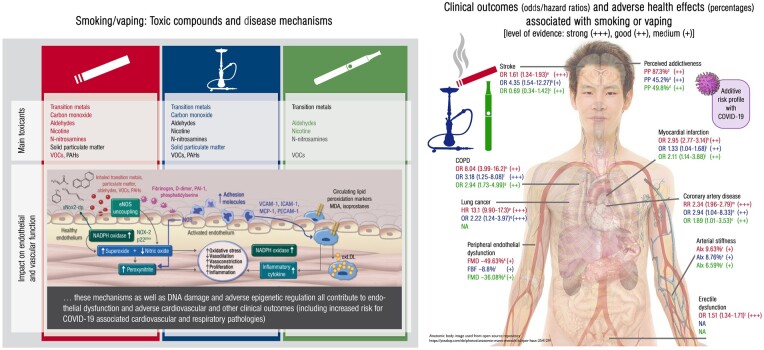
Abstract
Tobacco smoking is a leading cause of non-communicable disease globally and is a major risk factor for cardiovascular disease (CVD) and lung disease. Importantly, recent data by the World Health Organizations (WHO) indicate that in the last two decades global tobacco use has significantly dropped, which was largely driven by decreased numbers of female smokers. Despite such advances, the use of e-cigarettes and waterpipes (shisha, hookah, narghile) is an emerging trend, especially among younger generations. There is growing body of evidence that e-cigarettes are not a harm-free alternative to tobacco cigarettes and there is considerable debate as to whether e-cigarettes are saving smokers or generating new addicts. Here, we provide an updated overview of the impact of tobacco/waterpipe (shisha) smoking and e-cigarette vaping on endothelial function, a biomarker for early, subclinical, atherosclerosis from human and animal studies. Also their emerging adverse effects on the proteome, transcriptome, epigenome, microbiome, and the circadian clock are summarized. We briefly discuss heat-not-burn tobacco products and their cardiovascular health effects. We discuss the impact of the toxic constituents of these products on endothelial function and subsequent CVD and we also provide an update on current recommendations, regulation and advertising with focus on the USA and Europe. As outlined by the WHO, tobacco cigarette, waterpipe, and e-cigarette smoking/vaping may contribute to an increased burden of symptoms due to coronavirus disease 2019 (COVID-19) and to severe health consequences.
Keywords: Tobacco smoking, Shisha/waterpipe smoking, E-cigarette vaping, Endothelial function, Oxidative stress, Inflammation
Graphical Abstract
Graphical Abstract.
Introduction
‘Tobacco is a legal drug that kills many of its users when used exactly as intended by manufacturers’. That is the beginning sentence of the World Health Organizations (WHO) global report on trends in tobacco smoking from 2015.1 Indeed, in 2019, the WHO announced that tobacco (i.e. tobacco cigarettes, pipes, cigars, waterpipes, smokeless tobacco products, and heated tobacco products, but not e-cigarettes) kills up to half of its users and more than 8 million people each year, of which 7 million are the result of direct tobacco use, while exposure to second-hand smoke accounts for 1.2 million deaths among non-smokers.2 Thus, the tobacco epidemic represents one of the biggest threats for public health as a major but also entirely preventable cause of cardiovascular morbidity and mortality (summarized in Figure 1). Recent data indicate that in the last two decades global tobacco use has dropped from 1.397 billion in 2000 to 1.337 billion in 2018, which was largely driven by decreased numbers of female smokers (a decline of around 100 million).2 The WHO projects for the first time a decline of more than 1 million male smokers by 2020 compared to 2018.2
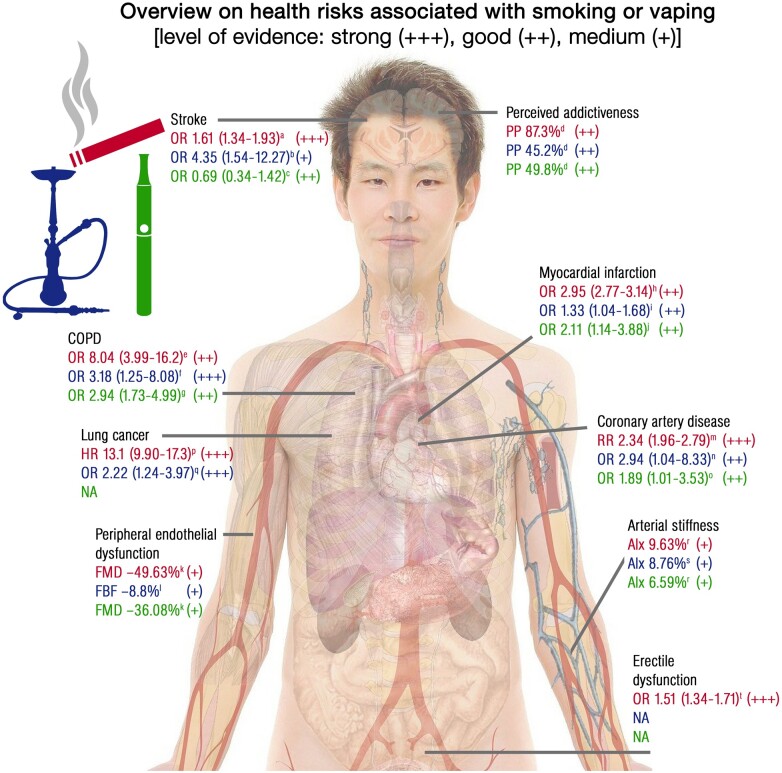
Figure 1.
Hazard/odds ratio (HR/OR) or adverse effects (percentages) for smoking or vaping associated health risks or complications based on selected representative studies. All alphabetical cross references in this figure (a, b, c…) are linked to literature reference numbers in the figure legend that can be found in the Supplementary material online with exactly identical numbering. Stroke source reports: a165 (+++), b166 (+), and c167 (++). Perceived addictiveness source reports: d168 (++). Percentages refer to the proportion of participants (PP) who believe that smoking/vaping is addictive. Chronic obstructive pulmonary disease (COPD) source reports: e169 (++), f170a (+++), and g170b (++) Myocardial infarction source reports: h,171 i,172 and j173 (++). Peripheral endothelial dysfunction source reports: k94 (+) and l143 (+). Percentages denote (k) relative difference in flow-mediated dilation (FMD) of the brachial artery in response to acute tobacco cigarette and e-cigarette use and (l) relative decrease in arterial forearm blood flow (FBF) in response to acute waterpipe smoking. Coronary artery disease source reports: m174 (+++), n175 (++), and o173 (++). Lung cancer source reports: p176 (+++) and q177 (+++). Arterial stiffness source reports: r178a (+) and s178b (+). Percentages denote relative increase in augmentation index (AIx) measured by tonometry in response to chronic tobacco cigarette and e-cigarette use or acute waterpipe smoking. Erectile dysfunction source reports: t179 (+++). NA means not available. + means medium (single cohort study <1000 subjects), ++ means good (single cohort study >1000 subjects) and +++ means strong (large scale meta-analysis) clinical evidence. Open access source for body image can be found at Pixabay (https://pixabay.com/de/photos/anatomie-mann-mensch-körper-haut-254129/). Note of caution: The presented values are based on selected representative studies with different quality levels of evidence highlighting the need for more research.
Despite such advances, the use of e-cigarettes and waterpipe products (shisha, hookah, narghile) is an emerging trend, especially among younger generations. The misperception that these products are substantially less harmful than tobacco cigarettes or even represent healthier alternatives, provides an explanation for this trend.3 The claim of being less harmful, the availability of countless ‘appealing’ flavours and the lack of regulations after their introduction to the broader market make e-cigarettes and waterpipe products an attractive gateway drug for adults and adolescents who have not previously smoked. Consequently, the number of e-cigarette and waterpipe users has dramatically increased, with e-cigarettes being the most commonly used smoking products in 2014 in the USA (>9-fold increase in usage from 2011 to 2015) and a projected global sales volume of US$26.84 billion by 2023.4–6 Also the prevalence of lifetime waterpipe use is of concern, ranging from 2.1% to 44.0% in the USA, 11.6% to 40.1% in the UK, and 20.0% to 28.9% in Germany.7 As a consequence, the USA recently announced a countrywide ban on flavoured e-cigarettes, which may also include the ban of flavoured waterpipe tobacco in the future, following the lead of other countries with a strict prohibition of the use and sale of e-cigarettes (see last sections for details).8
The mechanisms leading to cardiovascular diseases (CVDs) and mortality in smokers are multifactorial and not fully understood. Impaired endothelial function is an early pathophysiological biomarker in cigarette smokers as shown in 1993 by Celermajer et al.9 Importantly, endothelial dysfunction has been shown to be an early critical event in the pathogenesis of most CVD.10 , 11 In this review, we will discuss the current state of literature on how smoking and/or vaping induces endothelial dysfunction and atherogenesis with focus on tobacco cigarettes, e-cigarettes, and waterpipe.
