1. Invited essay
The world is presently grappling with a global pandemic known as coronavirus (i.e., COVID-19; Guan et al., 2020). At the time of this writing, 18.6 million confirmed cases and over 700,000 deaths due to COVID-19 have been reported globally (Sun, Chen, & Viboud, 2020). Although most individuals infected with COVID-19 present with minimal symptoms, 15–20% of those who contract the disease experience severe symptoms that require medical intervention and hospitalizations (Guan et al., 2020; W. Liu et al., 2020; Olds & Kabbani, 2020; Sun, Chen, & Viboud, 2020). Select segments of the population are at greater risk for more severe COVID-19 symptoms, including older persons and those with underlying medical conditions (e.g., heart disease, diabetes, liver disease, chronic lung disease, and being immunocompromised; Jiang, Zhou, & Tang, 2020; W. Liu et al., 2020; Shiau, Krause, Valera, Swaminathan, & Halkitis, 2020; J. Zhang, Yang, et al., 2020). At the present time there is no treatment or vaccine for COVID-19 and many parts of the world, including the United States (U.S.), continue to experience daily increases in cases and deaths.
The pandemic has affected the world in a deep and far reaching manner across nearly every level of analysis, ranging from personal (e.g., death, developing a COVID-19 illness, elevated poor mental health) to economic (e.g., economic recession, financial insecurity, bankruptcy) to social (e.g., isolation, inability to attend work or school, loss of developmental milestones such as graduation) (Guo et al., 2020; Yao, Chen, & Xu, 2020; Zhou et al., 2020). The psychological and lifestyle adjustment needed to cope with COVID-19 has offered a significant obstacle for many families and individuals. For example, many caregivers have had to balance working at home with the additional responsibility of homeschooling their children. Additionally, the stress-related burden of COVID-19 has negatively impacted interpersonal relationships (e.g., conflict with a partner, domestic violence) due to close living circumstances, social restriction, and heightened worries. These pandemic-related issues sit in the larger context of the lack of information about the ‘true’ nature of COVID-19 (i.e., its etiology; Guan et al., 2020) and misinformation across media and social outlets. COVID-19 has not affected all persons equally and many vulnerable subgroups are differentially exposed to risks. For example, racial/ethnic minority groups, individuals who are homeless, incarcerated or detained (e.g., immigration detention centers), or live-in poverty-stricken environments are particularly susceptible to COVID-19 related problems even when not directly infected (Bhopal, 2020; Rimmer, 2020; Yancy, 2020).
Governments around the world, including state and local constituents, are attempting to respond to the pandemic via various measures, ranging from the implementation of social distancing protocols, initiation of efforts to rapidly develop an efficacious COVID-19 vaccine, use of protective gear (e.g., face masks), public health messaging (e.g., how to wash hands properly to remove virus potential), restricting travel, closing borders, and shutting down major parts of the economy, among other tactics (Hale, Petherick, Phillips, & Webster, 2020, p. 31; Paterlini, 2020). Many governments have had to issue emergency declarations and offer some form of immediate financial assistance to businesses and families.
Many countries and some U.S. states are opening some aspect up their economies following relative success of social distancing. From countries to state and local municipalities, ‘opening up’ can involve different strategies (e.g., staggering the opening of businesses and schools) and mean different things (e.g., availability of medical supplies and COVID-19 testing capabilities, sanitation protocols), creating the potential for wide-spread confusion. During this time, there simultaneously remains a serious concern about the onslaught of future pandemic waves and the lack of an effective treatment for COVID-19. The uncontrollability and unpredictability related to COVID-19 and its health, social, and economic consequences is apt to elicit acute stress and continued worry about what the future may hold for individuals, families, communities, businesses, and countries (C. Wang, Luo, Chen, Chen, & Li, 2020). In many respects, the COVID-19 pandemic serves as a broad-based stressor and may interact with diatheses represented at the individual, familial, community, and population level. Behavioral scientists play an integral role in the acute and longer-term management of negative psychological, addictive, and health behavior consequences associated with the COVID-19 pandemic. Indeed, behavioral scientists currently are and will continue to work with public health, medicine, and governmental bodies to manage the psychological, addictive behavior, and health behavior aftermath of COVID-19 for youth, adults, and older adults. These activities include direct assessment and clinical care as well as policy-level actions. Like the work of other allied disciplines (e.g., public health, medicine, social work), behavioral scientists help determine the short- and longer-term impact of COVID-19 and play an integral role in shaping how we – as a global community – respond to the most certain risk of future pandemics.
The aim of this invited article in Behaviour Research and Therapy is to offer a conceptual essay that discusses the psychological, addictive, and health behavior correlates/issues related to the COVID-19 pandemic from a behavioral science perspective. The limited empirical study of psychologically-based behavioral outcomes associated with COVID-19 lends to the current essay necessarily maintaining a commentary function, but with the clear objective of detailing the scientifically informed implications for mental health, addiction, and health behavior; many of which will have ‘downstream’ effects. We have organized the article around some of the most clinically important psychological disorders, addictive behaviors, and health behaviors for well-being.
In the first section, we describe the COVID-19 implications for mental health focusing on (a) anxiety/stress and mood disturbance, (b) obsessive compulsive symptoms and disorders, and (c) posttraumatic stress. Such mental health problems, although certainly not exhaustive of the scope of psychological disorders impacted by COVID-19, are some of the most common mental health issues in the general population and are frequently comorbid with chronic illness. In the second section, we focus on addictive behaviors, including (d) tobacco (combustible and electronic), (e) alcohol use and misuse, and (e) cannabis. These forms of drug use represent the most prevalent types of substance use and are frequently associated with chronic illness and premature death. In the third section, we spotlight health behavior and chronic illness by discussing the role of (f) sleep health and behavior, (g) chronic illness using the example of HIV/AIDS as an illustrative model, and (h) physical activity. Health behaviors represent vital targets for the mitigation of COVID-related disease and may play a key role in psychological adjustment and recovery. In the final section, we highlight sociocultural factors (e.g., race/ethnicity, economic adversity), developmental considerations, and the role of individual difference factors for psychological, addictive, and health behavior and chronic illness. We conclude by offering an integrative COVID-19 stress-based model that could be used to guide research focused on the stress-related burden of the pandemic.
2. Section one
2.1. Anxiety, stress, and mood
Fear is an adaptive defense mechanism that is fundamental for survival and involves several psychological and biological processes of preparation for a response to potentially threatening events. COVID-19 represents a true threat, with many unknowns. If you are infected, there is a chance you could die, regardless of your current age, sex, or health status. As such, fear is a natural and adaptive response to this pandemic. On the other hand, every year tens of thousands die from influenza as well as many other preventable or unexpected causes. This raises the key question regarding the degree to which we should be anxious and fearful of COVID-19. How much anxiety is reasonable? Since even basic knowledge about COVID-19 is undeveloped, it will be difficult to clearly discriminate between normal, adaptive fear responses and less adaptive responses. That said, such an overarching true threat and the concomitant stressors such as social isolation, economic uncertainty and so forth could in fact recalibrate what is considered a normal level of anxiety in the general population. Research has demonstrated that trait levels of anxiety have increased in the US in recent decades, though the cause of such increases is unknown (Twenge, 2000). The COVID-19 pandemic is likely to contribute to these basic levels of trait anxiety, thus creating a “new normal” level of anxiety.
If we consider the likely general increase in anxiety and stress in the context of diathesis-stress conceptualizations of mental illness, we expect that such a salient and broad reaching stressor to increase the incidence of pathological anxiety. Anxiety conditions are already highly prevalent (Bandelow & Michaelis, 2015), and we may see an increased incidence of anxiety psychopathology if the pandemic serves to push vulnerable individuals toward the expression of maladaptive levels of anxiety. Moreover, those with preexisting conditions are likely to have their symptoms intensify. One could further speculate that forms of pathological anxiety will increase. First responders and hospital personnel, particularly in affected areas are already showing troubling signs of stress and psychopathology (Joob & Wiwanitkit, 2020). It is highly likely that we will see increased rates of generalized anxiety and posttraumatic stress related to the pandemic and its sequelae.
Beyond the somewhat vague notion of COVID-19 acting as a stressor to increase both normal and pathological anxiety, it is interesting to consider the specific mechanisms that play a role in this process. There are several well-established parameters that relate to the genesis and maintenance of anxiety that seem highly relevant to the current situation. These processes include perceptions relating to predictability/certainty and controllability of threat (Barlow, 2004). Coming across a shark while swimming is quite different from viewing the same shark in an aquarium since a potential threat in the wild is far less predictable or controllable than one in an enclosure. Historically, epidemics and pandemics were considered divine punishments that were essentially uncontrollable. Although medical understanding of pathogens has advanced, globalization now facilitates the spread of pathological agents, which diminishes the degree to which we can control them. Similarly, naturally occurring mutations and adaptation of viruses ensure that novel pathogens like COVID-19 will emerge and spread. These conditions leave us in a state of uncertainty, except that we can be certain that COVID-19 and other infectious agents will persist. Thus, COVID-19 affects many of the core anxiety generating mechanisms since it leads to a sense of diminished predictability and controllability along with increased uncertainty relating to a true threat. Ultimately, the COVID-19 pandemic creates an ideal environment for the onset, maintenance, and exacerbation of anxiety symptoms and syndromes.
2.2. Trauma and posttraumatic stress
The DSM-5 posttraumatic stress disorder (PTSD) Criterion A (American Psychiatric Association [APA], 2013, p.271) defines trauma as “exposure to actual or threatened death.” Individuals who are closer to that exposure -- providing healthcare to those infected, witnessing the deleterious and perhaps deadly effects of the virus on patients or loved ones, enduring losses of patients, family, or friends -- might experience the crisis as potentially traumatic. People on the frontlines of the pandemic, including healthcare personnel, first responders, grocery store clerks, and other essential workers, encounter the threat of possible exposure to the virus regularly and on an ongoing basis. Similarly, incarcerated populations and those who might feel compelled, financially or otherwise, to work in close quarters without adequate personal protective equipment (e.g., factory workers) may be exposed to the COVID-19 virus for extended periods without perceived or actual recourse and suffer negative mental health repercussions as a result. COVID-19 survivors, particularly those who might have struggled through various medical procedures and prolonged hospitalizations, may emerge with unique or shared constellations of mental health reactions from risk to resilience. Additional high-risk groups include healthcare professionals or first responders who may have experienced significant moral injuries (Jinkerson, 2016; Joannou, Besemann, & Kriellaars, 2017; Williamson, Stevelink, & Greenberg, 2018) as a result of making unfathomable decisions on the job (e.g., providing admission or ventilator access to one patient at the sacrifice of another).
Yet, in addition to considering direct impacts of the novel COVID-19 virus on our population, it is imperative to understand the secondary potentially traumatic effects of the pandemic on individuals and communities. The combination of prolonged stress, close quarters, and self-isolation guidelines has increased risk of domestic violence, child abuse, and substance use (Abramson, 2020; National Institute on Drug Abuse, 2020; Santhanam, 2020; Taub, 2020). Indeed, physical and sexual violence may escalate without the regular societal checks provided by employers, schools, and loved ones. Furthermore, such violence may stem from and/or intensify more unbridled substance use (Carter et al., 2020) emerging from a context where uncertainty and unpredictability are high, practical stressors (e.g., unemployment, financial stress, food insecurity) may be difficult to problem-solve, and social supports may be distant. Furthermore, in this pandemic, issues of grief and loss are inevitably interwoven with those of potential trauma. Spiritual and emotional grief processes to honor and emotionally mourn the losses of loved ones may be interrupted by this pandemic, potentially exacerbating or prolonging grief, traumatic bereavement, or PTSD reactions.
To understand the effects of COVID-19 on the mental health of those who experience it as potentially traumatic, we need to recognize first that the impacts of trauma may not be fully determined nor completely recognizable until after the traumatic stressor has concluded. The COVID-19 crisis is going to have a long, yet undetermined course, and thus our ongoing reactions to it are dynamic but indicative of peri-traumatic rather than post-traumatic coping (Bell, Boden, Horwood, & Mulder, 2017; Lapid Pickman, Greene, & Gelkopf, 2017). Based upon decades of research, we can expect the majority of the population, regardless of level of proximity to or interaction with COVID-19, to demonstrate resilience and to recover psychologically in the aftermath of the pandemic (Alisic et al., 2014; Kilpatrick et al., 2013). A relative minority, the proportions of which are unknown, may emerge from the crisis with clinical or subclinical PTSD or with exacerbation in pre-existing PTSD symptoms and related mental health conditions (e.g., depression, substance use disorder). Women are at heightened risk of PTSD following potentially traumatic events (Gaffey et al., 2019; Rattel et al., 2019) and racial/ethnic minority populations may be especially impacted due to socioeconomic inequities and health-related disparities with regard to financial security and access to healthcare and treatment (Asnaani & Hall-Clark, 2017; Cross et al., 2018; Sibrava et al., 2019).
The intersections of trauma and the COVID-19 pandemic are complex. Many constellations of interweaving risk and protective factors, learning histories, and life circumstances can affect how trauma histories and potentially traumatic experiences during the COVID-19 crisis can affect individual journeys of recovery. For example, more unbalanced, negative individual interpretations of the COVID-19 crisis and related changes in beliefs about oneself, others, or the world may have lasting deleterious effects (e.g., “I am damaged”; “People cannot be trusted”; “The world is dangerous and unsafe”; Beierl, Böllinghaus, Clark, Glucksman, & Ehlers, 2019; Bernardi & Jobson, 2019; Köhler, Goebel, & Pedersen, 2019; LoSavio, Dillon, & Resick, 2017; Scher, Suvak, & Resick, 2017). Similarly, avoidance of thoughts or emotions related to the COVID-19 crisis may increase the risk of developing PTSD symptoms and/or exacerbating or maintaining pre-existing trauma-related symptoms (e.g., Orcutt, Reffi, & Ellis, 2020, pp. 409–436). Additional risk factors for the development or exacerbation of PTSD symptoms include a prior history of trauma or mental health disturbances, depressed or anxious mood, significant concurrent life stressors (e.g., financial problems, job loss, relationship stress), low social connectedness or support, sleep disturbance, substance use, and emotional numbing or detachment (Colvonen, Straus, Acheson, & Gehrman, 2019; Cusack et al., 2019; Germain, McKeon, & Campbell, 2017; Hancock & Bryant, 2018; Shalev et al., 2019; Steenkamp et al., 2017; Vujanovic & Back, 2019). Navigating the COVID-19 crisis requires a tolerance of uncertainty that is challenging for all, but especially trauma survivors who may have endured, sometimes over months or years (e.g., combat, childhood abuse), unfathomable circumstances that were, by definition, unpredictable and uncontrollable (e.g., Raines, Oglesby, Walton, True, & Franklin, 2019; Vujanovic & Zegel, 2020). Undoubtedly, social connection and a sense of community and collectivism, hope, psychological awareness, and healthy coping will differentiate risk versus resilience trajectories during and after this crisis (Bernardi & Jobson, 2019; Long & Gallagher, 2018, pp. 233–242; Thompson, Fiorillo, Rothbaum, Ressler, & Michopoulos, 2018). Learning who suffers long-term negative effects of the COVID-19 pandemic, why, and under what circumstances will help us to understand how to intervene most effectively to psychologically support trauma survivors in the aftermath of this and future societal crises.
