Abstract
Liver fibrosis results from chronic damages together with an accumulation of extracellular matrix, and no specific medical therapy is approved for that until now. Due to liver metabolic capacity for drugs, the fragility of drugs, and the presence of insurmountable physiological obstacles in the way of targeting, the development of efficient drug delivery systems for anti-fibrotics seems vital. We have explored articles with a different perspective on liver fibrosis over the two decades, then collected and summarized the information by providing corresponding in vitro and in vivo cases. We have discussed the mechanism of hepatic fibrogenesis with different ways of fibrosis induction in animals. Furthermore, the critical chemical and herbal anti-fibrotics, biological molecules such as micro-RNAs, siRNAs, and growth factors, which can affect cell division and differentiation, are mentioned. Likewise, drug and gene delivery and therapeutic systems on in vitro and in vivo models are summarized in the data tables. This review article enlightens recent advances in emerging drugs and nanocarriers and represents perspectives on targeting strategies employed in liver fibrosis treatment.
Key Words: Liver fibrosis, Hepatic stellate cell, Drug delivery, Gene therapy, Lipid nanoparticle, Viral and non-viral vector, Herbal anti-fibrotic
Graphical abstract
Resolution of liver fibrosis could be the result of a proper drug delivery system and antifibrotic agent. During resolution process, liver restores its healthy texture with hepatocytes which have microvillus, hepatic stellate cells with retinol, liver sinusoidal endothelial cells with fenestra and space of Disse with reduced extracellular matrix.
1. Relevance and mechanism of hepatic fibrogenesis
Liver disease and cirrhosis together were the 12th leading cause of mortality, accounting for 40,545 cases or 1.5% of all deaths in the United States in 20161. Chronic liver diseases (CLDs) represent a significant world public health problem, and hepatic fibrosis is a common protective response to CLD of various etiologies, such as persistent viral hepatitis B and C, non-alcoholic fatty liver disease (NAFLD), alcohol overload, and autoimmune liver disease. When injury and inflammation become chronic and untreated, the cellular responses get dysregulated. The imbalance between augmented synthesis and decreased degradation causes an excess of extracellular matrix (ECM) proteins deposition and finally scar tissue formation or fibrosis development, which may eventually progress to cirrhosis and its associated complications2,3.
Liver resident mesenchymal cells, particularly hepatic stellate cells (HSCs), are the major source of fibrogenic myofibroblasts. HSCs as vitamin A (retinoid)-storing cells comprise approximately 15% of total liver cells residing in the space between hepatocytes and liver sinusoidal endothelial cells (LSECs), named space of Disse4. Other cells, such as portal fibroblasts, mesothelial cells, and bone-marrow-derived fibrocytes, also contribute, and their participation depends on the etiology of liver fibrosis. For instance, a previous study revealed that HSCs are the source of myofibroblasts in a carbon tetrachloride (CCl4)-induced liver fibrosis model. In contrast, portal fibroblasts give rise to myofibroblasts in the cholestatic liver5. Bone marrow-derived cells represent a substantial fraction of the total fibrogenic population in a more chronic injury.
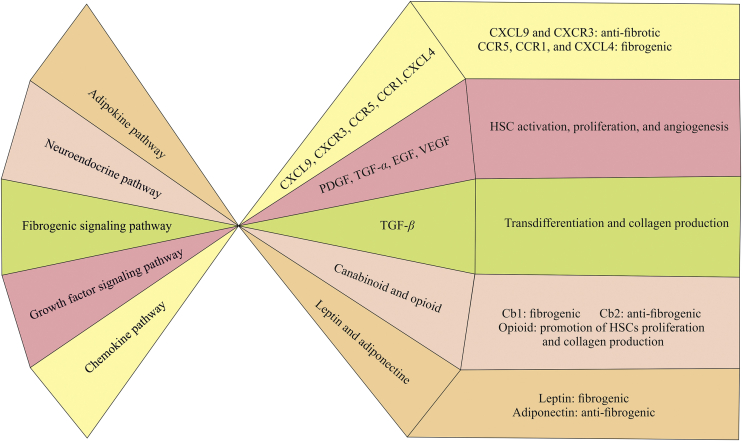
The literature on fibrosis demonstrated different pathways involved in the fibrogenesis (Fig. 1). Among them, fibrogenic signaling pathways, chemokine pathways, adipokine pathways, and neuroendocrine pathways have a significant role6.
Figure 1.
Major signaling pathways in liver fibrosis.
In the healthy liver, collagen types IV and VI are the major components of ECM. In contrast, proliferating myofibroblasts or activated HSCs as the vital sources of excess ECM molecules, give rise to collagen types I and III during fibrogenesis7, and augmented and accumulated ECM serves as reservoir for growth factors, cytokines, and chemokines, then this cycle perpetuates8. The changes in healthy tissue during fibrogenesis are summarized in Fig. 2.
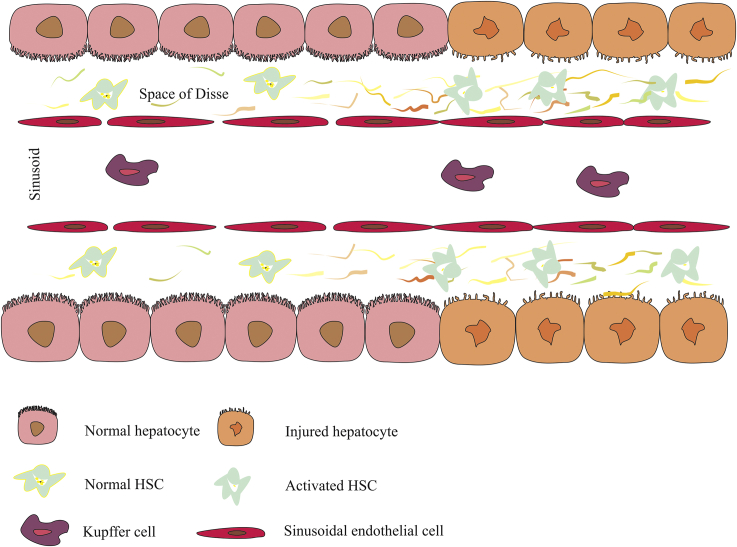
Figure 2.
Overview of liver fibrosis progression. Prolonged damage to hepatocytes triggers activation of HSCs (decreasing the amount of retinol in their cytoplasm is one manifestation of the activation), which increases ECM consequently; increased ECM applies extra pressure to sinusoids, that causes portal hypertension in some patients. Also, holes that are in the membrane of sinusoids get lost or tightened; therefore, the amount of nutrients and oxygen transportation comes down. As injury remains untreated, the situation gets worse, and the recruitment of Kupffer cells and other elements of the defending system in the injured area increases. The perpetuation of this process leads to scar tissue formation or fibrosis. ECM, extracellular matrix; HSC, hepatic stellate cell; Space of Disse, space between hepatocytes and liver sinusoidal endothelial cells.
For determining mechanisms of fibrosis and developing novel therapies, the use of animal models is crucial. To date, no animal model has recapitulated all features of liver fibrosis. However, in comparison to in vitro and clinical studies, animal model studies have several merits, including the possibility of collection of multiple samples, a shorter time for disease development, the ability to control and reduce variables that cannot be closely followed in humans, the ability to study the implication of genes/signaling pathways, and the study of the liver as a complete organ in crosslink with the entire body9. Although animal models have several benefits, they cannot answer all questions. Because they do not develop human diseases, and some liver pathologies occur in a specific metabolic or immune context. Also large variations in responses to noxious agents exist between humans and animals at several levels. First, some hepatic diseases like hepatitis C virus (HCV) do not exist in rodents. Second, animals may be less or more susceptible to toxic agents than humans. Therefore, alcoholic liver disease (ALD) is particularly difficult to induce in rodents and severe liver fibrosis does not develop by chronic alcohol feeding. In contrast to alcohol, common bile duct ligation (BDL) results in secondary biliary cirrhosis after only a few weeks in rodents, whereas month-long impairment of the bile flow is needed to cause severe liver fibrosis in humans10. In spite of mentioned limitations, animal models are being used for several decades and they have been discussed thoroughly9,11. Hepatotoxin-induced liver fibrosis, biliary fibrosis, autoimmune fibrosis, alcohol-induced fibrosis, non-alcoholic steatohepatitis (NASH)-associated fibrosis are mentioned as the prominent models of liver fibrosis. In hepatotoxin-induced fibrosis, repeated usage of toxins like CCl4, thioacetamide (TAA), dimethyl or diethyl nitrosamine (DMN or DEN), result in fibrosis that first appears in the perivenular area and then extends to portal areas. In humans, fibrosis is frequently distributed in periportal and lobular areas and central fibrosis is only seen in vascular disorders10. Biliary fibrosis develops within weeks. In BDL, rats are specially adapted due to the lack of gall bladder, and bile acid toxicity, stimulating the proliferation of bile duct epithelial cells, portal inflammation, and fibrosis12. Infection of mice with Schistosoma and prolonged administration of heterologous serum, are two ways to induce fibrosis immunologically13,14. Achieving sustained high alcohol level in blood causes liver injury and fibrosis, but in this method fibrosis is rather moderate and never evolves to cirrhosis15. In NASH-associated fibrosis, a high fat diet induces steatosis with fibrosis in rats16.
2. Rational therapeutic measures
Treatment options for liver fibrosis depend upon underlying causes can be different. The main factors contributing to diseases worsening are injury progression and withdrawal of the healing process. Drugs can be used to affect CLD progression and decrease parenchymal liver injury. In this context, pathways or signals causing recruitment/activation of Kupffer cells (KCs), monocyte-derived macrophages, and hepatic myofibroblasts could be targeted. The promotion of the healing process first could be achieved by the elimination of profibrogenic cells, and the reversion or senescence of them. Enhancing ECM degradation and transplantation of bone marrow-derived cells (like macrophages) are considered as the second approach17.
Medical intervention to cease the alterations which occur during fibrosis had limited therapeutic efficacy. Off-targeting, immensity of underlying factors at the same time and the conflict between interferences, and defection in delivery systems or the lack of suitable carrier, especially about plant extracts, are among the principal limiting steps. It seems unique physicochemical properties of nanoparticles (NPs) can increase solubility and half-life of drugs, facilitate their specific uptake and accumulation in the target site, and limit systemic side effects18,19. However, still there are some important facts that are neglected and prone drug delivery system (DDS) or gene delivery system (GDS) to failure. For instance, numerous cell types and fibrogenic activators are responsible for the fibrosis progression and each of them could be a complementary targeting site. In most of studies, targeting is done according to the characteristics of one special cell type, and other communicating factors and complexity of in vivo models were not considered. The immensity of conflicting factors makes the optimization process inefficient. For example, compared to hydrophilic NPs, hydrophobic ones are more rapidly removed from circulation by KCs. PEGylation, as the most used method decreases the uptake of NPs by KCs and increases the uptake by hepatocytes, while there is report on the detrimental effect of PEGylation on bioactivity and this fact is neglected in some studies20. KCs and LSECs specifically recognize oxidized low-density lipoprotein, human serum albumin (HSA), and negatively charged NPs by scavenger receptors, while hepatocytes are more likely to take up NPs with positive surface charge. According to the mentioned data in Table 121, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, almost all of drug-loaded NPs for liver fibrosis possess a negative charge which is in favor of KCs and LSECs, while the most favored cells in fibrosis targeting seem to be HSCs. Even though current therapies are not sufficient enough to completely cure of hepatic fibrosis, numerous drugs which include pioglitazone, obeticholic acid, losartan, candesartan, glycyrrhizin, pentoxifylline, everolimus, and simtuzumab have been registered/continued in clinical trials34,35 (Table 2), and at the same time, lipid- and polymer-based drug delivery carriers have gained much more attention for targeting liver fibrosis36 (Table 1).
