Abstract
Non-targeted analysis (NTA) methods are being increasingly used to aid in the identification of unknown compounds in the environment, a problem that has challenged environmental chemists for decades. Despite its increased use, quality assurance practices for NTA have not been well established. Furthermore, capabilities and limitations of certain NTA methods have not been thoroughly evaluated. Standard reference material dust (SRM 2585) was used here to evaluate the ability of NTA to identify previously reported compounds, as well as a suite of 365 chemicals that were spiked at various stages of the analytical procedure. Analysis of the unaltered SRM 2585 extracts revealed that several previously reported compounds can be identified by NTA, and that correct identification was dependent on concentration. A manual inspection of unknown features in SRM 2585 revealed the presence of two chlorinated and fluorinated compounds in high abundance, likely precursors to perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS). A retrospective analysis of data from the American Healthy Homes Survey revealed that these compounds were present in 42% of sampled homes. Spiking the dust at various stages of sample preparation revealed losses from extraction, cleanup, and instrumental analysis; the log Kow for individual compounds influenced the overall recovery levels but no pattern could be discerned from the various degrees of interference that the matrix had on the ionization efficiency of the spiked chemicals. Analysis of the matrix-free chemical mixture at low, medium, and high concentrations led to more correct identifications than analysis at one, very high concentration. Varying the spiked amount and identifying reported compounds at known concentrations allowed an estimation of the lower limits of identification (LOIs) for NTA, analogous to limits of detection in targeted analysis. The LOIs were much lower than levels in dust that would be likely to cause bioactivity in humans, indicating that NTA is useful for identifying and monitoring compounds that may be of toxicological concern. Graphical abstract.
Introduction
Identification of emerging environmental contaminants has long been a challenge to analytical chemists and all indications point to more contaminants existing in the environment that have yet to be identified [1,2,3,4]. Most environmental monitoring methods target known compounds and require class-specific methods, limiting studies to relatively small, selected panels of compounds. While these targeted methods have become exceedingly accurate and sensitive, they are unable to anticipate unknown chemicals of potential concern. A new era in analytical chemistry has therefore emerged with a dramatic rise in the use of non-selective high-resolution mass spectrometry (HRMS) platforms. HRMS instruments, most notably the time-of-flight (TOF) and Orbitrap instruments, allow analysts to simultaneously collect accurate mass measurements across a wide mass range. They further allow collection of MS/MS spectra for unknowns to enable compound identification. These enhancements effectively eliminate the need to target only a limited number of compounds in a given monitoring study.
The use of HRMS for non-targeted analysis and suspect screening (collectively referred to as “NTA”) has increased dramatically in recent years, enabling structural elucidation for emerging contaminants [5,6,7], as well as rapid identification of many known contaminants (from multiple compound classes) within a single analysis [3, 8, 9]. As a burgeoning field of science, NTA faces challenges regarding standard practices of quality assurance/quality control, acceptable reporting criteria, performance benchmarks, etc. [10]. To help address these challenges, the U.S. Environmental Protection Agency (EPA) is leading a multi-lab study called EPA’s Non-Targeted Analysis Collaborative Trial (ENTACT) [11]. ENTACT was first conceived by NTA experts convened at an EPA workshop in 2015 [12, 13]. Laboratories from government, academia, and industry in North America and Europe have since taken part in ENTACT sample analyses. Samples are partitioned across three research phases. Phase 1 included ten prepared mixtures of 95–365 chemical substances from EPA’s ToxCast library [12,13,14]. Phase 2 included extracts of standardized (and in some cases fortified) dust, human serum, and silicone bands. Finally, phase 3 included ~ 1200 or ~ 4200 individual chemical substances, from EPA’s ToxCast library, prepared in 384-well plates. Preliminary ENTACT results were presented and discussed at an EPA workshop in 2018. Further details regarding the genesis and design of ENTACT can be found in Ulrich et al. [12]
While ENTACT phase 1 was extremely useful for evaluating NTA methodologies using prepared mixtures of chemical standards (representing a “best-case” scenario for NTA), ENTACT phase 2 was designed to evaluate the abilities of NTA methods to identify chemicals in real-world samples at environmentally relevant concentrations. Dust was of particular interest, as it is known to be an important sink for many household contaminants [14]. A standardized and well-characterized dust sample was chosen for the dust portion of ENTACT phase 2: Standard Reference Material (SRM) 2585 “Organic Contaminants in House Dust”, sold by the National Institute of Standards and Technology (NIST) [11]. To reduce variability from the extraction and cleanup process, it was decided that dust extracts would be sent to ENTACT participants rather than aliquots of unextracted dust. There is still much interest, however, in characterizing the effectiveness of the utilized dust extraction procedures.
The current study focuses on the analysis of SRM 2585 extracts that were distributed as part of ENTACT. It further introduces a separate recovery experiment, using SRM 2585, designed to shed light on factors that affect analyte loss during extraction, cleanup, instrumental analysis, and data processing. Many compounds have been measured and reported in SRM 2585 to date; cross-referencing these compounds against those detected via NTA provides a unique means with which to critically evaluate NTA performance in a real-world context. The recovery experiment described herein further informs factors (e.g., matrix, extraction procedures) that influence compound identification using NTA. Finally, by analyzing the same mixture of compounds at different concentrations and in the presence and absence of dust matrix, the performance of NTA is evaluated here from a quantitative perspective, rather than a typical qualitative perspective. This article is meant to be a contribution, among several other articles, that helps address the challenges NTA faces as it moves towards becoming a critical part of twenty-first century exposure science [15, 16].
Methods
Materials
The dust used in this study was Standard Reference Material (SRM) 2585 “Organic Contaminants in House Dust” purchased from the NIST. Liquid chromatography (LC)/mass spectrometry (MS) grade acetonitrile and formic acid were purchased from Fisher Scientific (NJ, USA). Methanol (B&J Brand High Purity Solvent) was purchased from Honeywell Burdick & Jackson (Muskegon, MI, USA). Liquid chromatography/silica (LC-Si, 3 cm3) solid-phase extraction cartridges were obtained from Supelco (Bellefont, PA, USA).
