Abstract
Background
Early pubertal maturation has been posited to be a biopsychosocial risk factor for the onset of internalizing psychopathology in adolescence; further, early-maturing youths exhibit heightened reactivity to stressful events. School closures and enforced social distancing, as well as health and financial uncertainties, during the COVID-19 pandemic are expected to adversely affect mental health in youths, particularly adolescents who are already at risk for experiencing emotional difficulties. The executive control network (ECN) supports cognitive processes required to successfully navigate novel challenges and regulate emotions in stressful contexts.
Methods
We examined whether functional coherence of the ECN, measured using resting-state functional magnetic resonance imaging 5 years before the pandemic (T1), is a neurobiological marker of resilience to increases in the severity of internalizing symptoms during COVID-19 in adolescents who were in more advanced stages of puberty at T1 relative to their same-age peers (N = 85, 49 female).
Results
On average, participants reported an increase in symptoms from the 3 months before pandemic to the 2 most recent weeks during the pandemic. We found that early-maturing youths exhibited greater increases in internalizing symptoms during the pandemic if their ECN coherence was low; in contrast, relative pubertal stage was not associated with changes in internalizing symptoms in adolescents with higher ECN coherence at T1.
Conclusions
These findings highlight the role of the functional architecture of the brain that supports executive functioning in protecting against risk factors that may exacerbate symptoms of internalizing psychopathology during periods of stress and uncertainty.
Keywords: Adolescence, COVID-19, Executive control network, Internalizing, Puberty, Stress
The incidence of internalizing disorders, such as depression and anxiety, rises sharply throughout adolescence (1). Even at subclinical levels, these often co-occurring emotional difficulties in adolescence (2) predict persistent symptoms (3), poorer physical health (4), and increased risk for suicidal behaviors (5) in adulthood. Importantly, adolescents often report an increase in internalizing symptoms during stressful experiences, particularly those involving health and family discord (6). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or COVID-19) pandemic is a period of uncertainty, financial and familial distress, and enforced social isolation. Financial and health-related ambiguities during COVID-19 are expected to be detrimental to mental health both during and after the pandemic (7,8). Given the added stress of academic disruptions and social distancing during a developmental period in which peers and school environments are often sources of connectedness and support (9), adolescents may be especially susceptible to the stress resulting from COVID-19–related changes and restrictions (10). It is important that we examine factors that contribute to greater risk or vulnerability to the adverse effects of the pandemic. While resilience is typically characterized as the ability to achieve relatively “good” outcomes following risk experiences (11), resilience is also a dynamic process (12) that may be reflected in neural circuits (13). Given restricted access to social support, it may be even more crucial to identify neurobiological protective factors that buffer the influence of adversity and predict positive outcomes.
One risk factor for internalizing psychopathology in adolescence is earlier pubertal maturation (14,15), which could present as earlier pubertal timing (i.e., the estimated chronological age at which a pubertal milestone is reached) or more advanced pubertal staging relative to one’s same-age peers. Particularly, earlier-developing girls, as well as boys (16), have been found to have a higher risk for internalizing symptoms (17), due in part to an interplay between biological and social changes (18) that deviate in timing from those of their typically developing peers (19). Supporting this biopsychosocial interpretation of advanced pubertal maturation as a risk factor for internalizing psychopathology, researchers have found that early-maturing adolescents are more sensitive to interpersonal stress and rejection (20,21) and exhibit heightened responsiveness to stress (22, 23, 24). Similarly, adolescents who have experienced significant life stress tend to have earlier pubertal onset than do their same-age peers (25). It is important to note, however, that not all adolescents exhibit depression and anxiety following earlier pubertal maturation. Rather, the psychosocial consequences of off-time puberty appear to be context dependent. For example, early-maturing adolescents who experience stressful events (vs. those who do not) are more vulnerable to depressive symptoms (26). In addition, high-stress contexts related to peer and family relationships, as well as neighborhood quality (e.g., crime and lower resources), have been found to amplify the association between early pubertal maturation and psychopathology (27, 28, 29). It is clear, however, that more research is needed to examine protective factors that may buffer against the negative effects of advanced pubertal staging.
In this context, researchers are beginning to use neuroimaging to identify biomarkers of risk and resilience related to the onset and course of psychopathology (30). Emerging research suggests that the intrinsic functional architecture of brain networks (i.e., ensembles of brain regions that coactivate when a person is at “rest,” or not engaged in a task) may reflect differential adaptation to stress (31), particularly in individuals at risk for psychopathology (32). One resting-state network that has received attention as a possible neurobiological marker of resilience to risk factors of mental and physical health difficulties is the executive control network (ECN). The ECN includes frontoparietal brain regions (e.g., dorsolateral prefrontal and parietal cortices) that support executive functioning (33, 34, 35), which includes cognitive processes that allow one to successfully navigate and adapt to novel challenges in order to attain long-term goals (36).
However, adolescent studies of internalizing disorders consistently report disruptions in executive functioning, including goal-directed attention and cognitive flexibility (37, 38, 39, 40, 41, 42); these cognitive deficits are shown to underlie concurrent mood states and predict future symptoms (43,44). At the neurobiological level, adolescents with depression (45,46) and anxiety (47) show lower connectivity within the ECN compared with nondepressed peers. Emerging evidence also suggests that more advanced pubertal staging is associated with reduced functional connectivity between brain regions involved in cognitive and emotional processing (48). In addition, girls who experienced earlier pubertal timing have been shown to have lower levels of self-control relative to on-time peers (49). Thus, lower executive functioning and alterations in the ECN may contribute to the increased risk for internalizing symptoms observed in early-developing youths.
The ECN and executive functioning is particularly important during periods of ambiguity or stress, when adaptive reorientation of cognitive processes is necessary to successfully regulate emotional responses to stressors and meet situational demands (50). Miller et al. (51) found that youths with lower within-ECN resting-state connectivity showed an association between higher neighborhood murder rate and greater cardiometabolic risk, but youths with higher ECN connectivity did not exhibit this neighborhood–health risk association. Furthermore, resilient (i.e., nondepressed) adolescent girls at familial risk for depression have been found to have higher within-network ECN connectivity than do both high-risk peers with depression and low-risk peers (52). These ECN-related differences in resilience and susceptibility to stress and psychopathology (53) are likely attributed to behavioral variability in coping and self-regulation, strategies that are supported by executive processes (54).
In the present study, we used independent component analysis (ICA) to spatially identify the ECN based on temporal correlations among voxels in the brain. This data-driven approach has been shown to have higher test-retest reliability than does seed-based analyses (55,56). We tested whether ECN network coherence (the average temporal correlation among voxels in a brain network) 5 years before COVID-19 (T1) moderated the association between more advanced pubertal staging relative to same-age peers at baseline and increases in internalizing symptoms reported by adolescents during COVID-19. Based on initial research showing that youths are reporting more severe symptoms of psychopathology during COVID-19 (57), we expected that, on average, adolescents in our sample would report higher internalizing symptoms during the pandemic compared with the 3 months before the pandemic (hypothesis 1). Given that relatively advanced adolescents in pubertal staging are at greater risk for internalizing psychopathology (17), we expected that, on average, increases in internalizing symptoms during COVID-19 would be larger for those who were in later stages of puberty relative to their age group at T1 (hypothesis 2). Importantly, however, we expected ECN coherence to moderate this association, whereby more advanced pubertal staging would predict greater increases in internalizing symptoms during COVID-19 only in adolescents with lower ECN coherence, but not in those with higher ECN coherence (hypothesis 3).
