Abstract
Seven new genera, 26 new species, 10 new combinations, two epitypes, one new name, and 20 interesting new host and / or geographical records are introduced in this study. New genera are: Italiofungus (based on Italiofungus phillyreae) on leaves of Phillyrea latifolia (Italy); Neolamproconium (based on Neolamproconium silvestre) on branch of Tilia sp. (Ukraine); Neosorocybe (based on Neosorocybe pini) on trunk of Pinus sylvestris (Ukraine); Nothoseptoria (based on Nothoseptoria caraganae) on leaves of Caragana arborescens (Russia); Pruniphilomyces (based on Pruniphilomyces circumscissus) on Prunus cerasus (Russia); Vesiculozygosporium (based on Vesiculozygosporium echinosporum) on leaves of Muntingia calabura (Malaysia); Longiseptatispora (based on Longiseptatispora curvata) on leaves of Lonicera tatarica (Russia). New species are: Barrmaelia serenoae on leaf of Serenoa repens (USA); Chaetopsina gautengina on leaves of unidentified grass (South Africa); Chloridium pini on fallen trunk of Pinus sylvestris (Ukraine); Cadophora fallopiae on stems of Reynoutria sachalinensis (Poland); Coleophoma eucalyptigena on leaf litter of Eucalyptus sp. (Spain); Cylindrium corymbiae on leaves of Corymbia maculata (Australia); Diaporthe tarchonanthi on leaves of Tarchonanthus littoralis (South Africa); Elsinoe eucalyptorum on leaves of Eucalyptus propinqua (Australia); Exophiala quercina on dead wood of Quercus sp., (Germany); Fusarium californicum on cambium of budwood of Prunus dulcis (USA); Hypomyces gamsii on wood of Alnus glutinosa (Ukraine); Kalmusia araucariae on leaves of Araucaria bidwillii (USA); Lectera sambuci on leaves of Sambucus nigra (Russia); Melanomma populicola on fallen twig of Populus canadensis (Netherlands), Neocladosporium syringae on branches of Syringa vulgarishorus (Ukraine); Paraconiothyrium iridis on leaves of Iris pseudacorus (Ukraine); Pararoussoella quercina on branch of Quercus robur (Ukraine); Phialemonium pulveris from bore dust of deathwatch beetle (France); Polyscytalum pinicola on needles of Pinus tecunumanii (Malaysia); Acervuloseptoria fraxini on Fraxinus pennsylvanica (Russia); Roussoella arundinacea on culms of Arundo donax (Spain); Sphaerulina neoaceris on leaves of Acer negundo (Russia); Sphaerulina salicicola on leaves of Salix fragilis (Russia); Trichomerium syzygii on leaves of Syzygium cordatum (South Africa); Uzbekistanica vitis-viniferae on dead stem of Vitis vinifera (Ukraine); Vermiculariopsiella eucalyptigena on leaves of Eucalyptus sp. (Australia).
Keywords: biodiversity, ITS barcodes, multi-gene phylogeny, new taxa, systematics, typification
Introduction
New and Interesting Fungi (NIF) is a series that aims to capture knowledge of fungal biodiversity, focussing on new records, new sexual-asexual connections, the consolidation of generic names following the end of dual nomenclature for fungi (Hawksworth et al. 2011, Wingfield et al. 2012). It also includes the description of new fungal taxa or interesting observations relating to the biology or taxonomy of fungi (Crous et al. 2018). The series represents a regular feature in the journal Fungal Systematics and Evolution (www.FUSE-journal.org). Mycologists and other researchers wishing to contribute to future issues of NIF are encouraged to contact the Editor-in-Chief (p.crous@wi.knaw.nl).
MaterialS and Methods
Isolates
Samples were placed in moist chambers and treated as explained previously (Crous et al. 2019c). Single conidial colonies were grown from sporulating conidiomata in Petri dishes containing 2 % malt extract agar (MEA) as described by Crous et al. (1991), and single ascospore cultures established following the method described by (Crous 1998). Colonies were sub-cultured on 2 % potato-dextrose agar (PDA), oatmeal agar (OA), MEA (Crous et al. 2019c), or autoclaved pine needles on 2 % tap water agar (PNA) (Smith et al. 1996), and incubated at 25 °C under continuous near-ultraviolet light to promote sporulation. Reference strains and specimens of the studied fungi are maintained in the culture collection (CBS) of the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands.
DNA extraction, amplification (PCR) and phylogeny
Fungal mycelium (Table 1) was scraped from the surface of agar cultures with a sterile scalpel and the genomic DNA was isolated using the Wizard® Genomic DNA Purification Kit (Promega Corporation, WI, USA) following the manufacturers’ protocols. Nine loci were amplified following previously published protocols. First, the 28S nrRNA gene (LSU) and internal transcribed spacer regions with intervening 5.8S nrRNA gene (ITS) of the nrDNA operon were sequenced for all the isolates included in this study (for amplification conditions, see Fan et al. 2018). Other loci were sequenced for various species or genera using primers and conditions specific for those groups of fungi. Amplification of the partial DNA-directed RNA polymerase II second largest subunit gene (rpb2), the partial translation elongation factor 1-alpha gene (tef1) and the partial beta-tubulin gene (tub2) followed Braun et al. (2018), while amplification of the partial actin gene (act), the partial calmodulin gene (cmdA), the partial glyceraldehyde-3-phosphate dehydrogenase gene (gapdh) and the partial histone H3 gene (his3) followed Videira et al. (2016). The resulting fragments were sequenced in both directions using the respective PCR primers and the BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems Life Technologies, Carlsbad, CA, USA); DNA sequencing amplicons were purified through Sephadex G-50 Superfine columns (Sigma-Aldrich, St. Louis, MO) in MultiScreen HV plates (Millipore, Billerica, MA). Purified sequence reactions were analysed on an Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA). The DNA sequences were analysed and consensus sequences were computed using SeqMan Pro v. 13 (DNASTAR, Madison, WI, USA).
Table 1.
Collection details and GenBank accession numbers of isolates treated in this study, and associated ex-type strains where available. Novel sequences span MT223773.1–MT223869.1 (ITS), MT223870.1–MT223940.1 (LSU) and MT223673.1–MT223764.1 (protein-coding loci).
| Species | Culture or voucher accession number(s)1 | Locality and Substrate | Collector(s) |
GenBank accession number2 |
||||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tef1 | Other loci | ||||
| Acervuloseptoria fraxini, sp. nov. | CPC 36558 = CBS 145992, ex-type | Russia: Fraxinus (cf. pennsylvanica?) | T.S. Bulgakov | MT223773.1 | MT223870.1 | MT223673.1 | – | – |
| Amphobotrys ricini | CPC 36573 = CBS 145995 = EMSL 3787, ex-epitype | USA: Settle plate in bedroom | Ž. Jurjević | MT223774.1 | MT223871.1 | MT223674.1 | – | – |
| CPC 36709 | USA: Air from hospital | Ž. Jurjević | MT223775.1 | MT223872.1 | MT223675.1 | – | – | |
| CPC 36710 | USA: Air from hospital | Ž. Jurjević | MT223776.1 | MT223873.1 | MT223676.1 | – | – | |
| CPC 36719 | USA: Air from basement | Ž. Jurjević | MT223777.1 | MT223874.1 | MT223677.1 | – | – | |
| CPC 38557 = MUCL 001030 | USA: – | – | MT223778.1 | – | – | – | – | |
| CPC 38558 = MUCL 002165 | USA: – | – | MT223779.1 | – | – | – | – | |
| Arcticomyces warmingii | CPC 36560 = CBS 146033 | UK: Frullania dilatata | G. Greiff | MT223780.1 | MT223875.1 | – | MT223708.1 | cmdA: MT223762.1 |
| Barrmaelia serenoae, sp. nov. | CPC 37572 = CBS 146017, ex-type | USA: Serenoa repens, leaf | M.J. Wingfield | MT223781.1 | MT223876.1 | – | MT223709.1 | tub2: MT223730.1 |
| Cadophora fallopiae, sp. nov. | CPC 35742 = CBS 145565 | Germany: Reynoutria japonica, stem | Th. Hulsewig | MT223782.1 | MT223877.1 | – | – | – |
| CPC 38011 | Poland: Reynoutria sachalinensis | A. Akulov | MT223783.1 | MT223878.1 | – | – | – | |
| CPC 38013 = CBS 146083, ex-type | Poland: Reynoutria sachalinensis | A. Akulov | MT223784.1 | MT223879.1 | – | – | – | |
| Chaetopsina gautengina, sp. nov. | CPC 34896 = CBS 145535, ex-type | South Africa: Unidentified grass (Poaceae), leaves | A. Cilliers | MT223785.1 | MT223880.1 | – | – | – |
| Chaetospermum camelliae | CPC 34736 = CBS 145580 | South Africa: Trichilia emetica, leaves | P.W. Crous | MT223786.1 | MT223881.1 | – | – | – |
| MFLUCC 12-0436 = NTCL097-3 = ICMP 20008, ex-epitype | Thailand: Unidentified dead leaves | N. Tangthirasunun | KF516966.1 | KF516972.1 | KF516990.1 | – | rpb1: KF516984.1, SSU: KF516978.1 | |
| Chloridium pini, sp. nov. | CPC 36627 = CBS 146011, ex-type | Ukraine: Pinus sylvestris, fallen trunk | A. Akulov | MT223787.1 | MT223882.1 | – | – | tub2: MT223731.1 |
| Cladoriella paleospora | CPC 14646 = CBS 124761, ex-type | Australia: Eucalyptus oblonga | B.A. Summerell | GQ303272.1 | GQ303303.2 | – | – | – |
| CPC 34730 | South Africa: Eucalyptus sp., leaves | P.W. Crous | MT223788.1 | MT223883.1 | – | – | – | |
| CPC 34746 = CBS 144495 | South Africa: Eucalyptus sp., leaves | P.W. Crous | MT223789.1 | MT223884.1 | – | – | – | |
| Cladosporium ramotenellum | CPC 34845 = CBS 145592 | UK: Eucalyptus gunnii, leaves | S. Denman | MT223790.1 | MT223885.1 | MT223678.1 | MT223710.1 | act: MT223748.1 |
| Coleophoma eucalyptigena, sp. nov. | CPC 37589 = CBS 146018, ex-type | Spain: Eucalyptus sp., leaf litter | M. Deldgado | MT223791.1 | MT223886.1 | – | MT223711.1 | act: MT223749.1 |
| Coleophoma sp. | CPC 37437 = CBS 146128 | Germany: Hedera helix, leaves | R.K. Schumacher | MT223866.1 | MT223937.1 | – | MT223729.1 | tub2: MT223747.1 |
| Cylindrium corymbiae, sp. nov. | CPC 35637 = CBS 146087, ex-type | Australia: Corymbia maculata, leaves | A.J. Carnegie | MT223792.1 | MT223887.1 | MT223679.1 | MT223712.1 | tub2: MT223732.1, act: MT223750.1, cmdA: MT223763.1 |
| Dendryphiella eucalyptorum | CPC 22927 = CBS 137987, ex-type | Spain: Eucalyptus globulus, branches | E. Rubio | KJ869139.1 | KJ869196.1 | – | – | – |
| CPC 34855 = CBS 145593 | South Africa: Unidentified grass (Poaceae), leaves | P.W. Crous | MT223793.1 | MT223888.1 | – | – | – | |
| Diaporthe tarchonanthi, sp. nov. | CPC 37479 = CBS 146073, ex-type | South Africa: Tarchonanthus littoralis | L. Lombard | MT223794.1 | MT223889.1 | – | – | tub2: MT223733.1, his3: MT223759.1 |
| Elsinoe eucalyptorum, sp. nov. | CPC 13052 = CBS 120084, ex-type | Australia: Eucalyptus propinqua, leaves | B.A. Summerell | DQ923530.1 | DQ923530.1 | KX887098.1 | KX886862.1 | – |
| Eriospora leucostoma | CPC 35594 = CBS 145556 | Germany: Typha sp., attached leave | R.K. Schumacher | MT223795.1 | MT223890.1 | – | – | – |
| CPC 35598 | Germany: Juncus effusus, culm | R.K. Schumacher | MT223796.1 | MT223891.1 | – | – | – | |
| Exophiala quercina, sp. nov. | CPC 33408 = CBS 146024, ex-type | Germany: Quercus sp., dead wood | R.K. Schumacher | MT223797.1 | MT223892.1 | – | MT223713.1 | – |
| Fusarium californicum, sp. nov. | BL24 | USA: Prunus dulcis, necrotic inner bark | A.J. Stack | MK880134.1 | MK878585.1 | MK878565.1 | MK878575.1 | tub2: MK878570.1, rpb1: MK878580.1 |
| BL26 | USA: Prunus dulcis, necrotic inner bark | A.J. Stack | MK880135.1 | MK878586.1 | MK878566.1 | MK878576.1 | tub2: MK878571.1, rpb1: MK878581.1 | |
| BL28 | USA: Prunus dulcis, necrotic inner bark | A.J. Stack | MK880136.1 | MK878587.1 | MK878567.1 | MK878577.1 | tub2: MK878572.1, rpb1: MK878582.1 | |
| BL29 | USA: Prunus dulcis, necrotic inner bark | A.J. Stack | MK880137.1 | MK878588.1 | MK878568.1 | MK878578.1 | tub2: MK878573.1, rpb1: MK878583.1 | |
| CBS 145796 = BL30 | USA: Prunus dulcis, necrotic inner bark | A.J. Stack | MK880138.1 | MK878589.1 | MK878569.1 | MK878579.1 | tub2: MK878574.1, rpb1: MK878584.1 | |
| Gonatobotrys simplex | CMW 55931 = CN008I7 | South Africa: Blueberry, growing on Cladosporium sp. | C.M. Visagie | MT110303.1 | – | – | – | – |
| CMW 55932 = CN008I8 | South Africa: Blueberry, growing on Cladosporium sp. | C.M. Visagie | MT110304.1 | MT110305.1 | – | – | – | |
| Gonatophragmium epilobii | CBS 122271 = ICMP 17170, ex-type | New Zealand: Epilobium ciliatum, leaf spot | C.F. Hill | MH863183.1 | MH874728.1 | – | – | – |
| CPC 34889 = CBS 145594 = T17_03052C | New Zealand: Rhopalostylis sapida, leaves | R. Thangavel | MT223798.1 | MT223893.1 | – | – | – | |
| Graphostroma platystomum | CPC 37153 = CBS 146066 | USA: Lindera benzoin, fallen and corticated branch | E. Crenson & R.K. Schumacher | MT223799.1 | MT223894.1 | MT223680.1 | – | tub2: MT223734.1 |
| Hypomyces gamsii, sp. nov. | CPC 36232 = CBS 146044, ex-type | Ukraine: Alnus glutinosa, wood | A. Akulov | MT223800.1 | MT223895.1 | MT223681.1 | MT223714.1 | – |
| Hypsotheca eucalyptorum | CPC 35659 = CBS 146027 | Australia: Eucalyptus grandis × camaldulensis, leaves | A.J. Carnegie | MT223801.1 | MT223896.1 | – | – | – |
| CPC 35734 = CBS 145576, ex-type | Australia: Eucalyptus grandis × camaldulensis, leaves | A.J. Carnegie | MK876393.1 | MK876434.1 | – | – | – | |
| CPC 37928 = CBS 146089 | Australia: Eucalyptus grandis × camaldulensis, leaves | A.J. Carnegie | MT223802.1 | MT223897.1 | – | – | – | |
| CPC 37930 = CBS 146031 | Australia: Eucalyptus dunnii, branch | A.J. Carnegie | MT223803.1 | MT223898.1 | – | – | – | |
| Italiofungus phillyreae, gen. et comb. nov. | CPC 35566 = CBS 145537 | Italy: Phillyrea latifolia, leaves | P.W. Crous | MT223804.1 | MT223899.1 | – | – | – |
| Kalmusia araucariae, sp. nov. | CPC 37475 = CBS 146063, ex-type | USA: Araucaria bidwillii, leaf blight | M.J. Wingfield | MT223805.1 | MT223900.1 | – | – | – |
| Keissleriella phragmiticola | CPC 19311 = CBS 135473 | USA: Phragmites sp. | – | KF251241.1 | KF251744.1 | KF252246.1 | KF253194.1 | tub2: KF252726.1 |
| CPC 24110 | Netherlands: Phragmites sp. | W. Quaedvlieg | MT223806.1 | MT223901.1 | MT223682.1 | – | – | |
| CPC 27402 | Germany: Phragmites australis, culm | R.K. Schumacher | MT223807.1 | MT223902.1 | MT223683.1 | – | – | |
| CPC 33249 | Germany: Phragmites australis, leaf sheath | R.K. Schumacher | MT223808.1 | MT223903.1 | MT223684.1 | MT223715.1 | – | |
| CPC 33251 = CBS 144989 | Germany: Phragmitis australis, dead and attached leaf sheath | R.K. Schumacher | MT223809.1 | MT223904.1 | MT223685.1 | MT223716.1 | – | |
| MFLUCC 17-0779, ex-type | UK: Phragmites sp. | E.B.G. Jones | MG828904.1 | MG829014.1 | – | – | – | |
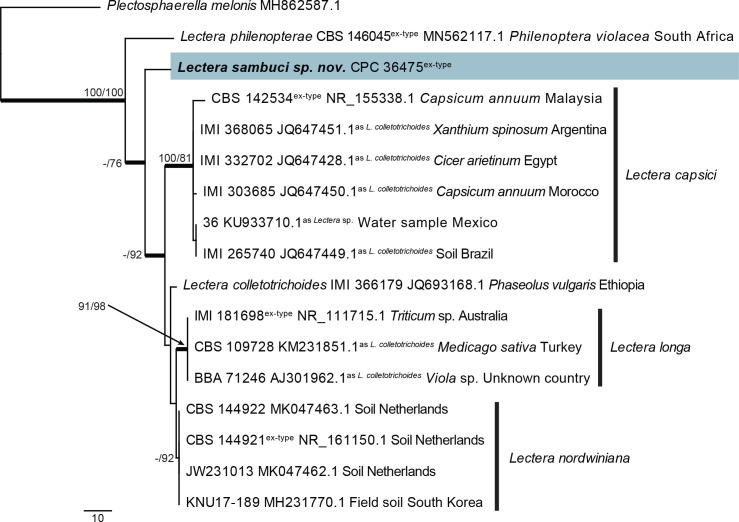
| Lectera sambuci, sp. nov. | CPC 36475 = CBS 145986, ex-type | Russia: Sambucus nigra | T.S. Bulgakov | MT223810.1 | MT223905.1 | MT223686.1 | – | tub2: MT223735.1 |
| Leptoxyphium fumago, comb. nov. | CPC 34802 = CBS 145579 | Thailand: Annona squamosa, leaves | R. Cheewangkoon | MT223811.1 | MT223906.1 | MT223687.1 | – | – |
| IFRDCC 2651, ex-type of Leptoxyphium glochidion | China: Glochidion wrightii, living leaf | Hui Yang | KF982307.1 | KF982308.1 | – | – | SSU: KF982309.1 | |
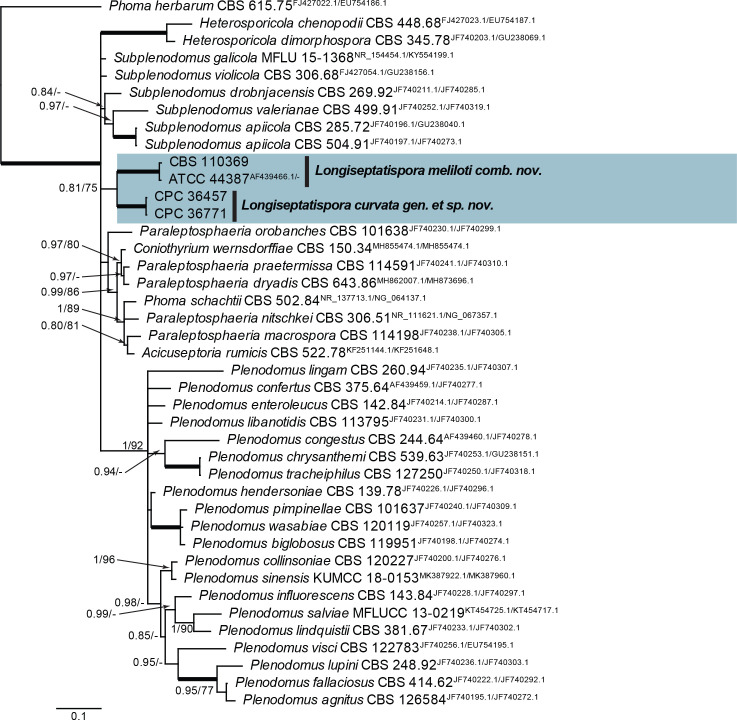
| Longiseptatispora curvata, gen. et sp. nov. | CPC 36457 = CBS 146035, ex-type | Russia: Lonicera tatarica | T.S. Bulgakov | MT223812.1 | MT223907.1 | MT223688.1 | MT223717.1 | tub2: MT223736.1 |
| CPC 36771 | Russia: Lonicera tatarica | T.S. Bulgakov | MT223813.1 | MT223908.1 | – | MT223718.1 | tub2: MT223737.1 | |
| ATCC 4438 | USA: Medicago sp. | – | AF439466.1 | – | – | – | – | |
| CBS 110369 | Netherlands: Melilotus sp. | G. Verkley | MT223814.1 | MT223909.1 | MT223689.1 | – | tub2: MT223738.1 | |
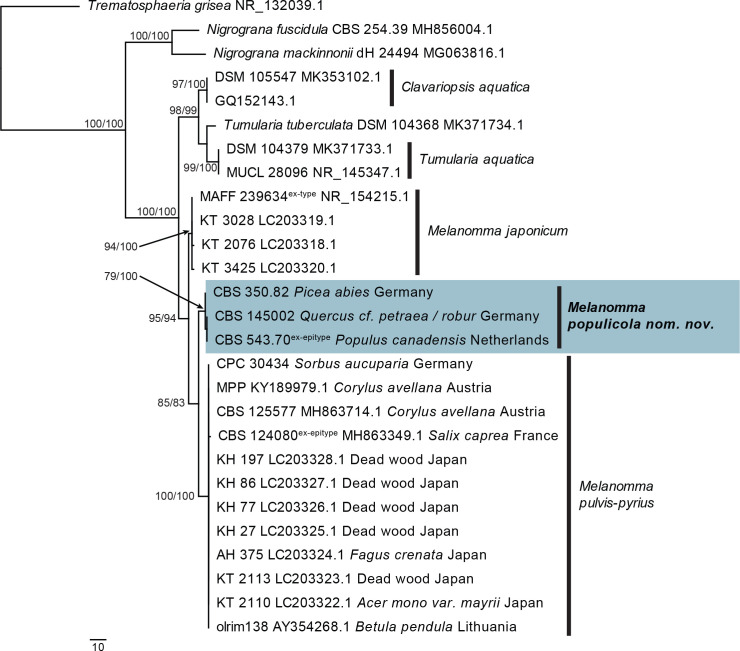
| Melanomma populicola, nom. nov. | CBS 350.82 | Germany: Picea abies, branch scars | – | MT223815.1 | JF740265.1 | – | – | – |
| CBS 543.70 | Netherlands: Populus canadensis, fallen twig | H.A. van der Aa | MT223816.1 | MH871620.1 | – | – | SSU: EU754031.1 | |
| CPC 27203 = CBS 145002 | Germany: Quercus cf. petraea / robur | R.K. Schumacher | MT223817.1 | MT223910.1 | – | – | – | |
| Melanomma pulvis-pyrius | CPC 30434 | Germany: Sorbus aucuparia, twig | R.K. Schumacher | MT223818.1 | MT223911.1 | MT223690.1 | – | – |
| IFRDCC 2044 = CBS 124080, ex-epitype | France: Salix caprea, bark | J. Fournier | MH863349.1 | FJ201984.1 | FJ795474.1 | – | SSU: FJ201985.1 | |
| Neocladosporium syringae, sp. nov. | CPC 35750 = CBS 145544, ex-type | Ukraine: Syringa vulgarishorus, overwintered dead branches | A. Akulov | MT223819.1 | MT223912.1 | – | – | – |
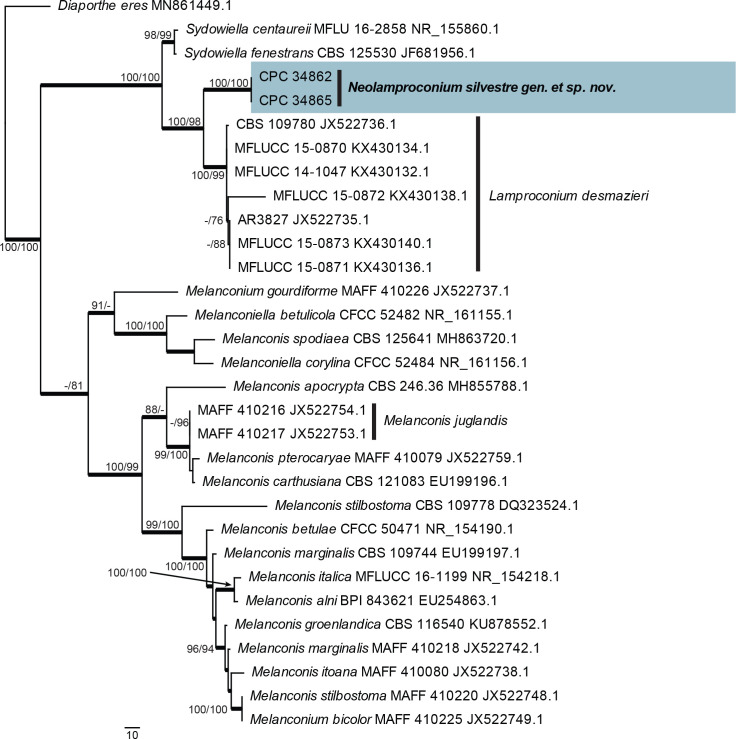
| Neolamproconium silvestre, gen. et sp. nov. | CPC 34862 | Ukraine: Quercus robur, branch | A. Akulov | MT223820.1 | MT223913.1 | – | – | – |
| CPC 34865 = CBS 145566, ex-type | Ukraine: Tilia sp., branch | A. Akulov | MT223821.1 | MT223914.1 | – | – | – | |
| Neosetophoma cerealis, comb. nov. | CBS 120096 | Spain: Composted livestock manure | – | MH863077.1 | MH874630.1 | – | – | – |
| CBS 157.78 | Germany: Triticum aestivum | P. Reinecke | MH861123.1 | JX681080.1 | – | – | – | |
| CBS 158.78 | Germany: Apera spica-venti, culm base | P. Reinecke | MH861124.1 | MH872883.1 | – | – | – | |
| CBS 232.77 | Netherlands: Agricultural soil | W. Verkerke | MH861054.1 | MH872823.1 | – | – | – | |
| CBS 443.82 | Netherlands: Mixed Beta and Hordeum vulgare soil sample | H. Nielander | MT223822.1 | MH873258.1 | MT223691.1 | – | tub2: MT223739.1 | |
| CBS 518.74 | Norway: Phleum pratense | K. Årsvoll | MT223823.1 | JX681079.1 | MT223692.1 | – | tub2: MT223740.1 | |
| CBS 672.68 | Germany: Wheat field soil | – | AJ293812.1 | MT223915.1 | – | – | – | |
| CBS 963.68 | Germany: Wheat field soil | – | MH859255.1 | MH870983.1 | – | – | – | |
| Neosorocybe pini, gen. et sp. nov. | CPC 36628 = CBS 146085, ex-type | Ukraine: Pinus sylvestris, fallen trunk | A. Akulov | MT223824.1 | MT223916.1 | – | – | – |
| Nothoseptoria caraganae, gen. et comb. nov. | CPC 36563 = CBS 145993 | Russia: Caragana arborescens | T.S. Bulgakov | MT223825.1 | MT223917.1 | MT223693.1 | – | tub2: MT223741.1 |
| CPC 36565 | Russia: Caragana arborescens | T.S. Bulgakov | MT223826.1 | MT223918.1 | MT223694.1 | – | tub2: MT223742.1 | |
| Paraconiothyrium iridis, sp. nov. | CPC 36281 = CBS 146036, ex-type | Ukraine: Iris pseudacorus, living leaf | A. Akulov | MT223827.1 | MT223919.1 | MT223695.1 | MT223719.1 | tub2: MT223743.1 |
| Pararoussoella quercina, sp. nov. | CPC 34864 = CBS 145567, ex-type | Ukraine: Quercus robur, branch | A. Akulov | MT223828.1 | MT223920.1 | – | – | – |
| Phialemonium pulveris, sp. nov. | CPC 37955 = MUCL 57769 = CBS 146072, ex-type | France: Bore dust of deathwatch beetle infesting Quercus floor | C. Decock | MT223829.1 | MT223921.1 | – | – | tub2: MT223744.1, act: MT223751.1 |
| Plenodomus visci | CBS 122783 = CBS 4130 = PD 74/1021, ex-epitype of Plectophomella visci | France: Viscum album | M.M.J. Dorenbosch | JF740256.1 | EU754195.1 | KY064050.1 | – | tub2: KY064063.1, SSU: EU754096.1 |
| CPC 35314 = CBS 145000 | France: Viscum album, attached, corticated and dead twig | G. Moyne & R.K. Schumacher | MT223830.1 | MT223922.1 | MT223696.1 | MT223720.1 | – | |
| CPC 35315 | France: Viscum album, attached, corticated and dead twig | G. Moyne & R.K. Schumacher | MT223831.1 | MT223923.1 | – | – | – | |
| CPC 35316 | France: Viscum album, attached, corticated and dead twig | G. Moyne & R.K. Schumacher | MT223832.1 | MT223924.1 | – | MT223721.1 | – | |
| Polyscytalum pinicola, sp. nov. | CPC 36759 = CBS 146015, ex-type | Malaysia: Pinus tecunumanii, needles | M.J. Wingfield | MT223833.1 | MT223925.1 | – | – | – |
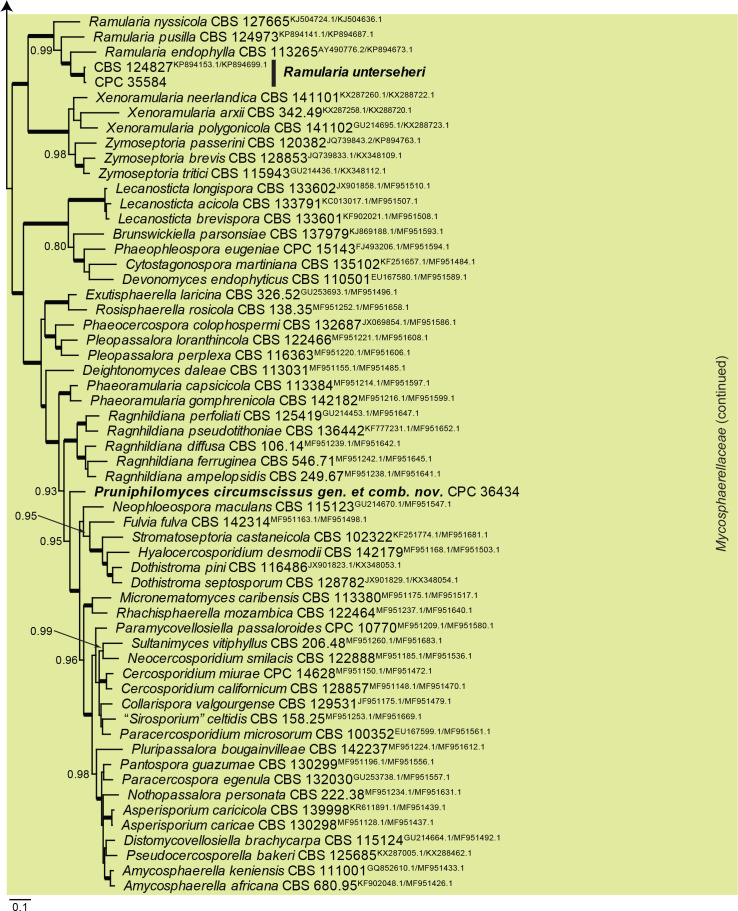
| Pruniphilomyces circumscissus, gen. et comb. nov. | CPC 36434 = CBS 145985 | Russia: Prunus cerasus | T.S. Bulgakov | MT223834.1 | MT223926.1 | MT223697.1 | – | – |
| Rachicladosporium iridis, comb. nov. | CBS 282.49, ex-epitype | Netherlands: Iris pseudacorus, leaf spots | J.A. von Arx | EU167586.1 | EU167586.1 | – | – | SSU: EU167586.1 |
| CPC 10020 | Netherlands: Iris sp., leaf | R. Summerbell | MT223835.1 | – | – | – | – | |
| CPC 34129 | Netherlands: Iris sp., leaf | P.W. Crous | MT223836.1 | – | – | – | – | |
| Ramularia unterseheri | CBS 124884, ex-type | Germany: Fagus sylvatica, leaf litter | M. Unterseher | KP894270.1 | KP894163.1 | KP894709.1 | KP894488.1 | tub2: KP895002.1, act: KP894378.1, his3: KP894820.1, cmdA: KP894923.1, gapdh: KP894598.1 |
| CPC 35584 | Germany: Fagus sylvatica, leaf litter | R.K. Schumacher | MT223837.1 | MT223927.1 | MT223698.1 | MT223722.1 | act: MT223752.1, his3: MT223760.1, gapdh: MT223764.1 | |
| Roussoella arundinacea, sp. nov. | CPC 35554 = CBS 146088, ex-type | Spain: Arundo donax, dead and standing culm | M.A. Delgado | MT223838.1 | MT223928.1 | MT223699.1 | MT223723.1 | – |
| Sphaerulina neoaceris, sp. nov. | CPC 36481 = CBS 145987 | Russia: Acer negundo | T.S. Bulgakov | MT223839.1 | MT223929.1 | MT223700.1 | MT223724.1 | act: MT223753.1, his3: MT223761.1 |
| CPC 36488 = CBS 145988, ex-type | Russia: Acer negundo | T.S. Bulgakov | MT223840.1 | MT223930.1 | MT223701.1 | MT223725.1 | act: MT223754.1 | |
| Sphaerulina salicicola, sp. nov. | CPC 36494 = CBS 146034 | Russia: Salix fragilis | T.S. Bulgakov | MT223841.1 | MT223931.1 | MT223702.1 | MT223726.1 | act: MT223755.1 |
| CPC 36561 = CBS 146032, ex-type | Russia: Salix fragilis | T.S. Bulgakov | MT223842.1 | MT223932.1 | MT223703.1 | MT223727.1 | act: MT223756.1 | |
| CPC 36569 | Russia: Caragana arborescens | T.S. Bulgakov | MT223843.1 | MT223933.1 | MT223704.1 | MT223728.1 | act: MT223757.1 | |
| CPC 36770 | Russia: Phytolacca sp. | T.S. Bulgakov | MT223844.1 | – | – | – | – | |
| Sporidesmiella hyalosperma | CPC 37552 = CBS 146016 | Spain: Zea mays, dead root | M. Deldgado | MT223845.1 | MT223934.1 | MT223705.1 | – | – |
| Sporocadus trimorphus | CBS 114203 = UPSC 2430, ex-type | Sweden: Rosa canina | K. & L. Holm | MH553977.1 | MH554196.1 | MH554876.1 | MH554395.1 | tub2: MH554636.1 |
| CPC 35675 = CBS 145550 | Germany: Rosa canina | Th. Hulsewig | MT223846.1 | MT223935.1 | MT223706.1 | – | tub2: MT223745.1 | |
| Teratosphaeria agapanthi | CPC 18266 | South Africa: Agapanthus umbellatus, leaf spots | P.W. Crous | JF770458.1 | JF770471.1 | – | – | – |
| CPC 18304 = CBS 129192, ex-epitype | South Africa: Agapanthus umbellatus, leaf spots | P.W. Crous | JF770457.1 | JF770470.1 | – | – | – | |
| CPC 18332 = CBS 129064 | South Africa: Agapanthus umbellatus, leaf spots | P.W. Crous | JF770456.1 | JF770469.1 | KF902406.1 | – | – | |
| CPC 19665 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223847.1 | – | – | – | – | |
| CPC 19673 | South Africa: Agapanthus praecox, leaf spots | P.W. Crous | MT223848.1 | – | – | – | – | |
| CPC 19689 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223849.1 | – | – | – | – | |
| CPC 19750 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223850.1 | – | – | – | – | |
| CPC 19769 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223851.1 | – | – | – | – | |
| CPC 19774 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223852.1 | – | – | – | – | |
| CPC 19828 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223853.1 | – | – | – | – | |
| CPC 20396 | USA: Agapanthus sp., leaves | P.W. Crous | MT223854.1 | – | – | – | – | |
| CPC 20402 | USA: Agapanthus sp., leaves | P.W. Crous | MT223855.1 | – | – | – | – | |
| CPC 23526 | France: Agapanthus sp., leaves | P.W. Crous | MT223856.1 | – | – | – | – | |
| CPC 25550 | Australia: Agapanthus sp., leaves | P.W. Crous | MT223857.1 | – | – | – | – | |
| CPC 26305 | La Reunion (France): Agapanthus sp., leaves | P.W. Crous | MT223858.1 | – | – | – | – | |
| CPC 27350 | South Africa: Agapanthus sp., leaves | P.W. Crous | MT223859.1 | – | – | – | – | |
| CPC 28986 | Australia: Agapanthus sp., leaves | P.W. Crous | MT223860.1 | – | – | – | – | |
| CPC 29126 | Australia: Agapanthus sp., leaves | P.W. Crous | MT223861.1 | – | – | – | – | |
| CPC 32077 | Australia: Agapanthus sp., leaves | P.W. Crous | MT223862.1 | – | – | – | – | |
| CPC 32107 | Australia: Agapanthus sp., leaves | P.W. Crous | MT223863.1 | – | – | – | – | |
| CPC 32200 | Australia: Agapanthus sp., leaves | P.W. Crous | MT223864.1 | – | – | – | – | |
| Trichomerium syzygii, sp. nov. | CPC 37184 = CBS 146074, ex-type | South Africa: Syzygium cordatum, leaves | M.J. Wingfield | MT223865.1 | MT223936.1 | – | – | tub2: MT223746.1, rpb1: MT223758.1 |
| Trochila craterium | CPC 37493 = CBS 146632 | Germany: Hedera helix, leaves | R.K. Schumacher | MT223866.1 | MT223937.1 | – | MT223729.1 | tub2: MT223747.1 |
| Uzbekistanica vitis-viniferae, sp. nov. | CPC 35793 = CBS 145545, ex-type | Ukraine: Vitis vinifera, dead stem | O. Ternovska | MT223867.1 | MT223938.1 | MT223707.1 | – | – |
| Vermiculariopsiella eucalyptigena, sp. nov. | CPC 36373 = CBS 146091, ex-type | Australia: Eucalyptus sp., leaves | A.J. Carnegie | MT223868.1 | MT223939.1 | – | – | – |
| Vesiculozygosporium echinosporum, gen. et comb. nov. | CPC 35607 = CBS 145807 | Malaysia: Muntingia calabura, leaves | M.J. Wingfield | MT223869.1 | MT223940.1 | – | – | – |
1 CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands; CMV: Culture collection of Cobus Visagie; CPC: Culture collection of Pedro Crous, housed at CBS; EMSL: EMSL Analytical, Inc., Cinnaminson, New Jersey, USA; ICMP: International Collection of Micro-organisms from Plants, Landcare Research, Private Bag 92170, Auckland, New Zealand; IFRDCC: International Fungal Research and Development Culture Collection, Chinese Academy of Forestry, Kunming, China; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; UPSC: Uppsala University Culture Collection of Fungi, Botanical Museum University of Uppsala, Uppsala, Sweden.
2 ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: large subunit (28S) of the nrRNA gene operon; act: partial actin gene; cmdA: partial calmodulin gene; gapdh: partial glyceraldehyde-3-phosphate dehydrogenase gene; his3: partial histone H3 gene; rpb1: partial DNA-directed RNA polymerase II largest subunit gene; rpb2: partial DNA-directed RNA polymerase II second largest subunit gene; SSU: small subunit (18S) of the nrRNA gene operon; tef1: partial translation elongation factor 1-alpha gene; tub2: partial beta-tubulin gene.
The sequences for each gene region were subjected to megablast searches (Zhang et al. 2000) to identify closely related sequences in the NCBI’s GenBank nucleotide database. The results are provided as part of the species notes or as selected phylogenetic trees. Phylogenetic trees were generated using Bayesian analyses performed with MrBayes v. 3.2.6 (Ronquist et al. 2012) for the overview trees and Maximum Parsimony analyses performed with PAUP v. 4.0b10 (Swofford 2003) as explained in Braun et al. (2018) for the genus and species trees. All resulting trees were printed with Geneious v. 11.0.3 (http://www.geneious.com, Kearse et al. 2012) and the layout of the trees was done in Adobe Illustrator v. CC 2017. Statistical measures calculated included tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC).
Morphology
Slide preparations were mounted in lactic acid, Shear’s mounting fluid or water, from colonies sporulating on MEA, PDA, PNA or OA. Observations were made with a Nikon SMZ25 dissection-microscope, and with a Zeiss Axio Imager 2 light microscope using differential interference contrast (DIC) illumination and images recorded on a Nikon DS-Ri2 camera with associated software. Colony characters and pigment production were noted after 2–4 wk of growth on MEA, PDA and OA (Crous et al. 2019c) incubated at 25 °C. Colony colours (surface and reverse) were scored using the colour charts of Rayner (1970). Sequences derived in this study were deposited in GenBank (Table 1), the alignment in TreeBASE (www.treebase.org; study number S25829), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004a).