Table 1.
| Carrier | Drug | Targeting agent | Size (nm) | Zeta potential (mV) | Effect and mechanism of action | Ref. |
|---|---|---|---|---|---|---|
| Bovine serum albumin | Berberine | – | 394.9 ± 102.03 | ‒30 to 30 | The LX-2 cell growth inhibition, stronger CASP3 activation at lower dose, and in vivo anti-hepatotoxicity effect at 1 and 2 μg/g | 21 |
| Bovine serum albumin | Sodium ferulate | M6P | 100 to 200 | −2.73 to −35.85 | Specific uptake by HSC (less distribution to the kidneys), slower elimination rate, and much higher drug concentration | 23 |
| Liposome | Vismodegib | Cyclic peptides (cRGDyK) | 75.6 ± 2.4 | −24.8 ± 1.8 | Inhibited hedgehog pathway signaling in HSCs, and alleviated hepatotoxin-induced fibrosis in mice | 24 |
| M6P-HSA-conjugated liposome | Rosiglitazone | M6P-HSA | 135.1 ± 3.74 | −30.5 ± 2.64 | Increased liver uptake (2.61-fold), improved biochemical markers level and histopathological morphology, and decreased fibrosis grade | 25 |
| cRGD-modified liposome | (IFN)-α1b | cRGD | 101 ± 17.7 | – | Reduction in the extent of liver fibrosis in BDL rats | 26 |
| Liposome | IFN-γ | Cyclic peptides | ≤100 | – | Extended circulation half-life, reduced side-effects in rats with hepatic fibrosis due to selective delivery to activated HSCs | 27 |
| Mesoporous silica NPs-RhB | Salvianolic acid B | – | 400 | – | Remarkable inhibiting effect on reactive oxygen species level and on the proliferation activity of LX-2 cells | 28 |
| NLC | Curcumin | Phosphatidylserine | 204.6 ± 1.97 | −46.29 ± 0.48 | Prolonged retention time, and enhanced bioavailability and delivery efficiency | 22 |
| Hyaluronic acid-polylactide NPs | Curcumin | Hyaluronic acid | 60–70 | −30 | Improved drug efficiency, reduced drug dosage, and attenuated tissue collagen production and cell proliferation | 29 |
| PLGA | Phyllanthin | – | 187.6 ± 5.0 | −24.6 ± 0.5 | Increased aqueous drug loading, and anti-fibrotic efficacy at lower doses | 30 |
| PEG−PLGA | Sorafenib | – | 100–300 | −10 to −15 | Ameliorated liver fibrosis, decreased α-SMA and collagen production in the livers of CCl4-treated mice, and decreased microvascular density | 31 |
| Micelles | Losartan | Hyaluronic acid | 300 ± 25 | −40 ± 5 | Reduction of α-SMA and collagen deposition, and reduction of serum enzyme level in mice | 32 |
| Iron oxide | – | Citrate | 12 | – | Production of good magnetic resonance contrast in liver diseases imaging | 33 |
−Not applicable.
Table 2.
Clinical trials for liver fibrosis.
| Drug | Study phase | Status | Clinical trials identifier |
|---|---|---|---|
| Simtuzumab | II | Terminated |
NCT01672853 NCT01672866 NCT01452308 |
| GS-4997 alone or in combination with simtuzumab | II | Completed | NCT02466516 |
| Peginterferon α-2b and glycyrrhizin in interferon | III | Terminated | NCT00686881 |
| Peginterferon α-2b and ribavirin | III | Completed | NCT00323804 |
| Cyclosporine A and tacrolimus | IV | Terminated | NCT00260208 |
| Entecavir and peg-interferon | IV | Completed | NCT01938781 |
| Entecavir and anluohuaxian | – | Recruiting | NCT03568578 |
| Entecavir + Fuzheng Huayu + TCM granule | IV | Recruiting | NCT02241616 |
| Candesartan and ramipril | III | Recruiting | NCT03770936 |
| Candesartan | I, II | Completed | NCT00990639 |
| Pirfenidone | II | Recruiting | NCT04099407 |
| Oltipraz | II | Completed | NCT00956098 |
| Methotrexate | – | Completed | NCT00673101 |
| Raltegravir and ritonavir-boosted protease inhibitor | II | Completed | NCT01231685 |
| Losartan | IV | Completed | NCT00298714 |
| GB1211 | I | Recruiting | NCT03809052 |
| BLD-2660 | I | Recruiting | NCT03559166 |
| Spirulina | – | Completed | NCT02744105 |
| GR-MD-02 | II | Completed | NCT02421094 |
| ND-L02-s0201 injection | I | Completed | NCT02227459 |
| Selonsertib | III | Completed | NCT03053050 |
| MGL-3196 | III | Recruiting | NCT03900429 |
| Emricasan | II | Completed | NCT02686762 |
| Silymarin, ursodeoxycholic acid, and colchicine | – | Completed | NCT03659058 |
| Silybin + vitamin E + phospholipids complex | III | Completed | NCT01935817 |
| Tacrolimus, antithymocyte globulins + mycophenolate mofetil, tacrolimus + antithymocyte globulins | IV | Completed | NCT00538265 |
| Irbesartan | III | Completed | NCT00265642 |
| Synbiotic | II, III | Completed | NCT01791959 |
| Tropifexor and cenicriviroc | II | Recruiting | NCT03517540 |
| Cenicriviroc | III | Recruiting | NCT03028740 |
| Pentoxyphilline and tocopherol | III | Terminated | NCT00119119 |
| Resveratrol | II, III | Completed | NCT02030977 |
−Not applicable.
In this review, we discuss the pros and cons of DDS containing conventional drugs and plant extracts, also GDS (non-coding RNA, DNA, and mRNA) with their perspectives.
3. Anti-fibrotic agent and delivery system
Several therapeutic agents, including chemical compounds, plant extracts, and nanotherapeutics like gold NPs, show potent anti-fibrotic activities in experimental models of hepatic fibrosis by targeting different pathways37. Gliptins, as an example of the chemical compound series, with anti-diabetic and anti-inflammatory activity, has been used for type 2 diabetes treatment. A study in 2018 revealed the effectiveness of linagliptin and sitagliptin on liver fibrosis and NASH38. Inflammation and steatosis regression were proved by suppression of tumor necrosis factor-α (TNFα), inducible nitric oxide synthase (iNOS), α-smooth muscle actin (α-SMA), procollagen α1(I) (Col1α1), and matrix metalloproteinase (MMP)-12. Moreover, the decrease in the ratio of liver-infiltrating pro-inflammatory monocytes/macrophages to anti-inflammatory macrophages mitigated vascular dysfunction and liver fibrosis. Another chemical compound is ethyl pyruvate that blocks TLR4 signal and NF-κB transcription and phosphorylation. It also reduces IL-6, TNF-α, IFN-γ, and HMGB1 and increases the ratio of MMPs to the tissue inhibitor of metalloproteinase (TIMPs). These changes result in the inhibition of HSCs activation and facilitate the degradation of ECM39,40.
Along with chemical compounds, herbal medicine has increasingly been prescribed for the treatment of liver fibrosis. About 50 plants have been monitored in vivo for their potential effect on fibrosis. Silymarin, armepavine, plumbagin, rhein, glycyrrhetic acid, ginseng, epigallocatechin-3-gallate, curcumin, salvianolic acid, and osthole have been studied and documented as the active ingredients of phytomedicine, which have been used in the treatment of liver fibrosis more than others41. However, due to the lack of scientific justification and processing difficulties, such as standardization and identification of individual drug components in complex polyherbal systems, they were not considered for developing novel formulations. Nevertheless, in the last decade, significant advances have been made in the development of plant-based hepatoprotective drugs, mostly on account of their lower toxicity42. For instance, the effect of S-allyl-cysteine (SAC), one of the major compounds in aged garlic extract, was examined in fibrotic rats in 201843. SAC, as an endogenous donor of hydrogen sulfide (H2S), plays emerging roles in the gastrointestinal tract and liver. Treatment with SAC improved semi-quantitative scores of fibrosis severity, reduced the mRNA expression of inflammatory and fibrogenic cytokines, and induced the mRNA expression of antioxidant enzymes. Moreover, the mRNA expression of liver fibrosis biomarkers, including α-SMA, fibronectin, and collagen, were also decreased after SAC treatment. Umbelliferone (UMB) is a natural coumarin with diverse biological activities. The anti-fibrotic efficacy of UMB was revealed in 201944, by attenuating oxidative stress, inflammation, TGF-β1/SMAD 3 signaling, and upregulation of PPARγ. Therefore, UMB may be a candidate for preventing hepatic fibrogenesis; however, further research is needed to determine the exact molecular mechanisms underlying its anti-fibrotic efficacy.
Although several chemical compounds and plant extracts have been tested for their efficacy in liver fibrosis, pharmaceutical interventions were not effective enough on account of the insufficient supply of drugs into the diseased tissue and the adverse effects of miss targeting or off-targeting. Using nano-drug delivery systems (NDDS) may be an effective solution for solving these drawbacks. For example, anti-inflammatory, immune-modulatory, and chemopreventive properties of carvacrol have been shown in vivo and in vitro 45,46. Carvacrol is highly volatile and lipophilic with low water solubility and a strong odor of essential oils; therefore, its application in the food industry is difficult. The research revealed that the encapsulation of phytochemicals could cause a decrease in size and an increase in their bioavailability47. Rely on that, a study conducted in 2017 to define the efficacy of carvacrol nanoencapsulation and nanoemulsion48. The results showed the potential of both formulations in bioavailability improvement and overcoming any drawbacks of carvacrol application. However, the efficiency of nano-encapsulated carvacrol in amelioration of the TAA model of liver fibrosis was more prominent than nanoemulsion form.
NDDS are not only capable carriers, but also may be an efficient therapeutic agent too. Inorganic NPs including, iron oxide, gold, cerium oxide, titanium oxide, and manganese oxide NPs can be used as a diagnostic, anti-inflammatory, anti-oxidant, and DDS to a fibrotic liver. However, their entrapment in the liver may pose health risks, mainly due to non-biodegradability and potential toxicity. Thus, before the application, assessment of health risks to beneficial effects seems necessary49.
