Abstract
Aging is a modifiable risk factor for most chronic diseases and an inevitable process in humans. The development of pharmacological interventions aimed at delaying or preventing the onset of chronic conditions and other age-related diseases has been at the forefront of the aging field. Preclinical findings have demonstrated that species, sex and strain confer significant heterogeneity on reaching the desired health- and lifespan-promoting pharmacological responses in model organisms. Translating the safety and efficacy of these interventions to humans and the lack of reliable biomarkers that serve as predictors of health outcomes remain a challenge. Here, we will survey current pharmacological interventions that promote lifespan extension and/or increased healthspan in animals and humans, and review the various anti-aging interventions selected for inclusion in the NIA’s Interventions Testing Program as well as the ClinicalTrials.gov database that target aging or age-related diseases in humans.
Keywords: Lifespan, Healthspan, Aging, “anti-aging”, Longevity, Frailty, Translation
1. Introduction
Aging has been recognized as a risk factor for most chronic diseases. It is an inevitable progression towards dysfunction and ultimately death across most living organisms, especially mammals. With aging, there is accumulation of damage that leads to an increase in disease vulnerability and death. However, despite years of intense research, the exact underlying mechanisms that govern aging processes remain poorly understood. Why and how we age still remains a mystery.
Most chronic diseases are multifactorial, polygenic and their clinical manifestations tend to emerge late in life (Fabbri et al., 2015; Ferrucci et al., 2018). The recent increase in life expectancy and the decline in birthrates account for the sharp rise in the number and proportion of older adults around the globe. In 2014, people 65 and older represented 14.5 % of the population, but by 2060, the number of elderly persons will double and outpace the number of children under age 5 (World Health Organization, 2015), which will cause a significant burden of age-related diseases on the economy in both developed and developing countries. This demographic change is already having an impact in individual healthcare costs, which will soon surpass 400$ billion dollars per year in the U.S. alone. In order to contain the escalating increase in health care spending, we must reduce the overall burden of disability and chronic disease.
Aging has long been considered a stochastic, inevitable process towards dyfunction and ultimately death (Hayflick, 2000). However, recent advances in the field of gerontology are showing that there are deterministic mechanisms that might be driving aging with some people aging at a slower rate than others. Therefore, aging should be viewed as adaptive and amenable to interventions aimed at extending health span and life span. Individuals with exceptional longevity often have delayed onset of age-related diseases and disabilities (Evert et al., 2003; Lipton et al., 2010), with a compression of morbidity and increased lifespan, living longer and healthier lives. Exceptional longevity and successful aging are only 20 % heritable (Murabito et al., 2012), while some of the main age-related chronic diseases, such as cancer (∼33 %) (Mucci et al., 2016), cardiovascular diseases (∼25–35 %) (Gluckman et al., 2016), dementia (i.e: Alzheimer’s Disease ∼70–79 %) (Selkoe, 1996; Barber, 2012) and others, are highly heritable (Zenin et al., 2019). External factors including environment, psychosocial impact, nutrition, and physical fitness all contribute to deterministic mechanisms of slower/faster aging (Shiels et al., 2019). As we age, our diminished ability to respond to stress renders us more susceptible to adverse health outcomes, leading to declining health and ultimately death. Rockwood and Mitnitski (2011) proposed that this increase in deficit accumulation could represent another way to define frailty.
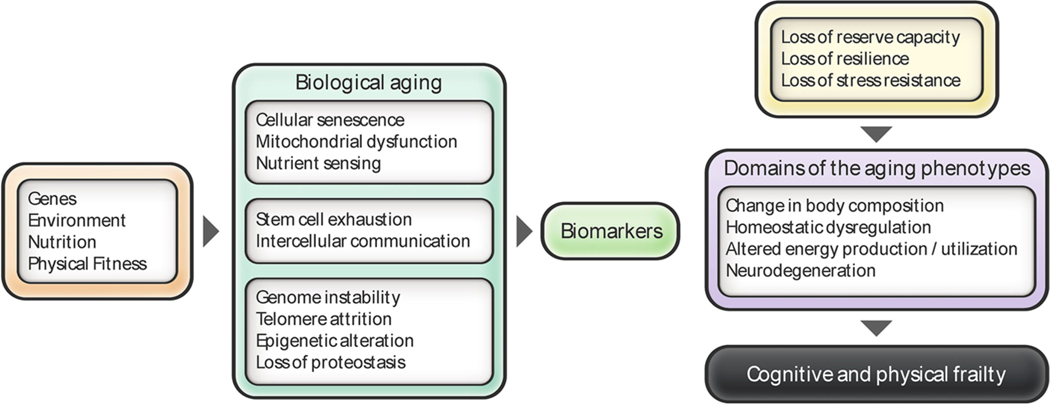
Ferrucci and colleagues clustered the systemic consequences of the aging process into four main domains (Ferrucci et al., 2010): i) Body composition; ii) balance between energy availability and energy demand; iii) homeostatic dysregulation; and iv) neurodegeneration. Changes in these four domains of the aging phenotypes increase the susceptibility to diseases and reduce the resilience or functional reserve capacity, leading to a condition that is known as frailty and the development of the so-called “geriatric syndromes” (Ferrucci and Studenski, 2012). These syndromes, which include delirium, cognitive impairment, falls, muscle atrophy (e.g. sarcopenia) and disability, are multifactorial and involve systemic changes in many parts of the body with adverse association of comorbidity with mortality. Some of the interventions presented herein target these four domains.
Recently, the landmark review by López-Otín helped conceptualize the hallmarks of aging by grouping of age-associated cellular and molecular mechanisms into three major categories known as ‘primary’, ‘antagonistic’, and ‘integrative’ hallmarks (Lopez-Otin et al., 2013) (Fig. 1). Genomic instability, telomere attrition, epigenetic alteration, and loss of proteostasis are deemed as primary hallmarks, causally related to molecular damage during aging. Antagonistic hallmarks have a beneficial hormetic function and protective role when expressed at low levels, but detrimental effects might occur at high levels. These hallmarks include deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence. Lastly, stem cell exhaustion and altered intercellular communication, known as integrative hallmarks, are indicators of impaired processes at the molecular and cellular levels, and both indicate a loss of reserve capacity or resilience, ultimately producing the aging phenotypes described by Ferrucci et al. (2010) (Fig. 1). The integration of all these hierarchical levels has been defined as metrics of aging (Ferrucci et al., 2018). Therefore, the geriatric syndromes would be considered ‘phenotypic aging’, and the hallmarks of aging should be viewed as ‘biological aging’. Ultimately a desequilibrum in both levels will lead to changes in cognition and physical performance, known as ‘functional aging’, ending in frailty and a loss of resilience.
Fig. 1. Hallmarks of aging and the four domains of aging phenotypes.
Integrative view of the hallmarks of aging described by Lopez-Otin et al. (2013) and the domains of the aging phenotypes described by Ferrucci et al. (2010). Different factors (genes, environment, exercise and nutrition) contribute to the rate of biological aging. The loss of reserve capacity or resilience, at a molecular and cellular level, ultimately leads to the development of the aging phenotypes.
In the last two decades, major scientific advances in our understanding of aging processes were achieved in model organisms, which led to the discovery of conserved longevity pathways, as well as the development of genetic, nutritional, and pharmacological interventions that target them. It is likely that the same interventions may provide benefits only in select tissues, organisms, or individuals based on age, sex, and ethnicity (Bartke et al., 2019). In model organisms, lifespan extension is often accompanied by a reduction or delay in morbidity (Fontana et al., 2010). Many of the pro-longevity pathways are also implicated in tissue development and metabolic regulation, as restriction in calorie intake is considered a key modulator in the aging process (de Cabo et al., 2014; Mercken et al., 2012). Drugs that mimic the effects of calorie restriction (CR) have shown life- and healthspan-extending properties through modulation of nutrient-sensing pathways, mitochondrial stress and antioxidant responses, and chromatin silencing (de Cabo et al., 2014; Lopez-Otin et al., 2016).
One of the most active areas of research in aging focuses on the identification of novel pathways that regulate the underlying processes of aging in order to develop interventions aimed at delaying the onset and progression of chronic diseases, preservation of functional capacity, and postponing death. We surmise that increases in mean lifespan with compression of morbidity is an ambitious, yet achievable goal within our reach (Fries et al., 2011; Ebeling et al., 2018), although translation of the advances made from model organisms to human clinical trials still remains a major challenge (Campisi et al., 2019). Nevertheless, there are multiple questions that persist on how to reverse or delay the dysregulation of biological systems that are implicated in age-associated phenotypic changes that lead to frailty and death. In this review, we considered pharmacological interventions that lead to lifespan extension and/or increase or preservation of function in mammals and human by targeting the hallmarks of aging.
2. Interventions that delay aging
Several small molecules and dietary manipulations based on CR have been developed during the last two decades that target processes of aging. A number of compounds have been found to delay the onset of age-related diseases and increase healthspan and lifespan from yeast to mammals, including nonhuman primates (Fontana et al., 2010; Ravussin et al., 2015; Mattison et al., 2017). These interventions target major signaling pathways whose dysregulation contributes to the emergence of aging phenotypes and disease. These compounds are generally considered CR mimetics that extend lifespan through an improvement of metabolic function, especially via mitochondrial metabolic reprogramming, and include drugs that target i) various growth factor signaling pathways; ii) insulin signaling pathway implicated in carbohydrate and fat metabolism; iii) NAD+-dependent sirtuins; iv) amino acid pathway; v) autophagy; vi) senescence; and vii) stem cells and rejuvenation factors. There are excellent recent reviews that have extensively covered the benefits of these CR-mimetic compounds and interventions (Fontana et al., 2010; Longo et al., 2015; Rizza et al., 2014; Fontana et al., 2012; Fontana and Partridge, 2015; de Cabo et al., 2014; Martin-Montalvo and de Cabo, 2013; Baur et al., 2012; Novelle et al., 2015; Pan and Finkel, 2017; Custodero et al., 2018; Gurau et al., 2018). Here, we present a brief overview of the beneficial effects that these drugs and interventions confer on the different hallmarks of aging.
2.1. Drugs targeting growth factor pathways
2.1.1. mTOR inhibitors, rapamycin, rapalogs and other
Rapamycin is an antifungal antibiotic that was first isolated from an Easter Island soil sample by Chang et al. (1991). Rapamycin has been approved by FDA for its immunosuppressive and anti-rejection properties (Camardo, 2003). Novel mTOR inhibitors, known as rapalogues, have the same molecular scaffold as rapamycin, but with different physiochemical properties (Lamming and Sabatini, 2013). The anti-cancer properties of rapamycin are associated with inhibition of the mammalian target of rapamycin (mTOR) through interaction with immunophilin FKBP12, which binds next to the kinase region of TOR, a serine/threonine kinase that is regulated by nutrients, growth factors, and the cellular energy status. TOR signals through two multiprotein complexes, termed mTORC1 and mTORC2, with distinct biological outcomes. Acute and chronic exposure to rapamycin has been shown to inhibit mTORC1 whereas inhibition of mTORC2 requires long-term treatment with the drug (for review, see Li et al., 2014). The pro-longevity effects of rapamycin are conveyed through mTORC1 whereas mTORC2 inactivation is believed to be responsible of the insulin resistance phenotype associated with rapamycin treatment (Saxton and Sabatini, 2017).
mTOR is considered a “metabolic master regulator” through its ability to regulate metabolism across metabolically active tissues, such as skeletal muscle, adipose tissue, liver and brain (Sengupta et al., 2010; Tsai et al., 2015; Lamming and Sabatini, 2013; Garelick and Kennedy, 2011). Inhibition of mTORC signaling can also have protective effects during obesity and type 2 diabetes (Reifsnyder et al., 2018). The healthspan and lifespan extension properties of rapalogs stem from the lowering in mTOR signaling pathway activation triggered by insulin/IGF-1 axis, amino acids and glucose levels, all of which acting in concert to influence the cellular energy status (for review see Kennedy and Lamming, 2016). Pharmacological inhibition of TOR with rapamycin or other mTOR inhibitors promotes lifespan extension in yeast, worm, flies, and mice (Vellai et al., 2003; Cao et al., 2010; Robida-Stubbs et al., 2012), notably by impacting downstream mTORC1-regulated processes that include autophagy, lipid synthesis, mitochondrial metabolism, ribosomal biogenesis, and modulation of the senescence-associated secretory phenotype among others (Pan and Finkel, 2017). The pro-longevity effects of rapamycin are seen preferentially in females than males (Miller et al., 2014). Recently, it has been suggested that the effects of mTORC1 inhibitors, such as the rapalog RAD001, could be mediated by regulation of c-Myc protein, with subsequent reduction in nephropathy lesions found in aged rats (Shavlakadze et al., 2018).
2.2. Drugs targeting insulin signaling pathways, carbohydrates and fat metabolism
2.2.1. Metformin
Metformin (N,N-dimethylbiguanide) belongs to the biguanide class of anti-diabetic drugs and is well tolerated compared to other drugs. Metformin is most commonly used as a first-line medication for type 2 diabetes by lowering hepatic glucose production and insulin resistance. The underlying mechanisms by which metformin inhibits hepatic gluconeogenesis remains unknown, although recent studies have shown that metformin could suppress gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase (Madiraju et al., 2014) and by activation of a duodenal pathway dependent on AMP-activated protein kinase (AMPK) (Duca et al., 2015). One of the major clinical advantages of metformin is that it does not induce hypoglycemia or weight gain while correcting hyperglycemia and conferring CR-like benefits such as improvement in insulin sensitivity and AMPK activity and better antioxidant protection. The ability of metformin at improving healthspan and lifespan of drosophila, C. elegans and mice raises the possibility of metformin-based interventions to promote healthy aging in humans (Slack et al., 2012; Cabreiro et al., 2013; Martin-Montalvo et al., 2013; Novelle et al., 2016; Alfaras et al., 2017). Metformin has been reported to abrogate DICER1-mediated cellular senescence by altering microRNA expression (Noren Hooten et al., 2016) and producing epigenetic modifications that regulate the expression levels of several microRNAs to confer protection against diabetes and cancer (Bridgeman et al., 2018). A 6-week treatment with metformin regulates several cellular processes (e.g., DNA repair) and metabolic pathways that include pyruvate metabolism, PPAR and SREBP signaling, and mitochondrial fatty oxidation in skeletal muscle and subcutaneous adipose tissue of older adults (Kulkarni et al., 2018). While neonatal exposure to metformin appears to slow aging down and prolongs lifespan in male mice (Anisimov et al., 2015), administration of the biguanide every other week when initiated in late-life leads to an overall improvement on health without an extension in lifespan as compared to control mice (Alfaras et al., 2017). Long-term metformin treatment has been found to lower the expression of the antioxidant regulator Nrf2 (Nfe2l2) and that of neurotrophic factors in the brain of old mice (Allard et al., 2016).
Based on experimental evidences from cellular and animal studies, a recent clinical trial known as TAME (Targeting Aging with Metformin) will assess whether metformin can delay the onset and/or progression of age-related diseases beyond its effects on glucose metabolism. TAME plans to enroll 3000 subjects, ages 65–79, in 14 different centers across the U.S. (Barzilai et al., 2016). Intriguingly, recent studies have found that the combination of metformin with exercise opposes the exercise-induced benefits in insulin sensitivity, cardiorespiratory fitness, and mitochondrial adaptations to aerobic exercise in older adults (Malin et al., 2012; Konopka et al., 2019).
2.2.2. 17α -Estradiol
While the feminizing hormone 17β-estradiol (17β-E2) is the most biologically active and abundant estrogen in circulation, 17α-estradiol (17α-E2) is considered a non-feminizing hormone with reduced affinity for the estrogen receptor. Previous studies have shown that 17α-E2 can confer protection against oxidative stress and age-related degenerative brain disorders, such as Parkinson’s and Alzheimer’s diseases, and age related inflammation (Dykens et al., 2005; Santos et al., 2017). Exposure to 17α-E2 reduces body weight and extends lifespan in male mice while mitigating metabolic and age-related chronic inflammation (Stout et al., 2017). The fact that 17α-E2 extends longevity of male but not female mice suggests a sexual dimorphism in its effect on lifespan (Strong et al., 2016; Harrison et al., 2014) that is reminiscent of acarbose (Harrison et al., 2014). Gonadectomised male and female mice have shown the contribution of sex hormones as main regulators of sexual dimorphism toward the lifespan extension properties of 17α-E2 and acarbose (Garratt et al., 2017). A recent study has shown that 17α-E2 treatment in male mice does not increase the contribution of protein synthesis to proteostatic processes in metabolically active tissues, contrary to what has been shown in energy-restricted models or long-lived organisms (Miller et al., 2019). Further studies are required to elucidate the molecular mechanism of 17α-E2 action.
