Abstract
Chronic non-specific inflammatory cell infiltration of the colon is generally considered to be the cause of ulcerative colitis (UC). Gloeostereum incarnatum (GI), a fungus rich in amino acids and fatty acids, exhibits a variety of biological functions. In the present study, GI was identified to contain 15 fatty acids, 17 amino acids and 11 metallic elements. The protective effect of GI against UC was investigated in C57BL/6 mice with UC induced by free drinking 3.5% dextran sulfate sodium (DSS). After a 21-day oral administration, GI prevented weight loss, enhancement of the disease activity index and colonic pathological alterations in mice with UC. GI reduced the levels of pro-inflammatory factors including interleukin (IL)-1β, IL-2, IL-6 and IL-12, tumor necrosis factor α and -β, interferon α and -γ, and pro-oxidative factors including reactive oxygen species and nitric oxide. In addition, it enhanced the levels of immunological factors including immunoglobulin (Ig)A, IgM and IgG, and antioxidative factors including superoxide dismutase and catalase in the serum and/or colon tissues. GI enhanced the expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream proteins and suppressed the phosphorylation of NF-κB signaling in colon tissues. Together, GI was shown to alleviate the physiological and pathological state of DSS-induced UC in mice via its antioxidant and anti-inflammatory functions, which may be associated with its modulation of the activation of Nrf2/NF-κB signaling.
Keywords: Gloeostereum incarnatum, ulcerative colitis, inflammation, oxidative stress, Nrf2/NF-κB
Introduction
Inflammatory bowel disease is a chronic, repetitive and non-specific gastrointestinal inflammatory disease comprising two conditions: Ulcerative colitis (UC) and Crohn's disease (1). As a chronic intestinal disease, UC produces inflammatory reactions and immune response at the colonic mucosa (2), accompanied by weight loss, diarrhea and bloody stools (3). Long-term severe colitis is known to cause colorectal cancer (4).
The pathogenesis of UC involves tissue damage in the colon and deterioration of the digestive and absorptive functions (5). Inflammation of the colon is due to an imbalance between the expression of pro-inflammatory and anti-inflammatory factors, and the migration of neutrophils to the damaged site, leading to the accumulation of inflammatory factors (6). Interleukin (IL)-6, closely associated with the differentiation and maturation of B cells, promotes the function of pro-inflammatory cells and inhibits the regulation of immune-related cells including T cells (7), both of which increase the levels of pro-inflammatory factors including IL-1 and IL-17 (8). Therefore, blocking the expression of pro-inflammatory cytokines may be a method for the potential treatment of UC (9). NF-κB inhibitor α is involved in the regulation of the development of early inflammation (10). The inflammatory response leads to the aggregation of peroxides and the imbalance of oxidation and antioxidation in the colon, particularly the over-accumulation of reactive oxygen species (ROS), causes oxidative damage (11). Antioxidant enzymes, including superoxide dismutase (SOD), are the first line of defense against oxidative stress, helping to remove peroxides from the body, but generally tend to promote oxidation in UC (12). During this process, the nuclear factor erythroid-2-related factor 2 (Nrf2) recognizes the transcription-enhancing sequence associated with antioxidation and repairs bodily damage by enhancing the expression of antioxidant enzymes (13).
Dextran sulfate sodium (DSS) affects the normal replication of DNA in cells, causes the aggregation of inflammatory factors and destruction of the intestinal microenvironment via the overproduction of ROS and eventually leads to the onset of UC (14). DSS was used to induce the occurrence of UC in the present study. Sulfasalazine (SASP), which reduces the infiltration of inflammatory monocytes, has been used to treat UC in clinics (15) and was applied as a positive control agent in this study.
Gloeostereum incarnatum (GI) is an edible and medicinal fungus, commonly cultivated in north-central China and Hokkaido, Japan (16). The anti-inflammatory activities of GI extracts were found to reduce nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW264.7 cells and delay melanin synthesis in B16/F10 cells (17). In our previous study, GI was confirmed to show immunomodulatory function in a cyclophosphamide monohydrate-induced mouse model by increasing immune-related factors including immunoglobulin (Ig) (7). Due to its anti-microbial activities, GI shows beneficial effects on gastrointestinal diseases including gastric ulcer and enteritis (18). However, thus far, there has been no systematic research on the protective effect of GI against UC.
In the present study, based on the detection of the main components of GI, its anti-inflammation and antioxidative properties were successfully confirmed in C57BL/6 mice with UC induced via oral administration of 3.5% DSS. The data provide experimental evidence for the applicability of GI for protection against UC.
Materials and methods
Detection of GI components
GI was purchased from TengHui Agriculture, identified by Professor Yu Li (Jilin Agricultural University, China) and pulverized by a pulverizer (XL-06A, Guangzhou Xulang Machinery Equipment Co., Ltd.).
Detection of main components
In GI powder, the contents of total sugar, reducing sugar, mannitol, total ash, total flavonoids, total triterpenoids, crude fat and crude fiber were detected using phenol-sulfuric acid assay (19), 3,5-dinitrosalicylic acid colorimetry (20), iodometry (21), regression analysis (22), the alumina colorimetric method (23), vanillin-glacial acetic acid-perchloric acid colorimetry (24), petroleum ether extraction (25) and the Ankom filter bag method (26), respectively.
Detection of minerals
Inductively coupled plasma optical emission spectrometry was used to detect the contents of mercury (Hg), lead (Pb), selenium (Se), arsenic (As), cadmium (Cd), zinc (Zn), iron (Fe), manganese (Mn), chromium (Cr), calcium (Ca), copper (Cu), sodium (Na) and potassium (K) in GI (27).
Detection of fatty acids
An oil sample of GI was extracted using a chloroform and methanol solution (2:1) and treated by alkaline hydrolysis to prepare the corresponding fatty acid methyl esters. Fatty acids were detected using gas chromatography-mass spectrometry (QP2010, Shimadzu Corporation) based on retention times (28).
Detection of amino acids
The protein in GI was hydrolyzed to single amino acid residues by hydrochloric acid hydrolysis and the contents of different amino acids were analyzed using an automatic amino acid analyzer (L-8900, Hitachi, Ltd.) (29).
Establishment of UC model mice and agent administration
A total of 48 male C57BL/6 mice [22–25 g; 8–10 weeks old; SPF grade, SCXK (LIAO) 2015-001], purchased from Liaoning Changsheng Biotechnology Co., Ltd., were housed in an exhaust ventilation cage system with a temperature of 22±2°C and humidity of 50±10%, with ad libitum access to food and water and a 12-h light/dark cycle. All animal experiments were approved by the Experimental Animal Ethics Committee of Jilin University [approval no. SYXK(JI)2014-0013]. The study was conducted using the Laboratory Guidelines for Animal Care (30,31) and the Guide for the Care and Use of Laboratory Animals (eighth edition) (32).
After 7 days of adaptive feeding, all mice with the exception of the control mice drank freely available clean water containing 3.5% DSS (S14048, Shanghai Yuanye Biological Technology Co., Ltd.) every day for 7 days. From the 8th to the 28th day, the mice drank 3.5% DSS on 1 day out of every 3 days. At the 7th day, the mice were randomly divided into 6 groups: The control group (n=8) that orally received double distilled (D.D.) water, the model group (n=8) that orally received D.D. water, the positive control group (n=8) that orally received 0.6 g/kg of SASP dissolved in D.D. water and the GI-treated groups that orally received 1.0 g/kg (n=8), 2.0 g/kg (n=8) and 4.0 g/kg (n=8) of GI suspended in D.D. water for the remaining 21 days. The doses of GI and SASP were selected based on previous studies (7,33) and our preliminary experiments (data not shown). On the 28th day, blood samples were collected from the orbital venous plexus of the mice and the mice were then euthanized by intraperitoneal injection of 100 mg/kg sodium pentobarbital (Xiya Reagent Co., Ltd.); mortality was characterized by cessation of heartbeat (34). Tissues including the colon, liver, spleen and kidney of each mouse were collected for further detection. The length of the colon and the organ index of the liver, spleen and kidney were calculated. The following formula was used to calculate the organ index (35): Organ index (%)=mean organ weight/mean body weight ×100.
