Abstract
Calcium (Ca2+) and reactive oxygen species (ROS) are versatile signaling molecules coordinating physiological and pathophysiological processes. While channels and pumps shuttle Ca2+ ions between extracellular space, cytosol and cellular compartments, short-lived and highly reactive ROS are constantly generated by various production sites within the cell. Ca2+ controls membrane potential, modulates mitochondrial adenosine triphosphate (ATP) production and affects proteins like calcineurin (CaN) or calmodulin (CaM), which, in turn, have a wide area of action. Overwhelming Ca2+ levels within mitochondria efficiently induce and trigger cell death. In contrast, ROS comprise a diverse group of relatively unstable molecules with an odd number of electrons that abstract electrons from other molecules to gain stability. Depending on the type and produced amount, ROS act either as signaling molecules by affecting target proteins or as harmful oxidative stressors by damaging cellular components. Due to their wide range of actions, it is little wonder that Ca2+ and ROS signaling pathways overlap and impact one another. Growing evidence suggests a crucial implication of this mutual interplay on the development and enhancement of age-related disorders, including cardiovascular and neurodegenerative diseases as well as cancer.
Keywords: Aging, ROS homeostasis, Ca2+ homeostasis, Cardiovascular diseases, Type 2 diabetes mellitus, Neurodegenerative diseases, Malignant diseases
1. The aging process
Life expectancy has steadily increased and approximately doubled during the last 200 years due to improvements in health care, housing, labor standards and education. The development of vaccines and antibiotics helped to drastically reduce communicable diseases, the main cause of early- and mid-life mortalities until the 1950s. Since then, researchers have searched for methods to delay late-life mortality [1], including behavioral strategies such as physical activity or caloric restriction as well as pharmacological and medical interventions [2]. Current statistics from Eurostat reveal that life expectancy at birth is 83.5 years for females and 78.3 years for males in Europe. It is an ongoing matter of debate whether life expectancy can be pushed even further. Since a constantly increasing number of people reach old age, more disabling conditions associated with the aging process are emerging. Consequently, modern medicine aims to find strategies to prevent or at least delay the onset of chronic age-related diseases [3] in order to prolong the so-called healthspan, defined as the period of life free from any chronic diseases and age-associated disabilities [4]. The most common age-related diseases include cardiovascular and metabolic diseases like atherosclerosis and diabetes, neurodegenerative diseases such as Alzheimer's or Parkinson's as well as cancer [5].
Organismal aging is a complex process driven by progressive impairment of the functionality and regenerative potential of tissues. One hallmark of organismal aging is the accumulation of senescent cells in organs, resulting in tissue dysfunction and frailty [6]. Due to damage and stress, senescent cells enter cell division arrest and release degradative proteases, growth factors and inflammatory cytokines. Aged cells crucially compromise the function of neighboring cells through the so-called senescence-associated secretory phenotype (SASP) [7]. Cellular senescence can be induced by a wide range of environmental and internal stressors. Reactive oxygen species (ROS) as well as Ca2+ are ubiquitous second messengers fine-tuning a variety of physiological and pathological processes within the cell. Although their characteristics are entirely different, they are both in crucial positions to modulate the process of aging [8]. For instance, lifespan and stress resistance of Caenorhabditis elegans (C. elegans) are also strongly dependent on redox and thiol homeostasis [9] as well as cellular Ca2+ homeostasis [10]. In turn, the process of aging causes alterations in ROS and Ca2+ homeostasis too. Experiments in rats revealed that changes in ROS homeostasis are measureable already at middle age. Plasma ROS levels raised, oxidative damage of proteins and DNA increased and DNA damage repairing capacity declined in middle-aged rats. These processes advanced with further aging [11]. Moreover, increased production of ROS as well as decreased antioxidant scavenging was found to change morphological arrangement, Ca2+ homeostasis and metabolism of neurons during aging [12]. Growing evidence also suggests that aging is associated with the increased oxidative damage of mitochondrial DNA (mtDNA) in cardiovascular diseases. In turn, overexpression of ROS scavenging enzymes resulted in enhanced resistance to fibrosis, cardiac hypertrophy and heart failure in mice [13]. Notably, fortification of the organism's antioxidant potential ahead of time by low levels of ROS that boost cellular ROS defense mechanisms may be a powerful strategy to counteract ROS-induced damage during aging. This so-called mitohormesis may be provoked by glucose restriction [14], physical activity [15] or endogenous metabolites [16].
Ca2+ changes by the aging process as well. For instance, aged rat neurons exhibit increased cytosolic resting Ca2+ levels, enhanced Ca2+ release from the endoplasmic reticulum (ER), elevated Ca2+ flux between ER and mitochondria and changed expression pattern of key proteins involved in cellular Ca2+ homeostasis [17]. Experiments in C. elegans revealed that a defected ER to mitochondrial Ca2+ signaling resulted in increased mitochondrial Ca2+ levels, causing oxidative stress and proteostatic collapse [18]. Furthermore, enhanced inter-compartmental Ca2+ flux between ER and mitochondria due to closer proximity between these organelles was found to modulate mitochondrial metabolism and apoptotic threshold in aged endothelial cells [19]. In addition, experiments in aged human atrial myocytes showed reduction of Ca2+ currents and a decrease in the sarcoplasmic reticulum (SR) Ca2+ content, associated with diminished expression of key proteins ensuring Ca2+ uptake into the SR [20].
The interrelation between ROS and Ca2+ during aging is evidenced by the fact that many proteins involved in Ca2+ signaling are modified by ROS, and Ca2+, in turn, regulates ROS generation sites [8]. The current review aims to provide an up-to-date overview of the mutual interplay between Ca2+ and ROS during aging and the possible potential for new treatment strategies utilizing these direct or indirect interactions.
2. ROS homeostasis
The group of ROS comprises oxygen-containing molecules with an odd number of electrons, causing ROS to be unstable, short-lived and highly reactive. Since ROS abstract electrons from other molecules to gain stability, free radicals are constantly evolving and taking action on themselves [21]. ROS occur either as so-called free radicals with unpaired electrons such as superoxide (O2• −) and hydroxyl radical (•OH) or as “non-radical derivatives” like hydrogen peroxide (H2O2).
Depending on the type, amount and reactivity toward specific cellular components, ROS either work as signaling molecules or introduce potentially harmful oxidative stress to the cell [22]. Redox equilibrium has to be kept under tight control to avoid excessive accumulation as well as depletion of ROS, resulting in cellular dysfunction and various pathologies [23]. Hydroxyl radicals are extremely reactive toward all biomolecules and migrate within a range of a few nanometers. Superoxide and singlet oxygen diffuse up to 30 nm and affect iron-sulfur proteins or lipids, proteins and DNA, respectively. In contrast, hydrogen peroxide diffuses more than 1 μm and oxidizes predominantly cysteine and methionine residues, heme proteins and DNA [24]. In summary, the less reactive a radical is with respect to surrounding molecules, the further it migrates and the larger its place of action as signaling molecule. ROS-induced oxidation, for instance of thiol groups on cysteine residues, may serve as a tool to control the activity of proteins and downstream pathways that contribute to regulation of cell proliferation, immune response and aging [25]. Moreover, low levels of ROS are able to promote health and lifespan by boosting cellular defense mechanisms via an adaptive up-regulation of antioxidant enzymes [26]. However, overwhelming ROS levels results in damage of proteins, lipids and DNA, causing massive oxidative stress and triggering the development of pathological states such as neurodegenerative disorders [27] and cancer [28]. Mitochondrial DNA (mtDNA) is especially vulnerable to ROS-induced damage due to the lack of repair mechanisms and histones, which pack and order nuclear DNA [29].
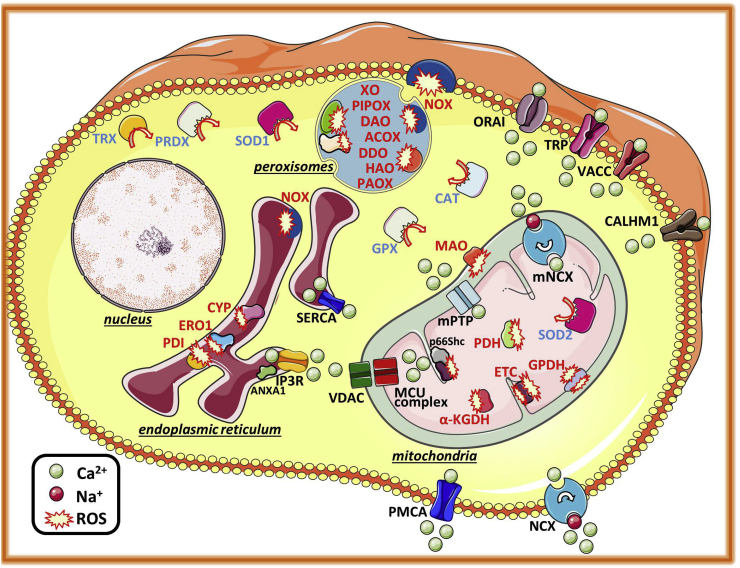
There are numerous endogenous ROS production sites (Fig. 1). Most endogenous ROS evolve from a leaky electron transport chain (ETC) during oxidative phosphorylation (OXPHOS) in mitochondria. Especially at complex I (NADH dehydrogenase) and complex III (ubiquinone cytochrome c reductase), electrons escape the pathway and partially reduce oxygen to superoxide anions. In addition, the major linker between carbohydrate and lipid metabolism, glycerol-3-phosphate dehydrogenase (GPDH), generates mitochondrial ROS. Notably, complex III and GPDH seem to produce ROS on both sides of the IMM, releasing ROS into the mitochondrial matrix as well as into the intermembrane space (IMS). Additional ROS production sites within mitochondria include pyruvate dehydrogenase (PDH), which converts pyruvate into acetyl-coenzyme A (CoA), the Krebs cycle enzyme α-ketoglutarate dehydrogenase (α-KGDH) and monoamino oxidase (MAO) [30,31]. Notably, superoxide generated within the mitochondrial matrix does not cross the IMM. However, as soon as mitochondrial superoxide dismutase 2 (SOD2) converts superoxide to hydrogen peroxide, diffusion across the membrane is possible [31]. In addition to mitochondria, enzymes within peroxisomes generate various types of ROS as by-products of their catalytic actions in metabolic pathways, including acyl-CoA oxidases (ACOX 1–3), d-amino acid oxidase (DAO), d-aspartate oxidase (DDO), l-pipecolic acid oxidase (PIPOX), L-alpha-hydroxyacid oxidase (HAO 1 and 2), polyamine oxidase (PAOX) and xanthine oxidase (XO) [32]. Further, ROS are generated as by-products of a disulfide bond formation by oxidoreductase ERO1 [33] and its thiol redox partner protein disulfide isomerase (PDI) in the lumen of the ER [34]. Moreover, cytochrome P450 (CYP) enzymes, well-known for their role in metabolism and biotransformation of drugs, as well as members of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family, contribute to ROS production within the ER. Membrane-bound NOX enzymes comprising NOX1 – NOX5, dual oxidase 1 (DUOX1) and dual oxidase 2 (DUOX2) catalyze the production of superoxide or hydrogen peroxide as part of the immune response. Notably, the action sites of NOX enzymes, which are missing any localization signal in their structure, are dynamic and stimulus-related. The location of the single isoforms is a matter of debate [35]. Besides endogenous ROS production, various exogenous sources such as air pollutants, tobacco smoke, heavy metals, ultraviolet light, drugs and alcohol also provoke cellular processes that generate ROS as part of signal transduction or cell defense mechanisms [21].
Fig. 1.