2.2.3. Acarbose
Acarbose is an alpha-glucosidase inhibitor that inhibits intestinal digestion of carbohydrates. Used for more than 20 years to treat hyperglycemia and type 2 diabetes, acarbose is considered a CR mimetic capable of lowering postprandial blood glucose levels as well as total cholesterol, triglycerides and low-density lipoprotein cholesterol levels while enhancing insulin sensitivity in mice (Yamamoto and Otsuki, 2006; Gentilcore et al., 2011; Santilli et al., 2010). As indicated above, acarbose extends median and maximal lifespan, and improves healthspan preferentially in male mice through an increase in fibroblast growth factor-21 (FGF21) and a decrease in insulin-like growth factor 1 (IGF1) (Harrison et al., 2014). The exact mechanisms of acarbose action toward healthspan remain unclear.
2.2.4. Fibroblast growth factor-21
FGF21 is a member of the FGF superfamily involved in the endogenous regulation of glucose, lipid metabolism, and inflammation (Nies et al., 2016). This protein hormone attenuates growth hormone (GH)/IGF1 signaling and has been proposed as a therapeutic target for aging and age-related incidence of diabetes and obesity (Mendelsohn and Larrick, 2012). FGF21 delays endothelial replicative senescence by protecting cells from DNA damage and premature senescence through SIRT1 (Yan et al., 2017). FGF21, via its co-receptor β-Klotho, crosses the blood brain barrier to reduce the levels of insulin, inhibit growth, and increase corticosterone levels, thus potentially leading to the development of new treatments for obesity and metabolic disorders (Hsuchou et al., 2007; Bookout et al., 2013). Transgenic overexpression of FGF21 extends lifespan in mice without reducing food intake or affecting either NAD+ metabolism or the regulation of mTOR signaling by AMPK (Zhang et al., 2012). Although the precise mechanism of action is poorly understood, FGF21 could affect longevity and healthspan through alterations of key metabolic pathways reminiscent of CR mimetics, e.g., improvement in cellular longevity through activation of autophagy, stress resistance, and survival signals while attenuating cellular growth and protein synthesis (Xie and Leung, 2017).
2.3. Drugs targeting the NAD+-dependent sirtuins
2.3.1. Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenol abundant in mulberries, peanuts, and the skin of red grapes. Supplementation of the normal diet with resveratrol extends lifespan and healthspan across a variety of species from yeast, C. elegans and small mammals to non-human primates (McCormack et al., 2015; Fiori et al., 2013; Jimenez-Gomez et al., 2013; Mattison et al., 2014; Bernier et al., 2016). Resveratrol elicits beneficial health effects by suppressing inflammation, oxidative damage, tumorigenesis, and immunomodulatory activities, thereby leading to improvement of mitochondrial function and protection against obesity, cancer, and cardiovascular dysfunction (Xia et al., 2008; Shin et al., 2009; Cho et al., 2017; Wang et al., 2018a, b; Novelle et al., 2015). Recent evidence suggests an attenuation of the inflammatory response in immune and endothelial cells by resveratrol (Schwager et al., 2017), which occurs likely through activation of SIRT1 and AMPK (Ohtsu et al., 2017). Moreover, resveratrol confers neuroprotection in human neural stem cells via AMPK activation and subsequent reduction in β-amyloid-induced inflammation and oxidative stress (Chiang et al., 2018). The inhibition of high-fat diet-induced NFκB signaling pathway also explains the anti-inflammatory effect of resveratrol (Pearson et al., 2008).
A cellular model of Alzheimer’s disease has helped to demonstrate that resveratrol attenuates oxidative damage through mitophagy activation (Wang et al., 2018a). Resveratrol alleviates the development of alcoholic liver injury and progression to fatty liver disease by down-regulating hepatic HIF-1α expression and mitochondrial ROS production (Ma et al., 2017). Low dose of resveratrol improves mitochondrial respiratory function and enhances cellular reprogramming in patient-derived fibroblasts with mitochondrial DNA mutations (Mizuguchi et al., 2017). More recently, it has been shown that treatment of diabetic mice with resveratrol increases mitochondrial biogenesis and inhibits the activation of mitophagy in skeletal muscle, thus ameliorating diabetes-induced skeletal muscle atrophy (Wang et al., 2018b).
Although resveratrol exhibits some CR-like benefits on healthspan, its limited absorption and bioavailability are impediment to its effective use. Several studies have shown over the past years that the concentration of resveratrol and its metabolites in urine and plasma are very heterogeneous among individuals on resveratrol supplementation, suggesting that some genetic factors, especially genes from the CYP450 enzymes, could be affecting the response to resveratrol treatment (Walle et al., 2004; Ortuño et al., 2010; Chang et al., 2001). In older community-dwelling adults, total urinary resveratrol metabolite concentration derived from normal diet was not associated with inflammatory markers, cardiovascular disease, and/or cancer or predictive of all-cause mortality (Semba et al., 2014). McDermott et al. (2017) found that resveratrol supplementation didn’t improve walking performance in older people with peripheral arterial disease. Similarly, Pollack et al. (2017) showed that resveratrol treatment improved vascular function and mitochondrial number but not glucose metabolism or insulin sensitivity. Conflicting results have been shown about the effects of resveratrol in lifespan extension in mice, with some authors reporting no pro-longevity effect (Pearson et al., 2008; Miller et al., 2011; da Luz et al., 2012) while others found positive benefits of resveratrol when mice were fed high-fat diet or under intermittent fasting feeding protocols, but not on standard diet (Novelle et al., 2015; Strong et al., 2013; Pearson et al., 2008; Pallauf et al., 2016).
The molecular mechanisms of action of resveratrol remain elusive; however, AMPK and the NAD+-dependent deacetylase SIRT1 have been proposed to mediate the anti-aging response and disease protection of resveratrol, reminiscent of CR signaling (Baur et al., 2006; Park et al., 2012; Kulkarni and Canto, 2015). Understanding how resveratrol exerts its beneficial effects in healthspan will help to develop new drugs to treat age-associated metabolic disorders.
2.3.2. Sirtuin-activating compounds (STACs): SRT1720, SRT2104 and SRT3025
SIRT1 and the other six members (SIRT2–7) of the highly conserved class III histone deacetylase family are positively associated with lifespan (Hubbard and Sinclair, 2014). These seven mammalian sirtuins have different subcellular locations and functional properties, and their activation have been linked to delayed aging, improved metabolism, and oxidative stress resistance in different animal models (Sinclair and Guarente, 2014). Compared to resveratrol, sirtuin-activating compounds (STACs) show better potency, solubility, and target selectivity by binding to the N-terminal domain in SIRT1 (Sinclair and Guarante, 2014), which results in the activation of pro-longevity pathways that target oxidative stress, inflammation and mitochondrial function (Imai and Guarente, 2016; Nogueiras et al., 2012). Pharmacological activation of SIRT1 with SRT1720 and SRT2104 promotes healthspan and lifespan extension via reduction of inflammatory pathways (Minor et al., 2011; Mitchell et al., 2014; Mercken et al., 2014; Bonkowski and Sinclair, 2016). SRT1720 inhibits circulating TNF-α and IL-6 levels in a mouse model of estrogen-induced cholestatic liver injury (Yu et al., 2016), and postnatal administration of SRT1720 attenuates obesity and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy (Nguyen et al., 2018). SRT1720 treatment lowers multi-organ injury and inflammation in mice via reduction of sepsis-induced inflammasome activation, thus attenuating renal fibrosis through apoptosis and reduction of oxidative stress (Ren et al., 2017). Furthermore, SRT1720 confers protection against endothelial senescence, resulting in the maintenance of cellular function via Akt/eNOS/VEGF axis (Li et al., 2016). Aged human mesenchymal stem cells can be rejuvenated by SRT1720-mediated SIRT1 activation of apoptosis (Liu et al., 2017). Another characterized STAC, SRT2104, has been found to increase mitochondrial oxidative phosphorylation and to decrease serum cholesterol and triglycerides in older adults (Libri et al., 2012). Treatment of diet-induced obese mice with SRT2104 promotes body weight loss, improves insulin sensitivity, and increases exercise capacity (Qi et al., 2010); however, these benefits have not been reported in humans (Baksi et al., 2014). Short-term use of SRT2104 extends survival of male mice and preserves bone and muscle mass in an experimental model of atrophy (Mercken et al., 2014). Clinical trials involving patients with type 2 diabetes have shown SRT2104 to be associated with weight loss and improved glycemic control without effects on lipids or platelet function (Noh et al., 2017). Administration of SRT2104 in participants with diabetes lessened aortic endothelial dysfunction via inhibition of p53 (Wu et al., 2018). SRT2104 treatment confers neuroprotection and lifespan extension in a mouse model of Huntington’s disease by virtue of its ability to cross the blood-brain barrier and attenuate brain atrophy while improving motor function (Jiang et al., 2014). A third characterized and selective SIRT1 activator, SRT3025, has been linked to hematopoietic stem cell expansion (Zhang et al., 2015) and inhibition of osteoclast generation and function in bone marrow-derived macrophages, a finding suggestive of a role for STACs in combatting osteoporosis (Gurt et al., 2015).
Despite extensive evidence for the delay of phenotypic aging and age-related diseases, more research is needed to elucidate the positive benefits of STACs to treat inflammation, metabolic disorders, and neurodegenerative diseases. See the recent review on the role of STACS in aging by Bonkowski and Sinclair (2016).
2.3.3. Drugs targeting NAD biosynthesis
Interventions such as CR and exercise increase the levels of nicotinamide adenine dinucleotide (NAD+), thus resulting in improved mitochondrial function (Canto et al., 2010; Liu et al., 2008). The decline in cellular NAD+ concentrations with aging is associated with neurodegeneration and other pathologies that adversely impact healthspan and lifespan; conversely, modulation of NAD+ levels appears to be a key factor for successful aging (Gomes et al., 2013; Gong et al., 2013; Mouchiroud et al., 2013). NAD+ fuels reduction-oxidation reactions and regulates a variety of biological processes, including metabolism and stress response, and mediates also some of the beneficial pro-longevity effects of intermittent fasting and CR, possibly through sirtuin activation (Bonkowski and Sinclair, 2016; Rajman et al., 2018; Imai and Guarente, 2016). The circulating levels of extracellular nicotinamide phosphoribosyltransferase (eNAMPT) significantly decline with age in mice and humans. eNAMPT is carried in extracellular vesicles (EVs) and enhances NAD+ biosynthesis to increase lifespan in mice (Yoshida et al., 2019). Diet supplementation with nicotinamide, a NAD+ precursor, has been recently reported to protect liver function, glucose metabolism and overall health of old mice on high-fat diet without beneficial effect on lifespan (Mitchell et al., 2018). Earlier studies with two other NAD+ precursors, namely nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR), have been found to extend healthspan and lifespan in Drosophila and yeast (Anderson et al., 2003; Balan et al., 2008). Administration of NMN ameliorates the impact of maternal obesity on offspring liver health in mice (Uddin et al., 2017) as well as cognitive function via neurovascular coupling in old mice (Tarantini et al., 2019). Improvement in endothelial blood flow and physical endurance in old mice on NMN is the result of SIRT1-dependent increase in both capillary density and hydrogen sulfide production (Das et al., 2018). Renal SIRT1 activity and NAD+ content are restored upon NMN treatment of young and 20-month-old mice (Guan et al., 2017), and NMN can synergize with exercise to delay skeletal muscle dysfunction in aging rats via increase in SIRT1 activity (Pajk et al., 2017).
In a mouse model of Alzheimer’s disease, NR treatment reduces DNA damage, neuroinflammation, and apoptosis of hippocampal neurons while promoting an increase in brain SIRT3 activity that leads to an improvement in cognitive function and hippocampal synaptic plasticity (Hou et al., 2018). NR supplementation attenuates the development of heart failure in a mouse model of dilated cardiomyopathy (Diguet et al., 2017), and stimulates hematopoiesis through increased mitochondrial clearance in immunodeficient mice (Vannini et al., 2019).
In humans, a recent clinical trial showed that chronic NR supplementation is well tolerated and stimulates NAD+ metabolism in healthy middle-aged and older adults (Martens et al., 2018).
As a whole, these results indicate that supplementation with NAD+ precursors is a promising intervention strategy to delay aging and reduce age-associated ailments. However, pharmaco-kinetics or pharmacodynamics studies with these compounds are missing and more work is needed to elucidate the exact mechanisms by which these drugs can improve healthspan and lifespan.
2.4. Interventions targeting amino acid pathways
Although the reduction in calorie intake does not always extend lifespan (Mattison et al., 2012; Mitchell et al., 2016), restriction in specific dietary components, such as proteins or amino acids, has been linked to the control of lifespan from yeast to humans (Leto et al., 1976; Grandison et al., 2009; McIsaac et al., 2016). The mechanisms underlying the role of methionine and other amino acids in delaying aging remain unclear, but may involve reductions in serum IGF-1 coupled with lower oxidative stress and autophagy (Mirzaei et al., 2014; Ables and Johnson, 2017; Liu et al., 2015). Animals on methionine- or tryptophan-restricted diets live longer and show significant reduction in age-related diseases partly though detoxification of mitochondrial ROS (Gonzalez-Burgos et al., 1998; Edwards et al., 2015; Obata and Miura, 2015; Gomez et al., 2015). Adipose tissue and liver are particularly responsive to methionine restriction (Ghosh et al., 2014; Wanders et al., 2015, 2016; Ables and Johnson, 2017), although other organs such as brain, heart and kidneys also benefit from this intervention (Cooke et al., 2018; Grant et al., 2016; Vogel et al., 2017; Marti-Carvajal et al., 2017). One recent study shows clear modulation of gut hormones, weight loss, energy balance, and gut microbiota in rats subjected to tryptophan restriction (Zapata et al., 2018). Moreover, the circadian clock (Nascimento et al., 2013) as well as brain plasticity and normal development (Serfaty et al., 2008) are influenced by tryptophan supplementation in mice. It is imperative that nutritional intervention studies with amino acid restriction be performed in humans.
2.5. Drugs targeting autophagy
Autophagy is a recycling mechanism that helps maintain cellular homeostasis and energetic balance (Sridhar et al., 2012; Singh and Cuervo, 2011). Several types of autophagy have been described and include macroautophagy, microautophagy, and chaperone-mediated autophagy (Singh and Cuervo, 2011), whose effects on health and disease have garnered attention over the years. With aging, there is progressive loss of proteostasis characterized by dysregulation in autophagy, ubiquitin-mediated degradation, and protein synthesis (Lopez-Otin et al., 2013). Aging and age-related diseases have been associated with changes in polyamine levels (Minois, 2014; Gupta et al., 2013) and their role in autophagy (Basisty et al., 2018). The impact of polyamines on cell growth, survival and proliferation has been ascribed to the inhibition of DNA methylation and tumorigenesis in mice (Soda et al., 2013).
Spermidine is a naturally occurring polyamine that elicits beneficial anti-aging effects through regulation of autophagy and other mechanisms, including antioxidant protection (Madeo et al., 2018a). Moreover, spermidine mediates lifespan extension in yeast via inhibition of histone acetylases and activation of autophagy genes, such as atg7, atg11 or atg15 (Morselli et al., 2009). Spermidine extends lifespan in several animal species via MAPK pathway (Minois, 2014) and is effective in improving neurodegeneration and conferring cardioprotection through autophagy (LaRocca et al., 2013; Buttner et al., 2014; Sigrist et al., 2014; Eisenberg et al., 2016). Spermidine supplementation is safe in humans and has positive effects on cognitive function of older adults (Schwarz et al., 2018) and on blood pressure (Eisenberg et al., 2016). The recent review by Madeo et al. (2018b) provides in-depth information about the role of spermidine in aging and disease.
2.6. Drugs targeting senescence pathways
Age-related accumulation of senescent cells in various tissues and organs is associated with several deficiencies that include (1) the decline in the number of stem cells that rely on proliferation for their proper function, (2) weakening of the immune system, (3) inadequate repair capacity, and (4) reduced global and site-specific DNA methylation in aging tissues (Sidler et al., 2014; LeBrasseur et al., 2015). The clearance of p16Ink4a-positive senescent cells delays age-related disorders (Baker et al., 2011) and evidence suggests that genes implicated in cellular senescence are also linked to longevity and age-related diseases (Tacutu et al., 2011). Senolytics refer to small molecules that can induce apoptosis in senescent cells and capable of promoting lifespan extension while delaying the onset of age-related diseases (Kirkland et al., 2017). Originally developed as common anticancer drugs, navitoclax, quercetin, and dasatinib have senolytic properties (Ranganathan et al., 2015; Tolcher et al., 2015) that target BCL-2 and related anti-apoptotic pathways (Zhu et al., 2017). Alvespimycin is a potent HSP90 inhibitor with senolytic properties (Fuhrmann-Stroissnigg et al., 2017). Other senolytics include fisetin, a naturally-occurring flavone with low toxicity, and the BCL-XL inhibitors, A1331852 and A1155463, that have similar reactivity as navitoclax but with less hematological toxicity (Zhu et al., 2017). Clearance of senescent cells with chronic senolytic treatment improves age-related vascular conditions and reduces mortality from cardiovascular disease (Roos et al., 2016). For example, the treatment of aged mice with navitoclax eliminates senescent cardiomyocytes and attenuates profibrotic protein expression in aged mice (Walaszczyk et al., 2019). The combination of dasatinib plus quercitin has been recently shown to improve physical function and increase lifespan in old mice (Xu et al., 2018) and ameliorates Aβ plaque-associated inflammation and cognitive deficits in Alzheimer’s disease mice (Zhang et al., 2019).
