Abstract
Historically, research in ovarian biology has focused on folliculogenesis, but recently the ovarian stroma has become an exciting new frontier for research, holding critical keys to understanding complex ovarian dynamics. Ovarian follicles, which are the functional units of the ovary, comprise the ovarian parenchyma, while the ovarian stroma thus refers to the inverse, or the components of the ovary that are not ovarian follicles. The ovarian stroma includes more general components such as immune cells, blood vessels, nerves, and lymphatic vessels, as well as ovary-specific components including ovarian surface epithelium, tunica albuginea, intraovarian rete ovarii, hilar cells, stem cells, and a majority of incompletely characterized stromal cells including the fibroblast-like, spindle-shaped, and interstitial cells. The stroma also includes ovarian extracellular matrix components. This review combines foundational and emerging scholarship regarding the structures and roles of the different components of the ovarian stroma in normal physiology. This is followed by a discussion of key areas for further research regarding the ovarian stroma, including elucidating theca cell origins, understanding stromal cell hormone production and responsiveness, investigating pathological conditions such as polycystic ovary syndrome (PCOS), developing artificial ovary technology, and using technological advances to further delineate the multiple stromal cell types.
Keywords: ovarian stroma, extracellular matrix, polycystic ovary, theca cell origins, artificial ovary
WHAT IS THE OVARIAN STROMA AND WHAT DOES IT DO?
Organs are comprised of two components: (1) the parenchyma, or the specialized tissue that performs the function of the organ, and (2) the stroma, which is typically the supporting tissue (Young et al., 2014; Mescher, 2018). Ovarian follicles, which are the functional units of the ovary, comprise the ovarian parenchyma. Conceptualizing the stroma as the inverse of the parenchyma, the ovarian stroma thus refers to the components of the ovary that are not ovarian follicles. The ovarian stroma is comprised of general components such as immune cells (Wu et al., 2004), blood vessels (Reeves, 1971), nerves (Neilson et al., 1970), and lymphatic vessels (Brown et al., 2010), as well as ovary-specific components. These ovary-specific components include ovarian surface epithelium (Auersperg et al., 2001), tunica albuginea (Reeves, 1971), intraovarian rete ovarii (Wenzel and Odend’hal, 1985), hilar cells (Neilson et al., 1970), ovarian stem cells (Hummitzsch et al., 2015), a majority of incompletely characterized stromal cells that includes the fibroblast-like, spindle-shaped, and interstitial cells (Reeves, 1971), and possibly other cell types not included in this list. In addition to these cell types, ovarian extracellular matrix (ECM) provides structural and biochemical support to surrounding cells and is a key component of the stroma (Berkholtz et al., 2006) (Figure 1, Table I). Some studies have used the broad terms ‘ovarian interstitial stroma’ or ‘theca interstitial cells’ (TICs) to refer to the heterogeneous stromal compartment (e.g. Tingen et al., 2011; Hummitzsch et al., 2019). For the purpose of this review, we will interpret the ovarian stroma as the broadly inclusive non-follicular components of the ovary. We also want to highlight that the term ‘stromal cells’ does not refer to a single homogenous cell population. Instead, when feasible, we recommend more specific descriptions like ‘stromal macrophages’ to refer to individual components of the stromal compartment. What is known about the multiple cell types and components of the stroma is detailed below.
Figure 1.
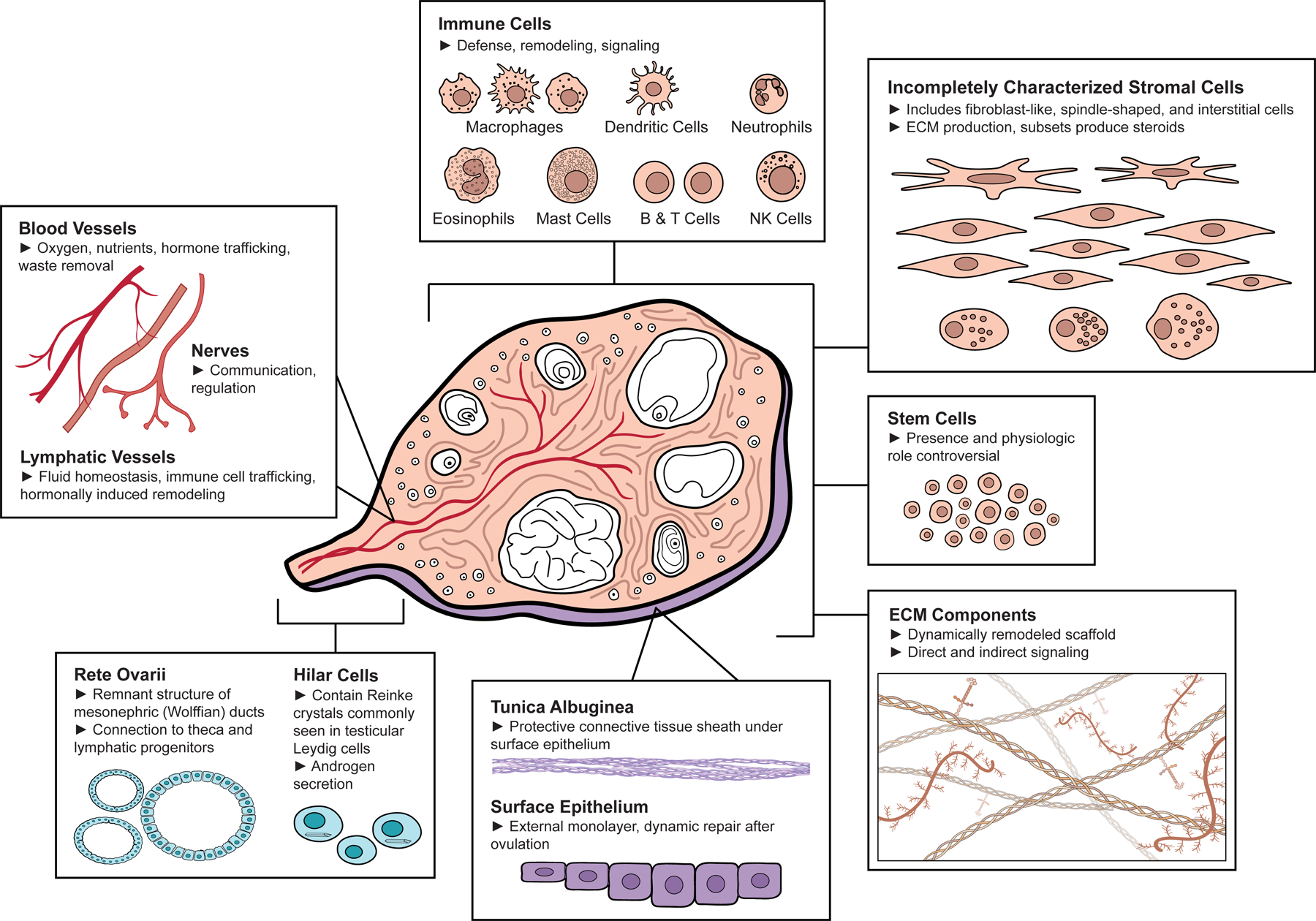
Components of the Ovarian Stroma. Central diagram of a human ovary (adapted from Gray, 1918) surrounded by boxes highlighting different ovarian stromal components including (clockwise from top center): immune cells including macrophages, dendritic cells, neutrophils, eosinophils, mast cells, B & T cells, and Natural Killer (NK) cells; incompletely characterized stromal cells (including fibroblast-like, spindle-shaped, and interstitial cells); stem cells; extracellular matrix (ECM) components; surface epithelium and tunica albuginea; rete ovarii and hilar cells; and blood vessels, lymphatic vessels, and nerves. Made using ©BioRender - biorender.com.
