Abstract
Objective
In the phase 3 CELESTIAL trial, cabozantinib improved overall survival (OS) and progression-free survival (PFS) compared with placebo in patients with previously treated advanced hepatocellular carcinoma (HCC). This subgroup analysis evaluated cabozantinib in patients who had received sorafenib as the only prior systemic therapy.
Methods
CELESTIAL randomised (2:1) patients with advanced HCC and Child–Pugh class A liver function to treatment with cabozantinib (60 mg daily) or placebo. Eligibility required prior treatment with sorafenib, and patients could have received ≤2 prior systemic regimens. The primary endpoint was OS. Outcomes in patients who had received sorafenib as the only prior therapy were analysed by duration of prior sorafenib (<3 months, 3 to <6 months and ≥6 months).
Results
Of patients who had received only prior sorafenib, 331 were randomised to cabozantinib and 164 to placebo; 136 patients had received sorafenib for <3 months, 141 for 3 to <6 months and 217 for ≥6 months. Cabozantinib improved OS relative to placebo in the overall second-line population who had received only prior sorafenib (median 11.3 vs 7.2 months; HR=0.70, 95% CI 0.55 to 0.88). This improvement was maintained in analyses by prior sorafenib duration with longer duration generally corresponding to longer median OS—median OS 8.9 vs 6.9 months (HR=0.72, 95% CI 0.47 to 1.10) for prior sorafenib <3 months, 11.5 vs 6.5 months (HR=0.65, 95% CI 0.43 to 1.00) for 3 to <6 months and 12.3 vs 9.2 months (HR=0.82, 95% CI 0.58 to 1.16) for ≥6 months. Cabozantinib also improved PFS in all duration subgroups. Safety data were consistent with the overall study population.
Conclusion
Cabozantinib improved efficacy outcomes versus placebo in the second-line population who had received only prior sorafenib irrespective of duration of prior sorafenib treatment, further supporting the utility of cabozantinib in the evolving treatment landscape of HCC.
Clinical trial number
Keywords: hepatocellular carcinoma, tyrosine kinase inhibitor, cabozantinib, sorafenib
Key questions.
What is already known about this subject?
Cabozantinib is a tyrosine kinase inhibitor with multiple targets, including VEGF receptors, MET and AXL.
Cabozantinib was recently approved for patients with advanced hepatocellular carcinoma (HCC) previously treated with sorafenib based on results of the phase 3 CELESTIAL trial.
Patients enrolled in CELESTIAL had received prior sorafenib treatment and could have received up to two previous lines of systemic therapy and were randomised to treatment with cabozantinib or placebo.
Cabozantinib significantly improved overall survival (OS), progression-free survival (PFS) and the objective response rate compared with placebo, with a manageable safety profile.
What does this study add?
The current subgroup analysis assesses outcomes in patients who received sorafenib as their only prior systemic therapy for HCC.
In patients who received sorafenib as their only prior therapy, cabozantinib improved OS and PFS compared with placebo, and improvement was more pronounced than in the broader overall study population.
Cabozantinib improved OS and PFS compared with placebo regardless of duration of prior sorafenib treatment.
The improvement in efficacy outcomes includes the subgroup who received prior sorafenib for <3 months, which likely included patients who were intolerant to sorafenib or had rapidly progressing disease.
How might this impact on clinical practice?
The results of this subgroup analysis further support the broad use of cabozantinib in patients who received prior sorafenib regardless of the duration of treatment with sorafenib or tolerance.
Introduction
Cabozantinib inhibits multiple tyrosine kinases including VEGF receptors, MET and AXL.1 2 MET and AXL have diverse roles in tumour biology, including promotion of invasion, metastasis and immunosuppression.3 4 They are also implicated in resistance to sorafenib,2 5 6 a standard of care for first-line advanced hepatocellular carcinoma (HCC), which targets VEGFR and other kinases.7 In particular, MET expression was shown to increase in HCC tumours after sorafenib exposure, supporting its role in resistance to VEGF pathway inhibition.6
Cabozantinib was recently approved for patients with advanced HCC previously treated with sorafenib, based on improvements in overall survival (OS) and progression-free survival (PFS) versus placebo in the randomised phase 3 CELESTIAL trial.8 Median OS was 10.2 months with cabozantinib and 8.0 months with placebo (HR 0.76; 95% CI, 0.63 to 0.92), with corresponding median PFS of 5.2 and 1.9 months (HR 0.44; 95% CI, 0.36 to 0.52). Eligible patients had received prior treatment with sorafenib and could have received up to two previous lines of systemic therapy. In contrast, other recent phase 3 studies with systemic agents restricted the study populations to patients who had received sorafenib as the only prior therapy.9–11 With subsequent lines of therapy, prognostic heterogeneity increases, owing to more advanced disease and comorbidities.12 13
Given the recent approval of second-line treatments and the inclusion of third-line patients in CELESTIAL, we provide further analyses of cabozantinib efficacy and safety in the subset of patients who received sorafenib as the only prior therapy, including outcomes based on the duration of prior sorafenib treatment.