Sample Preparation
Pure Mix 505 Dilutions
Ten ENTACT mixtures were designed by EPA scientists, each containing between 95 and 365 chemical substances from the ToxCast library [17]. One of these mixtures was chosen to fortify the ENTACT dust samples. This mixture was known as “Mix 505” and contained 365 chemical substances (see Electronic Supplementary Material (ESM) Table S1). Mix 505 was received at an approximate concentration of 0.05 mM per substance in dimethyl sulfoxide (DMSO). Dilutions were prepared at low, medium, and high concentrations corresponding to 0.02, 0.1, and 0.5 μM using methanol. An aliquot of each dilution was changed to 90:10 water:acetonitrile to match the starting mobile phase conditions of the LC method and a mixture of tracer compounds was added. The tracer mix consisted of 13C4 perfluorooctanesulfonic acid (PFOS), 13C4 perfluorooctanoic acid (PFOA), 13C2 perfluorodecanoic acid (PFDA), 15N2 fipronil sulfone, 13C3 atrazine, D3 pyriproxyfen, and D3 thiamethoxam (ESM Table S2).
ENTACT Dust Extracts
Description of the spiking and extraction procedures used for the original ENTACT dust samples (unfortified and fortified) can be found in Ulrich et al. [12]. Extracts of the original ENTACT dust samples (those which were sent to all participating labs) were examined here, along with additional dust extracts prepared to more fully examine factors affecting NTA performance. Procedures for the preparation of these additional dust extracts are described below.
Recovery Experiment
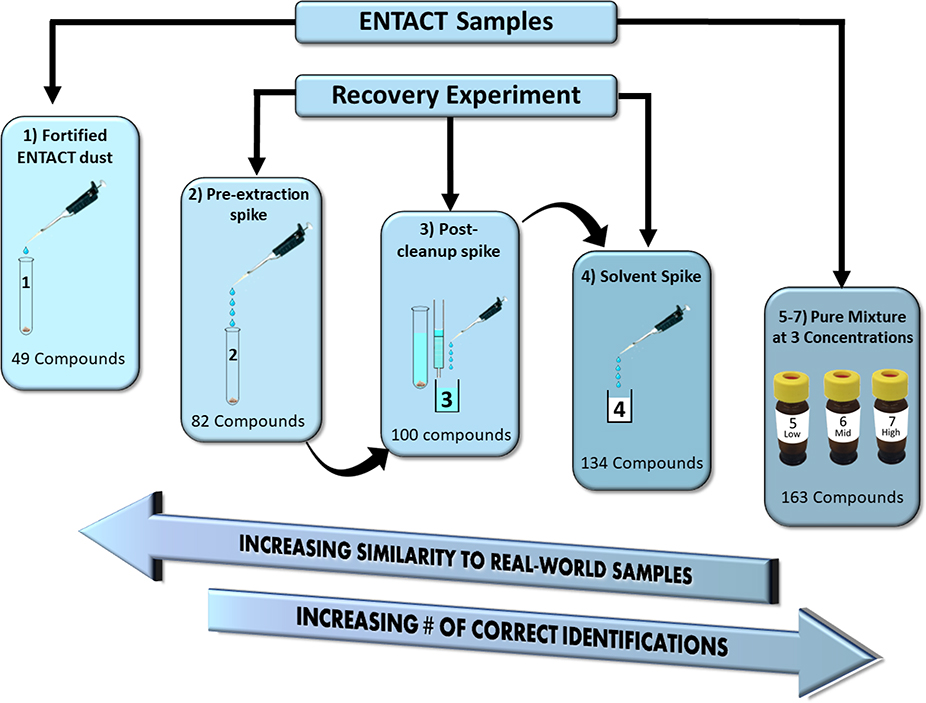
An additional recovery experiment was conducted for chemicals in Mix 505 that were spiked into the original ENTACT dust samples. Sample preparation procedures generally mimicked those used for the original ENTACT dust. In the current study, however, Mix 505 was used at four times higher concentration (1000–3000 ng/g for most compounds; ESM Table S1) and twice as much analyte was put on column. Six 50 mg aliquots of SRM 2585 were weighed into polypropylene centrifuge tubes. ENTACT Mix 505 in DMSO was diluted 2:1 in acetonitrile, and blank DMSO was diluted in the same manner. Three dust aliquots were spiked with 20 μL of diluted ENTACT Mix 505 (hereafter known as the “pre-extraction spiked samples”) and the other three were spiked with 20 μL of diluted DMSO (hereafter known as the “post-cleanup spiked samples”). Methanol (4 mL) was added to the samples and they were further prepared in the same manner as the original ENTACT dust samples. Briefly, the samples were extracted by vortex mixing, sonication for 30 min, and additional vortex mixing before being centrifuged. The entire supernatant was passed through pre-conditioned LC-Si cartridges and was collected. An additional 2 mL of fresh methanol was passed through the cartridges and combined with the original eluent. Cleaned extracts were reduced in volume to 0.5 mL under a gentle nitrogen stream at 40 °C. The pre-extraction spiked samples were then spiked with 20 μL of diluted DMSO and post-cleanup spiked samples were spiked with 20 μL of the diluted Mix 505. The purpose of adding the diluted DMSO was to ensure that any effects of DMSO on the extraction, cleanup, or instrumental analysis occurred to all samples equally. Blank solvent (0.5 mL acetonitrile) was also spiked with diluted ENTACT Mix 505 to investigate matrix effects on compound identification. This solvent spike sample is very similar to the mixture analysis samples in that it is simply comprised of the mixture diluted in solvent. This additional sample was necessary to include as part of the spiking experiment for direct comparison with the other spiking experiment samples as it contained the same spiked amount. In total, 20 prepared samples were included for this examination, including nine Mix 505 dilutions (three replicates at three concentrations), one extract of unfortified SRM 2585 (from ENTACT), one extract of fortified SRM 2585 (also from ENTACT), three extracts of pre-extraction spiked samples (four times greater concentration than ENTACT samples), three post-cleanup spiked samples (four times greater concentration than ENTACT samples), and three solvent spike samples (same spiking amount and volume of extract as the pre-extraction and post-cleanup spiked samples). A summary of the various sample types can be found in Table 1.
