Abstract
OBJECTIVE
To evaluate non-inferiority and diagnostic performance of an American College of Radiology compliant abbreviated MRI protocol (AB-MRI) compared with standard-of-care breast MRI (SOC-BMRI) in patients with increased breast cancer risk.
MATERIAL AND METHODS
Women with increased lifetime breast cancer risk by American Cancer Society guidelines underwent breast MRI at a single institution between October 2015 and February 2018. AB-MRI was acquired at 3.0T with T2-weighted extended fast spin echo triple-echo Dixon and pre- and post-contrast 3D dual-echo fast spoiled gradient echo two-point Dixon sequences with an 8-channel breast coil 1–7 days after SOC-BMRI. Three readers independently reviewed AB-MRI and assigned BI-RADS categories for maximum intensity projection images (AB1), dynamic contrast-enhanced (DCE) images (AB2), and DCE and non-contrast T2 and fat-only images (AB3). These scores were compared to those from SOC-BMRI.
RESULTS
Cancer yield was 14 per 1000 (women-years) in 73 women aged 26–75 years (mean 53.5 years). AB-MRI acquisition times (mean 9.63 minutes) and table times (mean 15.07 minutes) were significantly shorter than those of SOC-BMRI (means 19.46 and 36.3 minutes, respectively) (p<0.001). Accuracy, sensitivity, specificity, and positive and negative predictive values were identical for AB3 and SOC-BMRI (93%, 100%, 93%, 16.7%, and 100%, respectively). AB-MRI with AB1 and AB2 had significantly lower specificity (AB1=73.6%, AB2=77.8%), positive predictive values (AB1=5%, AB2=5.9%), and accuracy (AB1=74%, AB2=78%) than those of SOC-BMRI (p=0.002 for AB1, p=0.01 for AB2).
CONCLUSION
AB-MRI was acquired significantly faster than SOC-BMRI and its diagnostic performance was non-inferior. Inclusion of T2 and fat-only images was necessary to achieve non-inferiority by multireader evaluation.
Keywords: breast MRI, abbreviated protocol, Dixon, fast spin echo triple echo Dixon
1. Introduction
Breast magnetic resonance imaging (BMRI) is known as the most sensitive imaging test for breast cancer detection [1, 2]. American Cancer Society guidelines recommend yearly BMRI for women with a 20% or higher lifetime risk of breast cancer [3]. The American College of Radiology (ACR) further recommends including BMRI as a screening measure for women in whom breast cancer is diagnosed before age 50 and those with dense breast tissue, irrespective of age at diagnosis [4]. However, many factors such as cost-effectiveness and patient tolerance impede the acceptance of BMRI screening, and it is estimated that only ~2% of the eligible high-risk women actually undergo BMRI screening [5–7].
Most conventional BMRI protocols comprise at least a localizer and pre-contrast T1-weighted, T2-weighted, and dynamic fat-saturated pre/post-contrast T1-weighted sequences with subtraction images and kinetic enhancement curves [8]. Additional sequences such as diffusion-weighted sequences at different b-values may also be included. Such a protocol may require 30–60 minutes of scanner and technologist time. At most institutions, the BMRI scan protocol does not differ between screening and diagnostic examinations (e.g., those used in pre-operative staging). As a result, screening BMRI using a conventional protocol has the advantage of providing a complete diagnostic evaluation, allowing not only detection of the lesion but also its characterization and determination of appropriate management.
Abbreviating a BMRI protocol potentially helps decrease the costs and improve the patient tolerance associated with BMRI screening. Some investigators have proposed that a simplified and shortened BMRI protocol with a minimum number of the contrast-enhanced sequence to detect suspect enhancing lesions, referred to as abbreviated BMRI (AB-MRI), might be sufficient for screening [9–14]. Potential benefits of AB-MRI include greater patient throughput, earlier cancer identification than mammography, replacement of mammography as a primary screening method [15], and decreased screening frequency. However, the proposed AB-MRI protocols vary in the number of sequences performed, use of non-contrast series, and use of a dynamic versus contrast-enhanced scan. The potential implications of variations in AB-MRI protocols are unclear. For example, eliminating dynamic or multiphase contrast acquisition decreases scan time but also precludes the time-intensity curve, which is a useful diagnostic feature for the assessment of breast masses [16]. Also, reduced scan times do not necessarily translate to proportionally reduced table times, since a substantial portion of a BMRI study may be taken up by patient setup, manual prescan, and sequence repeat due to field shimming and fat suppression difficulties. Further, some investigators have suggested that abnormal AB-MRI findings would prompt a secondary diagnostic MRI to assure diagnostic and staging accuracy[14], doubling the work and the contrast exposure.
Further, ACR accreditation is recognized as the gold standard in medical imaging, and is commonly pursued in facilities seeking to acquire diagnostic imaging center of excellence in the United States. It also helps facilitate meeting governmental and third party payer criteria [17]. ACR accreditation manual advises that “a comprehensive breast MRI exam should include pulse sequences that provide more than one type of image contrast”, and cites T2 weighted/bright fluid series and multiphase T1-weighted series comprising pre, early and late contrast series with fat saturation or subtraction among the major categories of sequences to be assessed [18]. However, it is unclear how obtaining dynamic sequence and T2-weighted series contributes to the diagnostic performance of BMRI and whether it is possible to achieve the “abbreviated” timing if some or part of image contrast critical for diagnoses are eliminated. In a recent pilot study comparing complete standard-of-care BMRI (SOC-BMRI) and AB-MRI protocols comprising high-resolution T2-weighted and full T1-weighted dynamic sequences in a small group of women who underwent both studies, the AB-MRI protocol had a mean acquisition time of less than 10 minutes and a mean table time less than 14 minutes, both significantly shorter than the times for the SOC-BMRI [19]. Direct comparison of the imaging results in the same patients showed that the image quality was better for this AB-MRI protocol than for SOC-BMRI. We hypothesized that our AB-MRI technique is non-inferior in diagnostic performance (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV], and accuracy) to SOC-BMRI, which requires much longer magnet and table times. In addition, we sought to evaluate a full dynamic acquisition in comparison to a single, early timepoint acquisition as proposed by some investigators [20] and to determine the value of the additional T2-weighted series as required by the ACR for a complete diagnostic BMRI protocol.
