Abstract
BACKGROUND:
Thromboprophylaxis is routinely used with lenalidomide-based regimens in multiple myeloma because of a substantial risk of venous thromboembolism (VTE). However, little is known about the incidence of VTE with contemporary lenalidomide-based regimens. The objective of the current study was to estimate the incidence of VTE despite thromboprophylaxis with currently used lenalidomide-based regimens in patients with myeloma.
METHODS:
The Ovid MEDLINE, Embase, and Cochrane databases were queried from study inception to January 2019 for keywords to cover the following concepts: “lenalidomide,” “venous thromboembolism,” and “multiple myeloma.” Phase 1, 2, and 3 clinical trials evaluating lenalidomide-based regimens with thromboprophylaxis were included. The pooled incidence rate of VTE was estimated using a random-effects model.
RESULTS:
The search generated 1372 citations, with 51 clinical trials and 9069 patients included for analysis. The most common thromboprophylaxis agents were aspirin, low-molecular-weight heparin or warfarin, administered either per risk-stratification or at investigators’ discretion. The pooled incidence of VTE in trials of patients who had newly diagnosed and relapsed/refractory myeloma was 6.2% (95% CI, 5.4%–7.1%) over median treatment durations ranging from 2 to 34 cycles, which translated into 1.2 VTE events per 100 patient-cycles (95% CI, 0.9–1.7 VTE events per 100 patient-cycles). Among contemporary regimens, the risk of VTE was low with combined lenalidomide and low-dose dexamethasone (0.2 [95% CI, 0.1–0.6] events/100 patient-cycles) and lenalidomide maintenance (0.0 [95% CI, 0.0–0.7] events per 100 patient-cycles). VTE risk was higher with combined lenalidomide and low-dose dexamethasone plus proteasome inhibitors (1.3 [95% CI, 0.7–2.3] events per 100 patient-cycles).
CONCLUSIONS:
Despite adequate thromboprophylaxis, lenalidomide-based regimens have a substantial risk of VTE in controlled clinical trial settings. Further studies are needed on new thromboprophylaxis strategies with regimens that have a high VTE risk.
Keywords: lenalidomide, multiple myeloma, survivorship, thromboprophylaxis, venous thromboembolism
INTRODUCTION
Multiple myeloma (MM) constitutes 10% of all hematologic malignancies, with approximately 138,000 new cases globally per year.1 Compared with matched controls, patients with MM have a higher risk of venous thromboembolism (VTE).2–4 Immunomodulatory drugs (IMiDs) are highly active in MM and form the backbone of most combination regimens in patients with newly diagnosed (ND) and relapsed/refractory (R/R) MM. During the initial clinical experience with thalidomide, a first-generation IMiD, a signal for higher risk of VTE was identified in several studies.5–7 Lenalidomide (Len) is a second-generation IMiD that largely has replaced thalidomide in the United States because of its better toxicity profile and higher efficacy.8–10 However, Len is also associated with a high incidence of VTE. A pooled analysis of 3 seminal clinical trials testing Len-based combination regimens showed a VTE incidence of 13% without thromboprophylaxis.11 Hence, the International Myeloma Working Group (IMWG) recommends risk-stratified thromboprophylaxis with IMiD-based combination regimens.12
The treatment-related factors in the IMWG risk-stratification model are concomitant use of high-dose corticosteroids, doxorubicin, and multiagent chemotherapy.12,13 However, based on data from the E4A03 trial, which compared high-dose versus low-dose dexamethasone,14 and the emergence of novel agents, high-dose corticosteroids or multiagent cytotoxic chemotherapies are seldom used in combination with Len in the current era. Several combination regimens with Len and low-dose corticosteroids have emerged, including those with proteasome inhibitors, monoclonal antibodies, and alkylating agents. There is a lack of data on the cumulative risk of VTE with contemporary Len-based regimens in the context of IMWG-recommended thromboprophylaxis across the disease spectrum of MM.
We performed a systematic review and meta-analysis to estimate the cumulative incidence of VTE despite thromboprophylaxis with Len-based regimens. We also quantified the incidence of VTE in subgroups of commonly used Len-based regimens and single-agent Len maintenance.
MATERIALS AND METHODS
The systematic review and meta-analysis was conducted according to Cochrane Collaboration guidelines15 and is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).16
Data Sources and Search Strategy
A medical reference librarian searched the literature from the Ovid MEDLINE (1946-present), Embase (1947-present), and Cochrane Central Register of Controlled Trials (CENTRAL) databases. Two investigators (R.C. and N.M.) consulted with the medical librarian to identify Medical Subject Heading (MeSH) terms and other controlled vocabulary. Standardized terms and keywords were combined to search the following concepts: “lenalidomide,” “venous thromboembolism,” and “multiple myeloma.” The search terms used to identify the study drug were “Revlimid,” “lenalidomide,” “(CC adj2 “5013”)”, or “CC5013” (see Supporting Table 1 for detailed search strategy). All results were exported to EndNote X9 (Clarivate Analytics). The searches were executed twice. The first iteration was performed in April 2018, and the second was performed in January 2019 to ensure the inclusion of more recent publications.
Study Selection
Two investigators (R.C. and N.M.) independently screened the records at the title and abstract levels based on a priori inclusion criteria: 1) prospective clinical trials testing Len-based combination regimens with mandatory thromboprophylaxis per trial protocol or according to IMWG guidelines, and 2) studies with data on the duration of therapy and the number of VTE events with different Len-based regimens in study arm(s). Conference abstracts were excluded because discrepancies have been reported between abstract results and subsequent journal publications.17 Publications in languages other than English were excluded. Studies including trials of all phases, sample sizes, and follow-up durations were included.
Data Extraction and Outcome Measures
Data from each study were independently extracted by 2 investigators (R.C. and N.M.). Disagreements were resolved by consensus. We extracted the following key study-level information from trials: median age, performance status, prior lines of therapy, Len-based combination regimen in treatment arm(s), total number of VTE events, median duration of therapy, type of VTE prophylaxis used, and exclusion of patients with a prior history of VTE. Data were abstracted on an intention-to-treat principle.
