Abstract
Objective
Animal studies suggested that angiotensin-converting enzyme inhibitors (ACEi) and angiotensin-receptor blockers (ARB) facilitate the inoculation of potentially leading to a higher risk of infection and/or disease severity. We aimed to systematically evaluate the risk of COVID-19 infection and the risk of severe COVID-19 disease associated with previous exposure to (ACEi) and/or ARB).
Methods
MEDLINE, CENTRAL, PsycINFO, Web of Science Core Collection were searched in June 2020 for controlled studies. Eligible studies were included and random-effects meta-analyses were performed. The estimates were expressed as odds ratios (OR) and 95% confidence intervals (95%CI). Heterogeneity was assessed with I2 test. The confidence in the pooled evidence was appraised using the GRADE framework.
Results
Twenty-seven studies were included in the review. ACEi/ARB exposure did not increase the risk of having a positive test for COVID-19 infection (OR 0.99, 95%CI 0.89–1.11; I2 = 36%; 5 studies, GRADE confidence moderate). The exposure to ACEi/ARB did not increase the risk of all-cause mortality among patients with COVID-19 (OR 0.91, 95%CI 0.74–1.11; I2 = 20%; 17 studies; GRADE confidence low) nor severe/critical COVID-19 disease (OR 0.90, 95%CI 0.74–1.11; I2 = 55%; 17 studies; GRADE confidence very low). Exploratory analyses in studies enrolling hypertensive patients showed a association of ACEi/ARB with a significant decrease of mortality risk.
Conclusions
ACEi/ARB exposure does not seem to increase the risk of having the SARS-CoV-2 infection or developing severe stages of the disease including mortality. The potential benefits observed in mortality of hypertensive patients reassure safety, but robust studies are required to increase the confidence in the results.
Keywords: Coronavirus, SARS-CoV-2, Angiotensin-converting enzyme inhibitor, Angiotensin-receptor blocker, Acute respiratory distress syndrome, Acute lung injury
1. Introduction
The novel acute respiratory syndrome coronavirus 2 (SARS-CoV-2) firstly identified in Wuhan China lead to a world-wide outbreak pandemic situation with more than 350,000 related deaths [1]. The SARS-CoV-2 goes into the host cells through the angiotensin-converting enzyme (ACE) 2 (ACE2) receptor [2]. Some animal studies showed that angiotensin-converting enzyme inhibitors (ACEi) and angiotensin-receptor blockers (ARB) increase the ACE2, creating the hypothesis that these drugs could facilitate the inoculation of SARS-CoV-2 potentially leading to a higher risk of infection and/or disease severity [3]. The fragility of these assumptions led several medical societies to issue a recommendation for not withdrawing these drugs because the evidence was not compelling and due to the potential harms, as these drugs are effective treatments in the management of hypertension, diabetes mellitus, coronary heart disease, cerebrovascular disease and/or chronic kidney disease for many people. In this systematic review we aimed to assess the risk of infection by SARS-CoV-2 and the risk of mortality or respiratory complications in patients with symptomatic disease of SARS-CoV-2 (COVID-19) related to previous use of ACEi or ARBs.
2. Methods
This systematic review followed the reporting principles of MOOSE and PRISMA [4], [5]. The protocol is available at https://osf.io/6vf2w. Patients and public were not involved in this review.
2.1. Eligibility criteria
We included all controlled studies with information about risk of infection or the risk of disease complications associated with ACEi and/or ARBs.
For randomized controlled trials or cohort/nested case-control studies that evaluated the risk of infection (positive test), studies had to enrol a population submitted to tests and to report the risk of having a positive test associated with ACEi and/or ARB, or having raw data that enables these calculations.
Regarding the risk of disease complications, studies had to evaluate the risk of mortality/severe disease associated with ACEi and/or ARB use compared with patients not treated with these drugs, both from a population perspective or among population infected with SARS-CoV-2. ACEi or ARBs had to be reported by the investigators as a group (ACEi/ARB) or individually. We accepted controls treated with other antihypertensive drugs or without any antihypertensive drug.
In case-control studies, cases were patients with COVID-19 infection (positive test) irrespective of disease severity, and controls were matched individuals without the referred outcomes. Data about ACEi and/or ARB risks should be available.
The outcomes of interest were:
-
1)
COVID-19 infection documented by nasophaygeal or oropharyngeal swab tests or reported by authors as having COVID-19;
-
2)
All-cause Mortality;
-
3)
Severe/Critical Disease according with the World Health Organization and Chinese Centre of Disease Control [6], [7].
Whenever possible, if adequate, adjusted measures were retrieved particularly for observational studies, giving preference to propensity score matching or weighting.
2.2. Search methods for study identification
The reviewers performed an electronic database search through MEDLINE, CENTRAL, PsycINFO and Web of Science Core Collection databases for relevant studies (Search strategy at Supplementary Table 1). The database medRxiv was also searched for unpublished pre-print manuscripts for an exploratory analysis. Relevant reviews obtained in the searching process as well as the references of potentially included studies were analysed in order to search for potential eligible studies. There were no restrictions on language or publication date. The search lastly performed at 8th June 2020.
2.3. Study selection and data collection process
The title and abstract screening phase of records yielded by the search was performed independently by clusters of 2 reviewers. Disagreements were resolved through consensus or by a third reviewer (DC). The studies that were not excluded went to the full-text assessment phase.
The reasons for exclusion were recorded at this stage.
The reviewers extracted study data following a pre-established data collection form. When studies presented different estimates of the outcome of interest, we extracted the most precise or adjusted measures.
Risk of bias was independently evaluated by three authors (DC, MA, ANF) using the Cochrane Risk of Bias Tool for randomized controlled trials and ROBINS-I tool for observational studies [8], [9]. The studies were qualitatively classified as at critical, serious, moderate, or low risk of bias. Risk of bias graphs were derived from these tools.
2.4. Statistical analysis and pooled data evaluation
We used Review Manager for statistical analysis and to derive forest plots. We used the inverse variance method and random-effects model to pool data. We reported pooled dichotomous data using odds ratios (OR) with their 95% confidence intervals (95% CIs). Heterogeneity was assessed using I2 [10]. We present effect estimates as OR because relative estimates are more similar across studies with different designs, populations and lengths of follow-up than absolute effects [11]. We used the hazard ratio (HR) when OR was not available nor possible to calculate. Publication bias assessment was performed through funnel plot examination and Egger test providing that a sufficient number of studies were included [12].
Exploratory analyses were performed with adjusted estimates, and only those with data of hypertensive patients. We further performed an additional exploratory analysis including unpublished (Preprint) studies found in medRxiv.