To test our hypothesis, we conducted a multi-reader study to assess and compare an AB-MRI protocol with an SOC-BMRI protocol for screening in a single cohort of women with a high lifetime breast cancer risk. Further, we evaluated each independent reader’s Breast Imaging Reporting and Data System (BI-RADS) category assessment with and without the dynamic series and T2-weighted series available from our AB-MRI protocol.
2. Material and Methods
2.1. Patient selection and study design
Institutional Review Board approval was obtained for this single-center study, which was compliant with the U.S. Health Information Portability and Accountability Act.
The study participants were women aged 18 years and older who met the American Cancer Society’s guidelines for high-risk breast cancer screening [3] and were scheduled to undergo BMRI screening during the period from October 1, 2015, through February 8, 2018. All patients gave informed written consent after the nature of the study had been fully explained. Patients underwent dynamic contrast-enhanced (DCE) AB-MRI 1–7 business days after SOC-BMRI with DCE. Study data were collected and managed using REDCap (Vanderbilt University, Nashville, TN) electronic data capture tools that were installed at MD Anderson Cancer Center [21].
2.2. AB-MRI and SOC-BMRI protocols
SOC-BMRI was performed using a Signa Excite HDx 3T system (GE Healthcare, Waukesha, WI) with a bilateral 8-channel phased array breast coil, with the patient in the prone position. The SOC-BMRI study included a localizer sequence, axial T1-weighted sequence without fat saturation, axial T2-weighted sequence with fat saturation, one pre-contrast and four post-contrast fat-saturated axial or sagittal T1-weighted gradient echo images, a delayed axial or sagittal post-contrast T1-weighted sequence, and an axial diffusion-weighted sequence. The details of the scan parameters are described in a previous publication [19]. Post-contrast subtraction images and maximum intensity projection images (MIPs) from the first subtraction series were generated during image post-processing.
AB-MRI was performed using a Discovery MR750 3T system (GE Healthcare) with a bilateral 8-channel phased array breast coil, with the patient in the prone position. The study was performed with a T2-weighted extended fast spin echo triple-echo Dixon sequence [22] (imaging parameters: repetition time/echo time [TR/TE] ≈ 4500/90; flip angle, 90; matrix size, 384×320; field of view [FOV] ≈ 30–36; slice thickness, 4 mm; gap, 0 mm) and 3D dual-echo fast spoiled gradient echo two-point Dixon sequence [23] for volumetric T1-weighted imaging before and after contrast injection (imaging parameters: TR/TE, 5.3/1.5; flip angle, 10; matrix size, 320×512; FOV ≈ 30–36; slice thickness, 2 mm; gap, −1 mm). Compared to the T2-weighted and T1-weighted sequences that are used in SOC-BMRI, the two Dixon sequences produce similar image contrast but require no frequency-based RF pulses and no manual shimming or manual prescan to achieve uniform fat saturation. Further, the Dixon sequences generate both water-only and fat-only and water-only series in a single acquisition. The fat-only series is not available in SOC-BMRI but is evaluated in our study for its diagnostic value. There were four post-contrast acquisitions, identical to those of the SOC-BMRI protocol. Post-processing image reconstruction algorithms were performed with Orchestra SDK software (version 1.4, GE Healthcare).
For both SOC-BMRI and AB-MRI protocols, each patient received 0.1 mL/kg gadobutrol (Gadovist, Bayer HealthCare Pharmaceuticals, Berlin, Germany) at a rate of 2 mL/s through a power injector.
The acquisition times and table times were recorded for each scan. Acquisition time included the individual sequences in the SOC-BMRI and AB-MRI, excluding the delayed post-contrast T1-weighted sequence and the axial diffusion-weighted sequence from the SOC-BMRI. The table time included the interval from the start of the localizer sequence to the end of the last performed sequence.
2.3. SOC-BMRI interpretation
SOC-BMRI images were interpreted prospectively by one of sixteen breast radiologists with 1–27 years of experience (mean 12.2 years). The interpretation of these images was not part of this study. The BI-RADS category and any suspicious findings from the SOC-BMRI report were recorded in and extracted from the institutional electronic health record. BI-RADS assessments of 1, 2, or 3 were considered negative interpretation, while BI-RADS assessments of 0, 4, or 5 were considered positive interpretation.
2.4. AB-MRI interpretation
AB-MRI images were interpreted independently by three radiologists with a range of 6–15 years of experience (***, 8 years; ***, 15 years; ***, 13 years) on DynaCAD (v. 3.3, Invivo, Gainesville, FL). The reading radiologists were blinded to clinical history and results of other imaging studies. Readers initially evaluated serial subtraction MIPs, including the color-coding map (abbreviated breast protocol 1, or AB1), first; non-subtraction and subtraction DCE images (AB2) with reformatted multiplanar sagittal or coronal images as needed, second; and the triple-echo Dixon T2 series, including fluid-bright and fat-only images, which provide a contrast similar to that of T1-weighted non-contrast images without fat suppression (AB3), last. Since post processing was performed by DynaCAD software at viewing, post processing time was not included in scan or interpretation times.
Each radiologist reader assigned a BI-RADS category and recorded the interpretation time for each reading step in every patient. When there was a suspicious finding, lesion type (mass, nonmass enhancement, or focus), characteristics (shape, margin, and internal enhancement for mass; distribution and internal enhancement for nonmass enhancement), and delayed-phase kinetic curve were recorded using the BI-RADS lexicon [24]. After blinded readings were complete, the radiologists performed a consensus review and BI-RADS assessment of non-benign cases with comparison to prior imaging studies (AB4). Similar to SOC, each readout was categorized as negative (BI-RADS 1–3) or positive (BI-RADS 4–5) interpretation.
2.5. Follow-up
Biopsy pathology, surgical pathology, and imaging follow-up were recorded from the institutional electronic health record. For abnormal SOC-BMRI results, biopsy pathology and/or a minimum of 6-month breast imaging follow-up were used as the reference standard.