The outcome of interest was the incidence of VTE (all grade), which was reported as the cumulative incidence of VTE (percentage) and the incidence of VTE (number of events) per 100 patient-cycles to allow comparison with prior literature.18 VTE was defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events (versions 2, 3, or 4) or World Health Organization (WHO) grading. For clinical trials with incomplete or lack of reporting on the VTE event rate (eg, with only grade ≥3 events reported) or the duration of therapy, we searched adverse event data from the US National Library of Medicine’s clinicaltrials.gov registry of clinical trials (National Institutes of Health) through February 2019 or contacted the corresponding author by email. Of note, we excluded superficial thrombophlebitis and thrombosis events because of vascular access complications when that information was specifically available. A risk-of-bias assessment of phase 3 randomized clinical trials (RCTs) was performed independently by 2 investigators (R.C. and N.M.) using the Cochrane risk-of-bias tool.19
Data Synthesis and Meta-Analysis
VTE event rates were calculated by dividing the number of events by total sample size. The VTE incidence per 100 patient-cycles was calculated by dividing the total number of events by the product of the total sample size and the median number of treatment cycles. For studies in which the median duration of treatment was provided in months, that value was converted to the number of cycles based on the length of each cycle in the respective study. A fixed continuity correction was applied to studies with zero events.20 For each outcome measure, the 95% CI was calculated. Random-effects meta-analysis was performed by applying the DerSimonian and Laird method19 using Comprehensive Meta-Analysis software (version 3; Biostat). A random-effects model was selected a priori because of expected heterogeneity that may be caused using different Len-based regimens among included studies. To test the null hypothesis that there are no between-group differences, prespecified subgroup analyses were performed on different stages of MM therapy (ND, R/R, and maintenance), trial phases (phase 3 vs others), and different classes of Len-based combination regimens. Heterogeneity was assessed using the Cochrane Q statistic: I2 < 25% was consistent with negligible heterogeneity, I2 from 25% to 50% was consistent with moderate heterogeneity, and I2 ≥ 70% was considered substantial heterogeneity. Publication bias was assessed by using funnel plots and the Egger test. Statistical significance was set at 5% for all tests except the Egger regression test, for which the significance threshold was 10% because of the low power of the test. The systematic review is registered with PROSPERO, an international online prospective register of health-related systematic reviews (Centre for Reviews and Dissemination [CRD] 42018102971).
RESULTS
The search generated 1372 citations. In total, 245 duplicate records were removed, and 1127 records were screened. Ultimately, 51 clinical trials8–10,20–70 with 61 Len-containing treatment arms and 9069 patients were included for the analysis based on a priori selection criteria (Fig. 1).16
Figure 1.
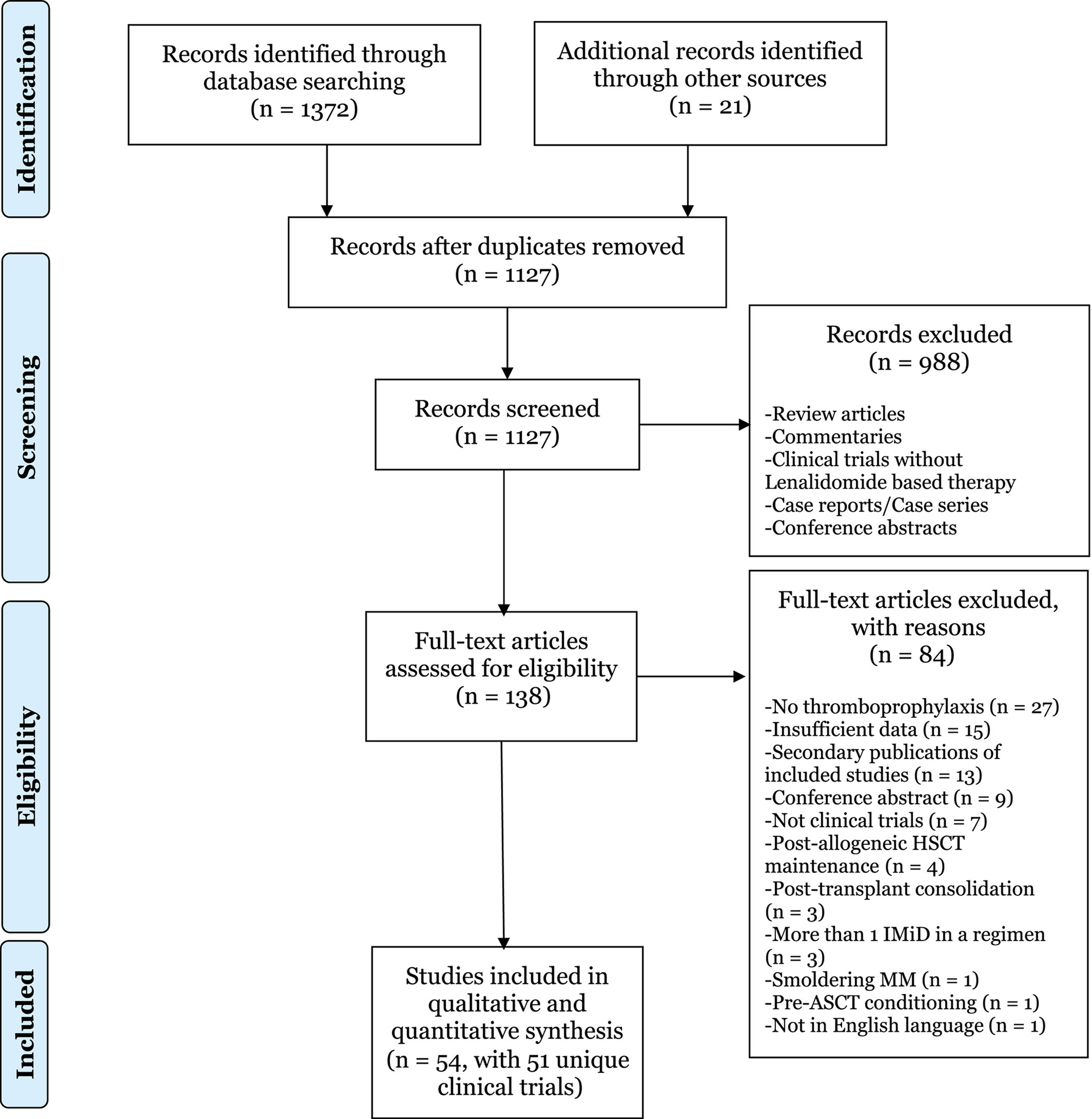
This is a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for study selection. ASCT indicates autologous stem-cell transplantation; HSCT hematopoietic stem cell transplantation; IMiD, immunomodulatory drug; MM, multiple myeloma.16
Study Characteristics
Of 51 clinical trials, 23 (n = 3633) were in the ND stage, 26 (n = 4068) were in the R/R stage, and 3 (n = 1557) were in the maintenance stage of therapy, including 1 trial incorporating both induction therapy for ND MM and subsequent maintenance with single-agent Len. Although phase 3 trials accounted for one-quarter of the total number of studies, it included approximately three-quarters of patients in the meta-analysis. Apart from phase 3 trials in the maintenance setting, none had a non-IMiD– containing control arm. In clinical trials of patients with R/R MM, approximately 60% had received a median of 1 or 2 prior lines of therapy. Patients with prior VTE were excluded in a minority of trials (12%). None of the trials used high-dose dexamethasone (>480 mg/cycle),12 which is consistent with current clinical practice. An anthracycline-based combination regimen, which is a known risk factor for VTE with Len, was received by 6% of patients. All trials in ND and R/R phase except 1 had used either low-dose dexamethasone or prednisone as the corticosteroid backbone. Most trials (75%) provided the options of aspirin (ASA), low-molecular-weight heparin (LMWH), or warfarin for thromboprophylaxis, either based on IMWG risk-stratification guidelines or at the investigators’ discretion. The most common Len-based combination regimen was Len plus low-dose dexamethasone (Rd), which was tested in approximately one-quarter of trials, representing 35% of the total patient population. Single-agent Len maintenance was received by 1557 patients (17%). Two maintenance trials did not mandate thromboprophylaxis in their protocol,9,70 which is in accordance with current IMWG guidelines. The combination of Rd with a proteasome inhibitor, which is the current standard of care in ND MM and is widely used in the R/R setting, was tested in one-quarter of trials, including 15% of patients. Among the phase 3 RCTs, investigators were masked to treatment allocation and outcome assessment in 3 of 12 trials. Allocation to treatment arm was concealed from investigators in 75% of RCTs. A summary of study-level and patient characteristics is provided in Table 1. Clinical and demographic characteristics of the individual studies included in the meta-analysis are provided in the Supporting Information (see Supporting Tables 2, 3, and 4 for ND, R/R, and maintenance trials, respectively). The risk of bias in phase 3 RCTs is also shown in the Supporting Information (see Supporting Table 5).