Physiology and prognostic implications of endothelial function
The endothelium is fundamental in the regulation of vascular tone, inflammation, vascular growth, platelet aggregation, and coagulation.10 , 11 The endothelium produces important vasodilators with anti-atherosclerotic and antiaggregatory properties such as nitric oxide (•NO) and prostacyclin. Endothelial dysfunction (impaired biochemical pathways in endothelial cells) is a characteristic feature of coronary artery disease,12 and a predictor of atherosclerosis13 and future cardiovascular events.14 Endothelial dysfunction is associated with traditional cardiovascular risk factors such as age, smoking, diabetes mellitus, hyperlipidaemia, genetic predisposition, and arterial hypertension.10 , 11 Endothelial dysfunction in humans is mostly measured by flow-mediated dilation (FMD) in the forearm, a technique where the reinitiated blood flow after occlusion (ischaemia) for some minutes causes endothelial •NO formation and vasodilation by mechanical and reoxygenation-mediated stimuli (called hyperaemia).15 Although the underlying pathophysiological mechanisms of endothelial dysfunction are likely to be multifactorial, an increased production of reactive oxygen species (ROS) within the vascular wall by systems such as the nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase, the mitochondrial electron transport chain, and uncoupled endothelial nitric oxide synthase (eNOS) are thought to contribute to this phenomenon.10 , 11 , 16 The extremely fast, diffusion-limited reaction of •NO with superoxide leads to formation of the highly reactive oxidant peroxynitrite (ONOO−), that has vasoconstrictive and cytotoxic effects, causing oxidative damage to proteins, lipids, and DNA. Oxidative degradation of •NO by superoxide, uncoupling of eNOS and tyrosine nitration/inactivation of prostacyclin synthase are three particular examples of how oxidative stress impairs endothelial function.
(Toxic) compounds in tobacco cigarette smoke, e-cigarette vapour, and waterpipe smoke
The literature on toxic compounds found in tobacco cigarette smoke, e-cigarette vapour, and waterpipe smoke is vast. Here, we present only the most important toxic components (summarized in Table 1), while a detailed overview can be found in the Supplementary material online. Some basic facts on heat-not-burn tobacco products (e.g. iQOS), their regulation and data on their toxicity were reviewed previously17 and more details can be found in the Supplementary material online.
Table 1.
Toxic compounds in smoke from tobacco cigarettes and waterpipe, and vapour from e-cigarettes (all references in this table can be found in the Supplementary material online with exactly identical numbering)
| Toxic compound type | Toxic compound | Concentration range cigarette | Concentration range e-cigarette | Concentration range waterpipe |
|---|---|---|---|---|
| Carbonyls | Formaldehyde | 7–10 μg/puff2,32,180 | 0.12–82 µg/puff181–184 | 0.21–0.65 µg/puff185,186 |
| Acetaldehyde | 50–140 μg/puff2,32,180 | 0.2–53 µg/puff181–184 | 2.0–5.5 µg/puff185,186 | |
| Acrolein | 6–14 μg/puff2,32,180 | 0.12–3.3 µg/puff181–184,187 | 0.06–1.19 µg/puff185,186 | |
| Propionaldehyde | 0.4–5.9 µg/puff2,32,180 | 0.057–1.79 µg/puff181,182 | 0.05–1.06 µg/puff185,186 | |
| Crotonaldehyde | 1–2 μg/puff2,32,180 | ND–0.04 µg/puff188 | 0.78–1.39 µg/puff189 | |
| N-Nitrosamines | N′-Nitrosonornicotine (NNN) | 0.5–370 ng/puff2,32,180 | ND–0.029 ng/puff22,183,190 | 0.2 ng/puff185,191 |
| N′-Nitrosoanabasine (NAB) | ND–15 ng/puff2,32,180 | ND–0.01 ng/puff22,190 | 0.05 ng/puff185,191 | |
| 4-(Methylnitrosamino)-1-(3-pyridyl)- 1-butanone (NNK) | 1.2–77 ng/puff2,32,180 | ND–0.019 ng/puff22,183,190 | 0.27 ng/puff185,191 | |
| N′-nitrosoanatabine (NAT) | 0.8–16 ng/puff2,32,180 | ND–0.085 ng/puff22,190 | 0.6 ng/puff185,191 | |
| VOCs | Toluene | 0.8–6.9 µg/puff2,32,180 | ND–1.53 µg/puff192 | 0.058 µg/puff185,193 |
| Benzene | 0.6–4.5 µg/puff2,32,180 | ND–0.41 µg/puff192 | 1.58 µg/puff185,193 | |
| Inorganic compounds | Nickel | ND–60 ng/puff2,32,180 | 0.1–6.4 ng/puff194 | 9.9 ng/puff185,195 |
| Cobalt | 0.013–0.02 ng/puff2,32,180 | 0.05–0.58 ng/puff196 | 0.7 ng/puff185,195 | |
| Chromium | 0.4–7 ng/puff2,32,180 | 0.05–9 ng/puff194 | 13.4 ng/puff185,195 | |
| Lead | 3.4–8.5 ng/puff2,32,180 | 0.16–3.8 ng/puff197 | 68.7 ng/puff185,195 | |
| Carbon monoxide (CO) | 1–2.3 mg puff2,32,180 | not applicable | 1.15–1.67 mg/puff40,185,186 | |
| PAHs | Benz[a]anthracene | 2-7 ng/puff2,32,180 | Not applicablea | 1.3–1.6 ng/puff40,185,191 |
| Benzo[b + k]fluoranthene | 1–3.4 ng/puff2,32,180 | Not applicablea | 0.13–2.16 ng/puff40,185,191 | |
| Benzo[a]pyrene | 2–4 ng/puff2,32,180 | Not applicablea | 0.78–1.79 ng/puff40,185,191 | |
| Dibenzo[a, h]anthracene | 0.06–0.4 ng/puff2,32,180 | Not applicablea | 0.86–ng/puff40,185,191 | |
| Nicotine | 0.1–0.3 mg/puff2,32,180 | 0–0.142 mg/puff196 | 0–0.058 mg/puff185,186,198 | |
| Particulate matter | TPM | 0.1–1.7 mg/puff32 | 0.87–5.8 mg/puff199,200,b | 1.8–9.3 mg/puff40,198,201 |
PAHs, polycyclic aromatic hydrocarbons and heterocyclic aromatic hydrocarbons; VOCs, volatile organic compounds.
Only a few studies find concentrations of PAHs in e-cigarette vapour that are close to the limit of quantification.
Liquid aerosol droplets.
Nicotine is the main reason why humans consume tobacco cigarettes and their alternatives. This alkaloid is known to increase blood pressure and heart rate, but it can also cause FMD due to the increased cardiac output.18 Effects of nicotine have been controversial since its consumption has been associated with lower incidence of Parkinson’s disease and Alzheimer’s disease,19 and also with protection against ulcerative colitis.20
Since classical tobacco cigarettes and waterpipe both burn tobacco, similar toxic compounds are produced. Most important classes of toxic compounds in tobacco-derived smoke are nitrosamines and polycyclic aromatic hydrocarbons. These two groups of compounds are known carcinogens and have been associated with many other complications including CVD.21 , 22 Tobacco specific nitrosamines have also been found in nicotine containing e-cigarette vapour.
E-cigarette liquid contains only nicotine, propylene glycol, and glycerine. Accordingly, most of the toxic compounds found in e-cigarette vapour are derived from the three ingredients. The main group of toxic compounds responsible for the observed health effects of e-cigarettes are carbonyl compounds. Toxic aldehydes and ketones that are generated as degradation products during heating of e-cigarette liquid have known negative health effects. Most notably, formaldehyde and acrolein exhibit their toxicity through adduct formation with proteins and DNA, causing oxidative stress and endoplasmic reticulum stress, mitochondrial dysfunction and inflammation.23
Other groups of toxic compounds found in both tobacco cigarette/waterpipe smoke and e-cigarette vapour are volatile organic compounds and inorganic compounds such as metals and carbon monoxide (CO). Carbon monoxide is a gaseous compound that is produced as a result of incomplete combustion of organic matter. Carbon monoxide is toxic to humans since it can irreversibly bind to haemoglobin and inhibit oxygen delivery and causes hypoxia.24 Although CO is toxic to humans, even at low concentrations, it is also an intrinsic signalling molecule produced by mammalian cells, with roles in vasodilation, regulation of leucocyte aggregation (anti-atherosclerotic effect), and reduction of ischaemia/reperfusion injury.25 Carbon monoxide can also play a role in ischaemic preconditioning, which could be one of the explanation for the ‘smoker’s paradox’.26 Since the effects of CO on the cardiovascular system are numerous and contradictory and have been explored before,27 we only summarized the main facts here and provide some more details in the Supplementary material online.
All of these compounds have known toxic effects on the human body including, but not limited to, inflammation, cancer, CVD, and impairment of cognitive ability.27–30
Details on the legal regulations of these toxic compounds as well as the selection criteria for the inclusion of literature on toxic compounds in tobacco smoke and e-cigarette vapour (Supplementary material online, Tables S1 and S2) can be found in the Supplementary material online.
Comparison of smoking/vaping patterns for tobacco cigarette, e-cigarette, and waterpipe
Smoking (puffing) topography is a set of parameters that describes how frequently and with what volume a person inhales during smoking. Smoking topographies of tobacco cigarette, e-cigarette, and waterpipe users are described in Table 2. When consuming their preferred device, users exhibit very different smoking topographies. Since puffing topography is an indirect method of determining exposure to smoke or vapour, the higher puff volumes and more frequent puffs observed in e-cigarette and waterpipe user could potentially increase the exposure to toxicants.33
Table 2.
