Abstract
Emotion processing is believed to dominate over other brain functions during adolescence, including inhibitory control. However, few studies have examined the neural underpinnings of affective states during cognitive control. Here, we characterized the brain in an affective state by cross-sectionally assessing age-related changes in amygdala background connectivity during an affective inhibitory control task. Participants completed an antisaccade (AS) fMRI task while affective auditory stimuli were presented, and a 5-minute resting state scan. Results showed that while adolescents reported similar arousal levels across emotional conditions, adults perceived negative sounds to be more “arousing” and performed better than adolescents in negative trials. Amygdala background connectivity showed age-related increases with brain regions related to attention and executive control, which were not evident during resting state. Together, results suggest that amygdala connectivity within an affective context is fairly low in mid-adolescence but much stronger in adulthood, supporting age-related improvements in inhibitory control within an affective state. These findings suggest limitations during adolescence in differentiating between the arousing effects of various emotions, potentially undermining the ability to optimally engage inhibitory control. Furthermore, the age-related fMRI findings suggest that low amygdala connectivity to brain areas involved in executive control may underlie these limited abilities during adolescence.
Keywords: Affect, Cognitive control, fMRI, Antisaccade, Background connectivity, Adolescence
1. Introduction
Adolescence is a period of heightened emotional reactivity (Guyer et al., 2016) believed to result from still-maturing neuroaffective (Crone and Dahl, 2012; Guyer et al., 2016; Pfeifer and Blakemore, 2012) and neurocognitive systems (Luna et al., 2015) that can undermine decision-making (Kann, 2016; Morris et al., 2018). Adolescence is also a period of heightened vulnerability to the emergence of mood disorders (Chambers et al., 2003; Paus et al., 2008; Pfeifer et al., 2011; Substance Abuse and Mental Health Services Administration, 2009), underscoring the importance of understanding the neurobiological basis of emotional processing during this time (Kann, 2016; Morris et al., 2018). Past studies have shown that the presence of emotional stimuli is associated with worse cognitive control performance in healthy individuals and in those with mood-related disorders (Arnsten and Rubia, 2012; Hare et al., 2008; Inzlicht et al., 2015; Yuan et al., 2012). Moreover, studies characterizing emotion regulation, a skill which engages cognitive systems to respond to emotional experiences, have found that this ability to regulate emotion is still maturing through adolescence and into early adulthood (Ahmed et al., 2015; Casey et al., 2017; Pitskel et al., 2011). Together, these findings indicate important changes to the emotional and cognitive systems during adolescence that position this period of development within a particularly vulnerable state of affective predominance over behavior.
Initial task fMRI studies have provided compelling evidence for greater adolescent brain reactivity to emotional stimuli (Hare et al., 2008; Rosen et al., 2018; Silvers et al., 2012). In particular, the amygdala, which is a core system for emotional processing (Mahler and Berridge, 2012; Pessoa, 2010; Todd et al., 2011), has been found to exhibit increased activity in adolescents compared to adults during the conscious or unconscious perception of emotional faces and pictures (Guyer et al., 2008; Killgore and Yurgelun-Todd, 2007; Vink et al., 2014). Regarding resting state connectivity, some past research has also shown age-related increases in amygdala-prefrontal connectivity from early childhood to young adulthood (Gabard-Durnam et al., 2014). In contrast, recent work from our group using large resting state samples for analysis and replication indicated that functional and structural amygdala/prefrontal resting state connectivity decreases during adolescent development, particularly through late childhood and early adolescence, suggesting a dampening of the amygdala’s influence on cognitive systems (Jalbrzikowski et al., 2017).
These studies have been a critical first step in providing evidence for heightened adolescent emotional reactivity in the brain. However, in real life situations, emotions often manifest as a state, beyond reactivity to discrete events, which may have a significant impact on cognition. For example, adolescents are known to persist in heightened affective states over an extended period of time and mood disorders that emerge in adolescence, such as depression, reflect a persistent state of heightened affective arousal as well as compromised cognitive control (Avenevoli et al., 2015; Costello et al., 2011; Rock et al., 2014; Schwartz et al., 2019). Based on this evidence, a critical next step in understanding the adolescent phenotype of emotional reactivity is to examine neurobiological underpinnings of cognitive performance in a persistent affective state, which is not yet well understood. Sustained emotional states operating beyond task-related effects may have unique influence on inhibitory control (Harlé et al., 2013), and because context can establish a response state that informs effects of emotion beyond the trial level, this may be more consistent with real life situations. Here, we examine age-related changes from mid-adolescence to adulthood in the influence of affective state on amygdala connectivity while engaging cognitive control. To this end, we applied background connectivity, which removes estimated trial-evoked neural activity to reveal sustained blood oxygen level-dependent (BOLD) responses that reflect brain systems underlying a state (Duncan et al., 2018; Frank et al., 2019; Murty et al., 2018; Tompary et al., 2015, 2018), to characterize age-related changes in amygdala connectivity during affective states. This technique assumes that task-evoked signals and spontaneous background fluctuations are linearly superimposed in task-based fMRI data (Al-Aidroos et al., 2012). As such, the removal of task-based signals should leave behind the sustained state-related activity that is modulated by the context of the task. Because prior evidence suggests that adolescents experience more extreme emotional states than adults and more negative emotional states than children (Larson et al., 1980; Larson and Lampman-Petraitis, 1989), and because negative emotion has been found to impair cognitive control abilities (Albert et al., 2009; Hare et al., 2008; Mueller, 2011; Patterson et al., 2016), background connectivity could reveal how amygdala functioning may be affected by emotional contexts.
We assessed age-related changes in (1) inhibitory control behavioral responses in an emotional context, (2) perception of emotional stimuli, and (3) background functional connectivity while performing an inhibitory control task in an emotional context. Given past studies indicating that adolescents display worse behavioral performance during emotion processing (Albert et al., 2009; Mueller, 2011; Patterson et al., 2016), greater overall emotional reactivity (Guyer et al., 2008; Larson et al., 1980; Monk et al., 2003), and decreasing overall amygdala connectivity relative to adults (Jalbrzikowski et al., 2017), we hypothesized that within an emotional context, adolescents would show (1) greater number of errors and slower responses, (2) higher self-reported reactivity to emotional stimuli, and (3) greater connectivity of amygdala to cortical regions in an affective context. Furthermore, given that the amygdala is predominantly engaged during threat and fear (Adolphs et al., 1995; Anderson et al., 2003), we also expected that (4) age-related changes would be more pronounced in the presence of negative emotion specifically.
