Abstract
Background
Dopamine receptors interact with other receptors to form heterooligomers. One such complex, the D1–D2 heteromer, demonstrated in cultured striatal neurons and rat striatum has been linked to drug addiction, Parkinson disease, schizophrenia, depression and anhedonia.
Methods
D1–D2 heteromer expression was evaluated using in situ proximity ligation assay, in parallel with cellular colocalization of D1 and D2 mRNA using in situ hybridization in nineteen different key rat brain regions. Expression in higher species and changes in rat striatum after repeated cocaine administration were evaluated.
Results
Differences in D1–D2 heteromer expression in striatal subregions are documented in higher species with nonhuman primate and human demonstrating higher density of heteromer-expressing neurons compared to rodents. All species had higher density of D1–D2 neurons in nucleus accumbens compared to dorsal striatum. Multiple other brain regions are identified where D1–D2 heteromer is expressed, prominently in cerebral cortical subregions including piriform, medial prefrontal, orbitofrontal and others; subcortical regions such as claustrum, amygdala and lateral habenula. Three categories of regions are identified: D1–D2 heteromer expressed despite little to no observed D1/D2 mRNA colocalization, likely representing heteromer on neuronal projections from other brain regions; D1–D2 heteromer originating locally with the density of neurons expressing heteromer matching neurons with colocalized D1/D2 mRNA; regions with both a local origin and targeted inputs projecting from other regions. Repeated cocaine administration significantly increased density of neurons expressing D1–D2 heteromer and D1/D2 mRNA colocalization in rat striatum, with changes in both direct and indirect pathway neurons.
Conclusion
The dopamine D1–D2 heteromer is expressed in key brain cortical and subcortical regions of all species examined. Species differences in striatum revealed greater abundance in human>nonhuman-primate>rat>mouse, suggesting an evolutionary biologic role for the D1–D2 heteromer in higher CNS function. Its upregulation in rat striatum following cocaine points to regulatory significance with possible relevance for clinical disorders such as drug addiction. The dopamine D1–D2 receptor heteromer may represent a potential target for neuropsychiatric and neurodegenerative disorders, given its distribution in highly relevant brain regions.
Background
In brain, dopamine (DA) is synthesized by specific DA neurons with the most important of them originating in the substantia nigra (SN) and ventral tegmental area (VTA). These dopaminergic neurons project to multiple brain regions, most notably to the ventral and dorsal striatum (vStr and dStr), the prefrontal cortex (PFC), the orbitofrontal cortex (OFC), and the amygdala (AMY). DA modulates many brain functions such as decision-making, reward, emotion, locomotion, cognition and memory, with dysfunction of the DA system cited as an important factor in multiple brain disorders such as schizophrenia, major depressive disorder (MDD), addiction, post-traumatic syndrome disease (PTSD), Parkinson disease, to cite a few.
Strong evidence has shown that the five G protein coupled receptors mediating DA actions (D1 to D5), are capable of interaction with other receptors to form heterooligomers (see reviews by Hasbi et al., 2011; Escuela-Boroto et al., 2017; Derouich and Massotte, 2019), with an important one being the D1–D2 receptor complex (Hasbi et al., 2009; 2018). The existence of direct receptor-receptor interaction generating the D1–D2 heteromer was shown in cells (Lee et al., 2004; Rashid et al., 2007), in striatal neurons in culture (Hasbi et al., 2009) and in rat (Rashid et al., 2007; Hasbi et al., 2009; 2018), mouse (Hasbi et al., 2018) and monkey (Rico et al., 2017; Hasbi et al., 2018) striatum. A variety of techniques was used to establish this, including co-immunoprecipitation, BRET (bioluminescence resonance energy transfer), in situ confocal FRET (Förster resonance energy transfer), in situ PLA (proximity ligation assay). Confocal in situ FRET has estimated the receptor-receptor distance between D1 and D2 receptors within the D1–D2 complex to be 60 angström (Hasbi et al., 2009; Perreault et al., 2010). The D1–D2 receptor heteromer has a unique pharmacological and signaling profile (George and O’Dowd, 2007; Rashid et al., 2007; Hasbi et al., 2009; 2011) and was shown to induce conditioned place aversion when activated (Hasbi et al., 2018). The D1–D2 heteromer was also implicated in rodent model systems for schizophrenia (Perreault et al., 2010), depression (Hasbi et al., 2014; Shen et al., 2015a), drug addiction (Hasbi et al., 2018; Shen et al., 2015b), anhedonia (Hasbi et al., 2014; 2018; Shen et al., 2015a), lack of motivation (Hasbi et al., 2018), as well as in nonhuman primate treated with MPTP, as a model for Parkinson disease (Rico et al., 2017).
Due to this large repertoire of functional implications, it is clear that dopamine D1–D2 heteromer involvement is likely not restricted to the ventral striatum or basal ganglia, where it has been the most studied. It is then imperative to examine the existence of this heteromer in other important brain regions and demonstrate its regulation in one of the disorders in which its involvement was implicated.
One aim of the present study was to investigate the density of dopamine D1–D2 heteromer expressing neurons in the striatum of various species, as well as its occurrence in other important regions of brain, primarily in rat. The second aim was to examine whether an experimental manipulation could lead to an alteration in the expression and localization of the D1–D2 heteromer showing evidence for dynamic regulation in brain. The investigations were performed in parallel at the protein level, using in situ proximity ligation assay (PLA) and at the mRNA level to measure D1 and D2 mRNA coexpression. The present results document a significant species difference in the expression of the D1–D2 heteromer in the striatum. It is also shown that beside the known existence of the D1–D2 heteromer in the ventral striatum, there are multiple brain regions where this heteromer is expressed, such as in different subregions of the cortex, notably the piriform cortex and the orbitofrontal cortex, as well as in subcortical areas such as the claustrum, amygdala, and lateral habenula. In total, nineteen regions were investigated in rat brain with corroboration of some of the major regions in nonhuman primate brain, with results identifying three categories of regional expression based on D1/D2 mRNA colocalization and D1–D2 heteromer localization analyzed in combination.
To establish evidence for D1–D2 heteromer regulation, exposure to a drug of abuse such as cocaine was selected, as drug addiction is one of the disorders in which the D1–D2 heteromer was postulated to be involved (Hasbi et al., 2018, Perreault et al., 2016). Cocaine causes molecular and cellular alterations in many regions of the brain, and in particular alters the activity of the medium spiny neurons (MSNs) in the striatum. For example, a single dose of cocaine was able to enhance both the rewarding and aversive forms of opioid place conditioning (Kim et al., 2004). During acute cocaine administration, the MSNs forming the D1-enriched direct and the D2-expressing indirect pathways (dMSNs and iMSNs) work together to exert balanced effects, modulating the rewarding and aversive actions of the drug. However, chronic cocaine reduces cocaine-induced DA signaling and disrupts this balance with a shift towards enhanced D1 dMSN activity which promotes compulsive drug intake (Park et al., 2013). Dopamine D1–D2 receptor heteromer has been shown to be involved in different aspects of cocaine addiction-like behavior and the associated neurochemical changes. Activation of the D1–D2 heteromer in rats blocks intravenous cocaine self-administration, cocaine-induced locomotor sensitization, cocaine-induced place preference (Hasbi et al., 2018), whereas selective disruption of the heteromer amplifies the cocaine-generated effects (Perreault et al., 2016; Hasbi et al., 2018). Biochemically, activation of D1–D2 heteromer bidirectionally modulates DARPP-32 by increasing the phosphorylation of Threonine-75 (pT75-DARPP-32) and inhibiting cocaine-induced phosphorylation of Threonine-34 (pT34-DARPP-32). Repeated D1–D2 heteromer activation also blocks cocaine-induced ΔFosB accumulation, and cocaine-induced ERK phosphorylation (Hasbi et al., 2018), these proteins representing hallmark changes for different stages of addiction. Here, we investigated the regulation of the D1–D2 heteromer and the mRNA coding for D1 and D2 receptors in the context of repeated cocaine administration to rats. Interestingly, important modifications in D1 and D2 mRNA coexpression and localization occurred after 8 days of cocaine exposure. Alterations at the translated protein levels were also observed, with cocaine also inducing significant increases in the number of D1–D2 heteromer expressing neurons in both dorsal and ventral striatum. These results indicate significant gene expression changes in direct and indirect pathway neurons induced by cocaine, with the majority of MSNs that previously expressed only D1 or D2 receptor now coexpressing D1 and D2 receptors as heteromers. These changes were accompanied by a parallel increase in the number of neurons expressing ΔFosB.
Material and Methods
Animals
Rats:
Adult male Sprague-Dawley rats (300–325 g; Charles River, Canada) were housed in pairs and maintained in a 12:12 h light:dark cycle with food and water available ad libitum. Rats were acclimatized for at least one week before being included in studies. Procedures in this study were carried out in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993). The protocol was approved by the University of Toronto Animal Use Protocol Committee.
Rhesus monkeys:
Adult male (n=2) and female (n=1) rhesus macaques (Macaca mulatta) weighing between 5–9 kg served as subjects in the present study. All were drug-free for a minimum of 2 months. Animals were in a behavioral study prior to euthanasia (Jacobs et al., 2016). All monkeys were housed singly in stainless steel cages that provided olfactory, visual, and auditory interaction with conspecifics. A nutritionally balanced diet (5045 High Protein Monkey Diet, Purina Mills International INC., Brentwood, MO) supplemented with fresh fruit or vegetables, banana pellets, vitamins, was provided daily. Water was available ad libitum from an automatic watering system. A 12-hr light-dark cycle was in effect (lights on 0800 – 2000 hr). Animal maintenance and research followed the guidelines provided by the Institute of Laboratory Animal Resources (ILAR-NRC, 2010) and the NIH Office of Laboratory Animal Welfare (OLAW). The Institutional Animal Care and Use Committee at McLean Hospital approved all experimental protocols. The research facility is licensed by the U.S. Department of Agriculture and consultant veterinarians monitored the health of the colony. Enrichment was provided through access to mirrors and toys in the home-cage, television or music, interaction with technical staff. Each of the animals received an IM injection of ketamine 10–20mg/kg followed by 5.0 ml IV Beuthanasia-D (pentobarbital-based euthanasia solution).
