Abstract
Purpose
To determine the effects of narrowband light exposure on choroidal thickness and the pupil response in humans.
Methods
Twenty subjects, ages 21 to 43 years, underwent 1 hour of exposure to broadband, short wavelength “blue,” or long wavelength “red” light, or darkness. Choroidal thickness, imaged with spectral domain optical coherence tomography, axial length, determined from biometry, and rod/cone- and intrinsically photosensitive retinal ganglion cell-driven pupil responses were measured before and after exposure. Pupil stimuli were six 1 second alternating red (651 nm) and blue (456 nm) stimuli, 60 seconds apart. Pupil metrics included maximum constriction and the 6 second post-illumination pupil response (PIPR).
Results
Compared with before exposure, the choroid significantly thinned after broadband light, red light, and dark exposure (all P < 0.05), but not after blue light exposure (P = 0.39). The maximum constriction to 1 second red stimuli significantly decreased after all light exposures (all P < 0.001), but increased after dark exposure (P = 0.02), compared with before exposure. Maximum constriction and 6-second PIPR to 1 second blue stimuli significantly decreased after all light exposures compared with before exposure (all P < 0.005), with no change after dark exposure (P > 0.05). There were no differences in axial length change or 6-second PIPR to red stimuli between exposures.
Conclusions
Narrowband blue and red light exposure induced differential changes in choroidal thickness. Maximum constriction, a function of rod/cone activity, and the intrinsically photosensitive retinal ganglion cell-mediated PIPR were attenuated after all light exposures. Findings demonstrate differing effects of short-term narrowband light and dark exposure on the choroid, rod/cone activity, and intrinsically photosensitive retinal ganglion cells.
Keywords: choroidal thickness, pupil, intrinsically photosensitive retinal ganglion cells, light exposure
Light exposure has been implicated in the regulation of eye growth and development of myopia.1,2 Increased duration of outdoor time and greater light exposure have been shown to decrease myopia onset and slow axial length growth in children.3,4 However, the mechanism underlying the protective effects of outdoor light has not yet been elucidated. Differences in the intensity and spectral composition of light between indoor and outdoor environments have been suggested to be involved in the protective effects of outdoor light.2 Outdoor environments consist of high-intensity broadband light, whereas indoor environments are composed of lower intensity light of varying spectrum, depending on the source of illumination.
Animal studies suggest that the chromaticity of light can guide eye growth, which may be attributed to longitudinal chromatic aberration. Longitudinal chromatic aberration results in wavelength-specific defocus, such that short wavelength (blue) light is refracted more than long wavelength (red) light. Thus, for an emmetropic eye, blue light tends to focus in front of the retina, whereas red light focuses behind the retina.5 Studies investigating the effects of short versus long wavelength light on eye growth have yielded contradicting results. Chicks and guinea pigs raised in long wavelength red light became more myopic compared with animals raised in short wavelength blue light.6−8 In contrast, tree shrews and rhesus monkeys raised in long wavelength light demonstrated slowed eye growth and hyperopia.9−12 Additionally, tree shrews raised in short wavelength light demonstrated relative myopia.11,13
The choroid may be involved in the regulation of eye growth. In experimental models, changes in choroidal thickness precede and predict changes in eye growth. For example, the choroid thickens in response to myopic defocus, followed by slowing of eye growth and hyperopia, whereas the choroid thins in response to hyperopic defocus, followed by increased eye growth and myopia.14−16 Choroidal thickness has been shown to be affected by light exposure. Red light rearing of tree shrews and rhesus monkeys that promoted hyperopia and slower eye growth also resulted in choroidal thickening compared with control animals raised in normal lighting.10,12 Changes in the choroid typically occur rapidly and are observed before long-term changes in eye growth.14−17
Another potential mechanism underlying the protective effects of light on eye growth may involve the intrinsically photosensitive retinal ganglion cells (ipRGCs). The ipRGCs are a class of retinal ganglion cells which express the photopigment melanopsin18 and are tuned to short wavelength light, with a peak sensitivity of approximately 482 nm.19,20 The ipRGCs are predominantly involved in non–image-forming functions and project to brain areas including the suprachiasmatic and olivary pretectal nuclei, which are involved in mediating circadian rhythm entrainment and the pupillary light reflex, respectively.18,21 ipRGC activity can be assessed in vivo through the pupillary light reflex. The initial, transient pupil constriction in response to a pulse of light is attributed to rods and cones, whereas the sustained pupil constriction after short wavelength light offset, known as the post-illumination pupil response (PIPR), is primarily attributed to the ipRGCs.22,23 Although the involvement of the ipRGCs in myopia development is speculative,1,24 their unique light-sensitive characteristics make them a potential candidate.
The contradictory effects of narrowband short and long wavelength light on eye growth in animals make it difficult to extrapolate findings to humans. Determining how the eye responds to short-term manipulations of light exposure is required before looking at long-term effects on eye growth to better understand the potential factors involved. Given the potential for both the choroid and ipRGCs to be involved in the protective effects of light, it is relevant to understand how they are affected by exposure to different wavelengths of light. The goal of this study was to determine the effects of short-term narrowband light exposure on choroidal thickness and the ipRGC-driven pupil response in humans.
Methods
Subjects
Twenty subjects, ages 21 to 43 years, participated in this study. The study was approved by the institutional review board at the University of Houston and procedures followed the tenets of the Declaration of Helsinki. Informed consent was obtained after explaining the nature of the study to subjects.
Visual acuity was measured with subject's habitual correction. All subjects had a best-corrected visual acuity of 20/25 or better. Noncycloplegic autorefraction was measured for both eyes (WAM-5000, Grand Seiko, Tokyo, Japan). Exclusion criteria included ocular pathology, prescription or over-the-counter medications known to affect sleep or the pupil, use of sleep aids such as melatonin, and shift work or travel across more than two time zones during the month before the first visit. No subjects had any systemic disease with ocular manifestations, such as diabetes or hypertension. No subjects reported being pregnant or breastfeeding.