Indeed, reactions of trauma survivors to the COVID-19 crisis are also likely to be as diverse as the traumas and individuals themselves with the possibility of emergent themes. Theoretically, individuals with histories of being directly impacted by natural disasters, people recovering from severe medical conditions, and those with histories of imprisonment or captivity may feel especially emotionally reactive to the large community-level impact, the social distancing and quarantining aspects of weathering COVID-19, and the continual perceived health threat inherent to the pandemic. Individuals with interpersonal trauma histories may experience a solidification or exacerbation of maladaptive beliefs relevant to trust, safety, or power. Others may feel increased social detachment or engage in increased harmful, self-injurious, or suicidal behaviors, particularly those with mood or substance use disorders. For some trauma survivors, following social distancing and self-quarantine guidelines may lead to less frequent exposure to trauma-related reminders in the outside world and/or a lower perceived interpersonal threat due to social-isolation, but increased trauma-related avoidance during the COVID-19 crisis in turn may exacerbate PTSD symptoms in the long-term. A high-risk subset may emerge who are slow or reluctant to heed public health guidelines due to a reaction against efforts to control, an increased risk-taking propensity, all-or-none thinking, or helplessness resulting from a history punctuated by traumatic, uncontrollable events. This may lead to incessant attempts, by some, to attain perceived control via closely monitoring news, stockpiling food, or supplies, and maintaining constant vigilance. For those affected by trauma prior to and/or during the COVID-19 crisis, the current, chronically stressful global atmosphere where uncertainty reigns may feel especially overwhelming. For others, this crisis may foster growth and resilience as they endure and overcome a crisis of epic and unimaginable proportions.
2.3. Obsessive compulsive symptoms and disorders
Obsessive-compulsive disorder (OCD) is a common (1–2% incidence; (Nestadt, Bienvenu, Cai, Samuels, & Eaton, 1998; Ruscio, Stein, Chiu, & Kessler, 2010), disabling mental health condition characterized by presence of obsessions and/or compulsions (American Psychiatric Association, 2013; Markarian et al., 2010). Symptoms present in a heterogeneous fashion across a number of dimensions, including contamination/cleaning, taboo obsessions (i.e., sexual, aggressive content), symmetry/repeating/ordering, and checking (McKay et al., 2006). Childhood onset occurs in over 50% of cases and symptoms run a chronic course without adequate intervention (Pinto, Mancebo, Eisen, Pagano, & Rasmussen, 2006). Clinical presentation is further characterized by frequent comorbidity (Stein et al., 2019) and variable degrees of insight (Hamblin, Park, Wu, & Storch, 2017). The COVID-19 pandemic is likely to have a number of effects on those with OCD, as well as those at risk. This includes the potential for symptom exacerbation and increased incidence of OCD cases, as well as having implications for assessment and treatment post-COVID-19.
Patients with OCD commonly present with contamination obsessions and associated cleaning compulsions (Mataix-Cols, do Rosario-Campos, & Leckman, 2005; Pinto et al., 2006). Some individuals with contamination related OCD have reported that their symptoms have worsened in light of public health recommendations for increased cleaning behaviors (e.g., washing, wearing masks) and other safety behaviors (e.g., social distancing, wearing masks), which may be difficult for some patients to maintain within recommended guidelines. COVID-19 has become a feared outcome for many patients with contamination-related OCD similar to other what has been observed with other infectious diseases (e.g., HIV). Outside of contamination-focused symptomology, other obsessive-compulsive symptoms may be affected such as harm obsessions whereby someone fears that they may have unintentionally spread COVID-19. Stress has an established relationship with worsened obsessive-compulsive symptoms (Adams et al., 2018; Brander, Perez-Vigil, Larsson, & Mataix-Cols, 2016), and availability of coping strategies is taxed for many; this may further impact OCD symptom presentation as well as comorbidity patterns. Although systematic data have not been presented, clinical accounts support symptom worsening for some affected individuals while, on balance, many others have not experienced negative symptomatic change.
Beyond worsening of symptoms in those with OCD, there is the possibility that there will be increased cases in the near future. This may involve those with subclinical symptoms or other risk factors experiencing onset or worsening of symptoms. The behavioral cycle of OCD/anxiety highlights the role of negative reinforcement in which rituals/avoidance are reinforced by distress reduction and creating a cognitive sense of control (i.e., not getting COVID-19 is due to compulsions; Rector, Wilde, & Richter, 2017). In this scenario, a person with or at risk for OCD may engage in rituals/safety behaviors in response to obsessional distress which in turn reduces anxiety and is perceived as reducing the risk. Reduction in distress may motivate further safety behaviors which, for some at risk, could begin to exceed recommended guidelines. While ordinary levels of risk have risen requiring increased hygiene, it remains to be seen what happens when risk levels decline. That is, do cleaning behaviors likewise decline or remain at elevated states thereby impacting diagnosis rates? Assessment approaches should continue to capture obsessive-compulsive symptoms that are impairing, distressing and excessive relative to current risk levels and not count symptoms that reflect behaviors consistent with accepted public health standards.
There are also treatment implications. The gold standard psychological treatment for adult and childhood OCD is cognitive behavioral therapy with exposure and response prevention (ERP; McGuire et al., 2015; Olatunji, Davis, Powers, & Smits, 2013). This treatment involves gradual exposure to triggers that evoke obsessive-compulsive symptoms while refraining from completing rituals or other avoidance behaviors. A core element to this treatment is that exposure to triggers involves exposure to ‘ordinary’ levels of risk. COVID-19 understandably has shaken what is perceived as ordinary; fortunately, adept therapists have shifted their practice to utilize exposures that reflect this new normal such as relying on imaginal exposures or exposures targeting rituals in excess of public health agency recommendations. At the same time, some clinicians have negative attitudes towards exposure (Meyer, Farrell, Kemp, Blakey, & Deacon, 2014) which is related to reduced practice of this core therapeutic technique (Farrell, Deacon, Kemp, Dixon, & Sy, 2013). It will be critical to provide guidelines established by expert ERP clinicians for how providers integrate realistic COVID-19 concerns into their ongoing practice, as well as that in the future. A concerning possibility is that ERP treatment post-COVID-19 is diluted by virtue of therapists not practicing exposures to the actual level of risk.
3. Section two
3.1. Tobacco
Cigarette smoking remains the leading cause of preventable death and disability globally. Smoking may confer worse COVID-19 outcomes given extensive evidence for the negative impact of smoking on lung health and respiratory function (Tonnesen, Marott, Nordestgaard, Bojesen, & Lange, 2019). Indeed, emerging evidence has identified smoking as a possible risk factor for adverse COVID-19 prognosis and disease progression (Patanavanich & Glantz, 2020; Vardavas & Nikitara, 2020). In the largest study of COVID-19 patients, 16.9% of severely affected patience were current smokers relative to 11.8% of non-severe patients (Guan et al., 2020). An inverse pattern emerged with non-smokers such that a greater proportion of non-severe patients identified as a non-smoker relative to severe patients. Moreover, 25.5% of COVID-19 patients who either needed mechanical ventilation, were admitted to an intensive care unit, or died from complications related to the disease were current smokers relative to 11.8% of those not experiencing these outcomes. Similar disparities in COVID-19 severity across smoking status have been observed in other samples (W. Liu et al., 2020; J. Zhang, Yang, et al., 2020). Thus, these data, albeit preliminary and limited by sample size, indicate that smoking is a risk factor for COVID-19 progression (W. Liu et al., 2020).
Taking a biological perspective to understand why smokers are more susceptible to severe COVID-19 symptoms, recent research has proposed that smoking and COVID-19 susceptibility and symptom severity may be related to an upregulation of the angiotensin-converting enzyme-2 (ACE2) receptor (Brake et al., 2020). ACE2, a membrane-bound aminopeptidase that plays a vital role in cardiovascular and immune systems, is highly expressed in the heart and the lungs (Turner, Hiscox, & Hooper, 2004; Wang, Luo, Chen, Chen, & Li, 2020). Studies have established that ACE2 is a receptor for the COVID-19 virus (J. Wang, Luo, et al., 2020), and greater ACE2 gene expression has been observed in smokers compared to non-smokers (Brake et al., 2020; Cai, 2020; Emami, Javanmardi, Pirbonyeh, & Akbari, 2020; Tian et al., 2020; Wan, Shang, Graham, Baric, & Li, 2020; Xu et al., 2020; Zhao et al., 2020; Zhou et al., 2020). The upregulation in ACE2 creates an environment that allows greater potential for COVID-19 to infect human cells among smokers through more opportunity to bind to this receptor (Olds & Kabbani, 2020; Zuluaga, Montoya-Giraldo, & Buendia, 2020). In part, this biological mechanism may help explain observed sex differences in COVID-19. Specifically, COVID-19 symptom severity and mortality rates in China indicate worse outcomes for men than for women, where 52.1% of men and 2.7% of women are current smokers (Parascandola & Xiao, 2019; Sun et al., 2020). It is possible that the elevated smoking rates among men in China, and therefore greater upregulation in ACE2, contributed to significant gender difference in COVID-19 incidence and severity (J. Wang, Luo, et al., 2020).
In addition to combustible cigarette smoking, there also is growing concern for the impact of electronic cigarette (e-cigarette) use on COVID-19 infection and disease progression (Lewis, 2020). Although it is believed that the worldwide distribution and adoption of e-cigarettes has the potential to increase population-level vulnerability to respiratory infecting diseases (Olds & Kabbani, 2020), such as COVID-19, no studies have assessed e-cigarette use among COVID-19 patients (Farsalinos, Barbouni, & Niaura, 2020). Given evidence for the impact of various e-cigarette formulations on lung health and functioning (Viswam, Trotter, Burge, & Walters, 2018) as well as the fact that most e-cigarette users are former or current combustible cigarette users (Mirbolouk et al., 2018), it is possible that product use will critically impact the course of COVID-19 among users. Additionally, similar to combustible cigarette use, it has been theorized that e-cigarette use may engage an upregulation in ACE2 that parallels that of combustible cigarette use and increases the likelihood of COVID-19 infection (Brake et al., 2020). Further research on these products and their influence on COVID-19 outcomes is urgently needed.
A final point to consider is the effect that the COVID-19 pandemic itself has on smoking. One of the leading reasons for smoking is stress management (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Garey et al., in pressGarey et al., ). The psychological effect of the current global environment, characterized by feelings of fear, uncertainty, isolation, and stress (Mertens, Gerritsen, Salemink, & Engelhard, 2020), coupled with limited availability of adaptive coping tools due to regulations and consequences of COVID-19 (i.e., social distancing, financial hardship) likely increases the risk for smoking onset, increased intensity, and relapse (Patwardhan, 2020; Stubbs et al., 2017). Smoking initiation and severity, in turn, increase susceptibility for COVID-19 and worse disease-related outcomes. Behavioral scientists must engage in targeted efforts to support current smokers and former smokers in achieving and maintaining cessation during this particularly challenging time. There are promising initial findings from smoking cessation programs implemented in smokers managing other infectious disease that may help guide some of these initiatives (O'Cleirigh et al., 2018). As more is learned about COVID-19, it is imperative that health care providers assess smoking (and e-cigarette) use status as well as relapse potential among former users and provide appropriate education and intervention to help mitigate the potential risk of this health behavior on disease infection and course.
3.2. Alcohol
The (mis)use of alcohol is a leading risk factor for global disease burden and preventable death (Degenhardt et al., 2018; Organization, 2019). Alarmingly, alcohol use, high-risk drinking, alcohol use disorder (AUD), and alcohol-related deaths were increasing before the COVID-19 pandemic (Grant et al., 2017; White, Castle, Hingson, & Powell, 2020). Despite the widespread belief that moderate alcohol consumption may confer health benefits (Diaz et al., 2002; Romeo et al., 2007), more recent work suggests that any alcohol consumption is associated with health risks (Griswold et al., 2018). In fact, given the immunosuppressing effects of alcohol both generally and in the respiratory system specifically (Molina, Happel, Zhang, Kolls, & Nelson, 2010; Szabo & Mandrekar, 2009), it is germane to consider the role that alcohol consumption, whether chronic or in acute response to the ongoing crisis, may have on contraction of the COVID-19 virus. In addition to the direct physiological impact of alcohol consumption on the body, the disinhibiting properties of alcohol (Kumar et al., 2009; Oscar-Berman & Marinković, 2007) may put individuals at risk for other risky/poor decisions (George, Rogers, & Duka, 2005). For example, those under the influence of alcohol may be more likely to violate social distance protocols, exhibit poor hand washing procedures, or refuse/forget to wear a face covering in public, leading to potential exposure to and/or spreading of the virus. Importantly, impulsivity has reciprocal relationships with alcohol such that consumption increases impulsive behaviors and individuals with greater trait impulsivity (mis)use alcohol to a greater extent (Dick et al., 2010).
Moreover, the effects of impulsivity on alcohol (mis)use can be amplified by other factors, such as stress, to confer greater risk for alcohol (mis)use (Fox, Bergquist, Gu, & Sinha, 2010). It is well-documented that stress, both acute and chronic, is a trigger for alcohol (mis)use (Becker, Lopez, & Doremus-Fitzwater, 2011; Blaine & Sinha, 2017). The COVID-19 pandemic has brought about both acute (e.g., work displacement, limited availability of cleaning supplies) and chronic stress (e.g., financial difficulty, isolation) that likely will contribute to alcohol (mis)use for coping. It also is reasonable to expect that alcohol (mis)use will worsen during the crisis in response to the stress and uncertainty. For example, during the 2008–2009 economic recession, although there was a decrease in prevalence of alcohol use overall (i.e., increase in abstainers), there was an increase in prevalence of binge drinking (Bor, Basu, Coutts, McKee, & Stuckler, 2013). This suggests that there may be a realignment/concentration of problematic drinking such that a greater segment of those who do consume alcohol may be doing so in a maladaptive or harmful way.
Although sales to restaurants and events have reduced markedly during the pandemic, sales of online and to-go alcohol have skyrocketed (Nielsen, 2020). Given shelter in place orders and limits on socializing, it is possible that greater amounts of alcohol are being consumed at home/solitarily relative to social contexts. Solitary drinking can, in some circumstances, lead to greater alcohol consumption than social drinking (Kuendig & Kuntsche, 2012) and is associated with greater alcohol-related consequences overall (Christiansen, Vik, & Jarchow, 2002). For many, the COVID-19 pandemic has led to significant social isolation with in-person socializing virtually eliminated and many working from home (if at all). These conditions may also exacerbate a common reason for alcohol-related relapse: boredom (Levy, 2008). Without other adaptive ways to manage stress, socialize, or simply occupy one's mind, it is possible that craving for alcohol may intensify.
Finally, there are important treatment implications for alcohol (mis)use during COVID-19. Individuals already report numerous barriers to seeking drug/alcohol treatment (McGovern, Xie, Segal, Siembab, & Drake, 2006). In the wake of the pandemic additional barriers may arise such as the perception that one's treatment is not a priority during a ‘life or death’ pandemic or not worth the risk of leaving one's home. Alternatively, for those seeking treatment, there may simply not be local resources available or treatment facilities may have waitlists. Although the use of telehealth services are growing in general (Dorsey & Topol, 2016), there is more work to be done, with specific considerations for low-income individuals (e.g. recently unemployed) who may be reluctant to spend money on treatment, perceive treatment to be a luxury, or not have technological resources or a private location to engage in telehealth. Affordable computer-based treatments without the need for a provider that focus on stress and alcohol use (Paulus, Gallagher, Neighbors, & Zvolensky, 2020) could be particularly pertinent during this pandemic.