Drugs
Cocaine (Cocaine hydrochloride, Medisca) was dissolved in saline and administered intraperitoneally (i.p.) once daily at 10 mg/kg for a total of 8 days. For control animals, an equivalent volume of saline was used.
Brain preparation and sectioning
One hour after the last injection, the rats were anaesthetized with isoflurane (induction 5%, maintenance 2%), and perfused with cold PBS and 4% PFA. The brains were then extracted and post-fixed in 4% PFA overnight, followed by sucrose solutions (10% and 30%), before being stored at −80 °C until use. In some cases where perfusion was not needed, the brains were flash-frozen and stored at −80 °C until use.
The brains were sectioned coronally at 20 µm per section using a cryostat (set at −23 °C), with adjacent sections serially used for PLA or mRNA evaluation.
Measure of D1 and D2 receptor mRNA
Fluorescent in situ hybridization (FISH) method was used to visualize Drd1 and Drd2 mRNA expression in rat brain tissue. The assay was performed according to RNAscope Multiplex Fluorescent v2 Kit user manual (NEL741001KT, ACD Bio). Briefly, 20 µm coronal brain sections mounted on charged slides were post-fixed with 4% PFA, dehydrated sequentially with 50, 70, and 100% ethanol and pretreated with hydrogen peroxide and protease III. Sections were then hybridized to rat Drd1 and Drd2 probes and probe hybridization signal was amplified using signal amplification reagents. Signal was developed using HRP channels and fluorophores (Perkin Elmer). HRP-C1 and TSA Plus Fluorescein (visualized as green signal using the 488 channel) were used for Drd1 and HRP-C2 and TSA Plus Cyanine 3 (visualized as red signal using the 568 channel) for Drd2 signal detection. Sections were stained with DAPI and mounted with Permafluor mountant (Thermo Scientific). Slides were imaged at 40X using an Olympus Fluoview-1000 microscope. Analysis was performed by two independent researchers, double blinded for both the region and treatment under investigation.
Co-Immunoprecipitation of the D1–D2 heteromer
Co-immunoprecipitation was performed as previously described (Hasbi et al., 2014; 2018). Protein homogenates (300 μg) obtained from tissue punches of different regions of rat brain were incubated with anti-D2R antibody (Alomone Laboratories) at 4°C overnight under gentle rotation. After adding 40–50 μl of protein G/A, the mixture was further incubated for 1 h. After 3 washes with PBS-Tween, Laemmli buffer was added, and the immunoprecipitate was incubated for 5 min at 95°C. Proteins were resolved by electrophoresis on 10% polyacrylamide gels under denaturing conditions (SDS-PAGE) and transferred onto nitrocellulose or PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA) using a semidry transfer system (Invitrogen, Carlsbad, CA, USA). Membranes were incubated in PBS-Tween (PBS-T)/10% nonfat milk for 1 h. After 3 washes, membranes were incubated with PBS-T/5% nonfat milk containing the anti-D1R antibody raised in rats (Sigma, D2944). Membranes were washed once in PBS-T and 2 times in PBS (10 min each) and incubated with the appropriate polyclonal secondary antibody for 2 h. After 3 washes as indicated above, signal detection was performed using a Li-Cor instrument (Odyssey).
Proximity Ligation Assay (PLA)
The in situ PLA was performed as described previously (Hasbi et al., 2018; 2020). The PLA probes were generated using a rat anti-D1R antibody (Sigma, D2944) conjugated directly with the PLUS oligonucleotide and a rabbit anti-D2R antibody (Millipore, AB5084P) conjugated directly with the MINUS oligonucleotide following manufacturer’s instructions (Duolink®, Sigma-Olink). The PLA protocol was performed as described by the manufacturer (Duolink®, Sigma-Olink). Briefly, coronal slices (20 µm) from rat brain were incubated for 1 h at 37 °C with the blocking solution in a pre-heated humidity chamber, followed by incubation with the generated PLA probes described above and washed with buffer A (DUO82047, Sigma-Olink). The PLA signal was detected using the Duolink II in situ PLA detection kit (DUO92008, Sigma-Olink) after the ligation-amplification steps. Nuclei were labeled by a DAPI solution. Positive PLA signals were identified as red dots using the confocal Fluoview Olympus microscope (FV 1000) with 40× or 60×/1.2 NA objectives. Z-stack images were taken to confirm that PLA signals were localized on cell bodies. Cell counting and analysis of the PLA signal were performed using Imagetool software (Duolink®) by two independent researchers, double blinded for both the region and treatment under investigation. The reported percentages were calculated from images taken by the 60×/1.2 NA objective. Appropriate negative control assays were performed to ensure the specificity of the PLA labeling and amplification. Further controls for antibodies and PLA specificity using dopamine D1 and D2 receptor gene-deleted mouse striata were performed previously (Hasbi et al., 2018; 2020). Schemes representing the regions are adapted from Paxinos and Watson Atlas (Paxinos and Watson 1988).
Data Analysis
For species and regional comparisons, two-way ANOVA followed by Bonferroni correction for multiple comparisons test was applied. For cocaine-induced changes in mRNA or PLA, the statistical significance of each dependent measure was evaluated using the appropriate ANOVA with a within-subjects factor of “treatment” and the between-subjects factors of “region” or “treatment” as required.
Results
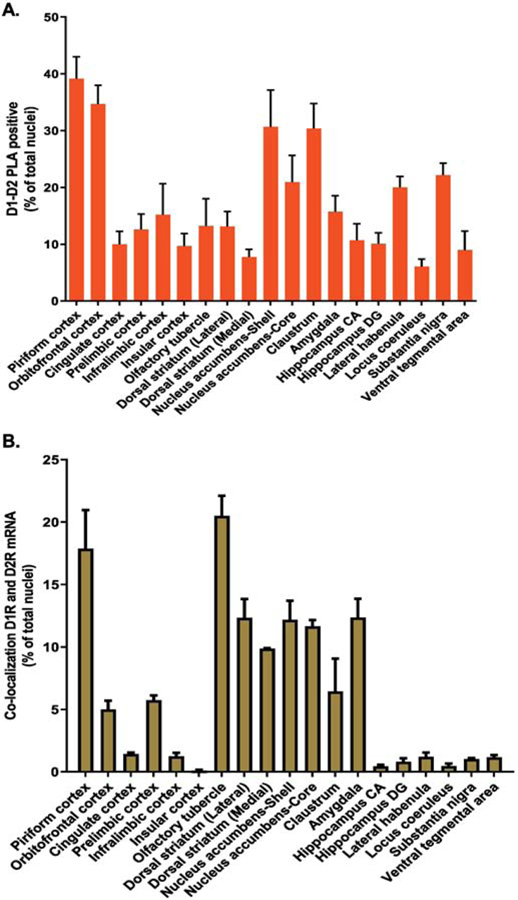
To investigate the expression profile of D1–D2 receptor heteromer expressing neurons in different regions of rat brain, two approaches were used: a) estimation of D1R and D2R mRNA expression and colocalization, using the FISH technique and b) the proximity ligation assay (PLA) to visualize and estimate the number of cells expressing the heteromer. Data resulting from both techniques are presented as the percentage of positive cells per total number of DAPI-labeled nuclei (total cells) and are summarized in Figure 1A and Table 1 for PLA analyses with Figure 1B and Table 2 for mRNA. Details are given in the following figures and supplementary figures.
Figure 1. Dopamine D1–D2 receptor heteromer distribution with D1R and D2R mRNA colocalization in rat brain regions.
Serial coronal slices from various rat brain regions were used for the estimation of cells expressing dopamine D1–D2 receptor heteromer using in situ PLA (A), and cells coexpressing D1 and D2 mRNAs using FISH (B). Data resulting from both techniques are presented as the percentage of positive cells per total number of DAPI-labeled nuclei from N=3 male rats.
Table 1.
Estimation of percent of cells expressing dopamine D1–D2 receptor heteromer using PLA technique. Numbers were round to the nearest value.
| Region | PLA (% of cells) | ± SEM |
|---|---|---|
| Piriform cortex | 39 | 4 |
| Orbitofrontal cortex | 35 | 3 |
| Cingulate cortex | 10 | 2 |
| Prelimbic cortex | 13 | 3 |
| Infralimbic cortex | 15 | 5 |
| Insular cortex | 10 | 2 |
| Olfactory tubercle | 13 | 5 |
| Dorsal striatum (Lateral) | 13 | 3 |
| Dorsal striatum (Medial) | 8 | 1 |
| Nucleus accumbens-Shell | 31 | 5 |
| Nucleus accumbens-Core | 21 | 5 |
| Claustrum | 30 | 4 |
| Amygdala | 16 | 3 |
| Hippocampus CA | 11 | 3 |
| Hippocampus DG | 10 | 2 |
| Lateral habenula | 20 | 2 |
| Locus coeruleus | 6 | 1 |
| Substantia nigra | 22 | 2 |
| Ventral tegmental area | 9 | 3 |
Table 2. Evaluation of percent of cells expressing mRNA for D1 receptor, D2 receptor or both using FISH technique.