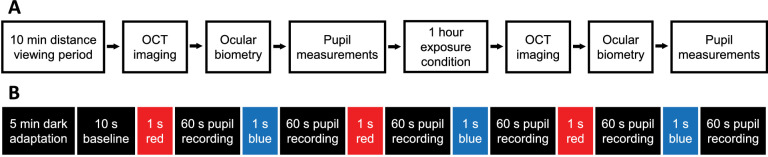
Each subject underwent four experimental sessions, in which ocular imaging and pupillometry were performed before and after 1 hour of exposure to either broadband light, narrowband long wavelength “red” or short wavelength “blue” light, or darkness, as described elsewhere in this article. The order of the conditions was randomized for each subject and each of the visits were scheduled 5 to 20 days apart. The dark condition was added to the protocol later, and therefore took place 5 to 190 days after visit 3. All experimental sessions occurred between 8:00 AM and 11:00 AM to minimize potential effects of circadian variation on choroidal thickness and the pupil.25−30 Subjects were asked to abstain from caffeinated and alcoholic beverages the morning of each laboratory visit, which have been shown to affect choroidal thickness.31−33
Ocular Imaging and Biometry
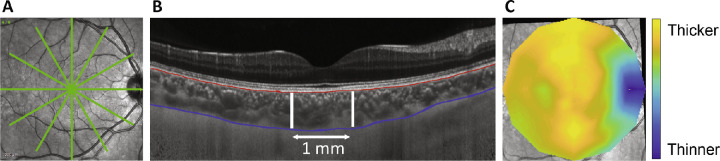
Before the ocular imaging measurements for each condition, subjects first underwent a 10-minute distance viewing period to minimize effects of prior ocular accommodation and physical activity. During this time, subjects viewed a television at 4 m under an illumination of 60 lux measured in the vertical plane at eye level (LX1330B; Dr. Meter, Union City, CA). Ocular imaging was then performed using spectral domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg, Germany). Two high-quality images (signal strength >35 dB) of the back of the right eye were collected. The scan protocol included a six-line 30° radial scan centered at the fovea (Fig. 1). For each subject, the first image at the first visit was set as the reference for subsequent imaging. Then, the axial length was measured for the right eye using a noncontact low-coherence optical biometer (LenStar, Haag-Streit, Köniz, Switzerland). Five measurements were collected and averaged. Axial length was measured as a correlate to choroidal thickness; previous studies have shown that short-term fluctuations in choroidal thickness are accompanied by fluctuations in axial length in the opposite direction.25−27 After these measurements, nonmydriatic pupillometry was performed (Fig. 2A).
Figure 1.
(A) A six-line 30° radial scan protocol used for SD-OCT imaging. (B) Segmentation of Bruch's membrane (red) and the choroid/sclera border (blue). Choroidal thickness was averaged for a 1-mm wide region centered at the fovea. (C) Representative choroidal thickness map generated from the segmentations. Yellow areas represent thicker regions of the choroid and blue areas represent thinner regions of the choroid.
Figure 2.
(A) Experimental protocol. Subjects first underwent a 10-minute distance viewing task under 60 lux room illumination. OCT imaging, ocular biometry, and pupil measurements were conducted before and after a 1-hour exposure condition. (B) Pupil stimulation protocol. Five minutes of dark adaptation was followed by 10 seconds of baseline pupil diameter recording. Three 1-second red and three 1-second blue stimuli were presented in alternating order, with a 60-second interstimulus interval.
Pupillometry
The pupillometry protocol has been described in detail previously.34,35 A frame mounted eye tracker with infrared illumination (Viewpoint EyeTracker, Arrington Research, Scottsdale, AZ) was used to record the pupil diameter of the right eye at 60 Hz. The infrared light-emitting diode light source has a lambda max of 943 nm with a half-max width of 46 nm (Ocean Optics Spectrometer, Largo, FL). The pupil diameter was calibrated for each subject by capturing an image of a 5-mm printed black circle positioned approximately at the subject's corneal plane. After calibration, subjects dark adapted for 5 minutes (<0.1 lux), then placed their head on a chinrest with an light-emitting diode-driven Ganzfeld system (Color Burst, Espion, Diagnosys LLC, Lowell, MA) centered 10 mm in front of the left eye, providing full-field stimulation. Subjects viewed a red fixation point located 3 m away with the right eye to maintain primary gaze and minimize accommodation. The baseline pupil diameter was recorded for 10 seconds before the onset of the first stimulus. Stimuli were presented to the left eye and consisted of six 1-second long pulses of either long wavelength red light or short wavelength blue light, presented in alternating order, with a 60-second interstimulus interval (Fig. 2B). The 60-second pupil recordings between stimuli were performed in the dark (<0.1 lux). The red stimulus, which was always presented first, was 651 nm with a half-max width of 25 nm (Spectroradiometer CS1W, Konica Minolta, Tokyo, Japan) and set to 33.3 cd/m2, with a measured corneal irradiance of 5.58 × 1013 photons/cm2/s (Power Meter, Newport Corporation, Irvine, CA). The blue stimulus was 456 nm with a half-max width of 20 nm and set to 16.67 cd/m2, with a measured corneal irradiance of 5.85 × 1013 photons/cm2/s, which is known to be above the melanopsin threshold.20,36 The stimulus intensities used have been shown to elicit similar pupil constriction.37 The same pupillometry protocol was repeated after the 1-hour exposure session, but without a 5-minute dark adaptation period.
Experimental Conditions
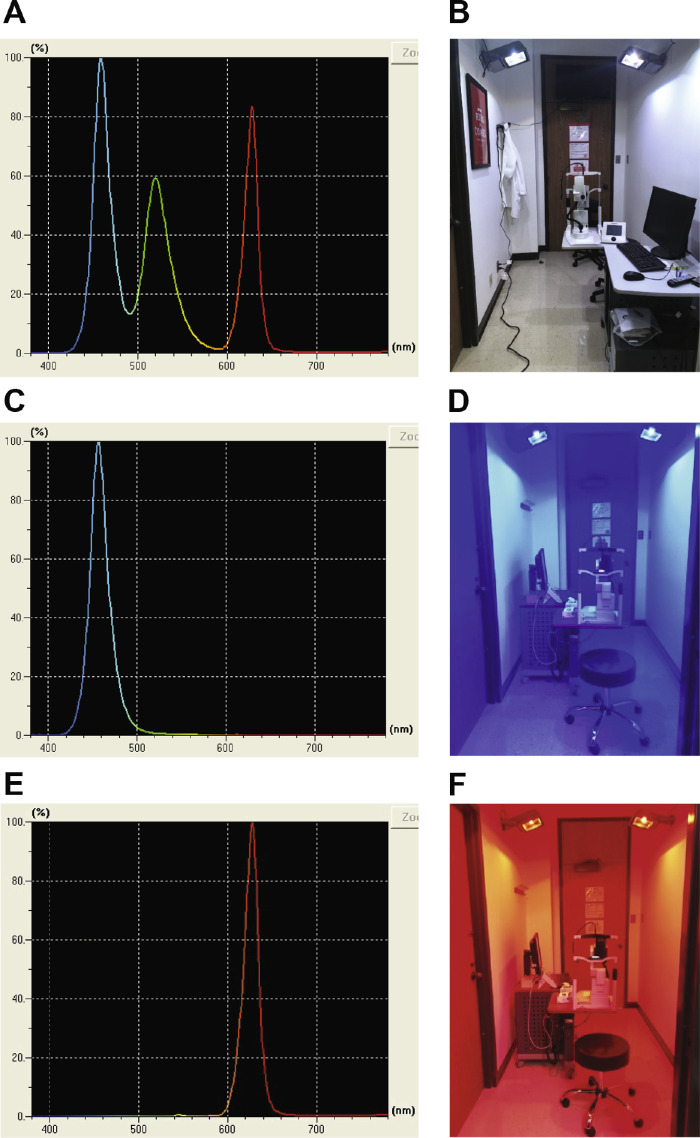
After SD-OCT imaging and pupillometry, subjects were exposed to one of four exposure conditions for 1 hour: narrowband blue light (456 nm, half-max width of 25 nm, 35 cd/m2, 1.0 W/m2), narrowband red light (630 nm, half-max width of 20 nm, 80 cd/m2, 1.0 W/m2), broadband light (1.0 W/m2; Spectroradiometer CS1W, Konica Minolta), or darkness (<0.1 lux). Spectral composition for each condition is shown in Figure 3. The alpha-opic irradiances for each photoreceptor are shown in Supplementary Table 1 and Supplementary Figure 1 and were calculated using the CIE S 026 Toolbox.38 Broadband light exposure was considered the control condition, such that any changes likely reflected normal diurnal rhythms known to occur in choroidal thickness and the pupil. Dark exposure was considered to be an extended dark adaptation period. The blue, red, and broadband lights were calibrated such that they were of similar irradiance, measured in the vertical plane at the subject's eye level when they were positioned for the experiment and viewing the target. The light sources consisted of five light-emitting diode panels mounted in a room that was approximately 2.5 m × 1 m and the lights illuminated the entire room. During the 1-hour exposure for broadband, blue, and red light, subjects viewed a television in a mirror, for a total viewing distance of 5 m, to maintain relaxed accommodation. The television was covered with a blue (LEE 172 Lagoon Blue, PNTA, Seattle, WA) or red filter (LEE 106 Primary Red) for the blue light exposure and red light exposure, respectively. During the dark condition, subjects listened to music or a podcast. Subjects were instructed not to use their personal electronic devices during any of the experimental sessions. After the 1-hour exposure, SD-OCT imaging, biometry, and pupillometry were repeated. The SD-OCT instrument was located in the exposure room so that imaging took place immediately after the 1-hour exposure, without the subjects needing to change position and still under the same conditions as the 1-hour exposure.