Cellular Ca2+and ROS homeostasis. Ca2+ ions enter the cell, among others, through transient receptor potential channels (TRP), voltage-activated Ca2+ channels (VACC), store-operated Ca2+ entry (SOCE)-induced ORAI channels or via the Ca2+ homeostasis modulator 1 (CALHM1). The Na+/Ca2+ exchanger (NCX) and the plasma membrane Ca2+ ATPase (PMCA) extrude Ca2+ from the cytosol into the extracellular space. Within the cell, the sarco/endoplasmic reticulum ATPase (SERCA) pumps Ca2+ into the lumen of the endoplasmic reticulum, from where it is released via inositol 1,4,5-trisphosphate receptor channels (IP3R), which is modulated by annexin 1 (ANXA1). Mitochondria take up Ca2+ through the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane and the mitochondrial Ca2+ uniporter (MCU) complex in the inner mitochondrial membrane, and extrude Ca2+ through the mitochondrial NCX (mNCX). Overwhelming Ca2+ accumulation within mitochondrial matrix provokes the formation of the mitochondrial permeability transition pore (mPTP), resulting in an uncontrolled release of Ca2+, apoptotic factors and reactive oxygen species (ROS). Main production sites of ROS are the mitochondrial electron transport chain (ETC), regulated among others by the cytochrome-c binding protein p66Shc, as well as nicotinamide adenine dinucleotide phosphate oxidases (NOX) at the plasma membrane and in peroxisomes. Besides NOX enzymes, various other enzymes contribute to ROS generation within peroxisomes, including xanthine oxidases (XO), l-pipecolic acid oxidase (PIPOX), d-amino acid oxidase (DAO), acyl-coenzyme A oxidases (ACOX), d-aspartate oxidase (DDO), L-alpha-hydroxyacid oxidase (HAO) and polyamine oxidase (PAOX). In mitochondria, monoaminoxidase (MAO), pyruvate dehydrogenase (PDH), glycerol-3-phosphate dehydrogenase (GPDH) and α-ketoglutarate dehydrogenase (α-KGDH) are further ROS production sites. In the lumen of the endoplasmic reticulum, enzymes like cytochrome P450 (CYP) as well as the protein disulfide isomerase (PDI) and oxidoreductase 1 (ERO1) produce ROS, often as by-product of protein folding. Cytosolic superoxide dismutase 1 (SOD1) as well as mitochondrial SOD2 are rapidly converting superoxide to the less reactive hydrogen peroxide, which is further processed to water and oxygen by catalases (CAT) or to water and oxidized glutathione by glutathione peroxidase (GPX). Moreover, reduced peroxiredoxins (PRDX) catalyze the reduction of hydrogen peroxide to water and are oxidized and restored in their catalytic activity by thioredoxin (TRX).
Since ROS most likely occurred together with the evolution of aerobic respiration, it seems logical that cells established sophisticated ways to keep ROS levels under control [24]. Cytosolic superoxide dismutase 1 (SOD1) as well as mitochondrial SOD2 rapidly convert superoxide to the less reactive hydrogen peroxide, which is further processed to water and oxygen by catalases (CAT) or to water and oxidized glutathione by glutathione peroxidase (GPX). Moreover, reduced peroxiredoxins (PRDX) catalyze the reduction of hydrogen peroxide to water and are oxidized and restored in their catalytic activity by thioredoxin (TRX). Notably, NADPH serves as an ultimate donor of reductive power for the majority of these detoxifying enzymes [36].
In short, ROS evolve from a leaky ETC from ROS-producing enzymes such as NOX enzymes or through external triggers inducing oxidative stress. To avoid excessive accumulation of ROS, enzymes such as SODs or CATs scavenge and detoxify high levels of ROS. Thus, a redox equilibrium is achieved, which allows ROS to operate as intracellular signaling molecules while their harmful potential is kept under control [23].
3. Ca2+ homeostasis
Ca2+ serves as a multifunctional second messenger participating in a broad range of processes within a cell, including signal transduction, neurotransmitter release, muscle contraction and fertilization. Together with potassium (K+), sodium (Na+) and chloride (Cl−), Ca2+ ions control membrane potential and shape the action potential [37,38]. Moreover, Ca2+ ions regulate the activity of enzymes such as mitochondrial dehydrogenases of the Krebs cycle [39]. In addition, Ca2+ ions convey their action by binding to proteins such as CaN or CaM, which, in turn, have a wide area of function [40]. For instance, Ca2+ is required for CaN-mediated dephosphorylation of the nuclear factor of an activated T cell (NFAT), which controls the expression of numerous genes and, thereby, processes including protein secretion, cell differentiation and immune response [41]. Moreover, binding of Ca2+ causes allosteric changes in CaM, modifying its interaction with target proteins, including kinases or phosphatases, and thereby affecting neurotransmitter secretion, transcription factor regulation, metabolism and muscle contraction [42]. Overwhelming Ca2+ accumulation within the mitochondrial matrix provokes the formation and opening of the mitochondrial permeability transition pore (mPTP), resulting in the release of Ca2+ as well as apoptotic factors into the cytoplasm [43].
To fulfill its versatile signaling function within physiological and pathophysiological processes, Ca2+ levels vary in amplitude and frequency within various cellular compartments. Therefore, Ca2+ homeostasis has to be tightly regulated by channels and pumps (Fig. 1) as well as by fine-tuning mechanisms affecting proteins involved in Ca2+ transport. Thus, Ca2+ is shuttled between extracellular space, cytosol, ER or SR, mitochondria and, to a lesser extent, to Golgi apparatus and lysosomes [44].
Cytosolic Ca2+ levels usually rank around 100 nM, but various signaling events cause temporal or local excess of this concentration. To overcome the activation threshold of certain proteins, Ca2+ levels even reach concentrations above 10 μM within certain cytosolic microdomains [45]. To keep Ca2+ on a generally low level in the cytosol, Ca2+ is actively transferred into ER and mitochondria, bound to proteins or shuttled outside the cell. Ca2+ levels reach concentrations of 1–2 mM in the extracellular space. The Na+/Ca2+ exchanger (NCX) and the plasma membrane Ca2+ ATPase (PMCA) actively extrude Ca2+ from the cytosol into the extracellular space. In turn, various channels ensure controlled Ca2+ uptake, including voltage-activated Ca2+ channels (VACC), receptor operated channels (ROC) and transient receptor potential channels (TRP), divided into six subfamilies (C, V, M, P, ML and A). The ER has by far the largest internal Ca2+ store with Ca2+ concentrations ranging between 100 and 800 μM [46]. Store-operated Ca2+ entry (SOCE) allows direct interplay between the ER and the plasma membrane to secure proper ER Ca2+ replenishment. The stromal interaction molecules 1 and 2 (STIM1 and STIM2) sense Ca2+ concentration within the ER. As soon as ER Ca2+ levels drop, STIM proteins aggregate and initiate the recruitment and opening of ORAI channels (ORAI1, ORAI2 and ORAI3) in the plasma membranes, which is followed by a rapid Ca2+ influx [44]. Ca2+ from the cytosol is taken up into the ER via the sarco/endoplasmic reticulum ATPase (SERCA) at the expense of ATP. Activation of the phospholipase C (PLC) by signals at the cell surface results in the formation of 1,4,5-trisphosphate (IP3), which diffuses to the ER and causes ER Ca2+ release by opening the inositol 1,4,5-trisphosphate receptor channels (IP3R1, IP3R2 and IP3R3). Like IP3Rs, the ryanodine receptor channels (RYR1, RYR2 and RYR3) mediate the release of Ca2+ ions from the SR in muscle cells [47]. Mitochondria take up Ca2+ irrespective of its source but show a large preference for Ca2+ uptake at ER-mitochondrial interaction sites. Specialized ER-membranes, the so-called mitochondria-associated ER membranes (MAMs), stretch closely to mitochondria, and cytosolic Ca2+ levels exceed levels of 10 μM in these areas [48]. While the outer mitochondrial membrane (OMM) is largely permeable to Ca2+ via the voltage-dependent anion channel (VDAC), Ca2+ transport across the inner mitochondrial membrane (IMM) is highly restricted. High Ca2+ concentrations within the MAM regions allow unblocking the gatekeeper proteins mitochondrial Ca2+ uptake 1 and 2 (MICU1 and MICU2) and the Ca2+ uptake via the mitochondrial Ca2+ uniporter (MCU), controlled by a variety of proteins [49]. This protein complex includes, among others, the essential MCU regulator (EMRE) linking MICU1 and MICU2 to MCU [50], the scaffold factor MCU regulator 1 (MCUR1) [51], the dominant-negative pore-forming subunit MCUb [52] and the mitochondrial Ca2+ uptake 3 (MICU3) acting as enhancer of MCU-dependent mitochondrial Ca2+ uptake [53].
Under controlled conditions, Ca2+ leaves mitochondria via the Na+/Ca2+ exchanger (mNCX) in exchange for Na+ [54]. Notably, this exchanger is also able to switch to a reversed transport mode following physiological stimulation of cell-surface receptors, thus generating oscillations in matrix Ca2+ [55].
All these mechanisms ensure proper handling of Ca2+ within physiological and pathophysiological processes. For instance, Ca2+ entry via VACC triggers Ca2+ release through the RYR on the SR and causes very rapid Ca2+ oscillations in cardiac myocytes and, thereby, muscle contraction by binding to troponin C [56]. Also in neurons, electrical or neurotransmitter stimulation causes Ca2+ entry via channels in the plasma membrane. In contrast, the binding of hormones to G-protein-coupled receptors (GPCRs) provokes slower Ca2+ signals by activation of the IP3R and depletion of ER Ca2+ in non-excitable cells [57].
In short, a sophisticated toolkit composed of channels, pumps and cytosolic buffers controls numerous biological processes by appropriately adjusting Ca2+ levels in various cell types. Electrical, hormonal or mechanical stimulation elicit Ca2+ signals by facilitating Ca2+ entry via the plasma membrane or Ca2+ release from intracellular stores. To prevent the induction of harmful processes, actions of Ca2+ are stopped by restoring resting Ca2+ concentrations within cellular compartments.
4. Mutual interplay between ROS and Ca2+
4.1. Ca2+ affects ROS homeostasis
Ca2+ signaling manipulates ROS levels by targeting major ROS generation sites, including mitochondrial respiration as well as NOX enzymes.
4.1.1. Mitochondrial ROS
About 0.15% of mitochondrial O2 consumption is assumed to be diverted to ROS under physiological conditions. In contrast, an increase of up to 1–4% has been reported in the presence of antimycin and a non-restricted amount of substrates and O2 [31]. Ca2+ boosts the activity of Krebs cycle dehydrogenases [39]; affects ATP synthase [58,59], adenine nucleotide translocase (ANT) [60] and glycerol-3-phosphate dehydrogenase [61]; and facilitates glutamate shuttle via the mitochondrial glutamate/aspartate carrier aralar [62]. Intra-as well as extra-mitochondrial Ca2+ thereby enhance the ETC activity, mitochondrial O2 consumption and ATP production. Whether enhanced mitochondrial metabolism does indeed go along with a higher probability of electron leakage and ROS production depends on substrate and O2 availability, functionality of the ETC, mitochondrial membrane potential and matrix pH [63]. Experimental observations suggest that Ca2+ diminishes ROS leakage from complex I and III under physiological conditions but enhances ROS production in the case of complex blockage [64]. Moreover, it must not be overlooked that mitochondrial Ca2+ uptake is linked to a mild dissipation of mitochondrial membrane potential (“uncoupling”), potentially enhancing ROS generation via perturbation of the pH gradient at the IMM, and thus affecting topology of ROS formation sites and mitochondrial respiration rates [65]. Notably, absence of MICU1, the gatekeeper for MCU-mediated mitochondrial Ca2+ uptake, was shown to trigger ROS production through a constitutive loading of mitochondria with Ca2+ [66]. Also, knockdown of the mNCX was associated with enhanced mitochondrial ROS production due to Ca2+ accumulation, causing impairment even of SOCE by ORAI1 inactivation through oxidation [67]. Several reported effects of the impact of mitochondrial Ca2+ on ROS levels might be linked to the formation of the mPTP. Stable opening of the mPTP involves a devastating ROS burst and may lead to necrotic cell death or apoptosis. In contrast, a brief and reversible mPTP opening plays a significant physiological role through short-term mitochondrial ROS formation and release, leading to activation of redox-sensitive enzymes involved in protective signaling pathways and removal of damaged cells or mitochondria [68].