Methods
Trial design
The randomised, double-blind, phase 3 CELESTIAL trial evaluated cabozantinib in patients previously treated for advanced HCC. Details of the study design have been reported.8 Briefly, patients ≥18 years of age with advanced HCC were eligible provided that they had Child–Pugh class A liver function, had received previous treatment with sorafenib and no more than two prior systemic therapies. The current analysis focused on patients who had received sorafenib as the only prior systemic treatment.
Patients were randomly assigned 2:1 to receive cabozantinib (60 mg daily) or matching placebo. Randomisation was stratified according to disease aetiology, geographic region and presence of extrahepatic spread or macrovascular invasion. Patients continued study treatment as long as they had clinical benefit, as judged by the investigator, or until unacceptable toxicity. Treatment interruptions and dose reductions (to 40 mg and then to 20 mg) were permitted to manage adverse events (AEs).
Endpoints and assessments
The primary endpoint was OS, defined as time from randomisation to death from any cause. Secondary endpoints were PFS and objective response rate (ORR). PFS was defined as time from randomisation to the earlier of disease progression or death from any cause. ORR was defined as the proportion of patients experiencing a confirmed complete or partial response.
Tumour assessments included CT or MRI at screening and every 8 weeks after randomisation continuing until 8 weeks after radiographic progression or discontinuation of study treatment. Response was determined by the investigator according to Response Evaluation Criteria in Solid Tumors (RECIST), V.1.1.14
AE severity was graded by investigators according to the National Cancer Institute Common Terminology Criteria for AEs, V.4.0. Safety was assessed every 2 weeks for the first 2 months and every 4 weeks thereafter until 30 days after discontinuation.
Statistical analysis
The primary and secondary endpoints for the overall study population have been reported previously.8 In the current analysis, efficacy and safety were assessed in the subset of patients who had received sorafenib as the only prior systemic therapy and included subgroups defined by the duration of prior sorafenib (<3 months, 3 to <6 months and ≥6 months). Efficacy was assessed in all randomly assigned patients, and safety was assessed in all patients who received ≥1 dose of study treatment. All subgroup analyses presented here are post hoc, and results are considered descriptive.
Time-to-event endpoints were assessed by the Kaplan–Meier method with no adjustments for multiplicity. HRs were unstratified and estimated using a Cox proportional hazards model. ORR was assessed by the Cochran–Mantel–Haenszel method. All analyses were performed with SAS software, V.9.1 or higher (SAS Institute). Data cut-off was 1 June 2017.
Results
Baseline characteristics
Of 707 patients randomised, 495 (70%) received sorafenib as the only prior systemic therapy, 331/470 (70%) in the cabozantinib arm and 164/237 (69%) in the placebo arm. For this subset of patients, median duration of prior sorafenib treatment was 5.0 months for cabozantinib and 4.8 months for placebo. Subgroups based on duration of prior sorafenib included 136 (27%) patients who received sorafenib for <3 months, 141 (28%) for 3 to <6 months and 217 (44%) for ≥6 months for the combined treatment arms; median duration of prior sorafenib in the cabozantinib-arm subgroups was 2.1, 3.9 and 11.0 months, respectively, with similar values in the placebo-arm subgroups. Median time from the end of sorafenib to study randomisation was 1.2 to 1.6 months across subgroups.
Baseline characteristics were generally balanced between arms, but some differences were noted by duration of prior sorafenib (table 1). Patients with <3 months of prior sorafenib were more likely to be hepatitis B virus-positive (49%) than patients with 3 to <6 months (32%) or ≥6 months of prior sorafenib (34%) and less likely to be hepatitis C virus-positive (17%, 29% and 27%, respectively).
Table 1.