Methods and Materials
Sample
We recruited 214 children and adolescents from 2013 to 2016 for a longitudinal study assessing the effects of early-life stress (ELS) on psychobiological development throughout the course of puberty (58, 59, 60). Of the 190 participants who successfully underwent resting-state functional magnetic resonance imaging (fMRI) scanning at baseline (T1), 17 were excluded from final analysis owing to motion and image quality constraints. In April 2020, T1 participants were invited to complete a COVID-19–related survey. Of the 102 who provided complete COVID-19 survey data, 86 had usable T1 resting-state data. One participant did not provide responses to the internalizing symptoms questionnaire, so the total sample for the present study consisted of 85 adolescents (49 female) ages 9–13 years (mean = 11.29, SD = 0.92) at T1 and ages 13–19 years (mean = 16.50, SD = 1.28) at the COVID-19 assessment, which was conducted from April 3 to April 20, 2020, ∼2.5–4.5 weeks after the start of the March 17, 2020, San Francisco Bay Area shelter-in-place directive. The interval between the T1 and COVID-19 assessments ranged from 3.72 to 6.54 years (mean = 5.20, SD = 0.70). Detailed information about recruitment procedures and a flowchart of the assessments and time-points are presented in the Supplement and Figure S1.
Measures (T1 and COVID-19 Assessments)
Internalizing Symptoms (T1 Assessment)
Participants completed the Youth Self-Report (61), a widely used measure designed to assess emotional and behavioral problems, including those related to internalizing (e.g., anxious and depressive) behaviors. Adolescents have been shown to provide reliable broadband reports of internalizing symptoms on the Youth Self-Report (62).
Pubertal Status (T1 Assessment)
Participants self-reported on their pubertal status using the Tanner Staging Questionnaire (63), which is significantly correlated with physician ratings of puberty-related physical development (64). We averaged the Tanner pubic hair and breast/testes ratings to compute an index of overall pubertal development (65).
Internalizing Symptom Severity, Stress, and Impact of COVID-19 (COVID-19 Assessment)
To assess the psychological impact of the COVID-19 pandemic and related school closure, social distancing, and shelter-in-place directives, participants completed the Coronavirus Health Impact Survey (v.0.2; https://github.com/nimh-mbdu/CRISIS), developed by researchers at the National Institute of Mental Health and the Child Mind Institute. Our analyses focused on the emotions and worries domain of the Coronavirus Health Impact Survey. Specifically, participants indicated on a 5-point scale (1 = “not at all,” 5 = “extremely”) their levels of 1) worry, 2) happiness versus sadness (reverse-scored), 3) enjoyment in usual activities (reverse-scored), 4) feeling relaxed versus anxious, 5) feeling fidgety or restless, 6) feeling fatigued or tired, 7) concentration, 8) irritability or anger, 9) loneliness, and 10) experiences of negative thoughts. Participants retrospectively rated their levels of emotions and worries in the 3 months before the onset of the COVID-19 crisis in the participants’ local area (pre–COVID-19 symptoms), as well as in the past 2 weeks (peri–COVID-19 symptoms) (10 questions per time interval) (Cronbach’s α = .81 and .87 for the past 3 months and past 2 weeks, respectively). Collectively, these questions relate to depression and general anxiety symptoms in DSM-5 (66); thus, we characterized this measure as an indicator of internalizing symptom severity. We used average severity scores to examine whether participants’ reports of internalizing severity increased significantly from pre– to peri–COVID-19 (hypothesis 1). Then we used the internalizing difference score (average peri–COVID-19 − average pre–COVID-19) to test relative pubertal stage and ECN effects in hypotheses 2 and 3.
A correlation matrix of the T1 and COVID assessment measures is presented in Figure S3; because of our study hypotheses, we focused on the Coronavirus Health Impact Survey internalizing scores.
Cumulative ELS Severity (T1 Assessment)
A modified version of the Traumatic Events Screening Inventory for Children (67) was used to assess the impact of more than 30 types of stressful life experiences (e.g., physical and emotional abuse, domestic violence). A cumulative ELS severity score was computed by summing the maximum objective severity scores (rated by a panel of coders) for each type of endorsed stressor. Full details about this measure are provided in the Supplement.
Socioeconomic Status (T1 Assessment)
To assess socioeconomic status, we estimated income-to-needs ratios for each participant by dividing the caregiver-reported total family income over the previous 12 months by the low-income limit for Santa Clara county (80% of the median income and based on number of people in the household) (59).
Neighborhood Disadvantage (T1 and COVID-19 Assessments)
We used participant-reported addresses to estimate neighborhood quality via census tract information provided by CalEnviroScreen (https://oehha.ca.gov/calenviroscreen), an online tool created by the Office of Environmental Health Hazard Assessment on behalf of the California Environmental Protection Agency. Neighborhood disadvantage was computed using average percentile values of housing burden, unemployment, education, and poverty relative to other census tracts in California (68). If participants moved between the T1 and the COVID-19 assessments, we used their updated addresses to estimate this information.
Resting-State fMRI Scan (T1 Assessment)
The resting-state scan was acquired with an axial echo-planar imaging T2∗-weighted sequence: 180 volumes; echo time = 30 ms, repetition time = 2000 ms, isometric voxel size = 3.2 mm3, slices = 37 (interleaved acquisition), field of view = 224 mm, flip angle = 77°, total scan time = 6 minutes. Details on scan data acquisition parameters and preprocessing [via fMRIPrep (69)] are included in the Supplement .
Coherence of Resting-State Networks
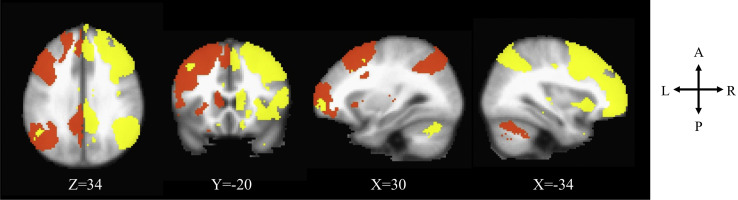
We conducted a group ICA using FSL’s MELODIC (70), specifying 25 components. The ICA decomposes 4-dimensional neuroimaging data into a set of spatial maps, each with associated time courses, yielding a series of 25 networks. We then visually identified the left and right ECNs based on their neuroanatomical components (included regions of the dorsolateral prefrontal cortex and the inferior parietal lobe) in the group-averaged spatial maps. To derive equivalent network components in individual participants, we then conducted a dual regression analysis (56,71,72). The first regression creates a time series for each individual associated with each group-averaged spatial map. The second regresses the participants’ time series into their own resting-state data to create a spatial map for each individual. Each individual’s spatial map is composed of regression weights that represent the functional connectivity between voxels in the group-defined network, regressing out shared variance with other networks. We normalized the functional connectivity values and computed average connectivity from network masks derived from the group-averaged map applied to the individual-level spatial maps, resulting in a network coherence score for each individual (73, 74, 75). Thus, network coherence reflects an average normalized correlation between the time-course of each voxel and all other voxels within the group-identified network. The left and right ECNs are visualized in Figure 1 .