3.3. Cannabis
Cannabis use rates continue to increase, with nearly 44 million individuals 12 years and older in the U.S. endorsing past year cannabis use in 2018, a rate greater than the rate in every year since 2002 (Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality, 2019). Further, rates of cannabis use disorder (CUD) remain greater than rates of all other federally illicit substance use disorders combined. Despite the high rates of CUD, perceived risk of cannabis use continues to decline (Okaneku, Vearrier, McKeever, LaSala, & Greenberg, 2015; Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality, 2019) presumably due at least in part to legalization of recreational and/or medical marijuana at the state level (Johnston, O'Malley, Miech, Bachman, & Schulenberg, 2015). Notably, cannabis users report using more cannabis during times of heightened distress following national disasters such as the September 11, 2001 terrorist attacks, a pattern that was especially prominent among individuals who experienced post-traumatic stress disorder and depression (Vlahov et al., 2002). It therefore follows that cannabis use and associated problems may increase during the COVID-19 pandemic.
Cannabis use increases during times of distress to manage negative affect. In support of this contention, cannabis users report relaxation and tension relief as one of the most common reasons for use (Copeland, Swift, & Rees, 2001; Hathaway, 2003; Reilly, Didcott, Swift, & Hall, 1998). Data from experimental studies support these self-reports. To illustrate, current cannabis users were randomly assigned to an anxiety-induction or non-anxious control condition and cannabis craving increased from before to during the task among participants in the anxiety condition, but not among those in the control condition (Buckner, Ecker, & Vinci, 2013). These data indicate that cannabis users were especially vulnerable to wanting to use cannabis during an anxiety-provoking situation, which has direct implications for the COVID-19 pandemic characterized by heightened stress. Notably, this effect was specific to cannabis craving and was not observed for craving for alcohol or cigarettes in this sample of cannabis users. Coping motives are the most common reasons cited for wanting to use during laboratory-induced anxiety (Buckner, Zvolensky, Ecker, & Jeffries, 2016). Prospective data collected via ecological momentary assessment also confirm that anxiety is positively, significantly related to cannabis craving at the momentary level, and is related to greater subsequent craving (Buckner, Crosby, Silgado, Wonderlich, & Schmidt, 2012). Further, although positive and negative affect were greater immediately prior to cannabis use compared to non-use episode, negative affect increased at a significant rate prior to cannabis use, and decreased at a significant rate following cannabis use; changes in positive affect were not significantly related to use (Buckner et al., 2015).
Further, the stress associated with the COVID-19 pandemic may serve as trigger for lapse and/or relapse among individuals undergoing a cannabis quit attempt. In a qualitative interview following cannabis quit attempts, situations involving negative affect and exposure to others smoking cannabis were among the most difficult situations individuals reported in which to abstain (Hughes, Peters, Callas, Budney, & Livingstone, 2008). Among cannabis users undoing a self-guided quit attempt, data from ecological momentary analysis indicated that although positive and negative affect were significantly higher during cannabis lapse episodes compared to non-use episodes, when negative and positive affect were analyzed simultaneously, negative affect, but not positive affect, remained significantly related to lapse (Buckner, Zvolensky, & Ecker, 2013). Again, the most common reason for use cited during lapse episodes was to cope with negative affect.
Not only could COVID-19 increase cannabis use, but cannabis use may exacerbate COVID-19 symptoms given that smoking cannabis damages the lungs. Respiratory toxins (including carcinogens) in cannabis smoke are similar to that of tobacco smoke but notably the smoking topography for cannabis leads to higher per-puff exposures to inhaled tar and gases (Tashkin & Roth, 2019). Further, respiratory symptoms such as chronic cough, sputum, and airway mucosal inflammation are also similar between cannabis smokers and tobacco smokers. The impact on respiratory functioning of cannabis smoke has led for the consideration of cannabis use as a pre-exiting condition that could increase the likelihood of more severe complications should one contract COVID-19 (National Institute on Drug Abuse, 2020).
4. Section three
4.1. Sleep health and behavior
Sleep is a fundamentally restorative process, but it is also highly responsive to stress (Irwin, 2015). During times of increased stress, sleep, quite paradoxically, serves both as a major line of defense and as a source of heightened vulnerability. These relationships derive from the fact that sleep and immunological functioning are reciprocally related: sleep promotes healthy immune responses and healthy immune responses (e.g., to infectious agents) promote deeper, more restorative sleep (Opp, 2005). Precise mechanisms are of course complex, but several specific links are noteworthy. Immune-signaling proteins called cytokines, such as tumor necrosis factor (TNF) and interleukin-1 (IL-1) directly target infection and inflammation but are also known to promote sleepiness and non-rapid eye movement (NREM) sleep (Jewett & Krueger, 2012). The hormone melatonin, which provides an endogenous marker of circadian phase peaks during the nocturnal sleep period but also has important immunomodulatory effects. Conversely, the hypothalamus–pituitary–adrenal (HPA) axis and the sympathetic nervous system (SNS), two primary stress response systems, are down-regulated during sleep, decreasing immune-regulating cortisol levels (Besedovsky, Lange, & Born, 2012). However, when sleep is inadequate or disrupted, alteration in these systems is readily observable. Experimental sleep research provides overwhelming evidence for the detrimental effects of chronic sleep disruption on immune responses including increases in multiple inflammatory markers such as C-reactive protein, diminished immune response to vaccination, and enhanced susceptibility to bacteria and toxins (Besedovsky et al., 2012). Rather than representing enhanced immunity, elevated levels of inflammation are associated with a range of health risks including cardio-pulmonary disease (Libby, 2006).
Sleep's inextricable role in human immunological functioning clearly place it at the forefront of critical behaviors during a pandemic. Unfortunately, multiple aspects of the COVID-19 pandemic threaten healthy sleep patterns which in turn endanger both physical and mental health. Widespread uncertainty, 24-h media coverage (including misinformation), fear for one's own health and the health of loved ones, and potential loss of employment/wages are but a few of the significant sources of stress present during these unprecedented times. Heighted psychological and physiological arousal elicited by such stress falls in direct odds with a calm, quiescent state necessary for sleep onset and maintenance. Further, common behaviors aimed at managing increased stress and anxiety such as smoking, alcohol consumption, and decreased physical activity can give rise to or worsen sleep disruption via known negative effects on sleep duration and quality (Irish, Kline, Gunn, Buysse, & Hall, 2015). Moreover, sleep deprivation can amplify inflammatory responses (Bollinger, Bollinger, Oster, & Solbach, 2010), increasing the risk for poor outcomes in COVID-19 as unrestrained inflammation is implicated in the pathophysiology of the disease (Gamaldo, Shaikh, & McArthur, 2012).
Although predisposing (e.g., genetics) and precipitating (e.g., trauma) factors play a role, stress is considered a primary cause of insomnia (Morin, Rodrigue, & Ivers, 2003) and among insomniacs, perceived inability to sleep often becomes a major source of stress in its own right. Studies that have systematically examined incidence and severity of insomnia symptoms during a global pandemic are unavailable despite ubiquitous anecdotal reports and cautions from health professional regarding the immunosuppressive effects of poor sleep. However, in a recently-published study conducted between January 29 and February 3, 2020, C. Zhang, Yang, et al. (2020) surveyed medical staff responding to the COVID-19 pandemic in China using the Insomnia Severity Index (ISI; Morin, Belleville, Bélanger, & Ivers, 2011). More than a third of workers (36.1%) endorsed symptoms indicative of clinical insomnia and those with insomnia reported elevated levels of depression. Insomnia is well-known to herald the onset of depression both acutely and years later even among those who have never been depressed (Baglioni et al., 2011). Studies directed at uncovering precise mechanisms of affective risk during the COVID-19 pandemic must therefore consider the presence and severity of insomnia symptoms.
The COVID-19 pandemic also has upended daily routines and associated ‘cues’ that serve to maintain regular sleep schedules. Working from home, altered mealtimes, increased sedentary behavior, social distancing, and increased “screen time” are only some of the changes that hold potential to disrupt circadian rhythms that govern sleep-wake patterns. Other factors such as social activities also can affect sleep-wake patterns. The human internal circadian clock runs slightly longer than 24 h and therefore needs to be ‘entrained’ to the 24-h day via internal and external cues (Czeisler et al., 1999). Sunlight is the most potent exogenous cue that aligns our internal rhythm to the external environment, but quarantine measures and greater time spent indoors means that many individuals are receiving inadequate dosages of light exposure. Although public health guidelines center on sufficient sleep duration (Watson et al., 2015), sleep timing is equally critical for overall health and well-being. Misalignment of the sleep period with the body's ‘biological night’ is routinely linked with a host of serious risks, including anxiety, depression, suicide, cardiac events, and several forms of cancer (Baron & Reid, 2014). Healthcare workers who are working long hours and night shifts during the COVID-19 pandemic are therefore a particularly high-risk group for circadian shifts and associated comorbidities. Considering sleep's role in immunological function, this represents an area of priority for future research.
4.2. Chronic illness: using HIV/AIDS as an exemplar
The intersection of COVID-19 with pre-existing chronic medical illness (e.g., cardiovascular disease, diabetes, HIV) raises additional challenges to the patient for managing multiple treatment cascades. These challenges are exacerbated by the poorer survival and disease course for patients with underlying medical conditions (Emami et al., 2020) which in turn seems to be driving, in part, the alarming COVID-19 racial disparity (Laurencin & McClinton, 2020). The overlapping epidemic of COVID-19 with HIV, for example, presents unique challenges for HIV access to care, HIV treatment engagement, and prevention.
At this time, it is not known whether HIV infection increases susceptibility to COVID-19 infection or if it exacerbates the likelihood of poor COVID-19 outcomes. However, people living with HIV may have other comorbidities, such as cardiovascular disease and chronic lung disease, that increase the risk for a more severe course of COVID-19 illness (Guaraldi et al., 2011; Guo et al., 2020). There is also a concern that individuals who are immunocompromised, such as those with HIV, may be at greater risk for severe COVID-19 symptoms (CDC, 2020a; Duffau et al., 2018).
In the U.S., most people living with HIV (PLWH) are tested, linked to HIV care, well engaged in antiretroviral treatment, and achieve HIV viral suppression thus ensuring their optimal health and protecting the public health by containing onward transmission (CDC, 2020b). However, structural and individual barriers to treatment and prevention create enduring inequalities and significantly increase the risk of infection, reduce access to, and engagement in, HIV care, and compromise participation in HIV biobehavioral prevention among particular risk groups. Gay and bisexual men (particularly Hispanic and African American men) are most impacted by HIV and account for nearly 70% of new HIV cases. HIV incidence rates in the U.S. are also significantly higher for those who are homeless or living in poverty (Denning & DiNenno, 2020).
With respect to individual barriers to care, PLWH are disproportionally affected by traumatic life experiences, anxiety, depression, and substance use (Brandt et al., 2017; Nanni, Caruso, Mitchell, Meggiolaro, & Grassi, 2015; C. O’Cleirigh, Magidson, Skeer, Mayer, & Safren, 2015). Each of these also have been associated with poorer engagement in HIV care, worse antiretroviral medication adherence, and poorer HIV disease course. Their co-occurrence and interaction significantly increases both the risk for HIV infection (Mimiaga et al., 2015) and poorer HIV disease management among those already infected (Harkness et al., 2018; Pantalone, Valentine, Woodward, & O'Cleirigh, 2018). These mental health barriers to full engagement in HIV care may well be exacerbated by increased levels of COVID-19 specific anxieties and increases in general health-related anxieties. The requirements of social distancing also may contribute to feelings of isolation and loneliness which may in turn contribute to increased depression or depression-related withdrawal. Both anxiety-related avoidance and depressive related withdrawal will likely have negative consequences for self-care generally and for HIV care specifically. These increases in distress will occur at a time when access to behavioral health services is already severely restricted. Some PLWH who become co-infected with COVID-19 will already be struggling with HIV disease management (e.g., missed medical appointments, sub-optimal medication adherence) and may require additional supports to manage COVID-19 care and treatment at a time when many routine supports may not be available due social distancing and lack of routine medical services.
Protecting access to care and treatment among those already struggling with the complexities of the HIV care cascade who must now manage the additional burdens of the COVID-19 illness is a robust clinical concern. Here, we underline the importance of community (Carrico et al., 2020) and health worker based approaches (Operario, King, & Gamarel, 2020) to HIV treatment and protecting access to care through innovative and virtual care models. Many of those at risk for being lost to care during this COVID-19 pandemic also may be vulnerable to perceived stigma (Krier, Bozich, Pompa, & Friedman, 2020; Logie, 2020). Many will have multiple stigmatized identities with respect to HIV status, COVID-19 status, substance use, sexual or gender minority status, and others. Keeping our community members and peers involved in our service delivery will help ensure our treatments are delivered in stigma-free contexts. Empirical support for integrated treatment platforms that address mental health (Ironson et al., 2013; Safren, O'Cleirigh, Skeer, Elsesser, & Mayer, 2013) and substance use issues (Mimiaga et al., 2019; Safren et al., 2012) to protect engagement in HIV treatment and prevention (Mayer et al., 2017; Conall O’Cleirigh et al., 2019) are available to guide these initiatives. In addition, protecting access and supporting engagement (virtual or otherwise), to mental health and substance use treatment will be critically important. These approaches may be particularly key for protecting access to HIV prevention services (i.e., HIV testing, access to Preexposure Prophylaxis [PrEP]) for those at risk for HIV. Access to these services may be particularly important for those whose behavioral risk profiles and risk appraisals may be disturbed because of the impact of social distancing on usual patterns of substance use or sexual behavior.
4.3. Physical activity
Although much remains unknown about COVID-19 and the mental health consequences of the pandemic, it is likely that regular physical activity offers protective effects. Regular physical activity reduces risk of and helps manage conditions that appear to increase risk of adverse outcomes of COVID-19 (e.g., obesity, cardiovascular disease, diabetes; Lee et al., 2012), and improves immune function (Nieman & Wentz, 2019) which likely positively affects the progression of COVID-19. It also buffers the effect of stressors and (in part thereby) can prevent the onset of mental health conditions (Harvey et al., 2018; Jacquart et al., 2019). Further, diminished physical activity can disrupt sleep quality (Buman & King, 2010; Youngstedt & Kline, 2006), which increases susceptibility to infection and mental and physical illness (see Sleep section). Hence, establishing or maintaining a regular physical activity habit has the potential to mitigate the impact of the pandemic both at a personal and societal level.
Establishing and maintaining a regular physical activity habit has proven to be challenging. Indeed, only 24% of adults meet the guidelines set forth by the Department of Health and Human Services (Whitfield et al., 2019). The COVID-19 pandemic has impacted several factors, including a change in the daily routine and increased stress and anxiety, that can affect the intent of or ability to engage in behavior change. It is important to acknowledge the relationship between factors such as stress or changes in routine and physical activity participation can vary in strength or direction (i.e., negative or positive) depending on the individual and their context. For example, for some routine changes have created barriers for exercise participation, while for others changes to the daily structure have opened opportunities to engage in regular exercise. Similarly, stress and anxiety at the “right” level can be motivating for some make exercise part of their daily routine, but when stress and anxiety become overwhelming, automated emotion action tendencies often cause people to move away from healthy (coping) behaviors such as exercise (Otto et al., 2016). Importantly, such relationships may further vary within and across individuals depending on other individual difference variables (e.g., risk factors, protective factors, [mental] health diagnosis) and contextual factors (e.g., job loss, financial stress, isolation).