TABLE 1.
Pooled Study and Patient Characteristics
| Study Characteristic | No. of Trials (%) | No. of Patients (%) |
|---|---|---|
| Total | 51 (100) | 9069 (100) |
| Phase of treatment | ||
| Newly diagnosed | 23 (45) | 3633 (40) |
| Relapsed/refractory | 26 (51) | 4068 (45) |
| Maintenance | 3 (6)a | 1557 (17)a |
| Trial phase | ||
| 1 | 8 (16) | 280 (3) |
| 1/2 | 14 (27) | 826 (99) |
| 2 | 17 (33) | 1237 (14) |
| 3 | 12 (24) | 6726 (74) |
| Median age, y | ||
| <65 | 29 (57) | 2735 (30) |
| ≥65 | 22 (43) | 6334 (70) |
| Median no. of prior lines of therapy for n = 26 trials in R/R MM, n = 4068 patients | ||
| 1–2 | 15 (58) | 3346 (82) |
| ≥3 | 11 (42) | 722 (18) |
| VTE prophylaxis | ||
| ASA/antiplatelet agent only | 9 (18) | 670 (7) |
| LMWH only | 2 (4) | 221 (2) |
| ASA/LMWH/warfarinb | 38 (75) | 6926 (76) |
| Mandatory but agent not specified | 1 (2) | 115 (1) |
| No VTE prophylaxisc | 1 (2) | 1137 (13) |
| Regimensd | ||
| Rd | 12 (24) | 3140 (35) |
| R maintenance | 3 (6) | 1557 (17) |
| Rd + PI | 14 (27) | 1385 (15) |
| MPR/CPR/BPR | 10 (20) | 1462 (16) |
| Rd + MoABs | 4 (8) | 693 (8) |
| Rd + anthracycline ± vincristine | 6 (12) | 468 (5) |
| Rd + AA | 4 (8) | 202 (2) |
| Rd + PI + anthracycline | 2 (4) | 112 (1) |
| Rd + PI + AA | 1 (2) | 48 (<1) |
| Rd + clarithromycin | 1 (2) | 72 (<1) |
| Rd + PI + HDACi | 1 (2) | 55 (<1) |
| Rd + HDACi | 1 (2) | 38 (<1) |
| R + mTORi | 1 (2) | 26 (<1) |
| Prior VTE excluded | ||
| Yes | 6 (12) | 502 (6) |
| No | 45 (88) | 8567 (94) |
| High-dose dexamethasonee | ||
| Yes | None | None |
| No | 51 (100) | 9069 (100) |
| Transplantation eligibility for n = 3 trials on maintenance, n = 1557 patients | ||
| Prior transplantation | 1 (2) | 231 (3) |
| No prior transplantation | 1 (2) | 1137 (13) |
| Both transplantation and nontransplantation | 1 (2) | 189 (2) |
Abbreviations: ±, with or without; AA, alkylating agent; ASA, aspirin; BPR, bendamustine, prednisone, and lenalidomide; CPR, cyclophosphamide, prednisone, and lenalidomide; HDACi, histone deacetylase inhibitor; LMWH, low-molecular-weight heparin; MM, multiple myeloma; MoABs, monoclonal antibodies; MPR, melphalan, prednisone, and lenalidomide; mTORi, mammalian target of rapamycin inhibitors; PI, proteasome inhibitor; R, lenalidomide; R/R, relapsed/refractory; Rd, lenalidomide and low-dose dexamethasone; VTE, venous thromboembolism.
One trial of single-agent lenalidomide maintenance was an extension of a trial in newly diagnosed MM (Zweegman et al9), hence it was counted twice, and the total percentage does not add up to 100.
This was based on IMWG guidelines or was decided at the investigator’s discretion.
According to 2008 International Myeloma Working Group (IMWG) guidelines, single-agent lenalidomide maintenance is associated with a minimal risk of VTE, and no thromboprophylaxis was recommended in this setting. Therefore, we included studies of single-agent lenalidomide maintenance that did not mandate thromboprophylaxis.
Trials with more than 1 arm tested different lenalidomide-based combination regimens, hence the total number of trials does not add up to 51.
High-dose dexamethasone was defined as 480 mg per month according to the IMWG 2008 definition.
Rates of VTE
The pooled incidence of VTE in the 9069 patients included in our meta-analysis was 6.0% (95% CI, 5.1%–7.1%; P < .001; I2 = 66.4) over median treatment durations ranging from 2 to 34 cycles. The incidence rate was 1.2 per 100 patient-cycles (95% CI, 0.8–1.6 per 100 patient-cycles; P = .041; I2 = 21.8). When we excluded maintenance trials, which did not mandate thromboprophylaxis for the entire treatment duration, the pooled incidence of VTE was 6.2% (95% CI, 5.4%–7.1%; P < .001; I2 = 60.5), and the VTE incidence was 1.2 per 100 patient-cycles (95% CI, 0.9–1.7 per 100 patient-cycles; P < .001; I2 = 19.2).
The incidence rate of VTE in different Len-based combination regimens is shown descriptively in Table 2. Notably, the incidence was low with Rd (0.2 [95% CI, 0.1–0.6] events per 100 patient-cycles) and single-agent Len maintenance (0.0 [95% CI, 0.0–0.7] events per 100 patient-cycles). Conversely, the incidence rate in patients who received Rd in combination with a proteasome inhibitor was higher at 1.3 events per 100 patient-cycles (95% CI, 0.7–2.3 events per 100 patient-cycles), with a cumulative rate of 8.4% (95% CI, 6.0%–11.6%). The incidence rate in trials on Rd with monoclonal antibodies was 7.1% (95% CI, 2.4%–19.3%), which translated into 0.9 events per 100 patient-cycles (95% CI, 0.1–9.9 events per 100 patient-cycles), with substantial heterogeneity noted within this subgroup. The risk of VTE in trials on Len and prednisone in combination with alkylating agents like melphalan and cyclophosphamide was 4.5% (95% CI, 3.5%–5.8%), with 0.5 events per 100 patient-cycles (95% CI, 0.2–1.3 events per 100 patient-cycles). The forest plot for event rate per 100 patient-cycles (the percentage of events per 100 patient-cycles for respective therapies) is shown in Figure 2.8–10,22–52,54–67
TABLE 2.