Figure 3.
Spectral composition and room set up for (A, B) broadband light (C, D) short wavelength blue light, and (E, F) long wavelength red light.
Data Analysis
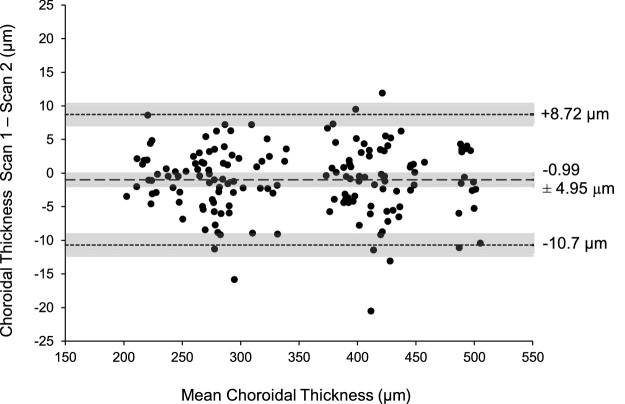
The SD-OCT images were exported and analyzed with a custom MATLAB program (Mathworks, Natick, MA) with a semiautomated procedure. Lateral magnification was adjusted based on each individual's axial length and corneal curvature data. A three-surface schematic eye was constructed for each subject based on Bennett and Rabbetts’ description.39,40 Transverse scaling was calculated for each subject as previously described.27 Bruch's membrane was segmented and manually corrected for any segmentation errors. The choroid/sclera border was manually segmented and axial thickness was determined for 1536 points along each of the six scan lines. The average thickness in a 1-mm wide region centered at the fovea was calculated as the choroidal thickness. The mean difference and 95% limits of agreement between the two images collected at both time points for each condition were determined with Bland-Altman analysis to assess within-session repeatability of choroidal thickness measurements in the central 1 mm region.41
Pupil data were analyzed offline with a custom MATLAB program. Blinks were identified as intervals of pupil aspect ratio outside six standard deviations of the mean pupil aspect ratio during stable fixation. Blinks and samples that were deemed poor quality by the instrument were removed from the pupil trace. For each pupillometry session, data for the three red stimuli were averaged, and data for the three blue stimuli were averaged. Pupil metrics included baseline pupil diameter, maximum constriction, and the 6-second PIPR. The baseline pupil diameter was calculated by averaging the pupil diameter during the 10-second recording period before the onset of the first stimulus. Relative pupil sizes were calculated by dividing the pupil diameter by the baseline pupil diameter. Maximum constriction was calculated as the difference between the minimum pupil diameter during light stimulation and the baseline pupil diameter, expressed in percent. The 6-second PIPR was calculated as the difference between the pupil diameter averaged over 6 to 7 seconds after stimulus offset and the baseline pupil diameter, expressed in percent. Data are presented as difference from baseline, such that a greater value for maximum constriction and the 6-second PIPR indicates a stronger pupil response.
Statistical Analysis
Statistical analyses were performed with IBM SPSS Statistics version 26 (IBM, Armonk, NY). Data are expressed as mean ± standard deviation unless otherwise indicated. Outliers were detected with box plots. For choroidal thickness, axial length, and pupil metrics, a two-way repeated measures ANOVA was performed with two within-subjects factors, condition and time, and Greenhouse-Geisser correction was applied when appropriate. Post hoc paired t-tests were performed on the difference values between before and after measurements to compare the change in choroidal thickness, axial length, and pupil metrics between conditions. All post hoc tests were corrected for multiple comparisons using the Benjamini-Hochberg correction and P < 0.05 was considered statistically significant.
Results
The mean subject age was 27.9 ± 6.1 years (12 females, 8 males). Mean spherical equivalent refraction of the right and left eyes was −1.47 ± 2.19 diopters and −1.59 ± 2.45 diopters, respectively. Right and left eyes were not significantly different from each other (P = 0.28).
Choroidal Thickness and Axial Length
For the assessment of within-session repeatability of choroidal thickness, Bland-Altman analysis showed that the mean difference between the two images collected within approximately 1 minute was −0.99 ± 4.95 µm, with a 95% limits of agreement from +8.72 to −10.70 µm (Fig. 4).
Figure 4.
Bland-Altman analysis of the difference between scan 1 and scan 2 against mean choroidal thickness to assess within-session repeatability. The dashed and dotted lines represent the mean difference and 95% limits of agreement, respectively. Shaded areas represent the 95% confidence intervals for the mean difference and 95% limits of agreement.
Two subjects were identified as extreme outliers for choroidal thickness and were excluded from the choroidal thickness analysis. Across the four conditions, choroidal thickness before the 1-hour exposure condition was not significantly different (P = 0.21). Repeated measures ANOVA for choroidal thickness revealed a significant main effect of time, F(1,17) = 18.40, P < 0.001, and a significant interaction effect of condition by time, F(3,51) = 5.88, P = 0.002. Post hoc analyses revealed that the choroid was significantly thinner after 1 hour of broadband light, red light, and dark exposure compared with before exposure (P < 0.05 for all), but not after blue light exposure (P = 0.39; Table 1). Paired t-tests comparing the change in choroidal thickness between conditions revealed that the choroid thinned more with red light and dark exposure compared with after blue light exposure (P = 0.006 and 0.006, respectively; Fig. 5A). There were no significant differences in the 1-hour choroidal thickness change between broadband light exposure and blue light exposure (P = 0.06), red light exposure (P = 0.35), or with darkness (P = 0.27). Similarly, there was no significant difference in the 1-hour choroidal thickness change between red light exposure and darkness (P = 0.80).