Research aimed at understanding the relationship between COVID-19 and physical activity mostly likely will benefit from considering the importance of individual differences and the influence of contextual factors. Comprehensive assessment batteries and statistical models that include the testing of these complex moderation effects are key. This perspective that acknowledges nuance in the relationship between COVID-19 (pandemic) and physical activity also will aid efforts to develop or fine-tune intervention programs for physical activity uptake.
5. Section four
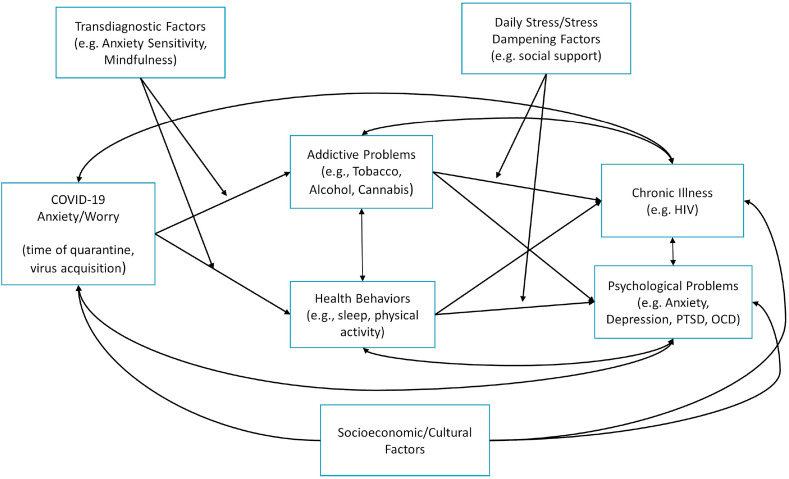
The COVID-19 pandemic, although still ongoing and presently under investigated from a behavioral health perspective, is apt to impart acute and potentially chronic exacerbations in psychological symptoms and disorders, addictive behavior, and health behavior and chronic illness. Across various phenotypes overviewed in the current essay, previous scientific work and theoretical models predict COVID-19, regardless of acquisition of the virus, has and will continue to have a strong negative psychological impact on negative mood states, various forms of substance use, and sleep, chronic illness, and physical activity. Although many of these relations would be expected, theoretically, to be negative, select subgroups will certainly adaptively respond to COVID-19 related stress (e.g., improve their physical fitness, improve self-care routines, quit/reduce maladaptive behaviors that place them at risk). In this final section of the paper, we discuss sociocultural considerations, developmental issues, and the role of individual difference factors for COVID-19-related psychological, addictive, health behavior and chronic illness. We conclude by offering an integrative COVID-19 model that could be used to guide research focused on the stress-related burden of the pandemic.
5.1. Sociocultural factors
Certain subpopulations and contextual factors (e.g., loss of work) are likely to signify a vulnerability gradient for COVID-19 in terms of mental health, addictive behavior, and health behavior. Although there are numerous possible sociocultural factors that could be relevant, we highlight first responders and medical professionals, economic adversity, and racial/ethnic factors as three prototypical factors of public health importance.
First responders and healthcare professionals. Of all the sectors of the population, first responders and front-line healthcare professionals are arguably at the greatest risk for at least acute disruptions in anxiety, stress, and negative mood. First responders and healthcare professionals at the front line of the COVID-19 pandemic have at their core mission to protect and preserve life (Prati & Pietrantoni, 2010). These groups, although engaging in a diverse range of specific occupational activities (e.g., direct medical care, transport, public safety enforcement), share in common that they are among the first to respond to the COVID-19 crisis and take primary responsibility for attending to COVID-19 related health issues.
First responders and healthcare professionals are undoubtedly experiencing emotionally challenging and unpredictable situations that can place their lives in danger. The acute emotional effects of managing COVID-19 cases is likely to be amplified by heavy work schedules and reduced access to and isolation from social support systems (e.g., self-isolation after finishing a shift). It is likely that first responders and healthcare professionals working with COVID-19 cases in hospitals will be exposed to potentially traumatic events, the greater-than-usual experience of life-threatening situations, working with emotional strain related to isolation of patients from their families (e.g., compassion stress in the form of offering emotional support to patients in a manner that family or caregiver of patients would typically offer), and exposure to the struggle to life and death. These experiences are apt to challenge the coping resources of even the most seasoned professionals, which can result in higher degrees of anxiety, stress, and depressed mood (LaFauci Schutt & Marotta, 2011). Such elevated stress levels are likely to be related to changes in cognition and physical health, including emotional exhaustion, fatigue, sleep dysfunction, and problems with interpersonal relationships (Kronenberg et al., 2008; Lane, Lating, Lowry, & Martino, 2010). Cognitive-based beliefs about personal safety and health can be altered and memories of potentially traumatic events engrained (Setti & Argentero, 2014).
Collectively, the COVID-19 related stress burden, as discussed in several sections of the current essay, will have a high likelihood of being related to increased risk of anxiety and depression for first responders and medical professionals working at the front line. Moreover, consistent with past literature of these populations, the regulation of affect will be associated with addictive and health behavior to modulate such affect (e.g., physical activity, substance use). Although some regulatory behavior will be adaptive (e.g., increasing sleep where possible to aid in recovery, engaging in regular physical exercise), others may be less adaptive (e.g., smoking to reduce stress) and promote the risk for other health problems (e.g., physical illness).
Economic adversity. Economic hardship related to COVID-19 is already evident at numerous levels of analysis, including job loss, reduced earnings, higher debt relative to assets ratio, inability to pay mortgage and bills, meeting governmental guidelines for poverty status, and worry about financials resources going forward due to the turbulent nature of the economy. Past work has shown that economic hardship is related to behavioral health problems, including psychological disorders, addictive behavior, physical health problems, and interpersonal dysfunction in adults and children (K. J. Conger et al., 2012; Sareen, Afifi, McMillan, & Asmundson, 2011). For instance, economic adversity has been linked to reduced social competence and elevated physiological markers of stress (K. E. Bolger, Patterson, Thompson, & Kupersmidt, 1995; Evans & English, 2002). Further, economic hardship is related to self-regulation capacity and the corresponding difficulty in dealing with additional responsibilities. For example, past work has found limited socioeconomic resources are related to harsher parenting behavior and greater substance use (R. D. Conger & Donnellan, 2007). The negative effects may be particularly profound when economic hardship is severe or chronic (Dearing, McCartney, & Taylor, 2001; Magnuson & Duncan, 2002). The totality of worsening economic conditions for individuals and families in the larger context of an uncertain economic future are apt to be related to elevations in anxiety, stress, and depression as well as other negative emotional states (e.g., anger, frustration, fatigue; Newland, Crnic, Cox, & Mills-Koonce, 2013). Such emotional symptoms and problems are likely to be related to elevations in substance use and other maladaptive behavior (e.g., less supportive interpersonal behavior, less affection) and may exacerbate chronic health conditions. Other work has found that these processes also disrupt social interconnections (Scaramella, Sohr-Preston, Callahan, & Mirabile, 2008).
Primary care givers who have children home from school, are unlikely to be able to work at their full capacity even with added flexibility in schedules. Although certain occupations have decreased activity, many have not. Therefore, it could be expected that for individuals with added responsibilities of educating their children at home occupational stress may be greater compared to those without such responsibilities. Further, it is possible that the accumulation of occupational responsibilities that are not addressed for persons with additional educational responsibilities will accumulate and make it more challenging to recover when going back to ‘normal,’ resulting in a greater degree of occupational stress.
Grappling with lower socioeconomic states related to COVID-19 will, for certain segments of the population, offer an additional psychological challenge. Indeed, past work has repeatedly documented that lower socioeconomic status is related to adverse health outcomes for chronic illness and mortality rates (Adler et al., 1994; Adler, Boyce, Chesney, Folkman, & Syme, 1993). Moreover, harms faced by people who cannot afford not to work in dangerous settings can exacerbate the psychological and health risk associated with COID-19. Further essential workers are more apt to be persons of color (Handerson, McCullough, & Treuhaft, 2020). Certain groups will be more likely to recover than others, which past work indicates is related to poorer health outcomes even at higher socioeconomic levels (Kraus, Borhani, & Franti, 1980). Moreover, research has found that lower socioeconomic persons experience more chronic stress and negative life events (Stansfeld, North, White, & Marmot, 1995). Additionally, lower socioeconomic status is related to cognitive biases for threat (Chen & Matthews, 2001), which engender greater degrees of interpersonal conflict and heightened negative emotional states (Matthews et al., 2000; Stansfeld, Head, & Marmot, 1997). It would be expected that such negative emotional experiences will be related to maintained direct relations with poorer health behavior and health outcomes (McEwen & Stellar, 1993). In fact, research has consistently found that lower socioeconomic status is related to greater degrees of anxiety, stress, and depression when compared to those higher in socioeconomic status (McLeod & Kessler, 1990). This heightened stress reactivity may be at least in part attributable to having fewer resources. Consequently, those struggling with a lower socioeconomic status due to COVD-19 may be more contexts in which they must utilize their emotional resources and be less likely to be in a sociocultural context wherein such resources can be replenished (Holahan, Moos, Holahan, & Cronkite, 1999). This perspective is in line with past work that has found that when persons are exposed to chronic stress, emotional resources are challenged, and there is a greater risk for future emotional distress (N. Bolger & Zuckerman, 1995; Ensel & Lin, 1991).
Racial/ethnic considerations. There is broad band evidence that significant health disparities exist for persons of racial/ethnic minority in the U.S. and beyond prior to COVID-19 for psychological, addictive behavior, and health behavior as well as chronic illness. For example, African American/Black individuals experience a disproportionate burden in disease morbidity, mortality, disability, and injury (Mechanic, 2005; Mensah, Mokdad, Ford, Greenlund, & Croft, 2005). Indeed, African American/Black individuals remain significantly and consistently more at risk for early death than do similar non-Latinx White individuals (Williams, Neighbors, & Jackson, 2003; Williams, Yu, Jackson, & Anderson, 1997); overall early death rates of African American/Black individuals are comparable to those observed among non-Latinx Whites in the U.S. decades ago (Levine et al., 2016; Williams & Jackson, 2005). Differences in prevalence and rate of growth of chronic illness are not accounted for solely by exposure to lower income environments (Franks, Muennig, Lubetkin, & Jia, 2006). Indeed, social determinants of health (e.g., racism; Krieger & Sidney, 1996), addictive behavior (e.g., tobacco use; Sakuma et al., 2015), and stress represent robust and consistent factors related to health inequalities among African American/Black individuals and those from other underrepresented racial/ethnic groups. The COVID-19 pandemic has appeared to strike racial and ethnic minority populations (e.g., African American/Black) hard and with possible longer-term consequences. For example, less access to health care services for chronic illness, addictive behavior, and mental illness could exacerbate COVID-19 related symptoms or promote a greater degree of stress-related burden associated with the pandemic (e.g., worry that loved ones, if infected, cannot access care). Consequently, addictive behaviors (e.g., smoking, alcohol misuse) and health behaviors (e.g., disrupted sleep, emotional eating) may be used in the short-term to cope with such COVID-19 related stress, increasing the longer-term risk for more severe negative emotional symptoms and health complaints (e.g., pain) and chronic health problems (e.g., obesity).
Additionally, situations characterized by mass fear and confusion, such as the current pandemic, also can elicit a human instinct to resolve the confusion and mitigate the fear by identifying a culprit for the introduction or spread of the disease (Bard, Verger, & Hubert, 1997; Bromet, 2011). Asian American persons are one group that has been singled out as responsible for the COVID-19. The misdirection of fear and/or anger related to COVID-19 toward a racial or ethnic group instead of the disease, however, can perpetuate fear and contribute to racism and stigma. Several reports have already documented the rise in violent crimes and discrimination experienced by Asian American persons related to COVID-19 beliefs (E. Liu, 2020). COVID-19 specific language, such as referring to COVID-19 as ‘the Chinese virus,’ has created a platform to propagate stigma and discrimination towards Asian Americans. It is likely that stigma and discrimination experienced by Asian Americans in response to COVID-19 will increase emotional distress, coping-oriented addictive behavior, and may alter health behavior or exacerbate chronic illness.
It would also be remiss to not call explicit attention to the fact that societies marked by greater economic and social inequality experience far more medical, psychological, and social pathology than do societies where such wealth inequalities are less pronounced (Wilkinson & Pickett, 2006, 2007). Further, such adverse effects occur across social classes, not merely among the most disadvantaged. Yet, the adverse effects of economic (and thus social) inequality hurt everyone, although the poorest or most marginalized are affected the most (Pickett, Kelly, Brunner, Lobstein, & Wilkinson, 2005; Wilkinson & Pickett, 2008).
5.2. Developmental considerations
There are far-reaching implications for psychological health, addictive behavior, and health behavior from a developmental perspective. For children, despite COVID-19 appearing to have less severe symptoms and lower mortality rates than other age groups, are among the highest risk groups (Sinha et al., 2020; Zimmermann & Curtis, 2020). Estimates suggests that there are over 1 billion children not in school (Cluver et al., 2020). The economic impact of COVID-19 will likely be related to greater risk for children to be utilized to offset such financial hardship (e.g., selling merchandise on the street, forced begging for food and goods) and be a more likely to be abused (Campbell, 2020). For example, it is possible that children will be more likely to be used for child labor and be exploited for sexual behavioral and experience corresponding risk for sexual disease and pregnancy as well as serious psychological distress. Interpersonal violence and child abuse will affect children at a significant rate, especially under conditions wherein there is no oversight from educational systems due to quarantine. World health organizations are already predicting an increase in children who will be orphaned and exposed to abuse and neglect (Cluver et al., 2020). Child abuse is less likely to be detected during the COVID-19 pandemic because the reduction or lack of child protection agencies monitoring cases, and teachers less able to detect signs of abuse. Further, children who received meals at school through government programs such as the National School Lunch Program may now no longer have access to nutritious food, which can negatively impact their development.
The lack of structure from schooling and missed education will have a lasting impact on well-being and apt to be related to increased anxiety, depression, and stress about educational attainment and progress going forward (Van Lancker & Parolin, 2020). Although on-line school may help offset some of these challenges, disparities will exist for those who are most vulnerable, including those who lack internet access or cannot afford technology. Older children and young adults may be more likely to drop out of school to help offset family needs. Children and youth also may be engaging in more on-line behavior in general or due to emotional distress (e.g., loneliness due to social isolation) and be increasing the chance for solicitation from others who prey on their emotional vulnerabilities (Peterman et al., 2020, p. 528). Lacking access to physical activity due to quarantine protocols may reduce fitness levels and immunological response as well as decrease psychological wellbeing (Rundle, Park, Herbstman, Kinsey, & Wang, 2020).
Children and youth in juvenile systems, such as orphanages, already were exposed to high density living conditions and often lack access to proper medical or psychological care. The COVID-19 pandemic is likely to place pressure on such systems (e.g., more children) and the physical environments of these settings may be amenable to the spread of infection. Likewise, refugee or otherwise displaced children and youth often live-in high-density environments wherein social distancing is challenging if not impossible. Further, lack of access in these settings to cleaning supplies and water can catalyze the spread of COVID-19 or even the basic fear of acquiring the virus. To the extent the COVID-19 challenges the medical system, it is possible other forms of medical care necessary for child welfare (e.g., routine exams, immunizations) will be reduced, as was the case during other pandemics such as Ebola (Mupere, Kaducu, & Yoti, 2001). Collectively, COVID-19 places an enormous stress on children and youth, placing them at an increased risk for psychological disturbances and physical health vulnerability (Liu, Bao, Huang, Shi, & Lu, 2020).
