Summary
Vitamin D deficiency is a pandemic disorder affecting over 1 billion of subjects worldwide and displaying a broad spectrum of implications on cardiovascular and inflammatory disorders. Since the initial reports of the association between hypovitaminosis D and COVID-19, Vitamin D has been pointed as a potentially interesting treatment for SARS-CoV-2 infection. We provide an overview on the current status of vitamin D deficiency, the mechanisms of action of vitamin D and the current literature on the topic, with a special focus on the potential implications for COVID-19 pandemic.
Background
SARS-CoV-2 is a beta coronavirus, it is a positive-sense, single-stranded RNA, enveloped virus that is 50–200 nm in diameter,1 probably derived from the bat and evolved to acquire the capability of human infection. Modes of transmission include droplet transmission, fecal–oral route, conjunctiva and fomites, either directly or through the contamination of objects with body fluids2 and the incubation period ranges from 2 to up to 14 days, leading to the presence of a large number of asymptomatic carriers favoring the spread of the virus.
The disease associated with SARS-CoV-2 infection, defined as COVID-19, is mainly represented by a bilateral interstitial pneumonia. In fact, the virus enters the respiratory cells through the angiotensin-converting enzyme 2 (ACE-2) receptor on type II pneumocytes.3 Clinical manifestations include a constellation of symptoms as fever, dyspnea, dry cough and more uncommonly (<5%) gastrointestinal manifestations. The pathognomonic radiological findings are represented by bilateral lung glassy opacities at thoracic CT scan.
The latest WHO data, updated at 1 May, reported 3 090 445 confirmed cases worldwide since the start of the outbreak, from 208 countries. Countries with the highest mortality rates are Italy, Spain, Iran, France and the USA,4 where it has reached proportions of >10% of the cases. In Italy, over 13% of the patients with a confirmed positive test have died for COVID-19.
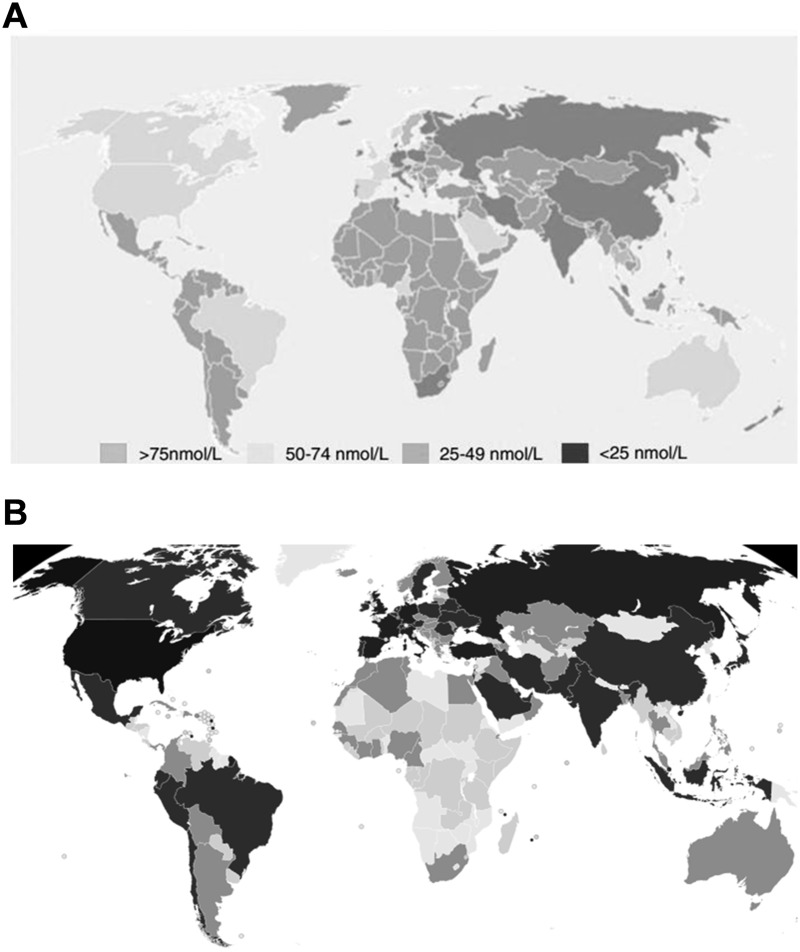
Despite the modest evidence on the topic, several studies have documented a negative prognosis and a higher vulnerability to the virus among older persons and those presenting with co-morbidities. In fact, although the presentation of COVID-19 is predominantly mild and asymptomatic in the age group <14 years, a more aggressive pulmonary damage, predisposing to the development of complications (acute respiratory distress syndrome (ARDS), septic shock) and death, has been described in advanced age and more fragile subsets of patients. In fact, patients dying for COVID-19 were at least 15 years older than survivors and in over 75% of the cases presented more than two associated medical conditions (https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf). The leading comorbid conditions reported included hypertension (about 70% of cases), cardiovascular disease, diabetes, obesity and a prior lung disorder, conditions communed by a more severe prevalence of vitamin D deficiency.5–7 In fact, previous studies have observed a significant relationships between vitamin D levels and the number COVID-19 cases and deaths, since the most vulnerable population and countries for COVID-19 are also the ones that have the most deficit in vitamin D, as shown in Figure 1.8,9 The aim of the current article is to provide an overview on the potential role of hypovitaminosis and the potential benefits from vitamin D supplementation during COVID-19 pandemic.