We used the Grading of Recommendations, Assessment, and Evaluation (GRADE) framework to report the overall quality of evidence. The certainty in the evidence for each outcome was graded as high, moderate, low, or very low [13].
3. Results
3.1. Included studies
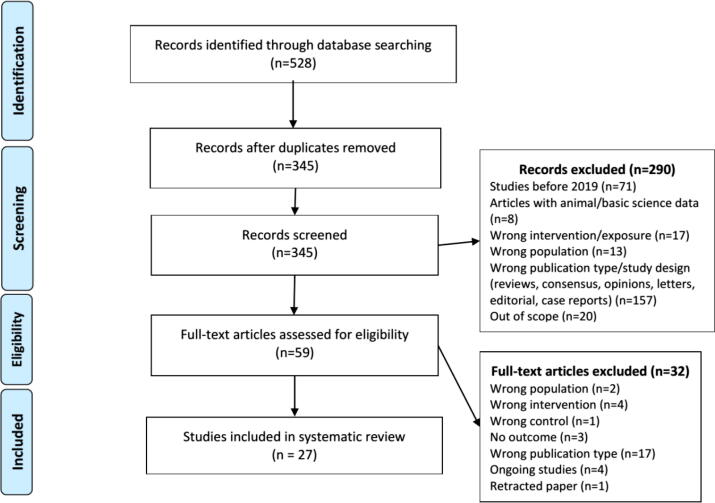
The search returned 528 records, resulting in 27 study records after the deduplication, title and abstract screening and full-text screening (Fig. 1; details of excluded studies at Supplementary Table 2) [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]. There was one randomized controlled trial (a non-prespecified interim analysis of an open-label trial), 4 case-control studies (two of them – Gnavi et al – were reported in the same article) and the remaining were cohort/nested case-control studies.
Fig. 1.
Flowchart of studies selection process.
The main characteristics of the included studies are depicted in Table 1. The median sample size was 522 [interquartile range 113–4051] and overall, there were 119,656 participants evaluated.
Table 1.
Main characteristics of included studies.
| Study Year | Design | Region | Population | Total/ ACEi/ARB | Control | Mean-median age / % female | Comorbidities | Outcome adjustments |
|---|---|---|---|---|---|---|---|---|
| RANDOMIZED CONTROLLED TRIAL | ||||||||
| Amat-Santos 2020 | Non-planned interim analysis of an open-label RCT | Spain | Patients with aortic stenosis successfully treated with transcatheter aortic valve replacement |
ACEi (ramipril): 52 | Placebo: 50 | 83 47% |
HTN: 54% CAD: 26% DM: 20% CKD: 33% |
– |
| CASE-CONTROL STUDIES | ||||||||
| de Abajo 2020 | Case-control study | Madrid, Spain | Case: ≥ 18 years with PCR-confirmed COVID-19 requiring hospital admission (n = 1139) Control: from database Investigación Farmacoepidemiológica en Atención Primaria (BIFAP), a Spanish primary health-care database (n = 11390) Matching 1:10 by sex and age |
COVID-19 positive Total: 497 ACEi: 240 ARB: 244 Aldosterone antagonists: 38 Renin inhibitors: 1 |
COVID-19 negative Total: 3822 ACEi: 2192 ARB: 1616 Aldosterone antagonists:218 Renin inhibitors: 8 |
69 61% |
HTN: 54% COVID-19+ 50% COVID-19 - CAD: 11% COVID-19+ 8% COVID-19 - DM: 27% COVID-19+ 20% COVID-19 - CKD: 8% COVID-19+ 5% COVID-19 - HF: 7% COVID-19+ 4% COVID-19 - |
Age, sex, diabetes, dyslipidemia, ischemic heart disease, heart failure, atrial fibrillation, thromboembolic disease, cerebrovascular accident, chronic obstructive pulmonary disease, asthma, cancer, and chronic kidney disease |
| Gnavi 2020 | Nested case-control study in 2 cohorts | Piedmont, Italy | Case: Discharged patients with confirmed COVID-19 infection (RT-PCR) in - Hypertensive patients (N = 316) - Cardiovasular disease* patients (N = 171) Control: Discharged patients without COVID-19 infection Matching 1:5 by sex and age |
COVID-19 positive Total: 215 ACEi: NR ARB: NR Total: 93 ACEi: NR ARB: NR |
COVID-19 negative Total: 1153 ACEi: NR ARB: NR Total: 475 ACEi: NR ARB: NR |
71 (hypertension cohort); 75 (cardiovascular disease cohort) 31% (hypertension cohort); 22% (cardiovascular disease cohort) |
HTN: 100% | Age, sex, and disease type (hypertension or cardiovascular disease) |
| Mancia 2020 | Population-based case–control study | Lombardy Italy | Case: Positive COVID-19 patients (≥40 years old) N = 6272 Control: beneficiaries of the Regional Health Service N = 30759 Matched 1:5 by sex, age at index date, and municipality of residence |
COVID-19 positive Total: 2896 ACEi: 1502 ARB: 1394 |
COVID-19 negative Total: 27863 ACEi: 6569 ARB: 5910 |
68 37% |
NR | Cardiovascular disease, respiratory disease, kidney disease, cancer, antihypertensive agents, lipid lowering agents, oral hypoglycemic agents, insulin, antiplatelet agents, antiarrhythmic agents, anticoagulant agents, digitalis, nitrates, inhaled glucocorticoids, nonsteroidal antiinflamatory drugs, immunosuppressive agents, beta agonists, other drugs for respiratory disease |
| COHORT/NESTED CASE-CONTROL STUDIES | ||||||||
| Argenziano 2020 | Single-center retrospective cohort study | New York, USA | Patients with hypertension and diabetes admitted in the emergency department or in the hospital for COVID-19 infection N = 1000 |
Total: 284 ACEI: NR ARB: NR |
Non-ACEi/ARB: 716 |
63 40% |
HTN: 60% CAD: 13% DM: 37% CKD: 14% HF: 10% |
– |
| Bean 2020 | Retrospective cohort study | London, UK |
Adult COVID 19 symptomatic patients N = 1200 |
Total: 399 ACEi: 260 ARB: 147 |
Non-ACEi/ARB: 801 |
68 43% |
HTN: 54% DM: 35% CKD: 17% HF: 9% |
Age, sex, hypertension, diabetes mellitus, chronic kidney disease, ischaemic heart disease, heart failure |
| Chen 2020 | Retrospective cohort study | Wuhan, China | Patients with hypertension and diabetes admitted in the hospital for symptomatic COVID-19 infection N = 71 |