4.1.2. NADPH oxidases
Besides affecting ROS levels by manipulating mitochondrial activity, Ca2+ also indirectly and directly affects NOX enzymes. For instance, Ca2+ modulates the activity of NOX2, a key element in the innate immune response, vasoconstriction and platelet aggregation. Patients with a loss of function of NOX2, a condition called “granulomatous disease” (CGD), have increased susceptibility to bacterial infections and increased flow-mediated dilation [69]. Ca2+ was shown to initiate the translocation of the cytosolic subunits of NOX2, namely p47phox, p67phox, p40phox and Rac GTPase, to the plasma membrane by activation of PKC [70]. Moreover, Ca2+ entry through the ORAI channels in the plasma membrane by SOCE causes Ca2+-induced recruitment of the cytosolic S100A8/A9 complex to the phagosomal membrane, resulting in a conformational change and induction of NOX2 [71]. In addition, it was shown that ensuring Ca2+ entry by charge compensation by the VSOP/Hv1 proton channel helps to sustain NOX2-induced ROS production and, thereby, motility and adhesion of neutrophils [72]. In addition to NOX2, the activity of NOX1 is also manipulated by Ca2+. NOX1 is a close structural homolog of NOX2 and contributes to innate immune defense and homeostasis as well as to inflammatory processes [73]. An ultraviolet A (UVA)-induced increase in intracellular Ca2+ of keratinocytes reportedly activated NOX1, potentially via Ca2+-induced phosphorylation by PKC and subsequent translocation of Rac GTPase to the plasma membrane [72]. In addition, direct phosphorylation by the Ca2+-dependent PKC-β1 [74] was associated with an activity boost of NOX1 [75]. Direct activation by Ca2+ was suggested for NOX5, DUOX1 and DUOX2 enzymes. NOX5 is largely expressed in vascular cell types, regulates vascular contraction and reactivity, and contains four Ca2+-binding EF hands that undergo conformational change in the case of Ca2+ binding, causing activation of NOX5 [76]. For instance, PKC-dependent phosphorylation of the C-terminus of the NOX5b-isoform was shown to increase the enzyme's sensitivity toward Ca2+ [77], while oxidation of the Ca2+-binding domain of NOX5 was associated with its inactivation [78]. Like NOX5, DUOX1 and DUOX2 contain an N-terminal extension comprising two EF-hand motifs and are directly activated by Ca2+ [79]. These enzymes are largely expressed in thyrocytes, where they support the production of hydrogen peroxide necessary for hormone biosynthesis [80]. In addition to this function, DUOX enzymes were also found to trigger hydrogen peroxide production and the inflammatory response involved in wound healing in response to wound-induced Ca2+ flashes in C. elegans, Drosophila embryos and zebrafish [81].
4.2. ROS affect Ca2+ homeostasis
ROS directly and indirectly affect Ca2+ transport proteins located in the plasma membrane, ER and mitochondria.
4.2.1. Plasma membrane
The plasma membrane serves as special platform for redox signaling. Enzymatic systems such as XO or nitric oxide synthase (NOS) as well as integral membrane proteins like NOX enzymes generate ROS at the outer or inner part of the membrane. Besides, hydrogen peroxide-transporting proteins or the formation of specific endosomes at the plasma membrane deliver ROS signals [82]. Indirect and direct interactions between Ca2+ transporters and ROS producing enzymes have been reported. For instance, physical interaction between TRPC3 and NOX2 contributes to ROS production under hypoxic stress and mediates cardiac plasticity [83]. Various Ca2+ transport proteins of the plasma membrane are affected by ROS. As discussed above, TRP channels are located in the plasma membrane and are activated and regulated by a variety of stimuli, ROS among others. For instance, the second messenger adenosine diphosphate (ADP)-ribose is generated in mitochondria in response to hydrogen peroxide and induces gating of TRPM2 [84]. In contrast to indirect regulation of TRPM2 by hydrogen peroxide, TRPC5, TRPV1 and TRPA1 are directly activated by ROS via modification of cysteine residues at the pore forming regions located within the plasma membrane [85]. Notably, TRPA1 seems to be triggered by hydroxyl radicals, while the activity of TRPC5 and TRPV1 are boosted by hydrogen peroxide [86]. Aside from TRP channels, ROS also affects the SOCE machinery, comprised of the ER Ca2+ sensors STIM1 and STIM2 and the plasma membrane Ca2+ channel ORAI, in a cell type-dependent manner. For instance, ROS leads to clustering of STIM1 and constitutive, store-independent Ca2+ entry by S-glutathionylating the conserved cysteine residue C56 of STIM1 in B lymphocytes [87]. Moreover, the ER oxidoreductase ERp57 was shown to inhibit STIM1 oligomerization and SOCE by oxidation of C56, which causes an intramolecular disulfide bond formation with C46 [88]. Also, STIM2, contributing to SOCE regulation under conditions of hypoxia, is apparently oxidized, possibly at C725 [89]. The ORAI1 channel is equipped with three cysteine residues at positions 126, 143 and 195 and has also been identified as a redox sensor inhibited by hydrogen peroxide [90]. Notably, ORAI2 also contains these three cysteine residues, though the hydrogen peroxide-insensitive ORAI3 is lacking C195. Consequently, the cysteine residue at 195 is assumed to be crucial for conveying the effect of ROS [91]. In addition, ROS seem to affect major Ca2+ extrusion proteins, too. For instance, hydrogen peroxide was found to induce thiol oxidation on NCX, causing an activity boost of this channel in rat myocytes [92]. Moreover, oxidative modifications were reported to affect PMCA, leading to rapid inactivation, conformational changes, aggregation, proteolytic degradation as well as internalization from the plasma membrane [93].
4.2.2. Endoplasmic reticulum
Within the ER, ROS are produced by catalytic processes of oxidoreductase ERO1 and PDI as by-product of protein folding [33,34]. Moreover, CYP enzymes as well as members of the NOX family contribute to ROS production within the ER. Several NOX enzymes and their regulators are located at the ER and function as integral signaling elements within the unfolded protein response (UPR) and ER stress. For instance, NOX2 enhances pro-apoptotic signals, NOX4 has a dual pro-survival and pro-apoptotic and the ER chaperone PDI was found to modulate NOX signals. The localization of these enzymes within the ER also affects the activity of proteins involved in ER Ca2+ homeostasis, potentially displaying additional signaling function [35]. Coupling between NOX activity and ER Ca2+ release was assumed to act as regulation point for actin dynamics in neurons [94]. Interestingly, it could be shown that NOX4 knockdown prevented SERCA oxidation and that NOX4-derived oxidants target the RYR, inducing an ER Ca2+ release [35]. However, further investigations are needed to pinpoint the key proteins in these ROS-Ca2+ interplay.
All three IP3R isoforms contain redox-sensitive cysteine residues, which are responsible for the heterogeneity of IP3Rs in their sensitivity towards IP3 and Ca2+ [95]. Similarly to various ORAI isoforms, IP3R isoforms are affected by ROS to a different extent in various cell types. The thiol-modifying agent thimerosal promotes Ca2+ release via IP3R1 at low μM concentrations but inhibits the channel activity at higher concentrations. In the same study, the IP3R3 channel function remained unchanged by thimerosal [96]. Another study suggested that thimerosal selectively sensitizes IP3R1 and IP3R2 to IP3 by stabilizing an active conformation of the receptors through modification of cysteine residues [97].
It was shown that the thiol-oxidizing agent diamine promotes Ca2+ release via IP3R from the ER Ca2+ store by glutathionylation in bovine aortic endothelial cells [98]. In addition, hydrogen peroxide was found to increase the level of glutathionylation of IP3R1 and the sensitivity of endothelial cells toward IP3-generating agonist histamine [99]. Furthermore, ROS derived from xanthine oxidase was also assumed to sensitize IP3Rs via oxidation of thiol groups [100]. Interestingly, the ROS-dependent interaction between ER luminal protein Erp44, a member of the thioredoxin family, with a luminal loop of IP3R1 inhibits the channel activity, showing that IP3Rs are not just controlled by cytosolic but also by intraluminal actions [101]. Liquid chromatography with tandem mass spectrometry revealed modifications at two specific cytosolic and two intraluminal cysteine residues of IP3R1 of HEK293 cells under basal conditions and additional eleven modified cysteine residues after thimerosal treatment [102]. Besides, the activity of IP3R channels was found to be strongly inhibited by high luminal ER Ca2+ content through an ER-luminal protein, likely to be annexin 1 (ANXA1) [103], pointing out that redox-mediated regulation on IP3R might also happen indirectly via regulatory proteins.
RyR channels, which serve as Ca2+ release channels in skeletal (RyR1) and cardiac (RyR2) muscle cells as well as in the brain (RyR3), contain numerous cysteine residues and are, consequently, prone to redox modifications [104]. As with IP3R, the impact of ROS on RyR might be concentration dependent. For instance, low concentrations of oxidizing agents boost the activity of RyR2, while persistent high oxidant concentrations cause irreversible inhibition. A different impact on individual cysteine residues is discussed as a possible reason [105]. The RyR2 appears to be the most redox sensitive RyR receptor. Several oxidative modifications such as S-glutathionlyation and S-nitrosylation have been shown to affect RyR2 activity. Furthermore, oxidative stress may trigger RyR2 activity by the formation of disulfide bonds between subunits, so-called “disulfide cross bridging”, which leads to structural rearrangement of the channel and an SR Ca2+ leak [106]. RyR2 are affected by ROS also indirectly via CaM, which binds to RyR2 under resting conditions and inhibits channel activity under low and high Ca2+ levels. Oxidative stress was shown to decrease the binding between CaM and RyR2 and, thereby, relieve RyR2 from the inhibitory effect of CaM [107].
S-glutathionlyation, S-nitrosylation as well as disulfide cross bridging in response to oxidative stress have been described for RyR1, too [108]. For instance, NOX4-induced ROS production was shown to boost RyR1 activity and Ca2+ release in cultured myofibers as well as contractility of skeletal muscle by oxidation of RyR1 cysteine thiols [109].
Also, SERCA exhibits oxidative-sensitive cysteine residues, which modulate the enzyme's activity upon oxidation and get partially lost during aging [110]. Notably, SERCA isoforms differ in their resistance to damage induced by various types of ROS [111]. Hydroxyl radicals were found to inhibit SERCA function by direct attack on the ATP binding site [112]. Moreover, micromolar pulses of peroxynitrite impair the ability of SR vesicles, isolated from muscles, to transport Ca2+ and to hydrolyze ATP by oxidation of thiol groups and tyrosine nitration [113]. In contrast, nitric oxide (NO)-induced reversible S-glutathionylation of cysteine-674 by peroxynitrite enhances the activity of SERCA, decreasing cytosolic Ca2+ levels and inducing muscle relaxation. Importantly, irreversible oxidation of SERCA's key cysteine residues was associated with the prevention of reversible S-glutathionylation and the subsequent activation of SERCA [114], pointing to a diverse range of ways to modulate SERCA by oxidation-induced mechanisms.
4.2.3. Mitochondria
The ETC is the main ROS production site within the cell. Like discussed above, additional ROS production sites within mitochondria include α-KGDH, GPDH, MAO and PDH [30,31]. The contribution of NOX enzymes to mitochondrial ROS production is still not fully clarified. NOX4 has been found in mitochondria of rat kidney as well as in cardiac myocytes. Moreover, cysteine residues of mitochondrial proteins are affected by modulation of NOX4 expression in mice. However, measurements directly assessing NOX4 activity in mitochondria are still missing and, consequently, it remains unclear to which extent NOX enzymes contribute to mitochondrial ROS production [115]. Interestingly, confocal microscopy revealed a co-localization of NOX4 with the ATP synthase [116], implying that NOX enzymes might at least indirectly affect mitochondrial Ca2+ homeostasis.
Activation of cytochrome c-binding protein p66Shc might be another mechanism to indirectly modulate mitochondrial Ca2+ homeostasis. Oxidative stress-induced phosphorylation of p66Shc and its subsequent translocation to mitochondria was found to promote ETC and OXPHOS activity as well as ROS production [117]. Mitochondrial import of p66Shc and its actions as oxidoreductase got associated with mitochondrial swelling, opening of the mPTP and release of pro-apoptotic factors [118]. These events are linked to a perturbation of mitochondrial structure and altered mitochondrial Ca2+ homeostasis. While oxidative stress cause a drastic reduction in mitochondrial Ca2+ uptake upon IP3-generating agonist stimulation in mouse embryonic fibroblasts (MEFs), mitochondrial Ca2+ response in MEFS depleted of p66Shc remains largely unaffected by ROS [118]. The physiological role of p66Shc seems to be controversial. Deletion of p66Shc was found to retard aging in mice [119]. Moreover, p66Shc expression contributes to liver fibrosis [120] and makes cells of the central nervous system prone to β-amyloid toxicity through regulation of mitochondrial ROS [117]. However, p66Shc was found to be highly expressed in fibroblasts from centenarians. A peculiar regulation of p66Shc in long-lived individuals was discussed in this context [121]. Moreover, p66shc-induced ROS formation might also contribute to self-endogenous defenses against mild ischemia/reperfusion injury [122], highlightening, once again, the controversial role of mitochondrial ROS and Ca2+ in cellular homeostasis.