COVID-19 also will affect ranges of the lifespan, including adults and older adults. The well-publicized health risks for older adults place an obvious psychological and health pressure on this group. Older adults are among the most likely to have a chronic illness (e.g., diabetes, cancer, cardiovascular disease) and consequently they maintain an increased vulnerability to deteriorating health and death from COVID-19. However, even in the absence of exposure to the virus, the fear and worry about contracting the disease is apt to be significant for this group, especially when in homecare facilities such as nursing homes or hospitals (Armitage & Nellums, 2020). This group also is at significant risk for lacking transportation for food, which could challenge the quality of nutrition and have a negative effect in immunological function. Similarly, older adults are among the least physical active groups, which again, will have the potential for decreasing psychological wellbeing and immunity.
Although not specific to older adults, the potential for disruption in grief and loss of others also is a significant psychological stressor. During the pandemic, regular methods of grieving such as funerals have been limited if not all together impossible. The inability to grieve with others or as traditionally done may spur escalation in psychological distress (e.g., sadness, depression) and complicate the grief process (Wallace, Wladkowski, Gibson, & White, 2020). To the extent that grief is impaired, individuals may engage in maladaptive addictive behaviors (e.g., alcohol misuse) to cope with the aversive experiences. Similar types of emotional reactions may occur when parents are separated from their children due to quarantine protocols and disruptions in travel (e.g., cannot travel to see children located in another region).
5.3. Individual differences relevant to psychological dysfunction, addiction, and health behavior
There are several individual difference factors at a psychological level of analysis that will place people at an increased or decreased risk for psychological problems, addiction, and poor health behavior, and chronic illness during and after the pandemic. Research over the past few decades has theorized and found consistent empirical support for emotional symptoms and disorders as well as addictive behavior being explained by individual differences in transdiagnostic processes (Sauer-Zavala et al., 2012). Transdiagnostic factors may contribute to onset, maintenance, and exacerbation of emotional symptomatology and addictive and health behavior. A core aspect of transdiagnostic models is that they seek to identify basic processes underlying multiple, usually comorbid, psychopathologies or addictive behavior.
One set of transdiagnostic factors relevant to COVID-19 may be those that are “reactive” vulnerabilities; that is, individual differences that reflect a heightened emotional response to stressful stimuli. Such vulnerabilities influence emotion experience by enhancing or diminishing the normative response to emotion stimuli and states, resulting in an excess or deficit, respectively, beyond typical emotional functioning; or altering the type of response to emotion stimuli and states (Gratz & Roemer, 2004; Reiss, 1991; Zvolensky, Bernstein, & Vujanovic, 2011). In both instances, such reactive processes may be maladaptive because they serve to reinforce the intensity and frequency of future emotional symptoms. For example, when faced with negative emotion states, individuals with an emotional vulnerability factor that limits their capacity to handle distress may be more apt to execute behaviors that preclude habituation to negative emotion states, which could ultimately increase the intensity of future negative affect and solidify beliefs and learned responses that interfere capacity to adaptively respond to distress.
To illustrate, a transdiagnostic factor that may be especially relevant to COVID-19 related stress responsivity, substance use, and physical health is anxiety sensitivity (Taylor, 2014). Anxiety sensitivity is a malleable, cognitive-affective factor reflecting the tendency to respond to interoceptive distress with anxiety (McNally, 1989). Anxiety sensitivity is related to, yet distinct from, negative affectivity and trait anxiety (Keough, Riccardi, Timpano, Mitchell, & Schmidt, 2010). Anxiety sensitivity has demonstrated racial/ethnic, gender, age, and time invariance (Ebesutani, McLeish, Luberto, Young, & Maack, 2014; Farris et al., 2015; Jardin et al., 2018). Given COVID-19 can produce physical sensations and even when not infected, COVID-related stress can elicit a range of interoceptive sensations, persons higher in anxiety sensitivity may be more be emotional reactive to such stimuli and engage in behavior to dampen stress symptoms (e.g., using tobacco, alcohol). For example, persons may interpret the onset of aversive bodily sensations (e.g., runny nose, cough, fever) as intolerable or catastrophic, exacerbating the experience of such interoceptive symptoms. Further, interoceptive symptoms might be particularly salient to persons with higher anxiety sensitivity who are prone to health inequalities (e.g., racial/ethnic minorities, persons in financial stress), as they may be more apt to perceive these internal sensations as uncontrollable because resources to regulate symptoms (i.e., adaptive cognitive and behavioral skills) are likely diminished due to chronic stress exposure (e.g., low socioeconomic status, discrimination). In turn, persons higher in anxiety sensitivity may be motivated to use substances to reduce emotional and interoceptive distress, elevating their chance for physical illness and compromised immune system function. Although this illustrative example represents only one of many possible transdiagnostic amplifying factors, it draws attention to the fact that individual differences in psychological processes are apt to play a central role in the relation between COVID-19 related stress and mental health, addictive behavior, health behavior, and chronic illness.
Individual difference factors also may play roles in offering resilience to COVID-19 related stress. That is, individual differences may contribute to the likelihood of a resilient response to COVID-19 in the short and long term. Thus, in addition to the many situational and contextual factors, individual difference factors will likely shape the level of resiliency to COVID-19 pandemic. Here, it is likely individual difference factors that de-amplify stress responses will play a central role in offsetting relative risk for psychological, addictive, and health behaviors problems and exacerbation of chronic illness (Pidgeon & Keye, 2014). As with affect amplifying factors, such as anxiety sensitivity, there most certainly is a range of factors of potential importance, including flexible coping repertoires, mindfulness, self-efficacy, self-compassion, and proneness to experience positive affect. To illustrate, individual difference in the capacity to accept difficult COVID-19 related stress may offset the potential escalation of anxiety, stress, and depression and mitigate the need for addictive or unhealthy behaviors (e.g., emotional eating) to delimit aversive internal experiences (Ranzijn & Luszcz, 1999). Consequently, the corresponding risk for health complaints or worsening of chronic health conditions can be offset. Indeed, there is a large theoretical and empirical literature that suggests the capacity to accept difficult emotions experiences is related to psychological well-being and adaptation. For example, one of the reasons meditative practice is related to decreased stress is via change emotional acceptance (Teasdale et al., 2002). This type of work has robust implications in efforts to intervene on COVID-19 related stress in the immediate context and for those that struggle to regain stability and growth in the future in terms of mental health, addictive behavior, and health behavior.
5.3.1. Theoretical model of COVID-19 stress burden
Despite the present lack of systematic empirical work on COVID-19 in terms of behavioral health problems, there is good theoretical basis from past scientific work to hypothesize that COVID-19 related stress burden, due to a myriad of sources, may play a major vulnerability role in terms of mental health, addictive disorders, and health behaviors as well as chronic illness. For some, the stress-related burden of COVID-19 may elicit fundamental changes in risk potential and serve as a fertile basis for future behavioral health problems. For others, the ability to adapt to COVD-19 will offer a different course; one that is characterized by greater stability, speed of recovery, and growth. Further, it is important to recognize that the adaptation process to COVID-19 related stress is apt to be non-linear in many instances. That is, contextual factors (e.g., future life stressors, access to social support) can influence the degree of risk for future problems.
Research described in this essay provides a basis to develop a theoretical model that could be used to evaluate COVID-19 related stress burden on psychological, addictive, and health behaviour problems. We therefore begin this section by briefly outlining a general model that can be used as a heuristic for understanding the complex issues at hand. See Fig. 1 for a graphical depiction of the model. In general, we predict individual differences in affect amplifying and de-amplifying factors will predict the course of psychological, addictive behavior and health behavior and chronic illness even when considering differences in exposure to COVID-19 experiences (e.g., time of quarantine, acquisition of virus). We would predict, based on past work that transdiagnostic affect amplifying factors will influence addictive and health behaviour, which in turn, will increase (or decrease if de-amplifying) the risk of chronic illness and psychological problems and their comorbidity. Further, we can expect that this type of perspective will be moderated by daily stress in the future and access to stress-dampening resources (e.g., social support). Accordingly, certain subgroups more prone to greater and more chronic stress, such as first responders and racial/ethnic minorities and orphaned children, may be particularly vulnerable. This conceptual model predicts that the associations which exist between are reciprocal and dynamic.
Fig. 1.
Theoretical Model for how COVID-19 stress burden may be associated with addictive problems and health behaviors, and how these may be associated with later chronic illness and psychological problems.
Although the model offered here is purposively general and is offered only as a heuristic, it is presumed that there is, in fact, specificity between specific affect amplifying and de-amplifying factors, moderators, mediators, and various forms of psychological and chronic illness. That is, a specific type of individual difference factor like anxiety sensitivity is linked to a particular type of problem (e.g., anxiety disorder, worsening of a chronic respiratory illness, severity of hazardous drinking) via a specified mediating process (e.g., smoking, sleep disruption) in the context of certain moderating variables (e.g., higher levels of COVID-19 stress burden). The core idea being that the underlying mechanism in this hypothetical example may be quite different from that explaining other problems.
The above theoretical model requires empirical testing, and if it is confirmed, one next logical step would be to intervene in it to reduce the burden of mental health, addictive disorders, poor health behaviours, and chronic health conditions related to COVID-19 stress burden. Ideally, this type of intervention approach would target the root of the pathway, including affect amplifying (i.e., decreasing levels) and de-amplifying (i.e., promoting growth). However, intervention efforts sit in the fact that the healthcare system will continue to shift and adapt to treatment delivery, including the uptake of digital health technologies. Digital health, including mobile health (mHealth), telemedicine/telehealth, and health information technology (e.g., mobile phones, wearable sensors), can be used to develop scalable interventions to promote adherence public health guidelines for mitigating the spread of COVID-19. They also can be combined with greater attention to affect amplifying (i.e., decreasing levels) and de-amplifying (i.e., promoting growth) factors that govern many psychological, addictive, and health behaviour processes. Here, there is great opportunity for growth of digital health interventions to offer standalone clinical grade therapeutic tactics and as an adjunct to face-to-face interventions. This type of work can close the gap in access to care and offer evidence-based interventions to large segment of society. For example, digital interventions can be used to combat resistance to public health measures at the level of individuals and institutions with a consideration of individual difference factors that affect emotional and behavioral self-regulation. Indeed, the public's response to public health measures is itself a potential risk and protective factor for many of the psychological, addictive, and health behavior problems reviewed in this essay.
6. Conclusion
The public health impact of COVID-19 on psychological symptoms and disorders, addiction, and health behavior is substantial and ongoing. There is a need for financial and social investment in research to better understand how COVID-19 affects the onset, maintenance, and relapse potential for some of the most common, costly, and chronic behavioral health conditions in the general population. Further, there is a need for the study of the role of psychological processes, addictive behavior, and health behavior in terms of the onset and maintenance of COVID-19 infection and stress burden. There most certainly will be a demand for preventative and intervention efforts for managing the impact of COVID-19 among individuals with elevated negative mood symptoms and disorders, addictive behavior, and certain health behaviors (e.g., sleep disorders) and chronic illness. This work is important to offset the current and projected burden to personal, system, and societal entities, and for providing a theoretical and empirical knowledge base for future pandemics. We presented a heuristic model, which posits that COVID-19 related stress and mood, addictive, and health behavior may, in fact, exacerbate each other via several distinct mechanisms. Future research in this emerging area has the potential to refine both theory and application with respect to COVID-19 and its relation to affect, addiction, and health behavior as well as chronic disease.