Total: 31 ACEi: NR ARB: NR |
Non-ACEi/ARB: 39 | 67 NR |
HTN: 100% DM: 100% |
– |
| Chodik 2020 | Cross sectional Cohort | Tel Aviv, Israel | individuals tested for SARS-COV-2 (RT-PCR) N = 1317 positive N = 13203 negative |
Total: 991 ARB 603 ACEI 388 |
Non-ACEi/ARB: 13,529 | 41 COVID-19+ 37 COVID-19– 40% COVID-19 + / 46% COVID-19 - |
HTN: 14% COVID-19+ 11% COVID-19 - DM: 9% COVID-19+ 5% COVID-19 - CKD: 8% COVID-19+ 6% COVID-19 - HF: 0.2% COVID-19+ 0.6% COVID-19 - |
Age, sex, SES, BMI, and co-morbidities |
| Yan, 2020 | Multicentre retrospective case-control study | Zhejiang, China | Case: Consecutive patients presenting to hospital with confirmed diagnosis of Covid-19 infection N = 610 Control: Population-based control group N = 48667 |
COVID-19+ Total: 58 ACEi: 5 ARB: 53 |
COVID.19 - Total: 8040 ACEi: 555 ARB: 7485 |
49 49% |
HTN: 22% DM: 10% CVD/cerebrovascular disease: 3% |
Age, sex, BMI |
| Felice 2020 | Single-centre retrospective cohort study | Treviso, Italy | Symptomatic COVID-19 hypertensive patients presenting to the emergency department N = 133 |
Total: 82 ACEI: 40 ARB: 42 |
Non-ACEi/ARB: 51 |
73 35% |
HTN: 100% DM: 26% HF: 24% |
– |
| Feng 2020 | Multi-center retrospective cohort study | Wuhan, Shangai, Tongling China |
Subgroup of hypertensive COVID 19 symptomatic patients admitted in 3 hospitals N = 113 |
Total: 33 ACEi: NR ARB: NR |
Non-ACEi/ARB: 62 | 53 45% |
HTN: 100% | – |
| Gao 2020 | Single-centre retrospective cohort study | Wuhan, China | Subgroup of hypertensive COVID 19 symptomatic patients admitted in the hospital N = 710 |
Total: 183 ACEi: NR ARB: NR |
Non-ACEi/ARB: 527 | 64 48% |
HTN: 100% DM: 28% HF: 3% CKD: 2% MI: 1% |
Propensity-matched score for mortality Age, sex, medical history of diabetes, insulin-treated diabetes, myocardial infarction, PCI/CABG, renal failure, stroke, heart failure, and COPD |
| Hu 2020 | Retrospective single-centre cohort | Zhejiang, China |
Subgroup of hypertensive COVID 19 symptomatic patients admitted in the hospital N = 149 |
Total: 65 ACEi: NR ARB: NR |
Non-ACEi/ARB: 84 | 57 41% |
HTN 100% DM: 20% CKD: 4% |
– |
| Huang 2020 | Retrospective single-centre cohort | Wuhan, China | Hypertensive COVID 19 symptomatic patients admitted in the hospital N = 50 |
Total: 20 ACEi: NR ARB: NR |
Non-ACEi/ARB: 30 |
62 45% |
HTN: 100% DM: 8% CAD: 2% |
– |
| Imam 2020 | Retrospective multicentre cohort | Detroit, USA | COVID-19 symptomatic patients N = 1305 |
Total: 565 ACEi: NR ARB: NR |
Non-ACEi/ARB: 740 | 61 46% |
HTN: 56% DM: 30% HF: 6% Vascular Disease: 16% |
Age, comorbidities, NSAID, ACEi/ARB |
| Jung 2020 | Cohort study | Seoul, Korea | Adult COVID 19 patients N = 5179 |
Total: 762 ACEI: 32 ARB: 730 |
Non-ACEi/ARB: 1577 |
45 56% |
HTN: 22% DM: 17% HF: 4% CAD: 1% CKD: 5% |
Age, sex, Charlson Comorbidity Index, immunosuppression, and hospital type. |
| Li 2020 | Retrospective, single-center cohort | Wuhan, China | Hypertensive COVID 19 symptomatic hospitalized patients N = 362 |
Total: 115 ACEi: NR ARB: NR |
Non-ACEI/ARB: 247 | 66 41-51% |
HTN: 100% DM 35% Cerebrovascular disease: 23% CHD: 18% HF 3% |
– |
| Mehra, 2020 | Cohort/Nested case-control | 169 hospitals in Asia, Europe, and North America |
Hospitalized patients from Surgical Outcomes Collaborative (Surgisphere), an international registry N = 8910 |
Total: 1326 ACEi: 770 ARB: 556 |
Non-ACEi/ARB: 7584 | 49 40% |
HTN: 26% CAD: 11% DM: 14% HF: 2% Dyslipidemia: 31% COPD: 3% |
Age, sex, hypertension |
| Mehta, 2020 | Retrospective cohort study | Ohio and Florida, USA | Patients tested for COVID-19 N = 18472 N positive = 1735 N negative = 16737 | Total: 2285 ACEi: 1322 ARB: 982 |
Non-ACEi/ARB: 16187 |
49 60% |
HTN: 93% DM: 46% CAD: 22% HF: 17% COPD: 14% |
Propensity score: Age, sex, and presence of hypertension, diabetes, coronary artery disease, heart failure, and COPD |
| Meng, 2020 | Retrospective single center case control | Shenzhen, China | Hospitalized patients with COVID-19 and receiving anti-hypertensive therapy N = 42 |
Total: 17 ACEi: NR ARB: NR |
Non-ACEi/ARB: 25 | 65 43% |
HTN: 100% DM: 24% ACEi/ARB 8% Non-ACEi/ARB CHD: 35% ACEi/ARB 8% Non-ACEi/ARB |
– |
| Million, 2020 | Retrospective cohort study | Marseille, France | COVID-19 positive tested patients N = 1061 |
ARB: 40 | Non-ARB: 1021 | 44 54% |
HTN: 14% DM: 7% CAD: 4% Obesity: 6% Chronic Respiratory Disease: 11% |
Age, comedications, COVID-19 severity score |
| Montastruc 2020 | Retrospective cohort study | Toulouse, France | Adult patients positive for COVID-19 admitted in the intensive care unit N = 96 |
Total: 35 ACEI: 12 ARB: 23 |
Non-ACEi/ARB: 61 | 63 21% |
HTN: 45% DM: 28% CKD: 10% Arrythmia: 6% |
– |
| Peng 2020 | Retrospective cohort study | Wuhan, China | Hospitalized COVID-19 patients with Cardiovascular disease N = 112 |