The main Ca2+ channel in the IMM, MCU, was found to be regulated by ROS via S-glutathionylation at cysteine 97, promoting the assembly of MCU channels into higher-order complexes with persistent activity. Oxidation as well as mutation of C97 caused enhanced MCU channel activity, leading to elevated ROS and Ca2+ levels within mitochondria [123]. Activation of redox-sensitive CaMKII by ischemia perfusion was reported to increase Ca2+ uptake via MCU, promoting mPTP opening and myocardial death [124]. However, a subsequent study failed to find direct electrophysiological evidence for CaMKII's regulation by MCU [125]. Notably, several other proteins involved in mitochondrial Ca2+ uptake, including MCUb, MCUR1, MICU1, MICU2, and EMRE, remained unaffected by oxidizing agents [123].
5. Crosstalk between ROS and Ca2+ in age-related diseases
Aging gives rise to cardiovascular diseases, type 2 diabetes, neurodegenerative and inflammatory conditions as well as cancer. The interplay between Ca2+ and ROS homeostasis crucially contributes to the development, enhancement and progression of these disorders. Important examples are discussed in the following chapter.
5.1. Cardiovascular diseases and type 2 diabetes mellitus
Mitochondrial ROS production is central to the development of age-related cardiovascular diseases, including arrhythmias, heart failure, atherosclerosis and type 2 diabetes mellitus [126]. The crucial role of ROS is highlighted by the occurrence of cardiovascular disorders and diseases in numerous animal models with modulation of mitochondrial ROS levels [127]. For instance, loss of mitochondrial respiration integrity was associated with ROS-induced mtDNA damage found in atherosclerotic plaques of apolipoprotein E-deficient mice as well as humans [128]. Moreover, impaired mitochondrial ROS and Ca2+ regulation, which facilitates opening of the mPTP and induces cell death, is also a common phenomenon in ischemia/reperfusion (I/R) injury, a tissue damage caused by a reestablished blood supply after stroke or cardiac arrest [129]. During heart failure, mitochondrial Ca2+ uptake is hampered by decreased open probability of MCU, accelerated mNCX activity and reduced SR Ca2+ load. Consequently, Krebs cycle activity was found to be diminished, resulting in limited availability of NADH for ATP production and of NADPH for detoxifying ROS. The resulting burst of mitochondrial ROS emission apparently contributed to cardiac failure. Indeed, ROS scavenging agents like MitoQ ameliorated cardiac damage in response to I/R injury and hypertension in preclinical studies [126].
In addition to mitochondrial ROS, NOX enzymes expressed in the cardiovascular system (NOX1 – NOX5) are also involved in the development and enhancement of cardiovascular diseases. NOX5 is widely expressed in various human tissues and is directly affected by Ca2+. The enzyme is constitutively active, triggered in its activity by vasoactive agents such as angiotensin II and expressed in the ER and perinuclear area of human microvascular endothelial and vascular smooth muscle cells. Notably, NOX5-induced ROS generation regulated by Ca2+ has been shown to be crucial in vascular contraction and cardiac function [130]. For instance, NOX5-expressing mice showed enhanced Ca2+-dependent agonist-induced vasoconstriction and increased Ca2+ and ROS levels in vascular smooth muscles. In addition, introduction of endothelial NOX5 increased risk for hypertension and stroke in mice [76]. Moreover, NOX5-derived ROS were shown to activate one of the most potent angiogenic chemokines, stromal cell-derived factor-1α (SDF-1α), which promotes endothelial cell migration and contributes to angiogenesis and atherosclerosis [131]. In addition, NOX5 activity was found to boost the expression of the intermediate-conductance Ca2+-activated K+ channel (KCNN4) in vascular smooth muscle cells. Notably, high activity of this channel has been associated with coronary artery smooth muscle contraction as well as proliferation and migration of smooth muscle cells during atherosclerotic plaque formation and restenosis [132]. Taking these implications into account, it makes sense that NOX5 expression is upregulated in human intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction [133]. In line with these findings, NOX5 expression increases in humans in the case of hypertension [134] and is currently being discussed as a blood pressure-associated gene [130]. Moreover, upregulation of NOX5 is associated with renal oxidative stress, inflammation and fibrosis, and it was found in human diabetic nephropathy [135].
In addition to NOX5, the activity of NOX2, which is dependent on Ca2+-induced arrangement of subunits at the plasma membrane, is also associated with atherothrombotic processes. Findings in knockout mice as well as in patients suffering from CGD suggest that NOX2, together with NOX1, has a vasoconstrictive effect by interfering with NO bioavailability. Consistent with these observations, upregulation of NOX2 or of its subunits was found in human atherosclerotic plaques in carotid and coronary arteries [69], and overexpression of the NOX2 subunit p22phox induced progression of carotid artery lesions in transgenic mice [136]. However, suppression of NOX2 in order to diminish atherosclerosis progression is not advisable, as it would be accompanied by chronic infection and inflammation [69]. Besides, activity of NOX2 broadly regulates lung function. For instance, NOX2-induced activation of TRPC and subsequent Ca2+ influx was found to trigger lung I/R-induced edema, a life-threating condition associated with endothelial dysfunction [137]. Moreover, NOX2 has also been shown to promote SOCE-associated Ca2+ entry, causing vascular barrier dysfunction as well as sepsis-induced acute lung injury [138]. NOX1, which is affected by Ca2+ in a similar way to its close homolog NOX2, is also potentially involved in the pathogenesis of cardiovascular diseases like atherosclerosis, hypertension and I/R injury. Increased expression of NOX1 was found in atherosclerotic arteries of humans, primates and mice already in an early disease state. Deficiency of NOX1 reduced infarct size after I/R injury and protected against angiotensin II-mediated hypertension [139].
The connection between mitochondrial ROS formation and the pathogenesis of diabetes and associated complications is well-established. Notably, cardiac mitochondria from diabetic patients were found to exhibit elevated hydrogen peroxide levels, impaired mitochondrial respiratory capacity and increased levels of oxidized proteins. This might lead to compromised ATP generation and increased propensity to mPTP opening [140]. Also NOX enzymes, including Ca2+ sensitive NOX1, NOX2 and NOX5, are a key source of ROS in the diabetic environment and able to enhance diabetic complications such as nephropathy, retinopathy and cardiomyopathy [141]. For instance, deletion as well as pharmacological inhibition of NOX1 had an antiatherosclerotic effect associated with reduced ROS formation in apolipoprotein E-deficient mice after induction of diabetes mellitus [139,142]. Experiments in NOX2 deficient mice revealed the requirement of NOX2 expression for diabetes-induced retinal vascular injury [143]. Moreover, NOX5 was shown to facilitate renal injury in diabetic nephropathy by enhanced ROS production associated with accelerated glomerulosclerosis and increased expression of proinflammatory chemokines [144].
Heart contractions are dependent on controlled intracellular Ca2+ homeostasis, largely regulated by Ca2+ released from the SR through RyR [104]. Consequently, redox-driven modifications of RyR2 are of major significance in the development of cardiac pathologies like arrhythmias as well as contractile dysfunction in infarcted and failing hearts [106]. Experiments in a non-ischemic canine heart failure model suggest that phosphorylation of RyR2 by CaMKII is associated with an early stage of heart failure, followed by RyR2 oxidation during a later stage [145]. Consequently, it appears reasonable that phosphorylation- and thiol oxidation-induced dysfunction of RyR2, accompanied by an enhanced SR Ca2+ leak, are linked to heart failure [146]. Notably, the β-blocker carvedilol that also serves as an antioxidant preventing thiol oxidation of RyR2 is significantly more effective in the treatment of heart failure than other β-blockers [147]. Further, ROS-mediated activity of TRP channels affects cardiac function and substantially contributes to I/R injury. For instance, ROS-induced activity of TRPM2 was found to contribute to cell death provoked by I/R injury in brain, heart and kidney. A combination of hydrogen peroxide and leukotriene B4 has been reported to trigger TRPM2 activity, facilitating Ca2+ flux and the exacerbation of myocardial reperfusion injury [148]. Furthermore, ROS-sensitive TRPA1 channel was found to contribute to Ca2+ overload and hypercontraction of cardiomyocytes. Genetic ablation of TRPA1 caused a significant decrease in myocardial infarction after I/R in mice, indicating that I/R activation of TRPA1 worsens myocardial infarction [149]. In addition to channels in the plasma membrane, Ca2+ transport proteins in ER and mitochondria are also crucial contributors to the development of cardiovascular diseases.
Treatment of adult rat ventricular myocytes with hydrogen peroxide caused a depletion of SR Ca2+ stores by inhibition of SERCA and activation of NCX. This resulted in diminished contractile function of cardio myocytes [92]. The loss of SERCA's cysteine residues is associated with an age-dependent decrease in SERCA activity [110]. NO-induced relaxation as well as activation of SERCA by S-glutathionylation was found to be decreased by atherosclerosis due to oxidized cysteine 674 residues [114]. Moreover, high glucose-induced oxidation prevents NO-induced activation of SERCA [150]. Besides, induction of endothelial cell migration, a critical step in physiological and pathophysiological angiogenesis, was found to be stimulated in a NOX4-dependent manner by the vascular endothelial growth factor through increased S-glutathionylation of SERCA and enhanced ER Ca2+ influx [151].
A cross-regulation between mitochondrial ROS and Ca2+ was also found in chronic ventricular remodeling during chronic ischemia. MAO-induced ROS thereby induced the generation of 4-hydroxynonenal (4-HNE), which provoked increased MCU activity, resulting in mitochondrial Ca2+ overload and mitochondrial dysfunction [152].
The interplay between Ca2+ and ROS may also be essential in the development or enhancement of type 2 diabetes. For instance, hydrogen peroxide-induced Ca2+ influx through TRPM2 has been shown to drive insulin secretion in pancreatic β cells [153]. Moreover, the redox-sensitive MCU was revealed as essential protein in glucose-stimulated insulin secretion in vitro and in vivo in mice [154].
5.2. Neurodegenerative diseases
Various neurodegenerative diseases such as Parkinson's disease, Alzheimer's disease, multiple sclerosis and dementia have been linked to biochemical alterations and cellular damage due to oxidative stress [155]. Furthermore, ROS also has an important signaling function in the central nervous system. For instance, Ca2+ was found to induce remodeling of mitochondrial morphology of primary astrocytes via ROS [156]. Numerous reports demonstrate that ROS derived from the main cellular production site, the ETC, play a critical role in neurodegeneration. However, the influence of Ca2+ homeostasis on the ETC under conditions of neurodegeneration is less well documented. But exaggerated Ca2+ signaling via IP3R and presenilins was, for instance, found to boost the generation of mitochondrial ROS in Alzheimer's disease models, a mechanism believed to be an important component in the pathogenesis of the disease [157]. ROS produced by NOX5 have been predominantly implicated in cardiovascular diseases, renal diseases and cancer, but little has been reported about their role in neurodegenerative diseases [130]. While NOX5 is nearly exclusively expressed in non-central nervous system tissues (CNS), NOX2 and NOX1 are both abundant in tissues of the CNS, and upregulation by a variety of neurodegenerative factors has been found [158]. For instance, NOX2 is significantly upregulated in humans with neurodegenerative disorders as well as in various neurodegenerative animal models, including models for Alzheimer's, Huntington's and Parkinson's disease as well as multiple sclerosis. Moreover, NOX2-derived ROS production and amyloid β deposition was found to be more prevalent in brain tissues of aged mice [159]. In addition, the NOX2 inhibitor apocynin was found to decrease neuroinflammation and to improve symptoms of neurodegeneration in preclinical studies [160].
