Abstract
The loss of nigrostriatal dopaminergic neurons containing neuromelanin underlies the motor symptoms of Parkinson’s disease. Neuromelanin accumulation into autophagic lysosomes is evidence of ongoing cytosolic dopamine stress in these neurons during normal aging. The formation of neuromelanin is likely neuroprotective, as oxidation of cytosolic dopamine to quinones and aldehydes, as reviewed here, can produce a host of neurotoxic sequela. In addition to sequestration of dopamine and its metabolites in autophagic lysosomes, the uptake of dopamine into monoaminergic neurons mediated by vesicular monoamine transporter-2 (VMAT- 2), prevents dopamine oxidation. Dopamine is stable in monoaminergic vesicles due to their low pH, and thus overexpression of VMAT-2 may provide a target for potential neuroprotective therapy in Parkinson’s disease.
Dopamine Synthesis and Accumulation in Synaptic Vesicles
Dopamine is required for the normal regulation of motor activity. Dopamine is synthesized by tyrosine (4-hydroxyphe-nylalanine) conversion to L-3,4- dihydroxyphenylalanine (L-DOPA) in a reaction catalyzed by the tyrosine hydroxylase. Tyrosine hydroxylase uses one oxygen atom to hydroxylate the carbon atom at position 3 in tyrosine to produce the catechol structure (hydroxyl groups in positions 3 and 4 of L-DOPA). L-DOPA is converted to dopamine in the cytosol by aromatic L-amino acid decarboxylase, which uses pyridoxal phosphate as a cofactor [1–6]. One important feature of dopamine synthesis in neurons is the rapid uptake of cytosolic dopamine into monoaminergic vesicles catalyzed by vesicular monoamine transporter-2 (VMAT-2; SLC18A2), which physically and functionally interacts with enzymes responsible for DA synthesis [7].
Dopamine is stored in synaptic vesicles for neurotransmission, where it is stable due to the low pH. Dopamine uptake into monoaminergic vesicles catalyzed by VMAT-2 is coupled to an H+-ATP dependent proton pump localized in monoaminergic vesicle membranes. The pumping of protons into the monoaminergic vesicles reduces the pH, generating an environment of around pH5, where dopamine is stable and cannot oxidize to o-quinones [5–10]. Dopamine is released from synaptic vesicles during neurotransmission, and particularly in the striatum, the dopamine transporter (DAT) takes up dopamine from the extracellular space to replenish synaptic vesicle stores for subsequent rounds of neurotransmission. Interesti-ngly, DAT, VMAT-2 and synaptogyrin-3 have been suggested to form a complex that could prevent oxidation of free dopamine in the cytosol [11] [Figure. 1].
Figure 1: Dopamine synthesis and uptake.
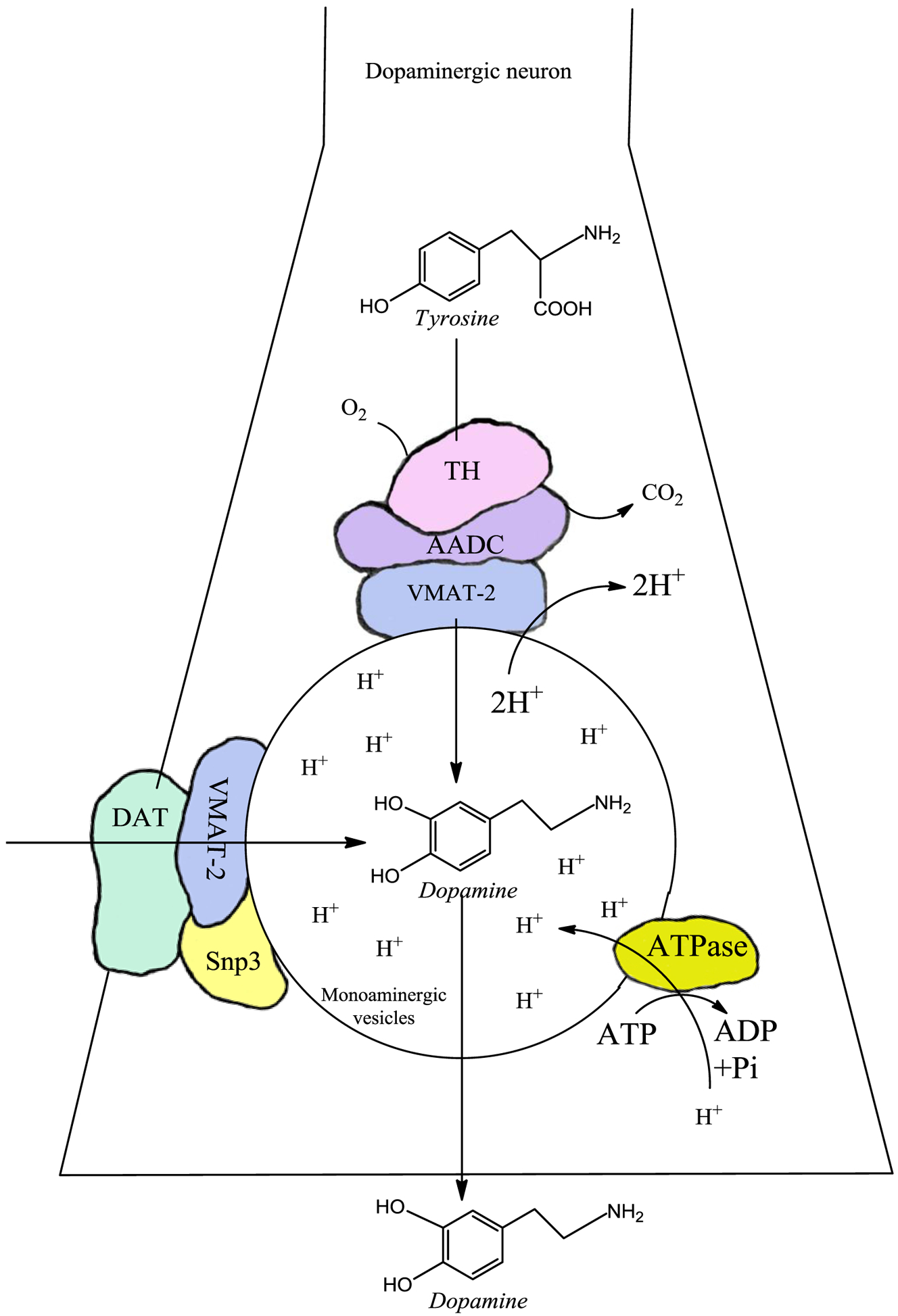
There are two sources of dopamine in dopaminergic neurons. (i) Synthesized dopamine. Dopamine is synthesized in the cytosol of dopaminergic neurons where the amino acid tyrosine is hydroxylated by the enzyme tyrosine hydroxylase, forming L-DOPA. Subsequently, L-DOPA is converted to dopamine by the aromatic L-amino acid decarboxylase. However, these reactions do not produce free dopamine in the cytosol, since it has been reported that VMAT-2 forms a complex with the aromatic L-amino acid decarboxylase and tyrosine hydroxylase. VMAT-2, expressed on the surface of the monoaminergic vesicles, immediately transports dopamine into these vesicles. Monoaminergic vesicles have a