During the entire experimental period, the activity, physiological status and body weight were monitored daily. The status of the UC mice was scored using the disease activity index (DAI) according to the degree of weight loss, fecal viscosity and bleeding (36).
Detection of biochemical factors in the serum and colon of UC mice
Colon tissue was homogenized in D.D. water contained 1.0% 50-mM phenylmethanesulfonyl fluoride (PMSF; Sigma-Aldrich; Merck KGaA) and 1.0% protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) using a homogenizer [S-18KS, Leopard scientific instruments (Beijing) Co., Ltd.]. After centrifuging the homogenate at 1,000 × g for 10 min at 4°C, the supernatant was collected, and the protein content was determined using a BCA protein assay kit (EMD Millipore). The levels of IL-1β (E20180501A), IL-2 (E20180501A), IL-6 (E20180501A), IL-12 (E20180501A), tumor necrosis factor (TNF)-α (E20180501A), TNF-β (E20180501A), interferon (IFN)-α (E20180501A) and IFN-γ (E20180501A) in the colon and IgA (E20180401A), IgM (E20180401A), IgG (E20170601A), SOD (E20180401A), catalase (CAT) (E20180401A), ROS (E20180401A) and NO (E20180401A) in the colon and serum were measured using ELISA kits purchased from Shanghai Yuanye Biological Technology Co., Ltd.
Histopathological examination of organs in the UC mice
The tissues were fixed in 4% paraformaldehyde at 37°C for 24 h and dehydrated in an ascending series of ethanol, embedded in paraffin and then cut into 5–8-µm-thick sections by a microtome (Leica Microsystems GmbH). The samples were dewaxed with xylene at 37°C and rehydrated in a descending series of ethanol. The samples were stained with 0.45% hematoxylin for 10 min and 0.5% eosin for 3 min, both at 37°C, and then observed and images captured using a light microscope (magnification, ×400; Olympus Corporation).
Western blot analysis of colon tissue
The proteins were extracted from colon tissue as detailed in our previous study (37). Total protein was quantified using a bicinchoninic acid protein assay kit (EMD Millipore) and 40 µg protein/well was separated by 12% SDS-PAGE and transferred to polyvinylidene fluoride membranes (EMD Millipore) using the same method as that used in our previous study (37). The membranes were blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) at 4°C for 4 h and then incubated overnight with primary antibodies against nuclear factor erythroid 2-related factor 2 (Nrf2; 1:2,000; cat. no. ab89443), CAT (1:2,000; cat. no. ab16731), heme oxygenase-1 (HO-1; 1:2,000; cat. no. ab137749), SOD-1 (1:2,000; cat. no. ab16831), SOD-2 (1:5,000; cat. no. ab13533), phosphorylated (p)-NF-κB (1:2,000; cat. no. ab86299), total (T)-NF-κB (1:1,000; cat. no. ab7970), p-inhibitor of NF-κB kinase α+β (IKKα+β; 1:1,000; cat. no. ab195907), T-IKKα+β (1:1,000; cat. no. ab178870), p-inhibitor of NF-κB (IκBα; 1:500; cat. no. ab12135), T-IκBα (1:1,000; cat. no. ab32518) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1,000; cat. no. ab8245; all from Abcam) at 4°C. The antibodies were diluted according to the ratio given in the manufacturer's protocols. Following washing with a Tris-buffered saline solution containing 0.05% Tween 20, the membranes were incubated with goat anti-rabbit (1:5,000; cat. no. IH-0011) or goat anti-mouse secondary antibody (1:5,000; cat. no. IH-0031; both Beijing Dingguo Changsheng Biotechnology Co Ltd.) at 4°C for 4 h. Electrochemiluminescence detection kits (EMD Millipore) and a Gel Imaging System (UVP, LLC) were used to detect changes in the protein content. The optical densities of bands were measured using ImageJ 1.48u software (National Institutes of Health).
Statistical analysis
Data are expressed as the mean ± standard error of the mean. One-way analysis of variance (ANOVA) and subsequent post-hoc multiple comparisons (Tukey's test) were performed using SPSS 16.0 software (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Evaluation of GI components
GI contained 16.6% of total sugar, 16.8% of crude fiber, 2.1% of mannitol, 13.9% of total ash, 6.3% of reducing sugar, 0.394% of total flavonoids, 5.328×10−3% of total triterpenes and 2.8% of crude fat (Table I). The presence of 35 fatty acids was detected, with linoleic acid (1.626%), oleic acid (0.236%) and hexadecenoic acid (0.229%) being the most abundant; by contrast, the fatty acids caprylic acid, undecanoic acid, translinoleic acid and docosadienoic acid were not detected (Table I). GI contained 17 amino acids and 11 minerals, with no harmful elements (Table I).
Table I.
Composition of Gloeostereum incarnatum.
| A, Main components | |
|---|---|
| Compounds | Contents, % |
| Total sugar | 16.6 |
| Total ash | 13.9 |
| Total triterpenes (×10−3) | 5.328 |
| Crude fiber | 16.8 |
| Reducing sugar | 6.3 |
| Crude fat | 2.8 |
| Mannitol | 2.1 |
| Total flavonoids | 0.394 |
| B, Fatty acids | |
| Compounds | Contents, % |
| Caprylic acid (C8:0) | ND |
| Capric acid (C10:0) | ND |
| Undecanoic acid (C11:0) | ND |
| Lauric acid (C12:0) | 0.015 |
| Tridecanoic acid (C13:0) | ND |
| Myristic acid (C14:0) | 0.003 |
| Myristoleic acid (C14:1) | ND |
| Pentadecanoic acid (C15:0) | 0.028 |
| Pentadecenoic acid (C15:1) | ND |
| Hexadecanoic acid (C16:0) | 0.229 |
| Palmitoleic acid (C16:1) | 0.021 |
| Heptadecanoic acid (C17:0) | 0.005 |
| Margaroleic acid (C17:1) | 0.004 |
| Stearic acid (C18:0) | 0.014 |
| Elaidic acid (C18:1n9t) | ND |
| Oleic acid (C18:1n9c) | 0.236 |
| Translinoleic acid (C18:2n6t) | ND |
| Linolic acid (C18:2n6c) | 1.626 |
| α-Linolenic acid (C18:3n3) | ND |
| γ-Linolenic acid (C18:3n6) | ND |
| Arachidic acid (C20:0) | ND |
| Cis-11-Eicosenoic acid C20:1n9) | 0.067 |
| Docosanoic acid (C22:0) | ND |
| Erucic acid (C22:1n9) | ND |
| Docosadienoic acid (C22:2n6) | ND |
| Docosahexaenoic acid (C22:6n3) | ND |
| Tricosanoic acid (C23:0) | 0.005 |
| Tetracosanoic acid (C24:0) | 0.008 |
| Nervonic acid (C24:1n9) | 0.016 |
| Dihomo-gamma-linolenic acid (C20:3n6) | ND |
| Arachidonic acid (C20:4n6) | ND |
| Eicosapentaenoic acid (C20:5n3) | ND |
| Heneicosanoic acid (C21:0) | ND |
| Eicosadienoic acid (C20:2) | 0.005 |
| Eicosatrienoic acid (C20:3n3) | ND |
| C, Amino acids | |
| Compounds | Contents, % |
| Aspartic acid | 0.728 |
| L-Threonine | 0.405 |
| Serine | 0.471 |
| Glutamic acid | 1.012 |
| Glycine | 0.400 |
| Alanine | 0.463 |
| Cystine | 0.266 |
| Valine | 0.406 |
| Methionine | 0.096 |
| Isoleucine | 0.343 |
| Leucine | 0.616 |
| Tyrosine | 0.248 |
| Phenylalanine | 0.335 |
| Lysine | 0.221 |
| Histidine | 0.188 |
| Arginine | 0.510 |
| Proline | 0.377 |
| D, Minerals | |
| Compounds | Contents, mg/kg |
| Mercury | ND |
| Lead | 0.11493 |
| Selenium | 0.03450 |
| Arsenic | 0.12343 |
| Cadmium | ND |
| Zinc | 44.64 |
| Iron | 105.9 |
| Manganese | 6.135 |
| Chromium | 3.15357 |
| Calcium | 601.5 |
| Copper | 9.230 |
| Sodium | 345.8 |
| Potassium | 48,605 |
ND, not detected.
