Abstract
The addition of lactoferrin and three unsaturated fatty acids, oleic acid, docosahexaenoic acid (DHA), and linolenic acid, to dairy products was approved in recent years. Research into the biological activities of lactoferrin and these three unsaturated fatty acids has revealed anti-inflammatory, antiviral, antioxidant, antitumor, antiparasitic, and antibiotic effects. However, investigations and comparisons of lactoferrin + oleic acid/DHA/linolenic acid combinations in an esophageal cancer cell model and in xenograft tumor models have not been extensively reported, and the related mechanism of these combinations remains elusive. In the present study, the effects of lactoferrin and the three fatty acids on KYSE450 cell viability, migration, and invasion were investigated to choose the proper doses and effective combination in vitro. A tumor-bearing nude mouse model was established to investigate the role of selected combinations in inhibiting esophageal tumor formation in vivo. Metabonomics detection and data analysis were performed to screen special metabolites and related pathways, which were validated by western blotting. The results demonstrated that lactoferrin, the three unsaturated fatty acids, and their combinations inhibited the viability, migration, and invasion of KYSE450 cells and induced apoptosis and the lactoferrin + linolenic acid combination exhibited the strongest activity in suppressing KYSE450 tumor formation in vivo. The lactoferrin + linolenic acid combination inhibited phosphorylation in the JAK2/STAT3-related pathway by downregulating the special metabolite lithocholyltaurine, thereby suppressing formation of KYSE450 tumors.
1. Introduction
As one of the ten most common causes of cancer death worldwide, esophageal cancer is a type of gastrointestinal cancer, and statistical data show that over 3,000,000 patients die from esophageal cancer every year.1 There are two main histological types, adenocarcinoma and squamous cell carcinoma. The histological former type is primarily a cancer affecting developed countries, and the epidemiology of this type of esophageal cancer differs markedly from those of other epithelial cancers, while the latter is the predominant histological type worldwide. There is a huge variation in the incidence of esophageal cancer worldwide, with greater than 100-fold differences observed between high-incidence areas, such as Iran and China, and low-incidence areas like western Africa. The average age of esophageal cancer patients is over 45 years, and the proportion of male patients is higher than that of females. The typical symptoms of this cancer include progressive dysphagia, esophagorrhagia, and severe pain.1 Studies have shown that the etiology of esophageal cancer is associated with dietary habits, hereditary factors, age, sex, occupation, region, and living environment susceptibility, which indicate that esophageal cancer is a multifactorial disease.
Although surgical treatments for esophageal cancer are well established in clinical treatment, the specific mechanism of its pathogenesis and especially the biomarkers of its appearance and development are still unclear. Therefore, preventative drugs, antitumor complexes in combination with adjunctive therapy, especially natural nutrients in foods, may play a role in preventing or inhibiting esophageal cancer and are urgently needed.
Regarding natural ingredients in foods, lactoferrin and three unsaturated fatty acids in dairy and meat products were examined in this study. Lactoferrin (80 kDa) is a single polypeptide chain glycoprotein with N- and C-terminal lobes connected by a short helix, which is mainly found in the milk, saliva, tears, seminal fluid, bile, and synovial fluid of mammals.2,3 In recent years, the biological activities of lactoferrin, including anti-inflammatory, antibacterial, antiviral, antioxidative, and anticarcinogenic effects have been widely reported.4−8
Oleic acid (C18:1, omega-9) is a monounsaturated fatty acid mainly found in olive oil. Linolenic acid (C18:3) and docosahexaenoic acid (DHA, C22:6) are two omega-3 polyunsaturated fatty acids enriched in meat, dairy products, and oils. Psaltopoulou et al. conducted a meta-analysis and found that a higher intake of olive oil could lead to a significantly lower probability of digestive system cancers by 30%,9 and this effect was mainly due to its large content of monounsatured fats, particularly oleic acid.10 The antitumor effects of vincristine carried by microemulsions composed of oleic acid and other components were evaluated and confirmed in a C57BL/6 mouse model bearing mouse murine histiocytoma M5076 tumors.11 Feng et al.12 demonstrated that DHA compounds could induce apoptosis of glioma cells (U251) and subsequently inhibit the growth of U251 tumors. Song et al.13 showed that DHA suppressed pancreatic cancer cell line (PANC-1 and SW1990) growth via downregulation of WNT/β-catenin signaling. The antitumor bioactivity of linolenic acid has been shown by Llor et al.14 who found that it could induce apoptosis and inhibit proliferation of HT-29 cells. The optimal amount to prevent colon carcinogenesis with perilla oil high in α-linolenic acid in a 12% medium-fat diet was investigated in female F344 rats, and it was concluded that a relatively small fraction of perilla oil, 25% of total dietary fat, may provide an appreciable beneficial effect in lowering the risk of colon cancer.15 A case–control study conducted in a homogeneous population from a central area in France was designed and proved the protective effect of α-linolenic acid in the risk of breast cancer.16 Fang et al.17 observed that β-lactoglobulin–oleic acid and β-lactoglobulin–linoleic acid complexes could inhibit the growth of human cervical carcinoma (HeLa) and human breast cancer cells (MCF-7) by inducing cell apoptosis. Research has also demonstrated that two unsaturated fatty acids, oleic acid and linoleic acid, induced intermediates of α-lactalbumin to form amorphous aggregates in a time- and concentration-dependent manner, and cytotoxic aggregates of α-lactalbumin produced by unsaturated fatty acids induced apoptosis of cancer cells.18 Especially, Xing et al.19 designed an ovalbumin–OA (oleic acid) complex, HAMLET similar, and then evaluated its antitumor activities via detecting cell growth inhibition, cell cycle, and apoptosis of Caco-2 cells (a colon cancer cell line). The results demonstrated that this ovalbumin–OA complex significantly inhibited cell growth rates, inducing cell cycle block and apoptosis, when compared with the ones in the OA alone group at the same concentration (273 μmol/L). They subsequently investigated the interaction between ovalbumin and OA and found that ovalbumin conformation was altered upon OA interaction driven by hydrophobic interaction and a hydrogen bond.20 Based on the previous research, the present study attempts to validate the effects of lactoferrin and oleic acid/DHA/linolenic acid combinations on esophageal cancer cells and xenograft esophageal tumors and investigates the underlying mechanism for these combinations, which may act as a novel antitumor complex in adjunctive therapy, as shown by pharmacokinetics research in mammals and clinical data.