RESULTS
Phylogeny
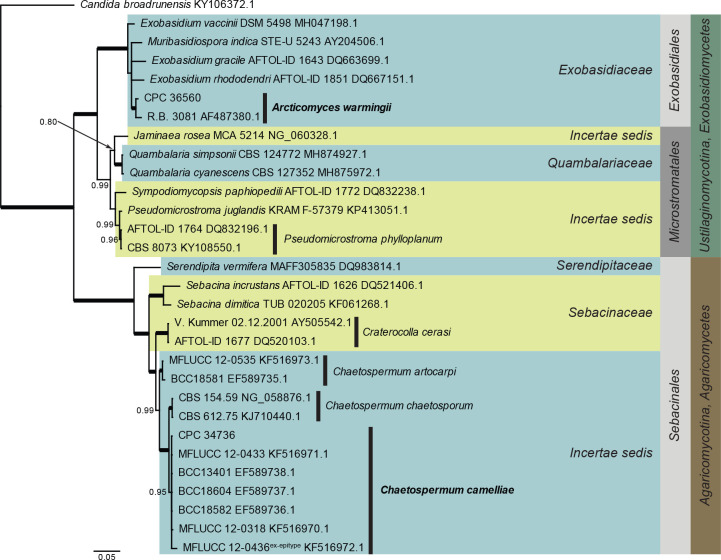
Agaricomycetes and Exobasidiomycetes LSU phylogeny (Fig. 1): The alignment contained 30 isolates and the tree was rooted to Candida broadrunensis (culture CBS 11838, GenBank KY106372.1). The final alignment contained a total of 839 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 294 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 902 trees (saved every 100 generations) from which 678 were sampled after 25 % of the trees were discarded as burn-in.
Fig. 1.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Agaricomycetes and Exobasidiomycetes LSU sequence alignment. Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. Classes, subdivisions, families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or culture collection numbers are indicated for all species. The tree was rooted to Candida broadrunensis (culture CBS 11838, GenBank KY106372.1) and the species treated in this study for which LSU sequence data were available are indicated in bold face.
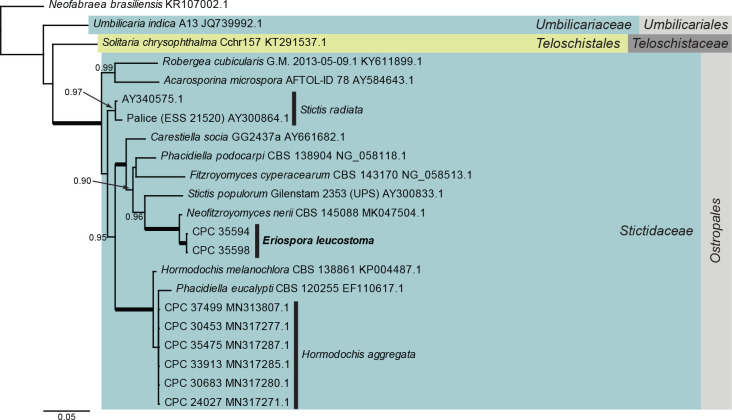
Lecanoromycetes LSU phylogeny (Fig. 2): The alignment contained 22 isolates and the tree was rooted to Neofabraea brasiliensis (voucher CNPUV499, GenBank KR107002.1). The final alignment contained a total of 793 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 183 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 3 002 trees (saved every 10 generations) from which 2 252 were sampled after 25 % of the trees were discarded as burn-in.
Fig. 2.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Lecanoromycetes LSU sequence alignment. Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or culture collection numbers are indicated for all species. The tree was rooted to Neofabraea brasiliensis (voucher CNPUV499, GenBank KR107002.1) and the species treated in this study for which LSU sequence data were available is indicated in bold face.
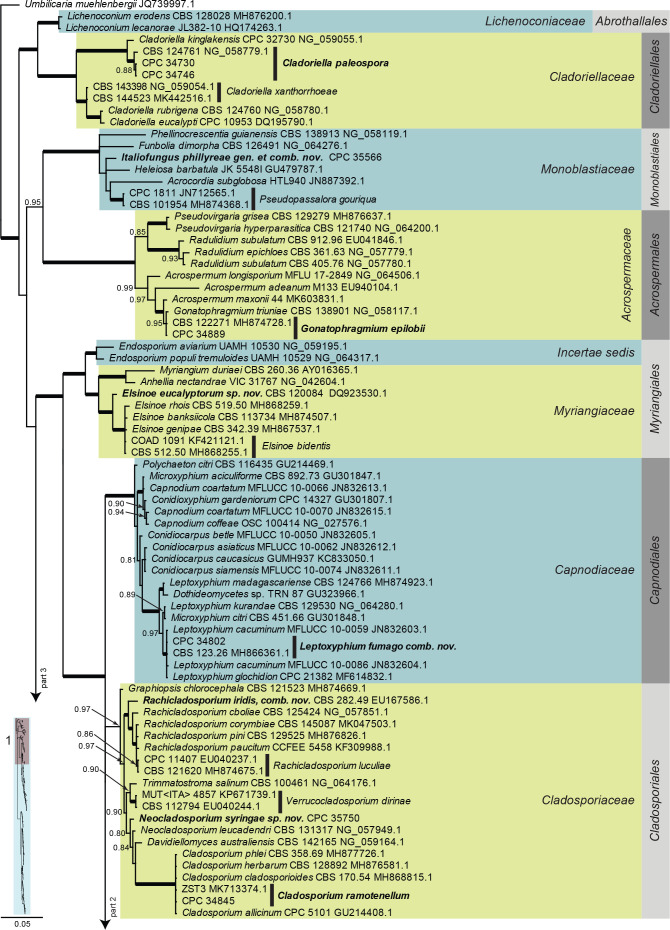
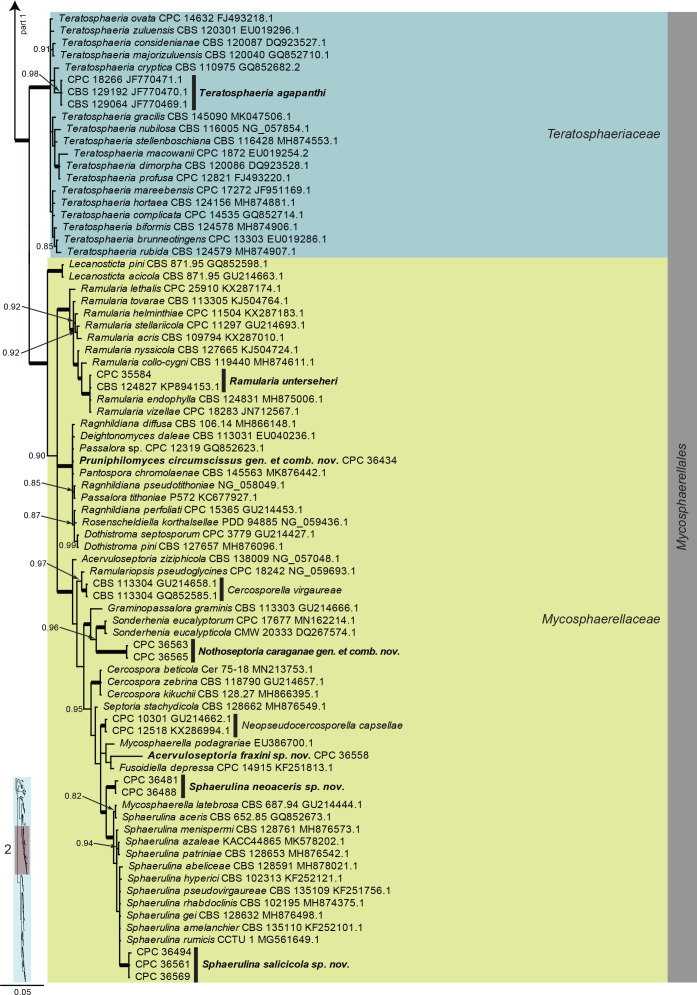
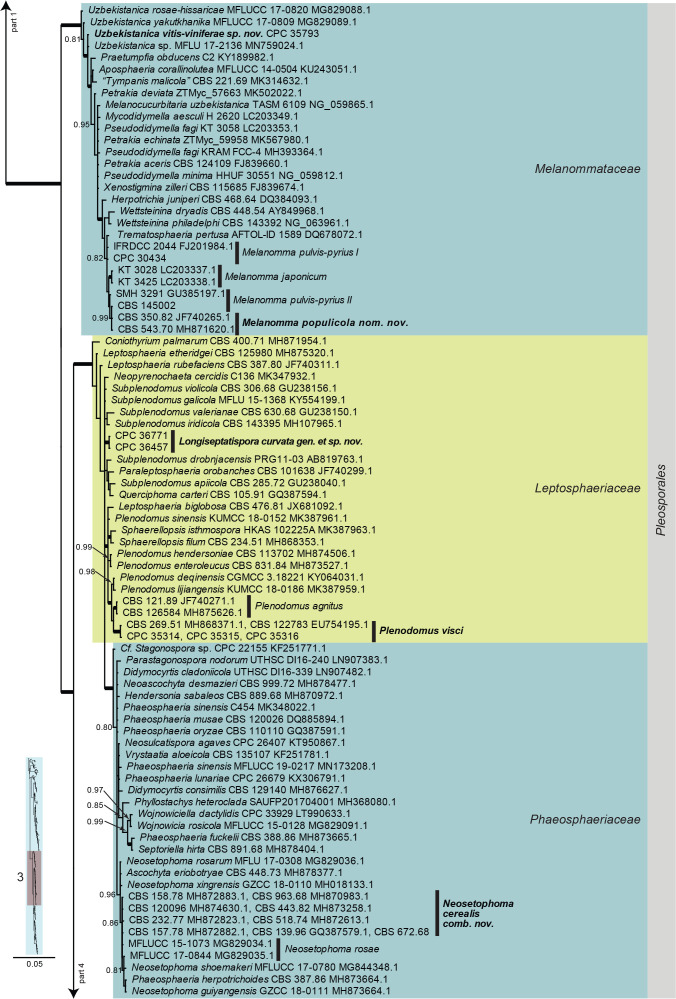
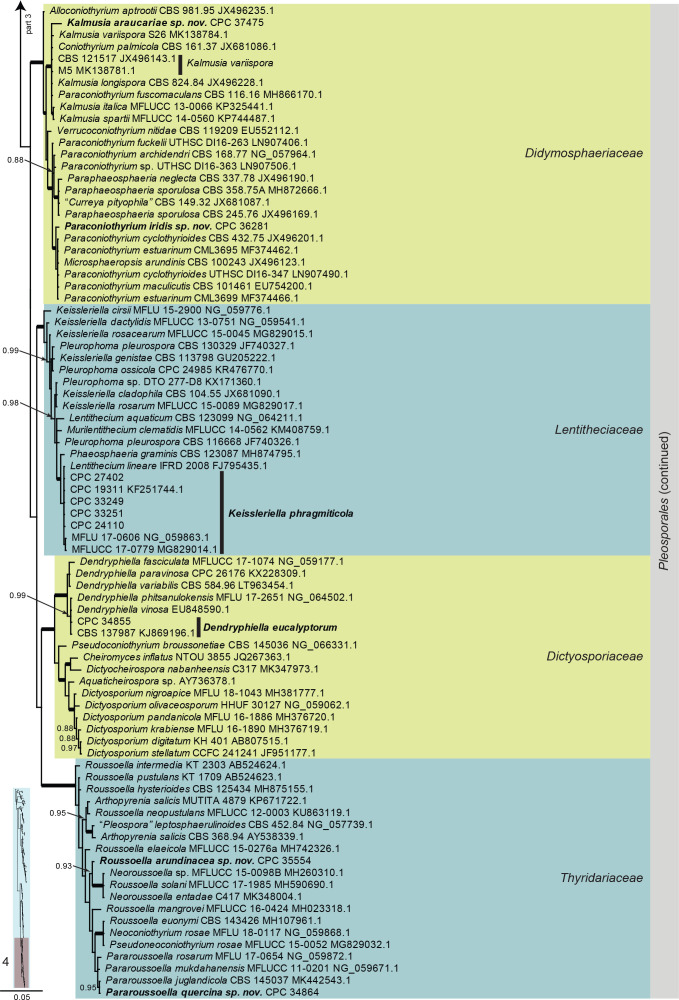
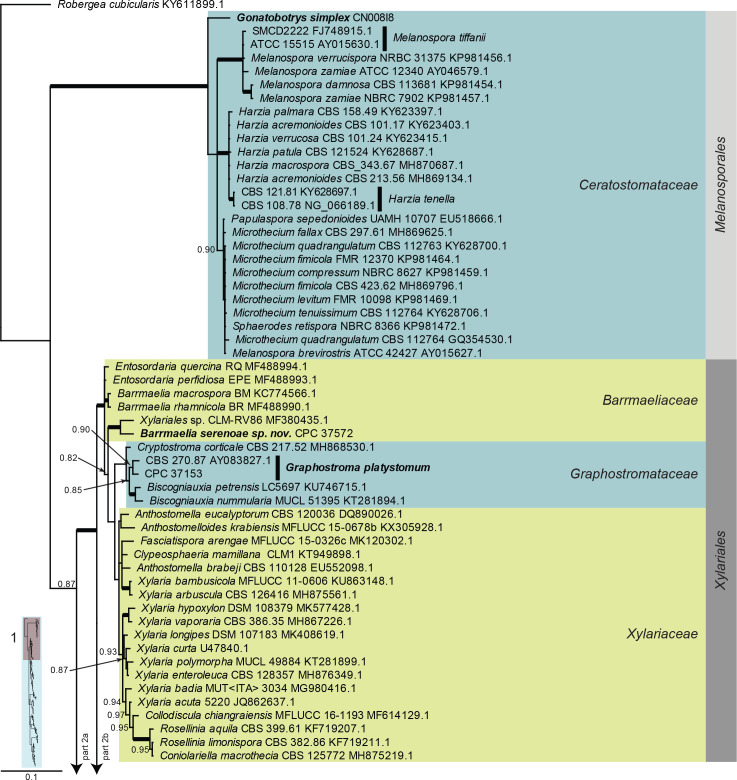
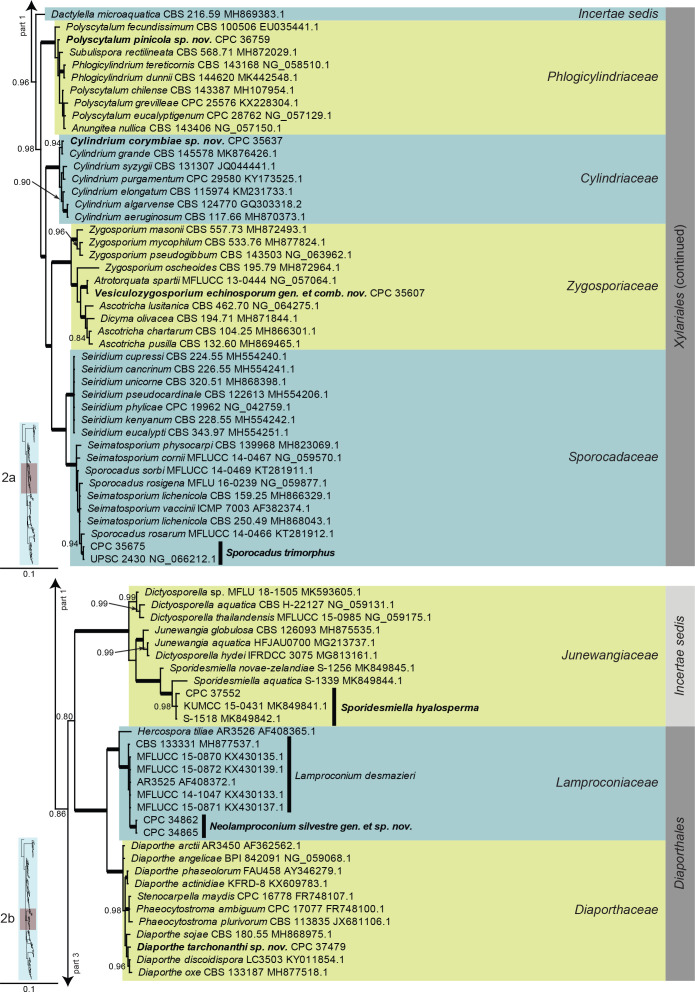
Dothideomycetes LSU phylogeny (Fig. 3, parts 1–4): The alignment contained 332 isolates and the tree was rooted to Umbilicaria muehlenbergii (strain A18, GenBank JQ739997.1). The final alignment contained a total of 808 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 391 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 6 502 trees (saved every 100 generations) from which 4 878 were sampled after 25 % of the trees were discarded as burn-in.
Fig. 3, parts 1–4.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Dothideomycetes LSU sequence alignment. Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or culture collection numbers are indicated for all species. The tree was rooted to Umbilicaria muehlenbergii (strain A18, GenBank JQ739997.1) and the species treated in this study for which LSU sequence data were available are indicated in bold face. A miniature overview tree is also presented to facilitate navigation along the tree topology.
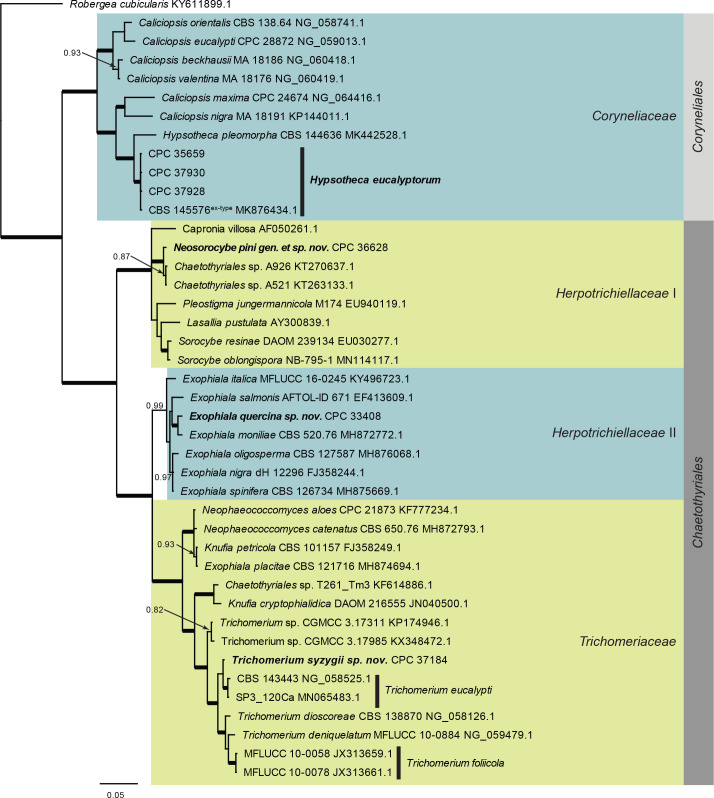
Eurotiomycetes LSU phylogeny (Fig. 4): The alignment contained 42 isolates and the tree was rooted to Robergea cubicularis (voucher G.M. 2013-05-09.1, GenBank KY611899.1). The final alignment contained a total of 773 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 226 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 302 trees (saved every 100 generations) from which 228 were sampled after 25 % of the trees were discarded as burn-in.
Fig. 4.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Eurotiomycetes LSU sequence alignment. Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or culture collection numbers are indicated for all species. The tree was rooted to Robergea cubicularis (voucher G.M. 2013-05-09.1, GenBank KY611899.1) and the species treated in this study for which LSU sequence data were available are indicated in bold face.
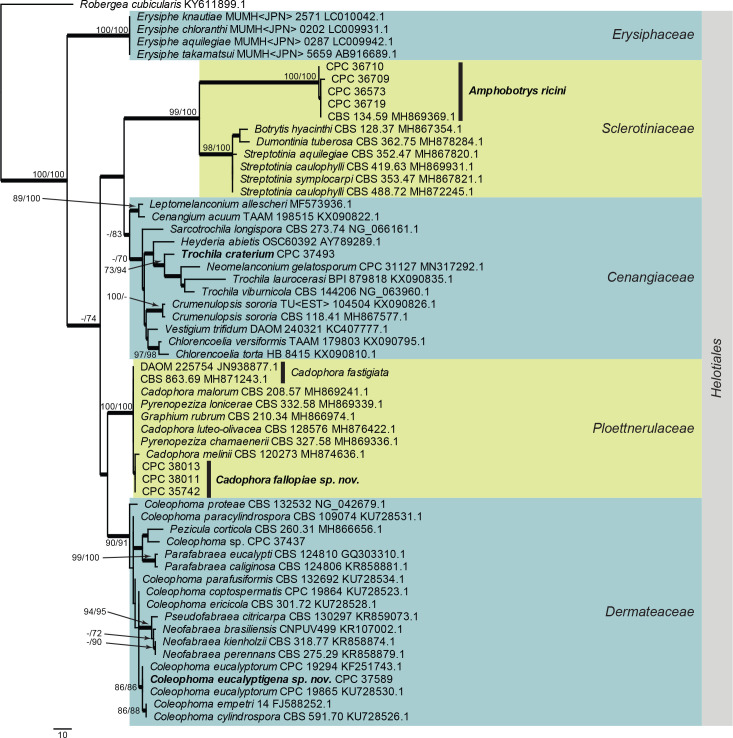
Leotiomycetes LSU phylogeny (Fig. 5): The alignment contained 41 isolates and the tree was rooted to Robergea cubicularis (voucher G.M. 2013-05-09.1, GenBank KY611899.1). The final alignment contained a total of 817 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 130 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 3 002 trees (saved every 10 generations) from which 2 252 were sampled after 25 % of the trees were discarded as burn-in.
Fig. 5.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Leotiomycetes LSU sequence alignment. Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1 and one branch was shortened four times to facilitate layout. Families and the order Helotiales are indicated with coloured blocks to the right of the tree. GenBank accession and/or culture collection numbers are indicated for all species. The tree was rooted to Robergea cubicularis (voucher G.M. 2013-05-09.1, GenBank KY611899.1) and the species treated in this study for which LSU sequence data were available are indicated in bold face.
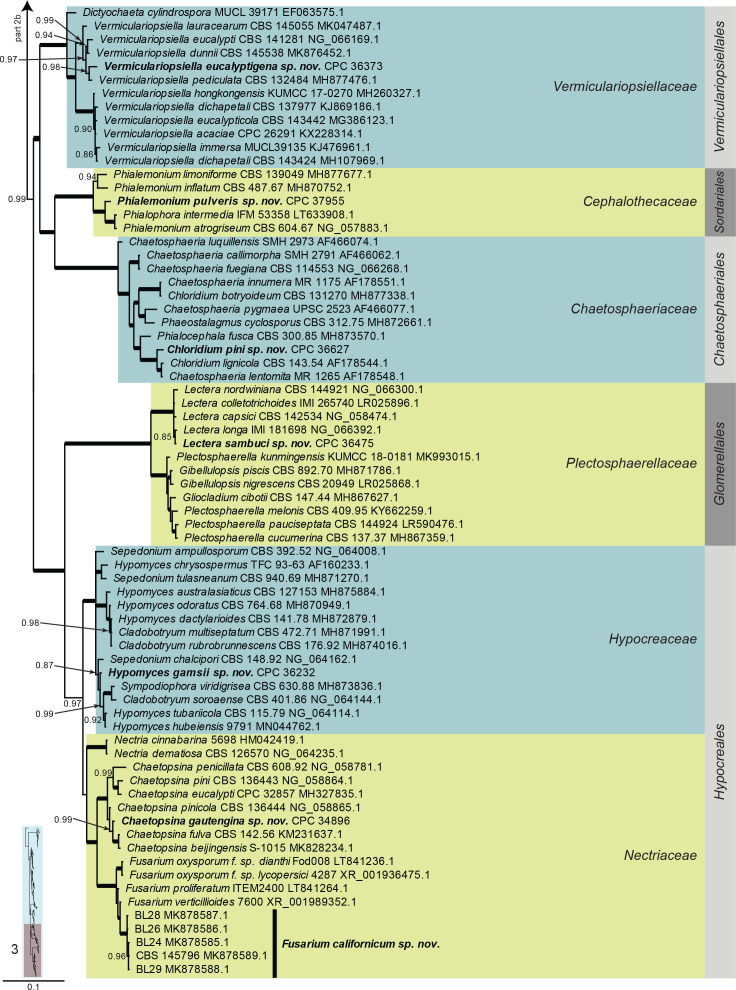
Sordariomycetes LSU phylogeny (Fig. 6, parts 1–3): The alignment contained 204 isolates and the tree was rooted to Robergea cubicularis (voucher G.M. 2013-05-09.1, GenBank KY611899.1). The final alignment contained a total of 825 characters used for the phylogenetic analyses, including alignment gaps. The alignment contained a total of 408 unique site patterns. Based on the results of MrModelTest, dirichlet base frequencies and the GTR+I+G model was used for the Bayesian analysis. The Bayesian analyses generated 117 502 trees (saved every 100 generations) from which 88 128 were sampled after 25 % of the trees were discarded as burn-in.
Fig. 6, parts 1–3.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Sordariomycetes LSU sequence alignment. Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. Families and orders are indicated with coloured blocks to the right of the tree. GenBank accession and/or culture collection numbers are indicated for all species. The tree was rooted to Robergea cubicularis (voucher G.M. 2013-05-09.1, GenBank KY611899.1) and the species treated in this study for which LSU sequence data were available are indicated in bold face. A miniature overview tree is also presented to facilitate navigation along the tree topology.
Species phylogenies: Specific phylogenetic analyses were run for selected species and the resulting phylogenies are discussed in the species notes where applicable. Statistics associated with those phylogenies are provided in the figure legends. The optimal identity thresholds to discriminate filamentous fungal species followed Vu et al. (2019), with secondary DNA barcodes generated where necessary (Stielow et al. 2015).
Taxonomy
Acervuloseptoria fraxini Crous & Bulgakov, sp. nov. MycoBank MB835067. Fig. 7.
Fig. 7.
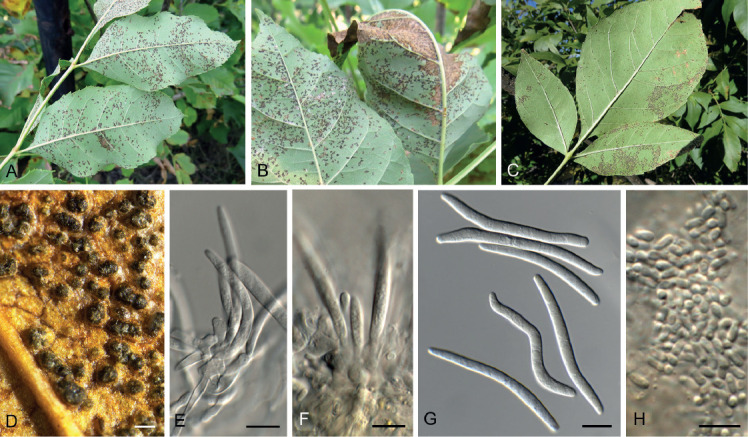
Acervuloseptoria fraxini (CPC 36558). A–C. Disease symptoms on Fraxinus pennsylvanica. D. Conidiomata. E, F. Conidiogenous cells. G. Conidia. H. Spermatia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Fraxinus from which the species was isolated.
Leaf spots hypophyllous, brown, 1–5 mm diam., appearing as small specks on leaf near nerves, later merging. Conidiomata pycnidial, intermingled among spermatogonia on leaves, 200–250 μm diam, black, aggregated in dense clusters, opening via central ostiole; wall of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity. Conidiogenous cells subcylindrical to ampulliform, hyaline, smooth, 6–10 × 3–4 μm, proliferating percurrently and sympodially at apex. Conidia solitary, subcylindrical, hyaline, smooth, granular, straight to curved, apex subobtuse, base truncate with basal marginal frill, medianly 1-septate, (45−)52–58(−60) × 4(−5) μm.
Culture characteristics: Colonies erumpent, with sparse aerial mycelium and smooth, lobate margin, reaching 5 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse cinnamon, but with diffuse scarlet pigment on OA.
Typus: Russia, Rostov region, Shakhty city district, trees near Atyukhta river, on living leaves of Fraxinus pennsylvanica, 7 Oct. 2018, T.S. Bulgakov, HPC 2609 = Myc-45 (holotype CBS H-24228, culture ex-type CPC 36558 = CBS 145992).
Notes: Acervuloseptoria has conidiomata that are black, erumpent, with the top layer disintegrating upon maturity, making the structures appear acervular (Quaedvlieg et al. 2013). Although this species clusters with A. ziziphicola, conidiomata did not appear acervular.
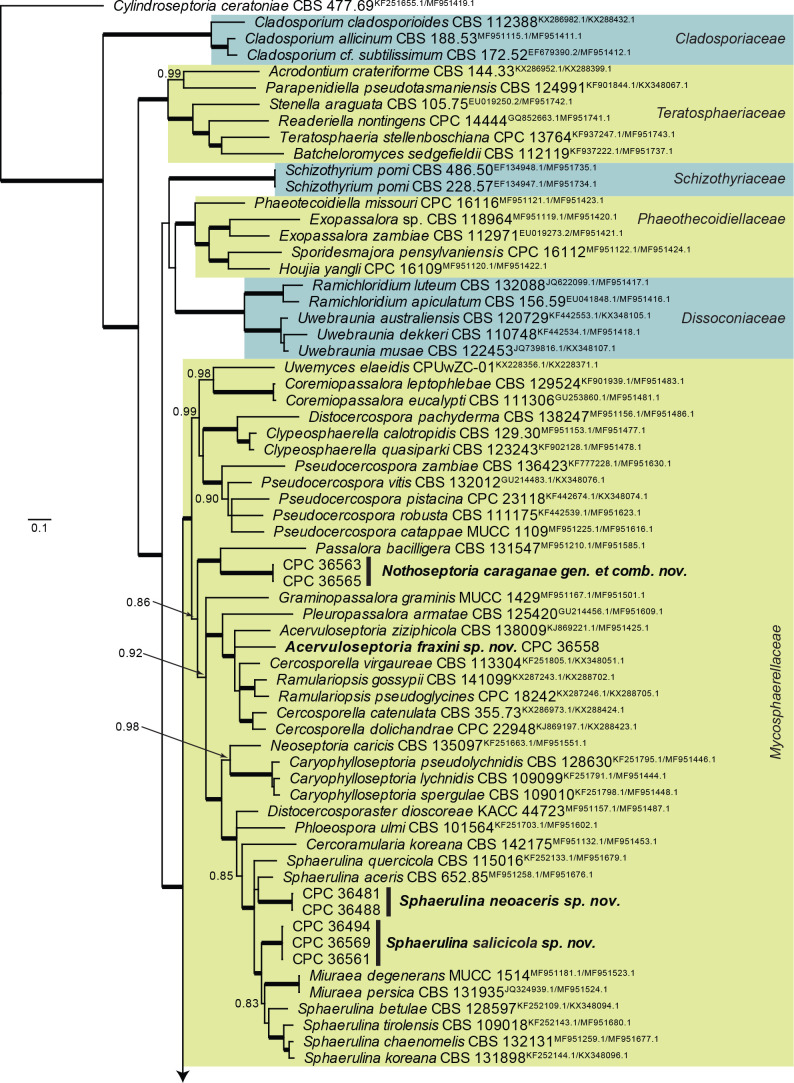
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Ascomycota sp. (as endophyte of healthy, mature leaves of Fraxinus velutina in Arizona, USA, strain ARIZ RTAsh3-1, GenBank JN120380.1; Identities = 471/472 (99 %), no gaps), Sonderhenia radiata (strain CBS 145600, GenBank MN162023.1; Identities = 487/542 (90 %), 16 gaps (2 %)), and Sonderhenia eucalyptorum (strain CPC 17677, GenBank MN162019.1; Identities = 487/542 (90 %), 16 gaps (2 %)). Closest hits using the LSU sequence are Cercosporella virgaureae (strain CBS 113304, GenBank GU214658.1; Identities = 814/847 (96%), 1 gap (0 %)), Graminopassalora graminis (strain CBS 113303, GenBank GU214666.1; Identities = 812/847 (96 %), 1 gap (0 %)), and Acervuloseptoria ziziphicola (strain CBS 138009, GenBank NG_057048.1; Identities = 808/843 (96 %), 1 gap (0 %)) – also see Fig. 3, part 2 and Fig. 8, part 1. Closest hits using the rpb2 sequence had highest similarity to Cercosporella dolichandrae (strain CBS 138101, GenBank KX288423.1; Identities = 713/851 (84 %), 2 gaps (0 %)), Ramulariopsis gossypii (strain CPC 25909, GenBank KX288702.1; Identities = 586/689 (85 %), 2 gaps (0 %)), and Neopseudocercosporella capsellae (strain MAFF 237605, GenBank MF951550.1; Identities = 672/877 (77 %), 6 gaps (0 %)) – also see Fig. 8, part 1.
Fig. 8, parts 1, 2.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Mycosphaerellaceae LSU/rpb2 sequence alignment (123 strains including the outgroup; 246 and 500 unique site patterns for LSU and rpb2, respectively; 35 928 sampled trees from 4 330 000 generations). The alignment is derived from the overview LSU/rpb2 alignment of Videira et al. (2017). Bayesian posterior probabilities (PP) > 0.79 are shown at the nodes and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. Families are indicated with coloured blocks to the right of the tree. GenBank accession (superscript text) and/or culture collection numbers are indicated for all species. The tree was rooted to Cylindroseptoria ceratoniae (culture CBS 477.69; GenBank KF251655.1 and MF951419.1 for LSU and rpb2, respectively) and the species treated in this study are indicated in bold face.
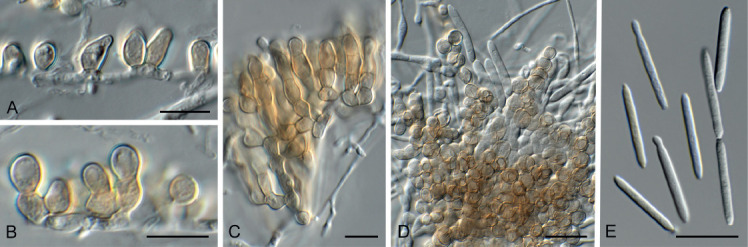
Amphobotrys ricini (N.F. Buchw.) Hennebert, Persoonia 7: 192. 1973. Fig. 9.
Fig. 9.
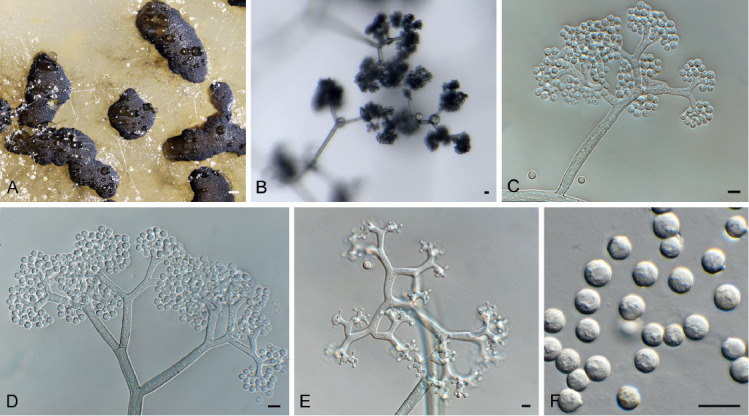
Amphobotrys ricini (CPC 36573). A. Sclerotia on OA. B–D. Conidiophores. E. Conidiogenous cells. F. Conidia. Scale bars: A = 4 mm, all others = 10 μm.
Basionym: Botrytis ricini N.F. Buchw., K. VetHojsk. Aarsskr. 32: 148. 1949.
Synonyms: Sclerotinia ricini G.H. Godfrey, Phytopathology 9: 565. 1919.
Botryotinia ricini (G.H. Godfrey) Whetzel, Mycologia 37: 680. 1945.
Colonies erumpent, grey olivaceous to brown, hyphae olivaceous brown, branched, septate. Conidiophores erect, solitary, with large conidial heads; stipe cylindrical, pale brown, septate, bifurcate, branches symmetrical, branching bifurcate at shorter intervals to produce groups of paired, globose, inflated terminal conidiogenous cells, each producing short pedicels that give rise to conidia, and collapse at maturity. Conidia holoblastic, globose, subhyaline to pale brown, smooth with inconspicuous basal marginal frill, 5–7 μm diam. Sclerotia black, solitary, irregular to curved, smooth, 1–4 mm diam. A Myrioconium synasexual morph could sometimes also develop in culture (adapted from Hennebert 1973, Soares 2012)
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, on CYA > 100 mm / 25 °C / 7 d, MEA > 100 mm / 25 °C / 7 d, CYA no growth / 37 °C / 7 d, CYA/MEA – abundant sclerotia, covering entire Petri dish. Very poor sporulation on MEA/CYA.
Typus: USA, Florida, on Ricinus communis Jun. 1919, G.H. Godfrey, holotype BPI 573856; Texas, Euless, settle plate in bedroom, 14 Dec. 2016, Ž. Jurjević (epitype designated here CBS H-24230 MBT391565, culture ex-type EMSL 3787 = CPC 36573 = CBS 145995).
Additional materials examined: USA, New Jersey, air from hospital, Oct. 2017, Ž. Jurjević, EMSL 4505 = CPC 36709; Illinois, Chicago, air from hospital, Oct. 2017, Ž. Jurjević, EMSL 4513 = CPC 36710; Massachusetts, Cohasset, air from basement, Sep. 2018, Ž. Jurjević, EMSL 4928 = CPC 36719; Georgia, CPC 38557 = MUCL 001030; USA but location unknown, CPC 38558 = MUCL 002165.
Notes: Grey mould of Ricinus communis was originally described as Sclerotinia ricini, which was later transferred to Botryotinia, with the asexual morph named as Botrytis ricini nom. nov. (Buchwald 1949). The fungus was later placed in the monotypic genus Amphobotrys by Hennebert (1973). This pathogen is frequently responsible for disease epidemics and heavy yield losses in castor crops (Soares 2012).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 36573 had highest similarity to Amphobotrys ricini (strain RWB 1595, GenBank JX961614.1; Identities = 460/471 (98 %), 4 gaps (0 %)), Monilinia fructicola (strain Victor, GenBank MN179292.1; Identities = 293/356 (82 %), 15 gaps (4 %)), and Peziza fascicularis (strain KL144, GenBank LT158418.1; Identities = 421/527 (80 %), 42 gaps (7 %)). The ITS sequence of CPC 36573 is identical to that of CPC 36719 (489/489 bases) and 38557 (488/488 bases) and differs with an extra T from CPC 36709 (481/482 bases) and 36710 (484/485 bases). The ITS sequence of CPC 38558 is not identical to the other sequences obtained here (474/488 (97 %) bayes including 4 gaps compared to CPC 36573) and has as highest similarity in GenBank Amphobotrys ricini (strain CopAr-5, GenBank JF433374.1; Identities = 476/488 (98 %), 4 gaps (0 %). Closest hits using the LSU sequence of CPC 36573 are Amphobotrys ricini (as Botryotinia ricini, strain CBS 134.59, GenBank MH869369.1; Identities = 877/877 (100 %), no gaps), Streptotinia symplocarpi (strain CBS 353.47, GenBank MH867821.1; Identities = 804/884 (91 %), 9 gaps (1 %)), and Streptotinia caulophylli (strain CBS 419.63, GenBank MH869931.1; Identities = 802/884 (91 %), 9 gaps (1 %)) – also see Fig. 5. The LSU sequence of CPC 36573 is identical to that of CPC 36719 (849/849 bases), differs with a single nucleotide from CPC 36710 (845/846 bases) and differs with 3 nucleotides from CPC 36709 (855/858 bases). Closest hits using the rpb2 sequence of CPC 36573 had highest similarity to Amphobotrys ricini (strain 14-21, GenBank KR183766.1; Identities = 782/783 (99 %), no gaps), Botrytis paeoniae (strain 0003, GenBank AJ745699.1; Identities = 870/910 (96 %), no gaps), and Botrytis convoluta (strain 9801, GenBank AJ745679.1; Identities = 862/910 (95 %), no gaps). The rpb2 sequence of CPC 36573 is identical to that of CPC 36719 (836/836 bases) and 36710 (846/846 bases) and differs with a single nucleotide from CPC 36709 (871/872 bases).
Arcticomyces warmingii (Rostr.) Savile, Canad. J. Bot. 37: 984. 1959. Fig. 10.
Fig. 10.
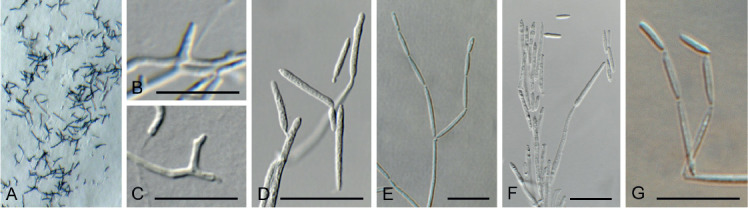
Arcticomyces warmingii (CPC 36560). A. Sporulation on SNA. B, C. Conidiophores. D-G. Chains of conidia. Scale bars = 10 μm.
Basionym: Exobasidium warmingii Rostr., Meddr Grønland, Biosc. 3: 530. 1888.
Mycelium consisting of hyaline, smooth, branched, septate, 1 μm diam hyphae. Conidiophores erect, hyaline, smooth, reduced to conidiogenous loci on hyphae, 1–5 × 1 μm; loci somewhat darkened but not refractive, 0.5 μm diam. Ramoconidia subcylindrical, hyaline, smooth, tapering at both ends, rarely indistinctly septate, slightly curved, 10–30 × 1 μm. Conidia hyaline, smooth, subcylindrical, apex obtuse, base truncate, aseptate, 5–8 × 1 μm; loci darkened not refractive, 0.5 μm diam.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and smooth to irregular, lobate margin, reaching 20 mm diam after 2 wk at 25 °C. On MEA surface white, reverse dark brick; on PDA surface white, reverse sepia; on OA surface white.