Figure 1.
Worldwide distribution of vitamin D deficiency in healthy population (A, upper graph, 9) and of COVID-19 pandemic (B, lower graph).
Vitamin D metabolism, mechanisms of action and prevalence of hypovitaminosis D
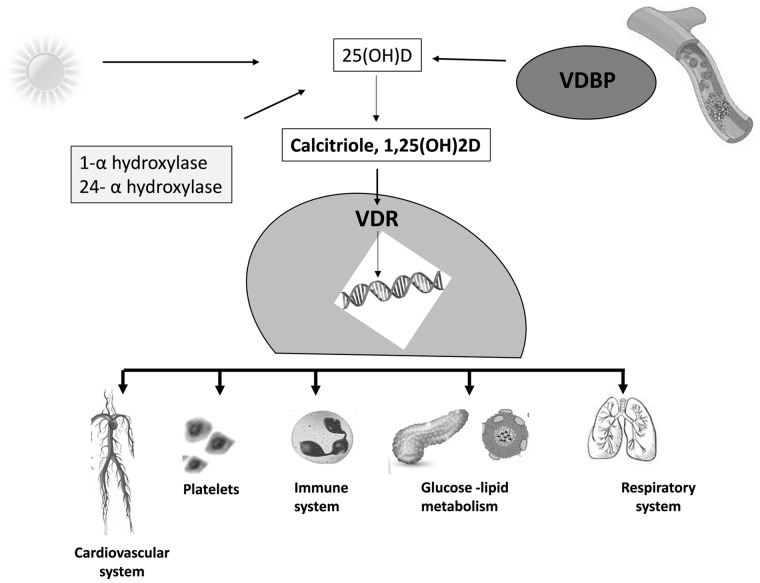
Vitamin D (25-hydroxycholecalciferol (25-(OH) D)) is a secosteroid that is mainly produced by the human body at the skin level, through sun exposure, and 20–30% introduced by the diet. Transformed by the organism into its active form (1,25-hydroxycholecalciferol or calcitriol (1,25(OH)2D)), it is not only the main regulator of calcium and bone homeostasis, but also a hormonal factor modulating about 3% of the human genome.10 Vitamin D receptor (VDR), in fact, is a nuclear factor, that is expressed in most of the organs and tissues and regulates genes transcription. The main pathways of vitamin D metabolism and function are displayed in Figure 2.
Figure 2.
Pathways of vitamin D metabolism and effectiveness.
About 85–90% of circulating vitamin D and its metabolites are linked to vitamin D-binding protein (VDBP), 10–15% to albumin, while generally <1%, is represented by the free active form.11
However, the steroid of cutaneous production requires two metabolic steps to become the biologically active hormone. The first occurs in the liver and consists of the hydroxylation of the vitamin in position 25 by the cytochrome P450 (CYP2R1), with the production of 25-OH D, that is the most represented form of circulating vitamin D. The second step involves another hydroxylation that takes place mainly in the kidneys via 1-α-hydroxylase, located in the cells of the proximal convoluted tubule and regulated by the parathyroid hormone. At this level, 25-OH D is converted into 1,25(OH)2D. However, in addition to being expressed by kidney cells, the enzyme 1-α-hydroxylase is also found in cardiomyocytes, endothelial cells and macrophages, capable of paracrine production of active vitamin D. On the contrary, the hydroxylation to 24,25(OH)2D produces an inactive form of vitamin D, which represents the major pathway of regulation of the effects of vitamin D.