Total: 22 ACEi: NR ARB: NR |
Non-ACEi/ARB: 90 | 61 53% |
DM: 33% HTN: 82% |
– |
| Reynolds 2020 | Retrospective cohort study | New York, USA | Patients tested for COVID-19 N = 12594 N positive = 5894 N negative = 6700 | Risk of infection (PSM): Total 1909 ACEi: 1137 ARB: 1044 Risk of severe infection (PSM): Total 1110 ACEi: 627 ARB: 664 |
Risk of infection (PSM): Non-ACEi/ARB: 4344 Risk of severe infection (PSM): Non-ACEi/ARB: 2453 |
49 59% |
HTN: 35% DM: 18% HF: 6% MI: 4% CKD: 18% COPD: 15% Current smoker: 5% Former smoker: 18% |
Age; sex; race; ethnic group; body-mass index; smoking history; history of hypertension, myocardial infarction, heart failure, diabetes, chronic kidney disease, and obstructive lung disease (e.g., asthma and obstructive pulmonary diseases) |
| Tan, 2020 | Retrospective cohort study | Wuhan, China | Subgroup of symptomatic COVID-19 patients with hypertension N = 100 |
Total: 31 ACEi: NR ARB: NR |
Non-ACEi/ARB: 69 | 67 NR |
HTN: 100% DM: 26% ACEi/ARB 29% Non-ACEi/ARB GI: 19% ACEi/ARB 25% Non-ACEi/ARB CKD: 7% ACEi/ARB 13% Non-ACEi/ARB CHD: 10% ACEi/ARB 16% Non-ACEi/ARB COPD: 7% ACEi/ARB 10% Non-ACEi/ARB Tumor: 3% ACEi/ARB 6% Non-ACEi/ARB |
– |
| Tedeschi, 2020 | Prospective cohort study | Bologna, Italy |
Hypertensive adult COVID 19 patients hospitalized N = 311 |
Total: 175 ACEI: 99 ARB: 76 |
Non-ACEi/ARB: 136 | 76 28% |
HTN: 100% CAD: 42% DM: 24% |
Age, gender, presence of CV comorbidities and COPD |
| Yang, 2020 | Retrospective, single-center study | Wuhan, China |
Subgroup of hypertensive patients with COVID-19 hospitalized N = 126 |
Total: 43 ACEi: NR ARB: NR |
Non-ACEi/ARB: 83 | Median 67 51% |
HTN: 100% DM: 30% Respiratory disease: 5% Kidney disease: 3% Hepatic disease: 6% Cardiopathy: 18% Neurological disease: 8% |
– |
| Zhang, 2020 | Retrospective cohort/nested case-control | Hubei, China | Hypertensive patients with COVID-19 hospitalized N = 522 matching 1:2 |
Total: 174 ACEi: NR ARB: NR |
Non-ACEi/ARB: 348 | median 64 47% |
HTN: 100% DM: 24% CAD: 12% CKD 3% CVD: 3% COPD: 1% |
Age, gender, fever, cough, dyspnea, comorbidities (diabetes, coronary heart disease, and chronic renal disease), CT-diagnosed bilateral lung lesions, and incidence of increased CRP and creatinine. |
| Zhou 2020 | Retrospective, single-center study | Wuhan, China |
Subgroup of hypertensive patients with symptomatic COVID-19 hospitalized N = 36 |
Total: 15 ACEi: NR ARB: NR |
Non-ACEi/ARB: 21 | 65 47% |
HTN:100% DM: 25% CVD: 19% |
Age, sex, hospitalization time, time from onset to hospital admission, and whether to take ACEi or ARB |
| UNPUBLISHED | ||||||||
| Rossi 2020 | Population-based prospective cohort study on archive data | Reggio Emilia, Italy |
COVID-19 symptomatic patients N = 2653 |
Total: 818 ACEi: 450 ARB: 368 |
Non-ACEi/ARB: 1835 | 63 50% |
HTN: 18% DM: 12% MI: 7% HF: 6% CKD: 3% COPD: 5% Vascular disease: 3% |
Age, sex and analysis restricted to subjects with ischemic heart disease, hypertension, or heart failure |
| Ip 2020 | Retrospective, Cohort, multicenter study | New Jersey, USA | Subgroup of hypertensive COVID 19 symptomatic hospitalized patients N = 1584 |
Total: 1231 ACEi: 688 ARB: 543 |
Non-ACEi/ARB: NR | NR | HTN: 100% | – |
| Liu 2020 | Multicentre retrospective cohort study | Shenzhen, Wuhan, and Beijing, China | Hypertensive COVID 19 symptomatic hospitalized elderly pts (greater than65 years-old) N = 78 |
Total: 12 ACEi: 2 ARB: 10 |
Non-ACEi/ARB: 8 | NR | HTN 100% | Gender |
| Rentsch 2020 | Retrospective cohort study | Connecticut, USA | Patients from National Veterans Affairs Healthcare System tested for COVID-19 N = 3789 | Total: 1532 ACEi: NR ARB: NR |
Non-ACEi/ARB: 2257 | 66 10% |
HTN: 65% DM: 38% Vascular disease: 29% COPD: 26% Alcohol use disorder: 14% |
Age, sex, race, medication, residence type, comorbidities |
| Zeng 2020 | Retrospective, single-center study | Wuhan, China |
Subgroup of hypertensive patients with clinically confirmed COVID-19 hospitalized N = 75 |
Total: 28 ACEi: NR ARB: NR |
Non-ACEi/ARB: 47 | 67 53% |
HTN: 100% DM: 31% CVD: 21% CKD: 5% |
– |
| Khera 2020 | Retrospective Cohort | Connecticut, USA | Hypertension patients hospitalized for COVID-19 N = 7933 | Total: 4587 ACEi: 2361 ARB: 2226 |
Non-ACEI/ARB: 3346 | Median 69 53% |
HTN 100% DM: 68% MI 4% HF 14% |
Propensity score: age, gender, race, insurance type, conditions that may lead to selective use of ACE inhibitors and ARBs each of the comorbidities in the Charlson Comorbidity Index, and the number of anti-hypertensive agents used for the patient |
*with a diagnosis of ischaemic heart disease (ICD9CM at discharge 410–414), cerebrovascular disease (430–438), or heart failure (428), and persons registered in the regional register of persons with diabetes.