Table I:
General and Ovary-Specific Components of the Ovarian Stroma
| Subsets | Regionality | Function | Example Cellular Markers† | Further Research Required | |
|---|---|---|---|---|---|
| General Components | |||||
| Immune Cells | Macrophages, dendritic cells, neutrophils, eosinophils, mast cells, B Lymphocytes, T Lymphocytes, Natural Killer cells | Throughout stroma, around theca vasculature | Defense, remodeling, signaling | Leukocyte: CD45 | Cyclic, hormonal, temporal dynamics, pathologic relevance |
| Myeloid: CD11b | |||||
| Macrophages: CD68 | |||||
| Dendritic Cells: CD11c | |||||
| Neutrophils: CD16 | |||||
| Eosinophils: CD193 | |||||
| Mast Cells: CD117 | |||||
| Lymphoid: | |||||
| B Lymphocytes: CD19 | |||||
| T Lymphocytes: CD3 | |||||
| Natural Killer: CD561 | |||||
| Blood Vessels | Endothelial cells, pericytes, smooth muscle cells | Branching medullary spirals to cortical arcades | Oxygen, nutrients, hormone trafficking | Endothelial cells: VE-Cadherin Pericytes: PDGFRB Smooth muscle cells: α-SMA2 |
Dynamic role of oxygen tension, pathologic role and management in PCOS |
| Nerves | Neurons, glial cells | Branching medulla to cortex | Communication, can regulate hormone secretion and vasoconstriction3 | Neurons: MAP2 Glia: SOX104 |
Neuronal regulation of stromal cell types, pathologic relevance |
| Lymphatic Vessels | Endothelial cells, smooth muscle cells | Branching medulla to cortex, vasculature association | Fluid homeostasis, immune cell trafficking, hormonally induced remodeling5 | Endothelial cells: LYVE16 Smooth muscle cells: α-SMA |
Dynamic regulation, pathologic relevance |
| Ovary-Specific Components | |||||
| Surface Epithelium | External monolayer of ovary | Supports repair after ovulation, dynamic | CK7, CK8, CK18, CK19, Plakophilin-2, Desmoglein-27 | Heterogeneity, pathologic contributions | |
| Tunica Albuginea | Outer layer under surface epithelium | Protection | Minimal cellularity | Physiologic and pathologic contributions | |
| Intraovarian Rete Ovarii | Hilar region, medulla | Connection to progenitor populations, including theca and lymphatics8 | CK19, Vimentin9 | Physiologic role in adults, pathologic relevance | |
| Hilar Cells | Hilar region, nerve trunk association | Contain Reinke crystals commonly seen in testicular Leydig cells, androgen secretion10 | Not established | Physiologic role, pathologic relevance | |
| Stem Cells | Physiologic role in adults not established | Oogonial: DDX4 or IFITM3 (controversial)11 | Presence and potential physiologic role in adults | ||
| Incompletely characterized stromal cells | Up to 5 different types of fibroblast-like/spindle-shaped/interstitial cells identified12 | Subsets mainly in cortex, subsets mainly in medulla | Subsets associated with production of collagens I or III, subsets are steroid producing with cytoplasmic lipids and vacuoles12 | COUP-TFII13; COUP-TFII and/or ARX14; DCN, LUM for theca/stroma15; some express PDGFRA, DCN, COL1A1, COL6A1, STAR, and/or CYP17A116; some TCF21, COL1A2, STAR17 | Cellular identification and ontology, regionality, steroid production, common and differentiating markers |
| Extracellular Matrix Components | Collagens, glycoproteins, proteoglycans, ECM-affiliated proteins, ECM regulators, secreted factors |
Stiff cortex with radially aligned collagen fibers and less dense medulla | Scaffold, direct and indirect signaling, dynamically remodeled, stiffness involved in follicular dormancy18 | Extracellular | ECM composition and structure not adjacent to follicles |
(http://docs.abcam.com/pdf/immunology/immune-cell-markers-poster.pdf, https://media.cellsignal.com/www/pdfs/science/pathways/Immune-Cell-Markers-Human.pdf, accessed March 2020);
(https://www.rndsystems.com/research-area/endothelial-progenitor-and-endothelial-cell-markers, accessed March 2020; Rensen et al., 2007; Kizuka-Shibuya et al., 2014);
(Uchida, 2015),
(https://docs.abcam.com/pdf/neuroscience/neural-markers-guide-web.pdf, accessed March 2020);
(Reeves, 1971);
Example cellular markers are presented for general components and possible cellular markers are presented for ovary-specific components
General Cell Types of the Ovarian Stroma
Immune Cells
Cells of the immune system appear to play critical roles in supporting ovarian physiologic processes. Immune cells, including macrophages, mast cells, and eosinophils, are present in immature or resting ovaries at low levels throughout the stroma. These levels tend to increase around ovulation, particularly near the theca vasculature, with subsequent migration into developing corpora lutea (Norman and Brannstrom, 1994). Ovarian immune cells serve multiple functions, including phagocytosis and antigen presentation, tissue remodeling via proteolytic enzymes, and secretion of soluble signals including cytokines, chemokines, and growth factors (Norman and Brannstrom, 1994; Wu et al., 2004). Macrophages are a predominant ovarian immune cell type, with other immune cells present including B and T lymphocytes, Natural Killer cells, dendritic cells, neutrophils, eosinophils, and mast cells (Norman and Brannstrom, 1994; Suzuki et al., 1998; Carlock et al., 2013; Kenngott et al., 2016; Fan et al., 2019; Zhang et al., 2020) (Figure 1, Table I). Ovarian macrophages have received ongoing attention with regard to their role in reproductive homeostasis and their regulation by estrogen (reviewed in Wu et al., 2004; Pepe et al., 2018). Ovaries may contain multiple macrophage subsets, and phenotypes can range from classical inflammatory (M1) to alternative tissue remodeling (M2) during different parts of the ovarian cycle (Carlock et al., 2013; Pepe et al., 2018). Increased proportions of M2 macrophages, monocyte-derived macrophages, and multinucleated macrophages have been seen with murine ovarian aging (Briley et al., 2016; Zhang et al., 2020). Macrophage and other myeloid cell depletion using the CD11b-DTR mouse model has resulted in infertility, with hemorrhagic ovaries, ovarian endothelial cell depletion, impaired corpora lutea formation, and diminished progesterone production (Turner et al., 2011; Care et al., 2013). Although ovarian immune cells, particularly macrophages, have been the subjects of ongoing research, gaps in knowledge remain regarding cyclic, hormonal, and temporal dynamics as well as contributions to ovarian pathologic conditions (Table I).
Blood Vessels
The vasculature of the ovary supports critical ovarian functions, and includes blood vessel endothelial cells, pericytes, and smooth muscle cells (Figure 1, Table I). Ovarian blood vessels travel through connective tissue to provide tissue oxygenation, hormone trafficking, and nutrients, in addition to supporting waste removal. The medulla of the human ovary typically contains the larger blood vessels and at the cortico-medullary junction, small medullary arteries branch to cortical arterioles (Reeves, 1971). These cortical arterioles form vascular arcades of interconnected short straight vessels of fixed length running along the connective tissue fascicles. With pressure, the cortical arterioles could be compressed to form avascular regions as part of the formation of stigma for ovulation (Reeves, 1971). Medullary vessels include spiraling arteries and arterioles, which may allow expansion with growth (Reeves, 1971). The microvasculature of the ovary contributes to folliculogenesis and corpora lutea formation. Follicles contain a basal lamina between their granulosa and theca cell compartments, allowing for a blood-follicle barrier (Siu and Cheng, 2012). With the formation of the theca cell layer, follicles develop microvasculature between the theca cells that supports the increased growth and development of the follicle, yet never passes beyond the basal lamina before ovulation. The formation of the corpus luteum, a highly vascular structure, occurs after theca microvasculature invades into the granulosa layer following ovulation (Rolaki et al., 2005). Gaps in knowledge remain around the role of oxygen tension, as regulated by ovarian vasculature. Oxygen tension may have regulatory effects in the ovary, with in vitro studies demonstrating that oxygen levels can impact bovine granulosa cell luteinization and rat corpora lutea progesterone production (Gafvels et al., 1987; Baddela et al., 2018). Dysfunction of ovarian vasculature has been implicated in the pathophysiology of PCOS (Di Pietro et al., 2018), and additional studies are needed to address the pathologic role and therapeutic management of altered ovarian angiogenesis (Table I).
Nerves
Neilson et al.’s (1970) review of ovarian innervation describes widespread innervation present in the ovarian stromal compartment, noting that some nerves follow blood vessels in the medulla while others branch among the cells in the stroma (Figure 1, Table I). In mouse gonadal development, neural crest neurons colonize the ovary, differentiate into neurons and glia, and form dense neural networks in the medulla that extend towards cortical regions (McKey et al., 2019). Functionally, both sympathetic and parasympathetic innervation of the ovary has been demonstrated, and regulation by the sympathetic nervous system has been shown to inhibit estradiol secretion and cause vasoconstriction (reviewed in Uchida, 2015). In a PCOS model, estradiol-treated rats demonstrated increased ovarian sympathetic activity and cystic anovulatory ovaries, with improvement noted in cyclicity and corpora lutea formation following superior ovarian nerve transection (Barria et al., 1993). Further study is warranted regarding the neuronal regulation of different cells types in the stroma, physiologic consequences of denervation, and neuronal contributions to pathology (Table I).
Lymphatic Vessels
Lymphatic vasculature includes small capillaries comprised of endothelial cells without a basement membrane that have large gaps between cells to allow fluid, cellular, and macromolecular transport. These capillaries feed into larger collecting vessels with basement membranes, valves, and smooth muscle (Figure 1, Table I). The ovary has a rich lymphatic network, closely associating with blood vasculature, extending from the medulla into the cortex adjacent to developing follicles, with some species variability in regard to presence in the corpus luteum (Brown and Russell, 2014). The lymphatic system typically helps to maintain fluid homeostasis by returning extravascular fluid and proteins back to the bloodstream and participating in immune cell trafficking. In the developing mouse ovary, lymphatic vessels only appeared postnatally, potentially arising from the extraovarian rete ovarii, as seen in a Prox1-EGFP mouse model, where Prox1 expression marks the commitment of endothelial cells to the lymphatic lineage (Svingen et al., 2012). Lymphatic vasculature has been shown to remodel in response to hormonal regulation in mouse ovaries (Brown et al., 2010). Although lymphatic vasculature plays essential physiologic roles in the ovary, the dynamic regulation and pathologic relevance of the ovarian lymphatics remains to be fully elucidated (Table I).
Ovary-Specific Cell Types of the Ovarian Stroma
Ovarian Surface Epithelium
The surface epithelium of the ovary is a heterogenous flat to cuboidal epithelial layer derived from the mesoderm, also called the “germinal epithelium” because of the false past belief that it contributed to germ cell formation (Auersperg et al., 2001) (Figure 1, Table I). The keratin-rich ovarian surface epithelial cell layer helps to facilitate repair after ovulation and dynamically expands and contracts with cyclic ovarian changes (Xu et al., 2018; Hartanti et al., 2020). Scanning electron microscopy and immunofluorescence of the surface epithelium of developing fetal bovine ovaries demonstrated expansion from the hilar region to surround the entire ovary, with changes corresponding to underlying stromal rearrangement (Hartanti et al., 2020). Although fetal ovarian surface epithelial cells had been previously thought to be a developmental source for granulosa cells, more recent studies suggest that ovarian surface epithelial cells instead share a common progenitor with granulosa cells, known as the Gonadal Ridge Epithelial-Like (GREL) cell (Auersperg et al., 2001; Hummitzsch et al., 2013). Although definitive markers have not been identified, surface epithelial cells have increased expression of the cytokeratins 7, 8, 18, and 19 as well as plakophillin-2 and desmoglein-2 (Hummitzsch et al., 2013; Hartanti et al., 2020) (Table I). Further work remains regarding identifying definitive markers, understanding heterogeneity, and clarifying the pathologic contributions of the ovarian surface epithelium.
Tunica Albuginea
The ovarian tunica albuginea, positioned beneath the surface epithelium, is a thin and hypocellular connective tissue sheath, which serves as a protective layer for the ovary (Reeves, 1971). The tunica albuginea is collagen-rich and undergoes remodeling prior to ovulation. Using electron microscopy, Okamura et al, (1980) observed a decrease in presence of collagen bundles at the human follicular apex as follicles reached the preovulatory stage. This degradation was paralleled by an increase in apical fibroblasts with developed cytoplasm and lysosome-like granules, which were suspected to contain collagenases for degradation of the tunica albuginea (Okamura et al., 1980). There has been limited study of the ovarian tunica albuginea and further work can help to clarify physiologic and pathologic roles and regulation (Figure 1, Table I).