References
- Abramson A. Paper presented at the American psychological association forum. 2020. How COVID-19 may increase domestic violence and child abuse.https://www.apa.org/topics/covid-19/domestic-violence-child-abuse [Google Scholar]
- Adams T.G., Kelmendi B., Brake C.A., Gruner P., Badour C.L., Pittenger C. The role of stress in the pathogenesis and maintenance of obsessive-compulsive disorder. Chronic Stress. 2018:2. doi: 10.1177/2470547018758043. Thousand Oaks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler N.E., Boyce T., Chesney M.A., Cohen S., Folkman S., Kahn R.L., et al. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49(1):15. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Adler N.E., Boyce W.T., Chesney M.A., Folkman S., Syme S.L. Socioeconomic inequalities in health: No easy solution. Jama. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- Alisic E., Zalta A.K., Van Wesel F., Larsen S.E., Hafstad G.S., Hassanpour K., et al. Rates of post-traumatic stress disorder in trauma-exposed children and adolescents: meta-analysis. The British Journal of Psychiatry. 2014;204(5):335–340. doi: 10.1192/bjp.bp.113.131227. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnositic and statistical manual of mental disorders. [Google Scholar]
- Armitage R., Nellums L.B. COVID-19 and the consequences of isolating the elderly. The Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnaani A., Hall-Clark B. Recent developments in understanding ethnocultural and race differences in trauma exposure and PTSD. Current opinion in psychology. 2017;14:96–101. doi: 10.1016/j.copsyc.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Battagliese G., Feige B., Spiegelhalder K., Nissen C., Voderholzer U., et al. Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Baker T.B., Piper M.E., McCarthy D.E., Majeskie M.R., Fiore M.C. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bandelow B., Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience. 2015;17(3):327. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard D., Verger P., Hubert P. Chernobyl, 10 years after: Health consequences. Epidemiologic Reviews. 1997;19(2):187–204. doi: 10.1093/oxfordjournals.epirev.a017952. [DOI] [PubMed] [Google Scholar]
- Barlow D.H. Guilford press; 2004. Anxiety and its disorders: The nature and treatment of anxiety and panic. [Google Scholar]
- Baron K.G., Reid K.J. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker H.C., Lopez M.F., Doremus-Fitzwater T.L. Effects of stress on alcohol drinking: A review of animal studies. Psychopharmacology. 2011;218(1):131. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierl E.T., Böllinghaus I., Clark D.M., Glucksman E., Ehlers A. Cognitive paths from trauma to posttraumatic stress disorder: A prospective study of Ehlers and clark's model in survivors of assaults or road traffic collisions. Psychological medicine. 2019:1–10. doi: 10.1017/S0033291719002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.J., Boden J.M., Horwood L.J., Mulder R.T. The role of peri-traumatic stress and disruption distress in predicting symptoms of major depression following exposure to a natural disaster. Aust N Z J Psychiatry. 2017;51(7):711–718. doi: 10.1177/0004867417691852. [DOI] [PubMed] [Google Scholar]
- Bernardi J., Jobson L. Investigating the moderating role of culture on the relationship between appraisals and symptoms of posttraumatic stress disorder. Clinical Psychological Science. 2019;7(5):1000–1013. [Google Scholar]
- Besedovsky L., Lange T., Born J. Sleep and immune function. Pflügers Archiv-European Journal of Physiology. 2012;463(1):121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal R. Covid-19: Undocumented migrants are probably at greatest risk. Bmj. 2020:369. doi: 10.1136/bmj.m1673. [DOI] [PubMed] [Google Scholar]
- Blaine S.K., Sinha R. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology. 2017;122:136–147. doi: 10.1016/j.neuropharm.2017.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger K.E., Patterson C.J., Thompson W.W., Kupersmidt J.B. Psychosocial adjustment among children experiencing persistent and intermittent family economic hardship. Child Development. 1995;66(4):1107–1129. [Google Scholar]
- Bolger N., Zuckerman A. A framework for studying personality in the stress process. Journal of Personality and Social Psychology. 1995;69(5):890. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Bollinger T., Bollinger A., Oster H., Solbach W. Sleep, immunity, and circadian clocks: A mechanistic model. Gerontology. 2010;56(6):574–580. doi: 10.1159/000281827. [DOI] [PubMed] [Google Scholar]
- Bor J., Basu S., Coutts A., McKee M., Stuckler D. Alcohol use during the great recession of 2008–2009. Alcohol and Alcoholism. 2013;48(3):343–348. doi: 10.1093/alcalc/agt002. [DOI] [PubMed] [Google Scholar]
- Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Multidisciplinary Digital Publishing Institute; 2020. Smoking upregulates angiotensin-converting enzyme-2 receptor: A potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander G., Perez-Vigil A., Larsson H., Mataix-Cols D. Systematic review of environmental risk factors for obsessive-compulsive disorder: A proposed roadmap from association to causation. Neuroscience & Biobehavioral Reviews. 2016;65:36–62. doi: 10.1016/j.neubiorev.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Brandt C., Zvolensky M.J., Woods S.P., Gonzalez A., Safren S.A., O'Cleirigh C.M. Anxiety symptoms and disorders among adults living with HIV and AIDS: A critical review and integrative synthesis of the empirical literature. Clinical Psychology Review. 2017;51:164–184. doi: 10.1016/j.cpr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet E.J. Lessons learned from radiation disasters. World Psychiatry. 2011;10(2):83. doi: 10.1002/j.2051-5545.2011.tb00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.D., Crosby R.D., Silgado J., Wonderlich S.A., Schmidt N.B. Immediate antecedents of marijuana use: An analysis from ecological momentary assessment. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43(1):297–304. doi: 10.1016/j.jbtep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.D., Ecker A.H., Vinci C. Cannabis use vulnerability among socially anxious users: Cannabis craving during a social interaction. Psychology of Addictive Behaviors. 2013;27(1):236–242. doi: 10.1037/a0029763. [DOI] [PubMed] [Google Scholar]
- Buckner J.D., Zvolensky M.J., Crosby R.D., Wonderlich S.A., Ecker A.H., Richter A.A. Antecedents and consequences of cannabis use among racially diverse cannabis users: An analysis from ecological momentary assessment. Drug and Alcohol Dependence. 2015;147:20–25. doi: 10.1016/j.drugalcdep.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.D., Zvolensky M.J., Ecker A.H. Cannabis use during a voluntary quit attempt: An analysis from ecological momentary assessment. Drug and Alcohol Dependence. 2013;132(3):610–616. doi: 10.1016/j.drugalcdep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.D., Zvolensky M.J., Ecker A.H., Jeffries E.R. Cannabis craving in response to laboratory-induced social stress among racially diverse cannabis users: The impact of social anxiety disorder. Journal of Psychopharmacology. 2016;30(4):363–369. doi: 10.1177/0269881116629115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman M.P., King A.C. Exercise as a treatment to enhance sleep. American Journal of Lifestyle Medicine. 2010;4(6):500–514. [Google Scholar]
- Cai G. 2020. Tobacco-use disparity in gene expression of ACE2, the receptor of 2019-nCov. [DOI] [Google Scholar]
- Campbell A.M. An increasing risk of family violence during the Covid-19 pandemic: Strengthening community collaborations to save lives. Forensic Science International. 2020:100089. doi: 10.1016/j.fsir.2020.100089. Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico A.W., Horvath K.J., Grov C., Moskowitz J.T., Pahwa S., Pallikkuth S., et al. Double jeopardy: Methamphetamine use and HIV as risk factors for COVID-19. AIDS and Behavior. 2020;1 doi: 10.1007/s10461-020-02854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P.M., Cranford J.A., Buu A., Walton M.A., Zimmerman M.A., Goldstick J., Cunningham R.M. Daily patterns of substance use and violence among a high-risk urban emerging adult sample: Results from the Flint Youth Injury Study. Addictive Behaviors. 2020;101:106127. doi: 10.1016/j.addbeh.2019.106127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2020. Coronavirus disease 2019 (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/hiv.html Retrieved from. [Google Scholar]
- CDC . 2020. HIV in the United States and dependent areas.https://www.cdc.gov/hiv/statistics/overview/ataglance.html Retrieved from. [Google Scholar]
- Chen E., Matthews K.A. Cognitive appraisal biases: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Annals of Behavioral Medicine. 2001;23(2):101–111. doi: 10.1207/S15324796ABM2302_4. [DOI] [PubMed] [Google Scholar]
- Christiansen M., Vik P.W., Jarchow A. College student heavy drinking in social contexts versus alone. Addictive Behaviors. 2002;27(3):393–404. doi: 10.1016/s0306-4603(01)00180-0. [DOI] [PubMed] [Google Scholar]
- Cluver L., Lachman J.M., Sherr L., Wessels I., Krug E., Rakotomalala S., et al. 2020. Parenting in a time of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvonen P.J., Straus L.D., Acheson D., Gehrman P. A review of the relationship between emotional learning and memory, sleep, and PTSD. Current Psychiatry Reports. 2019;21(1):2. doi: 10.1007/s11920-019-0987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger K.J., Martin M.J., Reeb B.T., Little W.M., Craine J.L., Shebloski B., et al. 2012. Economic hardship and its consequences across generations. [Google Scholar]
- Conger R.D., Donnellan M.B. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–199. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Copeland J., Swift W., Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;20(1):45–52. doi: 10.1016/S0740-5472(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Cross D., Vance L.A., Kim Y.J., Ruchard A.L., Fox N., Jovanovic T., et al. Trauma exposure, PTSD, and parenting in a community sample of low-income, predominantly African American mothers and children. Psychological trauma: Theory, Research, Practice, and Policy. 2018;10(3):327. doi: 10.1037/tra0000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack S.E., Hicks T.A., Bourdon J., Sheerin C.M., Overstreet C.M., Kendler K.S., et al. Prevalence and predictors of PTSD among a college sample. Journal of American College Health. 2019;67(2):123–131. doi: 10.1080/07448481.2018.1462824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler C.A., Duffy J.F., Shanahan T.L., Brown E.N., Mitchell J.F., Rimmer D.W., et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dearing E., McCartney K., Taylor B.A. Change in family income‐to‐needs matters more for children with less. Child Development. 2001;72(6):1779–1793. doi: 10.1111/1467-8624.00378. [DOI] [PubMed] [Google Scholar]
- Degenhardt L., Charlson F., Ferrari A., Santomauro D., Erskine H., Mantilla-Herrara A.…Griswold M. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet Psychiatry. 2018;5(12):987–1012. doi: 10.1016/S2215-0366(18)30337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning P., DiNenno E. Communities in crisis: Is there a generalized HIV epidemic in impoverished urban areas of the United States? 2020. https://www.cdc.gov/hiv/group/poverty.html Retrieved from.
- Diaz L., Montero A., Gonzalez-Gross M., Vallejo A., Romeo J., Marcos A. Influence of alcohol consumption on immunological status: A review. European Journal of Clinical Nutrition. 2002;56(3):S50–S53. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- Dick D.M., Smith G., Olausson P., Mitchell S.H., Leeman R.F., O'Malley S.S., et al. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15(2):217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey E.R., Topol E.J. State of telehealth. New England Journal of Medicine. 2016;375(2):154–161. doi: 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- Duffau P., Ozanne A., Bonnet F., Lazaro E., Cazanave C., Blanco P., et al. Multimorbidity, age-related comorbidities and mortality: Association of activation, senescence and inflammation markers in HIV adults. AIDS. 2018;32(12):1651–1660. doi: 10.1097/QAD.0000000000001875. [DOI] [PubMed] [Google Scholar]
- Ebesutani C., McLeish A.C., Luberto C.M., Young J., Maack D.J. A bifactor model of anxiety sensitivity: Analysis of the Anxiety Sensitivity Index-3. Journal of Psychopathology and Behavioral Assessment. 2014;36(3):452–464. [Google Scholar]
- Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: A systematic review and meta-analysis. Archives of academic emergency medicine. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- Ensel W.M., Lin N. The life stress paradigm and psychological distress. Journal of health and social behavior. 1991:321–341. [PubMed] [Google Scholar]
- Evans G.W., English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Farrell N.R., Deacon B.J., Kemp J.J., Dixon L.J., Sy J.T. Do negative beliefs about exposure therapy cause its suboptimal delivery? An experimental investigation. Journal of Anxiety Disorders. 2013;27(8):763–771. doi: 10.1016/j.janxdis.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Farris S.G., DiBello A.M., Allan N.P., Hogan J., Schmidt N.B., Zvolensky M.J. Evaluation of the anxiety sensitivity index-3 among treatment-seeking smokers. Psychological Assessment. 2015;27(3):1123. doi: 10.1037/pas0000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K., Barbouni A., Niaura R. Qeios; 2020. Smoking, vaping and hospitalization for COVID-19. [Google Scholar]
- Fox H.C., Bergquist K.L., Gu P., Sinha R. Interactive effects of cumulative stress and impulsivity on alcohol consumption. Alcoholism: Clinical and Experimental Research. 2010;34(8):1376–1385. doi: 10.1111/j.1530-0277.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P., Muennig P., Lubetkin E., Jia H. The burden of disease associated with being African-American in the United States and the contribution of socio-economic status. Social science & medicine. 2006;62(10):2469–2478. doi: 10.1016/j.socscimed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Gaffey A.E., Aranda F., Burns J.W., Purim-Shem-Tov Y.A., Burgess H.J., Beckham J.C., et al. Race, psychosocial vulnerability and social support differences in inner-city women's symptoms of posttraumatic stress disorder. Anxiety, Stress & Coping. 2019;32(1):18–31. doi: 10.1080/10615806.2018.1532078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaldo C.E., Shaikh A.K., McArthur J.C. The sleep-immunity relationship. Neurologic Clinics. 2012;30(4):1313–1343. doi: 10.1016/j.ncl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Garey, L., Olofsson, H., Garza, T., Shepherd, J. M., Smit, T., & Zvolensky, M. J. (in press). The role of anxiety in smoking onset, severity, and cessation-related outcomes: A review of recent literature. Current Psychiatry Reports. [DOI] [PubMed]
- George S., Rogers R.D., Duka T. The acute effect of alcohol on decision making in social drinkers. Psychopharmacology. 2005;182(1):160–169. doi: 10.1007/s00213-005-0057-9. [DOI] [PubMed] [Google Scholar]
- Germain A., McKeon A.B., Campbell R.L. Sleep in PTSD: Conceptual model and novel directions in brain-based research and interventions. Current opinion in psychology. 2017;14:84–89. doi: 10.1016/j.copsyc.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Grant B.F., Chou S.P., Saha T.D., Pickering R.P., Kerridge B.T., Ruan W.J., et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz K.L., Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment. 2004;26(1):41–54. [Google Scholar]
- Griswold M.G., Fullman N., Hawley C., Arian N., Zimsen S.R., Tymeson H.D., et al. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the global burden of disease study 2016. The Lancet. 2018;392(10152):1015–1035. doi: 10.1016/S0140-6736(18)31310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., et al. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Orlando G., Zona S., Menozzi M., Carli F., Garlassi E., et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical Infectious Diseases. 2011;53:1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T., Petherick A., Phillips T., Webster S. Blavatnik School of Government Working Paper; 2020. Variation in government responses to COVID-19. [Google Scholar]
- Hamblin R.J., Park J.M., Wu M.S., Storch E.A. In: Obsessive-compulsive disorder: Phenomenology, pathophysiology, and treatment. Pittenger C., editor. Oxford University Press; New York, NY: 2017. Variable insight in OCD; pp. 129–136. [Google Scholar]
- Hancock L., Bryant R.A. Perceived control and avoidance in posttraumatic stress. European Journal of Psychotraumatology. 2018;9(1):1468708. doi: 10.1080/20008198.2018.1468708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handerson J., McCullough E., Treuhaft S. A profile of frontline workers in the Bay area. Bay area equity Atlas. 2020. https://bayareaequityatlas.org/essential-workers Retrieved from.