4. Nanoparticles for liver targeting
The first role of NPs [liposome, solid lipid nanoparticles (SLNs), micelles, polymers] is to deliver compounds to the specific site of diseased tissue. Sometimes carriers, prepared from bioactive compounds, possess therapeutic properties themselves. Carriers with a homing device can be a two-edged sword, and sometimes adding a targeting moiety leads to the desired effect. However, on the other hand, it decreases or avoids targeting; therefore, the presence of a targeting ligand and its density may cause lots of conflicts that need to be controlled. In this respect, to clarify, the effect of liposome prepared from different kinds of lipids was studied on the activation of HSC and aggravation of liver fibrosis induced by BDL in rats50. Three types of liposome, including 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) liposome, 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLPC) liposome, and mannose 6-phosphate modified albumin (M6P-HSA)-DLPC liposome, were examined in this study. In cultured HSC, the anti-fibrotic effect of DLPC liposome containing M6P-HSA and plain DLPC liposome was noticeable, but liposome prepared by POPC did not decrease the mRNA level of fibrosis markers. However, in vivo results were not the same, and M6P-HSA DLPC liposome did not show an anti-fibrotic effect in the liver. In contrast, the accumulation of M6P-HSA DLPC liposomes in KCs and LSECs caused a pro-inflammatory trend in the liver. Since scavenger receptors on KCs and LSECs could recognize the introduced negative charge on HSA by M6P groups, in addition to the targeting ligand, its density is another important factor. The recent research emphasizes on the retinol density of NPs for active targeting of HSC51. In this respect, the research demonstrated that chitosan NPs modified with low retinol density has 2 times enhanced uptake in comparison to unmodified NPs. In contrast, NPs with a high retinol density showed approximately 0.8-fold change in uptake. Therefore, adding a targeting moiety, carrier, and its ingredients should be selected and optimized carefully for the density and orientation to achieve the best delivery device; otherwise, the concentration in undesirable areas will increase.
Lipid formulations can be modified by various methods to reduce toxicity, improve drug stability, and efficacy. Lipid-based NPs like liposomes are safe and effective, and it has been proven that they are valuable alternatives for the formulation of drugs, as well as vaccines, diagnostics, and nutraceuticals. Furthermore, liposomal DDS or GDS for the treatment of liver fibrosis is currently in the clinical stages, which indicate the efficiency of these NPs compared with other NPs in practice. However, the low solubility, the high cost, production complicity, and the probability of drug leakage are challenges ahead of the researches to clinical trials52. Imatinib is an inhibitor of two pro-fibrotic pathways, TGF-β and PDGF; liposomal imatinib not only improves liver fibrosis treatment but also resolves the drawbacks of conventional imatinib, which includes low concentration at target tissue and toxicity to other tissues, especially heart, lung, and liver. Preparation of new vitamin A coupled imatinib-loaded liposomes with the size smaller than 200 nm and their intraperitoneal (i.p.) injection showed 13.5-fold higher hepatic accumulation than conventional imatinib. Bio-distribution to other organs decreased too36. Since HSCs store 80% vitamin A of the body, and they are the main contributors to liver fibrosis pathogenesis, they could be actively targeted by coupling vitamin A to liposome.
SLNs as an alternative delivery system for carriers like liposomes and polymeric micro and nano NPs were introduced in 199153. The toxicity of SLN is lower because their lipid matrix was made from physiological lipids. Their other upsides are the enhancement of solubility and bioavailability of sparingly soluble drugs, site-specific delivery, and controlled release of the encapsulated drugs. The obstacles to their usage can be drug leakage during storage and insufficient total drug load. The effect of SLNs on liver targeting was studied in 2007 by compounds like silymarin and oxymatrine54,55. Measured factors, including relative exposure, targeting efficiency, and maximum drug concentration ratio in mice, verified SLN as a good liver-targeted DDS. Silibinin (SIL) is another compound that the anti-fibrotic effect of its SLN form is much higher than the suspension formulation56. Recent progress in this area is adding targeting ligand on the surface of the carrier to increase the efficacy of targeting and diminishing the side effects. Curcumin-NLC (nanostructured lipid carriers) modified with phosphatidylserine is an example that was able to overcome many defects of curcumin clinical application22.
Polymeric materials are another type of carrier used for preparing NPs for fibrosis targeting. They must be at least biocompatible and best biodegradable. Proteins are natural polymers with less possibility of opsonization by the reticuloendothelial system, so when the target cells are not KCs, these NPs can be useful. They also generate bioactive peptides through hydrolysis in the body that may exert some physiological effects in vivo. The other upsides of them are easy preparation and scaled up, creation of three-dimensional networks for protecting active compounds in a matrix, and specific targeting to the site of action57,58. The downside of protein-based NPs includes interruption of the scaling-up process due to heterogeneous size distribution and batch to batch variation. However, some researchers have studied the reproducibility of the process. For instance, monodispersed HSA and gelatin have been produced in 2008 and 2011, respectively59,60. The long half-life, biocompatibility, biodegradability, and non-immunogenicity make HSA an applicable protein-based carrier. This molecule can absorb negative and positive compounds. However, its hydrophilic nature and rapid solubilization do not allow sustained release of the drug. It is worth mentioning that this issue could be solved by using chemical crosslinkers and plant proteins61,62. HSA was the first carrier accumulated in HSC63 and the binding of this carrier to specific receptors, which are highly up-regulated on activated HSCs, brings out cell specificity64. In this regard, the M6P-HSA-losartan-Rho kinase inhibitor is a good example that shows the importance of NPs and targeting ligand. In the fibrotic liver, portal pressure is increased due to the increased intrahepatic resistance65,66. Thus, controlling the portal pressure without affecting mean arterial pressure and renal function is essential in end-stage liver failure management. For this purpose, M6P-HSA has been used for losartan and Rho-kinase inhibitors delivery. In vivo effectiveness of HSA-based drug delivery, on the fibrotic liver was shown by dexamethasone coupling to HSA. LSECs and KCs of the liver, which share specific characteristics like possession of scavenger receptors are targeted by this structure67.
5. Gene delivery
Liver-based gene therapy has been used to down regulate/block the expression of damaged genes, to deliver therapeutic genes, and to prevent allograft rejection68. According to possible changes in the liver during fibrosis, it seems genetic manipulation can modify myofibroblasts and convert hepatocytes to healthy liver cells and help liver regeneration69. In the gene therapy method first, the defective gene is identified and characterized; secondly, the extraction and mass production of healthy and the natural gene is conducted, and then this gene is placed in viral or non-viral vectors and delivered to target cells70. Several methods, such as clustered regularly interspaced short palindromic repeats (CRISPR), zink finger nucleases (ZFN), and transcription activator-like effector nucleases (TALEN), are common genome editing techniques that are considered as developments in genetic engineering and medical sciences71,72. CRISPR is the part of prokaryotes DNA that contains short alternating sequences, acts as a molecular scissors and makes specific cuts in regions of the genome. ZFNs, having two domains that bind to DNA and with the help of intracellular DNA reconstruction systems, alter the genome of evolved organisms accurately. TALENs as restriction enzymes with two domains like ZNFs, can be engineered to bind and cut any desired DNA. Since there are ethical restrictions on the manipulation of human reproductive cells, the maneuver can just be performed on somatic cells that are not passed on to the next generation73. Methods could be conducted in ex vivo and in vivo environment, and both of them are applicable potentially for liver cells. In the ex vivo method, individual cells are transmitted to the external environment; then, they are modified and transmitted to the body again. This method is invasive, but biocompatible and highly cell-specific. Advantages of the in vivo method are high repeatability and less aggression, although cell specificity of this process is low74.
5.1. DNA-based delivery
Drugs on the basis of DNA sequence and structure can control disease progression. Plasmids containing transgenes, oligonucleotides for antisense and antigene applications, aptamers, ribozymes, and DNAzymes are in this category. The high selectivity and specificity of molecules for recognition of their targets reduce their toxicity and side effects. However, poor cellular uptake and rapid in vivo degradation of DNA-based therapeutics necessitate the use of delivery systems75. Some of the ideal properties in a DNA delivery system for medical purposes include high transmission efficiency, low toxicity with high immunity, biodegradability, the stability of the pharmaceutical formulations, and convenience in manipulation75.
DNA delivery methods can be divided into three general types: electric techniques, mechanical transfection, and vector-related delivery systems. Mechanical and electrical methods for the transfer of naked DNA into the cells include microinjection76, particle bombardment under high vacuum pressure77, and electroporation78. The delivery system and method of transfer play a vital role; in this regard, the effect of ultrasound microbubble delivery on the efficacy of hepatocyte growth factor (HGF) delivery has been studied79. HGF is a cell growth factor with anti-fibrotic activity through apoptosis induction, regulation of inflammatory response, reduction in excessive collagen deposition, and stimulation of liver regeneration1. After transfection of HGF cationic liposomes with this method, the expression increased, lobules got their complete structure again, and the amount of fibrous septum went down79. In this process, the time and intensity of the ultrasound acted as the lever of the release process.
Oligonucleotides are short single-stranded segments of DNA that upon cellular internalization can selectively inhibit the expression of a single protein. For antisense applications, oligonucleotides form a duplex with the mRNA or the pre-mRNA and inhibit their translation and protein biosynthesis. TGF-β1 is a potent factor to enhance the synthesis and accumulation of ECM. Adenoviral expression of TGF-β1 antisense oligodeoxynucleotides (ODNs) resulted in an inhibition of HSC activation and liver fibrogenesis in rats80. In the other study hydrodynamic injection of Timp-2 antisense ODNs had a preventive effect on an immune-induced liver fibrosis in rats. Because ECM is degraded by a family of proteolytic enzymes called Mmps, and the activity of Mmps is regulated by Timps81.
Plasmid-based vectors are a circular, closed-loop DNA strand in which the desired gene is combined and used in different ways, such as direct injection into target tissues82. Although the plasmid vectors are relatively inexpensive, less-immunogenic, and more available, compared to viral vectors, they have some disadvantages that should be improved with changes, such as a reduction in their length83. Augmentation of liver regeneration (ALR) as a cytokine stimulates hepatic cell proliferation and inhibits hepatic natural killer cell activity in acute liver injury. In a previous research ALR recombinant plasmid reduced serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and expression of Timp-1, and collagen types I and III84. In the other study, transfection of a plasmid containing the soluble receptor type II TGF-β1 cDNA into skeletal muscle in hepatotoxin-induced fibrosis in rats, decreased hepatic fibrosis, hydroxyproline content, collagen and α-Sma expression85.
5.2. Coding and non-coding RNA delivery
In recent years, a large number of coding RNA (mRNA) and non-coding RNA, such as short non-coding RNA (miRNA & siRNA), have been identified through several screening programs for liver fibrosis research and clinical trials86. Unlike gene therapy based on the plasmid DNA, treatment with mRNA is a new approach and still in its infancy87. Compared to DNA, the use of mRNA for gene therapy has many advantages. First, mRNA applies its function in the cytoplasm, and its activity does not depend on the core membrane lysis, which is the principal intracellular barrier for DNA gene therapy88,89. Second, according to its location, treatment with mRNA does not require genomic integration; thus, the potential for the risk of an internal mutation reduces. Also, the process of production, raw material synthesis, and mRNA product quality relative to DNA can be easily customized, which makes mRNA gene therapy more advantageous90.
Micro RNAs (miRNAs) can affect liver fibrogenesis by TGFβ signaling modulation, since TGF is crucial for liver fibrogenesis. MicroRNA-101 family members act as suppressors of TGFβ signaling by targeting TβRI and its transcriptional activator Kruppel-like factor 6. In the liver, miR-101 weakened TGFβ and stopped the expression of profibrogenic cytokines, cell proliferation, and switched active HSCs to silent mode. So it seems blunt of TGFβ signaling in HSCs or hepatocytes could be one effective inhibitory factor for liver fibrosis91.