Schematic construction of tobacco cigarette, e-cigarette, and waterpipe with their corresponding puffing topographies
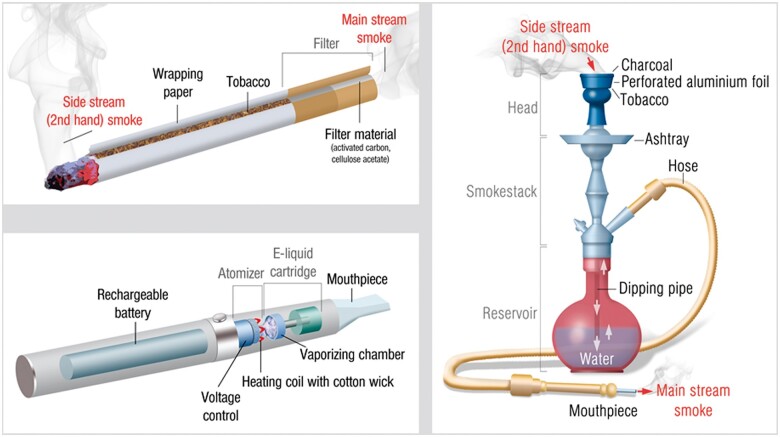
| ||
|---|---|---|
| Tobacco cigarette | Waterpipe | E-cigarette |
| Tobacco cigarette users display very little deviation in their smoking topography.31 This allowed for a standardized smoking protocol to be implemented in the form of an ISO 3308 standard (2 s puff duration, 35 mL puff volume, and 60 s duration between puffs). An average smoker consumes roughly between 10 and 20 cigarettes per day. | Waterpipe users usually attend only one smoking session per day which lasts for approximately 1 h. Puff volume, duration, and puffing frequency are all higher in waterpipe users in comparison to tobacco cigarette users. There is no ISO standard for waterpipe smoking, but most of the research groups use the ‘Beirut’ protocol (2.6 s puff duration, 530 mL puff volume, and 17 s duration between puffs).32 | E-cigarette users show a much more pronounced deviation in puffing parameters, making a standard vaping protocol hard to design.33 , 34 In 2019, an ISO 20768 standard was developed for routine analytical vaping experiments (3 s puff duration, 55 mL puff volume, and 30 s duration between puffs). |
Smoking/vaping and cardiovascular events
Over the last several decades, an undeniable body of evidence has emerged demonstrating the causal association between tobacco cigarette smoking and cardiovascular events including coronary heart disease, myocardial infarction, stroke, heart failure, and cardiovascular mortality.35 The relationship between tobacco cigarette smoking and CVD was first established in large epidemiological studies, in particular, the British Doctors Study36 and the Framingham Heart Study.37 These studies and the following examples provide evidence of a significant contribution of cigarette smoking on major atherosclerotic CVD outcomes and mortality. In the INTERHEART multicentre case–control study with 27 089 participants from 52 countries current smoking was associated with a higher risk of non-fatal acute myocardial infarction [odds ratio (OR) 2.95, 95% confidence interval (CI) 2.77–3-14] when compared with never smoking.38 The ARIC study showed (N = 13 355) over a median follow-up of 26 years a dose–response relationship between pack-years of smoking and the incidence of peripheral artery disease, coronary heart disease, and stroke.39 A meta-analysis of the association of cigarette smoking with cardiovascular mortality, including prospective studies from 25 cohorts (N = 503 905), found a hazard ratio of 2.07 (95% CI 1.82–2.36) and 1.37 (95% CI 1.25–1.49) for current and former smokers, respectively.40 Risk estimates in this study for acute coronary and stroke events followed a similar pattern. Also epigenetic changes may represent valuable risk markers in smokers as lower F2RL3 methylation was strongly related to higher mortality among a cohort of smokers with stable coronary heart disease41 (for more details see Supplementary material online).
Moreover, there is extensive evidence on the association between chronic waterpipe smoking and cardiovascular health.42 A recent systematic review and meta-analysis revealed an OR of 1.67 (95% CI 1.25–2.24) for the association of prevalent cardiovascular conditions such as ischaemic heart disease and heart failure with waterpipe smoking.43 In general, adverse cardiovascular effects of long-term waterpipe smoking are comparable to those associated with tobacco cigarette smoking, showing that chronic use exceeding 40 waterpipe-years is associated with a three-fold increase in the odds of coronary artery stenosis.44 Moreover, in a large prospective study (N = 20 033) from Bangladesh waterpipe smoking was shown to be associated with increased risk of death due to ischaemic heart disease in both men and women.45 More evidence for the adverse effects of waterpipe smoke on the heart was summarized in ref.46
Although there is a broad range of evidence for the adverse acute effects of e-cigarettes and their toxic properties on the cardiovascular system including oxidative stress and endothelial dysfunction,47 studies concerning the long-term use of e-cigarettes and CVD risk are limited and controversial. A large study based on cross-sectional NHIS data from 2016 (N = 33 028) and 2017 (N = 26 742) observed that some days use of e-cigarettes was associated with increased risk of myocardial infarction (OR 2.11, 95% CI 1.14–3.88) after adjustment for other cardiovascular risk factors.48 However, no statistically significant association was observed in the pooled analysis (daily e-cigarette use: OR 1.35, 95% CI 0.80–2.27, P = 0.267). Likewise, another study found daily e-cigarette use to be independently associated with increased odds of myocardial infarction (OR 1.79, 95% CI 1.20–2.66), as it was the case for daily tobacco cigarette smoking (OR 2.72, 95% CI 2.29–3.24).49 In contrast, former and some days use of e-cigarettes were not significantly associated with higher probability of myocardial infarction. In addition, a recent longitudinal study established an independent association between former and current e-cigarette use and incident respiratory disease after adjustment for tobacco cigarette smoking, demographic, and clinical variables.50 However, a recent systematic review and meta-analysis concluded that despite the adverse acute effects of e-cigarettes on heart rate, systolic, and diastolic blood pressure, benefits may occur concerning blood pressure regulation when switching from tobacco to chronic e-cigarette use.51
In line with this, a previous experimental study demonstrated that there was a significant improvement of endothelial function and vascular stiffness within 1 month of switching from tobacco to e-cigarettes in healthy smokers, whereas no clear trend in systolic blood pressure and heart rate was observed.52 This finding indicates that switching to e-cigarettes may reduce the CVD burden in former tobacco cigarette smokers. It is important to note that the majority of e-cigarette users are former smokers or dual users and that the availability of e-cigarettes may also promote the risk of smoking initiation in never smokers.53 Moreover, the potential efficacy of e-cigarettes as a cessation tool remains unclear. In a recent randomized trial (N = 886) evaluating different smoking cessation approaches, the 1-year abstinence rate in the e-cigarette group was 18.0% when compared with 9.9% in the nicotine replacement group.54 Among successful quitters, 80% of subjects in the e-cigarette group continued to use them a year later, compared to only 9% in the nicotine replacement group indicating that although the use of e-cigarettes was superior in terms of smoking cessation, quitting nicotine products was largely achieved in the nicotine replacement group.
Evidence of the association between smoking/vaping tobacco cigarette, e-cigarette, and waterpipe on vascular/endothelial function
A variety of different techniques have been employed to study the vascular consequences of smoking. Evidence for tobacco cigarette smoking-induced endothelial dysfunction and vascular damage stems from an array of studies, whereas data on the consequences of waterpipe and e-cigarette use are rather limited. Between and within these studies large differences in results may occur due to a variety of reasons such as differences in methods of measurements, time of assessment post-exposure, analysed populations, used tobacco/vaping products, and the amount and duration of inhaled smoke/vapour. Thus, difficulties in drawing absolute conclusions may arise, demanding caution in interpreting results. Here, a brief overview of the findings shall be provided. Supplementary material online, Table S3 summarizes selected studies to date that have provided insights on vascular/endothelial effects of smoking/vaping exposure.
Tobacco cigarettes
As mentioned before, Celermajer et al.9 provided early evidence that pack-years of smoking were dose-dependently associated with impaired FMD of the brachial artery. Likewise, long-term smoking was also associated with impaired endothelium-dependent coronary vasodilation.55 Importantly, decreased FMD of the brachial artery in chronic smokers was shown to increase by 1% after 1 year of smoking cessation.56 Impaired FMD was even measured in chronic smokers of light cigarettes (5.59%), showing no significant difference compared to chronic regular cigarette smokers (6.26%).57 Furthermore, smoking a single cigarette, irrespective of type, impaired FMD in chronic smokers, indicating analogous chronic, and acute effects. The association between exposure to second-hand smoke and FMD was assessed in further clinical studies revealing strong effects. Dose-dependent impairment of FMD in healthy young subjects in response to second-hand smoke exposure was reported,58 that may be reversible as demonstrated by a subsequent cross-sectional study.59 In that study, FMD in former and current passive smokers was significantly impaired when compared to non-smoking controls, while former passive smokers (5.1%) displayed better FMD than current passive smokers (2.3%).
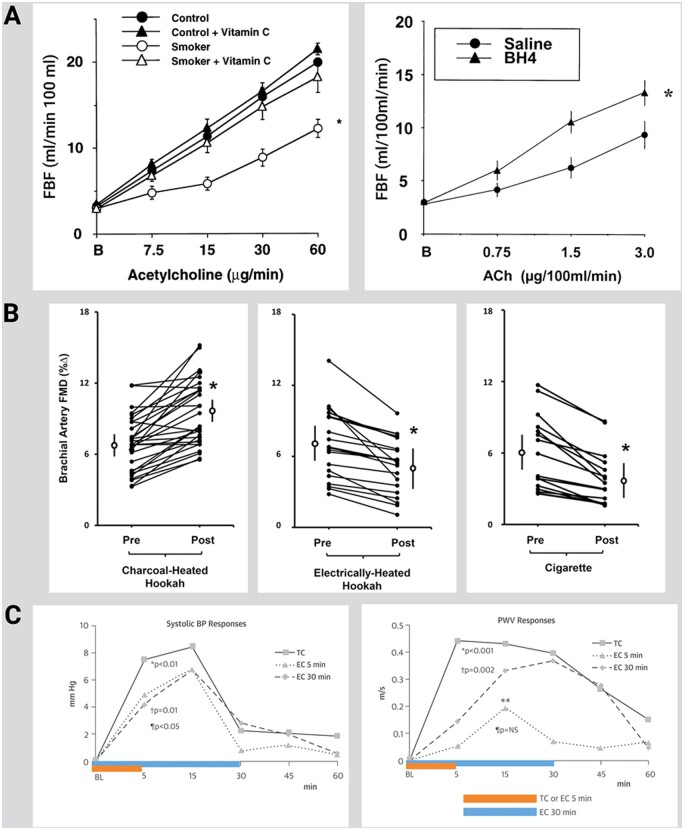
Heitzer et al.60 provided first evidence for the contribution of ROS formation and dysfunctional uncoupled eNOS in the pathogenesis of smoking-induced atherosclerosis. The authors showed that smoking strongly enhances endothelial dysfunction in patients with the cardiovascular risk factor hypercholesterolaemia and that the antioxidant vitamin C almost completely reversed endothelial dysfunction in chronic smokers, pointing to a crucial role of ROS in the decreased vascular NO bioavailability of endothelial dysfunction. The eNOS cofactor tetrahydrobiopterin (BH4, cofactor of eNOS), but not NH4 (tetrahydroneopterin)61 was associated with improved vasodilation in response to acetylcholine (Figure 2A), pointing to eNOS as a significant superoxide source in chronic smokers. In the ALSPAC study (n = 1266 teenagers) cigarette and alcohol use during adolescence showed additive cardiovascular health risk.64
Figure 2.