Pooled Incidence of Venous Thromboembolism With Lenalidomide-Based Regimens
| Regimen | No. of Trial Arms (No. of Patients) | Pooled Incidence of VTE [95% CI], % | Heterogeneity, I2 | Pooled Incidence of VTE, per 100 Patient-Cycles [95% CI] | Heterogeneity, I2 |
|---|---|---|---|---|---|
| Single-agent R maintenance | 3 (1557) | 3.2 [2.4–4.2] | 0.0 | 0.0 [0.0–0.7] | 0.0 |
| Rd | 12 (3140) | 5.5 [4.0–7.4] | 70.3 | 0.2 [0.1–0.6] | 0.0 |
| MPR/CPR | 9 (1416) | 4.5 [3.5–5.8] | 0.0 | 0.5 [0.2–1.3] | 0.0 |
| Rd + PI + AA | 1 (48) | 2.1 [0.3–13.4] | 0.0 | 0.7 [0.0–17.0] | 0.0 |
| Rd + PI + anthracycline | 2 (112) | 4.7 [2.0–10.9] | 0.0 | 1.6 [0.4–6.6] | 0.0 |
| R + mTORi | 1 (26) | 1.9 [0.1–24.4] | 0.0 | 1.9 [0.1–24.4] | 0.0 |
| Rd + PI | 14 (1385) | 8.4 [6.0–11.6] | 56.1 | 1.3 [0.7–2.3] | 0.0 |
| Rd + MoAbs | 4 (693) | 7.1 [2.4–19.3] | 88.3 | 0.9 [0.1–9.9] | 68.2 |
| Rd + anthracycline + vincristine | 2 (138) | 7.6 [4.1–13.5] | 0.0 | 2.3 [0.8–7.0] | 0.0 |
| Rd + anthracycline | 4 (330) | 5.9 [3.8–9.1] | 0.0 | 2.7 [0.5–12.4] | 72.0 |
| Rd + AA | 4 (202) | 9.6 [6.0–15.1] | 3.9 | 2.3 [0.9–5.6] | 0.0 |
| BPR | 2 (46) | 3.4 [0.7–15.1] | 0.0 | 3.4 [0.7–15.1] | 0.0 |
| Rd + PI + HDACi | 1 (55) | 9.1 [3.8–20.0] | 0.0 | 4.1 [1.1–14.0] | 0.0 |
| Rd + clarithromycin | 1 (72) | 12.5 [6.6–22.3] | 0.0 | 1.3 [0.2–9.2] | 0.0 |
| Rd + HDACi | 1 (38) | 10.5 [4.0–24.9] | 0.0 | 2.0 [0.2–16.5] | 0.0 |
| Combined | 61 (9069) | 6.0 [5.1–7.1] | 66.4 | 1.2 [0.8–1.6] | 21.8 |
Abbreviations: AA, alkylating agent; BPR, bendamustine, prednisone, and lenalidomide; CPR, cyclophosphamide, prednisone, and lenalidomide; HDACi, histone deacetylase inhibitor; I2, Cochrane Q statistic; MoABs, monoclonal antibodies; MPR, melphalan, prednisone, and lenalidomide; mTORi, mammalian target of rapamycin inhibitors; PI, proteasome inhibitor; R, lenalidomide; Rd, lenalidomide and low-dose dexamethasone; VTE, venous thromboembolism.
Figure 2.
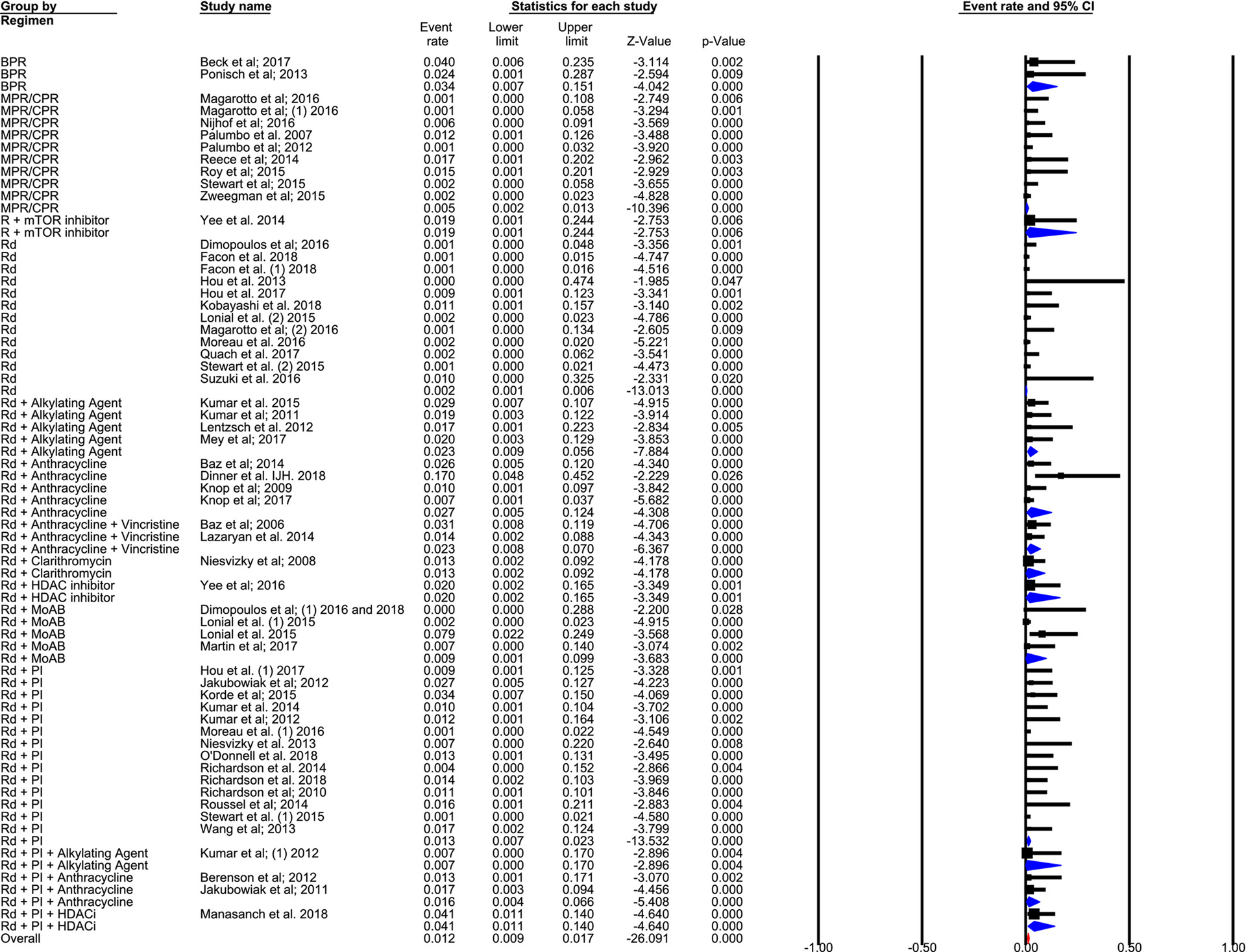
This forest plot illustrates the percentage of events per 100 patient-cycles for respective therapies. BPR indicates bendamustine, prednisone, and lenalidomide; CPR, cyclophosphamide, prednisone, and lenalidomide; HDACi, histone deacetylase inhibitor; MoAB, monoclonal antibody; MPR, melphalan, prednisone, and lenalidomide; mTORi, mammalian target of rapamycin inhibitor; PI, proteasome inhibitor; R, lenalidomide; Rd, lenalidomide and low-dose dexamethasone.8–10,22–52,54–67
Subgroup Analysis
Analyses in subgroups stratified by trial phase, median age, and stage of therapy (ND vs R/R vs maintenance) are shown in Supporting Table 6. The incidence of VTE was significantly lower in phase 3 trials compared with that in phase 1 and 2 trials. The VTE incidence per 100 patient-cycles was comparable in ND and R/R stages of treatment at 1.2 and 1.2, respectively (P for interaction was nonsignificant between ND and R/R), and both were significantly higher compared with single-agent Len maintenance. Trials in which the median patient age was ≤65 years had a significantly higher risk of VTE compared with those in which the median age was >65 years (2.1 vs 0.2 events per 100 patient-cycles, respectively; P for interaction <.001).