Figure 1.
Left (orange) and right (yellow) executive control networks identified through independent component analysis in Montreal Neurological Institute coordinates.
Data Analysis
We conducted a linear regression in R version 3.6.1 (76) with the internalizing severity difference score (peri–COVID-19 − pre–COVID-19 score) as the response variable, relative pubertal stage (T1) as the main effect, and ECN (average of bilateral ECN coherence values at T1) as a moderator of the effect of pubertal stage. The bilateral average of ECN coherence was used to reduce the number of interaction terms; however, a model testing left and right ECN values as two separate interaction terms with pubertal stage was also tested (Supplement). Additional covariates in our model included age (at T1 and COVID-19 assessments), internalizing symptom severity (T1 and pre–COVID-19), sex, head motion during the scan (i.e., mean framewise displacement), an identified “noise” component from the ICA, ELS severity (T1), socioeconomic status (T1), and neighborhood disadvantage (COVID-19). All variable values were standardized. We also tested the model using residual scores of peri– on pre–COVID-19 internalizing symptoms as the outcome variable, including all aforementioned covariates except pre–COVID-19 symptoms (Supplement).
To test whether other psychologically relevant networks, such as the default mode network (DMN) and salience network (SN) (Figure S2), might also moderate the expected association between more advanced pubertal stage at T1 and increases in internalizing severity from pre– to peri–COVID-19, we ran two additional regression models replacing the ECN with the SN and DMN as potential moderators (Supplement).
Results
Table 1 presents descriptive statistics of all variables; correlations among self-report variables are described in detail in the Supplement and depicted in Figure S3. Briefly, pubertal stage (at T1) was positively correlated with age at the T1 and COVID-19 assessments (both p < .05), boys and girls did not differ in pubertal stage (p > .05), girls showed a positive relation between pubertal stage and internalizing symptoms at T1 (p < .05), and pubertal stage was positively associated with internalizing symptom severity peri–COVID-19 (p < .05), though not pre–COVID-19 (p > .05). Girls reported greater internalizing severity pre– and peri–COVID-19 (both p < .05), though no sex differences were found in internalizing severity at T1. Girls also showed a positive association between ELS severity and pubertal stage at T1 (p < .05).
Table 1.
Sample and Variable Characteristics (N = 85, 49 Female)
| Variable | Mean | Median | Minimum | Maximum | SD |
|---|---|---|---|---|---|
| Age, Years (COVID-19) | 16.54 | 16.62 | 13.82 | 19.34 | 1.30 |
| Age, Years (T1) | 11.29 | 11.20 | 9.47 | 13.72 | 0.92 |
| Pubertal Stage (T1) | 1.84 | 1.50 | 1.00 | 3.50 | 0.67 |
| SES (COVID-19) | 25.15 | 21.88 | 0.75 | 79.50 | 18.79 |
| ELS Severity (T1) | 5.45 | 4.50 | 0.00 | 19.00 | 4.09 |
| Internalizing Symptoms (T1) | 10.18 | 8.00 | 0.00 | 39.00 | 8.24 |
| Internalizing Severity (Pre–COVID-19) | 2.33 | 2.20 | 1.10 | 4.00 | 0.63 |
| Internalizing Severity (Peri–COVID-19) | 2.75 | 2.80 | 1.30 | 4.80 | 0.76 |
| Internalizing Severity Difference (Pre-Peri–COVID-19) | 0.42 | 0.40 | −1.50 | 2.00 | 0.65 |
| ECN_L (T1) | 0.12 | 0.13 | −0.22 | 0.51 | 0.15 |
| ECN_R (T1) | 0.45 | 0.45 | 0.11 | 0.89 | 0.16 |
| ECN_B (T1) | 0.29 | 0.28 | 0.06 | 0.53 | 0.11 |
| Mean FD (T1) | 0.11 | 0.09 | 0.04 | 0.38 | 0.06 |
| Mental Health (Pre–COVID-19) | 2.62 | 2.00 | 1.00 | 5.00 | 1.10 |
| Worry re: Self Infection (COVID-19) | 2.18 | 2.00 | 1.00 | 4.00 | 0.94 |
| Worry re: Family Infection (COVID-19) | 2.69 | 3.00 | 1.00 | 5.00 | 1.13 |
| Worry re: Physical Health (COVID-19) | 2.12 | 2.00 | 1.00 | 5.00 | 1.02 |
| Worry re: Mental Health (COVID-19) | 2.56 | 3.00 | 1.00 | 5.00 | 1.23 |
| Reading and Talking About Virus (COVID-19) | 3.46 | 3.00 | 2.00 | 5.00 | 0.81 |
| Positive Changes (COVID-19) | 2.02 | 2.00 | 1.00 | 3.00 | 0.76 |
| Stress re: Restrictions (COVID-19) | 2.73 | 3.00 | 1.00 | 5.00 | 1.14 |
| Change in Contacts (COVID-19) | 2.54 | 2.00 | 1.00 | 5.00 | 1.46 |
| Difficulty With Restrictions (COVID-19) | 2.28 | 2.00 | 1.00 | 5.00 | 1.08 |
| Change in Family Quality (COVID-19) | 3.05 | 3.00 | 1.00 | 5.00 | 0.80 |
| Stress re: Family Change (COVID-19) | 2.14 | 2.00 | 1.00 | 5.00 | 1.06 |
| Change in Friends Quality (COVID-19) | 2.73 | 3.00 | 1.00 | 4.00 | 0.70 |
| Stress re: Friends Change (COVID-19) | 2.65 | 3.00 | 1.00 | 5.00 | 1.14 |
| Difficulty With Event Changes (COVID-19) | 2.95 | 3.00 | 1.00 | 5.00 | 1.28 |
| Stress re: Financial (COVID-19) | 1.87 | 2.00 | 1.00 | 5.00 | 0.99 |
| Worry re: Living Stability (COVID-19) | 1.59 | 1.00 | 1.00 | 5.00 | 0.89 |
| Hope re: Crisis Ending (COVID-19) | 3.11 | 3.00 | 1.00 | 5.00 | 1.21 |
COVID-19, assessment during the SARS-CoV-2 pandemic; ECN, executive control network; ECN_B, bilateral ECN coherence; ECN_L, left ECN coherence; ECN_R, right ECN coherence; ELS, early-life stress; FD, framewise displacement; SES, socioeconomic status; T1, assessment ∼5 years before COVID-19.
Tests of associations between bilateral ECN coherence and model covariates are described in the Supplement.