Effects of different forms of smoking and vaping on endothelial function in human subjects and antioxidant interventions. (A) Left, Vitamin C treatment markedly improves endothelium-dependent vasodilation to acetylcholine in chronic smokers while having no significant effect on the dose–response curve of control subjects. Mean ± SEM responses of forearm blood flow to intra-arterial acetylcholine in control subjects (n = 10) and chronic smokers (n = 10) with and without concomitant administration of vitamin C. *Significant difference in the overall dose–response relationship compared with control subjects with and without vitamin C and with smokers with vitamin C. Adopted from ref.60 with permission of the publisher. Copyright © 2000, Wolters Kluwer Health. Right, Effect of BH4 on ACh-induced vasodilation in chronic smokers. BH4 significantly improved ACh-mediated vasodilation in chronic smokers but failed to change the ACh dose–response relationship in control subjects (not shown). Data are presented as mean ± SEM. *Significant differences in the overall dose–response vs. saline treatment. Adopted from Ref.61 with permission of the publisher. Copyright © 2000, Wolters Kluwer Health. (B) Left, Individual and mean percentage changes before and after 30 minutes of charcoal-heated hookah smoking. Middle, Individual and mean percentage changes before and after 30 min of electrically heated hookah smoking. Right, Individual and mean percentage changes before and after smoking 1 cigarette. The circles with bars reflect the overall mean ± SEM. *P < 0.05 (pre- vs post-exposure). FMD indicates flow-mediated dilation. Adopted from ref.62 with permission of the publisher. Copyright © 2000, Wolters Kluwer Health. (C) Systolic BP (Left) and PWV (Right) responses. Each line represents response defined as net TC/EC smoking effect minus sham procedure effect at each time point. BL means baseline; NS means non-significant. The P-values refer to the composite effect of TC/EC at 5 and 30 min vs. sham during the whole study duration. The composite effect of TC/EC vs. sham was determined by using mean pressure as covariate. *TC vs. sham, ¶EC at 5 min vs. sham, †EC at 30 min vs. sham, **P < 0.001, PWV change between EC 5 min session and sham session after 15 min smoking using the Student’s t-test for paired measures. Adopted from ref.63 with permission of the publisher. © 2016 by the American College of Cardiology Foundation.
Waterpipe smoke
Several harmful substances present in tobacco cigarette smoke are also present in waterpipe smoke, even exceeding those established in cigarette smoke. Importantly, a recent statement from the American Heart Association (AHA) concluded that the acute cardiorespiratory toxicity of a single session of waterpipe smoking is worse than smoking a single tobacco cigarette due to the significantly higher levels of cardiorespiratory toxicants such as heavy metals and particulate matter.65 This is particularly concerning as there are general misperceptions about waterpipe smoke’s addictive potential and adverse health effects including that a majority of users believe it is less harmful and addictive than tobacco cigarette smoking.65 In addition to changes in cardiac function and blood pressure regulation as seen for tobacco cigarette smoking, a 30-min waterpipe smoking session in healthy young smokers was associated with increased vascular resistance and decreased forearm blood flow.66 These results were later extended to subjects with lower physical activity and fitness level exhibiting heightened vascular responses, probably due to the lack of beneficial effects of physical activity on endothelial function.67 In contrast, 30-min acute exposure to waterpipe smoke was not associated with changes in microvascular endothelial function using the Endo-PAT technique, which may indicate differences in the pathophysiological characteristics of endothelial dysfunction between circulatory beds.68
Smoking electrically heated waterpipe, similar to tobacco cigarettes, acutely impaired FMD (−27%), whereas smoking charcoal heated waterpipe led to increased FMD (Figure 2B).62 The authors concluded that the acute endothelial dysfunction was masked by the potent vasodilator CO, generated by the combustion of charcoal. A case–control study of healthy subjects comparing endothelial function in users of chronic tobacco cigarettes vs. waterpipes vs. non-users showed that chronic waterpipe use (7.9%) was more detrimental to FMD than cigarette use (12%) compared to non-smoking (21.5%). These findings may be explained by differences in the amount of exposure since most waterpipe users smoked three to five sessions per day, while the number of cigarettes smoked per day was 10–20 for more than 5 years.69 These findings are to be contrasted to a subsequent study that observed that chronic waterpipe and cigarette smoking caused a similar worsening of FMD as well as similar alterations of C-reactive protein levels.70
E-cigarettes
While studies providing evidence for effects of long-term e-cigarette use on endothelial function are generally limited, more studies evaluating their short-term consequences have been conducted. Importantly, difficulties in drawing absolute conclusions based on current evidence arise from a variety of different approaches used in studies including differences in methods of endothelial function measurement, study population, device voltage, liquid composition (nicotine content and flavouring), and the amount and duration of vapour inhaled. However, the potential harmful effects of e-cigarette use are widely acknowledged as outlined in a recent editorial by Eissenberg et al.3 that also called for adequate evaluation of the current evidence on the harms of e-cigarettes. In agreement with this, a recent systematic review and meta-analysis concluded that e-cigarettes should not be labelled as a safe product with respect to the cardiovascular system, in part due to the evidence linking e-cigarettes to impaired endothelial function.51
One study compared the acute impact of tobacco vs. e-cigarettes with same nominal nicotine content in healthy smokers and non-smokers on endothelial function, •NO bioavailability, markers of oxidative stress, and vitamin E levels. Both tobacco and e-cigarettes were shown to increase markers of oxidative stress and to decrease FMD, •NO bioavailability, and vitamin E levels in smokers and non-smokers, displaying no significant difference between tobacco and e-cigarettes.71 Our previous work demonstrated that short-term e-cigarette use in healthy smokers caused marked impairment of endothelial function and an increase in arterial stiffness.72 Others demonstrated acute microvascular endothelial dysfunction measured by acetylcholine-mediated vasodilation in smokers along with increased markers of oxidative stress and arterial stiffness after exposure to e-cigarettes with nicotine, but not after e-cigarettes without nicotine.73 A similar impairment of vascular compliance was observed following acute tobacco or e-cigarette use in smokers (Figure 2C),63 while a study of women smokers found a significant difference in stiffness after smoking just one tobacco cigarette, but not after use of e-cigarettes.74 In healthy seldom smokers, 10 puffs of e-cigarette vapour inhalation caused an increase in circulating endothelial progenitor cells measured by flow cytometry, qualitatively analogous to the changes seen after smoking one tobacco cigarette.75 A recent study comparing the acute effects of heat-not-burn devices, e-cigarettes, and tobacco cigarettes in healthy smokers revealed that single use of any product was associated with impaired FMD where heat-not-burn and e-cigarettes were equally less harmful than tobacco cigarettes.76
Evidence on adverse effects of smoking/vaping on endothelial function from animal studies Table S4
Prolonged exposure to tobacco cigarette smoke impaired acetylcholine-dependent vascular relaxation and coronary blood flow in rats that was associated with increased plasma cholesterol levels. Two weeks of tobacco cigarette smoke exposure caused mild hypertension and endothelial dysfunction in mice by down-regulation of sirtuin-3 and increased mitochondrial ROS formation that was improved by overexpression of mitochondria-targeted catalase.77 Chronic cigarette smoking (up to 32 weeks) also decreased NO• bioavailability and caused cardiac remodelling in mice that was also associated with structural damage of the endothelium. Genetic deletion of the detoxifying enzyme glutathione-S-transferase in mice aggravated tobacco smoking-induced endothelial dysfunction and increased acrolein-protein-adduct formation. Exposure of rabbits to chronic passive smoking for several weeks impaired endothelium-dependent relaxation of isolated rabbit arteries and caused left ventricular hypertrophy, but did not affect neurogenic contractions. Also mainstream smoke exposure of rabbits for several weeks impaired endothelial function of vascular tissues that was either corrected by ascorbic acid therapy or aggravated by a hypercholesterolaemic diet.