Since neurons use Ca2+ signals to control membrane excitability, neurotransmitter release, gene expression as well as neuronal cell cycle, these cells are especially prone to disturbances in Ca2+ homeostasis, often provoked in response to oxidative stress. For instance, neurodegeneration-associated ROS production was reported to disturb the Ca2+ flux between ER and mitochondria [161]. Furthermore, the Alzheimer's disease-associated amyloid β was found to promote cellular Ca2+ overload by diminishing the integrity of the plasma membrane via oxidative stress [162]. Proteins involved in Ca2+ homeostasis were also revealed to be directly affected under conditions of aging or neurodegeneration. For instance, mass spectrometry analysis revealed an age-dependent loss of SERCA's functional cysteine residues, which might be a reason for the progressive decline of SERCA activity during aging and disturbed Ca2+ homeostasis [110]. Notably, SERCA executes the reuptake of Ca2+ into the SR to initiate muscle relaxation. In SOD1−/− mice, restoration of SERCA function prevented oxidative stress-related muscle atrophy and weakness, suggesting that SERCA activity is necessary to control the redox state and muscle function [163].
The activity of PMCA is dependent on age and oxidation, too. Age-and oxidation-associated downregulation of PMCA and, thereby, disturbed cellular Ca2+ homeostasis is assumed to compromise neuronal function in the brain during aging and to increase the susceptibility to neurodegenerative diseases [93].
Besides, depletion of the Ca2+ homeostasis modulator 1 (CALHM1), a Ca2+ channel involved in the regulation of cytosolic Ca2+ levels, was found to downregulate ROS production, to increase the expression of HIF-1α and to display neuroprotective effects against ischemia in mice [164]. Notably, the mutation of this channel (P86L-CALHM1) got associated with Alzheimer's disease and mitochondrial Ca2+ overload, potentially causing increased vulnerability of these cells to apoptotic stimuli [[165], [166], [167]].
TRPM2, the most abundant TRP channel in the brain and an indirect target of ROS, is also associated with the development of neurodegenerative diseases. For instance, senescence-associated loss of glutathione was shown to facilitate TRPM2 channel activity, which is associated with an increased intracellular Ca2+-inducing toxicity in cultured hippocampal pyramide neurons [168,169]. In addition, TRPM2 seems to be involved in various aspects of immunity, boosting, for instance, production of pro-inflammatory cytokines in response to bacterial pathogens [170]. Besides, hydrogen peroxide-activated Ca2+-influx via TRMP2 was also associated with activation of macrophages [171].
5.3. Cancer/malignant diseases
Alterations in ROS homeostasis and enhanced resistance to oxidative stress due to upregulation of protective antioxidant pathways are hallmarks of cancer cells. ROS are assumed to serve as important signaling molecules in the proliferation of cancer cells. For instance, ROS stabilizes hypoxia-sensitive alpha subunits (HIF) and, thereby, ensures adaptation of cancer cells to an oxygen-reduced microenvironment [172]. Whether increased mitochondrial Ca2+ levels boost the activity of the TCA cycle dehydrogenases, namely pyruvate, isocitrate dehydrogenase and oxoglutarate dehydrogenases, in tumor cells requires further investigation. However, increased mitochondrial Ca2+ uptake is clearly associated with enhanced mitochondrial ROS production in cancer cells [172]. Also NOX2, indirectly affected by Ca2+ homeostasis, was found to be involved in cancer development by boosting angiogenesis as well as by silencing the immune response in cancer cells [173]. For instance, ROS derived from NOX2 helps to suppress an effective immune response against cancer cells in tumor-bearing mice. In turn, myeloid-derived suppressor cells lost the ability to inhibit T cell response in the absence of NOX2 activity. Thus, a proper immune reaction could be developed [174]. The expression of NOX5, shown to be directly affected by Ca2+, increased in several types of cancer, including gastric cancer, malignant melanoma, breast cancer, prostate cancer and esophageal cancer, and is associated with a loss in sensitivity to cisplatin in cancer cells [130]. In contrast, a downregulation of DUOX1 and DUOX2 was found in lung cancer cells [175] and DUOX downregulation correlated with cell dedifferentiation [176]. In addition, DUOX expression was proposed as a predictive marker for thyroid cancer, since high DUOX expression is correlated with a reduced death risk in poorly differentiated follicular thyroid carcinoma [176].
Elevation of mitochondrial Ca2+ levels is often required to boost mitochondrial respiration and mitochondrial ATP production in cancer cells to meet the energy demand of high proliferation activity. Notably, altered activity of mitochondrial respiration, in turn, affects the production of ROS [172]. Several studies suggest enhanced ER-mitochondrial Ca2+ flux as an essential mechanism for the proliferation of cancer cells [177]. Moreover, it has been shown that oncogene-driven senescence triggers ER Ca2+ release via IP3R2, which, in turn, facilitates mitochondrial Ca2+ accumulation via MCU, a subsequent decrease in mitochondrial membrane potential and the production of ROS [178]. Interestingly, the expression of MCU was found to positively correlate with tumor size and metastasis grade in patients suffering from triple-negative breast cancer. Thus, the interplay between Ca2+, ROS and HIF-1α seems to be crucial: Downregulation of MCU blunted ROS production and expression of the HIF-1α in the respective cancer cells and hampered cancer cell motility, invasiveness and tumor growth in xenografts [179]. Furthermore, ROS-induced overexpression of the chemokine CXCL14 caused cytosolic Ca2+ elevation by provoking an IP3R Ca2+ leak, which enhanced proliferation, motility and invasiveness in breast cancer cells [180]. IP3R activity is also boosted by association with the tumor suppressor PTEN, resulting in pro-apoptotic Ca2+ release and increased mitochondrial Ca2+. In cancer cells, PTEN is frequently inactivated by redox-mediated cysteine oxidation and, consequently, it might be possible to avoid IP3R-induced Ca2+ release, mitochondrial Ca2+ accumulation and mPTP-triggered apoptosis [181]. Redox-regulated Ca2+ signals other than ER-mitochondrial contact sites may serve as an additional driving force for the proliferation of cancer cells. For instance, TRPM2, indirectly affected by ROS, is highly expressed in many cancer cells, including breast, prostate and pancreatic cancer, and inhibition of TRPM2 function was shown to hamper the viability of cancer cells and tumor growth. The main reasons discussed for TRPM2′s ability to preserve cancer cell viability include the maintenance of mitochondrial function, cellular ATP production, autophagy as well as the reduction of ROS levels and DNA damage through promotion of expression of transcription factors such as HIF-1/2α, CREB and NRF2 [182]. The activity of another type of TRP channel, the TRPA1, seems to be enhanced in various cancer types, too. It was shown that ROS-induced oxidation of TRPA1 resulted in increased Ca2+ uptake, which promoted cancer cell survival as well as cell resistance to ROS-producing therapies like carboplatin [183]. In addition, the ratio between the redox-insensitive ORAI3 and redox-sensitive ORAI1 channel, surmised to form hetero-multimers together, appears to play a crucial role in different cancer cells. For instance, an increased ORAI1/ORAI3 ratio makes prostate cancer cells especially prone to hydrogen peroxide-induced SOCE inactivation [184]. The ratio between STIM1 and STIM2 might also be a crucial survival factor for cancer cells, but additional work is needed to gain a clearer picture [185].
6. Conclusion
As discussed in this review, Ca2+ and ROS homeostasis are inseparably connected and crucially control each other in numerous mechanisms feeding into the development and enhancement of age-related diseases. Ca2+ ions affect ROS production sites such as ETC, NOX1, NOX2 and NOX5, while ROS, in turn, modulate the activity of proteins ensuring and controlling Ca2+ flux between cellular compartments. Further in-depth understanding of these processes might enable us to find new treatment strategies to ameliorate cellular dysfunction and age-related diseases resulting from imbalanced homeostasis of Ca2+ or ROS.
Declaration of competing interest
We declare no conflict of interest.
Acknowledgement
C.T.M. is currently funded by an Erwin Schroedinger Abroad Fellowship (J4205-B27). The Ristow laboratory is supported by Swiss National Science Foundation (Schweizerischer Nationalfonds, SNF 31003A_176127) and the European Union Horizon 2020 program (Ageing with Elegans, Nr. 633589). We greatly thank Kimberly Swanson for proofreading our manuscript.
References
- 1.Ezzati M., Friedman A.B., Kulkarni S.C., Murray C.J. The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. PLoS Med. 2008;5(4):e66. doi: 10.1371/journal.pmed.0050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra S. Does modern medicine increase life-expectancy: quest for the Moon Rabbit? Indian Heart J. 2016;68(1):19–27. doi: 10.1016/j.ihj.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olshansky S.J. From lifespan to healthspan. J. Am. Med. Assoc. 2018;320(13):1323–1324. doi: 10.1001/jama.2018.12621. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein M. How healthy is the healthspan concept? Geroscience. 2018;40(4):361–364. doi: 10.1007/s11357-018-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaul E., Barron J. Age-related diseases and clinical and public health implications for the 85 Years old and over population. Front Public Health. 2017;5:335. doi: 10.3389/fpubh.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhinn M., Ritschka B., Keyes W.M. Cellular senescence in development, regeneration and disease. Development. 2019;146(20) doi: 10.1242/dev.151837. [DOI] [PubMed] [Google Scholar]
- 7.Basisty N., Kale A., Jeon O.H., Kuehnemann C., Payne T., Rao C., Holtz A., Shah S., Sharma V., Ferrucci L. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1) doi: 10.1371/journal.pbio.3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: a mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohnlein K., Urban N., Guerrero-Gomez D., Steinbrenner H., Urbanek P., Priebs J., Koch P., Kaether C., Miranda-Vizuete A., Klotz L.O. A Caenorhabditis elegans ortholog of human selenium-binding protein 1 is a pro-aging factor protecting against selenite toxicity. Redox Biol. 2020;28:101323. doi: 10.1016/j.redox.2019.101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Casas P., Arias-Del-Val J., Alvarez-Illera P., Fonteriz R.I., Montero M., Alvarez J. Inhibition of sarco-endoplasmic reticulum Ca(2+) ATPase extends the lifespan in C. elegans worms. Front. Pharmacol. 2018;9:669. doi: 10.3389/fphar.2018.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luceri C., Bigagli E., Femia A.P., Caderni G., Giovannelli L., Lodovici M. Aging related changes in circulating reactive oxygen species (ROS) and protein carbonyls are indicative of liver oxidative injury. Toxicol Rep. 2018;5:141–145. doi: 10.1016/j.toxrep.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelli V., Benedetti E., Antonosante A., Catanesi M., Pitari G., Ippoliti R., Cimini A., d'Angelo M. Neuronal cells rearrangement during aging and neurodegenerative disease: metabolism, oxidative stress and organelles dynamic. Front. Mol. Neurosci. 2019;12:132. doi: 10.3389/fnmol.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan Y., Xin Y., Tian G., Zhou J., Liu X. Mitochondrial ROS-modulated mtDNA: a potential target for cardiac aging. Oxid Med Cell Longev. 2020;2020:9423593. doi: 10.1155/2020/9423593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz T.J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metabol. 2007;6(4):280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Merry T.L., Ristow M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J Physiol. 2016;594(18):5195–5207. doi: 10.1113/JP271957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer F., Ristow M. Endogenous metabolites promote stress resistance through induction of mitohormesis. EMBO Rep. 2020;21(5) doi: 10.15252/embr.202050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvo-Rodriguez M., Hernando-Perez E., Lopez-Vazquez S., Nunez J., Villalobos C., Nunez L. Remodeling of intracellular Ca(2+) homeostasis in rat hippocampal neurons aged in vitro. Int. J. Mol. Sci. 2020;21(4) doi: 10.3390/ijms21041549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashkavand Z., Sarasija S., Ryan K.C., Laboy J.T., Norman K.R. Corrupted ER-mitochondrial calcium homeostasis promotes the collapse of proteostasis. Aging Cell. 2020;19(1) doi: 10.1111/acel.