2. Methods
2.1. Participants
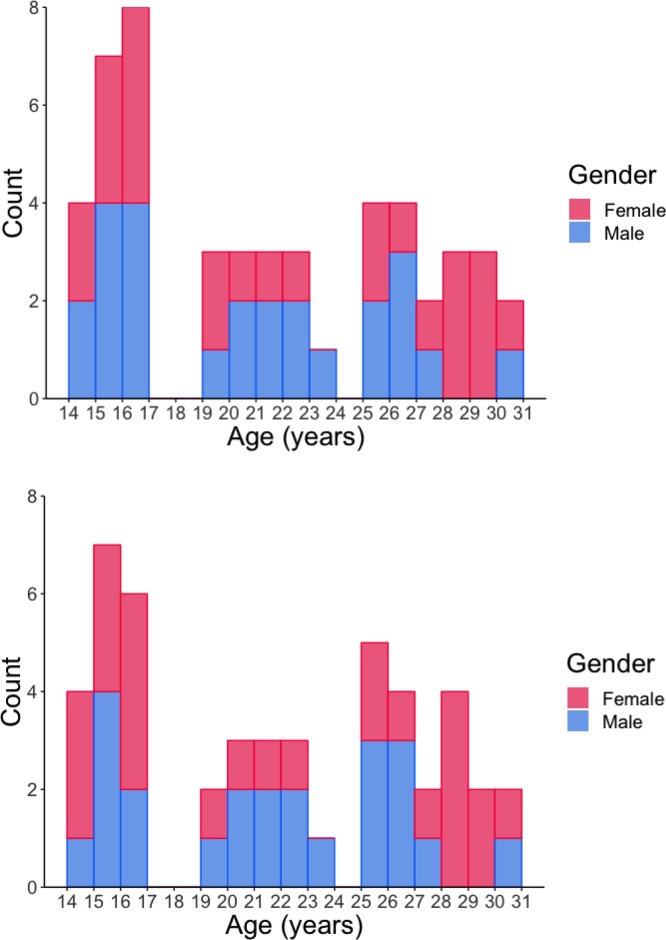
Neuroimaging (task and resting state) and behavioral data were collected on 66 participants (14–31 years old) as part of a multimodal cross-sectional study. Participants were recruited from the community and were excluded if they reported that they or a first-degree relative had been diagnosed with a psychiatric disorder or a neurological condition. Participants were also excluded if they reported MRI contraindications such as metal in the body. For all subjects under the age of 18, parental consent and assent from the participant were obtained before beginning data collection. For those over the age of 18, consent was obtained from the participant. The final sample included task data for 50 participants (25 female) and resting state data for 49 participants (25 female) with exclusions based on excessive head motion, defined by the censoring of more than 15 % of volumes during preprocessing (task N = 2, rest N = 7) and subjects who were unable to complete the scan or whose data files were missing/corrupt (task N = 12, rest N = 8). Subjects were similarly distributed across ages (Fig. 1). We note that the excluded subjects did not differ by age from the subjects included in the analyses. Two additional subjects were excluded from both datasets due to psychiatric diagnoses disclosed after data collection, resulting in the final sample given above. Additional demographic information about the subjects included in the final analyses can be found in Supplemental Table 1.
Fig. 1.
Histogram showing the age distribution of the task (top) and rest (bottom) subject pools, grouped by gender.
2.2. fMRI task design
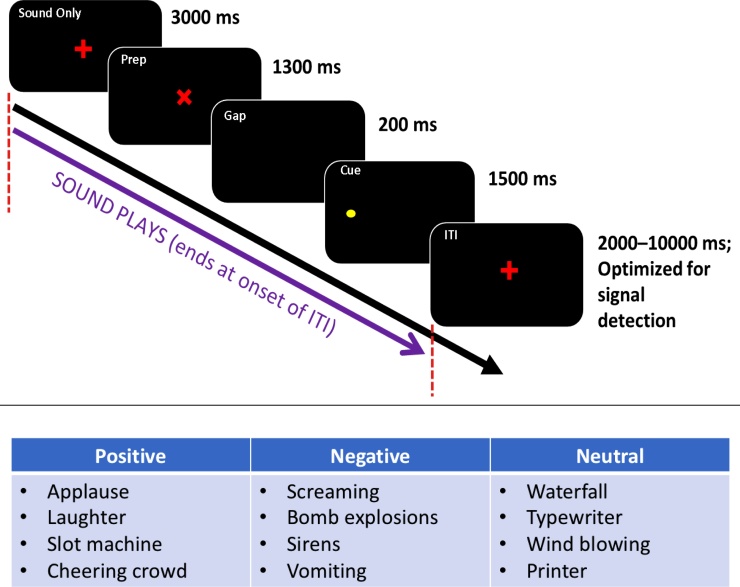
During fMRI scanning, participants completed a standard antisaccade inhibitory control task (Hwang et al., 2010; Ordaz et al., 2013; Velanova et al., 2008, 2009) with an added affective element (Fig. 2). Antisaccade trials began with a red fixation cross presented for 3000 ms on a black background, followed by a red “X”, indicating an upcoming peripheral yellow dot that appeared in an unpredictable location for 1500 ms along the horizontal meridian of the screen. Participants were instructed to avoid looking at this dot, and to instead look at the mirror location of the dot on the other side of this screen. Throughout this time, from the appearance of the red cross until the yellow dot disappeared from the screen, participants heard a 6-second positive, negative, or neutral sound. Examples of sounds chosen for this task within each category are shown in Fig. 2. Intertrial intervals consisted of a white central cross presented for 2–10 seconds. Participants completed four consecutive runs of this task, with 28 trials in each run (total trials: 112). Only trials with a correct inhibitory response were included in latency and fMRI analyses.
Fig. 2.
Diagram of affective antisaccade task design (top) and examples of positive, negative, and neutral sounds included in task (bottom).