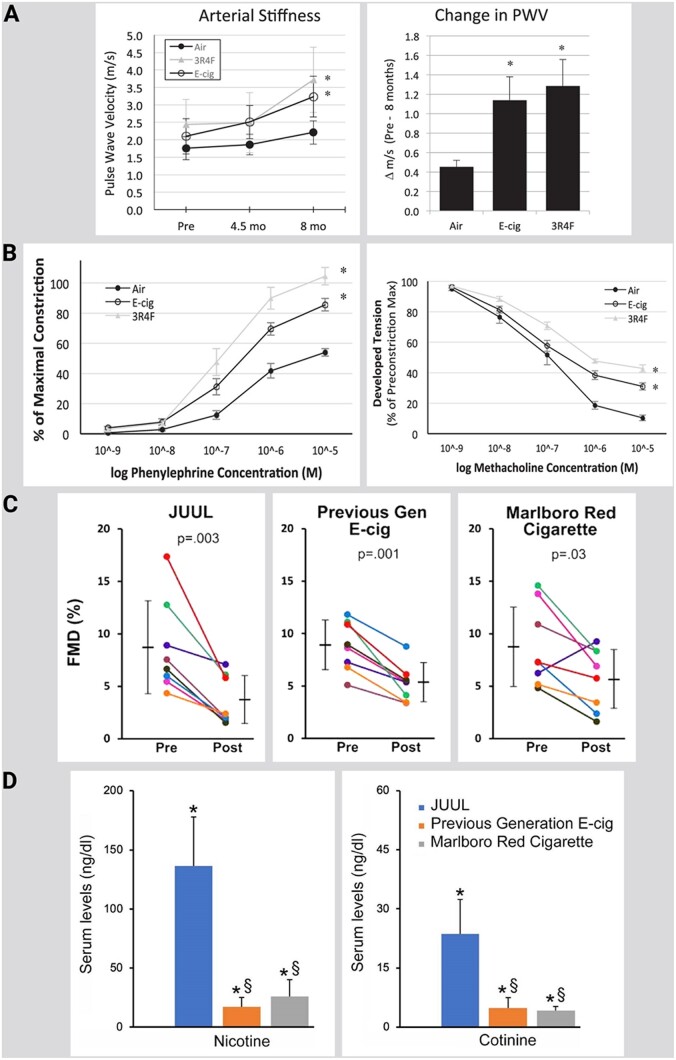
As indicated by our own results, e-cigarette vapour exposure (up to 5 days) caused endothelial dysfunction of isolated aortic segments of exposed mice that was associated with cardiovascular and cerebral oxidative stress, eNOS uncoupling, NOX-2 activation, endothelin-1 expression, as well as acrolein-adduct formation.72 These adverse effects were corrected by the endothelin-receptor blocker macitentan and genetic Nox2 deletion, as well as pharmacological NOX-2 inhibition. Studies on endothelial function in various animal models suggest comparable effects on smoking- or vaping-induced endothelial dysfunction. Also, chronic exposure of mice to e-cigarette vapour or tobacco smoke for 8 months induced a similar degree of arterial stiffness or endothelial dysfunction in isolated vessels (Figure 3A and B).78 Acute exposure of rats to the vapour of high nicotine containing e-cigarette liquid (JUUL), normal nicotine containing e-cigarette liquid, or traditional tobacco smoke (Marlboro red) resulted in similar decrease in endothelial function (Figure 3C and D).79 , 80 The nicotine uptake was measured by serum cotinine levels and correlated with FMD.
Figure 3.
Chronic and acute effects of different forms of smoking and vaping on endothelial function in animals. (A) B-mode Doppler ultrasound in vivo data from the carotid artery of mice under anaesthesia (inhaled isoflurane) before, during (at ∼4.5 months), and after 8 months of chronic exposure to electronic cigarette (E-cig) vapour and reference tobacco (3R4F) cigarette smoke. Left: significant increase in arterial stiffness [measured as pulse wave velocity (PWV)] for E-cig and 3R4F groups following 8-month exposure. Right: significantly greater change in PWV (translating to greater arterial stiffness) after 8 months in E-cig- and 3R4F-exposed than control (air-exposed) mice. Slight, non-significant, rise in PWV in control mice following 8 months is consistent with the normal aging effect. n = 5–8 mice/group. *P < 0.05 vs. air. (B) Ex vivo dose–response curves for phenylephrine (Left) and methacholine (Right), obtained from thoracic aorta ring segments following 8 months of exposure to E-cig vapour, reference tobacco (3R4F) cigarette smoke, and filtered air. α-Adrenergic vasoconstrictor response was greater (Left), while the endothelium-mediated vasodilatory response was impaired (Right), following 8 months of exposure to E-cig vapour and 3R4F cigarette smoke. Response to sodium nitroprusside was not altered or different between groups (not shown). n = 5 mice/group. *P < 0.05 vs. air. Adopted from ref.78 with permission of the publisher. Copyright © 2018, The American Physiological Society. (C) FMD was impaired by acute exposure to JUUL aerosol, previous generation e-cig aerosol, and Marlboro Red cigarette smoke. FMD after 5 min of exposure is shown. Coloured lines denote individual rats. Horizontal black bars denote the mean of the respective groups. P-values are derived from paired two-tailed t-tests. (D) Serum levels (ng/mL) of nicotine and cotinine from sera collected after 20 min of exposure. *P < 0.001 compared to air group. § P < 0.001 compared to JUUL. ‘Previous Gen’ means previous generation. Adopted from ref.79 Permission granted by Tobacco Regulatory Science Group to use figures. Rao P, Liu J, Springer ML. JUUL and combusted cigarettes comparably impair endothelial function. Tob Regul Sci 2020;6:30-37.
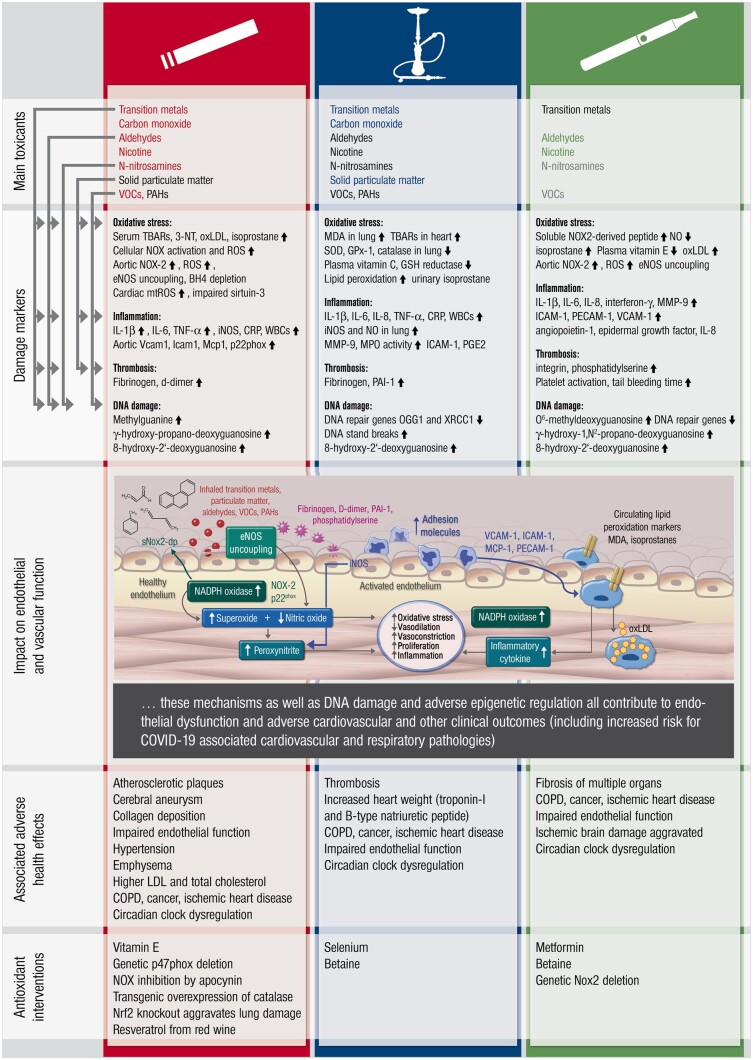
In summary, animal data suggest very similar extent of endothelial dysfunction by e-cigarette vaping or tobacco smoking, with oxidative stress and inflammation as central players of this process (Figure 4). These data are also in accordance with previous reviews on tobacco cigarette smoking,82 waterpipe smoking,42 , 83 and e-cigarette vaping.47 , 84 , 85 More details on the molecular mechanisms (e.g. the interplay of toxic compounds, oxidative stress and inflammation as well as epigenetic/circadian regulations) of the induction of endothelial dysfunction by e-cigarette vaping or tobacco smoking can be found in the Supplementary material online (summarized in Supplementary Table S4). Epigenetic regulations (e.g. via sirtuin-1) may also negatively affect endothelial function and life span of smokers, e.g. by activation of the mitochondrial adaptor protein p66Shc and subsequent mitochondrial dysfunction and oxidative stress.86
Figure 4.
Effects of different forms of smoking and vaping as summarized from human and animal studies. The major toxicants (red, blue and green = high quantity, black = intermediate quantity, grey = trace amounts) for tobacco cigarette and waterpipe smoking as well as e-cigarette vaping are listed. The molecular link of these toxicants on major damage markers reported for the different forms of smoking and vaping with respect to oxidative stress is shown on the left side. The effects of these toxicants on endothelial (vascular) function are summarized in the inserted scheme (modified from ref.81 with permission). The associated adverse health effects as well as antioxidant interventions are also shown.