2.6. Statistical analysis
Imaging diagnoses were summarized by frequencies and percentages. Table time, acquisition time, and interpretation time were summarized by calculating mean, standard deviation (SD), and range. Accuracy was estimated along with 95% confidence interval (CI), and accuracies of AB-MRI and SOC-BMRI were compared by using the McNemar test. Sensitivity, specificity, PPV, and NPV were estimated by modality. Kappa statistics were used to assess inter-reader agreement. A Wilcoxon signed-rank test was used to compare table time and acquisition time between AB-MRI and SOC-BMRI. All tests were two-sided, and p-values of 0.05 or less were considered statistically significant. Statistical analysis was performed using SAS version 9.4 software (SAS Institute, Cary, NC).
3. Results
3.1. Patient cohort
From October 1, 2015, through February 8, 2018, 131 patients were prospectively registered and underwent SOC-BMRI including DCE (Figure 1). Of those, 97 patients underwent DCE AB-MRI after SOC-BMRI. Because of a technical error by the technologist, one patient’s AB-MRI images were lost; this patient was excluded from analysis. A total of 73 women (age range 26–75 years, mean age 53.5 years, median 53 years) with pathology or imaging follow-up information were included in the analysis. Screening BMRI indications are shown in Table 1.
Figure 1:
Patient eligibility, enrollment and inclusion in reader study.
Table 1.
Indications for screening BMRI (N=73)
| Indication | Number (%) |
|---|---|
| BRCA1 | |
| Pathologic variant | 2 (2.7) |
| VUS | 3 (4.1) |
| BRCA2 | |
| Pathologic variant | 9 (12.3) |
| VUS | 2 (2.7) |
| Other gene mutation | |
| CDH1 | 1 (1.3) |
| PALB2 | 1 (1.3) |
| PALB2 VUS | 1 (1.3) |
| Chest radiation therapy before age 30 | 2 (2.7) |
| Risk model with LTR calculation ≥20% | |
| Gail | 22 (30.1) |
| Tyrer-Cuzick | 18 (24.7) |
| Claus | 12 (16.4) |
BMRI: breast magnetic resonance imaging; VUS: variant of uncertain significance; LTR: lifetime risk.
3.2. Pathology results and follow-up
One breast cancer was diagnosed among these 73 women, for a cancer yield of 14 per 1000 women-years. The woman in whom cancer was found was 67 years old; MRI-guided biopsy of nonmass enhancement in the right breast of this patient revealed low-grade cribriform ductal carcinoma in situ (DCIS), for which the patient underwent mastectomy (Fig. 2). The same patient concurrently underwent contralateral MRI-guided biopsy, also of a nonmass enhancement, which yielded a single focus of atypical ductal hyperplasia. Three patients underwent bilateral prophylactic mastectomy with benign results (Fig. 3). Imaging follow-up for the remaining patients ranged from 6–31 months (mean 23 months, median 24 months). No other cancers were diagnosed during the follow-up period.
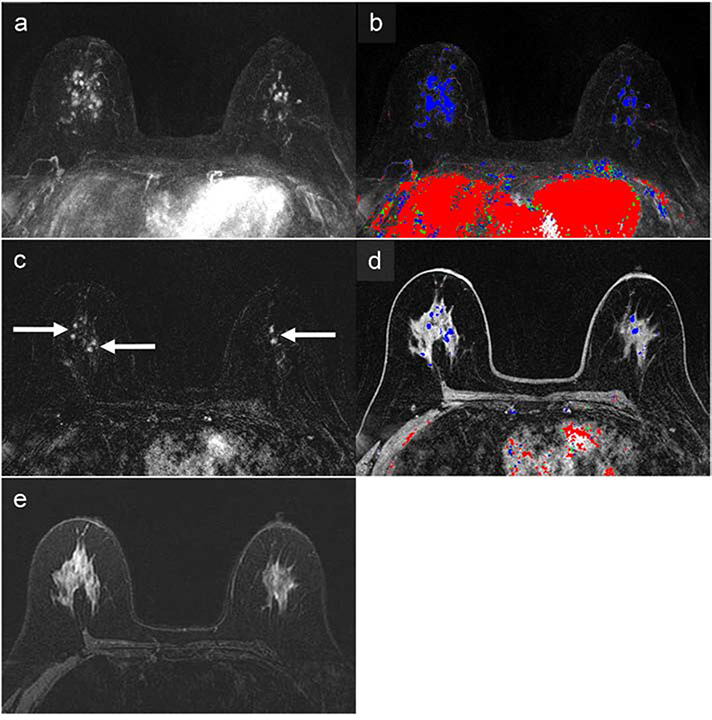
Figure 2:
67-year-old woman with a 41% lifetime breast cancer risk based on family history, as determined by the Gail model, and a variant of unknown significance in the PALB2 gene. The abbreviated breast MRI (AB-MRI) first post-contrast subtraction MIP (AB1) (a) with time-intensity color map (b) shows multiple areas of enhancement (right greater than left) in both breasts with associated persistent delayed kinetics indicated by predominantly blue coloration on the color map. The AB-MRI first post-contrast subtraction image (AB2) (c) shows a representative area centrally with multiple bilateral nonmass enhancements (arrows), right greater than left. The corresponding AB-MRI first post-contrast T1-weighted image (AB2) with a color map overlay shows associated delayed persistent kinetics (d). There was no associated increase in T2 signal on the AB-MRI T2-weighted image (AB3) (e). Both SOC-BMRI and AB-MRI exams were classified as BI-RADS 4, suspicious. MRI-guided biopsy of the right breast showed low-grade cribriform ductal carcinoma in situ (DCIS) without comedonecrosis and lobular carcinoma in situ with pagetoid extension. Subsequent right mastectomy showed cribriform atypical ductal proliferation similar to the DCIS in the MRI-guided biopsy, as well as atypical lobular hyperplasia and radial scar. Left breast MRI-guided biopsy showed a single minute focus of atypical ductal hyperplasia, as well as usual ductal hyperplasia, columnar cell change, and sclerosing adenosis. The left breast is undergoing imaging follow-up, showing benign results.