NL: unlabeled; NA: Not Applicable
| Region | D1 | D2 | D1+D2 | NL |
|---|---|---|---|---|
| Piriform cortex | 27.19 ± 0.65 | 9.35 ± 0.98 | 17.86 ± 3.09 | 50.38 ± 5.13 |
| Orbitofrontal cortex | 16.43 ± 3.91 | 7.15 ± 0.22 | 4.99 ± 0.71 | 71.41 ± 4.40 |
| Cingulate cortex | 6.11 ± 2.15 | 9.70 ± 1.61 | 1.41 ± 0.13 | 82.76 ± 0.68 |
| Prelimbic cortex | 10.16 ± 1.28 | 10.36 ± 1.75 | 5.72 ± 0.4 | 73.73 ± 1.03 |
| Infralimbic cortex | 8.29 ± 3.04 | 6.58 ± 0.95 | 1.24 ± 0.29 | 83.87 ± 4.25 |
| Insular cortex | 11.37 ± 2.35 | 1.66 ± 0.51 | 0.10 ± 0.05 | 86.86 ± 1.80 |
| Olfactory tubercle | 29.99 ± 3.17 | 13.37 ± 1.66 | 20.49 ± 1.61 | 36.13 ± 3.15 |
| Dorsal striatum (Lateral) | 38.46 ± 5.80 | 47.09 ± 4.91 | 15.26 ± 2.77 | NA |
| Dorsal striatum (Medial) | 38.27 ± 7.49 | 49.87 ± 8.32 | 12.79 ± 2.17 | NA |
| Nucleus accumbens-Shell | 45.82 ±5.86 | 40.98 ± 4.95 | 13.73 ± 4.10 | NA |
| Nucleus accumbens-Core | 40.97 ± 4.53 | 30.26 ± 9.24 | 14.03 ± 2.18 | NA |
| Claustrum | 17.99 ± 4.36 | 4.39 ± 0.53 | 6.43 ± 2.64 | 71.17 ± 7.52 |
| Amygdala | 27.89 ± 0.8 | 17.54 ± 2.61 | 12.35 ± 1.5 | 42.20 ± 3.32 |
| Hippocampus CA | 8.29 ± 0.05 | 2.41 ± 0.47 | 0.44 ± 0.12 | 90.70 ± 1.54 |
| Hippocampus DG | 16.08 ± 0.83 | 2.34 ± 0.73 | 0.80 ± 0.28 | 81.05 ± 1.99 |
| Lateral habenula | 6.19 ± 0.66 | 8.74 ± 1.97 | 1.19 ± 0.35 | 83.86 ± 2.97 |
| Locus coeruleus | 1.89 ± 0.94 | 9.11 ± 2.04 | 0.46 ± 0.2 | 88.52 ± 2.97 |
| Substantia nigra | 20.43 ± 0.04 | 1.12 ± 1.12 | 1.01 ± 0.08 | 77.42 ± 1.12 |
| Ventral tegmental area | 3.83 ± 0.48 | 23.27 ± 6.69 | 1.15 ± 0.20 | 71.73 ± 6.52 |
The in situ PLA was performed as described previously (Hasbi et al., 2018; 2020). As detailed in the materials and methods section, the PLA probes were generated by directly conjugating specific anti-D1R (Sigma, D2944) and anti-D2R (Millipore, AB5084P) antibodies with a PLUS and a MINUS oligonucleotide, respectively. Cell counting and analysis of the PLA signal were performed using Imagetool software (Duolink®), by two independent researchers, double blinded for both the region and treatment under investigation. Appropriate negative control assays were performed to ensure the specificity of the PLA labeling and amplification, including the use of dopamine D1 and D2 receptor gene-deleted mouse striata as described previously (Hasbi et al., 2018; 2020). The FISH data analysis was also performed by two independent, double blinded researchers, and the results were averaged.
1. Estimations of D1–D2 heteromer expression in rat brain regions using PLA
The regions expressing the highest level of heteromer (25%−40% of total cells) were the piriform cortex (39%), orbitofrontal cortex (35%), nucleus accumbens shell (31%) and claustrum (30%) (Figure 1A and Table 1).
These regions were followed by others where cells expressing the heteromer were relatively high (15–25%) and included the substantia nigra (22%), nucleus accumbens core (21%), lateral habenula (20%), amygdala (16%) and infralimbic region (15%) of the medial prefrontal cortex.
There were regions with intermediate expression of the heteromer in >10% to 14% of cells such as the olfactory tubercle (13%), dorsolateral caudate nucleus (13%), hippocampus CA (Cornu Ammonis) region (11%), and prelimbic region (13%) of the medial prefrontal cortex.
Finally, there were regions or subregions where 10% or fewer of cells expressed the heteromer, which included the hippocampus dentate gyrus (DG) region (10%), cingulate cortex (10%), insular cortex (10%), ventral tegmental area (9%), dorsomedial caudate nucleus (8%) and locus coeruleus (6%).
2. D1R and D2R mRNA expression
For each region, the results from FISH are presented as the percentage of nuclei (cells) expressing mRNA for D1R-alone, D2R-alone, colocalized D1/D2 or unlabelled (NL) nuclei, except for the striatum, in which ~95% of cells are MSNs expressing either D1, D2 or both mRNA species. The remainder of neurons in striatum being interneurons, from among which only cholinergic interneurons (~1%) express the D2 receptor (Rico et al., 2017). The results are reported in Figure 1B and Table 2.
For example, in the ventral striatum, the nucleus accumbens shell and core subregions showed similar expression of D1R mRNA (46±6% and 41±5%, respectively), D2R mRNA (41±5% and 30±9%, respectively), as well as D1 and D2 mRNA colocalization (~14.0%) (Table 2).
The overall expression of neurons with individual D1 or D2 receptor mRNAs in the different regions varied widely (Table 2) as expected, and in accordance with the literature. Some regions, such as piriform cortex, orbitofrontal cortex, insular cortex, nucleus accumbens core, amygdala, olfactory tubercle, hippocampus, and substantia nigra had a higher number of neurons that expressed D1 mRNA compared to D2 mRNA. Other regions, such as the dorsal striatum (or caudate putamen [CPu]), locus coeruleus and ventral tegmental area had a higher number of neurons that expressed D2 mRNA compared to D1 mRNA. Equivalent levels of neurons expressing either D1 mRNA or D2 mRNA were observed in the nucleus accumbens shell, lateral habenula, cingulate cortex, infralimbic cortex and prelimbic cortex (Table 2).
The colocalization of D1 and D2 mRNA reported in percent of total nuclei (Figure 1B and Table 2) demonstrated a higher percentage of D1 and D2 mRNA colocalization in the olfactory tubercle (21%), piriform cortex (18%), ventral striatum [nucleus accumbens shell (14%), nucleus accumbens core (14%)], dorsal striatum [lateral (15%), medial (13%)] and amygdala (12%).
D1 and D2 mRNA colocalized in 5–10% of total cells in the claustrum (7%), prelimbic cortex (6%) and orbitofrontal cortex (5%). There was very low to almost no colocalization of D1 and D2 mRNA in cingulate cortex (1.4%), lateral habenula (1.2%), ventral tegmental area (1.2%), substantia nigra (1%), with hippocampal subregions (CA and DG), locus coeruleus and insular cortex all <1%.
3. Regional distribution of D1/D2 mRNA colocalization and D1–D2 heteromer expression
We then analyzed the concordance between D1/D2 mRNA colocalization with D1–D2 heteromer expression for each region.
3.1. Striatum
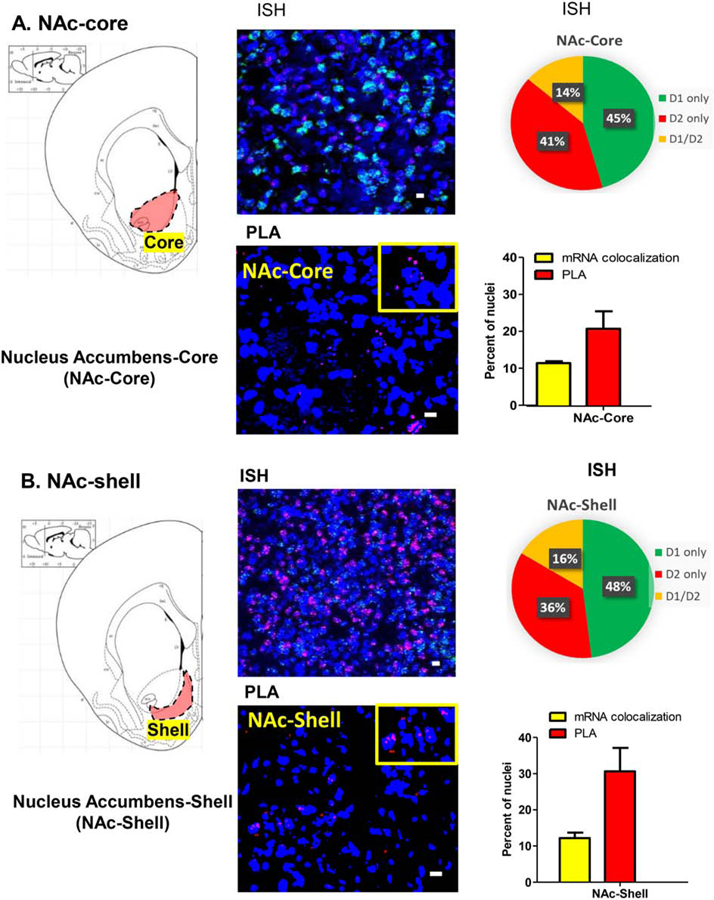
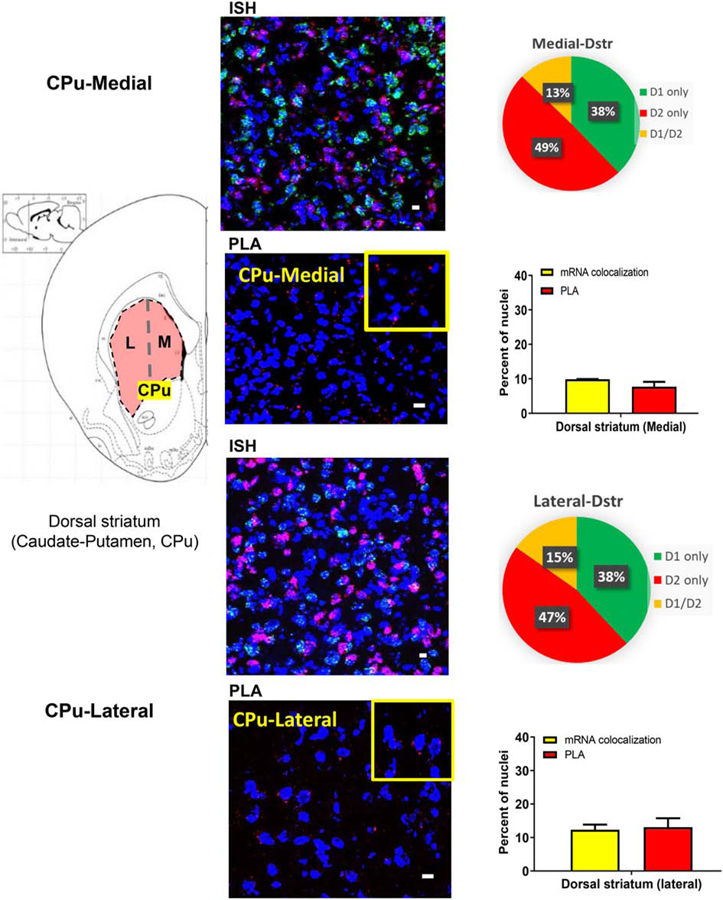
In rat striatal subregions (Figures 2 and 3), D1 and D2 mRNA colocalization was observed in 12 to 15% of total nuclei and was similar between the nucleus accumbens (NAc) (Figure 2) and the caudate putamen (CPu) (Figure 3). The number of neurons with PLA signal, however, was higher in the NAc (Figure 2) than in the CPu (Figure 3) regions. In CPu (Figure 3; Supplementary Figure 1A), PLA results showing density of neurons expressing D1–D2 heteromer (average 14%) coincided reasonably with the number of neurons where mRNA colocalization occurred (~13%), whereas in the NAc (Figure 2), the PLA-positive neuron numbers (21% and 31% in NAc core and NAc shell, respectively) were much higher than the mRNA colocalization percentages (14%). These results suggest that while there are a lower number of neurons expressing heteromer in the dorsal striatum, they are generated from receptors locally expressed, whereas in the NAc, notably in the shell subregion, more than half of the D1–D2 heteromers were probably expressed on terminals projecting to and targeting the MSNs of this region.