The ability of senolytic drugs to reduce the number of senescent cells and combat inflammatory diseases, such as obesity and other metabolic disorders, constitutes a major therapeutic approach aimed at ensuring healthy aging (Palmer et al., 2019). In fact, an open-label pilot clinical study has recently demonstrated that quercitin could alleviate idiopathic pulmonary fibrosis (Justice et al., 2019). The recent review of Kirkland et al. (2017) provides additional insight into this important class of compounds.
2.7. Rejuvenation factors (GDF11, GDF8)
Studies of heterochronic parabiosis have provided evidence of rejuvenation factors present in the blood of young mice (e.g., cells and proteins) that provide benefits in aged animals (Conboy et al., 2005). Systemic administration of young blood counteracts age-related decline in cognitive function and synaptic plasticity in mice (Villeda et al., 2014). Growth/differentiation factor 11 (GDF11) and GDF8, both members of the transforming growth factor (TGF)-β superfamily, have been identified as rejuvenation factors (Loffredo et al., 2013; Sinha et al., 2014). Circulating concentrations of GDF11 correlate with lifespan in mice (Zhou et al., 2016), and several studies have shown that the decline in circulating GDF11 in old mice can be restored, via parabaiosis or injection of the recombinant form, with concomitant reduction in age-related cardiac hypertrophy (Loffredo et al., 2013; Sinha et al., 2014). Daily injections of GDF11 also improves cerebral vasculature and increases the number of brain stem cells (Katsimpardi et al., 2014). However, other studies have reported the lack of pro-longevity effects of GDF11 in a mouse model of premature aging (Freitas-Rodriguez et al., 2016), and GDF11 treatment does not appear to rejuvenate skeletal muscle stem cells in old mice (Hinken et al., 2016). In humans, there is no evidence to suggest that GDF11 levels decline with age, although low GDF11 has been associated with frailty and morbidity in older adults with cardiovascular disease (Schafer et al., 2016).
The contribution of these rejuvenation factors in aging research has been controversial (Egerman et al., 2015; Smith et al., 2015), and it is based mostly on the lack of accuracy in quantifying GDF11 and GDF8. More work is needed to assess whether these and other rejuvenation factors can reverse aging phenotypes in both mice and its potential translation into humans.
3. Where are we now?
3.1. Compounds tested for anti-aging activity in mice via the National Institute on Aging (NIA) Interventions testing Program (ITP)
The Interventions Testing Program (ITP), developed in 2003 by the NIA at the National Institutes of Health (NIH), was designed to capitalize on the information previously reported on identified genes and gene products that impact healthy lifespan in non-mammalian species, and to rigorously evaluate in mice any agent that when taken in food or water could potentially delay aging rates or prevent late-life diseases (Warner, 2015). To achieve this goal, interventions are selected from the annual solicitation for collaborators by a two-level approval process and tested at three different sites simultaneously (University of Michigan, The Jackson Laboratory, and University of Texas Health Science Center in San Antonio (UTHSCSA) (Miller et al., 2007)). Key central features of this program include 1) the use of genetically heterogeneous mice (UM-HET3) obtained by a specific four-way crossbreeding scheme (CB6F1 females bred to C3D2F1 males), 2) the use of sufficient statistical power to detect 10 % changes in lifespan, in either sex, 3) the use of pair-fed and untreated controls, 4) the minimal inclusion of age-sensitive traits (i.e: locomotor activity, T-lymphocyte subtypes, and hormornes such as insulin, IGF-1, leptin and thyroxine), 5) plans for necropsy analysis, and 6) plan for interim analyses of survival (Miller et al., 2007). Grounded on these unique characteristics, the ITP has tested so far 67 interventions with 42 different compounds (Table 1). The efficiency/effects of the compounds are presented in Fig. 2 and have been published in several articles (https://www.nia.nih.gov/research/dab/interventions-testing-program-itp/publications-nia-interventions-testing-program).
Table 1.
Compounds under testing in the NIA-ITP. The ITP has tested so far 67 interventions with 42 different compounds.
| COMPOUND | CONCENTRATION IN FOOD (PPM) | AGE AT TREATMENT INTIATION (MONTHS) | Effect on lifespan |
|---|---|---|---|
| Aspirin | 20 | 4 | |
| 60 | 11 | Males | |
| 200 | 11 | ||
| NFP | 200 | 4 | None |
| NDGA | 2500 | 9 | |
| 800 | 6-male | ||
| 2500 | 6-male | Males | |
| 5000 | 6 | ||
| 2500 | 13 | ||
| 5000 | 13 | ||
| 4-OH-PBN | 315 | 4 | None |
| CAPE | 30 | 4 | |
| 300 | 4 | None | |
| Enalapril Maleate | 120 | 4 | None |
| Rapamycin | 14 | 20 | |
| 14 | 9 | ||
| 4.7 | 9 | Males | |
| Females | |||
| 14 | 9 | ||
| 42 | 9 | ||
| 42 | 20 | ||
| Simvastatin | 12 | 10 | |
| 120 | 10 | None | |
| Resveratrol | 300 | 12 | |
| 1200 | 12 | None | |
| 300 | 4 | ||
| Oxaloacetic acid | 2200 | 4 | None |
| Green tea extract | 2000 | 4 | None |
| Curcumin | 2000 | 4 | None |
| Medium Chain Triglyceride Oil | 60000 | 4 | None |
| 17a-Estradiol | 4.8 | 10 | |
| 14.4 | 10 | Males | |
| 14.4 | 16 and 20 | ||
| Methylene Blue | 28 | 4 | Females |
| Acarbose | 1000 | 4 | |
| 1000 | 16 | ||
| 2500 | 8 | Males | |
| Females | |||
| 1000 | 8 | ||
| 400 | 8 | ||
| Fish Oil | 15000 | 9 | None |
| 50000 | 9 | ||
| Bile acids | 5000 | 5 | - |
| Metformin | 1000 | 9 | |
| Protandim** | 600 | 10 | Male |
| INT-767 FXR/TG5R agonist | 180 | 10 | - |
| HBX | 1 | 15 | Males |
| Ursolic acid | 2000 | 10 | - |
| Glycine | 80000 | 9 | - |
| TM5441-inhibitor of PAI-1 | 60 | 11 | Males |
| Inulin | 600 | 11 | Males |
| 17-DMAG | 30 | 6 | - |
| MitoQ | 100 | 7 | Males |
| Minocycline | 300 | 6 | - |
| B-GPA | 3300 | 6 | - |
| MIF098 | 240 | 8 | Males |
| Nicotinamide Riboside | 1000 | 8 | None |
| Canagliflozin | 180 | 7 | - |
| Candesartan cilexetil | 30 | 8 | - |
| Geranylgeranylacetone | 600 | 9 | - |
| Hydrogen Sulfide - SG1002 | 240 | TBD | - |
| 1,3-butanediol | 100000 | 6 | Males |
| Captopril | 180 | 5 | - |
| L-leucine | 40000 | 5 | Males |
| Females | |||
| PB125 | 100 | 5 | - |
| Sulindac | 5 | 5 | - |
| Syringaresinol | 300 | 5 | - |
| Metformin + Rapamycin | 1000 / 14 | 9 | Males |
| Females | |||
| Rapamycin + Acarbose | 14.7 / 1000 | 9 and 16 | - |
no data available.
ppm (part per million by weight).
the Protandim® was increased from 600 ppm to 1200 ppm when the mice reached 17 months of age.
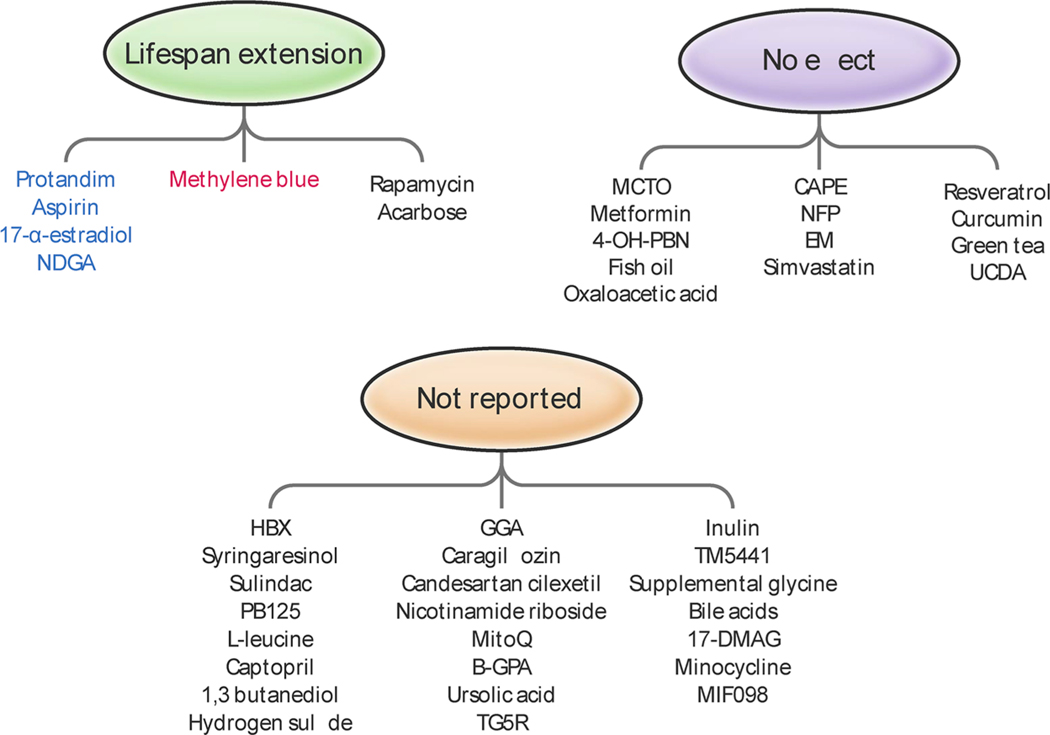
Fig. 2. Compounds tested by the National Institute on Aging (NIA) Interventions Testing Program (ITP) for their anti-aging activity and effects on lifespan extension in mice.
Some compounds elicit anti-aging effects on lifespan that are sex-dependent. For instance, rapamycin and acarbose extend lifespan in both sexes whereas methylene blue (in red) has pro-longevity effects only in females. Four compounds (in blue) extend lifespan in males. Most of the compounds are still being tested and there is no data available.
Perhaps the best known finding by the ITP is that rapamycin increases lifespan of both male and female mice (Harrison et al., 2009; Miller et al., 2011). The beneficial effects of rapamycin are seen when administration began at 270 days and later at 600 days of age, which suggests that it could have therapeutic potential in humans even if started late in life. Most recently, the ITP has conducted a dose-response study and found that concentrations of rapamycin higher than those used in the original studies can actually increase the maximal lifespan of mice (Miller et al., 2014). Rapamycin delays the progression of multiple age-related pathologies including liver degeneration, endometrial hyperplasia in females, and the appearance of abnormal cell nuclei in the heart (Wilkinson et al., 2012). However, negative effects of rapamycin, such as testicular degeneration, increased severity of cataracts, and insulin-resistant phenotype, were reported indicating that caution must be used when assessing the potential role of rapamycin in lifespan extension (Wilkinson et al., 2012; Miller et al., 2014). Surprisingly, in the context of type 2 diabetes, rapamycin has cardioprotective effects and positive benefits on glucose metabolism (Azar et al., 2018; Reifsnyder et al., 2016).
Acarbose has also been found to increase median and maximal lifespan in both sexes when administered early in life, although the effects of acarbose were much larger in males (Harrison et al., 2014, 2019). Starting the acarbose treatment at 16 months of age resulted in an extension of maximum lifespan for both sexes, with an increase in median longevity observed only in males (Strong et al., 2016).
Other ITP studies have shown that only males have increased median lifespan when given either aspirin, nordihydroguaiaretic acid (NDGA), 17-α-estradiol, or protandim (Harrison et al., 2014; Strong et al., 2016, 2008). Originally, it was thought that differences in the metabolism of aspirin and NDGA could be responsible for their sex-specific effects on lifespan; however, administration of high dose NDGA failed to improve the lifespan of females despite achieving plasma NDGA concentrations similar to the males in the original study (Harrison et al., 2014). Therefore, the refractoriness of female mice to NDGA cannot simply be accounted for by differences in pharmacodynamics. Gonadal hormones underlie male-specific metabolomics response to 17-α-estradiol and improvements in glucose tolerance and mTORC2 signaling (Garratt et al., 2017).
The control male mice at UTHSCSA and The Jackson Laboratory have shorter lifespan than at the University of Michigan, while the females have relatively similar lifespans at all three sites (Harrison et al., 2014). This fact may partially explain why in general the tested interventions preferentially benefit males compared to females. Nevertheless, other complex variables can be at play.
Most of the interventions tested by the ITP, as well as those reported here, did not produce significant effects on lifespan in mice regardless of sex (Fig. 3). These include 4–OH-α-phenyl-N-tert-butyl nitrone (4–OH-PBN), nitroflurbiprofen (NFP), caffeic acid phenethyl ester (CAPE), enalapril maleate, simvastatin, resveratrol, green tea extract, curcumin, oxaloacetic acid, medium-chain triglyceride oil, metformin, fish oil, and ursodeoxycholic acid (UDCA) (Harrison et al., 2009; Miller et al., 2011; Strong et al., 2016, 2008; Strong et al., 2013). Methylene blue increases maximal, but not median, lifespan only in female mice (Harrison et al., 2014). Some of these findings are inconsistent with reports from other laboratories (Baur et al., 2006; Kitani et al., 2007; Martin-Montalvo et al., 2013; Anisimov et al., 2015; Bartke et al., 2019) and variables such as the age of onset, genetic background, dosage, mode of delivery, diet composition, and treatment regimen could account for these discrepancies. It is also possible that some of these interventions had other long-term effects, positive or negative, on other measurements of health, which were not detected due to the sole focus of the ITP on lifespan.
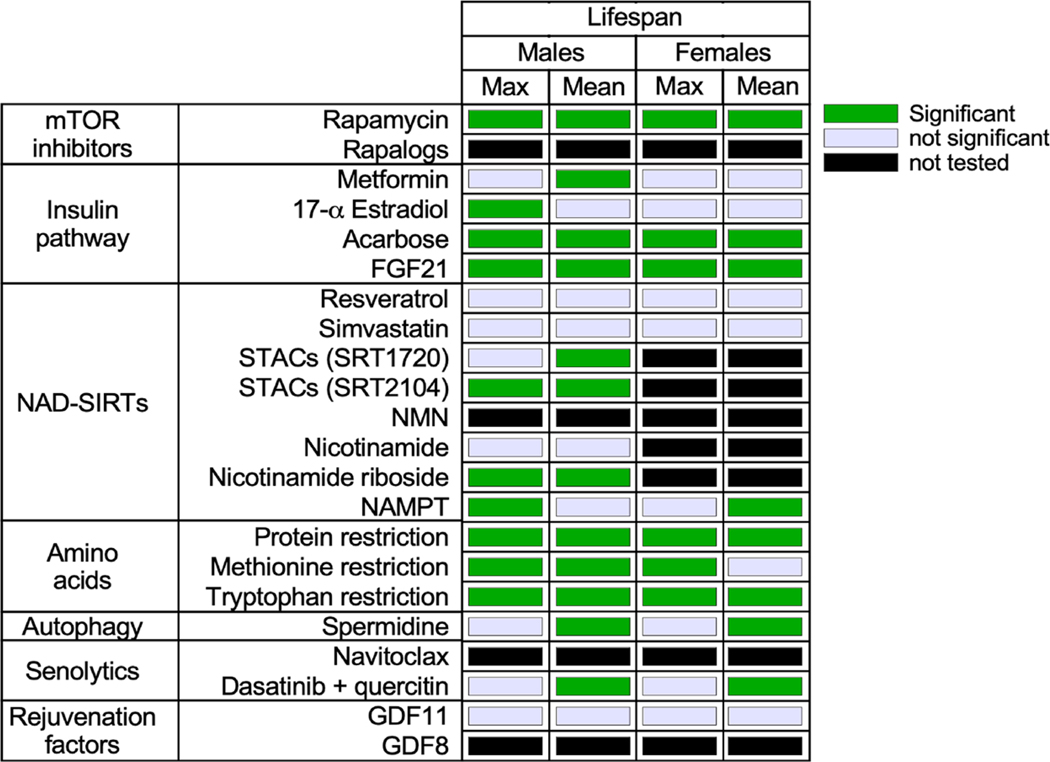
Fig. 3. Heatmap summarizing the different interventions and their effect in maximum and mean lifespan extension in mice.