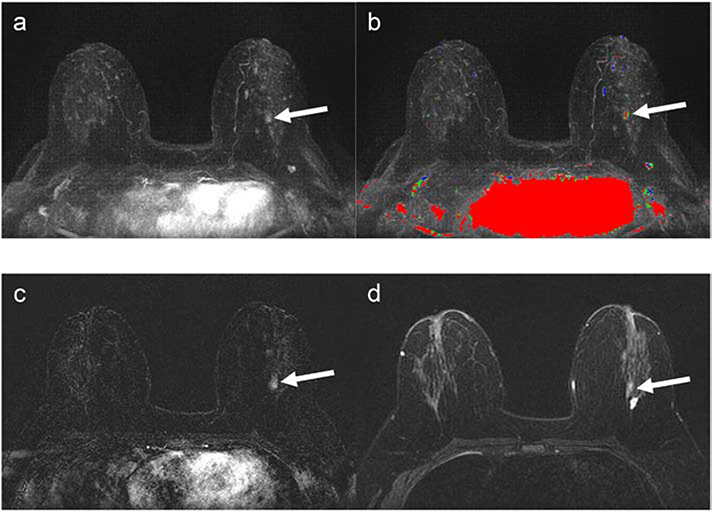
Figure 3:
42-year-old woman with 28.9% lifetime breast cancer risk based on family history, as determined by the Claus model. The abbreviated breast (AB-MRI) first subtraction MIP (a) with time-intensity color map (AB1) (b) shows an asymmetric lesion with washout in the posterior left breast (arrows). The AB-MRI first post-contrast subtraction (AB2) (c) shows focal nonmass enhancement (arrow) in the posterior central left breast. The corresponding AB-MRI T2-weighted image (AB3) (d) of the nonmass lesion shows intermediate T2 signal (arrow). This lesion was identified and classified as BI-RADS Category 4, suspicious, on both SOC-BMRI and AB-MRI exams. MR-guided biopsy showed atypical ductal hyperplasia, columnar cell change, and proliferative fibrocystic change. Subsequent bilateral skin-sparing mastectomies showed benign findings.
3.3. SOC-BMRI and AB-MRI table and acquisition times
Acquisition and table times are summarized in Table 2. SOC-BMRI had significantly longer acquisition and table times than AB-MRI (both p<0.001).
Table 2.
Acquisition and table times for AB-MRI and SOC-BMRI
| Time | N | Mean (range) | SD | p-valuea |
|---|---|---|---|---|
| Acquisition time, minutes | ||||
| AB-MRI | 73 | 9.63 (7.7–11.88) | 0.91 | |
| SOC-BMRI | 73 | 19.46 (14.68–26.18) | 2.91 | |
| SOC-BMRI+AB-MRI | 73 | 9.83 (4.77–16.32) | 3.31 | <0.0001 |
| Table time, minutes | ||||
| AB-MRI | 73 | 15.07 (11.62–20.47) | 2.01 | |
| SOC-BMRI | 73 | 36.3 (25.78–55.33) | 6.91 | |
| SOC-BMRI+AB-MRI | 73 | 21.23 (8.16–40.42) | 7.69 | <0.0001 |
AB-MRI: abbreviated-breast MRI; SOC: standard of care; BMRI: breast magnetic resonance imaging; SD: standard deviation.
P-value determined by Wilcoxon signed-rank test.
3.4. SOC-BMRI interpretation
Of the 73 women included in the analysis, 67 (91.8%) had a negative interpretation and 6 (8.2%) a positive interpretation. The positive interpretations from SOC-BMRI are shown in Table 3. The average number of prior BMRIs in the cohort was 2.99 (median 2, range 0–9). The SOC-BMRI was the baseline BMRI in 15 (20.5%). The accuracy was 93.2% (68/73, 95% confidence interval [CI] 85–98%). Other diagnostic indices are shown in Table 4.
Table 3.
Patients with positive interpretation on SOC-BMRI or AB-MRI
| Patient No. | Prior MRI Exams (n) | SOC BI-RADS | Outcome | AB-MRI BI-RADS | Lesion Typea | MRI-Guided Biopsy | Surgical Pathology | |||
|---|---|---|---|---|---|---|---|---|---|---|
| AB1 | AB2 | AB3 | AB4 | |||||||
| 10 | 4 | 1/2 | TN | 4 | 4 | 4 | 1/2 | Mass | ||
| 11 | 7 | 1/2 | TN | 4 | 4 | 1/2 | NME | |||
| 26 | 1 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 27 | 0 | 4 | FP | 1/2 | 1/2 | 1/2 | NME | FCC, IDP | ||
| 30 | 0 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 37 | 1 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 39 | 9 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 41 | 8 | 1/2 | TN | 4 | 1/2 | 1/2 | ||||
| 49 | 5 | 1/2 | TN | 4 | 4 | 1/2 | NME | |||
| 53 | 0 | 4 | FP | 4 | 1/2 | 1/2 | Mass | FCC, IDP | ||
| 62 | 2 | 1/2 | TN | 1/2 | 4 | 1/2 | NME | |||
| 64 | 5 | 1/2 | TN | 4 | 1/2 | 1/2 | ||||
| 69 | 0 | 4 | FP | 1/2 | 1/2 | 1/2 | NME | ALH, CCC, stromal fibrosis | ||
| 70b | 0 | 0 | FP | 1/2 | 1/2 | 1/2 | Mass | |||
| 73 | 1 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 78c (lesion 1) | 8 | 4 | TP | 4 | 4 | 4 | 4 | NME (right) | DCIS low grade | ADH (cribriform pattern), ALH |
| 78c (lesion 2) | 8 | 4 | FP | 4 | 4 | 4 | 4 | NME (left) | ADH, single focus | |
| 79 | 3 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 93 | 3 | 1/2 | TN | 4 | 4 | 4 | 1/2 | NME and mass | ||
| 94 | 0 | 3 | TN | 4 | 4 | 1/2 | Mass | |||
| 101 | 9 | 1/2 | TN | 4 | 4 | 1/2 | Mass | |||
| 103 | 9 | 1/2 | TN | 4 | 4 | 4 | 1/2 | Focus | ||
| 109 | 0 | 1/2 | TN | 4 | 4 | 4 | NME | |||
| 122 | 4 | 1/2 | TN | 4 | 1/2 | 1/2 | ||||
| 125d | 3 | 4 | FP | 4 | 4 | 4 | 4 | NME | ADH | Benign |
SOC: standard of care; BMRI: breast MRI; AB-MRI: abbreviated-breast MRI; BMRI: breast MRI; AB1: evaluation of maximum intensity projective images, including color-coded map; AB2: evaluation of dynamic contrast-enhanced images; AB3: evaluation of T2-weighted and fat-only images; AB4: non-blinded comparison performed only if AB3 was BI-RADS 4 and prior MRI exams were available; TN: true negative; FP: false positive; TP: true positive; NME: nonmass enhancement; FCC: fibrocystic changes; IDP: intraductal papilloma; ALH: atypical lobular hyperplasia; CCC: columnar cell change: DCIS: ductal carcinoma in situ; ADH: atypical ductal hyperplasia.