Figure 2. D1–D2 receptor heteromer expression in the nucleus accumbens with D1R and D2R mRNA expression and colocalization.
Coronal sections were used to estimate mRNA expression of D1R and D2R (3 sections/rat) using FISH technique and to visualize and estimate the number of cells expressing the D1–D2 heteromer (3 sections/rat) using PLA in the nucleus accumbens core (A) and the nucleus accumbens shell (B). Data resulting from both techniques are presented as percentage of positive cells per total DAPI-labeled nuclei. A scheme delimiting each subregion is shown on the left, followed by a representative FISH image, an estimation of mRNA expression and a graph summarizing the colocalization of mRNAs and PLA results. N=3 male rats. Scale bars: 10 µm.
Figure 3. D1–D2 receptor heteromer expression in the dorsal striatum with D1R and D2R mRNA expression and colocalization.
Coronal sections were used to estimate mRNA expression of D1R and D2R (3 sections/rat) using FISH technique and to visualize and estimate the number of cells expressing the D1–D2 heteromer (3 sections/rat) using PLA in the medial (A) and the lateral caudate-putamen (CPu) (B). Data resulting from both techniques are presented as percentage of positive cells per total DAPI-labeled nuclei. A scheme delimiting each subregion is shown on the left, followed by a representative FISH image, an estimation of mRNA expression and a graph summarizing the colocalization of mRNAs and PLA results. N=3 male rats. Scale bars: 10 µm.
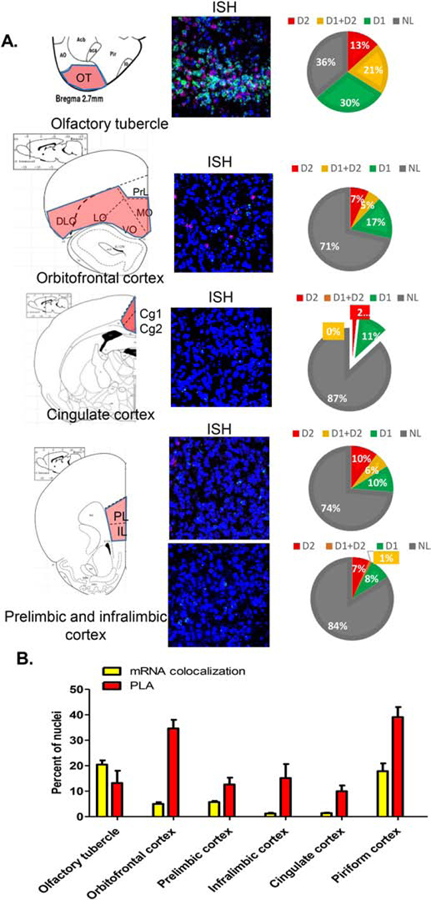
3.2. Cortical regions and Olfactory Tubercle
In the cerebral cortical regions (Figure 4), the highest number of nuclei showing PLA was observed in the pirifom cortex and orbitofrontal cortex (39 and 35%, respectively). The other cortical regions investigated, namely prelimbic, infralimbic and cingulate cortices, as well as the olfactory tubercle, all showed moderate numbers of cells positive for PLA in the range of 10–14% of total nuclei. These PLA results contrasted with the colocalization of mRNAs, which, except for the olfactory tubercle (21%) and piriform cortex (18%), was very low in the other regions, ranging from 0.1% in the insular cortex to 5.7% in the prelimbic cortex (Figure 4; Supplementary Figure 1B).
Figure 4. Estimation of D1R and D2R mRNA expression, colocalization, and D1–D2 receptor heteromer expression in different cerebral cortical regions and olfactory tubercle.
Coronal slices were used to estimate D1R and D2R mRNA expression (3 sections/rat) using FISH technique and to visualize and estimate the number of cells expressing the D1–D2 heteromer (3 sections/rat) using PLA in the olfactory tubercle and the indicated cortical regions (A). The graph (B) summarizes the colocalization of D1/D2 mRNAs and PLA results for each subregion. Data are presented as the percentage of positive cells per total DAPI-labeled nuclei. A scheme delimiting each subregion is shown on the left, followed by a representative FISH image and an estimation of mRNA expression. N=3 male rats.
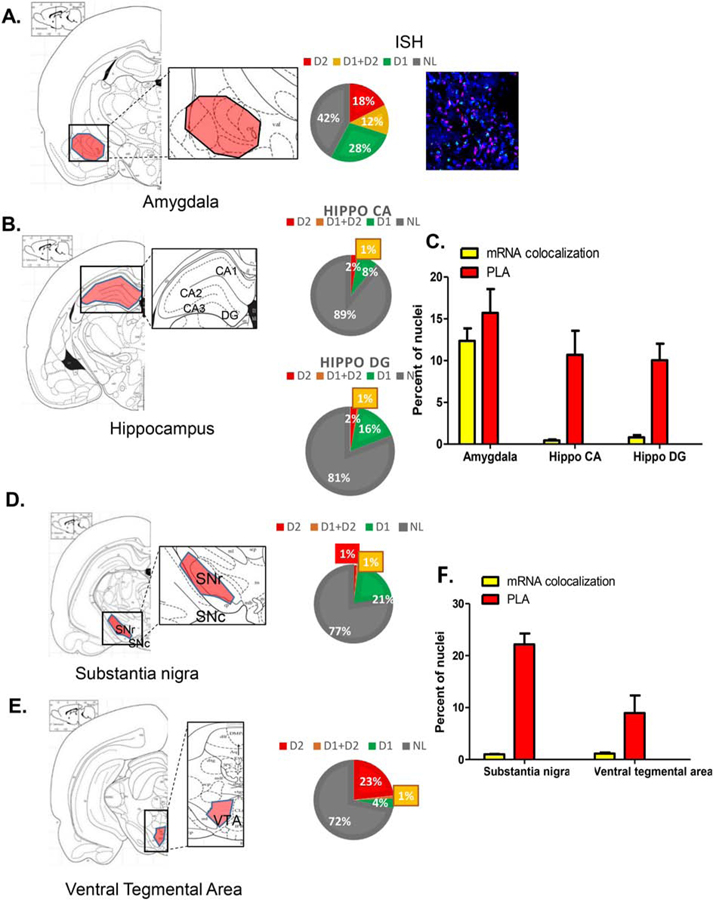
3.3. Amygdala and Hippocampus
In amygdala (Figures 5A and 5C; Supplementary Figure 2A), neurons demonstrating mRNA colocalization (12%) approximately matched the density of neurons expressing D1–D2 heteromer with PLA documented in ~16% of total cells. In contrast, there was a discrepancy in the hippocampal CA and DG regions between D1–D2 PLA expression in 10–11% of cells and almost no colocalization (<1%) of mRNAs (Figure 5B–5C; Supplementary Figure 2B).
Figure 5. Estimation of D1R and D2R mRNA expression, colocalization, and D1–D2 heteromer expression in amygdala, hippocampus, substantia nigra and ventral tegmental area.
Coronal slices were used to estimate D1 and D2 mRNA expression (3 sections/rat) using FISH technique and to visualize and estimate the number of cells expressing the D1–D2 heteromer (3 sections/rat) using PLA in the amygdala (A) and the hippocampus CA and DG subregions (B). the substantia nigra (D) and the ventral tegmental area (F). A scheme delimiting each subregion is shown on the left, followed by a representative FISH image, and an estimation of mRNA expression. The graphs in (C) and (E) summarize the colocalization of D1/D2 mRNAs and PLA results for each indicated subregion. Data are presented as percentage of positive cells per total DAPI-labeled nuclei, obtained from male rats (N=3).
3.4. Substantia Nigra and the Ventral Tegmental Area
Similar discordance was observed in the substantia nigra and the ventral tegmental area (Figure 5D–5F; Supplementary Figure 2B), where D1–D2 PLA was seen in 22% and 9% of cells respectively, whereas, there was almost no overlap between neurons expressing D1 mRNA and D2 mRNA (~1%). These results suggest that virtually all of the D1–D2 heteromers were likely expressed on terminals of neurons projecting to and targeting these regions rather than within neurons intrinsic to these areas.
