Abstract
Rigorous testing is the way forward to fight the coronavirus disease 2019 pandemic. Here we show that the currently used and most reliable reverse transcription-polymerase chain reaction-based severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) procedure can be further simplified to make it faster, safer, and economical by eliminating the RNA isolation step. The modified method is not only fast and convenient but also at par with the traditional method in terms of accuracy, and therefore can be used for mass screening. Our method takes about half the time and is cheaper by ∼40% compared to the currently used method. We also provide a variant of the new method that increases the efficiency of detection by ∼30% compared to the existing procedure. Taken together, we demonstrate a more effective and reliable method of SARS-CoV-2 detection.
Keywords: SARS-CoV-2 diagnosis, false-negative, COVID-19, cost-effective, direct RT-PCR, dry swab
Introduction
Efficient diagnosis of an infectious pandemic carries inherent challenges such as biosafety during sample handling, skilled manpower, time consumption for testing, sensitivity of the testing method, and significant economic burden, irrespective of the nations. The present severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is no exception for this, where the most efficient/reliable screening method is reverse transcription–polymerase chain reaction (RT–PCR)-based detection of viral nucleic acid from the patient sample [1, 2]. Rapidly growing number of coronavirus disease 2019 (COVID-19) cases warrant reliable and quicker testing methods [3]. In the absence of specific drug and/or vaccine, the only way to control SARS-CoV-2 spread is large-scale screening and isolation of the infected individuals at early stages of infection. Screening using antibody-based methods is rapid but cannot be used for early-stage detection [4]. Despite being a superior method, RT-PCR demands significant amount of time due to a laborious and expensive RNA isolation step from the viral transport medium (VTM) containing the swab samples. Currently, the challenge is to adapt a detection method which is quicker and still retaining the sensitivity of the standard RT-PCR-based method. Different studies have previously reported inexpensive, nucleic-acid extraction-free methods for PCR-based clinical diagnosis [5, 6].
Here we show that the need for VTM as well as RNA isolation step for performing RT-PCR can be completely eliminated by extracting biological samples from dry swabs using Tris EDTA (TE) buffer, which is cost-effective and can be used as a quick screening procedure. In addition, we also show that the sensitivity of the entire RT-PCR based detection is enhanced by ∼30%, when using RNA isolated from TE buffer extract compared to the traditional method.
Materials and methods
Sample collection and transport
The swab samples were collected from voluntary patients at Gandhi Medical College & Hospital, Secunderabad, India. Two nasopharyngeal swabs were collected from each patient and one was transported as dry swab and another in 1 ml VTM (HiMedia Labs, Mumbai), respectively, and the samples were kept at 4°C till further processing.
Sample processing
Complete sample processing was done in the Biosafety level-3 (BSL-3) facility of Council of Scientific and Industrial Research – Centre for Cellular and Molecular Biology (CSIR-CCMB) by following Standard Operating Procedures.
Resuspension/extraction of biological material from dry swabs The dry swabs were transferred to 1.5 ml microfuge tubes containing 400 μl of TE buffer (10 mM Tris pH-7.4, 0.1 mM EDTA 0.1 mM). The swabs were cut to make them fit into the tubes and incubated at room temperature for 30 min to ensure the release of biological material.
Heat Inactivation For direct VTM to RT-PCR, an aliquot of 1 ml VTM samples was diluted three times before processing (as the existing recommendation suggests using 3 ml VTM for sample collection). An aliquot of 50 µl of the VTM (for direct VTM to RT-PCR) and TE extract was aliquoted from the respective vials containing swabs in to separate vials and heated at 98°C for 6 min on a dry heat block. The inactivated samples were directly used as a template for RT-PCR.
RNA isolation
The RNA isolation from 3 ml VTM and TE-buffer (containing dry swab) was performed using the QIAamp Viral RNA isolation kit (Qiagen, Germany) according the manufacturer’s protocol. In both cases, 150 μl of the sample was processed for RNA isolation.