Material examined: UK, Scotland, Perthshire, Kindrogan Field Study Centre, on Frullania dilatata, 25 Aug. 2018, G. Greiff, HPC 2525 (CBS H-24168, culture CPC 36560 = CBS 146033).
Notes: Arcticomyces warmingii is a foliar pathogen of Saxifragaceae with a neo- and paleartic distribution. Phylogenetically, it is closely related to species of Exobasidium and Muribasidiospora, a relationship that requires further study (Wang et al. 2015).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Arcticomyces warmingii (voucher GLM-F105799, GenBank KY424481.1; Identities = 573/586 (98 %), 4 gaps (0 %)), Exobasidium rhododendri (strain CBS 101457, GenBank DQ667153.1; Identities = 446/477 (94 %), 11 gaps (2 %)), and Exobasidium japonicum (voucher GLM-F105792, GenBank KY424480.1; Identities = 422/453 (93 %), 12 gaps (2 %)). Closest hits using the LSU sequence are Exobasidium vaccinii (strain DSM 5498, GenBank MH047198.1; Identities = 890/914 (97 %), 1 gap (0 %)), Muribasidiospora indica (strain STE-U 5243, GenBank AY204506.1; Identities = 866/893 (97 %), 3 gaps (0 %)), and Exobasidium rhododendri (strain CBS 101457, GenBank DQ667151.1; Identities = 887/915 (97 %), 2 gaps (0 %)) – also see Fig. 1. Closest hits using the tef1 (second part) sequence had highest similarity to Exobasidium gracile (strain AFTOL-ID 1643, GenBank DQ663703.1; Identities = 429/468 (92 %), no gaps), Exobasidium rostrupii (strain CNJ2-3, GenBank KR262257.1; Identities = 427/466 (92 %), no gaps), and Exobasidium maculosum (strain D1-2, GenBank KR262207.1; Identities = 420/466 (90 %), no gaps). No significant hits were obtained when the cmdA sequence was used in blastn and megablast searches.
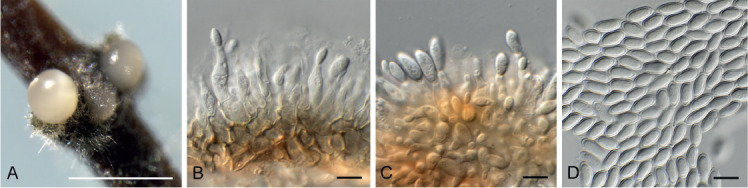
Barrmaelia serenoae Crous, sp. nov. MycoBank MB835068. Fig. 11.
Fig. 11.
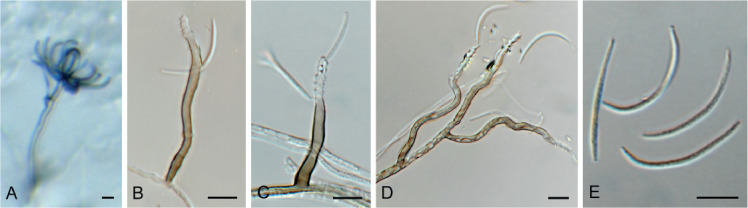
Barrmaelia serenoae (CPC 37572). A–D. Conidiophores giving rise to curved conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Serenoa from which the species was isolated.
Mycelium consisting of hyaline, smooth, septate, branched, 1.5–2 μm diam hyphae. Conidiophores solitary, erect, subcylindrical, brown, smooth, or base slightly roughened, unbranched, 1–6-septate, 30–100 × 2.5–3.5 μm. Conidiogenous cells terminal, integrated, subcylindrical, brown, smooth, 30–40 × 2 μm; apex forming a short rachis with subdenticulate loci, 0.5 μm diam. Conidia solitary, hyaline, smooth, aseptate, strongly curved, subcylindrical, apex subobtuse, base truncate, (20−)25–30 × 1.5 μm.
Culture characteristics: Colonies flat, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 40 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface buff, reverse cinnamon to buff.
Typus: USA, Florida, Gainesville, on leaf of Serenoa repens (Arecaeae), 24 Feb. 2019, M.J. Wingfield, HPC 2792 (holotype CBS H-24201, culture ex-type CPC 37572 = CBS 146017).
Notes: Barrmaelia serenoae, which is only known from its asexual morph, clusters close to sexual species of Barrmaelia, which in turn have asexual morphs corresponding in morphology to that of our fungus. For this reason, we choose to tentatively name the fungus from Serenoa repens in Barrmaelia (Voglmayr et al. 2018).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Fasciatispora arengae (strain MFLUCC 15-0326c, GenBank MK120277.1; Identities = 394/454 (87 %), 20 gaps (4 %)), Virgaria boninensis (strain JCM 18623, GenBank AB670710.1; Identities = 391/454 (86 %), 16 gaps (3 %)), and Virgaria nigra (strain NBRC 9453, GenBank AB670716.1; Identities = 350/395 (89 %), 11 gaps (2 %)). Closest hits using the LSU sequence are Xylaria enteroleuca (strain CBS 128357, GenBank MH876349.1; Identities = 786/819 (96 %), 6 gaps (0 %)), Xylaria longipes (strain DSM 107183, GenBank MK408619.1; Identities = 786/819 (96 %), 6 gaps (0 %)), and Xylaria badia (strain MUT 3034, GenBank MG980416.1; Identities = 784/817 (96 %), 2 gaps (0 %)) – also see Fig. 6, part 1. Closest hits using the tef1 (second part) sequence had highest similarity to Stegonsporium protopyriforme (strain D80, GenBank KF570212.1; Identities = 447/473 (95 %), no gaps), Stegonsporium acerinum (strain D43, GenBank EU040024.1; Identities = 443/470 (94 %), no gaps), and Stegonsporium acerophilum (strain D45, GenBank EU040030.1; Identities = 442/470 (94 %), no gaps). No significant hits were obtained when the tub2 sequence was used in blastn and megablast searches.
Cadophora fallopiae Crous & Akulov, sp. nov. MycoBank MB835069. Fig. 12.
Fig. 12.
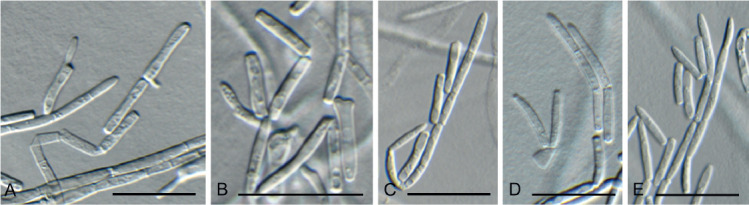
Cadophora fallopiae (CPC 38013). A–E. Subcylindrical conidiogenous cells giving rise to chains of subcylindrical conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Fallopia from which the species was isolated.
Conidiomata (in vivo) solitary, applanate, black, round, subepidermal, 200–400 μm diam, opening by irregular rupture; wall of 3–6 layers of pale brown textura angularis. Conidiophores lining the inner cavity, base pale brown, smooth, ampulliform to subcylindrical, 1-septate, branched or not, giving rise to 1–2 conidiogenous cells, 7–15 × 4–6 μm. Conidiogenous cells hyaline, smooth, subcylindrical, proliferating sympodially, 4–12 × 1.5 μm. Conidia solitary, subcylindrical, flexuous, apex subobtuse, base truncate, multiseptate, hyaline, smooth-walled, granular, (10−)40–75 × 1.5 μm. In culture conidiomatal initials develop, but remain infertile. However, a cladophialophora-like genus develops on SNA. Conidiophores arise from superficial hyphae, subcylindrical, pale brown, smooth. Conidiogenous cells subcylindrical, 10–20 × 1.5–2 μm, proliferating sympodially. Conidia similar to those observed in vivo, except that they disarticulate at septa into phragmospores, 6–11 μm long.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 40 mm diam after 2 wk at 25 °C. On MEA and PDA surface and reverse olivaceous grey; on OA surface iron-grey.
Typus: Poland, Krakow, city park, on Reynoutria sachalinensis (Polygonaceae) overwintered stems, 30 Mar. 2019, A. Akulov, HPC 2923 = CWU (Myc) AS 7061 (holotype CBS H-24251, culture ex-type CPC 38013 = CBS 146083), idem., culture CPC 38011.
Additional material examined: Germany, Witten, recreational area Hohenstein, on stem of Reynoutria japonica, 9 Feb. 2018, Th. Hülsewig, HPC 2440 (culture CPC 35742 = CBS 145565).
Notes: Although Cadophora fallopiae clusters among species of Cadophora, only a cladophialophora-like synasexual morph was observed in culture.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to numerous sequences of Cadophora luteo-olivacea (e.g. strain CBS 140.41, GenBank MH856091.1; Identities = 520/541 (96 %), 7 gaps (1 %)). The ITS sequence of CPC 38013 is identical to that of CPC 38011 (631/631 bases) and 99 % similar to that of CPC 35742 (627/631 bases, no gaps). Closest hits using the LSU sequence are Cadophora luteo-olivacea (strain CBS 128576, GenBank MH876422.1; Identities = 849/850 (99 %), no gaps), Pyrenopeziza compressula (strain CBS 359.63, GenBank MH869921.1; Identities = 849/850 (99 %), no gaps), and Pyrenopeziza lonicerae (strain CBS 332.58, GenBank MH869339.1; Identities = 849/850 (99 %), no gaps) – also see Fig. 5. The LSU sequence of CPC 38013 is identical (848/848 bases) to those of CPC 38011 and CPC 35742.
Chaetopsina gautengina Crous, sp. nov. MycoBank MB835070. Fig. 13.
Fig. 13.
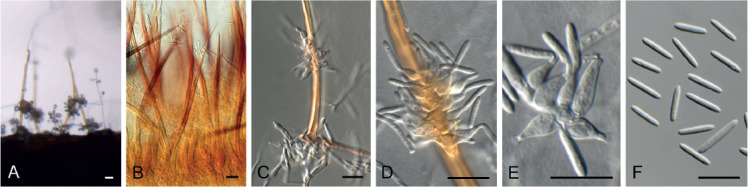
Chaetopsina gautengina (CPC 34896). A, B. Erect, setiform conidophores. C–E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the Gauteng Province, South Africa, where this fungus was collected.
Conidiophores erect, setiform, tapering gradually towards acutely rounded apex, flexuous, medium brown, turning red-brown in 3 % KOH, fertile mid region unbranched, verruculose, 100–300 × 5–8 μm, 8–16-septate, thick-walled (2 μm diam), base bulbous, 9–12 μm diam; fertile region consisting of irregularly branched, densely aggregated conidiogenous cells. Conidiogenous cells ampulliform to subcylindrical, hyaline to pale brown, smooth, mono- to polyphialidic, 6–10 × 2.5–4 μm. Conidia hyaline, smooth, guttulate, subcylindrical, aseptate, apex and base bluntly rounded, (8−)11–14(−20) × 2(−2.5) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 30 mm diam after 2 wk at 25 °C. On MEA surface sienna, reverse ochreous; on PDA surface and reverse ochreous; on OA surface apricot.
Typus: South Africa, Gauteng Province, on leaves of unidentified grass (Poaceae), 2016, A. Cilliers (holotype specimen CBS 145535, preserved as metabolically inactive culture, culture ex-type CPC 34896 = CBS 145535).
Notes: Chaetopsina gautengina is phylogenetically closely related to C. pinicola (on Pinus needles, Thailand, conidia (11−)13–15(−17) × 2(−2.5) μm; Crous et al. 2013), but can be distinguished based on its narrower setae, those of C. piniciola being 130–250 × 8–12 μm.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Chaetopsina pinicola (strain CBS 136444, GenBank NR_137823.1; Identities = 571/591 (97 %), 5 gaps (0 %)), Chaetopsina beijingensis (strain CBS 138004, GenBank KJ869159.1; Identities = 571/596 (96 %), 7 gaps (1 %)), and Chaetopsina fulva (strain FMR_13129, GenBank KY853432.1; Identities = 527/552 (95 %), 8 gaps (1 %)). Closest hits using the LSU sequence are Chaetopsina pinicola (strain CBS 136444, GenBank NG_058865.1; Identities = 825/829 (99 %), no gaps), Chaetopsina fulva (strain CBS 142.56, GenBank KM231637.1; Identities = 806/813 (99 %), no gaps), and Chaetopsina beijingensis (strain S-1015, GenBank MK828234.1; Identities = 796/804 (99 %), no gaps) – also see Fig. 6, part 3.
Chaetospermum camelliae Agnihothr., Mycopath. Mycol. appl. 16: 115. 1962. Fig. 14.
Fig. 14.
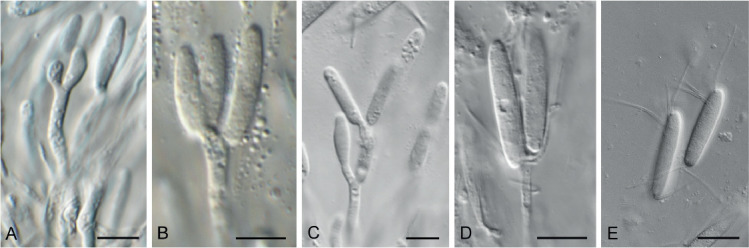
Chaetospermum camelliae (CPC 34736). A–D. Conidiogenous cells giving rise to conidia. E. Conidia with appendages. Scale bars = 10 μm.
Description and illustration: Crous (1993), Marincowitz et al. (2010).
Material examined: South Africa, Mpumalanga Province, Kruger National Park, on leaves of Trichilia emetica (Meliaceae), 2010, P.W. Crous (HPC 2260, culture CPC 34736 = CBS 145580).
Notes: This species has previously been reported from living leaves of Syzygium cordatum in South Africa (Crous 1993, Marincowitz et al. 2010), as saprobe on unidentified dead leaves in Thailand (Tangthirasunun et al. 2014; epitype designated by authors) and leaves of Typha angustifolia and Pandanus in Thailand (Rungjindamai et al. 2008). Trichilia emetica represents a new host record for this fungus.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Chaetospermum camelliae (as Chaetospermum chaetosporum, strain CBS 612.75, GenBank KJ710462.1; Identities = 560/560 (100 %), no gaps), Chaetospermum camelliae (strain MFLUCC 12-0318, GenBank KF516964.1; Identities = 558/559 (99 %), 1 gap (0 %)), and Chaetospermum chaetosporum (strain CBS 154.59, GenBank NR_126146.1; Identities = 583/615 (95 %), 12 gaps (1 %)). Closest hits using the LSU sequence are Chaetospermum camelliae (strain BCC18604, GenBank EF589737.1; Identities = 872/872 (100 %), no gaps), Chaetospermum chaetosporum (strain CBS 154.59, GenBank NG_058876.1; Identities = 835/845 (99 %), 1 gap (0 %)), and Chaetospermum artocarpi (strain MFLUCC 12-0535, GenBank KF516973.1; Identities = 855/878 (97 %), 1 gap (0 %)) – also see Fig. 1.
Chloridium pini Crous & Akulov, sp. nov. MycoBank MB835071. Fig. 15.
Fig. 15.
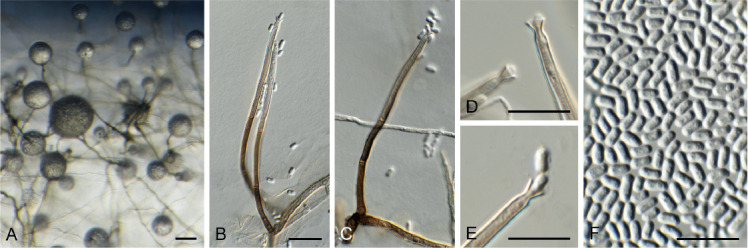
Chloridium pini (CPC 36627). A–C. Conidiophores. D, E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Pinus from which the fungus was isolated.
Mycelium consisting of pale brown, smooth, branched, septate, 1.5–2.5 μm diam hyphae. Conidiophores arising from superficial hyphae, erect, subcylindrical, flexuous, brown, smooth, 40–150 × 2–3 μm; at times rejuvenating percurrently. Conidiogenous cells terminal, integrated, subcylindrical, pale brown, smooth, 25–40 × 2.5–3 μm, phialidic, with flared collarette, 2–3 μm long. Conidia aseptate, solitary, hyaline, smooth, granular, subcylindrical, ends truncate to bluntly rounded, (3−)4–5 × 2(−2.5) μm.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 12 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Ukraine, Sumy region, Okhtyrka district, Klymentove village, NNP Hetmanskyi, on fallen trunk of Pinus sylvestris (Pinaceae), 3 Aug. 2018, A. Akulov, HPC 2548 = CWU (Myc) AS 6816 (holotype CBS H-24171, culture ex-type CPC 36627 = CBS 146011).
Notes: Chloridium pini is phylogenetically closely related to C. lignicola [conidia oblong-ellipsoidal, sometimes slightly allantoid, 3.5–5(−7) × 1.5–2 μm; Gams & Holubová-Jechová 1976], but is morphologically distinct from that species.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Chloridium lignicola (strain CBS 143.54, GenBank AF178544.1; Identities = 470/493 (95 %), 6 gaps (1 %)), Chaetosphaeria lentomita (strain MR 1265, GenBank AF178548.1; Identities = 468/492 (95 %), 4 gaps (0 %)), and Phialocephala fusca (strain CBS 300.85, GenBank AB671500.2; Identities = 488/538 (91 %), 12 gaps (2 %)). Closest hits using the LSU sequence are Chloridium lignicola (strain CBS 143.54, GenBank MH868806.1; Identities = 816/825 (99 %), no gaps), Chaetosphaeria lentomita (strain MR 1265, GenBank AF178548.1; Identities = 815/825 (99 %), no gaps), and Phialocephala fusca (strain CBS 300.85, GenBank MH873570.1; Identities = 795/823 (97 %), no gaps) – also see Fig. 6, part 3. No significant hits were obtained when the tub2 sequence was used in blastn and megablast searches.
Cladoriella paleospora Cheew. & Crous, Persoonia 23: 63. 2009.
Description and illustration: Cheewangkoon et al. (2009).
Material examined: South Africa, Western Cape Province, Stellenbosch, on leaves Eucalyptus sp. (Myrtaceae), 1 Nov. 2016, P.W. Crous, cultures CPC 34746 = CBS 144495, CPC 34730.
Notes: Cladoriella paleospora was originally described from Eucalyptus globulus leaves collected in Australia (Cheewangkoon et al. 2009).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 34746 had highest similarity to Cladoriella paleospora (strain CBS 124761, GenBank MH863405.1; Identities = 488/493 (99 %), 3 gaps (0 %)), Cladoriella kinglakensis (strain CPC 32730, GenBank NR_156396.1; Identities = 484/492 (98 %), 1 gap (0 %)), and Cladoriella eucalypti (strain CBS 115899, GenBank EU040224.1; Identities = 246/264 (93 %), 3 gaps (1 %)). The ITS sequences of CPC 34746 and CPC 34730 are identical (480/480 bases). Closest hits using the LSU sequence of CPC 34746 are Cladoriella paleospora (strain CBS 124761, GenBank NG_058779.1; Identities = 617/621 (99 %), no gaps), Cladoriella kinglakensis (strain CPC 32730, GenBank NG_059055.1; Identities = 831/843 (99 %), no gaps), and Cladoriella xanthorrhoeae (strain CBS 144523, GenBank MK442516.1; Identities = 805/862 (93 %), 1 gap (0 %)) – also see Fig. 3, part 1. The LSU sequences of CPC 34746 and CPC 34730 are identical (831/831 bases).
Cladosporium ramotenellum K. Schub. et al., Stud. Mycol. 58: 137. 2007.
Description and illustration: Schubert et al. (2007).
Material examined: UK, on leaves Eucalyptus gunnii (Myrtaceae), 17 Dec. 2017, S. Denman, culture CPC 34845 = CBS 145592.
Notes: Originally described from hypersaline water in a saltern in Slovenia (Schubert et al. 2007), but now known globally from a wide range of hosts and substrates (Bensch et al. 2015).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Cladosporium ramotenellum (strain BC2, GenBank MK910072.1; Identities = 544/544 (100 %), no gaps), Cladosporium puyae (strain CBS 274.80A, GenBank NR_152298.1; Identities = 534/534 (100 %), no gaps), and Cladosporium phaenocomae (strain CBS 128769, GenBank MH865096.1; Identities = 544/544 (100 %), no gaps). Closest hits using the LSU sequence are Cladosporium ramotenellum (strain ZST3, GenBank MK713374.1; Identities = 860/860 (100 %), no gaps), Cladosporium phlei (strain CBS 358.69, GenBank MH877726.1; Identities = 860/860 (100 %), no gaps), and Cladosporium herbarum (strain CBS 128892, GenBank MH876581.1; Identities = 860/860 (100 %), no gaps) – also see Fig. 3, part 1. Closest hits using the actA sequence had highest similarity to Cladosporium ramotenellum (strain CPC 19119, GenBank MF474080.1; Identities = 368/373 (99 %), no gaps), Cladosporium allicinum (strain CPC 21906, GenBank KT600573.1; Identities = 356/362 (98 %), no gaps), and Cladosporium wyomingense (strain CPC 22310, GenBank MF474165.1; Identities = 420/430 (98 %), no gaps). Closest hits using the rpb2 sequence had highest similarity to Cladosporium sphaerospermum (strain BAM 599, GenBank LN833570.1; Identities = 874/875 (99 %), no gaps), Cladosporium cladosporioides (strain AFTOL-ID 1289 (= CBS 170.54), GenBank DQ677952.1; Identities = 864/868 (99 %), 1 gap (0 %)), and Cladosporium ramotenellum (strain ATCC 36970, GenBank MF951413.1; Identities = 673/679 (99 %), no gaps). Closest hits using the tef1 sequence had highest similarity to Cladosporium ramotenellum (strain CPC 11395, GenBank KT600523.1; Identities = 293/293 (100 %), no gaps), Cladosporium basi-inflatum (strain CBS 822.84, GenBank HM148241.1; Identities = 424/433 (98 %), 1 gap (0 %)), and Cladosporium cladosporioides (strain CBS 170.54, GenBank FJ936162.1; Identities = 423/432 (98 %), 1 gap (0 %)).
Coleophoma eucalyptigena Crous & R.K. Schumach., sp. nov. MycoBank MB835072. Fig. 16.
Fig. 16.
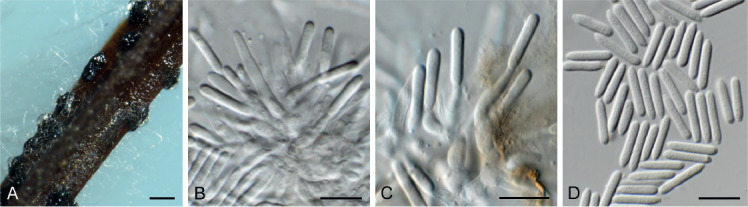
Coleophoma eucalyptigena (CPC 37589). A. Conidiomata forming on PNA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Etymology: Name refers to the host genus Eucalyptus from which the species was isolated.
Conidiomata pycnidial, brown, globose, 200–300 μm diam; wall of 2–6 layers of brown textura angularis. Paraphyses intermingled among conidiophores, hyaline, smooth, subcylindrical with apical taper towards obtusely rounded apex, 30–50 × 2.5–3.5 μm. Conidiophores hyaline, smooth, branched, subcylindrical, 0–3-septate, 10–20 × 3–4 μm. Conidiogenous cells hyaline, smooth, subcylindrical to ampulliform or doliiform, apical and intercalary, 5–10 × 3–4 μm, phialidic with periclinal thickening. Conidia solitary, hyaline, smooth, aseptate, guttulate, subcylindrical, apex obtuse, base tapering to flattened hilum, 1.5 μm diam, (10−)11–12(−14) × (2−)2.5(−3) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery margin, reaching 30 mm diam on MEA and PDA, 50 mm diam on OA after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Spain, Pontevedra, O Grove, on leaf litter of Eucalyptus sp. (Myrtaceae), 24 Feb. 2019, M. Deldgado, HPC 2826 = RKS 210 (holotype CBS H-24202, culture ex-type CPC 37589 = CBS 146018).
Notes: Coleophoma eucalyptigena is morphologically similar to C. eucalyptorum (Australia, leaves of Eucalyptus piperita, conidia (10−)11–12(−14) × (2−)2.5 μm; Crous et al. 2011), but is phylogenetically distinct from that species.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Coleophoma eucalyptorum (strain CPC 19865, GenBank KU728490.1; Identities = 537/538 (99 %), no gaps), Coleophoma paracylindrospora (strain CBS 109074, GenBank NR_154806.1; Identities = 529/539 (98 %), 1 gap (0 %)), and Coleophoma cylindrospora (strain BP-6252, GenBank MH762908.1; Identities = 528/538 (98 %), 1 gap (0 %)). Closest hits using the LSU sequence are “Septoria” sp. (strain CPC 19294, GenBank KF251743.1; Identities = 837/837 (100 %), no gaps), Coleophoma eucalyptorum (strain CPC 19865, GenBank KU728530.1; Identities = 867/868 (99 %), 1 gap (0 %)), and Coleophoma ericicola (strain CBS 301.72, GenBank KU728528.1; Identities = 865/868 (99 %), 1 gap (0 %)) – also see Fig. 5. Closest hits using the tef1 sequence had highest similarity to Coleophoma eucalyptorum (strain CPC 19865, GenBank KU728569.1; Identities = 435/457 (95 %), 1 gap (0 %)), Coleophoma camelliae (strain CBS 101376, GenBank KU728558.1; Identities = 379/437 (87 %), 14 gaps (3 %)), and Coleophoma paracylindrospora (strain CBS 109074, GenBank KU728570.1; Identities = 364/426 (85 %), 28 gaps (6 %)). No significant hits were obtained when the actA sequence was used in blastn and megablast searches.
Cylindrium corymbiae Crous, sp. nov. MycoBank MB835073. Fig. 17.
Fig. 17.
Cylindrium corymbiae (CPC 35637). A–D. Clusters of conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Etymology: Name reflects the genus Corymbia from which the species was isolated.
Mycelium consisting of branched, septate, hyaline, smooth, 1.5–2 μm diam hyphae. Conidiophores developing along hyphae, initially appearing as globose, brown, chlamydospore-like cells, intercalary on hyphae, later developing either a conidiogenous locus or additional brown cells forming a stroma (microsclerotium) with terminal conidiogenous cells. Conidiogenous cells medium brown, smooth, ampulliform, 7–13 × 3–5 μm, apex truncate, pale brown, 2 μm diam. Conidia aseptate, cylindrical, smooth, guttulate, ends obtuse with flattened hilum, in branched chains on host, but unbranched in culture, (19−)20–22 × 2–2.5 μm.
Culture characteristics: Colonies flat, spreading, surface folded with sparse aerial mycelium and smooth, lobate margin, reaching 50 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface umber, reverse isabelline.
Material examined: Australia, New South Wales, Mandalong, on leaves of Corymbia maculata (Myrtaceae), 16 Apr. 2018, A.J. Carnegie, HPC 2427 (holotype CBS H-24154, culture CPC 35637 = CBS 146087).
Notes: Cylindrium corymbiae is morphologically similar to Cylindrium grande [conidia (13−)18–20(−22) × (2−)2.5–3 μm; Crous et al. 2019a], but is phylogenetically distinct from that species.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Cylindrium grande (strain CPC 35403, GenBank MK876384.1; Identities = 540/542 (99 %), 1 gap (0 %)), Cylindrium elongatum (strain CBS 685.83A, GenBank KM231852.1; Identities = 531/545 (97 %), 2 gaps (0 %)), and Cylindrium syzygii (strain CBS 131307, GenBank NR_157430.1; Identities = 520/547 (95 %), 17 gaps (3 %)). Closest hits using the LSU sequence are Cylindrium grande (strain CBS 145578, GenBank MK876426.1; Identities = 833/834 (99 %), no gaps), Cylindrium elongatum (strain CBS 115974, GenBank KM231733.1; Identities = 824/832 (99 %), 1 gap (0 %)), and Cylindrium purgamentum (strain CPC 29580, GenBank KY173525.1; Identities = 814/822 (99 %), no gaps) – also see Fig. 6, part 2a. Closest hits using the actA sequence had highest similarity to Cylindrium grande (strain CBS 145578, GenBank MK876456.1; Identities = 618/640 (97 %), 5 gaps (0 %)), Cylindrium elongatum (strain CBS 685.83A, GenBank KM231264.1; Identities = 575/622 (92 %), 10 gaps (1 %)), and Cylindrium aeruginosum (strain CBS 693.83, GenBank KM231265.1; Identities = 511/557 (92 %), 11 gaps (1 %)). Closest hits using the cmdA sequence had highest similarity to Cylindrium grande (strain CBS 145578, GenBank MK876468.1; Identities = 671/707 (95 %), 5 gaps (0 %)), Cylindrium elongatum (strain CBS 685.83A, GenBank KM231448.1; Identities = 558/685 (81 %), 34 gaps (4 %)), and Cylindrium aeruginosum (strain CBS 693.83, GenBank KM231450.1; Identities = 393/481 (82 %), 34 gaps (7 %)). Closest hits using the rpb2 sequence had highest similarity to Cylindrium grande (strain CBS 145578, GenBank MK876482.1; Identities = 899/924 (97 %), 1 gap (0 %)), Lopadostoma turgidum (strain LT3, GenBank KC774564.1; Identities = 716/896 (80 %), 15 gaps (1 %)), and Lopadostoma fagi (strain LF, GenBank KC774530.1; Identities = 712/894 (80 %), 11 gaps (1 %)). Closest hits using the tef1 sequence had highest similarity to Cylindrium grande (strain CBS 145578, GenBank MK876496.1; Identities = 437/471 (93 %), 3 gaps (0 %)), and Cylindrium elongatum (strain CBS 685.83A, GenBank KM231988.1; Identities = 353/412 (86 %), 25 gaps (6 %)). Closest hits using the tub2 sequence had highest similarity to Cylindrium elongatum (strain CBS 115974, GenBank KM232123.1; Identities = 609/732 (83 %), 31 gaps (4 %)), Cylindrium grande (strain CPC 35403, GenBank MK876502.1; Identities = 533/629 (85 %), 12 gaps (1 %)), and Cylindrium aeruginosum (strain CBS 693.83, GenBank KM232124.1; Identities = 574/729 (79 %), 40 gaps (5 %)).
Dendryphiella eucalyptorum Crous & E. Rubio, Persoonia 32: 231. 2014.
Description and illustration: Crous et al. (2014).
Material examined: South Africa, Gauteng Province, University of Pretoria, FABI, in courtyard, on leaves of unidentified grass (Poaceae), 2016, P.W. Crous, culture CPC 34855 = CBS 145593.
Notes: Originally described from branches of Eucalyptus globulus collected in Spain (Crous et al. 2014).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Dendryphiella eucalyptorum (strain CBS 137987, GenBank KJ869139.1; Identities = 524/528 (99 %), 1 gap (0 %)), Dendryphiella phitsanulokensis (strain MFLU 17-2651, GenBank NR_159827.1; Identities = 575/591 (97 %), no gaps), and Dendryphiella sp. (strain MFLUCC 17-2242, GenBank MH118115.1; Identities = 510/522 (98 %), no gaps). Closest hits using the LSU sequence are Dendryphiella vinosa (GenBank EU848590.1; Identities = 832/832 (100 %), no gaps), Dendryphiella phitsanulokensis (strain MFLU 17-2651, GenBank NG_064502.1; Identities = 827/827 (100 %), no gaps), and Dendryphiella eucalyptorum (strain CBS 137987, GenBank KJ869196.1; Identities = 821/821 (100 %), no gaps) – also see Fig. 3, part 4.
Diaporthe tarchonanthi Crous, sp. nov. MycoBank MB835074. Fig. 18.
Fig. 18.
Diaporthe tarchonanthi (CPC 37479). A. Conidiomata on PNA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Etymology: Name refers to the host genus Tarchonanthus from which the species was isolated.
Conidiomata pycnidial, globose, up to 300 μm diam, black, erumpent; cream conidial droplets exuding from central ostioles; walls consisting of 3–6 layers of brown textura angularis. Conidiophores hyaline, smooth, 0–2-septate, branched, densely aggregated, subcylindrical, straight to sinuous, 10–20 × 3–4 μm. Conidiogenous cells 7–15 × 2.5–3 μm, phialidic, subcylindrical, terminal and intercalary, with slight apical taper, apex 1–2 μm diam, with visible periclinal thickening; collarette not flared, up to 2 μm long. Paraphyses not observed. Alpha conidia aseptate, hyaline, smooth, guttulate, fusoid to ellipsoid, tapering towards both ends, apex subobtuse, base truncate, 1.5 μm diam, (9.5−) 10–11(−13) × (4−)4.5(−5) μm. Gamma and beta conidia not observed.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium, covering dish after 2 wk at 25 °C. On MEA, PDA and OA surface dirty white, reverse cinnamon.
Typus: South Africa, Western Cape Province, Mossel Bay, on leaves of Tarchonanthus littoralis (Asteraceae), 2016, L. Lombard, HPC 2800 (holotype CBS H-24196, culture ex-type CPC 37479 = CBS 146073).
Notes: Diaporthe was recently treated by Marin-Felix et al. (2019), listing all accepted species. Diaporthe tarchonanthi is closely related to D. oxe (on petiole of Maytenus ilicifolia, Brazil, alpha conidia (5−)6–7(−8) × (2−)3 μm; Gomes et al. 2013), but can be distinguished based on its larger conidia.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Diaporthe oxe (strain LGMF939, GenBank KC343167.1; Identities = 548/574 (95 %), 6 gaps (1 %)), Diaporthe lithocarpus (strain TS-120, GenBank MG832540.1; Identities = 547/574 (95 %), 5 gaps (0 %)), and Diaporthe eucalyptorum (strain KACC 48653, GenBank MK396569.1; Identities = 537/567 (95 %), 8 gaps (1 %)). Closest hits using the LSU sequence are Diaporthe discoidispora (strain LC3503, GenBank KY011854.1; Identities = 827/830 (99 %), no gaps), Phaeocytostroma ambiguum (strain CPC 17077, GenBank FR748100.1; Identities = 851/857 (99 %), no gaps), and Diaporthe oxe (strain CBS 133187, GenBank MH877518.1; Identities = 851/857 (99 %), 1 gap (0 %)) – also see Fig. 6, part 2b. Closest hits using the his3 sequence had highest similarity to Diaporthe paranensis (strain CBS 133184, GenBank KC343655.1; Identities = 360/379 (95 %), 6 gaps (1 %)), Diaporthe impulsa (strain CBS 114434, GenBank KC343605.1; Identities = 358/378 (95 %), 7 gaps (1 %)), and Diaporthe detrusa (strain CBS 140.27, GenBank KC343547.1; Identities = 356/379 (94 %), 9 gaps (2 %)). Closest hits using the tub2 sequence had highest similarity to Diaporthe paranensis (strain CBS 133184, GenBank KC344139.1; Identities = 648/708 (92 %), 11 gaps (1 %)), Diaporthe oxe (strain CBS 133187, GenBank KC344133.1; Identities = 644/707 (91 %), 13 gaps (1 %)), and Diaporthe caatingaensis (strain URM7486, GenBank KY115600.1; Identities = 638/708 (90 %), 18 gaps (2 %)).
Diplocarpon coronariae (Ellis & Davis) Wöhner & Rossman, comb. nov. MycoBank MB834830.
Basionym: Ascochyta coronariae Ellis & Davis, Trans. Wisconsin Acad. Sci. 14: 94. 1903.
Synonyms: Marssonina coronariae (Ellis & Davis) Davis, Trans. Wisconsin Acad. Sci. 17: 881. 1914.
Marssonia coronariae Sacc. & Dearn., Ann. Mycol. 10: 313. 1912.
Marssonia mali Henn., Bot. Jb. 37: 164. 1905.
Marssonina mali (Henn.) S. Ito, Bot. Mag. (Tokyo) 32: 206. 1918.
Diplocarpon mali Y. Harada & Sawamura, Ann. Phytopathol. Soc. Japan 40: 415. 1974.
Notes: Apple blotch is a severe disease of apples that was initially described from North America (Davis 1903, 1913) and has caused enormous losses to apple production in Asia (Lee et al. 2011). It is also becoming more problematic in Europe, possibly due to climate change (Wöhner & Emeriewen 2019). Known primarily in the asexual morph, the causal fungus has long been known as Marssonina coronariae (Ellis & Davis) Davis. The sexual morph was discovered and described as Diplocarpon mali and there seems to be no doubt that M. coronariae and D. mali both refer to the fungal species causing apple blotch.
Starting with the International Code of Nomenclature for algae, fungi and plants (McNeill et al. 2012), only one scientific name may be used for a single species of fungus. At present, both Marssonina coronariae for the asexual morph and Diplocarpon mali for the sexual morph have been applied to this fungus as shown in the literature. In deciding which scientific name to use, one must determine the correct generic placement of the species. Johnston et al. (2014) recommended generic names of pleomorphic fungi for members of the Leotiomycetes. They determined that the type species of Diplocarpon F.A. Wolfe 1912, specifically D. rosae F.A. Wolfe 1912, was congeneric with the type species of Marssonina Magnus 1906, typified by M. potentillae (Desm.) Magnus 1906 (basionym: Phyllosticta potentillae Desm. 1847). Thus, Diplocarpon and Marssonina are synonyms. Because Diplocarpon is “more widely known for the serious, widespread diseases of rosaceous plants” (Johnston et al. 2014), they recommended that Diplocarpon should be protected and used rather than Marssonina. Once the correct generic name for a species has been determined, in this case Diplocarpon rather than Marssonina, the oldest epithet for a species must be placed in the correct genus regardless of whether the name has been used for a sexual or asexual morph. After reviewing all of the known synonyms that have been applied to this species, the oldest epithet is Ascochyta coronaria 1903. Thus, the name Ascochyta coronariae should be placed in Diplocarpon.
Several studies have confirmed that Diplocarpon coronariae is congeneric with D. rosae, type of Diplocarpon. Lee et al. (2011) showed that 21 isolates of D. coronariae (as M. coronariae) from China and Korea belonged to the same clade and that this species was related to D. rosae (as M. rosae) and D. mespili. Similarly, Johnston et al. (2019) recognised D. rosae and D. coronariae (as D. mali) as a single clade in the Drepanopezizaceae.
Elsinoe eucalyptorum Crous & Summerell, sp. nov. MycoBank MB835075.
Synonym: Elsinoe eucalyptorum Crous & Summerell, Fungal Diversity 23: 332. 2006. Nom. inval., Art. 40.6 (Melbourne).
Etymology: Name refers to the host genus Eucalyptus from which the species was isolated.
Description and illustration: Summerell et al. (2006).
Diagnosis: Ascospores hyaline, smooth, thin-walled, broadly ellipsoidal with rounded ends, with 1(−3) transverse septa, and 1–2 vertical or oblique septa; constricted at median septum, (11−)13–15 × (4−)5(−6) μm.
Typus: Australia, New South Wales, 0.9 km west of Pacific Highway on Middle Brother Road, ca. 11 km south of Kew, North Coast NSW, 31°42′38″ S, 152°42′20″ E, alt. 40 m; on leaves of Eucalyptus propinqua (Myrtaceae), Feb. 2006, B.A. Summerell (holotype CBS H-19746, culture ex-type CBS 120084 = CPC 13052).
Notes: Elsinoe eucalyptorum was invalidly described (Summerell et al. 2006), because the word “holotype” was omitted in the final publication. The taxon is thus validated here.
Eriospora leucostoma Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 5: 455. 1850. Fig. 19.
Fig. 19.
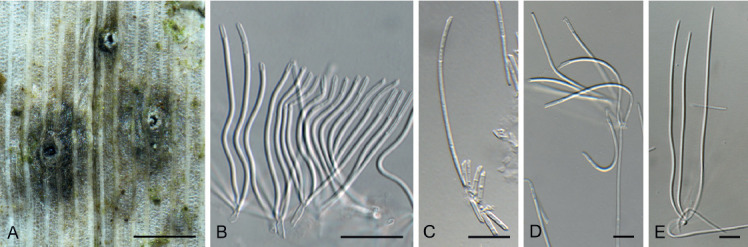
Eriospora leucostoma (CPC 35594). A. Immersed conidiomata. B–E. Conidiogenous cells giving rise to conidia. Scale bars: A = 300 μm, all others = 10 μm.
Conidiomata pycnidial, immersed, separate, dark brown, globose, unilocular, thick-walled; neck somewhat papillate, ostiole central, circular, appearing white to grey. Conidiophores branched, forming a reticulate layer lining the inner wall, hyaline, septate. Conidiogenous cells hyaline, smooth, integrated, proliferating sympodially with several unthickened conidiogenous loci in the apical region, 5–10 × 1.5 μm. Conidia frequently remaining attached, hyaline, smooth, curved, up to 8-septate, cylindrical with obtuse ends, 50–100 × 1 μm.
Culture characteristics: Colonies flat, spreading, surface folded, with sparse aerial mycelium and smooth, lobate margin, reaching 40 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface ochreous, reverse umber to sienna.
Materials examined: Germany, near Berlin, on attached leaf of Typha sp. (Typhaceae), 6 May 2018, R.K. Schumacher, RKS 172 = HPC 2389, culture CPC 35594 = CBS 145556; near Berlin, on culm of Juncus effusus (Juncaceae), 4 May 2018, R.K. Schumacher, RKS 165 = HPC 2391, culture CPC 35598, 35599.