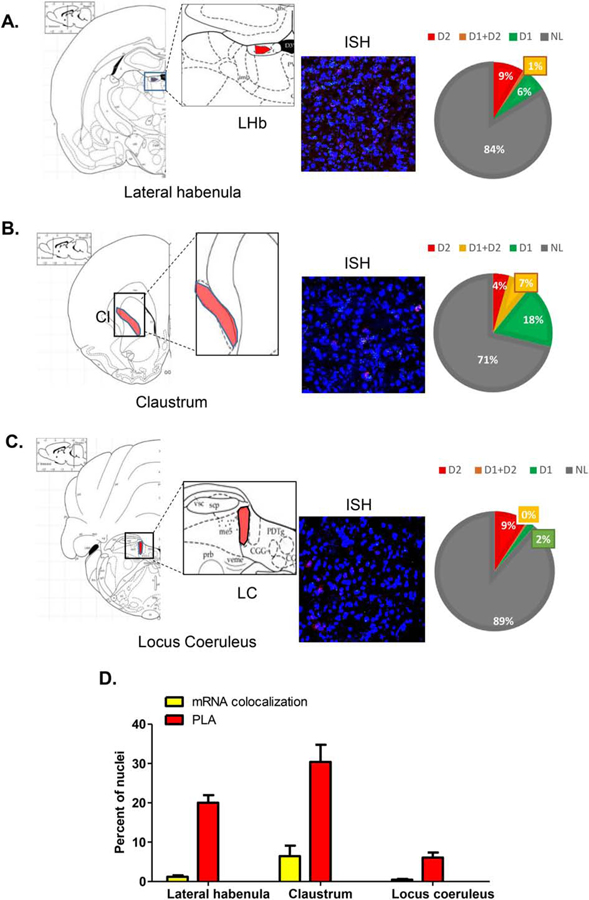
3.5. Other regions
Other regions (Figure 6; Supplementary Figure 2C), such as lateral habenula (Figure 6A) and claustrum (Figure 6B), showed low level of colocalization of D1 mRNA and D2 mRNA (1.2% and 6.4% of total nuclei, respectively), but much higher D1–D2 PLA values (20% and 30% of cells, respectively). In the locus coeruleus, a low number of cells expressed D1–D2 PLA (6%) with almost no colocalization between D1 and D2 mRNAs (0.5% of total nuclei). Again, according to these results, at least a significant proportion of the D1–D2 heteromers must be expressed on terminals of neurons targeting these regions.
Figure 6. Analysis of D1R and D2R mRNA expression and D1–D2 heteromer expression in lateral habenula, claustrum and locus coeruleus.
Coronal slices were used to estimate the D1 and D2 mRNA expression (3 sections/rat) using FISH technique and to visualize and estimate the number of cells expressing the D1–D2 heteromer (3 sections/rat) using PLA in lateral habenula (A), claustrum (B) and locus coeruleus (C). For each region, a scheme delimiting the region is shown on the left, followed by a representative FISH image, and an estimation of mRNA expression. The graph in (D) summarizes the colocalization of D1/D2 mRNAs and PLA results for each subregion. Data are presented as percentage of positive cells per total DAPI-labeled nuclei, obtained from male rats (N=3).
It is worth noting, that in all rat brain regions documented to have D1–D2 heteromer by PLA, D1 receptors were co-immunoprecipitated with the D2 receptor antibody, confirming the formation of receptor complexes between the dopamine D1 and D2 receptors. Shown are the examples for medial PFC regions, NAc, amygdala and lateral habenula (Supplementary Figure 3)
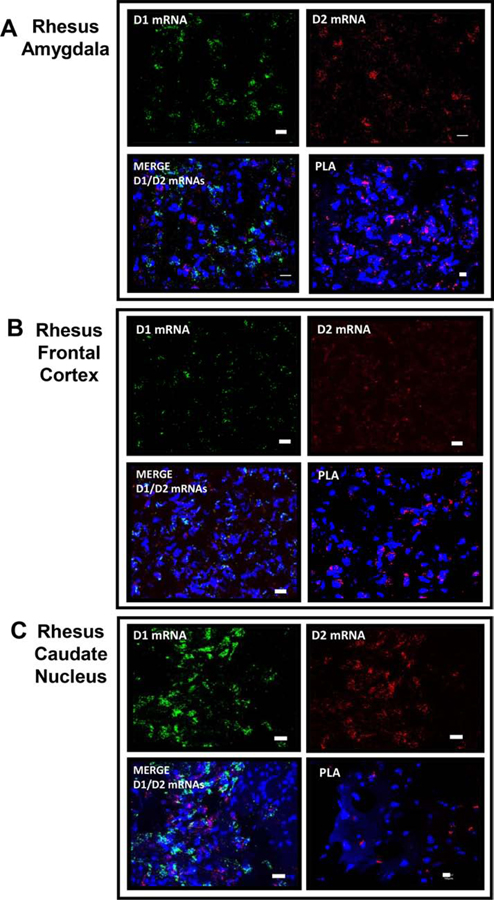
The D1–D2 heteromer was also documented in rhesus monkey brain regions (Figure 7) investigated such as amygdala (Figure 7A), frontal cortex (Figure 7B), and caudate nucleus (Figure 7C).
Figure 7. Representative images of D1 / D2 mRNA expression and D1–D2 PLA from different brain regions of rhesus monkey.
Coronal slices from rhesus monkey were used to visualize D1 and D2 mRNA expression using FISH technique and to visualize D1–D2 heteromer using PLA in amygdala (A), frontal cortex (B) and caudate (C). N=2.
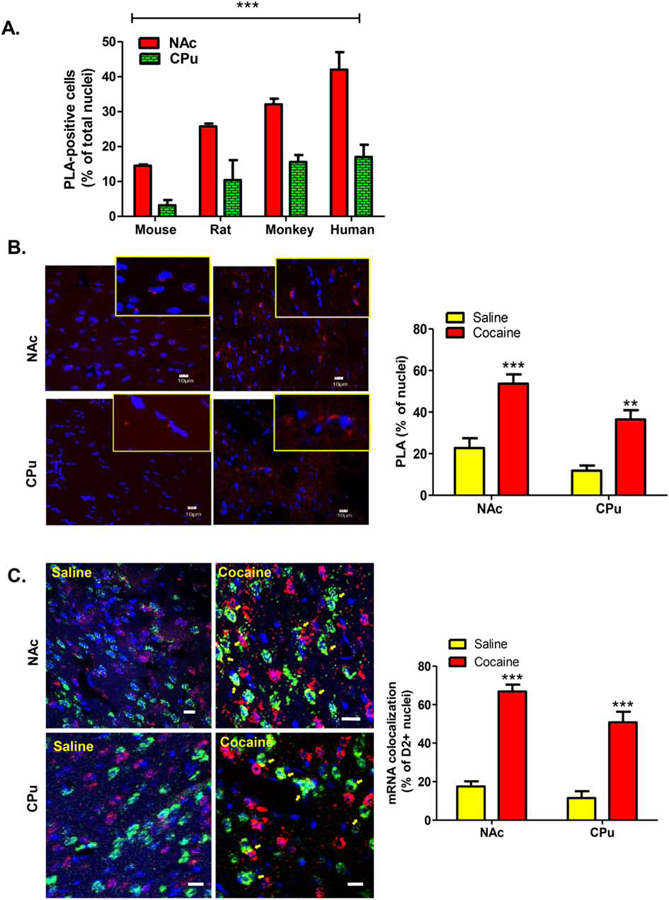
Species differences in the expression of D1–D2 heteromer in striatal nuclei
It has been shown that the D1–D2 receptor heteromer is expressed at a higher density in the NAc than in the CPu in rat (Hasbi et al., 2009; 2018; Perreault et al., 2010), mouse (Hasbi et al., 2018) and monkey (Rico et al., 2017; Hasbi et al., 2018), with the possibility of a species difference (Hasbi et al., 2020). The density of neurons expressing the D1–D2 heteromer was compared in the NAc and caudate nucleus (CN) of male mouse, rat, monkey and human to evaluate species differences to include human (Figure 8A). Data analyses using Two-way ANOVA showed a significant species difference (F(3,20) = 41.92, p<0.0001) and regional (NAc versus CN) difference (F(1,20) = 156.9, p<0.0001). An interaction [species X region] was also observed (F(3,20) = 4.48, p=0.015). These data indicated that the number of D1–D2 heteromer positive neurons is linked to the species order, with the highest number of heteromer-positive neurons in human, followed by monkey, then rat and finally mouse. These data also underlined the difference between NAc and CN in terms of heteromer expression, with the NAc containing a consistently greater number of cells expressing the D1–D2 heteromer compared to the caudate-putamen nuclei throughout the various species investigated.
Figure 8. Analysis of differential species expression of the D1–D2 heteromer in the striatum and its modulation by chronic cocaine in rat.
(A). Coronal sections from adult males from the indicated species were used to estimate D1–D2 heteromer expression in the NAc or the dorsal striatum (CPu). Three to four sections per individual (2 only for human) were used. Individuals used: N=3 for rodents and monkey, and N=2 for human. Two-way Anova (Region and Species). ***p<0.0001; **p<0.001. MK: monkey.
(B). Adult male rats (n=6 per group) were treated daily with cocaine (10 mg/Kg; i.p.) or vehicle (saline) for 8 days. Striatal coronal slices from these rats were used to visualize and estimate the number of cells expressing the heteromer (3 sections/rat) using PLA in the ventral striatum (NAc), and the dorsal striatum. Representative images are shown and the graph summarizing the PLA data is shown in the right.
(C) Representative images and estimates of D1 and D2 mRNA expression using FISH technique from rats treated as in (B). Three striatal coronal sections/rat were used. Data are presented as the percentage of positive cells per total DAPI-labeled nuclei, obtained from male rats (N=6 rats per condition). Two-way ANOVA (Region and Treatment). ***p<0.0001. Yellow arrows indicate cells with colocalized D1 and D2 mRNAs. Scale bars: 10 µm.