Baseline characteristics
| Overall | Duration of prior sorafenib* | |||||||
| <3 months | 3 to <6 months | ≥6 months | ||||||
| Cabozantinib (N=331) |
Placebo (N=164) |
Cabozantinib (N=89) |
Placebo (N=47) |
Cabozantinib (N=98) |
Placebo (N=43) |
Cabozantinib (N=143) |
Placebo (N=74) |
|
| Age, median (range), years | 63.0 (22–86) | 63.5 (34–86) | 62.0 (33–86) | 61.0 (34–83) | 62.0 (22–85) | 63.0 (41–82) | 65.0 (28–86) | 67.5 (36–86) |
| Male, n (%) | 264 (80) | 144 (88) | 71 (80) | 39 (83) | 79 (81) | 38 (88) | 113 (79) | 67 (91) |
| ECOG performance status, n (%) | ||||||||
| 0 | 186 (56) | 96 (59) | 52 (58) | 29 (62) | 52 (53) | 24 (56) | 82 (57) | 43 (58) |
| 1 | 144 (44) | 68 (41) | 36 (40) | 18 (38) | 46 (47) | 19 (44) | 61 (43) | 31 (42) |
| 2 | 1 (<1) | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| AFP ≥400 ng/mL, n (%) | 129 (39) | 73 (45) | 37 (42) | 28 (60) | 36 (37) | 24 (56) | 56 (39) | 21 (28) |
| Enrolment region, n (%) | ||||||||
| Asia | 78 (24) | 35 (21) | 29 (33) | 14 (30) | 18 (18) | 3 (7) | 31 (22) | 18 (24) |
| Europe | 175 (53) | 79 (48) | 44 (49) | 21 (45) | 55 (56) | 20 (47) | 75 (52) | 38 (51) |
| North America | 64 (19) | 39 (24) | 14 (16) | 8 (17) | 20 (20) | 18 (42) | 30 (21) | 13 (18) |
| Pacific | 14 (4) | 11 (7) | 2 (2) | 4 (9) | 5 (5) | 2 (5) | 7 (5) | 5 (7) |
| Race, n (%) | ||||||||
| White | 195 (59) | 93 (57) | 44 (49) | 20 (43) | 59 (60) | 26 (60) | 91 (64) | 47 (64) |
| Asian | 103 (31) | 53 (32) | 36 (40) | 24 (51) | 25 (26) | 9 (21) | 42 (29) | 20 (27) |
| Black | 6 (2) | 10 (6) | 1 (1) | 2 (4) | 4 (4) | 4 (9) | 1 (1) | 4 (5) |
| Other | 6 (2) | 1 (1) | 1 (1) | 0 | 4 (4) | 0 | 1 (1) | 1 (1) |
| Not reported | 21 (6) | 7 (4) | 7 (8) | 1 (2) | 6 (6) | 4 (9) | 8 (6) | 2 (3) |
| Aetiology of HCC, n (%)† | ||||||||
| HBV | 125 (38) | 60 (37) | 46 (52) | 20 (43) | 32 (33) | 13 (30) | 47 (33) | 27 (36) |
| HCV | 81 (24) | 43 (26) | 15 (17) | 8 (17) | 25 (26) | 16 (37) | 40 (28) | 19 (26) |
| Dual HBV and HCV infection | 4 (1) | 2 (1) | 2 (2) | 0 | 0 | 1 (2) | 2 (1) | 1 (1) |
| Alcohol | 80 (24) | 25 (15) | 21 (24) | 9 (19) | 22 (22) | 5 (12) | 37 (26) | 11 (15) |
| Non-alcoholic steatohepatitis | 25 (8) | 15 (9) | 6 (7) | 6 (13) | 9 (9) | 2 (5) | 10 (7) | 7 (9) |
| Other/not reported | 70 (21) | 42 (26) | 19 (21) | 9 (19) | 22 (22) | 12 (28) | 28 (20) | 21 (28) |
| Extrahepatic spread of disease and/or macrovascular invasion, n (%) | 278 (84) | 137 (84) | 77 (87) | 41 (87) | 83 (85) | 40 (93) | 117 (82) | 56 (76) |
| Extrahepatic spread | 255 (77) | 122 (74) | 72 (81) | 34 (72) | 75 (77) | 37 (86) | 108 (76) | 51 (69) |
| Macrovascular invasion | 84 (25) | 61 (37) | 31 (35) | 21 (45) | 18 (18) | 19 (44) | 34 (24) | 21 (28) |
| Total duration of prior sorafenib, median (range), months | 5.0 (0.4–46.5) |
4.8 (0.3–76.8) |
2.1 (0.4–3.0) |
1.9 (0.3–3.0) |
3.9 (3.0–6.0) |
4.0 (3.2–5.8) |
11.0 (6.1–46.5) |
11.3 (6.0–76.8) |
| Chemoembolisation for HCC, n (%) | 134 (40) | 75 (46) | 34 (38) | 23 (49) | 38 (39) | 11 (26) | 62 (43) | 41 (55) |
| Time from the end of sorafenib to randomisation, median (range), months | 1.4 (0–95.7) |
1.3 (0.3–16.3) |
1.5 (0.4–60.1) |
1.2 (0.5–12.2) |
1.6 (0–63.0) |
1.1 (0.3–11.8) |
1.2 (0–95.7) |
1.5 (0.5–16.3) |
| Sites of disease per investigator, n (%) | ||||||||
| Liver | 282 (85) | 148 (90) | 79 (89) | 45 (96) | 82 (84) | 39 (91) | 120 (84) | 64 (86) |
| Visceral (excluding liver) | 140 (42) | 65 (40) | 32 (36) | 16 (34) | 37 (38) | 21 (49) | 71 (50) | 28 (38) |
| Lymph node | 107 (32) | 50 (30) | 29 (33) | 14 (30) | 27 (28) | 15 (35) | 51 (36) | 21 (28) |
| Bone | 35 (11) | 24 (15) | 11 (12) | 8 (17) | 11 (11) | 9 (21) | 13 (9) | 7 (9) |
*Patients who received prior sorafenib as the only prior systemic therapy for HCC. Duration of prior sorafenib was known for 494 of 495 patients.