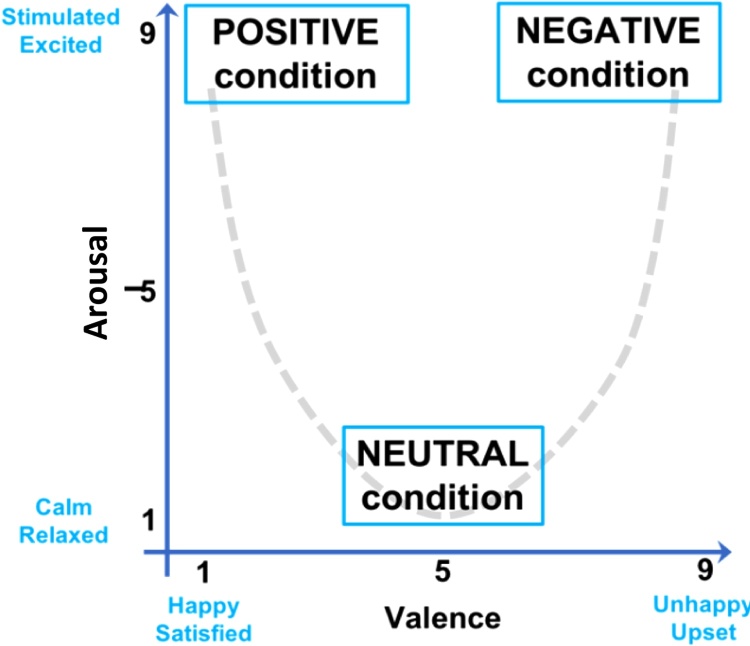
Sounds used during the task were taken from the International Affective Digitized Sounds (IADS), an established set of affective sounds that have been tested in a reference group of healthy college-age adults for consistency in perception of valence and arousal (Bradley and Lang, 2000). From the full database, sounds that were inappropriate for children (e.g., “erotic” sounds) or difficult to identify (e.g., “engine failure”, “jackhammer”) were excluded. Twenty-eight sounds were chosen for each run based on their average valence and arousal ratings provided in the IADS database (Bradley and Lang, 2007) (See Supplementary Materials for full list of sounds used). Arousal ratings were given on a scale of 1 (lowest arousal) to 9 (highest arousal). Similarly, the valence scale also spanned the 1–9 range, with 1 representing sounds that produced strong feelings of unhappiness and a 9 representing sounds producing very happy feelings (Fig. 3). In the reference group established during the creation of the IADS database, positive sounds had a positive valence rating (7–9) and a high arousal rating (7–9), while negative sounds also had a high arousal rating but a negative valence rating (1–3). Neutral sounds had a medium valence rating (4–6) and a low arousal rating (1–3). An equal number of trials were also included in which no sound played before the corresponding antisaccade trial. Each run of the task included seven trials of each type (positive, negative, neutral, or silent) for a total of 28 trials per condition.
Fig. 3.
Representation of the arousal-by-valence relationship. The positive condition represents sounds evoking high arousal and low valence, while the negative condition includes sounds with high arousal and high valence ratings. Neutral sounds were rated low on arousal, and somewhere in the middle on the valence scale.
2.3. MR data acquisition
Data were acquired using a Siemens 3 T Magnetom Trio scanner (Siemens Medical Solutions, Erlangen, Germany) at the University of Pittsburgh Medical Center Magnetic Resonance Research Center using a 12-channel phased-array head coil. Structural images, for use in aligning fMRI data, were obtained using an MPRAGE sequence (TR = 2100 ms, flip angle = 8°, inversion time =1050 ms, voxel size = 1 × 1 mm, 192 contiguous 1-mm slices).
Functional images were acquired using an echoplanar sequence sensitive to BOLD contrast (T2*). Four runs of task-based fMRI data were collected (each run: three minutes, two seconds). The fMRI scan parameters for the task were the following: TR = 2000 ms, flip angle = 80°, voxel size = 1.71875 × 1.71875 mm in plane, 33 3-mm axial slices separated by gaps of 0.75 mm, 728 TRs. We collected five minutes of resting-state data with eyes closed while awake. rsfMRI parameters were the following: TR = 2000 ms, flip angle = 80°, voxel size = 1.719 × 1.719 mm in plane, 33 contiguous 3.75-mm axial slices, 150 TRs.
2.4. Eye tracking data acquisition
Eye-tracking data were collected using a long-range optics eye-tracking system from Applied Science Laboratories (Model 504LRO; Bedford, MA). Eye position was obtained via pupil-corneal reflection on a head coil-mounted mirror. Video monitoring was also used to ensure compliance. A 9-point calibration was performed prior to the experimental session and between runs when necessary. Stimuli were presented using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) and projected onto a flat screen behind the scanner, visible to the subject through a coil-mounted mirror. Eye data were scored off-line using ILAB and MATLAB software (MathWorks, Inc.).
2.5. Eye tracking data scoring
Correct responses in the antisaccade task were defined as those in which the first eye movement during the saccade epoch with velocity greater than or equal to 30°/sec was made toward the mirror location of the peripheral cue and extended beyond a 2.5°/ visual angle from central fixation. Incorrect responses were defined by evidence that the first saccade during the saccade epoch was directed toward the peripheral stimulus and exceeded the 2.5°/ visual angle central fixation zone but were subsequently directed to the correct location, indicating that the instructions were being followed but the participant was unable to suppress their response. Trials in which no eye movements were generated, or in which the tracker lost fixation, were excluded from analyses. On average, 12.25 % of trials were omitted per subject, and the percent of omitted trials per subject did not significantly differ by age or sex. This scoring system was automated using custom software which has been made publicly available on GitHub (https://github.com/LabNeuroCogDevel/autoeyescore).
2.6. fMRI preprocessing
Preprocessing of task and resting state fMRI data followed our in-house standard protocol that incorporates tools from AFNI, NiPy, FSL and BrainWavelet (Cox, 1996; Gorgolewski et al., 2011; Jenkinson et al., 2012; Paulsen et al., 2015). Preprocessing included the following steps. The first four TRs from all scans were removed to allow for BOLD signal normalization. Functional images were warped into MNI standard space using a series of affine and nonlinear transforms. Next, all functional images were spatially smoothed using a 5-mm full width at half maximum Gaussian kernel. Removal of non-stationary events in the fMRI time series was conducted using wavelet despiking (Patel and Bullmore, 2016). For the resting state scans, we then conducted simultaneous multiple regression of nuisance variables and bandpass filtering using 3dTproject at 0.009 Hz < f < 0.08 Hz to better control nuisance-related variability (Hallquist et al., 2013). Nuisance regressors included were non-brain tissue (NBT), average white matter signal, average ventricular signal, six head realignment parameters obtained by rigid body head motion correction, and the first derivatives of these measures. Average white matter and average ventricular signal nuisance regressors were created based on segmentation of the MNI template (Collins et al., 1999). For all subjects, we calculated two quality control measures with respect to head motion: volume-to-volume frame displacement (FD) and the RMS derivative of fMRI time series (DVARS). Similar to previous publications from our group (Marek et al., 2015), we censored and removed volumes that had an FD > 0.5 mm and/or DVARS > 5 (computed after wavelet despiking), as well as the frame preceding the motion artifacts and the two subsequent frames. By first implementing wavelet despiking, we can use most of the time series data to provide a more reliable estimate of the true correlation between two regions-of-interest (ROIs) (Patel and Bullmore, 2016). However, because motion is a critical issue in developmental studies and there were some remaining DVARS values over the identified threshold after wavelet despiking, these volumes were censored as extra validation to ensure that motion was not contaminating the signal. Subjects were dropped from each fMRI analysis if more than 15 % of their volumes were removed via this censoring process. Resting state analyses shown in the results did not incorporate global signal regression; however, the same analyses were carried out with global signal regression and no notable differences in connectivity results were found (Supplemental Fig. 5).