Legend: RT-CPR reverse transcriptase-polymerase chain reaction; ACEi Angiotensin-converting-enzyme inhibitors; ARB angiotensin receptor blocker; RAAS renin-angiotensin-aldosterone system; SES socioeconomic status; BMI body mass index; HTN hypertension; CAD coronary artery disease; HF heart failure; DM diabetes mellitus; CKD chronic kidney disease; MI myocardial infarction; COPD Chronic obstructive pulmonary disease; CHD chronic heart disease; PCI percutaneous coronary intervention; PSM: Propensity-score matching CABG coronary artery bypass graft; NSAID Nonsteroidal anti-inflammatory drugs; GI gastrointestinal; CT computed tomography; CRP c-reactive protein; pts patients.
3.2. Risk of bias
The risk of bias in the included studies was moderate for studies evaluating the risk of infection, while those assessing the infection severity/mortality were classified as serious. The only randomized controlled trial had an open-label design, a small sample size (n = 102) and was not designed to assess COVID-19 outcomes as the reported results were from a non-prespecified interim analysis. The lack of outcome adjustments for important clinical factors was the main source of risk of bias. Supplementary Table 3 details the risk of bias for each study according with the outcome. Supplementary Fig. 1 overviews the proportions of risk of bias categories.
3.3. Risk of COVID-19 infection (positive test) associated with ACEi/ARB
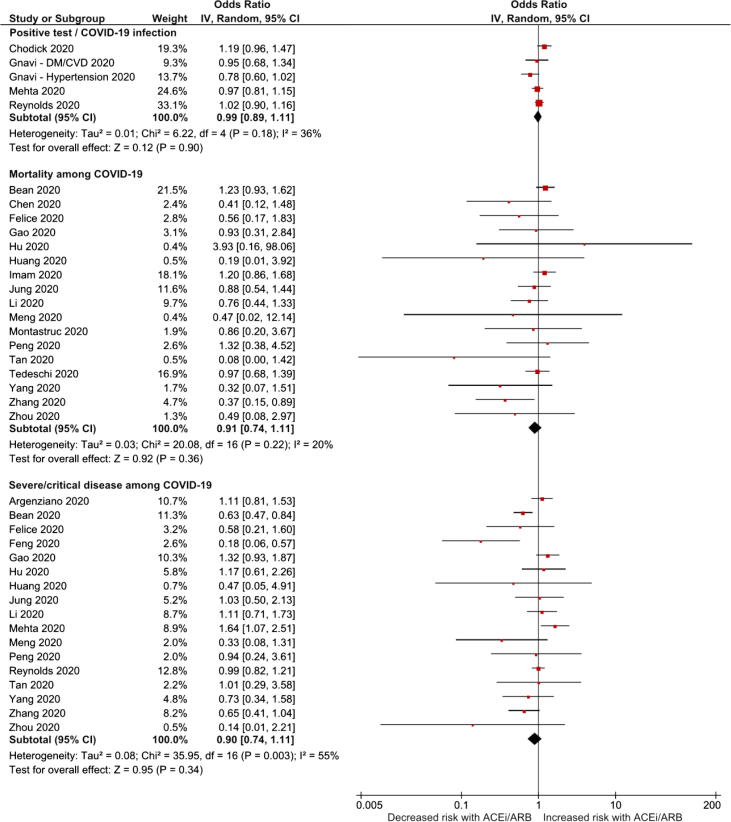
Six cohorts had information about COVID-19 infection (positive test) and ACEi and/or ARB. In the meta-analysis the ACEi/ARB group was not associated with increased risk of having a positive test for COVID-19 infection (OR 0.99, 95%CI 0.91–1.11; I2 = 36%; 6 studies; Fig. 2), nor ACEi (OR 0.94, 95%CI 0.87–1.02; I2 = 0%; 7 studies) or ARB (OR 1.01, 95%CI 0.93–1.10; I2 = 11%; 6 studies) (Supplementary Figs. 2 and 3), individually.
Fig. 2.
Forest plots of ACEi/ARB association with the risk of COVID-19 infection and disease severity.
3.4. Mortality risk associated with ACEi/ARB among patients with COVID-19 infection
Regarding all-cause mortality, ACEi or ARB were associated with neither an increased nor reduction in the risk this outcome: ACEi/ARB, OR 0.91, 95%CI 0.74–1.11, I2 = 20%, 17 studies, Fig. 2); ACEi, OR 0.85, 95%CI 0.40–1.78, I2 = 0%, 4 studies; and ARB OR 0.80, 95%CI 0.47–1.35, I2 = 0%, 3 studies (Fig. 3; Supplementary Figs. 2 and 3).
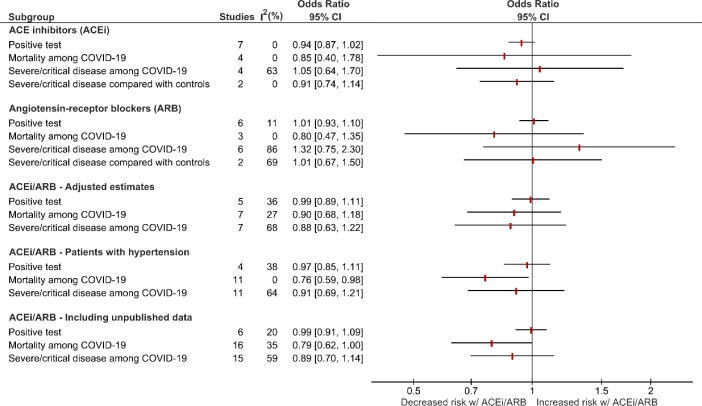
Fig. 3.
Forest plots of ACEi or ARB association with the risk of COVID-19 infection and disease severity, and the results of subanalyses of ACEi/ARB.
3.5. Risk of severe disease associated with ACEi/ARB among patients with COVID-19 infection
The risk of severe COVID-19 disease associated with ACEi/ARB (OR 0.90, 95%CI 0.74–1.11; I2 = 55%; 17 studies; Fig. 2), ACEi (OR 1.05, 95%CI 0.64–1.70; I2 = 63%; 4 studies) or ARB (OR 1.32, 95%CI 0.75–2.30; I2 = 86%; 6 studies) individually was not significantly increased nor decreased (Fig. 3; Supplementary Figs. 2 and 3).