†Some patients had >1 disease aetiology category.
AFP, alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Efficacy outcomes for patients receiving only prior sorafenib
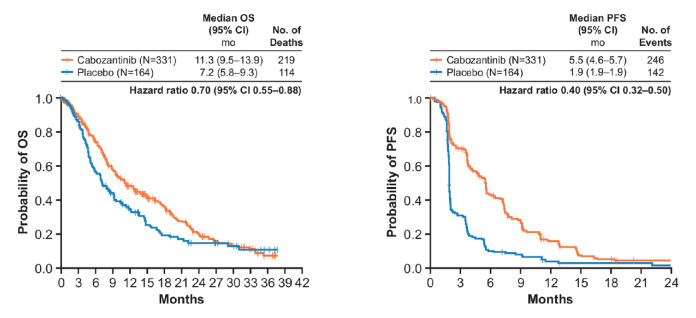
Cabozantinib improved OS relative to placebo in patients who received sorafenib as the only prior systemic therapy (figure 1), with a median OS from randomisation of 11.3 vs 7.2 months (HR 0.70, 95% CI 0.55 to 0.88). Median OS from the start of prior sorafenib was 24.5 (95% CI, 21.2 to 26.9) vs 18.8 months (95% CI 16.1 to 22.3).
Figure 1.
OS and PFS in patients who received only prior sorafenib. OS, overall survival; PFS, progression-free survival.
Cabozantinib improved PFS relative to placebo in patients who received only prior sorafenib (5.5 vs 1.9 months; HR 0.40, 95% CI 0.32 to 0.50). ORR was 5% (95% CI 2.8 to 7.7) with cabozantinib and 0.6% (95% CI 0 to 3.4) with placebo (online supplementary table S1). All were partial responses. The rate of stable disease was also higher with cabozantinib (62%) than with placebo (30%).
esmoopen-2020-000714supp001.pdf (82.4KB, pdf)
Efficacy outcomes based on duration of prior sorafenib
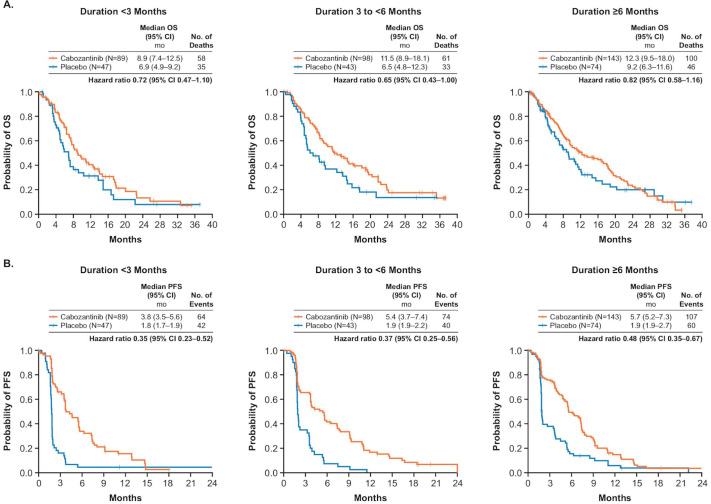
Cabozantinib prolonged OS and PFS relative to placebo across subgroups defined by duration of prior sorafenib (figure 2). Longer durations of prior sorafenib generally corresponded to longer median OS. Median OS was 8.9 months with cabozantinib and 6.9 months with placebo (HR 0.72, 95% CI 0.47 to 1.10) for patients who received sorafenib for <3 months, 11.5 and 6.5 months (HR 0.65, 95% CI 0.43 to 1.00) for those who received prior sorafenib for 3 to <6 months, and 12.3 and 9.2 months (HR 0.82, 95% CI 0.58 to 1.16) for those with ≥6 months of prior sorafenib. Longer durations of prior sorafenib also corresponded to longer median OS from the start of prior sorafenib (online supplementary table S2).