Differences Between Pre- and Peri–COVID-19 Internalizing Severity
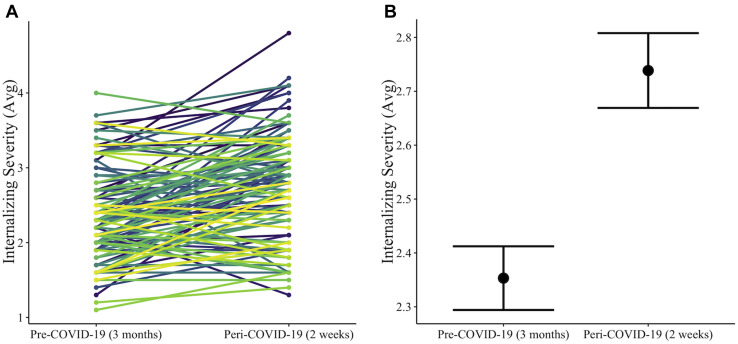
Participants reported an average internalizing severity of 2.33 (on a scale of 1–5) in the 3 months before COVID-19 (pre–COVID-19) and an average 0.42 increase in internalizing severity in the recent 2 weeks (peri–COVID-19) (Figure 2 ). A paired-sample t test revealed a statistically significant difference in means between the pre– and peri–COVID-19 internalizing scores (t 84 = 6.00, p < .0001).
Figure 2.
Differences between participant reports of internalizing severity pre– to peri–COVID-19 showing (A) individual and (B) average differences in severity between the time periods that participants were asked to reflect upon. Colored lines represent individual trajectories of internalizing symptom changes from pre– to peri–COVID-19, and error bars represent standard errors of means.
Main and Interaction Effects of Pubertal Stage and ECN Coherence (at T1) on Differences From Pre- to Peri–COVID-19 Internalizing Symptom Severity
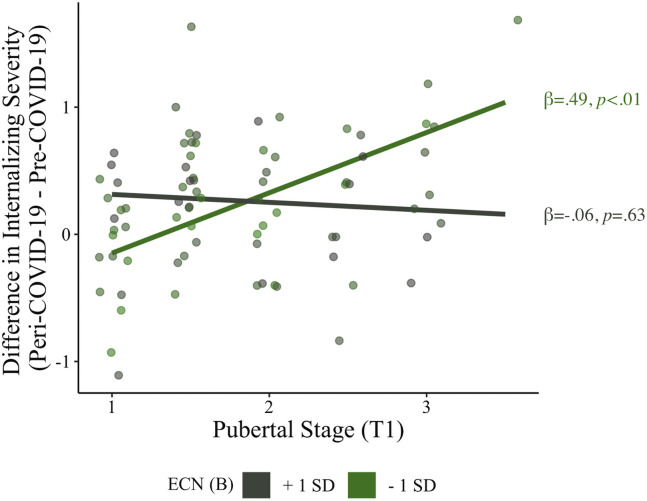
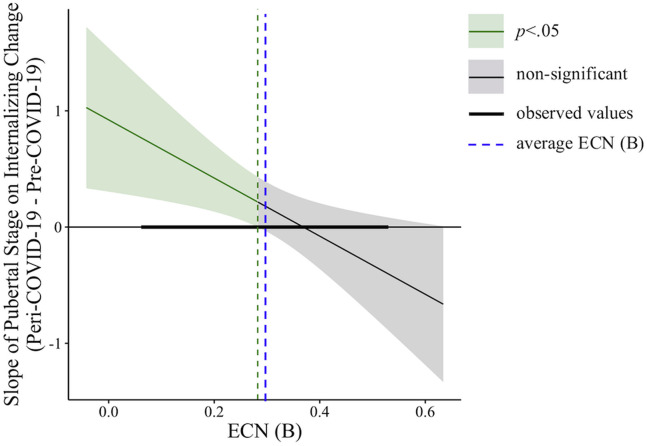
Participants in more advanced stages of puberty at T1 (controlling for age) reported greater increases in internalizing severity from pre– to peri–COVID-19 (β = .21, t 65 = 2.07, p = .04). Importantly, bilateral ECN coherence moderated the association between relative pubertal stage and the difference in internalizing severity between pre– and peri–COVID-19 (β = −.28, t 65 = −2.81, p = .007) (Table 2 ). Simple slope analyses revealed that the positive association between pubertal stage and the difference in internalizing severity from pre– to peri–COVID-19 was significant when bilateral ECN coherence was low (β = .49, p < .01), though not when ECN coherence was high (β = −.06, p = .63) (Figure 3 ; ECN is grouped only for visualization). A Johnson-Neyman plot of continuous predictors showed that more advanced pubertal staging was related to greater internalizing severity increases during the COVID-19 pandemic when ECN coherence was below the average bilateral ECN coherence of the sample (mean = 0.29); participants with higher ECN coherence (i.e., above average) did not show an association between advanced pubertal staging and increased internalizing severity during the pandemic (Figure 4 ). All statistical assumptions of linear regression were met in this model. A model using the pre– to peri–COVID-19 internalizing symptom residual score as the outcome variable yielded the same results as the model described above (Table S1; Figure S4).
Table 2.
Effects of Pubertal Stage and ECN Coherence (at T1) on Internalizing Severity Differences Between Pre– and Peri–COVID-19
| Effect | βa | SE | t | p Value |
|---|---|---|---|---|
| Pubertal Stage (T1) | .21 | .10 | 2.07 | .043 |
| ECN (B) (T1) | .01 | .10 | 0.08 | .938 |
| Scan Noise (T1) | −.11 | .10 | −1.10 | .274 |
| Internalizing Severity (Pre–COVID-19) | −.46 | .09 | −4.87 | .000 |
| Age (COVID-19) | .38 | .19 | 2.00 | .049 |
| Sexb | .53 | .22 | 2.45 | .017 |
| Head Motion During Scan (T1)c | −.08 | .10 | −0.89 | .378 |
| ELS Severity (T1) | .36 | .10 | 3.55 | .001 |
| Neighborhood Disadvantage (COVID-19) | −.13 | .10 | −1.31 | .194 |
| Internalizing Symptoms (T1) | −.16 | .10 | −1.69 | .095 |
| Age (T1) | −.46 | .19 | −2.45 | .017 |
| SES/Income to Needs (T1) | .02 | .10 | 0.18 | .857 |
| Pubertal Stage × ECN (B) | −.28 | .10 | −2.81 | .007 |
| Simple Slopes of Pubertal Stage | ||||
| ECN (B) − 1 SD | .49 | .15 | 3.21 | <.001 |
| ECN (B) + 1 SD | −.06 | .13 | −0.49 | .630 |
COVID-19, assessment during the SARS-CoV-2 pandemic; ECN (B), bilateral executive control network coherence; ELS, early-life stress; SES, socioeconomic status; T1, assessment ∼5 years before COVID-19.
Beta estimates are standardized.
Sex was coded as 1 = males and 2 = females.
Head motion based on mean framewise displacement (mm) during scan.
Figure 3.
Executive control network (ECN) coherence moderates the association between early pubertal maturation and reported differences in pre– to peri–COVID-19 internalizing severity. Pubertal stage and internalizing difference scores are not standardized for visualization. Pubertal stage is relative to same-age peers. ECN is grouped (mean + 1 SD/− 1 SD) only for visualization. The regression model included the interaction of pubertal stage (at T1) and ECN (at T1) (both continuous variables) and the following covariates: age (at T1 and COVID-19 assessments), internalizing severity (at T1 and pre–COVID-19), sex, early-life stress severity (at T1), head motion during the scan (i.e., mean framewise displacement), an identified “noise” component from the independent component analysis (at T1), as well as socioeconomic status and neighborhood disadvantage (at T1). ECN (B), bilateral ECN coherence.