4. Effect of cocaine on D1–D2 heteromer expression in rat striatum
As mentioned, the dopamine D1–D2 heteromer appears to be involved functionally in certain aspects of cocaine addiction as shown in a rat model system (Hasbi et al., 2018; Perreault et al., 2016). The effect(s) of repeated treatment with cocaine (10 mg/kg, daily i.p., for 8 days) on the D1–D2 heteromer and on the colocalization of D1 and D2 mRNAs were investigated. The present study was however, limited to the caudate-putamen and the nucleus accumbens. As noted, in rat the CPu has a lower density of D1–D2 heteromer expressing neurons (5–10% of neurons) than the NAc, with the higher incidence of heteromer expressing neurons validated by different methodologies including in situ FRET and PLA (20–25% in the NAc core and up to 28–30% in the NAc shell) (Hasbi et al., 2009; 2018; Perreault et al., 2010; 2016; present data).
Chronic cocaine treatment induced a significant increase in the expression of the D1–D2 heteromer (F(1,18) = 45.43, p<0.0001) in both regions of the striatum. In the NAc, the number of cells expressing the heteromer more than doubled (Figure 8B), significantly increasing from 22–23% after vehicle treatment to more than 50% in the NAc core, and approximating ~60% PLA-positive cells in the NAc shell subregion after cocaine administration (Supplementary Figure 4A; Bonferroni’s post-hoc correction p<0.001). In the CPu, the number of cells expressing the heteromer also increased by three-fold from 10–12% after vehicle to 35–37% of total cells in the lateral and medial dorsal striatum after cocaine (Bonferroni’s post-hoc correction: p<0.01; Figure 8B; Supplementary Figure 4A).
5. Rostro-caudal gradient in the expression of the D1–D2 heteromer
It was also apparent that the cocaine-induced increase in D1–D2 heteromer expression was more dramatic in the rostral rather than caudal regions of both NAc and CPu (Supplementary Figure 4B). Repeated treatment with cocaine increased the number of cells expressing the heteromer to ~52% in the medial-caudal NAc, and values reached more than 70% in the rostral region of the NAc, with a higher density of heteromers per cell than in the caudal regions (Supplementary Figure 4B). Similar results were observed in the CPu with D1–D2 heteromer-expressing neurons increasing dramatically after cocaine and with the increase more striking in the rostral region than in the caudal part of the CPu.
6. Effect of cocaine on D1R and D2R mRNA in the striatum
The analysis of the expression and distribution of D1 and D2 receptor mRNA in vehicle-treated male rats using FISH showed that there was a greater preponderance of D2 mRNA-expressing neurons among total nuclei in the CPu (D1 mRNA: 51±6% and D2 mRNA: 62±4%) and in the NAc (D1 mRNA 49±4% and D2 mRNA: 65±2%) (Supplementary Figure 5A). The colocalization of D1 and D2 mRNAs in the same cells was assessed by counting the presence of D1 mRNA in the total population of neurons expressing D2 mRNA. These estimations showed that D1 mRNA was observed in ~20% of D2 mRNA-positive nuclei in both striatal regions (Figure 8C, saline). In contrast, cocaine treatment changed the proportion of each type of cell (D1 mRNA+ and D2 mRNA+). In cocaine-treated rats D1 mRNA positive cells was increased in both CPu (66±7% of total nuclei) and NAc (88±4%), whereas D2 mRNA positive cells showed a decrease in the CPu, (46±4% of total nuclei) and no changes in the NAc (62±2% of total nuclei) (Supplementary Figure 5B). These results showed that D1 mRNA-positive cells were more preponderant than the D2 mRNA-expressing neurons after cocaine treatment in comparison with vehicle-treated rats. These data also showed that D1 mRNA expression was increased in both CPu and NAc of male rats after treatment with cocaine, unlike D2 mRNA which showed a decrease in the CPu and no change in the NAc (Supplementary Figure 5C). Importantly, the colocalization of D1 and D2 mRNAs in the same cells, assessed by counting the presence of D1 mRNA in neurons expressing D2 mRNA, showed also that cocaine treatment led to an increase in D2 mRNA-positive cells that co-expressed D1 mRNA, reaching close to ~60–67% in the NAc and almost 60% in the CPu (Figure 8C). Statistical analysis using a Two-way ANOVA test, with “treatment” and “region” as factors of variation [F(1,18) = 132.86; p<0.0001], confirmed that cocaine treatment increased the number of D2 mRNA-positive cells that co-expressed D1 mRNA in the NAc and the CPu (Figure 8C; Bonferroni’s post-hoc correction: p<0.001).
Overall, these results showed that cocaine induced an increase in the neurons coexpressing both D1 mRNA and D2 mRNA, which paralleled an increase in the number of neurons expressing the D1–D2 receptor heteromer.
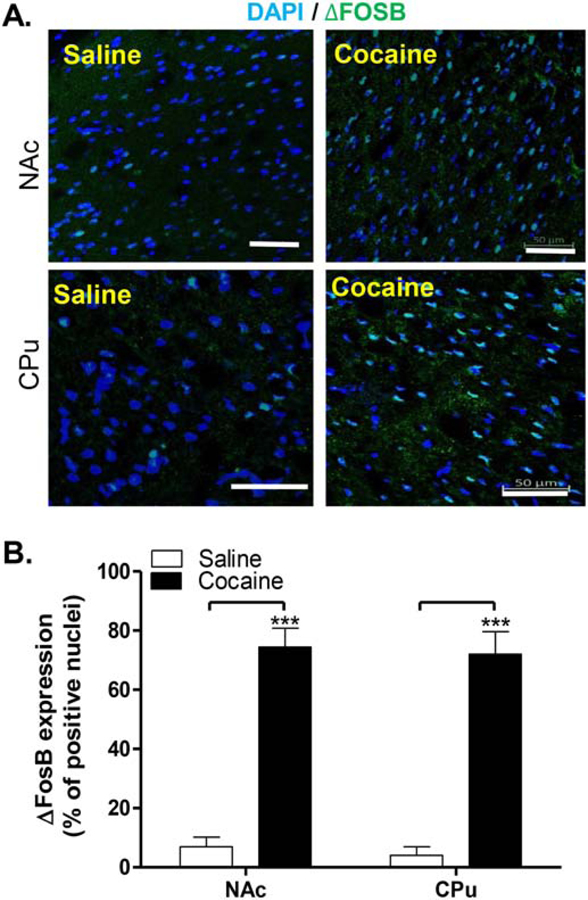
7. Delta FosB
Cocaine administration increased the expression of ΔFosB, as was observed by immunohistochemistry (Figure 9). The estimation of the number of cells expressing ΔFosB showed that cocaine increased the expression of ΔFosB from a mere ~5% of cells after vehicle treatment to almost 70% of total cells in both the NAc and CPu. Two-way ANOVA using treatment and region as factors, showed that Treatment accounts for 81.45% of the total variance [F(1, 20) = 88.33; p< 0.0001]. Bonferroni’s post-hoc corrections revealed that both regions were significantly affected by the treatment, with p<0.001 for increases in both NAc and CPu.
Figure 9. Analysis of deltaFosB expression in rat striatum and its modulation by chronic cocaine.
Adult male rats were treated daily with cocaine (10 mg/kg; i.p.) or vehicle (saline) for 8 days. Striatal coronal sections were used to visualize and estimate the number of cells expressing ΔFosB. Representative images from saline and cocaine treated rats are shown (A). The graph (B) represents the quantification of ΔFosB expression (mean±SEM). Data are presented as percentage of positive cells per total DAPI-labeled nuclei, obtained from male rats (N=6 rats per condition). Scale bars: 50 µm
These results suggest that cocaine induces ΔFosB in both the NAc and CPu. The high percentage of MSNs (70%) expressing ΔFosB after subchronic treatment with cocaine was higher than the percentage of D1-MSNs described in the literature as the cell type where ΔFosB is activated in the mouse striatum after chronic cocaine (Lobo et al., 2013). This may suggest that ΔFosB was in fact activated not only in D1-MSNs, but in D1–D2 expressing neurons as well.
Discussion
The present study highlights findings relevant to the dopamine D1–D2 receptor heteromer complex, its regional localization in parts of forebrain and midbrain, and its modulation by repeated cocaine administration. Data from evaluation of mRNA expression in situ using a RNA-FISH technique showed significant colocalization of D1 and D2 receptor mRNA in the piriform cortex, nucleus accumbens, amygdala, olfactory tubercle, claustrum, prelimbic cortex and orbitofrontal cortex. Data evaluating D1–D2 receptor heteromer expression in rat by in situ PLA validated that the mRNA coexpression was accompanied by coexpression of the receptor proteins and showed that D1 and D2 receptors form heteromers in most of the 19 investigated regions to a variable degree, with higher incidence of expression observed in the piriform cortex, NAc core and shell, amygdala, claustrum, orbitofrontal cortex, lateral habenula, cingulate cortex and substantia nigra. The D1–D2 heteromer was also observed in different brain regions of nonhuman primate, in rhesus monkey frontal cortex, amygdala, hippocampus and caudate nucleus.
The combined analyses of PLA and FISH data indicate that the D1–D2 heteromer has an extensive brain wide distribution that differed according to the regions examined, which could be subdivided into three strata of possibilities according to the following: 1)-Regions with strong D1–D2 by PLA but very low mRNA colocalization, suggesting that these regions do not express the heteromer in local neurons, but receive projections expressing the D1–D2 heteromer from other parts of the brain. This first category of regions includes the hippocampus CA region, hippocampus dentate gyrus, substantia nigra, ventral tegmental area, insular cortex, infralimbic cortex, lateral habenula, cingulate cortex and locus coeruleus. 2)-Regions where the PLA results matched closely with the mRNA colocalization data, suggesting that the D1–D2 heteromer is synthesized locally by the neurons intrinsic to these regions. This second category includes the lateral and medial dorsal striatum, amygdala and olfactory tubercle. 3)-Regions characterized by significant PLA and mRNA colocalization data, but with the PLA results indicating a much higher expression of D1–D2 heteromer than the proportion of cells with D1 and D2 mRNA colocalization. In this category of regions, it is suggested that a proportion of the heteromers are synthesized locally, whereas a significant proportion of heteromers are present on terminals projecting to the area from other brain regions. This third category of regions includes NAc core, NAc shell, piriform cortex, orbitofrontal cortex, claustrum and prelimbic cortex.