low pH because they have an H+-ATPase that pumps protons inward, generating an acid environment where dopamine accumulates without risk of oxidation because it is completely stable. (ii) The other source of cytosolic dopamine is by reuptake via DAT to the cytosol. Interestingly, DAT has been suggested to form a complex with synaptogyrin-3 and VMAT-2 that immediately transports imported extracellular dopamine towards the monoaminergic vesicles, preventing the existence of free cytosolic dopamine and its autoxidation to neurotoxic o-quinones.
The basis for selective death of specific neuronal populations in neurodegenerative diseases remains unclear. Parkinson’s disease (PD) is a synucleinopathy characterized by a preferential loss of dopaminergic neurons containing neuromelanin in the substantia nigra (SN), whereas neurons of the ventral tegmental area (VTA) that do not contain neuromelanin are quite spared until the late stage of PD. Using intracellular patch electrochemistry to directly measure cytosolic dopamine (DAcyt) in cultured midbrain neurons, we confirmed that elevated DAcyt and its metabolites are neurotoxic and that genetic and pharmacological interventions that decrease DAcyt provide neuroprotection. L-DOPA increased DAcyt in SN neurons to levels 2–3-fold higher than in VTA neurons, a response dependent on dihydropyridine-sensitive Ca2+ channels, resulting in greater susceptibility of SN neurons to L-DOPA-induced neurotoxicity. DAcyt was not altered by α-synuclein deletion, although dopaminergic neurons lacking α-synuclein were resistant to L-DOPA induced cell death. Thus, an interaction between Ca2+, DAcyt and α-synuclein may underlie the susceptibility of SN neurons in PD, suggesting directions for multiple therapeutic targets [12].
Dopamine metabolism to quinones
While dopamine is stable inside synaptic vesicles, cytosolic dopamine can autoxidize and be neurotoxic [12]. Dopamine can oxidize to dopamine ortho(o)-quinone, which cyclizes to aminochrome with a rate of s−1 as dopamine o-quinone is stable at a pH lower than 2 [13, 14]. Aminochrome is the most stable o-quinone because its rearrangement to 5,6-indolequinone has a rate of 0.06 m−1, and likely polymerizes after reaction with proteins to form the pigment neuromelanin [15] [Figure 2]. Dopamine oxidation, its reaction with proteins and lipids that form neuromelanin pigment inside autophagic-lysosomal organelles occurs during normal aging as neuroprotective process [16–18]. Neuromelanin accumulation in dopamine neurons of human SN can be visualized by MRI during aging, and in PD neuromelanin loss can be imaged [1, 17, 19–21]. However, o-quinones formed by dopamine oxidation can be neurotoxic, as dopamine o-quinone forms adducts with proteins including ubiquitin C-terminal hydrolase-L1, Parkinson protein 7, mortalin/GRP75/mthsp70 and actin, in cell cultures [22]. Dopamine oxidation also produces an adduct with parkin [23]. Accumulation of cytosolic or extraneuronal DA and subsequent oxidation to quinone species can induce nonspecific modifications on proteins. These processes require the presence of redox active metals such as iron and copper, both abundant in dopaminergic brain areas. The reaction between dopamine quinone species and proteins occurs on specific amino acids according to the reactivity order, cysteine >> histidine > lysine [24].