Figure 4.
Johnson-Neyman plot of executive control network (ECN) coherence values (at T1) where the slope between pubertal stage (at T1) and internalizing severity change (during COVID-19) is significant. The positive association between pubertal stage and internalizing severity change was significant (shaded green area) only at values of ECN coherence (shown here as a continuous predictor, as in the model) that were below the sample average (blue dashed line). At above-average ECN values, the association between pubertal stage and internalizing change was not significant (shaded gray area). The green dashed line is the ECN value at which the association between pubertal stage and internalizing severity change goes from significant to nonsignificant. ECN (B), bilateral ECN coherence.
Sensitivity Analyses
Detailed results of sensitivity analyses are described in the Supplement. There was no significant main effect of the DMN or SN or interactions with pubertal stage on the difference between pre– and peri–COVID-19 internalizing severity (Tables S2 and S3). A regression model including separate left and right ECN components revealed that only the left ECN moderated the association between puberty and internalizing symptom severity changes during COVID-19 (Table S4). Finally, as an exploratory analysis given the limited sample size, we tested possible sex effects in our main findings (described in the preceding section) using a regression model including a third interaction term of sex. Boys and girls showed differences in the moderating effects of the ECN on the association between pubertal stage and internalizing changes during COVID-19 (Table S5; Figure S5).
Discussion
Adolescents who experience earlier pubertal maturation relative to their same-age peers are at heightened risk for internalizing psychopathology in adolescence; stressful social contexts, such as a prolonged period of isolation and uncertainty during COVID-19, may be particularly difficult for these high-risk youths. In this study, we identified coherence of the ECN as a potential neurobiological marker of resilience to increases in internalizing symptoms in early-maturing adolescents during COVID-19. As expected, boys and girls who were in more advanced stages of puberty ∼5 years earlier exhibited steeper increases in the severity of internalizing symptoms from before to during COVID-19; however, this effect was present only in youths who had below-average ECN coherence 5 years earlier, in contrast to adolescents with higher ECN coherence. Importantly, the buffering effect of higher ECN coherence on increases in internalizing symptoms during COVID-19 was observable even 3–6 years later and remained significant even after including several related covariates, including baseline internalizing symptoms (at both T1 and pre–COVID-19), age, sex, and familial and neighborhood measures of socioeconomic status in our analyses. Our findings underscore the importance of considering neurobiological indicators of resilience to understand individual differences in susceptibility to psychopathology during periods of stress.
More Advanced Relative Pubertal Staging Is a Risk Factor for Greater Increases in Internalizing Severity During the Pandemic
Consistent with other early reports (57), adolescents in our sample, on average, reported that their internalizing symptoms had worsened from the 3-month period before the crisis to the most recent 2 weeks during the pandemic. A number of reports warned that the combination of disruptions to daily life (e.g., stay-at-home orders and enforced physical distancing when outside of the home), uncertainties related to personal and familial health, and financial stability would contribute to a surge in mental health difficulties during the pandemic (7,8,77), particularly in adolescents with existing mental health difficulties (10). Uniquely, the present study is the first to identify a predictor of risk (i.e., advanced puberty) and a neurobiological marker of resilience (i.e., the ECN) for changes in internalizing symptoms during COVID-19—a sudden and severe period of distress—in a community sample of adolescents.
As we hypothesized, adolescents who were in more advanced stages of puberty reported larger increases in internalizing symptoms during COVID-19. Girls and boys who experience earlier pubertal maturation have been found to be at heightened risk for developing internalizing psychopathology (17,20,21), potentially through interacting biological (e.g., hormonal sensitivity) and psychosocial (e.g., peer sensitivity) processes (22,24,29,78,79). Furthermore, early-maturing adolescents have been shown to exhibit heightened reactivity to stressful situations occurring after puberty (24), suggesting that precocious development is a risk factor for dysregulated stress responses and negative emotionality even after the pubertal phase. Given that the association between off-time maturation and risk for depression has been shown to be mediated by both biological and social processes (22), the steeper increase in COVID-19–related internalizing symptoms in adolescents who were in more advanced stages of puberty relative to their same-age peers at baseline may be a product of both hormonal reactivity to stress and social withdrawal (e.g., via school closure) during the pandemic. Future studies are needed to definitively test these possibilities.
Higher ECN Coherence at T1 Buffers the Risk of More Advanced Relative Pubertal Staging on Internalizing Severity Increases During the Pandemic
The association between more advanced pubertal staging and greater increases in internalizing severity during the pandemic was not significant in adolescents with above-average ECN coherence. Our findings are consistent with previous work showing that higher ECN connectivity protected youths against cardiometabolic risks associated with neighborhood violence (51) and was a marker of resilience to depression in adolescent girls with mothers who have a history of recurrent depression (52). In both of those studies, as well as in our own findings, connectivity of ECN regions differentiated resilient and susceptible adolescents with similar levels of risk for either physical or mental health difficulties. These results demonstrate the specific role of the ECN in promoting resilience to maladaptive outcomes, as neither the DMN nor the SN exhibited effects consistent with a role in buffering against risk factors in any of these studies (including ours). Importantly, while Miller et al. (51) focused on an environmental risk factor and Fischer et al. (52) focused on familial risk for depression, our study is the first to examine a biopsychosocial risk factor—off-time pubertal development—and the buffering role of the ECN against mental health difficulties during a period of major disruption and uncertainty.
The ECN may be a source and/or a reflection of how adolescents respond to novel challenges and stressful situations. For example, the recruitment of ECN regions has been shown to be necessary for cognitive reappraisal (80), an emotional regulatory strategy that involves adaptively reframing an emotion-evoking situation in nonemotional terms (81). In depressed adolescents, these same ECN regions are not as sufficiently recruited or functionally connected during cognitive reappraisal (82) or at rest (46,47), likely contributing to behavioral abnormalities in executive functioning [e.g., (37,44)]. Adolescents with preexisting risk factors for internalizing disorders (e.g., advanced pubertal maturation) may need to strategically recruit ECN regions to navigate stressful situations and dampen emotional responses during challenging and novel contexts.
These findings also have implications for the study of other groups of vulnerable adolescents, including those who have been exposed to ELS. Some work has suggested that among maltreated youths, those with higher prefrontal activation during a cognitive reappraisal task were at lower risk for depression (83). Our supplemental findings also revealed that ELS was associated with more advanced pubertal staging in girls as well as with lower ECN coherence; both of these results are consistent with past research in maltreated adolescents (84,85). Further work is needed to elucidate the mechanisms linking ELS, pubertal development, ECN connectivity, and executive functioning. This research would also clarify the extent to which ECN coherence and executive functioning are markers of resilience in youths who have experienced ELS or more extreme forms of adversity, who may also experience precocious pubertal development, and who are thus at risk for developing internalizing disorders.