Table 1.
Choroidal Thickness (µm) Before and After Exposure for Each Condition (mean ± standard deviation)
| Exposure Condition | ||||
|---|---|---|---|---|
| Broadband Light | Blue Light | Red Light | Darkness | |
| Before exposure | 346.97 ± 91.34 µm | 346.92 ± 86.68 µm | 350.57 ± 89.68 µm | 349.74 ± 89.00 µm |
| After exposure | 342.74 ± 91.12 µm | 346.13 ± 88.23 µm | 344.66 ± 88.77 µm | 343.45 ± 91.73 µm |
| Difference | −4.23 ± 6.61 µm | −0.79 ± 3.79 µm | −5.91 ± 5.72 µm | −6.29 ± 6.30 µm |
| P value | 0.015* | 0.39 | <0.001* | 0.001* |
P values are shown for before exposure vs after exposure.
* P < 0.05.
Figure 5.
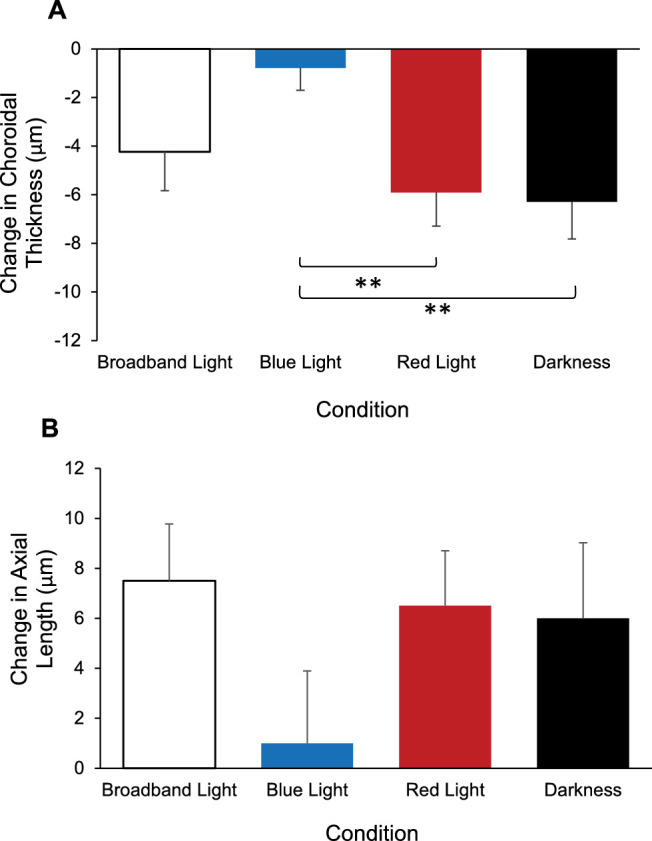
Change in (A) choroidal thickness and (B) axial length after 1 hour of exposure for each condition; broadband light (open bars), blue light (blue bars), red light (red bars), and darkness (black bars). Error bars represent standard error of the mean. **P < 0.01.
For axial length, repeated measures ANOVA revealed a significant main effect of time, F(1,19) = 9.64, P = 0.006, indicating that axial length increased after 1 hour compared with before exposure, regardless of condition (Fig. 5B). However, there was no significant interaction effect of condition by time, F(3,57) = 1.56, P = 0.21 (Table 2).
Table 2.
Results From Two-Way Repeated Measures ANOVA for Within-Subjects Factors (Condition and Time), and the Interaction of Condition by Time
| Condition | Time | Condition × Time | ||||
|---|---|---|---|---|---|---|
| Parameter | F-Value (Degrees of Freedom) | P Value | F-Value (Degrees of Freedom) | P Value | F-Value (Degrees of Freedom) | P Value |
| Choroidal thickness | 0.607 (3,51) | 0.61 | 18.40 (1,17) | <0.001* | 5.88 (3,51) | 0.002* |
| Axial length | 0.203 (3,57) | 0.89 | 9.64 (1,19) | 0.006* | 1.56 (3,57) | 0.21 |
| Pupil response to red stimulus | ||||||
| Maximum constriction | 7.82 (3,51) | <0.001* | 36.28 (1,17) | <0.001* | 26.87 (3,51) | <0.001* |
| 6 s PIPR | 1.31 (2.13,36.23) | 0.28 | 13.15 (1,17) | 0.002* | 0.913 (3,51) | 0.44 |
| Pupil response to blue stimulus | ||||||
| Maximum constriction | 9.19 (2.24,38.13) | <0.001* | 58.82 (1,17) | <0.001* | 23.38 (3,51) | <0.001* |
| 6 s PIPR | 8.60 (3,51) | <0.001* | 51.75 (1,17) | <0.001* | 13.77 (3,51) | <0.001* |
P < 0.05.
Pupil Responses
Two subjects were excluded from pupil analysis owing to excessive fluctuations in the pupil boundary and blinks. Pupil traces for the remaining 18 subjects for each condition are shown in Supplementary Figure 2. Baseline pupil diameter, that is, the pupil diameter recorded over 10 seconds before the first red stimulus, was not significantly different between conditions (P = 0.35) or before and after exposure for all conditions (P = 0.55; Table 3).
Table 3.
Baseline Pupil Diameter (mm) Before and After Exposure for Each Condition (mean ± standard deviation)
| Exposure Condition | ||||
|---|---|---|---|---|
| Broadband Light | Blue Light | Red Light | Darkness | |
| Before exposure | 6.39 ± 0.81 mm | 6.15 ± 0.76 mm | 6.27 ± 0.92 mm | 6.31 ± 0.75 mm |
| After exposure | 6.52 ± 0.79 mm | 6.09 ± 0.74 mm | 6.22 ± 0.86 mm | 6.14 ± 0.67 mm |
There were no significant differences in baseline pupil diameter across condition (P = 0.35) or across time (P = 0.55).