2. Materials and Methods
2.1. Chemicals
Bovine LF (lactoferrin) (>95% purity), linolenic acid (C18:3; cis,cis,cis-9,12,15; ≥99% purity), DHA (C22:6, ≥98% purity), oleic acid (C18:1, ≥99% purity), lithocholyltaurine standard sample, and the JAK2 inhibitor ruxolitinib were purchased from Sigma (St. Louis, MO, USA). Lactoferrin was diluted in ddH2O. Oleic acid, DHA, and linolenic acid were diluted with absolute ethyl alcohol (ETOH) to 10 mg/kg stocking solution. Roswell Park Memorial Institute (RPMI) 1640 Medium and fetal bovine serum (FBS) were obtained from GIBCO (Waltham, MA, USA), and 1% penicillin/streptomycin was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The human esophageal cancer cell line (KYSE450) was purchased from the Chinese Academy of Science (Shanghai, China). A cell counting kit-8 (CCK-8 kit), apoptosis detection kit with annexin V/FITC staining, and cell cycle detection kit with PI staining were purchased from Solarbio (Beijing, China). Transwell chambers were purchased from Corning (Franklin Lakes, NJ, USA). Primary antibodies against β-actin, (p)-JAK2, (p)-STAT3, (p)-Erk, and (p)-AKT, and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Reagents required for western blotting were purchased from Solarbio. The enhanced chemiluminescence (ECL) reagent was purchased from Tanon (Shanghai, China).
2.2. Cell Culture and Viability Detection
KYSE450 cells were grown in a RPMI-1640 medium containing 10% FBS in an incubator (5% CO2) at a constant temperature of 37 °C. KYSE450 cells (1 × 104 cells in 100 μL of growth medium per well) were plated in a 96 well plate and incubated for 24 h. When the medium was replaced with 100 μL of fresh medium containing increasing concentrations of lactoferrin, oleic acid, DHA, or linolenic acid (0, 0.01, 0.05, 0.1, 0.5, 1, and 5 g/L) and the combinations of lactoferrin + oleic acid (with a ratio of 1:1), lactoferrin + DHA (with a ratio of 1:1), and lactoferrin + linolenic acid (with a ratio of 1:1), then the cells were cultured for another 48 h, and the ethyl alcohol (ETOH) group was added as another control group to investigate the effect of ETOH on KYSE450 cell survival. The CCK-8 kit was utilized according to the manufacturer. The absorbances at 490 nm were measured using a Microplate Reader (Thermo). The cell survival rate (viability) = (A test – A blank)/(A control – A blank) × 100%. The dosages with viabilities greater than 80% and those significantly different from control cells (p < 0.05) were selected as the final concentrations of lactoferrin and fatty acids used in the experiments.
2.3. Cell Invasion Detection
The effects of lactoferrin, unsaturated fatty acids, or their combinations on the migratory capacity of KYSE450 cells were detected utilizing transwell chambers. The upper chambers were seeded with 5 × 103 KYSE450 cells in 150 μL of serum-free medium, and 500 μL of medium containing 10% FBS was added to the lower chambers. Samples of lactoferrin (final concentration, 0.5 g/L), oleic acid (0.05 g/L), DHA (0.05 g/L), linolenic acid (0.05 g/L), or the combinations lactoferrin (0.5 g/L) + oleic acid (0.05 g/L), lactoferrin (0.5 g/L) + DHA (0.05 g/L), and lactoferrin (0.5 g/L) + linolenic acid (0.05 g/L) were added to the upper chamber, and cells were cultured for 24 h. The top surface of the filter was scrubbed gently with cotton swabs, and cells that had invaded the undersurface were fixed with 20% ice-cold methanol for 15 min and then stained with 0.1% crystal violet for 20 min prior to washing with icy PBS buffer (5 min × 3). The stained cells were then photographed and counted, the mean number of stained cells was calculated in three random fields on each undersurface, and the number of invasive cells in the control and treatment groups were compared and analyzed.
2.4. Cell Scratch Analysis
KYSE450 cells were plated in a 6 well plate and incubated for 24 h to achieve a cell density greater than 90%. A single lesion approximately 5.0 mm wide was scratched across the cell monolayer by mechanical scraping. The cells were then incubated with lactoferrin (0.5 g/L), oleic acid (0.05 g/L), DHA (0.05 g/L), linolenic acid (0.05 g/L), lactoferrin (0.5 g/L) + oleic acid (0.05 g/L), lactoferrin (0.5 g/L) + DHA (0.05 g/L), or lactoferrin (0.5 g/L) + linolenic acid (0.05 g/L). The width of the scratch wound was photographed and scanned 24 h later. The scratch width at a timepoint of 0 h was chosen as the primary scratch width (control, 0 h), and the scratch width in the treatment groups represented the inhibitory activity of lactoferrin, unsaturated fatty acids, or combinations on cell invasion. The recovery rate (%) = the scratch width of the denuded area in the treatment groups/the scratch width of the denuded area in the control group (0 h) × 100%.
2.5. Animal Model Construction
Twenty male BALB/c nude mice (18–22 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The animal experiments were approved by the Ethics Committee of Chinese Academy of Agriculture Sciences (Beijing, China; permission number: CAS20190525, 25th May, 2019). KYSE450 cells in 20 dishes were cultured, and then 5 × 107 cells in 200 μL of Matrigel medium (BD, Franklin Lakes, NJ, USA) were subcutaneously injected into the left flank of each nude mouse. When the tumor volume reached 90–110 mm3, the nude mice were randomly divided into four groups (four mice per group): control (without treatment), lactoferrin group (50 mg/kg), linolenic acid group (5 mg/kg), and lactoferrin (50 mg/kg) + linolenic acid (5 mg/kg) group. LF was diluted in ddH2O, and OA, DHA, and LA (linolenic acid) were diluted in edible peanut oil (Luhua, China). All mice were euthanized on the 25th day, and the tumors were weighed. Tumor diameters were recorded with a caliper every 4 days, and tumor volume was calculated using the following formula: tumor volume (mm3) = 0.5 × width (mm)2 × length (mm). Individual tumor suppression rate (%) = (the average tumor weight in the control group – the individual tumor weight in the treatment groups)/the average tumor weight in the control group × 100%, where the average tumor weight in the control group was calculated from each tumor weight in the control group. Relative tumor volume (RTV, %) = detected volume/volume before dosing × 100%. Relative tumor proliferation rate (%) = RTV of each tumor in treatment groups/the average RTV in the control group × 100%, and the average RTV in the control group was calculated from the RTV of each tumor in the control group.
2.6. Metabonomics Analysis of KYSE450 Cells
Special metabolites in KYSE450 cells treated with lactoferrin (0.5 g/L), linolenic acid (0.05 g/L), or lactoferrin (0.5 g/L) + linolenic acid (0.05 g/L) were separated using a UHPLC system Dionex Ultimate 3000 (Thermo Fisher Scientific) equipped with a Waters column (Acquity BEH C18 1.7 μm, 2.1 × 50 mm) (Milford, Massachusetts, USA) at 40 °C. The mobile phase consisted of water containing 2 mM ammonium formate, 0.1% formic acid (solvent a, v/v), and acetonitrile containing 0.1% formic acid and 2 mM ammonium formate (solvent b, v/v), with a flow rate of 200 μL/min, and the gradient elution program is as follows: 0–1.0 min, 5% b; 1.0–5.0 min, 5–60% b; 5.0–8.0 min, 60–100% b; 8.0–11.0 min, 100% b; 11.0–14.0 min, 100–60% b; 14.0–15.0 min, 60–5% b; 15.0–18.0 min, 5% b. The Q-Exactive instrument (Thermo Fisher Scientific) equipped with electrospray ionization in positive and negative switching modes was utilized to detect the above samples, and the system was calibrated and controlled with X Calibur 3.1 and Q-Exactive Tune software. The UHPLC Q-Orbitrap analysis can produce large amounts of raw data using TraceFinder software (Thermo Fisher Scientific). The data were exported into Excel spreadsheets by Simca-P for the following analyses: PCA (principle components analysis), PLS-DA (partial least squares discriminant analysis), t-test, volcano plot, and VIP (variable importance in projection) plot analysis.