Limitations
This study is the first to examine the role of a neurobiological indicator of resilience in understanding the link between advanced pubertal staging (relative to same-age peers)—an established risk factor for psychopathology—and adolescents’ internalizing symptoms during an unprecedented period of stress and uncertainty. Nevertheless, we should note a few limitations of this study. First, our measure of internalizing symptom change was computed based on differences between retrospective self-reports of the 3 months before the pandemic and the 2 most recent weeks during the pandemic. Adolescents likely reported having better mental health before the public health crisis because of the emergence of new stressors related to the pandemic (e.g., having to shelter in place in stressful households or being without face-to-face peer interactions). Despite the limitations in retrospective reporting of symptoms, 3 months is arguably a sufficiently short interval for adolescents to accurately report on their mood states. Moreover, pre–COVID-19 symptoms were correlated with internalizing symptoms at baseline, whereas peri–COVID-19 symptoms were not, suggesting that adolescents’ retrospective reports were valid and reflective of baseline levels. Second, we did not directly measure executive functioning, which would have increased our understanding of the role of the ECN in predicting youths who experienced worsening internalizing symptoms during the pandemic. Third, our pubertal staging measure was administered at baseline but not at the COVID-19 assessment, in part because the sample had a mean age of 16.54 years during the COVID-19 assessment, and, thus, most adolescents would have reported that the pubertal process was complete. Similarly, our ECN coherence measure was obtained only at baseline. Although it would be helpful to also test the concurrent impact of the ECN on puberty-related differences in internalizing symptom changes during the pandemic, we were not permitted to scan during the shelter-in-place directive. However, our findings are consistent with those reported in previous work showing that even in early adolescence, connectivity within this network predicts later psychological and cognitive functioning (86, 87, 88). Finally, our resting-state fMRI scan was 6 minutes. Although the reliability of resting-state fMRI connectivity has been shown to improve with longer scan times [e.g., 12–16 minutes (89)], other work suggests that connectivity estimates stabilize with acquisition times as brief as 5 minutes (90). Future work will benefit from conducting longer scan times to estimate more reliable individual-level neuroimaging predictors of resilience.
Conclusions
Early pubertal maturation is a well-known biopsychosocial risk factor for the onset of internalizing psychopathology in adolescence: even after puberty, early-maturing youths exhibit heightened reactivity to stressful events. The COVID-19 pandemic is an unprecedented period of stress, particularly for adolescents, with school closures, social distancing directives, and health and financial uncertainties presenting novel challenges for youths who are now experiencing a deficit in social connectedness and academic stimulation. We examined whether ECN coherence, measured 5 years before the pandemic, serves as a potential marker of resilience to increases in internalizing symptom severity during COVID-19 in adolescents who were in more advanced stages of puberty relative to their same-age peers. Youths who were in more advanced stages of puberty for their age exhibited greater increases in internalizing symptoms during the pandemic only if their ECN coherence was below average; however, this association was absent in adolescents with higher ECN coherence. These findings highlight the role of the ECN in buffering against risk factors that may exacerbate symptoms of internalizing psychopathology during periods of stress and uncertainty.
Acknowledgments and Disclosures
This research was supported by the National Institutes of Health (Grant Nos. R37MH101495 [to IHG], F32MH120975 [to RC], K01MH117442 [to TCH], and T32MH019908 [Allan Reiss, principal investigator (funding JGM)]) and the Stanford University Precision Health and Integrated Diagnostics Center (to IHG, JSK, and TCH). The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
IHG designed the research; RC, JSK, JM, TCH, and IHG performed the research; RC analyzed the data; and RC, JSK, JM, TCH, and IHG wrote the paper.
We thank Rachel Weisenburger and Johanna Walker for their assistance with data collection and organization. We also thank the participants and their families for participating in this study.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsc.2020.08.010.
Supplementary Material
References
- 1.Petersen I.T., Lindhiem O., LeBeau B., Bates J.E., Pettit G.S., Lansford J.E., Dodge K.A. Development of internalizing problems from adolescence to emerging adulthood: Accounting for heterotypic continuity with vertical scaling. Dev Psychol. 2018;54:586–599. doi: 10.1037/dev0000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hankin B.L., Snyder H.R., Gulley L.D., Schweizer T.H., Bijttebier P., Nelis S., et al. Understanding comorbidity among internalizing problems: Integrating latent structural models of psychopathology and risk mechanisms. Dev Psychopathol. 2016;28:987–1012. doi: 10.1017/S0954579416000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winefield H.R., Hammarström A., Nygren K., Hägglöf B. Internalized symptoms in adolescence as predictors of mental health in adulthood in the Northern Swedish cohort. Health. 2013;5:1164–1171. [Google Scholar]
- 4.Jamnik M.R., DiLalla L.F. Health outcomes associated with internalizing problems in early childhood and adolescence. Front Psychol. 2019;10:60. doi: 10.3389/fpsyg.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fergusson D.M., Horwood L.J., Ridder E.M., Beautrais A.L. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin K.A., Hatzenbuehler M.L. Stressful life events, anxiety sensitivity, and internalizing symptoms in adolescents. J Abnorm Psychol. 2009;118:659–669. doi: 10.1037/a0016499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galea S., Merchant R.M., Lurie N. The mental health consequences of COVID-19 and physical distancing: The need for prevention and early intervention. JAMA Intern Med. 2020;180:817–818. doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- 8.Holmes E.A., O’Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore G.F., Cox R., Evans R.E., Hallingberg B., Hawkins J., Littlecott H.J., et al. School, peer and family relationships and adolescent substance use, subjective wellbeing and mental health symptoms in Wales: A cross sectional study. Child Indic Res. 2018;11:1951–1965. doi: 10.1007/s12187-017-9524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golberstein E., Wen H., Miller B.F. Coronavirus disease 2019 (COVID-19) and mental health for children and adolescents. JAMA Pediatr. 2020;174:819–820. doi: 10.1001/jamapediatrics.2020.1456. [DOI] [PubMed] [Google Scholar]
- 11.Rutter M. Implications of resilience concepts for scientific understanding. Ann N Y Acad Sci. 2006;1094:1–12. doi: 10.1196/annals.1376.002. [DOI] [PubMed] [Google Scholar]
- 12.Rutter M. Resilience as a dynamic concept. Dev Psychopathol. 2012;24:335–344. doi: 10.1017/S0954579412000028. [DOI] [PubMed] [Google Scholar]