Unmodified oligonucleotides are not stable in circulation; they can be attacked by the immune system and hardly penetrate the cells. Cationic modification can increase stability; however, most oligonucleotide treatments require an optimized delivery system to achieve the desired biological effects. In selection of a delivery system, several aspects should be considered, including stability against serum nucleases, escape from the inherent immune system, avoidance of unspecified interactions with serum proteins and non-target cells, prevention of kidney secretion, releasing from blood vessels, entrance to the desired cell, and attachment to RNAi (RNA interference)92. Additionally, diseases affect the performance of gene delivery; for instance, hydrodynamic gene transfer is a common method for gene transfer to the animal liver. Kobayashi et al.93 tested the effects of hepatic fibrosis on the performance of hydrodynamic gene delivery using rat liver fibrosis model. By using pCMV-Luc plasmid, they reported that this method is safe, but the amount of fibrotic tissue in the liver reduced gene transfer efficiency. They showed that anti-fibrotic gene therapy with the Mmp-13 gene decreased the hepatic fibrosis and improved the efficiency of the hydrodynamic gene transfer.
Gene expression regulation by small interfering RNAs (siRNAs) is a new and powerful tool that was recently used for therapeutic purposes94. siRNAs can induce silencing of any gene at the post-transcriptional levels, which do this by cleaving transcripts of homologous targets. In vivo barriers to siRNAs delivery are instability due to exposure to nucleases and toxicity at high doses95. Two major approaches are available to overcome these obstacles. The first one is the chemical modification of siRNA molecules, including backbone, nucleobase, and/or sugar modification and the latter is conjugation with vector or carrier molecules, such as lipophilic carriers, cationic polymers, and PEG conjugates. Besides, the using of NPs like lipid and lipid-like materials, and CPP-based NPs as a carrier has attracted much attention recently92.
Most of anti-fibrotic therapies mainly focus on HSCs. However, hepatocytes consist up to 80% of the liver mass and mediate a broad range of interactions among different cells. In a recent study miR-221-3p which is upregulated in hepatocytes during liver fibrosis has been targeted. Researchers showed that in vivo knockdown of miR-221-3p by AAV TuD suppressed HSC activation and alleviated hepatotoxin-induced liver fibrosis in mice. Unlike other methods of RNA silencing that lead to systemic effects, this method specifically targeted hepatocytes and decreased off target effects. This study introduced hepatocytes as a regulator of HSC activation and a therapeutic target of liver fibrosis96. The effect of hydrodynamic transfection of PDGFR-β siRNA plasmids has been studied in vitro and in vivo. Down regulation of Pdgfrb expression caused suppression of activated HSCs and improvement of liver function97. The effectiveness of siRNAs among the various gene therapies has been revealed by different researcher and the prominent studies are summarized in Table 376,80,91,93,96,98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121.
Table 3.
Gene delivery system for liver fibrosis76,80,91,93,96,98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121.
| Carrier | Gene | Target | Effect and mechanism of action | Ref. |
|---|---|---|---|---|
| Adenoviral vector + cytomegalovirus (Ad5-CMV) | Antisense-TGF-β1 mRNA | TGF-β synthesis in cultured HSCs | Abrogates TGF-β enhanced production of collagen and α-SMA | 80 |
| Adenoviral vector | Human urokinase plasminogen activator cDNA | Latent hepatic collagenases | Reduced α-Sma, increased Mmp-2, stimulated liver regeneration | 98 |
| Adenovirus + HBV vector (chimeric Ad-HBV shuttle vector) | Truncated MMP-8 gene (tMMP8) | HGF | Induced hepatocyte proliferation in liver cells without affecting other tissues | 99 |
| Adenoviral vector | HGF-encoding cDNA | Fibrogenic cytokines PDGF-bb and TGF-β1 | Elevated HGF levels in the portal vein, decreased collagen level | 100 |
| Recombinant lentivirus particles | Artificial miRNAs | Pdgfrβ and Tgfbr2 | Co-knockdowns the expressions of Tgfbr2 and Pdgfrβ, suppressed expressions of α-Sma, Col1a1, Mmps and Timp1 | 101 |
| Recombinant adenoviral vector | β-Galactosidase | Hepatocytes | Decreased the expression of hepatocytes | 102 |
| Recombinant adenoviral vector | MMP-1 | Hepatocytes | Decreased the number of activated HSCs, and increased hepatocyte proliferation | 103 |
| Recombinant adenoviral vector | MMP-8 | Hepatocytes | Diminished hydroxyproline content, and up regulated expression of MMP-2 and MMP-3 | 104 |
| Adeno-associated virus | ACE2 | Hepatic ACE2 and angiotensin II | Reduction of angiotensin II, and inflammatory cytokine expression | 105 |
| Adeno-associated virus | Bone morphogenetic protein-7 (Bmp-7) | Tgfb | Long-term elevation of serum Bmp-7 concentrations and amelioration of CCl4-induced hepatic fibrosis | 106 |
| Adeno-associated virus | miR-221-3p | Hepatocytes | Faster resolution of the deposited ECM, and reduced secretion of C-C motif chemokine ligand 2 | |
| Hemagglutinating virus of Japan (HVJ) | Oncostatin M (OSM) cDNA | OSM protein in KCs | Reduced centrilobular necrosis and inflammatory cell infiltration, augmented hepatocyte proliferation, and suppressed hepatocytes apoptosis and fibrosis | 107 |
| Recombinant simian virus 40 vector (rSV40) | Recombinant insulin-like growth factor I (rIGF-I) | rIGF-I receptor | Reduced serum bilirubin, transaminases and liver fibrosis score, and increased expression of Hgf and Mmps | 108 |
| Minicircle vector (MC-hALR) | Regeneration/growth factor ERV1-like (ALR/GFER) gene | Tgfb, Pdgfb, α-Sma | Suppressed production of collagen I and α-Sma, Tgfb, Pdgfb, alleviated liver injury and fibrosis in rats | 109 |
| Plasmid | Artificial miRNA | CTGF, TGF-β1 | Reduced hepatic fibrosis, and decreased levels of collagen I and α-SMA | 110 |
| Graphene-dendrimer nanostar | Plasmid encoding for the collagenase MMP-9 | Macrophages | Promoted macrophage switch from inflammatory M1 to pro-regenerative M2 in three days | 111 |
| pCMV-Luc plasmid | Mmp-13 gene | Hepatocytes | Reduced liver fibrosis, and improved efficiency of hydrodynamic gene delivery | 93 |
| psiCHECK-2 | miR-378a-3p, miR-378b, miR-378d | Gli3 in activated HSC | Accelerated expression of fibrotic genes and hedgehog signaling pathway | 112 |
| Lentivirus | miR-122 | Type I collagen | Decreased collagen, Fn1 and Srf levels in the liver of CCl4-treated mice | 113 |
| Lentivirus | miRNA-101 family members | – | Attenuated profibrogenic Tgfb signalling and suppressed Tgfb-induced hepatocyte apoptosis and the inhibited cell proliferation | 91 |
| Lentivirus | Atp7b gene | Copper transport protein | Lowered liver copper levels, and decreased fibrotic tissue | 114 |
| Lentivirus | MiR-542-3p | BMP-7 | Reduced liver fibrosis | 115 |
| pPB-modified stable nucleic acid LNPs | siRNA | Heat shock protein 47 | Inhibitory effect on TAA-induced hepatic fibrosis with high gp46 mRNA expression | 116 |
| Poly (lactide-co-glycolide)-polyspermine-poly (ethylene glycol)-vitamin A (PLGA-PSPE-PEG-VA) | Co-delivery of silibinin and siCol1α1 | Activated HSCs | Targeted activated HSCs specifically, decreased collagen I production and ameliorated liver fibrosis | 117 |
| CXCR4-targeted NPs | CXCR4+ sorafenib and MEK inhibitor | ERK in activated HSCs | Prevented activation of ERK in activated HSCs, anti-fibrotic effects in the CCl4-induced murine model | 118 |
| Ultrasound-targeted microbubbles | HGF | Collagen I and HGF | HGF delivery into the fibrotic liver and production of an anti-fibrosis effect | 79 |
| CSLNs | siCtgf | Pro-fibrotic genes in HSCs | Reduced collagen content Tnfa, Tgfb, IL-6, and Ctgf significantly, improved pathophysiological symptoms in rats | 119 |
| VA-polyethylene glycol polyethyleneimine-poly(N-(N′,N′-diisopropylaminoethyl)-co-benzylamino) aspartamide | miRNA-29b and miRNA-122 | HSC | Improved liver function, and relieved hepatic fibrosis | 120 |
| Poly(amine-co-ester) NPs | Nogo-B siRNA | Liver | Suppressed Nogo-B protein in the liver up to 60% after systemic administration | 121 |
6. Vectors
Viral and non-viral vectors, as the most relevant vectors in liver fibrosis context, are introduced and described here.
6.1. Viral vectors
According to studies, viral vectors are the best and most reliable carriers for gene transfer; these vectors have been modified in some specific genomic regions so that they cannot be replicated, and their immunity is increased. The benefits of these delivery systems are the high infection transmission rate and high expressing levels122. Retrovirus, lentivirus, adenovirus, or adeno-dependent viruses (AVVs) are among them.
Retroviruses are generally animal types and they are not pathogenic to humans. They can carry the gene into target cells without side effects or disease. Retroviruses have an RNA genome, which after infecting the cells, convert with the reverse transfusion enzyme into DNA and by integrase enzyme integrate within the host cell genome. Lentiviruses, belonging to this family, are widely used in gene delivery123.
Adenoviruses are viruses with genomic DNA, which cause lung, digestive, and respiratory infections in humans. The advantages of this category are relative immunity (even weakened viruses lead to mild respiratory infections), easy production, purification and condensation in high amounts, and the ability of gene delivery into the silent and dividing cells. They are the most common vectors for gene transfer with the most popular Ad5 and Ad1, 2 and 6 serotypes124. Nevertheless, the lack of optimum gene delivery to specific cells and the antiviral inflammatory responses of the immune system and, consequently, the lack of continuity of gene expression limit the application of these viruses125.
Despite the limitations, since 1990, adenoviruses have been the preferred option for gene therapy applications, especially in cancer. Reforms to solve the problems of this category have led to the creation of different generations of adenoviruses126. In fact, until 2003, 600 gene therapy protocols have been reported, 27% of which used adenovirus as carriers127. Currently, the third generation of adenoviruses, called helper-dependent vector or gutless carriers, has been developed that is free of viral proteins and causes long-term gene expression128. One of their applications was introduced in 2003 by human MMP1 on the liver fibrosis induced through either TAA or BDL. According to the findings, 14 days after the transmission, in Ad5MMP-1-injected, but not in Ad5LacZ-injected rats, fibrosis was moderated, and the number of active HSCs decreased. After a few weeks, the reproductive influence of the human MMP1 approximately disappeared; however, liver fibrosis remained attenuated in Ad5MMP-1-injected rats, which was in contrast with the situation of Ad5LacZ-injected rats103. In new therapeutic approaches for hepatic fibrosis with gene therapy, Ad vectors are used to deliver genes abundantly. Some applications of Ad in gene delivery are given in Table 3.
