Abstract
Leukotriene B4 (LTB4) is a major proinflammatory mediator important in host defense, whereas resolvins (Rvs) are produced during the resolution phase of inflammation. The authors determined the actions of both RvE1 and RvD1 on LTB4–induced responses of goblet cells cultured from rat conjunctiva. The responses measured were an increase in the intracellular [Ca2+] ([Ca2+]i) and high–molecular-weight glycoprotein secretion. Treatment with RvE1 or RvD1 for 30 minutes significantly blocked the LTB4–induced [Ca2+]i increase. The actions of RvE1 on LTB4–induced [Ca2+]i increase were reversed by siRNA for the RvE1 receptor, and the actions of RvD1 were reversed by an RvD1 receptor inhibitor. The RvE1 and RvD1 block of LTB4-stimulated increase in [Ca2+]i was also reversed by an inhibitory peptide to β-adrenergic receptor kinase. LTB4 and block of the LTB4–stimulated increase in [Ca2+]i by RvE1 and RvD1 were partially mediated by the depletion of intracellular Ca2+ stores. RvE1, but not RvD1, counterregulated the LTB4–induced high–molecular-weight glycoprotein secretion. Thus, both RvE1 and RvD1 receptors directly inhibit LTB4 by phosphorylating the LTB4 receptor using β adrenergic receptor kinase. RvE1 receptor counterregulates the LTB4–induced increase in [Ca2+]i and secretion, whereas RvD1 receptor only counterregulates LTB4–induced [Ca2+]i increase.
The conjunctiva is a mucous membrane that encircles the cornea and covers the sclera, fornix, and inner side of the eyelids. One of its major functions is to protect the eye from the environment, including exogenous allergens, pathogens, and pollutants. The epithelium of conjunctiva is made up of three main cell types: goblet cells, fibroblasts, and stratified squamous epithelial cells. Goblet cells synthesize, store, and secrete the large gel-forming mucin MUC5AC, which contributes to the inner layer of the tear film. The main functions of the tear film mucin are: i) moisturize the ocular surface (cornea and conjunctiva); ii) lubricate the ocular surface during blinking; iii) prevent the binding of pathogens onto the ocular surface epithelium; and iv) participate in the epithelial cell innate immune response.1 Physiological regulation of mucin production by the goblet cells is critical for ocular surface health. A decrease or an increase in mucin production leads to dysfunction of the ocular surface and degradation of vision.
Mucin production by goblet cells increases during ocular inflammatory diseases such as early dry eye2 and allergic conjunctivitis.3 Proinflammatory mediators such as histamine, prostaglandins, cysteinyl leukotrienes (cysLTs), and leukotriene B4 (LTB4) directly stimulate secretion including mucins from conjunctival goblet cells.4, 5, 6 The disturbance of normal structure of tear film caused by oversecretion of mucin together with inflammatory edema of the conjunctiva caused by proinflammatory allergic mediators leads to discomfort and pain.
Two of the most important inflammatory mediators: prostaglandins and leukotrienes (LTs) are biosynthesized from arachidonic acid, a polyunsaturated fatty acid.7,8 When cells are challenged by external stimuli, they release arachidonic acid from the cell membrane by activation of phospholipase A2. Activation of cyclooxygenase (COX) in most cell types produces prostaglandins, which cause pain, swelling, and fever.9 Activation of 5-lipoxygenase (LOX-5) biosynthesizes LTA4, which undergoes enzymatic hydrolysis to LTB4, conjugation with glutathione to LTC4, or via transcellular metabolism to produce LTB4.7 LTB4 triggers a proinflammatory cascade that promotes chemotaxis and adhesion to vascular endothelium by neutrophils, causes plasma leakage,10 and enhances proinflammatory effects caused by prostaglandins.11 Inflammatory cells (eg, neutrophils, macrophages, mast cells) biosynthesize LTB4.7 Ocular surface epithelial cells also express LOX-5, and LTB4 is present in human tears.12 On the ocular surface, LTB4 triggers allergic conjunctivitis,11 giant papillary conjunctivitis,12 and vernal keratoconjunctivitis.13
Resolvins, including RvE1 and RvD1 used in the present study, are endogenous mediators that are biosynthesized from omega-3 fatty acids. The production of resolvins increases temporally during the resolution phase of inflammation. Each resolvin is produced at a different time during resolution and uses different receptor-mediated mechanisms to resolve inflammation.14 RvE1 is derived from eicosapentaenoic acid15 and acts via two receptors: the chemokine-like receptor CMKLR1/RVER1 and the LTB4 receptor BLT1.15 RvD1 is derived from docosahexaenoic acid, activates the lipoxin A4 receptor/formyl peptide receptor (ALX/FPR) 2 in murine tissues (ie, rats and mice), and in humans, also activates the GPR32 receptor.16 RvD1 and RvE1 function by decreasing the production of proinflammatory cytokines in multiple tissues.16, 17, 18, 19 The mechanisms, however, by which RvE1 and RvD1 each interact with leukotrienes to terminate their proinflammatory actions are not completely known.
In conjunctival goblet cells during physiological homeostasis, both RvD1 and RvE1 trigger mucin secretion.17,18 In mouse models of ocular allergy, the level of LTB4 in conjunctiva is markedly increased,20 and excessive mucin is produced.21 To date, there are no reports on the interactions between RvE1, RvD1, and the inflammatory mediator LTB4 in conjunctival goblet cells as could occur during ocular inflammatory diseases, especially ocular allergy. In the present study, rat conjunctival goblet cells in culture were used to investigate the interaction of the RvE1 and RvD1 with LTB4 stimulation of goblet cell function and determine the underlying mechanisms of these interactions.
Materials and Methods
Materials
RPMI-1640 cell culture medium, penicillin/streptomycin, and l-glutamine were purchased from Lonza (Walkerville, IL). Fetal bovine serum was from Atlanta Biologicals (Norcross, GA). Histamine, histamine dimaleate (H1), and 2-aminoethoxydiphenyl borate (2-APB) were obtained from Sigma-Aldrich (St. Louis, MO). BARK1 inhibitor was from EMD Millipore (Billerica, MA). Fura-2/AM was purchased from Life Technologies (Grand Island, NY). RvD1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid), RvE1 (5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid), and LTB4 (5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid) were stored in an ethanol solution at −80°C as supplied by the manufacturer (Cayman Chemical, Ann Arbor, MI). The solution was diluted immediately before use in Krebs-Ringer bicarbonate buffer with HEPES [KRB-HEPES, 119 mmol/L NaCl, 4.8 mmol/L KCl, 1.0 mmol/L CaCl2, 1.2 mmol/L MgSO4,1.2 mmol/L KH2PO4, 25 mmol/L NaHCO3, 10 mmol/L HEPES, and 5.5 mmol/L glucose (pH 7.40 to 7.45)] to the desired concentrations and added to the cells.
Inhibitors were initially diluted and stored in dimethyl sulfoxide at −20°C. Immediately before use, the inhibitors were diluted to desired concentrations in KRB-HEPES and added to the cells.