- 13.Holz N.E., Tost H., Meyer-Lindenberg A. Resilience and the brain: A key role for regulatory circuits linked to social stress and support. Mol Psychiatry. 2020;25:379–396. doi: 10.1038/s41380-019-0551-9. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton J.L., Hamlat E.J., Stange J.P., Abramson L.Y., Alloy L.B. Pubertal timing and vulnerabilities to depression in early adolescence: Differential pathways to depressive symptoms by sex. J Adolesc. 2014;37:165–174. doi: 10.1016/j.adolescence.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y., Styne D. Influences on the onset and tempo of puberty in human beings and implications for adolescent psychological development. Horm Behav. 2013;64:250–261. doi: 10.1016/j.yhbeh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Galvao T.F., Silva M.T., Zimmermann I.R., Souza K.M., Martins S.S., Pereira M.G. Pubertal timing in girls and depression: A systematic review. J Affect Disord. 2014;155:13–19. doi: 10.1016/j.jad.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Ullsperger J.M., Nikolas M.A. A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk? Psychol Bull. 2017;143:903–938. doi: 10.1037/bul0000106. [DOI] [PubMed] [Google Scholar]
- 18.Berenbaum S.A., Beltz A.M., Corley R. The importance of puberty for adolescent development. Adv Child Dev Behav. 2015;48:53–92. doi: 10.1016/bs.acdb.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Petersen A.C., Taylor B. In: Handbook of Adolescent Psychology. Adelson J., editor. Wiley; New York: 1980. The biological approach to adolescence: Biological change and psychological adaptation; pp. 117–155. [Google Scholar]
- 20.McGuire T.C., McCormick K.C., Koch M.K., Mendle J. Pubertal maturation and trajectories of depression during early adolescence. Front Psychol. 2019;10:1362. doi: 10.3389/fpsyg.2019.01362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natsuaki M.N., Klimes-Dougan B., Xiaojia G., Shirtcliff E.A., Hastings P.D., Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. J Clin Child Adolesc Psychol. 2009;38:513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copeland W.E., Worthman C., Shanahan L., Costello E.J., Angold A. Early pubertal timing and testosterone associated with higher levels of adolescent depression in girls. J Am Acad Child Adolesc Psychiatry. 2019;58:1197–1206. doi: 10.1016/j.jaac.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahl R.E., Gunnar M.R. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- 24.Smith A.E., Powers S.I. Off-time pubertal timing predicts physiological reactivity to post-puberty interpersonal stress. J Res Adolesc. 2009;19:441–458. doi: 10.1111/j.1532-7795.2009.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gur R.E., Moore T.M., Rosen A.F.G., Barzilay R., Roalf D.R., Calkins M.E., et al. Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry. 2019;76:966–975. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge X., Conger R.D., Elder G.H. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–417. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- 27.Natsuaki M.N. Puberty in context: Toward a more nuanced understanding of early maturation. J Adolesc Health. 2013;53:677–678. doi: 10.1016/j.jadohealth.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Obeidallah D., Brennan R.T., Brooks-gunn J., Earls F. Links between pubertal timing and neighborhood contexts: Implications for girls’ violent behavior. J Am Acad Child Adolesc Psychiatry. 2004;43:1460–1468. doi: 10.1097/01.chi.0000142667.52062.1e. [DOI] [PubMed] [Google Scholar]
- 29.Winer J.P., Parent J., Forehand R., Breslend N.L. Interactive effects of psychosocial stress and early pubertal timing on youth depression and anxiety: Contextual amplification in family and peer environments. J Child Fam Stud. 2016;25:1375–1384. doi: 10.1007/s10826-015-0318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chahal R., Gotlib I.H., Guyer A.E. Research Review: Brain network connectivity and the heterogeneity of depression in adolescence: A precision mental health perspective. J Child Psychol Psychiatry. 2020;61:1282–1298. doi: 10.1111/jcpp.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iadipaolo A.S., Marusak H.A., Paulisin S.M., Sala-Hamrick K., Crespo L.M., Elrahal F., et al. Distinct neural correlates of trait resilience within core neurocognitive networks in at-risk children and adolescents. Neuroimage Clin. 2018;20:24–34. doi: 10.1016/j.nicl.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cisler J.M., James G.A., Tripathi S., Mletzko T., Heim C., Hu X.P., et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013;43:507–518. doi: 10.1017/S0033291712001390. [DOI] [PubMed] [Google Scholar]
- 33.Cole M.W., Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 34.Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullin B.C., Perks E.L., Haraden D.A., Snyder H.R., Hankin B.L. Subjective executive function weaknesses are linked to elevated internalizing symptoms among community adolescents. Assessment. 2018;27:560–571. doi: 10.1177/1073191118820133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller A.S., Leikauf J.E., Holt-Gosselin B., Staveland B.R., Williams L.M. Paying attention to attention in depression. Transl Psychiatry. 2019;9:1–12. doi: 10.1038/s41398-019-0616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joormann J., Levens S.M., Gotlib I.H. Sticky thoughts: Depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychol Sci. 2011;22:979–983. doi: 10.1177/0956797611415539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everaert J., Grahek I., Koster E.H.W. Individual differences in cognitive control over emotional material modulate cognitive biases linked to depressive symptoms. Cogn Emot. 2017;31:736–746. doi: 10.1080/02699931.2016.1144562. [DOI] [PubMed] [Google Scholar]
- 41.Pe M.L., Brose A., Gotlib I.H., Kuppens P. Affective updating ability and stressful events interact to prospectively predict increases in depressive symptoms over time. Emotion. 2016;16:73–82. doi: 10.1037/emo0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner S., Müller C., Helmreich I., Huss M., Tadić A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur Child Adolesc Psychiatry. 2015;24:5–19. doi: 10.1007/s00787-014-0559-2. [DOI] [PubMed] [Google Scholar]
- 43.Raes F., Verstraeten K., Bijttebier P., Vasey M.W., Dalgleish T. Inhibitory control mediates the relationship between depressed mood and overgeneral memory recall in children. J Clin Child Adolesc Psychol. 2010;39:276–281. doi: 10.1080/15374410903532684. [DOI] [PubMed] [Google Scholar]
- 44.Joormann J., Gotlib I.H. Emotion regulation in depression: Relation to cognitive inhibition. Cogn Emot. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang S., Lu L., Zhang L., Hu X., Bu X., Li H., et al. Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine. 2018;36:436–445. doi: 10.1016/j.ebiom.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sacchet M.D., Ho T.C., Connolly C.G., Tymofiyeva O., Lewinn K.Z., Han L.K., et al. Large-scale hypoconnectivity between resting-state functional networks in unmedicated adolescent major depressive disorder. Neuropsychopharmacology. 2016;41:2951–2960. doi: 10.1038/npp.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang F., Fan L., Zhai T., Lin Y., Wang Y., Ma J., et al. Decreased intrinsic functional connectivity in first-episode, drug-naive adolescents with generalized anxiety disorder. Front Hum Neurosci. 2019;12:539. doi: 10.3389/fnhum.2018.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T., Lu Y., Wang Y., Guo A., Xie X., Fu Y., et al. Altered brain structure and functional connectivity associated with pubertal hormones in girls with precocious puberty. Neural Plast. 2019;2019:1465632. doi: 10.1155/2019/1465632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaku N., Hoyt L.T. Developmental trajectories of executive functioning and puberty in boys and girls. J Youth Adolesc. 2019;48:1365–1378. doi: 10.1007/s10964-019-01021-2. [DOI] [PubMed] [Google Scholar]