2.7. Statistical analysis
Behavioral data were analyzed with a linear mixed-effects regression using the lme4 package in R (R Development Core Team, Vienna, Austria) (Bates et al., 2014). A linear-mixed effects model was used to examine the fixed effects of condition, age, sex, and their interactions on the four dependent variables: error rate (inhibitory failures), response latency, arousal ratings, and valence ratings. Subject was included in the model as a random effect. Within this model, linear, inverse, and quadratic forms of age were examined and Akaike’s Information Criterion (AIC) (Akaike, 1974) was used to determine the model with the best fit (i.e., the lowest AIC). Significant main effects or interactions were further disambiguated by performing post hoc analyses using the R package ‘lsmeans’ (Lenth, 2016).
Background connectivity data were derived by using the residuals obtained from the deconvolution model used to estimate task effects (see Supplementary Materials), which provided an estimate of the data after eliminating the effects of task stimuli on the hemodynamic response. Because these data resemble resting state fMRI data, the residuals were bandpass filtered (0.009 Hz < f < 0.08 Hz) to allow for comparison of age-related change with the resting state dataset and eliminate extreme values in the estimated hemodynamic response (Geerligs et al., 2015).
Left and right amygdala ROIs were obtained from the Harvard-Oxford Subcortical Atlas, distributed with FSL (http://www.fmrib.ox.ac.uk/fsl/), and a bilateral amygdala ROI was created by combining these two ROIs using the 3dCalc tool in AFNI. For both the resting state data and residuals from the task, mean time courses for the amygdala ROI were created for each individual using AFNI’s 3dROIstats. Voxelwise regressions were performed on the preprocessed data using AFNI’s 3dDeconvolve to compare the average amygdala ROI time series with each timeseries for all other voxels in the brain. These analyses resulted in voxelwise subject-level maps of Pearson correlations (r) between the average amygdala ROI time course and each voxel’s time course. For all statistical analyses, r-values were first normalized using the Fisher r-to-z transformation.
3dMVM was then applied to test for the group-level voxelwise effects of age and sex on amygdala background connectivity in a multiple linear regression. This analysis was masked to only consider voxels with a 50 % or greater probability of being grey matter in the MNI-152 template. Significant clusters with main effects of age were identified and mean parameter estimates were subsequently extracted for these clusters with 3dROIStats, followed by post hoc testing to determine the nature of these age effects. Results were corrected for multiple comparisons using a combination of cluster size and voxel probability, with parameters determined through a Monte Carlo simulation using AFNI’s 3dFWHMx and 3dClustSim program on randomly generated data within the task-related activation mask with the same smoothness as the group mean smoothness estimated from first-level residuals for each region. The autocorrelation function (ACF) option was used when running these scripts, with mean ACF values of 0.724, 3.060, and 10.809. This analysis specified the cluster size threshold (in this data, 17 voxels) applied with a single voxel threshold of p < 0.005 that was required to achieve a cluster-wise corrected p < 0.05. This implementation is the most current, stringent procedure recommended by the AFNI developers to prevent against obtaining false positive clusters of connectivity (Chen et al., 2015). Significant clusters with main effects of age, condition, or interactions were identified. Mean parameter estimates were extracted for these clusters with 3dROIStats and follow-up post hoc tests were performed.
3. Results
3.1. Behavioral results
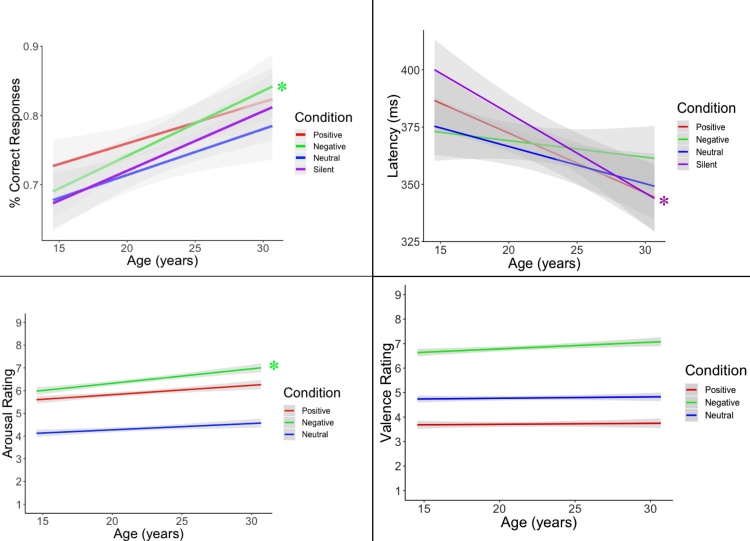
Linear mixed-effects models were used to examine the effects of age, emotional condition, and their interaction on each behavioral outcome, with sex as a covariate and subject as a random effect (Fig. 4). When examining these effects on percentage of correct responses, there was a significant effect of condition (χ2 = 9.251, p = 0.0261), with the best performance occurring in the positive condition, followed by the negative and neutral conditions. Fixed effects of age (χ2 = 3.632, p = 0.0567) and sex (χ2 = 3.608, p = 0.0575) were nonsignificant, as were the age-by-condition (χ2 = 1.758, p = 0.724) and age-by-sex (χ2 = 0.0006, p = 0.981) interaction effects. However, because we hypothesized that differing effects may occur based on emotional valence, we also tested these age effects within each condition. In these analyses, the effect of age was significant within the negative condition (p = 0.027). There were no significant effects of age (χ2 = 2.581, p = 0.108), condition (χ2 = 5.528, p = 0.137), sex (χ2 = 0.840, p = 0.359), or age-by-sex interaction (χ2 = 0.0001, p = 0.991) on response latency, but there was a significant age-by-condition interaction (χ2 = 11.684, p = 0.009). This interaction may have been driven by a significant association between increasing age and decreases in response latency within the silent condition (p = 0.024).
Fig. 4.
Line graphs showing the association between age and percentage of correct responses (top left), response latency (top right), individual arousal ratings (bottom left) and individual valence ratings (bottom right).