RT-PCR
All the RT-PCR work was carried out in a BSL-2 facility of CSIR-CCMB, Hyderabad, India.
Heat inactivated VTM (direct VTM), TE buffer extract, and RNA isolated from TE buffer extract (TE-RNA) and VTM (VTM-RNA) from appropriate samples were tested using the Food and Drug Administration (FDA) approved LabGun COVID-19 detection RT-PCR kit (LabGenomics Co., Ltd., Republic of Korea). The primer–probe mix targets Envelope (E) gene (Cy5-labeled) and RNA-dependent RNA polymerase (RdRP; FAM-labeled) in the viral genome. The RT-PCR was performed according to the manufacturer protocol. The reactions were multiplexed after performing an in-house standardization (Supplementary data, Table S5). RT–PCR was performed in duplicates using LightCycler® 480 II (Roche Life Science, Germany) and the average values of two technical replicates were used for the analysis. For plotting purposes mean CT or dCT values of E or RdRP genes from both the replicates were used (Fig. 2 and Supplementary data, Fig. S2). RNase P primers (Forward-5′-AGATTTGGACCTGCGAGCG-3′ and Reverse 5′-GAGCGGCTGTCTCCACAAGT-3′) and RNaseP Probe (5′-FAM-TTCTGACCTGAAGGCTCTGCGCG-BHQ-3′) were synthesized by as per ICMR, India guidelines (https://www.icmr.gov.in/pdf/covid/labs/1_SOP_for_First_Line_Screening_Assay_for_2019_nCoV.pdf). RNaseP oligos were synthesized at Eurofins Scientific, India. RT-PCR with RNase P primer–probe mix was performed only once.
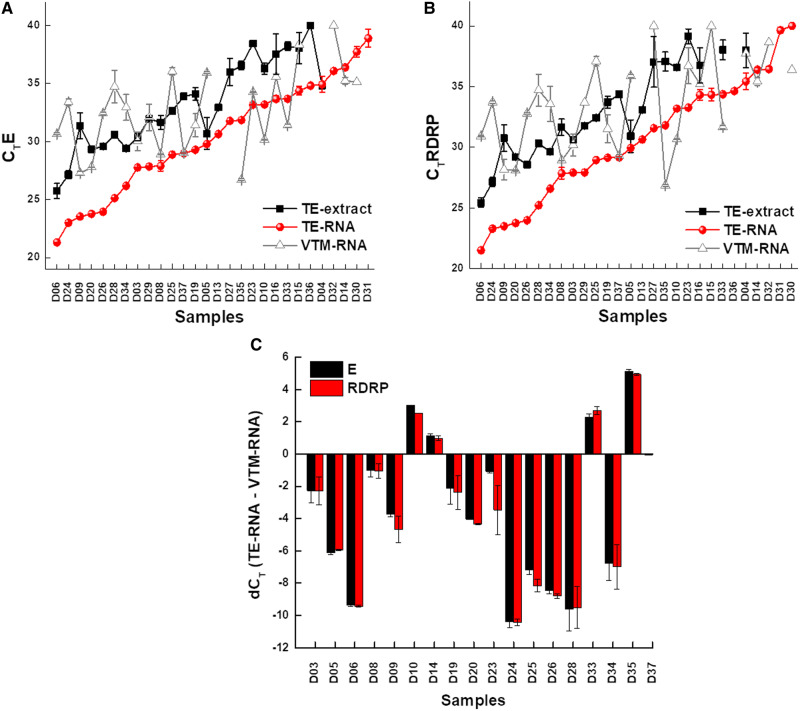
Figure 2:
Increased sensitivity of COVID-19 diagnosis using TE-extracted RNA. (A) and (B) Scatter plot of the CT values for each sample obtained using different methods as labeled in the Figure and the data points represents the average of CT values of E gene (A) and RdRP (B); error bars represent the standard error of mean for the data obtained from two experiments. (C) Bar graph representing the difference in CT values of E gene and RdRP between TE-based RNA and traditional (VTM-RNA) method. Negative and positive values indicate increased and reduced sensitivity, respectively. The data points are average of two technical replicates; error bars represent the standard error of mean for the data obtained from two experiments.