- 50.Gabrys R.L., Tabri N., Anisman H., Matheson K. Cognitive control and flexibility in the context of stress and depressive symptoms: The cognitive control and flexibility questionnaire. Front Psychol. 2018;9:2219. doi: 10.3389/fpsyg.2018.02219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller G.E., Chen E., Armstrong C.C., Carroll A.L., Ozturk S., Rydland K.J., et al. Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proc Natl Acad Sci U S A. 2018;115:12063–12068. doi: 10.1073/pnas.1810067115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischer A.S., Camacho M.C., Ho T.C., Whitfield-Gabrieli S., Gotlib I.H. Neural markers of resilience in adolescent females at familial risk for major depressive disorder. JAMA Psychiatry. 2018;75:493–502. doi: 10.1001/jamapsychiatry.2017.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisenberg N., Spinrad T.L., Eggum N.D. Emotion-related self-regulation and its relation to children’s maladjustment. Annu Rev Clin Psychol. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reising M.M., Bettis A.H., Dunbar J.P., Watson K.H., Gruhn M., Hoskinson K.R., Compas B.E. Stress, coping, executive function, and brain activation in adolescent offspring of depressed and nondepressed mothers. Child Neuropsychol. 2018;24:638–656. doi: 10.1080/09297049.2017.1307950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith D.V., Utevsky A.V., Bland A.R., Clement N., Clithero J.A., Harsch A.E.W., et al. Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. Neuroimage. 2014;95:1–12. doi: 10.1016/j.neuroimage.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo X.-N., Kelly C., Adelstein J.S., Klein D.F., Castellanos F.X., Milham M.P. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee J. Mental health effects of school closures during COVID-19. Lancet Child Adolesc Health. 2020;4:421. doi: 10.1016/S2352-4642(20)30109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humphreys K.L., Kircanski K., Colich N.L., Gotlib I.H. Attentional avoidance of fearful facial expressions following early life stress is associated with impaired social functioning. J Child Psychol Psychiatry. 2016;57:1174–1182. doi: 10.1111/jcpp.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King L.S., Humphreys K.L., Camacho M.C., Gotlib I.H. A person-centered approach to the assessment of early life stress: Associations with the volume of stress-sensitive brain regions in early adolescence. Dev Psychopathol. 2019;31:643–655. doi: 10.1017/S0954579418000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller J.G., Ho T.C., Humphreys K.L., King L.S., Foland-Ross L.C., Colich N.L., et al. Early life stress, frontoamygdala connectivity, and biological aging in adolescence: A longitudinal investigation. Cereb Cortex. 2020;30:4269–4280. doi: 10.1093/cercor/bhaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Achenbach T.M. Department of Psychiatry, University of Vermont; Burlington: 1991. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. [Google Scholar]
- 62.Ebesutani C., Bernstein A., Martinez J.I., Chorpita B.F., Weisz J.R. The Youth Self Report: Applicability and validity across younger and older youths. J Clin Child Adolesc Psychol. 2011;40:338–346. doi: 10.1080/15374416.2011.546041. [DOI] [PubMed] [Google Scholar]
- 63.Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 64.Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dorn L.D., Dahl R., Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl Dev Sci. 2006;10:30–56. [Google Scholar]
- 66.American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 67.Ribbe D. In: Measurement of Stress, Trauma, and Adaptation. Stamm B.H., editor. Sidran Press; Lutherville, MD: 1996. Psychometric review of Traumatic Events Screening Inventory for Children (TESI-C) pp. 386–387. [Google Scholar]
- 68.Miller J.G., Gillette J.S., Manczak E.M., Kircanski K., Gotlib I.H. Fine particle air pollution and physiological reactivity to social stress in adolescence: The moderating role of anxiety and depression. Psychosom Med. 2019;81:641–648. doi: 10.1097/PSY.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat Meth. 2019;16:111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Beckmann C.F. Modelling with independent components. Neuroimage. 2012;62:891–901. doi: 10.1016/j.neuroimage.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 72.Filippini N., MacIntosh B.J., Hough M.G., Goodwin G.M., Frisoni G.B., Smith S.M., et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ordaz S.J., Goyer M.S., Ho T.C., Singh M.K., Gotlib I.H. Network basis of suicidal ideation in depressed adolescents. J Affect Disord. 2018;226:92–99. doi: 10.1016/j.jad.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ordaz S.J., LeMoult J., Colich N.L., Prasad G., Pollak M., Popolizio M., et al. Ruminative brooding is associated with salience network coherence in early pubertal youth. Soc Cogn Affect Neurosci. 2016;12:298–310. doi: 10.1093/scan/nsw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz J., Ordaz S.J., Ho T.C., Gotlib I.H. Longitudinal decreases in suicidal ideation are associated with increases in salience network coherence in depressed adolescents. J Affect Disord. 2019;245:545–552. doi: 10.1016/j.jad.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing.https://www.R-project.org Available at: [Google Scholar]
- 77.Gunnell D., Appleby L., Arensman E., Hawton K., John A., Kapur N., et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry. 2020;7:468–471. doi: 10.1016/S2215-0366(20)30171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marceau K., Dorn L.D., Susman E.J. Stress and puberty-related hormone reactivity, negative emotionality, and parent-adolescent relationships. Psychoneuroendocrinology. 2012;37:1286–1298. doi: 10.1016/j.psyneuen.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Sontag-Padilla L.M., Dorn L.D., Tissot A., Susman E.J., Beers S.R., Rose S.R. Executive functioning, cortisol reactivity, and symptoms of psychopathology in girls with premature adrenarche. Dev Psychopathol. 2012;24:211–223. doi: 10.1017/S0954579411000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Messina I., Bianco S., Sambin M., Viviani R. Executive and semantic processes in reappraisal of negative stimuli: Insights from a meta-analysis of neuroimaging studies. Front Psychol. 2015;6:956. doi: 10.3389/fpsyg.2015.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gross J.J. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 82.LeWinn K.Z., Strigo I.A., Connolly C.G., Ho T.C., Tymofiyeva O., Sacchet M.D., et al. An exploratory examination of reappraisal success in depressed adolescents: Preliminary evidence of functional differences in cognitive control brain regions. J Affect Disord. 2018;240:155–164. doi: 10.1016/j.jad.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 83.Rodman A.M., Jenness J.L., Weissman D.G., Pine D.S., McLaughlin K.A. Neurobiological markers of resilience to depression following childhood maltreatment: The role of neural circuits supporting the cognitive control of emotion. Biol Psychiatry. 2019;86:464–473. doi: 10.1016/j.biopsych.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hart H., Lim L., Mehta M.A., Chatzieffraimidou A., Curtis C., Xu X., et al. Reduced functional connectivity of fronto-parietal sustained attention networks in severe childhood abuse. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mendle, Leve L.D., Van Ryzin M., Natsuaki M.N., Ge X. Associations between early life stress, child maltreatment, and pubertal development among girls in foster care. J Res Adolesc. 2011;21:871–880. doi: 10.1111/j.1532-7795.2011.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chahal R., Weissman D.G., Hallquist M.N., Robins R.W., Hastings P.D., Guyer A.E. Neural connectivity biotypes: Associations with internalizing problems throughout adolescence [published online ahead of print May 29] Psychol Med. 2020 doi: 10.1017/S003329172000149X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui Z., Li H., Xia C.H., Larsen B., Adebimpe A., Baum G.L., et al. Individual variation in functional topography of association networks in youth. Neuron. 2020;106:340–353.e8. doi: 10.1016/j.neuron.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jalbrzikowski M., Liu F., Foran W., Calabro F., Roeder K., Devlin B., Luna B. Cognitive and default mode networks support developmental stability in functional connectome fingerprinting through adolescence. bioRxiv. 2019 doi: 10.1101/812719. [DOI] [Google Scholar]
- 89.Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.