2.7. Western Blotting Detection
A total of 106 KYSE450 cells were lysed in 0.5 mL of RIPA buffer (Solarbio), and the total protein concentration was detected using a BCA kit (Solarbio). After a 5 min heat treatment, the protein samples were loaded onto a 12% SDS-polyacrylamide gel for electrophoresis, then the proteins were transferred onto nitrocellulose filters using Trans-blot machines (Bio-Rad, Hercules, CA, USA), and the membrane was blocked with 2.5% BSA in TBST buffer (Solarbio) for 1 h at room temperature (RT). The proteins were then probed with primary antibodies against β-actin, (p)-JAK2 (Tyr1007 + Tyr1008), (p)-STAT3 (Tyr705), (p)-Erk (Thr202 + Tyr204), and (p)-AKT (Ser473 + Tyr474), for 2 h at RT, utilizing β-actin as an internal reference to ensure the equal loadings. After three washes with PBST buffer (3 × 5 min, Solarbio), the membrane was incubated with secondary antibodies for 1 h at RT and washed with PBST buffer (3 × 10 min). Finally, the proteins were visualized utilizing the ECL reagent (Solarbio) and analyzed by Image J software (Rawak Software, Inc. Germany).
2.8. Statistical Analysis
All data are expressed as means ± standard deviation (SD) from several independent experiments (n ≥ 3). Statistical analyses were performed using SPSS 13.0 software (IBM, Armonk, NY, USA). Analysis of variance coupled with Tukey’s multiple comparsions was used to determine the differences between treatments. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Dosage Selections of Lactoferrin and Three Unsaturated Fatty Acids
To observe the effect of lactoferrin and three unsaturated fatty acids on the viability of KYSE450 cells, CCK-8 detection was performed. Results in Figure 1 showed that lactoferrin at 0.5 g/L significantly inhibited the viability of KYSE450 cells to (86.4 ± 3.7)% of control cells, while oleic acid, DHA, and linolenic acid at 0.05 g/L significantly inhibited the viability of KYSE450 cells to (88.7 ± 3.6), (88.9 ± 3.9), and (86.1 ± 4.0)%, respectively, of control cells (all p < 0.05). Based on the two principles of dose selection, “viability > 80%” and “significant differences (compared with the control, p<0.05)”, thus, 0.5 g/L was selected as the starting dosage for lactoferrin, and 0.05 g/L was selected as the starting dosage for the three unsaturated fatty acids in the following experiments. Additionally, there seemed no obvious effect of ETOH on the viability of KYSE450 cells, indicating that ETOH was a safe solvent of the three fatty acids in the present study (Figure 1).
Figure 1.
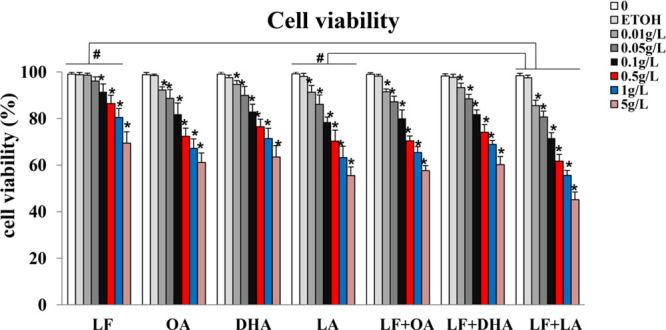
KYSE450 cell viability treated with lactoferrin and three unsaturated fatty acids and detected by CCK-8 kits. Lactoferrin and three unsaturated fatty acids showed different inhibitions on the KYSE450 cell survival rate, with 48 h treatments of lactoferrin and three unsaturated fatty acids as well as the combinations. The data are represented as mean ± SD (n = 8). *p < 0.05, compared with the control (0 g/L). #p < 0.05, compared with the lactoferrin (LF) group or linolenic acid (LA) group.
3.2. Lactoferrin and Three Unsaturated Fatty Acids Inhibited the Migration and Invasion of KYSE450 Cells to Different Degrees
To investigate the effect of lactoferrin and the three unsaturated fatty acids on the invasive capacity of KYSE450 cells, transwell assays were performed. By counting the cells invading the filters, results demonstrated that lactoferrin, the three unsaturated fatty acids alone, and various combinations of lactoferrin and the unsaturated fatty acids inhibited invasion of KYSE450 cells to different degrees, with the least number of invading cells in the lactoferrin + linolenic acid group when compared with control cells (p < 0.05, Figure 2).
Figure 2.
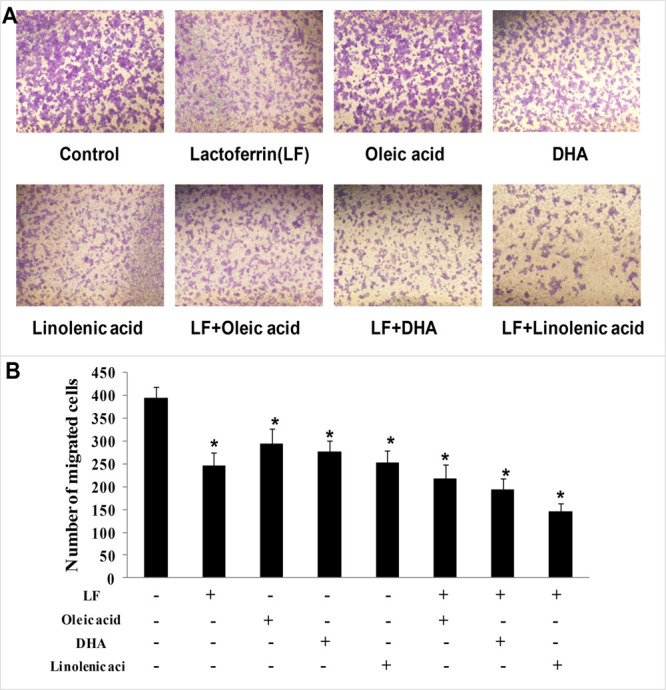
KYSE450 cell invasion treated with lactoferrin and three unsaturated fatty acids and detected by transwell. LF (0.5 g/L), three unsaturated fatty acids (0.05 g/L oleic acid (OA)/DHA/LA), and the combinations inhibited KYSE450 cell invasion in different degrees. (A) Invasive cells stained by crystal violet. (B) Statistical analysis of the invasive cells. The data are represented as mean ± SD (n = 3). *p < 0.05, compared with the control. 200× magnification.