* indicates significance at p < 0.05
Consistent with the valence-based categorization of the emotional conditions, there was a significant difference in valence ratings between conditions (χ2 = 2295.014, p = 2.000 × 10−16). There were no effects of age (χ2 = 0.540, p = 0.462), sex (χ2 = 0.434, p = 0.510), or interactions between age and sex (χ2 = 2.988, p = 0.0839) or age and condition (χ2 = 4.663, p = 0.0971) on these valence ratings. Similarly, arousal ratings, as expected, showed a significant effect of emotional condition (χ2 = 1032.317, p = 2.000 × 10−16), indicating that subjects perceived significant differences in the level of arousal elicited by stimuli from different emotional conditions. No significant sex (χ2 = 0.091, p = 0.763), age (χ2 = 3.512, p = 0.061), or age-by-sex interaction (χ2 = 0.0647, p = 0.799) effects were observed, but these analyses did yield a significant age-by-condition interaction (χ2 = 9.144, p = 0.010). Probing this interaction further revealed that it may have been driven by a significant age effect in the negative condition, such that increasing age was associated with higher arousal ratings (β = 0.063, p = 0.009).
3.2. Background connectivity
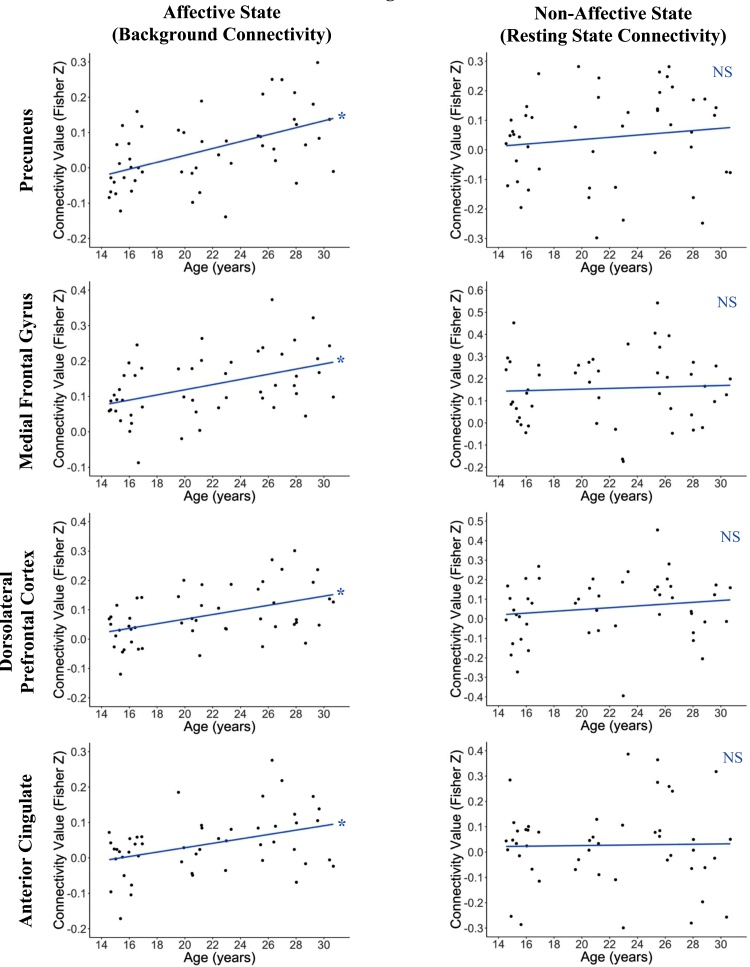
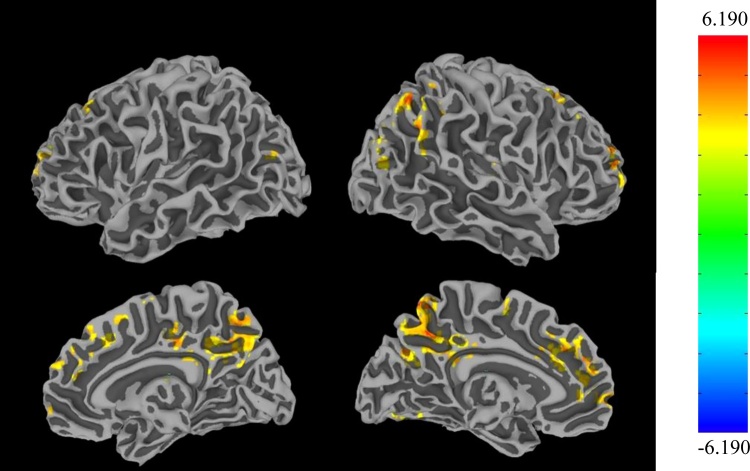
The overall pattern of amygdala background connectivity across all subjects is shown in Supplemental Fig. 4. Age-associated changes were observed in the background connectivity of the amygdala bilaterally to a wide range of cortical regions across the brain (Table 1, Figs. 5,6). At a voxelwise threshold of p < 0.005 and a cluster-corrected threshold of p < 0.05, amygdala connectivity was found to increase with age with the left dorsal anterior cingulate cortex (ACC), bilateral precuneus, bilateral medial prefrontal cortex (mPFC), bilateral dorsolateral prefrontal cortex (DLPFC), right insula, right thalamus, and throughout the parietal cortex (t = 2.952). In the resting state dataset, significant age-related increases in whole brain voxelwise amygdala connectivity were found only within the occipital cortex and cerebellum (p < 0.005 voxelwise, p < 0.05 corrected, Supplemental Table 4). To test the specificity of state-related connectivity changes to the task context, we first ran another seed-based voxelwise analysis, with both background connectivity and resting state in one model, to directly test for an interaction between age and context (affective vs. none). This analysis yielded no clusters of significance, likely due to the low sample size and short length of the resting state scan. Therefore, as another means to examine the specificity of the background connectivity findings, we performed a pairwise connectivity analysis in the resting state data, using the amygdala ROIs and all significant clusters derived from the amygdala background connectivity voxelwise analysis. Spherical ROIs of radius 9.38 mm (123 voxels per ROI) were generated centered on the peak voxel of each cluster, and pairwise connectivity values were calculated for these ROIs and the amygdala ROIs using the same methods within the resting state data. These analyses showed no significant correlations between the amygdala and the clusters, providing potential evidence that the pattern of age-related change in amygdala connectivity found in the background connectivity analysis may be specific to an affective state (Supplemental Table 5).
Fig. 6.
Graphs showing linear relationship between age and amygdala connectivity values in selected clusters. The comparison between the age relationship in background connectivity and the lack of an age relationship in resting state indicates that the increases in background connectivity seen with age are likely to be specific to the affective state, rather than an intrinsic connectivity change associated with maturation.