Intraovarian Rete Ovarii
The rete ovarii are remnants of the mesonephric (Wolffian) ducts that typically form part of the male reproductive tract and regress in the female reproductive tract. They are often found as groups of tubules lined by cuboidal or columnar epithelium in the hilus of the ovary or extending through the medulla, as well as in the extraovarian space (reviewed in Wenzel and Odend’hal, 1985) (Figure 1, Table I). There has been limited investigation into the function of the rete ovarii, particularly after development where they may play relevant roles. In a study of murine theca cell lineages, one of the two identified progenitor populations of theca cells migrated from the adjacent mesonephros and was potentially related to the rete ovarii (Liu et al., 2015; Rotgers et al., 2018). Ovarian lymphatic vasculature origins have also been connected to the rete ovarii (Svingen et al., 2012). Although not necessarily specific markers, increased levels of cytokeratin 19 and vimentin have been noted in human rete ovarii (Russo et al., 2000). Further study is needed to elucidate the physiologic role of the rete ovarii in adults as well as the pathologic relevance (Table I).
Hilar Cells
There are reports of distinct cells located in the ovarian hilus with Reinke crystals, which are commonly found in testicular Leydig cells (Neilson et al., 1970) (Figure 1, Table I). These cells are frequently located in clusters associated with a nerve trunk (Neilson et al., 1970), and synthesize and secrete androgens in response to LH stimulation, although their physiologic role has not been well-established (Erickson et al., 1985). Hyperplasia of these hilar cells has been implicated in virilization in postmenopausal women (Delibasi et al., 2007). Cellular markers have not been established and the physiologic role and pathologic relevance of these cells remains generally uncharacterized.
Ovarian Stem Cells
The ovary may contain stem cells for a variety of different cell types, including somatic (e.g., granulosa, surface epithelial, thecal, stromal) and germline stem cells (reviewed in Hummitzsch et al., 2015) (Figure 1, Table I). The presence and importance of ovarian germline (oogonial) stem cells has been a controversial topic, although the ovarian follicular reserve is generally lost with age without substantive renewal. Putative ovarian oogonial stem cells were first isolated through DEAD [Asp-Glu-Ala-Asp] box polypeptide 4 (DDX4, also known as VASA) tagging and cell sorting and have been shown to develop into oocytes, although isolation of DDX4 positive cells has been questioned, particularly related to assumptions about cytoplasmic versus surface expression and antibody cross-reactivity (Johnson et al., 2004; Zarate-Garcia et al., 2016). Others have disputed the presence of oogonial stem cells, noting that oogonial stem cells were not detectable using sensitive single-cell lineage-tracing in adult female mice (Lei and Spradling, 2013). Additionally, postnatal DDX4-expressing cells generated using a Rosa26rbw/+; Ddx4-Cre fluorescent reporter mouse were not seen to be mitotically active nor participating in follicular renewal (Zhang et al., 2012). A recent single-cell sequencing study isolated human Abcam DDX4-positive cells and concluded these cells were perivascular cells rather than oogonial stem cells (Wagner et al., 2020). In contrast, cell line establishment of female germline stem cells has been described using cells from human ovarian cortical tissue fragments present in follicular aspirates, which differentiated into oocyte-like cells (Ding et al., 2016). Isolated, purified, and cultured female germline stem cells from an EGFP-transgenic mouse were shown to differentiate into oocytes, capable of restoring function and generating offspring in a mouse model of premature ovarian failure (Wu et al., 2017). The presence of ovarian germline stem cells continues to be a highly contested topic, generally eclipsing the discussion of somatic stem cells. The addition of human mesenchymal stem cells originating from amniotic fluid has also been used to help restore ovarian function in mouse models of premature ovarian failure, suggesting a role for somatic stem cells in improving altered paracrine signaling and the stroma microenvironment (Liu et al., 2019).
Incompletely Characterized Stromal Cells
The majority of the ovarian stroma is comprised of a mixed population of incompletely characterized cells commonly referred to as stromal cells (Reeves, 1971). This includes the populations of cells also described as fibroblast-like, spindle-shaped cells, or interstitial cells (Figure 1, Table I). In general, fibroblasts secrete ECM proteins, such as collagen, for cellular support, scaffolding, and repair. A retrospective study of histologic sections from non-pathologic human ovaries from 167 women ages 17–79 carried out with the goal of describing the morphology of various types of stromal cells identified five types of fibroblast-like/interstitial stromal cells (Reeves, 1971). While recent human single-cell RNA-sequencing studies (e.g., Fan et al., 2019) confirm the presence of multiple stromal cell clusters, a comprehensive and complete characterization of stromal cell types throughout the ovary is lacking. The distribution and subtypes of stromal cells will likely differ with their location in the ovary (e.g. cortex vs. medulla). The stromal cell distribution is also likely to be affected by cyclic structural changes, as follicles grow and ovulate, and corpora lutea develop. Changes are also evident over the reproductive lifespan, including increases in fibrotic collagen as demonstrated in aging murine and primate ovaries (Briley et al., 2016; Wang et al., 2020). Some possible cellular markers that have been identified include COUP-TFII and/or ARX (Hummitzsch et al., 2013; Rotgers et al., 2018). Other studies have used DCN and LUM to identify populations of human theca/stroma cells (Fan et al., 2019). Higher expression in some of the human cells considered to be stroma was demonstrated for markers PDGFRA, DCN, COL1A1, COL6A1, STAR, and/or CYP17A1 (Wagner et al., 2020). A different study delineated nonhuman primate ovarian stroma by expression of TCF21, COL1A2, and/or STAR (Wang et al., 2020). For these incompletely characterized stromal cell types, careful ontology, further marker identification, and attention to nuances of regionality and steroid production are critical next steps (Table I).
Extracellular Matrix (ECM) Components
Structure & Definition
The ECM is composed of fibril- and network-forming proteins, proteoglycans, and glycosaminoglycans, the composition of which is unique to each tissue (Figure 1, Table I). Cells secrete soluble ECM components to the extracellular compartments where cell-secreted enzymes such as lysyl oxidase (LOX) crosslink the ECM precursors into large networks (Theocharis et al., 2016). These matrices regulate cellular functions including adhesion, migration, and proliferation through cell receptor interactions, mechanotransduction, and cell interaction with ECM-sequestered growth factors (Taipale and Keski-Oja, 1997).
Several reviews have covered the extensive list of ECM components that exist broadly in tissues and specifically in the ovary; most notably, collagen types I, III, IV, and VI, fibronectin, and laminin (Berkholtz et al., 2006; Irving-Rodgers and Rodgers, 2006). Collagens I and III have been shown to be distributed in concentric layers connected by bundles in human cortical stroma (Lind et al., 2006). A recent proteomic study examining the ECM of the human ovarian cortex revealed that collagens comprise nearly half of the ECM proteins and associated factors, the most dominant of which was collagen VI, a basement membrane-anchoring ECM protein (Ouni et al., 2019). Another recent proteomic study examined ECM compositional differences between porcine cortex and medulla, showing increased expression of collagen I, agrin, elastin microfibril interfacer 1, and fibronectin in the cortex compared to the medulla (Henning et al., 2019). These proteomic studies both identified over 80 ECM and ECM-associated proteins, in categories of collagens, glycoproteins, proteoglycans, ECM-affiliated proteins, ECM regulators, and secreted factors (Henning et al., 2019; Ouni et al., 2019).
Many studies of ECM have focused on matrix within follicles during development. Follicles have a unique pericellular matrix called the basal lamina, composed primarily of laminin and type IV collagen stabilized by nidogen and perlecan which separates the granulosa and theca cell compartments (Irving-Rodgers and Rodgers, 2006). As follicles grow, they continuously remodel the basal lamina to allow for expansion of the follicle as granulosa cells proliferate. Granulosa cells have been shown to produce the major components of the basal lamina, although theca and other cells in the ovarian stroma may contribute to basal lamina deposition in later stages (Rodgers et al., 1999). The basal lamina also plays a role in mediating granulosa cell growth and antrum formation through growth factor sequestration and signaling. Perlecan in the basal lamina is able to bind growth factors and is charge- and size-selective, serving as a barrier to diffusion of growth factors between the granulosa and theca cell compartments, allowing the follicular fluid and basal lamina to become reservoirs of factors to promote healthy folliculogenesis (McArthur et al., 2000).
Mechanics
The ovary has two major compartments which differ in their ECM composition and structure – a stiff cortex where primordial follicles reside in dormancy, and a less dense medulla where antral follicles vigorously remodel the ECM through proteolytic degradation as they reach preovulatory stages. Decellularized human and bovine ovarian tissue reveals radially-aligned collagen fibers in the cortex, lending to its increased stiffness, whereas the medulla is composed of a network of pores with anisotropic collagen fibers, suggesting differences between cortical and medullary ECM-producing stromal cells (Laronda et al., 2015; Chiti et al., 2018). The prominence of ovarian cortical and medullary regionalization can differ across species, and is notably reduced in rodent ovaries when compared to human ovaries (Jiménez, 2009). The mechanical properties of these regions have important roles in mechanotransduction for the follicles as they activate and develop. Primordial follicle dormancy has been shown to be regulated by the Hippo signaling pathway, where rigidity of the ovarian cortex inactivates yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) to inhibit growth (Kawamura et al., 2013). Follicle activation can be initiated with disruption of the Hippo signaling pathway, for example when follicles are isolated from the cortex, further illustrating the importance of ECM mechanical properties in maintenance of the follicular reserve (Kawamura et al., 2013). After activation, early stage follicle growth and survival is still dependent on a stiff matrix, as has been shown in vitro (Hornick et al., 2012). As follicles grow, they require a softer matrix for expansion as provided by the medullar region of the ovary, and in vitro studies have shown improved growth, survival, and steroidogenesis of later stage follicles in permissive matrices (West et al., 2007; West-Farrell et al., 2009).