Animals
Male Sprague-Dawley rats weighing between 125 and 150 g obtained from Taconic Farms (Germantown, NY) were anesthetized with carbon dioxide for 1 minute and then decapitated. The bulbar and forniceal conjunctiva were removed from both eyes. All experiments were performed according to the NIH guide for the Care and Use of Laboratory Animals22 and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Goblet Cell Culture
Goblet cells from rat conjunctiva were grown in organ culture as described previously.5,6,17,18,23,24 Pieces of minced conjunctival epithelium were placed on culture dishes with RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, and 100 μg/mL penicillin-streptomycin. The tissue pieces were removed after nodules of cells were observed. First-passage goblet cells were used in all of the experiments. Identity of cultured cells was periodically checked by evaluating staining with antibody to cytokeratin 7 (detects goblet cell bodies) and the lectin Ulex europaeus agglutinin (UEA)-1 (detects goblet cell secretory product) to ensure that goblet cells predominated.
Measurement of [Ca2+]i
First-passage rat conjunctival goblet cells were plated onto 35-mm glass-bottomed culture dishes and incubated at 37°C overnight. Cells were then incubated for 1 hour at 37°C with KRB-HEPES with 0.5% bovine serum albumin and 0.5 μmol/L fura-2/AM (Invitrogen, Grand Island, NY), 8 μmol/L pluronic acid F127 (Sigma-Aldrich), and 250 μmol/L sulfinpyrazone (Sigma-Aldrich). Before calcium measurements, cells were washed with KRB-HEPES containing sulfinpyrazone. Calcium measurements were conducted using a ratio imaging system (InCyt Im2 software version 5.33; Intracellular Imaging, Cincinnati, OH) using wavelengths of 340 and 380 nm, and an emission wavelength of 505 nm. Cells were stimulated with agonists, antagonists, and inhibitors. Intracellular [Ca2+] ([Ca2+]i) as a function of time was recorded, and the change in peak [Ca2+]i was calculated by subtracting the average of the basal value from the peak [Ca2+]i value. A duplicate was conducted for each experiment, and the mean value was recorded as a data point.
Knock-Down of Proteins Using siRNA
First-passage goblet cells were grown in 6-well plates. siRNA specific to the RvE1 receptor (Cmklr1) or negative control scrambled siRNA (scsiRNA), was added at a final concentration of 100 nmol/L in antibiotic-free RPMI 1640 as described previously.24 Media were removed after 18 hours and replaced with fresh, complete RPMI 1640 and incubated for 48 hours before use. To ensure the successful depletion of receptors from the goblet cells, one well per condition was scraped, and Western blotting analysis using an antibody against the receptor was performed. Use of 100 nmol/L Cmklr1 siRNA decreased Cmklr1 protein by 64%.4
Measurement of High–Molecular-Weight Glycoprotein Secretion
First-passage, cultured goblet cells were plated in 24-well plates and grown to confluence. Cells were serum starved for 2 hours before use and then stimulated with agonists for 2 hours in the serum-free RPMI 1640 supplemented with 0.5% bovine serum albumin. Goblet cell secretion was measured using an enzyme-linked lectin assay with the lectin UEA-I that detects rat conjunctival goblet cell mucins and other secreted high–molecular-weight glycoproteins. The media were collected and analyzed for the amount of lectin-detectable glycoprotein including the mucin MUC5AC.5,17,18 This will be referred to as glycoprotein secretion. The cells were scraped and analyzed for total protein using the Bradford assay. Glycoprotein secretion was standardized to the amount of protein in the cellular pellet and expressed as fold increase over basal that was set to 1.
Statistical Analysis
The data are presented as the fold-increase above basal as the averages ± SEM. The t-test was used for comparison of two groups, and one-way analysis of variance with Dunn multiple comparisons was used to perform statistical analysis in multiple groups. P < 0.05 was set as statistically significant.
Results
RvE1 and RvD1 Counterregulate LTB4-Stimulated Increase in [Ca2+]i in Conjunctival Goblet Cells
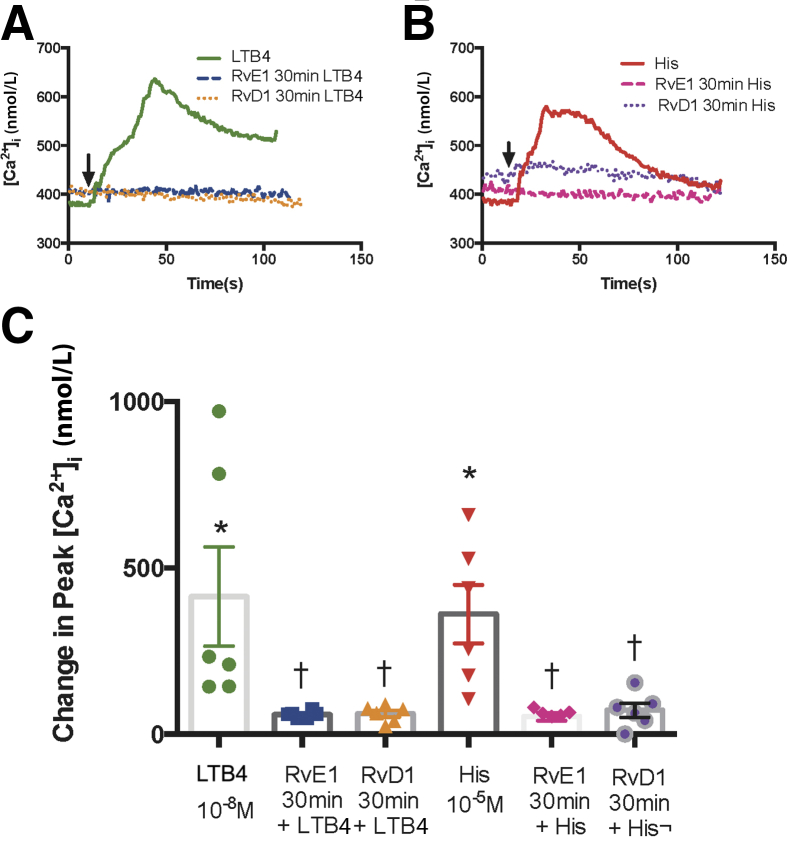
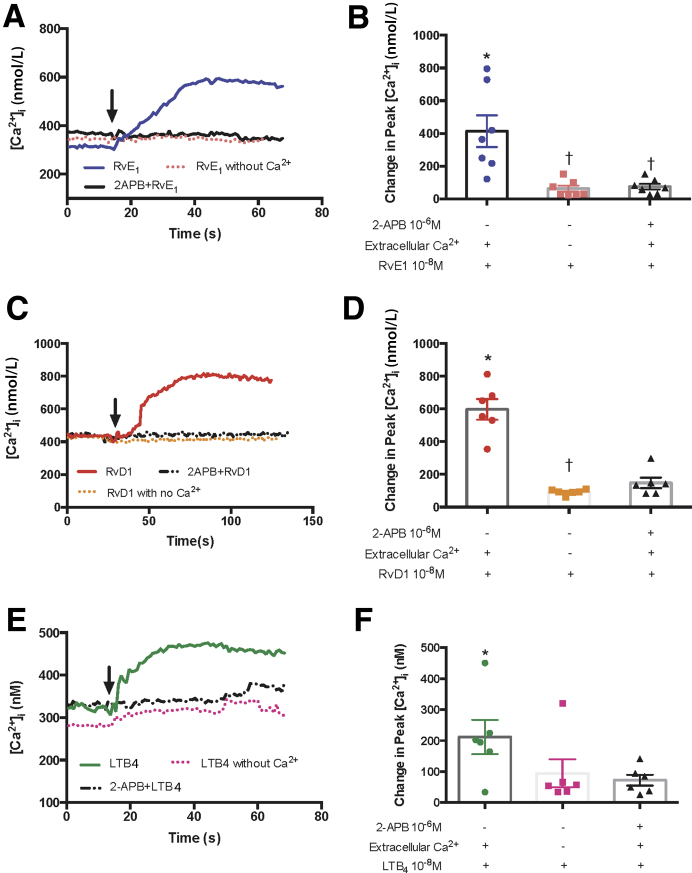
The LTB4-stimulated increase in [Ca2+]i was tested with or without RvE1 or RvD1. Goblet cells were incubated for 30 minutes with RvE1 (10−8 mol/L) or RvD1 (10−8 mol/L) before stimulation with LTB4 at (10−8 mol/L) (Figure 1). Histamine (10−5 mol/L) served as the positive control because previous studies showed that the response to histamine was attenuated after incubation with RvD1.24 LTB4 alone increased the [Ca2+]i by 413.81 ± 149.18 nmol/L, which is significantly increased from basal (P = 0.0098). Incubation with RvE1 decreased the LTB4–induced [Ca2+]i response to 59.15 ± 4.37 nmol/L (P = 0.019) (Figure 1, A and C). In the presence of RvD1, the LTB4–induced [Ca2+]i increase was significantly blocked to 61.13 ± 10.20 nmol/L (P = 0.019). The control histamine significantly increased the [Ca2+]i from basal to 361.05 ± 88.11 nmol/L (P = 0.0011). The histamine-induced response was blocked by RvE1 incubation decreasing it to 52.54 ± 11.71 nmol/L (P = 0.0078). Similarly, RvD1 incubation blocked the histamine-induced [Ca2+]i response decreasing it to 71.83 ± 21.08 nmol/L (P = 0.042) (Figure 1, B and C).