Lesions were not characterized if they were visualized only on maximum intensity projections.
Patient no. 70 underwent a second look for a circumscribed oval mass with dark internal septations. The second look determined that the mass was benign. A follow-up BMRI was performed 1 year later and had a benign result.
Patient no. 78 underwent a right mastectomy, showing atypia insufficient to meet DCIS criteria but morphologically similar to DCIS in the MR biopsy. She has undergone benign imaging follow -up with mammogram and ultrasound for the contralateral (left) breast.
Patient no. 125 underwent bilateral skin-sparing mastectomies with no evidence of atypia or carcinoma.
Table 4.
Diagnostic performance of SOC-BMRI and AB-MRI
| N | TN | TP | FN | FP | Sensitivity % |
Specificity | PPV | NPV | Accuracy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | p-value | % | % | % | p-valuea | |||||||
| SOC-BMRI images | 73 | 67 | 1 | 0 | 5 | 100 | 93 | 16.7 | 100 | 93 | ||
| AB-MRI MIP images (AB1) | 73 | 53 | 1 | 0 | 19 | 100 | 73.6 | p=0.002 | 5 | 100 | 74 | p=0.002 |
| AB-MRI MIP + DCE images (AB2) | 73 | 56 | 1 | 0 | 16 | 100 | 77.8 | p=0.01 | 5.9 | 100 | 78 | p=0.01 |
| AB MIP + DCE + T2 + fat-only images (AB3) | 73 | 67 | 1 | 0 | 5 | 100 | 93 | 16.7 | 100 | 93 | ||
SOC: standard of care; AB-MRI: abbreviated breast MRI; BMRI: breast magnetic resonance imaging; TN: true negative, TP: true positive; FN: false negative; FP: false positive; PPV: positive predictive value; NPV: negative predictive value; MIP maximum intensity projection; DCE: dynamic contrast-enhanced.
P-value was determined by McNemar test compared against SOC-BMRI diagnosis.
3.5. AB-MRI interpretation
The Kappa values for inter-reader agreement were 0.68 (95% CI 0.49–0.87) for AB1, 0.51 (95% CI 0.29–0.72) for AB2, and 0.37 (95% CI 0.03–0.71) for AB3. These Kappa values correspond to substantial agreement for AB1, moderate agreement for AB2, and fair agreement for AB3.
AB1 (MIP) assessment resulted in 53 (72.6%) negative and 20 (27.4%) positive interpretations. AB2 (DCE) assessment resulted in 56 (76.7%) negative and 17 (23.2%) positive interpretations. AB3 (MIPs, DCE, T2-weighted, and fat-only images) assessment resulted in 67 (91.8%) negative and 6 (8.2%) positive interpretations. The distribution of positive interpretations for each AB reading is illustrated in Table 3. Accuracies of AB1, AB2, and AB3 were 74% (95% CI 62–84%), 78.1% (95% CI 67–87%), and 93.2% (95% CI 85–98%), respectively.
Of the 20 positive interpretations on AB1, 4 (20%) were accurately recategorized as negative on AB2; 1 (5%) was incorrectly recategorized as positive on AB2. Eleven (55%) of the positive interpretations on AB2 were accurately re-categorized as negative interpretation on AB3 (Fig. 4). Comparison with prior exams on AB4 accurately recategorized 3 (15%) additional studies (Table 3) as negative.
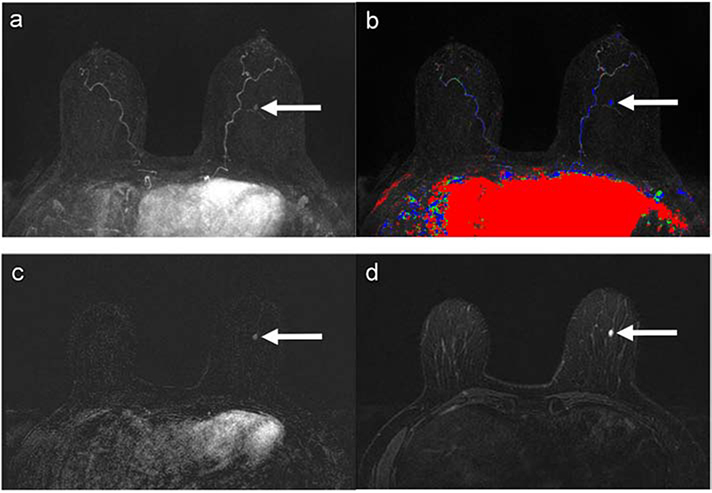
Figure 4:
58-year-old woman with 24.5% lifetime breast cancer risk by the Claus model. The AB-MRI first subtraction MIP (a) with time-intensity color map (AB1) (b) show an asymmetric mass with persistent enhancement kinetics in the middle central left breast (arrows). The AB-MRI first post-contrast subtraction (AB2) (c) shows an oval enhancing mass (arrow) in the inferior left breast, corresponding with the MIP finding. The corresponding AB-MRI T2-weighted image (AB3) (d) of the mass shows hyperintense T2 signal (arrow). This mass was identified and classified as BI-RADS Category 4, suspicious, on AB1 and AB2. The mass was downgraded to BI-RADS Category 2, benign, on AB3. The AB3 assessment matched the SOC-BMRI assessment of a benign, true-negative finding.