The endocrine society task force defines vitamin D deficiency values of 25 (OH) D <20 ng/ml, insufficiency if between 21 and 29 ng/ml and sufficiency when ≥30 ng/ml.12 However, a severe deficiency (<10 ng/ml) is common, especially in certain higher-risk subsets of patients, despite the minimum levels for maintaining body homeostasis are still debated.
The progressive aging of the population, sedentary lifestyle, obesity, chronic diseases such as kidney failure and also pollution have raised the prevalence of vitamin D deficiency to pandemic proportions, that now affects over 1 billion people worldwide, with peaks of over 50% of healthy people deficient in vitamin D in certain countries.13,14
In Italy, several studies on vitamin D status have been performed over the past 20 years. Isaia et al.15 reported 25(OH)D circulating levels <12 ng/ml (30 nmol/l) in 76% of Italian women over 70 years of age; the InChianti study, collecting information about diet, sun exposure, disability, kidney function, levels of 25(OH)D and PTH, revealed values of serum vitamin D, on average, higher than 20 ng/ml in healthy adults, but significantly reduced in males over 60 years and females >50 years.16 In fact, cholecalciferol represents the most commonly prescribed among drugs reimbursed by the healthcare system in Italy, accounting for a cost raising from 24 million Euro in 2006 to over 270 million in 2018 (+16.9% since 2017 according to the data of the Italian healthcare system).
Beyond cholecalciferol: calcitriol, VDBP and genetic polymorphisms
Differential activation of vitamin D to calcitriol can be held responsible for the clinical manifestations of vitamin D deficiency, against comparable levels of 25(OH)D. Genetic variants of VDBP and of the 24-α hydroxylase responsible for vitamin D inactivation (CYP24A1) have been claimed for explaining over 10% of the variability of vitamin D levels.17 In fact, carriers of the G allele of rs7041 of VDBP; a variant associated with an enhanced binding of the vitamin to its transport-protein, have been shown to display an increased thrombogenicity despite dual anti-platelet therapy, when this genetic status was associated with vitamin D deficiency.18
The association between 25-OH D levels, VDBP gene polymorphisms and coronary heart disease risk was also analysed by the ARIC study,19 which found that the risk of developing coronary heart disease increased in white subjects with levels of 25(OH)D <17 ng/ml.
Despite 25(OH)D represents the most stable and addressed parameter, it cannot be considered an equivalent measurement when compared with 1,25(OH)2D, since several factors, including genetics, can affect its transformation into the active hormonal form. In a previous study among over 5000 patients with heart failure, Zittermann et al.20 showed that calcitriol is a better predictor of short- and midterm survival than 25(OH)D, probably because the serum concentration of calcitriol not only related to substrate availability, but also depend on renal function and inflammatory processes.
In addition, in a study by Verdoia et al.,21 the wild-type genotype for rs2762939 of CYP24A1, responsible for a higher enzymatic activity and calcitriol inactivation, was associated with coronary calcifications.
Furthermore, the possibility of a differential, autocrine or paracrine production of calcitriol in different body districts where vitamin D exerts its effects, according to a local necessity, represents an intriguing process that is still largely unexplored, potentially playing a relevant role in the pathogenesis and modulation of acute events, especially for the cardiovascular and inflammatory disease, as the endothelium and the immune cells are expressing these hydroxylases.22 In fact, an acute, non-genomic-dependent effect of vitamin D has also been described, mediated by the regulation of intracellular calcium, and therefore potentially interfering with muscular contraction, protein bindings, macrophages degranulations and vesicles transportation and platelet aggregation. However, these mechanisms are still largely unexplored.23
Vitamin D and the immune system
Vitamin D represents a hormone with a broad-spectrum effect, modulating the homeostasis of several organs and districts, in particular the immune system.
Vitamin D receptor has been identified in most immune cells, notably in macrophages, dendritic cells and activated T cells. Overall, 1,25(OH)2D controls inflammatory and immune responses keeping them within physiological boundaries.