Taken together, these data widen the scope of the role of the D1–D2 heteromer in brain depending on the local regional expression as well as from projection neurons with possible differences based on the post- or pre-synaptic localization and mode of action of the heteromer. Indeed, we and others have shown previously that the D1 receptor forms receptor complexes with both splice variants of the D2 receptor, D2 long (D2L) and D2 short (D2S) isoforms (O’Dowd et al., 2012; Hasbi et al., 2014; Chun et al., 2013). We have also shown in rat striatum that the D1–D2 heteromer is localized on the cell body and proximal dendrites of MSNs (Hasbi et al., 2009), as well as being localized in the dendritic fields with synaptophysin, indicating expression on pre-synaptic terminals (Perreault et al., 2010), which likely represent projections from D1–D2 neurons to other neurons. More recently, Quake’s laboratory showed in an elegant manner, using single cell RNA sequencing in mouse striatum, a subpopulation of neurons that co-express D1 and D2 receptors (Gokce et al., 2016; Stanley et al., 2019). An important fraction (34%) were mapped to preferentially express D2S in neurons expressing D1 receptor and neurexophilin 4 (Nxph4), which showed the highest co-expression of Drd1 and Drd2 genes (Stanley et al., 2019).
Another interesting point is the clear species difference in the number of striatal neurons expressing the D1–D2 heteromer. These results, which need to be confirmed due to the limited number of human subjects (N=2) investigated, showed greater abundance in human > nonhuman primate > rat > mouse, suggesting an important evolutionary upregulation in the expression of dopamine D1–D2 heteromer. Furthermore, and as was reported previously in rat using different techniques (Hasbi et al., 2009; 2018), there was also a regional difference within the striatum, with a higher number of MSNs expressing the D1–D2 heteromer observed in the nucleus accumbens than in the dorsal striatum across all the species investigated. This distinction, dorsal versus ventral, is very important as the two regions of the striatum, caudate-putamen and nucleus accumbens, play very different functional roles (Calabresi et al., 2014 for review). According to the classically described segregation of the direct striatonigral and indirect striatopallidal pathways, there are mainly two types of neurons, MSNs expressing D1 receptor (dMSNs), and MSNs expressing D2 receptor (iMSNs) in the striatum (Gerfen et al., 1990). This discrete separation of neurons into direct and indirect pathways could be valid in the rodent dorsal striatum. Recent data using BAC transgenic mice along with optogenetics confirmed significant segregation in mouse dorsal striatum, with only a very low percentage of colocalization of D1 and D2 receptors (2–5%) observed in this region (for reviews, Lobo and Nestler, 2011; Soares-Cunha et al., 2016; Calabresi et al., 2014). However, this hypothesis is not completely valid in the ventral striatum, even in mice. Unlike dorsal striatum, a higher incidence of D1 and D2 receptor colocalization was observed in BAC transgenic mice in up to 15–17% of MSNs in NAc shell (Bertrand-Gonzales et al., 2008; Matemales et al., 2009; Gagnon et al., 2017), with comparable results observed in the expression of the D1–D2 heteromer using PLA in mouse NAc (present data and Hasbi et al., 2020). The recent single cell RNAseq analyses of mouse striatal neurons also show continuous spatial gradients of D1 and D2 receptor expression (Stanley et al., 2019). Indeed, several different authors have started to revisit the theory of exclusively segregated direct and indirect pathways of the basal ganglia function in both motor and behavioral activities (Calabresi et al., 2014; Soares-Cunha et al., 2016; Nadjar et al., 2006 and references herein).
Besides the striatum, the dopamine D1–D2 heteromer has been previously shown to exist in other regions, such as in the human globus pallidus (GP) (Perreault et al., 2010) or has been shown indirectly through c-Fos activation after either stimulation or specific disruption of the D1–D2 heteromer. Significant c-Fos immunoreactivity was observed in the anterior olfactory nucleus, lateral habenula, thalamic nuclei and in different cortical regions (Perreault et al., 2015). The expression of the D1–D2 heteromer in these regions and many others described in the present data may reflect its importance in normal physiological functions, and may as well prove to be significant in pathophysiological conditions, such as in Parkinson disease (Rico et al., 2017), schizophrenia (Perreault et al., 2010), drug addiction (Perreault et al., 2016; Hasbi et al., 2018; 2020), as well as in depression-like and anxiety-like behaviors (Shen et al., 2015; Hasbi et al., 2014). In rat models of addiction to cocaine, activation of dopamine D1–D2 heteromer blocks cocaine self-administration, cocaine-induced locomotor sensitization, cocaine-induced place preference, and cue-induced as well as drug priming-induced relapse to cocaine use (Hasbi et al., 2018). Selective disruption of the heteromer amplifies the above-mentioned behavioral effects resulting from cocaine actions (Hasbi et al., 2018; Perreault et al., 2016). Biochemical changes were also observed, with D1–D2 activation stimulating phosphorylation of Thr75-DARPP-32 and blocking cocaine-induced ΔFosB, cocaine-induced phosphorylation of ERK, and cocaine-induced phosphorylation of Thr34-DARPP-32 (Hasbi et al., 2018). In the present study, a cocaine administration regimen of 8 days induced a clear increase in the expression of ΔFosB from ~5% of cells to almost 70% of cells. These results suggest that cocaine induces ΔFosB in D1-expressing MSNs as was previously shown in mouse models (Nestler, 2008; Lobo et al., 2013; Perrotti et al., 2008), but probably also in the neurons expressing D1–D2 heteromer.
Interestingly, an increase in D1–D2 heteromer expression was observed after repeated cocaine administration in rat striatum (present data), and chronic low doses of THC in rhesus monkey (Hasbi et al., 2020), which may indicate profound genetic modifications occurring within striatal neurons after chronic drug intake. The detailed mechanisms that trigger these modifications are at the present time not known. Since activation of the D1–D2 heteromer has been shown to mediate aversion (Hasbi et al., 2018), the neurons expressing the heteromers may be part of a brain wide aversion network that includes not only nucleus accumbens and dorsal striatum, but possibly also regions such as prefrontal cortex, orbitofrontal cortex, amygdala, lateral habenula and ventral tegmental area.
Conclusions and perspectives
The widespread expression profile of the D1–D2 heteromer in very significant brain regions, and its evolutionary conservation with increased density of heteromer expressing neurons in striatum of higher species indicates an important potential role for the D1–D2 heteromer in the evolution of biological complexity and higher CNS function. Ongoing studies to elucidate the functional role of the D1–D2 heteromer and D1–D2 neuronal projections to define specific pathways will help delineate this more clearly. Its upregulation in striatum following drugs of abuse such as cocaine and THC points to potential involvement in clinical disorders, notably in drug addiction. The D1–D2 heteromer may also have unique and essential roles in depression, Parkinson disease and schizophrenia, placing it high on the list of targets for consideration in the development of novel therapeutics for neuropsychiatric disorders.
Supplementary Material
Supplementary Figure 1. Representative D1–D2 PLA images in the striatum and cortex of male rats
Brain sections from drug naïve rats were subjected to proximity ligation assay to detect D1–D2 receptor complex. A. Representative images of dopamine D1–D2 receptor complex in lateral and medial caudate-putamen (CPu), the nucleus accumbens-core (NAc-core) and the nucleus accumbens-shell subregions of the striatum. B. Representative images of dopamine D1–D2 receptor complex in different cortices: piriform cortex, prelimbic cortex, insular cortex, infralimbic cortex, orbitofrontal cortex, cingulate cotex. Scale bars: 10 µm.
Supplementary Figure 2. Representative D1–D2 PLA images from different rat brain regions
Proximity ligation assay to detect D1–D2 receptor complex was performed on brain sections from drug naive rats. Representative images of D1–D2 PLA are shown from A. hippocampus CA and DG regions and amygdala, B. substantia nigra, ventral tegmental area and locus coerulus, and C. olfactory tubercle, lateral habenula and claustrum. Scale bars: 10 µm.
Supplementary Figure 3. Co-Immunoprecipitation of D1R by D2R from different brain regions of male rats
Protein homogenates (300 μg) obtained from tissue punches from different regions of rat brain were immunoprecipitated with anti-D2R antibody and revealed by the anti-D1R antibody. Signal detection was performed using a Li-Cor instrument.
Supplementary Figure 4. Effect of cocaine treatment on D1–D2 heteromer
Rats were treated daily with vehicle or cocaine (10 mg/kg, i.p.) for eight days (Cocaine x8). Brain sections from these rats were subjected to proximity ligation assay to detect D1–D2 receptor complex. A. D1–D2 heteromer estimation by PLA in the dorso-medial (DMS), dorsolateral (DLS) striatum, as well as NAc core and NAc shell subregions. The graphs represent the percentage of PLA positive cells using DAPI-labeled nuclei to evaluate total number of cells. B. Representative images of rostro-caudal gradient of D1–D2 PLA expression in the dorsal (CPu) and ventral (NAc) regions of rat striatum. Scale bars: 10 µm.
Supplementary Figure 5. FISH analysis of D1 and D2 mRNA expression
Rats were treated daily with vehicle or cocaine (10 mg/kg, i.p.) for eight days (Cocaine x8). Striatal sections from these rats were subjected to fluorescent in situ hybridization (FISH) using RNAscope technology to detect D1 receptor-mRNA and D2 receptor-mRNA expression. A. Relative distribution of D1 and D2 mRNA-positive cells in the CPu (left) and NAc (right) of vehicle-treated rats. B. Relative distribution of D1 and D2 mRNA-positive cells in the striatum of cocaine-treated rats. C. Changes in D1 and D2 mRNA expression in cocaine-treated rats in comparison to vehicle-treated rats.
Highlights.