Table 1.
Clusters from Voxelwise Analysis of Age Effects on Amygdala Background Connectivity (p < 0.005 voxelwise, p < 0.05 corrected).
| Name | Brodmann Area | X | Y | Z | # of voxels |
|---|---|---|---|---|---|
| Bilateral Precuneus | BA 7 | +7.5 | +64.5 | +46.5 | 693 |
| Medial Frontal Gyrus | BA 9 | +1.5 | −52.5 | +16.5 | 249 |
| R Superior Frontal Gyrus | BA 10 | −25.5 | −55.5 | +19.5 | 96 |
| R Dorsolateral Prefrontal Cortex | BA 8 | −40.5 | −31.5 | +46.5 | 82 |
| L Medial Frontal Gyrus / Anterior Cingulate Cortex | BA 32 | +4.5 | −61.5 | −1.5 | 81 |
| R Inferior Parietal Lobule | BA 40 | −46.5 | +61.5 | +49.5 | 48 |
| R Angular Gyrus | BA 39 | −43.5 | +70.5 | +31.5 | 44 |
| L Dorsolateral Prefrontal Cortex | BA 8/9 | +37.5 | −28.5 | +46.5 | 43 |
| R Inferior Parietal Lobule | BA 40 | −58.5 | +58.5 | +31.5 | 38 |
| R Inferior Parietal Lobule | BA 40 | −46.5 | +43.5 | +40.5 | 36 |
| L Superior Frontal Gyrus | BA 10 | +25.5 | −58.5 | +7.5 | 35 |
| L Fusiform Gyrus | BA 18/19 | +25.5 | +76.5 | −4.5 | 33 |
| R Insula | BA 13 | −40.5 | +16.5 | +16.5 | 28 |
| L Middle Temporal Gyrus | BA 39 | +46.5 | +79.5 | +22.5 | 24 |
| R Thalamus | – | −10.5 | +16.5 | +19.5 | 22 |
| L Middle Frontal Gyrus | BA 6 | +1.5 | +1.5 | +61.5 | 22 |
Fig. 5.
Age-related changes in background connectivity with the amygdala were observed in areas including the dorsal ACC, BA 10, precuneus, DLPFC, medial PFC, right insula, and right thalamus, as well as various parietal areas (t = 2.952, p < 0.05 corrected).
In order to ensure that potential limitations in statistical power regarding sample size did not limit the ability to detect age-related changes in rsfMRI, the whole-brain voxelwise and pairwise connectivity analyses were repeated in a subset of resting state data (N = 395) from the Philadelphia Neurodevelopmental Cohort (Satterthwaite et al., 2014), including only healthy controls between 14 and 31 years old to match this study. In addition, these datasets were preprocessed using the same pipeline described above. The amygdala-cluster correlation results looked very similar to our resting state data, showing a predominant lack of age effects, with the exception of increased connectivity with the fusiform and thalamus clusters (Supplemental Table 6). This finding provides some preliminary support for our hypothesis that the age-related increase in amygdala connectivity found in the background connectivity analysis may be specific to the emotional context.
4. Discussion
This study aimed to understand age-related changes from mid-adolescence to adulthood in the effects of increased emotional state on cognitive control performance and amygdala connectivity. Overall, our results showed that from mid-adolescence to adulthood, there is increased differentiation of negative emotional stimuli by evoked arousal, along with improvements in inhibitory control and increased amygdala connectivity to key executive control regions, including the DLPFC and ACC, when performing a cognitive control task during an affective state. It is important to note that we did not find a context-by-age interaction in the voxelwise analysis including both data sets (background and resting state), which may be due to insufficient power. This somewhat tempers our findings of age-related increases in amygdala background connectivity and precludes us from determining whether these findings are specific to an affective state. However, these results may still provide insight into developmental enhancements that could be specific to an emotional state, which we have previously seen in reward state functional VTA/NAcc connectivity (Murty et al., 2018).
The increase in perceived arousal of negative stimuli with age seems counter to the notion that adolescents are believed to be more prone to experiencing negative emotions intensely, but may relate to known limitations in adolescent emotion regulation abilities, since awareness of one’s emotional state is critical for conscious emotion regulation (Guyer et al., 2016; Scheibe and Blanchard-Fields, 2009). Our findings also are supported by evidence indicating that increased arousal levels in response to emotional stimuli in adults are associated with greater amygdala-cortical connectivity (Mickley Steinmetz et al., 2010; Touroutoglou et al., 2014), suggesting that this may underlie our findings. These results are particularly striking given that the valence of the stimuli was rated similarly across all ages. In other words, participants on average perceived the same stimuli to be positive, negative, or neutral. Thus, while emotions are categorized similarly across adolescence and adulthood, they are perceived as having a different impact. In this case, adults were more likely to report that negative stimuli led to higher arousal levels than adolescents, suggesting potential limitations in the ability to accurately characterize one’s internal state during adolescence. Future studies could incorporate self-report measures of emotional awareness to explore this possibility empirically.
The background connectivity analysis sought to understand how emotional context may affect the integration of information from the amygdala, as a core region of emotional processing, with the rest of the brain. Results showed that across the brain, amygdala background connectivity increases with age, particularly with regions involved in general executive functioning, such as the DLPFC and dorsal ACC. Notably, a recent study looking at functional connectivity during emotional face perception found decreases in task-based connectivity from childhood (age 7) to adulthood (age 25) between the amygdala and executive system (Wu et al., 2016). While we did not perform any direct tests to reconcile these results with our own, one possible explanation may be that in adulthood, coupling of the amygdala and executive systems may be weaker compared to adolescents when emotion processing is the only task being performed, but when there is a need to utilize cognitive control simultaneously, adults may be more able to effectively engage this circuitry.
Our results here are in agreement with our past findings in subjects ages 10–22, indicating that after decreases in amygdala/vmPFC resting state functional connectivity through childhood and early adolescence, there is stabilization by later adolescence, which was replicated in the PNC cohort (Jalbrzikowski et al., 2017). Aligning with this, our current results in an older cohort spanning ages 14–31 also indicate a lack of age-related changes in amygdala/PFC resting state functional connectivity, implying again that stabilization occurs by this late adolescent period. Importantly, in this study we find evidence that suggests the occurrence of age-related increases in amygdala affective-state background functional connectivity with widespread cortical regions. While we did not directly test for the mechanism underlying this state-specific finding, it may reflect that in an emotional context, adults exhibit enhanced coupling in this circuitry that is stable at rest but may support the regulation of increased arousal levels in response to certain emotional stimuli.