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madreiter-Sokolowski C.T., Waldeck-Weiermair M., Bourguignon M.P., Villeneuve N., Gottschalk B., Klec C., Stryeck S., Radulovic S., Parichatikanond W., Frank S. Enhanced inter-compartmental Ca(2+) flux modulates mitochondrial metabolism and apoptotic threshold during aging. Redox Biol. 2019;20:458–466. doi: 10.1016/j.redox.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herraiz-Martinez A., Alvarez-Garcia J., Llach A., Molina C.E., Fernandes J., Ferrero-Gregori A., Rodriguez C., Vallmitjana A., Benitez R., Padro J.M. Ageing is associated with deterioration of calcium homeostasis in isolated human right atrial myocytes. Cardiovasc. Res. 2015;106(1):76–86. doi: 10.1093/cvr/cvv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehrer J.P., Klotz L.O. Free radicals and related reactive species as mediators of tissue injury and disease: implications for Health. Crit. Rev. Toxicol. 2015;45(9):765–798. doi: 10.3109/10408444.2015.1074159. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-mediated cellular signaling. Oxid Med Cell Longev. 2016;2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittler R. ROS are good. Trends Plant Sci. 2017;22(1):11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ristow M., Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Response. 2014;12(2):288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snezhkina A.V., Kudryavtseva A.V., Kardymon O.L., Savvateeva M.V., Melnikova N.V., Krasnov G.S., Dmitriev A.A. Vol. 2019. Oxid Med Cell Longev; 2019. ROS generation and antioxidant defense systems in normal and malignant cells; p. 6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hjelmeland A.B., Patel R.P. SOD2 acetylation and deacetylation: another tale of Jekyll and Hyde in cancer. Proc. Natl. Acad. Sci. U. S. A. 2019;116(47):23376–23378. doi: 10.1073/pnas.1916214116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kauppila J.H., Stewart J.B. Mitochondrial DNA: radically free of free-radical driven mutations. Biochim. Biophys. Acta. 2015;1847(11):1354–1361. doi: 10.1016/j.bbabio.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Pizzinat N., Copin N., Vindis C., Parini A., Cambon C. Reactive oxygen species production by monoamine oxidases in intact cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1999;359(5):428–431. doi: 10.1007/pl00005371. [DOI] [PubMed] [Google Scholar]
- 31.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45(7–8):466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fransen M., Nordgren M., Wang B., Apanasets O. Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim. Biophys. Acta. 2012;1822(9):1363–1373. doi: 10.1016/j.bbadis.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 33.McDonald K.R., Hernandez-Nichols A.L., Barnes J.W., Patel R.P. Hydrogen peroxide regulates endothelial surface N-glycoforms to control inflammatory monocyte rolling and adhesion. Redox Biol. 2020:101498. doi: 10.1016/j.redox.2020.101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoboue E.D., Sitia R., Simmen T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018;9(3):331. doi: 10.1038/s41419-017-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurindo F.R., Araujo T.L., Abrahao T.B. Nox NADPH oxidases and the endoplasmic reticulum. Antioxidants Redox Signal. 2014;20(17):2755–2775. doi: 10.1089/ars.2013.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Marcos P.J., Nobrega-Pereira S. NADPH: new oxygen for the ROS theory of aging. Oncotarget. 2016;7(32):50814–50815. doi: 10.18632/oncotarget.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iosub R., Avitabile D., Grant L., Tsaneva-Atanasova K., Kennedy H.J. Calcium-Induced calcium release during action potential firing in developing inner hair cells. Biophys. J. 2015;108(5):1003–1012. doi: 10.1016/j.bpj.2014.11.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayer M.L. A calcium-activated chloride current generates the after-depolarization of rat sensory neurones in culture. J Physiol. 1985;364:217–239. doi: 10.1113/jphysiol.1985.sp015740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787(11):1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Bootman M.D. Calcium signaling. Cold Spring Harb Perspect Biol. 2012;4(7):a011171. doi: 10.1101/cshperspect.a011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park Y.J., Yoo S.A., Kim M., Kim W.U. The role of calcium-calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front. Immunol. 2020;11:195. doi: 10.3389/fimmu.2020.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brzozowski J.S., Skelding K.A. The multi-functional calcium/calmodulin stimulated protein kinase (CaMK) family: emerging targets for anti-cancer therapeutic intervention. Pharmaceuticals. 2019;12(1) doi: 10.3390/ph12010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Marchi E., Bonora M., Giorgi C., Pinton P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium. 2014;56(1):1–13. doi: 10.1016/j.ceca.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierro C., Sneyers F., Bultynck G., Roderick H.L. ER Ca(2+) release and store-operated Ca(2+) entry - partners in crime or independent actors in oncogenic transformation? Cell Calcium. 2019;82:102061. doi: 10.1016/j.ceca.2019.102061. [DOI] [PubMed] [Google Scholar]
- 45.Raffaello A., Mammucari C., Gherardi G., Rizzuto R. Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem. Sci. 2016;41(12):1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samtleben S., Jaepel J., Fecher C., Andreska T., Rehberg M., Blum R. Direct imaging of ER calcium with targeted-esterase induced dye loading (TED) JoVE. 2013;(75) doi: 10.3791/50317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johny J.P., Plank M.J., David T. Importance of altered levels of SERCA, IP3R, and RyR in vascular smooth muscle cell. Biophys. J. 2017;112(2):265–287. doi: 10.1016/j.bpj.2016.11.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patergnani S., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Giorgi C., Marchi S., Missiroli S., Poletti F. Calcium signaling around mitochondria associated membranes (MAMs) Cell Commun. Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madreiter-Sokolowski C.T., Ramadani-Muja J., Ziomek G., Burgstaller S., Bischof H., Koshenov Z., Gottschalk B., Malli R., Graier W.F. Tracking intra- and inter-organelle signaling of mitochondria. FEBS J. 2019;286(22):4378–4401. doi: 10.1111/febs.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342(6164):1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomar D., Dong Z., Shanmughapriya S., Koch D.A., Thomas T., Hoffman N.E., Timbalia S.A., Goldman S.J., Breves S.L., Corbally D.P. MCUR1 is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Rep. 2016;15(8):1673–1685. doi: 10.1016/j.celrep.2016.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raffaello A., De Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabo I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32(17):2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patron M., Granatiero V., Espino J., Rizzuto R., De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26(1):179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demaurex N., Poburko D., Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim. Biophys. Acta. 2009;1787(11):1383–1394. doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 55.Samanta K., Mirams G.R., Parekh A.B. Sequential forward and reverse transport of the Na(+) Ca(2+) exchanger generates Ca(2+) oscillations within mitochondria. Nat. Commun. 2018;9(1):156. doi: 10.1038/s41467-017-02638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M.X., Hwang P.M. Structure and function of cardiac troponin C (TNNC1): implications for heart failure, cardiomyopathies, and troponin modulating drugs. Gene. 2015;571(2):153–166. doi: 10.1016/j.gene.2015.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21(1):13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 58.Huang G., Docampo R. The mitochondrial calcium uniporter interacts with subunit c of the ATP synthase of trypanosomes and humans. mBio. 2020;11(2) doi: 10.1128/mBio.00268-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hubbard M.J., McHugh N.J. Mitochondrial ATP synthase F1-beta-subunit is a calcium-binding protein. FEBS Lett. 1996;391(3):323–329. doi: 10.1016/0014-5793(96)00767-3. [DOI] [PubMed] [Google Scholar]
- 60.Beis I., Newsholme E.A. Effects of calcium ions on adenine nucleotide translocase from cardiac muscle. J. Mol. Cell. Cardiol. 1976;8(11):863–876. doi: 10.1016/0022-2828(76)90069-9. [DOI] [PubMed] [Google Scholar]
- 61.Lomax R.B., Robertson W.R. Mitochondrial alpha-glycerol phosphate dehydrogenase activity in IIA fibres of the rat lateral gastrocnemius muscle; the effect of Ca2+ and ATP. Histochem. J. 1990;22(2):119–124. doi: 10.1007/BF01885791. [DOI] [PubMed] [Google Scholar]
- 62.Gellerich F.N., Gizatullina Z., Trumbeckaite S., Nguyen H.P., Pallas T., Arandarcikaite O., Vielhaber S., Seppet E., Striggow F. The regulation of OXPHOS by extramitochondrial calcium. Biochim. Biophys. Acta. 2010;1797(6–7):1018–1027. doi: 10.1016/j.bbabio.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Aon M.A., Cortassa S., O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797(6–7):865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cadenas E., Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem. J. 1980;188(1):31–37. doi: 10.1042/bj1880031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287(4):C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 66.Mallilankaraman K., Doonan P., Cardenas C., Chandramoorthy H.C., Muller M., Miller R., Hoffman N.E., Gandhirajan R.K., Molgo J., Birnbaum M.J. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151(3):630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Kasus Nissim T., Zhang X., Elazar A., Roy S., Stolwijk J.A., Zhou Y., Motiani R.K., Gueguinou M., Hempel N., Hershfinkel M. Mitochondria control store-operated Ca(2+) entry through Na(+) and redox signals. EMBO J. 2017;36(6):797–815. doi: 10.15252/embj.201592481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Violi F., Carnevale R., Loffredo L., Pignatelli P., Gallin J.I. NADPH oxidase-2 and atherothrombosis: insight from chronic granulomatous disease. Arterioscler. Thromb. Vasc. Biol. 2017;37(2):218–225. doi: 10.1161/ATVBAHA.116.308351. [DOI] [PubMed] [Google Scholar]
- 70.Diaz-Vegas A., Campos C.A., Contreras-Ferrat A., Casas M., Buvinic S., Jaimovich E., Espinosa A. ROS production via P2Y1-PKC-NOX2 is triggered by extracellular ATP after electrical stimulation of skeletal muscle cells. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0129882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brechard S., Plancon S., Tschirhart E.J. New insights into the regulation of neutrophil NADPH oxidase activity in the phagosome: a focus on the role of lipid and Ca(2+) signaling. Antioxidants Redox Signal. 2013;18(6):661–676. doi: 10.1089/ars.2012.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Chemaly A., Okochi Y., Sasaki M., Arnaudeau S., Okamura Y., Demaurex N. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J. Exp. Med. 2010;207(1):129–139. doi: 10.1084/jem.20091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Makhezer N., Ben Khemis M., Liu D., Khichane Y., Marzaioli V., Tlili A., Mojallali M., Pintard C., Letteron P., Hurtado-Nedelec M. NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol. 2019;12(1):117–131. doi: 10.1038/s41385-018-0086-4. [DOI] [PubMed] [Google Scholar]