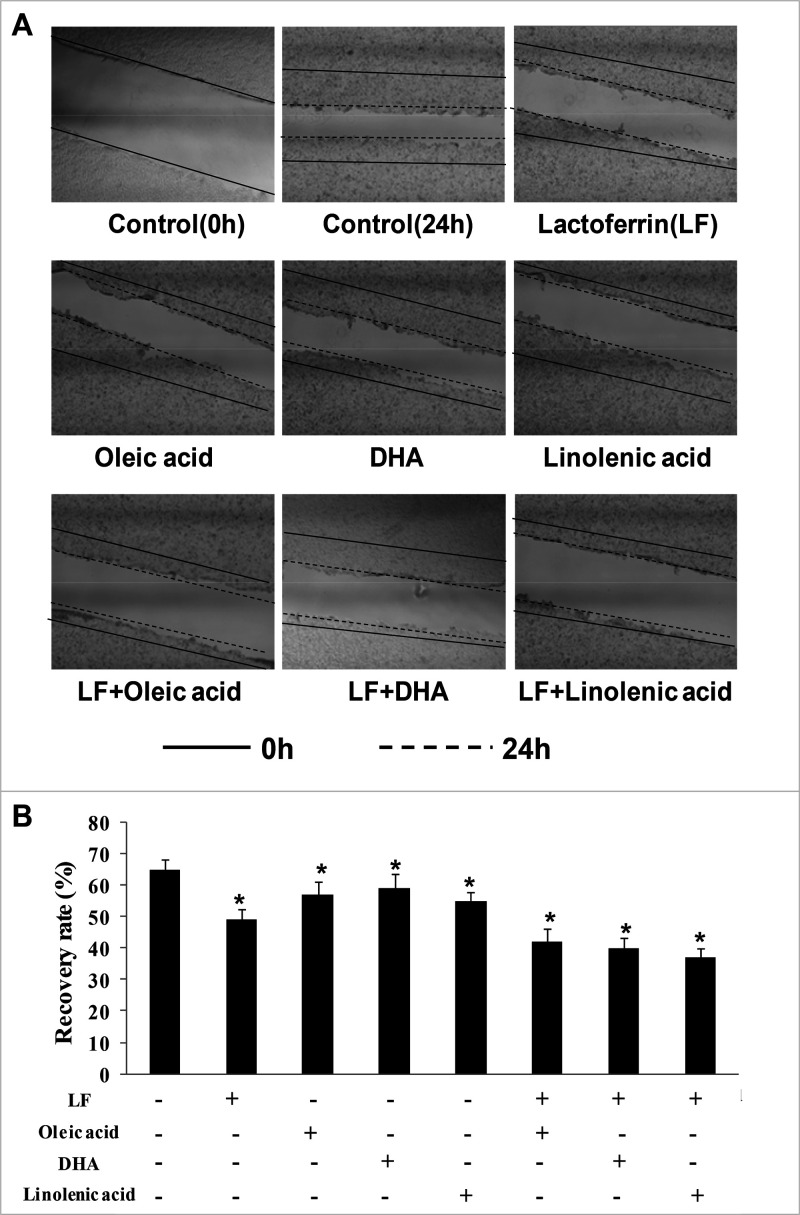
Additionally, to investigate the effect of lactoferrin and the three unsaturated fatty acids on the migratory capacity of KYSE450 cells, scratch analysis was performed to calculate the cell recovery rate, and the recovery rate (%) = the scratch width of the denuded area in the treatment groups/the scratch width of the denuded area in the control group (0 h) × 100%. Results showed that the scratch widths of the denuded area in the treatment groups were wider when compared with the control (p < 0.05), and the scratch widths in the lactoferrin + linolenic acid group was the biggest one, indicating that the migratory activity of KYSE450 cells was significantly inhibited by the lactoferrin + linolenic acid group (Figure 3).
Figure 3.
KYSE450 cell migration treated with lactoferrin and three unsaturated fatty acids and detected by scratch analysis. LF (0.5 g/L), three unsaturated fatty acids (0.05 g/L OA/DHA/LA), and the combinations inhibited KYSE450 cell migration in different degrees. (A) Cell migration photographs. (B) Statistical analysis of the recovery rate. The data are represented as mean ± SD (n = 3). *p < 0.05, compared with the control. 40× magnification.
3.3. Lactoferrin and Linolenic Acid Inhibited Tumor Growth in a Tumor-Bearing Nude Mouse Model
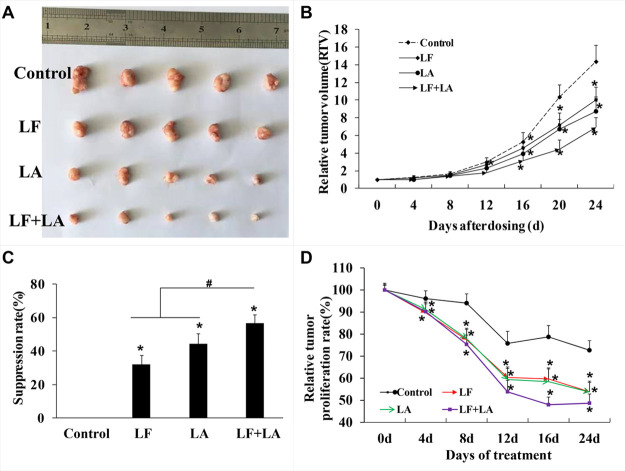
Based on the in vitro results, the combination of lactoferrin and linolenic acid was chosen for in vivo testing. To further validate the antitumor effects of lactoferrin and linolenic acid on KYSE450 tumors, a model employing xenografted tumor cells in BALB/c nude mice was established. As shown in Figure 4, the relative tumor volume (RTV) and the relative tumor proliferation rate in the treatment groups were reduced when compared with control animals (p < 0.05, Figure 4B,D). On the 25th day, KYSE450 tumor weights were significantly reduced in the treatment groups (compared with the control, p < 0.05), and there appeared to be a significant difference between the groups treated with lactoferrin or linolenic acid alone versus the combined lactoferrin + linolenic acid treatment group (p < 0.05, Figure 4A,C).
Figure 4.
In vivo effect of lactoferrin and linolenic acid on KYSE450 tumor-bearing nude mice. (A) Treatment of LF (50 mg/kg) and LA (5 mg/kg) decreased the size of KYSE450 tumors. (B) Relative tumor volume, which was calculated by each tumor volume. *p < 0.05, comparing with the control (no treatment) (n = 4). (C) Tumor suppression rate, which was calculated by each tumor weight. *p < 0.05, comparing with the control (no treatment) (n = 5). (D) Relative tumor proliferation rate, which was calculated by relative tumor volumes of different groups. *p < 0.05, comparing with the control (no treatment) (n = 5).