It is important to note that while these results suggest a possible developmental trajectory of amygdala connectivity, the lack of longitudinal data in this study precludes us from interpreting this as a developmental change. Furthermore, the small sample size of this study limited some interpretation of null findings, although the PNC dataset replication provides some support for our findings, particularly in the case of the null resting state findings. In addition, the resting state scan in this study was much shorter than the task fMRI scan to which it was being compared, undermining the ability to effectively test differences between both datasets within one statistical model, and was also on the lower end of what is currently recommended for reliable signal (Birn et al., 2013), a problem that is common in studies involving children and adolescents. Thus, the lack of significant age-effects in our resting state data could reflect noisy individual data resulting from short acquisitions. However, we believe this explanation is less likely given the similar pattern of results observed in the substantially larger PNC cohort. Incorporating novel methods for reducing in-scanner motion will be crucial in future studies to allow for longer scan times in developmental populations. In addition, past work has found that age-related differences in functional connectivity are only observable in resting state collected with eyes open or fixation, but not with eyes closed, as is the case in this study (Agcaoglu et al., 2019). However, the fact that the resting state results were replicated in the PNC resting state data, which is collected with eyes open, likely mitigates this issue. Finally, the lower end of the age range in this study is higher than would be ideal for this question, particularly since we know that the most significant changes in amygdala resting state connectivity occur earlier in development (Jalbrzikowski et al., 2017). Therefore, incorporating wider age ranges will be crucial to better characterize the developmental trajectory of amygdala-cortical connectivity.
Taken together, our results suggest that from mid-adolescence to adulthood, there is increased recognition of internal affective states, which may increase the engagement of relevant cortical regions by the amygdala and enhance cognitive control. While the directionality of this connectivity is not evident, it is often surmised that early in development, greater connectivity of the amygdala to executive regions reflects bottom-up predominance of amygdala on executive systems, undermining cognitive control. Here we find that greater connectivity of the amygdala to executive regions in adulthood occurs in the context of greater inhibitory control suggesting enhanced top-down control in adulthood supporting improved ability to regulate reactivity to emotionally salient stimuli. The greater awareness in adulthood of emotional arousal may signal the need to engage greater resources increasing cortico-amygdala engagement. Future longitudinal studies using larger sample sizes, wider age ranges, and data with higher spatial resolution, will be important to better elucidate the results suggested by the present study.
5. Conclusion
The current study found simultaneous age-related increases in arousal, background amygdala connectivity, and task performance during the transition from adolescence to adulthood. The results suggest that from mid-adolescence to adulthood, greater awareness of emotional arousal may lead to stronger integration of cortical and amygdala processing, enhancing behaviors that require executive control. Decreased processing of emotional arousal may undermine the capacity of adolescents to effectively engage control processes that may contribute to known poor decision-making in emotional circumstances.
Disclosures
All authors declare no conflict of interest.
Author contributions
OR designed and performed data analysis, wrote manuscript. SJO, AP, and BL designed study and data collection protocol. FC, MJ, and WF assisted in data analysis design. All authors contributed to the manuscript.
Acknowledgments
This work was supported by a National Institute of Mental Health grant (R01 MH067924) given to Dr. Beatriz Luna and a National Science Foundation Graduate Research Fellowship (1747452) given to Orma Ravindranath.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2020.100836.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adolphs R., Tranel D., Damasio H., Damasio A.R. Fear and the human amygdala. J. Neurosci. 1995;15(9):5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agcaoglu O., Wilson T.W., Wang Y.-P., Stephen J., Calhoun V.D. Resting state connectivity differences in eyes open versus eyes closed conditions. Hum. Brain Mapp. 2019;40(8):2488–2498. doi: 10.1002/hbm.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Al-Aidroos N., Said C.P., Turk-Browne N.B. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proc. Natl. Acad. Sci. 2012;109(36):14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert J., López-Martín S., Carretié L. Emotional context modulates response inhibition: neural and behavioral data. NeuroImage. 2009;49:914–921. doi: 10.1016/j.neuroimage.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Anderson A.K., Christoff K., Panitz D., De Rosa E., Gabrieli J.D.E. Neural correlates of the automatic processing of threat facial signals. J. Neurosci. 2003;23(13):5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T., Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Avenevoli S., Swendsen J., He J.-P., Burstein M., Merikangas K. Major depression in the national comorbidity survey- adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(1):37–44. doi: 10.1016/j.jaac.2014.10.010. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014;67:1–48. [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37(2):204–215. [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. 2nd ed. University of Florida; 2007. The International Affective Digitized Sounds (IADS-2): Affective Ratings of Sounds and Instruction Manual. [Google Scholar]
- Casey B.J., Heller A.S., Gee D.G., Cohen A.O. Development of the emotional brain. Neurosci. Lett. 2017 doi: 10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Saad Z.S., Adleman N.E., Leibenluft E., Cox R.W. Detecting the subtle shape differences in hemodynamic responses at the group level. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Zijdenbos A.P., Baaré W.F.C., Evans A.C. ANIMAL+INSECT: improved cortical structure segmentation. In: Kuba A., Šáamal M., Todd-Pokropek A., editors. Information Processing in Medical Imaging. Springer; Berlin Heidelberg: 1999. pp. 210–223. [Google Scholar]
- Costello E.J., Copeland W., Angold A. Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? J. Child Psychol. Psychiatry. 2011;52(10):1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Duncan K., Doll B.B., Daw N.D., Shohamy D. More than the sum of its parts: a role for the Hippocampus in configural reinforcement learning. Neuron. 2018;98(3):645–657. doi: 10.1016/j.neuron.2018.03.042. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L.E., Bowman C.R., Zeithamova D. Differential functional connectivity along the long Axis of the Hippocampus Aligns with differential role in memory specificity and generalization. J. Cogn. Neurosci. 2019;31(12):1958–1975. doi: 10.1162/jocn_a_01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L., Rubinov M., Cam C.A.N., Henson R.N. State and trait components of functional connectivity: individual differences vary with mental state. J. Neurosci. 2015;35(41):13949–13961. doi: 10.1523/JNEUROSCI.1324-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Front. Neuroinform. 2011;5 doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E.E., Roberson-Nay R., Adler A.D., Fromm S.J., Leibenluft E., Pine D.S., Ernst M. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Silk J.S., Nelson E.E. The neurobiology of the emotional adolescent: from the inside out. Neurosci. Biobehav. Rev. 2016;70:74–85. doi: 10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist M.N., Hwang K., Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–225. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-no go task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlé K.M., Shenoy P., Paulus M.P. The influence of emotions on cognitive control: Feelings and beliefs—where do they meet? Front. Hum. Neurosci. 2013;7:508. doi: 10.3389/fnhum.2013.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K., Velanova K., Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J. Neurosci. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzlicht M., Bartholow B.D., Hirsh J.B. Emotional foundations of cognitive control. Trends Cogn. Sci. 2015;19(3):126–132. doi: 10.1016/j.tics.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Larsen B., Hallquist M.N., Foran W., Calabro F., Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol. Psychiatry. 2017;82(7):511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kann L. Youth risk behavior surveillance—United States, 2015. Mmwr Surveill. Summ. 2016;65(6):1–174. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Yurgelun-Todd D.A. Unconscious processing of facial affect in children and adolescents. Soc. Neurosci. 2007;2(1):28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- Larson R., Lampman-Petraitis C. Daily emotional states as reported by children and adolescents. Child Dev. 1989;60(5):1250–1260. doi: 10.2307/1130798. [DOI] [PubMed] [Google Scholar]
- Larson R., Csikszentmihalyi M., Graef R. Mood variability and the psychosocial adjustment of adolescents. J. Youth Adolesc. 1980;9(6):469–490. doi: 10.1007/BF02089885. [DOI] [PubMed] [Google Scholar]
- Lenth Russell V. Least-squares means: the R Package lsmeans. J. Stat. Software. 2016;69(1):1–33. doi: 10.18637/jss.v069.i01. [DOI] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38(1):151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Berridge K.C. What and when to ‘Want’? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology. 2012;221(3):407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley Steinmetz K.R., Addis D.R., Kensinger E.A. The effect of arousal on the emotional memory network depends on valence. NeuroImage. 2010;53(1):318–324. doi: 10.1016/j.neuroimage.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Grillon C., Baas J.M., McClure E.B., Nelson E.E., Zarahn E., Charney D.S., Ernst M., Pine D.S. A neuroimaging method for the study of threat in adolescents. Dev. Psychobiol. 2003;43(4):359–366. doi: 10.1002/dev.10146. [DOI] [PubMed] [Google Scholar]
- Morris A.S., Squeglia L.M., Jacobus J., Silk J.S. Adolescent brain development: implications for understanding risk and resilience processes through neuroimaging research. J. Res. Adolesc. 2018;28(1):4–9. doi: 10.1111/jora.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S.C. The influence of emotion on cognitive control: relevance for development and adolescent psychopathology. Front. Psychol. 2011;2:1–21. doi: 10.3389/fpsyg.2011.00327. NOV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Shah H., Montez D., Foran W., Calabro F., Luna B. Age-related trajectories of functional coupling between the VTA and nucleus accumbens depend on motivational state. J. Neurosci. 2018;38(34):7420–7427. doi: 10.1523/JNEUROSCI.3508-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33(46):18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.X., Bullmore E.T. A wavelet-based estimator of the degrees of freedom in denoised fMRI time series for probabilistic testing of functional connectivity and brain graphs. NeuroImage. 2016;142:14–26. doi: 10.1016/j.neuroimage.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson T.K., Lenartowicz A., Berkman E.T., Ji D., Poldrack R.A., Knowlton B.J. Putting the brakes on the brakes: negative emotion disrupts cognitive control network functioning and alters subsequent stopping ability. Exp. Brain Res. 2016;234(11):3107–3118. doi: 10.1007/s00221-016-4709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D.J., Hallquist M.N., Geier C.F., Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev. Cogn. Neurosci. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?”. Neuropsychologia. 2010;48(12):3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Blakemore S.-J. Adolescent social cognitive and affective neuroscience: past, present, and future. Soc. Cogn. Affect. Neurosci. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., Oswald T.M., Mazziotta J.C., Iacoboni M., Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev. Cogn. Neurosci. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol. Med. 2014;44(10):2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- Rosen M.L., Sheridan M.A., Sambrook K.A., Dennison M.J., Jenness J.L., Askren M.K., Meltzoff A.N., McLaughlin K.A. Salience network response to changes in emotional expressions of others is heightened during early adolescence: relevance for social functioning. Dev. Sci. 2018;21(3):e12571. doi: 10.1111/desc.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Ruparel K., Loughead J., Prabhakaran K., Calkins M.E., Hopson R., Jackson C., Keefe J., Riley M., Mentch F.D., Sleiman P., Verma R., Davatzikos C., Hakonarson H., Gur R.C., Gur R.E. Neuroimaging of the Philadelphia neurodevelopmental cohort. NeuroImage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe S., Blanchard-Fields F. Effects of regulating emotions on cognitive performance: what is costly for young adults is not so costly for older adults. Psychol. Aging. 2009;24(1):217–223. doi: 10.1037/a0013807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Ordaz S.J., Kircanski K., Ho T.C., Davis E.G., Camacho M.C., Gotlib I.H. Resting-state functional connectivity and inflexibility of daily emotions in major depression. J. Affect. Disord. 2019;249:26–34. doi: 10.1016/j.jad.2019.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., McRae K., Gabrieli J.D.E., Gross J.J., Remy K.A., Ochsner K.N. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12(6):1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2009. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. (HHS Publication No. SMA 10-4856Findings; NSDUH Series H-38A, p. 104) [Google Scholar]
- Todd R.M., Evans J.W., Morris D., Lewis M.D., Taylor M.J. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Soc. Cogn. Affect. Neurosci. 2011;6(1):12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A., Duncan K., Davachi L. Consolidation of associative and item memory is related to post-encoding functional connectivity between the Ventral Tegmental Area and different medial temporal lobe subregions during an unrelated task. J. Neurosci. 2015;35(19):7326–7331. doi: 10.1523/JNEUROSCI.4816-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A., Al-Aidroos N., Turk-Browne N.B. Attending to what and where: background connectivity integrates categorical and spatial attention. J. Cogn. Neurosci. 2018;30(9):1281–1297. doi: 10.1162/jocn_a_01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Bickart K.C., Barrett L.F., Dickerson B.C. Amygdala task-evoked activity and task-free connectivity independently contribute to feelings of arousal. Hum. Brain Mapp. 2014;35(10):5316–5327. doi: 10.1002/hbm.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18(11):2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. Functional differences in emotion processing during adolescence and early adulthood. NeuroImage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D., Monk C.S., Phan K.L. Age-related changes in amygdala–frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37(5):1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Meng X., Yang J., Yao G., Hu L., Yuan H. The valence strength of unpleasant emotion modulates brain processing of behavioral inhibitory control: neural correlates. Biol. Psychol. 2012;89(1):240–251. doi: 10.1016/j.biopsycho.2011.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.