Figure 1.
Resolvin E1 (RvE1) and resolvin D1 (RvD1) counterregulate leukotriene B4 (LTB4)–stimulated increase in intracellular [Ca2+] ([Ca2+]i) in goblet cells. Cultured rat conjunctival goblet cells were stimulated with LTB4 (10−8 mol/L) or histamine (histamine 10−5 mol/L) alone or incubated with RvE1 or RvD1 (10−8 mol/L) or vehicle for 30 minutes prior to addition of LTB4 or histamine, and [Ca2+]i was measured. A and B: The average [Ca2+]i level over time is shown. Arrows indicate addition of LTB4 or histamine. C: Change in peak [Ca2+]i was calculated and is shown. One-way analysis of variance nonparametric with Dunn multiple comparisons was used. Data are expressed as individual data points from each animal and means ± SEM. n = 6 rats (C). ∗P < 0.05 versus basal; †P < 0.05 versus LTB4 or histamine alone.
RvE1 Counterregulation of the LTB4–Induced Increase in [Ca2+]i Is Receptor Mediated
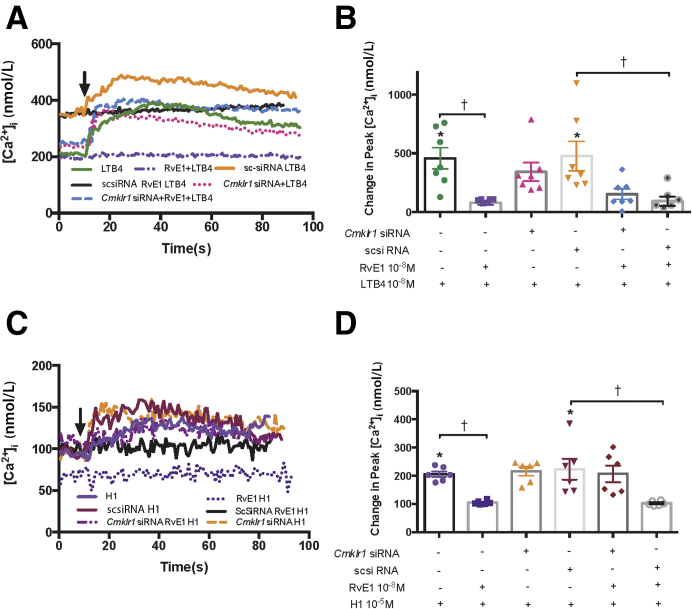
It was then tested whether the RvE1 receptor Cmklr1 mediated the RvE1 inhibition of the LTB4–induced Ca2+ response. The goblet cells were treated with vehicle, Cmklr1 siRNA (100 nmol/L), or scsiRNA. Use of 100 nmol/L Cmklr1 siRNA decreased Cmklr1 protein by 64%.4 LTB4 (10−8 mol/L) alone increased the [Ca2+]i significantly from basal by 457.10 ± 91.09 nmol/L (P = 0.0066) (Figure 2, A and B). When cells were treated with RvE1 (10−8 mol/L) for 30 minutes before LTB4 stimulation, the [Ca2+]i response to LTB4 was blocked and was 80.48 ± 17.56 nmol/L (P = 0.0027), thus showing counterregulation of LTB4 by RvE1. The LTB4 Ca2+ response was not significantly altered when incubated with either just the siRNA to Cmklr1 (343.10 ± 77.66 nmol/L; P > 0.99) or the scsiRNA (477.20 ± 125.50; P > 0.99) (Figure 2B). RvE1 pretreatment also blocked the [Ca2+]i response to LTB4 in scsiRNA–treated cells (P = 0.0066). When cells were incubated with Cmklr1 siRNA followed by RvE1 for 30 minutes, the LTB4-stimulated increase in [Ca2+]i was no longer inhibited and not different from the LTB4 response in both non–siRNA-treated (P = 0.32) and scsiRNA–treated cells (P = 0.28). When cells were treated with scsiRNA followed by addition of RvE1, the LTB4 response was still inhibited by RvE1 (P = 0.011) (Figure 2, A and B). Thus, the depletion of Cmklr1 blocked the counterregulatory actions of RvE1 on the LTB4-stimulated [Ca2+]i response.
Figure 2.
Resolvin E1 (RvE1) counterregulation of leukotriene B4 (LTB4)–induced intracellular [Ca2+] ([Ca2+]i) increase is reversed by depletion of CMKLR1 in goblet cells. A and C: Cultured rat conjunctival goblet cells were transfected with Cmklr1 siRNA (100 nmol/L) or scrambled (sc) siRNA (100 nmol/L) for 48 hours or were not transfected. All cells were stimulated with LTB4 (10−8 mol/L) alone or with RvE1 (10−8 mol/L) 30 minutes followed by LTB4. A and B: Cells transfected with Cmklr1 siRNA or scsiRNA were stimulated with LTB4 or with RvE1 for 30 minutes followed by LTB4. The average [Ca2+]i level over time is shown in A, and the change in peak [Ca2+]i is shown in B. C and D: Similar experiments were performed with histamine dimaleate (H1, 10−5 mol/L) replacing LTB4. Arrows indicate addition of LTB4 or histamine. One-way analysis of variance nonparametric with Dunn multiple comparisons was used. Data are expressed as individual data points from each animal and means ± SEM. n = 7 rats (B and D). ∗P < 0.05 versus basal; †P < 0.05.