Notes: Little is known regarding the ecology of Eriospora leucostoma, except that it appears to be a foliar pathogen with a wide host range.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Fitzroyomyces cyperacearum (strain CBS 143170, GenBank NR_156387.1; Identities = 377/417 (90 %), 10 gaps (2 %)), Fitzroyomyces cyperi (strain CBS 143170, GenBank MG386047.1; Identities = 377/417 (90 %), 10 gaps (2 %)), and Neofitzroyomyces nerii (strain CBS 145088, GenBank NR_161144.1; Identities = 569/646 (88 %), 29 gaps (4 %)). The ITS sequences of CPC 35594 and 35598 are 597/607 (98 %) similar. Closest hits using the LSU sequence are Neofitzroyomyces nerii (strain CBS 145088, GenBank MK047504.1; Identities = 807/813 (99 %), no gaps), Phacidiella podocarpi (strain CBS 138904, GenBank NG_058118.1; Identities = 783/827 (95 %), 5 gaps (0 %)), and Conotrema populorum (voucher Gilenstam 2353 (UPS), GenBank AY300833.1; Identities = 791/841 (94 %), 7 gaps (0 %)) – also see Fig. 2. The LSU sequences of CPC 35594 and 35598 are identical (837/837 bases) similar.
Exophiala quercina Crous & R.K. Schumach., sp. nov. MycoBank MB835076. Fig. 20.
Fig. 20.
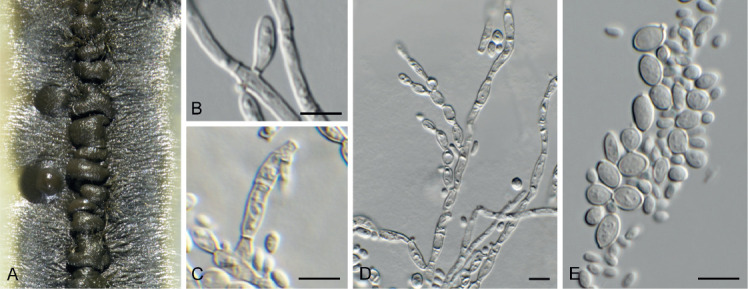
Exophiala quercina (CPC 33408). A. Colony on SNA. B–D. Conidiogenous cells giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Quercus from which the species was isolated.
Mycelium consisting of pale brown, smooth, branched, septate, 2–2.5 μm diam hyphae. Conidiophores reduced to conidiogenous cells on hyphae. Conidiogenous cells solitary hyphal pegs on hyphae, intercalary or terminal, cells at times constricted at septa, forming globose chains of brown cells that can develop conidiogenous loci, 1 × 1 μm, giving rise to mucoid conidial droplets. Conidia solitary, hyaline, smooth, guttulate, globose to ellipsoid, 2–2.5 × 1.5–2 μm, becoming swollen, globose or subcylindrical, pale brown, 3–5 × 2–4 μm.
Culture characteristics: Colonies erumpent, spreading, slimy, with sparse aerial mycelium, folded surface, and smooth, lobate margin, reaching 10 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse iron-grey.
Typus: Germany, near Berlin, on dead wood of Quercus sp. (Fagaceae), 6 Apr. 2017, R.K. Schumacher, RKS 89 = HPC 2042 (holotype CBS H-24150, culture ex-type CPC 33408 = CBS 146024).
Notes: Exophiala quercina is morphologically similar to E. capensis (South Africa, leaf bracts of Phaenocoma prolifera, conidia (2−)3–5(−6) × (2−)3–3.5(−4) μm; Crous & Groenewald 2011), but can be distinguished based on DNA sequence data.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Exophiala moniliae (strain LCM 953.01, GenBank MF495447.1; Identities = 599/615 (97 %), 6 gaps (0 %)), Exophiala capensis (strain CBS 128771, GenBank NR_121493.1; Identities = 488/548 (89 %), 20 gaps (3 %)), and Exophiala abietophila (strain CBS 145038, GenBank NR_163357.1; Identities = 571/643 (89 %), 34 gaps (5 %)). Closest hits using the LSU sequence are Exophiala moniliae (strain CBS 520.76, GenBank MH872772.1; Identities = 885/893 (99 %), 1 gap (0 %)), Exophiala spinifera (strain CBS 126734, GenBank MH875669.1; Identities = 884/897 (99 %), 4 gaps (0 %)), and Exophiala nigra (strain dH 12296, GenBank FJ358244.1; Identities = 868/881 (99 %), 4 gaps (0 %)) – also see Fig. 4. Closest hits using the tef1 sequence had highest similarity to Exophiala abietophila (strain HGUP-R300, GenBank MK887139.1; Identities = 175/186 (94 %), 2 gaps (1 %)), Exophiala salmonis (strain AFTOL-ID 671, GenBank EF413612.1; Identities = 173/184 (94 %), no gaps), and Capronia munkii (strain AFTOL-ID 656, GenBank EF413607.1; Identities = 179/192 (93 %), 3 gaps (1 %)).
Fusarium californicum D.P. Lawr., A.J. Stack, T.R. Gordon & R.M. Bostock, sp. nov. MycoBank MB830805. Fig. 21.
Fig. 21.
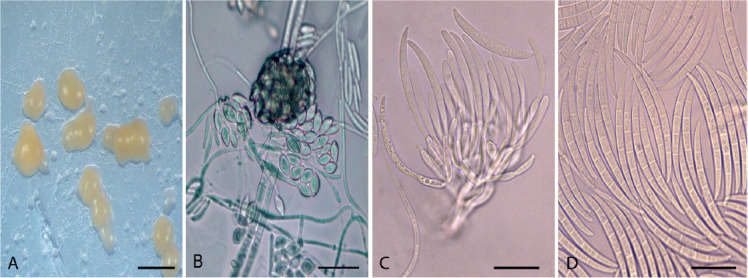
Fusarium californicum (CBS 145796). A. Sporodochia. B. Cylindrical and napiform microconidia. C. Conidiogenous cells. D. Macroconidia. Scale bars: A = 500 μm, B = 25 μm, C–D = 20 μm.
Etymology: Name refers to the state of California, where the ex-type strain was isolated.
Mycelium consisting of septate, straight, branched, smooth, hyaline, 3–4 μm diam hyphae. Aerial mycelium copious, commonly forming hyphal bundles. Abundant sporulation from 30–60 μm tall aerial conidiophores and sporodochia. Conidiophores of aerial mycelium commonly reduced to conidiogenous cells, 30–100 μm tall, irregularly or sympodially branched, bearing terminal or lateral monophialides; phialides ampulliform, subulate to subcylindrical, smooth- and thin-walled, (10−)12.5(−18.5) × (2−)2.5(−3.5) μm. Macroconidia multiseptate and falcate, morphologically similar to sporodochial conidia. Microconidia of two types formed singly or forming small false heads at the tip of blastic conidiogenous cells; a) hyaline, subcylindrical, smooth- and thin-walled, slightly curved with 0–1 septa, (11.5−)15.5(−20) × (1.5−)2.5(−3.5) μm; b) hyaline, napiform to pyriform, many with papilla at the base, 0–1-septate, (11.5−)14.5(−17.5) × (5−)6(−7.5) μm. Sporodochia abundantly produced on SNA, fleshy, cream to pale orange-colored; sporodochial conidiophores (20−)60–80(−85) μm tall, densely and irregularly branched, bearing 1–2 terminal, rarely lateral monophialides; sporodochial phialides subulate to subcylindrical, smooth- and thin-walled, (8.5−)14.5(−20.5) × (2−)2.5(−3.5) μm. Macroconidia on sporodochia falcate, apical cell conical with rounded apex, basal cell slightly papillate to foot-shaped, (3−)4–5 septate, hyaline, thin- and smooth-walled, 3-septate macroconidia (27−)32(−37.5) × (2−)3(−3.5) μm, 4-septate macroconidia (30−)34.5(−39.5) × (2.5−)3(−3.5) μm, and 5-septate macroconidia (29−)37(−44.5) × (2.5−)3(−4.5) μm. Chlamydospores not observed.
Culture characteristics: On PDA averaging 31.5 mm in 7 d at 25°C in the dark; colony surface initially white with flat to felty aerial mycelium with a torn margin, colony centre darkens slightly with age, reverse chestnut brown.
Typus: USA, California, Sutter County, Yuba City, 39°03′08″ N, 121°36′51″ W, 10 m a.s.l., isolated from necrotic inner bark and cambium of budwood of Prunus dulcis, Sep. 2013, A.J. Stack (holotype specimen CBS 145796, preserved as metabolically inactive culture, culture ex-type CBS 145796 = BL30).
Notes: Fusarium californicum produces predominately 5- but occasionally 3- to 4-septate macroconidia, whereas F. tricinctum mostly produces 3- but occasionally 4- to 5-septate macroconidia. Fusarium californicum differs from F. acuminatum by the latter forming cylindrical microconidia in culture, whereas F. californicum produces both cylindrical and napiform to pyriform papillate microconidia in culture. Based on the analysis of tef1 and rpb2 sequences, the newly proposed species Fusarium californicum clusters strongly (98 % / 100 %, parsimony and likelihood bootstrap support values, respectively) as an independent lineage within the ‘tricinctum‘ species complex. Within the ‘tricinctum’ complex F. acuminatum and F. tricinctum are the closest relatives to F. californicum. Isolates of this newly typified taxon have been previously reported from wheat (Triticum sp.) and the signal crayfish (Pacifastacus leniusculus) in North America.
Based on a megablast search of NCBI’s GenBank nucleotide database, the ITS sequence was 100 % identical to isolates named Fusarium lateritium (GenBank LC206632) and Fusarium avenaceum (GenBank MG274299). The LSU sequence closest matches included 99 % identity to Fusarium verticilliodes (GenBank XR_001989352) and Fusarium fujikuroi (GenBank KX375765) – also see Fig. 6, part 3. The closest matches for the rpb1 sequence included Fusarium tricinctum (GenBank JX171516) and Fusarium acuminatum (GenBank KC808324) with 98.8 % and 98.6 % identity, respectively. The closest matches for the rpb2 sequence included Fusarium tricinctum (GenBank HM068327) and Fusarium acuminatum (GenBank HM068334) with 98.4 % identity. The closest matches for the tef1 sequence included Fusarium tricinctum (GenBank AB674262) and Fusarium avenaceum (GenBank KX702713) with 100 % and 99.2 % identity, respectively. The closest matches for the tub2 sequence included Fusarium tricinctum (GenBank KU852605) and Fusarium acuminatum (GenBank KU852603) with 99.4 % and 98.5 % identity, respectively.
Gonatobotrys simplex Corda, Pracht-Fl. Eur. Schimmelbild.: 9. 1839. Fig. 22.
Fig. 22.

Gonatobotrys simplex. A. Corda (1839) Tafel V (lectotype). B–D. Colony texture and conidiophore appearance (CN008I7). E–G. Conidiophores. H, I. Conidia. Scale bars = 10 μm in E–I.
Synonyms: Melanospora simplex (Corda) D. Hawksw., IMA Fungus 7 (1): 137. 2016.
Desmotrichum simplex Lév., Annales des Sciences Naturelles Botanique 20: 217, t. 7: 8. 1843.
Gonatobotrys simplex var. leveillei Sacc., Sylloge Fungorum 4: 169. 1886.
Colonies white to cream. Conidiophores 250–1 500 × 4–10 μm, becoming longer after prolonged incubation. Conidiogenous cells with a swollen part 10–13 × 8–14 μm (fide Walker & Minter up to 18 μm), conspicuous denticles remaining after conidia break off; Conidia aseptate, smooth to finely roughened, 8–17 × 6–10 μm (fide Walker & Minter 10–22 × 6–12 μm) amended from Walker & Minter (1981).
Typus: Prague, on Helminthosporium on dead bark, 1839, Corda, in “Pracht-Flora Europaeischer Schimmelbildungen p. 9, (1839), tafel V (fig. 5)” (lectotype designated by Walker & Minter 1981), MBT391348). Germany, former West-Germany, Triticum aestivum seed, P. Reinecke, CBS 174.78 (with Alternaria alternata), (specimen CBS 174.78, preserved as metabolically inactive culture, epitype designated here, MBT391349, culture ex-epitype CBS 174.78).
Additional materials examined: Morocco, Moyen-Atlas, 800 m alt., L. Naijm, CBS 466.84 (with Alternaria alternata). Netherlands, Uden, on Betula verrucosa, Dec. 1973, W.M. Loerakker, CBS 281.74 (with Alternaria alternata). New Zealand, Seed Testing Station, Palmerston, Trifolium arvense seed, cv. Montgomery, 7 Jun. 1966, G.L. Hennebert, MUCL 9374 = CBS 111.67. South Africa, Pretoria, on Blueberry, C.M. Visagie, 2019, CMW 55931, CMW 55932 (with Cladosporium); Pretoria, on Blueberry, 2018, C.M. Visagie, PPRI 28268 (with Alternaria & Cladosporium).
Notes: Gonatobotrys Corda (1839) is a mycoparasite, with G. simplex as its generic type. Walker & Minter (1981) reviewed the genus and its morphologically similar asexual genera based on their morphology and modes of conidium development. They classified most described Gonatobotrys species in other genera, retaining only G. simplex while introducing the new species G. complex. Gonatobotrys africana Najim, Clauzet & Kadiri (nom. illegit., Art. 53.1) was subsequently introduced (Najim et al. 1984). However, this epithet had already been occupied by G. africana Saccas and the former species was renamed G. pyriformis V. Rao, de Hoog & Najim (Rao & de Hoog 1986). Comparing these species, G. simplex produces aseptate conidia that distinguishes it from the septate conidia produced by both G. complex and G. pyriformis. Gonatobotrys pyriformis was distinguished from G. complex based on a better developed proximal cell. From the original descriptions, G. africana conidiophores appear to be more regularly branched and are narrower (3–5 vs. 6–10 μm), while it generally produces smaller conidia (5–14 vs. 10–40 μm).
Vakili (1989) linked the sexual Melanospora damnosa morph with the asexual G. simplex morph and this resulted in Melanospora simplex (Corda) D. Hawksw. being introduced as a new combination (Reblova et al. 2016). Vakili’s (1989) conclusions were largely based on the fact that both fungi were found on moldy corn. They reported that single ascospore isolations of the sexual morph resulted in the Gonatobotrys morph occurring in pure culture, and subsequent crossings of compatible strains resulted in the sexual morph. Microconidiophores (solitary phialides) were also observed in their Melanospora and Gonatobotrys cultures. A phialidic asexual morph was reported for other Melanospora species (Marin-Felix et al. 2018), but the Gonatobotrys morph was never observed in any of their cultures. Based on the DNA sequences available for this group, our G. simplex strains formed a distinct clade in Melanosporales, sister to Vittatispora but distinct from Melanospora. It would thus appear that the link between Melanospora and Gonatobotrys was erroneous, and that the correct name for this fungus should be G. simplex.
Since Gonatobotrys is a mycoparasite, obtaining clean sequences in pure cultures is difficult. But we were fortunate to obtain these for some strains. BLAST searches against environmental sequences at NCBI and UNITE did not reveal any close matches (see Supplementary Fig. 1). However, the reference sequences deposited with this study should prove valuable for future studies on these mycoparasitic species, which have ecological and potential commercial value.
Gonatophragmium epilobii U. Braun & C.F. Hill, Australas. Mycol. 27(2): 46. 2008. Fig. 23.
Fig. 23.
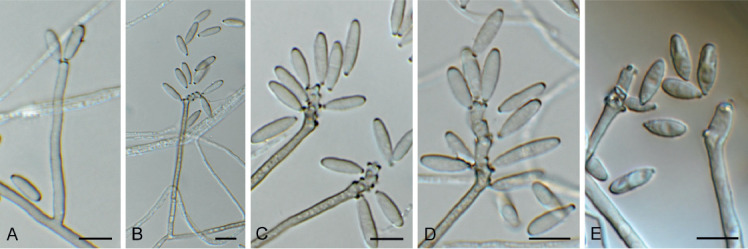
Gonatophragmium epilobii (CPC 34889). A–E. Conidiophores with conidiogenous cells giving rise to clusters of aseptate conidia. Scale bars = 10 μm.
Description and illustration: Braun & Hill (2008).
Material examined: New Zealand, Auckland, Auckland Botanical Garden, on leaves of Rhopalostylis sapida (Arecaceae), 30 Aug. 2017, R. Thangavel, culture T17_03052C = CPC 34889 = CBS 145594.
Notes: Gonatophragmium epilobii (CBS 122271 ex-type) was described from leaf spots on Epilobium ciliatum in northern New Zealand (Braun & Hill 2008). The present collection represents a new host record.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Gonatophragmium epilobii (strain CBS 122271, GenBank MH863183.1; Identities = 500/500 (100 %), no gaps), Acrospermum maxonii (as Acrospermum sp. VD-2019c, voucher 3108, GenBank MK562002.1; Identities = 521/536 (97 %), no gaps), and Gonatophragmium triuniae (strain CBS 138901, GenBank NR_137932.1; Identities = 514/540 (95 %), 9 gaps (1 %)). Closest hits using the LSU sequence are Gonatophragmium triuniae (strain CBS 138901, GenBank NG_058117.1; Identities = 824/825 (99 %), no gaps), Gonatophragmium epilobii (strain CBS 122271, GenBank MH874728.1; Identities = 842/845 (99 %), 3 gaps (0 %)), and Acrospermum maxonii (as Acrospermum sp. VD-2019c, voucher 44, GenBank MK603831.1; Identities = 829/833 (99 %), no gaps) – also see Fig. 3, part 1.
Graphostroma platystomum (Schwein.) Piroz. (as “platystoma”), Canad. J. Bot. 52: 2131. 1974. Fig. 24.
Fig. 24.

Graphostroma platystomum (CPC 37153). A–D. Conidiophores giving rise to aseptate conidia. E. Stroma with black ascomata. F, G. Asci. H. Paraphyses. I. Aseptate ascospores. Scale bars: E = 200 μm, all others = 10 μm.
Basionym: Sphaeria platystoma Schwein., Schr. naturf. Ges. Leipzig 1: 31 1822.
Synonym: Sphaeria platystoma Schwein. : Fr., Syst. Mycol. 2(2): 351. 1823. sanct.
Stroma intracorticolous (not superficial), erumpent, single, densely crowded to confluent, flat, black-brown, multilocular, loci in single level, margin not sterile, up to 4.4 × 3 × 0.1 cm. Ascomata perithecial, embedded, pyriform, black, ostioles as black, round discs, single, prominent. Paraphyses numerous, longer than most asci, unbranched, not anastomosed, multicelled, basally moniliform, upwards tapered, hyaline, thin-walled, smooth, with a few guttules per cell. Asci 8-spored, clavate, apically blunt, rounded, pedicel tapered, base truncate, apical apparatus faintly amyloid (water + lugol), 33–42 × 4.5–5.5 μm. Ascospores aseptate, allantoid, hyaline, thin-walled, smooth, eguttulate, 7–10 × 1–2 μm (mounted in water). Mycelium consisting of pale brown, smooth, septate, branched, 2.5–3 μm diam hyphae. Conidiophores arising in penicillate tufts up to 250 μm tall, consisting of a series of branches that are aggregated, subcylindrical, pale brown at base, hyaline at apex, smooth-walled, 2.5–3 μm diam. Conidiogenous cells terminal and intercalary, 20–40 × 2.5–3 μm, with aggregated pimple-like denticles giving rise to conidial clusters. Conidia hyaline, smooth, guttulate, aseptate, pyriform to obovoid, apex obtuse, tapering to narrowly truncate hilum, 0.5 μm diam, 4–6 × 2.5–3 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium, covering dish after 2 wk at 25 °C. On MEA surface and reverse cinnamon; on PDA surface and reverse vinaceous; on OA surface dirty white.
Material examined: USA, New York, New York City, Hill Park, on Lindera benzoin, fallen and corticated branch, 9 Dec. 2018, E. Crenson & R.K. Schumacher, HPC 2742 = RKS 182 (CBS H-24188, culture CPC 37153 = CBS 146066).
Notes: Graphostroma platystoma forms flat, perithecial stromata in the periderm of logs used for shiitake cultivation, destroying the bark, and causing a reduction in harvest (Tsunoda et al. 1996). The name G. platystoma is based on material collected in the USA (as is true for the culture used in this study), which appears phylogenetically distinct from material occurring in Europe (e.g. CBS 270.87) that could represent a distinct taxon.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Graphostroma platystoma (strain CBS 270.87, GenBank HG934115.1; Identities = 553/580 (95 %), 3 gaps (0 %)), Cryptostroma corticale (strain CBS 216.52, GenBank MH857008.1; Identities = 558/617 (90 %), 10 gaps (1 %)), and Biscogniauxia bartholomaei (strain ATCC 38992, GenBank AF201719.1; Identities = 548/609 (90 %), 13 gaps (2 %)). Closest hits using the LSU sequence are Graphostroma platystoma (strain CBS 270.87, GenBank AY083827.1; Identities = 821/834 (98 %), 1 gap (0 %)), Cryptostroma corticale (strain CBS 217.52, GenBank MH868530.1; Identities = 831/846 (98 %), 1 gap (0 %)), and Biscogniauxia petrensis (strain LC5697, GenBank KU746715.1; Identities = 828/846 (98 %), 1 gap (0 %)) – also see Fig. 6, part 1. Closest hits using the rpb2 sequence had highest similarity to Cryptostroma corticale (strain CBS 216.52, GenBank HG934116.1; Identities = 785/850 (92 %), no gaps), Biscogniauxia granmo (strain YMJ 135, GenBank JX507784.1; Identities = 789/856 (92 %), no gaps), and Biscogniauxia marginata (strain CBS 124505, GenBank KU684310.1; Identities = 753/826 (91 %), no gaps). Closest hits using the tub2 sequence had highest similarity to Graphostroma platystoma (strain CBS 270.87, GenBank HG934108.1; Identities = 440/485 (91 %), 5 gaps (1 %)), Cryptostroma corticale (strain Acer7, GenBank HG934105.1; Identities = 419/483 (87 %), 19 gaps (3 %)), and Biscogniauxia granmo (strain YMJ 135, GenBank AY951681.1; Identities = 414/484 (86 %), 21 gaps (4 %)).
Hypomyces gamsii Crous & Akulov, sp. nov. MycoBank MB835077. Fig. 25.
Fig. 25.
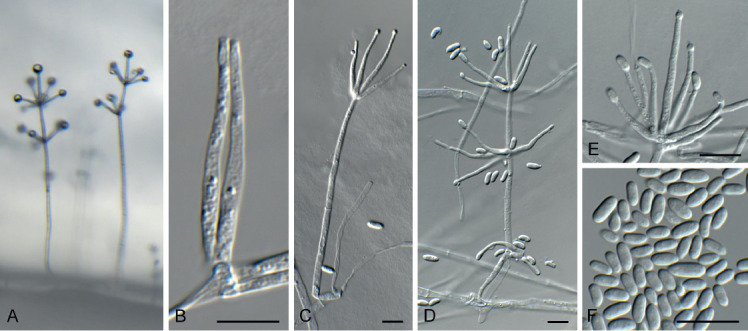
Hypomyces gamsii (CPC 36232). A, C, D. Verticillium-like conidiophores. B, E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Named after Konrad Walter Gams (1934–2017), in recognition of his contribution to our knowledge of verticillium-like fungi.
Mycelium consisting of hyaline, smooth, branched, septate, 1.5–3.5 μm diam hyphae. Conidiophores verticillium-like, erect, solitary, subcylindrical, flexuous, with whorls of 3–6 phialides along the length of the stipe; stipes 100–300 × 3–4 μm, hyaline, smooth. Conidiogenous cells phialidic, hyaline, smooth, 15–30 × 2.5–3 μm, subcylindrical or with apical taper, apex minute with non-flaring collarette, 1–2 μm long. Conidia aggregated in globoid mucoid mass, aseptate, hyaline, smooth, straight to somewhat curved, apex obtuse, tapering to truncate basal scar, subcylindrical to narrowly ellipsoid, guttulate, (4.5−)5–6(−9) × 2(−2.5) μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium on OA and PDA, fluffy aerial mycelium on MEA, and smooth, lobate margin, reaching 50 mm diam after 2 wk at 25 °C. On MEA surface dirty white, reverse buff; on PDA surface and reverse vinaceous, on OA surface hazel.
Typus: Ukraine, Sumy region, Okhtyrka district, Klymentove village, NNP Hetmanskyi, Vorskla river, on the strongly rotten basidiomata of not identified Polypore (cf. Hymenochaetaceae representative) on wood of Alnus glutinosa (Betulaceae), 6 Aug. 2018, A. Akulov, HPC 2532 = CWU (Myc) AS 6857 (holotype CBS H-24241, culture ex-type CPC 36232 = CBS 146044).
Notes: Species of Hypomyces are parasites on different macromycetes (mostly on Basidiomycota, less often on Ascomycota representatives). Hypomyces gamsii is phylogenetically distinct from other species that are known from DNA sequence data (Zare & Gams 2016).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Hypomyces subglobosus (strain CBS 543.86, GenBank NR_155169.1; Identities = 520/542 (96 %), 8 gaps (1 %)), Hypomyces tubariicola (strain CBS 115.79, GenBank NR_158483.1; Identities = 539/563 (96 %), 10 gaps (1 %)), and Nectriopsis tremellicola (voucher Mushroom Observer # 205859, GenBank MK607474.1; Identities = 566/593 (95 %), 8 gaps (1 %)). Closest hits using the LSU sequence are Hypomyces tubariicola (voucher NIBRFG0000501019, GenBank KY783351.1; Identities = 824/829 (99 %), no gaps), Hypomyces hubeiensis (as Hypomyces sp. WZ-2019a, strain 9791, GenBank MN044762.1; Identities = 793/799 (99 %), 1 gap (0 %)), and Sepedonium chalcipori (strain CBS 148.92, GenBank MH874014.1; Identities = 821/829 (99 %), no gaps) – also see Fig. 6, part 3. Closest hits using the rpb2 sequence had highest similarity to Hypomyces hubeiensis (strain 9791, GenBank MK484605.1; Identities = 757/872 (87 %), no gaps), Hypomyces tremellicola (strain TFC 97-50, GenBank EU710777.1; Identities = 696/814 (86 %), 9 gaps (1 %)), and Sepedonium chalcipori (strain KSH558, GenBank KU041495.1; Identities = 746/875 (85 %), 4 gaps (0 %)). Closest hits using the tef1 (second part) sequence had highest similarity to Trichoderma spirale (strain CBS 136472, GenBank KJ665740.1; Identities = 456/471 (97 %), no gaps), Trichoderma helicolixii (strain CBS 133499, GenBank KJ665517.1; Identities = 456/471 (97 %), no gaps), and Trichoderma concentricum (strain HMAS 248858, GenBank KY688028.1; Identities = 455/471 (97 %), no gaps).
Hypsotheca eucalyptorum Crous & Carnegie, Persoonia 42: 367. 2019.
Mycelium consisting of hyaline to pale brown, roughened, branched, septate, 2–2.5 μm diam hyphae. Conidiophores either reduced to conidiogenous cells on hyphae, or erect, flexuous, subcylindrical, up to 200 μm tall, brown, roughened to warty, multiseptate, with terminal and intercalary conidiogenous cells. Conidiogenous cells brown, verruculose to warty, subcylindrical to subulate with flared collarette, 1–2 μm long, 5–17 × 1.5–2.5 μm. Conidia hyaline, smooth, aseptate, subcylindrical, guttulate, apex obtuse, tapering to a truncate hilum, (3.5−)4–5(−6) × (1.5−)2(−2.5) μm.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 40 mm diam after 2 wk at 25 °C. On MEA surface brick with patches of vinaceous, reverse honey; on PDA surface cinnamon, margin isabelline; on OA surface Isabelline.
Materials examined: Australia, New South Wales, Booroobee SF, McCorquodale Plantation, on leaves of Eucalyptus grandis × camaldulensis (Myrtaceae), 20 Apr. 2018, A.J. Carnegie, HPC 2431 = CBS H-24155, culture CPC 35659 = CBS 146027; New South Wales, Booroobee SF, McCorquodale Plantation, on leaf of E. grandis × camaldulensis clone, 20 Apr. 2018, A.J. Carnegie, HPC 2431 = CBS H-24204, culture CPC 37928 = CBS 146089; New South Wales, Dilkoon, Hourn Plantation, on branch of E. dunnii, 10 Feb. 2019, A.J. Carnegie, HPC 2830 = AC 31384, culture CPC 37930 = CBS 146031.
Notes: Hypsotheca eucalyptorum has mainly been isolated from Eucalyptus leaves (Crous et al. 2019b), and its potential role as stem canker pathogen has yet to be clarified.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 35659 had highest similarity to Hypsotheca eucalyptorum (strain CBS 145576, GenBank MK876393.1; Identities = 547/547 (100 %), no gaps), Hypsotheca eucalyptorum (strain CBS 145577, GenBank MK876394.1; Identities = 541/549 (99 %), 2 gaps (0 %)), and Hypsotheca pleomorpha (as Caliciopsis pleomorpha, GenBank MG641785.1; Identities = 505/558 (91 %), 24 gaps (4 %)). The ITS sequences of CPC 35659, 37928 and 37930 are identical (553/553 bases). Closest hits using the LSU sequence of CPC 35659 are Hypsotheca eucalyptorum (strain CBS 145576, GenBank MK876434.1; Identities = 828/828 (100 %), no gaps), Hypsotheca pleomorpha (strain CBS 144636, GenBank MK442528.1; Identities = 822/852 (96 %), 3 gaps (0 %)), and Caliciopsis nigra (strain MA 18191, GenBank KP144011.1; Identities = 806/854 (94 %), 5 gaps (0 %)) – also see Fig. 4. The LSU sequence of CPC 35659 is identical to those of CPC 37928 (830/830 bases) and CPC 37930 (846/846 bases).
Italiofungus Crous, gen. nov. MycoBank MB835078.
Etymology: Name refers to Italy, the country of origin of this genus.
Conidiomata pycnidial, solitary, globose, brown with central ostiole; wall of several layers of brown textura intricata. Conidiophores arising from inner layer, hyaline, smooth, septate, branched, subcylindrical, straight to geniculate-sinuous. Conidiogenous cells integrated, terminal and intercalary, hyaline, smooth, subcylindrical with apical taper, proliferating sympodially at apex, scars not thickened nor darkened. Conidia solitary, hyaline, smooth, aseptate, subcylindrical, straight, apex obtuse, base truncate.
Type species: Italiofungus phillyreae (Thüm.) Crous
Italiofungus phillyreae (Thüm.) Crous, comb. nov. MycoBank MB835079. Fig. 26.
Fig. 26.
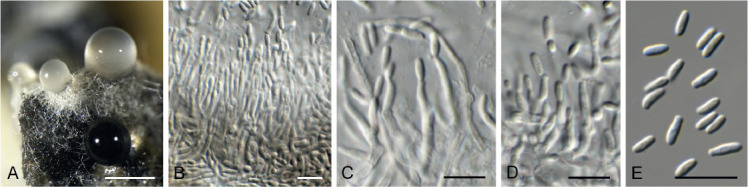
Italiofungus phillyreae (CPC 35566). A. Conidiomata on PDA. B–D. Conidiophores with conidiogenous cells. E. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Basionym: Depazea phillyreae Thüm., Boll. Soc. Adriatica Sci. Nat. Triests 2: 455. 1877.
Synonym: Phyllosticta goritiensis Sacc., Syll. Fung. (Abellini) 3: 22. 1884.
Conidiomata pycnidial, solitary, globose, brown with central ostiole, 200–300 μm diam; wall of several layers of brown textura intricata. Conidiophores arising from inner layer, hyaline, smooth, septate, branched, subcylindrical, straight to geniculate-sinuous, 10–30 × 2–3 μm. Conidiogenous cells integrated, terminal and intercalary, hyaline, smooth, subcylindrical with apical taper, proliferating sympodially at apex, scars not thickened nor darkened, 4–12 × 1.5–2 μm. Conidia solitary, hyaline, smooth, aseptate, subcylindrical, straight, apex obtuse, base truncate, (3.5−)4–5 × (1.5−)2 μm.
Culture characteristics: Colonies flat, spreading, surface folded with sparse aerial mycelium and smooth, lobate margin, reaching 30 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Material examined: Italy, Rome, on leaves Phillyrea latifolia (Oleaceae), 13 Apr. 2018, P.W. Crous, HPC 2336 (CBS H-23937, culture CPC 35566 = CBS 145537).
Notes: The oldest name that applies to the present collection is Depazea phillyreae, a microconidial morph described from leaves of Phillyrea in Italy. The genus Heleiosa (based on H. barbatula) applies to a bitunicate ascomycete described from leaves of Juncus roemerianus collected in North Carolina (Kohlmeyer et al. 1996), which is closely related to, but distinct from the new genus occurring on Phillyrea in Italy.
Based on a megablast search of NCBI’s GenBank nucleotide database, only distant hits were obtained using the ITS sequence, such as Didymosphaeria futilis (strain CMW 22186, GenBank EU552123.1; Identities = 482/546 (88 %), 21 gaps (3 %)), Pseudopassalora gouriqua (strain CBS 101954, GenBank NR_160207.1; Identities = 451/520 (87 %), 25 gaps (4 %)), and Funbolia dimorpha (strain CPC 14170, GenBank JF951136.1; Identities = 468/561 (83 %), 29 gaps (5 %)). Closest hits using the LSU sequence are Pseudopassalora gouriqua (strain CBS 101954, GenBank MH874368.1; Identities = 821/851 (96 %), 2 gaps (0 %)), Heleiosa barbatula (strain JK 5548I, GenBank GU479787.1; Identities = 814/853 (95 %), 5 gaps (0 %)), and Funbolia dimorpha (strain CPC 14170, GenBank JF951156.1; Identities = 806/851 (95 %), 3 gaps (0 %)) – also see Fig. 3, part 1.
Kalmusia araucariae Crous, sp. nov. MycoBank MB835080. Fig. 27.
Fig. 27.
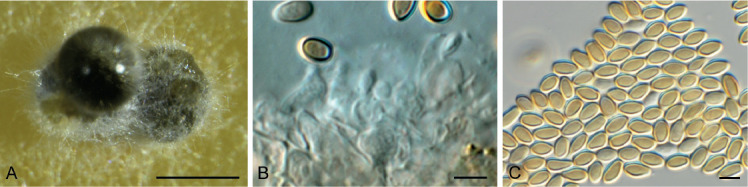
Kalmusia araucariae (CPC 37475). A. Conidiomata on OA. B. Conidiogenous cells. C. Conidia. Scale bars: A = 200 μm, all others = 10 μm.
Etymology: Name refers to the host genus Araucaria from which the species was isolated.
Conidiomata pycnidial, globose, brown, 180–200 μm diam, with central ostiole; wall of 6–8 layers of subhyaline to pale brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform, 4–6 × 3–5 μm, phialidic, with visible periclinal thickening. Conidia solitary, aseptate, golden brown, smooth, guttulate, thick-walled, ellipsoid, 4–5 × 2.5–3 μm.
Culture characteristics: Colonies flat, spreading, with moderate to abundant aerial mycelium, cover dish after 2 wk at 25 °C. On MEA and PDA surface dirty white, reverse cinnamon; on OA surface buff.
Typus: USA, Florida, Gainesville, associated with leaf blight of Araucaria bidwillii (Araucariaceae), 24 Feb. 2019, M.J. Wingfield, HPC 2797 (holotype CBS H-24195, culture ex-type CPC 37475 = CBS 146063).
Notes: Kalmusia araucariae resembles Alloconiothyrium aptrootii (conidia olivaceous brown, verruculose, 3–4(−5) × 2.5–3(−3.5) μm; Verkley et al. 2014), but the two species can easily be distinguished based on their conidial characteristics. Phylogenetically, K. auracariae clusters in a subclade along with several other species of Kalmusia, which also have coniothyrium-like asexual morphs. Hence Kalmusia appears to be the more appropriate genus to accommodate this fungus.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Alloconiothyrium aptrootii (strain SW129, GenBank MH469508.1; Identities = 428/454 (94 %), 6 gaps (1 %)), Paracamarosporium hawaiiense (as Microdiplodia hawaiiensis, strain CZ481, GenBank FJ755249.1; Identities = 435/466 (93 %), 8 gaps (1 %)), and Paraconiothyrium brasiliense (strain CBS 100299, GenBank NR_163552.1; Identities = 436/471 (93 %), 8 gaps (1 %)). Closest hits using the LSU sequence are Kalmusia variispora (strain S26, GenBank MK138784.1; Identities = 833/845 (99 %), 2 gaps (0 %)), Coniothyrium palmicola (strain CBS 161.37, GenBank JX681086.1; Identities = 833/845 (99 %), 2 gaps (0 %)), and Dendrothyrium longisporum (strain CBS 824.84, GenBank JX496228.1; Identities = 833/845 (99 %), 2 gaps (0 %)) – also see Fig. 3, part 4.
Keissleriella phragmiticola Wanas. et al., Fungal Diversity 89: 43. 2018. Fig. 28.
Fig. 28.
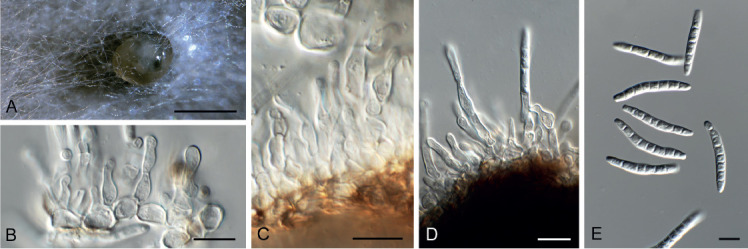
Keissleriella phragmiticola (CPC 33251). A. Conidioma on OA. B–D. Conidiogenous cells. E. Conidia. Scale bars: A = 200 μm, all others = 10 μm.
Description of sexual morph: Wanasinghe et al. (2018).
Conidiomata pycnidial, solitary, with central ostiole, 150–200 μm diam, exuding a slimy cream conidial mass; wall of 3–6 layers of brown textura angularis. Conidiophores lining the inner cavity, hyaline, smooth, ampulliform, 0–1-septate, 10–15 × 4–5 μm. Conidiogenous cells integrated, terminal, tapering, subcylindrical, proliferating percurrently, 4–7 × 3–4 μm. Conidia solitary, hyaline, guttulate, thick-walled, subcylindrical, irregularly curved, apex subobtuse, base truncate, 3–7-septate, (25−)30–35(−40) × (3−)4 μm in vitro, but 3–13-septate, 29–80 × 4–5.5 μm in vivo.
Culture characteristics: Colonies spreading with moderate to abundant aerial mycelium, and feathery, lobed margins. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Material examined: Germany, near Berlin, moist meadow, dead and attached leaf sheath of Phragmites australis (Poaceae), 11 Mar. 2017, R.K. Schumacher, RKS 60 = HPC 2000, cultures CPC 33251 = CBS 144989, CPC 33252; near Berlin, on Phragmites australis, 27 May 2015, R.K. Schumacher, RKS 719 = HPC 425, CPC 27402; near Berlin, on Phragmites australis, 11 Mar. 2017, R.K. Schumacher, RKS 58 = HPC 1998, CPC 33249. Netherlands, Oosterbeek, on Phragmites sp., 24 Jan. 2014, W. Quaedvlieg, CPC 24110. USA, on Phragmites sp., 20 Jul. 2011, P.W. Crous, CPC 19311 = CBS 135473.
Notes: Keissleriella phragmiticola was described based on the sexual morph from Phragmites collected in the UK (Wanasinghe et al. 2018), and this is the first report of its asexual morph.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 33251 had highest similarity to “Septoria” sp. (strain CPC 19311, GenBank KF251241.1; Identities = 559/560 (99 %), no gaps), Keissleriella phragmiticola (strain MFLUCC 17-0779, GenBank MG828904.1; Identities = 535/537 (99 %), 1 gap (0 %)), and Phaeosphaeria graminis (strain CBS 123087, GenBank MH863271.1; Identities = 558/577 (97 %), 3 gaps (0 %)). The ITS sequence of CPC 33251 is identical to CPC 24110 (574/574 bases), but differs with a single point mutation from CPC 33249 (573/574 bases), CPC 27402 (558/559 bases), and CPC 19311 (GenBank KF251241.1; 559/560 bases) and one point mutation and one indel from MFLUCC 17-0779 (GenBank MG828904.1; 535/537 bases, including 1 gap). Closest hits using the LSU sequence of CPC 33251 are Lentithecium lineare (strain IFRD 2008, GenBank FJ795435.1; Identities = 842/842 (100 %), no gaps), “Septoria” sp. (strain CPC 19311, GenBank KF251744.1; Identities = 840/840 (100 %), no gaps), and Keissleriella phragmiticola (strain MFLU 17-0606, GenBank NG_059863.1; Identities = 846/847 (99 %), no gaps) – also see Fig. 3, part 4. The LSU sequence of CPC 33251 is identical to CPC 24110 (827/827 bases), CPC 27402 (843/843 bases), CPC 33249 (815/815 bases) and CPC 19311 (GenBank KF251744.1; 840/840 bases) and differs with a single point mutation from MFLUCC 17-0779 (GenBank MG829014.1; 846/847 bases). Closest hits using the rpb2 sequence of CPC 33251 had highest similarity to Murilentithecium clematidis (strain MFLUCC 14-0561, GenBank KM454446.1; Identities = 662/752 (88 %), no gaps), Lentithecium carbonneanum (as Lentithecium sp. HAR-2018a; strain G951, GenBank MH037278.1; Identities = 730/842 (87 %), 2 gaps (0 %)), and Lentithecium cangshanense (as Lentithecium sp. LZ-2016a; strain DLUCC 0143, GenBank KU991151.1; Identities = 727/841 (86 %), no gaps). The rpb2 sequence of CPC 33251 is identical to CPC 19311 (GenBank KF252246.1; 109/109 bases), but differs with two point mutations from CPC 33249 (825/827 bases), three point mutations from CPC 27402 (818/821 bases), and four point mutations from CPC 24110 (821/825 bases). Closest hits using the tef1 sequence of CPC 33251 had highest similarity to Darksidea alpha (strain CBS 135654, GenBank KP184176.1; Identities = 331/383 (86 %), 18 gaps (4 %)), Darksidea beta (strain CBS 135637, GenBank KP184189.1; Identities = 327/381 (86 %), 19 gaps (4 %)), and Darksidea zeta (strain CBS 135640, GenBank KP184191.1; Identities = 327/382 (86 %), 18 gaps (4 %)). The tef1 sequence of CPC 33251 differs with two point mutations from CPC 33249 (561/563 bases), and with three point mutation from CPC 19311 (GenBank KF253194.1; 597/600 bases).