6.2. Non-viral vectors in liver fibrosis gene therapy
The increasing use of non-viral vectors has generally started in the last decade. Although there is less expression with these vectors, these carriers have more safety, less immunogenicity, and fewer restrictions than viral vectors129. Non-viral vectors including lipid NPs (LNPs)130, lipid-calcium-phosphate NPs (LCP NPs)131, lipoplexes132, polymeric NPs, and inorganic NPs, are used in gene therapy for liver disease130,133. Among them, LNPs and polyplexes are used in liver fibrosis gene therapy more than others (Table 3).
In recent years, lipid-based drug delivery systems (LBDDS) have become increasingly important because of their water-solubility and bioavailability. Liposome as an example of LBDDS was discovered as a DNA delivery system in 1979134, and between 1979 and 1980, the encapsulation of DNA plasmid and RNA poliovirus into liposome become possible135. MMP-2 is secreted by HSCs and it is important in the formation of liver fibrosis. Delivery of MMP-2 siRNA in vitamin A-coupled liposomes to the HSC-T6 cells reduced the mRNA expression and activity of MMP-2, and the protein expression levels of α-SMA and collagen type I decreased too. In addition, that liposomal delivery lowered cytotoxicity136.
Cationic solid lipid NPs (CSLNPs) are another non-genetic transfer techniques, and nuclease-resistant CSLNPs prepared from natural LDLs, have been applied as target specific systemic delivery of siRNA-connective tissue growth factor (siCTGF). CTGF is a secreted matricellular protein that induces formation and activation of myofibroblasts through trans-differentiation of epithelial cells, stellate cells, and resident fibroblasts. In 2006 intraportal vein siRNA injection targeting CTGF has shown inhibitory effect on CTGF expression137, and in 2013, specific delivery of CSLNPs/siCTGF complex to the liver, resulted in a significant reduction in collagen content and pro-fibrogenic parameters119.
Many cationic polymers are automatically connected to DNA for gene transfer in many cells, but also, the pharmaceutical state of the polyplexes limits the gene transfer. Poly 2-dimethylaminoethyl methacrylate (PDMAEMA) is a water-soluble cationic polymer, that can be linked to DNA by electrostatic bonding138. By reviewing the physiological and biological data of polyplexes, De Smedt, and colleagues139 created a new insight into this kind of gene delivery system. They reported surface features, solubility, agglomeration, fragmentation, and gene transfer methods as essential factors influencing the compression of DNA. Recently, various cationic polymers have been studied. Using nature's self-selective cellular uptake mechanisms for specific organ cells has enabled researchers to step closer to overcome some of the mentioned challenges on the way of optimal gene silencing140. MicroRNA-29b and miRNA-122 have great potential in treating liver fibrosis, but a specifically HSC targeted delivery system for in vivo applications was needed. This issue is solved by a pH-sensitive and vitamin A conjugated copolymer. Synthesized VA-PEG-PEI poly(N-(N′,N′-diisopropylamino ethyl)-co-benzyl amino)aspartamide (T-PBP) and its assembly into SPIO-decorated cationic micelle was able to transport the miRNA-29b and miRNA-122 to HSC in a magnetic resonance imaging-visible manner. Moreover, this combination improved liver function and alleviated hepatic fibrosis, whereas the non-targeting combination treatment showed almost no effect120.
7. Combination therapy
Although considerable emphasis has been placed on understanding the mechanism of liver fibrosis, strategies targeting a single receptor or pathway often exhibit limited efficacy in humans. Given such heterogeneity in response, combination therapy seems reasonable to treat the fibrotic liver comprehensively141. Combination therapy is a multipronged approach and in the simplest form targets two vital, however, very different pathways to reduce upstream (chronic) inflammation and downstream ECM deposition. A combination therapy may be more effective, given that crosstalk among different cell types, also it has the potential to decrease or eliminate the side effects that may result from targeting a single mechanism142. Despite its promise at present, significant expense and effort are required to validate efficacy of potential anti-fibrotics at different doses and in several rodent fibrosis models. In addition, noninvasive biomarkers is needed for the quantification of fibrogenesis, and liver function143. One of the difficulties on the way of the therapies is insufficient drug accumulation at the target site because of reduced hepatic blood flow144. Combination of sorafenib with mitogen-activated protein kinase kinase (MEK) inhibitors is a recent study showing the effectiveness of combination therapy. The drawback of RAF kinase inhibitors, such as sorafenib in anticancer studies, is the activation of the mitogen-activated protein kinase (MAPK) pathway in both malignant and normal stromal cells145,146, that leads to HSCs activation; however, the occurrence of this in activated HSCs during liver damage is unknown. Also, sorafenib often causes unwanted non-specific and off-target effects, leading to hand-foot syndrome, diarrhea, and hypertension147. The combination of sorafenib with MEK inhibitors on fibrosis pathogenesis is studied in vitro and in vivo, which showed suppression of both paradoxical MAPK and HSC activation in vitro, and alleviated liver fibrosis in murine models and prevented fibrosis-associated HCC development and liver metastasis118. In the other study in 2018117, poly (lactide-co-glycolide)-polyspermine-poly(ethylene glycol)-vitamin A, used for the transfer of a chemical drug (sylbinoin) and genetic (siCol1α1). This combination obstructed collagen I accumulation in fibrogenesis synergistically. These particles were about 151 nm with a positive charge, and they effectively accumulated in HSCs and decreased collagen I production in vitro and in vivo. Combination of statins and JQ1 (thienotriazolodiazepine inhibitor), which is an inhibitor of bromodomain-containing protein 4 (BRD4), has examined recently148,149. Statins apart from their anti-lipidemic properties have a proven role in the prevention/reduction of HSC activation, and fibrosis progression in vitro, and in vivo. They have also been reported to decrease hepatic venous pressure and improve liver perfusion in patients with cirrhosis150. It seems BRD4 plays a critical role in fibrosis through the intercession of pro-fibrotic gene expression in HSCs151. Thus, blocking its enhancer interactions is expected to reduce HSC activation, but its general inhibition would not be free of adverse off-target effects152, 153, 154, 155. Modified with chitosan NPs with different densities of retinol and its loading with JQ1 and atorvastatin, two drugs that prevent HSCs activation via different mechanisms, showed that NPs modified with a low density of retinol as a targeting ligand had increased uptake in primary HSCs and fibrotic liver in vitro and in vivo51.
8. Conclusions and perspectives
In this review, we covered and discussed most of the prominent chemical/herbal anti-fibrotics, genes, and delivery systems. Although many NDDSs enable us to overcome the deficiencies of conventional drugs and phytochemicals, there are still a vast number of unanswered questions. Some herbal ingredients like silymarin, salvianoic acid B, and adenosine are in clinical trials, but their effectiveness as anti-fibrotic medicine has not proven yet. Also, the safety of herbal anti-fibrotic for prolonged periods or chronic administrations has not discovered, and due to the legal problems like restrictions to liver biopsies, their effectiveness has not been documented in human so far. The lack of effective therapy for liver fibrosis shows the complexity of this disease and a variety of active factors in its progress. The chemicals and phytochemicals affect solving this problem; however, previous studies are commonly mono-mechanistical. Moreover, damaged cells depend on the underlying cause of the disease. In most studies, this fact has been neglected, thus designing a targeting device has been done based on the total features of the disease without a deep understanding of fibrosis. Although NPs demonstrated their positive outcomes in animals, there are still some difficulties avoid them reaching the clinic. One possible reason is that many specific ligands used for active targeting are exogenous products, so they are suspected to trigger immunological side effects in clinical applications. Endogenous products, such as apolipoprotein AI (apo AI), and small molecules, such as vitamin A or mannose, could be ideal surface ligands for active targeting and should not be detrimental to human immunity in clinical applications. Also, the amount and orientation of ligand on the surface of NPs have not been optimized in the majority of studies since they are crucial parameters to impress the uptake of NPs. Furthermore, as it is shown in Table 1, most NPs have been used in their negative form, so according to the mentioned reasons about the uptake of NPs in liver the investigation on positively charged NPs seems necessary. Another possible reason is that the ligands homing to target receptors in animal may not be able to bind to human receptors effectively. Thus, a proper experimental animal model should be chosen and maybe more than one animal model should be used to test the targeting efficacy and eliminate individual heterogeneity and sampling errors. Also the introduction of in vitro systems that more faithfully replicate the pro-fibrogenic microenvironment of human liver is really awaited. 2D and 3D ECMs have different effect on key biological features of fibroblasts like proliferation, matrix deposition and degradation. So suitable models should present a 3D structure and express a variety of ECM components. It is worth mentioning that about gene therapy, several therapies currently rely on viral vectors to deliver nucleic acid cargo into cells. However, there is significant interest in moving toward chemical-based methods, such as polymer-based vectors, and some modifications to create a compatible and capable vector seem necessary156.
Acknowledgments
We gratefully thank the financial support of Tabriz University of Medical Science (Iran). This article is based on a PhD thesis (Dissertation number 149) submitted by Somaye Mahdinloo in Faculty of Pharmacy, Tabriz University of Medical Sciences, Iran.