Table 1 —
Summary of the different samples included in this study.
| Sample Name | Spike Level | Sample Number in Figure 3 | Contains Dust Matrix? | Did Chemicals of Interest undergo Cleanup? |
|---|---|---|---|---|
| Mix 505 Analysis | Low, Medium, High | 5,6,7 | No | No |
| Solvent Spike | Very High | 4 | No | No |
| Post-cleanup Spike | Very High | 3 | Yes | No |
| Pre-extraction Spike | Very High | 2 | Yes | Yes |
| Fortified ENTACT | Low | 1 | Yes | Yes |
| Unfortified ENTACT | NA | Not Shown | Yes | Yes |
All samples, including the original ENTACT samples described above, were spiked with 50 ng of isotopically labeled tracers to track instrument performance. Tracer response did not exceed 20% relative standard deviation throughout the analysis for replicate samples. Samples spiked with tracers were vortexed before a 100 μL aliquot was removed and reduced to dryness with a gentle stream of nitrogen, followed by reconstitution in 100 μL 90:10 water:acetonitrile to match the starting mobile phase conditions of the LC method.
2.3. Instrumental Analysis
Instrumental analysis was performed using an Agilent 1290 Infinity II LC system coupled to a 6530B Accurate-Mass QTOF/MS (Santa Clara, CA) with a Dual AJS ionization source. For separation, a Waters Acquity UPLC® BEH C18 column (2.1 × 50 mm, 1.7 μm) was used. Mobile phase A was water with 0.1% formic acid and mobile phase B was acetonitrile with 0.1% formic acid. The flow rate was 0.2 mL/min and the gradient was as follows: 10% B hold for 2 min, 13 min linear gradient to 100% B, hold for 5 min, 1 min return to 10% B, hold for 9 min to equilibrate for next run. The total run time was 30 min per injection. Samples were analyzed in two batches, one for each polarity with electrospray ionization (ESI; details on settings can be found in Sobus et al. [18]). MS1 data was collected from 100 to 1000 m/z. ENTACT study samples were injected in triplicate, whereas samples from the recovery experiment were prepared in triplicate and injected only once. For MS/MS, a data-dependent acquisition (DDA) method was used in which a maximum of three of the most abundant precursors were selected for fragmentation at 10, 20, and 40 eV, cycling at 4 Hz.
2.4. Data Processing
The workflow used is described in detail in Sobus et al. [18]. Briefly, features were extracted recursively and aligned using Agilent Profinder B.08 (Santa Clara, CA). Noise thresholds were estimated by inspecting a spectrum from an area of the chromatogram with relatively little total ion current. Judgement was used to distinguish spectral noise from compound signal in the region near 300 Da by manually inspecting several samples; the noise threshold in Profinder was set approximately 25% above the noise height at 800 counts (ESM Table S3). Tracers were inspected across all runs to determine retention time shifts and the alignment setting was set to approximately double the maximum shift to ensure proper alignment. After processing, tracers were manually inspected for proper alignment and mass accuracy. Profinder settings were adjusted if not all tracers were properly aligned and the data was reprocessed. Mass error was never greater than 5 ppm for the tracers (ESM Table S2).
Aligned features were exported from Profinder as .cef files and subsequently imported into Agilent Mass Profiler Professional (Version B.14.9.1) where they were matched to a database of MS-Ready formulas [19] generated from the Distributed Structure-Searchable Toxicity (DSSTox) Database. The database contained ~ 149,000 unique MS-Ready formulas that were generated from ~ 776,000 unique substances. This database is updated frequently and the most current version is available for download in .csv format on the downloads page of the CompTox Chemicals Dashboard (hereafter, the “Dashboard”) (https://comptox.epa.gov/dashboard/downloads) [20]. Only formulas with a match score of 90 or greater were retained. Candidate compounds that mapped to MS-Ready formulas were retrieved from the Dashboard and ranked by number of data sources [21]. Custom scripts written in R were used to filter out features that were not present in all three replicates or had a coefficient of variation above 10% across the three replicates. MS/MS data were matched to the following Personal Compound Databases and Libraries (PCDLs) purchased from Agilent Technologies using Agilent Qualitative Workflows B.08.00: Environmental Water Screening; Pesticides; Forensic Toxicology; Metlin; Extractables and Leachables; and Veterinary drugs. The forward and reverse scores for MS/MS matching were set to 0 and 20, respectively. All MS/MS matches were manually inspected for accuracy.
Certain chemicals are known to be present in SRM 2585 based on previous targeted analyses, and others are expected to be in Mix 505. Each of these chemicals was scored from 0 to 5 stars in the unfortified SRM2585 and the pure Mix 505 analysis, according to the criteria described in Sobus et al. [18]. These stars convey the weight-of-evidence behind a feature’s match to a compound known to be present in the sample. This differs from the Schymanski scale [22] in that the Schymanski scale is designed to convey confidence in a tentative identification of an unknown compound. The five star levels, in descending order based on strength of identification, are as follows: 5 stars = PCDL match to MS/MS spectrum; 4 stars = correct formula and no. 1 ranked candidate compound; 3 stars = correct formula and ranked in the top 5 candidate compounds; 2 stars = correct formula but not in the top five candidate compounds; 1 star = mass match within 15 ppm; 0 stars = no mass detected. Only compounds identified with three stars or higher were evaluated for losses in the recovery experiment.
Sample features that had not been assigned a formula or identified by MS/MS in unfortified SRM 2585 were manually inspected. Specific focus was placed on features with a mass defect between − 0.2 and 0.1, which is often indicative of halogenated organic compounds [23], and the most abundant compounds were inspected first. Formula prediction was attempted using Agilent Qualitative Navigator B.08.00 and targeted MS/MS data was acquired at various collision energies to gather any information that is helpful in identification.
2.5. Literature Search for SRM 2585
A list of organic compounds reported to be present in SRM 2585 was compiled in April–May 2017 (ESM Table S4) starting with certified and reference values reported by the NIST [11]. The use of LC-ESI for these compounds in SRM 2585 was noted; however, a negative denotation on the use of LC-ESI does not necessary indicate the compounds are not amenable to LC-ESI, only that it was not used in the studies we found reporting on SRM 2585. For certified and reference concentrations, the value reported by the NIST was recorded above other values in the literature. For compounds reported in the literature but not by the NIST, average concentrations from studies reporting on multiple sources (e.g., an inter-lab study) were recorded. For all other compounds, the average concentration of all sources found during the literature search was recorded.