Figure 2.
OS (A) and PFS (B) based on duration of prior sorafenib. OS, overall survival; PFS, progression-free survival.
Patients in the cabozantinib arm received subsequent anticancer therapy with similar frequency to those in the placebo arm (25% vs 29%) regardless of duration of prior sorafenib (online supplementary table S3). The most common type of subsequent therapy was chemotherapy.
Cabozantinib improved PFS compared with placebo irrespective of duration of prior sorafenib (figure 2B). Median PFS was 3.8 for cabozantinib versus 1.8 months for placebo (HR 0.35, 95% CI 0.23 to 0.52) for patients who received sorafenib for <3 months, 5.4 vs 1.9 months (HR 0.37, 95% CI 0.25 to 0.56) for patients who received sorafenib for 3 to <6 months, and 5.7 vs 1.9 months (HR 0.48, 95% CI 0.35 to 0.67) for patients who received prior sorafenib for ≥6 months. In the cabozantinib arm, PFS was shorter for patients who received prior sorafenib for <3 months compared with longer durations. In the placebo arm, median PFS was similar across subgroups, with median PFS corresponding to approximately the time to the first tumour assessment.
In the cabozantinib arm, ORR was 4% (95% CI 1.2% to 11.1%), 2% (95% CI 0.2% to 7.2%) and 7% (95% CI 3.4% to 12.5%) for those who received sorafenib for <3 months, 3 to <6 months and ≥6 months, respectively (table 2). The rate of stable disease was higher with cabozantinib than with placebo, regardless of duration of prior sorafenib.
Table 2.
Best overall tumour response
| Duration of prior sorafenib* | ||||||
| <3 months | 3 to <6 months | ≥6 months | ||||
| Cabozantinib (N=89) |
Placebo (N=47) |
Cabozantinib (N=98) |
Placebo (N=43) |
Cabozantinib (N=143) |
Placebo (N=74) |
|
| ORR† (95% CI), n (%) | 4 (4) (1.2 to 11.1) |
0 | 2 (2) (0.2 to 7.2) |
0 | 10 (7) (3.4 to 12.5) |
1 (1) (0.0 to 7.3) |
| Best overall response, n (%) |
||||||
| Partial response | 4 (4) | 0 | 2 (2) | 0 | 10 (7) | 1 (1) |
| Stable disease | 53 (60) | 8 (17) | 61 (62) | 14 (33) | 91 (64) | 28 (38) |
| Progressive disease | 17 (19) | 34 (72) | 24 (24) | 22 (51) | 21 (15) | 35 (47) |
| Not evaluable/missing | 15 (17) | 5 (11) | 11 (11) | 7 (16) | 21 (15) | 10 (14) |
*Patients who received prior sorafenib as the only prior systemic therapy for hepatocellular carcinoma.
†All responses were partial responses.
ORR, overall response rate.
Safety and tolerability
The median average daily dose of cabozantinib was similar regardless of the duration of prior sorafenib (online supplementary table S4). Median duration of exposure for all patients who received only prior sorafenib was 4.0 months with cabozantinib versus 2.0 months with placebo. The duration of cabozantinib exposure increased with longer duration of prior sorafenib, with medians of 3.4, 3.9 and 5.1 months in patients with <3 months, 3 to <6 months and ≥6 months of prior sorafenib, respectively. For patients who received only prior sorafenib, the rate of dose reduction was 64% for cabozantinib and 10% for placebo. The rate of discontinuation due to treatment-related AEs was 17% and 2%, respectively; rates were similar across the duration of prior sorafenib subgroups.
For patients who received only prior sorafenib, grade 3/4 all-causality AEs were experienced by 68% (224/329) of patients receiving cabozantinib and 31% (51/164) of patients receiving placebo. The most common grade 3/4 AEs in the cabozantinib arm were palmar-plantar erythrodysesthesia (16%), hypertension (16%) and increased aspartate aminotransferase (12%). Duration of prior sorafenib treatment did not appear to impact AE rates (table 3).
Table 3.