Protective effect of GI against UC
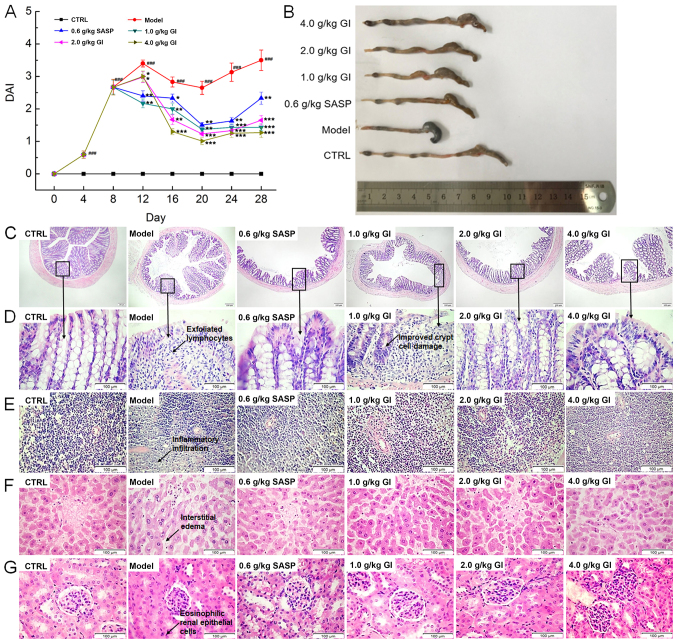
In the model mice with UC, DSS free-drinking caused marked body weight loss (P<0.05; Table II) and the worsening of the DAI (P<0.001; Fig. 1A), both of which were reversed after 21-day GI administration (P<0.05; Table II and Fig. 1A). Compared with the model mice, GI treatment in the UC mice prevented the increase of the spleen and liver indexes (P<0.05; Table II) and increased the kidney indexes (P<0.05; Table II); however, SASP demonstrated no significant effects on the liver indexes (Table II). Inflammatory cell infiltration and ulceration of the colon can lead to the shortening of the colon, which was noted in the model mice (Fig. 1B). SASP and GI notably prevented this pathological reduction of the colon length (Fig. 1B). In the colons of the model mice with UC, a large number of exfoliated lymphocytes were identified; meanwhile, the goblet cells were reduced and the crypt cells were damaged, which were all significantly alleviated following SASP and GI treatment (Fig. 1C and D). Furthermore, inflammatory cell infiltration in the spleen (Fig. 1E), interstitial edema in the liver (Fig. 1F) and eosinophilic renal tubular epithelial cells in the kidney (Fig. 1G) were all observed in the DSS-only-treated mice (the model mice). These pathological alterations were all alleviated following SASP and GI administration (Fig. 1E-G).
Table II.
Effect of GI on body weight and organ indexes.
| 3.5% dextran sulfate sodium | ||||||
|---|---|---|---|---|---|---|
| Gl, g/kg | ||||||
| Variable | Control | Model | Sulfasalazine, 0.6 g/kg | 1.0 | 2.0 | 4.0 |
| Body weight, g | ||||||
| Day 1 | 22.8±0.2 | 22.6±0.4 | 22.3±0.4 | 22.5±0.3 | 22.6±0.4 | 22.6±0.3 |
| Day 7 | 22.4±0.3 | 20.3±0.3a | 20.2±0.3 | 20.5±0.4 | 20.3±0.3 | 20.5±0.3 |
| Day 14 | 24.4±0.3 | 20.6±0.8b | 21.2±0.5 | 21.4±0.7 | 21.6±0.3 | 21.2±0.7 |
| Day 21 | 25.4±0.4 | 20.8±0.8b | 22.1±0.4 | 22.3±0.5 | 22.9±0.3d | 22.0±0.5 |
| Day 28 | 24.3±0.4 | 21.2±0.5b | 22.1±0.3 | 22.7±0.5d | 23.4±0.3d | 21.5±0.3 |
| Organ index, % | ||||||
| Spleen | 0.25±0.05 | 0.56±0.10c | 0.45±0.05d | 0.49±0.14 | 0.44±0.10d | 0.38±0.11e |
| Liver | 3.72±0.31 | 4.34±0.24a | 4.32±0.13 | 3.95±0.44d | 3.79±0.40d | 3.58±0.27e |
| Kidney | 1.16±0.05 | 1.03±0.05a | 1.13±0.06d | 1.10±0.10 | 1.11±0.05d | 1.01±0.07 |
Data were analyzed using a one-way ANOVA and are expressed as mean ± SEM (n=8).
P<0.05
P<0.01
P<0.001 vs. control mice
P<0.05
P<0.01 vs. DSS-induced UC mice. GI, Gloeostereum incarnatum.
Figure 1.
GI regulates the physiology and pathology of UC mice. The mice were continuously exposed to DSS (3.5% DSS dissolved in D.D. water) for 28 days, SASP (0.6 g/kg of SASP dissolved in D.D. water) and GI (1.0, 2.0 and 4.0 g/kg of GI suspended in D.D. water) were administered from the 7th day. (A) GI reduced the DAI index of UC mice and (B) ameliorated the shortening of colon length. Hematoxylin and eosin staining of (C) colon (scale bar, 100 µm; magnification, ×40), (D) colon, (E) spleen, (F) liver and (G) kidney tissues (scale bar, 100 µm; magnification, ×400) from C57BL/6 mice. ###P<0.001 vs. control mice; *P<0.05, **P<0.01 and ***P<0.001 vs. DSS-induced UC mice. GI, Gloeostereum incarnatum; UC, ulcerative colitis; DSS, dextran sulfate sodium; D.D., double distilled; SASP, sulfasalazine; DAI, disease activity index.
Anti-inflammatory effects of GI
Inflammation of the mucosal surface of the colon leads to the development of UC (38). The free-drinking of DSS in the present study increased the levels of pro-inflammatory factors including IL-1β, IL-2, IL-6, IL-12, TNF-α, TNF-β, IFN-α and IFN-γ (P<0.05; Table III). GI and SASP demonstrated strong anti-inflammatory effects, as evidenced by the regulation of inflammatory cytokines (P<0.05; Table III). Compared with the model mice, 21-day GI administration in the UC mice resulted in >19.3% (P<0.05), >31.7% (P<0.05), >31.5% (P<0.05), >30.8% (P<0.05), >23.4% (P<0.05), >30.2% (P<0.05), >28.1% (P<0.05) and >33.5% (P<0.05) reduction in IL-1β, IL-2, IL-6, IL-12, TNF-α, TNF-β, IFN-α and IFN-γ levels, respectively, in the colon (Table III).