2.6. Determining Toxicity Thresholds for Dust
Two approaches were used to estimate dust concentrations that may lead to a toxic response in sensitive populations. The first approach involves a simple application of thresholds of toxicological concern (TTCs), which are broad values below which there is a very low probability of an appreciable risk to human health [24]. Chemicals can fall into one of three categories based on a decision tree [25], and each category has a different TTC. The values for these three classes for chemicals in diet have been reported by Kroes et al. [24]. TTCs for the three classes, expressed as milligrams per kilogram body weight per day, were converted into dust concentrations (ng/g) using 11 kg body weight and a dust ingestion rate of 60 mg/day, which were taken from EPA’s Exposure Factor Handbook [26] and represent values for toddlers. Toddlers were chosen because they represent the highest risk age demographic for dust ingestion and these calculations assume ingestion is the main exposure pathway to dust for toddlers.
The second approach to determining levels of toxicological relevance in dust involves taking values derived from pharmacokinetic modeling using in vitro ToxCast data [27] that examined variability in the US population with regard to sensitivity to chemicals. We simulated the “worst-case scenario” and used exposure values needed in the most-sensitive 5% of the population to produce a steady-state plasma concentration equal to the 10th percentile of ToxCast half-maximal activity concentration (AC50) distribution across assays for the given chemical.
Results and Discussion
Unfortified SRM 2585
NTA of literature-reported chemicals
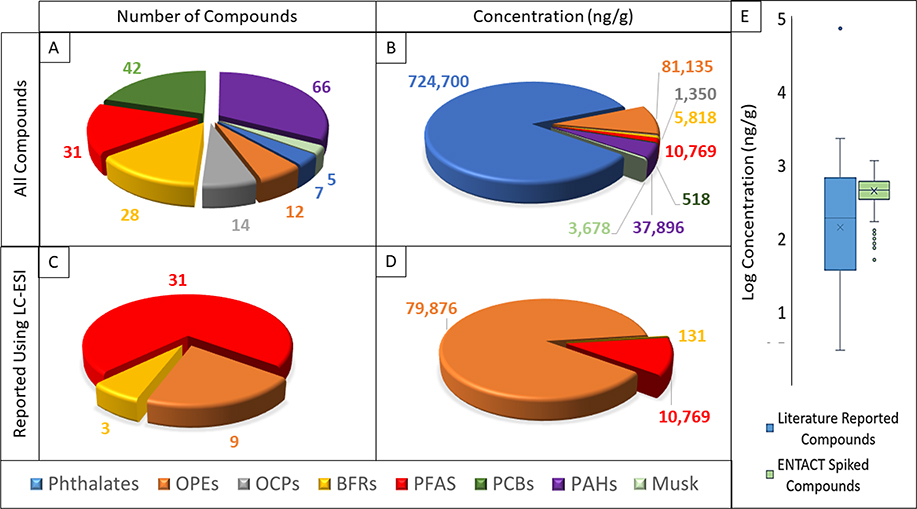
To evaluate NTA performance using known compounds in a real sample, a literature search was performed, yielding a list of organic compounds and their corresponding concentrations reported in SRM 2585 (ESM Table S4, also can be downloaded as a list from the Dashboard at https://comptox.epa.gov/dashboard/chemical_lists/SRM2585DUST). As of the time of this literature search, 212 organic compounds had been reported in SRM 2585 (shown in Fig. 1a), 14 of which could not be chromatographically separated from an isomer and are reported as a pair, leaving a total of 205 potential compounds or compound pairs to identify. These can be classified into seven categories: polyaromatic hydrocarbons (PAHs; n = 66); polychlorinated biphenyls (PCBs; n = 42); per- and polyfluorinated alkyl substances (PFAS; n = 31); brominated flame retardants (BFRs; n = 28, most of which are polybrominated diphenyl ethers [PBDEs]); organochlorine pesticides (OCPs; n = 14); organophosphate ethers (OPEs; n = 12); and phthalates (n = 7). While only 7 phthalates had been reported, they accounted for 84% of the total concentration of the 205 compounds (Fig. 1b), the bulk of which being attributed to di(2-ethylhexyl) phthalate (DEHP).
Figure 1 –
A) Number of compounds in chemical classes of all literature reported organic compounds in SRM 2585 B) Total concentration (ng/g) of all literature reported compounds in each compound class in SRM 2585 C) Number of compounds detected by LC-ESI in each chemical classes in SRM 2585 D) Total concentration (ng/g) of compounds detected by LC-ESI in each chemical class in SRM 2585 E) The range of concentrations for all literature reported compounds and spiking levels of Mix 505 in the ENTACT dust samples.
Only 43 compounds were reported as being detected in SRM 2585 via LC-ESI (Fig. 1c), with OPEs contributing to 88% of the total concentration (Fig. 1d). Of these 43 compounds, 10 were correctly identified at the formula level (score ≥ 3) using our NTA methodology (ESM Table S4). Further investigation into some compounds that were expected to be seen (higher concentrations and LC-ESI amenable) revealed identification of in-source fragments for three compounds: bis(2-butoxyethyl) hydrogen phosphate, which is believed to be a fragment of tris(2-butoxyethyl) phosphate (TBEP); dibutyl hydrogen phosphate, which is believed to be a fragment of tributyl phosphate (TBP); and mono(2-ethylhexyl) phthalate (MEHP), which is believed to be a fragment of DEHP (ESM Fig. S1). MEHP was observed at two retention times: the earlier eluting peak likely coming from MEHP as a sample degradant of DEHP [28] (as the loss of a carbon chain would make MEHP more polar than DEHP) and the later eluting peak likely coming from MEHP as an in-source fragment of DEHP.
Concentration was a major factor determining if previously reported compounds were observed or not, regardless of compound class (ESM Fig. S2). Compounds that were correctly identified via NTA had significantly higher concentrations than those that were not identified or not observed (Mann-Whitney test, p < 0.01).