- 74.Vuong P.T., Malik A.B., Nagpala P.G., Lum H. Protein kinase C beta modulates thrombin-induced Ca2+ signaling and endothelial permeability increase. J. Cell. Physiol. 1998;175(3):379–387. doi: 10.1002/(SICI)1097-4652(199806)175:3<379::AID-JCP16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 75.Streeter J., Schickling B.M., Jiang S., Stanic B., Thiel W.H., Gakhar L., Houtman J.C., Miller F.J., Jr. Phosphorylation of Nox1 regulates association with NoxA 1 activation domain. Circ. Res. 2014;115(11):911–918. doi: 10.1161/CIRCRESAHA.115.304267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montezano A.C., De Lucca Camargo L., Persson P., Rios F.J., Harvey A.P., Anagnostopoulou A., Palacios R., Gandara A.C.P., Alves-Lopes R., Neves K.B. NADPH oxidase 5 is a pro-contractile nox isoform and a point of cross-talk for calcium and redox signaling-implications in vascular function. J Am Heart Assoc. 2018;7(12) doi: 10.1161/JAHA.118.009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen F., Yu Y., Haigh S., Johnson J., Lucas R., Stepp D.W., Fulton D.J. Regulation of NADPH oxidase 5 by protein kinase C isoforms. PloS One. 2014;9(2) doi: 10.1371/journal.pone.0088405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrushanko I.Y., Lobachev V.M., Kononikhin A.S., Makarov A.A., Devred F., Kovacic H., Kubatiev A.A., Tsvetkov P.O. Oxidation of capital ES, cyrillicsmall a, cyrillic 2+-binding domain of NADPH oxidase 5 (NOX5): toward understanding the mechanism of inactivation of NOX5 by ROS. PloS One. 2016;11(7) doi: 10.1371/journal.pone.0158726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ameziane-El-Hassani R., Morand S., Boucher J.L., Frapart Y.M., Apostolou D., Agnandji D., Gnidehou S., Ohayon R., Noel-Hudson M.S., Francon J. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J. Biol. Chem. 2005;280(34):30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 80.Carvalho D.P., Dupuy C. Role of the NADPH oxidases DUOX and NOX4 in thyroid oxidative stress. Eur Thyroid J. 2013;2(3):160–167. doi: 10.1159/000354745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Razzell W., Evans I.R., Martin P., Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 2013;23(5):424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nordzieke D.E., Medrano-Fernandez I. The plasma membrane: a platform for intra- and intercellular redox signaling. Antioxidants. 2018;7(11) doi: 10.3390/antiox7110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Numaga-Tomita T., Oda S., Nishiyama K., Tanaka T., Nishimura A., Nishida M. TRPC channels in exercise-mimetic therapy. Pflügers Archiv. 2019;471(3):507–517. doi: 10.1007/s00424-018-2211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perraud A.L., Takanishi C.L., Shen B., Kang S., Smith M.K., Schmitz C., Knowles H.M., Ferraris D., Li W., Zhang J. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005;280(7):6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi N., Mori Y. TRP channels as sensors and signal integrators of redox status changes. Front. Pharmacol. 2011;2:58. doi: 10.3389/fphar.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto S., Shimizu S. Significance of TRP channels in oxidative stress. Eur. J. Pharmacol. 2016;793:109–111. doi: 10.1016/j.ejphar.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 87.Hawkins B.J., Irrinki K.M., Mallilankaraman K., Lien Y.C., Wang Y., Bhanumathy C.D., Subbiah R., Ritchie M.F., Soboloff J., Baba Y. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 2010;190(3):391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prins D., Groenendyk J., Touret N., Michalak M. Modulation of STIM1 and capacitative Ca2+ entry by the endoplasmic reticulum luminal oxidoreductase ERp57. EMBO Rep. 2011;12(11):1182–1188. doi: 10.1038/embor.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu W., Wang J., Peng G., Shimoda L.A., Sylvester J.T. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297(1):L17–L25. doi: 10.1152/ajplung.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bogeski I., Kummerow C., Al-Ansary D., Schwarz E.C., Koehler R., Kozai D., Takahashi N., Peinelt C., Griesemer D., Bozem M. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci. Signal. 2010;3(115):ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
- 91.Frisch J., Angenendt A., Hoth M., Prates Roma L., Lis A. STIM-orai channels and reactive oxygen species in the tumor microenvironment. Cancers. 2019;11(4) doi: 10.3390/cancers11040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuster G.M., Lancel S., Zhang J., Communal C., Trucillo M.P., Lim C.C., Pfister O., Weinberg E.O., Cohen R.A., Liao R. Redox-mediated reciprocal regulation of SERCA and Na+-Ca2+ exchanger contributes to sarcoplasmic reticulum Ca2+ depletion in cardiac myocytes. Free Radic. Biol. Med. 2010;48(9):1182–1187. doi: 10.1016/j.freeradbiomed.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaidi A. Plasma membrane Ca-ATPases: targets of oxidative stress in brain aging and neurodegeneration. World J. Biol. Chem. 2010;1(9):271–280. doi: 10.4331/wjbc.v1.i9.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilson C., Munoz-Palma E., Henriquez D.R., Palmisano I., Nunez M.T., Di Giovanni S., Gonzalez-Billault C. A feed-forward mechanism involving the NOX complex and RyR-mediated Ca2+ release during axonal specification. J. Neurosci. 2016;36(43):11107–11119. doi: 10.1523/JNEUROSCI.1455-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vais H., Siebert A.P., Ma Z., Fernandez-Mongil M., Foskett J.K., Mak D.O. Redox-regulated heterogeneous thresholds for ligand recruitment among InsP3R Ca2+-release channels. Biophys. J. 2010;99(2):407–416. doi: 10.1016/j.bpj.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ivanova H., Vervliet T., Missiaen L., Parys J.B., De Smedt H., Bultynck G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys. Acta. 2014;1843(10):2164–2183. doi: 10.1016/j.bbamcr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Khan S.A., Rossi A.M., Riley A.M., Potter B.V., Taylor C.W. Subtype-selective regulation of IP(3) receptors by thimerosal via cysteine residues within the IP(3)-binding core and suppressor domain. Biochem. J. 2013;451(2):177–184. doi: 10.1042/BJ20121600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lock J.T., Sinkins W.G., Schilling W.P. Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011;300(2):H493–H506. doi: 10.1152/ajpheart.01073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lock J.T., Sinkins W.G., Schilling W.P. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J Physiol. 2012;590(15):3431–3447. doi: 10.1113/jphysiol.2012.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bansaghi S., Golenar T., Madesh M., Csordas G., RamachandraRao S., Sharma K., Yule D.I., Joseph S.K., Hajnoczky G. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem. 2014;289(12):8170–8181. doi: 10.1074/jbc.M113.504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120(1):85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 102.Joseph S.K., Young M.P., Alzayady K., Yule D.I., Ali M., Booth D.M., Hajnoczky G. Redox regulation of type-I inositol trisphosphate receptors in intact mammalian cells. J. Biol. Chem. 2018;293(45):17464–17476. doi: 10.1074/jbc.RA118.005624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vais H., Wang M., Mallilankaraman K., Payne R., McKennan C., Lock J.T., Spruce L.A., Fiest C., Chan M.Y., Parker I. ER-luminal [Ca(2+)] regulation of InsP3 receptor gating mediated by an ER-luminal peripheral Ca(2+)-binding protein. Elife. 2020;9 doi: 10.7554/eLife.53531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nikolaienko R., Bovo E., Zima A.V. Redox dependent modifications of ryanodine receptor: basic mechanisms and implications in heart diseases. Front. Physiol. 2018;9:1775. doi: 10.3389/fphys.2018.01775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dulhunty A.F., Hewawasam R., Liu D., Casarotto M.G., Board P.G. Regulation of the cardiac muscle ryanodine receptor by glutathione transferases. Drug Metab. Rev. 2011;43(2):236–252. doi: 10.3109/03602532.2010.549134. [DOI] [PubMed] [Google Scholar]
- 106.Zima A.V., Mazurek S.R. Functional impact of ryanodine receptor oxidation on intracellular calcium regulation in the heart. Rev. Physiol. Biochem. Pharmacol. 2016;171:39–62. doi: 10.1007/112_2016_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balshaw D.M., Xu L., Yamaguchi N., Pasek D.A., Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J. Biol. Chem. 2001;276(23):20144–20153. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 108.Witherspoon J.W., Meilleur K.G. Review of RyR1 pathway and associated pathomechanisms. Acta Neuropathol Commun. 2016;4(1):121. doi: 10.1186/s40478-016-0392-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun Q.A., Hess D.T., Nogueira L., Yong S., Bowles D.E., Eu J., Laurita K.R., Meissner G., Stamler J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. U. S. A. 2011;108(38):16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharov V.S., Dremina E.S., Galeva N.A., Williams T.D., Schoneich C. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochem. J. 2006;394(Pt 3):605–615. doi: 10.1042/BJ20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barnes K.A., Samson S.E., Grover A.K. Sarco/endoplasmic reticulum Ca2+-pump isoform SERCA3a is more resistant to superoxide damage than SERCA2b. Mol. Cell. Biochem. 2000;203(1–2):17–21. doi: 10.1023/a:1007053802481. [DOI] [PubMed] [Google Scholar]
- 112.Xu K.Y., Zweier J.L., Becker L.C. Hydroxyl radical inhibits sarcoplasmic reticulum Ca(2+)-ATPase function by direct attack on the ATP binding site. Circ. Res. 1997;80(1):76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- 113.Gutierrez-Martin Y., Martin-Romero F.J., Inesta-Vaquera F.A., Gutierrez-Merino C., Henao F. Modulation of sarcoplasmic reticulum Ca(2+)-ATPase by chronic and acute exposure to peroxynitrite. Eur. J. Biochem. 2004;271(13):2647–2657. doi: 10.1111/j.1432-1033.2004.04193.x. [DOI] [PubMed] [Google Scholar]
- 114.Adachi T., Weisbrod R.M., Pimentel D.R., Ying J., Sharov V.S., Schoneich C., Cohen R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10(11):1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 115.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51(7):1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ago T., Kuroda J., Pain J., Fu C., Li H., Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2010;106(7):1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lone A., Harris R.A., Singh O., Betts D.H., Cumming R.C. p66Shc activation promotes increased oxidative phosphorylation and renders CNS cells more vulnerable to amyloid beta toxicity. Sci. Rep. 2018;8(1):17081. doi: 10.1038/s41598-018-35114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pinton P., Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle. 2008;7(3):304–308. doi: 10.4161/cc.7.3.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P.P., Lanfrancone L., Pelicci P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402(6759):309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Y., Wang Z., Feng D., Zhao H., Lin M., Hu Y., Zhang N., Lv L., Gao Z., Zhai X. p66Shc contributes to liver fibrosis through the regulation of mitochondrial reactive oxygen species. Theranostics. 2019;9(5):1510–1522. doi: 10.7150/thno.29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pandolfi S., Bonafe M., Di Tella L., Tiberi L., Salvioli S., Monti D., Sorbi S., Franceschi C. p66(shc) is highly expressed in fibroblasts from centenarians. Mech. Ageing Dev. 2005;126(8):839–844. doi: 10.1016/j.mad.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 122.Di Lisa F., Giorgio M., Ferdinandy P., Schulz R. New aspects of p66Shc in ischaemia reperfusion injury and other cardiovascular diseases. Br. J. Pharmacol. 2017;174(12):1690–1703. doi: 10.1111/bph.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dong Z., Shanmughapriya S., Tomar D., Siddiqui N., Lynch S., Nemani N., Breves S.L., Zhang X., Tripathi A., Palaniappan P. Mitochondrial Ca(2+) uniporter is a mitochondrial luminal redox sensor that augments MCU channel activity. Mol Cell. 2017;65(6):1014–1028 e7. doi: 10.1016/j.molcel.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joiner M.L., Koval O.M., Li J., He B.J., Allamargot C., Gao Z., Luczak E.D., Hall D.D., Fink B.D., Chen B. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491(7423):269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fieni F., Johnson D.E., Hudmon A., Kirichok Y. Mitochondrial Ca2+ uniporter and CaMKII in heart. Nature. 2014;513(7519):E1–E2. doi: 10.1038/nature13626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dietl A., Maack C. Targeting mitochondrial calcium handling and reactive oxygen species in heart failure. Curr. Heart Fail. Rep. 2017;14(4):338–349. doi: 10.1007/s11897-017-0347-7. [DOI] [PubMed] [Google Scholar]
- 127.Peoples J.N., Saraf A., Ghazal N., Pham T.T., Kwong J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019;51(12):1–13. doi: 10.1038/s12276-019-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu E.P.K., Reinhold J., Yu H., Starks L., Uryga A.K., Foote K., Finigan A., Figg N., Pung Y.F., Logan A. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler. Thromb. Vasc. Biol. 2017;37(12):2322–2332. doi: 10.1161/ATVBAHA.117.310042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou T., Chuang C.C., Zuo L. Molecular characterization of reactive oxygen species in myocardial ischemia-reperfusion injury. BioMed Res. Int. 2015;2015:864946. doi: 10.1155/2015/864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Touyz R.M., Anagnostopoulou A., Rios F., Montezano A.C., Camargo L.L. NOX5: molecular biology and pathophysiology. Exp. Physiol. 2019;104(5):605–616. doi: 10.1113/EP086204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pi X., Xie L., Portbury A.L., Kumar S., Lockyer P., Li X., Patterson C. NADPH oxidase-generated reactive oxygen species are required for stromal cell-derived factor-1alpha-stimulated angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34(9):2023–2032. doi: 10.1161/ATVBAHA.114.303733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gole H.K., Tharp D.L., Bowles D.K. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5) PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hahn N.E., Meischl C., Kawahara T., Musters R.J., Verhoef V.M., van der Velden J., Vonk A.B., Paulus W.J., van Rossum A.C., Niessen H.W. NOX5 expression is increased in intramyocardial blood vessels and cardiomyocytes after acute myocardial infarction in humans. Am. J. Pathol. 2012;180(6):2222–2229. doi: 10.1016/j.ajpath.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 134.Kleikers P.W., Dao V.T., Gob E., Hooijmans C., Debets J., van Essen H., Kleinschnitz C., Schmidt H.H. SFRR-E Young Investigator AwardeeNOXing out stroke: identification of NOX4 and 5as targets in blood-brain-barrier stabilisation and neuroprotection. Free Radic. Biol. Med. 2014;75(Suppl 1):S16. doi: 10.1016/j.freeradbiomed.2014.10.593. [DOI] [PubMed] [Google Scholar]