- Harkness A., Bainter S.A., O'Cleirigh C., Mendez N.A., Mayer K.H., Safren S.A. Longitudinal effects of syndemics on ART non-adherence among sexual minority men. AIDS and Behavior. 2018;22(8):2564–2574. doi: 10.1007/s10461-018-2180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S.B., Øverland S., Hatch S.L., Wessely S., Mykletun A., Hotopf M. Exercise and the prevention of depression: Results of the HUNT cohort study. American Journal of Psychiatry. 2018;175(1):28–36. doi: 10.1176/appi.ajp.2017.16111223. [DOI] [PubMed] [Google Scholar]
- Hathaway A.D. Cannabis effects and dependency concerns in long-term frequent users: A missing piece of the public health puzzle. Addiction Research and Theory. 2003;11(6):441–458. doi: 10.1080/1606635021000041807. [DOI] [Google Scholar]
- Holahan C.J., Moos R.H., Holahan C.K., Cronkite R.C. Resource loss, resource gain, and depressive symptoms: A 10-year model. Journal of Personality and Social Psychology. 1999;77(3):620. doi: 10.1037//0022-3514.77.3.620. [DOI] [PubMed] [Google Scholar]
- Hughes J.R., Peters E.N., Callas P.W., Budney A.J., Livingstone A.E. Attempts to stop or reduce marijuana use in non-treatment seekers. Drug and Alcohol Dependence. 2008;97(1–2):180–184. doi: 10.1016/j.drugalcdep.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish L.A., Kline C.E., Gunn H.E., Buysse D.J., Hall M.H. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Medicine Reviews. 2015;22:23–36. doi: 10.1016/j.smrv.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironson G., O'Cleirigh C., Leserman J., Stuetzle R., Fordiani J., Fletcher M., et al. Gender-specific effects of an augmented written emotional disclosure intervention on posttraumatic, depressive, and HIV-disease-related outcomes: A randomized, controlled trial. Journal of Consulting and Clinical Psychology. 2013;81(2):284. doi: 10.1037/a0030814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annual Review of Psychology. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquart J., Dutcher C.D., Freeman S.Z., Stein A.T., Dinh M., Carl E., et al. The effects of exercise on transdiagnostic treatment targets: A meta-analytic review. Behaviour Research and Therapy. 2019;115:19–37. doi: 10.1016/j.brat.2018.11.007. [DOI] [PubMed] [Google Scholar]
- Jardin C., Paulus D.J., Garey L., Kauffman B., Bakhshaie J., Manning K.…Zvolensky M.J. Towards a greater understanding of anxiety sensitivity across groups: The construct validity of the Anxiety Sensitivity Index-3. Psychiatry Research. 2018;268:72–81. doi: 10.1016/j.psychres.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett K.A., Krueger J.M. Vol 89. Elsevier; 2012. pp. 241–257. (Humoral sleep regulation; interleukin-1 and tumor necrosis factor Vitamins & hormones). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Zhou Y., Tang W. Maintaining HIV care during the COVID-19 pandemic. The Lancet HIV. 2020;7(5):e308–e309. doi: 10.1016/S2352-3018(20)30105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinkerson J.D. Defining and assessing moral injury: A syndrome perspective. Traumatology. 2016;22(2):122. [Google Scholar]
- Joannou M., Besemann M., Kriellaars D. Project trauma support: Addressing moral injury in first responders. Mental Health in Family Medicine. 2017;13:418–422. [Google Scholar]
- Johnston L.D., O'Malley P.M., Miech R.A., Bachman J.G., Schulenberg J.E. 2015. Monitoring the Future national survey results on drug use, 1975–2014: Overview, key findings on adolescent drug use. Retrieved from. [Google Scholar]
- Joob B., Wiwanitkit V. 2020. Traumatization in medical staff helping with COVID-19 control. Brain, Behavior, and Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough M.E., Riccardi C.J., Timpano K.R., Mitchell M.A., Schmidt N.B. Anxiety symptomatology: The association with distress tolerance and anxiety sensitivity. Behavior Therapy. 2010;41(4):567–574. doi: 10.1016/j.beth.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D.G., Resnick H.S., Milanak M.E., Miller M.W., Keyes K.M., Friedman M.J. National estimates of exposure to traumatic events and PTSD prevalence using DSM‐IV and DSM‐5 criteria. J Trauma Stress. 2013;26(5):537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M., Goebel S., Pedersen A. Psychological trauma: Theory, research, practice, and policy. 2019. PTSD severity among emergency personnel: An investigation based on the Ehlers and Clark cognitive model. [DOI] [PubMed] [Google Scholar]
- Kraus J.F., Borhani N.O., Franti C.E. Socioeconomic status, ethnicity, and risk of coronary heart disease. American Journal of Epidemiology. 1980;111(4):407–414. doi: 10.1093/oxfordjournals.aje.a112915. [DOI] [PubMed] [Google Scholar]
- Krieger N., Sidney S. Racial discrimination and blood pressure: The CARDIA study of young black and white adults. American journal of public health. 1996;86(10):1370–1378. doi: 10.2105/ajph.86.10.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krier S., Bozich C., Pompa R., Friedman M.R. Assessing HIV-related stigma in healthcare settings in the era of the COVID-19 pandemic. AIDS and Behavior. 2020;1 doi: 10.1007/s10461-020-02892-4. Pittsburgh, Pennsylvania. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Osofsky H.J., Osofsky J.D., Many M., Hardy M., Arey J. First responder culture: Implications for mental health professionals providing services following a natural disaster. Psychiatric Annals. 2008;38(2) [Google Scholar]
- Kuendig H., Kuntsche E. Solitary versus social drinking: An experimental study on effects of social exposures on in situ alcohol consumption. Alcoholism: Clinical and Experimental Research. 2012;36(4):732–738. doi: 10.1111/j.1530-0277.2011.01663.x. [DOI] [PubMed] [Google Scholar]
- Kumar S., Porcu P., Werner D.F., Matthews D.B., Diaz-Granados J.L., Helfand R.S., et al. The role of GABA A receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology. 2009;205(4):529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFauci Schutt J.M., Marotta S.A. Personal and environmental predictors of posttraumatic stress in emergency management professionals. Psychological trauma: Theory, Research, Practice, and Policy. 2011;3(1):8. [Google Scholar]
- Lane E.J., Lating J.M., Lowry J.L., Martino T.P. Differences in compassion fatigue, symptoms of posttraumatic stress disorder and relationship satisfaction, including sexual desire and functioning, between male and female detectives who investigate sexual offenses against children: A pilot study. International Journal of Emergency Mental Health. 2010;12(4):257–266. [PubMed] [Google Scholar]
- Lapid Pickman L., Greene T., Gelkopf M. Sense of threat as a mediator of peritraumatic stress symptom development during Wartime: An experience sampling study. J Trauma Stress. 2017;30(4):372–380. doi: 10.1002/jts.22207. [DOI] [PubMed] [Google Scholar]
- Laurencin C.T., McClinton A. 2020. The COVID-19 pandemic: A call to action to identify and. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.M., Shiroma E.J., Lobelo F., Puska P., Blair S.N., Katzmarzyk P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/s0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R.S., Foster J.E., Fullilove R.E., Fullilove M.T., Briggs N.C., Hull P.C.…Hennekens C.H. 2016. Black-white inequalities in mortality and life expectancy, 1933–1999: Implications for healthy people 2010. Public health reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M.S. Listening to our clients: The prevention of relapse. Journal of Psychoactive Drugs. 2008;40(2):167–172. doi: 10.1080/02791072.2008.10400627. [DOI] [PubMed] [Google Scholar]
- Lewis T. Smoking or vaping may increase the risk of severe coronavirus infection. Scientific American. 2020;17 [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. The American journal of clinical nutrition. 2006;83(2):456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Liu E. Opinions; 2020. April 11, 2020). Covid-19 has inflamed racism against Asian-Americans. Here's how to fight back.https://www.cnn.com/2020/04/10/opinions/how-to-fight-bias-against-asian-americans-covid-19-liu/index.html Retrieved from. [Google Scholar]
- Liu J.J., Bao Y., Huang X., Shi J., Lu L. Mental health considerations for children quarantined because of COVID-19. The Lancet Child & Adolescent Health. 2020;4(5):347–349. doi: 10.1016/S2352-4642(20)30096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tao Z.-W., Wang L., Yuan M.-L., Liu K., Zhou L., et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chinese medical journal. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie C.H. Lessons learned from HIV can inform our approach to COVID-19 stigma. J Int AIDS Soc. 2020;23(5) doi: 10.1002/jia2.25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L.J., Gallagher M.W. Oxford University Press; New York, NY: 2018. Hope and posttraumatic stress disorder the Oxford handbook of hope. [Google Scholar]
- LoSavio S.T., Dillon K.H., Resick P.A. Cognitive factors in the development, maintenance, and treatment of post-traumatic stress disorder. Current opinion in psychology. 2017;14:18–22. doi: 10.1016/j.copsyc.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Magnuson K.A., Duncan G.J. In: Bornstein M.H., editor. Vol. 4. 2002. Parents in poverty; pp. 95–121. (Handbook of parenting: Social conditions and applied parenting). Mahwah, NJ. [Google Scholar]
- Markarian Y., Larson M.J., Aldea M.A., Baldwin S.A., Good D., Berkeljon A., et al. Multiple pathways to functional impairment in obsessive-compulsive disorder. Clinical Psychology Review. 2010;30(1):78–88. doi: 10.1016/j.cpr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D., do Rosario-Campos M.C., Leckman J.F. A multidimensional model of obsessive-compulsive disorder. American Journal of Psychiatry. 2005;162(2):228–238. doi: 10.1176/appi.ajp.162.2.228. [DOI] [PubMed] [Google Scholar]
- Matthews K.A., Räikkönen K., Everson S.A., Flory J.D., Marco C.A., Owens J.F., et al. Do the daily experiences of healthy men and women vary according to occupational prestige and work strain? Psychosomatic Medicine. 2000;62(3):346–353. doi: 10.1097/00006842-200005000-00008. [DOI] [PubMed] [Google Scholar]
- Mayer K.H., Safren S.A., Elsesser S.A., Psaros C., Tinsley J.P., Marzinke M.…Haberer J. Optimizing pre-exposure antiretroviral prophylaxis adherence in men who have sex with men: Results of a pilot randomized controlled trial of “Life-steps for PrEP”. AIDS and Behavior. 2017;21(5):1350–1360. doi: 10.1007/s10461-016-1606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Stellar E. Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- McGovern M.P., Xie H., Segal S.R., Siembab L., Drake R.E. Addiction treatment services and co-occurring disorders: Prevalence estimates, treatment practices, and barriers. Journal of Substance Abuse Treatment. 2006;31(3):267–275. doi: 10.1016/j.jsat.2006.05.003. [DOI] [PubMed] [Google Scholar]
- McGuire J.F., Piacentini J., Lewin A.B., Brennan E.A., Murphy T.K., Storch E.A. A meta-analysis of cognitive behavior therapy and medication for child obsessive-compulsive disorder: Moderators of treatment efficacy, response, and remission. Depression and Anxiety. 2015;32(8):580–593. doi: 10.1002/da.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay D., Piacentini J., Greisberg S., Graae F., Jaffer M., Miller J. The structure of childhood obsessions and compulsions: Dimensions in an outpatient sample. Behavior, Research, and Therapy. 2006;44(1):137–146. doi: 10.1016/j.brat.2005.02.001. [DOI] [PubMed] [Google Scholar]
- McLeod J.D., Kessler R.C. Socioeconomic status differences in vulnerability to undesirable life events. Journal of health and social behavior. 1990:162–172. [PubMed] [Google Scholar]
- McNally R.J. 1989. Is anxiety sensitivity distinguishable from trait anxiety? Reply to lilienfeld. Jacob, and Turner (1989. [DOI] [PubMed] [Google Scholar]
- Mechanic D. Policy challenges in addressing racial disparities and improving population health. Health Affairs. 2005;24(2):335–338. doi: 10.1377/hlthaff.24.2.335. [DOI] [PubMed] [Google Scholar]
- Mensah G.A., Mokdad A.H., Ford E.S., Greenlund K.J., Croft J.B. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.cir.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- Mertens G., Gerritsen L., Salemink E., Engelhard I. 2020. Fear of the coronavirus (COVID-19): Predictors in an online study conducted in March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.M., Farrell N.R., Kemp J.J., Blakey S.M., Deacon B.J. Why do clinicians exclude anxious clients from exposure therapy? Behavior, Research, and Therapy. 2014;54:49–53. doi: 10.1016/j.brat.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Mimiaga M.J., O'Cleirigh C., Biello K.B., Robertson A.M., Safren S.A., Coates T.J., et al. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. Journal of acquired immune deficiency syndromes. 2015;68(3):329. doi: 10.1097/QAI.0000000000000475. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga M.J., Pantalone D.W., Biello K.B., Hughto J.M.W., Frank J., O'Cleirigh C., et al. An initial randomized controlled trial of behavioral activation for treatment of concurrent crystal methamphetamine dependence and sexual risk for HIV acquisition among men who have sex with men. AIDS Care. 2019;31(9):1083–1095. doi: 10.1080/09540121.2019.1595518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbolouk M., Charkhchi P., Kianoush S., Uddin S.M.I., Orimoloye O.A., Jaber R., et al. Prevalence and distribution of e-cigarette use among U.S. adults: Behavioral risk factor surveillance system, 2016. Annals of Internal Medicine. 2018;169(7):429–438. doi: 10.7326/M17-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina P.E., Happel K.I., Zhang P., Kolls J.K., Nelson S. Focus on: Alcohol and the immune system. Alcohol Research & Health. 2010;33(1–2):97. [PMC free article] [PubMed] [Google Scholar]
- Morin C.M., Belleville G., Bélanger L., Ivers H. The insomnia severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C.M., Rodrigue S., Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic Medicine. 2003;65(2):259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- Mupere E., Kaducu O., Yoti Z. Ebola haemorrhagic fever among hospitalised children and adolescents in nothern Uganda: Epidemiologic and clinical observations. African Health Sciences. 2001;1(2):60–65. [PMC free article] [PubMed] [Google Scholar]
- Nanni M.G., Caruso R., Mitchell A.J., Meggiolaro E., Grassi L. Depression in HIV infected patients: A review. Current Psychiatry Reports. 2015;17(1):530. doi: 10.1007/s11920-014-0530-4. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse . 2020. COVID-19: Potential implications for individuals with substance use disorders.https://www.drugabuse.gov/about-nida/noras-blog/2020/04/covid-19-potential-implications-individuals-substance-use-disorders Retrieved from. [Google Scholar]
- Nestadt G., Bienvenu O.J., Cai G., Samuels J., Eaton W.W. Incidence of obsessive-compulsive disorder in adults. The Journal of Nervous and Mental Disease. 1998;186(7):401–406. doi: 10.1097/00005053-199807000-00003. [DOI] [PubMed] [Google Scholar]
- Newland R.P., Crnic K.A., Cox M.J., Mills-Koonce W.R. The family model stress and maternal psychological symptoms: Mediated pathways from economic hardship to parenting. Journal of Family Psychology. 2013;27(1):96. doi: 10.1037/a0031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen . 2020. Rebalancing the 'COVID-19 effect' on alcohol sales.https://www.nielsen.com/us/en/insights/article/2020/rebalancing-the-covid-19-effect-on-alcohol-sales/ Retrieved from. [Google Scholar]
- Nieman D.C., Wentz L.M. The compelling link between physical activity and the body's defense system. Journal of sport and health science. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Cleirigh C., Zvolensky M.J., Smits J.A., Labbe A.K., Coleman J.N., Wilner J.G., et al. Integrated treatment for smoking cessation, anxiety, and depressed mood in people living with HIV: A randomized controlled trial. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2018;79(2):261–268. doi: 10.1097/QAI.0000000000001787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Cleirigh C., Magidson J.F., Skeer M., Mayer K.H., Safren S.A. Prevalence of psychiatric and substance abuse symptomatology among HIV-infected gay and bisexual men in HIV primary care. Psychosomatics. 2015;56(5):470–478. doi: 10.1016/j.psym.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Cleirigh C., Safren S.A., Taylor S.W., Goshe B.M., Bedoya C.A., Marquez S.M.…Shipherd J.C. Cognitive behavioral therapy for trauma and self-care (CBT-TSC) in men who have sex with men with a history of childhood sexual abuse: A randomized controlled trial. AIDS and Behavior. 2019;23(9):2421–2431. doi: 10.1007/s10461-019-02482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaneku J., Vearrier D., McKeever R.G., LaSala G.S., Greenberg M.I. Change in perceived risk associated with marijuana use in the United States from 2002 to 2012. Clinical Toxicology. 2015;53(3):151–155. doi: 10.3109/15563650.2015.1004581. [DOI] [PubMed] [Google Scholar]
- Olatunji B.O., Davis M.L., Powers M.B., Smits J.A. Cognitive-behavioral therapy for obsessive-compulsive disorder: A meta-analysis of treatment outcome and moderators. Journal of Psychiatric Research. 2013;47(1):33–41. doi: 10.1016/j.jpsychires.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Olds J.L., Kabbani N. Is nicotine exposure linked to cardiopulmonary vulnerability to COVID‐19 in the general population? FEBS Journal. 2020;Advance online publication doi: 10.1111/febs.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Operario D., King E.J., Gamarel K.E. Prioritizing community partners and community HIV workers in the COVID-19 pandemic. AIDS and Behavior. 2020;1 doi: 10.1007/s10461-020-02896-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp M.R. Cytokines and sleep. Sleep Medicine Reviews. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Orcutt H.K., Reffi A.N., Ellis R.A. Elsevier; 2020. Experiential avoidance and PTSD Emotion in posttraumatic stress disorder. [Google Scholar]