3.6. Risk of severe disease associated with ACEi/ARB compared with populational controls
Two case-control studies evaluated the risk of severe COVID-19 associated with ACEi/ARB using populational controls as reference [17], [27]. One only study had data about a grouped estimate of ACEi/ARB and the results did not support the hypothesis that ACEi/ARB was associated with severe COVID-19 (OR 1.08, 95%CI 0.79–1.47; 1 study) [27]. Two studies supplied data for ACEi and ARB individually [17], [27], and the pooled estimates for both evaluations showed no significant effects (ACEi: OR 0.91, 95% 0.72–1-14; I2 = 0%, 2 studies; ARB: 1.01, 95%CI 0.67–1.50; I2 = 69%; 2 studies; Fig. 3; Supplementary Figs. 2 and 3).
3.7. Publication bias risk assessment
We performed the Egger test in the evaluations of ACEi/ARB with more than 10 studies to determine whether publication bias exists. The Egger test was not statistically significant in the risk of having COVID-19 infection (p-value 0.64), risk of mortality among those symptomatic COVID-19 (p-value 0.09), and risk of severe disease among those with COVID (p-value 0.42). The funnel plots are depicted in Supplementary Figure 4.
3.8. Sub-analyses
We performed sub-analyses of ACEi/ARB association including only studies with adjusted estimates, hypertensive patients, and including unpublished data (Fig. 3).
The analysis of studies with adjusted estimates did not find any significant association between ACEi/ARB and risk of infection (OR 0.99, 95%CI 0.89–1.11, I2 = 35%, 5 studies), mortality (OR 0.90, 95%CI 0.68–1.18, I2 = 27%) and severe/critical disease (OR 0.88, 95%CI 0.63–1.22, I2 = 68%) among patients with COVID-19 (Fig. 3, Supplementary Figure 5).
Analysing only the data from hypertensive patients, the risk of developing the infection in patients treated with ACEi/ARB was not significantly increased (OR 0.97, 95%CI 0.85–1.11; I2 = 38%) (Fig. 3, Supplementary Figure 6). The mortality risk (OR 0.76, 95%CI 0.59–0.98; I2 = 0%) was significantly decreased in this population while the risk of developing severe disease (OR 0.91, 95%CI 0.69–1.21; I2 = 64%) was not statistically significant (Fig. 3, Supplementary Figure 6).
Considering both published and unpublished data retrieved from 7 additional studies (supplementary Table 4), there was a non-statistically significant association between ACEi/ARB and decreased mortality risk among COVID-19 patients (OR 0.79, 95%CI 0.62–1.00, I2 = 0%) (Fig. 3, Supplementary Figure 5). The risk of infection (OR 0.99, 95%CI 0.91–1.09, I2 = 20%) and the risk of severe/critical disease (OR 0.89, 95%CI 0.70–1.14, I2 = 59%) were neither significantly increased nor decreased (Fig. 3, Supplementary Figure 7).
3.8.1. Assessment of confidence in cumulative evidence
Table 2 presents a summary of findings table which summarizes the results obtained only for the associations found for grouped ACEi/ARB exposure, according to certainty of the evidence (GRADE). The current evidence is that ACEi/ARB use is not associated with increased clinically significant risk of having a positive test with moderate confidence. Mortality risk among COVID-19 patients was significantly decreased, but the confidence of these data was graded as low (Table 2). The confidence concerning the association of ACEi/ARB and risk severe/critical disease among COVID-19 patients was very low (Table 2).
Table 2.
Summary of finding table with the GRADE approach.
| Outcomes | № of studies | Certainty of the evidence (GRADE) for the lack of effect* | Relative effect (95% CI) |
|---|---|---|---|
| ACEi/ARB and COVID-19 | |||
| Positive test | 5 observational studies |  |
0.99 (0.89–1.11) |
| Mortality among COVID-19 patients | 17 observational studies | 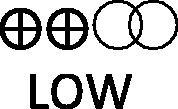 |
OR 0.91 (0.74–1.11) |
| Severe or critical disease | 17 observational studies | 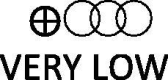 |
OR 0.90 (0.74–1.11) |
*The threshold for clinically significant effect (harm) was arbitrarily established as an increase of 25% in the odds of the outcome (a measure suggested by GRADE [43]).
CI: Confidence interval; OR: Odds ratio.
4. Discussion
The main finding of this systematic review was that ACEi/ARB were not associated with increased risk of being infected (moderate confidence), and among patients with COVID-19 the exposure to ACEi/ARB did not increase the risk of severe disease (very low confidence) or mortality (low confidence). In our exploratory analysis that only included hypertensive patients, ACEi/ARB were associated with a decreased mortality risk among COVID-19 patients however the data quality/risk of bias and the fragility of this exploratory analysis precludes definite and robust conclusions about the potential benefit. The other exploratory analyses also did not suggest harm, assuring the safety for the use of these drugs.
The rationale for this research was mainly based on the correspondence publication of Lancet Respiratory Medicine where Lei Fang and colleagues found that a significant number of patients with severe infection or death from SARS-CoV-2 were hypertensive, diabetic or had cardio-cerebrovascular disease and that these conditions are often treated with ACEi or ARB [3]. They hypothesized that the risk of infection or death might be increased in this group of patients due to an increase in the expression of ACE2 which can facilitate the entrance of SARS-CoV-2 into the cells [3]. The publication gained prominence in the scientific community and led to alarmism in the non-scientific community, given the high number of patients taking these drugs.
Given that the suspension of ACEI or ARBs can lead to decompensation of the underlying pathologies and there were no robust studies to corroborate the aforementioned hypothesis (data from only small preclinical studies), this led to some of the main scientific societies such as the American Heart Association, the American College of Cardiology, the Council on Hypertension of the European Society of Cardiology, and European Society of Hypertension, to publish recommendations to warn against discontinuing these drugs in the absence of clear clinical evidence of harm [41]. Our data are important because they validate these recommendations.
Despite ACEi and ARB having pharmacodynamic effects in the same pathway, the specific site of drug action may hypothetically lead to different effects, particularly in the risk of infectious diseases. Previous systematic review evaluating the potential role of ACEi in the prevention of pneumonia [42]. At that time the putative protective mechanism was thought to be related with enhanced cough reflex related to bradykinin and substance P, both derived from the inhibition of ACE [42]. Nowadays, the mechanisms are still speculative but hypothetically both ACEi and ARB may provide lung protection through the activation of angiotensin II-receptors type 2 (AT2R) and Mas receptors. The potential role of ACE2 in the case of SARS-CoV-2 infection is still ambiguous. While its increase may supply pathways for SARS-CoV-2 entrance into the cells [2], it is known that cleaved and shedded ACE2 leads to the breakdown of Angiotensin II to Angiotensin 1-7 (directly or indirectly increased with ARB or ACEi, respectively) have anti-inflammatory and anti-fibrotic effect through Mas receptors [41], [43]. The SARS-CoV-2 infection also leads to a downregulation of ACE2, that was associated with increased lung injury in animal models [44], [45]. Despite these ambiguous roles of ACE2, it is important to mention that relationship of serum/urinary ACE2 and tissue concentrations and use of ACEi/ARB is not well established, particularly in humans [46], [47], [48], and the clinical relevance of such relationships point towards a neutral effect according to our data. In order to further explore the potential role ACE2 and ACEi/ARB in the Influenza A infection, which share the same lung injury pathway as SARS-CoV-2, Chung et al analyzed the data of more than 5 million people in the UK followed for a median of 8.7 years and they found that ACEi and ARB exposure were associated with a decreased risk of Influenza A infection [49].