The average interpretation times of all readers for AB1, AB2, and AB3 assessments were 5.92 seconds (SD 2.93, range 2.67–21.33, median 5.67), 26.44 seconds (SD 7.4, range 17–25, median 25), and 21.68 seconds (SD 9.71, range 9.3–60, median 20), respectively. The average interpretation time for all readers to complete AB1, AB2, and AB3 was 54.04 seconds (SD 16.12, range 35.33–110.33, median 51.67).
3.6. Comparison between SOC-BMRI and AB-MRI interpretations
Diagnostic performances of SOC-BMRI and AB-MRI are summarized in Table 4. SOC-BMRI had significantly higher specificity and higher accuracy than AB1 and AB2 assessments from AB-MRI (p=0.002 and 0.01, respectively), but the two protocols did not differ on accuracy of AB3 assessment (both 93%).
4. Discussion
In our study, an AB-MRI protocol for high-risk breast cancer screening was significantly faster to perform than the SOC-BMRI protocol and required less than 1 minute to interpret. AB-MRI table time was almost 60% shorter than that of SOC-BMRI, and scan time was about 50% shorter. The cancer yield of BMRI in this high-risk cohort of 73 women was 14 per 1,000, compared with the reported cancer yield of 14.7 per 1000 for high-risk screening BMRI in ACRIN 6666 [25]. The accuracy, sensitivity, specificity, PPVs, and NPVs were identical for AB-MRI and SOC-BMRI when readers evaluated all AB images (AB3). Reader assessment of only abbreviated-protocol MIP images (AB1) showed 100% sensitivity and NPV; this agrees with the findings of Kuhl et al., which indicate that the MIP allows radiologists to quickly discern whether a significant lesion is present on MRI screening [9]. Interestingly, 2 of the false positive results from SOC-BMRI were interpreted as benign on AB-MRI, indicating that AB-MRI may have fewer false positives while still detecting clinically significant lesions. In our cohort, the clinically significant lesions included 1 cancer and 2 ADH lesions that were interpreted as positive on both SOC and AB-MRI.
The potential for a shortened BMRI was first demonstrated by Kuhl et al. [9]. That group compared the diagnostic performance from images acquired in 3 minutes, faster than their complete BMRI protocol by 14 minutes. The acquisition time of our AB study is within the range of 3 to 15 minutes that has been reported by various groups for AB-MRI [10, 14, 26–31]. Our average AB reading time was faster than the reading time of 5.8 minutes reported for BMRI with computer-aided detection [32] and within the range of 28 seconds to 2.98 minutes reported in AB-MRI studies [9, 12, 14, 26, 27, 29, 33, 34]. Time savings in acquisition and interpretation are important in terms of cost, patient throughput, and radiologist workflow. The acquisition time of our AB-MRI protocol is longer than the 4-minute acquisition time of a digital screening mammogram with tomosynthesis [35] but shorter than that of a bilateral screening breast ultrasound [36].
The PPV of AB3 was non-inferior to that of SOC-BMRI in our study but was also lower than the PPV range of 21.7% to 64% reported by others for AB-MRI [9, 29, 34]. Most likely our PPV is lower than those of prior studies because only one cancer was detected in our cohort. Our cohort included only women with high risk, most of whom had undergone previous MRI screening. Interestingly, there were no additional cancers in the second screening round in the Kuhl study [9] of 443 women with mostly a mild-moderate risk, indicating that even one previous BMRI screening affects the expected cancer yield in subsequent screening rounds and may explain why only one cancer was detected in the current study.
The time required to perform SOC-BMRI, which contributes to its high costs and poor patient tolerance, is one of the motivating factors in developing AB-MRI protocols. So far, most of the proposed AB-MRI protocols achieve scan time reduction through elimination of dynamic post-contrast imaging, T2-weighted imaging, or both. One consequence of such an approach is that the AB-MRI study is not compliant with the current ACR accreditation criteria and is not eligible for reimbursement in the United States [17, 37]. Perhaps more importantly, patients with a positive finding from the AB-MRI protocol will likely need to be recalled for a full diagnostic workup or require more frequent follow-up, increasing the overall cost of managing these cases.
In contrast, our AB-MRI protocol incorporates both high-resolution T2-weighted imaging and complete T1-weighted dynamic imaging sequences and is fully ACR-compliant. Our AB-MRI protocol achieves scan time and table time savings through the use of two sequences with the Dixon technique, which eliminates the need for manual tuning and repeats [19, 38]. The Dixon technique yields additional fat-only images without any extra scan time. Our study showed that the fat-only images are useful because they provide an image contrast that is similar to that of a conventional non–fat-suppressed T1-weighted sequence, which would have required several minutes of additional acquisition time. The images from our AB-MRI protocol allowed evaluation of both lesion morphology and enhancement characteristics and therefore a full characterization without a need for patients with positive interpretations to return for a second, diagnostic BMRI. In particular, our AB-MRI protocol included the dynamic pre-/post-contrast imaging that provides time-intensity curves and visualization of enhancement characteristics. The kinetic analysis is a standard part of BMRI interpretation as per the BI-RADS lexicon [24] and is essential for differentiation of benign and malignant lesions [16, 39]. Having multiple time points after contrast administration may also assist in identification of some malignancies, such as invasive lobular carcinoma or DCIS, that do not demonstrate rapid early enhancement. Although initial enhancement ratio and time to enhancement have been proposed in lieu of kinetic curve assessment [40, 41], the performance of these parameters for lesion characterization requires further validation.