All-causality grade 3/4 adverse events
| Overall | Duration of prior sorafenib* | |||||||
| <3 months | 3 to <6 months | ≥6 months | ||||||
| Cabozantinib (N=329) |
Placebo (N=164) |
Cabozantinib (N=88) |
Placebo (N=47) |
Cabozantinib (N=97) |
Placebo (N=43) |
Cabozantinib (N=143) |
Placebo (N=74) |
|
| Any grade 3/4 adverse event, n (%)† | 224 (68) | 51 (31) | 56 (64) | 16 (34) | 73 (75) | 13 (30) | 94 (66) | 22 (30) |
| PPE | 53 (16) | 0 | 17 (19) | 0 | 17 (18) | 0 | 18 (13) | 0 |
| Hypertension | 52 (16) | 3 (2) | 13 (15) | 0 | 20 (21) | 3 (7) | 18 (13) | 0 |
| AST increased | 39 (12) | 9 (6) | 15 (17) | 3 (6) | 10 (10) | 2 (5) | 14 (10) | 4 (5) |
| Diarrhoea | 37 (11) | 4 (2) | 5 (6) | 1 (2) | 11 (11) | 1 (2) | 21 (15) | 2 (3) |
| Fatigue | 34 (10) | 6 (4) | 6 (7) | 3 (6) | 10 (10) | 0 | 17 (12) | 3 (4) |
| Asthenia | 22 (7) | 3 (2) | 5 (6) | 0 | 7 (7) | 0 | 10 (7) | 3 (4) |
| Decreased appetite | 22 (7) | 1 (1) | 6 (7) | 0 | 8 (8) | 0 | 8 (6) | 1 (1) |
| Anaemia | 11 (3) | 8 (5) | 1 (1) | 1 (2) | 6 (6) | 2 (5) | 4 (3) | 5 (7) |
*Safety was assessed in all patients who received at least one dose of study treatment.
†Events that occurred at ≥5.0% frequency in either treatment arm in the overall safety population are summarised.
AST, aspartate aminotransferase; PPE, palmar-plantar erythrodysesthesia.
Discussion
In the phase 3 CELESTIAL trial, cabozantinib significantly prolonged OS and PFS in patients with advanced HCC previously treated with sorafenib who had received up to two prior lines of systemic therapy.8 In the current subgroup analysis of patients who had received only prior sorafenib (~70%), cabozantinib improved OS and PFS compared with placebo, demonstrating numerically lower HRs than for the more heterogeneous overall study population.8 These improvements were observed across subgroups defined by the duration of prior sorafenib treatment. Safety in patients who received only prior sorafenib was consistent with that previously reported for the overall study population.8
After over a decade with sorafenib as the only approved therapy for advanced HCC, the treatment landscape has expanded with the recent approvals of cabozantinib, ramucirumab, nivolumab and pembrolizumab after sorafenib failure or intolerance and regorafenib after sorafenib failure.9–11 15–17 No comparative data are available to guide choice of therapy in the second-line setting, requiring clinicians to carefully examine the study populations and outcomes from these trials for individualised treatment decisions.
Although prior sorafenib treatment was required for eligibility in all of the pivotal trials, the phase 3 trials for regorafenib, ramucirumab and pembrolizumab limited prior systemic therapy to sorafenib, unlike CELESTIAL, which allowed up to two prior systemic regimens. Prognostic heterogeneity arises from inclusion of both second-line and third-line patients, warranting the subgroup analysis reported; HRs favouring cabozantinib over placebo were generally lower in the strict second-line population of CELESTIAL compared with the overall study population.9 The duration of prior sorafenib differed across the pivotal studies with median duration ranging from 4.1 months in the REACH2 study11 to 7.8 months in the RESORCE study.10 The longer duration of prior sorafenib in the RESORCE trial is related to eligibility criteria, which required patients to tolerate sorafenib at a dose of ≥400 mg per day for at least 20 of the 28 days preceding discontinuation.10 In the second-line CELESTIAL subgroup analysed here, median duration of prior sorafenib was 5.0 months for the cabozantinib arm and 4.8 months for the placebo arm.