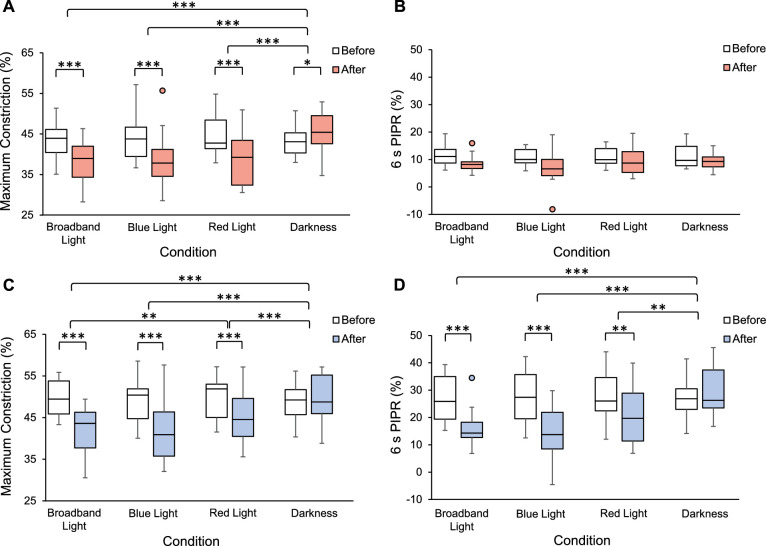
Maximum pupil constriction to a 1-second red stimulus showed significant main effects of condition, F(3,51) = 7.82, P < 0.001, and time, F(1,17) = 36.28, P < 0.001, and a significant interaction effect of condition by time, F(3,51) = 26.87, P < 0.001 (Table 2). Post hoc analyses revealed that, compared with before exposure, maximum constriction significantly decreased after broadband, blue, and red light exposure (P < 0.001 for all), but increased after dark exposure (P = 0.02; Table 4). These findings suggest that the rods and cones were less sensitive after the light exposure conditions and more sensitive after dark exposure. Paired t-tests revealed that the change in maximum constriction following broadband, blue, and red light exposure were significantly different than after dark exposure (P < 0.001 for all; Fig. 6A). There were no significant differences in the 1-hour change in maximum constriction between broadband, blue, and red light exposure conditions (P > 0.05 for all).
Table 4.
Maximum Constriction (% Change From Baseline) and the 6-second PIPR (% Change From Baseline) for 1 Second Red and 1 Second Blue Stimulations Before and After Exposure for Each Condition (mean ± standard deviation)
| Red Stimulus | Blue Stimulus | ||||||
|---|---|---|---|---|---|---|---|
| Exposure Condition | Parameter | Before Exposure | After Exposure | P Value | Before Exposure | After Exposure | P Value |
| Broadband light | Maximum constriction | 43.67 ± 4.17 | 37.84 ± 5.23 | <0.001* | 49.84 ± 4.34 | 41.82 ± 5.25 | <0.001* |
| 6 s PIPR | 11.62 ± 3.78 | 8.42 ± 2.96 | nsϮ | 27.02 ± 8.26 | 15.59 ± 6.27 | <0.001* | |
| Blue light | Maximum constriction | 43.89 ± 5.41 | 38.37 ± 6.40 | <0.001* | 49.26 ± 5.48 | 41.41 ± 6.87 | <0.001* |
| 6 s PIPR | 10.76 ± 3.04 | 7.13 ± 5.87 | nsϮ | 27.27 ± 9.82 | 14.69 ± 9.35 | <0.001* | |
| Red light | Maximum constriction | 44.40 ± 4.52 | 39.08 ± 6.53 | <0.001* | 49.77 ± 4.77 | 44.87 ± 5.94 | <0.001* |
| 6 s PIPR | 10.97 ± 3.28 | 9.54 ± 5.05 | nsϮ | 27.99 ± 8.85 | 20.88 ± 10.43 | 0.003* | |
| Darkness | Maximum constriction | 43.12 ± 3.58 | 45.30 ± 4.90 | 0.015* | 48.68 ± 4.88 | 49.47 ± 5.64 | 0.28 |
| 6 s PIPR | 11.20 ± 4.40 | 9.25 ± 2.89 | nsϮ | 27.14 ± 6.83 | 29.32 ± 9.20 | 0.073 | |
P values are shown for before exposure vs after exposure.
P < 0.05.
No significant effect of time.
Figure 6.
(A) Maximum constriction (%) and (B) 6-second PIPR (%) before (open bars) and after 1 hour of exposure (red bars) to broadband light, blue light, red light, or darkness in response to a 1-second red stimulus. (C) Maximum constriction (%) and (D) 6-second PIPR (%) before (open bars) and after 1 hour of exposure (blue bars) to broadband light, blue light, red light, or darkness in response to a 1 second blue stimulus. *P < 0.05, **P < 0.01, ***P < 0.001.
The 6-second PIPR to a 1-second red stimulus showed a significant main effect of time, F(1,17) = 13.15, P = 0.002, such that the 6-second PIPR decreased after 1 hour compared with before exposure, regardless of condition (Fig. 6B). However, there was no significant interaction effect of condition by time, F(3,51) = 0.91, P = 0.44 (Table 4).
Maximum pupil constriction to a 1-second blue stimulus showed significant main effects of condition, F(2.24,38.13) = 9.19, P < 0.001, and time, F(1,17) = 58.82, P < 0.001, and a significant interaction effect of condition by time, F(3,51) = 23.38, P < 0.001 (Table 2). Similar to what was observed in response to a 1-second red stimulus, post hoc analyses revealed that maximum constriction to a 1-second blue stimulus significantly decreased after broadband, blue, and red light exposure compared with before exposure (P < 0.001 for all), but not after dark exposure (P = 0.28; Table 4). Paired t-tests revealed that the change in maximum constriction after broadband, blue, and red light exposure were significantly different than after dark exposure (P < 0.001 for all). Additionally, the maximum constriction following broadband light exposure was significantly different than after red light exposure (P = 0.008; Fig. 6C).
The 6-second PIPR to a 1-second blue stimulus showed significant main effects of condition, F(3,51) = 8.60, P < 0.001, and time, F(1,17) = 51.75, P < 0.001, and a significant interaction effect of condition by time, F(3,51) = 13.77, P < 0.001 (Table 2). Post hoc analyses revealed that, compared with before exposure, the 6-second PIPR significantly decreased after broadband, blue, and red light exposure (P < 0.005 for all), indicating decreased sensitivity of the ipRGCs, whereas there was no significant difference in the 6-second PIPR after dark exposure (P = 0.07; Table 4). Paired t-tests revealed a significant difference in the change in the 6-second PIPR following broadband, blue, and red light exposure compared with after dark exposure (P < 0.005 for all; Fig. 6D). There were no significant differences in the decrease in the 6-second PIPR between broadband, blue, and red light exposure conditions (P > 0.05 for all).
Discussion
The aim of this study was to examine the effects of narrowband long and short wavelength light exposure, which have been shown, in animal studies, to have differential effects on eye growth.7,8,11,42 We examined the short-term effects of manipulation of light exposure on the human eye to begin to understand the potential mechanisms of the longer term observed effects on eye growth. This study demonstrates that 1 hour of narrowband light and dark exposure have differing effects on choroidal thickness and on rod/cone- and ipRGC-driven pupil responses. Previous studies show that the choroid undergoes thinning in the morning owing to normal diurnal variation.25−27,30 This expected choroidal thinning was observed after 1 hour of exposure to broadband and red light, as well as darkness. However, after the 1-hour exposure to blue light, choroidal thinning was not observed; rather, blue light exposure resulted in a relative choroidal thickening compared with red light and dark exposure. This finding suggests that short-term exposure to long and short wavelengths induces differing effects on choroidal thickness. Axial length change between conditions tended to mirror changes in choroidal thickness, although the differences did not reach significance. Given that changes in choroidal thickness tend to precede changes in eye growth, these results, although speculative, suggest that the wavelength of light may have differential effects on eye growth.