Microsoft Excel and Origin software were used to generate the plots; Microsoft PowerPoint was used to generate the images. Heatmaps were constructed using heatmapper.ca [7].
Ethical statement
The study follows the institutional ethics committee guidelines (IEC number: 82/2020).
Results and discussion
We first hypothesized that SARS-CoV-2 nucleic acid could be detected directly by using VTM containing swabs of COVID-19 patients. This methodology (direct VTM method) involves the lysis of the virions (in VTM) by heating a 50 µl aliquot of VTM at 98°C for 6 min, followed by using 4 µl of this as a template for subsequent RT–PCR reaction targeting. However, our results showed a 50% reduction in the detection efficiency of positive samples (n = 16) compared to the traditional RNA isolation-based method (Supplementary data, Fig. S1A and B; Supplementary data, Table S1).
Although our data put forth the feasibility of using VTM instead of extracted RNA, the detection ability of this method is limited to samples with moderate to high viral load. Probable reasons for this decreased efficiency could be dilution of the samples in 3 ml VTM or presence of PCR inhibitors in VTM, and to overcome this limitation, we changed our sample collection strategy. To test the new strategy, two nasopharyngeal swab samples were collected from each of the 14 patients with one swab transported dry and another in VTM and processed further (refer Methods for detail; Fig. 1A). Of the 14 patients, five were tested negative and the remaining nine positive samples were used for comparison. The results revealed that the performance of dry swab-TE buffer extracts in direct RT–PCR and the currently used standard method of detection which has the additional RNA extraction step from VTM samples were comparable (Supplementary data, Table S2 D1–D14), wherein, both the methods yielded same result for 11 out of 14 samples (six positives and five negatives) while the result differed for three samples.
Figure 1:
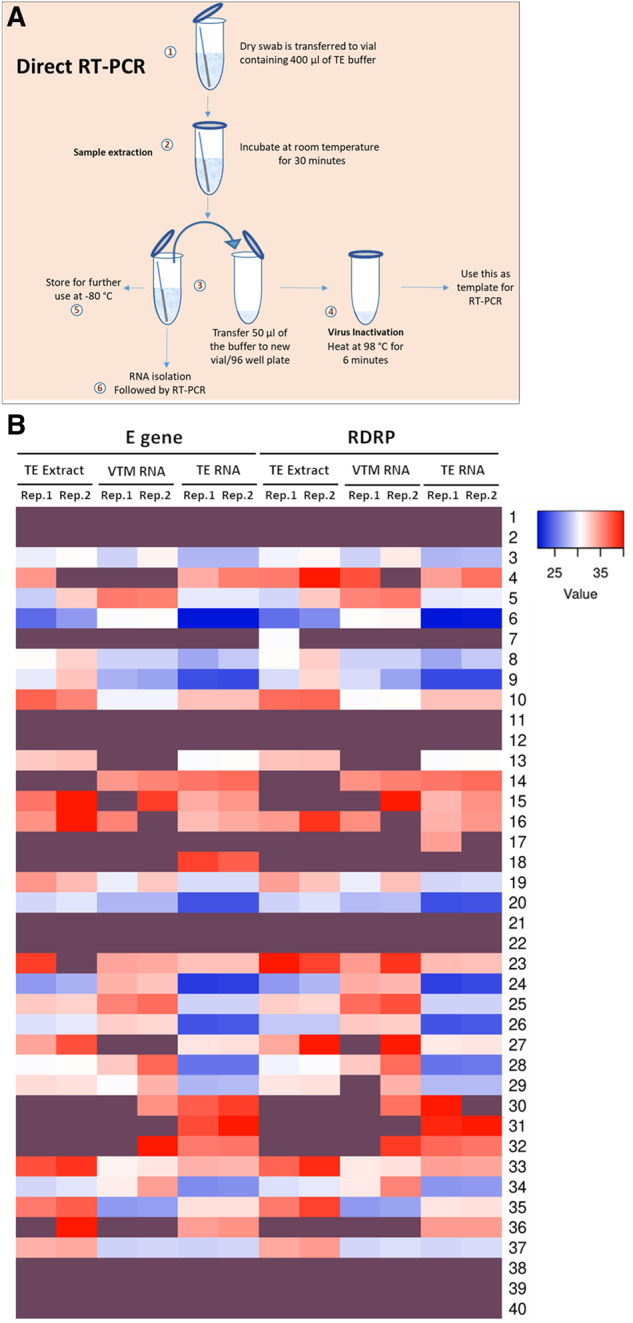
RNA extracted from TE buffer outperform other methods. (A) Schematic of the entire protocol for TE-based sample extraction and RT-PCR. (B) Heatmap representing the CT values of E and RdRP genes obtained in two replicates (Reps.1 and 2) of RT-PCR using TE extract, VTM-extracted RNA (VTM-RNA), and TE-extracted RNA (TE-RNA) as templates (n = 40). Details in Supplementary data, Table S2. Samples D1–D40 are represented as 1–40. Dark purple shade represents no signal detection.
To further validate the usage of TE buffer extract as a template for direct RT–PCR, we obtained similar samples from 26 patients, on the whole taking the sample size to 40. The results have further strengthened our observation that the TE buffer extract is as sensitive as the extracted RNA (n = 40; Fig. 1B). Overall, both the methods showed consistent results for 33 out of 40 samples (19 positives and 14 negatives) and differed for 7 samples. Also, the CT values of TE-based RT–PCR (TE extract) were comparable to that of the traditional method (VTM-RNA) and, therefore, can serve as an alternative method (Supplementary