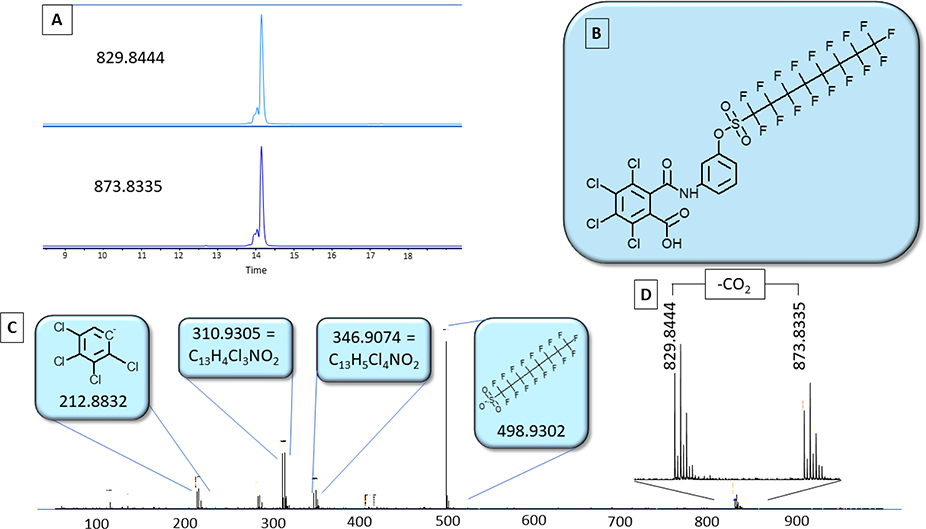
3.1.2. Discovery of a Chlorinated PFOS precursor
The two most abundant features that had not already been assigned a formula, identified by MS/MS, and were not recognized as in-source fragments of other compounds were at m/z 829.8444 and 729.8514. Both features exhibited the isotopic pattern indicative of four chlorines (Fig. 2, ESM Fig. S3), and the observed ions differed by 99.9945 Da, a common mass difference observed among homologues of PFAS as the mass of a C2F4 unit. These compounds were targeted for fragmentation to acquire MS/MS spectra (Fig. 2, ESM Fig. S4). The MS/MS spectra of the two compounds were similar except for diagnostic ions immediately recognizable as perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS), suggesting that the two unidentified compounds contain PFOS and PFHxS substructures. Each spectrum contained an isotope cluster with a four-chlorine pattern at m/z 212.8832, indicating four chlorines on one benzene ring. Formulas were predicted for other fragments leading to putative formulas for the molecules of C21H5O4SNCl4F17 and C19H5O4SNCl4F13.
Figure 2 –
A) Extracted ion chromatograms of TCBA-BA-PFOS and its decarboxylated version showing coelution B) The structure of TCBA-BA-PFOS C) MS/MS of TCBA-BA-PFOS with proposed structures or formulas for the fragments D) MS1 spectrum of TCBA-BA-PFOS (873.8335) and its decarboxylated fragment (829.8444)
Comparison of the detected species to SciFinder yielded no matches at the putative formula level, but structural similarity searches of hypothesized structures suggested 2,3,4,5-tetrachloro-6-[[[3-[[(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-heptadecafluorooctyl)sulfonyl]oxy]phenyl]amino]carbonyl]-benzoic acid (TCBA-BA-PFOS). This compound was a carboxylated version of the hypothesized structure and re-inspection of the original MS spectra revealed low abundance isotope clusters exhibiting the same chlorinated isotope pattern at m/z 873.8335 and 773.8369. It is believed that the peaks at m/z 873.8335 and 773.8369 are TCBA-BA-PFOS and a homologue containing PFHxS instead of PFOS. The originally observed peaks at m/z 829.8444 and 729.8514 are likely in-source fragments formed by decarboxylation. This is supported by the fact that the m/z 829.8444 and 873.8335 peaks co-elute and formation of the decarboxylated fragments is dependent on fragmentor voltages in the source.
Only recently have these two compounds been reported in the literature, by Zhang et al., who described the decarboxylated version of the two compounds and arrived at their structures by very different means [29]. Zhang et al. used a GC-QTOF for analysis and selected these peaks for closer analysis based on their proximity in chemical space to well-known persistent organic pollutants (POPs) and their high abundance in SRM 2585. These compounds are listed in several patents dating as far back as 1975, assigned to Minnesota Mining and Manufacturing Co. (3M) for textile treatment [30, 31] and hairspray [32]. They are also included in patents for insecticide use by the U.S. Department of Agriculture [33] and U.S. Department of Energy [34]. They were included, along with 74 other PFAS including PFOS, in a significant new use rule in 2002 by the EPA [35]. TCBA-BA-PFOS was also listed in a 2004 UK evaluation of PFOS as “Potentially Degrading to PFOS in the Environment” [36]. A retrospective analysis of the mass spectral data from 56 dust samples from the American Healthy Homes Survey revealed that TCBA-BA-PFOS was present in 23 of the 55 samples (42%) [3]. These samples were taken in 2005–2006 and it is unclear if TCBA-BA-PFOS presence in American homes has declined along with PFOS after being phased out of production starting in 2002. It is also unclear to what extent TCBA-BA-PFOS degrades into PFOS and contributes to PFOS contamination in the environment.
3.2. Spiked SRM 2585
Of the 365 compounds spiked into Mix 505, 71 were identified by matching to an MS/MS library (5 stars), 63 were assigned the correct formula and were the top ranked compound from the Dashboard search (4 stars), and 29 were assigned the correct formula but were not the top Dashboard hit (usually second or third, 3 stars). Thus, 163 compounds were assigned correct formulas or identified by MS/MS. Accurate mass matches (i.e., within 15 ppm) for an additional 72 compounds were also observed, but these were not included in further data analysis. The analysis of the fortified ENTACT sample identified 49 compounds at the formula level or greater, a stark contrast to the 163 identified in the Mix 505 analysis (Fig. 3).
Figure 3 –
The various types of spiked samples analyzed. The sample numbers correspond to those in Table 1.The complexity and difficulty in identifying compounds decreases from left to right as does the similarity to environmental concentrations and sample type. The number of compounds in each box represents the number of correct identifications out of 365 compounds that were spiked.
3.2.1. Multiple Concentrations
The spiked solvent sample from this experiment was double the concentration of the highest mix in the Mix 505 analysis, and yet 29 fewer compounds were identified (134 in the spiked solvent vs. 163 in the ENTACT mixture analysis). This challenges the assumption that more abundant features are easier to identify. However, the fact that the Mix 505 analysis was conducted at three different concentrations may offer some explanation. It is not only necessary for enough signal to be present to identify compounds using NTA, as was concluded previously in the section on literature-reported compounds in the non-spiked dust, but also necessary to not have too much signal. Too much signal may result in detector saturation, which has been observed to cause large errors in mass accuracy. An increased spike amount may also increase signals of interfering ions in the spectrum, leading to missed identifications.