It has been proposed that the wavelength of light can modulate eye growth in one of two mechanisms based on longitudinal chromatic aberration.11 First, the eye may use wavelength as a target. In this case, the eye would grow slower in short wavelength light and greater in long wavelength light to match the focal plane of the dominant wavelength. This case would be expected to cause an initial choroidal thickening (in short wavelength light) or thinning (in long wavelength light) to move the retina toward the focal plane. Alternatively, the eye may use wavelength as a cue, such that long wavelengths would signal that the eye is too long, causing choroidal thickening and slowed growth, and short wavelengths would signal that the eye is not long enough, causing choroidal thinning and accelerated eye growth to achieve emmetropia. Our results suggest that, for short-term exposure, the wavelength of light acted as a target; blue light resulted in relative choroidal thickening compared with red light exposure. These findings are similar to those reported by Read et al.43 The authors reported a small, but statistically significant, increase in choroidal thickness after light therapy for 30 minutes every morning for 1 week. Light therapy was delivered using glasses that emitted 506 lux of blue-green light with a peak wavelength of 500 nm, which is close to the peak sensitivity of ipRGCs.19,20
Berkowitz et al.44 showed that, in mice, melanopsin contributes to the choroidal thickening observed during the transition from dark to light. Here, we observed that exposure to blue light, which the ipRGCs are most sensitive to, resulted in relative choroidal thickening, suggesting a potential role of melanopsin in the response of the choroid to changes in light exposure. Further studies may be able to distinguish contributions of chromatic aberration- and ipRGC-mediated mechanisms of the effects of wavelength on choroidal thickness.
There were no differences in choroidal thickness between 1 hour of exposure to broadband light or 1 hour in darkness. Previous studies in humans have reported that 30 minutes of dark adaptation resulted in significant choroidal thickening, and this observed thickening was reversed after adapting to normal room lighting again.45 However, the experiments were conducted in the early evening, a time in which the choroid is expected to thicken owing to normal diurnal variation.25−27 A study in chicks showed that continuous long-term darkness results in choroidal thinning and greater axial elongation compared with chicks raised under normal light/dark cycles.46 Therefore, the timing and duration of dark exposure may also be important for modulating choroidal thickness and eye growth, and could account for differences observed between the current and previous studies.
In addition to choroidal thickness and axial length, we also assessed changes in the pupil response after light and dark exposure conditions. Maximum constriction to both a 1-second red and a 1-second blue stimulus was attenuated after 1 hour of exposure to broadband, blue, and red light. After 1 hour of exposure to darkness, the maximum constriction increased for a 1-second red stimulus and was not significantly different than before dark exposure for a 1-second blue stimulus. Maximum constriction represents the initial pupil constriction in response to a light stimulus and is primarily attributed to rod and cone activity.47,48 Therefore, decreased pupil constriction after 1 hour of light exposure indicates decreased sensitivity of the rods and cones, and increased pupil constriction after 1 hour of dark exposure indicates increased sensitivity of rods and cones. These findings are consistent with previous studies demonstrating the effects of light- and dark-adapted conditions on pupil constriction.49,50
We found that the PIPR to a 1-second blue, melanopsin-activating stimulus decreased after 1 hour of exposure to broadband, blue, and red light compared with after 1 hour of exposure to darkness. In contrast, the PIPR to a 1-second red stimulus decreased after 1 hour, independent of exposure condition. The PIPR, or sustained pupil constriction, observed after a blue stimulus offset is primarily attributed to intrinsic ipRGC activity.22 A larger sustained pupil constriction indicates a greater response from ipRGCs. Previous studies suggest that the PIPR is influenced by prior light exposure51,52 and is affected differently in light-adapted versus dark-adapted conditions.49,53 It has been previously shown that the PIPR in response to blue stimuli increases with increasing dark adaptation time, particularly in the first 20 minutes.50 However, we did not observe a significant increase in the 6-second PIPR to short wavelength stimuli after 1 hour of dark exposure. This could be due to a number of reasons. There was significant intersubject variability in the 6-second PIPR response; some subjects demonstrated a large increase in the 6-second PIPR after dark exposure, whereas other subjects demonstrated a decrease. This variation may be due to individual subjects state of arousal; studies have suggested that a correlation exists between the PIPR and subjective sleepiness, such that the PIPR is smaller when subjects are sleepier.49 Although we did not measure sleepiness, it is possible that exposure to 1 hour of darkness increased subjects’ sleepiness. Because we did not observe a significant difference in the 6-second PIPR after 1 hour of dark exposure, our results suggest that 5 minutes of dark adaptation should be sufficient to obtain a stable PIPR response.
Given the spectral sensitivity of ipRGCs and evidence of melanopsin bistability,51,54−56 we hypothesized that 1 hour of exposure to blue versus red light would have differential effects on the PIPR. Specifically, it was expected that 1 hour of exposure to blue light would attenuate the PIPR, whereas red light would increase the PIPR. However, the 6-second PIPR was attenuated after both blue and red light exposure with no significant difference between the two conditions; narrowband blue and red light did not have differential effects on ipRGC activity. Sustained pupil responses and maximum constriction tend to be correlated;50 thus, it is possible that the attenuated PIPR amplitudes after broadband and narrowband light exposures were due to the decrease in maximum constriction amplitudes. This may be a potential confounding factor in the interpretation of the effect of the light exposure conditions on the PIPR. Furthermore, in our pupillometry protocol, subjects dark adapted for 5 minutes before measuring pupil responses before the light exposure sessions. After the light exposure sessions, this 5-minute dark adaptation session was not included to ensure that subjects were still adapted to the exposure condition. Mure et al.57 demonstrated that prior exposure to long wavelength light enhances the pupillary constriction to short wavelength light in vivo, whereas pre-exposure to short wavelength light attenuates the response. In their protocol, they used a 40-minute dark adaptation period in between light exposures and measured the steady-state pupil constriction to a 5-minute short wavelength light stimulus. Thus, it is also important to consider potential differences in ipRGC responses between dark-adapted and light-adapted protocols.
Evidence that light exposure does affect the PIPR, and therefore ipRGC activity, has been demonstrated in previous studies. In children, objectively measured light exposure during the previous 24 hours was associated with the 6-second PIPR.34 In addition, Münch et al.58,59 demonstrated that seasonal differences in the melanopsin-mediated pupil response exist, suggesting that the responsiveness of the ipRGCs differs between winter and summer seasons. This finding is relevant because the spectral composition, intensity, and duration (i.e., photoperiod) of environmental light differ across seasons, particularly between winter and summer months. Overall light exposure, as well as the relative contribution of blue light, are both higher in the summer compared with the winter months.60−62 In contrast, Bruijel et al.63 found high test–retest reliability of the PIPR between the winter and summer months with no significant difference between seasons. The discrepancy between such studies could be due to different methods of assessing the PIPR. Whereas Münch et al. defined the PIPR as the relative pupil contraction 6 seconds after stimulus offset, similar to our protocol, Bruijel et al. calculated the PIPR as the average pupil diameter from 2 to 4 minutes after a 5-minute stimulus offset. It has been suggested that the PIPR measured 6 seconds after light offset to a 1-second stimulus is the optimum protocol for assessing ipRGC activity because it produces large PIPR amplitudes and shows lower intra-individual variability.23 Seasonal differences have also been observed in the rate of myopia progression and axial elongation, with slower progression and axial elongation observed in the summer compared with the winter months.64−66 Whether this difference in the rate of eye growth is associated with differences in photoperiod and the ipRGC-mediated pupil response has not been elucidated. Because ipRGCs are responsible for detecting environmental light, they may potentially be involved in the protective effects of light on myopia development. Further studies are required to confirm this hypothesis to determine whether long-term differences in the spectral composition or intensity of light affect the ipRGCs and how this relates to axial elongation and refractive development.