These interventions target major signaling pathways whose dysregulation contributes to the emergence of the aging phenotypes and disease.
3.2. Other compounds that are being tested in the NIA-ITP
The ITP is testing another 22 compounds, although no data has been reported yet (Table 2).
Table 2.
Compounds under testing in the NIA-ITP with no data reported to date.
| Compound | Classification/ Properties | Primary and secondary effects | Reference |
|---|---|---|---|
| 1,3-Butanediol | Precursor of the ketone body β-hydroxybutyrate (β-HB) | • Ketogenic diets increase levels of β-HB and have been shown to improve healthspan, memory, and longevity in mice | Newman et al., 2017; Roberts et al., 2017 |
| 17-DMAG | Derivative of the benzoquinone ansamycin antibiotic geldanamycin | • Inhibitor of the ATPase function of HSP90 with anti-inflammatory properties in autoimmune and neurodegenerative diseases, as well as obesity and metabolic diseases. • Prolongevity effects via reduction of acetyl-CoA levels with concomitant stimulation of autophagy. | Calamini and Morimoto, 2012; Tukaj and Węgrzyn (2016); Verma et al., 2016; Ambade et al., 2014; Madeo et al., 2014 |
| 2-(2-hydroxyphenyl)-benzoxazole (HBX) | Metal chelator and anti-aggregation properties | • Increases lifespan in C. elegans. • Increases lifespan in ALS mouse model. | Alavez et al., 2011; Evans et al., 2016 |
| β-guanidinopropionic acid (β-GPA) | Analogue of creatine that competitively inhibits creatine kinase and depletes intracellular phosphocreatine and ATP levels | • Activates AMPK and promotes mitochondrial biogenesis in skeletal muscle in rats. • Extends lifespan in flies via AMPK. | Oudman et al., 2013; Reznick et al., 2007; Yang et al., 2015 |
| Bile acids | Steroid acids | • Dietary absorption of lipids and the fat-soluble vitamins A, D, E and K. • Activation of nuclear hormone receptors, mainly the farnesoid X receptor (FXR), controlling hepatic lipid and glucose metabolism. • Activation of G protein-coupled receptor TGR5, acting on the immune system response, energy expenditure, glucose homeostasis, and insulin sensitivity. • Extends yeast chronological lifespan in a TOR-independent manner (lithocholic acid). • Modulates lifespan in C. elegans through nuclear receptor signaling (dafrachronic acid). | Russell, 2009; Trauner et al., 2010; Pols et al., 2011; Rizzo et al., 2010; Watanabe et al., 2012; Goldberg et al., 2010; Beach et al., 2013, 2015; Gerisch et al., 2007; Magner et al., 2013; Amador-Noguez et al., 2007 |
| Canagliflozin | Sodium-glucose co-transporter-2 (SGLT2) inhibitor convoluted tubule of the kidney, thereby facilitating the excretion of glucose in the urine. • Caloric restriction mimetic. | • Blocks the reabsorption of glucose in the proximal | Kalra, 2014; Kalra et al., 2016 |
| Candesartan cilexetil | Prodrug form of candesartan, an angiotensin II receptor antagonist | • Increases the lifespan of spontaneously hypertensive rats. | Baiardi et al., 2004 |
| Captopril | Angiotensin-converting enzyme (ACE) inhibitor | • Extends lifespan in nematodes via a mechanism that is distinct from calorie restriction. | Kumar et al., 2016 |
| Geranylgeranylacetone (GGA) | Inducer of the 70-kDa heat shock protein (HSP70) | • Prevents hearing loss and hair cell death in a mouse model of age-related hearing loss. Positive effects on cognition and Aβ pathology in the APP23 mouse model of Alzheimer’s disease. | Hirakawa et al., 1996; Hoshino et al., 2013 |
| Glycine | Methionine restriction mimetic | • Increases lifespan in flies and in rats. | Sugiyama et al., 1987; Orentreich et al., 1993; ;Obata and Miura, 2015 |
| Inulin | Bifidogenic and fiber-like compound | • Regulates GLP-1 and ghrelin levels for the control of food intake and appetite, stimulates the immune system, improves mineral absorption and bone mineralization, influences blood and liver lipid metabolism. • Reduces the risk of chronic gastrointestinal track and colon cancer. • Increases lifespan in rats. • Increase muscle strength in humans. • Anti-inflammatory effects via gut microbiota. | Milani et al., 2016; Tuohy, 2007; Shoaib et al., 2016; Rozan et al., 2008; Buigues et al., 2016 |
| L-Leucine | Branched chain amino acid (BCAAs) | • Extends lifespan in yeast, nematodes, and mice. • Stimulates protein synthesis in skeletal muscle via mTOR pathway. • Increases functional performance and lean tissue mass in elderly humans. | Alvers et al., 2009; D’Antona et al., 2010; Mansfeld et al., 2015; Norton and Layman, 2006; Ispoglou et al., 2016 |
| Minocycline | Semi-synthetic, second-generation tetracycline analogue | •Antioxidant properties, anti-inflammatory/immunomodulatory effects, and neuroprotection mainly by inhibition of microglia activation and attenuation of apoptosis. • Inhibits the formation of kynurenine from tryptophan, mechanism implicated in age-related metabolic disorders as well as psychiatric disorders • Improves healthspan and lifespan in Drosophila. | Garrido-Mesa et al., 2013; Oxenkrug et al., 2012; Oxenkrug, 2013; Mora et al., 2013 |
| MitoQ | Antioxidant ubiquinone conjugated to a lipophilic cation with anti-apoptotic effects | • Delays the onset of paralysis and increase lifespan in C. elegans model of AD.; • Increases lifespan in flies. • Increases lifespan in ALS mouse model. | Kelso et al., 2001; Ng et al., 2014; Magwere et al., 2006; Miquel et al., 2014 |
| MIF098 | Interferes with the ability of macrophage migration inhibitory factor (MIF) cytokine to bind to its receptor, CD74 | • Inhibition of MIF extends lifespan in mice. • Anti-inflammatory effects. | Harper et al., 2010; Weiser et al., 2015; Hare et al., 2010 |
| Nicotinamide riboside | NAD+ precursor | • Extends lifespan by increasing NAD + synthesis in yeast. | Belenky et al., 2007 |
| PB125 | Phytochemical compound consisting of carnosol, carnosic acid, shogaol, gingerol, luteolin, and withaferin A | • Activates NRF2 pathway in mice and cells. | Hybertson and McCord, 2017 (patent) |
| SG1002 (sodium polysulthionate) | Prodrug of the endogenous signaling gasotransmitter hydrogen sulfide (H2S) | • Increases lifespan in a Sir2-dependent manner in C. elegans. • Prevent oxidative stress and activates sirtuins affecting nutrient-sensing pathways. | Polhemus et al., 2015; Miller and Roth, 2007; Ng et al., 2018 |
| Sulindac | Nonsteroidal anti-inflammatory drug (NSAID) that inhibits both cyclooxygenase I and II | • Inhibit tumorigenesis, and might prevent sporadic colorectal adenomas in patients with a history of resected adenomas. • Attenuates age-related deficits in learning and memory in aged Fischer 344 rats. • Might extend lifespan in yeast, nematodes and flies (similar to ibuprofen). | Meyskens et al., 2008; Gurpinar et al., 2014; Mesches et al., 2004; He et al., 2014 |
| Syringaresinol | Phytochemical compound found in Panax ginseng berries | • Activate Sirt1 gene expression in human umbilical vein endothelial cells and delay senescence in a FOXO3-dependent manner. • Modulates gut integrity, microbiota diversity, and delays age-associated immunosenescence. • Induces vasorelaxation in endothelial cells by increasing nitric oxide production. | Cho et al., 2013, 2016; Chung et al., 2012 |
| TM5441 | Senolytic drug via plasminogen activator inhibitor-1 (PAI-1) | • Increases lifespan in the Klotho-deficient mice. • Protection against high-fat diet-induced obesity and insulin resistance. | Boe et al., 2013; Jeong et al., 2016; Piao et al., 2016; Eren et al., 2014; |
| Ursolic acid | Pentacyclic triterpenoid | • Increases lifespan in C. elegans. • Increases skeletal muscle mass in human and mouse • Reduces adiposity (mouse). • Stimulates IGF-1/ AKT signaling. | Vayndorf et al., 2013; Kunkel et al., 2011, 2012 |
3.3. Translation of the NIA-ITP findings toward the clinic in the treatment of age-associated chronic diseases
The goal of treating chronic conditions and common geriatric syndromes is to enhance the quality of life and reduce mortality in the elderly. Using ClinicalTrials.gov, a database of privately and publicly funded clinical studies conducted around the world, we surveyed the most recent number of clinical trials (until July 2019) that treat aging or age-related diseases with the compounds/drugs described in Section 2 of the manuscript. All these clinical trials are being tested in both male and female participants of various ages (Supplementary Table 1). The bulk of these studies are interventional or observational, and tackle a number of age-related medical conditions ranging from insulin resistance and diabetes to dementia and cancer. Drug combinations are also being tested, including rapamycin with acarbose or with metformin as well as interventions such as CR with exercise.
In the database “condition/disease” is defined as “The disease, disorder, syndrome, illness, or injury that is being studied”. On ClinicalTrials.gov, conditions may also include other health-related issues, such as “lifespan, quality of life, and health risks”, and “Intervention/Treatment” as “A process or action that is the focus of a clinical study. Interventions include drugs, medical devices, procedures, vaccines, and other products that are either investigational or already available. Interventions can also include noninvasive approaches, such as education or modifying diet and exercise”. Our search for conditions or disease included the terms: “age”, “aging,” “longevity”, “lifespan”, “senescence”, “frailty,” “sarcopenia”, “age-related atrophy”, “type 2 diabetes”, “metabolic syndrome,” “cardiovascular disease”, “age-related macular degeneration”, “age-related cognitive decline”, “dementia”, “mild cognitive impairment”, “Alzheimer Disease”, “Parkinson Disease”, “cancer”, “osteoporosis” and “osteoarthritis”. For interventions and treatments: “acarbose”, “aspirin”, “curcumin”, “estradiol”, “GDF8″, “myostatin”, “GDF11″, “green tea”, “metformin”, “methylene blue”, “NAD”, “nicotinamide”, “nicotinamide riboside, “NAD precursors”, “polyamines”, “quercitine”, “resveratrol”, “sirtuin-activating compounds”, “SRT2104″, “SRT3025″, “simvastatin”, “tryptophan”, “ketogenic diet”, “caloric restriction”, “dietary restriction”, “exercise” and “fasting”. We found ∼12,100 clinical trials that target age or age-related diseases with more than 538 clinical trials aimed toward “Aging as a condition or disease” Table 3). Table 3 describes all the clinical trials available by drug and age or age-related conditions. Many of these trials differ dramatically by sample size and, thus, interpreting the study results will require caution. Note the absence of GDF11 and 17 α-estradiol due to a lack of experimental intervention in clinical trials. Exercise, fasting and CR are the interventions with the highest number of clinical trials that target aging as a condition (435, 20 and 15 trials, respectively), followed by NAD precursors (12), metformin (11), and resveratrol (10).
Table 3.
Number of clinical trials available by drug and age or age-related condition. On ClinicalTrials.gov, “condition/disease” is defined as “the disease, disorder, syndrome, illness, or injury that is being studied”. We found ∼12,100 clinical trials that target age or age-related diseases with 510 clinical trials aimed toward “Aging as a condition or disease”. Of note, we have added anti-aging interventions such as CR, exercise, fasting, and ketogenic diet, although they are not the focus of the review. To date, exercise is the “anti-aging” intervention with the most on-going clinical trials.
| DRUGS | AGING* | AD** | AMD | CANCER | CDV | FRAILTY | MET.S** | OSTEOA | OSTEOP | PD | SARCOPENIA | T2D*** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACARBOSE | 2 | 0 | 0 | 4 | 19 | 0 | 1 | 0 | 0 | 0 | 0 | 67 |
| ASPIRIN | 1 | 0 | 3 | 161 | 1008 | 0 | 3 | 2 | 0 | 0 | 0 | 53 |
| CURCUMIN | 9 | 5 | 0 | 65 | 8 | 0 | 7 | 4 | 0 | 0 | 0 | 8 |
| GDF8 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| GREEN TEA | 5 | 0 | 0 | 97 | 31 | 0 | 2 | 1 | 1 | 1 | 0 | 17 |
| METFORMIN | 11 | 3 | 1 | 335 | 105 | 4 | 62 | 0 | 0 | 0 | 1 | 1337 |
| METHYLENE BLUE | 1 | 7 | 0 | 43 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NAD_NAD precursors | 12 | 2 | 0 | 13 | 57 | 1 | 9 | 0 | 0 | 0 | 2 | 9 |
| POLYAMINES | 1 | 2 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| QUERCETIN | 2 | 1 | 0 | 13 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| RAPAMYCIN | 11 | 0 | 12 | 1106 | 473 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| RESVERATROL | 10 | 5 | 2 | 15 | 10 | 0 | 13 | 1 | 2 | 6 | 1 | 15 |
| SRT2104 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 7 |
| SRT3025 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| SIMVASTATIN | 1 | 8 | 0 | 58 | 172 | 0 | 14 | 0 | 1 | 1 | 0 | 42 |
| TRYPTOPHAN | 0 | 2 | 0 | 3 | 9 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| INTERVENTIONS | ||||||||||||
| CALORIC RESTRICTION | 15 | 7 | 0 | 57 | 20 | 0 | 22 | 3 | 0 | 0 | 0 | 31 |
| EXERCISE | 435 | 167 | 0 | 1214 | 1979 | 80 | 229 | 331 | 93 | 179 | 121 | 628 |
| FASTING | 20 | 21 | 2 | 281 | 240 | 0 | 50 | 15 | 12 | 22 | 4 | 339 |
| KETOGENIC DIET | 0 | 9 | 0 | 26 | 4 | 0 | 2 | 0 | 0 | 2 | 0 | 7 |
| TOTAL | 538 | 239 | 20 | 3496 | 4141 | 85 | 414 | 357 | 109 | 212 | 134 | 2564 |
Includes age, aging and longevity.
Includes Alzheimer’s disease, dementia and mild cognitive impairment; AD (Alzheimer’s disease); CDV (cardiovascular disease); Met.S (metabolic syndrome); Osteoa (osteoarthritis); Osteop (osteoporosis); PD (Parkinson’s disease); T2D (type 2 diabetes).
4. Limitations and caveats
Older adults suffer from multiple medical conditions or co-morbidities and more than half take five or more medications, which raises the issue of polypharmacy leading to compliance concerns, adverse effects, and potential risk of drug-drug interactions. Moreover, environment, nutrition, age, ethnicity, and gender are important variables that contribute to differential responses to treatment or interventions. Indeed, there are substantial evidences pointing toward drug toxicity only at a certain age interval. Four possible outcomes have been ascribed within a cohort of patients having the same diagnosis and receiving the same prescription: 1) Drug toxic but beneficial; 2) drug toxic but not beneficial; 3) drug not toxic and not beneficial; and 4) drug not toxic and beneficial (Harrill, 2016). It would appear, therefore, that only few patients at a certain age range are expected to respond adequately to standard therapy, with most patients being either non-responders, develop resistance, or exhibit an inadequate response (Harrill, 2016). An example at hand is the hepatotoxicity to commonly used drugs, such as acetaminophen and ibuprofen, in sensitive individuals (Chitturi and George, 2002).
People age at different rates and the age-related deterioration and functional decline vary within the same individual in a tissue-specific manner: Epigenetic changes, such as DNA methylation and histone modification, largely account for the dynamic alteration in the transcriptional profile and/or cellular phenotype in a given tissue with age (Horvath, 2013; Melis et al., 2013). It has been assumed, wrongly perhaps, that the promotion of healthy aging, notably through delay in the aging phenotypes, frailty and associated geriatric syndromes, will impact every biological system to the same extent (Michel et al., 2016). However, whether changes in the number, dose frequency, and treatment length of medications influence physiological outcomes and cellular epigenetic landscapes within different types of tissues and organs remain to be answered. There is still a lack of clinical evidence for healthspan extension through compression of chronic disease in late life and it remains possible that some of the age–related phenotypic declines in function might not respond adequately to treatment (responders vs. non responders) (Evert et al., 2003; Atkinson et al., 2019). Therefore, successful assessment in the efficacy of an intervention that delays aging will require validation through stringent outcome measures of phenotypic enhancement of longevity in various biological systems. The emergence of age-related changes within each type of tissue, whether at the genome, transcriptome, proteome, or metabolome level, should enable the identification of a selective set of biomarkers of aging and validate the efficacy of an intervention aimed at preserving or maintaining tissue functionality. Yet, with the advances in the different fields of molecular biology, the search for biomarkers or predictors of biological and phenotypic age, rather than chronological age, remains unsuccessful, except the epigenetic clock developed by Horvath in 2013. Ideally, defining those predictors for ‘healthy aging’ will allow us to predict lifespan.