As the positive control, histamine receptor 1 agonist histamine dimaleate (H1, 10−5 mol/L) significantly increased the [Ca2+]i from basal by 205.6 ± 10.09 nmol/L (P = 0.019) (Figure 2, C and D). Incubation with RvE1 significantly blocked the histamine-induced [Ca2+]i increase (104.8 ± 2.8 nmol/L; P = 0.040). Neither CMKLR1 siRNA nor scsiRNA altered the H1-induced increase in [Ca2+]i (223.0 ± 37.05 nmol/L; P > 0.99) (Figure 2D). Treatment with CMKLR1 siRNA, but not scsiRNA (Figure 2D), followed by addition of RvE1 for 30 minutes, reversed the inhibition of the H1 response caused by RvE1 (Figure 2, C and D).
These results indicate that RvE1 is using the CMKLR1 receptor to counterregulate the LTB4–induced [Ca2+]i response in goblet cells.
RvD1 Counterregulation of the LTB4–Induced Increase in [Ca2+]i Is Receptor Mediated
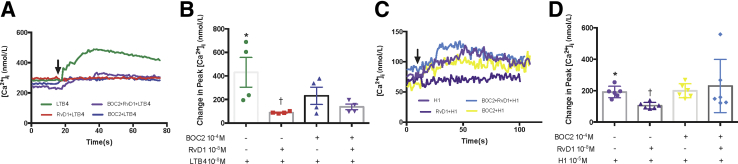
To determine the mechanism of the counter regulation of LTB4 by RvD1, it was investigated whether the RvD1 receptor ALX/FPR2 is involved in the RvD1-induced inhibition of the LTB4-stimulated increase in [Ca2+]i. To achieve this, goblet cells were incubated with the ALX/FPR2 inhibitor N-Boc−Phe-Leu-Phe-Leu-Phe (BOC2) at 10−4 mol/L (Figure 3). LTB4 (10−8 mol/L) stimulation alone increased the [Ca2+]i significantly from basal by 432.38 ± 87.49 nmol/L (P = 0.021) (Figure 3, A and B). BOC2 treatment alone did not alter the [Ca2+]i response to LTB4 (P > 0.99). RvD1 (10−8 mol/L) treatment for 30 minutes blocked the [Ca2+]i increase induced by LTB4, decreasing it to 84 ± 4.85 nmol/L (P = 0.041), demonstrating the counterregulatory effect. Blockage of the RvD1 receptor ALX/FPR2 by BOC2 prevented the counterregulatory actions of RvD1 on the LTB4 response (P = 0.82).
Figure 3.
Resolvin D1 (RvD1) counterregulation of leukotriene B4 (LTB4)–induced intracellular [Ca2+] ([Ca2+]i) increase is reversed by lipoxin A4 receptor/formyl peptide receptor ALX/FPR2 antagonist BOC2 in goblet cells. A and B: Cultured rat conjunctival goblet cells were stimulated with either of the following treatments: LTB4 (10−8 mol/L) alone; RvD1 (10−8 mol/L) for 30 minutes followed by LTB4; BOC2 (10−4 mol/L) for 15 minutes followed by LTB4; or BOC2 for 15 minutes, followed by RvD1 for 30 minutes followed by LTB4. The average [Ca2+]i level over time is shown (A). B: Change in peak [Ca2+]i was calculated and is shown. C and D: Similar experiments were performed with histamine dimaleate (H1, 10−5 mol/L) replacing LTB4. Arrows indicate addition of LTB4 or H1. One-way analysis of variance nonparametric with Dunn multiple comparisons was used. Data are expressed as individual data points from each animal and means ± SEM. n = 6 rats (B and D). ∗P < 0.05 versus basal; †P < 0.05 versus LTB4 or histamine alone.
As a positive control, histamine dimaleate (10−5 mol/L) significantly increased the [Ca2+]i from basal by 191.10 ± 14.92 nmol/L (P = 0.031), and preincubation with RvD1 significantly blocked the histamine-induced [Ca2+]i increase (104.5 ± 8.3 nmol/L; P = 0.042) (Figure 3, C and D). Treatment with BOC2 alone for 45 minutes did not change the H1-induced [Ca2+]i (P > 0.99). With BOC2 treatment for 15 minutes prior to RvD1 addition for 30 minutes, H1-induced [Ca2+]i increase was not altered compared with H1 alone (P > 0.99).
These results indicate that RvD1 is using the ALX/FPR2 receptor to counterregulate the LTB4–induced [Ca2+]i increase in goblet cells.
RvE1 and RvD1 Use BARK1 to Counterregulate the LTB4-Stimulated Increase in [Ca2+]i in Goblet Cells
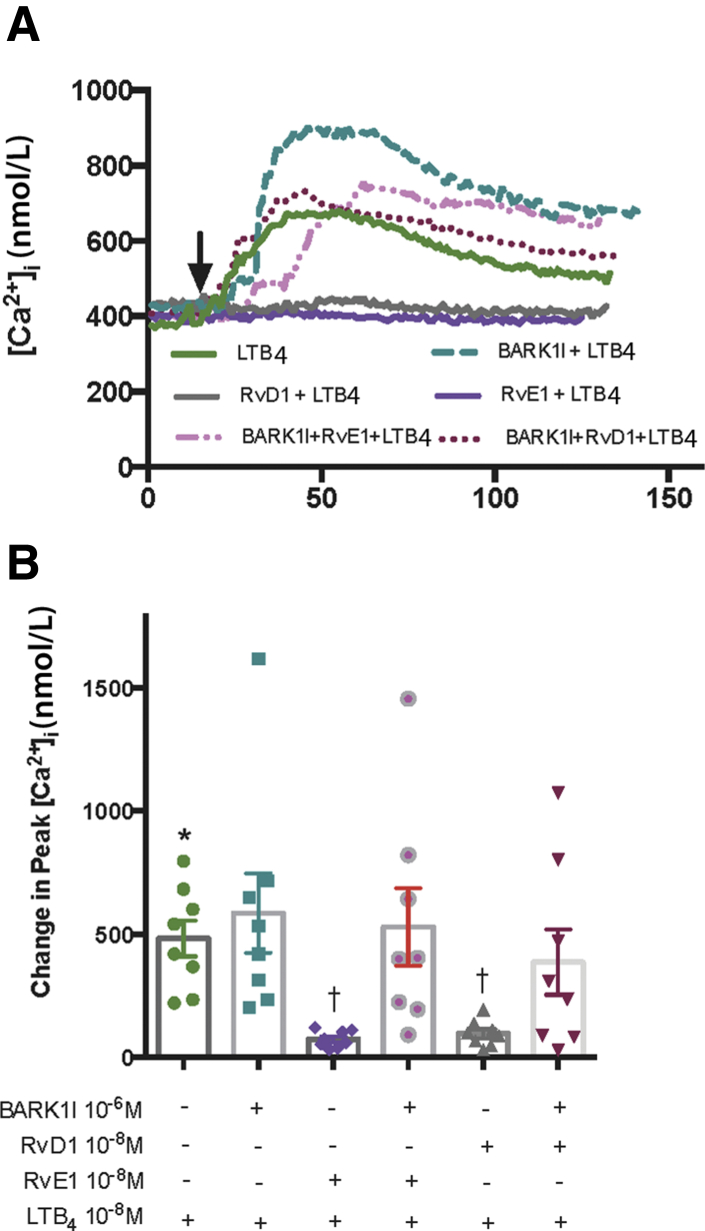
The authors found that RvD1 downregulates the histamine response by activating β-adrenergic receptor kinase (BARK1).24 Thus, the authors tested whether BARK1 is also used in the down-regulation of the LTB4 response. LTB4 (10−8 mol/L) stimulation alone increased the [Ca2+]i to 483.50 ± 73.57 nmol/L (Figure 4). The LTB4 response was not altered by incubation with the BARK1 inhibitor (10−6 mol/L) alone for 45 minutes. The response of LTB4 was significantly down-regulated to 74.86 ± 12.00 nmol/L (P = 0.011) by RvE1 (10−8 mol/L) added for 30 minutes. The counterregulation of LTB4-stimulated increase in [Ca2+]i was prevented by the addition of BARK1 inhibitor 15 minutes prior to RvE1 incubated for 30 minutes and was 529.70 ± 157.20 nmol/L (P > 0.99) (Figure 4). Similar results were obtained with RvD1(10−8 mol/L), which significantly blocked the LTB4 response to 96.92 ± 18.17 nmol/L (P = 0.011) and was reversed by the BARK1 inhibitor to 387.40 ± 132.80 nmol/L (P > 0.99) (Figure 4). As control, H1 (10−5 mol/L) was used to rule out effects from other histamine receptors. The H1 response was significantly blocked by RvE1 (P = 0.0016) and by RvD1 (P = 0.011). The BARK1 inhibitor treatment failed to prevent blocking the H1-induced [Ca2+]i increase with RvE1 or RvD1 (data not shown). These results indicate that both RvE1 and RvD1 used BARK1 to counterregulate LTB4-stimulated increases in [Ca2+]i in goblet cells.
