutant CHMP2B (Fig. 4a/vi,b). We also used two different RNAi lines to knockdown ctp expression, at least one of these RNAi lines has been previously characterized by others31. We found that RNAi knockdown of ctp activity also worsened this phenotype (Fig. 5). Thus, compromised early endosome transport contributes to neurodegeneration induced by FTD3-associated mutant CHMP2B.
Figure 4.
Partial loss of function of Hook enhances the neurotoxicity of CHMP2BIntron5 in vivo. (a) As judged by comparison with control flies (i), multiple hook genetic alleles (ii, iii) or RNAi knockdown (iv, v) in the eye did not cause any eye phenotypes. In contrast, comparison with flies expressing CHMP2BIntron5 in the eye (vi) showed that partial loss of function of hook through genetic alleles (vii, viii) or different RNAi lines (ix, x) greatly enhanced the toxicity of CHMP2BIntron5. The eye images were taken with a Nikon DS-Fi1 camera on a Nikon SMZ1500 stereomicroscope using the NIS-Element BR software version 3.10. This software requires purchase and is not freely available. (b) Quantification of eye phenotypes in panel a by categorical data analysis. **p < 0.01, ***p < 0.001, by chi-squared test for three categorical variables (Low, Medium and High).
Figure 5.
Partial loss of function of ctp also enhances the neurotoxicity of mutant CHMP2B in vivo. (a) Eye morphology in flies expressing two different ctp-specific RNAi lines (ii, iii) was indistinguishable from that of control flies (i). Comparison with flies expressing mutant CHMP2B (iv) showed that partial loss of function of ctp through RNAi-mediated knockdown (v, vi) enhanced mutant CHMP2B toxicity. The eye images were taken with a Nikon DS-Fi1 camera on a Nikon SMZ1500 stereomicroscope using the NIS-Element BR software version 3.10. This software requires purchase and is not freely available. (b) Quantification of eye phenotypes in (a) by categorical data analysis. ***p < 0.001, **p < 0.01, by Chi-square test for three categorical variables (Low, Medium and High).
Discussion
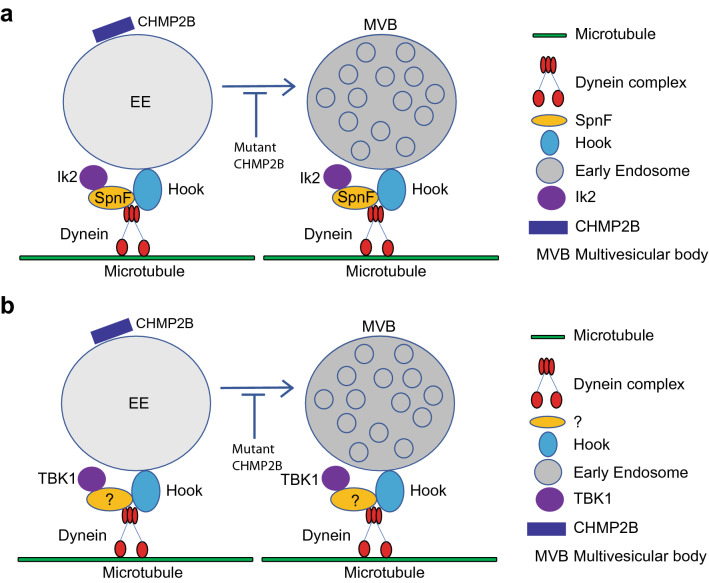
Through genetic interaction analysis, we first identified the genes encoding Ik2 and its known binding target, Spn-F, as strong genetic modifiers of mutant CHMP2B toxicity in Drosophila. We then identified Hook as another protein that interacts with Spn-F by immunoprecipitation and mass spectrometry analyses. Exactly how endogenous Ik2/TBK1 and Hook family proteins interact in flies and mammalian neurons needs to be further investigated (Fig. 6). Further genetic studies indicated that the genes encoding Hook, an adaptor molecule for early endosome transport, and its binding partner Dlc1 are also strong modifiers of mutant CHMP2B toxicity. Together, our studies identified three evolutionarily conserved genes, ik2, hook and ctp, as previously unknown genetic modifiers of FTD3-associated mutant CHMP2B and suggest that compromised early endosome transport contributes to neurodegeneration in FTD (Fig. 6).