The data of this review are also important to reassure the safety of ACEi/ARB after the retraction of a large observational study that supported the safety of ACEi/ARB and showed a potential association of ACEi with lower COVID-19 mortality (Mehra MR et al N Eng J Med 2020). The authors asked for paper retraction after some concerns about the study and the impossibility of having a third party review on their data and analyses. Therefore, and despite the retraction, considering our data (without the retracted study), it seems reasonable to claim that ACEi/ARB are not harmful, despite the limitations reflected in the GRADE confidence. This supports the recommendations for not stopping the therapeutic use of ACEi/ARB. For potential benefit assessment, as seen in the hypertensive subgroup, further studies, such as the Elimination or prolongation of ACE inhibitors and ARB in Coronavirus Disease 2019 (REPLACECOVID) or Stopping ACE-inhibitors in COVID-19 (ACEI-COVID), Coronavirus ACEi/ARB Investigation (CORONACION) will provide more insights.
Our data are limited by the studies risk of bias which includes their observational nature for most of them. Pooling data of studies with different designs that evaluated different populations should also be considered as a potential limitation. Nevertheless, it increases the power and external validity of obtained data. In some studies, the risk of severe/critical disease was retrieved from specific outcomes such as the need of mechanical invasive ventilation or acute respiratory distress syndrome. This could explain the heterogeneity found in this outcome, but exclusion of these studies did not decrease the statistical heterogeneity and it remained substantial in the sub-analyses (data not shown). Lastly in these results only reflect the impact of ACEi and/or ARB. Other modulators of the renin-angiotensin-aldosterone system such renin inhibitors (aliskiren), mineralocorticoid receptor antagonists (spironolactone or epleronone), or even sacubitril were not evaluated in this review. In fact these drugs are residual considering the prescription of ACEi or ARB that in the de Abajo study we used the odds ratio of renin-angiotensin-aldosterone inhibitors as ACEi and ARB represented more than 90% of patients treated with the drugs of this group [27].
5. Conclusions
Our systematic review with meta-analysis did not suggest that the exposure to ACEi/ARB increases the risk of having the SARS-CoV-2 infection or developing severe stages of the disease, which supports the position papers of several medical associations recommending for not withholding these drugs in people already treated with them. Our data also showed a statistically significant association between ACEi/ARB exposure and reduction in COVID-19 mortality in hypertensive patients, but the frailty of the data and analysis precludes definite conclusions and emphasizes the need of further robust data.
Declaration of Competing Interest
DC in the last 3 years has participated in educational conferences/congresses (including travel, accommodation, and/or hospitality) and has received speaker/consultant fees from Daiichi Sankyo, Menarini, Roche and Merck-Serono. FJP that has received speaker and consultant fees from Bayer, Boehringer Ingelheim, Daiichi, Sankyo and Astra Zeneca.
Acknowledgments
Acknowledgments
None.
Funding
This review was an academic project and was not supported by any funding.
Contributorship
DC is the guarantor and contributed for concept and design. MA, RGM, PSA, NC, ANF and LP searched the articles and retrieved the data. DC coordinated the data search and retrieval. DC, MA, ANF performed the risk of bias assessment. DC performed the statistical analysis and wrote the first draft. DA, MA, RGM, PSA, NC, ANF and LP were involved in the result interpretation, discussion and text writing. JC and FJP were involved in the analysis and interpretation of the data, critically revised the manuscript for important intellectual content. All the authors approved the version of the manuscript.
Ethics committee approval
Not required as this was a systematic review of publicly available studies.
Statement
Daniel Caldeira MD PhD is the guarantor and This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100627.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO. Coronavirus disease 2019 (COVID-19): situation report, vol. 129, 2020.
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respiratory Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 5.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed) 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization WH. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization, 2020.
- 7.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical research ed) 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.J.P.T. Higgins, D.G. Altman, J.A.C. Sterne, Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011), The Cochrane Collaboration, 2011.
- 10.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Statist. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Deeks J.J. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Statist. Med. 2002;21(11):1575–1600. doi: 10.1002/sim.1188. [DOI] [PubMed] [Google Scholar]
- 12.J.A.C.E.M. Sterne, D. Moher, I. Boutron (Eds.), Chapter 10: Addressing reporting biases, Cochrane Handbook for Systematic Reviews of Interventions version 520 (updated June 2017), Cochrane, 2017. Available from: www.training.cochrane.org/handbook.
- 13.H.J.O.A. Schünemann, J.P.T. Higgins, G.E. Vist, P. Glasziou, E. Akl, G.H. Guyatt, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence, in: J.P.T.C.R. Higgins, J. Chandler, M.S. Cumpston (Eds.), Cochrane Handbook for Systematic Reviews of Interventions version 520 (updated June 2017), Cochrane; 2017. Available from: www.training.cochrane.org/handbook.