Very few animal models, with the exception of non-human primates, adequately replicate physical and biochemical deficits in terms of human aging and age-related diseases. The rhesus monkey (Macaca mulatta) genome shares 93 % sequence identity with the human genome, and most of their anatomy, physiology, neurology, endocrinology, immunology an ddecline in function with age directly parallel those of humans, making this species an excellent model to study human aging. It is imperative to develop new animal models that better mimic key clinical features of human aging phenotypes and their environments for use in preclinical studies. Recently, researchers at university of Washington in Seatle, initiated an intervention study The Dog Aging Project, 2020 (http://dogagingproject.com) aiming to perform a longitudinal study of aging in dogs and an intervention trial to test whether rapamycin will prevent disease and extend healthy longevity in middle-aged dogs.
Pharmacological interventions need to satisfy the following conditions in order to be translatable: i) Low toxicity and few side effects; ii) effective via oral administration, iii) maximum dosing frequency of once-a-day; iv) stability; v) scalability and low manufacturing costs; vi) detectable in blood; and vii) effective when administered late in life or once symptoms have already started to develop (Kirkland, 2016). It remains to be seen whether long-term exposure of middle-aged adults to an ‘anti-aging drug’ will confer longevity dividend (described by Olshansky et al., 2016 as “the economic and health benefits that would accrue to individuals and societies if we extend healthy life by slowing the biological processes of aging”).
Perhaps, one of the main obstacles for the development of drugs to treat aging is that the FDA does not consider aging as a preventable condition. However, the FDA has recently agreed of a new clinical trial aimed at testing the ability of the diabetes drug metformin at delaying or preventing diseases associated with old age, including heart disease, cognitive impairment, and cancer (Barzilai et al., 2016). Metformin could be the first drug of its kind to be repurposed with the goal of targeting aging. If successful, similar studies will surely follow with other compounds, with the goal of maximizing the years that we live free of diseases or chronic conditions. This compression of morbidity should shorten the time spent with age-related syndromes as humans approach the limit of their lifespan (Fries et al., 2011).
5. Conclusions and future directions
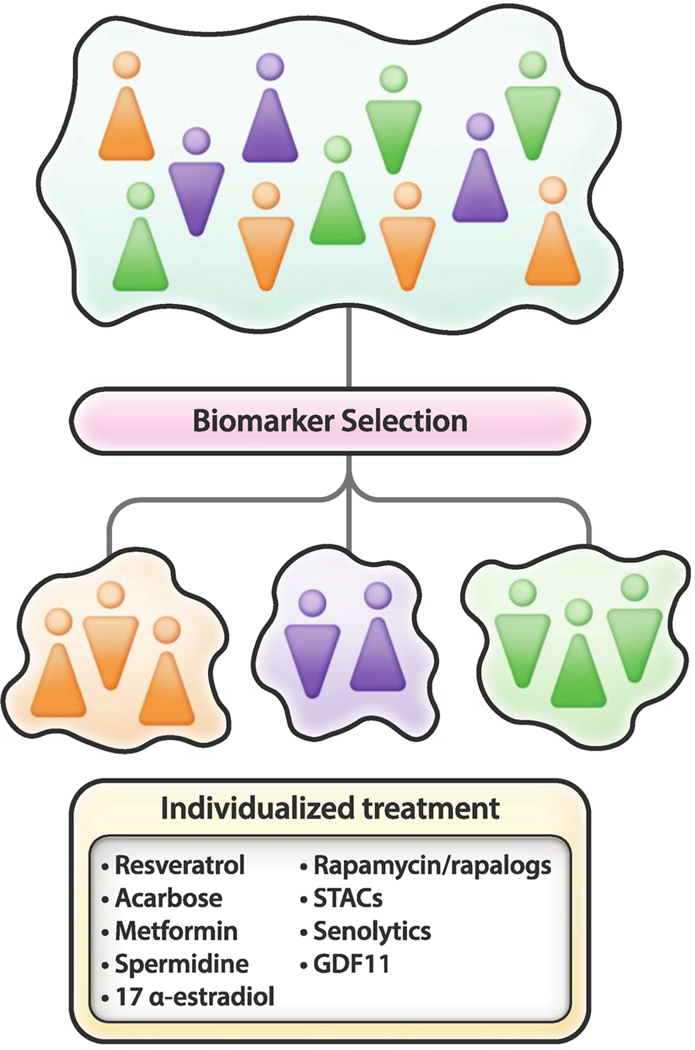
Aging is an intrinsic heterogenic feature of most living organisms, especially mammals, and recent research has provided clues about how to improve several converging cellular processes implicated in nutrient sensing, mitochondrial bioenergetics, and healthy senescence. Animal studies and human clinical trials have thought us that diet, environment, gender, ethnicity, polymorphisms, and age are among the variables that contribute to being responders or non-responders to any given therapy, whether it is cancer, cardiovascular disease, or other ailments. The extensive genetic diversity both within and among human populations generally leads to discordance in the safety and efficacy profile of a drug. It has become increasingly apparent that these different responses to treatment require the use of molecular profiling to determine the appropriate therapy. The tailoring of pharmacotherapy to the individual characteristics of each patient can be applied to areas as varied as cancer, coronary heart disease, diabetes, and neuropathological disorders to name of few (Vargas and Harris, 2016; Orho-Melander, 2015; Scheen, 2016; Golan et al., 2016). It is likely that the identification of relevant biomarkers of healthspan will enable to stratify subgroups of individuals that will respond favorably to interventions aimed at reducing age-related ailments and chronic disease (Fig. 4).
Fig. 4. Personalized approach for the treatment of age and age-related diseases.
The identification of relevant biomarkers of healthspan and the integration of ‘omics’ data sets from the genome, transcriptome, proteome, and metabolome, and machine learning approaches, will likely allow the development of personalized treatments aimed at reducing age-related diseases.
Hopefully, integration of intermediate phenotypes of different (genetic and non-genetic) origin along with ‘omics’ data sets from the genome, transcriptome, proteome, and metabolome (Williams et al., 2016), and machine learning approaches will soon enable identification of subgroups of elderly subjects with similar complex trait frequency and predict whether an individual might benefit from a treatment aimed at tackling age-associated cognitive deficits and physical frailty and geriatric syndromes. This approach should identify subgroups of the aging population that exhibit differential responses to treatment through selection of potential targetable biomarkers. It follows that the conditional probability that an elderly individual responds favorably to a given intervention will be measurably improved.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging (NIA), National Institutes of Health (NIH). We thank Mr. Marc Raley for generating the figures.
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest, financial or otherwise.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.arr.2020.101037.
References
- Ables GP, Johnson JE, 2017. Pleiotropic responses to methionine restriction. Exp. Gerontol. 94, 83–88. 10.1016/j.exger.2017.01.012. [DOI] [PubMed] [Google Scholar]
- Alavez S, Vantipalli MC, Zucker DJS, Klang IM, Lithgow GJ, 2011. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 472, 226–229. 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaras I, Mitchell SJ, Mora H, Lugo DR, Warren A, Navas-Enamorado I, Hoffmann V, Hine C, Mitchell JR, Le Couteur DG, Cogger VC, Bernier M, de Cabo R, 2017. Health benefits of late-onset metformin treatment every other week in mice. NPJ Aging Mech. Dis. 3, 16 10.1038/s41514-017-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R, 2016. Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav. Brain Res. 301, 1–9. 10.1016/j.bbr.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvers AL, Fishwick LK, Wood MS, Hu D, Chung HS, Dunn WAJ, Aris JP, 2009. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell 8, 353–369. 10.1111/j.1474-9726.2009.00469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Noguez D, Dean A, Huang W, Setchell K, Moore D, Darlington G, 2007. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell 6, 453–470. 10.1111/j.1474-9726.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA, Mandrekar P, 2014. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J. Hepatol. 61, 903–911. 10.1016/j.jhep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA, 2003. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423, 181–185. 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Popovich IG, Zabezhinski MA, Egormin PA, Yurova MN, Semenchenko AV, Tyndyk ML, Panchenko AV, Trashkov AP, Vasiliev AG, Khaitsev NV, 2015. Sex differences in aging, life span and spontaneous tumorigenesis in 129/Sv mice neonatally exposed to metformin. Cell Cycle 14, 46–55. 10.4161/15384101.2014.973308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson G, Williamson P, Batterham AM, 2019. Issues in the determination of’ responders’ and “non-responders” in physiological research. Exp. Physiol. 104, 1215–1225. 10.1113/EP087712. [DOI] [PubMed] [Google Scholar]
- Azar A, Lawrence I, Jofre S, Mell J, Sell C, 2018. Distinct patterns of gene expression in human cardiac fibroblasts exposed to rapamycin treatment or methionine restriction. Ann. N. Y. Acad. Sci. 1418, 95–105. 10.1111/nyas.13566. [DOI] [PubMed] [Google Scholar]
- Baiardi G, Bregonzio C, Jezova M, Armando I, Saavedra JM, 2004. Angiotensin II AT1 receptor blockade prolongs the lifespan of spontaneously hypertensive rats and reduces stress-induced release of catecholamines, glucocorticoids, and vasopressin. Ann. N. Y. Acad. Sci. 1018, 131–136. 10.1196/annals.1296.015. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM, 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksi A, Kraydashenko O, Zalevkaya A, Stets R, Elliott P, Haddad J, Hoffmann E, Vlasuk GP, Jacobson EW, 2014. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br. J. Clin. Pharmacol. 78, 69–77. 10.1111/bcp.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan V, Miller GS, Kaplun L, Balan K, Chong Z-Z, Li F, Kaplun A, VanBerkum MFA, Arking R, Freeman DC, Maiese K, Tzivion G, 2008. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J. Biol. Chem. 283, 27810–27819. 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RC, 2012. The genetics of Alzheimer’s disease. Scientifica (Cairo) 2012, 246210. 10.6064/2012/246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Evans TR, Musters C, 2019. Anti-aging interventions affect lifespan variability in sex, strain, diet and drug dependent fashion. Aging 11, 4066–4074. 10.18632/aging.102037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N, Meyer JG, Schilling B, 2018. Protein turnover in aging and longevity. Proteomics 18, e1700108. 10.1002/pmic.201700108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA, 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342. 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R, 2012. Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 11, 443–461. 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach A, Richard VR, Leonov A, Burstein MT, Bourque SD, Koupaki O, Juneau M, Feldman R, Iouk T, Titorenko VI, 2013. Mitochondrial membrane lipidome defines yeast longevity. Aging (Albany NY) 5, 551–574. 10.18632/aging.100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach A, Richard VR, Bourque S, Boukh-Viner T, Kyryakov P, Gomez-Perez A, Arlia-Ciommo A, Feldman R, Leonov A, Piano A, Svistkova V, Titorenko VI, 2015. Lithocholic bile acid accumulated in yeast mitochondria orchestrates a development of an anti-aging cellular pattern by causing age-related changes in cellular proteome. Cell Cycle 14, 1643–1656. 10.1080/15384101.2015.1026493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C, 2007. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD. Cell 129, 473–484. 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R, 2016. Resveratrol supplementation confers neuro-protection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 8, 899–916. 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, McAnally D, Smith LH, Miyata T, Vaughan DE, 2013. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nω-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 128, 2318–2324. 10.1161/CIRCULATIONAHA.113.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Sinclair DA, 2016. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 17, 679–690. 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MHM, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA, 2013. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152. 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgeman SC, Ellison GC, Melton PE, Newsholme P, Mamotte CDS, 2018. Epigenetic effects of metformin: from molecular mechanisms to clinical implications. Diabetes Obes. Metab. 20, 1553–1562. 10.1111/dom.13262. [DOI] [PubMed] [Google Scholar]
- Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, Verdejo Y, Mascarós MC, Peris C, Cauli O, 2016. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int. J. Mol. Sci. 17, 932 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner S, Broeskamp F, Sommer C, Markaki M, Habernig L, Alavian-Ghavanini A, Carmona-Gutierrez D, Eisenberg T, Michael E, Kroemer G, Tavernarakis N, Sigrist SJ, Madeo F, 2014. Spermidine protects against alpha-synuclein neurotoxicity. Cell Cycle 13, 3903–3908. 10.4161/15384101.2014.973309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung K-Y, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene NDE, Gems D, 2013. Metformin retards aging in C. Elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239. 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamini B, Morimoto RI, 2012. Protein homeostasis as a therapeutic target for diseases of protein conformation. Curr. Top. Med. Chem. 12, 2623–2640. 10.2174/1568026611212220014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camardo J, 2003. The Rapamune era of immunosuppression 2003: the journey from the laboratory to clinical transplantation. Transplant. Proc. 35, 18S–24S. 10.1016/s0041-1345(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E, 2019. From discoveries in ageing research to therapeutics for healthy ageing. Nature 571, 183–192. 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J, 2010. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 11, 213–219. 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Li A, Cho H-Y, Lee B, Obrietan K, 2010. Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock. J. Neurosci. 30, 6302–6314. 10.1523/JNEUROSCI.5482-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JY, Sehgal SN, Bansbach CC, 1991. FK506 and rapamycin: novel pharmacological probes of the immune response. Trends Pharmacol. Sci. 12, 218–223. 10.1016/0165-6147(91)90555-7. [DOI] [PubMed] [Google Scholar]
- Chang TK, Chen J, Lee WB, 2001. Differential inhibition and inactivation of human CYP1 enzymes by trans-resveratrol: evidence for mechanism-based inactivation of CYP1A2. J. Pharmacol. Exp. Ther. 299, 874–882 doi: (not available). [PubMed] [Google Scholar]
- Chiang M-C, Nicol CJ, Cheng Y-C, 2018. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem. Int. 115, 1–10. 10.1016/j.neuint.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Chitturi S, George J, 2002. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipid-lowering agents, psychotropic drugs. Semin. Liver Dis. 22, 169–183. 10.1055/s-2002-30102. [DOI] [PubMed] [Google Scholar]
- Cho S-Y, Cho M, Seo DB, Lee SJ, Suh Y, 2013. Identification of a small molecule activator of SIRT1 gene expression. Aging (Albany NY) 5, 174–182. 10.18632/aging.100539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-Y, Kim J, Lee JH, Sim JH, Cho D-H, Bae I-H, Lee H, Seol MA, Shin HM, Kim T-J, Kim D-Y, Lee S-H, Shin SS, lm S-H, Kim H-R, 2016. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci. Rep. 6, 39026. 10.1038/srep39026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Namkoong K, Shin M, Park J, Yang E, Ihm J, Thu VT, Kim HK, Han J, 2017. Cardiovascular protective effects and clinical applications of resveratrol. J. Med. Food 20, 323–334. 10.1089/jmf.2016.3856. [DOI] [PubMed] [Google Scholar]
- Chung BH, Kim S, Kim JD, Lee JJ, Baek YY, Jeoung D, Lee H, Choe J, Ha KS, Won MH, Kwon YG, Kim YM, 2012. Syringaresinol causes vasorelaxation by elevating nitric oxide production through the phosphorylation and dimerization of endothelial nitric oxide synthase. Exp. Mol. Med. 44, 191–201. 10.3858/emm.2012.44.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA, 2005. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Cooke D, Ouattara A, Ables GP, 2018. Dietary methionine restriction modulates renal response and attenuates kidney injury in mice. FASEB J. 32, 693–702. 10.1096/fj.201700419R. [DOI] [PubMed] [Google Scholar]
- Custodero C, Mankowski RT, Lee SA, Chen Z, Wu S, Manini TM, Hincapie Echeverri J, Sabba C, Beavers DP, Cauley JA, Espeland MA, Fielding RA, Kritchevsky SB, Liu CK, McDermott MM, Miller ME, Tracy RP, Newman AB, Ambrosius WT, Pahor M, Anton SD, 2018. Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res. Rev. 46, 42–59. 10.1016/j.arr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E, 2010. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 12, 362–372. 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- da Luz PL, Tanaka L, Brum PC, Dourado PMM, Favarato D, Krieger JE, Laurindo FRM, 2012. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis 224, 136–142. 10.1016/j.atherosclerosis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim L-J, Osborne B, Joshi S, Lu Y, Trevino-Villarreal JH, Kang M-J, Hung T-T, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA, 2018. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell 173, 74–89. 10.1016/j.cell.2018.02.008. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, Hall MN, Madeo F, 2014. The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515–1526. 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J, Breton M, Decaux J-F, Lavery G, Baczko I, Zoll J, Garnier A, Li Z, Brenner C, Mericskay M, 2017. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256–2273. 10.1161/CIRCULATIONAHA.116.026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TKT, 2015. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 21, 506–511. 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens JA, Moos WH, Howell N, 2005. Development of 17alpha-estradiol as a neuroprotective therapeutic agent: rationale and results from a phase I clinical study. Ann. N. Y. Acad. Sci. 1052, 116–135. 10.1196/annals.1347.008. [DOI] [PubMed] [Google Scholar]
- Ebeling M, Rau R, Baudisch A, 2018. Rectangularization of the survival curve reconsidered: the maximum inner rectangle approach. Popul. Stud. (Camb) 72, 369–379. 10.1080/00324728.2017.1414299. [DOI] [PubMed] [Google Scholar]
- Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, Brunquell J, Westerheide SD, Bradshaw PC, 2015. Mechanisms of amino acid-mediated life-span extension in Caenorhabditis elegans. BMC Genet. 16, 8 doi: 0.1186/s12863–015-0167–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg A-U, Brack AS, Glass DJ, 2015. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 22, 164–174. 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, Frieling-Salewsky von M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Muhlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F, 2016. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438. 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GRS, Mutlu GM, Miyata T, Vaughan DE, 2014. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A 111, 7090–7095. 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TM, Bhattacharya A, Shi Y, Qi W, Block TJ, Chaudhuri A, Chaudhuri AR, Hawker K, Van Remmen H, 2016. Moderate modulation of disease in the G93A model of ALS by the compound 2-(2-hydroxyphenyl)-benzoxazole (HBX). Neurosci. Lett. 624, 1–7. 10.1016/j.neulet.2016.04.035. [DOI] [PubMed] [Google Scholar]