Publication Bias Assessment
Visual examination of funnel plots revealed publication bias both for the cumulative incidence of events (%) and for the number of events per 100 patient-cycles (see Supporting Figs. 1 and 2). The event rate of VTE was asymmetrically distributed among published studies, which was statistically significant on the Egger regression test (P = .011 and P < .001 for cumulative incidence and the number or events per 100 patient-cycles, respectively).
DISCUSSION
The results of this meta-analysis have several implications for clinical practice and future research on VTEs with IMiDs in MM. First, the incidence of VTE events with Len-based regimens remains substantial at 6% and 1.2 events per 100 patient-cycles. Second, the risk is lowest with Rd and single-agent Len maintenance at 0.2 and 0.0 events per 100 patient-cycle, respectively. Conversely, the risk is substantially higher with Rd in combination with proteasome inhibitors at 1.3 events per 100 patient-cycles. Third, the rate of VTE was comparable in trials of ND and R/R MM, in contrast to results from prior studies, mostly before routine thromboprophylaxis, which had shown a higher incidence in ND patients.12,71
Proposed mechanisms for thrombosis with IMiDs include increased platelet aggregation,72 single-nucleotide sequence variants,73,74 and altered balance between procoagulant and anticoagulant proteins on the surface of endothelial cells.75 Literature on the effect of combining novel antimyeloma agents like proteasome inhibitors and monoclonal antibodies with IMiDs on the risk of VTE is limited. Bortezomib, a first-generation proteasome inhibitor, does not have a significant prothrombotic effect, as demonstrated in the randomized VISTA (Velcade as Initial Standard Therapy in Multiple Myeloma)76 and APEX (Assessment of Proteasome Inhibition for Extending Remission)77 trials as well as preclinical studies.78 A qualitative review had demonstrated a lower risk of VTE with IMiDs in conjunction with bortezomib-containing regimens versus bortezomib-free regimens.79 However, 2 RCTs comparing IMiDs and dexamethasone with versus without bortezomib did not show any significant difference in the risk of VTE between treatment arms.80,81 Notably, in the Southwest Oncology Group S0777 trial (Lenalidomide and Dexamethasone With or Without Bortezomib in Treating Patients With Previous Untreated Multiple Myeloma) comparing Rd with versus without bortezomib in a randomized fashion, the incidence of all-grade thrombosis/embolism was comparable in both arms.80 In our meta-analysis, the exposure-adjusted incidence of VTE was higher when Rd was combined with proteasome inhibitors versus Rd alone, with nonoverlap-ping confidence intervals. Notably, among proteasome inhibitors, >80% of patients had received carfilzomib or ixazomib, which may have influenced the incidence of VTE. The risk of VTE with Rd in combination with monoclonal antibodies also was higher at 7.1%, with a wide confidence interval. Notably, there was substantial heterogeneity (see Supporting Table 6), which was likely because of a higher risk of VTE with elotuzumab versus daratumumab. However, these data should be interpreted cautiously because of the low number of trials in this subgroup. The expression of SLAMF7, which is the target of elotuzumab, is decreased in patients who have pulmonary embolism compared with healthy controls.82 Further studies are required to investigate the increased risk, nature, and mechanism of vascular events when elotuzumab is combined with IMiDs.
How do the results of our meta-analysis compare with prior evidence on the risk of VTE using Len-based regimens? A qualitative summary of prior systematic reviews on the risk of VTE with Len-based regimens is provided in Table 3.11,18,83,84 Notably, an important caveat regarding the meta-analysis by Carrier et al is that they included studies with high-dose and low-dose dexamethasone, which could be responsible for the increased risk of VTE with Rd regimens compared with our meta-analysis. A study of patients with MM in the Veterans Administration database between 1999 and 2013 demonstrated a VTE risk of 13.4%.85 Another single-institution study demonstrated a VTE rate of 12.7%, despite all patients on IMiDs or corticosteroids receiving thromboprophylaxis.86 The rates of VTE in the aforementioned studies compare adversely with VTE risk estimates from large observational data sets in the United States4 and Sweden3 predominantly before the era of IMiDs.
TABLE 3.
Results From Prior Systematic Reviews and Meta-Analyses on Risk of Venous Thromboembolism With Lenalidomide-Based Regimens
| Study | Disease/Phase of Treatment | Len-based Combination Regimens Used, No. of Patients | Pooled VTE Rate | VTE Prophylaxis |
|---|---|---|---|---|
| Carrier 201118 | Newly diagnosed MM | • Rd (high-dose or low-dose Dex; n = 349) | • 0.7 Events per 100 patient-cycle | Yes (ASA) |
| • Rd (high-dose or low-dose Dex; n = 278) | • 0.8 Events per 100 patient-cycle | No | ||
| Previously Treated MM | • Rd (high-dose or low-dose Dex; n = 361) | • 0.7 Events per 100 patient-cycles | No | |
| • Rd + chemotherapy, including doxorubicin (n = 131) | • 0.6 Events per 100 patient-cycle | Yes (ASA/LMWH) | ||
| Menon 200811 | Newly diagnosed MM | Rd (34% with high-dose Dex; n = 125) | 8% Cumulative rate | Yes in 88% (ASA) |
| Al-Ani 201683 | Newly diagnosed MM | Rd (high-dose or low-dose Dex) or MPR (n = 915) | 1.5 Events per 100 patient-cycles | Yes (ASA) |
| Yamshon 201884 | B-cell NHL | • All patients (n = 1433) | • 0.77 Events per 100 patient-cycle | Mixed |
| • Single-agent Len (n = 698) | • 1.09 Events per 100 patient-cycles | Mixed | ||
| • Len + biologics (n = 357) | • 0.49 Events per 100 patient-cycles | Mixed | ||
| • Len + chemotherapy (n = 378) | • 0.89 Events per 100 patient-cycles | Mixed |
Abbreviations: ASA, aspirin; Len, lenalidomide; Dex, dexamethasone; MWH, low-molecular-weight heparin; MM, multiple myeloma; MPR, melphalan, prednisone, and lenalidomide; NHL, non-Hodgkin lymphoma; Rd, lenalidomide and dexamethasone; VTE, venous thromboembolism.