Figure 2: Neuroprotection in dopaminergic neurons.
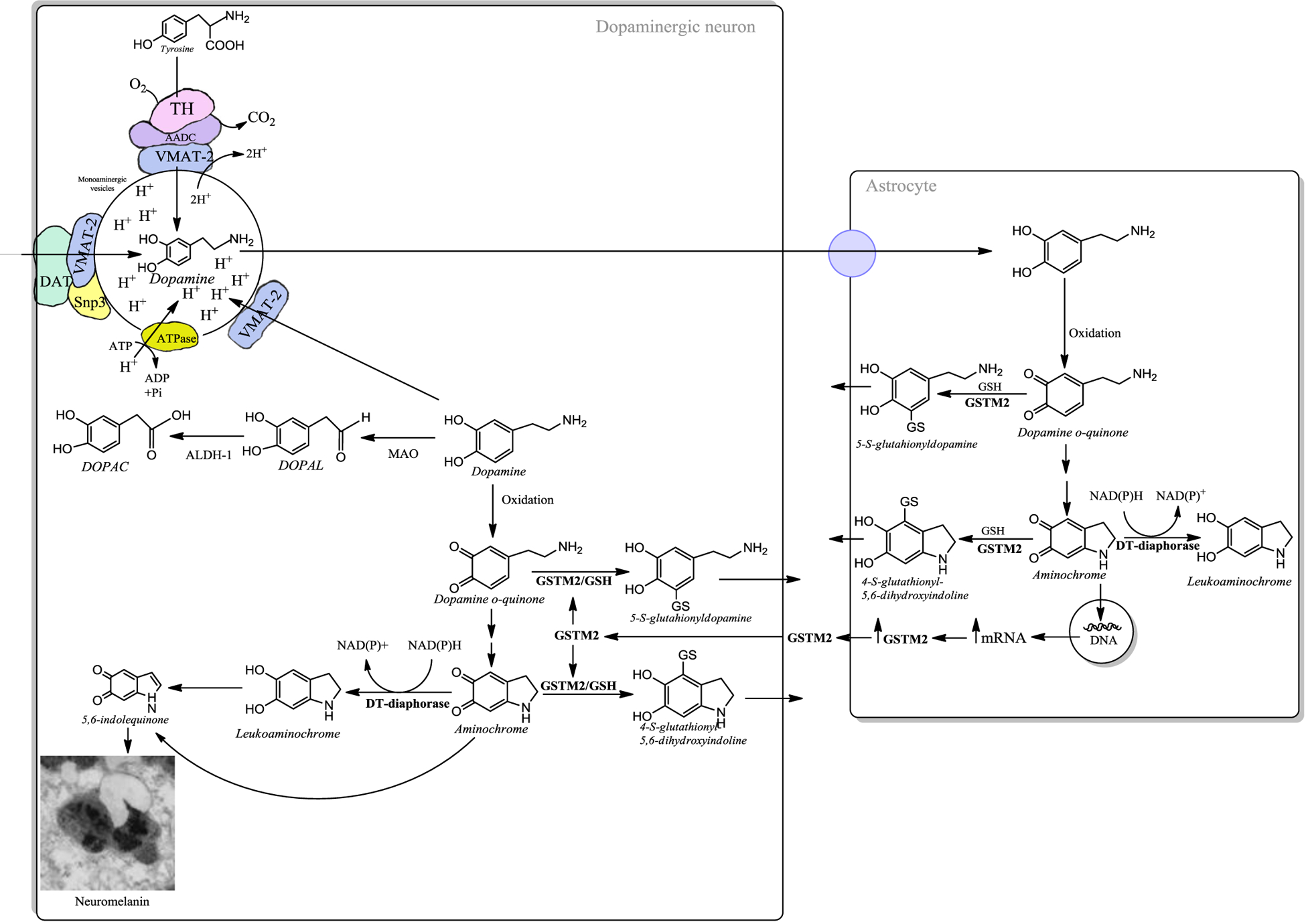
The presence of free dopamine in the cytosol is a risk, because during the oxidation of dopamine to neuromelanin (through a multi-step biosynthetic mechanism that involves iron-catalyzed oxidation, polymerization and reaction with proteins and lipids), or in its degradation catalyzed by monoamine oxidase (MAO in the Fig.), neurotoxic o-quinones can be generated. Therefore, VMAT-2 is the first line of neuroprotection against the neurotoxic effects of dopamine oxidation. VMAT-2 protects dopaminergic neurons by transporting dopamine towards the monoaminergic neurotransmission vesicles. Dopaminergic neurons are likely exposed to dopamine-derived damage when levels of VMAT-2 are not high enough to limit free dopamine and its autoxidation occurs in the cytosol. In this case neuromelanin biosynthesis provides an additional means to limit dopamine toxicity through a neuroprotective mechanism. There are two enzymes that prevent the neurotoxic effects of dopamine o-quinone and aminochrome in dopaminergic neurons. The enzyme DT-diaphorase catalyzes the two-electron reduction of aminochrome to leukoaminochrome, then leading to formation of neuromelanin precursors and the enzyme GSTM2, which can inactivate both dopamine o-quinone and aminochrome by conjugating these o-quinones with glutathione. GSTM2 is expressed only in human astrocytes, which secrete this enzyme. Dopaminergic neurons are able to internalize GSTM2 into the cytosol, where this enzyme conjugates these o-quinones with glutathione which undergo further degradation forming other probable precursors of neuromelanin pigment.