Figure 4.
Resolvin D1 (RvD1) and resolvin E1 (RvE1) use β-adrenergic receptor kinase (βARK) to counterregulate the leukotriene B4 (LTB4)–stimulated increase in intracellular [Ca2+] ([Ca2+]i) in goblet cells. A: Vehicle or β adrenergic receptor kinase (BARK1) inhibitor (BARK1I, 10−6 mol/L) (negative control) was added to cultured rat conjunctival goblet cells 15 minutes prior to addition of LTB4 (10−8 mol/L); RvD1 or RvE1 (10−8 mol/L) was added for 30 minutes followed by LTB4; or BARK1 inhibitor was added for 15 minutes followed by RvD1 or RvE1 for 30 minutes followed by LTB4. The average [Ca2+]i level over time is shown. Arrow indicates addition of LTB4. B: Change in peak [Ca2+]i was calculated and is shown. One-way analysis of variance nonparametric with Dunn multiple comparisons was used. Data are expressed as individual data points from each animal and means ± SEM. n = 8 rats (B). ∗P < 0.05 versus basal; †P < 0.05 versus LTB4 alone.
RvE1, RvD1, and LTB4 Use Overlapping Cellular Calcium Stores to Increase [Ca2+]i
Next, the cellular calcium stores used for the intracellular Ca2+ response stimulated by RvE1, RvD1, and LTB4, each at 10−8 mol/L, were investigated. It is well established that G protein–coupled receptors (GPCRs) release Ca2+ from intracellular Ca2+ stores located in the endoplasmic reticulum. Inositol trisphosphate (IP3) produced by GPCR activation phospholipase C binds to its receptors IP3RI, -II, and -III on the endoplasmic reticulum, releasing Ca2+ from the endoplasmic reticulum into the cytoplasm.25 The depletion of this Ca2+ store activates influx of extracellular Ca2+ using the proteins STIM1 and ORAI to refill the Ca2+ store. It was tested whether RvE1, RvD1, or LTB4 (all at 10−8 mol/L) use extracellular calcium to increase [Ca2+]i by using calcium-free KRB buffer. The goblet cells were also treated with the IP3R inhibitor 2-APB (10−6 mol/L) in the presence of extracellular calcium for 10 minutes, then stimulated with RvE1, RvD1, or LTB4 to test whether the IP3-dependent intracellular Ca2+ store was used. Histamine (10−5 mol/L) served as the control. RvE1 significantly increased the [Ca2+]i from baseline by 413.40 ± 97.37 nmol/L (P = 0.016), removal of extracellular Ca2+ blocked the response to 62.77 ± 18.44 nmol/L (P = 0.0027), whereas 2-APB inhibited the [Ca2+]i response to 75.21 ± 17.06 nmol/L (P = 0.032) (Figure 5, A and B). These data indicate that RvE1 uses both intracellular calcium released from IP3-dependent intracellular stores and subsequent increase in extracellular calcium influx. RvD1 significantly increased the [Ca2+]i from baseline to 597.10 ± 63.48 nmol/L (P = 0.00024); the removal of extracellular calcium decreased the [Ca2+]i response to 91.72 ± 7.00 nmol/L (P = 0.0030), whereas 2-APB treatment dampened the RvD1-induced [Ca2+]i response to 148.01 ± 32.00 nmol/L, but was not statistically significant (P = 0.087) (Figure 5, C and D). The LTB4-stimulated [Ca2+]i increase was significantly different from baseline (211.81 ± 55.20 nmol/L; P = 0.038) (Figure 5, E and F). The removal of extracellular calcium decreased the LTB4-stimulated [Ca2+]i response, but not significantly from LTB4 response in KRB with calcium (94.55 ± 45.35 nmol/L; P = 0.17). Similar results were obtained with 2-APB incubation which decreased the LTB4-stimulated [Ca2+]i response to 72.19 ± 17.59 nmol/L that did not prove to be statistically significant (P = 0.17) (Figure 5, E and F). As control, histamine increased the [Ca2+]i level from basal (P = 0.032), which was significantly blocked by removal of extracellular Ca2+ (P = 0.03), and 2-APB treatment (P = 0.047) (data not shown). The histamine result is consistent with previous findings.24 These results indicate that RvE1-induced [Ca2+]i increase depends on both extracellular calcium and intracellular calcium storage, RvD1-induced [Ca2+]i increase was less dependent on intracellular calcium, whereas LTB4–induced [Ca2+]i increase was less dependent on extracellular or intracellular calcium.
Figure 5.
Resolvin E1 (RvE1) and resolvin D1 (RvD1) use the same cellular Ca2+ stores as leukotriene B4 (LTB4) to increase the intracellular [Ca2+] ([Ca2+]i) in goblet cells. Cultured rat goblet cells were transferred into Ca2+-free or normal KRB buffer for 2 hours or IP3R inhibitor 2-APB (10−6 mol/L) or vehicle was added for 15 minutes followed by RvE1 (A and B), RvD1 (C and D), or LTB4 (E and F), each at 10−8 mol/L. A, C, and E: The average [Ca2+]i level over time is shown. B, D, and F: Change in peak [Ca2+]i was calculated and is shown. Arrows indicate addition of RvE1 (A), RvD1 (C), or LTB4 (E). One-way analysis of variance nonparametric with Dunn multiple comparisons was used. Data are expressed as individual data points from each animal and means ± SEM. n = 7 rats (B); n = 6 rats (D and F). ∗P < 0.05 versus basal; †P < 0.05 versus agonist alone.