To address the impact of prior sorafenib tolerability on outcomes in this CELESTIAL second-line subgroup analysis, outcomes were analysed according to duration of prior sorafenib (<3 months, 3 to <6 months and ≥6 months). Cut points were selected based on clinical relevance and sample size considerations. The <3 month subgroup likely included patients who were sorafenib intolerant or had rapidly progressing disease,18 whereas the ≥6 month subgroup likely included patients who tolerated and derived clinical benefit from VEGFR tyrosine kinase inhibitor therapy or had less aggressive disease. Cabozantinib improved OS and PFS compared with placebo in all three subgroups. Median OS in both treatment arms was generally longer in patients with longer durations of prior sorafenib, consistent with the premise that these patients may have tumours with greater responsiveness to VEGF-targeted therapies or other favourable prognostic characteristics. Importantly, the improvements observed in the <3 month subgroup suggest that cabozantinib has activity in patients who are intolerant or refractory to first-line sorafenib, a subgroup who would have been ineligible for RESORCE. Cabozantinib inhibits multiple receptor tyrosine kinases implicated in HCC pathogenesis and treatment resistance (MET, AXL and VEGF receptors),1–6 which may help to overcome both primary and secondary sorafenib resistance and could contribute to the observed efficacy across varying durations of prior sorafenib.
The analysis presented here has limitations. This was a post hoc subgroup analysis with relatively small sample sizes for the subgroups. CELESTIAL was not stratified by the number of lines of treatment nor the duration of prior sorafenib. Additionally, prior sorafenib dosage and reasons for sorafenib discontinuation were not collected during the study. Subgroups based on shorter durations of prior sorafenib treatment than 3 months, which may be more representative of sorafenib intolerance, were not explored owing to small sample sizes. Nonetheless, the current analysis supports that cabozantinib improves OS and PFS across all subgroups of patients based on duration of prior sorafenib.
In summary, this subgroup analysis of CELESTIAL examined patients who received sorafenib as the only prior systemic therapy. Cabozantinib improved efficacy outcomes versus placebo irrespective of duration of prior sorafenib treatment, further supporting the broad utility of cabozantinib in the complex and evolving treatment landscape of HCC.
Acknowledgments
We thank the patients, their families, the investigators and site staff, and the study teams who participated in the CELESTIAL trial. The CELESTIAL trial was sponsored by Exelixis (Alameda, California, USA). Medical writing assistance was provided by Bryan Thibodeau, PhD (Fishawack Communications, Conshohocken, Pennsylvania, USA), and was funded by Exelixis.
Footnotes
Presented at: These data were presented in part at the ASCO 2018 Annual Meeting, June 1–5, Chicago, Illinois, USA.
Contributors: All authors contributed to the conception of the manuscript and interpretation of data. RKK, JL and GKA-A contributed to the drafting of the manuscript. All authors provided critical review and revisions, and all authors approved the final version of the manuscript for submission and publication.
Competing interests: RKK: consulting or advisory role—Agios (Inst); AstraZeneca (Inst); Bayer (Inst); Bristol-Myers Squibb (Inst); Genentech/Roche; Gilead; Target Pharmasolutions; Target Pharmasolutions (Inst); research funding—Adaptimmune (Inst); Agios (Inst); AstraZeneca (Inst); Bayer (Inst); Bristol-Myers Squibb (Inst); Celgene (Inst); EMD Serono (Inst); Exelixis (Inst); Lilly (Inst); MedImmune (Inst); Merck Sharp & Dohme (Inst); Novartis (Inst); Taiho Pharmaceutical (Inst); PM: consulting or advisory role—Bayer; Bristol-Myers Squibb; Ipsen; MSD; Onxeo; Roche. LB: honoraria—Bayer; Bracco Diagnostics; Brystol-Myers-Squibb; Guerbet; Lilly; Meda Pharmaceuticals; Sirtex Medical; consulting or advisory role—Bayer; Brystol-Myers-Squibb; Guerbet; Sirtex medical speakers' bureau—Bayer; Bracco Diagnostics; Brystol-Myers-Squibb; Guerbet; Lilly; Meda Pharmaceuticals; Sirtex Medical; research funding—ArQule; Bayer; Brystol-Myers-Squibb; Daiichi Synkyo; travel, accommodations, expenses—Bayer; Bracco Diagnostics; Guerbet; Lilly. SLC: grants—Merck Sharp & Dohme (Asia) Ltd; personal fees—AstraZeneca Hong Kong Limited, Bayer HealthCare Limited. ADB: speakers' bureau—Amgen; Bristol-Myers Squibb; Genentech/Roche; Lilly; Merck. JK: honoraria—Novartis; consulting or advisory role—Lilly; Merck; research funding— AstraZeneca; Merck. SC: personal fees—Ipsen. TY: personal fees—BMS, MSD, Ipsen, Exelxis, Eisai, Bayer; grants