Vitamin D, in fact, has been shown to lower the serum levels of acute-phase reaction proteins and proinflammatory cytokines, as TNF-α, interleukin (IL-6), hs-CRP and to favor an increase in the anti-inflammatory cytokine IL-10 and a shift of the lymphocytes subpopulations toward a TH2/T-reg differentiation.24,25 Moreover, Calcitriol induces the differentiation, phagocytic capacity and anti-microbial activity of macrophages, resulting in a less tolerogenic status to foreign antigens.26
Experimental evidence demonstrating modulation of immune and inflammatory cell differentiation and related cytokine release suggests important roles for vitamin D metabolites in the pathogenesis of atherosclerosis, and other inflammatory chronic and acute disorders, including autoimmune disease and infections.
In fact, higher levels of vitamin D are associated with reduced risk for developing and with a lower clinical activity of multiple sclerosis, gastrointestinal inflammatory disorders, type 1 diabetes and other immune-mediated disease.27
In addition, it has been suggested that vitamin D could attenuate the exacerbation of the immune response responsible of the complications of infectious disorders,28 as the ARDS, therefore playing a promising role even for the most recent COVID-19, although the latter hypothesis still deserves confirmation.
Vitamin D and atherothrombosis
The protective role of vitamin D in cardiovascular disease is well established. In fact, it has been involved in the pathogenesis of the main cardiovascular risk factors, such as arterial hypertension, diabetes mellitus, the alteration of lipid metabolism, and also in the onset of vascular calcifications, intimal thickening, ventricular hypertrophy and in the regulation of thrombotic processes.29,30 Furthermore, vitamin D can display antioxidant, anti-inflammatory anti-thrombotic properties, that lead to endothelial dysfunction, lipid deposition and the formation of atherosclerotic plaques.31
In fact, vitamin D can modulate the production of nitric oxide (NO), prevent the expression of endothelial proteins for the adhesion of leukocytes, lower tissue factor, downregulate the pro-thrombotic plasminogen activator inhibitor-1 and thrombospondin-1 mRNA expression and upregulate thrombomodulin.32
Numerous studies have previously reported the association between hypovitaminosis D and global and cardiovascular mortality,33 highlighting an inverse linear relationship between increased cardiovascular risk and vitamin D for each reduction of 10 ng/ml of 25(OH)D. In addition, Verdoia et al.6,34 previously showed that lower levels of vitamin D were associated with a higher prevalence and severity of coronary atherosclerosis and with enhanced platelet reactivity.
However, vitamin D supplementation has not provided, so far, a significant prognostic and cardiovascular benefit in cardiovascular prevention, especially among healthy subjects.
In a meta-analysis of 11 randomized trials, Mao et al.35 showed that vitamin D supplementation did not have an effect on major cardiovascular events, myocardial infarction or stroke and similar conclusions were reached by Zhang et al.36 Moreover, in the recent VITAL trial,37 randomizing over 25 000 healthy subjects to a supplementation with either n-3 fatty acid or vitamin D3 at a dose of 2000 IU/day or placebo and reporting no prognostic difference at a 5-year follow-up.
However, few well-designed, large-scale studies, powered for the evaluation of cardiovascular endpoints have been conducted so far, and especially among higher-risk patients with cardiovascular disease. In the randomized evaluation of calcium or vitamin D randomized controlled trial (RCT), treatment with cholecalciferol prevented cardiac failure among 5292 older people but did not appear to protect against MI or stroke.38 Indeed, more promising results could be drawn by directly addressing calcitriol when compared with vitamin D. Bonakdaran et al.39 reported that calcitriol supplementation could improve metabolic parameters and the control of cardiovascular risk factors among 119 patients with diabetes. Another in vitro study showed that calcitriol could downregulate pro-inflammatory gene expression without affecting angiogenesis and anti-inflammatory gene expression in vascular endothelial cells.40
Thus, the cardioprotective role vitamin D, preventing platelets activation and improving endothelial function, could result potentially relevant in COVID-19, where thrombosis and panvascular inflammation have been claimed as major determinants of the pulmonary and systemic complications of the infection.41
Vitamin D and renin–angiotensin system inhibitors
An additional important function of vitamin D is the modulation of the renin–angiotensin system (RAS). Both renin activity and hypertension have been found to be inversely associated with 25(OH)D levels in clinical observational studies and additional reports have suggested that oral supplementation of vitamin D could lead to a decrease in the blood pressure.42,43
Moreover, in a cross-sectional study, Forman et al.44 reported that individuals with vitamin D deficiency and insufficiency had greater plasma angiotensin II levels and a trend for higher plasma renin activity.