Table III.
Effect of GI on inflammatory factors of colon tissues of mice with ulcerative colitis.
| 3.5% dextran sulfate sodium | ||||||
|---|---|---|---|---|---|---|
| GI, g/kg | ||||||
| Inflammatory factors | Control | Model | SASP, 0.6 g/kg | 1.0 | 2.0 | 4.0 |
| IL-1β, pg/mgprot | 45.7±3.9 | 60.0±3.5a | 44.1±3.1d | 48.4±1.4c | 42.2±3.8c | 39.7±3.7d |
| IL-2, pg/mgprot | 158.1±9.4 | 219.1±14.6a | 153.8±11.9c | 130.2±11.0d | 149.5±16.4c | 128.4±10.1d |
| IL-6, pg/mgprot | 61.6±3.7 | 83.7±6.3a | 54.5±8.2c | 57.3±1.4c | 62.1±6.8 | 50.0±4.8d |
| IL-12, pg/mgprot | 37.3±2.6 | 57.2±6.3a | 31.9±3.9d | 30.5±2.0d | 39.6±2.0c | 27.8±2.1d |
| TNF-α, pg/mgprot | 407.5±21.9 | 489±22.2a | 429.0±28.9 | 374.5±27.2c | 358.0±43.1c | 337.5±14.6d |
| TNF-β, pg/mgprot | 78.6±7.8 | 119.8±11.5b | 62.9±7.7d | 70.1±4.1d | 83.6±10.9c | 63.3±4.4d |
| IFN-α, pg/mgprot | 19.7±1.6 | 28.8±2.6b | 16.3±1.9d | 20.7±1.2c | 20.2±1.6c | 17.2±1.8d |
| IFN-γ, pg/mgprot | 72.4±6.6 | 126.6±14.8b | 72.9±9.5d | 75.3±5.4d | 84.2±5.5c | 69.8±7.0c |
Data were analyzed using a one-way ANOVA and are expressed as mean ± SEM (n=8).
P<0.05
P<0.01 vs. control mice
P<0.05
P<0.01 vs. DSS-induced UC mice. GI, Gloeostereum incarnatum; IL, interleukin; TNF, tumor necrosis factor; IFN, interferon.
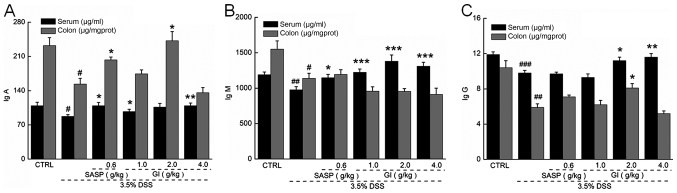
The immune response is involved in the pathogenesis of UC (39). Compared with the control mice, the UC mice (the model group) demonstrated lower levels of IgA, IgG and IgM in the colon and serum (P<0.05; Fig. 2). GI enhanced the levels of IgA (P<0.05) (Fig. 2A) and IgG (P<0.05; Fig. 2C) in the colon and serum and the levels of IgM (P<0.001) (Fig. 2B) in the serum of the UC mice, but did not enhance the levels of IgM in the colon (Fig. 2B).
Figure 2.
GI increases the levels of immune factors in serum and colon tissues of mice with UC. GI raised the levels of (A) IgA, (B) IgM and (C) IgG in serum and colon tissues. #P<0.05, ##P<0.01 and ###P<0.001 vs. control mice; *P<0.05, **P<0.01 and ***P<0.001 vs. DSS-induced UC mice. GI, Gloeostereum incarnatum; UC, ulcerative colitis; DSS, dextran sulfate sodium.
Effect of GI on oxidative factors
Oxidative stress destroys the cellular macromolecules of the colon, which is the key to the pathogenesis of UC (40). Compared with the control mice, the UC mice demonstrated lower levels of SOD and CAT and higher levels of ROS and NO in the serum and colon tissues (P<0.05; Table IV). In the serum, GI resulted in >14.8% (P<0.01) and >17.4% (P<0.05) increase in SOD and CAT levels and >12.9% (P<0.05) reduction in NO levels, but no effect on ROS levels (Table IV). In the colon tissues, GI failed to affect CAT levels, but resulted in >36.3% (P<0.05) increase in SOD levels and >25.8% (P<0.05) and >45.2% (P<0.05) reductions in ROS and NO levels respectively (Table IV). SASP significantly prevented the pathological alterations in the levels of anti- and pro-oxidative factors in the colon tissues (P<0.05; Table IV).
Table IV.
Effect of GI on oxidative factors in serum and colon tissues of mice with ulcerative colitis.
| A, Serum | ||||||
|---|---|---|---|---|---|---|
| DSS | ||||||
| GI, g/kg | ||||||
| Oxidative factors | CTRL | Model | SASP, 0.6 g/kg | 1.0 | 2.0 | 4.0 |
| SOD, U/ml | 99.7±3.3 | 81.7±2.6c | 94.8±3.1e | 83.9±3.1 | 93.8±2.2e | 98.2±1.9f |
| CAT, U/ml | 26.8±0.9 | 21.9±1.1b | 28.1±1.6e | 24.7±1.7 | 29.5±1.7e | 25.7±0.5d |
| ROS, U/ml | 177.1±3.1 | 174.2±3.9 | 165.6±2.8 | 167.3±5.7 | 172.4±2.1 | 179.9±0.7 |
| NO, µmol/l | 14.0±0.6 | 17.8±0.8b | 14.3±0.6d | 15.3±0.8d | 15.5±0.8d | 15.1±0.4d |
| B, Colon | ||||||
| DSS | ||||||
| GI, g/kg | ||||||
| Oxidative factors | CTRL | Model | SASP, 0.6 g/kg | 1.0 | 2.0 | 4.0 |
| SOD, U/mgprot | 152.0±9.6 | 104.5±9.1b | 145.0±11.2d | 113.8±3.7 | 119.7±8.6 | 142.4±4.3d |
| CAT, U/mgprot | 31.3±2.2 | 21.4±2.1a | 27.8±0.9d | 22.2±0.6 | 25.3±1.9 | 22.2±1.4 |
| ROS, U/mgprot | 310.7±23.9 | 418.2±33.7a | 308.5±22.7d | 292.2±19.3d | 310.3±13.2d | 284.1±13.0d |
| NO, µmol/gprot | 13.8±1.1 | 21.9±2.8a | 11.5±1.9e | 11.6±0.9d | 12.0±1.7d | 8.0±0.5e |
Data were analyzed using a one-way ANOVA and are expressed as the mean ± SEMs (n=8).
P<0.05
P<0.01
P<0.001 vs. control mice
P<0.05
P<0.01
P<0.001 vs. DSS-induced UC mice. GI, Gloeostereum incarnatum; CTRL, control; DSS, dextran sulfate sodium.