- Evert J, Lawler E, Bogan H, Perls T, 2003. Morbidity profiles of centenarians: survivors, delayers, and escapers. J. Gerontol. A Biol. Sci. Med. Sci. 58, 232–237. 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L, 2015. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J. Am. Med. Dir. Assoc. 16, 640–647. 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Studenski S, 2012. Clinical problems of aging. Harrison’s principles of internal medicine 18, 570–585. [Google Scholar]
- Ferrucci L, Hesdorffer C, Bandinelli S, Simonsick EM, 2010. Frailty as a nexus between the biology of aging, environmental conditions and clinical geriatrics. Public Health Rev. 32, 475–488 doi: (not available). [Google Scholar]
- Ferrucci L, Levine ME, Kuo P-L, Simonsick EM, 2018. Time and the metrics of aging. Circ. Res. 123, 740–744. 10.1161/CIRCRESAHA.118.312816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori JL, Shin Y-K, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, Doyle ME, Pearson KJ, Mattison JA, de Cabo R, Egan JM, 2013. Resveratrol prevents beta-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes 62, 3500–3513. 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, 2015. Promoting health and longevity through diet: from model organisms to humans. Cell 161, 106–118. 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD, 2010. Extending healthy life span–from yeast to humans. Science 328, 321–326. 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Vinciguerra M, Longo VD, 2012. Growth factors, nutrient signaling, and cardiovascular aging. Circ. Res. 110, 1139–1150. 10.1161/CIRCRESAHA.111.246470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Rodriguez S, Rodriguez F, Folgueras AR, 2016. GDF11 administration does not extend lifespan in a mouse model of premature aging. Oncotarget 7, 55951–55956. 10.18632/oncotarget.11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF, Bruce B, Chakravarty E, 2011. Compression of morbidity 1980–2011: a focused review of paradigms and progress. J. Aging Res. 2011, 261702. 10.4061/2011/261702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A, Corbo L, Tang P, Bukata C, Ring N, Giacca M, Li X, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD, 2017. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 8, 422 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick MG, Kennedy BK, 2011. TOR on the brain. Exp. Gerontol. 46, 155–163. 10.1016/j.exger.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt M, Bower B, Garcia GG, Miller RA, 2017. Sex differences in lifespan extension with acarbose and 17-alpha estradiol: gonadal hormones underlie male-specific improvements in glucose tolerance and mTORC2 signaling. Aging Cell 16, 1256–1266. 10.1111/acel.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Gálvez J, 2013. Minocycline: far beyond an antibiotic. Br. J. Pharmacol. 169, 337–352. 10.1111/bph.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilcore D, Vanis L, Teng JC, Wishart JM, Buckley JD, Rayner CK, Horowitz M, Jones KL, 2011. The oligosaccharide alpha-cyclodextrin has modest effects to slow gastric emptying and modify the glycaemic response to sucrose in healthy older adults. Br. J. Nutr. 106, 583–587. 10.1017/S0007114511000444. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A, 2007. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci U S A 104, 5014–5019. 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wanders D, Stone KP, Van NT, Cortez CC, Gettys TW, 2014. A systems biology analysis of the unique and overlapping transcriptional responses to caloric restriction and dietary methionine restriction in rats. FASEB J. 28, 2577–2590. 10.1096/fj.14-249458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman TJ, Kovacs RJ, Stone NJ, Damalas D, Mullen JB, Oetgen WJ, 2016. The ASCVD risk estimator app: from concept to the current state. J. Am. Coll. Cardiol. 67, 350–352. 10.1016/j.jacc.2015.10.068. [DOI] [PubMed] [Google Scholar]
- Golan D, Staun-Ram E, Miller A, 2016. Shifting paradigms in multiple sclerosis: from disease-specific, through population-specific toward patient-specific. Curr. Opin. Neurol. 29, 354–361. 10.1097/WCO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- Goldberg AA, Richard VR, Kyryakov P, Bourque SD, Beach A, Burstein MT, Glebov A, Koupaki O, Boukh-Viner T, Gregg C, Juneau M, English AM, Thomas DY, Titorenko VI, 2010. Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes. Aging (Albany NY) 2, 393–414. 10.18632/aging.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA, 2013. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638. 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Gomez J, Lopez Torres M, Naudi A, Mota-Martorell N, Pamplona R, Barja G, 2015. Cysteine dietary supplementation reverses the decrease in mitochondrial ROS production at complex I induced by methionine restriction. J. Bioenerg. Biomembr. 47, 199–208. 10.1007/s10863-015-9608-x. [DOI] [PubMed] [Google Scholar]
- Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM, 2013. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 34, 1581–1588. 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Perez-Vega MI, Del Angel-Meza AR, Feria-Velasco A, 1998. Effect of tryptophan restriction on short-term memory. Physiol. Behav. 63, 165–169. 10.1016/s0031-9384(97)00395-8. [DOI] [PubMed] [Google Scholar]
- Grandison RC, Piper MDW, Partridge L, 2009. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064. 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Lees EK, Forney LA, Mody N, Gettys T, Brown PAJ, Wilson HM, Delibegovic M, 2016. Methionine restriction improves renal insulin signalling in aged kidneys. Mech. Ageing Dev. 157, 35–43. 10.1016/j.mad.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Guan Y, Wang S-R, Huang X-Z, Xie Q-H, Xu Y-Y, Shang D, Hao C-M, 2017. Nicotinamide mononucleotide, an NAD(+) precursor, rescues age-associated susceptibility to AKI in a sirtuin 1-Dependent manner. J. Am. Soc. Nephrol. 28, 2337–2352. 10.1681/ASN.2016040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KSY, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ, 2013. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 16, 1453–1460. 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- Gurau F, Baldoni S, Prattichizzo F, Espinosa E, Amenta F, Procopio AD, Albertini MC, Bonafe M, Olivieri F, 2018. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res. Rev. 46, 14–31. 10.1016/j.arr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- Gurpinar E, Grizzle WE, Piazza GA, 2014. NSAIDs inhibit tumorigenesis, but how? Clin. Cancer Res. 20, 1104–1113. 10.1158/1078-0432.CCR-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurt I, Artsi H, Cohen-Kfir E, Hamdani G, Ben-Shalom G, Feinstein B, El-Haj M, Dresner-Pollak R, 2015. The Sirt1 activators SRT2183 and SRT3025 inhibit RANKL-Induced osteoclastogenesis in bone marrow-derived macrophages and down-regulate Sirt3 in Sirt1 null cells. PLoS One 10, e0134391. 10.1371/journal.pone.0134391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare AA, Leng L, Gandavadi S, Du X, Cournia Z, Bucala R, Jorgensen WL, 2010. Optimization of N-benzyl-benzoxazol-2-ones as receptor antagonists of macrophage migration inhibitory factor (MIF). Bioorg. Med. Chem. Lett. 20, 5811–5814. 10.1016/j.bmcl.2010.07.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JM, Wilkinson JE, Miller RA, 2010. Macrophage migration inhibitory factor-knockout mice are long lived and respond to caloric restriction. FASEB J. 24, 2436–2442. 10.1096/fj.09-152223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill AH, 2016. Mouse population-based toxicology for personalized medicine and improved safety prediction In: Will Y, McDuffie JE, Olaharski AJ, Jeffy BD (Eds.), ‘Drug Discovery Toxicology: From Target Assessment to Translational Biomarkers. John Wiley & Sons, Inc., pp. 314–329. [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA, 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA, 2014. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell 13, 273–282. 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Alavez S, Astle CM, DiGiovanni J, Fernandez E, Flurkey K, Garratt M, Gelfond JAL, Javors MA, Levi M, Lithgow GJ, Macchiarini F, Nelson JF, Sukoff Rizzo SJ, Slaga TJ, Stearns T, Wilkinson JE, Miller RA, 2019. Acarbose improves health and lifespan in aging HET3 mice. Aging Cell 18, e12898. 10.1111/acel.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, 2000. The future of ageing. Nature 408, 267–269. 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- He C, Tsuchiyama SK, Nguyen QT, Plyusnina EN, Terrill SR, Sahibzada S, Patel B, Faulkner AR, Shaposhnikov MV, Tian R, Tsuchiya M, Kaeberlein M, Moskalev AA, Kennedy BK, Polymenis M, 2014. Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import. PLoS Genet. 10, e1004860. 10.1371/journal.pgen.1004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ, 2016. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell 15, 582–584. 10.1111/acel.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T, Rokutan K, Nikawa T, Kishi K, 1996. Geranylgeranylacetone induces heat shock proteins in cultured guinea pig gastric mucosal cells and rat gastric mucosa. Gastroenterology 111, 345–357. 10.1053/gast.1996.v111.pm8690199. [DOI] [PubMed] [Google Scholar]
- Horvath S, 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Suzuki K, Matsushima T, Yamakawa N, Suzuki T, Mizushima T, 2013. Suppression of alzheimer’s disease-related phenotypes by Geranylgeranylacetone in mice. PLoS One 8, e76306. 10.1371/journal.pone.0076306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, Zhang Y, Moritoh K, O’Connell JF, Baptiste BA, Stevnsner TV, Mattson MP, Bohr VA, 2018. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A 115, E1876–E1885. 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Kastin AJ, 2007. The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386. 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard BP, Sinclair DA, 2014. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 35, 146–154. 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybertson BM, McCord JM, 2017. WO2017041054A1/en#patentCitations. https://patents.google.com/patent/.
- Imai S-I, Guarente L, 2016. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2, 16017. 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispoglou T, White H, Preston T, McElhone S, McKenna J, Hind K, 2016. Double-blind, placebo-controlled pilot trial of L-Leucine-enriched amino-acid mixtures on body composition and physical performance in men and women aged 65–75 years. Eur. J. Clin. Nutr. 70, 182–188. 10.1038/ejcn.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong BY, Uddin MJ, Park JH, Lee JH, Lee HB, Miyata T, Ha H, 2016. Novel plasminogen activator Inhibitor-1 inhibitors prevent diabetic kidney injury in a mouse model. PLoS One 11, e0157012. 10.1371/journal.pone.0157012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zheng J, Peng Q, Hou Z, Zhang J, Mori S, Ellis JL, Vlasuk GP, Fries H, Suri V, Duan W, 2014. Sirtuin 1 activator SRT2104 protects Huntington’s disease mice. Ann. Clin. Transl. Neurol. 1, 1047–1052. 10.1002/acn3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, Longo DL, Belman JP, Malagon MM, Navas P, Sanghvi M, Moaddel R, Tilmont EM, Herbert RL, Morrell CH, Egan JM, Baur JA, Ferrucci L, Bogan JS, Bernier M, de Cabo R, 2013. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 18, 533–545. 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL, 2019. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563. 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S, 2014. Sodium glucose Co-Transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 5, 355–366. 10.1007/s13300-014-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S, Jacob JJ, Gupta Y, 2016. Newer antidiabetic drugs and calorie restriction mimicry. Indian J. Endocrinol. Metab. 20, 142–146. 10.4103/2230-8210.172242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP, 2001. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J. Biol. Chem. 276, 4588–4596. 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Lamming DW, 2016. The mechanistic target of rapamycin: the grand ConducTOR of metabolism and aging. Cell Metab. 23, 990–1003. 10.1016/j.cmet.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, 2016. Translating the science of aging into therapeutic interventions. Cold Spring Harb. Perspect. Med. 6, a025908. 10.1101/cshperspect.a025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD, 2017. The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 65, 2297–2301. 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani K, Osawa T, Yokozawa T, 2007. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology 8, 567–573. 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, Musci RV, Safairad OD, Linden MA, Biela LM, Bailey SM, Hamilton KL, Miller BF, 2019. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 18, e12880. 10.1111/acel.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SS, Canto C, 2015. The molecular targets of resveratrol. Biochim. Biophys. Acta 1852, 1114–1123. 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Kulkarni AS, Brutsaert EF, Anghel V, Zhang K, Bloomgarden N, Pollak M, Mar JC, Hawkins M, Crandall JP, Barzilai N, 2018. Metformin regulates metabolic and nonmetabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 17 10.1111/acel.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Dietrich N, Kornfeld K, 2016. Angiotensin converting enzyme (ACE) inhibitor extends Caenorhabditis elegans life span. PLoS Genet. 12, e1005866 doi: 0.1371/journal.pgen.1005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel SD, Suneja M, Ebert SM, Bongers KS, Fox DK, Malmberg SE, Alipour F, Shields RK, Adams CM, 2011. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 13, 627–638. 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel SD, Elmore CJ, Bongers KS, Ebert SM, Fox DK, Dyle MC, Bullard SA, Adams CM, 2012. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS One 7, e39332. 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Sabatini DM, 2013. A Central role for mTOR in lipid homeostasis. Cell Metab. 18, 465–469. 10.1016/j.cmet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Gioscia-Ryan RA, Hearon CMJ, Seals DR, 2013. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 134, 314–320. 10.1016/j.mad.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrasseur NK, Tchkonia T, Kirkland JL, 2015. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr. Inst. Workshop Ser. 83, 11–18. 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto S, Kokkonen GC, Barrows CH Jr., 1976. Dietary protein, life-span, and biochemical variables in female mice. J. Gerontol. 31, 144–148. 10.1093/geronj/31.2.144. [DOI] [PubMed] [Google Scholar]
- Li J, Kim SG, Blenis J, 2014. Rapamycin: one drug, many effects. Cell Metab. 19, 373–379. 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R-L, Lu Z-Y, Huang J-J, Qi J, Hu A, Su Z-X, Zhang L, Li Y, Shi Y-Q, Hao C-N, Duan J-L, 2016. SRT1720, a SIRT1 specific activator, protected H2O2-induced senescent endothelium. Am. J. Transl. Res. 8, 2876–2888 doi: (not available). [PMC free article] [PubMed] [Google Scholar]
- Libri V, Brown AP, Gambarota G, Haddad J, Shields GS, Dawes H, Pinato DJ, Hoffman E, Elliot PJ, Vlasuk GP, Jacobson E, Wilkins MR, Matthews PM, 2012. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One 7, e51395. 10.1371/journal.pone.0051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Hirsch J, Katz MJ, Wang C, Sanders AE, Verghese J, Barzilai N, Derby CA, 2010. Exceptional parental longevity associated with lower risk of Alzheimer’s disease and memory decline. J. Am. Geriatr. Soc. 58, 1043–1049. 10.1111/j.1532-5415.2010.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Mattson MP, 2008. Preventing NAD(+) depletion protects neurons against excitotoxicity: bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. Ann. N. Y. Acad. Sci. 1147, 275–282. 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang W, Wang K, Wang X, Yin F, Li C, Wang C, Zhao B, Zhong C, Zhang J, Peng F, Bi Y, Shen C, Hou X, Zhang D, Liu Y, Ai J, Zhao S, 2015. Methionine and cystine double deprivation stress suppresses glioma proliferation via inducing ROS/autophagy. Toxicol. Lett. 232, 349–355. 10.1016/j.toxlet.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu D, Zeng Z, Zhu W, Zhang N, Yu H, Chen H, Wang K, Wang Y, Wang L, Zhao J, Zhang L, Wu R, Hu X, Wang J, 2017. SRT1720 promotes survival of aged human mesenchymal stem cells via FAIM: a pharmacological strategy to improve stem cell-based therapy for rat myocardial infarction. Cell Death Dis. 8, e2731. 10.1038/cddis.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim M-J, Serwold T, Wagers AJ, Lee RT, 2013. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839. 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L, 2015. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell 14, 497–510. 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Galluzzi L, Freije JMP, Madeo F, Kroemer G, 2016. Metabolic control of longevity. Cell 166, 802–821. 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- Ma Z, Zhang Y, Li Q, Xu M, Bai J, Wu S, 2017. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1alpha expression and mitochondrial ROS production. PLoS One 12, e0183426. 10.1371/journal.pone.0183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Pietrocola F, Eisenberg T, Kroemer G, 2014. Caloric restriction mimetics: towards a molecular definition. Nat. Rev. Drug Discov. 13, 727–740. 10.1038/nrd4391. [DOI] [PubMed] [Google Scholar]