Function: Signaling and Remodeling
ECM components play a large role in regulating cell functions through both direct and indirect signaling. Fibronectin and laminin contain integrin-binding sequences (most notably Arg-Gly-Asp, or RGD) which allow cells to directly interact with the ECM and initiate signaling cascades for proliferation and differentiation as follicles develop (Monniaux et al., 2006). ECM also has an indirect role in signaling as it acts as a reservoir of growth factors and cytokines and mediates their presentation to cells both when they are bound and when they are released upon ECM degradation. ECM is a dynamic structure in tissues, continuously being remodeled by the cells which reside in it through matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), and plasminogen activators (McIntush and Smith, 1998). Follicles and other ovarian stromal cells secrete these enzymes to soften the surrounding ECM and allow for follicular expansion, and in this process cytokines and growth factors bound to the ECM are released. Several growth factors known to be key regulatory molecules in folliculogenesis including fibroblast growth factor, transforming growth factor beta, platelet derived growth factor, hepatocyte growth factor, and insulin-like growth factor have ECM-binding motifs or can be sequestered within the ECM through binding factors such as follistatin (Logan and Hill, 1992). In this way, ECM remodeling is a mechanism by which growth factor bioavailability can be mediated or disrupted in some pathological conditions (McIntush and Smith, 1998). If dysregulated, ECM degradation may also trigger pathogen-free inflammation. For example, hyaluronan is a glycosaminoglycan that forms low molecular weight fragments during turnover, which have been shown in cultured murine stromal cells to increase the secretion of type 2 inflammatory cytokines and activate genes involved in eosinophil recruitment, while also leading to adverse effects on cultured follicles (Rowley et al., 2020).
At the final stages of follicular maturation the ECM again plays an important role in ovulation. Follicles are stimulated by the LH surge to produce large amounts of MMPs and plasminogen activator to degrade the ECM at the apical region of the follicle (Curry and Smith, 2006). This process is further amplified by the release of tumor necrosis factor-alpha (TNF-a) from the degraded ECM to promote collagenase production and apoptosis of ovarian epithelial cells (Curry and Smith, 2006). The weakened cellular and ECM components at the apical region, along with pressure from the follicular fluid and increased vascular pressure, facilitate follicular rupture and expulsion of the oocyte into the periovarian space (Matousek et al., 2001).
KEY AREAS FOR FURTHER RESEARCH AND FUTURE PERSPECTIVES
Understanding the origins of the theca cells
The theca cell layer is divided into the theca interna, with cytoplasmic lipid droplets characteristic of its role in steroid production, and the theca externa, which is a mix of fibroblasts and smooth muscle cells that are more contiguous with the broader ovarian stroma (reviewed in Young and McNeilly, 2010; Richards et al., 2018). The relationship between the supporting cells of the ovarian stroma and the theca cells has not been definitively established, although it is generally agreed that the theca cells originate at least in part from stromal cells (Young and McNeilly, 2010; Rotgers et al., 2018).
Murine theca cells have been shown to arise from two types of progenitors: Wt1-positive cells in the fetal ovary and Gli1-positive cells migrating from the mesonephros adjacent to the ovary (Liu et al., 2015). Near birth, desert hedgehog and Indian hedgehog paracrine signals from granulosa cells appear to prompt expression in undifferentiated stromal progenitor cells of the theca lineage marker Gli1. Microarray analysis suggested differences based on theca progenitor population, with increased steroidogenesis in the mesonephros-derived Gli1-positive cells (Liu et al., 2015). The steroidogenic androgen-producing theca cells may arise from the mesonephros derived progenitors, while the theca fibroblasts, perivascular smooth muscle cells, and possibly the interstitial ovarian cells may arise from the ovarian WT1+ progenitors (Richards et al., 2018).
Additional undifferentiated stromal cell progenitors (possibly positive for Lhx9, Mafb, Coup-tfII, and Arx) may yield a nonsteroidogenic stromal cell population, possibly expressing Coup-tfII and Arx. Overlapping expression of COUP-TFII and ARX in the same population of cells has not been established (Rotgers et al., 2018). Sonic hedgehog signaling has been shown to regulate expression of COUP-TFII, which was identified in murine theca interna cells and in mesenchymal cells around the corpus luteum (Krishnan et al., 1997; Takamoto et al., 2005). COUP-TFII is likely expressed in steroidogenic cells, as haploinsufficient female mice demonstrated altered reproduction function, including reduced expression of steroidogenic enzymes needed for progesterone synthesis and reduced vascularization (Takamoto et al., 2005). Three populations of somatic cell precursors have been demonstrated in murine fetal ovaries, marked by mutually exclusive expression of COUP-TFII and the granulosa cell markers FOXL2 and LGR5 (Rastetter et al., 2014). Mutually exclusive FOXL2 and COUP-TFII expression was also seen in early fetal human ovaries, with COUP-TFII expression in the stromal cell population. Several 46,XX SRY-negative children with mutations in the gene encoding COUP-TFII were virilized, with testicular tissue confirmed in one child, suggesting a “pro-ovary” and “anti-testis” role for COUP-TFII in developing human female gonads (Bashamboo et al., 2018).
A transgenic mouse study suggests the presence of at least two steroidogenic cell types for ovarian theca and interstitial gland cells. In postnatal mouse ovaries, only a portion of the steroidogenic theca and interstitial gland cells expressed enhanced green fluorescent protein (EGFP) as a reporter of the fetal Leydig enhancer (FLE) of the Nr5a1 gene (SF-1). SF-1 regulates expression of steroidogenic CYP genes. In testes, the FLE differentiates fetal from adult Leydig cells. In these ovaries only approximately 16% of the SF-1 postive cells were positive for EGFP, suggesting at least two cell populations (Miyabayashi et al., 2015).
A transcriptome analysis of the bovine ovarian stroma found that populations isolated by laser microdissection were similar between general interstitial stroma and what they labeled as pre-theca cells (stroma adjacent to preantral follicles). They combined them for the purpose of analysis, and the subsequent stroma was found to be different from both the tunica albuginea and the theca interna (Hummitzsch et al., 2019). The theca interna of small antral follicles had an upregulation of genes associated with steroid hormone and cholesterol synthesis as compared to the stroma (Hummitzsch et al., 2019).
Of note, the concept of theca interstitial cells (TICs) has been used as a catch-all for the residual ovarian tissue husk once follicles had been punctured (Tingen et al., 2011; Tian et al., 2015). When cultured, theca interstitial cells from mouse ovaries take on a fibroblast-like appearance that is distinct from granulosa cells (Tian et al., 2015). The heterogeneity of the TICs has been noted, with a reported shift in populations over a 12-day co-culture with follicles. At the beginning of the culture, the population contained predominantly lipid droplet-containing cells resembling theca cells as well as fibroblast-like cells, whereas the cells were mainly macrophages by day 12 (Tingen et al., 2011). This transition in cell phenotype may be due to differential survival in culture of the different starting cell populations, emphasizing that TICs are not a homogenous grouping.
Further understanding the stromal compartment may aid in better identification of theca progenitors (Figure 2). Additionally, studies using mixed populations of TICs may benefit from greater categorization of these non-follicular populations to aid in interpretation and reproducibility of findings.
Figure 2.
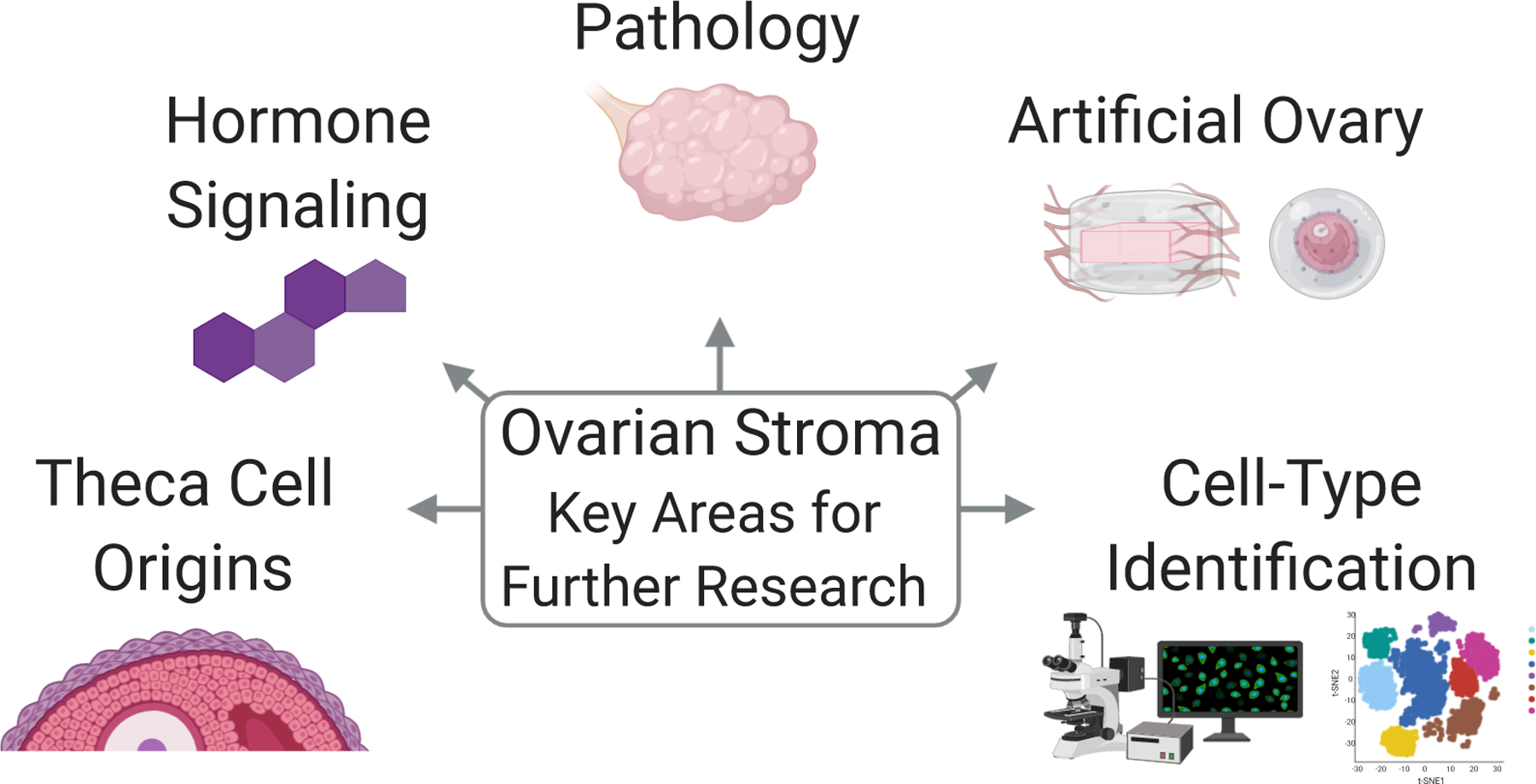
Ovarian Stroma Key Areas for Further Research. Includes (clockwise from left): theca cell origins, hormone signaling, pathology, artificial ovary, and cell-type identification. Made using ©BioRender - biorender.com.