Are current thromboprophylaxis strategies adequate in mitigating the risk of VTEs with IMiDs in MM? A large multicenter observational study MELISSE (Maladie thromboEmbolique dans le myéLome multIple SouS chimiothérapiE) in France of 524 patients with ND MM who initiated an IMiD-based regimen has demonstrated a VTE risk of 7% at 12 months of follow-up.87 Another study of 4892 patients with MM demonstrated a 12% rate of VTE.88 Notably, ASA use was not associated with a decreased risk of VTE on multivariable analysis in that study.88 In the Myeloma XI trial, the incidence of thrombosis was 11% in the first 6 months of induction therapy despite thromboprophylaxis.89 Two RCTs have addressed the question of the best thromboprophylaxis strategy with IMiDs in MM and compared ASA, LMWH, and vita-min K antagonist head-to-head.90,91 Both studies have yielded negative results; however, an important caveat is that those studies systematically excluded all high-risk patients, as exemplified by the low risk of VTE in the control arms. With multiple risk-stratification tools being developed currently for predicting VTE risk with IMiDs in MM,12 studies focusing on high-risk patients in the first 6 to 12 months of therapy are urgently needed.
Finally, phase 3 trials in our meta-analysis had a low VTE event rate likely because the regimens mostly comprised single-agent Len maintenance, Rd, and combined melphalan, prednisone, and Len (82% of patients in phase 3 trials), all of which are associated with a low risk of VTE. Furthermore, 30% of phase 3 trials reported grade 3 and 4 events only, compared with 10% of phase 1 and 2 trials, which could have affected the cumulative incidence of VTE. The VTE incidence was significantly higher in the subgroup aged ≤65 years despite no significant heterogeneity in exposure-adjusted incidence. Age is not incorporated as a risk factor in the IMWG risk-stratification model.12,92 It is possible that the subgroup analysis on age was influenced by the confounding effect of studies on Rd, single-agent Len maintenance, and combined melphalan, prednisone, and Len regimens, which comprised 85% of patients. Notably, patients with ND and R/R MM had comparable rates of exposure-adjusted VTE events, with moderate heterogeneity noted in the R/R subgroup. The incidence of VTE in different subgroups of Len-based regimens could be influenced by the use of certain regimens at distinct stages of disease (eg, single-agent Len in the maintenance setting and monoclonal antibodies in the R/R setting).
This study has limitations. First, while computing the event rate per 100 patient-cycles, there is an implicit assumption that the risk of VTE remains stable over time. However, studies have shown that, in ND MM, majority of VTE events happen in the first 6 months of therapy.11,71 To address this, we have reported both the cumulative incidence and the event rate per 100 patient-cycles. Second, because MM is associated with a high risk of VTE, especially in the first year after diagnosis,3,4 the magnitude of the additional contribution of Len-based regimens cannot be identified in this study. However, the cumulative incidence of VTE in ND and RR MM with Len-based regimens in controlled clinical trial settings, as noted in our meta-analysis (6.2%), is higher than estimates from large observational data sets in the United States (2.4%) and Sweden (4.2%), which were performed predominantly before the IMiD era. Third, we detected evidence of publication bias. Notably, statistical methods to assess publication bias have been developed for comparative analyses, whereas the current meta-analysis of incidence rates is a non-comparative one. Fourth, appropriate ascertainment of thromboembolic events (arterial vs venous) was not available in some studies. For studies with posted results in clinicaltrials.gov in which detailed report of adverse events (AEs) are available, we have verified the AEs from primary publication to limit our outcomes to VTEs (deep vein thrombosis or pulmonary embolism) whenever possible. Finally, in the current study, we could not address the effect of different thromboprophylaxis strategies on VTE risk. As noted in Table 1, approximately 75% of trials provided options of ASA, LMWH, and warfarin, based either on investigators’ discretion or IMWG guidelines. Hence, in the absence of patient-level data on thromboprophylaxis, we are unable to comment on the risk of VTE with different thromboprophylaxis agents.
In conclusion, our study demonstrates a cumulative VTE risk of 6.2% with Len-based regimens in patients with ND and R/R MM despite thromboprophylaxis. Apart from combination regimens known to have a high VTE risk, such as anthracycline or multiagent chemotherapy, regimens of Rd in combination with proteasome inhibitors also had a substantial VTE risk. These estimates can be used as a benchmark for the evaluation of VTE risk with future IMiD-based combination regimens and to test new thromboprophylaxis strategies in regimens with a high risk of VTE.
Supplementary Material
FUNDING SUPPORT
No specific funding was disclosed.
We acknowledge Loren Hackett, Medical Librarian at the Cleveland Clinic Floyd D. Loop Alumni Library, for performing the literature search for our study.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Faiz Anwer reports personal fees and reimbursement for travel and accommodation from InCyte and Seattle Genetics outside the submitted work. Alok A. Khorana reports institutional research funding from Array BioPharma, Bristol-Myers Squibb, Leap Oncology, and Merck; personal fees from AngioDynamics, Bayer, Halozyme, Janssen, Pfizer, Pharmacyclics, Pharmacyte Biotech, and Seattle Genetics; and reimbursement for travel and expenses from Bayer, Janssen, and Pfizer, all outside the submitted work. S. Vincent Rajkumar reports author royalties from UpToDate outside the submitted work. Shaji Kumar reports institutional research funding from AbbVie, Celgene, Janssen Oncology, Kite Pharma, MedImmune/ AstraZeneca, Merck, Novartis, Roche/Genentech, Sanofi, and Takeda; personal fees from AbbVie, Adaptive Biotechnologies, Amgen, Celgene, Genecentrix, Genentech/Roche, IRC, Janssen Oncology, Kite Pharma, MedImmune/AstraZeneca, Merck, Oncopeptides, Reddys Laboratories, and Takeda, all outside the submitted work. Navneet S. Majhail reports personal fees from Anthem Inc, Atara Biotherapeutics, Incyte, and Mallinckrodt, plc; and reimbursement for travel and expenses from Atara Biotherapeutics and Incyte, all outside the submitted work. The remaining authors made no disclosures.