- Madeo F, Carmona-Gutierrez D, Kepp O, Kroemer G, 2018a. Spermidine delays aging in humans. Aging (Albany NY) 10, 2209–2211. 10.18632/aging.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Pietrocola F, Kroemer G, 2018b. Spermidine in health and disease. Science 359 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez J-P, Lee H-Y, Cline GW, Samuel VT, Kibbey RG, Shulman GI, 2014. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546. 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magner DB, Wollam J, Shen Y, Hoppe C, Li D, Latza C, Rottiers V, Hutter H, Antebi A, 2013. The NHR-8 nuclear receptor regulates cholesterol and bile acid homeostasis in C. Elegans. Cell Metab. 18, 212–224. 10.1016/j.cmet.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magwere T, West M, Riyahi K, Murphy MP, Smith RAJ, Partridge L, 2006. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 127, 356–370. 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Malin SK, Gerber R, Chipkin SR, Braun B, 2012. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 35, 131–136. 10.2337/dc11-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J, Urban N, Priebe S, Groth M, Frahm C, Hartmann N, Gebauer J, Ravichandran M, Dommaschk A, Schmeisser S, Kuhlow D, Monajembashi S, Bremer-Streck S, Hemmerich P, Kiehntopf M, Zamboni N, Englert C, Guthke R, Kaleta C, Platzer M, Sühnel J, Witte OW, Zarse K, Ristow M, 2015. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun. 6, 10043. 10.1038/ncomms10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR, 2018. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat. Commun. 9, 1286 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Carvajal AJ, Sola I, Lathyris D, Dayer M, 2017. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 8 10.1002/14651858.CD006612.pub5. CD006612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, de Cabo R, 2013. Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid. Redox Signal. 19, 310–320. 10.1089/ars.2012.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin M-J, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R, 2013. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R, 2012. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489, 318–321. 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park S-S, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R, 2014. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 20, 183–190. 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM, 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063. 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S, Polyak E, Ostrovsky J, Dingley SD, Rao M, Kwon YJ, Xiao R, Zhang Z, Nakamaru-Ogiso E, Falk MJ, 2015. Pharmacologic targeting of sirtuin and PPAR signaling improves longevity and mitochondrial physiology in respiratory chain complex I mutant Caenorhabditis elegans. Mitochondrion 22, 45–59. 10.1016/j.mito.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Leeuwenburgh C, Guralnik JM, Tian L, Sufit R, Zhao L, Criqui MH, Kibbe MR, Stein JH, Lloyd-Jones D, Anton SD, Polonsky TS, Gao Y, de Cabo R, Ferrucci L, 2017. Effect of resveratrol on walking performance in older people with peripheral artery disease: the RESTORE randomized clinical trial. JAMA Cardiol. 2, 902–907. 10.1001/jamacardio.2017.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIsaac RS, Lewis KN, Gibney PA, Buffenstein R, 2016. From yeast to human: exploring the comparative biology of methionine restriction in extending eukaryotic life span. Ann. N. Y. Acad. Sci. 1363, 155–170. 10.1111/nyas.13032. [DOI] [PubMed] [Google Scholar]
- Melis JPM, Jonker MJ, Vijg J, Hoeijmakers JHJ, Breit TM, van Steeg H, 2013. Aging on a different scale–chronological versus pathology-related aging. Aging (Albany NY) 5, 782–788. 10.18632/aging.100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AR, Larrick JW, 2012. Fibroblast growth factor-21 is a promising dietary restriction mimetic. Rejuvenation Res. 15, 624–628. 10.1089/rej.2012.1392. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R, 2012. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 11, 390–398. 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R, 2014. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 13, 787–796. 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, Bickford PC, 2004. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol. Aging 25, 315–324. 10.1016/S0197-4580(03)00116-7. [DOI] [PubMed] [Google Scholar]
- Meyskens FLJJ, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ, Kidao J, McCracken J, Albers CG, Ahnen DJ, Turgeon DK, Goldschmid S, Lance P, Hagedorn CH, Gillen DL, Gerner EW, 2008. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. Phila. (Phila) 1, 32–38. 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel JP, Dreux C, Vacheron A, 2016. Healthy ageing: evidence that improvement is possible at every age. Eur. Geriatr. Med. 7, 298–305 doi: (not available). [Google Scholar]
- Milani C, Ferrario C, Turroni F, Duranti S, Mangifesta M, van Sinderen D, Ventura M, 2016. The human gut microbiota and its interactive connections to diet. J. Hum. Nutr. Diet. 29, 539–546. 10.1111/jhn.12371. [DOI] [PubMed] [Google Scholar]
- Miller DL, Roth MB, 2007. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A 104, 20618–20622. 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Strong R, 2007. An aging interventions testing program: study design and interim report. Aging Cell 6, 565–575. 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R, 2011. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 66, 191–201. 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L, Strong R, 2014. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477. 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Pharaoh GA, Hamilton KL, Peelor FF, Kirkland JL, Freeman WM, Mann SN, Kinter M, Price JC, Stout MB, 2019. Short-term calorie restriction and 17α-estradiol administration elicit divergent effects on proteostatic processes and protein content in metabolically active tissues. J. Gerontol. A Biol. Sci. Med. Sci. 10.1093/gerona/glz113. [ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minois N, 2014. Molecular basis of the “anti-aging” effect of spermidine and other natural polyamines - a mini-review. Gerontology 60, 319–326. 10.1159/000356748. [DOI] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin Y-K, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R, 2011. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 1, 70 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel E, Cassina A, Martínez-Palma L, Souza JM, Bolatto C, Rodríguez-Bottero S, Logan A, Smith RAJ, Murphy MP, Barbeito L, Radi R, Cassina P, 2014. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 70, 204–213. 10.1016/j.freeradbiomed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Mirzaei H, Suarez JA, Longo VD, 2014. Protein and amino acid restriction, aging and disease: from yeast to humans. Trends Endocrinol. Metab. 25, 558–566. 10.1016/j.tem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R, 2014. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 6, 836–843. 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, Gonzalez-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Buron MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R, 2016. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 23, 1093–1112. 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Bernier M, Aon MA, Cortassa S, Kim EY, Fang EF, Palacios HH, Ali A, Navas-Enamorado I, Di Francesco A, Kaiser TA, Waltz TB, Zhang N, Ellis JL, Elliott PJ, Frederick DW, Bohr VA, Schmidt MS, Brenner C, Sinclair DA, Sauve AA, Baur JA, de Cabo R, 2018. Nicotinamide improves aspects of Healthspan, but not lifespan, in mice. Cell Metab. 27, 667–676. 10.1016/j.cmet.2018.02.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi Y, Hatakeyama H, Sueoka K, Tanaka M, Goto Y-I, 2017. Low dose resveratrol ameliorates mitochondrial respiratory dysfunction and enhances cellular reprogramming. Mitochondrion 34, 43–48. 10.1016/j.mito.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Mora M, Medina-Leendertz SJ, Bonilla E, Terán RE, Paz MC, Arcaya JL, 2013. Minocycline, but not ascorbic acid, increases motor activity and extends the life span of Drosophila melanogaster. Invest. Clin. 54, 161–170 doi: (not available). [PubMed] [Google Scholar]
- Morselli E, Galluzzi L, Kepp O, Criollo A, Maiuri MC, Tavernarakis N, Madeo F, Kroemer G, 2009. Autophagy mediates pharmacological lifespan extension by spermidine and resveratrol. Aging (Albany NY) 1, 961–970. 10.18632/aging.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo Y-S, Viswanathan M, Schoonjans K, Guarente L, Auwerx J, 2013. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441. 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, Graff RE, Holst K, Möller S, Unger RH, McIntosh C, Nuttall E, Brandt I, Penney KL, Hartman M, Kraft P, Parmigiani G, Christensen K, Koskenvuo M, Holm NV, Heikkilä K, Pukkala E, Shytthe A, Adami HO, Kaprio J, 2016. Nordic Twin Study of Cancer (NorTwinCan) Collaboration, 2016. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 315, 68–76. 10.1001/jama.2015.17703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murabito JM, Yuan R, Lunetta KL, 2012. The search for longevity and healthy aging genes: insights from epidemiological studies and samples of long-lived individuals. J. Gerontol. A Biol. Sci. Med. Sci. 67, 470–479. 10.1093/gerona/gls089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento E, Guzman-Quevedo O, Delacourt N, da Silva Aragao R, Perez-Garcia G, de Souza SL, Manhaes-de-Castro R, Bolanos-Jimenez F, Kaeffer B, 2013. Long-lasting effect of perinatal exposure to L-tryptophan on circadian clock of primary cell lines established from male offspring born from mothers fed on dietary protein restriction. PLoS One 8, e56231. 10.1371/journal.pone.0056231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, Verdin E, 2017. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26, 547–557. 10.1016/j.cmet.2017.08.004. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B, 2014. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 71, 390–401. 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Ng LT, Gruber J, Moore PK, 2018. Is there a role of H2S in mediating health span benefits of caloric restriction? Biochem. Pharmacol. 149, 91–100. 10.1016/j.bcp.2018.01.030. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Chen H, Mak C, Zaky A, Pollock C, Saad S, 2018. SRT1720 attenuates obesity and insulin resistance but not liver damage in the offspring due to maternal and postnatal high-fat diet consumption. Am. J. Physiol. Endocrinol. Metab. 315, E196–E203. 10.1152/ajpendo.00472.2017. [DOI] [PubMed] [Google Scholar]
- Nies VJM, Sancar G, Liu W, van Zutphen T, Struik D, Yu RT, Atkins AR, Evans RM, Jonker JW, Downes MR, 2016. Fibroblast growth factor signaling in metabolic regulation. Front. Endocrinol. (Lausanne) 6, 193 10.3389/fendo.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH, 2012. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol. Rev. 92, 1479–1514. 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh RM, Venkatasubramanian S, Daga S, Langrish J, Mills NL, Lang NN, Hoffmann E, Waterhouse B, Newby DE, Frier BM, 2017. Cardiometabolic effects of a novel SIRT1 activator, SRT2104, in people with type 2 diabetes mellitus. Open Heart 4, e000647. 10.1136/openhrt-2017-000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Martin-Montalvo A, Dluzen DF, Zhang Y, Bernier M, Zonderman AB, Becker KG, Gorospe M, de Cabo R, Evans MK, 2016. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 15, 572–581. 10.1111/acel.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton LE, Layman DK, 2006. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 136, 533s–537s. 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- Novelle MG, Wahl D, Dieguez C, Bernier M, de Cabo R, 2015. Resveratrol supplementation: Where are we now and where should we go? Ageing Res. Rev. 21, 1–15. 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelle MG, Ali A, Dieguez C, Bernier M, de Cabo R, 2016. Metformin: a hopeful promise in aging research. Cold Spring Harb. Perspect. Med. 6, a025932. 10.1101/cshperspect.a025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F, Miura M, 2015. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat. Commun. 6, 8332 10.1038/ncomms9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsu A, Shibutani Y, Seno K, Iwata H, Kuwayama T, Shirasuna K, 2017. Advanced glycation end products and lipopolysaccharides stimulate interleukin-6 secretion via the RAGE/TLR4-NF-kappaB-ROS pathways and resveratrol attenuates these inflammatory responses in mouse macrophages. Exp. Ther. Med. 14, 4363–4370. 10.3892/etm.2017.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Martin GM, Kirkland JL, 2016. Aging: The Longevity Dividend, ed. Cold Spring Harbor Laboratory Press, New York. 10.1101/cshperspect.a025932. [DOI] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA, 1993. Low methionine ingestion by rats extends life span. J. Nutr. 123, 269–274. 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Orho-Melander M, 2015. Genetics of coronary heart disease: towards causal mechanisms, novel drug targets and more personalized prevention. J. Intern. Med. 278, 433–446. 10.1111/joim.12407. [DOI] [PubMed] [Google Scholar]
- Ortuño J, Covas M-I, Farre M, Pujadas M, Fito M, Khymenets O, Andres-Lacueva C, Roset P, Joglar J, Lamuela-Raventós RM, Torre R, 2010. Matrix effects on the bioavailability of resveratrol in humans. Food Chem. 120, 1123–1130. 10.1016/j.foodchem.2009.11.032. [DOI] [Google Scholar]
- Oudman I, Clark JF, Brewster LM, 2013. The effect of the creatine analogue beta-guanidinopropionic acid on energy metabolism: a systematic review. PLoS One 8, e52879. 10.1371/journal.pone.0052879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G, 2013. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 48, 294–301. 10.1007/s12035-013-8497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenkrug G, Navrotskaya V, Vorobyova L, Summergrad P, 2012. Minocycline effect on life and health span of Drosophila melanogaster. Aging Dis. 3, 352–359 doi: (not available). [PMC free article] [PubMed] [Google Scholar]
- Pajk M, Cselko A, Varga C, Posa A, Tokodi M, Boldogh I, Goto S, Radak Z, 2017. Exogenous nicotinamide supplementation and moderate physical exercise can attenuate the aging process in skeletal muscle of rats. Biogerontology 18, 593–600. 10.1007/s10522-017-9705-9. [DOI] [PubMed] [Google Scholar]
- Pallauf K, Rimbach G, Rupp PM, Chin D, Wolf IMA, 2016. Resveratrol and lifespan in model organisms. Curr. Med. Chem. 23, 4639–4680. 10.2174/0929867323666161024151233. [DOI] [PubMed] [Google Scholar]
- Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang-Verzosa G, Johnson KO, Cubro H, Doornebal EJ, Ogrodnik M, Jurk D, Jensen MD, Chini EN, Miller JD, Matveyenko A, Stout MB, Schafer MJ, White TA, Hickson LJ, Demaria M, Garovic V, Grande J, Arriaga EA, Kuipers F, Zglinicki von T, LeBrasseur NK, Campisi J, Tchkonia T, Kirkland JL, 2019. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18, e12950. 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Finkel T, 2017. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 292, 6452–6460. 10.1074/jbc.R116.771915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-J, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH, 2012. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433. 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R, 2008. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168. 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao L, Jung I, Huh JY, Miyata T, Ha H, 2016. A novel plasminogen activator inhibitor-1 inhibitor, TM5441, protects against high-fat diet-induced obesity and adipocyte injury in mice. Br. J. Pharmacol. 173, 2622–2632. https://doi.org/101111/bph.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhemus DJ, Li Z, Pattillo CB, Gojon GS, Gojon GJ, Giordano T, Krum H, 2015. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 33, 216–226. 10.1111/1755-5922.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack RM, Barzilai N, Anghel V, Kulkarni AS, Golden A, O’Broin P, Sinclair DA, Bonkowski MS, Coleville AJ, Powell D, Kim S, Moaddel R, Stein D, Zhang K, Hawkins M, Crandall JP, 2017. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1703–1709. 10.1093/gerona/glx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols TWH, Noriega LG, Nomura M, Auwerx J, Schoonjans K, 2011. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 54, 1263–1272. 10.1016/j.jhep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YDM, Hirsch ML, Cote AM, Lainez EO, Johnson MO, Gagne DJ, Vlasuk GP, Ellis JL, 2010. Activation of Sirtuin1 (SIRT1) by the Novel Small Molecule SRT2104 Promotes Body Weight Loss, Increases Exercise Capacity and Improves Insulin Sensitivity in Diet-induced Obese Mice. ADA, 70th Scientific Sessions, pp. 390 poster. [Google Scholar]