GI regulates the Nrf2/NF-κB signaling in the colon of UC mice
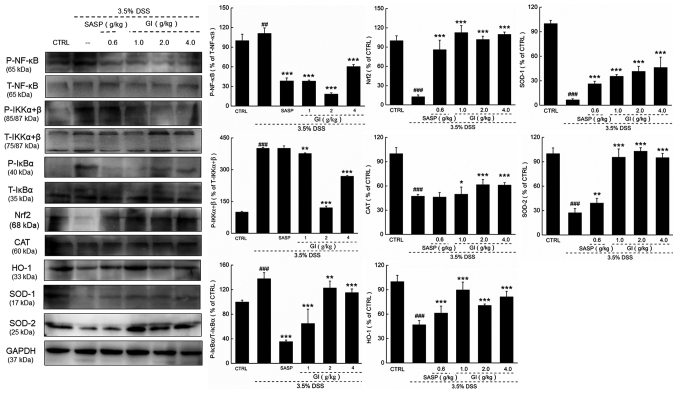
Compared with the model mice, the UC mice administered with GI demonstrated lower expression levels, in the colon, of Nrf2 and its downstream proteins, including CAT, HO-1, SOD-1 and SOD-2, and higher phosphorylated activities of NF-κB, IKKα/β and IκBα, all of which were prevented by GI administration (P<0.05; Fig. 3).
Figure 3.
GI demonstrates antioxidant and anti-inflammatory functions via modulation the activation of Nrf2/NF-κB signaling. GI enhanced the expression levels of Nrf2, CAT, HO-1, SOD-1 and SOD-2, and reduced the phosphorylation level of NF-κB, IKKα+β and IκBα. The quantitative expression of each protein was normalized using GAPDH. ##P<0.01 and ###P<0.001 vs. control mice; *P<0.05, **P<0.01 and ***P<0.001 vs. DSS-induced UC mice. GI, Gloeostereum incarnatum; SASP, sulfasalazine; P-, phosphorylated; T-, total; Nrf2, nuclear factor erythroid 2-related factor 2; CAT, catalase; HO-1, heme oxygenase-1; SOD, superoxide dismutase; IKK, inhibitor of NF-κB kinase; IκB, inhibitor of NF-κB; DSS, dextran sulfate sodium; CTRL, control.
Discussion
The commonly used anti-colitis drugs mainly include non-steroidal drugs, which exhibit side effects including intestinal ulcers (41,42). Recently, edible mushrooms have attracted researchers' attention due to their diverse pharmacological effects and minimal adverse effects. For example, Ganoderma lucidum alleviates intestinal damage induced by indomethacin in mice (43) and Hericium erinaceus improves testicular damage via its antioxidative effects and regulation of the GPR41/43 receptor in UC rats and Caco-2 cells (44). Based on the immunomodulatory and anti-inflammatory effects of GI reported previously (7), the present study successfully confirmed its protective effect against UC in C57BL/6 mice.
GI is a medicinal and edible fungus with complex ingredients and multiple nutritional value components. Its natural and crude character not only supports its low toxicity with various pharmacological efficacy, but also helps to explain its non-dose dependent effects during the experiments in the present study, which may show anti-UC effects via multiple targets. Indeed, the non-dose dependent manner can be noted in the research associated with Traditional Chinese Medicine (37). GI contains 6.3% crude fiber, which is beneficial for intestinal peristalsis and eases constipation (45). Mixed fiber can markedly regulate blood sugar levels, in addition to inflammatory factors, and exhibits a beneficial regulatory effect on the intestinal microbiota (46). GI contains 0.394% flavonoids, which exert antioxidant effects by regulating the body's oxygen free radical levels. In an LPS-damaged mice model, a flavonoid-rich fraction of Ocimum gratissimum leaves regulated oxidative stress and inflammation in the liver and brain by reducing the levels of TNF-α and malondialdehyde (47). The flavonoid apigenin can reduce the elevation of pro-inflammatory cytokines in the colon, reduce the density of eosinophils and transform M1 pro-inflammatory macrophages to the M2 anti-inflammatory phenotype in diet-induced obese mice (48). GI contains 5.328%x10−3 triterpenes, which trigger the anti-inflammatory response and reduce the expression of pro-inflammatory cytokines in a streptozotocin-induced diabetes model (49). Thus, GI shows a good nutritional foundation for its antioxidation and anti-inflammation activities.
UC is a process driven by T helper type 2 (Th-2)-like T-cells combined with the infiltration of lymphocytes and macrophages (50). DSS-induced damage to the colon tissue in mice causes the activation of macrophages, destruction of the composition of the lamina propria cells and aggregation of inflammatory factors, which eventually leads to imbalance in Th-1/2 cells (51). Cluster of differentiation 1a acts as an inflammatory mediator of UC, inducing T cell activation to elicit an immune response in the body (52). As a central factor in immune mediation, the elevated levels of secretory IgA in the colon help to prevent intestinal damage and restore mucosal barrier function (53). IgG exerts important protective and regulatory effects in the placental barrier (54) and IgM serves an important role in its own immune regulation (55). In the present study, GI increased the levels of Ig in the UC mice to stimulate an immune response.
Furthermore, TGF-β serves a role in pro-inflammatory mediator production during the development of UC, which can regulate the levels of ILs, TNFs and IFNs (56). During the development of UC, signaling of the receptor-interacting protein kinase 3 can upregulate the expression of repair-associated cytokines, including cyclooxygenase 2 and IL-22 (57). As a pro-inflammatory factor, abnormal release of TNF-α recruits more macrophages and neutrophils, which in turn increases inflammatory damage and intestinal permeability (58). In the morphological observations of the current study, reduced goblet cells, crypt abscesses and inflammatory cell infiltration were noted in the colon tissue following DSS administration; these were alleviated following GI administration. The overexpression of IL-6 may aggravate the inflammatory cell infiltration at the injured site and recruit more pro-inflammatory factors (8), which further activate the inducible nitric oxide synthase and cyclooxygenase-2 pathways, in turn increasing neutrophil aggregation (59). In the present study, the regulatory activity of GI on inflammatory factors may have been involved in its protective effect against UC in C57BL/6 mice.
As an upstream regulatory protein, NF-κB can also be activated by increased levels of pro-inflammatory factors (60). The IKKα/β complex is an aggregate composed of a catalytic subunit (61) and is essential for the initiation of the NF-κB pathway (62). In general, IκBα forms a dimer with NF-κB in the cytoplasm. When the inflammatory factor stimulates the IKKα/β complex, IKKα/β phosphorylates IκBα, thereby separating it from NF-κB. NF-κB is thus activated and enters the nucleus to induce the expression of relevant genes to reduce the expression of pro-inflammatory cytokines (63). In the present study, GI markedly reduced the phosphorylated activation of NF-κB by suppressing the phosphorylated IKKα/β and IκBα.
Inflammatory cells in the colon produce ROS and the accumulation of ROS damages proteins and nucleic acids, leading to oxidative stress (64). SOD and CAT are enzymatic antioxidants that catalyze the decomposition of oxides or peroxides to avoid oxidative damage (65). A previous study indicates that SOD reduces inflammation by enhancing the body's antioxidant function (66). SOD decomposes superoxide into hydrogen peroxide (H2O2) and H2O2 can be further decomposed into H2O by CAT to exert antioxidant effects (67). The abnormal levels of anti- and pro-oxidation factors caused by DSS in the present study were all restored by GI treatment. Nrf2 is an important starting element in antioxidant systems (68) that can counteract the damaging effect of peroxide by activating the peroxiredoxin-1 gene (69). Under peroxidative conditions, Nrf2 dissociates from the dimer and transfers to the nucleus, binding to the antioxidant responsive element (70). Binding to this promoter activates the expression of a cytoprotective enzyme, including HO-1, CAT, or SOD (71). Nrf2 upregulates the GST-A4 gene expression via the mitogen-activated protein kinase pathway to protect against UC (69). Nrf2 and NF-κB are inseparable in the process of anti-inflammation and antioxidation. The activation of Nrf2 attenuates inflammatory signaling by inhibiting NF-κB entry into the nucleus, whereas NF-κB directly inhibits the Nrf2 pathway at the RNA level (72). The data from the present study suggested that Nrf2/NF-κB signaling is involved in GI-mediated protection against UC; however, more experiments are required to investigate the synergistic process between Nrf2 and NF-κB signaling.