RvE1, but Not RvD1, Counterregulates LTB4–Induced Glycoprotein Secretion in Conjunctival Goblet Cells
Goblet cells were incubated with RvD1 (10−8 mol/L), RvE1 (10−8 mol/L), or vehicle for 30 minutes, then stimulated with LTB4 (10−10 mol/L). Goblet cells were also stimulated with LTB4 (10−10 mol/L), RvD1 (10−8 mol/L), or RvE1 (10−8 mol/L) separately. LTB4 stimulation increased glycoprotein secretion significantly from baseline by 1.65 ± 0.16-fold (P = 0.0038) (Figure 6). RvE1 alone increased the glycoprotein secretion significantly from baseline by 1.77 ± 0.32-fold (P = 0.041). Incubation with RvE1 for 30 minutes significantly blocked the LTB4–induced stimulation (0.65 ± 0.15-fold; P = 0.023). RvD1 stimulation alone increased the glycoprotein stimulation by 1.81 ± 0.34-fold. RvD1 at 30 minutes’ incubation did not block the LTB4–induced stimulation (1.40 ± 0.23-fold; P = 0.41) (Figure 6). These results suggest that RvE1 and RvD1 use different mechanisms when interacting with LTB4 to stimulate goblet cell secretion.
Figure 6.
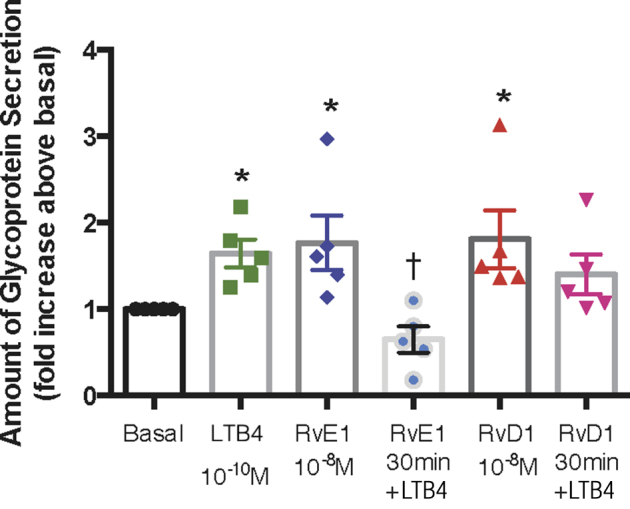
Resolvin E1 (RvE1), but not resolvin D1 (RvD1), counterregulates leukotriene B4 (LTB4)–induced glycoprotein secretion in goblet cells. Cultured rat goblet cells were stimulated with LTB4 (10−10 mol/L), RvE1 (10−8 mol/L), or RvD1 (10−8 mol/L) alone or incubated with RvE1 or RvD1 for 30 minutes prior to addition of LTB4 for 2 hours. Glycoprotein secretion was measured. The average fold increase above basal, which was set to 1, is shown. One-way analysis of variance nonparametric with Dunn multiple comparisons was used. Data are expressed as individual data points from each animal and means ± SEM. n = 5 rats. ∗P < 0.05 versus basal; †P < 0.05 versus LTB4 alone.
Discussion
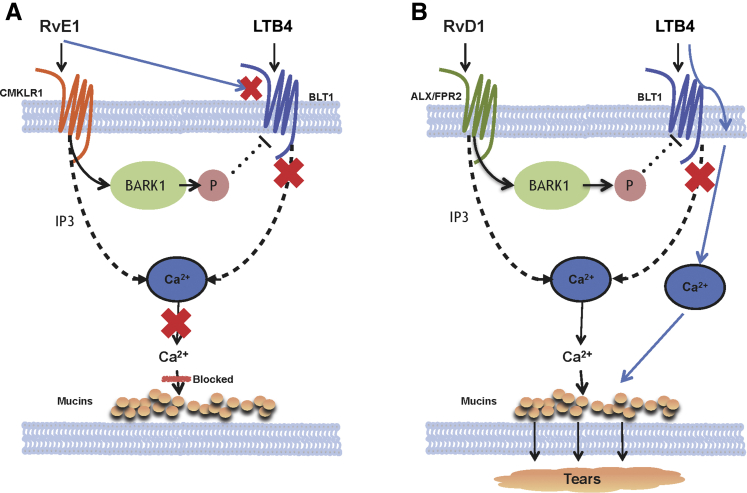
This study provides evidence that both RvE1 and RvD1 counterregulate the [Ca2+]i increase triggered by LTB4 (Figure 7). Combining results from Yang et al4 with those presented herein demonstrates that RvE1 employs two mechanisms to counterregulate the LTB4-stimulated response. First, RvE1 uses its receptor CMKLR1 to activate BARK1, which phosphorylates and down-regulates the BLT1 receptor, which prevented LTB4 from increasing [Ca2+]i and stimulating secretion. Second, RvE1 interacts with the BLT1 receptor directly preventing LTB4 from activating it and thereby blocking the increase in [Ca2+]i and stimulation of secretion.4 In contrast to RvE1, RvD1 uses only one mechanism to counterregulate the LTB4-stimulated response in this tissue. RvD1 interacts with its receptor ALX/FPR2 to activate BARK1, which phosphorylates and down-regulates the BLT1 receptor. Unlike RvE1, RvD1 only blocks the LTB4 increase in [Ca2+]i. RvE1, but not RvD1, blocks the excess goblet cell mucin secretion associated with LTB4-dependent inflammation and helps to balance mucin levels in the tear film.
Figure 7.
Schematic of the cellular mechanisms used by resolvin E1 (RvE1) and resolvin D1 (RvD1) to block leukotriene B4 (LTB4)–stimulated increase in intracellular [Ca2+] ([Ca2+]i) and mucin secretion in cultured conjunctival goblet cells. A: RvE1 blocks the effect of LTB4 on both intracellular Ca2+ and secretion by either interacting with the LTB4 receptor BLT1 receptor or by activating the β-adrenergic receptor kinase (BARK1) that phosphorylates BLT1, causing its internalization. B: RvD1 only blocks the effect of LTB4 on increasing intracellular Ca2+, but not on secretion, because RvD1 does not interact directly with the BLT1 receptor, but only activates the BARK1 that phosphorylates BLT1 causing its internalization. In this case, LTB4 interacts with the BLT1 receptor to release intracellular Ca2+ and stimulate secretion. ALX/FPR, lipoxin A4 receptor/formyl peptide receptor; CMKLR1, chemerin chemokine-like receptor; IP3, inositol trisphosphate.