The concentration range in which a compound can be identified by NTA varies dramatically for each compound as instrument response and matrix suppression vary dramatically for each compound. Thus, analyzing at multiple concentrations results in the largest number of identified compounds and this should be considered in studies where the greatest number of identifications is the goal. It remains to be seen how other instruments perform in this regard, particularly Orbitraps which have more control over the number of ions entering the detector. Many ENTACT participants used Orbitrap instruments, so this issue should be answerable in future manuscripts.
3.2.2. Matrix Effects
Comparison of the spiked solvent and the post-cleanup spike revealed that 34 compounds experienced matrix effects and were no longer identified. Matrix interference could happen in many places and in various ways, for example, (1) in the sample extract, chemical reactions or competitive solubility could result in compound loss; (2) on the analytical column, high carbon load or competing column interactions could cause peak dispersion; or (3) in the ion source, competitive ionization could lead to decreased signal or interfering ions in a compound’s spectrum could hinder correct identification. The degree of matrix interference for each compound was calculated as a percentage according to Eq. 1:
Where Peak Areamatrix = the average peak area of the compound in the three replicates of post-cleanup spikes and Peak Areasolvent = the average peak area of the compound in the three spiked solvent replicates. Compounds with % Matrix interference = 100 were detected in the spiked solvent but not in the spiked dust matrix above a signal-to-noise ratio of three. The percentages for matrix interference can be found in Table S1. In a few cases, a negative value was obtained for % Matrix interference, indicating the signal for that compound was enhanced in the presence of the dust matrix which has been known to occur [37]. It was hypothesized that competitive ionization is the main factor contributing most to ion suppression. However, if this were true, the degree of ion suppression would correlate with the total ion current at the time of elution; no such correlation was found. No meaningful trends were observed regarding chemical structure and matrix interference.
3.2.3. Extraction Efficiency
In the pre-extraction spike, 82 compounds were identified compared with 100 in the post-cleanup spike, meaning that 18 compounds were lost in the extraction and/or cleanup processes. To investigate these losses, octanol-water partition coefficients (log Kow), predicted by OPERA [38] and downloaded from the Dashboard, were examined. The 82 recovered compounds had significantly lower log Kow values (Mann-Whitney test, p < 0.01) than the 18 compounds that were not recovered, meaning that polar compounds were more likely to be recovered and non-polar compounds tended to be lost during extraction and cleanup (Fig. 3, ESM Fig. S5). The median recovery for the 82 compounds that were recovered was 93% as calculated by Eq. 2:
Where Peak areapre is the average peak area of the three pre-extraction spike replicates and Peak areapost is the average peak area of the three post-cleanup spike replicates.
There are two steps where compounds are likely lost between these two spiking points: (1) the extraction itself (i.e., compounds may not be soluble in methanol, or sorb more strongly to the dust) or (2) the silica column cleanup step. Given that silica is a polar stationary phase and dust is more likely to retain non-polar compounds, this difference in log Kow between recovered and non-recovered compounds indicates compounds are probably retained in the dust after extraction. Different extraction solvents (hexane, isopropanol) or schemes (sequential extraction) may recover more of the lost compounds, but care must be taken to retain polar compounds; we leave those experiments for future work. The goal of this experiment was to demonstrate the value of a comprehensive evaluation of an NTA method rather than to optimize that method.
3.2.4. Concentration Differences
The recovery experiment was designed so that there were minimal differences between the pre-extraction spiked dust and fortified ENTACT dust sample except the concentration of the spiked compounds. As mentioned, 82 of the spiked compounds were identified in the pre-extraction spiked dust and 49 were identified in the fortified ENTACT sample, suggesting that the loss of 32 compounds can be attributed to concentration differences alone. Upon manual inspection of the extracted ion chromatograms, it is evident that these lost 32 compounds were indeed in low abundance in the pre-extraction spike and exhibit a very small peak or no peak at all in the ENTACT sample.
3.3. Limits of identification
When performing targeted analysis, limits of detection (LODs) are typically defined as the amount of compound needed to produce a signal-to-noise ratio of 3:1 (or other defined threshold). When performing NTA, however, a higher concentration may be required to produce an isotope pattern for correct database matching or to trigger an MS/MS event and produce a quality fragmentation spectrum for library matching. Here, we define a limit of identification (LOI) as the minimum amount of compound required to result in a correct identification when performing NTA. It is more difficult to accurately determine these LOIs than it is to determine LODs when performing targeted analysis because extrapolation is not possible; i.e., for a compound that has been correctly identified, it cannot be estimated how much less analyte can be present and still result in a correct identification, though such estimation is possible for a signal-to-noise ratio. For practical purposes, we report our LOIs for spiked compounds as the minimum dust concentrations at which compounds were correctly identified, in units of nanograms per gram. The LOIs are specific to our NTA method and encompass the extraction efficiency, instrument performance, and data filters. This is a useful exercise, as it allows us to add a semi-quantitative approach to the typically qualitative practice of NTA.