There remains a limitation the present study. Although the constituents contained in GI, based on the present data, were detected, the active ingredients with the anti-UC effect were not isolated and purified; this will be further investigated.
In summary, GI was identified to alleviate the physiological and pathological state of DSS-induced UC in mice via its antioxidant and anti-inflammatory functions, which may be associated with its modulation of the activation of Nrf2/NF-κB signaling.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key Research & Development Program of China (grant no. 2018YFE0107800), the Science and Technology Development Program of Jilin Province in China (grant no. 20170623092-TC), the Science and Technology Bureau of Changchun in China (grant no. 15SS11) and the Special Projects of the Cooperation between Jilin University and Jilin Province of China (grant no. SXGJSF2017-1).
Availability of data and materials
All data generated and analyzed during this study are included in this published article.
Authors' contributions
DW and YL contributed to the conceptual design of the research. XiaL, XinL, YoZ, YaZ, SL and NZ performed the experiments. DW, YL, XiaL and XinL analyzed the data and wrote the manuscript. DW and YL helped perform the analysis with constructive discussions. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The experimental animal protocol was approved by the Animal Ethics Committee of Jilin University [approval no. SYXK (JI) 2014-0013]. The present study was carried out under relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Yue B, Ren YJ, Zhang JJ, Luo XP, Yu ZL, Ren GY, Sun AN, Deng C, Wang ZT, Dou W. Anti-inflammatory effects of fargesin on chemically induced inflammatory bowel disease in mice. Molecules. 2018;23:3418. doi: 10.3390/molecules23061380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cawthorpe D, Davidson M. Temporal comorbidity of mental disorder and ulcerative colitis. Perm J. 2015;19:52–57. doi: 10.7812/TPP/14-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vargas Robles H, Citalán Madrid AF, García Ponce A, Silva Olivares A, Shibayama M, Betanzos A, Del Valle Mondragón L, Nava P, Schnoor M. Experimental colitis is attenuated by cardioprotective diet supplementation that reduces oxidative stress, inflammation, and mucosal damage. Oxid Med Cell Longev. 2016;2016:8473242. doi: 10.1155/2016/8473242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:269–276. doi: 10.1016/S2468-1253(17)30004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. Immune response and inflammatory pathway of ulcerative colitis. J Basic Clin Physiol Pharmacol. 2018;30:1–10. doi: 10.1515/jbcpp-2018-0036. [DOI] [PubMed] [Google Scholar]
- 6.Chaussade S, Denizot Y. Mediators of inflammation and hemorrhagic rectocolitis. Ann Gastroenterol Hepatol (Paris) 1991;27:117–121. (In French) [PubMed] [Google Scholar]
- 7.Wang D, Li Q, Qu Y, Wang M, Li L, Liu Y, Li Y. The investigation of immunomodulatory activities of Gloeostereum incaratum polysaccharides in cyclophosphamide-induced immunosuppression mice. Exp Ther Med. 2018;15:3633–3638. doi: 10.3892/etm.2018.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Chen W. Interleukin 6 inhibition by triptolide prevents inflammation in a mouse model of ulcerative colitis. Exp Ther Med. 2017;14:2271–2276. doi: 10.3892/etm.2017.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon YD, Bang KS, Shin MK, Lee JH, Chang YN, Jin JS. Regulatory effects of Glycyrrhizae radix extract on DSS-induced ulcerative colitis. BMC Complement Altern Med. 2016;16:459. doi: 10.1186/s12906-016-1390-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Ai XY, Qin Y, Liu HJ, Cui ZH, Li M, Yang JH, Zhong WL, Liu YR, Chen S, Sun T, et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB signaling. Oncotarget. 2017;8:100216–100226. doi: 10.18632/oncotarget.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Li S, Cao Y, Tian X, Zeng R, Liao DF, Cao D. Oxidative stress and carbonyl lesions in ulcerative colitis and associated colorectal cancer. Oxid Med Cell Longev. 2016;2016:9875298. doi: 10.1155/2016/9875298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Yang J, Lin L, Lin Y, Zheng C. The attenuation of Scutellariae radix extract on oxidative stress for colon injury in lipopolysaccharide-induced RAW264.7 cell and 2,4,6-trinitrobenzene sulfonic acid-induced ulcerative colitis rats. Pharmacogn Mag. 2016;12:153–159. doi: 10.4103/0973-1296.177913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZL, Fan HY, Yang MY, Zhang ZK, Liu K. Therapeutic effect of a hydroxynaphthoquinone fraction on dextran sulfate sodium-induced ulcerative colitis. World J Gastroenterol. 2014;20:15310–15318. doi: 10.3748/wjg.v20.i41.15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin MR, Kim KJ, Kim SH, Kim SJ, Seo BI, An HJ, Roh SS. Comparative evaluation between sulfasalazine alone and in combination with herbal medicine on DSS-induced ulcerative colitis mice. BioMed Res Int. 2017;2017:6742652. doi: 10.1155/2017/6742652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asai R, Mitsuhashi S, Shigetomi K, Miyamoto T, Ubukata M. Absolute configurations of (−)-hirsutanol A and (−)-hirsutanol C produced by Gloeostereum incarnatum. J Antibiot (Tokyo) 2011;64:693–696. doi: 10.1038/ja.2011.73. [DOI] [PubMed] [Google Scholar]
- 17.Soo LT. Free radical scavenging, anti-inflammatory and melannin synthesis inhibitory activities of Gloeostereum incarnatum. J Mushrooms. 2014;12:107–116. doi: 10.14480/JM.2014.12.2.107. [DOI] [Google Scholar]
- 18.Bunbamrung N, Intaraudom C, Dramae A, Boonyuen N, Veeranondha S, Rachtawee P, Pittayakhajonwut P. Antimicrobial activity of illudalane and alliacane sesquiterpenes from the mushroom Gloeostereum incarnatum BCC41461. Phytochem Lett. 2017;20:274–281. doi: 10.1016/j.phytol.2017.05.017. [DOI] [Google Scholar]
- 19.Rover MR, Johnston PA, Lamsal BP, Brown RC. Total water-soluble sugars quantification in bio-oil using the phenol-sulfuric acid assay. J Anal Appl Pyrolysis. 2013;104:194–201. doi: 10.1016/j.jaap.2013.08.004. [DOI] [Google Scholar]
- 20.Lindsay H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973;16:176–179. doi: 10.1007/BF02356048. [DOI] [Google Scholar]
- 21.Devapriya F, Sajith P, Ranganathan R, Shanmugam J. Prevalence of biofilm and beta-lactamase producing Staphylococcus in nasal and throat isolates from healthy volunteers: A medical alert. Nepal J Med Sci. 2014;3:79–83. doi: 10.3126/njms.v3i2.13448. [DOI] [Google Scholar]
- 22.Popek S. Application of regression analysis as a method to determine total ash content in some selected nectar honeys. Nahrung. 2003;47:36–38. doi: 10.1002/food.200390006. [DOI] [PubMed] [Google Scholar]