REFERENCES
- 1.Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018;4:1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristinsson SY. Thrombosis in multiple myeloma. Hematology Am Soc Hematol Edu Program. 2010;2010:437–444. [DOI] [PubMed] [Google Scholar]
- 3.Kristinsson SY, Pfeiffer RM, Bjorkholm M, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010; 115:4991–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristinsson SY, Fears TR, Gridley G, et al. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood. 2008;112:3582–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma. N Engl J Med. 2001;344: 1951–1952. [DOI] [PubMed] [Google Scholar]
- 6.Zangari M, Siegel E, Barlogie B, et al. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: implications for therapy. Blood. 2002;100:1168–1171. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV. Thalidomide therapy and deep venous thrombosis in multiple myeloma. Mayo Clin Proc. 2005;80:1549–1551. [DOI] [PubMed] [Google Scholar]
- 8.Stewart AK, Jacobus S, Fonseca R, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015;126:1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zweegman S, van der Holt B, Mellqvist UH, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127:1109–1116. [DOI] [PubMed] [Google Scholar]
- 10.Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon SP, Rajkumar SV, Lacy M, Falco P, Palumbo A. Thromboembolic events with lenalidomide-based therapy for multiple myeloma. Cancer. 2008;112:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. [DOI] [PubMed] [Google Scholar]
- 13.Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine. 2003;28:1290–1299. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toma M, McAlister FA, Bialy L, Adams D, Vandermeer B, Armstrong PW. Transition from meeting abstract to full-length journal article for randomized controlled trials. JAMA. 2006;295:1281–1287. [DOI] [PubMed] [Google Scholar]
- 18.Carrier M, Le Gal G, Tay J, Wu C, Lee AY. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011;9:653–663. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 20.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351–1375. [DOI] [PubMed] [Google Scholar]
- 21.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–917. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Shinagawa A, Uchida T, et al. Lenalidomide and low-dose dexamethasone in Japanese patients with newly diagnosed multiple myeloma: a phase II study. Cancer Sci. 2016;107:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baz RC, Shain KH, Hussein MA, et al. Phase II study of pegylated liposomal doxorubicin, low-dose dexamethasone, and lenalidomide in patients with newly diagnosed multiple myeloma. Am J Hematol. 2014;89:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knop S, Langer C, Engelhardt M, et al. Lenalidomide, Adriamycin, dexamethasone for induction followed by stem-cell transplant in newly diagnosed myeloma. Leukemia. 2017;31:1816–1819. [DOI] [PubMed] [Google Scholar]
- 25.Kumar SK, Lacy MQ, Hayman SR, et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. Am J Hematol. 2011;86:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012; 120:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roussel M, Lauwers-Cances V, Robillard N, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol. 2014; 32:2712–2717. [DOI] [PubMed] [Google Scholar]
- 28.Korde N, Roschewski M, Zingone A, et al. Treatment With carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. [DOI] [PubMed] [Google Scholar]
- 31.Jakubowiak AJ, Griffith KA, Reece DE, et al. Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: a phase 1/2 Multiple Myeloma Research Consortium trial. Blood. 2011;118:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palumbo A, Falco P, Corradini P, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA—Italian Multiple Myeloma Network. J Clin Oncol. 2007;25:4459–4465. [DOI] [PubMed] [Google Scholar]
- 33.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma [erratum appears in N Engl J Med. 2012;367:285]. N Engl J Med. 2012;366:1759–1769. [DOI] [PubMed] [Google Scholar]
- 34.Roy V, Stewart AK, Bergsagel PL, et al. Phase I/II study of melphalan, prednisone and lenalidomide combination for patients with newly diagnosed multiple myeloma who are not candidates for stem cell transplantation. Blood Cancer J. 2015;5:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127:1102–1108. [DOI] [PubMed] [Google Scholar]
- 36.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1109. [DOI] [PubMed] [Google Scholar]
- 37.Richardson PG, Hofmeister CC, Rosenbaum CA, et al. Twice-weekly ixazomib in combination with lenalidomide-dexamethasone in patients with newly diagnosed multiple myeloma. Br J Haematol. 2018;182:231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi T, Miura M, Niioka T, et al. Phase II clinical trial of lenalidomide and dexamethasone therapy in Japanese elderly patients with newly diagnosed multiple myeloma to determine optimal plasma concentration of lenalidomide. Ther Drug Monit. 2018;40:301–309. [DOI] [PubMed] [Google Scholar]
- 39.Manasanch EE, Shah JJ, Lee HC, et al. Bortezomib, lenalidomide, and dexamethasone with panobinostat for front-line treatment of patients with multiple myeloma who are eligible for transplantation: a phase 1 trial. Lancet Haematol. 2018;5:e628–e640. [DOI] [PubMed] [Google Scholar]
- 40.O’Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15: 1503–1512. [DOI] [PubMed] [Google Scholar]
- 42.Baz R, Walker E, Karam MA, et al. Lenalidomide and pegylated liposomal doxorubicin-based chemotherapy for relapsed or refractory multiple myeloma: safety and efficacy. Ann Oncol. 2006;17:1766–1771. [DOI] [PubMed] [Google Scholar]
- 43.Beck J, Schwarzer A, Glaser D, et al. Lenalidomide in combination with bendamustine and prednisolone in relapsed/refractory multiple myeloma: results of a phase 2 clinical trial (OSHO-#077). J Cancer Res Clin Oncol. 2017;143:2545–2553. [DOI] [PubMed] [Google Scholar]
- 44.Berenson JR, Yellin O, Kazamel T, et al. A phase 2 study of pegylated liposomal doxorubicin, bortezomib, dexamethasone and lenalidomide for patients with relapsed/refractory multiple myeloma. Leukemia. 2012; 26:1675–1680. [DOI] [PubMed] [Google Scholar]
- 45.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. [DOI] [PubMed] [Google Scholar]
- 46.Hou J, Du X, Jin J, et al. A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol. 2013;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou J, Jin J, Xu Y, et al. Randomized, double-blind, placebo-controlled phase III study of ixazomib plus lenalidomide-dexamethasone in patients with relapsed/refractory multiple myeloma: China Continuation study. J Hematol Oncol. 2017;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knop S, Gerecke C, Liebisch P, et al. Lenalidomide, Adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: a report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom). Blood. 2009;113:4137–4143. [DOI] [PubMed] [Google Scholar]
- 49.Kumar SK, Krishnan A, Laplant B, et al. Bendamustine, lenalidomide, and dexamethasone (BRD) is highly effective with durable responses in relapsed multiple myeloma. Am J Hematol. 2015;90:1106–1110. [DOI] [PubMed] [Google Scholar]
- 50.Lazaryan A, Hussein MA, Reu FJ, et al. Mature results of MM-011: a phase I/II trial of liposomal doxorubicin, vincristine, dexamethasone, and lenalidomide combination therapy followed by lenalidomide maintenance for relapsed/refractory multiple myeloma. Am J Hematol. 2014;89:349–354. [DOI] [PubMed] [Google Scholar]