In fact, calcitriol is a negative endocrine regulator of the renin gene and it has been shown to downregulate the ACE-2.
Therefore, a potential interaction could be hypothesized between vitamin D and angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, representing commonly prescribed pharmacological treatments for hypertension and cardiovascular disease. In a previous study, rats treated with losartan during lactation showed alterations in renal function and structure, including hydronephrosis, papillary atrophy, endothelial dysfunction and aberrant endothelial structure, that were mitigated by the treatment with calcitriol.45 Similarly, Soares et al.46 among patients with end-stage renal failure, showed that the combination treatment of RASI with cholecalciferol could prevent the decline in glomerular filtration in the randomized VITAL Study paricalcitol was able to reduce albuminuria and blood pressure in patients with diabetic nephropathy who were already on RAS inhibitor therapy.47
This positive interaction could be of particular relevance among patients with COVID-19, where hypertension represents the most commonly associated comorbidity and angiotensin-converting enzyme inhibitor/angiotensin-receptor blockers the most common therapy. Furthermore, ACE-2 enzyme, the door for the viral infection and a mediator of acute lung injury in pulmonary infections,48 is hyper expressed among patients receiving RAS blockers, an event that could be counteracted by vitamin D.49
Evidence on vitamin D supplementation in sepsis and pulmonary disease
The modulatory effects of vitamin D on the immune and inflammatory response have emerged as a key point in respiratory and infectious disease.
Among 16 975 participants in NHANES III, 25(OH)D levels were inversely associated with history of community-acquired pneumonia and several additional studies and meta-analysis associated vitamin D deficiency with the risk of sepsis.50,51
Moreover, Dancer et al.52 showed that vitamin D deficiency resulted in exaggerated alveolar inflammation, epithelial damage and hypoxia in a murine model of ARDS and Park et al. reported that 25(OH)D3 levels were inversely related with length of hospital stay and intensive care unit stay among in-hospital survivors to ARDS.53
In a previous large-scale meta-analysis, vitamin D emerged as a beneficial add-on treatment for adult patients with asthma and a potent intervention to reduce exacerbations in patients with COPD.54 Additionally, the VITdAL-ICU randomized clinical trial showed lower hospital mortality with high-dose vitamin D in the severe vitamin D-deficient patients admitted to intensive care units.55
However, a large trial from the National Heart, Lung and Blood Institute PETAL Clinical Trials Network showed that a high-dose enteral vitamin D did not provide an advantage over placebo with respect to 90-day mortality or other, non-fatal outcomes among critically ill patients.56
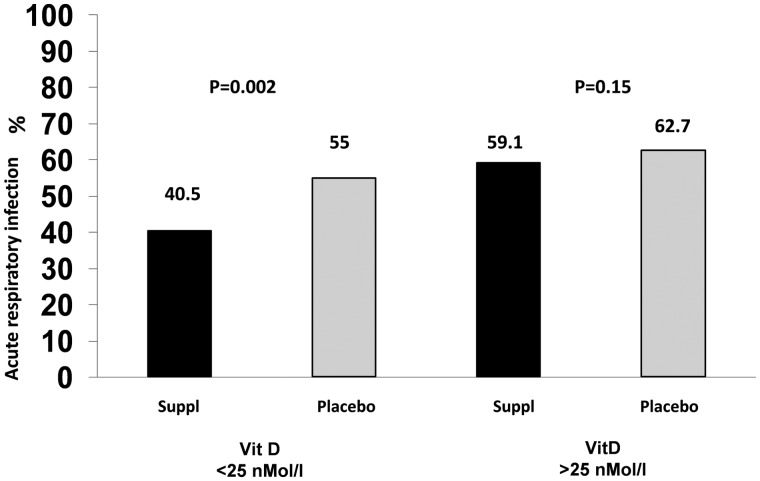
In a recent large-scale meta-analysis of individual participant data, Martineau et al.57 showed that vitamin D supplementation could lower the risk of acute respiratory infections, with the largest benefits being observed for daily/weekly vs. bolus doses and in patients who were very vitamin D deficient, where the risk of acute respiratory events was almost halved. Nevertheless, no data specific to the COVID-19 infection have been so far reported. Results are summarized in Figure 3.
Figure 3.
Impact of vitamin D supplementation on the risk of acute respiratory infections (meta-analysis data from Martineau et al., 57) (suppl = supplementation).