3.4. Lithocholyltaurine Was Isolated as a Special Metabolite of Lactoferrin and Linolenic Acid through Metabonomics Analysis
To elucidate the specific mechanism of lactoferrin and linolenic acid in inhibiting esophageal tumors, metabonomics analysis and data mining (literature survey) of KYSE450 cells treated with lactoferrin, linolenic acid, and lactoferrin + linolenic acid were performed. The results showed that 20 metabolites were screened out by overlapping three groups (control versus lactoferrin, control versus linolenic acid, and control versus (lactoferrin + linolenic acid)), which were with variable importance in projection (VIP) values of >1 and P values of <0.05 (VENN plot, Figure 5A), which are demonstrated in Supplementary Table 1. Referring to data mining of metabonomics detection, a literature survey was performed, and lithocholyltaurine was selected as a special metabolite of interest in the present study.21−23 As shown in Figure 5B, the level of lithocholyltaurine in the treatment groups was significantly lower than that in the control group (p < 0.05); in particular, lithocholyltaurine levels in the combination group were the lowest compared with the lactoferrin or linolenic acid group (p < 0.05, Figure 5B).
Figure 5.
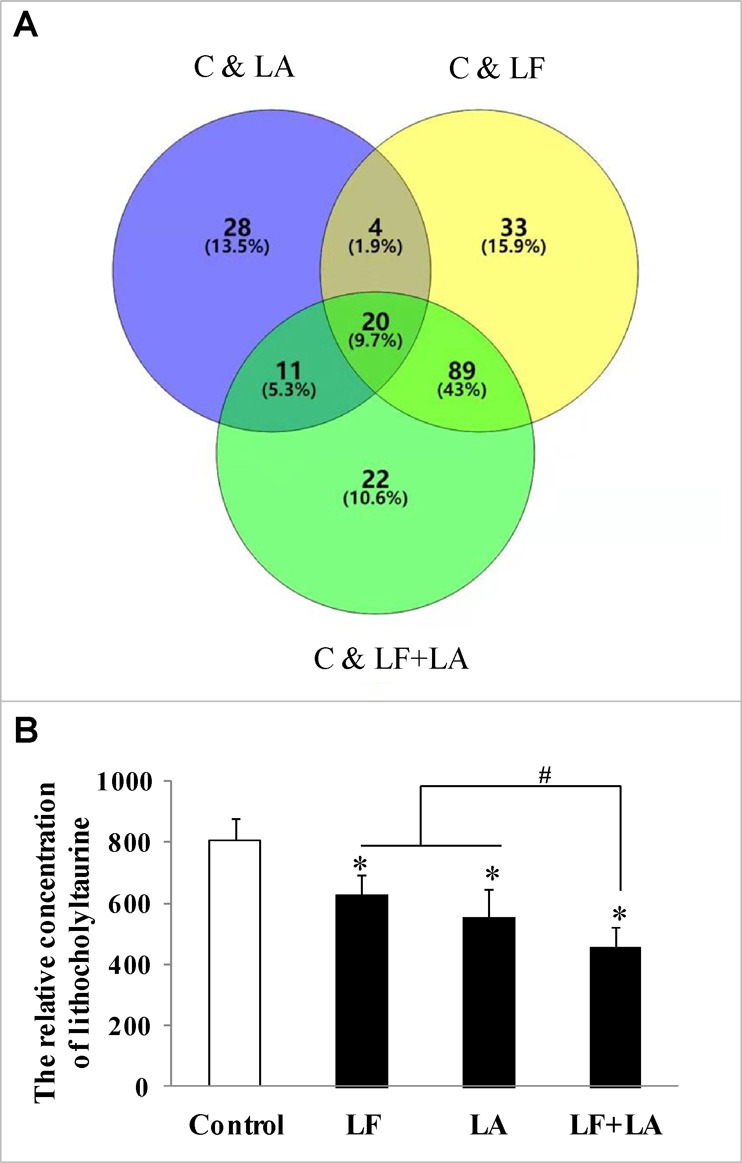
Metabonomics detection and expression of lithocholyltaurine in KYSE450 cells treated with lactoferrin and linolenic acid. (A) Overlapping of selected metabolites with changed expressions in control and LF, control and LA, and control and (LF + LA) (n = 6), with 0.5 g/L for LF and 0.05 g/L for LA. (B) Expression level of lithocholyltaurine in KYSE450 cells. The data are represented as mean ± SD. *p < 0.05, compared with the control. #p < 0.05, compared with LF or LA (n = 6).
3.5. Lactoferrin and Linolenic Acid Inhibited KYSE450 Tumors by Downregulating the Level of Lithocholyltaurine and Inhibiting Phosphorylation in the JAK2/STAT3-Related Pathway
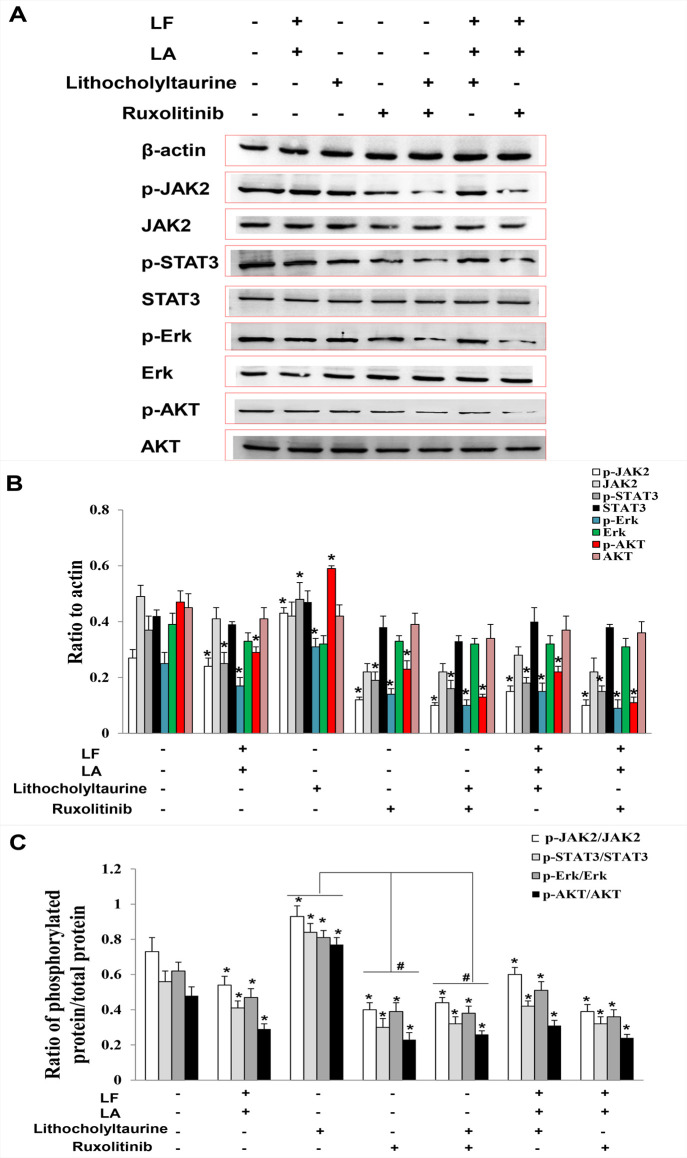
To investigate the role of lithocholyltaurine in regulating the JAK2/STAT3-related pathway, lithocholyltaurine and an inhibitor of JAK2 (ruxolitinib) were added to KYSE450 cells, and the expressions of JAK2/STAT3-related proteins were measured by western blotting. Results demonstrated that the levels of phosphorylated proteins including p-JAK2, p-STAT3, p-Erk, and p-AKT in lactoferrin + linolenic acid and ruxolitinib groups were inhibited compared with the control group (p < 0.05), and the levels of phosphorylated proteins in the lithocholyltaurine group were upregulated significantly (compared with the control, p < 0.05), suggesting that lithocholyltaurine played a role in activating JAK2/STAT3-related phosphorylated proteins. Either in the lactoferrin + linolenic acid + ruxolitinib group or in lactoferrin + linolenic acid + lithocholyltaurine group, the levels of phosphorylated proteins showed no obvious change when compared with the lactoferrin + linolenic acid group, which were all lower than the control ones (p < 0.05, Figure 6A,B), indicating that lactoferrin + linolenic acid could inhibit the phosphorylations of the JAK2/STAT3/Erk/AKT pathway. Treatment with lithocholyltaurine + ruxolitinib decreased the levels of the phosphorylated proteins when compared with the control (p < 0.05), and there were no significant differences in these proteins between the ruxolitinib group and lithocholyltaurine + ruxolitinib group, which were significantly lower than the ones in the lithocholyltaurine group (p < 0.05, Figure 6C), further proving that lithocholyltaurine was the upstream regulator of JAK2/STAT3-related proteins. The above data validated that lactoferrin + linolenic acid downregulated the level of lithocholyltaurine and subsequently inactivated the phosphorylation of JAK2/STAT3/Erk/AKT proteins, which further inhibited the development of KYSE450 tumors.