Author contributions
Parvin Zakeri-Milani and Somaye Mahdinloo designed the work. Somaye Mahdinloo, Seyed Hossein Kiaie, and Ala Amiri collected data and wrote the manuscript. Salar Hemmati, Hadi Valizadeh, and Parvin Zakeri-Milani checked and revised the article. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under the responsibility of Institute of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Xu J., Murphy S.L., Kochanek K.D., Bastian B., Arias E. Deaths: final data for 2016. Natl Vital Stat Rep. 2018;67:1–75. [PubMed] [Google Scholar]
- 2.Yu Y., Lu L., Qian X., Chen N., Yao A., Pu L. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2009;19:903–914. doi: 10.1089/scd.2009.0254. [DOI] [PubMed] [Google Scholar]
- 3.Henderson N.C., Iredale J.P. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci. 2007;112:265–280. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 4.Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. J Hepatol. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwaisako K., Jiang C., Zhang M., Cong M., Moore-Morris T.J., Park T.J. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A. 2014;111:3297–3305. doi: 10.1073/pnas.1400062111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aydın M.M., Akçalı K.C. Liver fibrosis. Turk J Gastroenterol. 2018;29:14–21. doi: 10.5152/tjg.2018.17330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown B., Lindberg K., Reing J., Stolz D.B., Badylak S.F. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–526. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 8.Wynn T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starkel P., Leclercq I. Animal models for the study of hepatic fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:319–333. doi: 10.1016/j.bpg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Delire B., Stärkel P., Leclercq I. Animal models for fibrotic liver diseases: what we have, what we need, and what is under development. J Clin Transl Hepatol. 2015;3:53–66. doi: 10.14218/JCTH.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liedtke C., Luedde T., Sauerbruch T., Scholten D., Streetz K., Tacke F. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6:19–43. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiev P., Jochum W., Heinrich S., Jang J., Nocito A., Dahm F. Characterization of time-related changes after experimental bile duct ligation. BJS. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 13.Baba Y., Saeki K., Onodera T., Doi K. Serological and immunohistochemical studies on porcine-serum-induced hepatic fibrosis in rats. Exp Mol Pathol. 2005;79:229–235. doi: 10.1016/j.yexmp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Bartley P., Ramm G.A., Jones M.K., Ruddell R.G., Li Y., McManus D.P. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Mathews S., Xu M., Wang H., Bertola A., Gao B. Animals models of gastrointestinal and liver diseases. Animal models of alcohol-induced liver disease: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;306:819–823. doi: 10.1152/ajpgi.00041.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z.J., Fan J.G., Ding X.D., Qiao L., Wang G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–940. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parola M., Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspect Med. 2019;65:37–55. doi: 10.1016/j.mam.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Wang H., Ong Z.Y., Xu K., Ee P.L.R., Zheng S. Polymer-and lipid-based nanoparticle therapeutics for the treatment of liver diseases. Nano Today. 2010;5:296–312. [Google Scholar]
- 20.Veronese F.M., Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22:315–329. doi: 10.2165/00063030-200822050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lam P.L., Kok S.L., Gambari R., Kok T.W., Leung H.Y., Choi K.L. Evaluation of berberine bovine serum albumin nanoparticles for liver fibrosis therapy. Green Chem. 2015;17:1640–1646. [Google Scholar]
- 22.Wang J., Pan W., Wang Y., Lei W., Feng B., Du C. Enhanced efficacy of curcumin with phosphatidylserine-decorated nanoparticles in the treatment of hepatic fibrosis. Drug Deliv. 2018;25:1–11. doi: 10.1080/10717544.2017.1399301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F.Q., Su H., Chen X., Qin X.J., Liu J.Y., Zhu Q.G. Mannose 6-phosphate-modified bovine serum albumin nanoparticles for controlled and targeted delivery of sodium ferulate for treatment of hepatic fibrosis. J Pharm Pharmacol. 2009;61:1155–1161. doi: 10.1211/jpp/61.09.0004. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Pu S., Liu Q., Li R., Zhang J., Wu T. An integrin-based nanoparticle that targets activated hepatic stellate cells and alleviates liver fibrosis. J Contr Release. 2019;303:77–90. doi: 10.1016/j.jconrel.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Patel G., Kher G., Misra A. Preparation and evaluation of hepatic stellate cell selective, surface conjugated, peroxisome proliferator-activated receptor-gamma ligand loaded liposomes. J Drug Target. 2012;20:155–165. doi: 10.3109/1061186X.2011.610800. [DOI] [PubMed] [Google Scholar]
- 26.Du S.L., Pan H., Lu W.Y., Wang J., Wu J., Wang J.Y. Cyclic Arg-Gly-Asp peptide-labeled liposomes for targeting drug therapy of hepatic fibrosis in rats. J Pharmacol exp ther. 2007;322:560–568. doi: 10.1124/jpet.107.122481. [DOI] [PubMed] [Google Scholar]
- 27.Li F., Li Qh, Wang Jy, Zhan Cy, Xie C., Lu Wy. Effects of interferon-gamma liposomes targeted to platelet-derived growth factor receptor-beta on hepatic fibrosis in rats. J Contr Release. 2012;159:261–270. doi: 10.1016/j.jconrel.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 28.He Q., Zhang J., Chen F., Guo L., Zhu Z., Shi J. An anti-ROS hepatic fibrosis drug delivery system based on salvianolic acid B loaded mesoporous silica nanoparticles. Biomaterials. 2010;31:7785–7796. doi: 10.1016/j.biomaterials.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y.N., Hsu S.L., Liao M.Y., Liu Y.T., Lai C.H., Chen J.F. Ameliorative effect of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. Int J Environ Res Publ Health. 2017;14:11–27. doi: 10.3390/ijerph14010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krithika R., Vhora I., Verma R.J. Preparation, toxicity analysis and in vivo protective effect of phyllanthin-loaded PLGA nanoparticles against CCl4-induced hepatic fibrosis. J Drug Deliv Sci Technol. 2019;51:364–371. [Google Scholar]
- 31.Lin T.T., Gao D.Y., Liu Y.C., Sung Y.C., Wan D., Liu J.Y. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J Contr Release. 2016;221:62–70. doi: 10.1016/j.jconrel.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Thomas R.G., Moon M.J., Kim J.H., Lee J.H., Jeong Y.Y. Effectiveness of losartan-loaded hyaluronic acid (HA) micelles for the reduction of advanced hepatic fibrosis in C3H/HeN mice model. PloS One. 2015;10 doi: 10.1371/journal.pone.0145512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saraswathy A., Nazeer S.S., Jeevan M., Nimi N., Arumugam S., Harikrishnan V.S. Citrate coated iron oxide nanoparticles with enhanced relaxivity for in vivo magnetic resonance imaging of liver fibrosis. Colloids Surf B Biointerfaces. 2014;117:216–224. doi: 10.1016/j.colsurfb.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Bansal R., Nagórniewicz B., Prakash J. Clinical advancements in the targeted therapies against liver fibrosis. Mediat Inflamm. 2016;2016:1–16. doi: 10.1155/2016/7629724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Surendran S.P., Thomas R.G., Moon M.J., Jeong Y.Y. Nanoparticles for the treatment of liver fibrosis. Int J Nanomed. 2017;12:6997–7006. doi: 10.2147/IJN.S145951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Mezayen N.S., El-Hadidy W.F., El-Refaie W.M., Shalaby T.I., Khattab M.M., El-Khatib A.S. Hepatic stellate cell-targeted imatinib nanomedicine versus conventional imatinib: a novel strategy with potent efficacy in experimental liver fibrosis. J Contr Release. 2017;266:226–237. doi: 10.1016/j.jconrel.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 37.Schon H.-T., Bartneck M., Borkham-Kamphorst E., Nattermann J., Lammers T., Tacke F. Pharmacological intervention in hepatic stellate cell activation and hepatic fibrosis. Front Pharmacol. 2016;7:33. doi: 10.3389/fphar.2016.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X., Hausding M., Weng S.Y., Kim Y.O., Steven S., Klein T. Gliptins suppress inflammatory macrophage activation to mitigate inflammation, fibrosis, oxidative stress, and vascular dysfunction in models of nonalcoholic steatohepatitis and liver fibrosis. Antioxidants Redox Signal. 2018;28:87–109. doi: 10.1089/ars.2016.6953. [DOI] [PubMed] [Google Scholar]
- 39.Wang L.K., Wang L.W., Li X., Han X.Q., Gong Z.J. Ethyl pyruvate prevents inflammatory factors release and decreases intestinal permeability in rats with d-galactosamine-induced acute liver failure. Hepatobiliary Pancreat Dis Int. 2013;12:180–188. doi: 10.1016/s1499-3872(13)60029-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M., Hu X., Li S., Lu C., Li J., Zong Y. Hepatoprotective effects of ethyl pyruvate against CCl4-induced hepatic fibrosis via inhibition of TLR4/NF-κB signaling and up-regulation of MMPs/TIMPs ratio. Clin Res Hepatol Gastroenterol. 2018;42:72–81. doi: 10.1016/j.clinre.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Latief U., Ahmad R. Herbal remedies for liver fibrosis: a review on the mode of action of fifty herbs. J Tradit Complement Med. 2018;8:352–360. doi: 10.1016/j.jtcme.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mishra N., Yadav N.P., Rai V.K., Sinha P., Yadav K.S., Jain S. Efficient hepatic delivery of drugs: novel strategies and their significance. BioMed Res Int. 2013;2013:1–20. doi: 10.1155/2013/382184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong Z., Ye H., Huo Y., Wang L., Huang Y., Huang M. S-Allyl-cysteine attenuates carbon tetrachloride-induced liver fibrosis in rats by targeting STAT3/SMAD3 pathway. Am J Transl Res. 2018;10:1337–1346. [PMC free article] [PubMed] [Google Scholar]
- 44.Mahmoud A.M., Hozayen W.G., Hasan I.H., Shaban E., Bin-Jumah M. Umbelliferone ameliorates CCl4-induced liver fibrosis in rats by upregulating PPARγ and attenuating oxidative stress, inflammation, and TGF-β1/Smad3 signaling. Inflammation. 2019;42:1103–1116. doi: 10.1007/s10753-019-00973-8. [DOI] [PubMed] [Google Scholar]
- 45.Kılıç Y., Geyikoglu F., Çolak S., Turkez H., Bakır M., Hsseinigouzdagani M. Carvacrol modulates oxidative stress and decreases cell injury in pancreas of rats with acute pancreatitis. Cytotechnology. 2016;68:1243–1256. doi: 10.1007/s10616-015-9885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.da Silva Lima M., Quintans-Júnior L.J., de Santana W.A., Kaneto C.M., Soares M.B.P., Villarreal C.F. Anti-inflammatory effects of carvacrol: evidence for a key role of interleukin-10. Eur J Pharmacol. 2013;699:112–117. doi: 10.1016/j.ejphar.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 47.Huang Q., Yu H., Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75:50–57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 48.Hussein J., El-Banna M., Mahmoud K.F., Morsy S., Latif Y.A., Medhat D. The therapeutic effect of nano-encapsulated and nano-emulsion forms of carvacrol on experimental liver fibrosis. Biomed Pharmacother. 2017;90:880–887. doi: 10.1016/j.biopha.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Tee J.K., Peng F., Ho H.K. Effects of inorganic nanoparticles on liver fibrosis: optimizing a double-edged sword for therapeutics. Biochem Pharmacol. 2019;160:24–33. doi: 10.1016/j.bcp.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Adrian J.E., Poelstra K., Kamps J.A. Addressing liver fibrosis with liposomes targeted to hepatic stellate cells. J Liposome Res. 2007;17:205–218. doi: 10.1080/08982100701528047. [DOI] [PubMed] [Google Scholar]