Figure 6.
Schematic representation of endosome transport on microtubules mediated by the Hook-Dynein complex in flies (a) and mammalian cells (b). The mammalian equivalent of Spn-F remains to be identified. Moreover, how endogenous Ik2/TBK1 and Hook family proteins interact in flies and mammalian neurons needs to be further investigated.
CHMP2B is a subunit of ESCRT-III required for the maturation of early endosomes into MVBs5. As expected, this process is disrupted by the ectopic expression of FTD3-associated mutant CHMP2B, leading to a disruption of intracellular sorting or degradation of cargo proteins such as EGF receptor13 and accumulation of aberrant Rab7-positive endosomal structures13, 14. Mutant CHMP2B acts through its failure to dissociate from ESCRT-III due to its lack of C-terminus that is required to interact with SKD1, an AAA family ATPase essential for ESCRT-III dissociation13, 32. Indeed, expression of a dominant-negative form of SKD1 caused a similar endosomal phenotype as that induced by mutant CHMP2B13. Moreover, mutant CHMP2B disrupts regulation of TGF-β and JNK signaling in the endosome18. Thus, abnormal endosomal function is a key pathological mechanism in FTD3. This notion is further supported by our finding here that partial loss of function in the Hook-Dlc1 complex required for early endosome transport greatly exacerbates mutant CHMP2B toxicity. Taken together, these studies suggest that normalizing endosomal function is a promising potential therapeutic approach for FTD3.
Conclusion
Genetic analyses in a Drosophila model of FTD3 identify several evolutionarily conserved genes, including ik2/TBK1, ctp/Dlc1 and hook, as genetic modifiers of mutant CHMP2B toxicity. These findings implicate early endosome transport as a potential contributing pathway and, together with earlier reports, suggest that normalizing endosomal function is a promising potential therapeutic approach for FTD3.
Materials and methods
Fly strains and maintenance
Flies were raised at 25 °C on a standard diet. GMR-Gal4, UAS-CHMP2BIntron5 recombined flies were generated and studied previously16. GMR-Gal4, UAS-GFP, ik2Alice, UAS-ik2 RNAi, ird5KG08072, UAS-Spn-F RNAi and Ter94K15502/CyO were from the Bloomington Drosophila Stock Center. GMR-Gal4, UAS-GFP recombined flies were generated in this study. UAS-hook RNAi (35483 and 35485), and UAS-ctp RNAi (43115 and 104084) were from the Vienna Drosophila RNAi Center. Spn-F2 and UAS-GFP-Spn-F fly lines33 were kindly provided by Dr. Hsiu-Hsiang Lee, and hook7 and hook11 fly lines34 were from Dr. Helmut Krämer. The genetic aberrations of these alleles are summarized in Supplementary 1. For genetic interaction studies, the recombined stock, GMR-Gal4, UAS-CHMP2BIntron5/CyO, was crossed with individual classic mutants or RNAi lines. To quantify the CHMP2BIntron5 eye phenotype, we arbitrarily classified the eye phenotype with or without enhancers into three groups based on the relative abundance of black spots on the surface of the eye.
Immunoprecipitation, SDS-PAGE, and silver stain
Adult Actin-Gal4/UAS-GFP and Actin-Gal4/UAS-GFP-Spn-F flies were frozen with dry ice and vortexed to remove the heads. Heads from each genotype were homogenized in lysis buffer (50 mM Tris–HCl, pH 7.5, 150-mM sodium chloride, 1% Nonidet P40, 0.5% sodium deoxycholate, 1 tablet of complete Mini protein inhibitor cocktail/10 mL). Homogenates were centrifuged at 4 °C for 20 min at 12,000g. Protein concentrations were determined with the Bradford assay (Bio-Rad). For co-immunoprecipitation experiments, supernatants of GFP and GFP-Spn-F with the same amount of total proteins were incubated with GFP magnetic beads (Chromoteck) overnight at 4 °C. The beads were incubated, washed three times for 15 min each with washing buffer (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.1% Nonidet P40), and then suspended in the gel loading buffer and boiled for 5 min. The co-immunoprecipitation samples were then run on a 10% polyacrylamide-SDS gel for a short time, and stained with a silver staining kit (Sigma) for subsequent digestion and downstream LC–MS/MS analysis (Proteomics and Mass Spectrometry Facility at UMass).