Figure 6.
Protein expression of (p)-JAK2, (p)-STAT3, (p)-Erk, and (p)-AKT in KYSE450 cells treated with lactoferrin and linolenic acid. (A) Protein bands in treatment groups (LF (0.5 g/L), LA (0.05 g/L), lithocholyltaurine (10 μM), and JAK2 inhibitor ruxolitnib (10 μM)) by western blotting detection. (B) Statistical analysis of the protein bands in (A). (C) Ratio of phosphorylated proteins to proteins. All the data are represented as mean ± SD (n = 3). *p < 0.05, compared with the control. #p < 0.05, compared with the lithocholyltaurine group or ruxolitinib group.
4. Discussion
As a major cause of morbidity and mortality in several types of gastrointestinal tumors, esophageal cancer is difficult to diagnose and cure at an early stage. Thus, a large number of patients must undergo surgery and chemotherapy because of tumor development and metastasis during esophageal cancer treatment, which brings economic burdens and diminishes survival. Chemotherapy drugs like 5-fluorouracil and taxol, commonly utilized in esophageal cancer treatment, impact human health and result in hair loss, nausea, fatigue, and vomiting, among other effects.24−28 Unlike chemotherapy drugs, natural ingredients with antitumor effects, especially food nutrients, have many advantages including inherent biological activities, less toxicity, easy availability, and affordability and have attracted much attention.
There have been several studies validating the antitumor effects of lactoferrin and these unsaturated fatty acids in esophageal tumor models. For example, Moradian et al.29 found that bovine lactoferrin inhibited human esophageal cancer cell growth using MTT and flow cytometric methods. Inamori et al.30 found that lactoferrin protected against esophageal mucosal damage caused by acute acid reflux through downregulation of inflammatory factors, including tumor necrosis factor-α and interleukin-1β. Patti et al.31 suggested that lactoferrin was effective in treating primary esophageal motility disorders after minimally invasive surgical therapy. Moon et al.32 showed that oleic acid and linolenic acid could downregulate the malignant potential of OE19 and OE33 esophageal cancer cells, mainly through regulating the AMPK/S6 axis. Kitagawa et al.33 found that γ-linolenic acid inhibited esophageal tumor growth based on clinical statistical data. The antitumor activities of lactoferrin or the three unsaturated fatty acids alone have been widely validated, but the effect of lactoferrin + oleic acid/DHA/linolenic combinations in inhibiting esophageal tumors has been rarely studied. Therefore, the present study aimed to investigate the effects of combinations of lactoferrin + these three unsaturated fatty acids on esophageal cancer both in vitro and in vivo. The results showed that lactoferrin, the three unsaturated fatty acids, and their combinations inhibited cell migration and invasion of KYSE450 cells to different degrees, and the potency of the lactoferrin + linolenic acid combination was the strongest. Additionally, results in the nude mouse model also demonstrated that RTV and average esophageal tumor weight in the lactoferrin + linolenic acid group were significantly reduced compared to control mice, further demonstrating that lactoferrin + linolenic acid could suppress the growth and development of esophageal tumors. Also, there seems to be an additive effect of lactoferrin and linolenic acid in inhibiting KYSE450 tumors when compared with lactoferrin or linolenic acid alone, which requires a further study and validation.
To investigate the molecular mechanism, metabonomics analysis of KYSE450 cells and data mining were performed and revealed the potential role of special metabolite lithocholyltaurine and the related JAK2/STAT3/Erk/AKT pathway. Cells were treated with lactoferrin + linolenic acid, lithocholyltaurine, or JAK2 inhibitor, and protein levels of JAK2, STAT3, Erk, and AKT were detected by western blotting. Results indicated that lithocholyltaurine activated phosphorylation in the JAK2/STAT3/Erk/AKT pathway, while lactoferrin + linolenic acid or JAK2 inhibitor inhibited expression of p-JAK2/p-STAT3/p-Erk/p-AKT to different degrees. Additionally, protein expression levels in the lithocholyltaurine + JAK2 inhibitor group were lower than those in the lithocholyltaurine group, validating that JAK2 was regulated by lithocholyltaurine, and lactoferrin + linolenic acid downregulated the level of lithocholyltaurine and subsequently inactivated the phosphorylation of JAK2/STAT3/Erk/AKT proteins, which further inhibited the development of KYSE450 tumors.
Lithocholyltaurine (C26H45NO5S; Mw, 483.7), a conjugated 3α-mono (hydroxy) bile acid, can bind to M3 subtype muscarinic cholinergic receptors.34,35 The activation of M3 receptors can induce cell proliferation,36 and lithocholytaurine has shown the ability to promote H508 colon cancer cell proliferation and pepsinogen secretion from gastric cells by activating the M3 receptor.36,37 Since the M3 receptor is expressed in various cells of human gastric and esophageal mucosa, lithocholyltaurine may induce activation of multiple cellular pathways when bile regurgitation occurs, promoting proliferation of esophageal squamous epithelial cells and mucus secretion, which may lead to reflux esophagitis, reflux gastritis, and eventually gastric cancer. In our study, we showed that lithocholyltaurine could be downregulated by lactoferrin and linolenic acid alone, but downregulation was greatest in cells treated with the lactoferrin + linolenic acid combination, indicating that lactoferrin and linolenic acid alone, especially the lactoferrin + linolenic acid combination, could inhibit esophageal cancer by suppressing the level of lithocholyltaurine. Additionally, lactoferrin and linolenic acid appeared to exert additive effects in inhibiting lithocholyltaurine.