Recommendations/regulatory updates for tobacco, e-cigarette, and waterpipe smoking
Most of the countries in the world have implemented some form of tobacco products regulation. In the USA, the FDA regulates all aspects of tobacco production and sales. In 2016, FDA has included waterpipe and e-cigarettes into the list of tobacco products, so they are regulated the same way as tobacco cigarettes. Although FDA allows sales of e-cigarettes, some organizations within the USA have called for their total ban. The Center for Disease Control (CDC) recommends that e-cigarettes of all kinds ‘never be used by youths’87 and the WHO expressed reservations about the value of e-cigarettes and grave concerns about their risks.88 The AHA has started a campaign to ban all e-cigarette sales.89 The AHA claims that there is enough evidence associating e-cigarettes with teenager’s addiction to nicotine and with seduction of non-smokers to smoking. A recent ‘epidemic’ of lung injury, and even deaths, resulting from use of e-cigarettes has caught attention of many health organizations.90 Although the majority of the reported cases of lung injury have been associated with use of e-cigarettes for THC consumption as well as vitamin E additives, the CDC has responded by recommending that people should avoid vaping altogether.91
Countries belonging to the WHO Framework Convention on Tobacco Control (FCTC—181 countries, excluding USA) have all agreed that they will implement the recommended guidelines on protection of public health policies, protection from exposure to tobacco smoke, regulation of tobacco products, advertising, sales, and also for reduction measures concerning tobacco dependence and cessation.92 The WHO does not have the power to create laws, but most of its member countries have implemented their recommendations to some extent. In the European Union (EU), tobacco products and smoking are heavily regulated. In most countries, there is a ban on smoking in the workplace and enclosed public spaces. Some countries (such as Hungary, UK, and Ireland) have a total ban on smoking in bars and hotels, while other countries (such as France, Germany, and Italy) allow for a designated smoking area in bars and hotels. Some countries have even banned smoking in outdoor areas near to hospitals, children’s playgrounds, and bus stops.93
Nevertheless, the position of Europe in particular with respect to e-cigarettes is not consistent at all. In the UK, the National Health Service (NHS) and the British Heart Foundation (BHF) strongly support the use of e-cigarettes in order to quit tobacco smoking. According to a 2019 YouGov survey,94 more than 3.6 million adults in Great Britain use e-cigarettes ∼7.1% of the adult population. Of these users, 54% are ex-smokers, suggesting they are trying to stop smoking. It is proposed that it might be easier to quit with an e-cigarette. They also recommend that any smoker with a heart condition should try e-cigarettes in case he has tried to quit in the past, and failed. They propose that it might be a lot easier to quit smoking with an e-cigarette although they are probably not completely safe.
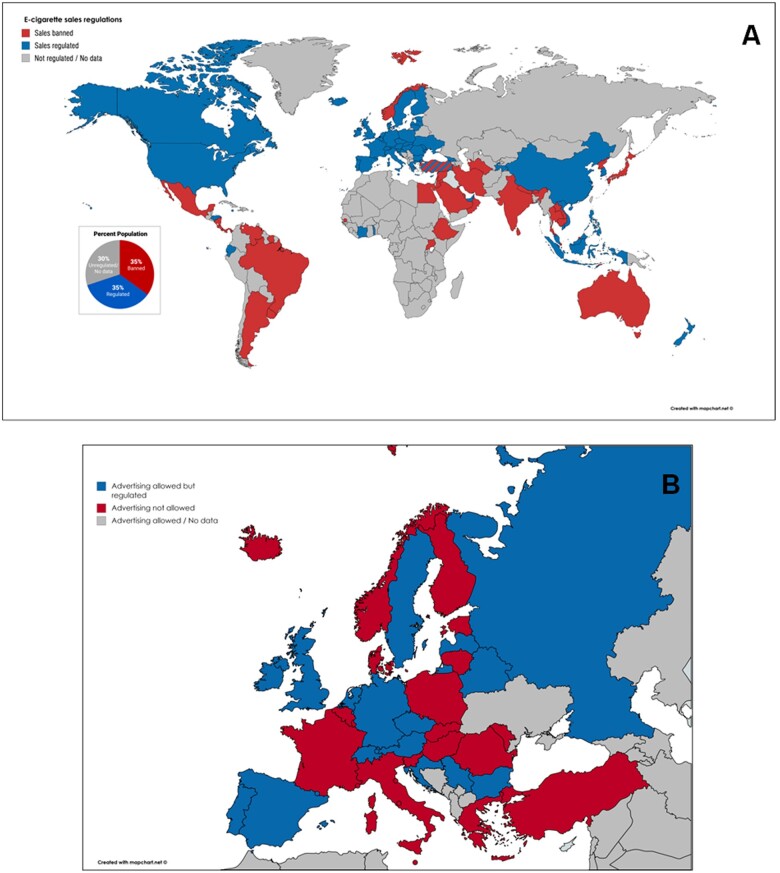
Waterpipe is considered a tobacco product in most countries and it also has to follow the no-smoking laws and regulations. Waterpipe bars are prohibited in some countries but can operate in others. Some countries have specific regulations for smoking establishments (usually no food or drinks can be served), and some treat them as regular smoking areas within a bar. E-cigarettes have been legally recognized as tobacco products in most countries, although individual countries regulate them differently. In some countries that ban indoor smoking (such as UK and Ireland), e-cigarettes could be used to circumvent no-smoking laws, since the ban does not apply to them. According to the 2019 report from the Global Center for Good Governance in Tobacco Control (GGTC), e-cigarette sales are banned in 42 countries and regulated in 56 countries.95 The sales regulation by country is shown in Figure 5A. The 42 countries that have banned e-cigarette sales, represent 35% of the world population with another 35% living in the countries where sales are regulated. Some countries, such as Turkey, have issued a statement that they will completely ban all e-cigarette sales, but haven’t done so at the present.
Figure 5.
Overview on global regulations on e-cigarette products sales (A) and on European regulations on tobacco product advertising (B). Maps were created using data from www.globaltobaccocontrol.org, www.tobaccocontrollaws.org and https://ggtc.world (last accessed 31/05/2020).
Advertising
In the EU, TV and radio advertising of cigarettes, and tobacco products is not permitted. Some countries (such as Slovenia and Norway) have strict laws that ban all types of advertising, even at the place of sale. Germany is unfortunately the only EU country where tobacco products, including e-cigarettes, can be advertised in public spaces via billboards. Also in the US advertising of both tobacco products and e-cigarettes through the medium of billboards is allowed. E-cigarettes are mostly considered as tobacco products, but the regulations on their advertising are not always clear. The map of Europe shows in which country advertising of e-cigarettes is legal (Figure 5B).
Impact of coronavirus disease 2019 (COVID-19) on cardiovascular risk in smokers and vapers
Recently, evidence has emerged indicating that tobacco use may increase the risk of adverse health outcomes in coronavirus disease 2019 (COVID-19) patients. As outlined by the WHO, tobacco cigarette and waterpipe smoking may contribute to increased burden of symptoms due to COVID-19 compared to non-smoking, including being admitted to intensive care, requiring mechanical ventilation, and suffering severe health consequences.96 Since smoking per se is a well-established risk factor for respiratory infections and increases the probability of having pre-existing conditions such as CVD, it could make COVID-19 patients more susceptible to severe symptoms, thus leading to increased mortality. Accordingly, considering the potential acute pulmonary and cardiovascular toxicity of e-cigarettes, the use of these products may put patients at higher risk of severe illness from COVID-19.97 As a potential mechanistic basis for COVID-19 associated cardiovascular complications, recent data by electron microscopy revealed viral inclusion structures in endothelial cells leading to endotheliitis that was associated with an accumulation of inflammatory cells in the endothelium as well as apoptotic bodies in the heart,98 with the consequence of impaired microcirculatory function. The authors explain that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects the host using the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed in several organs, including the lung, heart, kidney, and the intestine. Of note, ACE2 is also expressed by endothelial cells.99 Thus, the endothelium should be targeted with antiviral substances and cardiovascular drugs with anti-inflammatory pleiotropic effects such as angiotensin-converting enzyme inhibitors and statins. In addition, this therapeutic strategy may be implemented mainly in subjects with already existing endothelial dysfunction due to male sex, smoking, hypertension, diabetes, obesity, and established CVD, all of which are associated with adverse outcomes in COVID-19.98
Conclusions, clinical implications and gaps in current knowledge
Taken together, there is no doubt tobacco cigarette smoke has severe cardiovascular side effects leading to endothelial dysfunction, increased oxidative stress, and increased cardiovascular morbidity and mortality. There is also evidence that e-cigarette vapour is less toxic than tobacco smoke. Nevertheless, acute e-cigarette smoking increases blood pressure, causes endothelial dysfunction and increases vascular and cerebral oxidative stress. Despite being less toxic, the proposed 95% less harmful assertion by the Public Health England and the Royal College of Physician should be rapidly re-evaluated due to the growing body of hard evidence regarding harm caused by e-cigarettes. Waterpipe smoking is not less harmful than tobacco smoking and thus cannot be considered a healthy alternative. Hookah smoke has significant cardiovascular side effects, causes endothelial dysfunction, oxidative stress within the vasculature and arterial hypertension. The greater smoke volumes expelled from waterpipe sessions may lead to even higher exposure to toxicants as compared to tobacco cigarette smoking. In general, the increased use of e-cigarettes and waterpipe is concerning and as recently recommended broader tobacco control efforts by raising tobacco taxes, adopting smoke-free laws, conducting mass media campaigns, and restricting tobacco marketing should be implemented for better health protection of the general population.100 Future research should focus in particular on the long-term adverse effects of e-cigarette and waterpipe smoking on the cardiovascular system or respiratory diseases and cancer, as strong evidence is still missing (summarized in Figure 1). In particular, individuals exposed to second-hand smoke will benefit from any increased knowledge concerning the impact on CVD from tobacco/waterpipe (shisha) and e-cigarette smoking/vaping. There is no doubt, however, that smoking cessation is and will remain the most powerful approach to prevent smoking-induced cardiovascular and respiratory disease.101 This may be even more important in light of the actual COVID-19 pandemic as it increases the risk for COVID-19 associated cardiovascular and other severe complications in smokers and vapers.
Supplementary Material
Acknowledgements
We are indebted to the expert graphical assistance of Margot Neuser.