Neuromuscular junction (NMJ) analysis
For NMJ analysis, Drosophila were raised on standard cornmeal medium at 18 °C on a 12-h light:dark cycle. Immunohistochemistry was performed described20. Motor-neuronal expression was under the control of the OK6-Gal4 driver. NMJs were imaged at 40× with a Hamamatsu ORCA-R2 C10600-10B digital camera on a Leica DM6000B microscope fitted with Qioptiq OptiGrid Structured-Light system using Leica MM AF software. Muscles were imaged with the same system at 10x, without the OptiGrid. NMJ analysis was done as described18. Prism 7 (GraphPad Software) was used for statistical analysis.
LC–MS/MS protein identification
This analysis was performed by UMass Proteomics Core Facility and Dr. John Leszyk provided the method description that was previously published35, 36.
In gel digestion
Silver-stained gel bands were destained with a 1:1 ratio of potassium ferricyanide (30 mM) and sodium thiosulfate (100 mM). Gels were washed extensively with water to remove and destain the yellow color. Gel slices were cut into 1 × 1-mm pieces and placed in 1.5-ml eppendorf tubes with 1 ml of water for 30 min. The water was removed, and 200 µl of 250 mM ammonium bicarbonate was added. For reduction, 25 µl of a 45-mM solution of 1,4 dithiothreitol was added, and the samples were incubated at 50 °C for 30 min. After cooling to room temperature, the samples were alkylated by adding 25 µl of a 100-mM iodoacetamide solution for 30 min. The gel slices were washed twice with 1-ml aliquots of water. The water was removed, and 1 ml of a 50:50 mixture of 50-mM ammonium bicarbonate and acetonitrile was placed in each tube. After incubation at room temperature for 1 h, the solution was removed, and 200 µl of acetonitrile was added to each tube, turning the gel slices opaque white. The acetonitrile was removed, and the gel slices were further dried in a Speed Vac and rehydrated in 1,000 µl of 2 ng/µl trypsin (Sigma) in 0.01% ProteaseMAX Surfactant (Promega):50-mM ammonium bicarbonate. Samples were incubated at 37 °C for 21 h. The supernatant of each sample was removed and placed in a separate 1.5-ml eppendorf tube. Gel slices were further dehydrated with 100 µl of an 80:20 mixture of acetonitrile and 1% formic acid. The extract was combined with the supernatants of each sample. The samples were then dried in a Speed Vac.
LC/MS/MS on Q exactive
After reconstitution in 25 µl of 0.1% trifluoroacetic acid in 5% acetonitrile, a 3-µl aliquot of each sample was directly injected onto a custom-packed 2 cm × 100 µm C18 Magic 5-µm particle trap column. Peptides were eluted and sprayed from a custom-packed emitter (75 µm × 25 cm C18 Magic 3-µm particle) with a linear gradient from 95% solvent A (0.1% formic acid in water) to 35% solvent B (0.1% formic acid in acetonitrile) for 90 min at a flow rate of 300 nanoliters per minute on a Waters Nano Acquity UPLC system. Data-dependent acquisitions were done on a Q Exactive mass spectrometer (Thermo Scientific) according to an experiment in which full MS scans from 300 to 1,750 m/z were acquired at a resolution of 70,000 followed by 10 MS/MS scans acquired under higher-energy collisional dissociation (HCD) fragmentation at a resolution of 17,500 with an isolation width of 1.6 Da. Raw data files were processed with Proteome Discoverer (Thermo, version 1.4) and then searched with Mascot Server (Matrix Sciences, version 2.5) against the human index of Uniprot. The search parameters used were fully tryptic with 2 missed cleavages, parent mass tolerances of 10 ppm, and fragment mass tolerances of 0.05 Da. Variable modifications of acetyl (protein N-term), pyro glutamic for N-term glutamine, oxidation of methionine, and carboxymethyl cysteine were considered.
Statistical analysis
Significant difference between control and experimental groups were determined by using Chi-square tests to calculate P values for categorical data.
Other information
Detailed information is also provided regarding genotypes of flies (Supplementary 4) and reagents (Supplementary 5) as well as values of all the statistical rest results (Supplementary 6).