The dopaminochrome structure is unclear and it is unknown if its structure corresponds to one of the two other o-quinones reported, that is aminochrome or 5,6-indolequinone. Dopaminochrome has absorption maxima at 303 and 479 nm [25], but the structure has not been determined by NMR, while aminochrome has absorption maxima at 280 and 475 nm and its structure has been confirmed by NMR [26]. It is therefore possible that dopaminochrome corresponds to 5, 6-indolequinone or an unidentified o-quinone.
Aminochrome is also potentially neurotoxic, due to a host of potential mechanisms, like inducing alpha-synuclein aggregation to neurotoxic oligomers [27, 28], mitochondria dysfunction by decreasing mitochondrial membrane potential and ATP levels [29–34], autophagy dysfunction [35–37], increased lysosome pH [38, 39], disruption of cytoskeleton architecture [26, 40], inhibition of axonal transport of monoaminergic vesicles to the terminals in the striatum [34], decreased dopamine release [34], neuroinflammation [38, 39], proteasome dysfu-nction [43, 44], and endoplasmic reticulum and oxidative stress [45].
The 5,6-indolequinone has also been reported to form adducts with alpha-synuclein in studies performed with NMR[15], having toxic effects similar to those of aminochrome previously described. Dopaminochrome induces neurotoxicity in cells, with a slow and progressive loss of dopaminergic neurons after intranigral injection and it has been reported to form adducts with alpha-synuclein [46–50].
Dopamine metabolism to aldehydes
Free dopamine in cytosol is degraded through oxidative deamination catalyzed by monoamine oxidase to 3,4-dihydroxyphenylacetaldehyde (DOPAL), which is oxidized to 3,4-dihydroxyphenylacetic acid by aldehyde dehydrogenase-1 [5, 6] [Figure 2]. Two forms of aldehyde dehydrogenase are expressed in human SN (1 and 2), but only aldehyde dehydrogenase-1 expressed in the cytosol is decreased in PD patients [51–53] and roles for it have been proposed in the degeneration of the nigrostriatal system [54–56].
Intracerebral injection of DOPAL into the SN and the VTA induced loss of tyrosine hydroxylase positive staining [57]. DOPAL induces alpha-synuclein aggregation in vitro and in animals when injected into the SN [58]. Additionally, divalent metal ions can enhance DOPAL-induced aggregation of alpha-synuclein [59]. DOPAL induces aggregation of alpha-synuclein by generating dicatechol pyrrole adducts with lysine [60]. DOPAL-induced alpha- synuclein oligomers inhibit the formation of mature amyloid fibrils. DOPAL affects alpha-synuclein function by disturbing its interaction with lipid membranes and its role in the regulation of synaptic vesicle traffic in neurons. DOPAL-induced alpha-synuclein oligomers induce dopamine leakage in a cellular model and in an in vitro model of synaptic vesicles [61–63].
Inhibition of VMAT-2 by reserpine increases the level of DOPAL in PC12 cells, contributing to apoptosis [55].
Accumulation of cytosolic o-quinones into neuromelanin
The conversion of dopamine to neuromelanin requires the formation of dopamine o-quinone, aminochrome and 5,6-indolequinone sequentially in the cytosol. The oxidation of dopamine is catalyzed by iron, which is abundant in SN dopaminergic neurons, and is then bound into neuromelanin pigment during its biosynthesis [17]. As above, these o-quinones can be neurotoxic and induce the loss of dopaminergic neurons of the nigrostriatal system in PD if not efficiently removed. However, healthy seniors have viable dopaminergic neurons that contain high levels of neuromelanin. This apparent paradox can be explained by the existence of two enzymes that play a neuroprotective role against quinone toxicity in dopa-minergic neurons. DT-diaphorase, which is expressed in both dopaminergic neurons and astrocytes, prevents the neurotoxic effects of aminochrome by reducing aminochrome with two electrons to leukoaminochrome. Leukoaminochrome can rearrange its structure to form 5,6-dihydroxyindole, which can oxidize to one of the direct precursors of neuromelanin 5,6-indolequinone. These quinones can react with aggregated proteins to form adducts that undergo further reactions to generate neuromelanin pigment that immobilize potentially toxic dopamine-derived compounds along with metals, several proteins and lipids inside specific autolysosomes [16]. DT-diaphorase prevents aminochrome induced mitochondrial dysfu-nction [29–31], alpha-synuclein aggregation to neurotoxic oligomers [27,28], proteasome dysfunction [43], lysosome dysfunction [39], cytoskeleton architecture disruption [26] and cell death [64]. The other neuroprotective enzyme is glutathione transferase M2–2 (GSTM2), which can detoxify dopamine o-quinone and aminochro-me by conjugating these o-quinones with glutathione [65–67]. GSTM2 is produced only in astrocytes, but these cells secrete GSTM2 that dopaminergic neurons accumulate in their cytosol, protecting neurons from the neurotoxic effects of dopamine o-quinone and aminochrome [68–70].