Stromal cell hormone production and responsiveness
Some of the ovarian stromal cells are capable of steroid hormone production and contain hormone receptors. For instance, estrogen receptor alpha and beta have been identified in the cytoplasm and nucleus of bovine interstitial cells, which were described as oval cells with lipid droplets and vacuoles that were distinguishable from fibroblasts (Kenngott et al., 2016). Progesterone receptor alpha has been identified in stromal cells and interstitial cells of pregnant and post-partum rabbit ovaries (Abd-Elkareem, 2017). Interstitial cells with features of steroid production have been documented in early gestation in the human fetal ovary (Konishi et al., 1986). Postmenopausal ovarian stromal cells have been postulated to produce androgens, although a study of in vitro isolated postmenopausal human stromal cells found that the predominant population had negligible expression of a key steroidogenic enzyme in the androgen biosynthesis pathway, CYP17A1, and did not appear to have significant steroidogenic potential (Jabara et al., 2003). Additionally, they found that transcripts for certain steroidogenic enzymes (STAR, CYP11A1, and HSD3B) were much less abundant in the in vitro isolated stromal cells than in theca cells, with the exception of STAR which had more transcript abundance in stromal cells than in fibroblasts (Jabara et al., 2003). In contrast, localization of CYP17A1 shifted from exclusively the theca interna in control mice to patches in the interstitial stroma in DHT-treated mice, supporting a potential role for the stroma in androgen production following certain perturbations (Candelaria et al., 2019). Single-cell RNA sequencing studies have also demonstrated subpopulations of stromal cells expressing CYP17A1 and STAR (Wagner et al., 2020; Wang et al., 2020). Although stromal cells have demonstrated varied hormone production and responsiveness, definitive characterization of these dynamics and their functional significance remains to be established (Figure 2).
Pathological ovarian stromal changes: polycystic ovary syndrome as an example
Polycystic ovary syndrome (PCOS) has been defined by the Rotterdam Criteria (2004) as two of the three characteristics – hyperandrogenism, oligo or amenorrhea, and follicular cysts as noted on ultrasound (The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group., 2004). Polycystic ovarian morphology includes the following features – thickening of the tunica albuginea, ovarian stromal hyperplasia, stromal cell luteinization, and large cystic antral follicles (Hughesdon, 1982). The thickness of the cortical stroma is increased by one third and the subcortical stroma by five-fold (Hughesdon, 1982). In detailed ultrasound assessment, women with PCOS were found to have significantly increased ovarian volume, stromal volume, and stromal peak blood flow velocity as compared to controls (Buckett et al., 1999). In contrast, no difference was found in ovarian stromal blood flow between women with PCOS and a control group explicitly excluding patients with low ovarian reserve (Younis et al., 2011). The ratio of ovarian stromal area to total ovarian area (S/A) by ultrasound was a good predictor of hyperandrogenism in lean Italian women with PCOS, with increased ovarian vascularization and blood flow noted in PCOS patients as compared to controls (Battaglia et al., 2012), and S/A ratio has been proposed as a method to refine the Rotterdam PCOS classification (Belosi et al., 2006). In contrast, the S/A ratio was found to have limited predictive value as a PCOS diagnostic in reproductive-aged Thai women with PCOS (Leerasiri et al., 2015). Another study found increased ovarian stromal area with PCOS, but was unable to demonstrate a relationship between stromal area and PCOS hormonal characteristics (Kaleli et al., 1998). Ovarian angiogenesis dysfunction including increased ovarian stromal vascularization, lower impedance to flow (Alcázar and Kudla, 2012), and alterations in angiogenic factors levels in PCOS, have been further reviewed elsewhere (Di Pietro et al., 2018), with possible implications that restoration of appropriate vessel formation could improve folliculogenesis and ovulation. Inflammation-related gene expression was downregulated in the ovarian stroma and upregulated in granulosa cells for PCOS women as compared to controls, although the downregulation in the stroma may have been affected by a reduced abundance of leukocytes in the PCOS stroma as measured by CD45 mRNA levels (Schmidt et al., 2014). A reduction in theca-associated activated/memory T lymphocytes has also been seen in PCOS ovaries as compared to controls, without notable differences in macrophage or neutrophil levels across multiple ovarian compartments (Wu et al., 2007). Broadly, PCOS may impact stromal volume, tunica albuginea thickness, stromal luteinization, vascularization, blood flow, inflammation and immune cell distribution, although the causes and functional impacts of these stromal changes have not been fully elucidated.
Hyperandrogenism, one of the common aspects of PCOS, has been shown to drive certain stromal alterations. For instance, in transgender men given exogenous testosterone therapy, increases were noted in tunica albuginea collagenization, stromal hyperplasia, and stromal luteinization with clusters of luteinized stromal cells (Spinder et al., 1989; Ikeda et al., 2013), as well as increased stromal androgen receptor staining (Chadha et al., 1994). Multiple cell types in polycystic ovaries may produce androgens, as immunohistochemistry revealed the presence of steroidogenic enzymes for androgen synthesis in follicular theca cells, luteinized stromal cells, hilar cells, and sporadic non-luteinized stromal cells (Kaaijk et al., 2000). Mice treated with dihydrotestosterone (DHT) also demonstrated stromal changes, including less dense, hyperplastic, and lipid-filled stroma when compared to age-matched controls. These mice also had an overexpression of multiple genes in the mechanically-separated stroma between controls and DHT-treated mice (Candelaria et al., 2019). This included increased Vcam1 expression (which may impact vascular and immune responses) in thecal and stromal cells, while theca-specific androgen receptor knockout mice (ThARKO, Cyp17a1-iCre, ARf/f mice) demonstrated a lack of DHT-induced Vcam1 elevation (Richards et al., 2018; Candelaria et al., 2019). ThARKO mice were also shown to retain much of their reproductive function, including cyclicity and fertility as compared to controls when treated with DHT (Ma et al., 2017). For mice with DHT-induced stromal changes, superovulation rescued at least some of the abnormal stromal morphology (Candelaria et al., 2019).
Several changes occur in the ovarian ECM in polycystic ovary syndrome. The cortex and basal laminas of follicles thicken and become more collagenous with reduced glycosaminoglycan content (Salvetti et al., 2003). A comparison of human PCOS to control ovaries in both the follicular and luteal phases revealed significantly lower pro-collagen IV expression compared to control ovaries, and this decrease in collagen IV was postulated to contribute to premature luteinization (Oksjoki et al., 2004). PCOS patients tend to have increased MMP-9 secretion as well, which may be related to the inability of follicles to undergo normal atresia (Dambala et al., 2019).
Stromal contribution to artificial ovary technology
The term ‘artificial ovary’ typically references an ovary constructed using a combination of ovarian follicles (or hormone-producing cell types) within a supportive scaffold (Figure 2). The creation of an artificial ovary as a means of fertility preservation and endocrine support has been a persistent challenge from biological and engineering perspectives as follicle development requires a complex symphony of soluble signals and mechanical cues, some of which may derive from the ovarian stroma.
Co-culture of follicles with stromal feeder cells has shown promise for providing the key soluble factors to promote growth of early stage murine follicles in vitro (Tingen et al., 2011). With regard to directly sourcing ovarian stromal cells, ideal collection strategies may differ between stromal cells and follicles. Human stromal cells have been shown to be better preserved after vitrification than slow freezing, with slow freezing increasing necrosis and collagen bundle disruption in the stromal cells, while follicles were similarly preserved in both vitrification and slow freezing (Keros et al., 2009). Isolating human stromal cells from fresh medullary tissue was shown to be superior to isolation from ovarian cortex in slow frozen and fresh samples, and led to increased cell yield, better viability, and improved vascularization when encapsulated in fibrin and implanted in the peritoneal pockets of nude mice (Soares et al., 2015). For xenograft models, the importance of transplanting stromal endothelial cells has been demonstrated (Dath et al., 2011). Isolated human ovarian cortical stromal cell suspensions containing stromal endothelial cells yielded well-vascularized and organized grafts after one-week implantations in mice, in contrast to grafts depleted of stromal endothelial cells, which were smaller, necrotic, and poorly vascularized (Dath et al., 2011).
It is also challenging to develop a supportive scaffold that fully recapitulates the ovarian ECM. Multiple three-dimensional hydrogel culture systems such as alginate, fibrin, and poly(ethylene glycol) (PEG) aim to recapitulate the mechanical properties of the ovarian environment to maintain the spherical structure of follicles and allow for their expansion; however, these systems are lacking the biological functionality of ECM and the ability to sequester growth factors (Luyckx et al., 2014; Smith et al., 2014; Kniazeva et al., 2015; Kim et al., 2016; Chiti et al., 2018; Rios et al., 2018). Several groups have attempted to restore the biological function of ECM in these artificial ovaries by encapsulating follicles in ECM matrices such as Matrigel or decellularized tissues (Scott et al., 2004; Laronda et al., 2015). Unfortunately, these matrices do not include all of the components present in native ovarian ECM and also face challenges in translation in regard to availability of tissue and batch-to-batch variability.
While each of these systems incorporates key components necessary for follicle growth, there is yet to be a system that truly mimics the ovarian microenvironment in both complexity of cell populations and extracellular matrix composition which can be translated for clinical use. Part of this limitation relates to scarcity of knowledge as it pertains to the cell types and functions of the ovarian stroma.