The JAK2/STAT signaling pathway has been shown to participate in the development of several types of tumors. Phosphorylation of these factors was activated after translocation into the nucleus, which subsequently promoted tumor growth and metastasis.36−42 The aberrant activation of JAK2/STAT signaling contributes to tumor initiation and progression. Therefore, targeting JAK2/STAT signaling, especially blocking phosphorylation in the JAK2/STAT pathway, suppressed tumor progression and metastasis in ovarian cancer,36,37 melanoma,38 a glioma model,39 renal carcinoma,38 and esophageal squamous cell carcinoma.41,42 In our study, we found that JAK2/STAT3/Erk/AKT were highly expressed in KYSE450 cells and that lithocholyltaurine further increased the expression of these factors, validating that lactoferrin and linolenic acid could suppress the growth and metastasis of esophageal tumors by downregulating lithocholyltaurine and subsequently inhibiting phosphorylation of the JAK2/STAT3 pathway.
To conclude, the lactoferrin + linolenic acid combination had shown to exert inhibitive effects on KYSE450 cells and KYSE450 tumors via downregulation of lithocholyltaurine and by inhibiting the phosphorylation of the JAK2/STAT3/Erk/AKT pathway. Demonstrating the additive effect between lactoferrin and linolenic acid will require further pharmaceutical experiments. The potential development of the lactoferrin + linolenic acid combination for treatment of esophageal cancer should be evaluated with further toxicological assessments and more pharmacokinetics data, which may provide a novel perspective on other food nutrients.
Acknowledgments
This study was supported by the Scientific Research Project for Major Achievements of the Agricultural Science and Technology Innovation Program (ASTIP) (no. CAAS-ZDXT2019004), the Agricultural Science and Technology Innovation Program (ASTIP-IAS12), and Modern Agro-Industry Technology Research System of China (CARS-36).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01132.
Special metabolites screened by metabonomics detection (Supplementary Table 1) (PDF)
Author Contributions
⊥ H.L. and Q.Y. should be regarded as the first author.
The authors declare no competing financial interest.
Supplementary Material
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin 2018, 68, 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Campbell E. J. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc. Natl. Acad. Sci. U. S. A. 1982, 79, 6941–6945. 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małaczewska J.; Rotkiewicz Z. Lactoferrin - a multipotential protein. Med. Weter. 2007, 63, 136–139. [Google Scholar]
- Li H. Y.; Li P.; Yang H. G.; Wang Y. Z.; Huang G. X.; Wang J. Q. Investigation and comparison of the anti-tumor activities of lactoferrin, α-lactalbumin, and β-lactoglobulin in A549, HT29, HepG2, and MDA231-LM2 tumor models. J. Dairy Sci. 2019, 102, 9586–9597. 10.3168/jds.2019-16429. [DOI] [PubMed] [Google Scholar]
- Chung S. H.; Kang H. B.; Kim J. W.; Yoon S. S.; Nam M. S. The biological effects of bovine lactoferrin on inflammatory cytokine expression in the PMA stimulated cells. Korean J. Food Sci. Anim. Res. 2012, 32, 364–368. 10.5851/kosfa.2012.32.3.364. [DOI] [Google Scholar]
- Koshu O.; Mako K.; Yasuteru U.; Hiroshi N.; Jan M.; Herter J. M. Lactoferrin supresses neutrophil extracellular traps release in inflammation. EBio Med. 2016, 10, 204–215. 10.1016/j.ebiom.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwan E. M.; El-Baky N. A.; Al-Hejin A. M.; Baeshen M. N.; Almehdar H. A.; Elsaway A. Significant antibacterial activity and synergistic effects of camel lactoferrin with antibiotics against methicillin-resistant staphylococcus aureus (mRSA). Res. Microbiol. 2016, 167, 480–491. 10.1016/j.resmic.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Korpela R. Milk peptides and cardiovascular health: Effects on blood pressure and beyond. Aust. J. Dairy Technol. 2009, 64, 26–28. [Google Scholar]
- Psaltopoulou T.; Kosti R. I.; Haidopoulos D.; Dimopoulos M.; Panagiotakos D. B. Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. 10.1186/1476-511X-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill C. I.; Boyd A.; McDermott E.; McCann M.; Servili M.; Selvaggini R. Potential anticancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. 10.1002/ijc.21083. [DOI] [PubMed] [Google Scholar]
- Junping W.; Takayama K.; Nagai T.; Maitani Y. Pharmacokinetics and antitumor effects of vincristine carried by microemulsions composed of PEG-lipid, oleic acid, vitamin E and cholesterol. Int. J. Pharm. 2003, 251, 13–21. 10.1016/S0378-5173(02)00580-X. [DOI] [PubMed] [Google Scholar]
- Feng X. G.; Yao W. H.; Liu Y.; Sun K. R. Antitumor effect of DHA compound in vitro and in vivo and its mechanism. Chin. J. Oncol. 2010, 32, 415–419. [PubMed] [Google Scholar]
- Song K. S.; Jing K.; Kim J. S.; Yun E. J.; Shin S.; SSeo K. Omega-3-polyunsaturated fatty acids suppress pancreatic cancer cell growth in vitro and in vivo via downregulation of WNT/beta-catenin signaling. Pancreatology 2011, 11, 574–584. 10.1159/000334468. [DOI] [PubMed] [Google Scholar]
- Llor X.; Pons E.; Roca A.; Alvarez M.; Mañé J.; Fernández-Bañares F.; Gassull M. A. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 2003, 22, 71–79. 10.1054/clnu.2002.0627. [DOI] [PubMed] [Google Scholar]
- Narisawa T.; Fukaura Y.; Yazawa K.; Ishikawa C.; Isoda Y.; Nishizawa Y. Colon cancer prevention with a small amount of dietary perilla oil high in alpha-linolenic acid in an animal model. Cancer 1994, 73, 2069–2075. . [DOI] [PubMed] [Google Scholar]
- Klein V.; Chajès V.; Germain E.; Schulgen G.; Bougnoux P. Low alpha-linolenic acid content of adipose breast tissue is associated with an increased risk of breast cancer. Eur. J. Cancer 2000, 36, 335–340. 10.1016/S0959-8049(99)00254-3. [DOI] [PubMed] [Google Scholar]
- Fang B.; Zhang M.; Tian M.; Ren F. Z. Self-assembled β-lactoglobulin–oleic acid and β-lactoglobulin–linoleic acid complexes with antitumor activities. J. Dairy Sci. 2015, 98, 2898–2907. 10.3168/jds.2014-8993. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Yang F. Jr.; Yang F.; Chen J.; Zheng C. Y.; Liang Y. Cytotoxic aggregates of α-lactalbumin induced by unsaturated fatty acid induce apoptosis in tumor cells. Chem.-Biol. Interact. 2009, 180, 131–142. 10.1016/j.cbi.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Xing X. J.; Liu J. L.; Jing H. HAMLET-like cell-growth-inhibitory effect of ovalbumin-oleic acid complex on colon cancer cell. Sci. Technol. Food Ind. 2016, 37, 133–138. [Google Scholar]