- 135.Holterman C.E., Boisvert N.C., Thibodeau J.F., Kamto E., Novakovic M., Abd-Elrahman K.S., Ferguson S.S.G., Kennedy C.R.J. Podocyte NADPH oxidase 5 promotes renal inflammation regulated by the toll-like receptor pathway. Antioxidants Redox Signal. 2019;30(15):1817–1830. doi: 10.1089/ars.2017.7402. [DOI] [PubMed] [Google Scholar]
- 136.Khatri J.J., Johnson C., Magid R., Lessner S.M., Laude K.M., Dikalov S.I., Harrison D.G., Sung H.J., Rong Y., Galis Z.S. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation. 2004;109(4):520–525. doi: 10.1161/01.CIR.0000109698.70638.2B. [DOI] [PubMed] [Google Scholar]
- 137.Weissmann N., Sydykov A., Kalwa H., Storch U., Fuchs B., Mederos y Schnitzler M., Brandes R.P., Grimminger F., Meissner M., Freichel M. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gandhirajan R.K., Meng S., Chandramoorthy H.C., Mallilankaraman K., Mancarella S., Gao H., Razmpour R., Yang X.F., Houser S.R., Chen J. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Invest. 2013;123(2):887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gimenez M., Schickling B.M., Lopes L.R., Miller F.J., Jr. Nox1 in cardiovascular diseases: regulation and pathophysiology. Clin. Sci. (Lond.) 2016;130(3):151–165. doi: 10.1042/CS20150404. [DOI] [PubMed] [Google Scholar]
- 140.Kaludercic N., Di Lisa F. Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front Cardiovasc Med. 2020;7:12. doi: 10.3389/fcvm.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gorin Y., Block K. Nox as a target for diabetic complications. Clin. Sci. (Lond.) 2013;125(8):361–382. doi: 10.1042/CS20130065. [DOI] [PubMed] [Google Scholar]
- 142.Gray S.P., Di Marco E., Okabe J., Szyndralewiez C., Heitz F., Montezano A.C., de Haan J.B., Koulis C., El-Osta A., Andrews K.L. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation. 2013;127(18):1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- 143.Rojas M., Zhang W., Xu Z., Lemtalsi T., Chandler P., Toque H.A., Caldwell R.W., Caldwell R.B. Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0084357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jha J.C., Banal C., Okabe J., Gray S.P., Hettige T., Chow B.S.M., Thallas-Bonke V., De Vos L., Holterman C.E., Coughlan M.T. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66(10):2691–2703. doi: 10.2337/db16-1585. [DOI] [PubMed] [Google Scholar]
- 145.Belevych A.E., Terentyev D., Viatchenko-Karpinski S., Terentyeva R., Sridhar A., Nishijima Y., Wilson L.D., Cardounel A.J., Laurita K.R., Carnes C.A. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc. Res. 2009;84(3):387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walweel K., Molenaar P., Imtiaz M.S., Denniss A., Dos Remedios C., van Helden D.F., Dulhunty A.F., Laver D.R., Beard N.A. Ryanodine receptor modification and regulation by intracellular Ca(2+) and Mg(2+) in healthy and failing human hearts. J. Mol. Cell. Cardiol. 2017;104:53–62. doi: 10.1016/j.yjmcc.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 147.Mochizuki M., Yano M., Oda T., Tateishi H., Kobayashi S., Yamamoto T., Ikeda Y., Ohkusa T., Ikemoto N., Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 2007;49(16):1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 148.Hiroi T., Wajima T., Negoro T., Ishii M., Nakano Y., Kiuchi Y., Mori Y., Shimizu S. Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 2013;97(2):271–281. doi: 10.1093/cvr/cvs332. [DOI] [PubMed] [Google Scholar]
- 149.Conklin D.J., Guo Y., Nystoriak M.A., Jagatheesan G., Obal D., Kilfoil P.J., Hoetker J.D., Guo L., Bolli R., Bhatnagar A. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2019;316(4):H889–H899. doi: 10.1152/ajpheart.00106.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tong X., Ying J., Pimentel D.R., Trucillo M., Adachi T., Cohen R.A. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J. Mol. Cell. Cardiol. 2008;44(2):361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Evangelista A.M., Thompson M.D., Bolotina V.M., Tong X., Cohen R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic. Biol. Med. 2012;53(12):2327–2334. doi: 10.1016/j.freeradbiomed.2012.10.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mialet-Perez J., Parini A. Cardiac monoamine oxidases: at the heart of mitochondrial dysfunction. Cell Death Dis. 2020;11(1):54. doi: 10.1038/s41419-020-2251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Togashi K., Hara Y., Tominaga T., Higashi T., Konishi Y., Mori Y., Tominaga M. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25(9):1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Georgiadou E., Haythorne E., Dickerson M.T., Lopez-Noriega L., Pullen T.J., da Silva Xavier G., Davis S.P.X., Martinez-Sanchez A., Semplici F., Rizzuto R. Diabetologia; 2020. The Pore-Forming Subunit MCU of the Mitochondrial Ca(2+) Uniporter Is Required for Normal Glucose-Stimulated Insulin Secretion in Vitro and in Vivo in Mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8) doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Deheshi S., Dabiri B., Fan S., Tsang M., Rintoul G.L. Changes in mitochondrial morphology induced by calcium or rotenone in primary astrocytes occur predominantly through ros-mediated remodeling. J. Neurochem. 2015;133(5):684–699. doi: 10.1111/jnc.13090. [DOI] [PubMed] [Google Scholar]
- 157.Muller M., Cheung K.H., Foskett J.K. Enhanced ROS generation mediated by Alzheimer's disease presenilin regulation of InsP3R Ca2+ signaling. Antioxidants Redox Signal. 2011;14(7):1225–1235. doi: 10.1089/ars.2010.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K., Brann D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017;12(1):7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Geng L., Fan L.M., Liu F., Smith C., Li J. Nox2 dependent redox-regulation of microglial response to amyloid-beta stimulation and microgliosis in aging. Sci. Rep. 2020;10(1):1582. doi: 10.1038/s41598-020-58422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Loffredo L., Ettorre E., Zicari A.M., Inghilleri M., Nocella C., Perri L., Spalice A., Fossati C., De Lucia M.C., Pigozzi F. Oxidative stress and gut-derived lipopolysaccharides in neurodegenerative disease: role of NOX2. Oxid Med Cell Longev. 2020;2020:8630275. doi: 10.1155/2020/8630275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cali T., Ottolini D., Brini M. Calcium and endoplasmic reticulum-mitochondria tethering in neurodegeneration. DNA Cell Biol. 2013;32(4):140–146. doi: 10.1089/dna.2013.2011. [DOI] [PubMed] [Google Scholar]
- 162.Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31(9):454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qaisar R., Bhaskaran S., Ranjit R., Sataranatarajan K., Premkumar P., Huseman K., Van Remmen H. Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol. 2019;20:68–74. doi: 10.1016/j.redox.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Garrosa J., Paredes I., Marambaud P., Lopez M.G., Cano-Abad M.F. Molecular and pharmacological modulation of CALHM1 promote neuroprotection against oxygen and glucose deprivation in a model of hippocampal slices. Cells. 2020;9(3) doi: 10.3390/cells9030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moreno-Ortega A.J., Ruiz-Nuno A., Garcia A.G., Cano-Abad M.F. Mitochondria sense with different kinetics the calcium entering into HeLa cells through calcium channels CALHM1 and mutated P86L-CALHM1. Biochem. Biophys. Res. Commun. 2010;391(1):722–726. doi: 10.1016/j.bbrc.2009.11.127. [DOI] [PubMed] [Google Scholar]
- 166.Moreno-Ortega A.J., Buendia I., Mouhid L., Egea J., Lucea S., Ruiz-Nuno A., Lopez M.G., Cano-Abad M.F. CALHM1 and its polymorphism P86L differentially control Ca(2)(+)homeostasis, mitogen-activated protein kinase signaling, and cell vulnerability upon exposure to amyloid beta. Aging Cell. 2015;14(6):1094–1102. doi: 10.1111/acel.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rubio-Moscardo F., Seto-Salvia N., Pera M., Bosch-Morato M., Plata C., Belbin O., Gene G., Dols-Icardo O., Ingelsson M., Helisalmi S. Rare variants in calcium homeostasis modulator 1 (CALHM1) found in early onset Alzheimer's disease patients alter calcium homeostasis. PloS One. 2013;8(9) doi: 10.1371/journal.pone.0074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sita G., Hrelia P., Graziosi A., Ravegnini G., Morroni F. TRPM2 in the brain: role in health and disease. Cells. 2018;7(7) doi: 10.3390/cells7070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Belrose J.C., Xie Y.F., Gierszewski L.J., MacDonald J.F., Jackson M.F. Loss of glutathione homeostasis associated with neuronal senescence facilitates TRPM2 channel activation in cultured hippocampal pyramidal neurons. Mol. Brain. 2012;5:11. doi: 10.1186/1756-6606-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Knowles H., Li Y., Perraud A.L. The TRPM2 ion channel, an oxidative stress and metabolic sensor regulating innate immunity and inflammation. Immunol. Res. 2013;55(1–3):241–248. doi: 10.1007/s12026-012-8373-8. [DOI] [PubMed] [Google Scholar]
- 171.Mori Y., Takahashi N., Polat O.K., Kurokawa T., Takeda N., Inoue M. Redox-sensitive transient receptor potential channels in oxygen sensing and adaptation. Pflügers Archiv. 2016;468(1):85–97. doi: 10.1007/s00424-015-1716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Delierneux C., Kouba S., Shanmughapriya S., Potier-Cartereau M., Trebak M., Hempel N. Mitochondrial calcium regulation of redox signaling in cancer. Cells. 2020;9(2) doi: 10.3390/cells9020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weyemi U., Redon C.E., Parekh P.R., Dupuy C., Bonner W.M. NADPH Oxidases NOXs and DUOXs as putative targets for cancer therapy. Anticancer Agents Med Chem. 2013;13(3):502–514. [PMC free article] [PubMed] [Google Scholar]
- 174.Corzo C.A., Cotter M.J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T.V., McCaffrey J.C., Gabrilovich D.I. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 2009;182(9):5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Luxen S., Belinsky S.A., Knaus U.G. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Canc. Res. 2008;68(4):1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 176.Pulcrano M., Boukheris H., Talbot M., Caillou B., Dupuy C., Virion A., De Vathaire F., Schlumberger M. Poorly differentiated follicular thyroid carcinoma: prognostic factors and relevance of histological classification. Thyroid. 2007;17(7):639–646. doi: 10.1089/thy.2007.0029. [DOI] [PubMed] [Google Scholar]
- 177.Cardenas C., Muller M., McNeal A., Lovy A., Jana F., Bustos G., Urra F., Smith N., Molgo J., Diehl J.A. Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep. 2016;14(10):2313–2324. doi: 10.1016/j.celrep.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wiel C., Lallet-Daher H., Gitenay D., Gras B., Le Calve B., Augert A., Ferrand M., Prevarskaya N., Simonnet H., Vindrieux D. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat. Commun. 2014;5:3792. doi: 10.1038/ncomms4792. [DOI] [PubMed] [Google Scholar]
- 179.Tosatto A., Sommaggio R., Kummerow C., Bentham R.B., Blacker T.S., Berecz T., Duchen M.R., Rosato A., Bogeski I., Szabadkai G. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol. Med. 2016;8(5):569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pelicano H., Lu W., Zhou Y., Zhang W., Chen Z., Hu Y., Huang P. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Canc. Res. 2009;69(6):2375–2383. doi: 10.1158/0008-5472.CAN-08-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Szado T., Vanderheyden V., Parys J.B., De Smedt H., Rietdorf K., Kotelevets L., Chastre E., Khan F., Landegren U., Soderberg O. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105(7):2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miller B.A. TRPM2 in cancer. Cell Calcium. 2019;80:8–17. doi: 10.1016/j.ceca.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reczek C.R., Chandel N.S. ROS promotes cancer cell survival through calcium signaling. Canc. Cell. 2018;33(6):949–951. doi: 10.1016/j.ccell.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 184.Holzmann C., Kilch T., Kappel S., Dorr K., Jung V., Stockle M., Bogeski I., Peinelt C. Differential redox regulation of Ca(2)(+) signaling and viability in normal and malignant prostate cells. Biophys. J. 2015;109(7):1410–1419. doi: 10.1016/j.bpj.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aytes A., Mollevi D.G., Martinez-Iniesta M., Nadal M., Vidal A., Morales A., Salazar R., Capella G., Villanueva A. Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol. Carcinog. 2012;51(9):746–753. doi: 10.1002/mc.20843. [DOI] [PubMed] [Google Scholar]