Lectera sambuci Crous & Bulgakov, sp. nov. MycoBank MB835081. Fig. 29.
Fig. 29.
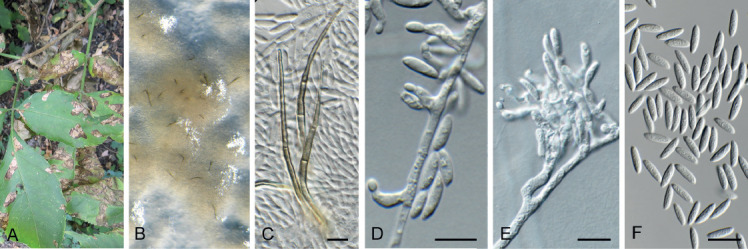
Lectera sambuci (CPC 36475). A. Disease symptoms. B. Sporodochia on SNA. C. Setae. D, E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Sambucus from which the species was isolated.
Leaf spots amphigenous, 3–12 mm diam, rounded, merging, firstly brown, later pale to gray in centre. Conidiomata sporodochial, erumpent, 80–150 μm diam. Setae distributed throughout sporodochium, erect, flexuous, unbranched, subcylindrical, tapering to subobtuse apex, brown, verruculose, 60–90 × 3–4 μm, 3–6-septate. Conidiophores reduced to conidiogenous cells or branched, 0–2-septate, subcylindrical, hyaline, smooth, 6–20 × 3–4 μm. Conidiogenous cells subcylindrical to reniform, phialidic, hyaline, smooth, 5–8 × 2.5–3 μm. Conidia hyaline, smooth, guttulate, inequilateral, inner plane flat, outer plane convex, straight, ends subacute, base with truncate hilum, (9−) 10–11(−12) × 2.5(−3) μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and smooth, lobate margin, reaching 20–25 mm diam after 2 wk at 25 °C. On MEA surface and reverse orange; on PDA and OA surface and reverse saffron.
Typus: Russia, Rostov region, Shakhty city district, shrubs near Atyukhta river, on living leaves of Sambucus nigra, 22 Sep. 2018, T.S. Bulgakov, HPC 2645 = Myc-15 (holotype CBS H-24227, culture ex-type CPC 36475 = CBS 145986).
Notes: Species of Lectera are commonly isolated from plant litter and soil, and generally regarded as plant pathogens (Giraldo & Crous 2019, Giraldo et al. 2019). Lectera sambuci is phylogenetically distinct from other members of the genus.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Lectera nordwiniana (strain CBS 144922, GenBank MK047463.1; Identities = 545/560 (97 %), 5 gaps (0 %)), Lectera colletotrichoides (voucher KNU17-189, GenBank MH231770.1; Identities = 521/536 (97 %), 5 gaps (0 %)), and Lectera longa (strain IMI 181698, GenBank NR_111715.1; Identities = 495/511 (97 %), 6 gaps (1 %)) – also see Fig. 30. Closest hits using the LSU sequence are Lectera longa (strain IMI 181698, GenBank NG_066392.1; Identities = 819/820 (99 %), no gaps), Lectera colletotrichoides (strain IMI 265740, GenBank LR025896.1; Identities = 819/820 (99 %), no gaps), and Lectera nordwiniana (strain JW231013, GenBank MK047512.1; Identities = 820/823 (99 %), 2 gaps (0 %)) – also see Fig. 6, part 3. Closest hits using the rpb2 sequence had highest similarity to Lectera nordwiniana (strain CBS 144922, GenBank MK047551.1; Identities = 831/867 (96 %), no gaps), Lectera colletotrichoides (strain IMI 265740, GenBank LR026169.1; Identities = 718/743 (97 %), no gaps), and Lectera longa (strain IMI 366179, GenBank LR026171.1; Identities = 712/743 (96 %), no gaps). Closest hits using the tub2 sequence had highest similarity to Lectera colletotrichoides (strain CBS 109728, GenBank KM232121.1; Identities = 656/729 (90 %), 5 gaps (0 %)), and Lectera capsici (strain CBS 142534, GenBank KY979934.1; Identities = 635/726 (87 %), 6 gaps (0 %)).
Fig. 30.
The first of 16 equally most parsimonious trees obtained from a phylogenetic analysis of the Lectera ITS alignment (17 strains including the outgroup; 476 characters including alignment gaps analysed: 371 constant, 80 variable and parsimony-uninformative and 25 parsimony-informative). The tree was rooted to Plectosphaerella melonis (strain CBS 489.96, GenBank MH862587.1) and the scale bar indicates the number of changes. Parsimony bootstrap (PBS) and neighbour joining bootstrap support (NJBS) values higher than 74 % are shown at the nodes (PBS/NJBS) and the treated species is highlighted with a coloured box and bold text. Species names are indicated to the right of the tree, or before the culture collection, GenBank accession numbers, substrate and country of origin. Type status and species name under which the sequence is deposited on GenBank are in superscript. Branches present in the strict consensus tree are thickened. Tree statistics: TL = 146, CI = 0.911, RI = 0.865, RC = 0.788.
Leptoxyphium fumago (Woron.) Crous, comb. nov. MycoBank MB835082. Fig. 31.
Fig. 31.
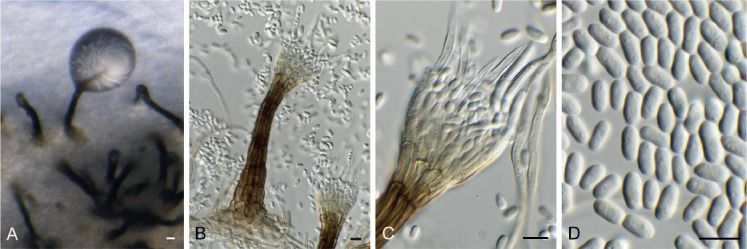
Leptoxyphium fumago (CPC 34802). A, B. Conidiophores. C. Conidiogenous cells. D. Conidia. Scale bars: A = 20 μm, all others = 10 μm.
Basionym: Caldariomyces fumago Woron., Annls mycol. 25(3/4): 261. 1926.
Synonyms: Leptoxyphium fumago (Woron.) R.C. Srivast., Arch. Protistenk. 125(1–4): 333. 1982. Nom. inval., Art. 41.4, Note 1 (Melbourne).
Leptoxyphium glochidion H. Yang & K.D. Hyde, Phytotaxa 178: 177. 2014.
Description and illustration: Yang et al. (2014).
Material examined: Thailand, Chiang Mai, on leaves of Annona squamosa (Annonaceae), 2008, R. Cheewangkoon, culture CPC 34802 = CBS 145579.
Notes: Leptoxyphium glochidion (conidia 6.5–9.5 × 3.4–4.8 μm) was described from leaves of Glochidion wrightii collected in China (Yang et al. 2014). Based on DNA sequence data, it is identical to an isolate we collected on Annona squamosa in Thailand, as well as isolates of Leptoxyphium fumago (nom. inval.) on Hibiscus tiliaceus from Indonesia (CBS 123.26 =ATCC 11925 = IMI 089363; Vu et al. 2019). Leptoxyphium fumago is based on Caldariomyces fumago, a fungus described from living leaves of an unknown host in Georgia, Armenia and Azerbaijan [conidia 5–6(−10) × 2.5(5–6) μm]. Because the name Leptoxyphium fumago is well established in the literature, and the basionym Caldariomyces fumago is older, a new combination is introduced for this taxon, and L. glochidion is reduced to synonymy with it.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Leptoxyphium fumago (strain CBS 123.26, GenBank MH854862.1; Identities = 535/535 (100 %), no gaps), Leptoxyphium glochidion (strain IFRDCC 2651, GenBank NR_155316.1; Identities = 533/533 (100 %), no gaps), and Leptoxyphium kurandae (strain OP193, GenBank JN604454.1; Identities = 463/463 (100 %), no gaps). Closest hits using the LSU sequence are Leptoxyphium fumago (strain CBS 123.26, GenBank MH866361.1; Identities = 853/853 (100 %), no gaps), Leptoxyphium cacuminum (strain MFLUCC 10-0059, GenBank JN832603.1; Identities = 853/853 (100 %), no gaps), and Leptoxyphium glochidion (strain CPC 21382, GenBank MF614832.1; Identities = 836/836 (100 %), no gaps) – also see Fig. 3, part 1. Closest hits using the rpb2 sequence had highest similarity to Leptoxyphium fumago (strain CBS 123.26, GenBank GU371741.1; Identities = 729/740 (99 %), no gaps)), Microxyphium citri (strain CBS 451.66, GenBank GU371727.1; Identities = 667/740 (90 %), no gaps), and Hormodochis aggregata (strain CPC 37499, GenBank MN313837.1; Identities = 117/141 (83 %), no gaps).
Longiseptatispora L.W. Hou & Crous, gen. nov. MycoBank MB835083.
Etymology: Name reflects the longer (= Longi) and septate (= septata) conidia produced by species in this genus.
Conidiomata pycnidial, (sub-)globose, immersed to erumpent, ostiolate; wall of 1–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, lining the inner cavity, doliiform, ampulliform, phialidic with percurrent proliferation. Conidia variable in shape and size, relatively longer, hyaline, smooth, granular, solitary, (0−)3-septate, (sub-)globose, elliopsoidal or subcylindrical, straight to irregularly curved.
Type species: Longiseptatispora curvata Crous & Bulgakov
Longiseptatispora curvata Crous & Bulgakov, sp. nov. MycoBank MB835084. Fig. 32.
Fig. 32.
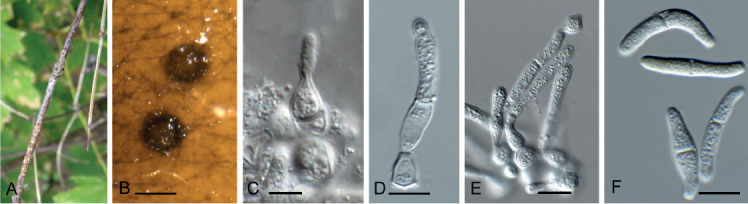
Longiseptatispora curvata (CPC 36457). A. Conidiomata on stems of Lonicera tatarica. B. Conidiomata on MEA. C–E. Conidiogenous cells giving rise to conidia. F. Conidia. Scale bars: B = 300 μm, all others = 10 μm.
Etymology: Name refers to the curved conidia of this species.
Conidiomata pycnidial, brown, globose, immersed to erumpent, 250–300 μm diam, with 1–2 ostioles; wall of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells (or with a supporting cell), lining the inner cavity, doliiform to ampulliform, 5–8 × 5–7 μm, phialidic with percurrent proliferation. Conidia hyaline, smooth, granular, solitary, subcylindrical with obtuse apex and truncate base, straight to irregularly curved, (0−)1-septate, (20−)25–32(−37) × 4(−5) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 60 mm diam after 2 wk at 25 °C. On MEA surface dirty white, reverse buff; on PDA surface dirty white in centre, grey olivaceous in outer region, reverse iron-grey; on OA surface dirty white in centre, grey olivaceous in outer region.
Typus: Russia, Rostov region, Shakhty city district, trees near Atyukhta river, on living and dying twigs of Lonicera tatarica, 22 Sep. 2018, T.S. Bulgakov, HPC 2616 = Myc-22 (holotype CBS H-24166, culture ex-type CPC 36457 = CBS 146035).
Additional material examined: Russia, Rostov region, Shakhty city district, Alexandrovsky park, on live and dying twigs of Lonicera tatarica (Caprifoliaceae), 3 Nov. 2018, T.S. Bulgakov, HPC 2684 = Myc-71, culture CPC 36771.
Notes: The DNA phylogeny (Fig. 33) showed that the two strains from Russia on Lonicera tatarica formed a fully supported clade.This was also true for an isolate collected from the Netherlands on Melilotus sp. with a sequence from GenBank (accession AF439466.1) deposited under the name Leptosphaeria weimeri. The association between these two lineages is not well-supported (PP = 0.81/ML-BS = 75 %), but they are clearly different from the other known genera in Leptosphaeriaceae. Therefore the new genus Longiseptatispora is proposed here to accommodate these taxa. A greater number of isolates and phylogenetically informative gene sequences are needed to determine whether these two lineages might represent distinct genera. Morphologically, Lo. curvata shares some characters with the other species (Lo. meliloti) in this genus by producing long, septate conidia, but could be differentiated from the latter by its longer and less septate conidia: conidia of Lo. curvata are (0−)1-septate, (20−)25–32(−37) × 4(−5) μm, while conidia of Lo. meliloti are (0−)3-septate, 15–26 × 3.5–5.5 μm. In addition, Lo. curvata also differs from the latter species by producing pycnidia with 1–2 ostioles while pycnidia of Lo. meliloti have a single central ostiole.
Fig. 33.
Consensus phylogram (50 % majority rule) resulting from a Bayesian analysis of the Leptosphaeriaceae ITS/LSU sequence alignment (41 strains including the outgroup; 83 and 228 unique site patterns for ITS and LSU, respectively; 275 252 sampled trees from 1 835 000 generations). The alignment is derived from the ITS/LSU alignment of De Gruyter et al. (2013). Bayesian posterior probabilities (PP) > 0.79 and Maximum likelihood (as implemented in MEGA v. 7, Kumar et al. 2016) bootstrap support values (ML-BS) > 74 % are shown at the nodes (PP/ML-BS) and the scale bar represents the expected changes per site. Thickened branches represent PP = 1. GenBank accession (superscript text) and/or culture collection numbers are indicated for all species. The tree was rooted to Phoma herbarum (culture CBS 615.75; GenBank FJ427022.1 and EU754186.1 for ITS and LSU, respectively) and the species treated in this study are indicated in bold face.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 36457 had highest similarity to Paraleptosphaeria dryadis (strain CBS 643.86, GenBank MH862007.1; Identities = 546/578 (94 %), 8 gaps (1 %)), Coniothyrium wernsdorffiae (strain CBS 150.34, GenBank MH855474.1; Identities = 546/579 (94 %), 9 gaps (1 %)), and Phoma schachtii (strain CBS 502.84, GenBank MH861770.1; Identities = 541/578 (94 %), 7 gaps (1 %)). The ITS sequences of CPC 36457 and 36771 are identical (573/573 bases) – also see Fig. 33. Closest hits using the LSU sequence of CPC 36457 are Subplenodomus galicola (voucher MFLU 15-1368, GenBank KY554199.1; Identities = 836/842 (99 %), no gaps), Subplenodomus iridicola (strain CBS 143395, GenBank MH107965.1; Identities = 825/832 (99 %), no gaps), and Subplenodomus violicola (strain CBS 306.68, GenBank GU238156.1; Identities = 825/832 (99 %), no gaps) – also see Fig. 3, part 3 and Fig. 33. The LSU sequences of CPC 36457 and 36771 are identical (815/815 bases). Closest hits using the rpb2 sequence of CPC 36457 had highest similarity to Neopyrenochaeta cercidis (as Neopyrenochaeta sp. SCJ-2019a; voucher MFLU 18-2089, GenBank MK434908.1; Identities = 645/775 (83 %), 2 gaps (0 %)), Curvularia hawaiiensis (strain CBS 727.96, GenBank HG779168.1; Identities = 580/697 (83 %), 8 gaps (1 %)), and Plenodomus hendersoniae (strain LTO, GenBank MF795832.1; Identities = 602/725 (83 %), 8 gaps (1 %)). Closest hits using the tef1 (second part) sequence of CPC 36457 had highest similarity to Stemphylium callistephi (strain EEB 1055, GenBank JQ672393.1; Identities = 403/418 (96 %), no gaps), Wojnowiciella dactylidis (strain CBS 145077, GenBank MK442724.1; Identities = 395/410 (96 %), no gaps), and Paraphoma radicina (strain UTHSC DI16-209, GenBank LT797075.1; Identities = 395/410 (96 %), no gaps). The tef1 sequences of CPC 36457 and 36771 are identical (418/418 bases). Closest hits using the tub2 sequence of CPC 36457 had highest similarity to Parafenestella salicis (strain C303, GenBank MK357628.1; Identities = 450/520 (87 %), 19 gaps (3 %)), Parafenestella pseudosalicis (strain C301, GenBank MK357620.1; Identities = 445/514 (87 %), 19 gaps (3 %)), and Parafenestella pseudoplatani (strain C26, GenBank MF795914.1; Identities = 444/515 (86%), 19 gaps (3 %)). The tub2 sequences of CPC 36457 and 36771 are identical (535/535 bases).
Longiseptatispora meliloti (Lasch ex Rabenh.) L.W. Hou & Crous, comb. nov. MycoBank MB835085. Fig. 34.
Fig. 34.
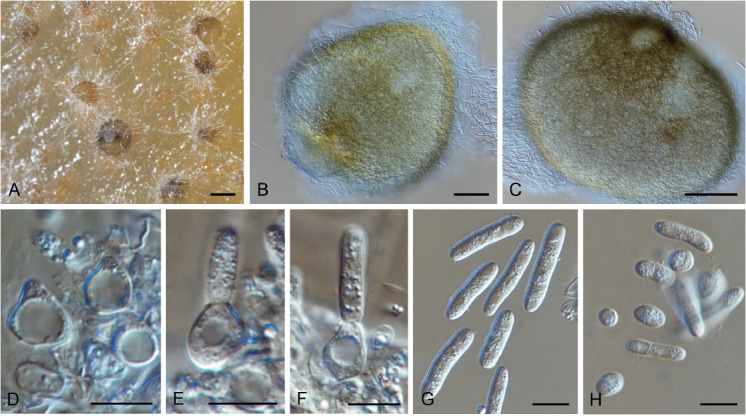
Longiseptatispora meliloti (CBS 110369). A–C. Conidiomata on OA. D–F. Conidiogenous cells. G, H. Dimorphic conidia. Scale bars: A = 300 μm, B, C = 40 μm, all others = 10 μm.
Basionym: Depazea meliloti (Lasch ex Rabenh.) Rabenh., Deutschl. Krypt.-Fl. (Leipzig) 1: 138. 1844.
Synonyms: Sphaeria (Depazea) meliloti Lasch, in Rabenh., Klotzschii Herb. Viv. Mycol., Cent. 4: no. 370. 1842 [and Bot. Zeitung 1: 517. 1843], nom. nud.
Septoria meliloti (Lasch ex Rabenh.) Sacc., Bull. Soc. Mycol. Fr. 5(4): 122. 1890.
Stagonospora meliloti (Lasch ex Rabenh.) Petr., Annls Mycol. 17(2/6): 66. 1920. [1919]
Phoma meliloti Allesch., Ber. bot. Ver. Landshut 12(1): 19. 1892.
Leptosphaeria weimeri Shoemaker et al., Canad. J. Bot. 69(3): 572. 1991.
Ascomata scattered, immersed, depressed, brown, globose with central ostiole; wall of several layers of brown polygonal cells. Pseudoparaphyses intermingled among asci, hyaline, septate. Asci fasciculate, stipitate, cylindrical, with biseriate ascospores. Ascospores fusoid, straight to slightly curved, 3-septate, pale yellowish brown, smooth, enclosed in mucoid sheath. Conidiomata pycnidial, pale brown to brown, (sub-)globose, immersed to erumpent, 190–480(−700) μm diam, with single ostioles; wall of 1–3 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells, lining the inner cavity, doliiform to ampulliform, 7–11 × 5.5–9 μm, phialidic with percurrent proliferation. Conidia dimorphic: large conidia hyaline, smooth, granular, solitary, subcylindrical with obtuse apex and base, straight to slightly curved, (0−)3-septate, 15–26 × 3.5–5.5 μm; small conidia hyaline, smooth, granular, solitary, (sub-)globose, ellipsoidal or subcylindrical, (0−)1-septate, 4.5–9 × 4–5.5 μm (CBS 110369).
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, regular margin, reaching 25 mm diam after 2 wk at 25 °C. On MEA surface rosy buff, reverse buff; on PDA surface rosy buff, reverse buff; on OA surface dirty white with pale grey olivaceous margin, reverse concolorous.
Material examined: Netherlands, Prov. Flevoland, Ketelmeer, IJsseloog, on Melilotus sp. (Leguminosae), 22 May 2002, G. Verkley, culture CBS 110369.
Notes: This species was originally described by Lasch as Sphaeria meliloti (Rabenhorst 1842) and was subsequently transferred to Stagonospora by Petrak as Stagonospora meliloti (Petrak 1919). Later, Stagonospora meliloti was considered as the syn-asexual morph of Phoma meliloti, which was described by Allescher from Meliloti altissimae in Germany. It was characterised by producing phoma-like conidia (Jones & Weimer 1938), but the sexual morph was confused with Leptosphaeria pratensis and Le. viridella (Lucas & Webster 1967). Shoemaker et al. (1991) described a new species Le. weimeri from Meliloti albae in USA and established the sexual/asexual connection with Stagonospora meliloti, which was generally accepted in subsequent studies (Boerema et al. 1994, 2004, Câmara et al. 2003). In the present study, isolate CBS 110369 collected from Melilotus sp. in the Netherlands was treated as a representative strain for the species. It is morphologically comparable with the description of Phoma meliloti in Boerema et al. (1994) producing both phoma-like and stagonospora-like conidia in the same pycnidia. The phylogenetic analysis, showed that CBS 110369 clustered with a reference strain of Leptosphaeria weimeri (ATCC 44387) to form a separate clade in the genus Longiseptatispora (Fig. 33).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Leptosphaeria weimeri (strain ATCC 44387, GenBank AF439466.1; Identities = 401/402 (99 %), no gaps), Monodictys arctica (strain A698, GenBank MK247468.1; Identities = 378/401 (94 %), 1 gap (0 %)), and Subplenodomus galicola (voucher MFLU 15-1368, GenBank NR_154454.1; Identities = 378/401 (94 %), 1 gap (0 %)). Closest hits using the LSU sequence are Subplenodomus iridicola (strain CBS 143395, GenBank NG_067334.1; Identities = 841/853 (99 %), 1 gap (0 %)), Plenodomus deqinensis (strain CGMCC 3.18221, GenBank NG_067547.1; Identities = 837/850 (98 %), 3 gaps (0 %)), and Plenodomus lijiangensis (strain KUMCC 18-0186, GenBank MK387959.1; Identities = 840/854 (98 %), 3 gaps (0 %)). No significant hits were obtained when the rpb2 and tub2 sequences were used in blastn and megablast searches.
Melanomma populicola Crous & R.K. Schumach., nom. nov. MycoBank MB835087. Fig. 35.
Fig. 35.
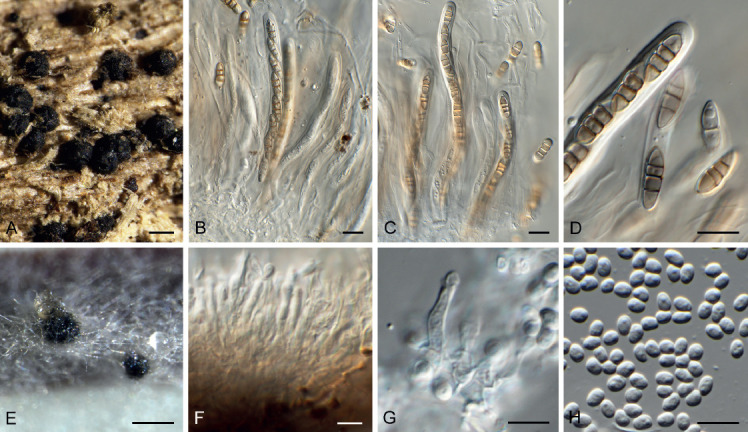
Melanomma populicola (CPC 27203). A. Ascomata on stems of Quercus sp. B–D. Asci with ascospores. E. Conidiomata on OA. F, G. Conidiogenous cells. H. Conidia. Scale bars: A, E = 300 μm, all others = 10 μm.
Basionym: Aposphaeria populina Died., Krypt.-Fl. Brandenburg (Leipzig) 9(1): 206. 1912.
Synonym: Melanomma populinum (Died.) Phukhams. & K.D. Hyde [as ‘populina’], Fungal Diversity 83: 49. 2017. Nom. illegit. Art. 53.1.
Occurring on dead twigs. Ascomata pseudothecial, lignicolous, caespitose, between green algae, superficial, globose to pyroid, ostiole central, terete and short papillate, black, +/-smooth, thick, hyphae and setae absent, 0.25–3 mm diam. Peridium multilayered, consisting of textura angularis with thick-walled and smooth cells, inner layer hyaline, outer layer red brown. Paraphysoids numerous, longer than the asci, filiform, branched, with anastomoses, hyaline, thin-walled, smooth, septa smooth and thin-walled, eguttulate, 1.5–2 μm diam. Asci 8-spored, cylindrical, apically rounded, pedicel short and furcate, thick-walled, bitunicate, fissitunicate, ocular chamber small, inamyloid (water plus Lugol), 91–106(−120) × 6.5–7.5(−9) μm, spores obliquely uniseriate. Ascospores 3-septate, ellipsoid, straight to slightly curved, end cells conical and longer than the middle-cells, wall thin and smooth, septa golden, thick-walled and constricted, plasma pale ochre with 1–3 middle-sized guttules per cell, examined in water, living and mature, (13−)15.1(−19) × (4.5−)5(−6.5) μm. Phoma-like morph formed in culture. Conidiomata solitary to aggregated, pycnidial, 200–300 μm diam, dark brown, globose, papillate with central ostiole, surface covered with brown hyphae; wall of 6–8 layers of brown textura angularis. Conidiophores lining the inner cavity, hyaline, smooth, reduced to conidiogenous cells, or with a supporting cell, branched at base, 10–15 × 3–4 μm. Conidiogenous cells terminal and intercalary, hyaline, smooth, subcylindrical, phialidic with minute periclinal thickening, 8–12 × 2.5–3 μm. Conidia aseptate, solitary, hyaline, smooth, guttulate, ellipsoid, 3–4 × 2.5–3 μm.
Culture characteristics: Colonies flat, spreading, covering dish in 2 wk, with smooth, even margins ad moderate aerial mycelium. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey.
Typus: Germany, Triglitz, from twigs of Populus canadensis (Salicaceae), Mar. 1904, O. Jaap, B, holotype; from branch scars of Picea abies (Pinaceae), Feb. 1982, H. von Aufess, CBS 350.82. Netherlands, Valkenswaard, from fallen twig of Populus canadensis (Salicaceae), 23 Mar. 1970, H.A. van der Aa, epitype CBS H-9336, culture ex-epitype CBS 543.70.
Additional materials examined: Germany, near Berlin, hornbeam wood (Carpinion), fallen and decorticated branch of Quercus cf. petraea / robur (Fagaceae), leg. et det. 7 Mar. 2015, R.K. Schumacher, RKS 831 = HPC 340, cultures CPC 27203 = CBS 145002, CPC 27204. Melanomma pulvis-pyrius: Germany, near Berlin, on branch of Sorbus aucuparia (Rosaceae), 17 Feb. 2016, R.K. Schumacher, RKS 970 = HPC 965-1, cultures CPC 30434.
Notes: Melanomma is known to have phoma-like asexual morphs (Hashimoto et al. 2017). Aposphaeria populina, is a species of Melanomma, was treated by de Gruyter et al. (2013).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CBS 543.70 (Melanomma populicola) had highest similarity to Melanomma pulvis-pyrius (strain CBS 124080, GenBank MH863349.1; Identities = 445/455 (98 %), 1 gap (0 %)), Melanomma japonicum (strain MAFF 239634, GenBank NR_154215.1; Identities = 458/469 (98 %), 3 gaps (0 %)), and Dendrophoma pleurospora (strain CBS 197.48, GenBank MH856307.1; Identities = 446/469 (95 %), 3 gaps (0 %)) – also see Fig. 36. The ITS sequence of CBS 543.70 is identical (466/466) to that of CBS 145002 and differs with a single nucleotide (468/469 similar) to that of CBS 350.82. Closest hits using the LSU sequence are Melanomma pulvis-pyrius (strain MPP, GenBank KY189979.1; Identities = 879/882 (99 %), 1 gap (0 %)), Trematosphaeria pertusa (strain AFTOL-ID 1589, GenBank DQ678072.1; Identities = 905/909 (99 %), 2 gaps (0 %)), and Melanomma japonicum (strain KT 3028, GenBank LC203337.1; Identities = 875/881 (99 %), 2 gaps (0 %)) – also see Fig. 3, part 3. The LSU sequence of CBS 543.70 differs with two gaps (877/879 similar) from that of CBS 350.82 and two nucleotide changes and two gaps (902/906 similar) to that of CBS 145002.
Fig. 36.
Single most parsimonious tree obtained from a phylogenetic analysis of the Melanomma ITS alignment (27 strains including the outgroup; 478 characters analysed: 289 constant, 104 variable and parsimony-uninformative and 85 parsimony-informative). The tree was rooted to Trematosphaeria grisea (strain CBS 332.50 GenBank NR_132039.1) and the scale bar indicates the number of changes. Parsimony bootstrap (PBS) and neighbour joining bootstrap support (NJBS) values higher than 74 % are shown at the nodes (PBS/NJBS) and the treated species is highlighted with a coloured box and bold text. Species names are indicated to the right of the tree, or before the culture collection, GenBank accession numbers, substrate and country of origin. Type status is indicated in superscript. Tree statistics: TL = 280, CI = 0.907, RI = 0.917, RC = 0.832.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 30434 (Melanomma pulvis-pyrius) had highest similarity to Melanomma pulvis-pyrius (strain MPP, GenBank KY189979.1; Identities = 1 013/1 013(100 %), no gaps), Melanomma japonicum (strain MAFF 239634, GenBank NR_154215.1; Identities = 460/474 (97 %), 1 gap (0 %)), and Melanodiplodia tianschanica (strain TASM 6110, GenBank NR_157520.1; Identities = 416/430 (97 %), 1 gap (0 %)) – also see Fig. 36. Closest hits using the LSU sequence of CPC 30434 are Melanomma pulvis-pyrius (strain CBS 125577, GenBank MH875177.1; Identities = 891/892 (99 %), no gaps), Trematosphaeria pertusa (strain AFTOL-ID 1589, GenBank DQ678072.1; Identities = 891/892 (99 %), no gaps), and Aposphaeria populina (strain CBS 350.82, GenBank JF740265.1; Identities = 870/873 (99 %), no gaps) – also see Fig. 3, part 3. Closest hits using the rpb2 sequence of CPC 30434 had highest similarity to Melanomma pulvis-pyrius (strain CBS 124080, GenBank GU456350.1; Identities = 887/887 (100 %), no gaps), Melanomma japonicum (strain KT 3028, GenBank LC203393.1; Identities = 813/850 (96 %), no gaps), and Lophiostoma rugulosum (strain CBS 123093, GenBank FJ795459.1; Identities = 404/463 (87 %), no gaps).
Neocladosporium syringae Crous & Akulov, sp. nov. MycoBank MB835088. Fig. 37.
Fig. 37.

Neocladosporium syringae (CPC 35750). A. Colony on SNA. B–E. Conidial chains. Scale bars = 10 μm.
Etymology: Name refers to Syringa, the host genus from which this fungus was isolated.
Mycelium consisting of pale brown, roughened, branched, septate, 3–4 μm diam hyphae. Conidiophores erect, subcylindrical, straight to flexuous, brown, smooth, 50–100 × 3–4 μm, 3–6-septate. Conidiogenous cells terminal, straight to geniculate-sinuous, brown, smooth, but with outer mucoid layer that gives a roughened appearance, 12–40 × 3.5–5 μm; scars thickened, darkened, 1.5–2 μm. Conidia occurring in branched chains, subcylindrical, fusoid-ellipsoid, brown, smooth but appearing roughened due to mucoid outer layer, hila thickened, darkened, 1.5–2 μm diam; ramoconidia 0–2-septate, 16–30 × 3.5–5 μm; conidia 0–1-septate, (13−)14–16(−18) × (3−)3.5–4 μm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium, folded surface and smooth, lobate margin, reaching 30 mm diam after 2 wk at 25 °C. On MEA surface umber to sienna, reverse umber; on PDA surface and reverse isabelline; on OA surface isabelline with diffuse sienna pigment.
Typus: Ukraine, Kharkiv region, Zolochiv district, Chepeline village, on overwintered dead branches of Syringa vulgaris (Oleaceae), 15 Apr. 2018, A. Akulov, HPC 2320 = CWU (Myc) AS 6728 (holotype specimen CBS 145544, preserved as metabolically inactive culture, culture ex-type CPC 35750 = CBS 145544).
Notes: Neocladosporium was previously monotypic, based on N. leucadendri (Bezerra et al. 2017). Both species are characterised by having conidia with a roughened (warty) mucoid outer layer. Neocladosporium syringae is distinguished from N. leucadendri by its larger ramoconidia (25–45 × 3–5 μm), and smaller terminal conidia (6–15 × 2.5–4 μm).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Davidiellomyces australiensis (strain CBS 142165, GenBank NR_154036.1; Identities = 604/679 (89 %), 21 gaps (3 %)), Neocladosporium leucadendri (strain CBS 131317, GenBank NR_152324.1; Identities = 491/521 (94 %), 12 gaps (2 %)), and Verrucocladosporium dirinae (strain CBS 112794, GenBank NR_152317.1; Identities = 460/498 (92 %), 9 gaps (1 %)). Closest hits using the LSU sequence are Neocladosporium leucadendri (strain CBS 131317, GenBank NG_057949.1; Identities = 828/834 (99 %), 1 gap (0 %)), Davidiellomyces australiensis (strain CBS 142165, GenBank NG_059164.1; Identities = 809/818 (99 %), 1 gap (0 %)), and Graphiopsis chlorocephala (strain CBS 121523, GenBank MH874669.1; Identities = 824/834 (99 %), 1 gap (0 %)) – also see Fig. 3, part 1.
Neolamproconium Crous & Akulov, gen. nov. MycoBank MB835089.
Etymology: Name refers to its morphological similarity to Lamproconium.
Ascomata immersed in host tissue, perithecial, black, erect to oblique, erumpent, in clusters, long necked with central ostioles, converging through a black stromatic disc; ascomata subglobose, in diatrypoid configuration, neck slightly swollen at the tip; ostiole with hyaline periphyses; peridium of thick-walled cells of brown textura angularis; hamathecium of hyaline, septate, branched, hyphae-like paraphyses. Asci 8-spored, subcylindrical with obtuse to slightly flattened apex and apical mechanism (not bluing in Meltzer’s reagent), stipitate, arranged in basal layer. Ascospores hyaline, smooth, guttulate, granular, fusoid to subcylindrical, ends subobtuse, slightly constricted at median septum, mostly straight. Conidiomata erumpent through bark, black, stromatic, with aggregated rosettes of pycnidia, upper part with slightly elongated black necks. Conidiomata erumpent, solitary, pycnidial, globose, 200–300 μm diam, with central ostiole; wall of 3–6 layers of pale brown textura angularis. Conidiophores lining inner layer, hyaline, smooth, septate, branched. Conidiogenous cells hyaline, smooth-walled, subcylindrical, terminal and intercalary, phialidic with prominent periclinal thickening. Conidia dimorphic. Macroconidia hyaline, smooth, guttulate, granular, fusoid, apex subobtuse, base truncate, septate. Microconidia developing in same conidioma as macroconidia, rod-shaped, ends obtuse, curved to straight, aseptate.
Type species: Neolamproconium silvestre Crous & Akulov
Neolamproconium silvestre Crous & Akulov, sp. nov. MycoBank MB835090. Fig. 38.
Fig. 38.

Neolamproconium silvestre (CPC 34865). A. Stroma with ascomata. B. Paraphyses. C–E. Asci. F. Ascospores. G. Conidioma on OA. H, I. Conidiophores with conidiogenous cells. J. Macroconidia. K. Microconidia. Scale bars: A = 350 μm, G = 300 μm, all others = 10 μm.
Etymology: Name refers to its location, collected in a forest.
In vivo: Ascomata immersed in host tissue, perithecial, black, erect to oblique, erumpent, in clusters of 6–15, long necked with central ostioles, converging through a black stromatic disc, 1–1.5 mm diam; ascomata subglobose, in diatrypoid configuration, up to 350 μm diam, neck up to 600 μm long, slightly swollen at the tips; ostiole with hyaline periphyses; peridium of thick-walled cells of brown textura angularis; hamathecium of hyaline, septate, branched, hyphae-like paraphyses, 2.5–5 μm diam. Asci 8-spored, subcylindrical with obtuse to slightly flattened apex and apical mechanism (not bluing in Meltzer’s reagent), stipitate, arranged in basal layer, 90–140 × 13–16 μm. Ascospores hyaline, smooth, guttulate, granular, fusoid to subcylindrical, ends subobtuse, slightly constricted at median septum, mostly straight, (27−) 30–35(−38) × (6−)7(−8) μm (in water). Conidiomata erumpent through bark, black, stromatic, with aggregated rosettes of up to 15 pycnidia, upper part with slightly elongated black necks. In vitro: Conidiomata erumpent, solitary, pycnidial, globose, 200–300 μm diam, with central ostiole; wall of 3–6 layers of pale brown textura angularis. Conidiophores lining inner layer, hyaline, smooth, septate, branched, 40–80 × 2.5–4 μm. Conidiogenous cells hyaline, smooth-walled, subcylindrical, terminal and intercalary, 15–25 × 2–2.5 μm, phialidic with prominent periclinal thickening. Conidia dimorphic. Macroconidia hyaline, smooth, guttulate, granular, fusoid, apex subobtuse, base truncate, 1(−3)-septate, (28−)34–38(−42) × 4(−4.5) μm. Microconidia developing in same conidioma as macroconidia on SNA, rod-shaped, ends obtuse, curved to straight, aseptate, (6−)7–8 × 1.5(−2) μm.
Culture characteristics: Colonies flat, spreading, with sparse to moderate aerial mycelium and smooth, lobate margin, reaching 40 mm diam after 2 wk at 25 °C. On MEA surface olivaceous grey with patches of honey, reverse isabelline to cinnamon; on PDA surface and reverse olivaceous grey; on OA surface ochreous.
Typus: Ukraine, Kharkiv, Forest-park, Sokol`niki-Pomerki, on branch of Tilia sp. (Malvaceae), 8 Oct. 2017, A. Akulov, HPC 2274 = CWU (Myc) AS 6601 (holotype specimen CBS 145566, preserved as metabolically inactive culture, culture ex-type CPC 34865 = CBS 145566).
Additional material examined: Ukraine, Kharkiv, Forest-park, Sokol`niki-Pomerki, on branch of Quercus robur (Fagaceae), 8 Oct. 2017, A. Akulov, HPC 2273 = CWU (Myc) AS 6660, culture CPC 34862.
Notes: Lamproconium, based on L. desmazieri, was recently treated by Norphanphoun et al. (2016), based on specimens on dead branches of Tilia cordata collected in Russia. Characteristic features include the fact that the fusoid-ellipsoid conidia are aseptate, and turn dark blue at maturity. Hercospora tiliae (also on Tilia cordata) represents a second genus in Lamproconiaceae, and is distinct in that conidia remain hyaline, and are aseptate. Our collections (on Tilia sp., Ukraine) differ in that the macroconidia are septate, and were not observed to become pigmented after 1 mo in culture.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 34865 had highest similarity to Lamproconium desmazieri (as Melanconis desmazieri, strain MFLUCC 14-1047, GenBank KX430132.1; Identities = 530/577 (92 %), 19 gaps (3 %)), Sydowiella centaureii (voucher MFLU 16-2858, GenBank NR_155860.1; Identities = 500/552 (91 %), 19 gaps (3 %)), and Sydowiella fenestrans (strain CBS 125530, GenBank JF681956.1; Identities = 499/552 (90 %), 20 gaps (3 %)) – also see Fig. 39. The ITS sequence of CPC 34865 is identical to that of CPC 34862 (582/582 bases). Closest hits using the LSU sequence of CPC 34865 are Lamproconium desmazieri (as Melanconis desmazieri, strain AR3525, GenBank AF408372.1; Identities = 813/820 (99 %), no gaps), Hercospora tiliae (strain AR3526, GenBank AF408365.1; Identities = 790/820 (96 %), no gaps), and Diaporthe pustulata (strain AR3430, GenBank AF408357.1; Identities = 790/820 (96 %), no gaps) – also see Fig. 6, part 2b. The LSU sequence of CPC 34865 is identical to that of CPC 34862 (820/820 bases).
Fig. 39.
The first of three equally most parsimonious trees obtained from a phylogenetic analysis of the “Lamproconium” ITS alignment (31 strains including the outgroup; 570 characters including alignment gaps analysed: 260 constant, 58 variable and parsimony-uninformative and 252 parsimony-informative). The tree was rooted to Diaporthe eres (strain UASWS2094, GenBank MN861449.1) and the scale bar indicates the number of changes. Parsimony bootstrap (PBS) and neighbour joining bootstrap support (NJBS) values higher than 74 % are shown at the nodes (PBS/NJBS) and the treated species is highlighted with a coloured box and bold text. Species names are indicated to the right of the tree, or before the culture collection and GenBank accession numbers. Branches present in the strict consensus tree are thickened. Tree statistics: TL = 686, CI = 0.724, RI = 0.917, RC = 0.664.
Neosetophoma cerealis (E. Müll.) Crous, comb. nov. MycoBank MB835091. Fig. 40.
Fig. 40.