- Organization W.H. World Health Organization; 2019. Global status report on alcohol and health 2018. [Google Scholar]
- Oscar-Berman M., Marinković K. Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review. 2007;17(3):239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M.W., Eastman A., Lo S., Hearon B.A., Bickel W.K., Zvolensky M., et al. Anxiety sensitivity and working memory capacity: Risk factors and targets for health behavior promotion. Clinical Psychology Review. 2016;49:67–78. doi: 10.1016/j.cpr.2016.07.003. [DOI] [PubMed] [Google Scholar]
- Pantalone D.W., Valentine S.E., Woodward E.N., O'Cleirigh C. Syndemic indicators predict poor medication adherence and increased healthcare utilization for urban HIV-positive men who have sex with men. Journal of Gay & Lesbian Mental Health. 2018;22(1):71–87. doi: 10.1080/19359705.2017.1389794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascandola M., Xiao L. Tobacco and the lung cancer epidemic in China. Translational Lung Cancer Research. 2019;8(Suppl 1):S21. doi: 10.21037/tlcr.2019.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanavanich R., Glantz S.A. medRxiv; 2020. Smoking is associated with COVID-19 progression: A meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini M. On the front lines of coronavirus: The Italian response to covid-19. Bmj. 2020:368. doi: 10.1136/bmj.m1065. [DOI] [PubMed] [Google Scholar]
- Patwardhan P. BJGP open; 2020. COVID-19: Risk of increase in smoking rates among England's 6 million smokers and relapse among England's 11 million ex-smokers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus D.J., Gallagher M.W., Neighbors C., Zvolensky M.J. Computer-delivered personalized feedback intervention for hazardous drinkers with elevated anxiety sensitivity: Study protocol for a randomized controlled trial. Journal of Health Psychology. 2020;1359105320909858 doi: 10.1177/1359105320909858. [DOI] [PubMed] [Google Scholar]
- Peterman A., Potts A., O'Donnell M., Thompson K., Shah N., Oertelt-Prigione S., et al. Center for Global Development Working Paper; 2020. Pandemics and violence against women and children. [Google Scholar]
- Pickett K.E., Kelly S., Brunner E., Lobstein T., Wilkinson R.G. Wider income gaps, wider waistbands? An ecological study of obesity and income inequality. Journal of Epidemiology & Community Health. 2005;59(8):670–674. doi: 10.1136/jech.2004.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidgeon A.M., Keye M. Relationship between resilience, mindfulness, and pyschological well-being in University students. International Journal of Liberal Arts and Social Science. 2014;2(5):27–32. [Google Scholar]
- Pinto A., Mancebo M.C., Eisen J.L., Pagano M.E., Rasmussen S.A. The Brown longitudinal obsessive compulsive study: Clinical features and symptoms of the sample at intake. Journal of Clinical Psychiatry. 2006;67(5):703–711. doi: 10.4088/jcp.v67n0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prati G., Pietrantoni L. The relation of perceived and received social support to mental health among first responders: A meta‐analytic review. Journal of Community Psychology. 2010;38(3):403–417. [Google Scholar]
- Raines A.M., Oglesby M.E., Walton J.L., True G., Franklin C.L. Intolerance of uncertainty and DSM-5 PTSD symptoms: Associations among a treatment seeking veteran sample. Journal of Anxiety Disorders. 2019;62:61–67. doi: 10.1016/j.janxdis.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Ranzijn R., Luszcz M. Acceptance: A key to wellbeing in older adults? Australian Psychologist. 1999;34(2):94–98. [Google Scholar]
- Rattel J.A., Wegerer M., Miedl S.F., Blechert J., Grünberger L.M., Craske M.G., et al. Peritraumatic unconditioned and conditioned responding explains sex differences in intrusions after analogue trauma. Behaviour Research and Therapy. 2019;116:19–29. doi: 10.1016/j.brat.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Rector N.A., Wilde J.L., Richter M.A. In: The Wiley handbook of obsessive compulsive disorders. Abramowitz J.S., McKay D., Storch E.A., editors. 2017. Obsessive compulsive disorder and comorbidity; pp. 697–725. [Google Scholar]
- Reilly D., Didcott P., Swift W., Hall W. Long-term cannabis use: Characteristics of users in an Australian rural area. Addiction. 1998;93(6):837–846. doi: 10.1080/09652149835350. [DOI] [PubMed] [Google Scholar]
- Reiss S. Expectancy model of fear, anxiety, and panic. Clinical Psychology Review. 1991;11(2):141–153. [Google Scholar]
- Rimmer A. British Medical Journal Publishing Group; 2020. Covid-19: Disproportionate impact on ethnic minority healthcare workers will be explored by government. [DOI] [PubMed] [Google Scholar]
- Romeo J., Wärnberg J., Nova E., Díaz L.E., Gómez-Martinez S., Marcos A. Moderate alcohol consumption and the immune system: A review. British Journal of Nutrition. 2007;98(S1):S111–S115. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID‐19 related school closings and risk of weight gain among children. Obesity. 2020;28(6):1008–1009. doi: 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio A.M., Stein D.J., Chiu W.T., Kessler R.C. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Molecular Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren S.A., O'Cleirigh C.M., Bullis J.R., Otto M.W., Stein M.D., Pollack M.H. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2012;80(3):404. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren S.A., O'Cleirigh C.M., Skeer M., Elsesser S.A., Mayer K.H. Project enhance: A randomized controlled trial of an individualized HIV prevention intervention for HIV-infected men who have sex with men conducted in a primary care setting. Health Psychology. 2013;32(2):171. doi: 10.1037/a0028581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma K.-L.K., Felicitas J., Fagan P., Gruder C.L., Blanco L., Cappelli C., et al. Smoking trends and disparities among black and non-Hispanic Whites in California. Nicotine & Tobacco Research. 2015;17(12):1491–1498. doi: 10.1093/ntr/ntv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam L. Why child welfare experts fear a spike of abuse during COVID-19. 2020. https://www.pbs.org/newshour/health/why-child-welfare-experts-fear-a-spike-of-abuse-during-covid-19 Retrieved from.
- Sareen J., Afifi T.O., McMillan K.A., Asmundson G.J. Relationship between household income and mental disorders: Findings from a population-based longitudinal study. Archives of General Psychiatry. 2011;68(4):419–427. doi: 10.1001/archgenpsychiatry.2011.15. [DOI] [PubMed] [Google Scholar]
- Sauer-Zavala S., Boswell J.F., Gallagher M.W., Bentley K.H., Ametaj A., Barlow D.H. The role of negative affectivity and negative reactivity to emotions in predicting outcomes in the unified protocol for the transdiagnostic treatment of emotional disorders. Behaviour Research and Therapy. 2012;50(9):551–557. doi: 10.1016/j.brat.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramella L.V., Sohr-Preston S.L., Callahan K.L., Mirabile S.P. A test of the Family Stress Model on toddler-aged children's adjustment among Hurricane Katrina impacted and nonimpacted low-income families. Journal of Clinical Child and Adolescent Psychology. 2008;37(3):530–541. doi: 10.1080/15374410802148202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C.D., Suvak M.K., Resick P.A. Trauma cognitions are related to symptoms up to 10 years after cognitive behavioral treatment for posttraumatic stress disorder. Psychological trauma: Theory, Research, Practice, and Policy. 2017;9(6):750. doi: 10.1037/tra0000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti I., Argentero P. The role of mindfulness in protecting firefighters from psychosomatic malaise. Traumatology: International Journal. 2014;20(2):134. [Google Scholar]
- Shalev A.Y., Gevonden M., Ratanatharathorn A., Laska E., Van Der Mei W.F., Qi W., et al. Estimating the risk of PTSD in recent trauma survivors: Results of the international Consortium to predict PTSD (ICPP) World Psychiatry. 2019;18(1):77–87. doi: 10.1002/wps.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S., Krause K.D., Valera P., Swaminathan S., Halkitis P.N. The burden of COVID-19 in people living with HIV: A syndemic perspective. AIDS and Behavior. 2020:1–6. doi: 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibrava N.J., Bjornsson A.S., Pérez Benítez A.C.I., Moitra E., Weisberg R.B., Keller M.B. Posttraumatic stress disorder in African American and Latinx adults: Clinical course and the role of racial and ethnic discrimination. American Psychologist. 2019;74(1):101. doi: 10.1037/amp0000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha I.P., Harwood R., Semple M.G., Hawcutt D.B., Thursfield R., Narayan O.…Southern K.W. COVID-19 infection in children. The Lancet. Respiratory Medicine. 2020;8(5):446–447. doi: 10.1016/S2213-2600(20)30152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld S.A., Head J., Marmot M. Explaining social class differences in depression and well-being. Social Psychiatry and Psychiatric Epidemiology. 1997;33(1):1–9. doi: 10.1007/s001270050014. [DOI] [PubMed] [Google Scholar]
- Stansfeld S.A., North F., White I., Marmot M.G. Work characteristics and psychiatric disorder in civil servants in London. Journal of Epidemiology & Community Health. 1995;49(1):48–53. doi: 10.1136/jech.49.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp M.M., Schlenger W.E., Corry N., Henn‐Haase C., Qian M., Li M., et al. Predictors of PTSD 40 years after combat: Findings from the national Vietnam Veterans longitudinal study. Depression and Anxiety. 2017;34(8):711–722. doi: 10.1002/da.22628. [DOI] [PubMed] [Google Scholar]
- Stein D.J., Costa D.L.C., Lochner C., Miguel E.C., Reddy Y.C.J., Shavitt R.G., et al. Obsessive–compulsive disorder. Nature Reviews Disease Primers. 2019;5(1):52. doi: 10.1038/s41572-019-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B., Veronese N., Vancampfort D., Prina A.M., Lin P.Y., Tseng P.T.…Koyanagi A. Perceived stress and smoking across 41 countries: A global perspective across europe, Africa, Asia and the Americas. Sci Rep. 2017;7(1):7597. doi: 10.1038/s41598-017-07579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality Results from the 2018 national survey on drug Use and health: Graphics from the key findings. 2019. https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHffrBriefingSlides2018_w-final-cover.pdf Report Retrieved from.
- Sun K., Chen J., Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: A population-level observational study. The Lancet Digital Health. 2020;2(4):e201–e208. doi: 10.1016/S2589-7500(20)30026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G., Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcoholism: Clinical and Experimental Research. 2009;33(2):220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D.P., Roth M.D. Pulmonary effects of inhaled cannabis smoke. The American Journal of Drug and Alcohol Abuse. 2019 doi: 10.1080/00952990.2019.1627366. [DOI] [PubMed] [Google Scholar]
- Taub A. Vol. 6. New York Times; 2020. (A new COVID-19 crisis: Domestic abuse rises worldwide). [Google Scholar]
- Taylor S. Routledge; 2014. Anxiety sensitivity: Theory, research, and treatment of the fear of anxiety. [Google Scholar]
- Teasdale J.D., Moore R.G., Hayhurst H., Pope M., Williams S., Segal Z.V. Metacognitive awareness and prevention of relapse in depression: Empirical evidence. Journal of Consulting and Clinical Psychology. 2002;70(2):275. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- Thompson N.J., Fiorillo D., Rothbaum B.O., Ressler K.J., Michopoulos V. Coping strategies as mediators in relation to resilience and posttraumatic stress disorder. Journal of Affective Disorders. 2018;225:153–159. doi: 10.1016/j.jad.2017.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerging Microbes & Infections. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen P., Marott J.L., Nordestgaard B., Bojesen S.E., Lange P. Secular trends in smoking in relation to prevalent and incident smoking-related disease: A prospective population-based study. Tobacco Induced Diseases. 2019;17 doi: 10.18332/tid/112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A.J., Hiscox J.A., Hooper N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends in pharmacological sciences. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge J.M. The age of anxiety? The birth cohort change in anxiety and neuroticism, 1952–1993. Journal of Personality and Social Psychology. 2000;79(6):1007. doi: 10.1037//0022-3514.79.6.1007. [DOI] [PubMed] [Google Scholar]
- Van Lancker W., Parolin Z. COVID-19, school closures, and child poverty: A social crisis in the making. The Lancet Public Health. 2020;5(5):e243–e244. doi: 10.1016/S2468-2667(20)30084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas C.I., Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tobacco Induced Diseases. 2020;18 doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswam D., Trotter S., Burge P.S., Walters G.I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Reports, 2018. 2018;bcr-2018-224350 doi: 10.1136/bcr-2018-224350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov D., Galea S., Resnick H., Ahern J., Boscarino J.A., Bucuvalas M., et al. Increased use of cigarettes, alcohol, and marijuana among Manhattan, New York, residents after the September 11th terrorist attacks. American Journal of Epidemiology. 2002;155(11):988–996. doi: 10.1093/aje/155.11.988. [DOI] [PubMed] [Google Scholar]
- Vujanovic A.A., Back S.E. Routledge; New York: 2019. Posttraumatic stress and substance use disorders: A comprehensive clinical handbook. [Google Scholar]
- Vujanovic A.A., Zegel M. In: Emotion in posttraumatic stress disorder. Tull M.T., Kimbrel N.A., editors. Guilford Press; New York: 2020. Distress tolerance and posttraumatic stress; pp. 343–376. [Google Scholar]
- Wallace C.L., Wladkowski S.P., Gibson A., White P. Grief during the COVID-19 pandemic: Considerations for palliative care providers. Journal of Pain and Symptom Management. 2020;60(1):e70–e76. doi: 10.1016/j.jpainsymman.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. Journal of Virology. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. International Journal of Environmental Research and Public Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Luo Q., Chen R., Chen T., Li J. 2020. Susceptibility analysis of COVID-19 in smokers based on ACE2. [Google Scholar]
- Watson N.F., Badr M.S., Belenky G., Bliwise D.L., Buxton O.M., Buysse D., et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of sleep medicine and sleep research society. Sleep. 2015;38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A.M., Castle I.J.P., Hingson R.W., Powell P.A. Vol. 44. Alcoholism Clinical and Experimental Research; 2020. Using death certificates to explore changes in alcohol‐related mortality in the United States, 1999 to 2017; pp. 178–187. [DOI] [PubMed] [Google Scholar]
- Whitfield G.P., Carlson S.A., Ussery E.N., Fulton J.E., Galuska D.A., Petersen R. Trends in meeting physical activity guidelines among urban and rural dwelling adults—United States, 2008–2017. Morbidity and Mortality Weekly Report. 2019;68(23):513. doi: 10.15585/mmwr.mm6823a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R.G., Pickett K.E. Income inequality and population health: A review and explanation of the evidence. Social science & medicine. 2006;62(7):1768–1784. doi: 10.1016/j.socscimed.2005.08.036. [DOI] [PubMed] [Google Scholar]
- Wilkinson R.G., Pickett K.E. The problems of relative deprivation: Why some societies do better than others. Social science & medicine. 2007;65(9):1965–1978. doi: 10.1016/j.socscimed.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Wilkinson R.G., Pickett K.E. Income inequality and socioeconomic gradients in mortality. American journal of public health. 2008;98(4):699–704. doi: 10.2105/AJPH.2007.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Jackson P.B. Social sources of racial disparities in health. Health Affairs. 2005;24(2):325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Neighbors H.W., Jackson J.S. Racial/ethnic discrimination and health: Findings from community studies. American journal of public health. 2003;93(2):200–208. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Yu Y., Jackson J.S., Anderson N.B. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Williamson V., Stevelink S.A., Greenberg N. Occupational moral injury and mental health: Systematic review and meta-analysis. The British Journal of Psychiatry. 2018;212(6):339–346. doi: 10.1192/bjp.2018.55. [DOI] [PubMed] [Google Scholar]
- Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China Life Sciences. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C.W. COVID-19 and African Americans. Jama. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- Yao H., Chen J.-H., Xu Y.-F. Patients with mental health disorders in the COVID-19 epidemic. The Lancet Psychiatry. 2020;7(4):e21. doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt S.D., Kline C.E. Epidemiology of exercise and sleep. Sleep and Biological Rhythms. 2006;4(3):215–221. doi: 10.1111/j.1479-8425.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yang L., Liu S., Ma S., Wang Y., Cai Z., et al. Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Frontiers in Psychiatry. 2020;11:306. doi: 10.3389/fpsyt.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y., et al. 2020. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. 2020. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. The Pediatric Infectious Disease Journal. 2020;39(5):355. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuluaga A.F., Montoya-Giraldo M.A., Buendia J.A. Are the high smoking rates related to COVID-19 outbreaks? Latin American Journal of Clinical Sciences and Medical Technology. 2020;2:25–27. [Google Scholar]
- Zvolensky M.J., Bernstein A., Vujanovic A.A. Guilford Press; 2011. Distress tolerance: Theory, research, and clinical applications. [Google Scholar]