Identification of ovarian stromal cells
The multiple populations of cells referred to as stromal cells are incompletely characterized and categorized, leading to confusion across studies that report findings about stromal cells without further identification (Figure 2). Regional differences (e.g. cortex vs. medulla) likely influence the distribution and subtypes of stromal cells. Immunofluorescent imaging using known markers for follicular or stromal cells has advanced our understanding of the ovarian stroma, including the delineation of at least two distinct populations of steroidogenic theca and interstitial gland cells in postnatal murine ovaries, as well as the identification of at least three different somatic cell lineages in murine fetal ovaries (Rastetter et al., 2014; Miyabayashi et al., 2015). With developments in single-cell sequencing technologies to complement these detailed imaging studies, we may soon have the ability to better characterize the cells commonly called stromal cells and refer to them with more precise names as we understand their individual roles in physiologic and pathologic processes.
Single-cell RNA-sequencing experiments have already made progress in identifying major ovarian cell types, transition stages, and markers for cell identification. These studies have significantly contributed to mapping the signatures of human and murine oocytes and granulosa cells from multiple follicular stages (Zhang et al., 2018). Yet, data about the ovarian stroma remain elusive and comparatively scarce. An investigation of somatic cells only in the inner cortex was performed in women undergoing fertility preservation procedures, detecting five clusters of granulosa cells, five clusters of theca and stromal cells, two clusters of smooth muscle cells, three clusters of endothelial cells, and four clusters of immune cells (Fan et al., 2019). They confirmed the presence of adaptive immune cells including T lymphocytes, Natural Killer cells, and B lymphocytes, as well as innate immune cells including monocytes and macrophages. This study also identified upregulation of the complement system (including C1R, C1S, and C7) by theca and stromal cells as a potential contributor to ovarian tissue remodeling (Fan et al., 2019). A subsequent single-cell analysis of the human ovarian cortex reported six clusters, including oocytes, granulosa cells, immune cells, endothelial cells, perivascular cells, and stromal cells. They classified a majority of cells (83%) as stroma, noting shared expression of mesodermal lineage markers (PDGFRA, DCN), ECM proteins (COL1A1, COL6A1), as well as expression of STAR and CYP17A1 by some cells in the stromal cluster. Although they isolated many stromal cells, their study mainly focused on discerning whether cells isolated using the Abcam DDX4 antibody were oogonial stem cells (Wagner et al., 2020). A single-cell transcriptomic study of ovarian aging in nonhuman primate ovaries identified seven ovarian cell types, including oocytes, granulosa cells, stromal cells, smooth muscle cells, endothelial cells, Natural Killer T cells, and macrophages (Wang et al., 2020). The stromal cell cluster specifically expressed TCF21 and COL1A2, with some cells in the stromal cluster expressing high levels of STAR (Wang et al., 2020). A time series single-cell RNA sequencing study was performed for cells labeled with the gonadal somatic cell marker Nr5a1 (steroidogenic factor 1, SF-1) in the developing mouse ovary from E10.5 to postnatal day 6. Four distinct populations, including early progenitors, stromal progenitors, pre-granulosa cells, and postnatal granulosa cells were identified from their sequencing. Using their time series, they analyzed cell conversion from early progenitors to both the stromal progenitor lineage (E13.5) and the granulosa cell lineages (E11.5-E12.5) (Stévant et al., 2019). These studies are supported by precise immunofluorescent characterization of at least three somatic cell populations in fetal mouse ovaries, including COUP-TFII-positive possible pre-theca progenitors, LGR5-positive cortical granulosa cell progenitors, and FOXL2-positive medullary granulosa cell progenitors (Rastetter et al., 2014). Although single-cell sequencing studies allow for greater granularity in understanding the nuance of different ovarian cellular populations, including the stroma, it remains important to continually reflect on the possible limitations of any starting cellular populations (e.g. inner cortex only), with the overall goal of broadening our understanding of the entire ovarian microenvironment.
Future perspectives
As the majority of ovarian research studies focus on the ovarian follicles, a thorough understanding of the components and functions of the ovarian stroma is an active area of current research. The support provided by the ovarian stroma is essential for three-dimensional follicular maintenance and the integration of signals to support folliculogenesis. The stromal compartment is heterogeneous and analyses using bulk methods or gross dissection may lose the granularity that could be observed between low density specialized cellular populations. In addition to precise immunohistochemical and immunofluorescent studies for specific stromal cell population identification and lineage tracing, single-cell sequencing studies will continue to allow for more in-depth analysis of physiologic and pathologic changes occurring to specific cell types that might otherwise be grouped together. These sequencing studies must be conducted with critical reflection on the specifics of the origin of the sequenced cells. Greater understanding and careful ontology of the different populations of stromal cells would reduce ambiguity between studies. Further study integrating phenotypic changes in specific stromal cellular populations with functional changes would also help determine how changes in the ovarian stroma occur over time and may interact with folliculogenesis, position, and hormone production.
Supplementary Material
FUNDING
This work was supported by the National Institutes of Health (R01-EB022033 to A.S., R01-HD098233 to M.B.M., P01-HD044232 to V.P., F30-HD100163 and T32-HD079342 to H.M.K., F31-HD100069 and T32-DE007057 to C.E.T.); NSF CAREER (1552580 to A.S.), American Society for Reproductive Medicine / Society for Reproductive Endocrinology and Infertility Grant to M.B.M., Chan Zuckerberg Initiative Human Cell Atlas of the Female Reproductive System to A.S., University of Michigan Office of Research funding (U058227) to M.B.M.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Vasantha Padmanabhan is an Associate Editor of Reproduction. Vasantha Padmanabhan was not involved in the review or editorial process for this paper, on which she is listed as an author
REFERENCES
- Abd-Elkareem M 2017. Cell-specific immuno-localization of progesterone receptor alpha in the rabbit ovary during pregnancy and after parturition. Animal Reproduction Science 180 100–120. [DOI] [PubMed] [Google Scholar]
- Alcázar JL and Kudla MJ 2012. Ovarian stromal vessels assessed by spatiotemporal image correlation-high definition flow in women with polycystic ovary syndrome: a case-control study. Ultrasound in Obstetrics and Gynecology 40 470–475. [DOI] [PubMed] [Google Scholar]
- Auersperg N, Wong AST, Choi K-C, Kang SK and Leung PCK 2001. Ovarian Surface Epithelium: Biology, Endocrinology, and Pathology. Endocrine Reviews 22 255–288. [DOI] [PubMed] [Google Scholar]
- Baddela VS, Sharma A, Viergutz T, Koczan D and Vanselow J 2018. Low Oxygen Levels Induce Early Luteinization Associated Changes in Bovine Granulosa Cells. Frontiers in Physiology 9 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Leyton V, Ojeda SR and Lara HE 1993. Ovarian steroidal response to gonadotropins and β-Adrenergic stimulation is enhanced in polycystic ovary syndrome: Role of sympathetic innervation. Endocrinology 133 2696–2703. [DOI] [PubMed] [Google Scholar]
- Bashamboo A, Eozenou C, Jorgensen A, Bignon-Topalovic J, Siffroi JP, Hyon C, Tar A, Nagy P, Sólyom J, Halász Z et al. 2018. Loss of Function of the Nuclear Receptor NR2F2, Encoding COUP-TF2, Causes Testis Development and Cardiac Defects in 46,XX Children. American Journal of Human Genetics 102 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia C, Battaglia B, Morotti E, Paradisi R, Zanetti I, Meriggiola MC and Venturoli S 2012. Two- and Three-Dimensional Sonographic and Color Doppler Techniques for Diagnosis of Polycystic Ovary Syndrome. J Ultrasound Med 31 1015–1024. [DOI] [PubMed] [Google Scholar]
- Belosi C, Selvaggi L, Apa R, Guido M, Romualdi D, Fulghesu AM and Lanzone A 2006. Is the PCOS diagnosis solved by ESHRE/ASRM 2003 consensus or could it include ultrasound examination of the ovarian stroma? Human Reproduction 21 3108–3115. [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Shea LD and Woodruff TK 2006. Extracellular matrix functions in follicle maturation. Seminars in Reproductive Medicine 24 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT and Duncan FE 2016. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 152 245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HM and Russell DL 2014. Blood and lymphatic vasculature in the ovary: Development, function and disease. Human Reproduction Update 20 29–39. [DOI] [PubMed] [Google Scholar]
- Brown HM, Robker RL and Russell DL 2010. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology 151 5446–5455. [DOI] [PubMed] [Google Scholar]
- Buckett WM, Bouzayen R, Watkin KL, Tulandi T and Tan SL 1999. Ovarian stromal echogenicity in women with normal and polycystic ovaries. Human Reproduction 14 618–621. [DOI] [PubMed] [Google Scholar]
- Candelaria NR, Padmanabhan A, Stossi F, Cecilia Ljungberg M, Shelly KE, Pew BK, Solis M, Rossano AM, McAllister JM, Wu S et al. 2019. VCAM1 is induced in ovarian theca and stromal cells in a mouse model of androgen excess. Endocrinology 160 1377–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV. and Robertson SA 2013. Macrophages regulate corpus luteum development during embryo implantation in mice. Journal of Clinical Investigation 123 3472–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlock C, Wu J, Zhou C, Ross A, Adams H and Lou Y 2013. Ovarian phagocyte subsets and their distinct tissue distribution patterns. Reproduction 146 491–500. [DOI] [PubMed] [Google Scholar]
- Chadha S, Pache TD, Huikeshoven FJM, Brinkmann AO and van der Kwast TH 1994. Androgen Receptor Expression in Human Ovarian and Uterine Tissue of Long Term Androgen-Treated Transsexual Women. Human Pathology 25 1198–1204. [DOI] [PubMed] [Google Scholar]