- 23.Bahloul N, Bellili S, Aazza S, Chérif A, Faleiro ML, Antunes MD, Miguel MG, Mnif W. Aqueous extracts from tunisian diplotaxis: Phenol content, antioxidant and anti-acetylcholinesterase activities, and impact of exposure to simulated gastrointestinal fluids. Antioxidants. 2016;5:12. doi: 10.3390/antiox5020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JJ, Hu XQ, Zhang XF, Liu JJ, Cao LS. Study on variation of main ingredients from spores and fruiting bodies of Ganoderma lucidum. Zhongguo Zhong Yao Za Zhi. 2014;39:4246–4251. (In Chinese) [PubMed] [Google Scholar]
- 25.Dong QF, Wang JL, Zhang SF, Wang Z, Zhang CX, Gao H, Zhang HM, Zhang L. Antifungal activity of crude extracts and fat-soluble constituents of Holotrichia diomphalia larvae. Bioresour Technol. 2008;99:8521–8523. doi: 10.1016/j.biortech.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Marichal MJ, Trujillo AI, Cadenazzi M, Arias G. Fiber analysis: Evaluation of screen printing fabric filters bags by three statistical approaches. Anim Feed Sci Technol. 2011;169:79–85. doi: 10.1016/j.anifeedsci.2011.06.005. [DOI] [Google Scholar]
- 27.da Costa WKOC, da Silva CS, Figueiredo JFD, Nóbrega JA, Paim APS. Direct analysis of deodorants for determination of metals by inductively coupled plasma optical emission spectrometry. J Pharm Biomed Anal. 2018;155:247–252. doi: 10.1016/j.jpba.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Utami PI, Rahayu WS, Nugraha I, Rochana AN. Fatty acid analysis of lipid extracted from rats by gas chromatography-mass spectrometry method. 2ND Annual applied science and engineering conference (AASEC 2017) IOP Conf Series Mater Sci Eng. 2018:288. [Google Scholar]
- 29.Paquin CS, Paquin R. Integration, identification and concentration measurement of amino acids in plant samples by means of an automatic amino acid analyzer linked to a mini-computer. J Chromatogr A. 1978;156:79–85. doi: 10.1016/S0021-9673(00)83128-3. [DOI] [Google Scholar]
- 30.Couto M, Cates C. Laboratory guidelines for animal care. Methods Mol Biol. 2019;1920:407–430. doi: 10.1007/978-1-4939-9009-2_25. [DOI] [PubMed] [Google Scholar]
- 31.China sets lab animal guidelines. Science. 2016;351:1372–1373. [Google Scholar]
- 32.The National Academies Press; Washington, DC: 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals: Guide for the Care and Use of Laboratory Animals: Eighth Edition. [Google Scholar]
- 33.Gan HT, Chen YQ, Ouyang Q. Sulfasalazine inhibits activation of nuclear factor-kappaB in patients with ulcerative colitis. J Gastroenterol Hepatol. 2005;20:1016–1024. doi: 10.1111/j.1440-1746.2005.03862.x. [DOI] [PubMed] [Google Scholar]
- 34.Cao Z, Dai W, Zhang R, Chen L, Yang X, Hu L, Chiang LY, Liu W. Opening of the adenosine triphosphate-sensitive potassium channel attenuates morphine tolerance by inhibiting JNK and astrocyte activation in the spinal cord. Clin J Pain. 2016;32:617–623. doi: 10.1097/AJP.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 35.Taba MY, Mohammadi S, Jalali M, Beheshti F, Attari SS. Effects of different doses of curcumin on testicular histopathology, apoptosis, and reproductive organs weight index in mice D-galactose-induced aging model. Comp Clin Pathol. 2019;28:997–1002. doi: 10.1007/s00580-018-2870-7. [DOI] [Google Scholar]
- 36.Wang R, Wang L, Luo Y, Wang D, Du R, Du J, Wang Y. Maggot protein ameliorates dextran sulphate sodium-induced ulcerative colitis in mice. Biosci Rep. 2018;38:BSR20181799. doi: 10.1042/BSR20181799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng B, Zhang Y, Wang Z, Ding Q, Song J, Wang D. Hepatoprotective effects of Morchella esculenta against alcohol-induced acute liver injury in the C57BL/6 mouse related to Nrf-2 and NF-κB signaling. Oxid Med Cell Longev. 2019;6029876:2019. doi: 10.1155/2019/6029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarlos P, Kovesdi E, Magyari L, Banfai Z, Szabo A, Javorhazy A, Melegh B. Genetic update on inflammatory factors in ulcerative colitis: Review of the current literature. World J Gastrointest Pathophysiol. 2014;5:304–321. doi: 10.4291/wjgp.v5.i3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Y, Niu W, Tang Y, Zhang Q, Liu S, Liu X, Wang X, Xu Y. Aggravated mucosal and immune damage in a mouse model of ulcerative colitis with stress. Exp Ther Med. 2019;17:2341–2348. doi: 10.3892/etm.2019.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan H, Wang H, Zhang X, Li X, Yu J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int J Clin Exp Med. 2015;8:20245–20253. [PMC free article] [PubMed] [Google Scholar]
- 41.Stadnicki A, Frysz-Naglak D. Non-steroidal anti-inflammatory drugs and intestinal side effects. Wiad Lek. 2007;60:286–290. (In Polish) [PubMed] [Google Scholar]
- 42.Lychkova AÉ, Puzikov AM. Non-steroidal anti-inflammatory drugs in the correction of experimental ulcerative colitis. Eksp Klin Gastroenterol. 2014;7:59–63. (In Russian) [PubMed] [Google Scholar]
- 43.Nagai K, Ueno Y, Tanaka S, Hayashi R, Shinagawa K, Chayama K. Polysaccharides derived from Ganoderma lucidum fungus mycelia ameliorate indomethacin-induced small intestinal injury via induction of GM-CSF from macrophages. Cell Immunol. 2017;320:20–28. doi: 10.1016/j.cellimm.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Zhang Y, Yang S, Zhao D, Wang M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int J Biol Macromol. 2019;125:572–579. doi: 10.1016/j.ijbiomac.2018.12.092. [DOI] [PubMed] [Google Scholar]
- 45.Mehmood MH, Aziz N, Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of Psyllium husk (Ispaghula) in constipation and diarrhea. Dig Dis Sci. 2011;56:1460–1471. doi: 10.1007/s10620-010-1466-0. [DOI] [PubMed] [Google Scholar]
- 46.Zhai X, Lin D, Zhao Y, Li W, Yang X. Effects of dietary fiber supplementation on fatty acid metabolism and intestinal microbiota diversity in C57BL/6J mice fed with a high-fat diet. J Agric Food Chem. 2018;66:12706–12718. doi: 10.1021/acs.jafc.8b05036. [DOI] [PubMed] [Google Scholar]
- 47.Ajayi AM, Ben-Azu B, Onasanwo SA, Adeoluwa O, Eduviere A, Ademowo OG. Flavonoid-rich fraction of Ocimum gratissimum attenuates lipopolysaccharide-induced sickness behavior, inflammatory and oxidative stress in mice. Drug Res (Stuttg) 2019;69:151–158. doi: 10.1055/a-0654-5042. [DOI] [PubMed] [Google Scholar]