and personal fees—BMS; personal fees—MSD, Ipsen, Exelixis, Bayer. JCL: employment and stock holdings—Exelixis, Inc. SM: employment—Exelixis, Inc; stock holdings—Amgen; Exelixis; FibroGen; GlaxoSmithKline. ABE-K: honoraria—AstraZeneca; Bayer; Brystol-Myers-Squibb; Genentech; GlaxoSmithKline; consulting or advisory role—AstraZeneca; Brystol-Myers-Squibb; Genentech/Roche; speakers' bureau—Merrimack; research funding—Astex Pharmaceuticals; travel, accommodations, expenses—AstraZeneca; Bayer; Brystol-Myers-Squibb; Genentech; GlaxoSmithKline. A-LC: personal fees (advisory board, honoraria, travel expenses)—Ono Pharmaceutical; Exelixis; Nucleix Ltd.; Roche/Genentech; IQVIA; Merck Sharp Dohme; Bayer Yakuhin; Amgen Taiwan; Ispen; advisor/board member—Bayer Schering Pharma; Bristol-Myers Squibb; Eisai; Merck Serono; Novartis. TM: consulting or advisory role—Beigene; Bristol-Myers Squibb; BTG; Eisai; Ipsen; MSD; Tarveda Therapeutics; research funding—Bayer (Inst); BTG (Inst); Ipsen (Inst). GKA-A: grants and personal fees—Bayer, Exelixis, BMS, Eisai, Lilly, Merck; patent: Articles and methods for preventing and treating dermatologic adverse events, identified by International Patent Application No PCT/US2014/031545 filed on 24 March 2014, and priority application serial no: 61/804,907; filed: 25 March 2013 issued. All other authors report no conflicts of interest.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Yakes FM, Chen J, Tan J, et al. . Cabozantinib (XL184), a novel Met and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298–308. 10.1158/1535-7163.MCT-11-0264 [DOI] [PubMed] [Google Scholar]
- 2.Xiang Q, Chen W, Ren M, et al. . Cabozantinib suppresses tumor growth and metastasis in hepatocellular carcinoma by a dual blockade of VEGFR2 and Met. Clin Cancer Res 2014;20:2959–70. 10.1158/1078-0432.CCR-13-2620 [DOI] [PubMed] [Google Scholar]
- 3.Niu Z-S, Niu X-J, Wang W-H. Role of the receptor tyrosine kinase Axl in hepatocellular carcinoma and its clinical relevance. Future Oncol 2019;15:653–62. 10.2217/fon-2018-0528 [DOI] [PubMed] [Google Scholar]
- 4.García-Vilas JA, Medina MA. Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J Gastroenterol 2018;24:3695–708. 10.3748/wjg.v24.i33.3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinato DJ, Brown MW, Trousil S, et al. . Integrated analysis of multiple receptor tyrosine kinases identifies Axl as a therapeutic target and mediator of resistance to sorafenib in hepatocellular carcinoma. Br J Cancer 2019;120:512–21. 10.1038/s41416-018-0373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rimassa L, Assenat E, Peck-Radosavljevic M, et al. . Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol 2018;19:682–93. 10.1016/S1470-2045(18)30146-3 [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, et al. . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Meyer T, Cheng A-L, et al. . Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54–63. 10.1056/NEJMoa1717002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Qin S, Merle P, et al. . Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56–66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 10.Zhu AX, Kang Y-K, Yen C-J, et al. . Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:282–96. 10.1016/S1470-2045(18)30937-9 [DOI] [PubMed] [Google Scholar]
- 11.Zhu AX, Park JO, Ryoo B-Y, et al. . Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (reach): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859–70. 10.1016/S1470-2045(15)00050-9 [DOI] [PubMed] [Google Scholar]
- 12.Iavarone M, Cabibbo G, Biolato M, et al. . Predictors of survival in patients with advanced hepatocellular carcinoma who permanently discontinued sorafenib. Hepatology 2015;62:784–91. 10.1002/hep.27729 [DOI] [PubMed] [Google Scholar]
- 13.Reig M, Rimola J, Torres F, et al. . Postprogression survival of patients with advanced hepatocellular carcinoma: rationale for second-line trial design. Hepatology 2013;58:2023–31. 10.1002/hep.26586 [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15.El-Khoueiry AB, Sangro B, Yau T, et al. . Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu AX, Finn RS, Edeline J, et al. . Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 17.Finn RS, Ryoo B-Y, Merle P, et al. . Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). JCO 2019;37:4004 10.1200/JCO.2019.37.15_suppl.4004 [DOI] [Google Scholar]
- 18.Llovet JM, Yen C-J, Finn RS, et al. . Ramucirumab (ram) for sorafenib intolerant patients with hepatocellular carcinoma (HCC) and elevated baseline alpha fetoprotein (AFP): outcomes from two randomized phase 3 studies (reach, REACH2). ASCO Annual Meeting 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000714supp001.pdf (82.4KB, pdf)