- 51.Hassan R., Tammam S.N., El Safy S., Abdel-Halim M., Asimakopoulou A., Weiskirchen R. Prevention of hepatic stellate cell activation using JQ1-and atorvastatin-loaded chitosan nanoparticles as a promising approach in therapy of liver fibrosis. Eur J Pharm Biopharm. 2019;134:96–106. doi: 10.1016/j.ejpb.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Noble G.T., Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Omwoyo W.N., Ogutu B., Oloo F., Swai H., Kalombo L., Melariri P. Preparation, characterization, and optimization of primaquine-loaded solid lipid nanoparticles. Int J Nanomed. 2014;9:3865–3874. doi: 10.2147/IJN.S62630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J., Zhou Z., Liu F., Chen G. Pharmacokinetics and tissue distribution of oxymatrine-SLN. Chin Pharm J. 2007;42:1091–1095. [Google Scholar]
- 55.Cengiz M., Kutlu H.M., Burukoglu D.D., Ayhancı A. A comparative study on the therapeutic effects of silymarin and silymarin-loaded solid lipid nanoparticles on d-GaIN/TNF-α-induced liver damage in balb c mice. Food Chem Toxicol. 2015;77:93–100. doi: 10.1016/j.fct.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Li Y., Dong L., Jia A., Chang X., Xue H. Preparation of solid lipid nanoparticles loaded with traditional Chinese medicine by high-pressure homogenization. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:541–544. [PubMed] [Google Scholar]
- 57.Malafaya P.B., Silva G.A., Reis R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Chen L., Remondetto G.E., Subirade M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci Technol. 2006;17:272–283. [Google Scholar]
- 59.Elzoghby A.O., El-Fotoh W.S.A., Elgindy N.A. Casein-based formulations as promising controlled release drug delivery systems. J Contr Release. 2011;153:206–216. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Langer K., Anhorn M., Steinhauser I., Dreis S., Celebi D., Schrickel N. Human serum albumin (HSA) nanoparticles: reproducibility of preparation process and kinetics of enzymatic degradation. Int J Pharm. 2008;347:109–117. doi: 10.1016/j.ijpharm.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 61.Elsadek B., Kratz F. Impact of albumin on drug delivery—new applications on the horizon. J Contr Release. 2012;157:4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 62.Park K. Albumin: a versatile carrier for drug delivery. J Contr Release. 2012;157:3. doi: 10.1016/j.jconrel.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Beljaars L., Molema G., Weert B., Bonnema H., Olinga P., Groothuis G.M. Albumin modified with mannose 6-phosphate: a potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology. 1999;29:1486–1493. doi: 10.1002/hep.510290526. [DOI] [PubMed] [Google Scholar]
- 64.Beljaars L., Molema G., Schuppan D., Geerts A., De Bleser P.J., Weert B. Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J Biol Chem. 2000;275:12743–12751. doi: 10.1074/jbc.275.17.12743. [DOI] [PubMed] [Google Scholar]
- 65.Moreno M., Gonzalo T., Kok R.J., Sancho-Bru P., Van Beuge M., Swart J. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942–952. doi: 10.1002/hep.23419. [DOI] [PubMed] [Google Scholar]
- 66.Van Beuge M., Prakash J., Lacombe M., Gosens R., Post E., Reker-Smit C. Reduction of fibrogenesis by selective delivery of a Rho kinase inhibitor to hepatic stellate cells in mice. J Pharmacol Exp Therapeut. 2011;337:628–635. doi: 10.1124/jpet.111.179143. [DOI] [PubMed] [Google Scholar]
- 67.Melgert B.N., Olinga P., Jack V.K., Molema G., Meijer D.K., Poelstra K. Dexamethasone coupled to albumin is selectively taken up by rat nonparenchymal liver cells and attenuates LPS-induced activation of hepatic cells. J Hepatol. 2000;32:603–611. doi: 10.1016/s0168-8278(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 68.Aravalli Raj. Gene therapy for liver disease. In: Muriel P., editor. Liver pathophysiology: therapies and antioxidants. Elsevier; Waltham: 2017. pp. 837–851. [Google Scholar]
- 69.Michalopoulos G.K. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mammen B., Ramakrishnan T., Sudhakar U. Principles of gene therapy. Indian J Dent Res. 2007;18:196–200. doi: 10.4103/0970-9290.35832. [DOI] [PubMed] [Google Scholar]
- 71.Gaj T., Gersbach C.A., Barbas C.F., III ZFN, TALEN, and CRISPR-Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin H., Song C.Q., Dorkin J.R., Zhu L.J., Li Y., Wu Q. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat Biotechnol. 2016;34:328–333. doi: 10.1038/nbt.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walters L., Palmer J.G., Palmer J.G. Oxford University Press; Oxford: 1997. The ethics of human gene therapy. [Google Scholar]
- 74.van Haasteren J., Hyde S.C., Gill D.R. Lessons learned from lung and liver in vivo gene therapy: implications for the future. Expet Opin Biol Ther. 2018;18:959–972. doi: 10.1080/14712598.2018.1506761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patil S.D., Rhodes D.G., Burgess D.J. DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J. 2005;7:61–77. doi: 10.1208/aapsj070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McAllister D.V., Allen M.G., Prausnitz M.R. Microfabricated microneedles for gene and drug delivery. Annu Rev Biomed Eng. 2000;2:289–313. doi: 10.1146/annurev.bioeng.2.1.289. [DOI] [PubMed] [Google Scholar]
- 77.Luo D., Saltzman W.M. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 78.Regnier V., Tahiri A., André N., Lemaître M., Le Doan T., Préat V. Electroporation-mediated delivery of 3′-protected phosphodiester oligodeoxynucleotides to the skin. J Contr Release. 2000;67:337–346. doi: 10.1016/s0168-3659(00)00223-6. [DOI] [PubMed] [Google Scholar]
- 79.Wang Z.X., Wang Z.G., Ran H.T., Ren J.L., Zhang Y., Li Q. The treatment of liver fibrosis induced by hepatocyte growth factor-directed, ultrasound-targeted microbubble destruction in rats. Clin Imag. 2009;33:454–461. doi: 10.1016/j.clinimag.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Arias M., Sauer-Lehnen S., Treptau J., Janoschek N., Theuerkauf I., Buettner R. Adenoviral expression of a transforming growth factor-β1 antisense mRNA is effective in preventing liver fibrosis in bile-duct ligated rats. BMC Gastroenterol. 2003;3:1–12. doi: 10.1186/1471-230X-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nie Q.H., Zhu C.L., Zhang Y.F., Yang J., Zhang J.C., Gao R.T. Inhibitory effect of antisense oligonucleotide targeting TIMP-2 on immune-induced liver fibrosis. Dig Dis Sci. 2010;55:1286–1295. doi: 10.1007/s10620-009-0858-5. [DOI] [PubMed] [Google Scholar]
- 82.Mali S. Delivery systems for gene therapy. Indian J Hum Genet. 2013;19:3–8. doi: 10.4103/0971-6866.112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardee C.L., Arévalo-Soliz L.M., Hornstein B.D., Zechiedrich L. Advances in non-viral DNA vectors for gene therapy. Genes. 2017;8:1–22. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Q., Liu D.W., Zhang L.M., Zhu B., He Y.T., Xiao Y.H. Effects of augmentation of liver regeneration recombinant plasmid on rat hepatic fibrosis. World J Gastroenterol. 2005;11:2438–2443. doi: 10.3748/wjg.v11.i16.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamuta M., Morizono S., Tsuruta S., Kohjima M., Kotoh K., Enjoji M. Remote delivery and expression of soluble type II TGF-β receptor in muscle prevents hepatic fibrosis in rats. Int J Mol Med. 2005;16:59–64. [PubMed] [Google Scholar]
- 86.Eddy S.R. Computational genomics of noncoding RNA genes. Cell. 2002;109:137–140. doi: 10.1016/s0092-8674(02)00727-4. [DOI] [PubMed] [Google Scholar]
- 87.Meng Z., O'Keeffe-Ahern J., Lyu J., Pierucci L., Zhou D., Wang W. A new developing class of gene delivery: messenger RNA-based therapeutics. Biomater Sci. 2017;5:2381–2392. doi: 10.1039/c7bm00712d. [DOI] [PubMed] [Google Scholar]
- 88.O'Keeffe Ahern J., Zhou D., Gao Y., Lyu J., Meng Z., Cutlar L. Brush like cationic polymers with low charge density for gene delivery. Biomacromolecules. 2017;19:1410–1415. doi: 10.1021/acs.biomac.7b01267. [DOI] [PubMed] [Google Scholar]
- 89.Li C., Zhou D., Hu Y., Zhou H., Chen J., Zhang Z. The target gene carrying validity to HepG2 cells with the brush-like glutathione modified chitosan compound. Carbohydr Polym. 2012;89:46–53. doi: 10.1016/j.carbpol.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 90.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 91.Tu X., Zhang H., Zhang J., Zhao S., Zheng X., Zhang Z. MicroRNA-101 suppresses liver fibrosis by targeting the TGF β signalling pathway. J Pathol. 2014;234:46–59. doi: 10.1002/path.4373. [DOI] [PubMed] [Google Scholar]
- 92.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 93.Kobayashi Y., Kamimura K., Abe H., Yokoo T., Ogawa K., Shinagawa-Kobayashi Y. Effects of fibrotic tissue on liver-targeted hydrodynamic gene delivery. Mol Ther Nucleic Acids. 2016;5:e359. doi: 10.1038/mtna.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao K., Huang L. Nonviral methods for siRNA delivery. Mol Pharm. 2008;6:651–658. doi: 10.1021/mp800134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shum K., Rossi J. Springer; New York: 2016. SiRNA delivery methods. [Google Scholar]
- 96.Tsay H.C., Yuan Q., Balakrishnan A., Kaiser M., Möbus S., Kozdrowska E. Hepatocyte-specific suppression of microRNA-221-3p mitigates liver fibrosis. J Hepatol. 2019;70:722–734. doi: 10.1016/j.jhep.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 97.Chen S.W., Zhang X.R., Wang C.Z., Chen W.Z., Xie W.F., Chen Y.X. RNA interference targeting the platelet-de-rived growth factor receptor β subunit ameliorates experimental hepatic fibrosis in rats. Liver Int. 2008;28:1446–1457. doi: 10.1111/j.1478-3231.2008.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salgado S., Garcia J., Vera J., Siller F., Bueno M., Miranda A. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol Ther. 2000;2:545–551. doi: 10.1006/mthe.2000.0210. [DOI] [PubMed] [Google Scholar]
- 99.Liu J., Cheng X., Guo Z., Wang Z., Li D., Kang F. Truncated active human matrix metalloproteinase-8 delivered by a chimeric adenovirus-hepatitis B virus vector ameliorates rat liver cirrhosis. PloS One. 2013;8 doi: 10.1371/journal.pone.0053392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim M.D., Kim S.S., Cha H.Y., Jang S.H., Chang D.Y., Kim W. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014;46:e110. doi: 10.1038/emm.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Y., Zhao Y., He F., Wang H. Artificial microRNA-mediated Tgfbr2 and Pdgfrb co-silencing ameliorates carbon tetrachloride-induced hepatic fibrosis in mice. Hum Gene Ther. 2018;30:179–196. doi: 10.1089/hum.2018.047. [DOI] [PubMed] [Google Scholar]