Preventive role of vitamin D supplementation during COVID pandemic
High cost-effectiveness, safety and tolerability are the key features of vitamin D supplementation. Therefore, it has been pointed as a promising preventive measure among healthy subjects, in particular accounting for the large prevalence of deficiency subjects and its beneficial effects on survival, quality of life and several chronic disorders, whose protective effects could be even more relevant in the context of COVID-19 pandemic.
The British Dietetic Association, in fact, currently recommends, in addition to lifestyle measures and sunlight exposure, to consider taking a daily supplement containing 10 µg to ensure a healthy vitamin D status (for adults and children over the age of one).
A similar conclusion has been reached in ‘The Irish Longitudinal Study on Ageing’, stating that vitamin D is a potent immune modifying micronutrient and if vitamin D status is sufficient, it could benefit vulnerable adults in particular those over 70 years, also considering the restrictions to outdoor living during the COVID-19 outbreak (https://tilda.tcd.ie/publications/reports/pdf/Report_Covid19VitaminD.pdf; 25 April 2020, date last accessed).
In addition to empowering the immune defense, in fact, vitamin D could prevent the possibility of access of the virus to respiratory cells, by lowering the ACE-2 enzyme expression.58 However, no evidence from dedicated studies currently supports such hypothesis.
Vitamin D supplementation for the treatment of SARS-CoV-2 infection
Since the initial reports of the association between hypovitaminosis D and COVID-19, vitamin D has been pointed as a potentially interesting treatment for SARS-CoV-2 infection, based on its established anti-inflammatory and anti-thrombotic properties, as summarized in Table 1.
Table 1.
Mechanisms of action of Vitamin D and potential interaction with SARS-CoV-2 infection
| Target site | Vitamin D mechanism | SARS-CoV-2 mechanism | Vitamin D effect |
|---|---|---|---|
| Immune system | |||
| Macrophage |
|
|
Anti-inflammatory, antioxidant |
| T lymphocytes |
|
||
| B lymphocytes |
|
||
| Cardiovascular system | |||
| Endothelial cells |
|
↑ Inflammation, endothelial damage |
|
| Cardiomyocytes |
|
Possible myocytes infection |
|
| Platelets |
|
|
↓ Thrombogenicity |
| Thrombosis |
|
↑ Inflammation, fibrinogen, TF, TNF-α | |
| Metabolism | |||
| Glucose |
|
Stress-induced hyperglycemia | ↓ Glycemia |
| Lipids |
|
Malnutrition, hyporexia, hepatic damage | ↓ Dyslipidemia |
| Blood pressure |
|
↓ Blood pressure and RAS-system | |
| Respiratory system | |||
| Lung cells | ↓ ACE-2 expression ↓ Bradykinins and cytokines |
|
|
Like tocilizumab, a drug with promising effects in the treatment of COVID-19, Vitamin D appears to modulate the activity of an IL-6, therefore reducing the acute-phase response associated to a larger pulmonary damage and complications as ARDS or sepsis.59
In addition, preventing malnutrition associated to hyporexia, which is common among infected people, and maintaining a diverse diet with a broad nutrient profile could aptly alleviate vulnerability to acute and chronic disease.
Ongoing RCTs and large population studies will certainly contribute to shed light on the potential benefits and the most effective therapeutic strategies for the management of patients acutely infected with SARS-CoV-2 virus (https://clinicaltrials.gov/ct2/show/NCT04334005; https://clinicaltrials.gov/ct2/show/NCT04344041).
Conflict of interest. None declared.
References
- 1. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020; 63:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kampf G, Todt D, Pfaender S, Steinmann E.. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song W, Gui M, Wang X, Xiang Y.. Xiang Y Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2 . PLOS Pathog 2018; 14:e1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html (22 March 2020, date last accessed).