Protective Roles of VMAT-2
VMAT-2 activity decreases the level of free cytosolic dopamine by sequestering it into synaptic and other secretory vesicles where it remains stable and can be used for neurotransmission. VMAT-2 expression has long been known to be neuroprotective, as it was originally cloned due to its role in protecting cells from MPP+ toxicity [71], and it also protects dopamine neurons from ampheta-mine neurotoxicity [72] and L-DOPA toxicity [12], presumably by decreasing cytosolic amphetamine and dopamine levels in the cytosol and preventing the formation of the toxic metabolites discussed above.
It has been suggested that the ventral SN neurons accumulate the most neuromelanin pigment because they have lower VMAT-2 expression, while the midbrain dopaminergic neurons of VTA, which produce larger amounts of dopamine, have more vesicular storage capacity for action potential-induced release of the neurotransmitter and then lower levels of neuromelanin [73]. This idea is supported by the observation that overexpression of VMAT-2 prevents neuromelanin biosynthesis [74].
Analysis of dopamine storage vesicles from the PD striatum revealed a significant decrease of VMAT-2 expression in patients’ caudate and putamen nuclei in comparison to control brains [75]. It therefore appears plausible that overexpression of VMAT-2 in dopaminergic neurons of the nigrostriatal system could provide a gene therapy aimed at preventing dopamine oxidation-induced neurotoxicity (Figure 2). In support of this hypothesis, it was suggested that variability in VMAT-2 promoter region may reduce the risk of developing PD, so that increased VMAT-2 levels may confer protection against the disease [76]. Additionally, targeted manipulation of VMAT-2 expression in PD patients could also improve the efficacy of dopamine derived from L-DOPA administrations, increasing dopamine availability for neurotransmission in the surviving nigrostriatal neurons. Therefore, future interventions on VMAT-2 may be a viable therapeutic approach to address both dopamine deficits in neurotransmission and dopamine-derived neurotoxicity.
Acknowledgments
Supported by FONDECYT 1170033 (JSA), by Italian Ministry of Education, University, and Research (MIUR) - National Research Programme (PNR) - National Research Council of Italy (CNR) Flagship “InterOmics” Project (PB.P05) (LZ, FAZ), by MIUR - PNR -CNR Aging program 2012-2014 (LZ, FAZ), by MIUR - Research Projects of National Interest (PRIN) 2015 prot. 2015T778JW (LZ, FAZ), and DS is supported by NIH R01s NS95435 and DA7418 and the JPB foundation. LZ was supported also by the Grigioni Foundation for Parkinson’s Disease (Milan, Italy).
References
- 1.Segura-Aguilar J, Paris I, Muñoz P, Ferrari E, Zecca L, Zucca FA. Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem 2014; 129: 898–915. [DOI] [PubMed] [Google Scholar]
- 2.Segura-Aguilar J, Muñoz P, Paris I. Aminochrome as New Preclinical Model to Find New Pharmacological Treatment that Stop the Development of Parkinson’s Disease. Curr Med Chem 2016; 23: 346–359. [DOI] [PubMed] [Google Scholar]
- 3.Herrera A, Muñoz P, Steinbusch HW, Segura-Aguilar J. Are Dopamine Oxidation Metabolites Involved in the Loss of Dopaminergic Neurons in the Nigrostriatal System in Parkinson’s Disease? ACS Chem Neurosci 2017; 8:702–711. [DOI] [PubMed] [Google Scholar]
- 4.Segura-Aguilar J On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease. Neural Regen Res 2017; 12:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segura-Aguilar J, Ahumada-Castro U, and Paris I. Dopamine and L-dopa as Selective 203 Endogenous Neurotoxins. In Kostrzewa RM (ed.), Handbook of Neurotoxicity, New York, Springer, 199–218. [Google Scholar]
- 6.Segura-Aguilar J, Paris I. Mechanisms of Dopamine Oxidation and Parkinson’s Disease. In Kostrzewa RM (ed.), Handbook of Neurotoxicity, New York, Springer. 865–883. [Google Scholar]
- 7.Cartier EA, Parra LA, Baust TB, Quiroz M, Salazar G, Faundez V, et al. A biochemical and functional protein complex involving dopamine synthesis and transport into synaptic vesicles. J Biol Chem 2010; 285:1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson RG, Carty SE, and Scarpa A. Proton: substrate stoichiometries during active transport of biogenic amines in chromaffin ghosts. J Biol Chem 1981; 256: 5773–5780. [PubMed] [Google Scholar]
- 9.Knoth J, Isaacs JM, and Njus D. Amine transport in chromaffin granule ghosts. pH dependence implies cationic form is translocated. J Biol Chem 1981; 256: 6541–6543. [PubMed] [Google Scholar]
- 10.Sulzer D, Sonders MS, Poulsen NW, and Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 2005; 75:406–433. [DOI] [PubMed] [Google Scholar]