Light exposure also affects circadian rhythms;67 therefore, it is possible that the narrowband light exposure induced a circadian phase shift. Light exposure in the morning tends to phase advance the circadian rhythm and light exposure in the evening tends to phase delay the rhythm. This study was conducted in the morning only. If the study had been conducted in the evening, different results might have been observed. Whether the results are due to a direct effect of light exposure on choroidal thickness and the pupil response or owing to a shift in circadian phase requires further research.
One limitation of this study is that the light sources used for the light exposure conditions were equated by irradiance. Irradiance measurements do not take into account the spectral sensitivity of the eye; thus, the perceived brightness of the lights were different. The luminance of the red light (80 cd/m2) was higher than the luminance of the blue light (35 cd/m2). If the differences in luminance significantly contributed to the observed effects on choroidal thickness and the pupil response, then we would have expected a greater difference between red light and dark exposure and not between blue light and dark exposure. Thus, the observed effects were likely due to the wavelength of light. A deeper understanding of the underlying mechanisms requires measuring the response to a range of narrowband wavelengths and light intensities, which is beyond the scope of the current study. Another limitation is that measurements were only conducted at two time points, before and after 1 hour of light exposure; therefore, the time course of the observed effects on choroidal thickness or the pupil and how long they last after exposure offset are unknown. It is possible that changes occur within the first few minutes of light exposure and then changes or reverses over time. Determining the timing or duration of the effects may also help to understand the underlying mechanisms involved in the choroid and pupil responses to different wavelengths of light.
In conclusion, this study demonstrates that there are differing effects of short-term 1 hour of narrowband and broadband light and dark exposure on the choroid and rod/cone and ipRGC activity. Short-term exposure to narrowband blue and red light induced differential responses on choroidal thickness, but similar changes on the melanopsin-driven pupil response. Future studies to determine whether this effect can be observed with longer term manipulation of light exposure are warranted to better understand the effects of the spectral composition of light on the eye.
Supplementary Material
Acknowledgments
The authors thank Hope Queener and Nimesh Patel for assistance in this study.
Supported by NIH R01EY030193 and NIH P30 EY007551.
Disclosure: L. Lou, None; L.A. Ostrin, None
References
- 1. Norton TT, Siegwart JT. Light levels, refractive development, and myopia – a speculative review. Exp Eye Res. 2013; 114: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramamurthy D, Lin Chua SY, Saw S-M. A review of environmental risk factors for myopia during early life, childhood and adolescence. Clin Exp Optom. 2015; 98: 497–506. [DOI] [PubMed] [Google Scholar]
- 3. Rose KA, Morgan IG, Ip J, et al.. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008; 115: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 4. Read SA, Collins MJ, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015; 56: 6779–6787. [DOI] [PubMed] [Google Scholar]
- 5. Rucker F. Monochromatic and white light and the regulation of eye growth. Exp Eye Res. 2019; 184: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long Q, Chen D, Chu R. Illumination with monochromatic long-wavelength light promotes myopic shift and ocular elongation in newborn pigmented guinea pigs. Cutaneous Ocular Toxicol. 2009; 28: 176–180. [DOI] [PubMed] [Google Scholar]
- 7. Foulds WS, Barathi VA, Luu CD. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Invest Ophthalmol Vis Sci. 2013; 54: 8004–8012. [DOI] [PubMed] [Google Scholar]
- 8. Jiang L, Zhang S, Schaeffel F, et al.. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Res. 2014; 94: 24–32. [DOI] [PubMed] [Google Scholar]
- 9. Smith EL 3rd, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015; 56: 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gawne TJ, Ward AH, Norton TT. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017; 140: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gawne TJ, Siegwart JT Jr., Ward AH, Norton TT. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017; 155: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hung L-F, Arumugam B, She Z, Ostrin L, Smith EL 3rd. Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Exp Eye Res. 2018; 176: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gawne TJ, Ward AH, Norton TT. Juvenile tree shrews do not maintain emmetropia in narrow-band blue light. Optom Vis Sci. 2018; 95: 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wallman J, Wildsoet C, Xu A, et al.. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995; 35: 37–50. [DOI] [PubMed] [Google Scholar]
- 15. Wildsoet C, Wallman J.. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995; 35: 1175–1194. [DOI] [PubMed] [Google Scholar]
- 16. Hung L-F, Wallman J, Smith EL III. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000; 41: 1259–1269. [PubMed] [Google Scholar]
- 17. Read SA, Fuss JA, Vincent SJ, Collins MJ, Alonso-Caneiro D. Choroidal changes in human myopia: insights from optical coherence tomography imaging. Clin Exp Optom. 2019; 102: 270. [DOI] [PubMed] [Google Scholar]
- 18. Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002; 295: 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002; 295: 1070–1073. [DOI] [PubMed] [Google Scholar]
- 20. Dacey DM, Liao H-W, Peterson BB, et al.. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005; 433: 749–754. [DOI] [PubMed] [Google Scholar]
- 21. Hattar S, Kumar M, Park A, et al.. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006; 497: 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gamlin PDR, McDougal DH, Pokorny J, Smith VC, Yau K-W, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007; 47: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adhikari P, Zele AJ, Feigl B. The post-illumination pupil response (PIPR). Invest Ophthalmol Vis Sci. 2015; 56: 3838–3849. [DOI] [PubMed] [Google Scholar]
- 24. Chakraborty R, Ostrin LA, Nickla DL, Iuvone PM, Pardue MT, Stone RA. Circadian rhythms, refractive development, and myopia. Ophthalmic Physiol Opt. 2018; 38: 217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown JS, Flitcroft DI, Ying GS, et al.. In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci. 2009; 50: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011; 52: 5121–5129. [DOI] [PubMed] [Google Scholar]
- 27. Burfield HJ, Patel NB, Ostrin LA. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest Ophthalmol Vis Sci. 2018; 59: 5176–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zele AJ, Feigl B, Smith SS, Markwell EL. The circadian response of intrinsically photosensitive retinal ganglion cells. PloS One. 2011; 6: e17860–e17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Münch M, Léon L, Crippa SV, Kawasaki A. Circadian and wake-dependent effects on the pupil light reflex in response to narrow-bandwidth light pulses. Invest Ophthalmol Vis Sci. 2012; 53: 4546–4555. [DOI] [PubMed] [Google Scholar]