Funding
A.D. and T.M. were supported by vascular biology research grants from the Boehringer Ingelheim Foundation for the collaborative research group ‘Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutics’. Our research was continuously supported by Foundation Heart of Mainz. T.M. is PI of the DZHK (German Center for Cardiovascular Research), Partner Site Rhine-Main, Mainz, Germany.
Conflict of interest: none declared.
Contributor Information
Thomas Münzel, Center for Cardiology—Cardiology I, University Medical Center of the Johannes Gutenberg-University Mainz, Langenbeckstraße 1, 55131 Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine-Main, Langenbeckstraße 1, 55131 Mainz, Germany.
Omar Hahad, Center for Cardiology—Cardiology I, University Medical Center of the Johannes Gutenberg-University Mainz, Langenbeckstraße 1, 55131 Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine-Main, Langenbeckstraße 1, 55131 Mainz, Germany.
Marin Kuntic, Center for Cardiology—Cardiology I, University Medical Center of the Johannes Gutenberg-University Mainz, Langenbeckstraße 1, 55131 Mainz, Germany.
John F Keaney, Jr, Division of Cardiovascular Medicine, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655, USA.
John E Deanfield, Institute of Cardiovascular Science, University College London, 1 St Martin’s le Grand, London EC1A 4NP, UK.
Andreas Daiber, Center for Cardiology—Cardiology I, University Medical Center of the Johannes Gutenberg-University Mainz, Langenbeckstraße 1, 55131 Mainz, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Rhine-Main, Langenbeckstraße 1, 55131 Mainz, Germany.
References
- 1.World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2015. Geneva; 2015. https://apps.who.int/iris/bitstream/handle/10665/156262/9789241564922_eng.pdf?sequence=1 (31 May 2020).
- 2.World Health Organization. WHO global report on trends in prevalence of tobacco use 2000-2025, 3rd ed. Geneva; 2019. https://www.who.int/publications-detail/who-global-report-on-trends-in-prevalence-of-tobacco-use-2000-2025-third-edition (31 May 2020).
- 3. Eissenberg T, Bhatnagar A, Chapman S, Jordt SE, Shihadeh A, Soule EK. Invalidity of an oft-cited estimate of the relative harms of electronic cigarettes. Am J Public Health 2020;110:161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014;129:1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corey CG, Ambrose BK, Apelberg BJ, King BA. Flavored tobacco product use among middle and high school students–United States, 2014. MMWR Morb Mortal Wkly Rep 2015;64:1066–1070. [DOI] [PubMed] [Google Scholar]
- 6.Electronic cigarette market by product type, flavor and distribution channel—global opportunity analysis and industry forecast, 2017-2023. 2018. https://www.researchandmarkets.com/research/pjkd84/global_electronic? w=5 (31 May 2020).
- 7. Jawad M, Charide R, Waziry R, Darzi A, Ballout RA, Akl EA. The prevalence and trends of waterpipe tobacco smoking: a systematic review. PLoS One 2018;13:e0192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint. 2020. https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children (31 May 2020).
- 9. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149–2155. [DOI] [PubMed] [Google Scholar]
- 10. Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Munzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol 2017;174:1591–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med 2008;40:180–196. [DOI] [PubMed] [Google Scholar]
- 12. Gori T, Muxel S, Damaske A, Radmacher MC, Fasola F, Schaefer S, Schulz A, Jabs A, Parker JD, Munzel T. Endothelial function assessment: flow-mediated dilation and constriction provide different and complementary information on the presence of coronary artery disease. Eur Heart J 2012;33:363–371. [DOI] [PubMed] [Google Scholar]
- 13. Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990;323:22–27. [DOI] [PubMed] [Google Scholar]
- 14. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation 2002;105:1567–1572. [DOI] [PubMed] [Google Scholar]
- 15. Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T, Green DJ, Ghiadoni L. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 2019;40:2534–2547. [DOI] [PubMed] [Google Scholar]
- 16. Forstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 2017;120:713–735. [DOI] [PubMed] [Google Scholar]
- 17. Jankowski M, Brożek G, Lawson J, Skoczyński S, Majek P, Zejda J. New ideas, old problems? Heated tobacco products—a systematic review. Int J Occup Med Environ Health 2019;32:595–634. [DOI] [PubMed] [Google Scholar]
- 18. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med 2016;26:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD. Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin 2009;30:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guslandi M. Nicotine treatment for ulcerative colitis. Br J Clin Pharmacol 1999;48:481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, Kyrklund T, Rannug A, Tornqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 2002;110:451–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konstantinou E, Fotopoulou F, Drosos A, Dimakopoulou N, Zagoriti Z, Niarchos A, Makrynioti D, Kouretas D, Farsalinos K, Lagoumintzis G, Poulas K. Tobacco-specific nitrosamines: a literature review. Food Chem Toxicol 2018;118:198–203. [DOI] [PubMed] [Google Scholar]
- 23. Moghe A, Ghare S, Lamoreau B, Mohammad M, Barve S, McClain C, Joshi-Barve S. Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol Sci 2015;143:242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prockop LD, Chichkova RI. Carbon monoxide intoxication: an updated review. J Neurol Sci 2007;262:122–130. [DOI] [PubMed] [Google Scholar]
- 25. Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol 2006;46:411–449. [DOI] [PubMed] [Google Scholar]
- 26. Stein AB, Bolli R, Dawn B, Sanganalmath SK, Zhu Y, Wang OL, Guo Y, Motterlini R, Xuan YT. Carbon monoxide induces a late preconditioning-mimetic cardioprotective and antiapoptotic milieu in the myocardium. J Mol Cell Cardiol 2012;52:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim HH, Choi S. Therapeutic aspects of carbon monoxide in cardiovascular disease. Int J Mol Sci 2018;19:2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McHale CM, Zhang L, Smith MT. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis 2012;33:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Camara-Lemarroy CR, Rodríguez-Gutiérrez R, Monreal-Robles R, González-González JG. Acute toluene intoxication–clinical presentation, management and prognosis: a prospective observational study. BMC Emerg Med 2015;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl 2012;101:133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burling TA, Stitzer ML, Bigelow GE, Mead AM. Smoking topography and carbon monoxide levels in smokers. Addict Behav 1985;10:319–323. [DOI] [PubMed] [Google Scholar]
- 32. Shihadeh A, Azar S, Antonios C, Haddad A. Towards a topographical model of narghile water-pipe cafe smoking: a pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol Biochem Behav 2004;79:75–82. [DOI] [PubMed] [Google Scholar]
- 33. Blank MD, Pearson J, Cobb CO, Felicione NJ, Hiler MM, Spindle TR, Breland A. What factors reliably predict electronic cigarette nicotine delivery? Tob Control 2019;doi: 10.1136/tobaccocontrol-2019-055193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic cigarette topography in the natural environment. PLoS One 2015;10:e0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress. A report of the surgeon general. 2014. https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm#report ( 31 May 2020). [PubMed]
- 36. Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. Br Med J 1954;1:1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doyle JT, Dawber TR, Kannel WB, Heslin AS, Kahn HA. Cigarette smoking and coronary heart disease. Combined experience of the Albany and Framingham studies. N Engl J Med 1962;266:796–801. [DOI] [PubMed] [Google Scholar]
- 38. Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, Zhang X, Yusuf S INTERHEART Study Investigators. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet 2006;368:647–658. [DOI] [PubMed] [Google Scholar]
- 39. Ding N, Sang Y, Chen J, Ballew SH, Kalbaugh CA, Salameh MJ, Blaha MJ, Allison M, Heiss G, Selvin E, Coresh J, Matsushita K. Cigarette smoking, smoking cessation, and long-term risk of 3 major atherosclerotic diseases. J Am Coll Cardiol 2019;74:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mons U, Muezzinler A, Gellert C, Schottker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O'Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H; CHANCES consortium. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350:h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breitling LP, Salzmann K, Rothenbacher D, Burwinkel B, Brenner H. Smoking, F2RL3 methylation, and prognosis in stable coronary heart disease. Eur Heart J 2012;33:2841–2848. [DOI] [PubMed] [Google Scholar]
- 42. Qasim H, Alarabi AB, Alzoubi KH, Karim ZA, Alshbool FZ, Khasawneh FT. The effects of hookah/waterpipe smoking on general health and the cardiovascular system. Environ Health Prev Med 2019;24:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waziry R, Jawad M, Ballout RA, Al Akel M, Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol 2017;46:32–43. [DOI] [PubMed] [Google Scholar]
- 44. Sibai AM, Tohme RA, Almedawar MM, Itani T, Yassine SI, Nohra EA, Isma'eel HA. Lifetime cumulative exposure to waterpipe smoking is associated with coronary artery disease. Atherosclerosis 2014;234:454–460. [DOI] [PubMed] [Google Scholar]
- 45. Wu F, Chen Y, Parvez F, Segers S, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Ahsan H. A prospective study of tobacco smoking and mortality in Bangladesh. PLoS One 2013;8:e58516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sudano I, Barthelmes J. Is waterpipe-smoking bad for your heart? Eur Heart J 2018;39:3016–3017. [DOI] [PubMed] [Google Scholar]