- 48.Gentile D, Fornai M, Colucci R, Pellegrini C, Tirotta E, Benvenuti L, Segnani C, Ippolito C, Duranti E, Virdis A, et al. The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS One. 2018;13:e0195502. doi: 10.1371/journal.pone.0195502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu YS, Chen SN. Extracted triterpenes from Antrodia cinnamomea reduce the inflammation to promote the wound healing via the STZ inducing hyperglycemia-diabetes mice model. Front Pharmacol. 2016;7:154. doi: 10.3389/fphar.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Majewska-Szczepanik M, Góralska M, Marcińska K, Zemelka-Wiącek M, Strzępa A, Dorożyńska I, Szczepanik M. Epicutaneous immunization with protein antigen TNP-Ig alleviates TNBS-induced colitis in mice. Pharmacol Rep. 2012;64:1497–1504. doi: 10.1016/S1734-1140(12)70947-7. [DOI] [PubMed] [Google Scholar]
- 51.Queen D, Hedayat AA, Magro C, Geskin LJ. An unusual cause of bilateral orbital swelling: Immunoglobulin G4-related orbital disease arising in a patient with ulcerative colitis. JAAD Case Rep. 2019;5:634–638. doi: 10.1016/j.jdcr.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Amodi O, Jodeleit H, Beigel F, Wolf E, Siebeck M, Gropp R. CD1a-expressing monocytes as mediators of inflammation in ulcerative colitis. Inflamm Bowel Dis. 2018;24:1225–1236. doi: 10.1093/ibd/izy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui Y, Zhu C, Ming Z, Cao J, Yan Y, Zhao P, Pang G, Deng Z, Yao Y, Chen Q. Molecular mechanisms by which casein glycomacropeptide maintains internal homeostasis in mice with experimental ulcerative colitis. PLoS One. 2017;12:e0181075. doi: 10.1371/journal.pone.0181075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ignat'eva NV, Ziganshina MM, Shilova NV, Khasbiullina NR, Bovin NV, Tyutyunnik VL, Sukhikh GT. Isolation of IgG associated with human placenta. Bull Exp Biol Med. 2019;167:120–122. doi: 10.1007/s10517-019-04474-4. [DOI] [PubMed] [Google Scholar]
- 55.Kubagawa H, Honjo K, Ohkura N, Sakaguchi S, Radbruch A, Melchers F, Jani PK. Functional roles of the IgM Fc receptor in the immune system. Front Immunol. 2019;10:945. doi: 10.3389/fimmu.2019.00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Binabaj MM, Asgharzadeh F, Avan A, Rahmani F, Soleimani A, Parizadeh MR, Ferns GA, Ryzhikov M, Khazaei M, Hassanian SM. EW-7197 prevents ulcerative colitis-associated fibrosis and inflammation. J Cell Physiol. 2019;234:11654–11661. doi: 10.1002/jcp.27823. [DOI] [PubMed] [Google Scholar]
- 57.Xu YL, Tang HL, Zhu SY, Peng HR, Qi ZT, Wang W. RIP3 deficiency exacerbates inflammation in dextran sodium sulfate-induced ulcerative colitis mice model. Cell Biochem Funct. 2017;35:156–163. doi: 10.1002/cbf.3257. [DOI] [PubMed] [Google Scholar]
- 58.Liang J, Liang J, Hao H, Lin H, Wang P, Wu Y, Jiang X, Fu C, Li Q, Ding P, et al. The extracts of Morinda officinalis and its hairy roots attenuate dextran sodium sulfate-induced chronic ulcerative colitis in mice by regulating inflammation and lymphocyte apoptosis. Front Immunol. 2017;8:905. doi: 10.3389/fimmu.2017.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, He J, Suo Y, Lv L, Wang J, Huo C, Zheng Z, Wang Z, Li J, Sun W, Zhang Y. Anti-inflammatory effect of taurocholate on TNBS-induced ulcerative colitis in mice. Biomed Pharmacother. 2016;81:424–430. doi: 10.1016/j.biopha.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 60.Gupta RA, Motiwala MN, Dumore NG, Danao KR, Ganjare AB. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J Ethnopharmacol. 2015;164:239–246. doi: 10.1016/j.jep.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 61.Koppe C, Verheugd P, Gautheron J, Reisinger F, Kreggenwinkel K, Roderburg C, Quagliata L, Terracciano L, Gassler N, Tolba RH, et al. IκB kinaseα/β control biliary homeostasis and hepatocarcinogenesis in mice by phosphorylating the cell-death mediator receptor-interacting protein kinase 1. Hepatology. 2016;64:1217–1231. doi: 10.1002/hep.28723. [DOI] [PubMed] [Google Scholar]
- 62.Li Y, Li J, Li B, Qin H, Peng X, Zhao Y, Chen Y. Anthocyanin suppresses CoCrMo particle-induced osteolysis by inhibiting IKKα/β mediated NF-κB signaling in a mouse calvarial model. Mol Immunol. 2017;85:27–34. doi: 10.1016/j.molimm.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Reale C, Iervolino A, Scudiero I, Ferravante A, D'Andrea LE, Mazzone P, Zotti T, Leonardi A, Roberto L, Zannini M, et al. NF-κB essential modulator (NEMO) is critical for thyroid function. J Biol Chem. 2016;291:5765–5773. doi: 10.1074/jbc.M115.711697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nikkhah-Bodaghi M, Maleki I, Agah S, Hekmatdoost A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: A randomized, placebo-controlled, clinical trial. Complement Ther Med. 2019;43:1–6. doi: 10.1016/j.ctim.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 65.Meurer MC, Mees M, Mariano LNB, Boeing T, Somensi LB, Mariott M, da Silva RCMVAF, Dos Santos AC, Longo B, Santos França TC, et al. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr Res. 2019;66:95–106. doi: 10.1016/j.nutres.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Kang JE, Kim HD, Park SY, Pan JG, Kim JH, Yum DY. Dietary supplementation with a Bacillus superoxide dismutase protects against γ-radiation-induced oxidative stress and ameliorates dextran sulphate sodium-induced ulcerative colitis in mice. J Crohn's Colitis. 2018;12:860–869. doi: 10.1093/ecco-jcc/jjy034. [DOI] [PubMed] [Google Scholar]
- 67.Rana SV, Sharma S, Prasad KK, Sinha SK, Singh K. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J Med Res. 2014;139:568–571. [PMC free article] [PubMed] [Google Scholar]
- 68.Almeer RS, Mahmoud SM, Amin HK, Abdel Moneim AE. Ziziphus spina-christi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem Toxicol. 2018;115:49–62. doi: 10.1016/j.fct.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Sabzevary-Ghahfarokhi M, Shohan M, Shirzad H, Rahimian G, Soltani A, Ghatreh-Samani M, Deris F, Bagheri N, Shafigh M, Tahmasbi K. The regulatory role of Nrf2 in antioxidants phase2 enzymes and IL-17A expression in patients with ulcerative colitis. Pathol Res Pract. 2018;214:1149–1155. doi: 10.1016/j.prp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Liu D, Huo X, Gao L, Zhang J, Ni H, Cao L. NF-κB and Nrf2 pathways contribute to the protective effect of Licochalcone A on dextran sulphate sodium-induced ulcerative colitis in mice. Biomed Pharmacother. 2018;102:922–929. doi: 10.1016/j.biopha.2018.03.130. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Wang H, Zheng Z, Luo L, Wang P, Liu K, Namani A, Jiang Z, Wang XJ, Tang X. Mkp-1 cross-talks with Nrf2/Ho-1 pathway protecting against intestinal inflammation. Free Radic Biol Med. 2018;124:541–549. doi: 10.1016/j.freeradbiomed.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Saber S, Khalil RM, Abdo WS, Nassif D, El-Ahwany E. Olmesartan ameliorates chemically-induced ulcerative colitis in rats via modulating NFκB and Nrf-2/HO-1 signaling crosstalk. Toxicol Appl Pharmacol. 2019;364:120–132. doi: 10.1016/j.taap.2018.12.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in this published article.