- Xing X. J.; Liu J. L.; Jing H. Spectroscopic analysis of the interaction between ovalbumin and oleic acid. Food Sci. 2016, 37, 38–44. [Google Scholar]
- Bartoszko-Malik A.; Zimniak P.; Raufman J. P. Lithocholyltaurine (LCT), a conjugated 3α-mono (hydroxy) bile acid, binds to M3 subtype muscarinic cholinergic receptors. Gastroenterology 1998, 114, A1126. 10.1016/S0016-5085(98)84580-0. [DOI] [Google Scholar]
- Raufrnan J. P.; Cheng K.; Zimniak P.; Xiao Y.; Frucht H. Interaction of taurine conjugates of lithocholic acid with M3 muscarinic receptors on a colon cancer cell line. Gastroenterology 2000, 118, A558. 10.1016/S0016-5085(00)84365-6. [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhi X.; Zhang Q.; Wei S.; Xu Z. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumor Biol. 2016, 37, 2105–2117. 10.1007/s13277-015-4011-0. [DOI] [PubMed] [Google Scholar]
- Chang W. L.; Chapkin R. S.; Lupton J. R. Fish oil blocks azoxymethane-induced rat colon tumorigenesis by increasing cell differentiation and apoptosis rather than decreasing cell proliferation. J. Nutr. 1998, 128, 491–497. 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- Calviello G.; Palozza P.; Maggiano N.; Piccioni E.; Franceschelli P.; Frattucci A. Cell proliferation, differentiation, and apoptosis are modified by n-3 polyunsaturated fatty acids in normal colonic mucosa. Lipids 1999, 34, 599–604. 10.1007/s11745-999-0404-6. [DOI] [PubMed] [Google Scholar]
- Hu Y.; McIntosh G. H.; Leu R. K.; Nyskohus L. S.; Woodman R. J.; Young G. P. Combination of selenium and green tea improves the efficacy of chemoprevention in a rat colorectal cancer model by modulating genetic and epigenetic biomarkers. PLoS One 2013, 8, e64362. 10.1371/journal.pone.0064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambara-Murthy K. N.; Jayaprakasha G. K.; Patil B. S. Citrus limonoids and curcumin additively inhibit human colon cancer cells. Food Funct. 2013, 4, 803–810. 10.1039/c3fo30325j. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Dacosta C.; Wang W.; Zhou Z.; Liu M.; Bao Y. Synergy between sulforaphane and selenium in protection against oxidative damage in colonic CCD841 cells. Nutr. Res. 2015, 35, 610–617. 10.1016/j.nutres.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Moradian F.; Farziyan M. A.; Rafiei A. R. Anticancer effect of bovine lactoferrin on human esophagus cancer cell line. Res. Mol. Med. 2016, 4, 18–23. [Google Scholar]
- Inamori M.; Togawa J.; Matsumoto S.; Harad K.; Matsuura M.; Iida H. Protective effect of lactoferrin on acute acid reflux-induced esophageal mucosal damage. Hepato-Gastroenterol. 2014, 61, 1595–1600. [PubMed] [Google Scholar]
- Patti M. G.; Pellegrini C. A.; Arcerito M.; Tong J.; Mulvihill S. J.; Way L. W. Comparison of medical and minimally invasive surgical therapy for primary esophageal motility disorders. Arch. Surg. 1995, 130, 609. 10.1001/archsurg.1995.01430060047009. [DOI] [PubMed] [Google Scholar]
- Moon H. S.; Batirel S.; Mantzoros C. S. Alpha linolenic acid and oleic acid additively down-regulate malignant potential and positively cross-regulate AMPK/S6 axis in OE19 and OE33 esophageal cancer cells. Metabolism 2014, 63, 1447–1454. 10.1016/j.metabol.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Kitagawa H.; Namikawa T.; Yatabe T.; Munekage M.; Yamasaki F.; Kobayashi M. Effects of a preoperative immune-modulating diet in patients with esophageal cancer: a prospective parallel group randomized study. Langenbeck’s Arch. Surg. 2017, 402, 531–538. 10.1007/s00423-016-1538-5. [DOI] [PubMed] [Google Scholar]
- Cheng K.; Chen Y.; Zimniak P.; Raufman J. P.; Xiao Y.; Frucht H. Functional interaction of lithocholic acid conjugates with m3 muscarinic receptors on a human colon cancer cell line. Biochim. Biophys. Acta 2002, 1588, 48–55. 10.1016/S0925-4439(02)00115-1. [DOI] [PubMed] [Google Scholar]
- Cheng K.; Khurana S.; Chen Y.; Kennedy R. H.; Zimniak P.; Raufman J. P. Lithocholylcholine, a bile acid/acetylcholine hybrid, is a muscarinic receptor antagonist. J. Pharmacol. Exp. Ther. 2002, 303, 29–35. 10.1124/jpet.102.036376. [DOI] [PubMed] [Google Scholar]
- Wen W.; Liang W.; Wu J.; Kowolik C. M.; Buettner R.; Scuto A. Targeting JAK1/STAT3 signaling suppresses tumor progression and metastasis in a peritoneal model of human ovarian cancer. Mol. Cancer Ther. 2014, 13, 3037–3048. 10.1158/1535-7163.MCT-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsina G.; Xiao F.; O’Brien S. W.; Gabbasov R.; Maglaty M. A.; Xu R. H. Targeted blockade of JAK/STAT3 signaling inhibits ovarian carcinoma growth. Mol. Cancer Ther. 2015, 14, 1035–1047. 10.1158/1535-7163.MCT-14-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.; Nam S.; Tian Y.; Yang F.; Wu J.; Wang Y. 6-bromoindirubin-3-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Res. 2011, 71, 3972–3979. 10.1158/0008-5472.CAN-10-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek-Machado K.; Mieczkowski J.; Ellert-Miklaszewska A.; Swierk P.; Fokt I.; Szymanski S. Novel small molecular inhibitors disrupt the JAK/STAT3 and fak signaling pathways and exhibit a potent antitumor activity in glioma cells. Cancer Biol. Ther. 2012, 13, 658–670. 10.4161/cbt.20083. [DOI] [PubMed] [Google Scholar]
- Um H. J.; Min K. J.; Kim D. E.; Kwon T. K. Withaferin a inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma caki cells. Biochem. Bioph. Res. Co. 2012, 427, 24–29. 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Jin G.; Liu H.; Liu K.; Zhao J.; Chen X. Metformin inhibits esophageal squamous cell carcinoma-induced angiogenesis by suppressing JAK/STAT3 signaling pathway. Oncotarget 2017, 8, 74673–74687. 10.18632/oncotarget.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl C. D. EGFR-induced cell migration is mediated predominantly by the jak-stat pathway in primary esophageal keratinocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, 1227–1237. 10.1152/ajpgi.00253.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.