Our study also revealed that review of T2-weighted and fat-only images was necessary to achieve non-inferior diagnostic performance compared with SOC-BMRI. Without these sequences, the recall rate for additional imaging or biopsy would have been 23% or 8%, which compares favorably to a typical screening mammography recall rate. Our findings are consistent with those of Panigrahi et al., who reported that some potential recall cases would be resolved using T1-weighted or T2-weighted non-contrast sequences [14]. Lack of specificity is a concern for BMRI and can result in women being recalled for a second BMRI for lesion characterization and possibly a third BMRI to guide a biopsy. In our opinion, T1-weighted non-contrast images are helpful in evaluating benign enhancing findings such as lymph nodes and fat necrosis and T2-weighted sequences are valuable in combination with morphologic and kinetic features in differentiating benign and malignant lesions [42, 43]. These valuable sequences are omitted from most AB-MRI protocols because they would usually take up to 10 minutes to acquire [9, 10, 12, 14, 28, 31, 34, 44]. Some investigators have reported that including a T2-weighted sequence in an AB-MRI protocol increases lesion conspicuity [26], but there has been no evaluation of the added value or contribution to the lesion specificity [30, 33]. The T2-weighted sequence in our study had an average scan time of less than 2 minutes and was found to add value to the diagnostic performance. We therefore believe that inclusion of a T2-weighted or fluid bright sequence is both practical and worthwhile in screening BMRI to minimize false-positive findings. Further, a T2-weighted sequence is an essential requirement for ACR accreditation. Our study has some limitations. First, the number of patients in our study is small. Our recruitment of participants was affected by the study requirement that participants return on a separate date for AB-MRI, and some participants were lost to follow-up. Additionally, most of the study participants had had at least one prior BMRI exam, which likely decreased the number of cancers present in this cohort and may explain why only one cancer was detected in this high-risk cohort. Second, the Dixon technique used in our AB-MRI study is not an FDA-cleared commercial product, and it results in occasional artifacts at fat and water boundaries. These artifacts did not negatively affect image interpretation. The image generation was not fully automatic and required some post-processing, which added some time delay for image interpretation. Finally, our study may not be directly applicable to a community practice because it was performed at a single high-volume tertiary care center and on 3.0-T scanners, and the images were interpreted by radiologists specialized in breast imaging with >5 years of experience. Finally, the interpretation time reported may not be closely reproducible in an actual clinical setting, as the blinded reading in our study did not reflect standard practice.
5. Conclusions
In conclusion, our study demonstrates that an AB-MRI protocol consisting of two Dixon sequences can achieve significant timing savings for high-risk breast cancer screening in direct comparison to SOC-BMRI and yet is in full compliance with ACR accreditation. The kinetic curve from the full dynamic imaging and T2-weighted images as well as the fat-only images in the AB-MRI are important for BI-RADS category and lesion characterization and are needed to maintain the non-inferiority in performance when compared to SOC-BMRI. In view of the mounting evidence to expand the use of AB-MRI to routinely screen average risk women or breast cancer survivors, our protocol that significantly decreases scan and table time, minimizes false positive rate with no impact on cancer detection is a viable technique to scan and interpret large patient populations.
Acknowledgements
We thank the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing this manuscript.
Grant Support: This research was conducted at the Center for Advanced Biomedical Imaging at The University of Texas MD Anderson Cancer Center with support from General Electric Healthcare. This study was supported by MD Anderson Cancer Support grant NIH/NCI P30CA016672.
Funding
This study received equipment support from General Electric and was supported by MD Anderson Cancer Support grant NIH/NCI P30CA016672.
Footnotes
Disclosures:
The following authors have no disclosures: M.E.S., R.P.C., M.J.D., W.W., J.B.S B.K.A.: Astra Zeneca research paid to the institution; AbbVie research paid to the institution; Invitae research paid to the institution; AbbVie steering committee member: non-paid; Bright Pink: medical committee member, non-paid.
J.M.: IP licensing with Siemens Healthineers and GE Healthcare Technologies; consultant for C4 Imaging.
B.E.D.: Consultant, Endomag, Cambridge, UK
IRB Statement: The appropriate institutional review board approved the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: Using magnetic resonance imaging to screen women at high risk for breast cancer. Annals of Internal Medicine 2008;148(9):671–9. [DOI] [PubMed] [Google Scholar]
- [2].Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- [4].Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J Am Coll Radiol 2018;15(3 Pt A):408–14. [DOI] [PubMed] [Google Scholar]
- [5].Berg WA, Blume JD, Adams AM, Jong RA, Barr RG, Lehrer DE, et al. Reasons women at elevated risk of breast cancer refuse breast MR imaging screening: ACRIN 6666. Radiology 2010;254(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wernli KJ, DeMartini WB, Ichikawa L, Lehman CD, Onega T, Kerlikowske K, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med 2014;174(1):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deike-Hofmann K, Koenig F, Paech D, Dreher C, Delorme S, Schlemmer H-P, et al. Abbreviated MRI Protocols in Breast Cancer Diagnostics. Journal of magnetic resonance imaging : JMRI 2019;49(3):647–58. [DOI] [PubMed] [Google Scholar]
- [8].Harms SE. Technical report of the international working group on breast MRI. J Magn Reson Imaging 1999;10(6):979. [DOI] [PubMed] [Google Scholar]
- [9].Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers R-DD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI . Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(22):2304–10. [DOI] [PubMed] [Google Scholar]
- [10].Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol 2015;84(1):65–70. [DOI] [PubMed] [Google Scholar]
- [11].Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Academic radiology 2015;22(9):1157–62. [DOI] [PubMed] [Google Scholar]
- [12].Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An Abbreviated Protocol for High-Risk Screening Breast MRI Saves Time and Resources. J Am Coll Radiol 2016;13(4):374–80. [DOI] [PubMed] [Google Scholar]
- [13].Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated Combined MR Protocol: A New Faster Strategy for Characterizing Breast Lesions . Clinical breast cancer 2016;16(3):207–11. [DOI] [PubMed] [Google Scholar]
- [14].Panigrahi B, Mullen L, Falomo E, Panigrahi B, Harvey S. An Abbreviated Protocol for High-risk Screening Breast Magnetic Resonance Imaging: Impact on Performance Metrics and BI-RADS Assessment. Acad Radiol 2017;24(9):1132–8. [DOI] [PubMed] [Google Scholar]
- [15].Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental Breast MR Imaging Screening of Women with Average Risk of Breast Cancer. Radiology 2017;283(2):361–70. [DOI] [PubMed] [Google Scholar]
- [16].Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology 1999;211(1):101–10. [DOI] [PubMed] [Google Scholar]
- [17].Covington MF, Young CA, Appleton CM. American College of Radiology Accreditation, Performance Metrics, Reimbursement, and Economic Considerations in Breast MR Imaging. Magn Reson Imaging Clin N Am 2018;26(2):303–14. [DOI] [PubMed] [Google Scholar]
- [18].Radiology ACo. Breast MRI Clinical Image Review Category A: Pulse Sequences and Image Contrast (Revised 12–12-19); 2019. Available from: https://accreditationsupport.acr.org/support/solutions/articles/11000070271-breast-mri-clinical-image-review-category-a-pulse-sequences-and-image-contrast-revised-12-12-19- [Accessed 02/06/2020.