- 11.Egaña LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, et al. Physical and functional interaction 219 between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci 2009; 29:4592–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009; 62:218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segura-Aguilar J, Lind C. On the mechanism of the Mn3(+)-induced neurotoxicity of 225 dopamine: prevention of quinone-derived oxygen toxicity by DT diaphorase and superoxide dismutase. Chem Biol Interact 1989; 72:309–324. [DOI] [PubMed] [Google Scholar]
- 14.Tse DCS, McCreery RL, and Adams RN. Potential oxidative pathways of brain catecholamines. J Med Chem 1976; 19, 37–34. [DOI] [PubMed] [Google Scholar]
- 15.Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with alpha-synuclein. J Biol Chem 2007; 282:15597–15605. [DOI] [PubMed] [Google Scholar]
- 16.Zucca FA, Vanna R, Cupaioli FA, Bellei C, De Palma A, Di Silvestre D, et al. Neuromelanin organelles are specialized autolysosomes that 234 accumulate undegraded proteins and lipids in aging human brain and are likely involved in 235 Parkinson’s disease. NPJ Parkinsons Dis 2018; 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zucca FA, Segura-Aguilar J, Ferrari E, Muñoz P, Paris I, Sulzer D, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 2017, 155; 96–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engelen M, Vanna R, Bellei C, Zucca FA, Wakamatsu K, Monzani E, et al. Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS One 2012; 7:e48490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci U S A 2019; 5108–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett 2002; 510:216–220. [DOI] [PubMed] [Google Scholar]
- 21.Sulzer D, Cassidy C, Horga G, Kang UJ, Fahn S, Casella L, Pezzoli G, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis 2018; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Laar VS, Mishizen AJ, Cascio M, Hastings TG. Proteomic identification of dopamine- conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiol Dis 2009; 34:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently 256 modifies and functionally inactivates parkin. Nat Med 2005; 11:1214–1221. [DOI] [PubMed] [Google Scholar]
- 24.Monzani E, Nicolis S, Dell’Acqua S, Capucciati A, Bacchella C, Zucca FA, et al. Dopamine, oxidative stress and protein-quinone modifications in Parkinson’s 259 and other neurodegenerative diseases. Angew Chem Int Ed. Engl 2018; 260 10.1002/anie.201811122. [DOI] [PubMed] [Google Scholar]
- 25.Ochs SD, Westfall TC and Macarthur H The separation and quantification of aminochromes using high-pressure liquid chromatography with electrochemical detection. J Neurosci Methods 2005; 142: 201–208. [DOI] [PubMed] [Google Scholar]
- 26.Paris I, Perez-Pastene C, Cardenas S, Iturriaga-Vasquez P, Muñoz P, Couve E, Caviedes P, Segura-Aguilar J. Aminochrome induces disruption of actin, alpha-, and beta-tubulin cytoskeleton networks in substantia-nigra-derived cell line. Neurotox Res 2010; 18:82–92. [DOI] [PubMed] [Google Scholar]
- 27.Muñoz P, Cardenas S, Huenchuguala S, Briceño A, Couve E, Paris I, Segura-Aguilar J. DT Diaphorase Prevents Aminochrome-Induced Alpha-Synuclein Oligomer Formation and Neurotoxicity. Toxicol Sci 2015; 145:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muñoz PS, Segura-Aguilar J. DT-diaphorase Protects Against Autophagy Induced by 271 Aminochrome-Dependent Alpha-Synuclein Oligomers. Neurotox Res 2017; 32:362–367. [DOI] [PubMed] [Google Scholar]
- 29.Arriagada C, Paris I, Sanchez de las Matas MJ, Martinez-Alvarado P, Cardenas S, Castañeda P, et al. On the neurotoxicity mechanism of leukoaminochrome o-semiquinone radical derived from dopamine oxidation: mitochondria damage, necrosis, and hydroxyl radical formation. Neurobiol Dis 2004; 16:468–477. [DOI] [PubMed] [Google Scholar]
- 30.Fuentes P, Paris I, Nassif M, Caviedes P, Segura-Aguilar J. inhibition of VMAT-2 and DT-277 diaphorase induce cell death in a substantia nigra-derived cell line--an experimental cell model for dopamine toxicity studies. Chem Res Toxicol 2007; 20:776–783. [DOI] [PubMed] [Google Scholar]
- 31.Paris I, Muñoz P, Huenchuguala S, Couve E, Sanders LH, Greenamyre JT, Caviedes P, Segura-Aguilar J. Autophagy protects against aminochrome-induced cell death in substantia nigra-derived cell line. Toxicol Sci 2011; 121:376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguirre P, Urrutia P, Tapia V, Villa M, Paris I, Segura- Aguilar J, and Nuñez MT. The dopamine metabolite aminochromeinhibits mitochondrial complex I and modifies the expression of iron transportersDMT1and FPN1. Biometals 2012; 25,795–803. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz P, Paris I, Sanders LH, Greenamyre JT, Segura-Aguilar J. Overexpression of VMAT-2 ana DT-diaphorase protects substantia nigra-derived cells against aminochrome neurotoxicity. Biochim Biophys Acta 2012; 1822:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrera A, Muñoz P, Paris I, Díaz-Veliz G, Mora S, Inzunza J, Hultenby K, Cardenas C, Jaña F, 289 Raisman-Vozari R, Gysling K, Abarca J, Steinbusch HW, Segura-Aguilar J. Aminochrome 290 induces dopaminergic neuronal dysfunction: a new animal model for Parkinson’s disease. Cell Mol Life Sci 2016; 73:3583–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segura-Aguilar J, Huenchuguala S. Aminochrome Induces Irreversible Mitochondrial Dysfunction by Inducing Autophagy Dysfunction in Parkinson’s Disease. Front Neurosci 2018; 12:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huenchuguala S, Muñoz P, Segura-Aguilar J. The Importance of Mitophagy in Maintaining Mitochondrial Function in U373MG Cells. Bafilomycin A1 Restores Aminochrome-Induced Mitochondrial Damage. ACS Chem Neurosci 2017; 8:2247–2253. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinsons Dis 2012; 920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huenchuguala S, Muñoz P, Zavala P, Villa M, Cuevas C, Ahumada U, et al. Glutathione transferase mu 2 protects glioblastoma cells against aminochrome toxicity by preventing autophagy and lysosome dysfunction. Autophagy 2014; 10:618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meléndez C, Muñoz P & Segura-Aguilar J. DT-Diaphorase Prevents Aminochrome-Induced Lysosome Dysfunction in SH-SY5Y Cells. Neurotox Res 2019; 35:255–259. [DOI] [PubMed] [Google Scholar]
- 40.Briceño A, Muñoz P, Brito P, Huenchuguala S, Segura-Aguilar J, Paris IB. Aminochrome toxicity is mediated by inhibition of microtubules polymerization through the formation of adducts with tubulin. Neorotox Res 2016; 29: 381–393. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.Santos CC, Araújo FM, Ferreira RS, Silva VB, Silva JH, Grangeiro MS, et al. Aminochrome induces microglia and astrocyte activation. Toxicol In Vitro 2017; 42: 54–60. [DOI] [PubMed] [Google Scholar]
- 42.de Araújo FM, Ferreira RS, Souza CS, Dos Santos CC, Rodrigues TLRS, E Silva JHC, Aminochrome decreases NGF, GDNF and induces neuroinflammation in organotypic midbrain slice cultures. Neurotoxicol 2018; 66:98–106. [DOI] [PubMed] [Google Scholar]
- 43.Zafar KS, Siegel D, and Ross D. A potential role for cyclized quinones derived from dopamine, DOPA, and 3,4- dihydroxyphenylacetic acid in proteasomal inhibition. Mol Pharmacol 2006; 70: 1079–1086. [DOI] [PubMed] [Google Scholar]
- 44.Zhou ZD, Lim TM. Dopamine (DA) induced irreversible proteasome inhibition via DA derived quinones. Free Radic Res 2009; 43:417–430. [DOI] [PubMed] [Google Scholar]
- 45.Xiong R, Siegel D, Ross D. Quinone-induced protein handling changes: implications for major protein handling systems in quinone-mediated toxicity. Toxicol Appl Pharmacol 2014; 280:285–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, et al. Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem 2005; 280: 21212–21219. [DOI] [PubMed] [Google Scholar]
- 47.Touchette JC, Breckenridge JM, Wilken GH, Macarthur H. Direct intranigral injection of dopaminochrome causes degeneration of dopamine neurons. Neurosci Lett 2016; 612:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linsenbardt AJ, Breckenridge JM, Wilken GH, Macarthur H. Dopaminochrome induces caspase-independent apoptosis in the mesencephalic cell line, MN9D. J Neurochem 2012; 122:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoccarato F, Toscano P, Alexandre A. Dopamine-derived dopaminochrome promotes H(2)O(2) release at mitochondrial complex I: stimulation by rotenone, control by Ca(2+), and relevance to Parkinson disease. J Biol Chem 2005; 280:15587–15594. [DOI] [PubMed] [Google Scholar]
- 50.Galzigna L, Zanatta L, Esposito N. Toxicity of dopamine and dopaminochrome on cultured cells. Neurotox Res 1999; 1: 149–152. [DOI] [PubMed] [Google Scholar]
- 51.Grünblatt E, Mandel S, Jacob-Hirsch J, Zeligson S, Amariglo N, Rechavi G, et al. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in ubiquitin-proteasome, heat shock protein, iron and oxidative stress regulated proteins, cell adhesion/cellular matrix and vesicle trafficking genes. J Neural Transm (Vienna) 2004. 111: 1543–1573. [DOI] [PubMed] [Google Scholar]
- 52.Grünblatt E, Riederer P. Aldehyde dehydrogenase (ALDH) in Alzheimer’s and Parkinson’s disease. J Neural Transm (Vienna) 2016. 123: 83–90. [DOI] [PubMed] [Google Scholar]
- 53.Mandel S, Grunblatt E, Riederer P, Amariglio N, Jacob-Hirsch J, Rechavi G, et al. Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin-proteasome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann N Y Acad Sci 2005. 1053: 356–375. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein DS, Kopin IJ, Sharabi Y. Catecholamine autotoxicity Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol Ther 2014; 144:268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Sullivan R, Gross DJ, et al. Vesicular uptake blockade generates the toxic dopamine metabolite 3,4-dihydroxypheny lacetaldehyde in PC12 cells: relevance to the pathogenesis of Parkinson’s disease. J Neurochem 2012; 123:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deza-Ponzio R, Herrera ML, Bellini MJ, Virgolini MB, Hereñú CB. Aldehyde dehydrogenase 2 in the spotlight: The link between mitochondria and neurodegeneration. Neurotoxicol 2018; 356 68:19–24. [DOI] [PubMed] [Google Scholar]