- 102.Li Q., Kay M.A., Finegold M., Stratford-Perricaudet L.D., Woo S.L. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 103.Iimuro Y., Nishio T., Morimoto T., Nitta T., Stefanovic B., Choi S.K. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- 104.Siller-López F., Sandoval A., Salgado S., Salazar A., Bueno M., Garcia J. Treatment with human metalloproteinase-8 gene delivery ameliorates experimental rat liver cirrhosis. Gastroenterology. 2004;126:1122–1133. doi: 10.1053/j.gastro.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 105.Mak K.Y., Chin R., Cunningham S.C., Habib M.R., Torresi J., Sharland A.F. ACE2 therapy using adeno-associated viral vector inhibits liver fibrosis in mice. Mol Ther. 2015;23:1434–1443. doi: 10.1038/mt.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hao Z.M., Cai M., Lv Y.F., Huang Y.H., Li H.H. Oral administration of recombinant adeno-associated virus-mediated bone morphogenetic protein-7 suppresses CCl4-induced hepatic fibrosis in mice. Mol Ther. 2012;20:2043–2051. doi: 10.1038/mt.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamada T., Sato A., Hirano T., Yamamoto T., Son G., Onodera M. Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats. Am J Pathol. 2007;171:872–881. doi: 10.2353/ajpath.2007.060972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vera M., Sobrevals L., Zaratiegui M., Martinez L., Palencia B., Rodriguez C. Liver transduction with a simian virus 40 vector encoding insulin-like growth factor I reduces hepatic damage and the development of liver cirrhosis. Gene Ther. 2007;14:203–210. doi: 10.1038/sj.gt.3302858. [DOI] [PubMed] [Google Scholar]
- 109.Wu X., Liu G., Mu M., Peng Y., Li X., Deng L. Augmenter of liver regeneration gene therapy using a novel minicircle DNA vector alleviates liver fibrosis in rats. Hum Gene Ther. 2016;27:880–891. doi: 10.1089/hum.2016.006. [DOI] [PubMed] [Google Scholar]
- 110.Yang D., Gao Y., Tan K., Zuo Z., Yang W., Hua X. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene Ther. 2013;20:1140–1148. doi: 10.1038/gt.2013.41. [DOI] [PubMed] [Google Scholar]
- 111.Melgar-Lesmes P., Luquero A., Parra-Robert M., Mora A., Ribera J., Edelman E.R. Graphene-dendrimer nanostars for targeted macrophage overexpression of metalloproteinase 9 and hepatic fibrosis precision therapy. Nano Lett. 2018;18:5839–5845. doi: 10.1021/acs.nanolett.8b02498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hyun J., Wang S., Kim J., Rao K.M., Park S.Y., Chung I. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat Commun. 2016;7:10993–10996. doi: 10.1038/ncomms10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zeng C., Wang Y.L., Xie C., Sang Y., Li T.J., Zhang M. Identification of a novel TGF-β-miR-122-fibronectin 1 serum response factor signaling cascade and its implication in hepatic fibrogenesis. Oncotarget. 2015;6:12224–12233. doi: 10.18632/oncotarget.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Merle U., Enckea J., Tuma S., Volkmann M., Naldini L., Stremmel W. Lentiviral gene transfer ameliorates disease progression in Long-Evans cinnamon rats: an animal model for Wilson disease. Scand J Gastroenterol. 2006;41:974–982. doi: 10.1080/00365520600554790. [DOI] [PubMed] [Google Scholar]
- 115.Ji F., Wang K., Zhang Y., Mao X.L., Huang Q., Wang J. MiR-542-3p controls hepatic stellate cell activation and fibrosis via targeting BMP-7. Mol Cell Biochem. 2019;120:4573–4581. doi: 10.1002/jcb.27746. [DOI] [PubMed] [Google Scholar]
- 116.Jia Z., Gong Y., Pi Y., Liu X., Gao L., Kang L. pPB peptide-mediated siRNA-loaded stable nucleic acid lipid nanoparticles on targeting therapy of hepatic fibrosis. Mol Pharm. 2017;15:53–62. doi: 10.1021/acs.molpharmaceut.7b00709. [DOI] [PubMed] [Google Scholar]
- 117.Qiao J.B., Fan Q.Q., Xing L., Cui P.F., He Y.J., Zhu J.C. Vitamin A-decorated biocompatible micelles for chemogene therapy of liver fibrosis. J Contr Release. 2018;283:113–125. doi: 10.1016/j.jconrel.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 118.Sung Y.C., Liu Y.C., Chao P.H., Chang C.C., Jin P.R., Lin T.T. Combined delivery of sorafenib and a MEK inhibitor using CXCR4-targeted nanoparticles reduces hepatic fibrosis and prevents tumor development. Theranostics. 2018;8:894–905. doi: 10.7150/thno.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kong W.H., Park K., Lee M.Y., Lee H., Sung D.K., Hahn S.K. Cationic solid lipid nanoparticles derived from apolipoprotein-free LDLs for target specific systemic treatment of liver fibrosis. Biomaterials. 2013;34:542–551. doi: 10.1016/j.biomaterials.2012.09.067. [DOI] [PubMed] [Google Scholar]
- 120.Wu J., Huang J., Kuang S., Chen J., Li X., Chen B. Synergistic microRNA therapy in liver fibrotic rat using MRI-visible nanocarrier targeting hepatic stellate cells. Adv Sci. 2019;6:180–189. doi: 10.1002/advs.201801809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cui J., Piotrowski-Daspit A.S., Zhang J., Shao M., Bracaglia L.G., Utsumi T. Poly(amine-co-ester) nanoparticles for effective Nogo-B knockdown in the liver. J Contr Release. 2019;28:259–267. doi: 10.1016/j.jconrel.2019.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lundstrom K. Viral vectors in gene therapy. J Dis. 2018;6:42. doi: 10.3390/diseases6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Daya S., Berns K.I. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miravet S., Ontiveros M., Piedra J., Penalva C., Monfar M., Chillón M. Construction, production, and purification of recombinant adenovirus vectors. Adenovirus. 2014;1089:159–173. doi: 10.1007/978-1-62703-679-5_12. [DOI] [PubMed] [Google Scholar]
- 126.Alemany R., Balagué C., Curiel D.T. Replicative adenoviruses for cancer therapy. Nat Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 127.Nadeau I., Kamen A. Production of adenovirus vector for gene therapy. Biotechnol Adv. 2003;20:475–489. doi: 10.1016/s0734-9750(02)00030-7. [DOI] [PubMed] [Google Scholar]
- 128.Burroughs K.D., Kayda D.B., Sakhuja K., Hudson Y., Jakubczak J., Bristol J.A. Potentiation of oncolytic adenoviral vector efficacy with gutless vectors encoding GMCSF or TRAIL. Cancer Gene Ther. 2004;11:92–107. doi: 10.1038/sj.cgt.7700660. [DOI] [PubMed] [Google Scholar]
- 129.Salazar-Montes A.M., Hernández-Ortega L.D., Lucano-Landeros M.S., Armendariz-Borunda J. New gene therapy strategies for hepatic fibrosis. World J Gastroenterol. 2015;21:3813–3825. doi: 10.3748/wjg.v21.i13.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fan Y., Wu J. Poly Lipid nanoparticles, a novel lipid-based vector for liver gene transfer. In: Molina F.M., editor. Gene therapy—tools and potential applications. InTechOpen; London: 2013. pp. 91–107. [Google Scholar]
- 131.Haynes M.T., Huang L. Lipid-coated calcium phosphate nanoparticles for nonviral gene therapy. Adv Genet. 2014;88:205–229. doi: 10.1016/B978-0-12-800148-6.00007-9. [DOI] [PubMed] [Google Scholar]
- 132.Zhao Y., Huang L. Lipid nanoparticles for gene delivery. Adv Genet. 2014;88:13–36. doi: 10.1016/B978-0-12-800148-6.00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pathak A., Vyas S.P., Gupta K.C. Nano-vectors for efficient liver specific gene transfer. Int J Nanomed. 2008;3:31–49. [PMC free article] [PubMed] [Google Scholar]
- 134.Dimitriadis G.J. Entrapment of plasmid DNA in liposomes. Nucleic Acids Res. 1979;6:2697–2705. doi: 10.1093/nar/6.8.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wilson T., Papahadjopoulos D., Taber R. The introduction of poliovirus RNA into cells via lipid vesicles (liposomes) Cell. 1979;17:77–84. doi: 10.1016/0092-8674(79)90296-4. [DOI] [PubMed] [Google Scholar]
- 136.Li Y., Liu F., Ding F., Chen P., Tang M. Inhibition of liver fibrosis using vitamin A-coupled liposomes to deliver matrix metalloproteinase-2 siRNA in vitro. Mol Med Rep. 2015;12:3453–3461. doi: 10.3892/mmr.2015.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li G., Xie Q., Shi Y., Li D., Zhang M., Jiang S. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- 138.Lungwitz U., Breunig M., Blunk T., Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 139.De Smedt S.C., Demeester J., Hennink W.E. Cationic polymer based gene delivery systems. Pharm Res (N Y) 2000;17:113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 140.Siller-López F., Sandoval A., Salgado S., Salazar A., Bueno M., Garcia J. Glycosylated reversible addition-fragmentation chain transfer polymers with varying polyethylene glycol linkers produce different short interfering RNA uptake, gene silencing, and toxicity profiles. Biomacromolecules. 2017;18:4099–4112. doi: 10.1021/acs.biomac.7b01168. [DOI] [PubMed] [Google Scholar]
- 141.Mehal W., Schuppan D. Antifibrotic therapies in the liver. Semin Liver Dis. 2015;35:184–198. doi: 10.1055/s-0035-1550055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhuo L., Liao M., Zheng L., He M., Huang Q., Wei L. Combination therapy with taurine, epigallocatechin gallate and genistein for protection against hepatic fibrosis induced by alcohol in rats. Biol Pharm Bull. 2012;35:1802–1810. doi: 10.1248/bpb.b12-00548. [DOI] [PubMed] [Google Scholar]
- 143.Li Y., Zhu M., Huo Y., Zhang X., Liao M. Anti-fibrosis activity of combination therapy with epigallocatechin gallate, taurine and genistein by regulating glycolysis, gluconeogenesis, and ribosomal and lysosomal signaling pathways in HSC-T6 cells. Exp Ther Med. 2018;16:4329–4338. doi: 10.3892/etm.2018.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giannitrapani L., Soresi M., Bondì M.L., Montalto G., Cervello M. Nanotechnology applications for the therapy of liver fibrosis. World J Gastroenterol. 2014;20:7242–7251. doi: 10.3748/wjg.v20.i23.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Y., Liu Y.C., Sung Y.C., Ramjiawan R.R., Lin T.T., Chang C.C. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep. 2017;7:1–12. doi: 10.1038/srep44123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alcalá A.M., Flaherty K.T. BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res. 2012;18:33–39. doi: 10.1158/1078-0432.CCR-11-0997. [DOI] [PubMed] [Google Scholar]
- 147.Cheng A.-L., Kang Y.-K., Chen Z., Tsao C.-J., Qin S., Kim J.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 148.Trebicka J., Hennenberg M., Odenthal M., Shir K., Klein S., Granzow M. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53:702–712. doi: 10.1016/j.jhep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 149.Simon T.G., King L.Y., Zheng H., Chung R.T. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abraldes J.G., Albillos A., Bañares R., Turnes J., González R., García-Pagán J.C. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 151.Ding N., Hah N., Ruth T.Y., Sherman M.H., Benner C., Leblanc M. BRD4 is a novel therapeutic target for liver fibrosis. Proc Natl Acad Sci India B Biol Sci. 2015;112:15713–15718. doi: 10.1073/pnas.1522163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Korb E., Herre M., Zucker-Scharff I., Darnell R.B., Allis C.D. BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat Neurosci. 2015;18:1464–1473. doi: 10.1038/nn.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jostes S., Nettersheim D., Fellermeyer M., Schneider S., Hafezi F., Honecker F. The bromodomain inhibitor JQ1 triggers growth arrest and apoptosis in testicular germ cell tumours in vitro and in vivo. J Cell Mol Med. 2017;21:1300–1314. doi: 10.1111/jcmm.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Andrieu G., Belkina A.C., Denis G.V. Clinical trials for BET inhibitors run ahead of the science. Drug Discov Today Technol. 2016;19:45–50. doi: 10.1016/j.ddtec.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alghamdi S., Khan I., Beeravolu N., McKee C., Thibodeau B., Wilson G. BET protein inhibitor JQ1 inhibits growth and modulates WNT signaling in mesenchymal stem cells. Stem Cell Res Ther. 2016;7:22. doi: 10.1186/s13287-016-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Bruggen C., Hexum J.K., Tan Z., Dalal R.J., Reineke T.M. Nonviral gene delivery with cationic glycopolymers. Acc Chem Res. 2019;52:1347–1358. doi: 10.1021/acs.accounts.8b00665. [DOI] [PubMed] [Google Scholar]