data, Fig. S2). This approach can be employed as rapid and economical method for diagnosis which does not require an RNA extraction step. Our results are in line with the earlier reports of RNA extraction-free RT–PCR [8–10], but here we introduce a sample collection strategy in the form of dry swabs, which enhances biosafety during collection, transportation, and processing of samples, as there is no scope of spillage. Also, this method drastically reduces the cost incurred by eliminating VTM and RNA-extraction step. We have standardized this procedure which is now consistent and compelling. In addition, we have calculated the limit of detection of the TE buffer extract in comparison to RNA isolated from VTM samples using the LabGun kit, i.e. sensitive enough to detect as low as 100 copies/μl. We obtained comparable detection efficiencies between TE buffer extract and VTM RNA samples (Supplementary data, Table S3). One of the biggest challenges in diagnostics is overcoming the problem of false-negatives, and SARS-CoV-2 is not an exception to this. Recent reports have shown that the percentage of false negative reported for SARS-CoV-2 is between 20% and 40% with the onset of symptoms and varies with respect to the phase of infection [11, 12], which is alarming and calls for immediate improvements in the detection methodology. To address this issue, we have combined the TE-extraction method with traditional method that includes RNA-extraction. Here RNA was first isolated from TE extract (described in methodology), followed by RT–PCR. We were pleasantly surprised that almost one-third of the samples (∼30%) which were consistently negative with traditional VTM-based method and also direct-RT-PCR method turned out to be positive for SARS-CoV-2. This observation was reproducible in multiple rounds of testing (Figs. 1B, 2A and B). Upon a further closer look at the overall data, it was intriguing to note that the samples which were positive in the TE-based RNA extraction (and negative in other two methods) had a CT value for only one of the two gene (E gene and RdRP), therefore, possibly hinting at the low viral load which can be now picked by the new method. The dCT values indicate the increased detection limits of this method as in majority of the samples CT difference between TE-RNA and VTM-RNA was <0 (Fig. 2B). To rule out any discrepancies in the sample processing, we have used RNaseP as an internal control (Supplementary data, Table S4). Interestingly as an indication of RNA amount and quality, the RNase P CT values in case of TE-based approach had lower values compared to RNA isolated from VTM, thereby proving the higher efficiency of the TE-based approach (Supplementary data, Table S4). Therefore, the new hybrid method of TE-based sample extraction results in increasing the overall efficiency by ∼30%. These results put forth a remarkable improvement in the detection of SARS-CoV-2 patients with less viral load and, therefore, provide a better opportunity to manage the pandemic. Furthermore, an improved detection efficiency provides an avenue for adapting this method in combination with pooling strategies.