- 57.Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS. 3,4-Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res 2003; 989:205–213. [DOI] [PubMed] [Google Scholar]
- 58.Burke WJ, Kumar VB, Pandey N, Panneton WM, Gan Q, Franko MW, et al. Aggregation of alpha-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol 2008; 115:193–203. [DOI] [PubMed] [Google Scholar]
- 59.Jinsmaa Y, Sullivan P, Gross D, Cooney A, Sharabi Y, Goldstein DS. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neurosci Lett 2014. 21; 569:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Werner-Allen JW, DuMond JF, Levine RL, Bax A. Toxic Dopamine Metabolite DOPAL Forms an Unexpected Dicatechol Pyrrole Adduct with Lysines of α-Synuclein. Angew Chem Int Ed Engl 2016; 55:7374–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Follmer C, Coelho-Cerqueira E, Yatabe-Franco DY, Araujo GD, Pinheiro AS, Domont GB, et al. Oligomerization and Membrane-binding Properties of Covalent Adducts Formed by the Interaction of α-Synuclein with the Toxic Dopamine Metabolite 3,4-Dihydroxyphenylacetaldehyde 372 (DOPAL). J Biol Chem 2015; 290:27660–27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werner-Allen JW, Monti S, DuMond JF, Levine RL, Bax A. Isoindole Linkages Provide a Pathway for DOPAL-Mediated Cross-Linking of α-Synuclein. Biochem 2018; 57:1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plotegher N, Berti G, Ferrari E, Tessari I, Zanetti M, Lunelli L, et al. DOPAL derived alpha-synuclein 377 oligomers impair synaptic vesicles physiological function. Sci Rep 2017; 7:40699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lozano J, Muñoz P, Nore BF, Ledoux S, Segura-Aguilar J. Stable expression of short interfering 379 RNA for DT-diaphorase induces neurotoxicity. Chem Res Toxicol 2010; 23:1492–1496. [DOI] [PubMed] [Google Scholar]
- 65.Segura-Aguilar J, Baez S, Widersten M, Welch CJ, Mannervik B. Human class Mu glutathione 381 transferases, in particular isoenzyme M2–2, catalyze detoxication of the dopamine metabolite 382 aminochrome. J Biol Chem 1997; 272:5727–5731. [DOI] [PubMed] [Google Scholar]
- 66.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases 384 catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as 385 an antioxidant system preventing degenerative cellular processes. Biochem J 1997; 324:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dagnino-Subiabre A, Cassels BK, Baez S, Johansson AS, Mannervik B, Segura-Aguilar J. Glutathione transferase M2–2 catalyzes conjugation of dopamine and dopa o-quinones. Biochem Biophys Res Commun 2000; 274:32–36. [DOI] [PubMed] [Google Scholar]
- 68.Cuevas C, Huenchuguala S, Muñoz P, Villa M, Paris I, Mannervik B, et al. Glutathione transferase-M2–2 secreted from glioblastoma cell protects SH-SY5Y cells from aminochrome neurotoxicity. Neurotox Res 2015. 27; 217–228. [DOI] [PubMed] [Google Scholar]
- 69.Segura-Aguilar J On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease. Neural Regen Res 2017. 12:897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segura-Aguilar J A new mechanism for protection of dopaminergic neurons mediated by astrocytes. Neural Regen Res 2015; 10:1225–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adam Y, Edwards RH, Schuldiner S. Expression and function of the rat vesicular monoamine transporter 2. Am J Physiol Cell Physiol 2008; 294:C1004–1011. [DOI] [PubMed] [Google Scholar]
- 72.Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci 2002; 22:8951–8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liang CL, Nelson O, Yazdani U, Pasbakhsh P, German DC. Inverse relationship between the contents of neuromelanin pigment and the vesicular monoamine transporter-2: human midbrain dopamine neurons. J Comp Neurol 2004; 473:97–106. [DOI] [PubMed] [Google Scholar]
- 74.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, et al. Neuromelanin biosynthesis is driven by excess cytosolic 407 catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A 2000; 97:11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pifl C, Rajput A, Reither H, Blesa J, Cavada C, Obeso JA, et al. Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human 411 and nonhuman primate striatum. J. Neurosci 2014; 34: 8210–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brighina L, Riva C, Bertola F, Saracchi E, Fermi S, Goldwurm S, Ferrarese C. Analysis of vesicular monoamine transporter 2 polymorphisms in Parkinson’s disease. Neurobiol Aging 2013; 34:1712.e9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