- 14.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. COVID-19 with Different Severity: A Multi-center Study of Clinical Features. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z., Cao J., Yao Y., Jin X., Luo Z., Xue Y., Zhu C., Song Y., Wang Y., Zou Y., Qian J., Yu K., Gong H., Ge J. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann. Transl. Med. 2020;8(7):430. doi: 10.21037/atm.2020.03.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Wang X., Chen J., Zhang H., Deng A. Association of Renin-Angiotensin System Inhibitors With Severity or Risk of Death in Patients With Hypertension Hospitalized for Coronavirus Disease 2019 (COVID-19) Infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of Covid-19. N. Engl. J. Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta N., Kalra A., Nowacki A.S., Anjewierden S., Han Z., Bhat P. Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon A., Klein E.J., Kollars K., Kleinhenz A.L.W. Medical Students Are Not Essential Workers: Examining Institutional Responsibility During the COVID-19 Pandemic. Academic Med.: J. Assoc. American Med. Colleges. 2020 doi: 10.1097/ACM.0000000000003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., Hausvater A., Newman J.D., Berger J.S., Bangalore S., Katz S.D., Fishman G.I., Kunichoff D., Chen Y.u., Ogedegbe G., Hochman J.S. Renin–Angiotensin–Aldosterone System Inhibitors and Risk of Covid-19. N. Engl. J. Med. 2020;382(25):2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.G. Yang, Z. Tan, L. Zhou, M. Yang, L. Peng, J. Liu, et al., Effects Of ARBs And ACEIs On Virus Infection, Inflammatory Status And Clinical Outcomes In COVID-19 Patients With Hypertension: A Single Center Retrospective Study, Hypertension (Dallas, Tex : 1979). 2020. [DOI] [PubMed]
- 22.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.S. Tedeschi, M. Giannella, M. Bartoletti, F. Trapani, M. Tadolini, C. Borghi, et al., Clinical impact of renin-angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for COVID-19, Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 2020. [DOI] [PMC free article] [PubMed]
- 24.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., Aubry C., Correard F., Giraud-Gatineau A., Roussel Y., Berenger C., Cassir N., Seng P., Zandotti C., Dhiver C., Ravaux I., Tomei C., Eldin C., Tissot-Dupont H., Honoré S., Stein A., Jacquier A., Deharo J.-C., Chabrière E., Levasseur A., Fenollar F., Rolain J.-M., Obadia Y., Brouqui P., Drancourt M., La Scola B., Parola P., Raoult D. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chodick G., Nutman A., Yiekutiel N., Shalev V. Angiotension-converting enzyme inhibitors and angiotensin-receptor blockers are not associated with increased risk of SARS-CoV-2 infection. J. Travel Med. 2020 doi: 10.1093/jtm/taaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X., Zhu J., Xu T. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients with hypertension on renin–angiotensin system inhibitors. Clin. Exp. Hypertens. 2020;42(7):656–660. doi: 10.1080/10641963.2020.1764018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.F.J. de Abajo, S. Rodriguez-Martin, V. Lerma, G. Mejia-Abril, M. Aguilar, A. Garcia-Luque, et al., Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study, Lancet (London, England), 2020. [DOI] [PMC free article] [PubMed]
- 28.R. Gnavi, M. Demaria, R. Picariello, M. Dalmasso, F. Ricceri, G. Costa, Therapy with agents acting on the renin-angiotensin system and risk of SARS-CoV-2 infection, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 2020. [DOI] [PMC free article] [PubMed]
- 29.S.Y. Jung, J.C. Choi, S.H. You, W.Y. Kim, Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 2020. [DOI] [PMC free article] [PubMed]
- 30.Montastruc F., Romano C., Montastruc J.-L., Silva S., Seguin T., Minville V., Georges B., Riu-Poulenc B., Fourcade O. Pharmacological characteristics of patients infected with SARS-Cov-2 admitted to Intensive Care Unit in South of France. Therapies. 2020;75(4):381–384. doi: 10.1016/j.therap.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua xin xue guan bing za zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 32.Tan N.-D., Qiu Y., Xing X.-B., Ghosh S., Chen M.-H., Mao R. Associations Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blocker Use, Gastrointestinal Symptoms, and Mortality Among Patients With COVID-19. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., Liu C., Xiong M., Deng A., Zhang Y.u., Zheng L., Huang K. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Dia Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 34.Bean D.M., Kraljevic Z., Searle T., Bendayan R., Kevin O.G., Pickles A. ACE-inhibitors and Angiotensin-2 Receptor Blockers are not associated with severe SARS-COVID19 infection in a multi-site UK acute Hospital Trust. Eur. J. Heart Fail. 2020 doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amat-Santos I.J., Santos-Martinez S., López-Otero D., Nombela-Franco L., Gutiérrez-Ibanes E., Del Valle R. Ramipril in High Risk Patients with COVID-19. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu J., Zhang X., Zhang X., Zhao H., Lian J., Hao S., Jia H., Yang M., Lu Y., Xiang D., Cai H., Zhang S., Gu J., Ye C., Yu G., Jin C., Zheng L., Yang Y., Sheng J. COVID-19 patients with hypertension have more severity condition, and ACEI/ARB treatment have no infulence on the clinical severity and outcome. J. Infect. 2020 doi: 10.1016/j.jinf.2020.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imam Z., Odish F., Gill I., O'Connor D., Armstrong J., Vanood A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J. Internal Med. 2020 doi: 10.1111/joim.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. European heart journal. 2020;41:2058-66. [DOI] [PMC free article] [PubMed]
- 39.Felice C, Nardin C, Di Tanna GL, Grossi U, Bernardi E, Scaldaferri L, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. American journal of hypertension. 2020. [DOI] [PMC free article] [PubMed]
- 40.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. 2020;369:m1996. [DOI] [PMC free article] [PubMed]
- 41.Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovascular research. 2020. [DOI] [PMC free article] [PubMed]
- 42.Caldeira D., Alarcao J., Vaz-Carneiro A., Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ. 2012;345(jul11 1):e4260. doi: 10.1136/bmj.e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lumbers E.R., Delforce S.J., Pringle K.G., Smith G.R. The Lung, the Heart, the Novel Coronavirus, and the Renin-Angiotensin System; The Need for Clinical Trials. Frontiers in medicine. 2020;7:248. doi: 10.3389/fmed.2020.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imai Y., Kuba K., Rao S., Huan Y.i., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020;113:104350. doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- 46.Sama IE, Ravera A, Santema BT, van Goor H, Ter Maaten JM, Cleland JGF, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. European heart journal. 2020;41:1810-7. [DOI] [PMC free article] [PubMed]
- 47.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. 2018;13:e0198144. [DOI] [PMC free article] [PubMed]
- 48.Furuhashi M., Moniwa N., Mita T., Fuseya T., Ishimura S., Ohno K., Shibata S., Tanaka M., Watanabe Y., Akasaka H., Ohnishi H., Yoshida H., Takizawa H., Saitoh S., Ura N., Shimamoto K., Miura T. Urinary Angiotensin-Converting Enzyme 2 in Hypertensive Patients May Be Increased by Olmesartan, an Angiotensin II Receptor Blocker. Am. J. Hypertens. 2015;28(1):15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 49.Chung S.-C., Providencia R., Sofat R. Association between Angiotensin Blockade and Incidence of Influenza in the United Kingdom. N. Engl. J. Med. 2020;383(4):397–400. doi: 10.1056/NEJMc2005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.