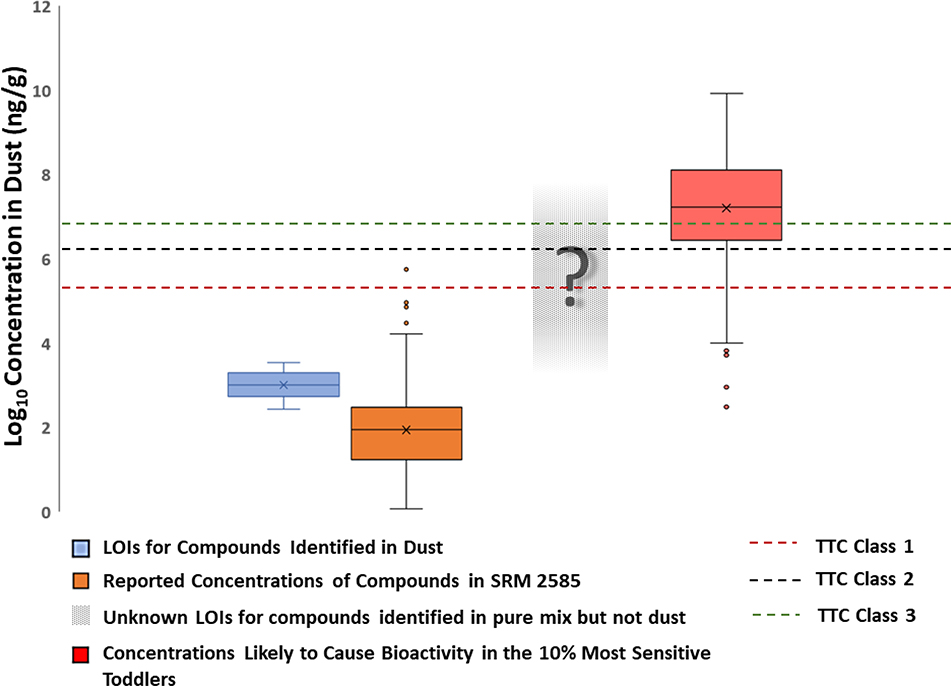
LOIs, as determined from the fortified ENTACT dust and the pre-extraction spike, could be determined for 100 compounds. The LOIs ranged from 280 to 3500 ng/g (ESM Table S1). While these values are much higher than typical limits of detection for targeted methods, a more relevant comparison for exposure science is to determine if these values are low enough to identify compounds in dust that could possibly cause a toxic response. LOIs that could be determined in this study generally fall about two orders of magnitude below the lowest TTC (Fig. 4).
Figure 4 –
Limits of Identification compared with dust concentration likely to cause bioactivity in sensitive populations and thresholds of toxicological concern
There is little overlap in the distribution of the worst-case scenario chemicals and our LOIs or with reported concentrations in SRM 2585 (Fig. 4). This indicates that concentration is unlikely to be a limiting factor in our ability to identify unknown chemicals using NTA that are present in dust at concentrations likely to be of toxicological concern. This finding indicates that many of the thousands of unknown features observed in a given NTA experiment are individually likely of little toxicological consequence. While researchers should not discard these features altogether, they would be wise to prioritize the more abundant features in a sample set for further analysis, such as in Rager et al. [3] and Newton et al. [2]. It is important to note that the comparison here uses only the LOIs that could be determined and are specific to the methods used in this study. There were many chemicals identified in the pure mixture analysis but not the dust samples, so LOIs for these compounds are likely to be higher than the spiked concentrations. Furthermore, the Kow evaluation from the recovery study revealed that this method likely loses compounds with a log Kow greater than 3.5 which, coincidentally, are more likely to be bioaccumulative and, thus, of greater toxicological concern.
Conclusion
Here we have characterized the types of compounds likely to be identified using our method of NTA, as well as the concentration ranges where those compounds can be identified. We must emphasize that this evaluation is specific to the extraction and cleanup methods, the chromatography, and the instrument used. For labs performing NTA on dust, the list of reported compounds in SRM 2585 can be used for self-evaluation. Weaknesses in NTA were identified, such as the inability to identify in-source fragmentation. The contemporary reports of a chlorinated PFOS precursor by two separate groups, in spite of decades of use, highlight two facts: (1) in the past, emerging chemicals of concern have remained in use for long periods of time before their discovery due to the challenges involved with elucidating unknown compounds and (2) NTA is becoming known as a useful tool to discover these chemicals.
The recovery experiment conducted allowed us to evaluate the chemical space covered by our own NTA method, report the recovery of spiked compounds, and challenge our assumptions about ionization changes due to matrix effects (i.e., ion suppression should correlate with total ion current). It also provides a framework for self-evaluation for labs performing NTA that can be applied to other matrices. As the use of NTA increases, we encourage laboratories to perform similar types of self-evaluation and hope to make this kind of evaluation standard practice before publishing NTA data. The concentrations at which a variety of compounds could be identified (i.e., LOIs) were mostly below concentrations likely to cause some toxicological effect in toddlers, but some overlap did exist. Thus, analysts performing NTA in dust are advised to prioritize identifying a smaller number of more abundant features than a larger number of less abundant features.
Supplementary Material
Acknowledgements
The authors would like to acknowledge John Wambaugh for providing pharmacokinetic modeled data. We would also like to acknowledge Paul Price for first applying the concept of Thresholds of Toxicological Concern to NTA data. Matthew Scott Clifton is acknowledged for his valuable input, particularly regarding mechanisms of matrix interference.
References
- 1.Alygizakis NA, Samanipour S, Hollender J, Ibáñez M, Kaserzon S, Kokkali V et al. Exploring the potential of a global emerging contaminant early warning network through the use of retrospective suspect screening with high-resolution mass spectrometry. Environmental Science & Technology. 2018;52(9):5135–44. doi: 10.1021/acs.est.8b00365. [DOI] [PubMed] [Google Scholar]
- 2.Newton SR, McMahen RL, Sobus JR, Mansouri K, Williams AJ, McEachran AD et al. Suspect screening and non-targeted analysis of drinking water using point-of-use filters. Environmental Pollution. 2018;234:297–306. doi: 10.1016/j.envpol.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rager JE, Strynar MJ, Liang S, McMahen RL, Richard AM, Grulke CM et al. Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environment International. 2016;88:269–80. doi: 10.1016/j.envint.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Phillips KA, Yau A, Favela KA, Isaacs KK, McEachran A, Grulke C et al. Suspect screening analysis of chemicals in consumer products. Environmental Science & Technology. 2018;52(5):3125–35. doi: 10.1021/acs.est.7b04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E et al. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environmental Science & Technology. 2015;49(19):11622–30. [DOI] [PubMed] [Google Scholar]
- 6.Newton S, McMahen R, Stoeckel JA, Chislock M, Lindstrom A, Strynar M. Novel polyfluorinated compounds identified using high resolution mass spectrometry downstream of manufacturing facilities near Decatur, Alabama. Environmental Science & Technology. 2017;51(3):1544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagnino S, Strynar MJ, McMahen RL, Lau CS, Ball C, Garantziotis S et al. Identification of biomarkers of exposure to FTOHs and PAPs in humans using a targeted and nontargeted analysis approach. Environmental Science & Technology. 2016;50(18):10216–25. doi: 10.1021/acs.est.6b01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerona RR, Schwartz JM, Pan J, Friesen MM, Lin T, Woodruff TJ. Suspect screening of maternal serum to identify new environmental chemical biomonitoring targets using liquid chromatography–quadrupole time-of-flight mass spectrometry. Journal of Exposure Science And Environmental Epidemiology. 2017;28:101. doi: 10.1038/jes.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andra SS, Austin C, Wright RO, Arora M. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: Towards a retrospective temporal exposome. Environment International. 2015;83:137–45. doi: 10.1016/j.envint.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hites RA, Jobst KJ. Is nontargeted screening reproducible? Environmental Science & Technology. 2018;52(21):11975–6. doi: 10.1021/acs.est.8b05671. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich EM, Sobus JR, Grulke CM, Richard AM, Newton SR, Strynar MJ et al. EPA’s non-targeted analysis collaborative trial (ENTACT): genesis, design, and initial findings. Analytical and Bioanalytical Chemistry. 2019;411(4):853–66. doi: 10.1007/s00216-018-1435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobus JR, Wambaugh JF, Isaacs KK, Williams AJ, McEachran AD, Richard AM et al. Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. Journal of Exposure Science and Environmental Epidemiology. 2018;28(5):411–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moschet C, Anumol T, Lew BM, Bennett DH, Young TM. Household dust as a repository of chemical accumulation: new insights from a comprehensive high-resolution mass spectrometric study. Environmental Science & Technology. 2018;52(5):2878–87. doi: 10.1021/acs.est.7b05767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Standards and Technology. SRM 2585 - organic contaminants in house dust. 2019. https://www-s.nist.gov/srmors/view_detail.cfm?srm=2585. Accessed 8/22/2019 2019.