- 5. Kakodkar P, Kaka N, Baig MA.. Comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus 2020; 12:e7560. doi: 10.7759/cureus.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Verdoia M, Schaffer A, Sartori C, Barbieri L, Cassetti E, Marino P, et al. Vitamin D deficiency is independently associated with the extent of coronary artery disease .Eur J Clin Invest 2014; 44:634–42. [DOI] [PubMed] [Google Scholar]
- 7. Nardin M, Verdoia M, Schaffer A, Barbieri L, Marino P, De Luca G.. Vitamin D status, diabetes mellitus and coronary artery disease in patients undergoing coronary angiography. Atherosclerosis 2016; 250:114–21. [DOI] [PubMed] [Google Scholar]
- 8. Illie PC, Stefanescu C, Smith L.. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 2020; 32:1195–8. doi: 10.21203/rs.3.rs-21211/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, et al. A global representation of vitamin D status in healthy populations. Arch Osteoporos 2012; 7:155–72. [DOI] [PubMed] [Google Scholar]
- 10. Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, et al. The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev 2012; 33:456–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schneider ALC, Lutsey PL, Selvin E, Mosley TH, Sharrett AR, Carson KA, et al. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Neurol 2015; 22:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holick MF, Binkley NC, Bischoff-Ferrari HA, ordon CM, Hanley DA, Heaney R, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–30. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 13. Palacios C, Gonzalez L.. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 2014; 144:138–45. doi: 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavie CJ, DiNicolantonio JJ, Milani RV, O'Keefe JH, Vitamin D, Health C.. Vitamin D and cardiovascular health. Circulation 2013; 128:2404–6. [DOI] [PubMed] [Google Scholar]
- 15. Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, Adami S.. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporos Int 2003; 14:577–82. [DOI] [PubMed] [Google Scholar]
- 16. Shardell M, Semba RD, Kalyani RR, Hicks GE, Bandinelli S, Ferrucci L.. Serum 25-hydroxyvitamin D, plasma klotho, and lower-extremity physical performance among older adults: findings from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 2015; 70:1156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, et al. Vitamin D–binding protein and vitamin d status of black Americans and white Americans. N Engl J Med 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verdoia M, Daffara V, Pergolini P, Olla R, Marino P, Bellomo G, et al. Vitamin D binding protein rs7041 polymorphism and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Vascul Pharmacol 2017; 93–95:42–7. [DOI] [PubMed] [Google Scholar]
- 19. Michos ED, Misialek JR, Selvin E, Folsom AR, Pankow JS, Post WS, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: the ARIC study. Atherosclerosis 2015; 241:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zittermann A, Schleithoff SS, Frisch S, Götting C, Kuhn J, Koertke H, et al. Circulating calcitriol concentrations and total mortality. Clin Chem 2009; 55:1163–70. [DOI] [PubMed] [Google Scholar]
- 21. Verdoia M, Ceccon C, Nardin M, Negro F, Marcolongo M, De Luca G, et al. Polymorphism rs2762939 of CYP24A1 enzyme and coronary artery disease: angiographic results from a large prospective cohort of patients. Blood Coagul Fibrinolysis 2020. doi:10.1097/MBC.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 22. Norman PE, Powel JT.. Vitamin D and cardiovascular disease. Circ Res 2014; 114:379–93. [DOI] [PubMed] [Google Scholar]
- 23. Lajdova I, Chorvat D Jr, Chorvatova A.. Rapid effects of 1alpha, 25(OH)2D3 in resting human peripheral blood mononuclear cells. Eur J Pharmacol 2008; 586:14–23. [DOI] [PubMed] [Google Scholar]
- 24. Shab-Bidar S, Neyestani TR, Djazayery A, Eshraghian M-R, Houshiarrad A, Kalayi A, et al. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 2012; 28:424–30. [DOI] [PubMed] [Google Scholar]
- 25. Mao X, Hu B, Zhou Z, Xing X, Wu Y, Gao J, et al. Vitamin D levels correlate with lymphocyte subsets in elderly patients with age-related diseases. Sci Rep 2018; 8:7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeLuca HF. Evolution of our understanding of vitamin D. Nutr Rev 2008; 66:S73–87. [DOI] [PubMed] [Google Scholar]
- 27. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev 2019; 18:102350. [DOI] [PubMed] [Google Scholar]
- 28. Khare D, Godbole NM, Pawar SD, Mohan V, Pandey G, Gupta S, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr 2013; 52:1405–15. [DOI] [PubMed] [Google Scholar]
- 29. Ameri P, Giusti A, Boschetti M, Murialdo G, Minuto F, Ferone D.. Interactions between vitamin D and IGF-1: from physiology to clinical practice. Clin Endocrinol 2013; 79:457–63. [DOI] [PubMed] [Google Scholar]
- 30. Stach K, Kalsch AI, Nguyen XD, Elmas E, Kralev S, Lang S, et al. 1alpha, 25-Dihydroxyvitamin D3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells. Cardiology 2011; 118:107–15. [DOI] [PubMed] [Google Scholar]