LTB4 is an important mediator of primary chemoattractants during neutrophil chemotaxis,26 and is the proinflammatory mediator in multiple mucosal diseases such as asthma,25,27 peritonitis,28 and allergic conjunctivitis.29 In an allergic conjunctivitis animal model induced by short ragweed, the level of LTB4 in conjunctiva was markedly increased with the induction of the allergic response.29 LTB4 directly stimulates glycoprotein, including mucin secretion of conjunctival goblet cells,5 which is correlated with the increase in the symptoms of allergic conjunctivitis.21 An increase in goblet cell secretion as occurs from proinflammatory mediators in ocular allergy can disturb the tear film structure leading to dryness, sticky, and itching symptoms during ocular surface inflammation.30
Protective roles for RvE1 or RvD1 were observed in mucosal tissues other than conjunctiva. In the lung during allergy or asthma, RvE1 reduces cytokine levels; reduces eosinophil and lymphocyte recruitment; and decreases airway mucus exudation and tracheal goblet cell ratio in mouse.31,32 RvE1 also accelerates the clearance of airway inflammation and hyperreactivity in response to methacholine by suppressing IL-23 and IL-6 production.33 RvD1 along with its 17R-epimer aspirin-triggered RvD1 protects the airway by reducing oxidative stress via NRF2/KEAP1 pathway,34 decreasing the proinflammatory cytokines35 and up-regulating the anti-inflammatory cytokines.36 In the gastrointestinal tract, RvE1 prevents colitis by increasing the expression of a protective mediator alkaline phosphatase.37 RvD1 prevents colitis in an inflammatory bowel disease model.38
Results from many studies have now demonstrated a protective role for both RvE1 and RvD1 in the ocular surface including: decreasing inflammatory cytokines,39, 40, 41 preventing goblet cell loss,39 and blocking mucin secretion induced by LTD4 or histamine.5,24 It was observed that RvE1 and RvD1 alone can stimulate goblet cell mucin secretion.17,18 To maintain ocular surface health, the right amount of MUC5AC secretion must be maintained. In healthy, noninflamed conjunctiva, RvD1 was found in tears and functions to maintain an optimal mucin layer needed to keep the ocular surface healthy. Minimal to undetectable LTB4 or other proinflammatory mediators were detected during the noninflammatory phase.20 By contrast, during inflammation, with the increase of invading leukocytes, the amount of LTB4 along with other proinflammatory mediators increases and causes the excess mucin secretion associated with ocular allergic disease.20 With resolution and mediator class switching, RvE1 and RvD1 are produced and counterregulate the action of LTB4 on its receptor, returning the tear mucin level to homeostasis.
In the present study, the authors found that in conjunctival goblet cells, pretreatment with RvE1 and RvD1 counterregulates the action of LTB4 by activating BARK1. BARK1 is a member of the family of GPCR kinases. Upon activation, it phosphorylates GPCRs and promotes interaction of the receptor with β-arrestin to prevent signaling by the receptor via protein kinase A (PKA), protein kinase C, p44/p42 MAPK, p38 MAPK, or a variety of other signaling proteins.42,43 The BARK1 inhibitor used in this study selectively blocks the phosphorylation of the BLT1 receptor without inhibiting PKA.44 It is known that the RvD1 pathway uses BARK1 to inhibit histamine response,24 which is consistent with the current findings.
Pre-treatment with RvE1 inhibited LTB4-triggered [Ca2+]i increase and mucin secretion in conjunctival goblet cells by cross-talk between the CMKLR1 and BLT1 receptors. It was observed that either CMKLR1 or BLT1 knockdown decreases the RvE1-induced [Ca2+]i increase as well as mucin secretion.4 Decreasing the amount of the CMKLR1 did not fully reverse the effect of RvE1 on the increase in [Ca2+]i, either because CMKLR1 was not completely depleted or activation of the BLT1 receptor by RvE1 could also contribute to the counterregulatory actions of RvE1 on LTB4–induced goblet cell response. Similarly to the lack of complete inhibition of RvE1 on the effect of LTB4, pharmacologic inhibition of the RvD1 receptor ALX/FPR2 did not fully reverse the RvD1-mediated decrease in LTB4 stimulation of the [Ca2+]i. The pharmacologic inhibitor BOC2 is an antagonist for FPR1 and ALX/FPR2.45 The partial reversal of the LTB4 response in this tissue may be due to the lack of complete inhibition of ALX/FPR2 receptor by BOC2. In addition, BOC2 may have off-target effects in this tissue. So far, however, there is no published evidence that BOC2 inhibits LTB4 receptors, and the authors’ unpublished data suggest that BOC2 does not alter the LTB4–induced [Ca2+]i increase.
In contrast to the similar actions of both RvE1 and RvD1 on the LTB4 increase in [Ca2+]i, RvE1, but not RvD1, inhibited LTB4-stimulated secretion. This difference could be explained by the interaction of RvE1 with both CMKLR1 and BLT1 compared with the interaction of RvD1 with only the ALX/FPR2 receptor. Furthermore different receptors are activated by RvE1 and RvD1. Although RvD1 failed to block LTB4–induced secretion, RvE1, RvD1, and LTB4 used the same intracellular Ca2+ source to increase [Ca2+]i. Ca2+ is a major second messenger in the LTB4 intracellular signaling pathway for secretion. Use of distinct cellular Ca2+ stores by RvE1 and RvD1 to block the LTB4–induced [Ca2+]i increase, but only RvE1 to inhibit LTB4–induced secretion, requires further study.
In conclusion, our findings suggest a mechanism for the protective role that Rvs play in conjunctival epithelial inflammation. RvE1 counterregulates the LTB4–induced [Ca2+]i increase and secretion in conjunctival goblet cells via interactions with both CMKLR1 and BLT1. By contrast, RvD1 blocks only the LTB4–induced [Ca2+]i increase via the ALX/FPR2 receptor. Both RvE1 and RvD1 use BARK1 to counterregulate the actions of LTB4 on goblet cell function. Since goblet cells from different mucosal epithelial share common characteristics, this result may also apply to other mucosal epithelial such as airway and gastrointestinal tract. Because resolvins are not immunosuppressive, resolvins could be a promising treatment for inflammatory diseases of the ocular and other mucosal surfaces.
Footnotes
Supported by NIH grants RO1EY019470 (D.A.D.) and RO1GM038765 (C.N.S.).
Disclosures: None declared.