- 47. Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. Impact of electronic cigarettes on the cardiovascular system. J Am Heart Assoc 2017;6:e006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farsalinos KE, Polosa R, Cibella F, Niaura R. Is e-cigarette use associated with coronary heart disease and myocardial infarction? Insights from the 2016 and 2017 National Health Interview Surveys. Ther Adv Chronic Dis 2019;10:204062231987774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alzahrani T, Pena I, Temesgen N, Glantz SA. Association between electronic cigarette use and myocardial infarction. Am J Prev Med 2018;55:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhatta DN, Glantz SA. Association of E-cigarette use with respiratory disease among adults: a longitudinal analysis. Am J Prev Med 2020;58:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, Charalambous G, Galiatsatos N, Vlachopoulos C, Tousoulis D. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol 2019;26:1219–1228. [DOI] [PubMed] [Google Scholar]
- 52. George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, Donnan PT, Khan F, Lang CC. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. J Am Coll Cardiol 2019;74:3112–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag 2019;15:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, Li J, Parrott S, Sasieni P, Dawkins L, Ross L, Goniewicz M, Wu Q, McRobbie HJ. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019;380:629–637. [DOI] [PubMed] [Google Scholar]
- 55. Zeiher AM, SchäChinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation 1995;92:1094–1100. [DOI] [PubMed] [Google Scholar]
- 56. Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol 2010;55:1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amato M, Frigerio B, Castelnuovo S, Ravani A, Sansaro D, Tremoli E, Squellerio I, Cavalca V, Veglia F, Sirtori CR, Werba JP, Baldassarre D. Effects of smoking regular or light cigarettes on brachial artery flow-mediated dilation. Atherosclerosis 2013;228:153–160. [DOI] [PubMed] [Google Scholar]
- 58. Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 1996;334:150–154. [DOI] [PubMed] [Google Scholar]
- 59. Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Celermajer DS. Arterial endothelial dysfunction related to passive smoking is potentially reversible in healthy young adults. Ann Intern Med 1999;130:578–581. [DOI] [PubMed] [Google Scholar]
- 60. Heitzer T, Just H¨R, MüNzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 1996;94:6–9. [DOI] [PubMed] [Google Scholar]
- 61. Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Munzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: evidence for a dysfunctional nitric oxide synthase. Circ Res 2000;86:E36–E41. [DOI] [PubMed] [Google Scholar]
- 62. Rezk-Hanna M, Mosenifar Z, Benowitz NL, Rader F, Rashid M, Davoren K, Moy NB, Doering L, Robbins W, Sarna L, Li N, Chang LC, Elashoff RM, Victor RG. High carbon monoxide levels from charcoal combustion mask acute endothelial dysfunction induced by hookah (waterpipe) smoking in young adults. Circulation 2019;139:2215–2224. [DOI] [PubMed] [Google Scholar]
- 63. Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, Stefanadis C, Tousoulis D. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol 2016;67:2802–2803. [DOI] [PubMed] [Google Scholar]
- 64. Charakida M, Georgiopoulos G, Dangardt F, Chiesa ST, Hughes AD, Rapala A, Davey Smith G, Lawlor D, Finer N, Deanfield JE. Early vascular damage from smoking and alcohol in teenage years: the ALSPAC study. Eur Heart J 2019;40:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bhatnagar A, Maziak W, Eissenberg T, Ward KD, Thurston G, King BA, Sutfin EL, Cobb CO, Griffiths M, Goldstein LB, Rezk-Hanna M. Water pipe (Hookah) smoking and cardiovascular disease risk: a scientific statement from the American Heart Association. Circulation 2019;139:e917–e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alomari MA, Khabour OF, Alzoubi KH, Shqair DM, Eissenberg T. Central and peripheral cardiovascular changes immediately after waterpipe smoking. Inhal Toxicol 2014;26:579–587. [DOI] [PubMed] [Google Scholar]
- 67. Alomari MA, Khabour OF, Alzoubi KH, Shqair DM, Stoner L. Acute vascular effects of waterpipe smoking: importance of physical activity and fitness status. Atherosclerosis 2015;240:472–476. [DOI] [PubMed] [Google Scholar]
- 68. Bentur L, Hellou E, Goldbart A, Pillar G, Monovich E, Salameh M, Scherb I, Bentur Y. Laboratory and clinical acute effects of active and passive indoor group water-pipe (narghile) smoking. Chest 2014;145:803–809. [DOI] [PubMed] [Google Scholar]
- 69. Selim GM, Elia RZ, El Bohey AS, El Meniawy KA. Effect of shisha vs. cigarette smoking on endothelial function by brachial artery duplex ultrasonography: an observational study. Anadolu Kardiyol Derg 2013;13:759–765. [DOI] [PubMed] [Google Scholar]
- 70. Diab OA, Abdelrahim EM, Esmail M. Effect of water pipe tobacco smoking on plasma high sensitivity C reactive protein level and endothelial function compared to cigarette smoking. Egypt Heart J 2015;67:233–241. [Google Scholar]
- 71. Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 2016;150:606–612. [DOI] [PubMed] [Google Scholar]
- 72. Kuntic M, Oelze M, Steven S, Kroller-Schon S, Stamm P, Kalinovic S, Frenis K, Vujacic-Mirski K, Jimenez B, Kvandova MT, Filippou M, Al Zuabi K, Bruckl A, Hahad V, Daub O, Varveri S, Gori F, Huesmann T, Hoffmann R, Schmidt T, Keaney FP, Daiber JF, Munzel A. T. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). Eur Heart J 2019;doi:10.1093/eurheartj/ehz772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chaumont M, de Becker B, Zaher W, Culie A, Deprez G, Melot C, Reye F, Van Antwerpen P, Delporte C, Debbas N, Boudjeltia KZ, van de Borne P. Differential effects of e-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep 2018;8:10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Szołtysek-Bołdys I, Sobczak A, Zielińska-Danch W, Bartoń A, Koszowski B, Kośmider L. Influence of inhaled nicotine source on arterial stiffness. Przegl Lek 2014;71:572–575. [PubMed] [Google Scholar]
- 75. Antoniewicz L, Bosson JA, Kuhl J, Abdel-Halim SM, Kiessling A, Mobarrez F, Lundbäck M. Electronic cigarettes increase endothelial progenitor cells in the blood of healthy volunteers. Atherosclerosis 2016;255:179–185. [DOI] [PubMed] [Google Scholar]
- 76. Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, Falco E, Chimenti I, Cammisotto V, Valenti V, Coluzzi F, Cavarretta E, Carrizzo A, Prati F, Carnevale R, Frati G. Acute effects of heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: the Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR - VAPES) 2 randomized trial. J Am Heart Assoc 2019;8:e010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dikalov S, Itani H, Richmond B, Arslanbaeva L, Vergeade A, Rahman SMJ, Boutaud O, Blackwell T, Massion PP, Harrison DG, Dikalova A. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am J Physiol Heart Circ Physiol 2019;316:H639–H646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, Mandler WK, Erdreich BH, Ducatman BS, Bryner RW, Dasgupta P, Chantler PD. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985) 2018;124:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rao P, Liu J, Springer ML. JUUL and combusted cigarettes comparably impair endothelial function. Tob Regul Sci 2020;6:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nabavizadeh P, Liu J, Havel CM, Ibrahim S, Derakhshandeh R, Jacob Iii P, Springer ML. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control 2018;27:s13–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017;38:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014;34:509–515. [DOI] [PubMed] [Google Scholar]
- 83. Golbidi S, Li H, Laher I. Oxidative stress: a unifying mechanism for cell damage induced by noise, (water-pipe) smoking, and emotional stress-therapeutic strategies targeting redox imbalance. Antioxid Redox Signal 2018;28:741–759. [DOI] [PubMed] [Google Scholar]
- 84. Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BA, Benowitz NL. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the American College of Cardiology. J Am Coll Cardiol 2015;66:1378–1391. [DOI] [PubMed] [Google Scholar]
- 85. Buchanan ND, Grimmer JA, Tanwar V, Schwieterman N, Mohler PJ, Wold LE. Cardiovascular risk of electronic cigarettes: a review of preclinical and clinical studies. Cardiovasc Res 2020;116:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi Y, Camici GG, Luscher TF. Cardiovascular determinants of life span. Pflugers Arch 2010;459:315–324. [DOI] [PubMed] [Google Scholar]
- 87.Centers for Disease Control and Prevention report. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease/faq/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ftobacco%2Fbasic_information%2Fe-cigarettes%2Fsevere-lung-disease%2Fneed-to-know%2Findex.html (31 May 2020).
- 88.WHO report. https://www.who.int/news-room/q-a-detail/e-cigarettes-how-risky-are-they (31 May 2020).
- 89.American Heart Association news item. 2019. https://pace-cme.org/2019/11/16/aha-launches-anti-e-cigarette-campaign-with-hashtag-quitlying-addressed-to-big-vape/. (31 May 2020).
- 90. Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde M, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin—preliminary report. N Engl J Med 2020;382:903–916. [DOI] [PubMed] [Google Scholar]
- 91.Centers for Disease Control and Prevention report. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (31 May 2020).
- 92.WHO report. https://www.who.int/fctc/treaty_instruments/adopted/en/ (31 May 2020).
- 93.European Commission report. https://ec.europa.eu/health/tobacco/smoke-free_environments_en (31 May 2020).
- 94.Action on Smoking and Health. https://ash.org.uk/wp-content/uploads/2019/09/Use-of-e-cigarettes-among-adults-2019.pdf (31 May 2020).
- 95.E-cigarette ban & regulation Global Status 2019. https://ggtc.world/dmdocuments/E-cigarette%20ban%26regulation-Global-Status-as-of-October-2019.pdf (31 May 2020).
- 96.World Health Organization. Tobacco and waterpipe use increases the risk of suffering from COVID-19. http://www.emro.who.int/tfi/know-the-truth/tobacco-and-waterpipe-users-are-at-increased-risk-of-covid-19-infection.html (31 May 2020).
- 97. Javelle E. Electronic cigarette and vaping should be discouraged during the new coronavirus SARS-CoV-2 pandemic. Arch Toxicol 2020;doi: 10.1007/s00204-020-02744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;39:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 100. Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1030–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; Group ESCSD. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.