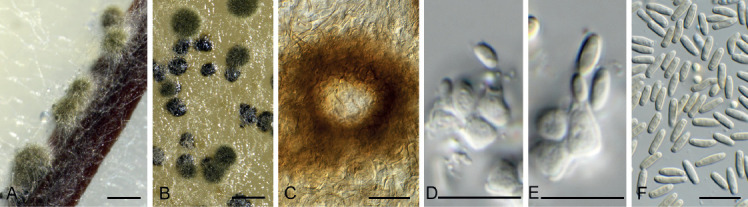
Neosetophoma cerealis (CBS 232.77). A. Conidiomata on PNA. B. Conidiomata on OA. C. Conidiomatal ostiole. D, E. Conidiogenous cells. F. Conidia. Scale bars: A, B = 300 μm, all others = 10 μm.
Basionym: Coniothyrium cerealis E. Müll., Phytopath. Z. 18: 11. 1951.
Synonym: Neosetophoma phragmitis Crous et al., Stud. Mycol. 94: 48. 2019.
Conidiomata solitary, globose, erumpent, brown, 150–200 μm diam (up to 350 μm diam in CBS 120096), with central ostiole exuding a brown mucoid conidial mass; wall of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to doliiform, phialidic with prominent periclinal thickening, 4–6 × 4–6 μm. Conidia aseptate, pale brown, smooth-walled, subcylindrical, straight, apex obtuse, base truncate, 1.5–2 μm diam, guttulate, (5−)6–8(−10) × 2(−2.5) μm (based on CBS 232.77).
Culture characteristics: Colonies flat, spreading, surface folded with moderate aerial mycelium and smooth, lobate margin, covering dish in 2 wk at 25 °C. On MEA surface and reverse isabelline; on PDA surface hazel, reverse brown vinaceous; on OA surface saffron.
Materials examined: Germany, near Götingen, Apera spica-venti (Poaceae), Jul. 1977, P. Reinecke, CBS H-10762, culture CBS 158.78; Kiel-Kitzeberg, wheat field soil, W. Gams, No. C 743, culture CBS 672.68; Kiel-Kitzeberg, wheat field soil, W. Gams, No. C 455, culture CBS 963.68; near Götingen, Triticum aestivum (Poaceae), Jul. 1977, P. Reinecke, CBS H-10761, culture CBS 157.78. Netherlands, N. O. Polder, Parcel “Klaverland Nagele”, agricultural soil, Nov. 1976, W. Verkerke, No. 53a, culture CBS 232.77; Noord Oost Polder, Nagele, soil sample mixed with Beta vulgaris and Hordeum vulgare, Feb. 1982, H. Nielander, CBS H-10766, CBS H-10771, culture CBS 443.82. Norway, Bilrum, Phleum pratense (Poaceae), 1974, K. Årsvoll, Norw. Plantprot. Inst., CBS H-10757; CBS H-10758, culture CBS 518.74. Spain, Sierra de Bulejo, Guadalajara, composted livestock manure, 5 Aug. 2002, G.F. Bills, culture CBS 120096.
Notes: Coniothyrium cerealis was originally described from decaying wheat stubble collected in Switzerland, with brown, aseptate conidia, 4–6 × 1 μm (Zogg 1951). Domsch et al. (2007) examined the type specimen, and cited conidia as being 5.5–8(−11) × 1.5–2.5 μm. Although the isolates studied here showed some variation with regards to their conidial dimensions, this appeared to be an intraspecific trait. Phylogenetically, C. cerealis clustered in Neosetophoma (see Fig. 3, part 3), and a new combination is therefore introduced for this fungus. Neosetophoma phragmitis (leaf sheath of Phragmitis australis, Germany, conidia (3−)4–5(−6) × 2 μm; Marin-Felix et al. 2019) appears to be a recent synonym (see Fig. 41, part 2).
Fig. 41, parts 1, 2.
The first of 132 equally most parsimonious trees obtained from a phylogenetic analysis of the Neosetophoma ITS alignment (74 strains including the outgroup; 472 characters including alignment gaps analysed: 310 constant, 103 variable and parsimony-uninformative and 59 parsimony-informative). The tree was rooted to Kalmusia longispora (strain CBS 824.84, GenBank JX496115.1) and the scale bar indicates the number of changes. Parsimony bootstrap (PBS) and neighbour joining bootstrap support (NJBS) values higher than 74 % are shown at the nodes (PBS/NJBS) and the treated species is highlighted with a coloured box and bold text. Species names are indicated to the right of the tree, or before the culture collection and GenBank accession numbers. The substrate and country of origin are also provided for strains of Neosetophoma cereale. Type status is indicated in superscript. Branches present in the strict consensus tree are thickened and the most basal branch was shortened four times to facilitate layout. Tree statistics: TL = 283, CI = 0.756, RI = 0.880, RC = 0.665.
Closest hits using the rpb2 sequence had highest similarity to Neosetophoma sp. 1 (strain UTHSC DI16-191, GenBank LT796991.1; Identities = 586/652 (90 %), no gaps), Brunneomurispora lonicerae (strain KUMCC 18-0157, GenBank MK359079.1; Identities = 572/651 (88 %), no gaps), and Septoriella pseudophragmitis (strain CBS 145417, GenBank MK559450.1; Identities = 536/641 (84 %), no gaps). The rpb2 sequences of CBS 443.82 and CBS 518.74 are 97 % (634/652 bases, no gaps) similar. Closest hits using the tub2 sequence of CBS 443.82 had highest similarity to Neosetophoma sp. (strain ICMP 6864, GenBank KT309580.1; Identities = 255/260 (98 %), 1 gap (0 %)), Neosetophoma samarorum (strain CBS 139.96, GenBank KF252656.1; Identities = 293/299 (98 %), 1 gap (0 %)), Neosetophoma sp. 1 (strain UTHSC DI16-337, GenBank LT796970.1; Identities = 238/261 (91 %), 2 gaps (0 %)), and Diederichomyces xanthomendozae (strain CBS 129666, GenBank KP170701.1; Identities = 190/209 (91 %), 8 gaps (3 %)). The tub2 sequences of CBS 443.82 and CBS 518.74 are 98 % (357/364 bases, no gaps) similar.
Neosorocybe Crous & Akulov, gen. nov. MycoBank MB835092.
Etymology: Name refers to the fact that it is allied to Sorocybe.
Conidiomata synnematous, consisting of conidiophores that are brown, smooth, multiseptate, branched, subcylindrical. Conidiogenous cells integrated on conidiophores, terminal and intercalary, giving rise to branched conidial chains via sympodial proliferation; hila truncate, not thickened nor darkened. Conidia subcylindrical, medium brown, smooth, guttulate, septate, forming branched chains of disarticulating conidia; hila not thickened nor darkened.
Type species: Neosorocybe pini Crous & Akulov
Neosorocybe pini Crous & Akulov, sp. nov. MycoBank MB835093. Fig. 42.
Fig. 42.
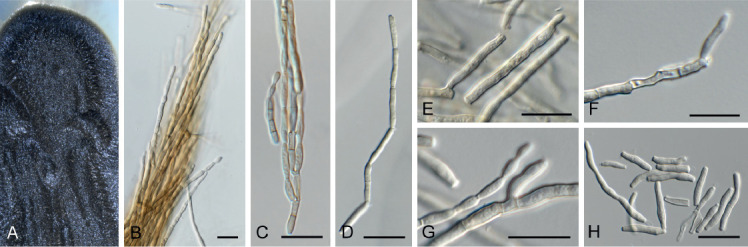
Neosorocybe pini (CPC 36628). A. Colony on SNA. B. Synnema. C, D. Conidial chains. E–G. Conidiogenous cells. H. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Pinus from which the species was isolated.
Conidiomata synnematous, consisting of conidiophores that are brown, smooth, multiseptate, branched, subcylindrical, up to 300 μm tall. Conidiogenous cells integrated on conidiophores, terminal and intercalary, giving rise to branched conidial chains via sympodial proliferation, 7–12 × 2.5–3.5 μm, hila truncate, 2 μm diam, not thickened nor darkened. Conidia subcylindrical, medium brown, smooth, guttulate, 0–1(−2)-septate, forming branched chains of disarticulating conidia; ramoconidia 10–20 × 2–3 μm; conidia 5–20 × 2–3 μm; hila 1.5–2 μm diam, not thickened nor darkened.
Culture characteristics: Colonies flat, spreading, slimy, with sparse aerial mycelium and smooth, lobate margin, reaching 7 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse iron-grey.
Typus: Ukraine, Sumy region, Okhtyrka district, Klymentove village, NNP Hetmanskyi, on Pinus sylvestris (Pinaceae), fallen trunk, 3 Aug. 2018, A. Akulov, HPC 2548 = CWU (Myc) AS 6816 (holotype CBS H-24172, culture ex-type CPC 36628 = CBS 146085).
Notes: Neosorocybe pini is phylogenetically allied to Sorocybe, but appears to represent a distinct genus, for which the name Neosorocybe is introduced. Neosorocybe has synnemata with chains of pigmented, cylindrical conidia, with typical culture characteristics of Chaetothyriales, with slimy, iron-grey colonies on MEA and PDA.
Based on a megablast search of NCBI’s GenBank nucleotide database, only distant hits were obtained using the ITS sequence, e.g. to Capronia villosa (strain ATCC 56206, GenBank AF050261.1; Identities = 397/454 (87 %), 23 gaps (5 %)), Sorocybe oblongispora (as Sorocybe sp. JBT-2019, strain NB-795-1, GenBank MN114115.1; Identities = 419/487 (86 %), 26 gaps (5 %)), and Sorocybe resinae (strain DAOM 239134, GenBank EU030275.1; Identities = 412/480 (86 %), 21 gaps (4 %)). Closest hits using the LSU sequence are Sorocybe resinae (strain DAOM 239134, GenBank EU030277.1; Identities = 803/826 (97 %), 1 gap (0 %)), Sorocybe oblongispora (as Sorocybe sp. JBT-2019a, strain NB-795-1, GenBank MN114117.1; Identities = 828/852 (97 %), 1 gap (0 %)), and Lasallia pustulata (voucher 5 June 01 Wedin (UPS), GenBank AY300839.1; Identities = 821/852 (96 %), 3 gaps (0 %)) – also see Fig. 4.
Nothoseptoria Crous & Bulgakov, gen. nov. MycoBank MB835094.
Etymology: Name refers to its similarity to Septoria.
Conidiomata pycnidial, hypophyllous on leaves, oozing a creamy conidial cirrhus. Conidiomata brown, pycnidial, opening via central ostiole; wall of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to subcylindrical, proliferating percurrently at apex. Conidia solitary, straight to slightly curved, subcylindrical, apex subobtuse, base truncate with minute marginal frill, septate, prominently guttulate.
Type species: Nothoseptoria caraganae (Henn.) Crous & Bulgakov
Nothoseptoria caraganae (Henn.) Crous & Bulgakov, comb. nov. MycoBank MB835095. Fig. 43.
Fig. 43.
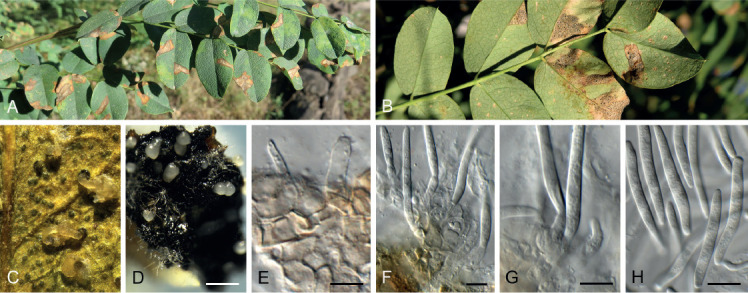
Nothoseptoria caraganae (CPC 36563). A, B. Leaf spots on Caragana arborescens. C. Immersed conidiomata with conidial cirrus. D. Conidiomata with conidial cirri on SNA. E–G. Conidiogenous cells giving rise to conidia. H. Conidia. Scale bars: D = 250 μm, all others = 10 μm.
Basionym: Septoria caraganae Henn., Z. PflKrankh. 12: 15. 1902.
Synonym: Mycosphaerella jaczewskii Potebnia, Annls mycol. 8(1): 50. 1910.
Leaf spots pale brown, confined by leaf veins, angular, leading to leaf blight. Conidiomata pycnidial, hypophyllous on leaves, oozing a creamy conidial cirrhus. Conidiomata brown, pycnidial, 250–300 μm diam, opening via central ostiole; wall of 3–6 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to subcylindrical, 6–10 × 3–5 μm, proliferating percurrently at apex. Conidia solitary, straight to slightly curved, subcylindrical, apex subobtuse, base truncate with minute marginal frill, 1(−3)-septate, prominently guttulate, (35−)41–50(−60) × (3.5−)4(−5.5) μm, in culture (32−)35–40(−45) × (3−)4 μm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and smooth, lobate margin, reaching 3 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Materials examined: Russia, Rostov region, Shakhty city district, Hospital park, on live leaves of Caragana arborescens (Leguminosae), 21 Sep. 2018, T.S. Bulgakov, HPC 2631 = Myc-01 = CBS H-24229, culture CPC 36563 = CBS 145993; Rostov region, Shakhty city district, street shrubs, on live leaves of Caragana arborescens, 21 Sep. 2018, T.S. Bulgakov, HPC 2632 = Myc-02, culture CPC 36565.
Notes: Septoria caraganae was described from leaves of Caragana arborescens collected in Germany, with conidia being 1–3-septate, 30–50 × 3–4 μm (von Hennings 1902). The link to the sexual morph, Mycosphaerella jaczewskii, requires confirmation in culture.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 36563 had highest similarity to Sonderhenia radiata (strain CBS 145600, GenBank MN162023.1; Identities = 435/490 (89 %), 10 gaps (2 %)), Sonderhenia eucalyptorum (strain CPC 32601, GenBank MN162022.1; Identities = 435/490 (89 %), 10 gaps (2 %)), and Sonderhenia eucalypticola (strain CPC 31596, GenBank MN162018.1; Identities = 434/490 (89 %), 10 gaps (2 %)). The ITS sequences of CPC 36563 and 36565 are identical (485/485 bases). Closest hits using the LSU sequence of CPC 36563 are Sonderhenia eucalyptorum (strain CPC 17677, GenBank MN162214.1; Identities = 805/836 (96 %), 9 gaps (1 %)), Sonderhenia eucalypticola (as Mycosphaerella walkeri, strain CMW 20333, GenBank DQ267574.1; Identities = 803/834 (96 %), 5 gaps (0 %)), and Fusoidiella depressa (as Passalora depressa, strain CPC 14915, GenBank KF251813.1; Identities = 790/823 (96 %), 8 gaps (0 %)) – also Fig. 3, part 2 and Fig. 8, part 1. The LSU sequences of CPC 36563 and 36565 are identical (834/834 bases). Closest hits using the rpb2 sequence of CPC 36563 had highest similarity to Septoria protearum (strain CBS 778.97, GenBank KT216543.1; Identities = 750/924 (81 %), 12 gaps (1 %)), Septoria bupleuricola (strain TCM-5, GenBank KY798146.1; Identities = 730/921 (79 %), 6 gaps (0 %)), and Mycovellosiella cajani (strain CBS 113999, GenBank MF951528.1; Identities = 732/928 (79 %), 18 gaps (1 %)). The rpb2 sequences of CPC 36563 and 36565 are identical (892/892 bases). No significant hits were obtained when the tub2 sequence of CPC 36563 was used in blastn and megablast searches. The tub2 sequences of CPC 36563 and 36565 are identical (436/436 bases).
Paraconiothyrium iridis Crous & Akulov, sp. nov. MycoBank MB835096. Fig. 44.
Fig. 44.
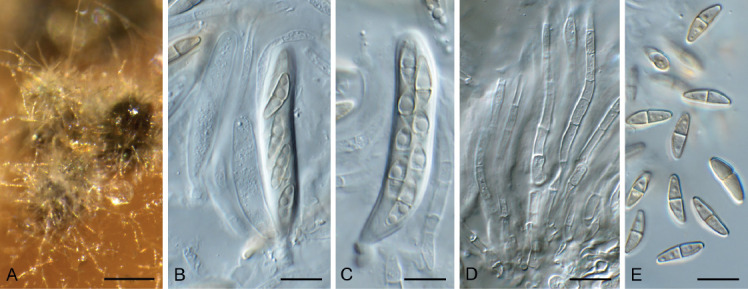
Paraconiothyrium iridis (CPC 36281). A. Ascomata on MEA. B, C. Asci. D. Pseudoparaphyses. E. Ascospores. Scale bars: A = 150 μm, all others = 10 μm.
Etymology: Name refers to the host genus Iris from which the species was isolated.
Ascomata pseudothecial, aggregated in clusters, linked by a brown stroma, globose, 70–150 μm diam, brown, surface covered in hyphae; wall of 6–8 layers of brown textura angularis. Pseudoparaphyses intermixed between asci, hyphae-like, hyaline, smooth, septate, 2.5–3 μm diam. Asci bitunicate, broadly ellipsoid, stipitate, 8-spored, inconspicuous ocular chamber, 35–45 × 8–9 μm. Ascospores tri- to multiseriate, fusoid-ellipsoid, widest just above median septum, constricted at septum, ends subobtuse, slightly curved, brown verruculose, guttulate, 12–13(−14) × (3−)3.5–4 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery margin, reaching 50 mm diam after 2 wk at 25 °C. On MEA surface cinnamon, reverse isabelline in centre, cinnamon in outer region; on PDA surface buff, reverse cinnamon to buff; on OA surface honey.
Typus: Ukraine, Chernihiv region, Kozelets district, Otrokhy village, Bondarivske bog, Regional landscape park Mizhrichynskyi, on living leaves of Iris pseudacorus (Iridaceae), 8 Aug. 2018, A. Akulov, HPC 2547 = CWU (Myc) AS 6830 (holotype CBS H-24164, culture ex-type CPC 36281 = CBS 146036).
Notes: Paraconiothyrium iris is closely related to P. cyclothyrioides (Verkley et al. 2004), but these species cannot be compared morphologically because P. iris produced only a sexual morph in culture.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Paraconiothyrium cyclothyrioides (strain UTHSC DI16-328, GenBank LT796885.1; Identities = 574/591 (97 %), 6 gaps (1 %)), and Microsphaeropsis arundinis (strain CX, GenBank KU212359.1; Identities = 574/591 (97 %), 6 gaps (1 %)). Closest hits using the LSU sequence are Paraconiothyrium cyclothyrioides (strain CBS 432.75, GenBank JX496201.1; Identities = 884/887 (99 %), no gaps), Microsphaeropsis arundinis (strain CBS 100243, GenBank JX496123.1; Identities = 883/886 (99 %), no gaps), and Paraconiothyrium estuarinum (strain CML3695, GenBank MF374462.1; Identities = 870/873 (99 %), no gaps (0 %)) – also see Fig. 3, part 4. Closest hits using the rpb2 sequence had highest similarity to Paraconiothyrium cyclothyrioides (strain UTHSC DI16-327, GenBank LT797044.1; Identities = 908/937 (97 %), no gaps), Paraconiothyrium sp. 1 NV-2015 (strain UTHSC DI16-349, GenBank LT797056.1; Identities = 895/937 (96 %), no gaps), and Paraconiothyrium estuarinum (strain CBS 109850, GenBank LT854937.1; Identities = 886/937 (95 %), no gaps). Closest hits using the tef1 sequence had highest similarity to Paraconiothyrium hakeae (strain CBS 142521, GenBank KY979892.1; Identities = 323/383 (84 %), 16 gaps (4 %)), Paraphaeosphaeria parmeliae (strain CBS 131728, GenBank KP170679.1; Identities = 300/367 (82 %), 19 gaps (5 %)), and Paraphaeosphaeria xanthorrhoeae (strain CBS 142164, GenBank KY979888.1; Identities = 302/377 (80 %), 24 gaps (6 %)). Closest hits using the tub2 sequence had highest similarity to Paraconiothyrium cyclothyrioides (strain UTHSC DI16-243, GenBank LT796929.1; Identities = 461/494 (93 %), 4 gaps (0 %)), Paraconiothyrium sp. 1 NV-2015 (strain UTHSC DI16-349, GenBank LT796976.1; Identities = 446/481 (93 %), 2 gaps (0 %)), and Paraconiothyrium estuarinum (strain CBS 109850, GenBank JX496355.1; Identities = 421/456 (92 %), 6 gaps (1 %)).
Pararoussoella quercina Crous & Akulov, sp. nov. MycoBank MB835097. Fig. 45.
Fig. 45.
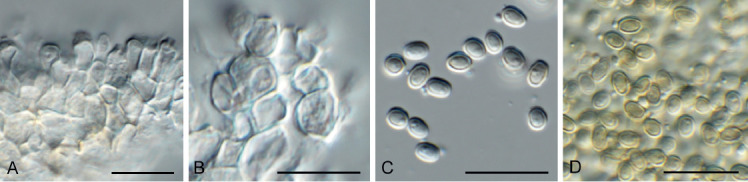
Pararoussoella quercina (CPC 34864). A, B. Conidiogenous cells. C, D. Conidia. Scale bars = 10 μm.
Etymology: Name refers to Quercus, the host genus from which this fungus was isolated.
Cultures sporulating sparsely. Conidiomata immersed in agar, globose, brown, 200–250 μm diam. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, doliiform, 4–6 × 3–4.5 μm, phialidic with prominent periclinal thickening. Conidia solitary, aseptate, thick-walled, brown, smooth, guttulate, broadly ellipsoid, apex obtuse, base bluntly rounded, (3−)4(−5) × 3 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery margin, reaching 30 mm diam after 2 wk at 25 °C on PDA, covering dish on OA and MEA. On MEA surface smoke grey, reverse umber; on PDA surface and reverse olivaceous grey; on OA surface olivaceous grey.
Typus: Ukraine, Kharkiv, Forest-park, Sokol`niki-Pomerki, on branch of Quercus robur (Fagaceae), 8 Oct. 2017, A. Akulov, HPC 2273 = CWU (Myc) AS 6660 (holotype specimen CBS 145567, preserved as metabolically inactive culture, culture ex-type CPC 34864 = CBS 145567).
Notes: Pararoussoella quercina is related to P. mukdahanensis (on dead culms of bamboo, Thailand) (Crous et al. 2019b), but is phylogenetically distinct.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Pararoussoella juglandicola (strain CBS 145037, GenBank MK442607.1; Identities = 567/568 (99 %), no gaps), Pararoussoella mukdahanensis (as Roussoella mukdahanensis; voucher MFLU 11-0237, GenBank NR_155722.1; Identities = 488/497 (98 %), 1 gap (0 %)), and Pararoussoella rosarum (voucher MFLU 17-0654, GenBank NR_157529.1; Identities = 491/505 (97 %), 4 gaps (0 %)). Closest hits using the LSU sequence are Pararoussoella juglandicola (strain CBS 145037, GenBank MK442543.1; Identities = 836/837 (99 %), 1 gap (0 %)), Pararoussoella mukdahanensis (as Roussoella mukdahanensis; strain MFLUCC 11-0201, GenBank NG_059671.1; Identities = 825/827 (99 %), no gaps), and Pararoussoella rosarum (voucher MFLU 17-0654, GenBank NG_059872.1; Identities = 828/832 (99 %), no gaps) – also see Fig. 3, part 4.
Phialemonium pulveris Crous & Decock, sp. nov. MycoBank MB835098. Fig. 46.
Fig. 46.
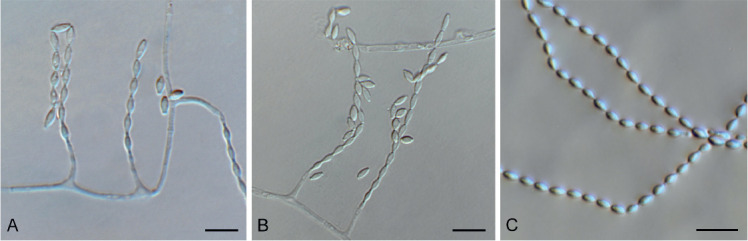
Phialemonium pulveris (CPC 37955). A, B. Conidiophores giving rise to conidia arranged in chains. C. Conidial chains. Scale bars = 10 μm.
Etymology: Name refers to the fact that this species was isolated from dust = pulveris.
Mycelium consisting of hyaline, smooth, branched, septate, 1.5–2 μm diam hyphae. Conidiophores reduced to conidiogenous cells arranged solitary on superficial hyphae, hyaline, smooth, subcylindrical with apical taper, having a truncate phialidic apex, 7–15 × 1.5–2.5 μm. Conidia occurring in long, unbranched chains that remain attached after secession, aseptate, hyaline, smooth, ellipsoid with truncate hila, 0.5 μm diam, (3.5−)4(−5) × (1.5−)2(−2.5) μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 25 mm diam after 2 wk at 25 °C. On MEA surface rosy buff with isabelline pigment in agar, and reverse dark brick; on PDA surface hazel, and reverse isabelline; on OA surface isabelline with diffuse isabelline pigment in agar.
Typus: France, Rouen, house, from bore dust of deathwatch beetle (Xestobium rufovillosum) infesting floor (Quercus wood), May 2019, C. Decock (holotype CBS H-24250, culture ex-type CPC 37955 = MUCL 57769 = CBS 146072).
Notes: Phialemonium pulveris is related to Phialemonium atrogriseum (phialides 7–11 × 1.5–2 μm, conidia ellipsoidal or obovoid, 3–5 × 2–3 μm; Perdomo et al. 2013), but is phylogenetically distinct.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Phialemonium atrogriseum (strain NWHC 24728_17 3SD, GenBank MK793843.1; Identities = 497/531 (94 %), 8 gaps (1 %)), Phialophora intermedia (strain IFM 53358, GenBank AB190399.1; Identities = 518/556 (93 %), 6 gaps (1 %)), and Phialemonium inflatum (strain MUT 5272, GenBank KT715721.1; Identities = 420/466 (90 %), 6 gaps (1 %)). Closest hits using the LSU sequence are Phialemonium atrogriseum (strain CBS 604.67, GenBank MH870783.1; Identities = 818/834 (98 %), no gaps), Phialophora intermedia (strain IFM 53358, GenBank LT633908.1; Identities = 799/815 (98 %), no gaps), and Phialemonium limoniforme (strain CBS 139049, GenBank MH877677.1; Identities = 710/733 (97 %), no gaps) – also see Fig. 6, part 3. Closest hits using the actA sequence had highest similarity to Volutella rosea (strain CBS 128258, GenBank KM231162.1; Identities = 372/410 (91 %), no gaps), Microdochium rhopalostylidis (strain CBS 145125, GenBank MK442636.1; Identities = 373/413 (90 %), no gaps), and Gliocephalotrichum longibrachium (strain CBS 126571, GenBank KM231117.1; Identities = 371/410 (90 %), no gaps). Closest hits using the tub2 sequence had highest similarity to Phialemonium atrogriseum (strain CBS 604.67, GenBank LT633955.1; Identities = 412/469 (88 %), 11 gaps (2 %)), Phialophora intermedia (strain IFM 53358, GenBank LT633960.1; Identities = 404/462 (87 %), 11 gaps (2 %)), and Phialemonium obovatum (strain CBS 279.76, GenBank LT634002.1; Identities = 394/472 (83 %), 20 gaps (4 %)).
Plenodomus visci (Sacc.) Gruyter, Aveskamp & Verkley, comb. nov. MycoBank MB835099. Fig. 47.
Fig. 47.
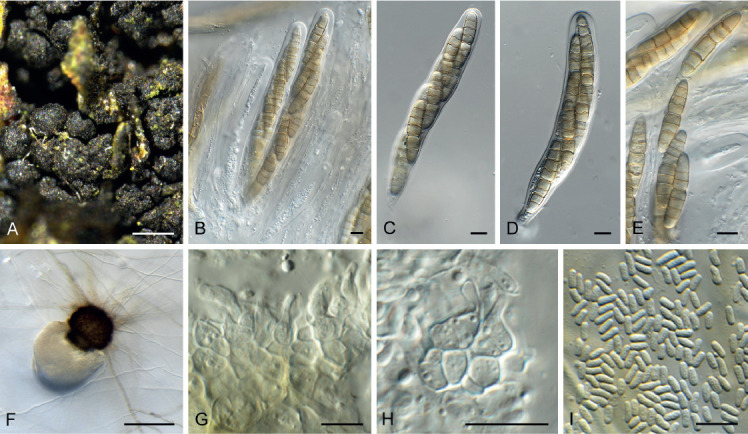
Plenodomus visci (CPC 35314). A. Ascomata. B–D. Asci. E. Ascospores. F. Conidioma on SNA. G, H. Conidiogenous cells. I. Conidia. Scale bars: A = 300 μm, F = 150 μm, all others = 10 μm.
Basionym: Phoma visci Sacc., Michelia 1(no. 2): 125. 1878.
Synonyms: Phyllosticta visci (Sacc.) Allesch., Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 1(6): 96. 1898.
Plectophomella visci (Sacc.) Moesz, Magyar Bot. Lapok 21: 13. 1922.
Plenodomus visci (Moesz) Gruyter et al., Stud. Mycol. 75: 22. 2012 (2013). Nom. inval., Art. 41.5
? Apocytospora visci Höhn., Mitteilungen aus dem Botanischen Institut der Technischen Hochschule in Wien 1(3): 43. 1924.
Pleurostromella visci Petr., Ann. Mycol. 23(1–2): 61 (1925).
Gibberidea visci Fuckel, Jb. nassau. Ver. Naturk. 23–24: 168. 1870, Nom. illeg., Art. 53.1 based on Fr. (1849) and Kuntze (1898).
Ascomata pseudothecial, saprobic, subcorticolous, later erumpent, singly, later densely crowded, globose, ostiole central, short papillate, black, thick, smooth, about 350 μm diam. Asci 8-spored, cylindrical to subclavate, apically blunt rounded with a small ocular chamber, bitunicate, inamyloid, pedicel short and furcate, 110–140 × 13–17 μm, spores biseriate. Pseudoparaphyses numerous, filiform, branched, with anastomoses, short-celled, hyaline, thin-walled, smooth, eguttulate, up to 3 μm broad. Ascospores (5−)7-septate, dothiora-like, cylindrical to slightly clavate, upper part somewhat broader, lower part slightly tapered, end cells conical to rounded, olive, thin-walled, smooth, eguttulate to a few small guttules per cell, septa +/- thick-walled, smooth and dark brown, prominently constricted in the middle, without longitudinal septa, no mucilaginous sheath or appendages, (28−)32–36(−38) × (6−) 7–8 μm. Conidiomata developing in culture on SNA; pycnidial, globose, dark brown, 100–150 μm diam, somewhat papillate, with a central darker ostiolar area. Conidia bacilliform, hyaline, guttulate, apex obtuse, base truncate, 4–6 × 2 μm.
Culture characteristics: Colonies reaching 50 mm diam after 2 wk at 25 °C, with moderate, fluffy aerial mycelium and smooth, lobate margins. On MEA surface umber with patches of cinnamon and buff, reverse sepia; on PDA surface fuscous black, reverse dark mouse grey; on OA surface olivaceous with patches of dirty white, and diffuse yellow pigment.
Material examined: France, Chassagne-Saint-Denis, Rocher de Cul Blanc, on an attached, corticated and dead twig of Viscum album (Santalaceae), 14 Mar. 2018, G. Moyne & R.K. Schumacher RKS143 = HPC 2281, cultures CPC 35314 = CBS 145000, CPC 35315, 35316.
Notes: The combination Plenodomus visci is reintroduced here, as de Gruyter et al. (2013) proposed it based on the incorrect basionym. The collection of Gibberidea visci (nom. illeg.), which is the type species of Gibberidea (nom. illeg.), appears to be a fresh collection of Plenodomus visci. This is the first record of a sexual morph for this taxon.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 35314 had highest similarity to Plenodomus visci (strain CBS 122783, GenBank NR_119957.1; Identities = 532/532 (100 %), no gaps), Plenodomus sinensis (strain KUMCC 18-0152, GenBank MK387923.1; Identities = 525/579 (91 %), 12 gaps (2 %)), and Plenodomus hendersoniae (strain CBS 113702, GenBank MH862939.1; Identities = 525/579 (91 %), 13 gaps (2 %)). The ITS sequence of CPC 35314 is identical to that of CPC 35316 (775/775 bases). Closest hits using the LSU sequence of CPC 35314 are Plenodomus visci (strain CBS 122783, GenBank EU754195.1; Identities = 775/775 (100 %), no gaps), Sphaerellopsis isthmospora (strain HKAS 102225A, GenBank MK387963.1; Identities = 767/775 (99 %), 1 gap (0 %)), and Plenodomus agnitus (strain CBS 126584, GenBank MH875626.1; Identities = 766/775 (99 %), 1 gap (0 %)). The LSU sequence of CPC 35314 is identical to those of CPC 35315 (572/572 bases) and 35316 (570/570 bases). Closest hits using the rpb2 sequence of CPC 35314 had highest similarity to Plenodomus visci (strain CBS 122783, GenBank KY064050.1; Identities = 702/702 (100 %), no gaps), Plenodomus collinsoniae (strain CBS 120227, GenBank KY064039.1; Identities = 643/702 (92 %), no gaps), and Plenodomus influorescens (strain CBS 143.84, GenBank KY064045.1; Identities = 624/700 (89 %), no gaps). Closest hits using the tef1 of CPC 35314 sequence had highest similarity to Plenodomus hendersoniae (strain LTO, GenBank MF795878.1; Identities = 322/403 (80 %), 42 gaps (10 %)), and Septoriella germanica (strain CBS 145372, GenBank MK540159.1; Identities = 217/251 (86 %), 8 gaps (3 %)). The tef1 sequence of CPC 35314 is identical to that of 35316 (441/441 bases).
Polyscytalum pinicola Crous, sp. nov. MycoBank MB835100. Fig. 48.
Fig. 48.
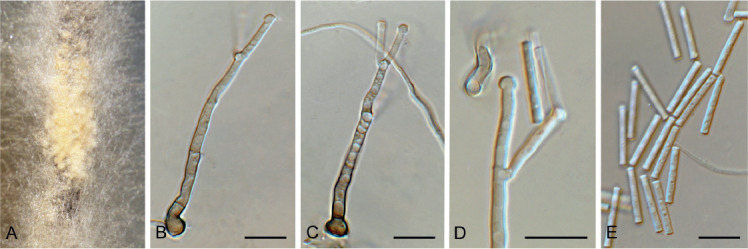
Polyscytalum pinicola (CPC 36759). A. Colony on PDA. B–D. Conidiophores with conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Pinus from which the species was isolated.
Conidiophores solitary, erect (aggregating to form a pale brown cushion of conidia on pine needle), brown, smooth, subcylindrical, branched or not, 2–4-septate, straight to flexuous, base swollen, 4–6 μm diam, stipe 40–80 × 2–3 μm. Conidiogenous cells terminal and intercalary, subcylindrical, pale brown, smooth, 10–15 × 2 μm, apex swollen with several flat-tipped scars, 1 μm diam, proliferating sympodially. Conidia hyaline, smooth, guttulate, (0−)1-septate, occurring in long unbranched chains, apical conidium with obtuse apex, all others with truncate ends, (13−)14–15(−16) × 2 μm; hila somewhat refractive.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 25 mm diam after 2 wk at 25 °C. On MEA surface isabelline, reverse sepia; on PDA surface and reverse isabelline; on OA surface isabelline with diffuse isabelline pigment in agar.
Typus: Malaysia, on needles of Pinus tecunumanii (Pinaceae), 1 Oct. 2018, M.J. Wingfield, HPC 2657 (holotype CBS H-24176, culture ex-type CPC 36759 = CBS 146015).
Notes: Polyscytalum pinicola should be compared to Polyscytalum pini (on Pinus sylvestris, UK; conidia (0−)1(−2)-septate, 7–12(−14) × 1.5–2(−2.5) μm, conidiophores 50–110(−140) μm; Kirk 1983) (erroneously later regarded as a species of Pseudopenidiella). The two species can be distinguished by the slightly longer, thinner conidia and shorter conidiophores in P. pinicola. Polyscytalum pini is currently not known from DNA sequence data.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Polyscytalum neofecundissimum (strain CBS 143390, GenBank NR_158959.1; Identities = 529/579 (91 %), 5 gaps (0 %)), Polyscytalum fecundissimum (strain CBS 100506, GenBank EU035441.1; Identities = 525/581 (90 %), 5 gaps (0 %)), and Polyscytalum eucalyptorum (strain CBS 137967, GenBank NR_132904.1; Identities = 362/386 (94 %), 2 gaps (0 %)). Closest hits using the LSU sequence are Polyscytalum chilense (strain CBS 143387, GenBank MH107954.1; Identities = 819/826 (99 %), 1 gap (0 %)), Subulispora rectilineata (strain CBS 568.71, GenBank MH872029.1; Identities = 819/827 (99 %), 2 gaps (0 %)), and Anungitea nullica (strain CBS 143406, GenBank NG_057150.1; Identities = 814/823 (99 %), 1 gap (0 %)) – also see Fig. 6, part 2a.
Pruniphilomyces Crous & Bulgakov, gen. nov. MycoBank MB835101.
Etymology: Name reflects the fact that the fungus occurs on Prunus.
Plant pathogenic. Ascomata pseudothecial, densely aggregated, amphigenous, immersed to erumpent, globose, black. Asci aparaphysate, stipitate, clavate-cylindrical, with eight biseriate, slightly curved, hyaline, unequally bicellular ascospores. Caespituli hypophyllous, fasciculate, arising from an erumpent, brown pseudoparenchymatal stroma. Conidiophores subcylindrical, medium brown, thick-walled, finely verruculose, unbranched, straight to geniculate-sinuous, septate. Conidiogenous cells integrated, terminal and intercalary, subcylindrical with obtuse ends, brown, finely verruculose; scars, thickened, darkened, with visible pore. Conidia solitary, brown, thin-walled, guttulate, finely verruculose, gently curved or straight, obclavate, apex subobtuse, base obconically truncate, euseptate; hilum thickened and darkened.
Type species: Pruniphilomyces circumscissus (Sacc.) Crous & Bulgakov
Pruniphilomyces circumscissus (Sacc.) Crous & Bulgakov, comb. nov. MycoBank MB835102. Fig. 49.
Fig. 49.

Pruniphilomyces circumscissus (CPC 36434). A. Leaf spots on Prunus cerasus. B. Sporulation on SNA. C–E. Conidiophores and conidia in culture. F. Leaf spot. G, H. Stroma with conidiophores. I. Conidia. Scale bars = 10 μm.
Basionym: Cercospora circumscissa Sacc., Fungi venet. nov. vel. Crit., Sér. 5: 189. 1878.
Synonyms: Passalora circumscissa (Sacc.) U. Braun [as ‘circumcissa’], Mycotaxon 55: 230. 1995.
Mycosphaerella cerasella Aderh., Ber. dt. bot. Ges. 18: 246. 1900.
Leaf spots amphigenous, medium brown, with raised dark brown border, circular to irregular, 2–7 mm diam. Caespituli hypophyllous, fasciculate, forming an erumpent brown pseudoparenchymatal stroma, up to 60 μm wide, 30 μm high, bursting through stromata. Conidiophores subcylindrical, medium brown, thick-walled, finely verruculose, unbranched, straight to geniculate-sinuous, 1–2-septate, 25–50 × 4–6 μm. Conidiogenous cells integrated, terminal and intercalary, subcylindrical with obtuse ends, brown, finely verruculose, 20–30 × 3–4 μm; with multiple round scars, thickened, darkened, with visible pore, 2 μm diam. Conidia (30−)55–65(−70) × (3.5−)4(−5) μm, solitary, brown, thin-walled, guttulate, finely verruculose, gently curved or straight, obclavate, apex subobtuse, base obconically truncate, (2−)3(−4)-euseptate; hilum thickened and darkened, 2 μm diam. When sporulating in culture, conidiophores tend to be much larger, and also branched, but conidial dimensions are similar, with older conidia undergoing microcyclic conidiation.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 8 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Typus: Russia, Rostov region, Shakhty city district, private orchard, on live leaves of Prunus cerasus (Rosaceae), 7 Oct. 2018, T.S. Bulgakov, HPC 2607 = Myc-52, CBS H-24226, culture CPC 36434 = CBS 145985.
Notes: Passalora circumscissa is a foliar pathogen of Prunus. It clusters apart from the type species of Passalora (P. bacilligera), and is sister to the genus Ragnhildiana (Videira et al. 2017) – see Fig. 3, part 2 and Fig. 8, part 2.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Passalora circumscissa (as Passalora sp., strain CHTA-03, GenBank KT428056.1; Identities = 525/525 (100 %), no gaps), Paracercosporidium microsorum (as Mycosphaerella microsora, strain CBS 100352, GenBank EU167599.1; Identities = 522/537 (97 %), 3 gaps (0 %)), and Passalora arctostaphyli (strain CPC 22067, GenBank NR_155638.1; Identities = 521/536 (97 %), 2 gaps (0 %)). Closest hits using the LSU sequence are Ragnhildiana diffusa (strain CBS 106.14, GenBank MH866148.1; Identities = 861/862 (99 %), no gaps), Ragnhildiana pseudotithoniae (strain CBS 136442, GenBank NG_058049.1; Identities = 861/862 (99 %), no gaps), and Ragnhildiana pseudotithoniae (as Passalora pseudotithoniae, strain CPC 21688, GenBank KF777231.1; Identities = 861/862 (99 %), no gaps) – also see Fig. 3, part 2 and Fig. 8, part 2. Closest hits using the rpb2 sequence had highest similarity to Cercosporidium californicum (strain CPC 18390, GenBank MF951471.1; Identities = 488/580 (84 %), no gaps), Pantospora guazumae (strain CBS 130299, GenBank MF951556.1; Identities = 485/577 (84 %), no gaps), and Cercosporidium miurae (strain CPC 14643, GenBank MF951473.1; Identities = 487/580 (84 %), no gaps). The closest hit in the genus Ragnhildiana was with Ragnhildiana ferruginea (strain CBS 546.71, GenBank MF951645.1; Identities = 479/577 (83 %), no gaps).
Rachicladosporium iridis (Auersw.) Crous, comb. nov. MycoBank MB835103. Fig. 50.