- 30. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 261–266. [DOI] [PubMed] [Google Scholar]
- 31. Kang HM, Woo YJ, Koh HJ, Lee CS, Lee SC. The effect of consumption of ethanol on subfoveal choroidal thickness in acute phase. Br J Ophthalmol. 2016; 100: 383. [DOI] [PubMed] [Google Scholar]
- 32. Vural DA, Kara AN, Sayin AN, Pirhan AD, Ersan AHB. Choroidal thickness changes after a single administration of coffee in healthy subjects. Retina. 2014; 34: 1223–1228. [DOI] [PubMed] [Google Scholar]
- 33. Altinkaynak H, Ceylan E, Kartal B, Keleş S, Ekinci M, Olcaysu OO. Measurement of choroidal thickness following caffeine intake in healthy subjects. Curr Eye Res. 2016; 41: 708–714. [DOI] [PubMed] [Google Scholar]
- 34. Ostrin LA. The ipRGC-driven pupil response with light exposure and refractive error in children. Ophthalmic Physiol Opt. 2018; 38: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ostrin LA, Abbott KS, Queener HM. Attenuation of short wavelengths alters sleep and the ipRGC pupil response. Ophthalmic Physiol Opt. 2017; 37: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barrionuevo PA, Nicandro N, McAnany JJ, Zele AJ, Gamlin P, Cao D. Assessing rod, cone, and melanopsin contributions to human pupil flicker responses. Invest Ophthalmol Vis Sci. 2014; 55: 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abbott KS, Queener HM, Ostrin LA. The ipRGC-driven pupil response with light exposure, refractive error, and sleep. Optom Vis Sci. 2018; 95: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. CIE System for Metrology of Optical Radiation for ipRGC-influenced Responses to Light. CIE. Vienna, Austria: CIE Central Bureau; 2018. [Google Scholar]
- 39. Bennett AG, Rabbetts RB. The schematic eye. In: Bennett AG,, Rabbetts RB, eds. Clinical visual optics. London, UK: Butterworths; 1989: 249–274. [Google Scholar]
- 40. Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994; 232: 361–367. [DOI] [PubMed] [Google Scholar]
- 41. Bland JM, Altman DG.. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 327: 307–310. [PubMed] [Google Scholar]
- 42. Liu R, Hu M, He JC, et al.. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci. 2014; 55: 1901–1909. [DOI] [PubMed] [Google Scholar]
- 43. Read SA, Pieterse EC, Alonso-Caneiro D, et al.. Daily morning light therapy is associated with an increase in choroidal thickness in healthy young adults. Sci Rep. 2018; 8: 8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berkowitz BA, Schmidt T, Podolsky RH, Roberts R. Melanopsin phototransduction contributes to light-evoked choroidal expansion and rod L-type calcium channel function in vivo. Invest Ophthalmol Vis Sci. 2016; 57: 5314–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alagöz C, Pekel G, Alagöz N, et al.. Choroidal thickness, photoreceptor thickness, and retinal vascular caliber alterations in dark adaptation. Curr Eye Res. 2016; 41: 1608–1613. [DOI] [PubMed] [Google Scholar]
- 46. Nickla DL, Wildsoet CF, Troilo D. Endogenous rhythms in axial length and choroidal thickness in chicks: implications for ocular growth regulation. Invest Ophthalmol Vis Sci. 2001; 42: 584–588. [PubMed] [Google Scholar]
- 47. Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology. 2009; 116: 1564–1573. [DOI] [PubMed] [Google Scholar]
- 48. Markwell EL, Feigl B, Zele AJ. Intrinsically photosensitive melanopsin retinal ganglion cell contributions to the pupillary light reflex and circadian rhythm. Clin Exp Optom. 2010; 93: 137–149. [DOI] [PubMed] [Google Scholar]
- 49. de Zeeuw J, Papakonstantinou A, Nowozin C, et al.. Living in biological darkness: objective sleepiness and the pupillary light responses are affected by different metameric lighting conditions during daytime. J Biol Rhythms. 2019; 34: 410–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang B, Shen C, Zhang L, et al.. Dark adaptation-induced changes in rod, cone and intrinsically photosensitive retinal ganglion cell (ipRGC) sensitivity differentially affect the pupil light response (PLR). Graefes Arch Clin Exp Ophthalmol. 2015; 253: 1997–2005. [DOI] [PubMed] [Google Scholar]
- 51. Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: evidence for photopigment bistability in vivo. J Biol Rhythms. 2007; 22: 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hansen MS, Sander B, Kawasaki A, Brondsted AE, Nissen C, Lund-Andersen H. Prior light exposure enhances the pupil response to subsequent short wavelength (blue) light. J Clin Exp Ophthalmol. 2011; 2. [Google Scholar]
- 53. Joyce DS, Feigl B, Zele AJ. The effects of short-term light adaptation on the human post-illumination pupil response. Invest Ophthalmol Vis Sci. 2016; 57: 5672–5680. [DOI] [PubMed] [Google Scholar]
- 54. Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005; 15: 1065–1069. [DOI] [PubMed] [Google Scholar]
- 55. Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005; 433: 741–745. [DOI] [PubMed] [Google Scholar]
- 56. Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005; 307: 600–604. [DOI] [PubMed] [Google Scholar]
- 57. Mure LS, Cornut P-L, Rieux C, et al.. Melanopsin bistability: a fly's eye technology in the human retina. PLoS One. 2009; 4: e5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Münch M, Kourti P, Brouzas D, Kawasaki A. Variation in the pupil light reflex between winter and summer seasons. Acta Ophthalmol. 2016; 94: e244–e246. [DOI] [PubMed] [Google Scholar]
- 59. Münch M, Ladaique M, Roemer S, Hashemi K, Kawasaki A. Melanopsin-mediated acute light responses measured in winter and in summer: seasonal variations in adults with and without cataracts. Front Neurol. 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thorne HC, Jones KH, Peters SP, Archer SN, Dijk D-J. Daily and seasonal variation in the spectral composition of light exposure in humans. Chronobiol Int. 2009; 26: 854–866. [DOI] [PubMed] [Google Scholar]
- 61. Ostrin LA, Sajjadi A, Benoit JS. Objectively measured light exposure during school and summer in children. Optom Vis Sci. 2018; 95: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ostrin LA. Objectively measured light exposure in emmetropic and myopic adults. Optom Vis Sci. 2017; 94: 229–238. [DOI] [PubMed] [Google Scholar]
- 63. Bruijel J, van der Meijden WP, Bijlenga D, et al.. Individual differences in the post-illumination pupil response to blue light: assessment without mydriatics. Biology (Basel). 2016; 5: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fulk WG, Cyert AL, Parker AD. Seasonal variation in myopia progression and ocular elongation. Optom Vis Sci. 2002; 79: 46–51. [DOI] [PubMed] [Google Scholar]
- 65. Donovan L, Sankaridurg P, Ho A, et al.. Myopia progression in Chinese children is slower in summer than in winter. Optom Vis Sci. 2012; 89: 1196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gwiazda J, Deng L, Manny R, Norton TT. Seasonal variations in the progression of myopia in children enrolled in the Correction of Myopia Evaluation Trial. Invest Ophthalmol Vis Sci. 2014; 55: 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tähkämö L, Partonen T, Pesonen A-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. 2019; 36: 151–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.