- 15.National Academies of Sciences E, Medicine. Using 21st century science to improve risk-related evaluations. National Academies Press; 2017. [PubMed] [Google Scholar]
- 16.Thomas RS, Bahadori T, Buckley TJ, Cowden J, Deisenroth C, Dionisio KL et al. The next generation blueprint of computational toxicology at the US Environmental Protection Agency. Toxicological Sciences. 2019;169(2):317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I et al. ToxCast chemical landscape: paving the road to 21st century toxicology. Chemical Research in Toxicology. 2016;29(8):1225–51. doi: 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- 18.Sobus JR, Grossman JN, Chao A, Singh R, Williams AJ, Grulke CM et al. Using prepared mixtures of ToxCast chemicals to evaluate non-targeted analysis (NTA) method performance. Analytical and Bioanalytical Chemistry. 2019;411(4):835–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McEachran AD, Mansouri K, Grulke C, Schymanski EL, Ruttkies C, Williams AJ. “MS-Ready” structures for non-targeted high-resolution mass spectrometry screening studies. Journal of Cheminformatics. 2018;10(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. Journal of Cheminformatics. 2017;9(1):61. doi: 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEachran AD, Sobus JR, Williams AJ. Identifying known unknowns using the US EPA’s CompTox Chemistry Dashboard. Analytical and Bioanalytical Chemistry. 2017;409(7):1729–35. doi: 10.1007/s00216-016-0139-z. [DOI] [PubMed] [Google Scholar]
- 22.Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP et al. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environmental Science & Technology. 2014;48(4):2097–8. [DOI] [PubMed] [Google Scholar]
- 23.Jobst KJ, Shen L, Reiner EJ, Taguchi VY, Helm PA, McCrindle R et al. The use of mass defect plots for the identification of (novel) halogenated contaminants in the environment. Analytical and Bioanalytical Chemistry. 2013;405(10):3289–97. doi: 10.1007/s00216-013-6735-2. [DOI] [PubMed] [Google Scholar]
- 24.Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A et al. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food and Chemical Toxicology. 2004;42(1):65–83. doi: 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Cramer GM, Ford RA, Hall RL. Estimation of toxic hazard—A decision tree approach. Food and Cosmetics Toxicology. 1976;16(3):255–76. doi: 10.1016/S0015-6264(76)80522-6. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Environmental Protection Agency (EPA). Exposure Factors Handbook: 2011 Edition. In: Assessment NCfE, editor. Washington, DC [Google Scholar]
- 27.Ring CL, Pearce RG, Setzer RW, Wetmore BA, Wambaugh JF. Identifying populations sensitive to environmental chemicals by simulating toxicokinetic variability. Environment International. 2017;106:105–18. doi: 10.1016/j.envint.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staples CA, Peterson DR, Parkerton TF, Adams WJ. The environmental fate of phthalate esters: A literature review. Chemosphere. 1997;35(4):667–749. doi: 10.1016/S0045-6535(97)00195-1. [DOI] [Google Scholar]
- 29.Zhang X, Di Lorenzo RA, Helm PA, Reiner EJ, Howard PH, Muir DCG et al. Compositional space: A guide for environmental chemists on the identification of persistent and bioaccumulative organics using mass spectrometry. Environment International. 2019. doi: 10.1016/j.envint.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loudas BL, inventor Minnesota Mining and Manufacturing Co., USA: assignee. Textile treatment patent DE2508537A1 1975.
- 31.Fong JJ, inventor Minnesota Mining and Manufacturing Co., USA: assignee. Treating composition containing fluorochemical compound mixture and textiles treated therewith patent US4681790A 1987.
- 32.Ko SS, inventor Minnesota Mining and Manufacturing Co., USA: assignee. Hair spray containing fluorocarbon compounds as additives patent US4044121A 1977.
- 33.Vander Meer RK, inventor United States Dept. of Agriculture, USA: assignee. Insecticidal compositions patent US455727A0 1983.
- 34.Vander Meer RK, Lofgren CS, Williams DF, inventors; United States Dept. of Energy, USA. assignee. Control of insects with fluorocarbons patent US758856A0 1986.
- 35.Perfluoroalkyl sulfonates; significant new use rule. Fed Regist. 2002;67(47):11008–13. [Google Scholar]
- 36.Brooke D, Footitt A, A Nwaogu T. Environmental Risk Evaluation Report: Perflurooctanesulphonate (PFOS). 2004. [Google Scholar]
- 37.Mallet CR, Lu Z, Mazzeo JR. A study of ion suppression effects in electrospray ionization from mobile phase additives and solid‐phase extracts. Rapid Communications in Mass Spectrometry. 2004;18(1):49–58. [DOI] [PubMed] [Google Scholar]
- 38.Mansouri K, Grulke CM, Judson RS, Williams AJ. OPERA models for predicting physicochemical properties and environmental fate endpoints. Journal of Cheminformatics. 2018;10(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.