Fig. 50.
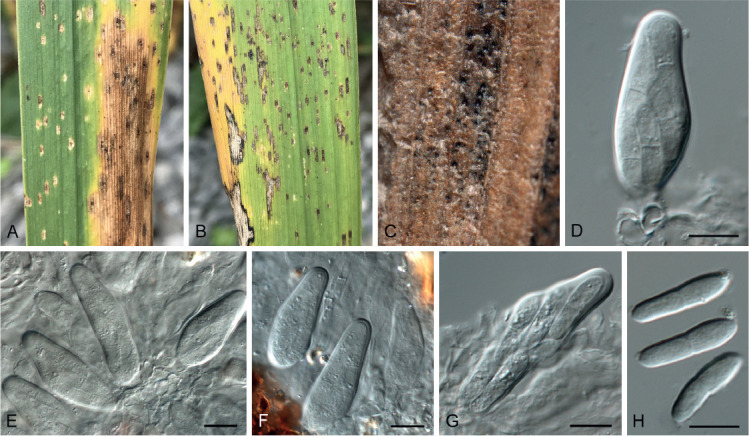
Rachicladosporium iridis (CBS H-4908). A, B. Leaf spots on Iris sp. C. Leaf with immersed ascomata. D–G. Asci. H. Ascospores. Scale bars = 10 μm.
Basionym: Sphaerella iridis Auersw., In Gonnermann & Rabenhorst, Mycol. Europaea 5–6: 18. 1869.
Synonym: Mycosphaerella iridis (Auersw.) J. Schröt., In Cohn, Kryptog.-Fl. Schlesien 3(2): 339. 1894 (1893).
Ascomata occurring on leaves, amphigenous, black, globose, subepidermal to erumpent, up to 90 μm diam, arranged in angular clusters, confined by leaf veins; wall of 2–3 layers of brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, obovoid to broadly ellipsoid, straight to slightly curved, apiculus not prominent, 8-spored, 35–50 × 12–15 μm. Ascospores tri- to multiseriate, hyaline, guttulate, thick-walled, straight to slightly curved, fusoid-ellipsoid with obtuse ends, medianly 1-septate, widest in middle of apical cell, somewhat constricted at the septum, tapering towards both ends, but slightly more to lower end, 16–22 × 4.5–5 μm; ascospores germinating from polar ends, with germ tubes parallel to the long axis.
Typus: Germany, on leaves of Iris pumila (Iridaceae), holotype in B lost, original description Sphaerella iridis Auersw., In Gonnermann & Rabenhorst, Mycol. Europaea 5–6: 18. 1869, tab. 5, fig. 71 designated here as lectotype, MBT391587. Netherlands, Baarn, Eemnes-Buiten, symptomatic leaf of Iris pseudacorus, 10 May 1949, J.A. von Arx, epitype designated here CBS H-4908, MBT391588, culture ex-epitype CBS 282.49.
Additional material examined: Netherlands, Bilthoven, Evert Cornelislaan 11, on leaves of Iris sp., 13 Aug. 2017, P.W. Crous, HPC 2181, cultures CPC 34129, 34130.
Notes: Although the type of Mycosphaerella iridis in B has been lost (ascospores 17 × 4–5 μm, illustrated in original publication as fusoid-ellipsoidal with broadly ellipsoid asci; see Corlett 1991), Aptroot (2006) examined several specimens, and found that this taxon is a species of Davidiella, and that it had Cladosporium iridis as asexual morph. This is incorrect, as C. iridis is linked to Davidiella macrospora (Braun et al. 2003). Although this is a true sexual morph of Rachicladosporium, it is presently not known to have any names in Rachicladosporium, and thus a new combination is proposed.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Rachicladosporium eucalypti (strain CBS 138900, GenBank NR_155718.1; Identities = 434/463 (94 %), 13 gaps (2 %)), Rachicladosporium pini (strain CPC 16770, GenBank JF951145.1; Identities = 431/464 (93 %), 14 gaps (3 %)), and Rachicladosporium luculiae (strain CBS 121620, GenBank NR_160222.1; Identities = 427/465 (92 %), 15 gaps (3 %)). The ITS sequence of CBS 282.49 (GenBank EU167586.1) is identical to the sequences of CPC 10020 (556/556 bp) and 34129 (563/563 bp). Closest hits using the LSU sequence are Rachicladosporium luculiae (strain CPC 11407, GenBank EU040237.1; Identities = 876/891 (98 %), 2 gaps (0 %)), Rachicladosporium corymbiae (strain CBS 145087, GenBank MK047503.1; Identities = 805/820 (98 %), no gaps), and Rachicladosporium luculiae (strain CBS 121620, GenBank MH874675.1; Identities = 837/853 (98 %), 3 gaps (0 %)) – also see Fig. 3, part 1.
Ramularia unterseheri Videira & Crous, Fungal Biology 119(9): 836. 2015.
Description and illustration: Videira et al. (2015).
Material examined: Germany, near Berlin, on fallen leaves of Fagus sylvatica (Fagaceae), 30 Apr. 2018, R.K. Schumacher, RKS 171 = HPC 2380, culture CPC 35584.
Notes: Ramularia unterseheri was described from a sexual morph occurring on leaves of Acer, Alnus and Fagus collected in Germany and the Netherlands (Videira et al. 2015).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Ramularia vizellae (strain IGB-KR56, GenBank MK012421.1; Identities = 497/498 (99 %), no gaps), Ramularia endophylla (strain CBS 124827, GenBank MH863519.1; Identities = 497/498 (99 %), no gaps), and Ramularia unterseheri (strain CBS 124884, GenBank NR_154918.1; Identities = 497/498 (99 %), no gaps). Closest hits using the LSU sequence are Ramularia endophylla (strain CBS 124831, GenBank MH875006.1; Identities = 831/831 (100 %), no gaps), Ramularia vizellae (strain CPC 18283, GenBank JN712567.1; Identities = 831/831 (100 %), no gaps), and Ramularia unterseheri (strain CBS 124827, GenBank KP894153.1; Identities = 813/813 (100 %), no gaps) – also see Fig. 3, part 2 and Fig. 8, part 2. Closest hits using the actA sequence had highest similarity to Ramularia unterseheri (strain CBS 130721, GenBank KP894379.1; Identities = 581/581 (100 %), no gaps), Ramularia vizellae (strain CBS 115980, GenBank KP894390.1; Identities = 567/582 (97 %), 1 gap (0 %)), and Ramularia inaequalis (strain CPC 25741, GenBank KP894335.1; Identities = 547/582 (94 %), 2 gaps (0 %)). Closest hits using the gapdh sequence had highest similarity to Ramularia unterseheri (strain CBS 117879, GenBank KP894585.1; Identities = 520/520 (100 %), no gaps), Ramularia vizellae (strain CBS 185.97, GenBank KP894632.1; Identities = 492/520 (95 %), no gaps), and Ramularia gaultheriae (strain CBS 299.80, GenBank KX288250.1; Identities = 419/451 (93 %), 3 gaps (0 %)). Closest hits using the his3 sequence had highest similarity to Ramularia unterseheri (strain CBS 130721, GenBank KP894821.1; Identities = 381/381 (100 %), no gaps), Ramularia vizellae (strain CPC 25736, GenBank KP894871.1; Identities = 377/381 (99 %), no gaps), and Ramularia haroldporteri (strain CPC 16296, GenBank KJ504593.1; Identities = 362/379 (96 %), no gaps). Closest hits using the rpb2 sequence had highest similarity to Ramularia unterseheri (strain CBS 355.90, GenBank KP894711.1; Identities = 844/859 (98 %), no gaps), Ramularia vizellae (strain CBS 117798, GenBank KP894728.1; Identities = 816/853 (96 %), no gaps), and Ramularia plurivora (strain CPC 11517, GenBank KJ504652.1; Identities = 797/903 (88 %), no gaps) – also see Fig. 8, part 2. Closest hits using the tef1 sequence had highest similarity to Ramularia unterseheri (strain CPC 25740, GenBank KP894492.1; Identities = 379/379 (100 %), no gaps), Ramularia vizellae (strain CPC 25734, GenBank KP894537.1; Identities = 271/290 (93 %), 5 gaps (1 %)), and Ramularia abscondita (strain CBS 114727, GenBank KX287864.1; Identities = 267/302 (88 %), 14 gaps (4 %)).
Roussoella arundinacea Crous & R.K. Schumach., sp. nov. MycoBank MB835104. Fig. 51.
Fig. 51.
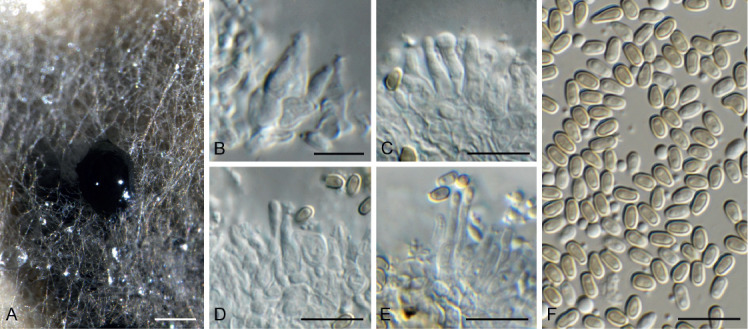
Roussoella arundinacea (CPC 35554). A. Conidioma on PDA. B–E. Conidiogenous cells. F. Conidia. Scale bars: A = 250 μm, all others = 10 μm.
Etymology: Name refers to the host genus Arundo from which the species was isolated.
Conidiomata erumpent, globose, brown, 200–250 μm diam, with central ostiole; wall of 6–8 layers of brown textura angularis. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform, phialidic, 7–10 × 2.5–4 μm. Conidia solitary, aseptate, brown, smooth, guttulate, subcylindrical, apex obtuse, base truncate, (3−)3.5(−4) × 2 μm.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 25 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface smoke grey, and reverse smoke grey to olivaceous grey.
Typus: Spain, Pontevedra, O Grove, on Arundo donax (Poaceae), dead and standing culm, 16 Mar. 2018, M.A. Delgado, RKS 154 = HPC 2314 (holotype CBS H-24153, culture ex-type CPC 35554 = CBS 146088).
Notes: Species of Roussoella are usually saprobic on dead twigs and branches. Roussoella arundinacea is closely related to Roussoella intermedia, a species known only by its sexual morph (Liu et al. 2014).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Roussoella hysterioides (strain CBS 125434, GenBank MH863689.1; Identities = 626/751 (83 %), 67 gaps (8 %)), Thyridaria broussonetiae (strain TB1a, GenBank KX650569.1; Identities = 601/731 (82 %), 44 gaps (6 %)), and Roussoella acaciae (strain CBS 138873, GenBank KP004469.1; Identities = 561/686 (82 %), 39 gaps (5 %)). Closest hits using the LSU sequence are Roussoella mangrovei (voucher MFLU 17-1542, GenBank MH023318.1; Identities = 797/805 (99 %), no gaps), Roussoella mukdahanensis (strain MFLUCC 11-0201, GenBank NG_059671.1; Identities = 866/876 (99 %), no gaps), and Pararoussoella rosarum (voucher MFLU 17-0654, GenBank NG_059872.1; Identities = 860/870 (99 %), no gaps) – also see Fig. 3, part 4. Closest hits using the rpb2 sequence had highest similarity to Thyridaria broussonetiae (strain TB1, GenBank KX650586.1; Identities = 724/866 (84 %), no gaps), Roussoella hysterioides (strain HH 26988, GenBank AB539102.1; Identities = 723/866 (83 %), no gaps), and Roussoella pseudohysterioides (strain HKAS 101758, GenBank MH453483.1; Identities = 731/879 (8 3%), 2 gaps (0 %)). No significant hits were obtained when the tef1 sequence was used in blastn and megablast searches.
Sphaerulina neoaceris Crous & Bulgakov, sp. nov. MycoBank MB835105. Fig. 52.
Fig. 52.
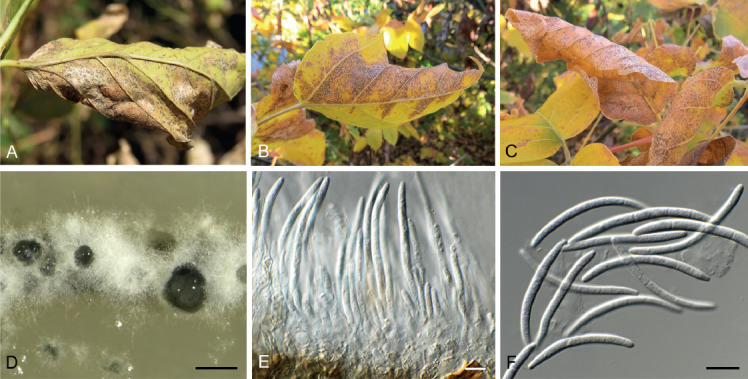
Sphaerulina neoaceris (CPC 36488). A–C. Leaf spots on Acer negundo. D. Conidiomata on OA. E. Conidiogenous cells. F. Conidia. Scale bars: D = 250 μm, all others = 10 μm.
Etymology: Name reflects its morphological similarity to Sphaerulina aceris.
Leaf spots amphigenous, brown, firstly 1–2 mm diam., appearing as small specks on leaf, later merging, up to 5–7 mm diam. Conidiomata acervular, dark brown, up to 250 μm diam, releasing conidia in white mucoid masses; conidiomatal wall mainly consisting of 3–6 layers of brown textura angularis. Conidiogenous cells hyaline, discrete or integrated in 1(−3)-septate conidiophores, subglobose, doliiform to ampulliform, proliferating percurrently or sympodially, 7–15(−20) × 3–4 μm. Conidia cylindrical, straight or slightly curved, attenuated to a broadly rounded apex, and to a truncate base, 3(−6)-septate, conspicuously constricted at the septa hyaline, granular, (35−)40–45(−55) × 3(−4) μm.
Culture characteristics: Colonies erumpent, spreading, with sparse aerial mycelium and smooth, lobate margin, reaching 6 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface dirty white, reverse isabelline in middle, honey in outer region.
Typus: Russia, Rostov region, Ust-Donetsky district, floodplain forest near Atyukhta river, on living and dying leaves of Acer negundo (Sapindaceae), 6 Oct. 2018, T.S. Bulgakov, Myc-35 = HPC 2629 (holotype specimen CBS 145988, preserved as metabolically inactive culture, cultures ex-type CPC 36488 = CBS 145988).
Additional material examined: Russia, Ust-Donetsky district, near Mostovoy village, floodplain forest of Kundryuchya river, on living and dying leaves of A. negundo, 6 Oct. 2018, T.S. Bulgakov, HPC 2603 = Myc-48, culture CPC 36481 = CBS 145987.
Notes: Sphaerulina aceris has been reported on leaves of several Acer spp. (A. campestre, A. circinatum, A. hyrcanum and A. pseudoplatanus) in Europe and the USA (Verkley et al. 2013). Sphaerulina neoaceris occurs on Acer negundo in Russia. It causes similar leaf spots, and has conidia that resemble those of S. aceris [1–3-sepate, (32−)37–47(−50) × 3–4 μm; Verkley et al. 2013], but can be distinguished by having more conidial septa than generally seen in S. aceris. The two species are phylogenetically distinct (Fig. 3, part 2 and Fig. 8, part 1).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 36481 had highest similarity to Sphaerulina aceris (strain CBS 183.97, GenBank KF251593.1; Identities = 474/494 (96 %), 3 gaps (0 %)), Mycosphaerella latebrosa (strain CBS 183.97, GenBank AF362051.1; Identities = 479/502 (95 %), 4 gaps (0 %)), and Neopseudocercosporella brassicae (strain CBS 173.88, GenBank KX287293.1; Identities = 511/539 (95 %), 4 gaps (0 %)). The ITS sequences of CPC 36481 and 36488 differ with a single substitution (533/534 bases). Closest hits using the LSU sequence of CPC 36481 are Mycosphaerella latebrosa (strain CBS 687.94, GenBank GU214444.1; Identities = 824/838 (98 %), no gaps), Sphaerulina aceris (strain CBS 652.85, GenBank GQ852673.1; Identities = 824/838 (98 %), no gaps), and Neopseudocercosporella capsellae (strain CPC 12518, GenBank KX286994.1; Identities = 813/827 (98 %), no gaps) – also see Fig. 3, part 2. The LSU sequences of CPC 36481 and 36488 differ with a single substitution (837/838 bases). Closest hits using the actA sequence of CPC 36481 had highest similarity to Pseudophaeophleospora phormii (strain CBS 144606, GenBank MK442643.1; Identities = 400/428 (93 %), no gaps), Sphaerulina quercicola (strain CBS 663.94, GenBank JQ325032.1; Identities = 467/526 (89 %), 12 gaps (2 %)), and Ramularia eucalypti (strain CPC 13044, GenBank KJ504458.1; Identities = 457/513 (89 %), 6 gaps (1 %)). The actA sequences of CPC 36481 and 36488 are identical (598/598 bases). Closest hits using the his3 sequence of CPC 36481 had highest similarity to Paramycosphaerella marksii (as Mycosphaerella marksii; strain CPC 11795, GenBank JX500169.1; Identities = 336/369 (91 %), 5 gaps (1 %)), Mycosphaerella musae (strain CBS 121386, GenBank EU514376.1; Identities = 336/369 (91 %), 5 gaps (1 %)), and Ramularia osterici (strain CPC 10750, GenBank KX288931.1; Identities = 339/374 (91 %), 10 gaps (2 %)). Closest hits using the rpb2 sequence of CPC 36481 had highest similarity to Sphaerulina chaenomelis (strain CBS 132131, GenBank MF951677.1; Identities = 744/898 (83 %), 2 gaps (0 %)), Sphaerulina gei (strain CBS 128632, GenBank KX348095.1; Identities = 712/862 (83 %), 2 gaps (0 %)), and Sphaerulina koreana (strain CBS 131898, GenBank KX348096.1; Identities = 711/862 (82 %), 2 gaps (0 %)). The rpb2 sequences of CPC 36481 and 36488 are identical (912/912 bases). No significant hits were obtained when the tef1 sequence of CPC 36481 was used in blastn and megablast searches. The tef1 sequences of CPC 36481 and 36488 are identical (568/568 bases).
Sphaerulina salicicola Crous & Bulgakov, sp. nov. MycoBank MB835106. Fig. 53.
Fig. 53.
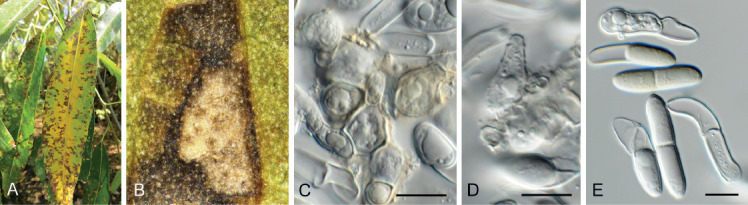
Sphaerulina salicicola (CPC 36561). A. Leaf spots on Salix fragilis. B. Leaf spot. C, D. Conidiogenous cells. E. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Salix from which the species was isolated.
Leaf spots amphigenous 1–5 mm diam, angular, merging, firstly medium brown, later become pale to almost white with raised, dark brown border. Conidiomata immersed, chiefly epiphyllous, globose, 150–200 μm diam, with central ostiole. Conidiophores reduced to conidiogenous cells lining the inner cavity, hyaline, smooth, ampulliform to doliiform, 7–12 × 5–8 μm, proliferating percurrently at apex. Conidia solitary, hyaline, smooth, granular to guttulate, subcylindrical, straight to slightly curved, medianly 1(−3)-septate, apex subobtuse, base truncate, (23−)27–40(−50) × (4−)5–6(−7) μm.
Culture characteristics: Colonies erumpent, slow growing, with sparse aerial mycelium and smooth, lobate margin, reaching 12 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface grey olivaceous, reverse iron-grey.
Typus: Russia, Rostov region, Shakhty city district, willow trees near Atyukhta river, on living leaves of Salix fragilis (Salicaceae), 21 Sep. 2018, T.S. Bulgakov, Myc-34 = HPC 2628 (holotype CBS H-24169, culture ex-type CPC 36561 = CBS 146032).
Additional materials examined: Russia, Rostov region, Shakhty city district, street trees, on live leaves of Salix fragilis, 21 Sep. 2018, T.S. Bulgakov, HPC 2634 = Myc-12, culture CPC 36569; Rosstov region, Shakhty, Atukhta, on Salix fragilis, 21 Sep. 2018, T.S. Bulgakov, HPC 2641 = Myc-11 (CBS H-24167, culture CPC 36494 = CBS 146034).
Notes: Sphaerulina salicicola is morphologically and phylogenetically distinct from S. salicina (USA, on Salix longifolia, conidia 1-septate, 15–18 × 5.5–7.5 μm; Sydow & Sydow 1913). The ITS sequence of S. salicinomyces (CPC 36561) is 91 % similar (472/520 bases, including 23 gaps) to that of S. salicina (CPC 36481, present study). The occurrence of Sphaerulina salicicola on Phytolacca americana is unclear, and will require further collections to resolve.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence of CPC 36561 had highest similarity to Sphaerulina menispermi (strain CBS 128666, GenBank MH865112.1; Identities = 486/521 (93 %), 22 gaps (4 %)), Sphaerulina cercidis (strain CBS 128634, GenBank MH865061.1; Identities = 485/520 (93 %), 21 gaps (4 %)), and Sphaerulina rhododendricola (strain CBS 136435, GenBank NR_137839.1; Identities = 485/520 (93 %), 21 gaps (4 %)). The ITS sequence of CPC 36561 differs with a single substitution from CPC 36770 (498/499 bases) and two substitutions (497/499 bases) from CPC 36494 and 36569. Closest hits using the LSU sequence of CPC 36561 are Sphaerulina gei (strain CBS 128632, GenBank MH876498.1; Identities = 881/888 (99 %), no gaps), Sphaerulina rhabdoclinis (strain CBS 102195, GenBank MH874375.1; Identities = 875/882 (99 %), no gaps), and Mycosphaerella latebrosa (strain CBS 687.94, GenBank GU214444.1; Identities = 882/890 (99 %), no gaps) – also see Fig. 3, part 2 and Fig. 8, part 1. The LSU sequence of CPC 36561 is identical to that of CPC 36569 (868/868 bases) differs with a single substitution from CPC 36494 (840/841 bases). Closest hits using the actA sequence of CPC 36561 had highest similarity to Sphaerulina azaleae (strain KACC44227, GenBank MK584565.1; Identities = 531/573 (93 %), 2 gaps (0 %)), Sphaerulina cercidis (strain CBS 118910, GenBank KF903507.1; Identities = 485/522 (93 %), 2 gaps (0 %)), and Sphaerulina quercicola (strain CBS 663.94, GenBank JQ325032.1; Identities = 515/582 (88 %), 12 gaps (2 %)). The actA sequence of CPC 36561 is identical to those of CPC 36569 (575/575 bases) and CPC 36494 (574/574 bases). Closest hits using the rpb2 sequence of CPC 36561 had highest similarity to Sphaerulina betulae (strain CBS 128597, GenBank KX348094.1; Identities = 699/804 (87 %), no gaps), Sphaerulina chaenomelis (strain CBS 132131, GenBank MF951677; Identities = 728/857 (85 %), no gaps), and “Mycosphaerella” harthensis (strain CBS 325.52, GenBank MF951675.1; Identities = 649/741 (88 %), no gaps). The rpb2 sequence of CPC 36561 is identical to that of CPC 36569 (860/860 bases) and CPC 36494 (858/858 bases). No significant hits were obtained when the tef1 sequence was used in blastn and megablast searches. The tef1 sequence of CPC 36561 is identical (421/421 bases) to those of CPC 36569 and 36494.
Sporidesmiella hyalosperma (Corda) P.M. Kirk, Trans. Br. mycol. Soc. 79: 481. 1982. Fig. 54.
Fig. 54.
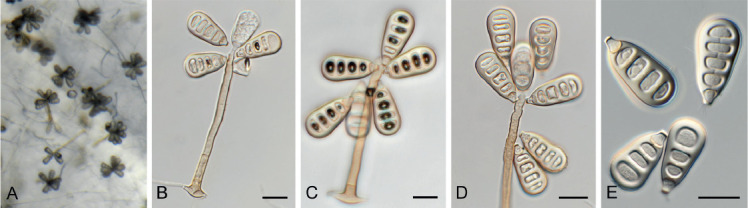
Sporidesmiella hyalosperma (CPC 37552). A. Sporulation on SNA. B–D. Conidiophores giving rise to conidia. E. Conidia. Scale bars = 10 μm.
Basionym: Helminthosporium hyalospermum Corda, Icon. fung. (Prague) 1: 13. 1837.
Mycelium consisting of hyaline, smooth, branched, septate, 1.5–2 μm diam hyphae. Conidiophores solitary, erect, brown, straight, arising from superficial hyphae, brown, thick-walled, smooth, 1–3-septate, unbranched, 60–90 × 3–4 μm; base swollen, 4–7 μm diam. Conidiogenous cells integrated, terminal, subcylindrical, brown, smooth, proliferating percurrently, 20–40 × 3–4 μm. Conidia brown, smooth, thick-walled, 4-distoseptate, septa with central pore, clavate to obovoid, basal cell darker brown, with dark brown truncate hilum, 2–2.5 μm diam, (19−) 20–22(−24) × (9−)10(−11) μm.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 10 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse isabelline, with diffuse scarlet pigment in agar.
Material examined: Spain, Pontevedra, on dead root of Zea mays (Poaceae), 18 Feb. 2019, M. Deldgado, RKS 205 = HPC 2785 = CBS H-24200, culture CPC 37552 = CBS 146016.
Notes: Sporidesmiella hyalosperma is common saprobic fungus in Europe on many substrates (conidia (17−)22–25(−29) × 9–12(−13) μm, 4-septate; Kirk 1982). Sporidesmiella novae-zelandiae is morphologically similar, but has wider conidia (20.5–28 × 11.5–15 μm; Hernández-Restrepo et al. 2018).
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Sporidesmiella hyalosperma (strain MFLUCC 18-1312, GenBank MK828688.1; Identities = 509/526 (97 %), 5 gaps (0 %)), Sporidesmiella novae-zelandiae (strain S-1256, GenBank MK828693.1; Identities = 478/536 (89 %), 26 gaps (4 %)), and Sporidesmiella aquatica (as Sporidesmiella sp. ZLL-2019a, strain S-777, GenBank MK828692.1; Identities = 443/510 (87 %), 28 gaps (5 %)). Closest hits using the LSU sequence are Sporidesmiella hyalosperma (strain S-1518, GenBank MK849842.1; Identities = 803/808 (99 %), 1 gap (0 %)), Sporidesmiella aquatica (as Sporidesmiella sp. ZLL-2019a, strain S-777, GenBank MK849843.1; Identities = 748/768 (97 %), no gaps), and Sporidesmiella novae-zelandiae (strain S-1256, GenBank MK849845.1; Identities = 783/808 (97 %), 2 gaps (0 %)) – also see Fig. 6, part 2b. Closest hits using the rpb2 sequence had highest similarity to Sporidesmiella hyalosperma (strain HKAS 92987, GenBank MN124522.1; Identities = 583/606 (96 %), no gaps), Sporidesmiella novae-zelandiae (voucher MFLU 18-1604, GenBank MN124526.1; Identities = 692/789 (88 %), no gaps), and Sporidesmiella aquatica (voucher MFLU 18-2331, GenBank MN124524.1; Identities = 638/735 (87 %), 1 gap (0 %)).
Sporocadus trimorphus F. Liu et al., Stud. Mycol. 92: 406. 2018 (2019). Fig. 55.
Fig. 55.
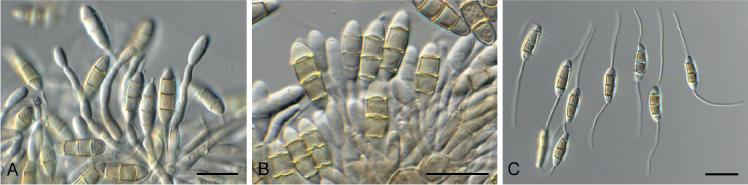
Sporocadus trimorphus (CPC 35675). A, B. Conidiogenous cells giving rise to conidia. C. Conidia. Scale bars = 10 μm.
Conidiomata acervular, scattered on agar surface, 150–350 μm diam, oval, dark brown. Conidiophores arising from upper cells of basal stroma, conidiophores pale brown and roughened, becoming hyaline and smooth above, branched, septate, 30–50 × 2.5–3.5 μm. Conidiogenous cells terminal and intercalary, subcylindrical, 10–16 × 2.5–3 μm, proliferating percurrently at apex. Conidia fusoid to ellipsoid, 3-septate, walls thin, smooth, at times collapsed, (13−)14–15(−18) × 4(−4.5) μm, mostly with appendages; basal cell obconic with truncate base, pale brown, 2.5–3.5 μm long; two median cells doliiform to subcylindrical, medium brown with dark septa, (8−)8.5–9(−10) μm; apical cell with acute apex, pale brown, 3–4 μm long; appendages single, unbranched, filiform, flexuous, (13−)23–27(−35) μm long; basal appendage excentric, apical appendage central.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and feathery margin, reaching 40 mm diam after 2 wk at 25 °C. On MEA surface and reverse umber; on PDA surface and reverse isabelline; on OA surface dark mouse grey.
Material examined: Germany, Witten, on stems of Rosa canina (Rosaceae), 4 May 2018, Th. Hülsewig, HPC 2441, culture CPC 35675 = CBS 145550.
Notes: Sporocadus trimorphus was recently described from Rosa canina collected in Sweden (Liu et al. 2019), while the collection in this study was made from the same host in Germany.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Sporocadus trimorphus (strain UPSC 2430, GenBank NR_161079.1; Identities = 513/514 (99 %), 1 gap (0 %)), Seimatosporium pseudorosarum (strain MFLUCC 14-0466, GenBank KT284775.1; Identities = 583/587 (99 %), no gaps), and Discostroma fuscellum (strain GSAA-0182, GenBank JF320818.1; Identities = 559/563 (99 %), 1 gap (0 %)). Closest hits using the LSU sequence are Sporocadus trimorphus (strain UPSC 2430, GenBank NG_066212.1; Identities = 797/797 (100 %), no gaps)), Seimatosporium lichenicola (strain CBS 159.25, GenBank MH866329.1; Identities = 802/806 (99 %), no gaps), and Sporocadus rosigena (strain CBS 116498, GenBank MH554200.1; Identities = 793/797 (99 %), no gaps) – also see Fig. 6, part 2a. Closest hits using the rpb2 sequence had highest similarity to Sporocadus trimorphus (strain CBS 114203, GenBank MH554876.1; Identities = 832/832 (100 %), no gaps), Sporocadus rosarum (strain CBS 113832, GenBank MH554864.1; Identities = 796/829 (96 %), no gaps), and Sporocadus mali (strain CBS 446.70, GenBank MH554960.1; Identities = 702/752 (93 %), no gaps). Closest hits using the tub2 sequence had highest similarity to Sporocadus trimorphus (strain CBS 114203, GenBank MH554636.1; Identities = 669/677 (99 %), 1 gap (0 %)), Sporocadus rosarum (strain CBS 113832, GenBank MH554629.1; Identities = 628/679 (92 %), 6 gaps (0 %)), and Sporocadus incanus (strain CBS 123003, GenBank MH554659.1; Identities = 612/686 (89 %), 12 gaps (1 %)).
Teratosphaeria agapanthi (Kalchbr. & Cooke) Crous, IMA Fungus 2: 61. 2011. Fig. 56.
Fig. 56.
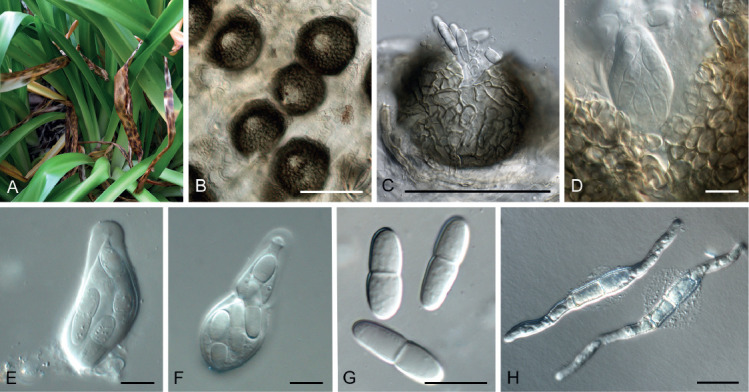
Teratosphaeria agapanthi (CPC 18304). A. Disease symptoms. B, C. Ascomata on leaf tissue. D–F. Asci. G. Ascospores. H. Germinating ascospores. Scale bars: B, C = 150 μm, all others = 10 μm.
Basionym: Sphaerella agapanthi Kalchbr. & Cooke, Grevillea 9(49): 31. 1880.
Synonym: Mycosphaerella agapanthi (Kalchbr. & Cooke) Lindau, in Engl. & Prantl, Natürlichen Pflanzenf. 1(1): 426. 1897.
Leaf spots amphigenous, ellipsoid, large, developing where plants are grown in wet, shady areas, pale to medium brown, with a red-brown border, coalescing, becoming visible as leaf tip blight symptoms. Ascomata amphigenous, black, substomatal, erumpent, predominantly arranged in tight, round clusters, 2–6 mm diam; ascomata 80–150 μm diam, with central, periphysate ostiole, 10–15 μm diam; periphyses 0–1-septate, 10–15 × 1.5–2 μm; ascomatal wall of 3–4 layers of brown textura angularis. Asci aparaphysate, fasciculate, bitunicate, subsessile, fusoid-ellipsoid, straight to slightly curved, 8-spored, 25–45 × 15–25 μm, with visible apical apiculus. Ascospores multi-seriate, hyaline, thick-walled, with angular cellular inclusions, fusoid-ellipsoid, medianly 1-septate, becoming constricted at the septum, widest in middle of apical cell, with rounded ends, granular, (17−)18–20(−21) × 4.5–5(−6) μm; ascospore germination irregular, from both ends or middle of the cell, parallel or at angle to the long axis; spore becoming brown, verruculose, 6–7 μm wide, but not swelling and distorting after 24 h on MEA.
Culture characteristics: Colonies after 14 d at 24 °C spreading, with sparse aerial mycelium and even, smooth, lobate margins, reaching 30 mm diam. On MEA surface dark mouse grey, reverse greenish black. On OA surface olivaceous-grey. On PDA surface olivaceous-grey, reverse iron-grey.
Typus: South Africa, Western Cape Province, Kirstenbosch Botanical Garden, at entrance to lower gate, on leaf spots of Agapanthus africanus (= A. umbellatus) (Amaryllidaceae), 8 May 2010, P.W. Crous, epitype CBS H-20552, cultures ex-epitype CPC 18304–18305 = CBS 129192.
Material examined: Australia, Melbourne, on leaves of Agapanthus sp., 2 Nov. 2014, P.W. Crous, cultures CPC 25550, 25551; Western Australia, Middleton beach, Albany, on leaves of Agapanthus sp., 19 Sept. 2015, P.W. Crous, cultures CPC 29126, 29127; Western Australia, Porongurup, Karribank Country Retreat, on leaves of Agapanthus sp., 22 Sept. 2015, P.W. Crous, culture CPC 28986; New South Wales, Sydney, Meriton Hotel, on leaves of Agapanthus sp., 25 Nov. 2016, P.W. Crous, HPC 1726, CPC 32077, cultures CPC 32107, CPC 32200. France, Nice, on leaves of Agapanthus sp., 29 Jul. 2013, P.W. Crous, cultures CPC 23526, 23527. La Réunion (France), on leaves of Agapanthus sp., 8 Mar. 2015, P.W. Crous, cultures CPC 26305, 26306. Portugal, Braga, Ria do Souto, on dead leaf of A. umbellatus, 8 Jun. 2010, P.W. Crous, CBS H-20553, cultures CPC 18332 = CBS 129064, 18333. South Africa, KwaZulu-Natal, Hilton, on leaves of Agapanthus sp., 19 Jul. 2011, P.W. Crous, cultures CPC 19665, 19666; KwaZulu-Natal, Dundee, on leaves of Agapanthus sp., 17 Jul. 2011, P.W. Crous, cultures CPC 19769; Mpumalanga, Nelspruit, Lowveld Botanical Garden, on leaves of Agapanthus praecox, 16 Jul. 2011, P.W. Crous, cultures CPC 19673, 19674; Mpumalanga, Pilgrim’s Rest, on leaves of Agapanthus sp., 15 Jul. 2011, P.W. Crous, cultures CPC 19774, 19775, 19828, 19829; Western Cape Province, Robben Island, on leaves of Agapanthus sp., 24 May 2015, P.W. Crous, cultures CPC 27350, 27351; Western Cape Province, entrance to Kirstenbosch Botanical Garden, on leaves of Agapanthus sp., 8 May 2010, P.W. Crous, culture CPC 18266; KwaZulu-Natal Province, Hilton, on leaves of Agapanthus sp., 19 July 2011, P.W. Crous, CPC 19689; Western Cape Province, Knysna Pledge Nature Reserve, on leaves of Agapanthus sp., 28 July 2011, P.W. Crous, culture CPC 19750. USA, California, Walnut Creek, Marriot Hotel, on leaves of Agapanthus sp., 18 Mar. 2012, P.W. Crous, cultures CPC 20396, 20397; California, Walnut Creek, Ruth Bancroft Botanical Garden, on leaves of Agapanthus sp., 20 Mar. 2012, P.W. Crous, cultures CPC 20402, 20403.
Notes: Based on the phylogenies generated here (Fig. 3, part 2, and Fig. 57), the fungus associated with Teratosphaeria leaf blight of Agapanthus appears to be a single, well-defined species, T. agapanthi. All isolates clustered with the ex-epitype strain of T. agapanthi (CBS 129192) in a well-defined clade. Under moist, humid conditions, the terminal ends of infected leaves become water-soaked and brown, with the circular arrangement or clusters of black pseudothecia that become visible to the naked eye on the dead leaf parts, a diagnostic character. In dry areas, the symptomatic leaves are restricted to the lower parts of the plants.
Fig. 57.
The first of seven equally most parsimonious trees obtained from a phylogenetic analysis of the Teratosphaeria agapanthi ITS alignment (27 strains including the outgroup; 489 characters including alignment gaps analysed: 378 constant, 68 variable and parsimony-uninformative and 43 parsimony-informative). The tree was rooted to Ramularia endophylla (strain CBS 113265, GenBank AY490763.1) and the scale bar indicates the number of changes. Parsimony bootstrap (PBS) and neighbour joining bootstrap support (NJBS) values higher than 74 % are shown at the nodes (PBS/NJBS) and the treated species is highlighted with a coloured box and bold text. Species names are indicated to the right of the tree, or before the GenBank accession numbers. The substrate and country of origin are also provided for strains of Teratosphaeria agapanthi. Type status is indicated in superscript. Branches present in the strict consensus tree are thickened. Tree statistics: TL = 150, CI = 0.900, RI = 0.857, RC = 0.771.
Although species of Agapanthus (Amaryllidaceae) are native to southern Africa, they are commonly planted as ornamentals worldwide. In a recent treatment, Zonneveld & Duncan (2003) recognised six species, although a great many hybrids and cultivars are commonly cultivated. Agapanthus is generally regarded as disease and pest free, but species in South Africa have in recent years suffered from infestation by the Agapanthus Borer, Neuranethes spodopterodes. Agapanthus leaf blight caused by T. agapanthi has largely been overlooked in South Africa due to the damage caused by N. spodoterodes.
Results of this study suggest that leaf blight caused by T. agapanthi occurs throughout the native range of the Agapanthus in South Africa, including the Western, Eastern and Northern Cape, Free State, Mpumalanga and KwaZulu-Natal provinces. It is also reported here for the first time from La Réunion Island, which is geographically close to South Africa. Furthermore, our collections include those from France and Portugal, showing that it is well established in Europe. The fungus is also newly reported from California (USA), and as well as Western Australia (Perth), New South Wales (Sydney) and Victoria (Melbourne), Australia. The record of Mycosphaerella agapanthi-umbellati on Agapanthus umbellatus from India (asci 25–50 μm, ascospores 14–25 × 4–7 μm; Aptroot 2006), suggests that it could be a synonym of T. agapanthi, and that it is probably also present in Asia. Further collections from Asia would be required, to confirm this supposition.
In recent years there have been several important accidental introductions of Teratosphaeria spp. pathogenic on Eucalyptus spp. into different countries. The most notable has been the introduction of Teratosphaeria nubilosa from Australia to Africa (Crous 1998), Europe (Crous & Wingfield 1997, Crous et al. 2004b, 2006) and South America (Crous et al. 1993, 2007, Perez et al. 2009, Perez et al. 2010, Hunter et al. 2011), T. pseudoeucalypti from Australia to Uruguay (Soria et al. 2014), and T. destructans from Asia (Wingfield et al. 1996, Burgess et al. 2006, Barber et al. 2012) to South Africa (Greyling et al. 2016).
Species of Teratosphaeria are commonly isolated as endophytes (Sánchez Márquez et al. 2011, Marsberg et al. 2014, Crous et al. 2019d), and thus we suspect that inadvertent introductions commonly occur via asymptomatic plant material. Infected seed may also be tested for the presence of Teratosphaeria spp., and several species-specific primers have been developed for species of Teratosphaeria that infect Eucalyptus (Kularatne et al. 2004, Maxwell et al. 2005). Agapanthus is commonly propagated via seed and cuttings, and we suspect that this has also been its pathway of introduction to different countries and continents (Jimu et al. 2016, Andjic et al. 2019).
Although unwanted introductions are usually complicated by the fact that many plant pathogens have wide host ranges, this does not presently appear to be the case for Teratosphaeria spp. Species studied to date on Proteaceae and Myrtaceae appear to be highly host specific, and likewise, T. agapanthi has thus far only been encountered on Agapanthus.