- [19].Dogan BE, Scoggins ME, Son JB, Wei W, Candelaria R, Yang WT, et al. American College of Radiology-Compliant Short Protocol Breast MRI for High-Risk Breast Cancer Screening: A Prospective Feasibility Study. AJR Am J Roentgenol 2018;210(1):214–21. [DOI] [PubMed] [Google Scholar]
- [20].Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32(22):2304–10. [DOI] [PubMed] [Google Scholar]
- [21].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Son JB, Hwang K-P, Madewell JE, Bayram E, Hazle JD, Low RN, et al. A flexible fast spin echo triple-echo Dixon technique. Magnetic resonance in medicine 2017;77(3):1049–57. [DOI] [PubMed] [Google Scholar]
- [23].Ma J, Vu AT, Son JB, Choi H, Hazle JD. Fat-suppressed three-dimensional dual echo Dixon technique for contrast agent enhanced MRI. Journal of magnetic resonance imaging : JMRI 2006;23(1):36–41. [DOI] [PubMed] [Google Scholar]
- [24].Morris E, Comstock C, Lee C, Lehman C, Ikeda D, Newstead G, et al. ACR BI-RADS ® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- [25].Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: Correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol 2016;85(4):815–23. [DOI] [PubMed] [Google Scholar]
- [27].Bickelhaupt S, Laun FB, Tesdorff J, Lederer W, Daniel H, Stieber A, et al. Fast and Noninvasive Characterization of Suspicious Lesions Detected at Breast Cancer X-Ray Screening: Capability of Diffusion-weighted MR Imaging with MIPs. Radiology 2016;278(3):689–97. [DOI] [PubMed] [Google Scholar]
- [28].Romeo V, Cuocolo R, Liuzzi R, Riccardi A, Accurso A, Acquaviva A, et al. Preliminary Results of a Simplified Breast MRI Protocol to Characterize Breast Lesions: Comparison with a Full Diagnostic Protocol and a Review of the Current Literature. Acad Radiol 2017;24(11):1387–94. [DOI] [PubMed] [Google Scholar]
- [29].Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated Combined MR Protocol: A New Faster Strategy for Characterizing Breast Lesions. Clin Breast Cancer 2016;16(3):207–11. [DOI] [PubMed] [Google Scholar]
- [30].Choi BH, Choi N, Kim MY, Yang JH, Yoo YB, Jung HK. Usefulness of abbreviated breast MRI screening for women with a history of breast cancer surgery. Breast Cancer Res Treat 2018;167(2):495–502. [DOI] [PubMed] [Google Scholar]
- [31].Petrillo A, Fusco R, Sansone M, Cerbone M, Filice S, Porto A, et al. Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat 2017;164(2):401–10. [DOI] [PubMed] [Google Scholar]
- [32].Lehman CD, Blume JD, DeMartini WB, Hylton NM, Herman B, Schnall MD. Accuracy and interpretation time of computer-aided detection among novice and experienced breast MRI readers. AJR Am J Roentgenol 2013;200(6):W683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 2015;22(9):1157–62. [DOI] [PubMed] [Google Scholar]
- [34].Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of Abbreviated Protocol of Magnetic Resonance Imaging for Breast Cancer Screening in Dense Breast Tissue. Acad Radiol 2017;24(3):316–20. [DOI] [PubMed] [Google Scholar]
- [35].Bernardi D, Ciatto S, Pellegrini M, Anesi V, Burlon S, Cauli E, et al. Application of breast tomosynthesis in screening: incremental effect on mammography acquisition and reading time. Br J Radiol 2012;85(1020):e1174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Böhm-Vélez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA 2008;299(18):2151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Radiology ACo . Breast Magnetic Resonance Imaging (MRI) Accreditation Program Requirements; 2017. Available from: https://www.acraccreditation.org/-/media/ACRAccreditation/Documents/Breast-MRI/Requirements.pdf?la=en [Accessed 05/22/2018.
- [38].Son JB, Hwang KP, Madewell JE, Bayram E, Hazle JD, Low RN, et al. A flexible fast spin echo triple-echo Dixon technique. Magn Reson Med 2017;77(3):1049–57. [DOI] [PubMed] [Google Scholar]
- [39].Kinkel K, Helbich TH, Esserman LJ, Barclay J, Schwerin EH, Sickles EA, et al. Dynamic high-spatial-resolution MR imaging of suspicious breast lesions: diagnostic criteria and interobserver variability. AJR Am J Roentgenol 2000;175(1):35–43. [DOI] [PubMed] [Google Scholar]
- [40].Heacock L, Lewin AA, Gao Y, Babb JS, Heller SL, Melsaether AN, et al. Feasibility analysis of early temporal kinetics as a surrogate marker for breast tumor type, grade, and aggressiveness. J Magn Reson Imaging 2018;47(6):1692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mus RD, Borelli C, Bult P, Weiland E, Karssemeijer N, Barentsz JO, et al. Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol 2017;89:90–6. [DOI] [PubMed] [Google Scholar]
- [42].Kuhl CK, Klaschik S, Mielcarek P, Gieseke J, Wardelmann E, Schild HH. Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging 1999;9(2):187–96. [DOI] [PubMed] [Google Scholar]
- [43].Ballesio L, Savelli S, Angeletti M, Porfiri LM, D’Ambrosio I, Maggi C, et al. Breast MRI: Are T2 IR sequences useful in the evaluation of breast lesions? Eur J Radiol 2009;71(1):96–101. [DOI] [PubMed] [Google Scholar]
- [44].Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer 2017;24(3):411–9. [DOI] [PubMed] [Google Scholar]