References
- 1.Singh P.K., Hollingsworth M.A. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 3.Dartt D.A., Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol. 2014;14:464–470. doi: 10.1097/ACI.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang M., Lippestad M., Hodges R.R., Fjaervoll H.K., Fjaervoll K.A., Bair J.A., Utheim T.P., Serhan C.N., Dartt D.A. RvE1 uses the LTB4 receptor BLT1 to increase [Ca(2+)]i and stimulate mucin secretion in cultured rat and human conjunctival goblet cells. Ocul Surf. 2020;18:470–482.4. doi: 10.1016/j.jtos.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dartt D.A., Hodges R.R., Li D., Shatos M.A., Lashkari K., Serhan C.N. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186:4455–4466. doi: 10.4049/jimmunol.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi D., Li D., Hayashi C., Shatos M., Hodges R.R., Dartt D.A. Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest Ophthalmol Vis Sci. 2012;53:2993–3003. doi: 10.1167/iovs.11-8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 8.Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 9.Williams T.J., Jose P.J., Wedmore C.V., Peck M.J., Forrest M.J. Mechanisms underlying inflammatory edema: the importance of synergism between prostaglandins, leukotrienes, and complement-derived peptides. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:33–37. [PubMed] [Google Scholar]
- 10.Sahin A., Kam W.R., Darabad R.R., Topilow K., Sullivan D.A. Regulation of leukotriene B4 secretion by human corneal, conjunctival, and meibomian gland epithelial cells. Arch Ophthalmol. 2012;130:1013–1018. doi: 10.1001/archophthalmol.2012.1067. [DOI] [PubMed] [Google Scholar]
- 11.Garceau D., Ford-Hutchinson A.W., Charleson S. 5-Lipoxygenase inhibitors and allergic conjunctivitis reactions in the guinea-pig. Eur J Pharmacol. 1987;143:1–7. doi: 10.1016/0014-2999(87)90728-x. [DOI] [PubMed] [Google Scholar]
- 12.Akman A., Irkec M., Orhan M., Erdener U. Effect of lodoxamide on tear leukotriene levels in giant papillary conjunctivitis associated with ocular prosthesis. Ocul Immunol Inflamm. 1998;6:179–184. doi: 10.1076/ocii.6.3.179.4042. [DOI] [PubMed] [Google Scholar]
- 13.Akman A., Irkec M., Orhan M. Effects of lodoxamide, disodium cromoglycate and fluorometholone on tear leukotriene levels in vernal keratoconjunctivitis. Eye (Lond) 1998;12:291–295. doi: 10.1038/eye.1998.67. [DOI] [PubMed] [Google Scholar]
- 14.Serhan C.N., Clish C.B., Brannon J., Colgan S.P., Chiang N., Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita M., Ohira T., Sun Y.P., Elangovan S., Chiang N., Serhan C.N. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 16.Hong S., Gronert K., Devchand P.R., Moussignac R.-L., Serhan C.N. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 17.Lippestad M., Hodges R.R., Utheim T.P., Serhan C.N., Dartt D.A. Resolvin D1 increases mucin secretion in cultured rat conjunctival goblet cells via multiple signaling pathways. Invest Ophthalmol Vis Sci. 2017;58:4530–4544. doi: 10.1167/iovs.17-21914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippestad M., Hodges R.R., Utheim T.P., Serhan C.N., Dartt D.A. Signaling pathways activated by resolvin E1 to stimulate mucin secretion and increase intracellular Ca(2+) in cultured rat conjunctival goblet cells. Exp Eye Res. 2018;173:64–72. doi: 10.1016/j.exer.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan C.N. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008;79:157–163. doi: 10.1016/j.plefa.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirakata T., Lee H.-C., Ohba M., Saeki K., Okuno T., Murakami A., Matsuda A., Yokomizo T. Dietary omega-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating Th2 immune responses. FASEB J. 2019;33:3392–3403. doi: 10.1096/fj.201801805R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saban D.R., Hodges R.R., Mathew R., Reyes N.J., Yu C., Kaye R., Swift W., Botten N., Serhan C.N., Dartt D.A. Resolvin D1 treatment on goblet cell mucin and immune responses in the chronic allergic eye disease (AED) model. Mucosal Immunol. 2019;12:145–153. doi: 10.1038/s41385-018-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council . National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 23.Li D., Carozza R.B., Shatos M.A., Hodges R.R., Dartt D.A. Effect of histamine on Ca(2+)-dependent signaling pathways in rat conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2012;53:6928–6938. doi: 10.1167/iovs.12-10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D., Hodges R.R., Jiao J., Carozza R.B., Shatos M.A., Chiang N., Serhan C.N., Dartt D.A. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013;6:1119–1130. doi: 10.1038/mi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taube C., Miyahara N., Ott V., Swanson B., Takeda K., Loader J., Shultz L.D., Tager A.M., Luster A.D., Dakhama A., Gelfand E.W. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian B.C., Moissoglu K., Parent C.A. The LTB4-BLT1 axis regulates the polarized trafficking of chemoattractant GPCRs during neutrophil chemotaxis. J Cell Sci. 2018;131:jcs217422. doi: 10.1242/jcs.217422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terawaki K., Yokomizo T., Nagase T., Toda A., Taniguchi M., Hashizume K., Yagi T., Shimizu T. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol. 2005;175:4217–4225. doi: 10.4049/jimmunol.175.7.4217. [DOI] [PubMed] [Google Scholar]
- 28.Tager A.M., Dufour J.H., Goodarzi K., Bercury S.D., von Andrian U.H., Luster A.D. BLTR mediates leukotriene B(4)-induced chemotaxis and adhesion and plays a dominant role in eosinophil accumulation in a murine model of peritonitis. J Exp Med. 2000;192:439–446. doi: 10.1084/jem.192.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azari A.A., Barney N.P. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310:1721–1729. doi: 10.1001/jama.2013.280318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelikan Z. Inflammatory mediator profiles in tears accompanying keratoconjunctival responses induced by nasal allergy. Br J Ophthalmol. 2013;97:820–828. doi: 10.1136/bjophthalmol-2012-302829. [DOI] [PubMed] [Google Scholar]
- 31.Aoki H., Hisada T., Ishizuka T., Utsugi M., Kawata T., Shimizu Y., Okajima F., Dobashi K., Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Levy B.D. Resolvin D1 and resolvin E1 promote the resolution of allergic airway inflammation via shared and distinct molecular counter-regulatory pathways. Front Immunol. 2012;3:390. doi: 10.3389/fimmu.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haworth O., Cernadas M., Yang R., Serhan C.N., Levy B.D. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Posso S.V., Quesnot N., Moraes J.A., Brito-Gitirana L., Kennedy-Feitosa E., Barroso M.V., Porto L.C., Lanzetti M., Valenca S.S. AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int Immunopharmacol. 2018;56:330–338. doi: 10.1016/j.intimp.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 35.Rogerio A.P., Haworth O., Croze R., Oh S.F., Uddin M., Carlo T., Pfeffer M.A., Priluck R., Serhan C.N., Levy B.D. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsiao H.-M., Sapinoro R.E., Thatcher T.H., Croasdell A., Levy E.P., Fulton R.A., Olsen K.C., Pollock S.J., Serhan C.N., Phipps R.P., Sime P.J. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One. 2013;8:e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell E.L., MacManus C.F., Kominsky D.J., Keely S., Glover L.E., Bowers B.E., Scully M., Bruyninckx W.J., Colgan S.P. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107:14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bento A.F., Claudino R.F., Dutra R.C., Marcon R., Calixto J.B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol. 2011;187:1957–1969. doi: 10.4049/jimmunol.1101305. [DOI] [PubMed] [Google Scholar]
- 39.de Paiva C.S., Schwartz C.E., Gjorstrup P., Pflugfelder S.C. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31:1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- 40.Erdinest N., Ovadia H., Kormas R., Solomon A. Anti-inflammatory effects of resolvin-D1 on human corneal epithelial cells: in vitro study. J Inflamm (Lond) 2014;11:6. doi: 10.1186/1476-9255-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajasagi N.K., Reddy P.B.J., Suryawanshi A., Mulik S., Gjorstrup P., Rouse B.T. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benovic J.L., Kuhn H., Weyand I., Codina J., Caron M.G., Lefkowitz R.J. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci U S A. 1987;84:8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jean-Charles P.-Y., Kaur S., Shenoy S.K. G protein-coupled receptor signaling through beta-arrestin-dependent mechanisms. J Cardiovasc Pharmacol. 2017;70:142–158. doi: 10.1097/FJC.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iino M., Furugori T., Mori T., Moriyama S., Fukuzawa A., Shibano T. Rational design and evaluation of new lead compound structures for selective betaARK1 inhibitors. J Med Chem. 2002;45:2150–2159. doi: 10.1021/jm010093a. [DOI] [PubMed] [Google Scholar]
- 45.Gastardelo T.S., Damazo A.S., Dalli J., Flower R.J., Perretti M., Oliani S.M. Functional and ultrastructural analysis of annexin A1 and its receptor in extravasating neutrophils during acute inflammation. Am J Pathol. 2009;174:177–183. doi: 10.2353/ajpath.2009.080342. [DOI] [PMC free article] [PubMed] [Google Scholar]