Supplementary information
Acknowledgements
We thank the UMMS Proteomics Core facility for help with the Mass Spec analysis. We also thanks Dr. Hsiu-Hsiang Lee for Spn-F2 and UAS-GFP-Spn-F flies, Dr. Helmut Krämer for hook7 and hook11 flies, and the Bloomington Drosophila Stock Center and the Vienna Drosophila RNAi Center for other fly lines. This work was supported by grants from the NIH (R37NS057553, R01NS101986, and R01NS093097 to F.B.G.), Alzheimer’s Society UK (AS-JF-16b-004 to RJHW and AS-PG-2013-005 to STS), and MRC UK (MR/M013596/1 to STS).
Author contributions
Y.L., R.J.H.W. and M.P. did the experiments. Y.L., R.J.H.W., S.T.S., and F.B.G. analyzed the data and wrote the manuscript. FBG supervised the project.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71097-5.
References
- 1.Deleon J, Miller BL. Frontotemporal dementia. Handb. Clin. Neurol. 2018;148:409–430. doi: 10.1016/B978-0-444-64076-5.00027-2. [DOI] [PubMed] [Google Scholar]
- 2.Sleegers K, Cruts M, Van Broeckhoven C. Molecular pathways of frontotemporal lobar degeneration. Annu. Rev. Neurosci. 2010;33:71–88. doi: 10.1146/annurev-neuro-060909-153144. [DOI] [PubMed] [Google Scholar]
- 3.Gao FB, Almeida S, Lopez-Gonzalez R. Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder. EMBO J. 2017;36:2931–2950. doi: 10.15252/embj.201797568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann JW, Seeley WW, Huang EJ. RNA binding proteins and the pathogenesis of frontotemporal lobar degeneration. Ann. Rev. Pathol. 2019;14:469–495. doi: 10.1146/annurev-pathmechdis-012418-012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020;21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 6.Skibinski G, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 7.Parkinson N, et al. ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B) Neurology. 2006;67:1074–1077. doi: 10.1212/01.wnl.0000231510.89311.8b. [DOI] [PubMed] [Google Scholar]
- 8.Cox LE, et al. Mutations in CHMP2B in lower motor neuron predominant amyotrophic lateral sclerosis (ALS) PLoS ONE. 2010;5:e9872. doi: 10.1371/journal.pone.0009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari R, et al. Novel missense mutation in charged multivesicular body protein 2B in a patient with frontotemporal dementia. Alzheimer Dis. Assoc. Disord. 2010;24:397–401. doi: 10.1097/WAD.0b013e3181df20c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momeni P, et al. Sequence analysis of all identified open reading frames on the frontal temporal dementia haplotype on chromosome 3 fails to identify unique coding variants except in CHMP2B. Neurosci. Lett. 2006;410:77–79. doi: 10.1016/j.neulet.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 11.Momeni P, et al. Genetic variability in CHMP2B and frontotemporal dementia. Neurodegener. Dis. 2006;3:129–133. doi: 10.1159/000094771. [DOI] [PubMed] [Google Scholar]
- 12.van Blitterswijk M, et al. Genetic overlap between apparently sporadic motor neuron diseases. PLoS ONE. 2012;7:e48983. doi: 10.1371/journal.pone.0048983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr. Biol. 2007;17:1561–1567. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 14.van der Zee J, et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum. Mol. Genet. 2008;17:313–322. doi: 10.1093/hmg/ddm309. [DOI] [PubMed] [Google Scholar]
- 15.Clayton EL, et al. Frontotemporal dementia causative CHMP2B impairs neuronal endolysosomal traffic-rescue by TMEM106B knockdown. Brain J. Neurol. 2018;141:3428–3442. doi: 10.1093/brain/awy284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad ST, Sweeney ST, Lee JA, Sweeney NT, Gao FB. Genetic screen identifies serpin5 as a regulator of the toll pathway and CHMP2B toxicity associated with frontotemporal dementia. Proc. Natl. Acad. Sci. USA. 2009;106:12168–12173. doi: 10.1073/pnas.0903134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y, Zhang Z, Sun D, Sweeney ST, Gao FB. Syntaxin 13, a genetic modifier of mutant CHMP2B in frontotemporal dementia, is required for autophagosome maturation. Mol. Cell. 2013;52:264–271. doi: 10.1016/j.molcel.