Based on the above results, we recommend a two-tier screening method for SARS-CoV-2 management. Since, TE buffer extracts can be used for direct RT–PCR without compromising the sensitivity of detection, we strongly recommend that this method be employed as a first line of SARS-CoV-2 for large-scale screening, while the TE buffer extract-based RNA could be employed if the former method yields an ambiguous result. TE buffer extract-based detection can probably be expanded for screening other respiratory viral infections that are diagnosed using RT–PCR as well [13]. Finally, we also recommend sample collection using dry swab approach which not only eliminates the need of VTM, but also makes the sample handling, transporting, and testing more convenient and safer for the frontline healthcare workers and technicians.
Supplementary data
Supplementary data is available at Biology Methods and Protocols online.
Supplementary Material
Acknowledgments
We acknowledge the state government official and the Department of Medical Education, Telangana for providing the samples for this study. U.K., C.G.G., and S.K.K. thank the financial support received from University Grants Commission (UGC), Council of Scientific and Industrial Research (CSIR), Department of Science and Technology (DST) and Innovation in Science Pursuit for Inspired Research (INSPIRE), India, respectively. All the authors acknowledge the support received from CSIR, India. The authors also acknowledge V Devi Prasad (CSIR-CCMB) and Divya Gupta (CSIR-CCMB) for their helpful suggestions.
Conflicts of interest
The authors declare no conflict of interests.
References
- 1. Al-Tawfiq JA, Memish ZA, Diagnosis of SARS-CoV-2 infection based on CT scan vs RT-PCR: reflecting on experience from MERS-CoV. J Hosp Infect 2020;105:154–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emery SL, Erdman DD, Bowen MD. et al. Real-time reverse transcription–polymerase chain reaction assay for SARS-associated coronavirus. Emerg Infect Dis 2004;10:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu F, Zhao S, Yu B. et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carter LJ, Garner LV, Smoot JW. et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci 2020;6:591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freeman JD, Rosman LM, Ratcliff JD. et al. State of the science in dried blood spots. Clin Chem 2018;64:656–79. [DOI] [PubMed] [Google Scholar]
- 6. Simon N, Shallat J, Williams Wietzikoski C. et al. Optimization of Chelex 100 resin-based extraction of genomic DNA from dried blood spots. Biol Methods Protocol 2020;5:bpaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babicki S, Arndt D, Marcu A. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res 2016;44:W147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruce EA, Huang ML, Perchetti GA. et al. Direct RT-qPCR detection of SARS-CoV-2 RNA from patient nasopharyngeal swabs without an RNA extraction step. bioRxiv 2020. doi: 10.1101/2020.03.20.001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smyrlaki I, Ekman M, Lentini A. et al. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. medRxiv 2020. doi: 10.1101/2020.04.17.20067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alcoba-Florez J, González-Montelongo R, Íñigo-Campos A. et al. Fast SARS-CoV-2 detection by RT-qPCR in preheated nasopharyngeal swab samples. Int J Infect Dis 2020;97:66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kucirka LM, Lauer SA, Laeyendecker O. et al. Variation in falsenegative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Inern Med2020; 173:4, 262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Yao L, Li J. et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol 2020;92:903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boncristiani HF, Criado MF, Arruda E. Respiratory viruses In: Schaechter M. (ed.), Encyclopedia of Microbiology, 3rd edn.Oxford: Academic Press, 2009, 500–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.