- 31. Kunadian V, Ford GA, Bawamia B, Qiu W, Manson JE.. Vitamin D deficiency and coronary artery disease: a review of the evidence. Am Heart J 2014; 167:283–91. [DOI] [PubMed] [Google Scholar]
- 32. Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, Sudo T, et al. Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem 2004; 279:35798–802. [DOI] [PubMed] [Google Scholar]
- 33. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008; 117:503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verdoia M, Pergolini P, Rolla R, Sartori C, Nardin M, Schaffer A, et al. ; Novara Atherosclerosis Study Group (NAS). Vitamin D levels and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Platelets 2016; 27:576–82. [DOI] [PubMed] [Google Scholar]
- 35. Mao PJ, Zhang C, Tang L, Xian YQ, Li YS, Wang W, et al. Effect of calcium or vitamin D supplementation on vascular outcomes: a meta-analysis of randomized controlled trials. Int J Cardiol 2013; 169:106–1119. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 2019; 366:l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019; 380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M.. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr 2014; 100:746–55. [DOI] [PubMed] [Google Scholar]
- 39. Bonakdaran S, Nejad AF, Abdol-Reza V, Hatefi A, Shakeri M.. Impact of oral 1,25-dihydroxy vitamin D (calcitriol) replacement therapy on coronary artery risk factors in type 2 diabetic patients. Endocr Metab Immune Disord Drug Targets 2014; 13:295–300. [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez-Curiel I, Marin-Luevano P, Trujillo V, Enciso-Moreno JA, Gonzalez-Castillo C, Rivas-Santiago B.. Calcitriol prevents inflammatory gene expression in macrovascular endothelial cells. Br J Biomed Sci 2016; 73:74–8. [DOI] [PubMed] [Google Scholar]
- 41. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and Thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 2020; 75:2950–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pilz S, Tomaschitz A, Ritz E, Pieber TR.. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–30. [DOI] [PubMed] [Google Scholar]
- 43. Nasri H, Behradmanesh S, Ahmadi A, Rafieian-Kopaei M.. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J Nephropathol 2014; 3:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forman JP, Williams JS, Fisher ND.. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin systemin humans. Hypertension 2010; 55:1283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deluque AL, de Almeida LF, Francescato HDC, da Silva C, Costa RS, Antunes-Rodrigues J, et al. Effect of calcitriol on the renal microvasculature differentiation disturbances induced by AT1 blockade during nephrogenesis in rats. Front Med 2020; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soares AE, Maes M, Godeny P, Matsumoto AK, Barbosa DS, da Silva T, et al. Addition of vitamin D reverses the decline in GFR following treatment with ACE inhibitors/angiotensin receptor blockers in patients with chronic kidney disease. Life Sci 2017; 191:175–9. [DOI] [PubMed] [Google Scholar]
- 47. Azizi M, Menard J, Bissery A, Guyenne TT, Bura-Rivière A, Vaidyanathan S, et al. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol 2004; 15:3126–33. [DOI] [PubMed] [Google Scholar]
- 48. Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H.. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep 2017; 16:7432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res 2020; 116:1688–99. doi: 10.1093/cvr/cvaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quraishi SA, Bittner EA, Christopher KB, Camargo CA Jr.. Vitamin D status and community-acquired pneumonia: results from the third National Health and Nutrition Examination Survey. PLoS One 2013; 8:e81120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Upala S, Sanguankeo A, Permpalung N.. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol 2015; 15:84.doi: 10.1186/s12871-015-0063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dancer RC, Parekh D, Lax S, D'Souza V, Zheng S, Bassford CR, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015; 70:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park S, Lee MG, Hong SB, Lim CM, Koh Y, Huh JW.. Effect of vitamin D deficiency in Korean patients with acute respiratory distress syndrome. Korean J Int Med 2018; 33:1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mathyssen C, Gayan-Ramirez G, Bouillon R, Janssens W.. Vitamin D supplementation in respiratory diseases: evidence from randomized controlled trials. Pol Arch Intern Med 2017; 127:775–84. [DOI] [PubMed] [Google Scholar]
- 55. Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 2014; 312:1932. [DOI] [PubMed] [Google Scholar]
- 56. Ginde AA, Brower RG, National Heart, Lung, and Blood Institute PETAL Clinical Trials Network, et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N Engl J Med 2019; 381:2529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017; 356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111:2605–10. [DOI] [PubMed] [Google Scholar]
- 59. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J.. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020; 92: 814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]