Expression of DA D1–D2 heteromer in cortical and subcortical regions of rat and monkey (87)
Heteromer expression and mRNA colocalization identified three categories of regions (86)
Species difference observed in striatum: human>nonhuman-primate>rat>mouse (75)
D1–D2 heteromer upregulation in rat striatum after chronic cocaine administration (84)
Relevance for clinical disorders such as drug addiction (58)
Suggest important evolutionary role for D1–D2 heteromer in higher CNS function (81)
Acknowledgments
Funding: This project was funded by grants from NIDA DA042178 (to BKM and SRG), CIHR PJT-148633 (to SRG).
Abbreviations
- OFC
orbitofrontal cortex
- OFT
olfactory tubercle
- LHb
lateral habenula
- SN
substantia nigra
- Dstr
dorsal striatum
- Hipp
hippocampus
- VTA
ventral tegmental area
- IL
infralimbic cortex
- PL
prelimbic cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
Ethics approval and consent to participate
All procedures in this study were carried out in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care, 1993). The protocol was approved by the University of Toronto Animal Use Protocol Committee. The Institutional Animal Care and Use Committee at McLean Hospital approved all experimental protocols on rhesus monkeys. The research facility housing the monkeys is licensed by the U.S. Department of Agriculture and consultant veterinarians monitored the health of the colony.
Consent for publication: All authors read and approved the manuscript.
Availability of supporting data
All data and supportive data are presented. Any reasonable request should be addressed to the corresponding authors.
Competing interests: All Authors declare no conflict of interest.
REFERENCES
- Albin RL, Young AB, Penney JB (1995). The functional anatomy of disorders of the basal ganglia. Trends Neurosci 18:63–64. [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, 2008. Opposing patterns of signaling activation in dopamine D1 and D2 receptorexpressing striatal neurons in response to cocaine and haloperidol. J. Neurosci 28, 5671–5685. 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014). Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat. Neurosci 17, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (1993). Guide to the Care and Use of Experimental Animals, 2nd Edn., Vol. 1, eds Olfert ED, Cross BM, and McWilliam AA. Ottawa, ON. [Google Scholar]
- Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, Sibley DR. D1–D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol Pharmacol 2013;84(2):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouiche L, Massotte D. (2018). G protein-coupled receptor heteromers are key players in substance use disorder. Neurosci Biobehav Rev S0149–7634, 30158–1. [DOI] [PubMed] [Google Scholar]
- Borroto-Escuela DO, Perez De La Mora M, Manger P, Narváez M, Beggiato S, Crespo-Ramírez M, Navarro G, Wydra K, Díaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Filip M, Franco R, Fuxe K (2018). Brain Dopamine Transmission in Health and Parkinson’s Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors. Front Synaptic Neurosci 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, Parent M (2017). Striatal Neurons Expressing D(1) and D(2) Receptors are morphologically distinct and differently affected by dopamine denervation in Mice. Sci Rep 7, 41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, O’Dowd BF (2007). A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. Scientific World Journal 7: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–32. [DOI] [PubMed] [Google Scholar]
- Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, Rothwell PE, Fuccillo MV, Südhof TC, Quake SR (2016). Cellular Taxonomy of the Mouse Striatum as Revealed by Single-Cell RNA-Seq. Cell Rep 16(4), 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR (2011). Dopamine D1–D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Molecular Brain, 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Madras BK, Bergman J, Kohut S, Lin Z, Withey SL, George SR, 2020. Δ9-tetrahydrocannabinol increases dopamine D1-D2 receptor Heteromer and elicits phenotypic reprogramming in adult primate striatal neurons. ISCIENCE 23, 100794 10.1016/j.isci.2019.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O’Dowd BF, George SR (2009). Calcium signaling cascade links dopamine D1–D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U S A 106, 21377–21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Perreault ML, Shen MY, Zhang L, To R, Fan T, Nguyen T, Ji X, O’Dowd BF, George SR (2014). A peptide targeting an interaction interface disrupts the dopamine D1–D2 receptor heteromer to block signaling and function in vitro and in vivo: effective selective antagonism. FASEB J 28, 4806–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Perreault ML, Shen MYF, Fan T, Nguyen T, Alijaniaram M, Banasikowski TJ, Grace AA, O’Dowd BF, Fletcher PJ, George SR (2018). Activation of Dopamine D1–D2 Receptor Complex Attenuates Cocaine Reward and Reinstatement of Cocaine-Seeking through Inhibition of DARPP-32, ERK, and ΔFosB. Front Pharmacol 4, 8:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Pollak KA, Hjelmstad GO, Fields HL (2004). A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci U S A 101(15), 5664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lança AJ, O’Dowd BF, George SR (2004). Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem 279, 35671–35678. [DOI] [PubMed] [Google Scholar]
- Levesque M, Parent A (2005). The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci USA 102:11888–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, and Nestler EJ (2011). The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. (2013). ΔFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci 33, 18381–18395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau J, Valjent E, 2009. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS ONE 4 (e4770). 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou SB, Wang GJ, Ravenscroft P, Georges F, Crossman AR, Bezard E (2006) Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J Neurosci 26(34):8653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2008). Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci 363, 3245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd BF, Ji X, Nguyen T, George SR, 2012. Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation. Biochem Biophys Res Commun. 417 (1), 23–28. 10.1016/j.bbrc.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Volkow ND, Pan Y, Du C (2013). Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci 33, 15827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, and Watson C (1988). The Rat Brain in Stereotaxic Coordinates San Diego, CA: Academic Press. [Google Scholar]
- Perreault ML, Shen MY, Fan T, George SR (2015). Regulation of c-fos expression by the dopamine D1–D2 receptor heteromer. Neuroscience 285: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O’Dowd BF, George SR (2010). The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: Increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem 285, 36625–36634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Shen MY, Fan T, Navarro G, Fletcher PJ, Franco R, Lanciego JL, George SR (2016). Disruption of a dopamine receptor complex amplifies the actions of cocaine. Eur Neuropsychopharmacol 26, 1366–77. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, et al. (2008). Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse 62, 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR (2007). D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U.S.A 104, 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico AJ, Dopeso-Reyes IG, Martínez-Pinilla E, Sucunza D, Pignataro D, Roda E, Marín-Ramos D, Labandeira-García JL, George SR, Franco R, Lanciego JL (2017). Neurochemical evidence supporting dopamine D1–D2 receptor heteromers in the striatum of the long-tailed macaque: changes following dopaminergic manipulation. Brain Struct Funct 222, 1767–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MY, Perreault ML, Bambico FR, Jones-Tabah J, Cheung M, Fan T, Nobrega JN, George SR (2015a). Rapid anti-depressant and anxiolytic actions following dopamine D1–D2 receptor heteromer inactivation. Eur Neuropsychopharmacol 25, 2437–48. [DOI] [PubMed] [Google Scholar]
- Shen MY, Perreault ML, Fan T, George SR (2015b). The dopamine D1–D2 receptor heteromer exerts a tonic inhibitory effect on the expression of amphetamine-induced locomotor sensitization. Pharmacol Biochem Behav 128, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Sousa N, Rodrigues AJ (2016). Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci Biobehav Rev 68, 370–386. [DOI] [PubMed] [Google Scholar]
- Stanley G, Gokce O, Malenka RC, Südhof TC, Quake SR, 2019. Discrete and continuous cell identities of the adult murine striatum. bioRxiv. 10.1101/591396. [DOI] [PubMed]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F (2011). Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci U S A, 108, 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative D1–D2 PLA images in the striatum and cortex of male rats
Brain sections from drug naïve rats were subjected to proximity ligation assay to detect D1–D2 receptor complex. A. Representative images of dopamine D1–D2 receptor complex in lateral and medial caudate-putamen (CPu), the nucleus accumbens-core (NAc-core) and the nucleus accumbens-shell subregions of the striatum. B. Representative images of dopamine D1–D2 receptor complex in different cortices: piriform cortex, prelimbic cortex, insular cortex, infralimbic cortex, orbitofrontal cortex, cingulate cotex. Scale bars: 10 µm.
Supplementary Figure 2. Representative D1–D2 PLA images from different rat brain regions
Proximity ligation assay to detect D1–D2 receptor complex was performed on brain sections from drug naive rats. Representative images of D1–D2 PLA are shown from A. hippocampus CA and DG regions and amygdala, B. substantia nigra, ventral tegmental area and locus coerulus, and C. olfactory tubercle, lateral habenula and claustrum. Scale bars: 10 µm.
Supplementary Figure 3. Co-Immunoprecipitation of D1R by D2R from different brain regions of male rats
Protein homogenates (300 μg) obtained from tissue punches from different regions of rat brain were immunoprecipitated with anti-D2R antibody and revealed by the anti-D1R antibody. Signal detection was performed using a Li-Cor instrument.
Supplementary Figure 4. Effect of cocaine treatment on D1–D2 heteromer
Rats were treated daily with vehicle or cocaine (10 mg/kg, i.p.) for eight days (Cocaine x8). Brain sections from these rats were subjected to proximity ligation assay to detect D1–D2 receptor complex. A. D1–D2 heteromer estimation by PLA in the dorso-medial (DMS), dorsolateral (DLS) striatum, as well as NAc core and NAc shell subregions. The graphs represent the percentage of PLA positive cells using DAPI-labeled nuclei to evaluate total number of cells. B. Representative images of rostro-caudal gradient of D1–D2 PLA expression in the dorsal (CPu) and ventral (NAc) regions of rat striatum. Scale bars: 10 µm.
Supplementary Figure 5. FISH analysis of D1 and D2 mRNA expression
Rats were treated daily with vehicle or cocaine (10 mg/kg, i.p.) for eight days (Cocaine x8). Striatal sections from these rats were subjected to fluorescent in situ hybridization (FISH) using RNAscope technology to detect D1 receptor-mRNA and D2 receptor-mRNA expression. A. Relative distribution of D1 and D2 mRNA-positive cells in the CPu (left) and NAc (right) of vehicle-treated rats. B. Relative distribution of D1 and D2 mRNA-positive cells in the striatum of cocaine-treated rats. C. Changes in D1 and D2 mRNA expression in cocaine-treated rats in comparison to vehicle-treated rats.