- Chiti MC, Dolmans M-M, Mortiaux L, Zhuge F, Ouni E, Shahri PAK, Van Ruymbeke E, Champagne S-D, Donnez J and Amorim CA 2018. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. Journal of Assisted Reproduction and Genetics 35 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry T and Smith M 2006. Impact of Extracellular Matrix Remodeling on Ovulation and the Folliculo-Luteal Transition. Seminars in Reproductive Medicine 24 228–241. [DOI] [PubMed] [Google Scholar]
- Dambala K, Paschou SA, Michopoulos A, Siasos G, Goulis DG, Vavilis D and Tarlatzis BC 2019. Biomarkers of Endothelial Dysfunction in Women With Polycystic Ovary Syndrome. Angiology 70 797–801. [DOI] [PubMed] [Google Scholar]
- Dath C, Dethy A, Van Langendonckt A, Van Eyck AS, Amorim CA, Luyckx V, Donnez J and Dolmans MM 2011. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Human Reproduction 26 1431–1439. [DOI] [PubMed] [Google Scholar]
- Delibasi T, Erdogan MF, Serinsöz E, Kaygusuz G, Erdogan G and Sertçelik A 2007. Ovarian hilus-cell hyperplasia and high serum testosterone in a patient with postmenopausal virilization. Endocrine Practice 13 472–475. [DOI] [PubMed] [Google Scholar]
- Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, Wang J, Du M, Teng X and Wu J 2016. Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Scientific Reports 6 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Dyer CA and Hofeditz C 1985. The ovarian androgen producing cells: A review of structure/function relationships. Endocrine Reviews 6 371–399. [DOI] [PubMed] [Google Scholar]
- Fan X, Bialecka M, Moustakas I, Lam E, Torrens-Juaneda V, Borggreven NV., Trouw L, Louwe LA, Pilgram GSK, Mei H et al. 2019. Single-cell reconstruction of follicular remodeling in the human adult ovary. Nature Communications 10 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafvels M, Selstam G and Damber JE 1987. Influence of oxygen tension and substrates on basal and luteinizing hormone stimulated progesterone production and energy metabolism by isolated corpora lutea of adult pseudopregnant rats. Acta Physiologica Scandinavica 130 475–482. [DOI] [PubMed] [Google Scholar]
- Gray H 1918. Anatomy of the Human Body. Philadelphia and New York: Lea & Febiger. [Google Scholar]
- Hartanti MD, Hummitzsch K, Bonner WM, Bastian NA, Irving-Rodgers HF and Rodgers RJ 2020. Formation of the Bovine Ovarian Surface Epithelium during Fetal Development. Journal of Histochemistry and Cytochemistry 68 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning NF, LeDuc RD, Even KA and Laronda MM 2019. Proteomic analyses of decellularized porcine ovaries identified new matrisome proteins and spatial differences across and within ovarian compartments. Scientific Reports 9 20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD and Woodruff TK 2012. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Human Reproduction 27 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughesdon PE 1982. Morphology and Morphogenesis of the Stein-Leventhal Ovary and of So-called “Hyperthecosis”. Obstetrical and Gynecological Survey 37 59–77. [DOI] [PubMed] [Google Scholar]
- Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D and Rodgers RJ 2013. A New Model of Development of the Mammalian Ovary and Follicles. PLoS ONE 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummitzsch K, Anderson RA, Wilhelm D, Wu J, Telfer EE, Russell DL, Robertson SA and Rodgers RJ 2015. Stem cells, progenitor cells, and lineage decisions in the ovary. Endocrine Reviews 36 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummitzsch K, Hatzirodos N, Macpherson AM, Schwartz J, Rodgers RJ and Irving-Rodgers HF 2019. Transcriptome analyses of ovarian stroma: tunica albuginea, interstitium and theca interna. Reproduction 157 545–565. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T and Saito T 2013. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Human Reproduction 28 453–461. [DOI] [PubMed] [Google Scholar]
- Irving-Rodgers HF and Rodgers RJ 2006. Extracellular matrix of the developing ovarian follicle. Seminars in Reproductive Medicine 24 195–203. [DOI] [PubMed] [Google Scholar]
- Jabara S, Christenson LK, Wang CY, McAllister JM, Javitt NB, Dunaif A and Strauss JF 2003. Stromal cells of the human postmenopausal ovary display a distinctive biochemical and molecular phenotype. Journal of Clinical Endocrinology and Metabolism 88 484–492. [DOI] [PubMed] [Google Scholar]
- Jiménez R 2009. Ovarian Organogenesis in Mammals: Mice Cannot Tell Us Everything. Sexual Development 3 291–301. [DOI] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK and Tilly JL 2004. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428 145–150. [DOI] [PubMed] [Google Scholar]
- Kaaijk EM, Sasano H, Suzuki T, Beek JF and van der Veen F 2000. Distribution of steroidogenic enzymes involved in androgen synthesis in polycystic ovaries: an immunohistochemical study. Molecular Human Reproduction 6 443–447. [DOI] [PubMed] [Google Scholar]
- Kaleli S, Erel CT, Oral E, Elter K, Akman C and Colgar U 1998. Ovarian stromal hypertrophy in polycystic ovary syndrome. Journal of Reproductive Medicine for the Obstetrician and Gynecologist 43 893–897. [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho C-h., Kawamura N, Tamura M, Hashimoto S et al. 2013. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proceedings of the National Academy of Sciences 110 17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenngott RAM, Scholz W and Sinowatz F 2016. Ultrastructural Aspects of the Prenatal Bovine Ovary Differentiation with a Special Focus on the Interstitial Cells. Anatomia Histologia Embryologia 45 357–366. [DOI] [PubMed] [Google Scholar]
- Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J and Hovatta O 2009. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Human Reproduction 24 1670–1683. [DOI] [PubMed] [Google Scholar]
- Kim J, Perez AS, Claflin J, David A, Zhou H and Shikanov A 2016. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. Npj Regenerative Medicine 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizuka-Shibuya F, Tokuda N, Takagi K, Adachi Y, Lee L, Tamura I, Maekawa R, Tamura H, Suzuki T, Owada Y et al. 2014. Locally existing endothelial cells and pericytes in ovarian stroma, but not bone marrow-derived vascular progenitor cells, play a central role in neovascularization during follicular development in mice. Journal of Ovarian Research 7 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva E, Hardy AN, Boukaidi SA, Woodruff TK, Jeruss JS and Shea LD 2015. Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model. Scientific Reports 5 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L-L, Yang N-Z, Shi L-H, Zhao G-H, Zhou W, Ding Q, Wang M-H and Zhang Y-S 2017. The optimum marker for the detection of lymphatic vessels. Molecular and Clinical Oncology 7 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi I, Fujii S, Okamura H, Parmley T and Mori T 1986. Development of interstitial cells and ovigerous cords in the human fetal ovary: an ultrastructural study. Journal of Anatomy 148 121–135. [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Pereira FA, Qiu Y, Chen CH, Beachy PA, Tsai SY and Tsai MJ 1997. Mediation of sonic hedgehog-induced expression of COUP-TFII by a protein phosphatase. Science 278 1947–1950. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN and Woodruff TK 2015. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerasiri P, Wongwananuruk T, Rattanachaiyanont M, Indhavivadhana S, Techatraisak K and Angsuwathana S 2015. Ratio of ovarian stroma and total ovarian area by ultrasound in prediction of hyperandrogenemia in reproductive-aged Thai women with polycystic ovary syndrome: A diagnostic test. Journal of Obstetrics and Gynaecology Research 41 248–253. [DOI] [PubMed] [Google Scholar]
- Lei L and Spradling AC 2013. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proceedings of the National Academy of Sciences of the United States of America 110 8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind A-K, Weijdegård B, Dahm-Kähler P, Mölne J, Sundfeldt K and Brännström M 2006. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstetricia et Gynecologica Scandinavica 85 1476–1484. [DOI] [PubMed] [Google Scholar]
- Liu C, Peng J, Matzuk MM and Yao HHC 2015. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nature Communications 6 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhang X, Fan Z, Wang Y, Yao G, Wan X, Liu Z, Yang B and Yu L 2019. Human amniotic mesenchymal stem cells improve the follicular microenvironment to recover ovarian function in premature ovarian failure mice. Stem Cell Research & Therapy 10 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A and Hill DJ 1992. Bioavailability: Is this a key event in regulating the actions of peptide growth factors? Journal of Endocrinology 134 157–161. [DOI] [PubMed] [Google Scholar]
- Luyckx V, Dolmans M-M, Vanacker J, Legat C, Fortuño Moya C, Donnez J and Amorim CA 2014. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertility and Sterility 101 1149–1156. [DOI] [PubMed] [Google Scholar]
- Ma Y, Andrisse S, Chen Y, Childress S, Xue P, Wang Z, Jones D, Ko C, Divall S and Wu S 2017. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology 158 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matousek M, Carati C, Gannon B and Brännström M 2001. Novel method for intrafollicular pressure measurements in the rat ovary: increased intrafollicular pressure after hCG stimulation. Reproduction 121 307–314. [DOI] [PubMed] [Google Scholar]
- McArthur ME, Irving-Rodgers HF, Byers S and Rodgers RJ 2000. Identification and Immunolocalization of Decorin, Versican, Perlecan, Nidogen, and Chondroitin Sulfate Proteoglycans in Bovine Small-Antral Ovarian Follicles1. Biology of Reproduction 63 913–924. [DOI] [PubMed] [Google Scholar]
- McIntush EW and Smith MF 1998. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in ovarian function. Reviews of Reproduction 3 23–30. [DOI] [PubMed] [Google Scholar]
- McKey J, Bunce C, Batchvarov IS, Ornitz DM and Capel B 2019. Neural crest-derived neurons invade the ovary but not the testis during mouse gonad development. Proceedings of the National Academy of Sciences 116 5570–5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL 2018. Epithelial Tissue. New York, NY: McGraw-Hill Education. [Google Scholar]
- Miyabayashi K, Tokunaga K, Otake H, Baba T, Shima Y and Morohashi K 2015. Heterogeneity of ovarian theca and interstitial gland cells in mice. PLoS ONE 10 e0128352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monniaux D, Huet-Calderwood C, Le Bellego F, Fabre S, Monget P and Calderwood DA 2006. Integrins in the Ovary. Seminars in Reproductive Medicine 24 251–261. [DOI] [PubMed] [Google Scholar]