Trichomerium syzygii Crous, sp. nov. MycoBank MB835107. Fig. 58.
Fig. 58.
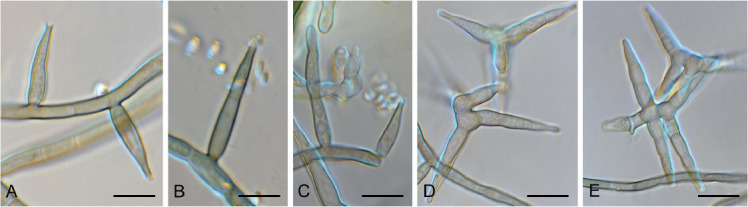
Trichomerium syzygii (CPC 37184). A–C. Microconidiogenous cells. D, E. Macroconidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Syzygium from which the species was isolated.
Mycelium consisting of smooth, branched, septate, 4–5 μm diam, brown hyphae. Conidiophores reduced to conidiogenous loci on hyphae, inconspicuous, 1–5 × 2 μm, not thickened nor darkened. Macroconidia solitary, medium brown, smooth, guttulate, star-shaped, with two globose central cells giving rise to four irregular radiating arms of 3–4(−5) cells, tapering from point of attachment to subobtuse apices (with mucoid caps), constricted at septa, 15–60 × 5–7 μm; conidia also have a fifth branch of 1–2 cells tapering to a subobtuse apex, 9–20 × 4.5–6 μm, which is the branch that attaches to the conidiogenous locus on hyphae. Microconidiogenous cells on hyphae solitary, ampulliform, medium brown, smooth, phialidic, 10–15 × 3–4 μm. Microconidia bacilliform, hyaline, smooth, aseptate, ends rounded, 2–3 × 2 μm.
Culture characteristics: Colonies erumpent, spreading, with moderate aerial mycelium and even, lobate margins, reaching 10–20 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface olivaceous grey, reverse iron-grey
Typus: South Africa, Mpumalanga, Hendriksdal, Mbulwa estate, on leaves of Syzygium cordatum (Myrtaceae), 2016, M.J. Wingfield, HPC 2760 (holotype CBS H-24190, culture ex-type CPC 37184 = CBS 146074).
Notes: Trichomerium syzygii, which is closely related to T. eucalypti (on Eucalyptus tereticornis in Australia; Crous et al. 2017), occurs on leaves of Syzygium cordatum in South Africa. As was the case for T. eucalypti, both conidial morphs were also observed to form in culture. Trichomerium syzygii is distinguished from T. eucalypti [conidia with 4–5(−7) arms, 30–80 × 8–10 μm] in having smaller conidia.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Trichomerium eucalypti (strain CBS 143443, GenBank NR_156672.1; Identities = 623/644 (97 %), 12 gaps (1 %)), Trimmatostroma cordae (strain CBS 418.93, GenBank AJ244263.1; Identities = 582/608 (96 %), 11 gaps (1 %)), and Trichomerium foliicola (strain MFLUCC 10-0058, GenBank JX313653.1; Identities = 611/659 (93 %), 13 gaps (1 %)). Closest hits using the LSU sequence are Trichomerium eucalypti (strain CBS 143443, GenBank NG_058525.1; Identities = 835/842 (99 %), no gaps), Trichomerium dioscoreae (strain CBS 138870, GenBank NG_058126.1; Identities = 825/841 (98 %), no gaps), and Trichomerium deniquelatum (strain MFLUCC 10-0884, GenBank NG_059479.1; Identities = 837/854 (98 %), no gaps) – also see Fig. 4. Closest hits using the rpb1 sequence had highest similarity to Trichomerium sp. LS-2015b (strain CGMCC 3.17307, GenBank KP226531.1; Identities = 567/653 (87 %), 11 gaps (1 %)), Trichomerium sp. WS-2017a (strain CGMCC 3.17988, GenBank KX348490.1; Identities = 556/652 (85 %), 9 gaps (1 %)), and Trichomerium sp. LS-2015a (strain CGMCC 3.17309, GenBank KP226534.1; Identities = 482/616 (78 %), 15 gaps (2 %)). Closest hits using the tub2 sequence had highest similarity to Chaetothyriales sp. (strain CBS 128947, GenBank KX822372.1; Identities = 409/476 (86 %), 18 gaps (3 %)), Trichomerium sp. LS-2015b (strain CGMCC 3.17344, GenBank KP226541.1; Identities = 189/208 (91 %), 1 gap (0 %)), and Trichomerium sp. WS-2017a (strain CGMCC 3.17988, GenBank KX348496.1; Identities = 166/181 (92 %), 4 gaps (2 %)).
Trochila craterium (DC.) Fr., Summa veg. Scand., Sectio Post. (Stockholm): 367. 1849. Fig. 59.
Fig. 59.
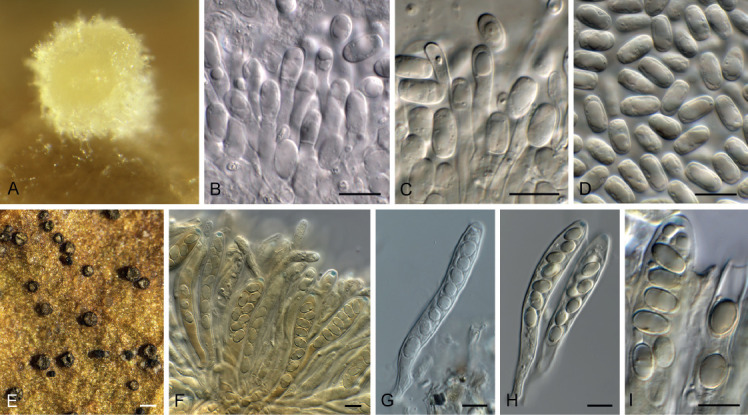
Trochila craterium (CPC 37493). A. Conidiomata on MEA. B, C. Conidiogenous cells and conidia. D. Conidia. E. Ascomata on leaf surface. F–I. Asci with ascospores. Scale bars: A, E = 200 μm, all others = 10 μm.
Basionym: Sphaeria craterium DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 298. 1805.
Occurring on dead leaves. Ascomata single, gregarious, intraepidermal, hypophyllous, erumpent, opening via 3–4 black flaps, cushion-shaped, greenish and soft at maturity, 300–400 μm diam. Asci 8-spored, clavate, apex amyloid in Melzer’s reagent, pedicel short and furcate, 65–80 × 10–12 μm. Paraphyses not longer than asci, cylindrical, hyphae-like, basally branched, anastomosed, apically clavate, plasma yellow, thin-walled, smooth, 5–7 μm diam at apex. Ascospores aseptate, ellipsoid, hyaline, thin-walled, smooth, guttulate, (8−)9(−10) × (4.5−) 5(−6) μm; older ascospores become brown and verruculose. Conidiomata solitary, superficial on agar, but intraepidermal, becoming erumpent on host tissue, acervular, opening by irregular rupture, 80–150(−300) μm diam, cream coloured with mucoid conidial mass. Conidiophores subcylindrical, branched, septate, hyaline, smooth, 20–50 × 3–4 μm. Conidiogenous cells integrated, subcylindrical, hyaline, smooth, terminal and intercalary, 10–20 × 3–4 μm, proliferating percurrently with several inconspicuous annellations. Conidia solitary, hyaline, smooth, with large central guttula and several small guttules, subcylindrical, apex obtuse, base truncate with minute marginal frill, aseptate, thin-walled, (7.5−)8–9(−12) × (4−)4.5–5(−6) μm; conidia become medium brown with age, but remain smooth.
Culture characteristics: Colonies erumpent, spreading, with folded surface and moderate aerial mycelium and feathery, lobate margin, reaching up to 30 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse buff.
Material examined: Germany, near Berlin, on dead and attached leaves of Hedera helix (Araliaceae), 5 Feb. 2019, R.K. Schumacher, HPC 2762 = RKS195 = CBS H-24366, culture CPC 37493 = CBS 146632).
Notes: Ascospores of Trochila craterium gave rise to a coelomycetous asexual morph in culture. Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Trochila viburnicola (strain CBS 144206, GenBank NR_159070.1; Identities = 470/502 (94 %), 4 gaps (0 %)), Vestigium trifidum (strain DAOM 240321, GenBank NR_121556.1; Identities = 461/501 (92 %), 3 gaps (0 %)), and Chlorencoelia versiformis (voucher TAAM 179803, GenBank LT158427.1; Identities = 461/504 (91 %), 6 gaps (1 %)). Closest hits using the LSU sequence are Trochila viburnicola (strain CBS 144206, GenBank NR_159070.1, GenBank NG_063960.1; Identities = 814/832 (98 %), no gaps), Vestigium trifidum (strain DAOM 240321, GenBank KC407777.1; Identities = 816/838 (97 %), 4 gaps (0 %)), and Heyderia abietis (strain OSC60392, GenBank AY789289.1; Identities = 796/818 (97 %), 1 gap (0 %)). Closest hits using the rpb1 sequence had highest similarity to Trochila craterium (voucher BPI 880163, GenBank KX090779.1; Identities = 707/707 (100 %), no gaps), and partial hits to Acarospora laqueata (strain AFTOL-ID 1007, GenBank DQ782860.1; Identities = 130/156 (83 %), no gaps), and Geopora cooperi (voucher BAP 517 (FH), GenBank JX943681.1; Identities = 118/143 (83 %), no gaps). Closest hits using the rpb2 sequence had highest similarity to Chlorociboria aeruginascens (strain KL152, GenBank KX090706.1; Identities = 749/894 (84 %), 1 gaps (0 %)), Velutarina alpestris (strain KL378, GenBank KX090738.1; Identities = 748/893 (84 %), 6 gaps (0 %)), and Chlorociboria duriligna (strain D1814, GenBank JN985500.1; Identities = 749/895 (84 %), 1 gap (0 %)).
Uzbekistanica vitis-viniferae Crous & Akulov, sp. nov. MycoBank MB835108. Fig. 60.
Fig. 60.
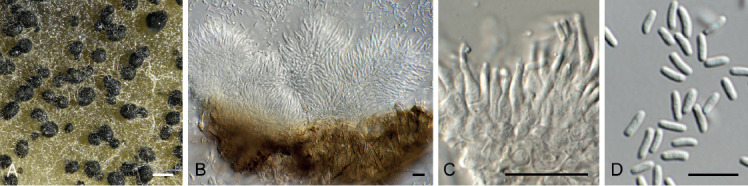
Uzbekistanica vitis-viniferae (CPC 35793). A. Conidiomata on OA. B, C. Conidiogenous cells. D. Conidia. Scale bars: A = 300 μm, all others = 10 μm.
Etymology: Name refers to Vitis vinifera, the host from which this fungus was isolated.
Conidiomata pycnidial, solitary, globose, dark brown, smooth, 300–350 μm diam, somewhat papillate, with central ostiole, 10–15 μm diam; wall of 6–10 layers of brown textura angularis, becoming hyaline towards inner fertile region. Conidiophores densely aggregated, arising from the inner layer of cavity, hyaline, smooth, subcylindrical, branched, 1–2-septate, 15–25 × 2.5–3.5 μm. Conidiogenous cells in clusters of 2–6, phialidic, subcylindrical, tapered towards apex with periclinal thickening, terminal and intercalary, 7–10 × 2–2.5 μm. Conidia cytospora-like, aseptate, hyaline, smooth, subcylindrical with obtuse ends, straight to slightly curved, 4–5(−6) × 1.5 μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and smooth, lobate margin, reaching 25 mm diam after 2 wk at 25 °C. On MEA surface olivaceous grey, reverse umber; on PDA surface iron-grey and reverse olivaceous grey; on OA surface umber.
Typus: Ukraine, Kharkiv district, Nova Vodolaha, on dead stem of Vitis vinifera (Vitaceae) soc. Hendersonia sp., 22 Apr. 2018, O. Ternovska, HPC 2437 = CWU (Myc) AS 6746 (holotype specimen CBS 145545, preserved as metabolically inactive culture, culture ex-type CPC 35793 = CBS 145545).
Notes: Uzbekistanica was introduced as a new genus to accommodate species with broadly oblong ascomata, filamentous, pseudoparaphyses, cylindrical to cylindrical-clavate asci, muriform, ellipsoidal, yellowish brown to brown ascospores, and globose conidiomata with hyaline 1-septate, sepia or brown conidia (Wanasinghe et al. 2018). Two species were described from Uzbekistan, namely U. yakutkhanika and U. rosae-hissaricae. Uzbekistanica vitis-viniferae (from Ukraine) adds a third species to this complex.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Uzbekistanica sp. DB-2019a (voucher MFLU 17-2136, GenBank MN758893.1; Identities = 463/465 (99 %), no gaps), Uzbekistanica yakutkhanika (strain MFLUCC 17-0809, GenBank MG828977.1; Identities = 470/491 (96 %), 2 gaps (0 %)), and Uzbekistanica rosae-hissaricae (strain MFLUCC 17-0820, GenBank MG828976.1; Identities = 470/491 (96 %), 2 gaps (0 %)). Closest hits using the LSU sequence are Uzbekistanica sp. DB-2019a (voucher MFLU 17-2136, GenBank MN759024.1; Identities = 811/811 (100 %), no gaps), Uzbekistanica yakutkhanika (strain MFLUCC 17-0809, GenBank MG829089.1; Identities = 848/851 (99 %), no gaps), and Aposphaeria corallinolutea (strain MFLUCC 14-0504, GenBank KU243051.1; Identities = 847/851 (99 %), no gaps) – also see Fig. 3, part 3. Closest hits using the rpb2 sequence had highest similarity to Uzbekistanica yakutkhanika (strain MFLUCC 17-0842, GenBank MG829265.1; Identities = 858/921 (93 %), no gaps), Uzbekistanica rosae-hissaricae (strain MFLUCC 17-0819, GenBank MG829262.1; Identities = 857/921 (93 %), no gaps), and Praetumpfia obducens (strain C56, GenBank KY190001.1; Identities = 836/915 (91 %), no gaps).
Vermiculariopsiella eucalyptigena Crous, sp. nov. MycoBank MB835109. Fig. 61.
Fig. 61.
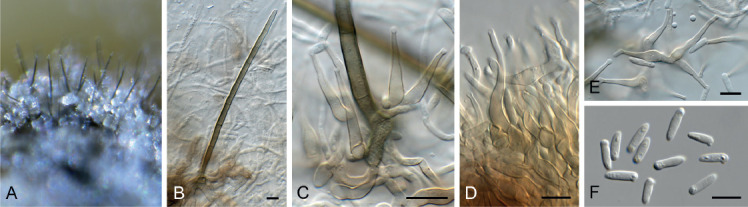
Vermiculariopsiella eucalyptigena (CPC 36373). A. Conidiophores and setae on MEA. B. Seta. C. Setal base. D, E. Conidiogenous cells. F. Conidia. Scale bars = 10 μm.
Etymology: Name refers to the host genus Eucalyptus from which the species was isolated.
Mycelium consisting of hyaline, smooth, branched, septate, 1.5–2 μm diam hyphae. Setae erect, cylindrical, 60–160 × 4–5(−7) μm, unbranched, (2−)6–8-septate, straight to gently curved, brown, smooth, but verruculose at base, apex obtusely rounded. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, discrete, ampulliform to subulate, pale brown, smooth, aggregated around base of setae, forming a mucoid conidial mass, 15–30 × 3.5–4 μm, with distinct apical collarette. Conidia asymmetrical, subfusiform to oblong, straight, irregular (depending on medium), fimbrillate at base, forming a small lateral protrusion (on SNA), aseptate, hyaline, smooth, guttulate, (6−)10–12(−13) × (2.5−)3(−4) μm.
Culture characteristics: Colonies flat, spreading, with sparse aerial mycelium and smooth, lobate margin, reaching 15 mm diam after 2 wk at 25 °C. On MEA surface and reverse isabelline; on PDA surface grey olivaceous, reverse olivaceous grey; on OA surface olivaceous grey.
Typus: Australia, New South Wales, Royal National Park, Winifred Falls, fire trail, on leaves of Eucalyptus sp. (Myrtaceae), 1 Jun. 2016, A.J. Carnegie, HPC 2542 (holotype CBS H-24252, culture ex-type CPC 36373 = CBS 146091).
Notes: Species of Vermiculariopsiella are commonly isolated from leaf and twig litter. The genus is characterised by sporodochia with brown, erect setae (branched or not), subhyaline conidiophores, phialidic conidiogenous cells, and hyaline, aseptate conidia (Crous et al. 2014, Hernández-Restrepo et al. 2017). Vermiculariopsiella eucalyptigena is phylogenetically related to V. pediculata (conidia 19–28 × 3–4 μm on potato carrot agar; Hernández-Restrepo et al. 2012), but is distinguished by its smaller conidia and unbranched setae.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Vermiculariopsiella pediculata (strain CBS 132484, GenBank MH866028.1; Identities = 537/553 (97 %), 5 gaps (0 %)), Vermiculariopsiella microsperma (strain CBS 140231, GenBank KY853478.1; Identities = 525/545 (96 %), 5 gaps (0 %)), and Vermiculariopsiella lauracearum (strain CBS 145055, GenBank NR_164441; Identities = 528/556 (95 %), 10 gaps (1 %)). Closest hits using the LSU sequence are Vermiculariopsiella hubeiensis (strain CBS 141281, GenBank NG_066169.1; Identities = 816/827 (99 %), no gaps), Vermiculariopsiella pediculata (strain CBS 132484, GenBank MH877476.1; Identities = 827/840 (98 %), 3 gaps (0 %)), and Vermiculariopsiella dunnii (strain CBS 145538, GenBank MK876452.1; Identities = 799/812 (98 %), no gaps) – also see Fig. 6, part 3.
Vesiculozygosporium Crous, gen. nov. MycoBank MB835110.
Etymology: Name refers to species of Zygosporium with tubular appendages terminating in vesicles.
Mycelium consisting of hyaline, smooth, branched, septate hyphae. Conidiophores arising directly from superficial mycelium, or from a cylindrical stipe, erect, medium brown, smooth, subcylindrical, unbranched, septate, giving rise to conidiophore as lateral branch, forming a stipe extension, septate, terminating in a vesicle, pale brown, finely roughened. Vesicular cell dark brown, smooth, clavate, curved (hooked), giving rise to 3–4 conidiogenous cells. Conidiogenous cells terminal and lateral on apex of hooked vesicular cell, doliiform, pale brown, smooth, phialidic, apex with cylindrical collarette. Conidia solitary, globose, verruculose, thick-walled, pale brown, guttulate, scar visible.
Type species: Vesiculozygosporium echinosporum (Bunting & E.W. Mason) Crous
Vesiculozygosporium echinosporum (Bunting & E.W. Mason) Crous, comb. nov. MycoBank MB835111. Fig. 62.
Fig. 62.
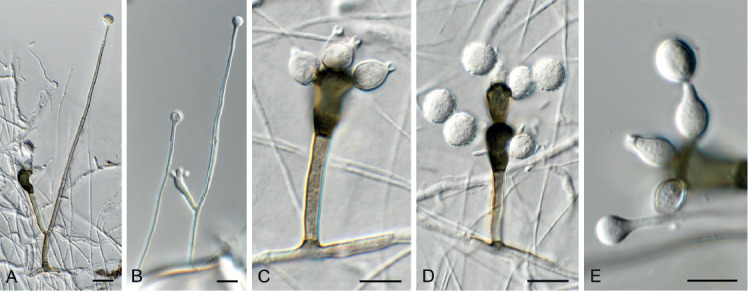
Vesiculozygosporium echinosporum (CPC 35607). A, B. Conidiophores. C–E. Conidiogenous cells and conidia. Scale bars = 10 μm.
Basionym: Zygosporium echinosporum Bunting & E.W. Mason, In Mason, Annot. Acct Fungi rec’d Imp. Mycol. Inst. 2: 135. 1941.
Mycelium consisting of hyaline, smooth, branched, septate, 2–2.5 μm diam hyphae. Conidiophores arising directly from superficial mycelium, or from a cylindrical stipe, erect, medium brown, smooth, subcylindrical, unbranched, 2–3-septate, 20–60 × 3.5–4 μm, giving rise to conidiophore as lateral branch, forming a stipe extension, 100–160 × 2–3 μm, 3–4-septate, terminating in a sphaeropedunculate vesicle, pale brown, finely roughened, (6−)6.5(−7) μm. Vesicular cell dark brown, smooth, clavate, curved (hooked), 13–15 × 8–10 μm, giving rise to 3–4 conidiogenous cells. Conidiogenous cells terminal and lateral on apex of hooked vesicular cell, doliiform, pale brown, smooth, phialidic, 7–9 × 5–6 μm, apex with cylindrical collarette, 1–2 × 1.5–2 μm. Conidia solitary, globose, verruculose, thick-walled, pale brown, guttulate, scar visible, (8−)9(−10) μm diam.
Culture characteristics: Colonies flat, spreading, with moderate aerial mycelium and smooth, lobate margin, reaching 10 mm diam after 2 wk at 25 °C. On MEA, PDA and OA surface and reverse olivaceous grey.
Material examined: Malaysia, on leaves of Muntingia calabura (Muntingiaceae), 29 Mar. 2018, M.J. Wingfield, HPC 2395 = CBS H-24047, culture CPC 35607 = CBS 145807.
Notes: Zygosporium has three generic synonyms, namely Pimina, Urobasidium and Urophiala, but as the type species of these genera lack setiform conidiophores, they do not apply to Zygosporium echinosporum. Zygosporium echinosporum is phylogenetically identical to an LSU sequence deposited for Atrotorquata spartii. However, the ITS (from MFLU 14-0821/MFLUCC 13-0445; Jian-Kui Liu, pers. comm.) and LSU (from holotype MFLU 14-0738 / ex-type culture MFLUCC 13-0444; GenBank KP325443.1) sequences for this taxon are incongruent, and as the type species of Atrotorquata is A. lineata (not known from DNA sequence), we rather choose to introduce a new genus, Vesiculozygosporium, to accommodate Z. echinosporum. It could well be that other species of Zygosporium with vesiculate setae and setiform conidiophores also belong to Vesiculozygosporium, but cultures and additional phylogenetic studies would be required to confirm this hypothesis.
Based on a megablast search of NCBI’s GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to Zygosporium echinosporum (strain CBS 247.72, GenBank MH860466.1; Identities = 518/519 (99 %), 1 gap (0 %)), Ascotricha chartarum (strain BPS247, GenBank MH700426.1; Identities = 403/452 (89 %), 13 gaps (2 %)), and Ascotricha erinacea (strain CBS 535.73, GenBank KF893285.1; Identities = 469/528 (89 %), 17 gaps (3 %)). Closest hits using the LSU sequence are Atrotorquata spartii (strain MFLUCC 13-0444, GenBank NG_057064.1; Identities = 851/851 (100 %), no gaps), Ascotricha chartarum (strain HMCK-30, GenBank MK051166.1; Identities = 835/852 (98 %), 1 gap (0 %)), and Dicyma olivacea (strain CBS 116.31, GenBank MH866600.1; Identities = 832/851 (98 %), no gaps) – also see Fig. 6, part 2a.
ACKNOWLEDGEMENTS
We are grateful to Arien van Iperen (cultures), Mieke Starink-Willemse (DNA isolation, amplification, and sequencing), and Marjan Vermaas (photographic plates) for their technical assistance. Keith A. Seifert and Yasmina Marin Felix provided assistance with text related to Gonatobotrys simplex.
Supplementary Material: http://fuse-journal.org/
REFERENCES
- Andjic V, Carnegie AJ, Pegg GS, et al. , (2019). 23 years of research on Teratosphaeria leaf blight of Eucalyptus. Forest Ecology and Management 443: 19–27. [Google Scholar]
- Aptroot A. (2006) Mycosphaerella and its anamorphs: 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5: 1–231. Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. [Google Scholar]
- Barber PA, Thu PQ, Hardy GE, et al. , (2012). Emerging disease problems in eucalypt plantations in Lao PDR. In: Proceeding of International Conference on the Impacts of Climate Change to Forest Pests and Diseases in the Tropics, (Mohammed C, Beadle C, Roux J, et al., , eds), 8–10 October 2012, Yogyakarta, Indonesia: 79–84. [Google Scholar]
- Bensch K, Groenewald JZ, Braun U, et al. , (2015). Common but different: The expanding realm of Cladosporium. Studies in Mycology 82: 23–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra JDP, Sandoval-Denis M, Paiva LM, et al. , (2017). New endophytic Toxicocladosporium species from cacti in Brazil, and description of Neocladosporium gen. nov. IMA Fungus 8: 77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerema GH, de Gruyter J, Noordeloos ME, et al. , (2004). Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. CABI Publishing, Wallingford, UK. [Google Scholar]
- Boerema GH, de Gruyter J, van Kesteren HA. (1994). Contributions towards a monograph of Phoma (Coelomycetes)—III. 1. Section Plenodomus: Taxa often with a Leptosphaeria teleomorph. Persoonia 15: 431–487. [Google Scholar]
- Braun U, Crous PW, Dugan F, et al. , (2003). Phylogeny and taxonomy of cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3–18. [Google Scholar]
- Braun U, Hill CF. (2008). New species and new records of foliicolous hyphomycetes from New Zealand. Australasian Mycologist 27: 45–56. [Google Scholar]
- Braun U, Nakashima C, Crous PW, et al. , (2018). Phylogeny and taxonomy of the genus Tubakia s. lat. Fungal Systematics and Evolution 1: 41–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald NF. (1949). Studies in the Scletoriniaceae: I. Taxonomy of the Sclerotiniaceae. Kongelige Veterinær- og Landbohøjskole, Aarsskrift 32: 1–116. [Google Scholar]
- Burgess TI, Andjic V, Hardy GEStJ, et al. , (2006). First report of Phaeophleospora destructans in China. Journal of Tropical Forest Science 18: 144–146. [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, et al. , (2009). Myrtaceae, a cache of fungal biodiversity. Persoonia 23: 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda ACJ. (1839). Pracht-Flora Europaeischer Schimmelbildungen. edn Gerhard Fleischer, Leipzig, Berlin. [Google Scholar]
- Corlett M. (1991). An annotated list of the published names in Mycosphaerella and Sphaerella. Mycologia Memoirs 18: 1–328. [Google Scholar]
- Crous PW. (1993). New and interesting fungi. 13. Foliicolous microfungi. South African Journal of Botany 59: 602–610. [Google Scholar]
- Crous PW. (1998). Mycosphaerella spp. and their anamorphs associated with leaf spot diseases of Eucalyptus. Mycologia Memoir 21: 1–170. APS Press, MN, USA. [Google Scholar]
- Crous PW, Braun U, Groenewald JZ. (2007). Mycosphaerella is polyphyletic. Studies in Mycology 58: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Carnegie AJ, Wingfield MJ, et al. , (2019a). Fungal Planet description sheets: 868–950. Persoonia 42: 291–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Ferreira FA, Alfenas A, et al. , (1993). Mycosphaerella suberosa associated with corky leaf spots on Eucalyptus in Brazil. Mycologia 85: 705–710. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. , (2004a). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Groenewald JZ, Mansilla JP, et al. , (2004b) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Studies in Mycology 50: 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. (2011). Why everlastings don’t last. Persoonia 26: 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Akulov A, et al. , (2019b). New and Interesting Fungi. 2. Fungal Systematics and Evolution 3: 57–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. , (2018). New and interesting fungi. 1. Fungal Systematics and Evolution 1: 169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. , (2014). Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. , (2011). Fungal Planet description sheets: 92–106. Persoonia 27: 130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al., (eds) (2019c). Fungal Biodiversity. [Westerdijk Laboratory Manual Series no. 1.] Utrecht: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands. [Google Scholar]
- Crous PW, Wingfield MJ. (1997). New species of Mycosphaerella occurring on Eucalyptus leaves in Indonesia and Africa. Canadian Journal of Botany 75: 781–790. [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. , (2017). Fungal Planet description sheets: 625–715. Persoonia 39: 270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Cheewangkoon R, et al. , (2019d). Foliar pathogens of eucalypts. Studies in Mycology 94: 125–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. , (2013). Fungal Planet description sheets: 154–213. Persoonia 31: 188–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, et al. , (2006) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Park RF. (1991). Mycosphaerella nubilosa a synonym of M. molleriana. Mycological Research 95: 628–632. [Google Scholar]
- Davis JJ. (1903). Third supplementary list of parasitic fungi of Wisconsin. Transactions of the Wisconsin Academy of Sciences, Arts and Letters 14: 83–106. [Google Scholar]
- Davis JJ. (1914). A provisional list of parasitic fungi of Wisconsin. Transactions of the Wisconsin Academy of Sciences, Arts and Letters 17: 846–984. [Google Scholar]
- De Gruyter J, Woudenberg JHC, Aveskamp MM, et al. , (2013). Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domsch KH, Gams W, Anderson T-H. (2007). Compendium of soil fungi. 2nd Ed IHW-Verlag, Eching. [Google Scholar]
- Fan XL, Bezerra JDP, Tian CM, et al. , (2018). Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Persoonia 40: 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams W, Holubová-Jechová V. (1976). Chloridium and some other dematiaceous hyphomycetes growing on decaying wood. Studies in Mycology 13: 1–99. [Google Scholar]
- Giraldo A, Crous PW. (2019). Inside Plectosphaerellaceae. Studies in Mycology 92: 227–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo A, Hernández-Restrepo M, Crous PW. (2019). New plectosphaerellaceous species from Dutch garden soil. Mycological Progress 18: 1135–1154. [Google Scholar]
- Gomes RR, Glienke C, Videira CIR, et al. , (2013). Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greyling I, Wingfield MJ, Coetzee MPA, et al. , (2016). The Eucalyptus shoot and leaf pathogen Teratosphaeria destructans recorded in South Africa. Southern Forests: a Journal of Forest Science 78: 123–129. [Google Scholar]
- Hashimoto A, Matsumura M, Hirayama K. (2017). Pseudodidymellaceae fam. nov.: phylogenetic affiliations of mycopappus-like genera in Dothideomycetes. Studies in Mycology 87: 187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL, Crous PW, Redhead SA, et al. , (2011). The Amsterdam Declaration on Fungal Nomenclature. IMA Fungus 2: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebert GL. (1973). Botrytis and botrytis-like genera. Persoonia 7: 183–204. [Google Scholar]
- Hernández-Restrepo M, Castañeda-Ruiz RF, Gené J, et al. , (2012). Microfungi from Portugal: Minimelanolocus manifestus sp. nov. and Vermiculariopsiella pediculata comb. nov. Mycotaxon 122: 135–143. [Google Scholar]
- Hernández-Restrepo M, Gené J, Castañeda-Ruiz RF, et al. , (2017). Phylogeny of saprobic microfungi from Southern Europe. Studies in Mycology 86: 53–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Madrid H, Tan YP, et al. , (2018). Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia 41: 71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GC, Crous PW, Carnegie AJ, et al. , (2011). Mycosphaerella and Teratosphaeria diseases of Eucalyptus; easily confused and with serious consequences. Fungal Diversity 50: 145–166. [Google Scholar]
- Jimu L, Kemler M, Wingfield MJ, et al. , (2015). The Eucalyptus stem canker pathogen Teratosphaeria zuluensis detected in seed samples. Forestry 89: 316–324. [Google Scholar]
- Johnston RP, Seifert K, Stone JK, et al. , (2014). Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 5: 91–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PR, Quijada L, Smith CA, et al. , (2019). A multigene phylogeny toward a new phylogenetic classification of Leotiomycetes. IMA Fungus 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FR, JL Weimer. (1938). Stagonospora leaf spot and root rot of forage legumes. Journal of Agricultural Research 57: 791–812. [Google Scholar]
- Kearse M, Moir R, Wilson A, et al. , (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM. (1982). New or interesting microfungi VI. Sporidesmiella gen. nov. (hyphomycetes). Transactions of the British Mycological Society 79: 479–489. [Google Scholar]
- Kirk PM. (1983). New or interesting microfungi IX. Dematiaceous hyphomycetes from Esher Common. Transactions of the British Mycological Society 80: 449–467. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE. (1996). Fungi on Juncus roemerianus. 8. New bitunicate ascomycetes. Canadian Journal of Botany 74: 1830–1840. [Google Scholar]
- Kularatne HAGC, Lawrie AC, Barber PA, et al. , (2004). A specific primer PCR and RFLP assay for the rapid identification and differentiation in planta of some Mycosphaerella species associated with foliar diseases of Eucalyptus globulus. Mycological Research 108: 1476–1493. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-T, Shin H-D. (2000). Taxonomic studies on the genus Marssonina in Korea. Mycobiology 281: 39–46. [Google Scholar]
- Liu F, Bonthond G, Groenewald JZ, et al. , (2019). Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Studies in Mycology 92: 287–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Phookamsak R, Dai DQ, et al. , (2014). Roussoellaceae, a new pleosporalean family to accommodate the genera Neoroussoella gen. nov., Roussoella and Roussoellopsis. Phytotaxa 181: 1–33. [Google Scholar]
- Lucas MT, Webster J. (1967). Conidial states of British species of Leptosphaeria. Transactions of the British Mycological Society 50: 85–121. [Google Scholar]
- Marin-Felix Y, Guarro J, Ano-Lira JF, et al. , (2018). Melanospora (Sordariomycetes, Ascomycota) and its relatives. MycoKeys 44: 81–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Felix Y, Hernández-Restrepo M, Iturrieta-González I, et al. , (2019). Genera of phytopathogenic fungi: GOPHY 3. Studies in Mycology 94: 1–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincowitz S, Gryzenhout M, Wingfield MJ. (2010). New and rare coelomycetes with appendage-bearing conidia from Pondoland, South Africa. Mycotaxon 111: 309–322. [Google Scholar]
- Marsberg A, Slippers B, Wingfield MJ, et al. , (2014) Endophyte isolations from Syzygium cordatum and a Eucalyptus clone (Myrtaceae) reveal new host and geographical reports for the Mycosphaerellaceae and Teratosphaeriaceae. Australasian Plant Pathology 43: 503–512. [Google Scholar]
- Maxwell A, Jackson SL, Dell B, et al. , (2005). PCR-identification of Mycosphaerella species associated with leaf diseases of Eucalyptus. Mycological Research 109: 992–1004. [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FF, Buck WR, et al., (eds). (2012). International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) [Regnum vegetable no. 154.] Königstein: Koeltz Scientific Books. [Google Scholar]
- Najim L, Clauzet JP, Kadii M. (1984). Contribution à l’étude de la flore fongique microscopique du Maroc. I. - Le genre Gonatobotrys: quelques aspects morphologiques et physiologiques. Cryptogamie Mycologie 5: 109–120. [Google Scholar]
- Norphanphoun C, Hongsanan S, Doilom M, et al. , (2016). Lamproconiaceae fam. nov. to accommodate Lamproconium desmazieri. Phytotaxa 270: 89–102. [Google Scholar]
- Perdomo H, García D, Gené J, et al. , (2013). Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia 105: 398–421. [DOI] [PubMed] [Google Scholar]
- Pérez G, Hunter GC, Slippers B, et al. , (2009). Teratosphaeria (Mycosphaerella) nubilosa, the causal agent of Mycosphaerella Leaf Disease (MLD), recently introduced into Uruguay. European Journal of Plant Pathology 125: 109–118. [Google Scholar]
- Pérez G, Slippers B, Wingfield BD, et al. , (2010). Micro- and macro spatial scale analyses illustrates mixed mating strategies and extensive geneflow in populations of an invasive haploid pathogen. Molecular Ecology 19: 1801–1813. [DOI] [PubMed] [Google Scholar]
- Petrak F. (1919). Mykologische Notizen. I. Annales Mycologici 17(2/6): 59–100. [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, et al. , (2013). Sizing up Septoria. Studies in Mycology 75: 307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenhorst GL. (1844). Deutschlands Kryptogamen - Flora 1 (Pilze). Leipzig. [Google Scholar]
- Rao VG, de Hoog GS. (1986). New or critical Hyphomycetes from India. Studies in Mycology 28: 1–84. [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society; Kew, Surrey, UK. [Google Scholar]
- Reblova M, Miller AN, Rossman AY, et al. , (2016). Recommendations for competing sexual-asexually typified generic names in Sordariomycetes (except Diaporthales, Hypocreales, and Magnaporthales). IMA Fungus 7: 131–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. , (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungjindamai N, Sakayaroj J, Plaingam N, et al. , (2008). Putative basidiomycete teleomorphs and phylogenetic placement of the coelomycete genera: Chaetospermum, Giulia and Mycotribulus based on nu-rDNA sequences. Mycological Research 112: 802–810. [DOI] [PubMed] [Google Scholar]
- Sánchez Márquez S, Bills GF, Zabalgogeazcoa I. (2011). Fungal species diversity in juvenile and adult leaves of Eucalyptus globulus from plantations affected by Mycosphaerella leaf disease. Annals of Applied Biology 158: 177–187. [Google Scholar]
- Schubert K, Groenewald JZ, Braun U, et al. , (2007). Biodiversity in the Cladosporium herbarum complex (Davidiellaceae, Capnodiales), with standardisation of methods for Cladosporium taxonomy and diagnostics. Studies in Mycology 58: 105–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RA, Babcock CE, Irwin JAG. (1991). Massarina walkeri n. sp., the teleomorph of Acrocalymma medicaginis from Medicago sativa contrasted with Leptosphaeria pratensis, L. weimeri n. sp., and L. viridella. Canadian Journal of Botany 69: 569–573. [Google Scholar]
- Smith H, Wingfield MJ, Crous PW, et al. , (1996). Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. South African Journal of Botany 62: 86–88. [Google Scholar]
- Soares DJ. (2012). Gray mold of castor: a review. In: Plant Pathology (Cumagun CJR, ed.). Ntech publisher, Rijeka, Croatia: 219–240. [Google Scholar]
- Soria S, Alonso R, Bettucci L, et al. , (2014) First report of Teratosphaeria pseudoeucalypti in Uruguay. Australasian Plant Disease Note 9: 146. [Google Scholar]
- Stielow JB, Lévesque CA, Seifert KA, et al. , (2015). One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia 35: 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, et al. , (2006). Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23: 323–350. [Google Scholar]
- Swofford DL. (2003). PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0b10. Sinauer Associates, Sunderland. [Google Scholar]
- Sydow H, Sydow P. (1913). Novae fungorum species - X. Annales Mycologici 11: 254–271. [Google Scholar]
- Tangthirasunun N, Silar P, Bhat D, et al. , (2014). Morphology and phylogeny of Chaetospermum (asexual coelomycetous Basidiomycota). Phytotaxa 175: 61–72. [Google Scholar]
- Tsunoda M, Hidaka T, Taniguchi M. (1996). Development of Graphostroma platystoma on bed-logs of shiitake (Lentinus edodes) and assessment of its injurious effects (in Japanese). Transactions of the Mycological Society of Japan 37: 81–89. [Google Scholar]
- Vakili NG. (1989). Gonatobotrys simplex and its teleomorph, Melanospora damnosa. Mycological Research 93: 67–74. [Google Scholar]
- Verkley GJM, Dukik K, Renfurm R, et al. , (2014). Novel genera and species of coniothyrium-like fungi in Montagnulaceae (Ascomycota). Persoonia 32: 25–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin HD, et al. , (2013). A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkley GJM, Silva da M, Wicklow DT, Crous PW. (2004). Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50: 323–335. [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. , (2016). All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Nakashima C, et al. , (2017). Mycosphaerellaceae – chaos or clarity? Studies in Mycology 87: 257–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Verkley GJM, et al. , (2015). The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology 119: 823–843. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Friebes G, Gardiennet A, et al. , (2018). Barrmaelia and Entosordaria in Barrmaeliaceae (fam. nov., Xylariales) and critical notes on anthostomella-like genera based on multigene phylogenies. Mycological Progress 17: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hennings P. (1902). Zwei neue parasitische blattpilze auf Laubhölzern. Zeitschrift für Pflanzenkrankheiten 12: 14–16. [Google Scholar]
- Vu D, Groenewald M, de Vries M, et al. , (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JC, Minter DW. (1981). Taxonomy of Nematogonum, Gonatobotrys, Gonatobotryum and Gonatorrhodiella. Transactions of the British Mycological Society 77: 299–319. [Google Scholar]
- Wanasinghe DN, Phukhamsakda C, Hyde KD, et al. , (2018). Fungal diversity notes 709–839: taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Diversity 89: 1–236. [Google Scholar]
- Wang Q-M, Begerow D, Groenewald M, et al. , (2015). Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Studies in Mycology 81: 55–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield MJ, Crous PW, Boden D. (1996). Kirramyces destructans sp. nov., a serious leaf pathogen of Eucalyptus in Indonesia. South African Journal of Botany 62: 325–327. [Google Scholar]
- Wingfield MJ, De Beer ZW, Slippers B, et al. , (2012). One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology 13: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhner T, Emeriewen OF. (2019). Apple blotch disease (Marssonina coronaria (Ellis & Davis) Davis) review and research prospects. European Journal of Plant Pathology 153: 657–669. [Google Scholar]
- Yang H, Ariyanwansa HA, Wu H-X, et al. , (2014). The genus Leptoxyphium (Capnodiaceae) from China. Phytotaxa 176: 174–183. [Google Scholar]
- Zhang Z, Schwartz S, Wagner L, et al. , (2000). A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7: 203–214. [DOI] [PubMed] [Google Scholar]
- Zare R, Gams W. (2016). More white verticillium-like anamorphs with erect conidiophores. Mycological Progress 15: 993–1030. [Google Scholar]
- Zogg H. (1951). Studien über die pathogenität von Erregergemischen bei Getreidefusskrankheiten. Phytopathologische Zeitschrift 18: 1–54. [Google Scholar]
- Zonneveld BJM, Duncan GD. (2003). Taxonomic implications of genome size and pollen colour and vitality for species of Agapanthus L’Héritier (Agapanthaceae). Plant Systematics and Evolution 241: 115–123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.