- 51.Lentzsch S, O’Sullivan A, Kennedy RC, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119:4608–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015; 373:621–631. [DOI] [PubMed] [Google Scholar]
- 53.Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30:1953–1959. [DOI] [PubMed] [Google Scholar]
- 54.Martin T, Baz R, Benson DM, et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017;129:3294–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016; 374:1621–1634. [DOI] [PubMed] [Google Scholar]
- 56.Mey UJ, Brugger W, Schwarb H, et al. Bendamustine, lenalidomide and dexamethasone (BRd) has high activity as 2nd-line therapy for relapsed and refractory multiple myeloma—a phase II trial. Br J Haematol. 2017;176:770–782. [DOI] [PubMed] [Google Scholar]
- 57.Niesvizky R, Martin TG 3rd, Bensinger WI, et al. Phase Ib dose-escalation study (PX-171–006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res. 2013;19:2248–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nijhof IS, Franssen LE, Levin MD, et al. Phase 1/2 study of lenalidomide combined with low-dose cyclophosphamide and prednisone in lenalidomide-refractory multiple myeloma. Blood. 2016;128:2297–2306. [DOI] [PubMed] [Google Scholar]
- 59.Ponisch W, Heyn S, Beck J, et al. Lenalidomide, bendamustine and prednisolone exhibits a favourable safety and efficacy profile in relapsed or refractory multiple myeloma: final results of a phase 1 clinical trial OSHO-#077. Br J Haematol. 2013;162:202–209. [DOI] [PubMed] [Google Scholar]
- 60.Reece DE, Masih-Khan E, Atenafu EG, et al. Phase I-II trial of oral cyclophosphamide, prednisone and lenalidomide for the treatment of patients with relapsed and refractory multiple myeloma. Br J Haematol. 2015;168:46–54. [DOI] [PubMed] [Google Scholar]
- 61.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Martin T, Bensinger W, et al. Phase 2 dose-expansion study (PX-171–006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122: 3122–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yee AJ, Bensinger WI, Supko JG, et al. Ricolinostat plus lenalidomide, and dexamethasone in relapsed or refractory multiple myeloma: a multicentre phase 1b trial. Lancet Oncol. 2016;17:1569–1578. [DOI] [PubMed] [Google Scholar]
- 65.Yee AJ, Hari P, Marcheselli R, et al. Outcomes in patients with relapsed or refractory multiple myeloma in a phase I study of everolimus in combination with lenalidomide. Br J Haematol. 2014;166:401–409. [DOI] [PubMed] [Google Scholar]
- 66.Quach H, Fernyhough L, Henderson R, et al. Upfront lower dose lenalidomide is less toxic and does not compromise efficacy for vulnerable patients with relapsed refractory multiple myeloma: final analysis of the phase II RevLite study. Br J Haematol. 2017;177:441–448. [DOI] [PubMed] [Google Scholar]
- 67.Dinner S, Dunn TJ, Price E, et al. A phase I, open-label, dose-escalation study of amrubicin in combination with lenalidomide and weekly dexamethasone in previously treated adults with relapsed or refractory multiple myeloma. Int J Hematol. 2018;108:267–273. [DOI] [PubMed] [Google Scholar]
- 68.Holstein SA, Jung SH, Richardson PG, et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: a randomised, double-blind, phase 3 trial. Lancet Haematol. 2017;4:e431–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012; 366:1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20:57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swan D, Rocci A, Bradbury C, Thachil J. Venous thromboembolism in multiple myeloma—choice of prophylaxis, role of direct oral anticoagulants and special considerations. Br J Haematol. 2018;183:538–556. [DOI] [PubMed] [Google Scholar]
- 72.Baz R, Li L, Kottke-Marchant K, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc. 2005;80:1568–1574. [DOI] [PubMed] [Google Scholar]
- 73.Bagratuni T, Kastritis E, Politou M, et al. Clinical and genetic factors associated with venous thromboembolism in myeloma patients treated with lenalidomide-based regimens. Am J Hematol. 2013;88:765–770. [DOI] [PubMed] [Google Scholar]
- 74.Johnson DC, Corthals S, Ramos C, et al. Genetic associations with thalidomide mediated venous thrombotic events in myeloma identified using targeted genotyping. Blood. 2008;112:4924–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li W, Garcia D, Cornell RF, et al. Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. 2017;3:980–988. [DOI] [PubMed] [Google Scholar]
- 76.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. [DOI] [PubMed] [Google Scholar]
- 77.Lonial S, Richardson PG, San Miguel J, et al. Characterisation of haematological profiles and low risk of thromboembolic events with bortezomib in patients with relapsed multiple myeloma. Br J Haematol. 2008;143:222–229. [DOI] [PubMed] [Google Scholar]
- 78.Zangari M, Guerrero J, Cavallo F, Prasad HK, Esseltine D, Fink L. Hemostatic effects of bortezomib treatment in patients with relapsed or refractory multiple myeloma. Haematologica. 2008;93:953–954. [DOI] [PubMed] [Google Scholar]
- 79.Zangari M, Fink L, Zhan F, Tricot G. Low venous thromboembolic risk with bortezomib in multiple myeloma and potential protective effect with thalidomide/lenalidomide-based therapy: review of data from phase 3 trials and studies of novel combination regimens. Clin Lymphoma Myeloma Leuk. 2011;11:228–236. [DOI] [PubMed] [Google Scholar]
- 80.Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075–2085. [DOI] [PubMed] [Google Scholar]
- 82.Lv W, Duan Q, Wang L, Gong Z, Yang F, Song Y. Expression of B-cell-associated genes in peripheral blood mononuclear cells of patients with symptomatic pulmonary embolism. Mol Med Rep. 2015;11:2299–2305. [DOI] [PubMed] [Google Scholar]
- 83.Al-Ani F, Bermejo JM, Mateos MV, Louzada M. Thromboprophylaxis in multiple myeloma patients treated with lenalidomide—a systematic review. Thromb Res. 2016;141:84–90. [DOI] [PubMed] [Google Scholar]
- 84.Yamshon S, Christos PJ, Demetres M, Hammad H, Leonard JP, Ruan J. Venous thromboembolism in patients with B-cell non-Hodgkin lymphoma treated with lenalidomide: a systematic review and meta- analysis. Blood Adv. 2018;2:1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoen MW, Luo S, Gage B, Carson KR, Sanfilippo KM. Association of venous thromboembolism with increased mortality in patients with multiple myeloma [abstract]. J Clin Oncol. 2018;36(15 suppl):8051. [Google Scholar]
- 86.Barrett A, Quinn J, Glavey S, et al. Younger age at diagnosis is associated with an increased risk of venous thromboembolism in multiple myeloma [abstract]. Blood. 2018;132(suppl 1):1223. [Google Scholar]
- 87.Leleu X, Rodon P, Hulin C, et al. MELISSE, a large multicentric observational study to determine risk factors of venous thromboembolism in patients with multiple myeloma treated with immunomodulatory drugs. Thromb Haemost. 2013;110:844–851. [DOI] [PubMed] [Google Scholar]
- 88.Sanfilippo KM, Luo S, Carson KR, Gage BF. Aspirin may be inadequate thromboprophylaxis in multiple myeloma [abstract]. Blood. 2017;130(suppl 1):3419. [Google Scholar]
- 89.Bradbury CA, Jenner M, Striha A, et al. Thrombotic events in patients with myeloma treated with immunomodulatory drugs; results of the Myeloma XI study. Br J Haematol. 2018;181(suppl 1):19–20. [Google Scholar]
- 90.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–939; quiz 1093. [DOI] [PubMed] [Google Scholar]
- 91.Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986–993. [DOI] [PubMed] [Google Scholar]
- 92.Li A, Wu Q, Luo S, et al. Derivation and validation of a risk assessment model for immunomodulatory drug-associated thrombosis among patients with multiple myeloma. J Natl Compr Canc Netw. 2019;17:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