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West RJH, Lu YB, Marie B, Gao FB, Sweeney ST. Rab8, POSH, and TAK1 regulate synaptic growth in a Drosophila model of frontotemporal dementia. J. Cell. Biol. 2015;208:931–947. doi: 10.1083/jcb.201404066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West RJH, Ugbode C, Gao FB, Sweeney ST. The pro-apoptotic JNK scaffold POSH/SH3RF1 mediates CHMP2BIntron5-associated toxicity in animal models of frontotemporal dementia. Hum. Mol. Genet. 2018;27:1382–1395. doi: 10.1093/hmg/ddy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro RS, Anderson KV. Drosophila Ik2, a member of the I kappa B kinase family, is required for mRNA localization during oogenesis. Development. 2006;133:1467–1475. doi: 10.1242/dev.02318. [DOI] [PubMed] [Google Scholar]
- 21.Bergalet J, et al. Inter-dependent centrosomal co-localization of the cen and ik2 cis-natural antisense mRNAs in Drosophila. Cell Rep. 2020;30:3339–3352.e3336. doi: 10.1016/j.celrep.2020.02.047. [DOI] [PubMed] [Google Scholar]
- 22.Freischmidt A, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 2015;18:631. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- 23.Cirulli ET, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubin-Bar D, et al. The Drosophila IKK-related kinase (Ik2) and Spindle-F proteins are part of a complex that regulates cytoskeleton organization during oogenesis. BMC Cell Biol. 2008;9:1–4. doi: 10.1186/1471-2121-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amsalem S, et al. Drosophila oocyte polarity and cytoskeleton organization require regulation of Ik2 activity by Spn-F and Javelin-Like. Mol. Cell Biol. 2013;33:4371–4380. doi: 10.1128/MCB.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otani T, et al. A transport and retention mechanism for the sustained distal localization of Spn-F-IKKepsilon during Drosophila bristle elongation. Development. 2015;142:3612. doi: 10.1242/dev.130674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang X, et al. Cytoplasmic dynein and early endosome transport. Cell Mol. Life Sci. 2015;72:3267–3280. doi: 10.1007/s00018-015-1926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reck-Peterson SL, Redwine WB, Vale RD, Carter AP. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 2018;19:479–479. doi: 10.1038/s41580-018-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdu U, Bar D, Schupbach T. spn-F encodes a novel protein that affects oocyte patterning and bristle morphology in Drosophila. Development. 2006;133:1477–1484. doi: 10.1242/dev.02319. [DOI] [PubMed] [Google Scholar]
- 30.Dwivedi D, Kumari A, Rathi S, Mylavarapu SVS, Sharma M. The dynein adaptor Hook2 plays essential roles in mitotic progression and cytokinesis. J. Cell Biol. 2019;218:3526–3527. doi: 10.1083/jcb.20180418308222019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaytseva O, et al. The novel zinc finger protein dASCIZ regulates mitosis in Drosophila via an essential role in dynein light-chain expression. Genetics. 2014;196:443–453. doi: 10.1534/genetics.113.159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JA, Liu L, Gao FB. Autophagy defects contribute to neurodegeneration induced by dysfunctional ESCRT-III. Autophagy. 2009;5:1070–1072. doi: 10.4161/auto.5.7.9823. [DOI] [PubMed] [Google Scholar]
- 33.Lin T, et al. Spindle-F Is the central mediator of Ik2 kinase-dependent dendrite pruning in Drosophila sensory neurons. PLoS Genet. 2015;11:e1005642. doi: 10.1371/journal.pgen.1005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunio A, Metcalf AB, Kramer H. Genetic dissection of endocytic trafficking in Drosophila using a horseradish peroxidase bride of sevenless chimera: Hook is required for normal maturation of multivesicular endosomes. Mol. Biol. Cell. 1999;10:847–859. doi: 10.1091/mbc.10.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao KN, Li L, Anand M, Khanna H. Ablation of retinal ciliopathy protein RPGR results in altered photoreceptor ciliary composition. Sci. Rep. 2015;5:11137. doi: 10.1038/srep11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalski A, et al. Mass spectrometry-based proteomics using Q exactive, a high-performance benchtop quadrupole orbitrap mass spectrometer. Mol. Cell Proteomics. 2011;10:M111.011015. doi: 10.1074/mcp.M111.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.