- Rajman L, Chwalek K, Sinclair DA, 2018. Therapeutic potential of NAD-Boosting molecules: the in vivo evidence. Cell Metab. 27, 529–547. 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan S, Halagowder D, Sivasithambaram ND, 2015. Quercetin suppresses twist to induce apoptosis in MCF-7 breast Cancer cells. PLoS One 10, e0141370. 10.1371/journal.pone.0141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB, 2015. A 2-Year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1097–1104. 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder PC, Flurkey K, Te A, Harrison DE, 2016. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY) 8, 3120–3130. 10.18632/aging.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder PC, Ryzhov S, Flurkey K, Anunciado-Koza RP, Mills I, Harrison DE, Koza RA, 2018. Cardioprotective effects of dietary rapamycin on adult female C57BLKS/J-Lepr(db) mice. Ann. N. Y. Acad. Sci. 1418, 106–117. 10.1111/nyas.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Du C, Shi Y, Wei J, Wu H, Cui H, 2017. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int. J. Mol. Med. 39, 1317–1324. 10.3892/ijmm.2017.2931. [DOI] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu Z-X, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI, 2007. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 5, 151–156. 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza W, Veronese N, Fontana L, 2014. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res. Rev. 13, 38–45. 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Passeri D, De Franco F, Ciaccioli G, Donadio L, Rizzo G, Orlandi S, Sadeghpour B, Wang XX, Jiang T, Levi M, Pruzanski M, Adorini L, 2010. Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol. Pharmacol. 78, 617–630. 10.1124/mol.110.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, McMackin MZ, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA, 2017. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 26, 539–546. 10.1016/j.cmet.2017.08.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK, 2012. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15, 713–724. 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A, 2011. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin. Geriatr. Med. 27, 17–26. 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD, 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977. 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozan P, Nejdi A, Hidalgo S, Bisson J-F, Desor D, Messaoudi M, 2008. Effects of lifelong intervention with an oligofructose-enriched inulin in rats on general health and lifespan. Br. J. Nutr. 100, 1192–1199. 10.1017/S0007114508975607. [DOI] [PubMed] [Google Scholar]
- Russell DW, 2009. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. (50 Suppl), S120–S125. 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli F, Formoso G, Sbraccia P, Averna M, Miccoli R, Di Fulvio P, Ganci A, Pulizzi N, Lattanzio S, Ciabattoni G, Consoli A, Lauro R, Patrono C, Davi G, 2010. Postprandial hyperglycemia is a determinant of platelet activation in early type 2 diabetes mellitus. J. Thromb. Haemost. 8, 828–837. 10.1111/j.1538-7836.2010.03742.x. [DOI] [PubMed] [Google Scholar]
- Santos RS, de Fatima LA, Frank AP, Carneiro EM, Clegg DJ, 2017. The effects of 17 alpha-estradiol to inhibit inflammation in vitro. Biol. Sex Differ. 8, 30 10.1186/s13293-017-0151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, Sabatini DM, 2017. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371. 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- Schafer MJ, Atkinson EJ, Vanderboom PM, Kotajarvi B, White TA, Moore MM, Bruce CJ, Greason KL, Suri RM, Khosla S, Miller JD, Bergen HR, LeBrasseur NK, 2016. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 23, 1207–1215. 10.1016/j.cmet.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, 2016. Precision medicine: The future in diabetes care? Diabetes Res. Clin. Pract. 117, 12–21. 10.1016/j.diabres.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Schwager J, Richard N, Widmer F, Raederstorff D, 2017. Resveratrol distinctively modulates the inflammatory profiles of immune and endothelial cells. BMC Complement. Altern. Med. 17, 309 10.1186/s12906-017-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Stekovic S, Wirth M, Benson G, Royer P, Sigrist SJ, Pieber T, Dammbrueck C, Magnes C, Eisenberg T, Pendl T, Bohlken J, Kobe T, Madeo F, Floel A, 2018. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY) 10, 19–33. 10.18632/aging.101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, 1996. Amyloid β-protein and the genetics of Alzheimer’s disease. J. Biol. Chem. 271, 18295–18298. [DOI] [PubMed] [Google Scholar]
- Semba RD, Ferrucci L, Bartali B, Urpi-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, Andres-Lacueva C, 2014. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern. Med. 174, 1077–1084. 10.1001/jamainternmed.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM, 2010. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468, 1100–1104. 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Serfaty CA, Oliveira-Silva P, Faria Melibeu ADC, Campello-Costa P, 2008. Nutritional tryptophan restriction and the role of serotonin in development and plasticity of central visual connections. Neuroimmunomodulation 15, 170–175. 10.1159/000153421. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, Zhu J, Wang S, Zhou W, Morin B, Egerman MA, Fan L, Wang Y, Iartchouk O, Meyer A, Valdez RA, Mannick JB, Klickstein LB, Glass DJ, 2018. Short-term low-dose mTORC1 inhibition in aged rats counter-regulates age-related gene changes and blocks age-related kidney pathology. J. Gerontol. A Biol. Sci. Med. Sci. 73, 845–852. 10.1093/gerona/glx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, Buchanan S, Selman C, Stenvinkel P, 2019. Allostatic load and ageing: linking the microbiome and nutrition with age-related health. Biochem. Soc. Trans. 47, 1165–1172. 10.1042/BST20190110. [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG, 2009. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol. Pharmacol. 76, 884–895. 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Shehzad A, Omar M, Rakha A, Raza H, Sharif HR, Shakeel A, Ansari A, Niazi S, 2016. Inulin: properties, health benefits and food applications. Carbohydr. Polym. 147, 444–454. 10.1016/j.carbpol.2016.04.020. [DOI] [PubMed] [Google Scholar]
- Sidler C, Woycicki R, Kovalchuk I, Kovalchuk O, 2014. WI-38 senescence is associated with global and site-specific hypomethylation. Aging (Albany NY) 6, 564–574. 10.18632/aging.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist SJ, Carmona-Gutierrez D, Gupta VK, Bhukel A, Mertel S, Eisenberg T, Madeo F, 2014. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy 10, 178–179. 10.4161/auto.26918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L, 2014. Small-molecule allosteric activators of sirtuins. Annu. Rev. Pharmacol. Toxicol. 54, 363–380. 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Cuervo AM, 2011. Autophagy in the cellular energetic balance. Cell Metab. 13, 495–504. 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim M-J, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ, 2014. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344, 649–652. 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C, Foley A, Partridge L, 2012. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS One 7, e47699. 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR, 2015. GDF11 does not rescue aging-related pathological hypertrophy. Circ. Res. 117, 926–932. 10.1161/CIRCRESAHA.115.307527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K, Kano Y, Chiba F, Koizumi K, Miyaki Y, 2013. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS One 8, e64357. 10.1371/journal.pone.0064357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S, Botbol Y, Macian F, Cuervo AM, 2012. Autophagy and disease: always two sides to a problem. J. Pathol. 226, 255–273. 10.1002/path.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Steyn FJ, Jurczak MJ, Camporez J-PG, Zhu Y, Hawse JR, Jurk D, Palmer AK, Xu M, Pirtskhalava T, Evans GL, de Souza Santos R, Frank AP, White TA, Monroe DG, Singh RJ, Casaclang-Verzosa G, Miller JD, Clegg DJ, LeBrasseur NK, Zglinicki von T, Shulman GI, Tchkonia T, Kirkland JL, 2017. 17alpha-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J. Gerontol. A Biol. Sci. Med. Sci. 72, 3–15. 10.1093/gerona/glv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE, 2008. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell 7, 641–650. 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE, 2013. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 68, 6–16. 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhaes JP, Martinez PA, McCord JM, Miller BF, Muller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE, 2016. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an alpha-glucosidase inhibitor or a Nrf2-inducer. Aging Cell 15, 872–884. 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K, Kushima Y, Muramatsu K, 1987. Effect of dietary glycine on methionine metabolism in rats fed a high-methionine diet. J. Nutr. Sci. Vitaminol. 33, 195–205. 10.3177/jnsv.33.195. [DOI] [PubMed] [Google Scholar]
- Tacutu R, Budovsky A, Yanai H, Fraifeld VE, 2011. Molecular links between cellular senescence, longevity and age-related diseases - a systems biology perspective. Aging (Albany NY) 3, 1178–1191. 10.18632/aging.100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Toth P, Yabluchanskiy A, Tucsek Z, Kiss T, Hertelendy P, Kinter M, Ballabh P, Sule Z, Farkas E, Baur JA, Sinclair DA, Csiszar A, Ungvari Z, 2019. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192. 10.1016/j.redox.2019.101192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Dog Aging Project. http://dogagingproject.com.
- Tolcher AW, LoRusso P, Arzt J, Busman TA, Lian G, Rudersdorf NS, Vanderwal CA, Waring JF, Yang J, Holen KD, Rosen LS, 2015. Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with irinotecan: results of an open-label, phase 1 study. Cancer Chemother. Pharmacol. 76, 1041–1049. 10.1016/j.redox.2019.101192. [DOI] [PubMed] [Google Scholar]
- Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M, 2010. Bile acids as regulators of hepatic lipid and glucose metabolism. Dig. Dis. 28, 220–224. 10.1159/000282091. [DOI] [PubMed] [Google Scholar]
- Tsai S, Sitzmann JM, Dastidar SG, Rodriguez AA, Vu SL, McDonald CE, Academia EC, O’Leary MN, Ashe TD, La Spada AR, Kennedy BK, 2015. Muscle-specific 4E-BP1 signaling activation improves metabolic parameters during aging and obesity. J. Clin. Invest. 125, 2952–2964. 10.1172/JCI77361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukaj S, Węgrzyn G, 2016. Anti-Hsp90 therapy in autoimmune and inflammatory diseases: a review of preclinical studies. Cell Stress Chaperones 21, 213–218. 10.1007/s12192-016-0670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy KM, 2007. Inulin-type fructans in healthy aging. J. Nutr. 137, 2590S–2593S. 10.1093/jn/137.11.2590S. [DOI] [PubMed] [Google Scholar]
- Uddin GM, Youngson NA, Doyle BM, Sinclair DA, Morris MJ, 2017. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Sci. Rep. 7, 15063. 10.1038/s41598-017-14866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, Ragusa S, Stefanidis E, Ryu D, Rainer PY, Nikitin G, Giger S, Li TY, Semilietof A, Oggier A, Yersin Y, Tauzin L, Pirinen E, Cheng W-C, Ratajczak J, Canto C, Ehrbar M, Sizzano F, Petrova TV, Vanhecke D, Zhang L, Romero P, Nahimana A, Cherix S, Duchosal MA, Ho P-C, Deplancke B, Coukos G, Auwerx J, Lutolf MP, Naveiras O, 2019. The NAD-Booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell 24, 405–418. 10.1016/j.stem.2019.02.012. e7. [DOI] [PubMed] [Google Scholar]
- Vargas AJ, Harris CC, 2016. Biomarker development in the precision medicine era: lung cancer as a case study. Nat. Rev. Cancer 16, 525–537. 10.1038/nrc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayndorf EM, Lee SS, Liu RH, 2013. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J. Funct. Foods 5, 1236–1243. 10.1016/j.jff.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F, 2003. Genetics: influence of TOR kinase on lifespan in C. Elegans. Nature 426, 620 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Verma S, Goyal S, Jamal S, Singh A, Grover A, 2016. Hsp90: friends, clients and natural foes. Biochimie 127, 227–240. 10.1016/j.biochi.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T, 2014. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663. 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Arning E, Bottiglieri T, Gibson KM, 2017. Multicompartment analysis of protein-restricted phenylketonuric mice reveals amino acid imbalances in brain. J. Inherit. Metab. Dis. 40, 227–235. 10.1007/s10545-016-9984-3. [DOI] [PubMed] [Google Scholar]
- Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD, 2019. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945. 10.1111/acel.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JEJ, Walle UK, 2004. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 32, 1377–1382. 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- Wanders D, Burk DH, Cortez CC, Van NT, Stone KP, Baker M, Mendoza T, Mynatt RL, Gettys TW, 2015. UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J. 29, 2603–2615. 10.1096/fj.14-270348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders D, Stone KP, Forney LA, Cortez CC, Dille KN, Simon J, Xu M, Hotard EC, Nikonorova IA, Pettit AP, Anthony TG, Gettys TW, 2016. Role of GCN2-Independent signaling through a noncanonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes 65, 1499–1510. 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun H, Song G, Yang Y, Zou X, Han P, Li S, 2018a. Resveratrol improves muscle atrophy by modulating mitochondrial quality control in STZ-Induced diabetic mice. Mol. Nutr. Food Res. 62, e1700941. 10.1002/mnfr.201700941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Jiang T, Li W, Gao N, Zhang T, 2018b. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 282, 100–108. 10.1016/j.toxlet.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Warner HR, 2015. NIA’s Intervention Testing Program at 10 years of age. Age Dordr. (Dordr) 37, 22 10.1007/s11357-015-9761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Morimoto K, Houten SM, Kaneko-Iwasaki N, Sugizaki T, Horai Y, Mataki C, Sato H, Murahashi K, Arita E, Schoonjans K, Suzuki T, Itoh H, Auwerx J, 2012. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS One 7, e38286. 10.1371/journal.pone.0038286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser JN, Roche AM, Hergott CB, LaRose MI, Connolly T, Jorgensen WL, Leng L, Bucala R, Das R, 2015. Macrophage migration inhibitory factor is detrimental in pneumococcal pneumonia and a target for therapeutic immunomodulation. J. Infect. Dis. 212, 1677–1682. 10.1093/infdis/jiv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan C-C, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA, 2012. Rapamycin slows aging in mice. Aging Cell 11, 675–682 doi:0.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, Houten SM, Amariuta T, Wolski W, Zamboni N, Aebersold R, Auwerx J, 2016. Systems proteomics of liver mitochondria function. Science 352 10.1126/science.aad0189. aad0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2015. World report on ageing and health. ISBN 978 (92), 4 156504 2. [Google Scholar]
- Wu H, Wu J, Zhou S, Huang W, Li Y, Zhang H, Wang J, Jia Y, 2018. SRT2104 attenuates diabetes-induced aortic endothelial dysfunction via inhibition of P53. J. Endocrinol. 237, 1–14. 10.1530/JOE-17-0672. [DOI] [PubMed] [Google Scholar]
- Xia L, Wang XX, Hu XS, Guo XG, Shang YP, Chen HJ, Zeng CL, Zhang FR, Chen JZ, 2008. Resveratrol reduces endothelial progenitor cells senescence through augmentation of telomerase activity by Akt-dependent mechanisms. Br. J. Pharmacol. 155, 387–394. 10.1038/bjp.2008.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Leung PS, 2017. Fibroblast growth factor 21: a regulator of metabolic disease and health span. Am. J. Physiol. Endocrinol. Metab. 313, E292–E302. 10.1152/ajpendo.00101.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, 2018. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256. 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Otsuki M, 2006. Effect of inhibition of alpha-glucosidase on age-related glucose intolerance and pancreatic atrophy in rats. Metabolism 55, 533–540. 10.1016/j.metabol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Yan J, Wang J, Huang H, Huang Y, Mi T, Zhang C, Zhang L, 2017. Fibroblast growth factor 21 delayed endothelial replicative senescence and protected cells from H2O2-induced premature senescence through SIRT1. Am. J. Transl. Res. 9, 4492–4501 doi: (not available). [PMC free article] [PubMed] [Google Scholar]
- Yang S, Long L-H, Li D, Zhang J-K, Jin S, Wang F, Chen J-G, 2015. β-Guanidinopropionic acid extends the lifespan of Drosophila melanogaster via an AMP-activated protein kinase-dependent increase in autophagy. Aging Cell 14, 1024–1033. 10.1111/acel.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Wong M, Apte RS, Imai S-I, 2019. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 30, 329–342. 10.1016/j.cmet.2019.05.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Liu X, Li X, Yuan Z, Yang H, Zhang L, Jiang Z, 2016. Protective effects of SRT1720 via the HNF1alpha/FXR signalling pathway and anti-inflammatory mechanisms in mice with estrogen-induced cholestatic liver injury. Toxicol. Lett. 264, 1–11. 10.1016/j.toxlet.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Zapata RC, Singh A, Ajdari NM, Chelikani PK, 2018. Dietary tryptophan restriction dose-dependently modulates energy balance Gut Hormones, and Microbiota in Obesity-Prone Rats. Obesity 26 Silver Spring, pp. 730–739. 10.1002/oby.22136. [DOI] [PubMed] [Google Scholar]
- Zenin A, Tsepilov Y, Sharapov S, Getmantsev E, Menshikov LI, Fedichev PO, Aulchenko Y, 2019. Identification of 12 genetic loci associated with human healthspan. Commun. Biol. 2 (1), 41 10.1038/s42003-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ, 2012. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1, e00065. 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q-S, Deater M, Schubert K, Marquez-Loza L, Pelz C, Sinclair DA, Grompe M, 2015. The Sirt1 activator SRT3025 expands hematopoietic stem and progenitor cells and improves hematopoiesis in Fanconi anemia mice. Stem Cell Res. 15, 130–140. 10.1016/j.scr.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP, 2019. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22, 719–728. 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Jiang Z, Harris EC, Reeves J, Chen X, Pazdro R, 2016. Circulating concentrations of growth differentiation factor 11 are heritable and correlate with life span. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1560–1563. 10.1093/gerona/glv308. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL, 2017. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 9, 955–963. 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.