- Neilson D, Seegar Jones G, Woodruff JD and Goldberg B 1970. The innervation of the ovary. Obstetrical and Gynecological Survey 25 889–904. [Google Scholar]
- Norman RJ and Brannstrom M 1994. White cells and the ovary – incidental invaders or essential effectors? Journal of Endocrinology 140 333–336. [DOI] [PubMed] [Google Scholar]
- Okamura H, Takenaka A, Yajima Y and Nishimura T 1980. Ovulatory changes in the wall at the apex of the human Graafian follicle. J Reprod Fert 58 153–155. [DOI] [PubMed] [Google Scholar]
- Oksjoki S, Rahkonen O, Haarala M, Vuorio E and Anttila L 2004. Differences in connective tissue gene expression between normally functioning, polycystic and post-menopausal ovaries. Molecular Human Reproduction 10 7–14. [DOI] [PubMed] [Google Scholar]
- Ouni E, Vertommen D, Chiti MC, Dolmans M-M and Amorim CA 2019. A Draft Map of the Human Ovarian Proteome for Tissue Engineering and Clinical Applications. Molecular & Cellular Proteomics 18 S159–S173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G, Locati M, Della Torre S, Mornata F, Cignarella A, Maggi A and Vegeto E 2018. The estrogen-macrophage interplay in the homeostasis of the female reproductive tract. Human Reproduction Update 24 652–672. [DOI] [PubMed] [Google Scholar]
- Di Pietro M, Pascuali N, Parborell F and Abramovich D 2018. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction 155 R199–R209. [DOI] [PubMed] [Google Scholar]
- Rastetter RH, Bernard P, Palmer JS, Chassot A-A, Chen H, Western PS, Ramsay RG, Chaboissier M-C and Wilhelm D 2014. Marker genes identify three somatic cell types in the fetal mouse ovary. Developmental Biology 394 242–252. [DOI] [PubMed] [Google Scholar]
- Reeves G 1971. Specific Stroma in the Cortex and Medulla of the Ovary. Obstetrics & Gynecology 37 832–844. [PubMed] [Google Scholar]
- Rensen SSM, Doevendans PAFM and Van Eys GJJM 2007. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart Journal 15 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JAS, Ren YA, Candelaria N, Adams JE and Rajkovic A 2018. Ovarian follicular theca cell recruitment, differentiation, and impact on fertility: 2017 update. Endocrine Reviews 39 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios PD, Kniazeva E, Lee HC, Xiao S, Oakes RS, Saito E, Jeruss JS, Shikanov A, Woodruff TK and Shea LD 2018. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnology and Bioengineering 115 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Lavranos TC, van Wezel IL and Irving-Rodgers HF 1999. Development of the ovarian follicular epithelium. Molecular and Cellular Endocrinology 151 171–179. [DOI] [PubMed] [Google Scholar]
- Rolaki A, Drakakis P, Millingos S, Loutradis D and Makrigiannakis A 2005. Novel trends in follicular development, atresia and corpus luteum regression: A role for apoptosis. Reproductive BioMedicine Online 11 93–103. [DOI] [PubMed] [Google Scholar]
- Rotgers E, Jørgensen A and Yao HH-C 2018. At the crossroads of fate - somatic cell lineage specification in the fetal gonad. Endocrine Reviews 39 739–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JE, Amargant F, Zhou LT, Galligos A, Simon LE, Pritchard MT and Duncan FE 2020. Low molecular weight hyaluronan induces an inflammatory response in ovarian stromal cells and impairs gamete development in vitro. International Journal of Molecular Sciences 21 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo L, Woolmough E and Heatley MK 2000. Structural and cell surface antigen expression in the rete ovarii and epoophoron differs from that in the Fallopian tube and in endometriosis. Histopathology 37 64–69. [DOI] [PubMed] [Google Scholar]
- Salvetti NR, Gimeno EJ, Canal AM, Lorente JA and Ortega HH 2003. Histochemical Study of the Extracellular Matrix Components in the Follicular Wall of Induced Polycystic Ovaries. Braz. J. Morphol. Sci 20 93–100. [Google Scholar]
- Schmidt J, Weijdegård B, Mikkelsen AL, Lindenberg S, Nilsson L and Brännström M 2014. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Molecular Human Reproduction 20 49–58. [DOI] [PubMed] [Google Scholar]
- Scott JE, Carlsson IB, Bavister BD and Hovatta O 2004. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reproductive BioMedicine Online 9 287–293. [DOI] [PubMed] [Google Scholar]
- Siu MKY and Cheng CY 2012. The blood-follicle barrier BFB in disease and in ovarian function. Adv Exp Med Biol 763 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Shikanov A, Kniazeva E, Ramadurai D, Woodruff TK and Shea LD 2014. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Engineering - Part A 20 3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares M, Sahrari K, Chiti MC, Amorim CA, Ambroise J, Donnez J and Dolmans MM 2015. The best source of isolated stromal cells for the artificial ovary: medulla or cortex, cryopreserved or fresh? Human Reproduction 30 1589–1598. [DOI] [PubMed] [Google Scholar]
- Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PGA and Gooren LJG 1989. The Effects of Long Term Testosterone Administration on Pulsatile Luteinizing Hormone Secretion and on Ovarian Histology in Eugonadal Female to Male Transsexual Subjects. Journal of Clinical Endocrinology and Metabolism 69 151–157. [DOI] [PubMed] [Google Scholar]
- Stévant I, Kühne F, Greenfield A, Chaboissier MC, Dermitzakis ET and Nef S 2019. Dissecting Cell Lineage Specification and Sex Fate Determination in Gonadal Somatic Cells Using Single-Cell Transcriptomics. Cell Reports 26 3272–3283.e3. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Date F and Nagura H 1998. Leukocytes in normal-cycling human ovaries: immunohistochemical distribution and characterization. Human Reproduction 13 2186–2191. [DOI] [PubMed] [Google Scholar]
- Svingen T, François M, Wilhelm D and Koopman P 2012. Three-Dimensional Imaging of Prox1-EGFP Transgenic Mouse Gonads Reveals Divergent Modes of Lymphangiogenesis in the Testis and Ovary. PLoS ONE 7 e52620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J and Keski-Oja J 1997. Growth factors in the extracellular matrix. The FASEB Journal 11 51–59. [DOI] [PubMed] [Google Scholar]
- Takamoto N, Kurihara I, Lee K, DeMayo FJ, Tsai MJ and Tsai SY 2005. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Molecular Endocrinology 19 2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reproduction 19 41–47. [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C and Karamanos NK 2016. Extracellular matrix structure. Advanced Drug Delivery Reviews 97 4–27. [DOI] [PubMed] [Google Scholar]
- Tian Y, Shen W, Lai Z, Shi L, Yang S, Ding T, Wang S and Luo A 2015. Isolation and identification of ovarian theca-interstitial cells and granulose cells of immature female mice. Cell Biology International 39 584–590. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L and Woodruff TK 2011. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction 141 809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EC, Hughes J, Wilson H, Clay M, Mylonas KJ, Kipari T, Duncan WC and Fraser HM 2011. Conditional ablation of macrophages disrupts ovarian vasculature. Reproduction 141 821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S 2015. Sympathetic regulation of estradiol secretion from the ovary. Autonomic Neuroscience: Basic and Clinical 187 27–35. [DOI] [PubMed] [Google Scholar]
- Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S et al. 2020. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nature Communications 11 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, Liu Z, Min Z, Hu H, Jing Y et al. 2020. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell 180 585–600.e19. [DOI] [PubMed] [Google Scholar]
- Wenzel JGW and Odend’hal S 1985. The mammalian rete ovarii: a literature review. Cornell Vet 75 411–425. [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK and Shea LD 2009. The Mouse Follicle Microenvironment Regulates Antrum Formation and Steroid Production: Alterations in Gene Expression Profiles1. Biology of Reproduction 80 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK and Shea LD 2007. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 28 4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Van der Hoek KH, Ryan NK, Norman RJ and Robker RL 2004. Macrophage contributions to ovarian function. Human Reproduction Update 10 119–133. [DOI] [PubMed] [Google Scholar]
- Wu R, Fujii S, Ryan NK, Van der Hoek KH, Jasper MJ, Sini I, Robertson SA, Robker RL and Norman RJ 2007. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Human Reproduction 22 527–535. [DOI] [PubMed] [Google Scholar]
- Wu C, Xu B, Li X, Ma W, Zhang P, Chen X and Wu J 2017. Tracing and Characterizing the Development of Transplanted Female Germline Stem Cells In Vivo. Molecular Therapy 25 1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zheng T, Hong W, Ye H, Hu C and Zheng Y 2018. Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo-Oogenesis or Ovarian Tumorigenesis. Cellular Physiology and Biochemistry 50 214–232. [DOI] [PubMed] [Google Scholar]
- Young JM and McNeilly AS 2010. Theca: the forgotten cell of the ovarian follicle. Reproduction 140 489–504. [DOI] [PubMed] [Google Scholar]
- Young B, O’Dowd G and Woodford P 2014. Cell Structure and Function. Philadephia:Churchill Livingstone. [Google Scholar]
- Younis JS, Jadaon JE, Haddad S, Izhaki I and Ben-Ami M 2011. Prospective evaluation of basal stromal Doppler studies in women with good ovarian reserve and infertility undergoing in vitro fertilization-embryo transfer treatment: patients with polycystic ovary syndrome versus ovulatory patients. Fertility and Sterility 95 1754–1758. [DOI] [PubMed] [Google Scholar]
- Zarate-Garcia L, Lane SIR, Merriman JA and Jones KT 2016. FACS-sorted putative oogonial stem cells from the ovary are neither DDX4-positive nor germ cells. Scientific Reports 6 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H and Liu K 2012. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proceedings of the National Academy of Sciences of the United States of America 109 12580–12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan Z, Qin Q, Nisenblat V, Chang HM, Yu Y, Wang T, Lu C, Yang M, Yang S et al. 2018. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Molecular Cell 72 1021–1034.e4. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schlamp F, Huang L, Clark H and Brayboy L 2020. Inflammaging is associated with shifted macrophage ontogeny and polarization in the aging mouse ovary. Reproduction 159 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


